0001837607
false
Priveterra Acquisition Corp.
0001837607
2023-07-24
2023-07-24
0001837607
dei:FormerAddressMember
2023-07-24
2023-07-24
0001837607
us-gaap:CommonStockMember
2023-07-24
2023-07-24
0001837607
us-gaap:WarrantMember
2023-07-24
2023-07-24
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
July 24, 2023
AEON BIOPHARMA, INC.
(Exact Name of Registrant as Specified in Charter)
| Delaware |
|
001-40021 |
|
85-3940478 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
5 Park Plaza, Suite 1750
Irvine, California
(Address of Principal Executive Offices) |
92614
(Zip Code) |
(949)
354-6499
(Registrant’s telephone number, including area code)
Priveterra Acquisition Corp.
300 SE 2nd Street, Suite 600
Fort
Lauderdale, Florida 33301
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of
the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which
registered |
| Class
A Common Stock, $0.0001 par value per share |
AEON |
The New York Stock Exchange American |
| Warrants,
each whole warrant exercisable to purchase one share of Class A Common Stock at an exercise price of $11.50 per
share |
AEON WS |
The New York Stock Exchange American |
Indicate by check mark whether the registrant is an emerging growth
company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities
Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate
by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial
accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 7.01 |
Regulation FD Disclosure. |
Representatives of AEON Biopharma, Inc. (the
“Company”) intend to make presentations at investor conferences and in other forums and these presentations may include the
information contained in Exhibit 99.1 attached to this Current Report on Form 8-K (the “Investor Presentation”).
A copy of the Investor Presentation containing such information that may be disclosed by the Company is attached as Exhibit 99.1
to this report and the information set forth therein is incorporated herein by reference and constitutes a part of this report. The Company
intends to disclose the information contained in the Investor Presentation, in whole or in part, and with updates and possibly modifications,
in connection with presentations to investors, analysts and others and on its corporate website.
The Company is furnishing the information contained
in Exhibit 99.1 pursuant to Regulation FD and Item 7.01 of Form 8-K promulgated by the Securities and Exchange Commission (“SEC”).
This information shall not be deemed to be “filed” with the SEC for the purposes of Section 18 of the Securities Exchange
Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed
to be incorporated by reference into any registration statement or other document filed under the Securities Act of 1933, as amended,
or the Exchange Act, except as expressly set forth by specific reference in such filing.
The information contained in Exhibit 99.1
is summary information that is intended to be considered in the context of the Company’s SEC filings and other public announcements
that the Company may make, by press release or otherwise, from time to time. The Company undertakes no duty or obligation to publicly
update or revise the information contained in Exhibit 99.1, although it may do so from time to time as its management believes is
warranted. Any such updating may be made through the filing of other reports or documents with the SEC, through press releases or through
other public disclosure. By filing this report and furnishing this information, the Company makes no admission as to the materiality of
any information contained in this report, including Exhibit 99.1.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURE
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Dated: July 24, 2023 |
|
AEON BIOPHARMA, INC. |
| |
|
|
|
| |
|
By: |
/s/ Marc Forth |
| |
|
Name: |
Marc Forth |
| |
|
Title: |
Chief Executive Officer |
Exhibit 99.1

Corporate Presentation July 2023

2 Forward Looking Statements This presentation includes forward - looking statements . All statements other than statements of historical facts contained in this presentation, including statements concerning possible or assumed future actions, business strategies, events or results of operations, and any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward - looking statements . These statements may involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements . These statements may be preceded by, followed by or include the words “believes”, “estimates”, “expects”, “projects”, “forecasts”, “may”, “will”, “should”, “seeks”, “plans”, “scheduled”, “anticipates” or “intends” or similar expressions . The forward - looking statements in this presentation are only predictions . We have based these forward - looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations . These forward - looking statements are based upon estimates and assumptions that, while considered reasonable by AEON and its management, are inherently uncertain . Factors that may cause actual results to differ materially from current expectations include, but are not limited to : (i) the outcome of any legal proceedings that may be instituted against AEON or others following the announcement of the business combination and any definitive agreements with respect thereto ; (ii) the ability to meet continued stock exchange listing standards following the consummation of the business combination ; (iii) the risk that the business combination disrupts current plans and operations of AEON as a result of the announcement and consummation of the business combination ; (iv) the ability to recognize the anticipated benefits of the business combination, which may be affected by, among other things, competition, the ability of AEON to grow and manage growth profitably, maintain relationships with customers and suppliers and retain its management and key employees ; (v) costs related to the business combination and being a public company ; (vi) changes in applicable laws or regulations ; (vii) the possibility that AEON may be adversely affected by other economic, business, regulatory, and/or competitive factors ; (viii) AEON’s estimates of expenses and profitability ; (ix) the evolution of the markets in which AEON competes ; (x) the ability of AEON to implement its strategic initiatives, including the continued development of ABP - 450 ; (xi) the ability of AEON to defend its intellectual property ; (xii) the ability of AEON to satisfy regulatory requirements ; (xiii) the impact of the COVID - 19 pandemic on AEON’s business ; (xiv) AEON’s expected capital resources and liquidity needs ; and (xv) other risks and uncertainties set forth in the section entitled "Risk Factors" and "Cautionary Note Regarding Forward - Looking Statements" in the company’s final proxy statement/prospectus relating to the business combination . Because forward - looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward - looking statements as predictions of future events . The events and circumstances reflected in our forward - looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward - looking statements . Moreover, we operate in an evolving environment and a competitive industry . New risks and uncertainties may emerge from time to time, and it is not possible for management to predict all risks and uncertainties, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward - looking statements we may make in this presentation . As a result of these factors, although we believe that the expectations reflected in our forward - looking statements are reasonable, we cannot assure you that the forward - looking statements in this presentation will prove to be accurate . Except as required by applicable law, we do not plan to publicly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances, or otherwise . We qualify all of our forward - looking statements by these cautionary statements . You should view this presentation completely and with the understanding that the actual future results, levels of activity, performance, events and circumstances of AEON may be materially different from what is expected .

3 Investment Highlights Botulinum toxin has demonstrated utility across dozens of published uses expected to be accessible to ABP - 450; accelerated and simplified commercialization timeline due to existing aesthetic BLA (separately held by Evolus) and manufacturing site approved by FDA, EMA and Health Canada Episodic migraine approval would nearly triple the current addressable patient population (Botox ® indicated for chronic migraine only); ABP - 450 Phase 2 migraine study includes chronic and episodic migraine patients with a streamlined injection protocol with potential to enhance safety and tolerability Potential to pursue traditional pharma pricing model currently not pursued by therapeutic toxin competition with a 900 kDa botulinum toxin expected to facilitate physician adoption Significant neurotoxin commercial and clinical development experience among management team translating to approximately $162M invested in AEON since 2019 Strong Potential Episodic Migraine Market Expansion Business Model Advantage Strong Leadership & Sponsorship Market Ripe for Competition Single existing competitor for key target markets (episodic & chronic migraine); unlike in aesthetic market, ABP - 450’s therapeutic focus will compete with market leader on a product - to - product basis, not against a portfolio of products PTSD Opportunity Ongoing pre - clinical program utilizing proprietary injection paradigm in a novel part of the anatomy is designed to provide IND supporting safety and efficacy data; this innovative approach has the potential for utility across a broad Neuro/Psych portfolio including PTSD

4 Leadership Team with Highly Relevant Industry Experience and Track Record of Success Experienced Management Team Marc Forth Chad Oh, MD Chief Executive Officer Chief Medical Officer • 25+ years of Biopharma experience • Former US Business Lead for BOTOX ® Therapeutic • 16 years at Allergan dedicated to the entire BOTOX ® franchise • 7 years at TAP Pharmaceuticals responsible for Lupron Depot (Urology, Oncology and Gynecology) • 30+ years of combined experience in academia and the pharmaceutical industry • Responsible for multiple IND, NDA, and BLA submissions • Chief, Division of Allergy and Immunology at Harbor - UCLA Medical Center • Associate Professor, Department of Pediatrics at UCLA School of Medicine • Published multiple scientific papers, books, book chapters, and abstracts, including 38 peer - reviewed original scientific papers • 10+ years of legal experience in corporate governance, mergers & acquisitions and capital markets • Associate General Counsel of Glaukos Corporation, responsible for business development activities, capital markets, corporate governance and SEC reporting • Counsel at O’Melveny & Myers • B.S. in Business Management, Brigham Young University and J.D. from the UCLA School of Law EVP, Chief Legal Officer & Secretary Alex Wilson Peter Reynolds Acting Chief Financial Officer • 25+ years of financial experience in Healthcare, Medical Device, and Aesthetic Toxins • Senior Finance roles with both Publicly Traded and Privately held companies • Significant experience in mergers, acquisitions and public offerings

5 Botulinum Toxin: The Ultimate Platform Product

6 Global Therapeutic Toxin Landscape AbbVie Inc. Merz Pharma Ipsen Group Molecular Size 900 kDa 900 kDa 150 kDa ~400 kDa 150 kDa Approved Indications 1. Blepharospasm 2. Strabismus 3. Cervical Dystonia 4. Hyperhidrosis 5. Spasticity 6. Chronic Migraine 7. Overactive Bladder 8. Neurogenic Detrusor Overactivity (adult and pediatric) None 1. Blepharospasm 2. Cervical Dystonia 3. Adult Spasticity (Upper Limb) 4. Chronic Sialorrhea 1. Cervical Dystonia 2. Spasticity None In Development 1. Atrial Fibrillation 2. Episodic Migraine 3. Essential Tremor 4. IC/BPS 1. Migraine (episodic & chronic) 2. Cervical Dystonia 3. Gastroparesis 4. Posttraumatic Stress Disorder (PTSD) Undisclosed 1. Neurogenic Detrusor Overactivity 1. Cervical Dystonia 2. Adult Spasticity (Upper Limb) FDA Approved US Share 95% 2% 2% Sources: Decision Resources Group Therapeutic Botulinum Toxin Market Analysis Global 2021

7 Indication Current Phase 2020 2021 2022 2023 2024 Recent/Upcoming Milestones 1H 2H 1H 2H 1H 2H 1H 2H 1H 2H ABP - 450 Episodic Migraine 1 Phase 2 ongoing x IND accepted Oct 2020 x First patient treated Mar 2021 x Enrollment completed Dec 2022 • P2 data anticipated - Fall 2023 Chronic Migraine 2 x IND accepted Oct 2020 x First patient treated Mar 2021 • P2 data anticipated 2024 Cervical Dystonia Phase 2 completed x IND accepted Sep 2020 x First patient treated Apr 2021; last patient last visit Jul 2022 x P2 data reported Sep 2022 • P3 initiation anticipated 2024 Gastroparesis Phase 2 ready x IND accepted 2022 Posttraumatic Stress Disorder Preclinical x Safety assessment ongoing ABP - 450 Indications: Pipeline in a Product 1. Episodic: <15 headache days AND 6 - 14 migraine days / month 2. Chronic: >15 headache days AND >8 migraine days / month

8 Global Therapeutic Toxin Market – 9.9% Projected Growth Through 2027 • Current indications: Organic growth in current indications driven primarily by continued investment in disease awareness and growing patient populations • New indications: Development in therapeutic specialties that do not currently have a toxin treatment option • Improved reimbursement: Favorable dynamics to facilitate coverage at current and projected pricing levels Anticipated Volume Growth Drivers $2.3B $2.7B $3.0B $3.2B $3.5B $3.8B $4.1B $4.4B 2020A 2021E 2022E 2023E 2024E 2025E 2026E 2027E 2.7M procedures $557 ASP (US) 5.0M procedures $619 ASP (US) CGAR: 1.5% CGAR: 8.8% 9% 7% 84% EU Asia Pacific US 6% 13% 17% 28% 36% Overactive Bladder Other Cervical Dystonia Spasticity Migraine Global Therapeutic Toxin Market Share Breakdown By Geography By Indication Global Therapeutic Toxin Market Projections Source: Decision Resources Group Therapeutic Botulinum Toxin Market Analysis Global 2021

9 No competitive product has therapeutic indications under separate BLA Model Allows Reimbursement Based Solely on Therapeutic Pricing *Average selling price Value to Physician Consistent, predictable reimbursement Value to Payor Potential to offer financial incentives • Original BLA expected to allow AEON’s ASP* to be unencumbered by pricing pressures from aesthetic indications that hamper the competition’s reimbursement structure • Physicians to receive consistent and favorable reimbursement from payors • Flexibility to provide targeted economic incentives to payors and/or providers that competition cannot

(episodic and chronic prophylaxis) Migraine

11 Migraine Market Resurgence From New Players is Benefitting Toxins New CGRPs are rapidly expanding the market driven by new product launches and promotional investment Migraine affects ~40M people in the United States & ~1B people worldwide every year, making it the third most prevalent illness in the world 1 Chronic Migraine • 15+ headache days/month • ~4.0M patients ($11.2B market) 2 Episodic Migraine • 6 - 14 headache days/month • ~9.4M patients ($18.5B market) 3 Accelerating Migraine Market • Botox ® in chronic migraine returned to double digit growth after a brief flat period due to CGRP launches and COVID challenges • Botox ® only has a claim for chronic migraine vs. CGRPs with both chronic and episodic indications Toxins are Faring Well Despite Competition • Pursuing label for broader patient population than Botox ® with inclusion of both episodic and chronic migraine patients in Phase 2 • Novel injection paradigm with fewer injections and differentiated injection locations for ABP - 450 • Potential for increased safety and tolerability • High overlap in commercial audience between migraine and cervical dystonia ABP - 450 Opportunity 1. Migraine Research Foundation. 2. Company estimates based on 2017 US Census Projections; Diamond, Patterns of Diagnosis and Acute and Preventive Treatment for Mig raine in the United States: Results from the American Migraine Prevalence and Prevention Study (2006); Lipton, Barriers to the Diagnosis and Treatment of Migraine (2012) and assumes 2.2 million diagn ose d patients receiving four treatments per year (two vials) at a cost of $634 per vial 3. Messali, Direct and Indirect Costs of Chronic and Episodic Migraine in the United States: A Web - Based Survey (2016) and assumes 3.65 million diagnosed patients receiving four treatments per year (two vials) at a cost of $634 per vial. 4. DRG report, Migraine Disease Landscape & Forecast, December 2020

12 Q1 2019 Q2 2019 Q3 2019 Q4 2019 Q1 2020 Q2 2020 Q3 2020 Q4 2020 Q1 2021 Q2 2021 Q3 2021 Q4 2021 Q1 2022 Q2 2022 Q3 2022 Q4 2022 Normalized Botox 560,178 591,747 596,955 603,957 571,818 562,773 617,901 638,988 656,238 709,350 728,967 733,767 755,091 777,729 797,475 769,050 CGRPs 252,687 365,078 466,273 530,906 565,375 656,832 678,583 704,068 725,935 773,386 784,708 792,707 804,160 856,029 897,106 924,526 K 100K 200K 300K 400K 500K 600K 700K 800K 900K 1000K Volume (# of Claims) NORMALIZED Botox and CGRPs in Preventive Migraine Treatment [Q1’19 - Q4’22] Botox Still Showing Impressive YoY Growth Despite New CGRPs Source: KOMODO Claims Data (2015 - Dec'2022) CGRP includes Aimovig, Ajovy, Emgality,Vyepti, Qulipta Normalized Botox claims = Botox claims * 3 Competitive products listed at time of FDA approval +9% +10% +15% +15% +18% +26% +15% +6% +4% - 5% +2% Qulipta Vyepti +5% Nurtec ODT Prophylaxis

13 Addressable Patient Population ~3x Higher with Episodic Indication 1.6M Total Addressable Patients 260M People Projected 2020 US 18+ Population 40M Patients (15%) Estimated Migraine and severe HA Patients 9.4M Patients (24%) Episodic Migraine (6 - 14 HA/month) 4.0M Patients (10%) Chronic Migraine (15+ HA/month) 2.2M Patients (56%) Diagnosed Prevalence 3.7M Patients (39%) Diagnosed Prevalence 820k Patients (37%) Treated Patients (Prophylactic) 740k Patients (20%) Treated Patients (Prophylactic) Chronic Migraine Episodic Migraine Sources: 2017 US Census Projections Burch, The Prevalence and Impact of Migraine and Severe Headache in the United States: Figures and Trends From Government Hea lth Studies (2018) Lipton, Migraine in America Symptoms and Treatment (MAST) Study: Baseline Study Methods, Treatment Patterns, and Gender Diffe ren ces (2018) Buse, Impact of Migraine on the Family: Perspectives of People With Migraine and Their Spouse/Domestic Partner in the CaMEO S tud y (2016) Diamond, Patterns of Diagnosis and Acute and Preventive Treatment for Migraine in the United States: Results from the America n M igraine Prevalence and Prevention Study (2006) Messali, Direct and Indirect Costs of Chronic and Episodic Migraine in the United States: A Web - Based Survey (2016)

14 • N = 765 • ~60 sites in US, Canada & Australia • Includes both EM & CM patients • # headache days and migraine days/month established during 4 - week baseline prior to randomization • 3 arms: 150 Units, 195 Units, PBO • 2 injection cycles, 3 months apart • 6 - month treatment duration • Novel injection paradigm: - 22 injection sites (ABP - 450 low dose) - vs. 31 injections for Botox Primary: • Mean change of monthly migraine days (MMD) at Week 24 • Incidence of TEAEs throughout study compared to placebo Secondary: • Percentage of patients with reduction from baseline of ≥50%, 75%, 100% in average number of MMD throughout the study • Change in use of rescue medications from baseline • Safety endpoints of change in laboratory tests, ECG, etc. Exploratory: • Change in PGI - S, PGI - C, MIDAS, etc. Placebo n= 255 195 Units ABP - 450 n= 255 24 weeks Primary Efficacy Endpoint 150 Units ABP - 450 n= 255 4 - week Baseline 4 - week Screening Randomized R Endpoints Randomized Double - Blinded Placebo - Controlled Study Open Label Extension Study 4 treatments of ABP - 450 52 weeks Study Design 12 weeks Phase 2 Design: Episodic Migraine (EM) and Chronic Migraine (CM)

15 Independent Analysis of Phase 2 Episodic and Chronic Cohorts Randomization and Stratified for Episodic Migraine and Chronic Migraine Episodic Migraine N=~300 <15 headache days / month And 6 - 14 migraine days / month 150 U N=100 195 U N=100 Placebo N=100 Chronic Migraine N=~465 ≥ 15 headache days / month And >8 migraine days / month 150 U N=155 195 U N=155 Placebo N=155 Final Analysis of the Episodic Cohort Anticipated Fall 2023 Final Analysis of the Chronic Cohort Anticipated 2024

16 22 injection sites (low dose) in P3 migraine protocol focused on specific nerves vs. 31 with Botox targeting muscles Smart Injection Paradigm Targeting Nerves - Differentiation from Botox

17 BOTOX PREEMPT2 1 Phase 3 in chronic migraine • Baseline migraine days were 19.8 days • Change from baseline (CFB) for Botox and placebo was 45% and 33%, respectively ABP - 450 Phase 2 assumptions: • Projected CFB for ABP - 450 and placebo is 45% and 33%, respectively • Treatment effect between placebo and active is ~1.3 days • Standard deviation (SD) of 3.1 days • Sample size of 91 patients/arm (n=273 for all 3 arms) is needed to achieve 80% statistical power Endpoint Expectations for Phase 2 Episodic Migraine 1 https://headachejournal.onlinelibrary.wiley.com/doi/epdf/10.1111/j.1526 - 4610.2010.01678.x Statistical Powering & Sample Size Assumptions Expected Baseline (days) Projected CFB ABP - 450 Projected CFB Placebo Effect Size Stnd. Dev. Evaluable Subjects/Arm TOTAL N Power 10.5 - 4.73 (45%) - 3.47 (33%) - 1.3 3.1 91 273 80%

18 Phase 3 Trials for CGRPs in Episodic Migraine Not intended for cross - trial comparisons; Sources: package inserts; Neurol Ther (2021) 10:469 – 497 Brand Generic Company Trial Duration Dose Primary Endpoint N Change (days) Difference from placebo p - value Aimovig erenumab STRIVE 24 Weeks 70 mg SC QM Monthly migraine days (change from baseline to months 4 through 6) 312 - 3.2 - 1.4 <0.001 140 mg SC QM 318 - 3.7 - 1.9 <0.001 Placebo 316 - 1.8 — — ARISE 12 weeks 70 mg SC QM Monthly migraine days (change from baseline in the last 4 weeks of the trial) 282 - 2.9 - 1.1 <0.001 Placebo 288 - 1.8 — — Ajovy fremanezumab 12 weeks 225 mg SC QM Monthly migraine days (change during the 12 - week trial period, after first dose) 287 - 3.7 - 1.5 <0.001 675 mg SC Q3M 288 - 3.4 - 1.2 <0.001 Placebo 290 - 2.2 — — Emgality galcanezumab EVOLVE - 1 6 months 120 mg SC QM Monthly migraine days (change from baseline during the treatment period) 210 - 4.7 - 1.9 <0.001 Placebo 425 - 2.8 — — EVOLVE - 2 6 months 120 mg SC QM Monthly migraine days (change from baseline during the treatment period) 231 - 4.3 - 2.0 <0.001 Placebo 461 - 2.3 — — Nurtec ODT rimegepant Study 1 12 weeks 75 mg ODT QOD Monthly migraine days (change from baseline during the treatment period) 348 - 4.3 - 0.8 0.010 Placebo 347 - 3.5 — — QULIPTA atogepant ADVANCE 12 weeks 10 mg ODT OD Monthly migraine days (change from baseline across 12 - week treatment period) 214 - 3.7 - 1.2 <0.001 30 mg ODT OD 223 - 3.9 - 1.4 <0.001 60 mg ODT OD 222 - 4.2 - 1.7 <0.001 Placebo 214 - 2.5 — —

19 Tolerability parameter (% of pts) Pooled PREEMPT studies 1 COMPEL 2 REPOSE 3 CM - PASS 4 ABP - 450 (EM) ABP - 450 (CM) DB OLE OL OL OL DB DB BoNT/A ( n = 687) PL ( n = 692) BoNT/A ( n = 1205) BoNT/A ( n = 716) BoNT/A ( n = 633) BoNT/A ( n = 1160) TEAEs 62.4** 51.7 58.3 60.9 – 41.2 Serious TEAEs 4.8* 2.3 3.8 10.5 – 5.3 TRAEs a 29.4** 12.7 20.3 18.3 18.3 25.1 Serious TRAEs a 0.1 0.0 0.1 0.1 1.3 < ௗ 0.1 Common TRAEs a Blinded Data ABP - 450 (as of Feb 10, 2023) Neck pain 6.7 2.2 4.6 4.1 2.8 4.4 2.1 (4/190 completers) 1.6 (2/128 completers) Muscular weakness 5.5 0.3 3.9 1.4 – 2.7 0 0 Eyelid ptosis 3.3 0.3 2.5 2.5 5.4 4.1 0 0 Injection site pain 3.2 2.0 2.0 2.0 – – Headache 2.9 1.6 1.4 1.4 – 2.2 Myalgia 2.6 0.3 1.2 – – 0.9 Musculoskeletal stiffness 2.3 0.7 1.7 1.7 2.7 2.0 Musculoskeletal pain 2.2 0.7 1.1 – – 0.9 Facial paresis 2.2 b – 1.2 b 1.3 – 1.3 Discontinuations due to AEs 3.8 c 1.2 c 2.6 c 4.5 c (1.8 d ) 1.6 d 4.4 c 1. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56 - week PREEMPT clinical program. Headache. 2011;51(9):1358 – 1373. 2. Blumenfeld AM, Stark RJ, Freeman MC, et al. Long - term study of the efficacy and safety of onabotulinumtoxinA for the preventi on of chronic migraine: COMPEL study. J Headache Pain. 2018;19(1):13. 3. Ahmed F, Gaul C, Garcia - Monco JC, et al. An open - label prospective study of the real - life use of onabotulinumtoxinA for the t reatment of chronic migraine: the REPOSE study. J Headache Pain. 2019;20(26):1 – 14. 4. Matharu M, Pascual J, Nilsson Remahl I, et al. Utilization and safety of onabotulinumtoxinA for the prophylactic treatment of chronic migraine from an observational study in Europe. Cephalalgia. 2017;37(14):1384 – 97. AEs adverse events, ADR adverse drug reactions, BoNT/A onabotulinumtoxinA (Botox ® ), DB double - blind phase, OL(E) open - label (extension phase), PL placebo, pts patients, TEAEs treatment - emergent AEs, TRAEs treatment - related AEs, – information not available (e.g. only ADRs occurring in > ௗ 2% of pts in REPOSE were reported). * p = 0.0133, ** p < 0.0001 vs PL a ADRs (REPOSE) b Included in muscular weakness in PREEMPT 1 and 2 c Discontinuations due to TEAEs d Discontinuations due to TRAEs Blinded Safety from Phase 2 Migraine Compares Favorably to Botox

Gastroparesis

21 Gastroparesis Opportunity for ABP - 450 • 2019 FDA Guidance for Industry 1 • Since a well - defined and reliable patient reported outcome (PRO) is not yet available, FDA recommends that the core signs and symptoms be included as endpoints. • Anticipate initiating a Phase 2a study in 2024 • Our primary endpoint measures change in core signs and symptoms from baseline over a 12 - week treatment period • We plan to assess idiopathic and diabetic patients in separate trials Potential Regulatory Pathway • Defined by delayed gastric emptying • Symptoms are chronic with episodic exacerbations • Nausea, vomiting and pain are most troubling symptoms • Both idiopathic and diabetic (nerve damage leads to impaired intestinal muscle function) • Very limited therapeutic options; Reglan ® and Gimoti tm (metoclopramide) limited by AEs The Disorder • ~400,000 patients 2 • ~$900M market • High unmet need, low competitive intensity • We are aware of no other neurotoxin pursuing gastroparesis indication • Data from investigator - sponsored trials show positive response to neurotoxins with endoscopic intrasphincter injection of the pylorus in patients with idiopathic and diabetic gastroparesis ABP - 450 Opportunity 1. https://www.fda.gov/media/129880/download 2. Company estimates based on 2017 US Census Projections; Syed, Epidemiology and Diagnosis of Gastroparesis in the United States : A Population - based Study. (2019); and Parkman, Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity (2011).

22 Provides an Opportunity for a Targeted Treatment for Gastroparesis Injections in the Pylorus and Pyloric Sphincter Region Sclerotherapy needle Esophagogastroduodenoscopy Plan to administer injections of ABP - 450 using a standard sclerotherapy needle in the pylorus and pyloric sphincter region Esophagus Lower Esophageal Sphincter Pyloric Sphincter Duodenum • Initiated preclinical study Dec 2020; completed Sep 2021 • IND accepted May 2022

Posttraumatic Stress Disorder (PTSD)

24 Posttraumatic Stress Disorder (PTSD) • PTSD is well defined with broad disease awareness • A psychiatric disorder that may occur in people who experience or witness a traumatic event or events • Categories of symptoms include intrusion (involuntary memories), avoidance, alterations in cognition and mood (negative thoughts and feelings), and alterations in arousal and reactivity (angry outbursts) The Disorder 1 • Current t reatments include cognitive behavioral therapy, medication (e.g., SSRIs, SNRIs), and other alternative therapies • Established FDA regulatory pathway for clinical development with several medications approved for use, including Zoloft and Paxil • Numerous validated scales are already widely accepted (e.g., CAPS, PCL - 5, BDI) Current Treatments & Regulatory Pathway 1. American Psychiatric Association. https://www.psychiatry.org/patients - families/ptsd/what - is - ptsd .

25 Posttraumatic Stress Disorder Associated with the Stellate Ganglion Source: Image Left: Rae Olmsted KL, Bartoszek M, Mulvaney S, et al. Effect of Stellate Ganglion Block Treatment on Posttraumatic Stre ss Disorder Symptoms: A Randomized Clinical Trial. JAMA Psychiatry. 2020;77(2):130 – 138. doi:10.1001/jamapsychiatry.2019.3474. Image Right: Levi, Ofir, Ariel Ben Yehuda, Daniel S. Pine, and Yair Bar - Haim. “A Sobering Look at Treatment Effectiveness of Mil itary - Related Posttraumatic Stress Disorder.” Clinical Psychological Science 10, no. 4 (2021): 690 – 99. https://doi.org/10.1177/21677026211051314. Effect of Stellate Ganglion Block Treatment on Posttraumatic Stress Disorder Symptoms A Randomized Clinical Trial Kristine L. Rae Olmsted, MSPH; Michael Bartoszek, MD; Sean Mulvaney, MD; et al JAMA Psychiatry. 2020;77(2):130 - 138. Key Points Question : How does stellate ganglion block compare with sham treatment in reducing the severity of posttraumatic stress disorder symptoms over 8 weeks? Findings : In this sham - controlled randomized clinical trial, 2 stellate ganglion block treatments 2 weeks apart were effective in reducing Clinician - Administered PTSD Scale for DSM - 5 total symptom severity scores over 8 weeks . The adjusted mean symptom change was − 12 . 6 points for the group receiving stellate ganglion blocks, compared with − 6 . 1 points for those receiving sham treatment, a significant difference . Meaning : Stellate ganglion block treatment warrants further study as a posttraumatic stress disorder treatment adjunct . A Sobering Look at Treatment Effectiveness of Military - Related Posttraumatic Stress Disorder A Randomized Clinical Trial Ofir Levi, Ariel Ben Yehuda, Yair Bar - Haim; et al Clinical Psychological Science. 2021; 10 , no.4 Abstract Approximately two thirds of veterans with posttraumatic stress disorder (PTSD) remain with the disorder following treatment . Pinpointing the per - symptom effectiveness of treatments in real - world clinical settings can highlight relevant domains for treatment augmentation and development . Baseline and posttreatment assessments of PTSD and depression were performed in 709 veterans with PTSD . PTSD remission was 39 . 4 % . Treatment was least effective for intrusion symptoms and had no effect on flashbacks or on poor recall of traumatic features . Of veterans who remitted, 72 . 8 % still met diagnostic criteria for at least one cluster . Poor clinical effectiveness was noted for depression ; only 4 . 1 % of the patients remitted following treatment . Treatments for veterans with PTSD show limited overall effectiveness in real - world settings . Enhancing treatment response may require enhancing provider fidelity and patient compliance with extant treatments or the development of new treatments that specifically target the symptoms of PTSD that do not respond well to extant treatments .

26 Stellate ganglion (sympathetic) block using ultrasound guidance • Establish safety and preliminary efficacy signal • Rats placed in supine position under anesthesia • Administer approximately 10 - 20 µl of active, following standard protocols • Observe for 1 - 2 weeks after the injection • Monitor heart rate and blood pressure • Assess effect on sympathetic nervous pathway ABP - 450 Pre - Clinical PTSD Study

Cervical Dystonia

28 Cervical Dystonia Opportunity for ABP - 450 • Potential for efficient development • Potential to allow participation in segment of on - label movement disorders • Potential to leverage into a broader muscle spasticity indication and label with a focused clinical program Important Indication for Muscle Movement Disorders • Cervical dystonia is a chronic condition with no cure • Painful and debilitating twisting movements of neck and shoulders • Botulinum toxin injection is the standard of care • Established outcome measures and regulatory pathway The Disorder • ~50,000 patients • ~$360M market • Approximately 40% of therapeutic toxin market sales in 2019 were for various muscle movement disorders • Anticipate that Phase 3 program will include a head - to - head comparison to Botox ® to demonstrate non - inferiority US Market Opportunity For ABP - 450 Sources: Company estimates based on 2017 US Census Projections Defazio, Descriptive Epidemiology of Cervical Dystonia (2013) Richardson, American Academy of Neurology - Dystonia Treatment (2017)
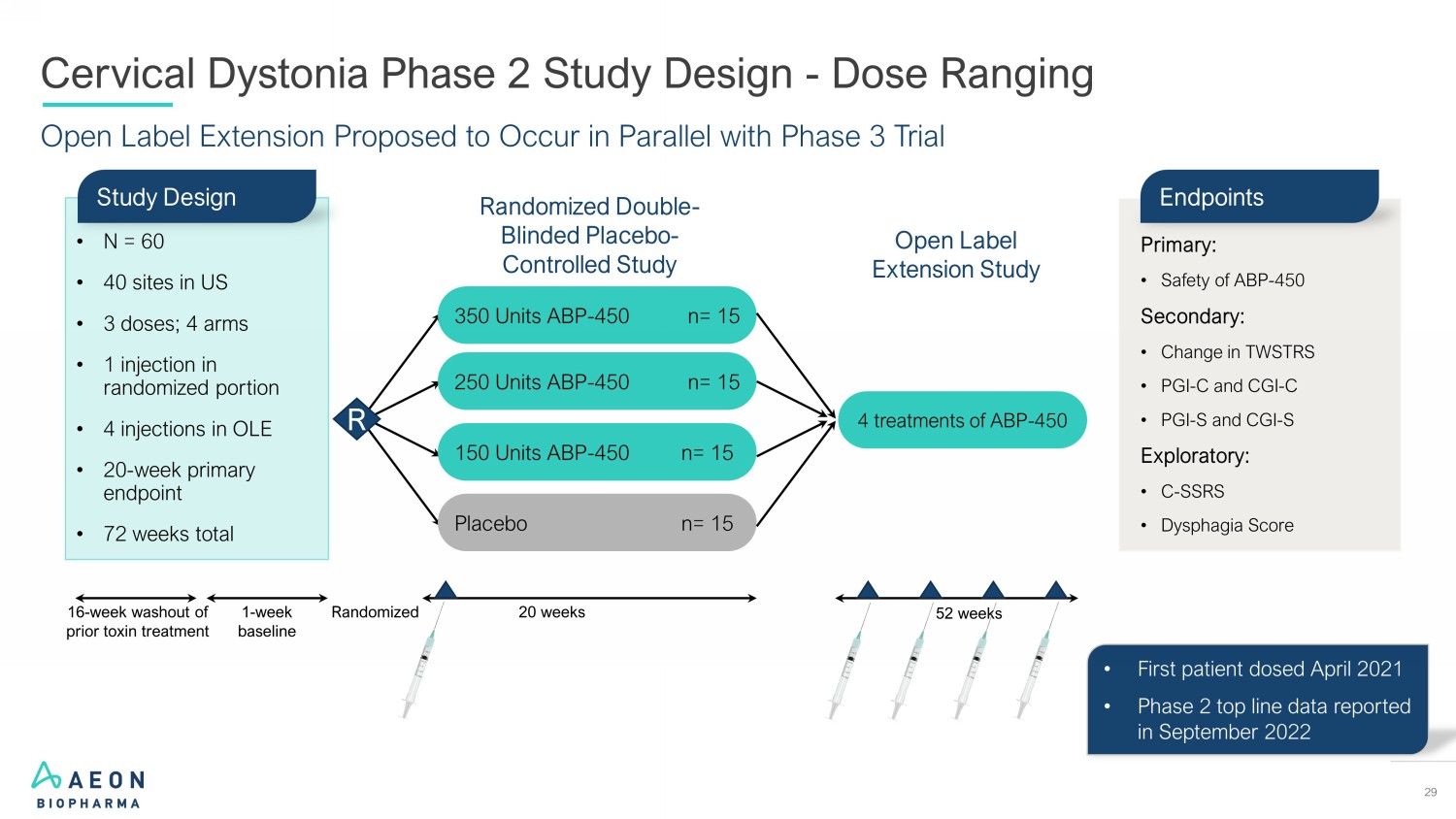
29 Open Label Extension Proposed to Occur in Parallel with Phase 3 Trial Cervical Dystonia Phase 2 Study Design - Dose Ranging • First patient dosed April 2021 • Phase 2 top line data reported in September 2022 Randomized Double - Blinded Placebo - Controlled Study 20 weeks Randomized R 1 - week baseline 16 - week washout of prior toxin treatment Open Label Extension Study Placebo n= 15 150 Units ABP - 450 n= 15 250 Units ABP - 450 n= 15 350 Units ABP - 450 n= 15 4 treatments of ABP - 450 52 weeks Primary: • Safety of ABP - 450 Secondary: • Change in TWSTRS • PGI - C and CGI - C • PGI - S and CGI - S Exploratory: • C - SSRS • Dysphagia Score Endpoints • N = 60 • 40 sites in US • 3 doses; 4 arms • 1 injection in randomized portion • 4 injections in OLE • 20 - week primary endpoint • 72 weeks total Study Design

30 Phase 2 Data Conclusions • Phase 2 trial met primary and other key endpoints, supporting the safety and efficacy of ABP - 450 in reducing signs and symptoms associated with CD. • ABP - 450 demonstrated adverse event rates similar to, or lower than, other botulinum toxin products for the treatment of CD. • Zero discontinuations due to Treatment - Emergent Adverse Events (TEAEs) • Low rate of treatment - related TEAEs (TRAEs) • Zero dysphagia cases in the 150 U arm and low rate of dysphagia (11%) and muscle weakness (6.7%) overall • All TRAEs were mild to moderate in severity and transient in nature • ABP - 450 demonstrated efficacy similar to, or better than, other botulinum toxin products for the treatment of CD. • TWSTRS at Week 4 improved 14.01 points in 150 U; 11.28 points in 250 U; 9.92 points in 350 U; 3.57 points in placebo – Statistical significance in lower dose arms (150 U and 250 U) vs. placebo and numerical improvement in high dose arm (350 U) vs. placebo • Patient Global Impression of Change (PGI - C) demonstrated statistically significant improvement in all three ABP - 450 dose groups over placebo • Clinical Global Impression of Change (CGI - C) demonstrated statistically significant improvement in all three ABP - 450 dose groups over placebo • Median duration of treatment effect was at least 20 weeks for 150 U, 20 weeks for 250 U, and 20 weeks for 350U.

Financial Overview

32 Financial Overview Sufficient capital to fund operations beyond topline data from ongoing Phase 2 study of ABP - 450 for episodic migraine expected in Fall 2023 $0 D ebt 27.4M Basic Shares Outstanding $25M Cash at Close 1 14.48M Warrants 2 19.45M Total Potential Earnout Shares 1. Proforma as of 7 - 24 - 23 2. 9.2M warrants with $11.50 strike and 5.28M warrants with $8.05 strike 3. Approximately 7.5M shares subject to forward purchase agreement; for purchase details, see SEC filings ~7.5M Shares in Forward Purchase Agreement for potential purchase 3

33 ABP - 450 – Clinical and Regulatory Timeline 2025 2H 1H 2026 2H 1H 2027 2H 1H 2028 2H 1H 2029 2H 1H Earnout Deadline Chronic Migraines Episodic Migraines Cervical Dystonia 14.45M 5 M 2M shares - upon first patient dosed in Phase 3 study in the first to occur between CM or EM prior to 06/30/25 2024 2H 1H Aligns AEON and SPAC Sponsor Incentives with Public Market Investors Earnout Structure Note: CM = Chronic Migraine, EM = Episodic Migraine, CD = Cervical Dystonia. Please refer to “ Forward - Looking Statements” in th e slide titled "Disclaimer" for important information you should consider regarding the timing of earn - out milestone achievement, as no assurance can be given that any such indicated earn - out milestone will be achieved on the timeline indicated or at all. 1. If BLA acceptance for episodic migraine occurs prior to BLA acceptance for chronic migraine, earnout for episodic migraine BL A a cceptance will increase from 4M shares to 11M shares and there shall be no further earnout issuance for chronic migraine. In the event that AEON licenses any of its products (except for Migraine or CD indications) to a third - party licensor for distribution in the U.S. market prior to the time that the 11M shares have been issued upon achievement of either the BLA acceptance for episodic migraine or chronic migraine (as described above), then 2M shares shall be issued upon the entry int o such license agreement and the remaining shares to be issued for achievement of the BLA acceptance for episodic migraine or chronic migraine, as applicable, will be correspondingly reduced. 2. 1.45M founder shares shall vest upon the earlier to occur of (i) BLA acceptance for chronic migraine or (ii) BLA acceptance f or episodic migraine, subject to potential forfeiture of sponsor shares pursuant to the Business Combination Agreement. 7M shares - upon BLA acceptance for CM prior to 06/30/28 4M shares 1 - upon BLA acceptance for EM prior to 06/30/29 1.45M shares – upon earlier of: 1) BLA acceptance for CM prior to 06/30/28 or 2) BLA acceptance for EM prior to 06/30/29 2 5M shares - upon BLA acceptance for CD prior to 11/30/26

34 Investment Highlights Botulinum toxin has demonstrated utility across dozens of published uses expected to be accessible to ABP - 450; accelerated and simplified commercialization timeline due to existing aesthetic BLA (separately held by Evolus) and manufacturing site approved by FDA, EMA and Health Canada Episodic migraine approval would nearly triple the current addressable patient population (Botox ® indicated for chronic migraine only); ABP - 450 Phase 2 migraine study includes chronic and episodic migraine patients with a streamlined injection protocol with potential to enhance safety and tolerability Potential to pursue traditional pharma pricing model currently not pursued by therapeutic toxin competition with a 900 kDa botulinum toxin expected to facilitate physician adoption Significant neurotoxin commercial and clinical development experience among management team translating to approximately $162M invested in AEON since 2019 Strong Potential Episodic Migraine Market Expansion Business Model Advantage Strong Leadership & Sponsorship Market Ripe for Competition Single existing competitor for key target markets (episodic & chronic migraine); unlike in aesthetic market, ABP - 450’s therapeutic focus will compete with market leader on a product - to - product basis, not against a portfolio of products PTSD Opportunity Ongoing pre - clinical program utilizing proprietary injection paradigm in a novel part of the anatomy is designed to provide IND supporting safety and efficacy data; this innovative approach has the potential for utility across a broad Neuro/Psych portfolio including PTSD

Thank you
v3.23.2
Cover
|
Jul. 24, 2023 |
| Entity Addresses [Line Items] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jul. 24, 2023
|
| Entity File Number |
001-40021
|
| Entity Registrant Name |
Priveterra Acquisition Corp.
|
| Entity Central Index Key |
0001837607
|
| Entity Tax Identification Number |
85-3940478
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
5 Park Plaza,
|
| Entity Address, Address Line Two |
Suite 1750
|
| Entity Address, City or Town |
Irvine
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
92614
|
| City Area Code |
949
|
| Local Phone Number |
354-6499
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| Entity Information, Former Legal or Registered Name |
Priveterra Acquisition Corp.
|
| Common Stock [Member] |
|
| Entity Addresses [Line Items] |
|
| Title of 12(b) Security |
Class
A Common Stock, $0.0001 par value per share
|
| Trading Symbol |
AEON
|
| Security Exchange Name |
NYSE
|
| Warrant [Member] |
|
| Entity Addresses [Line Items] |
|
| Title of 12(b) Security |
Warrants,
each whole warrant exercisable to purchase one share of Class A Common Stock at an exercise price of $11.50 per
share
|
| Trading Symbol |
AEON WS
|
| Security Exchange Name |
NYSE
|
| Former Address [Member] |
|
| Entity Addresses [Line Items] |
|
| Entity Address, Address Line One |
300 SE 2nd Street
|
| Entity Address, Address Line Two |
Suite 600
|
| Entity Address, City or Town |
Fort
Lauderdale
|
| Entity Address, State or Province |
FL
|
| Entity Address, Postal Zip Code |
33301
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
dei_EntityAddressesLineItems |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Priveterra Acquisition C... (NASDAQ:PMGMU)
Historical Stock Chart
From Apr 2024 to May 2024
Priveterra Acquisition C... (NASDAQ:PMGMU)
Historical Stock Chart
From May 2023 to May 2024