false000181056000018105602024-01-302024-01-300001810560revb:CommonStockParValue0001PerShareMember2024-01-302024-01-300001810560revb:RedeemableWarrantsEachExercisableForA11050thShareOfCommonStockAtAnExercisePriceOf1207500PerShareMember2024-01-302024-01-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): January 30, 2024 |
REVELATION BIOSCIENCES, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-39603 |
84-3898466 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
4660 La Jolla Village Drive Suite 100 |
|
San Diego, California |
|
92122 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (650) 800-3717 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common stock, par value $0.001 per share |
|
REVB |
|
The Nasdaq Stock Market LLC |
Redeemable warrants, each exercisable for a 1/1,050th share of common stock at an exercise price of $12,075.00 per share |
|
REVBW |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On January 30, 2024, Revelation Biosciences, Inc. (the “Company”) made an updated corporate presentation available to the public, the corporate presentation can be found on the Company’s website. A copy of the presentation is attached to this Current Report on Form 8-K as Exhibit 99.1 and is incorporated herein by reference.
The information in Item 7.01 and in Exhibit 99.1 will not be treated as “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section. This information will not be incorporated by reference into any filing under the Securities Act of 1933, as amended, or into another filing under the Exchange Act, unless that filing expressly incorporates this information by reference.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
REVELATION BIOSCIENCES, INC. |
|
|
|
|
Date: |
January 30, 2024 |
By: |
/s/ Chester S. Zygmont, III |
|
|
|
Chester S. Zygmont, III
Chief Financial Officer
(principal financial and accounting officer)
|

Developing innovative therapeutics to address unmet needs Corporate Presentation / January 2024 www.revbiosciences.com

Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These forward-looking statements are generally identified by the words "anticipate", "believe", "expect", "estimate", "plan", "outlook", and "project" and other similar expressions. We caution investors that forward-looking statements are based on management’s expectations and are only predictions or statements of current expectations and involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from those anticipated by the forward-looking statements. Revelation cautions investors not to place undue reliance on any such forward-looking statements, which speak only as of the date they were made. The following factors, among others, could cause actual results to differ materially from those described in these forward-looking statements: the ability of Revelation to meet its financial and strategic goals, due to, among other things, competition; the ability of Revelation to grow and manage growth profitability and retain its key employees; the possibility that the Revelation may be adversely affected by other economic, business, and/or competitive factors; risks relating to the successful development of Revelation’s product candidates; the risk that our preclinical studies will not demonstrate sufficient positive data to support commencement of clinical trials; the risk that we may not fully enroll our clinical studies or enrollment will take longer than expected; risks relating to the occurrence of adverse safety events and/or unexpected concerns that may arise from data or analysis from our clinical studies; changes in applicable laws or regulations; expected initiation of the clinical studies, the timing of clinical data; the outcome of the clinical data, including whether the results of such study is positive or whether it can be replicated; the outcome of data collected, including whether the results of such data and/or correlation can be replicated; the timing, costs, conduct and outcome of our other clinical studies; the anticipated treatment of future clinical data by the FDA, the EMA or other regulatory authorities, including whether such data will be sufficient for approval; the success of future development activities for Gemini or any other product candidates; potential indications for which product candidates may be developed; the ability of Revelation to maintain the listing of its securities on NASDAQ; the expected duration over which Revelation’s balances will fund its operations; the ability of Revelation to obtain further financing and other risks and uncertainties described herein, as well as those risks and uncertainties discussed from time to time in other reports and other public filings with the SEC by Revelation.
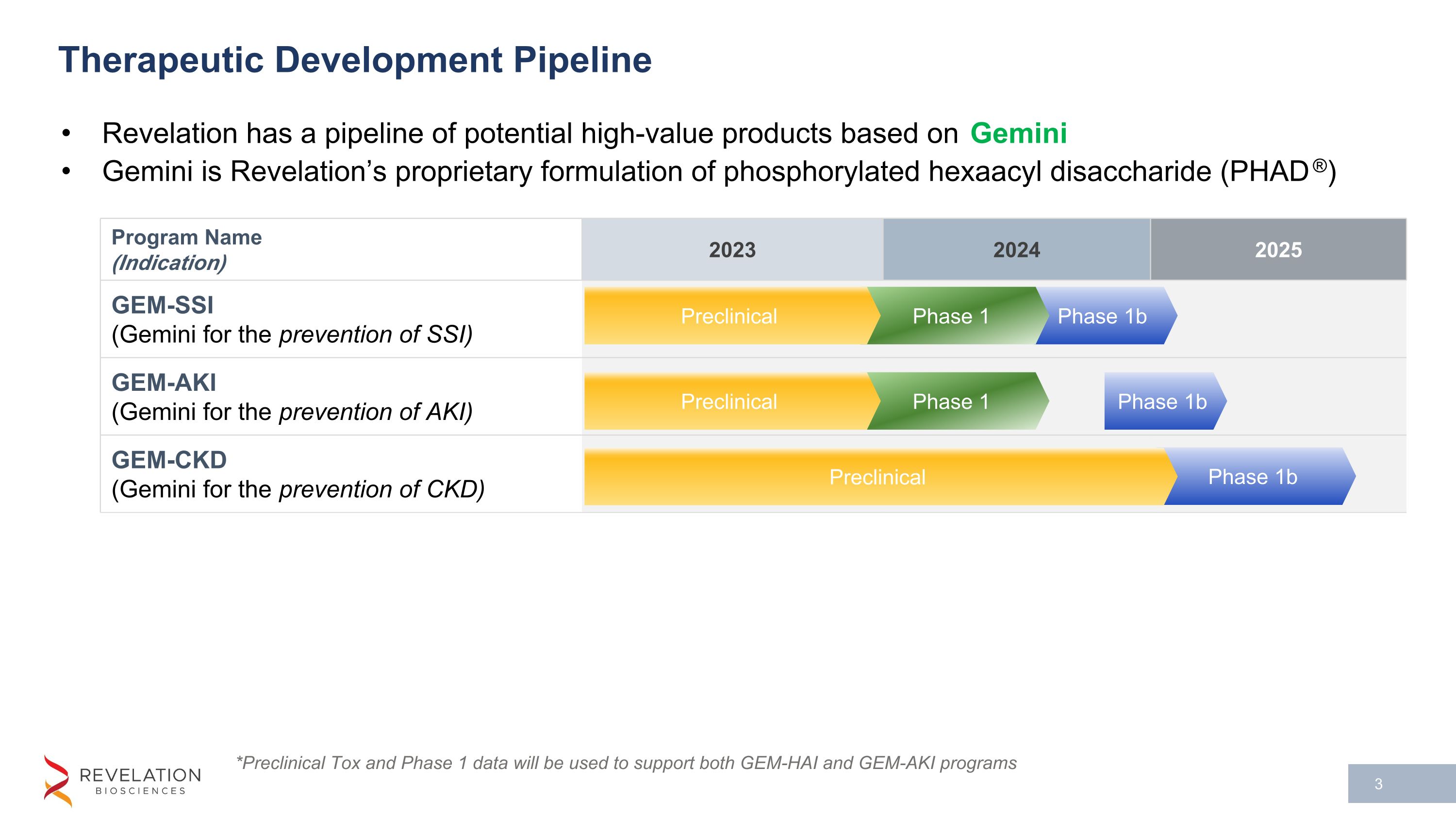
Program Name (Indication) 2023 2024 2025 GEM-SSI (Gemini for the prevention of SSI) GEM-AKI (Gemini for the prevention of AKI) GEM-CKD (Gemini for the prevention of CKD) Therapeutic Development Pipeline Revelation has a pipeline of potential high-value products based on Gemini Gemini is Revelation’s proprietary formulation of phosphorylated hexaacyl disaccharide (PHAD®) *Preclinical Tox and Phase 1 data will be used to support both GEM-HAI and GEM-AKI programs Phase 1b Phase 1 Phase 1b Phase 1b Phase 1 Preclinical Preclinical Preclinical
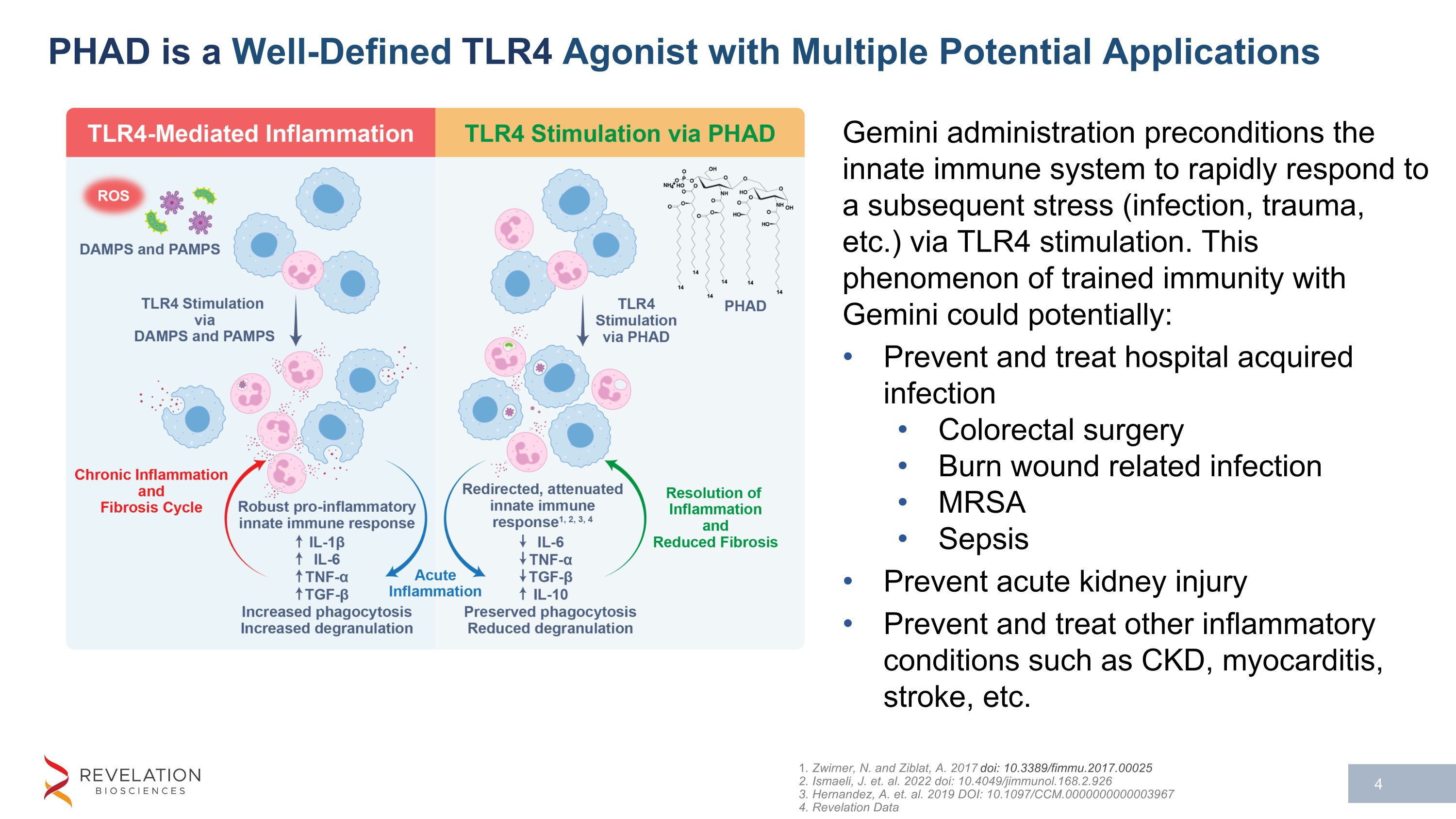
PHAD is a Well-Defined TLR4 Agonist with Multiple Potential Applications 1. Zwirner, N. and Ziblat, A. 2017 doi: 10.3389/fimmu.2017.00025 2. Ismaeli, J. et. al. 2022 doi: 10.4049/jimmunol.168.2.926 3. Hernandez, A. et. al. 2019 DOI: 10.1097/CCM.0000000000003967 4. Revelation Data Gemini administration preconditions the innate immune system to rapidly respond to a subsequent stress (infection, trauma, etc.) via TLR4 stimulation. This phenomenon of trained immunity with Gemini could potentially: Prevent and treat hospital acquired infection Colorectal surgery Burn wound related infection MRSA Sepsis Prevent acute kidney injury Prevent and treat other inflammatory conditions such as CKD, myocarditis, stroke, etc.

GEM-HAI Program Gemini for the Prevention of Hospital Acquired Infection
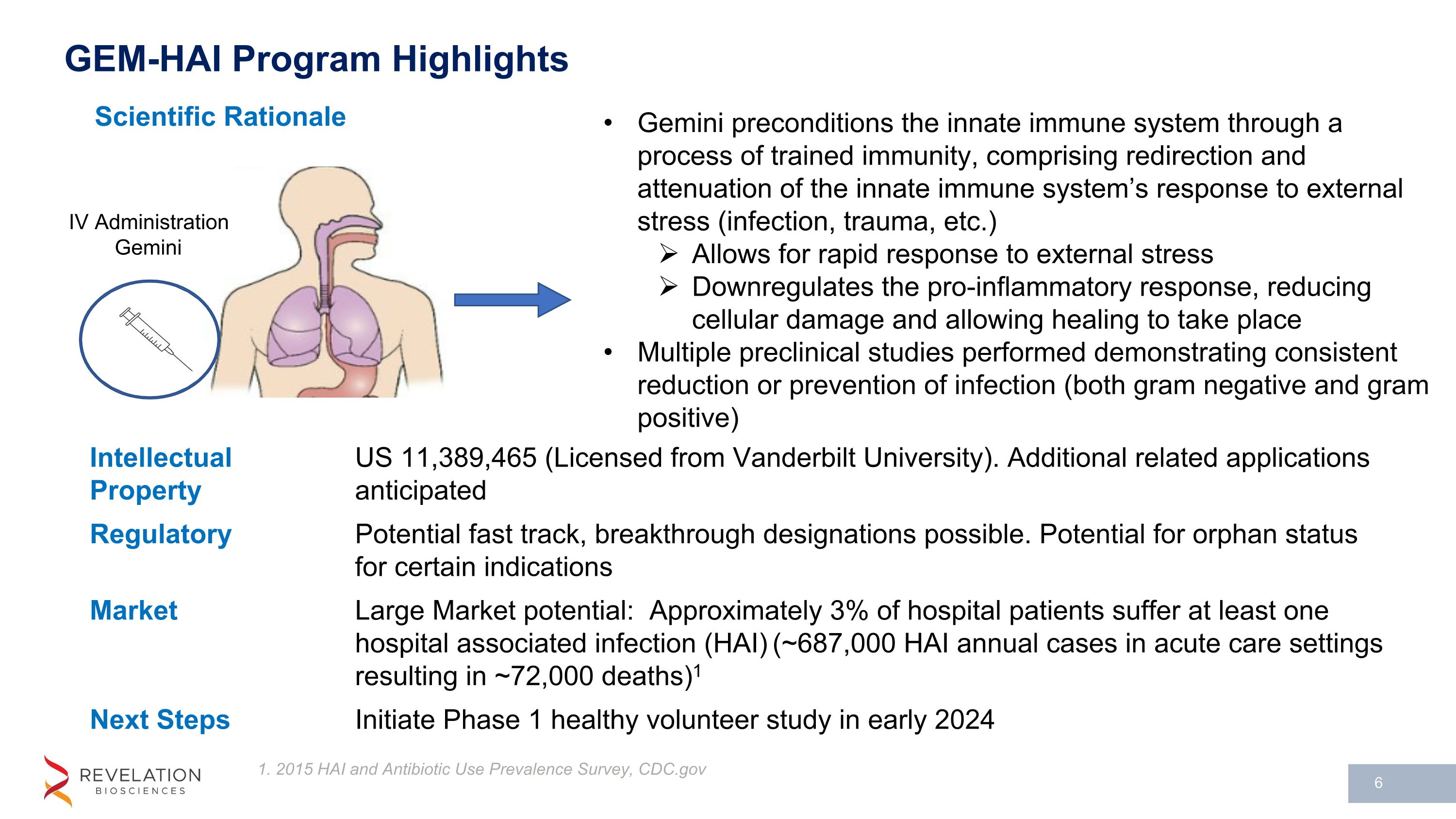
GEM-HAI Program Highlights Scientific Rationale 1. 2015 HAI and Antibiotic Use Prevalence Survey, CDC.gov Gemini preconditions the innate immune system through a process of trained immunity, comprising redirection and attenuation of the innate immune system’s response to external stress (infection, trauma, etc.) Allows for rapid response to external stress Downregulates the pro-inflammatory response, reducing cellular damage and allowing healing to take place Multiple preclinical studies performed demonstrating consistent reduction or prevention of infection (both gram negative and gram positive) IV Administration Gemini Intellectual Property US 11,389,465 (Licensed from Vanderbilt University). Additional related applications anticipated Regulatory Potential fast track, breakthrough designations possible. Potential for orphan status for certain indications Market Large Market potential: Approximately 3% of hospital patients suffer at least one hospital associated infection (HAI) (~687,000 HAI annual cases in acute care settings resulting in ~72,000 deaths)1 Next Steps Initiate Phase 1 healthy volunteer study in early 2024
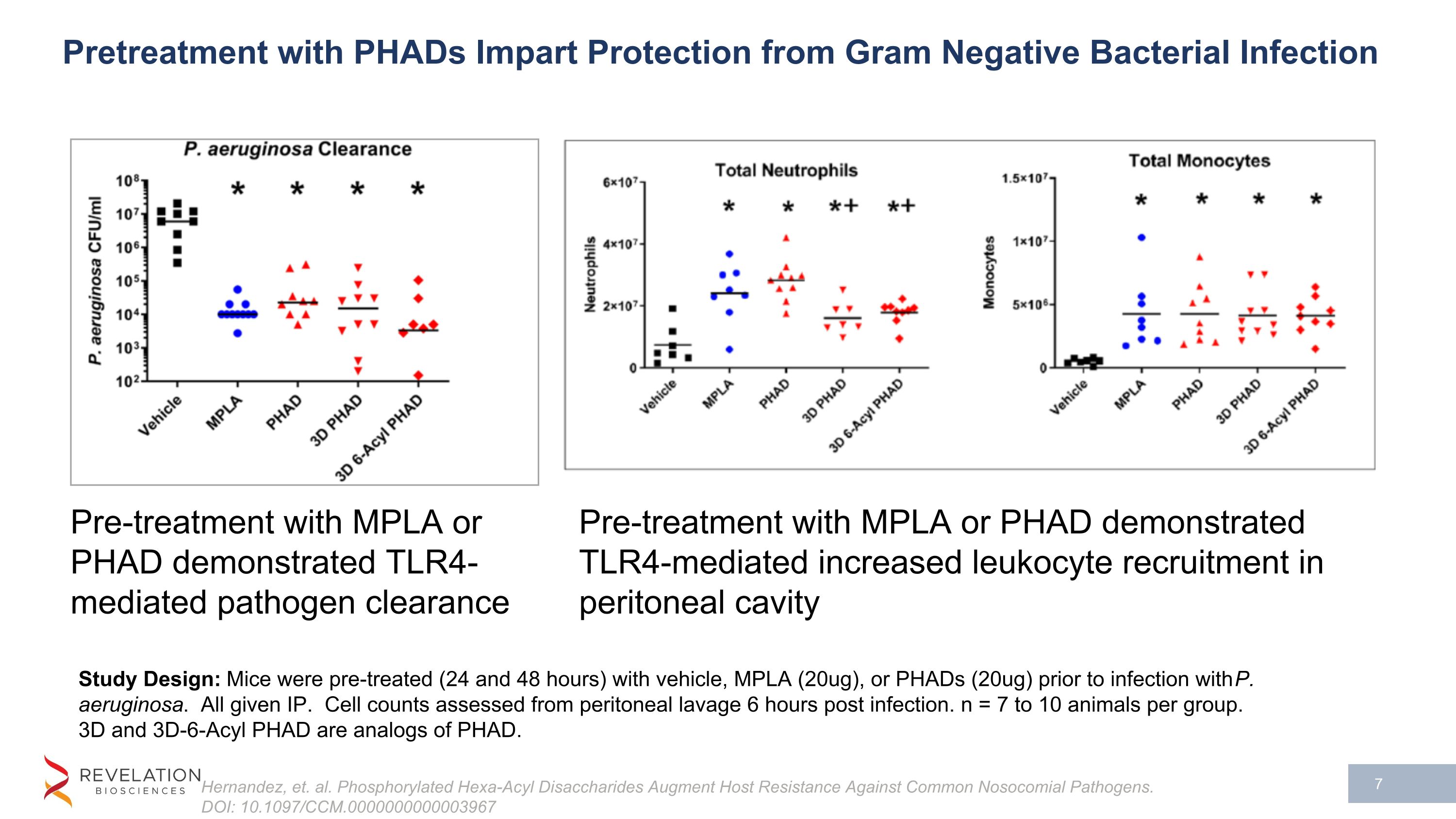
Pretreatment with PHADs Impart Protection from Gram Negative Bacterial Infection Hernandez, et. al. Phosphorylated Hexa-Acyl Disaccharides Augment Host Resistance Against Common Nosocomial Pathogens. DOI: 10.1097/CCM.0000000000003967 Study Design: Mice were pre-treated (24 and 48 hours) with vehicle, MPLA (20ug), or PHADs (20ug) prior to infection with P. aeruginosa. All given IP. Cell counts assessed from peritoneal lavage 6 hours post infection. n = 7 to 10 animals per group. 3D and 3D-6-Acyl PHAD are analogs of PHAD. Pre-treatment with MPLA or PHAD demonstrated TLR4-mediated pathogen clearance Pre-treatment with MPLA or PHAD demonstrated TLR4-mediated increased leukocyte recruitment in peritoneal cavity
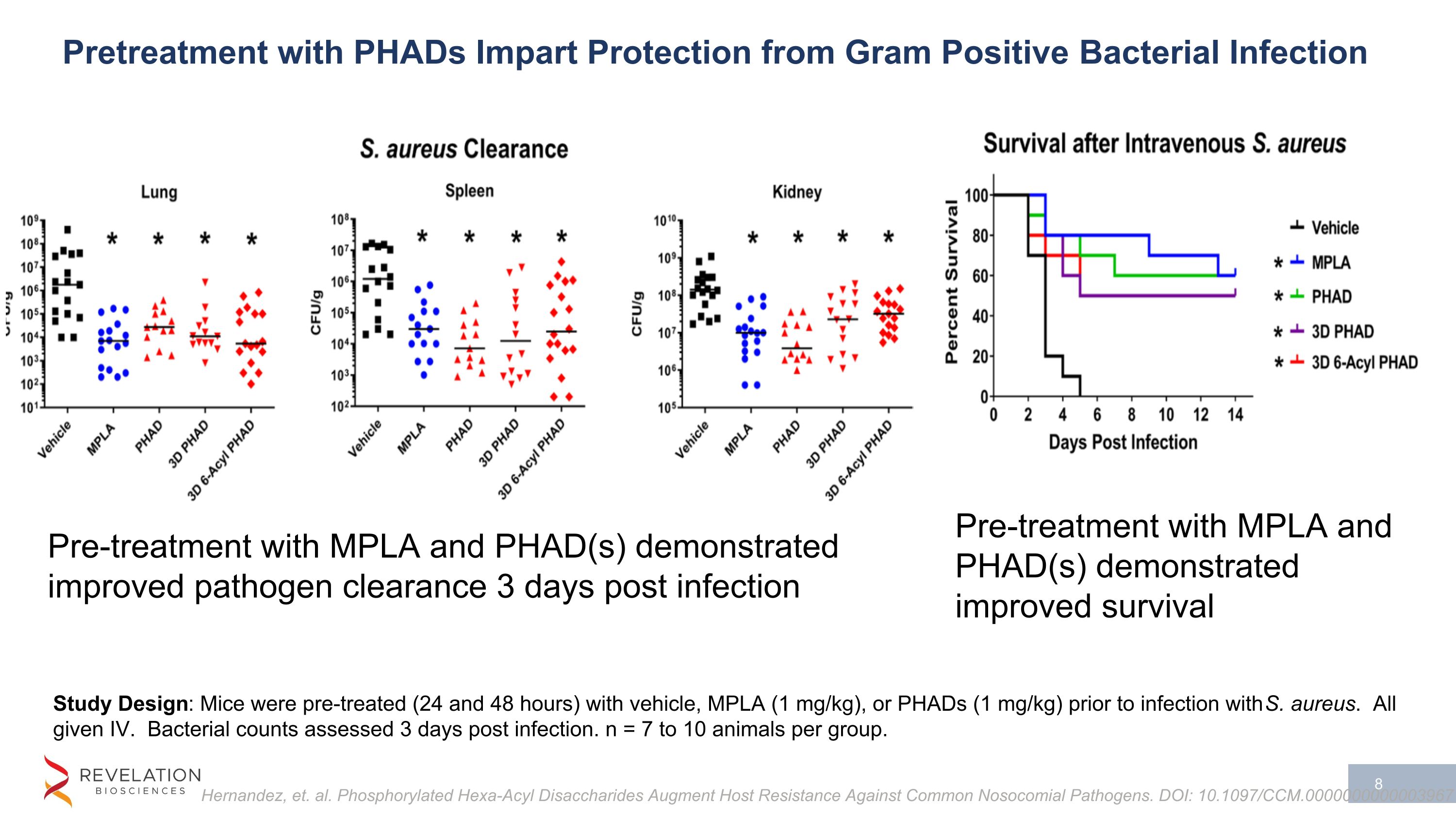
Study Design: Mice were pre-treated (24 and 48 hours) with vehicle, MPLA (1 mg/kg), or PHADs (1 mg/kg) prior to infection with S. aureus. All given IV. Bacterial counts assessed 3 days post infection. n = 7 to 10 animals per group. Pre-treatment with MPLA and PHAD(s) demonstrated improved pathogen clearance 3 days post infection Pre-treatment with MPLA and PHAD(s) demonstrated improved survival Hernandez, et. al. Phosphorylated Hexa-Acyl Disaccharides Augment Host Resistance Against Common Nosocomial Pathogens. DOI: 10.1097/CCM.0000000000003967 Pretreatment with PHADs Impart Protection from Gram Positive Bacterial Infection
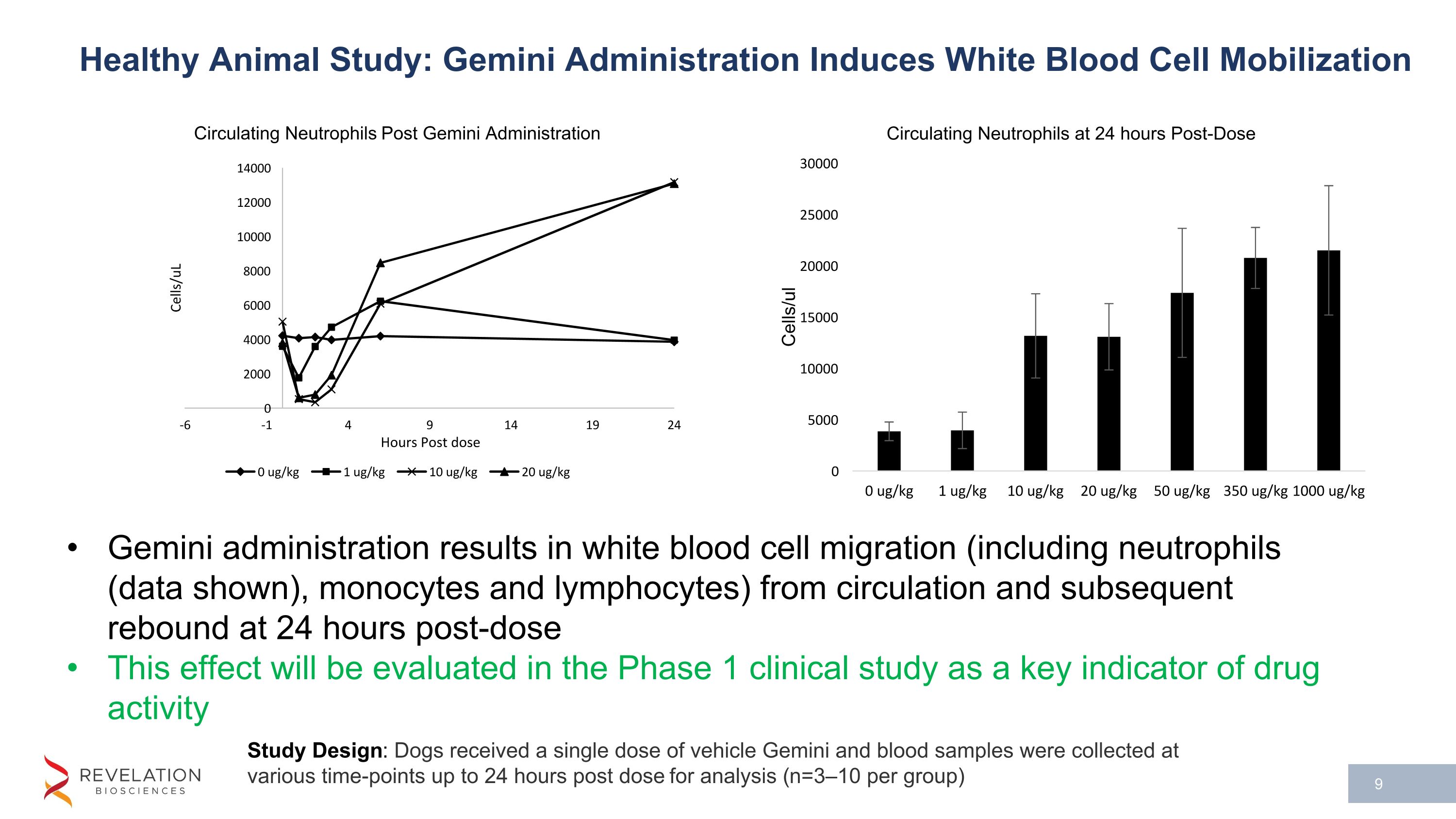
Study Design: Dogs received a single dose of vehicle Gemini and blood samples were collected at various time-points up to 24 hours post dose for analysis (n=3–10 per group) Healthy Animal Study: Gemini Administration Induces White Blood Cell Mobilization Gemini administration results in white blood cell migration (including neutrophils (data shown), monocytes and lymphocytes) from circulation and subsequent rebound at 24 hours post-dose This effect will be evaluated in the Phase 1 clinical study as a key indicator of drug activity
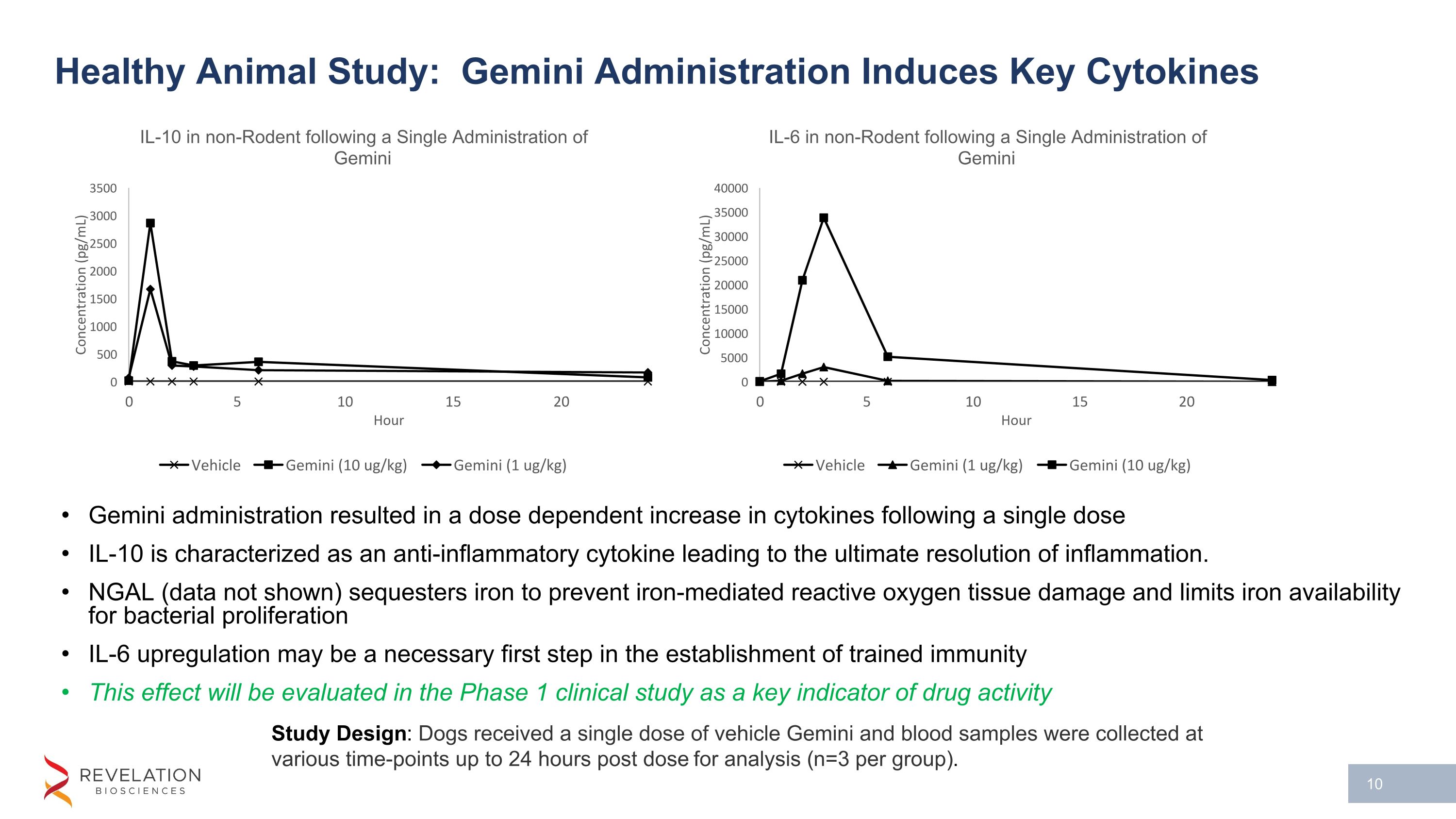
Healthy Animal Study: Gemini Administration Induces Key Cytokines Gemini administration resulted in a dose dependent increase in cytokines following a single dose IL-10 is characterized as an anti-inflammatory cytokine leading to the ultimate resolution of inflammation. NGAL (data not shown) sequesters iron to prevent iron-mediated reactive oxygen tissue damage and limits iron availability for bacterial proliferation IL-6 upregulation may be a necessary first step in the establishment of trained immunity This effect will be evaluated in the Phase 1 clinical study as a key indicator of drug activity Study Design: Dogs received a single dose of vehicle Gemini and blood samples were collected at various time-points up to 24 hours post dose for analysis (n=3 per group).
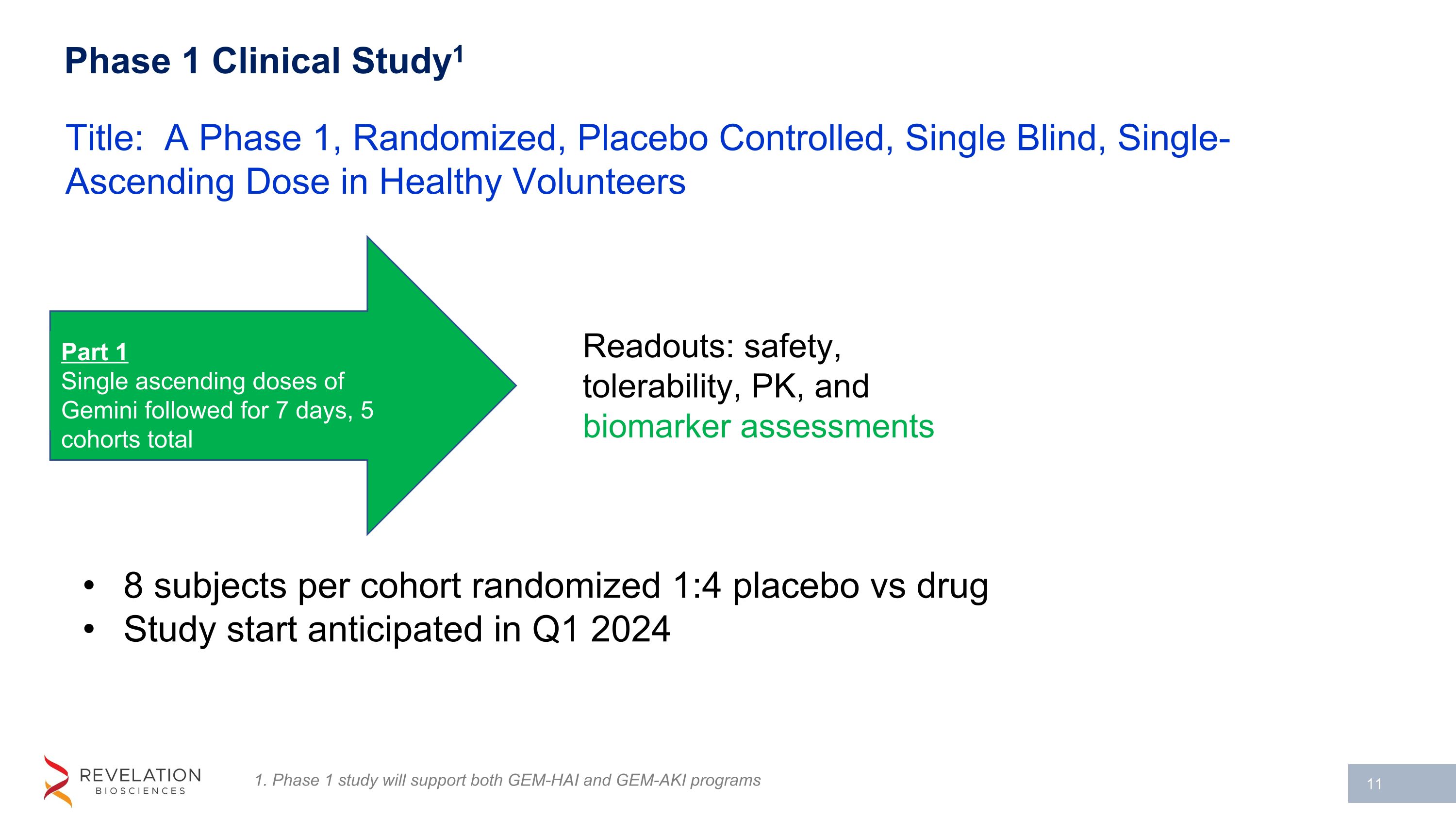
Phase 1 Clinical Study1 8 subjects per cohort randomized 1:4 placebo vs drug Study start anticipated in Q1 2024 Title: A Phase 1, Randomized, Placebo Controlled, Single Blind, Single-Ascending Dose in Healthy Volunteers Part 1 Single ascending doses of Gemini followed for 7 days, 5 cohorts total Readouts: safety, tolerability, PK, and biomarker assessments 1. Phase 1 study will support both GEM-HAI and GEM-AKI programs
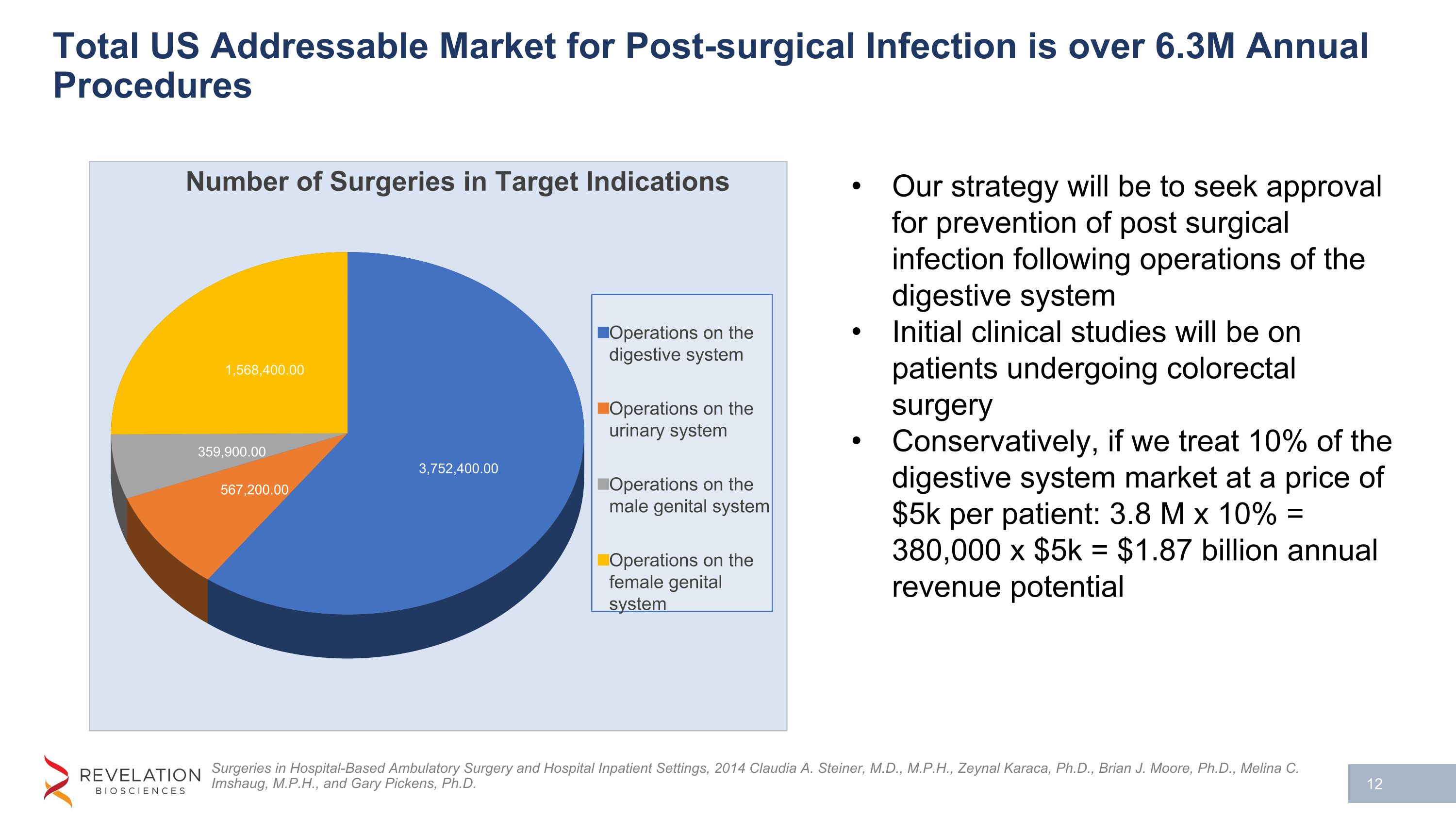
Total US Addressable Market for Post-surgical Infection is over 6.3M Annual Procedures Surgeries in Hospital-Based Ambulatory Surgery and Hospital Inpatient Settings, 2014 Claudia A. Steiner, M.D., M.P.H., Zeynal Karaca, Ph.D., Brian J. Moore, Ph.D., Melina C. Imshaug, M.P.H., and Gary Pickens, Ph.D. Our strategy will be to seek approval for prevention of post surgical infection following operations of the digestive system Initial clinical studies will be on patients undergoing colorectal surgery Conservatively, if we treat 10% of the digestive system market at a price of $5k per patient: 3.8 M x 10% = 380,000 x $5k = $1.87 billion annual revenue potential
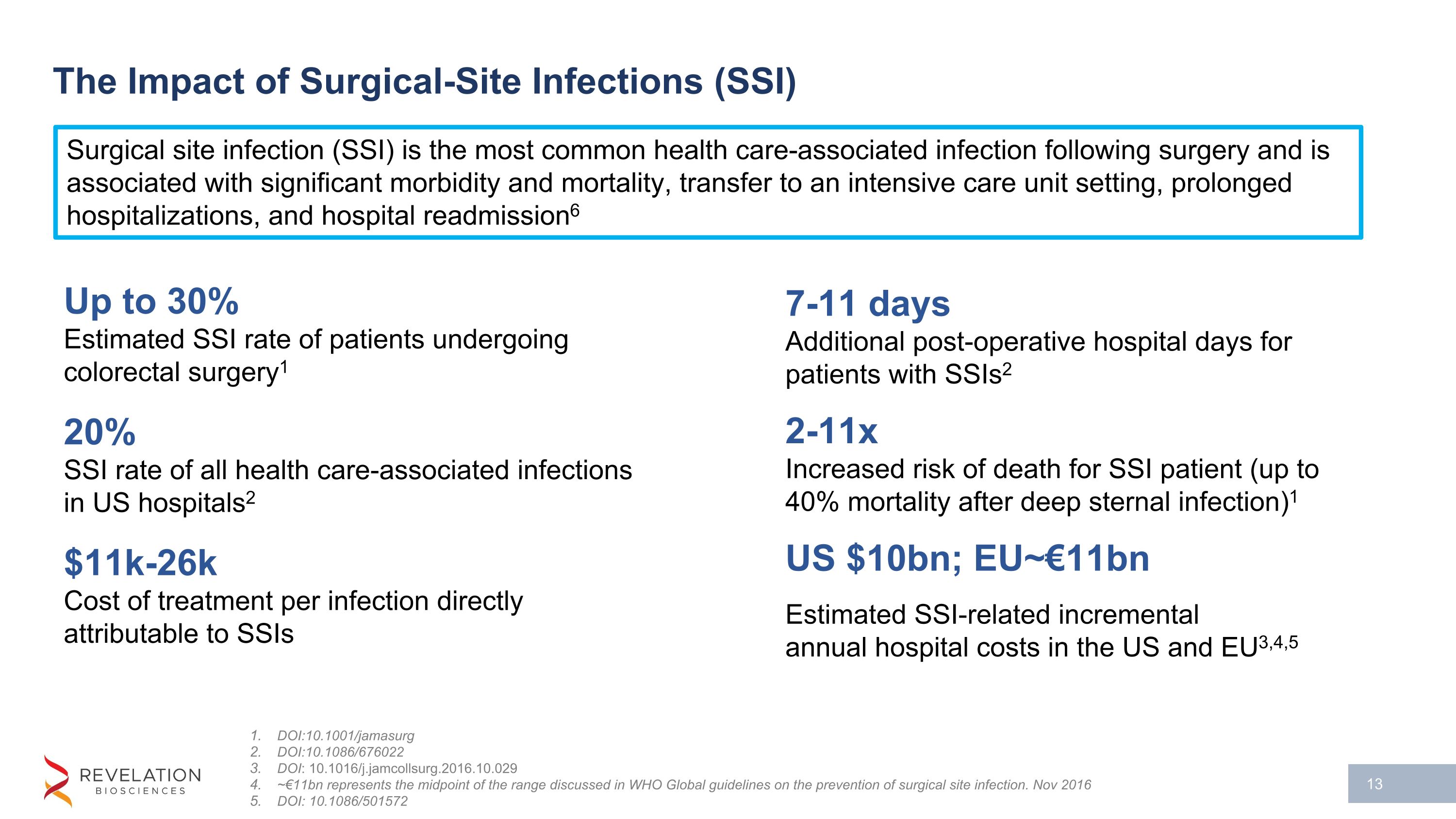
The Impact of Surgical-Site Infections (SSI) DOI:10.1001/jamasurg DOI:10.1086/676022 DOI: 10.1016/j.jamcollsurg.2016.10.029 ~€11bn represents the midpoint of the range discussed in WHO Global guidelines on the prevention of surgical site infection. Nov 2016 DOI: 10.1086/501572 Up to 30% Estimated SSI rate of patients undergoing colorectal surgery1 20% SSI rate of all health care-associated infections in US hospitals2 $11k-26k Cost of treatment per infection directly attributable to SSIs 7-11 days Additional post-operative hospital days for patients with SSIs2 2-11x Increased risk of death for SSI patient (up to 40% mortality after deep sternal infection)1 US $10bn; EU~€11bn Estimated SSI-related incremental�annual hospital costs in the US and EU3,4,5 Surgical site infection (SSI) is the most common health care-associated infection following surgery and is associated with significant morbidity and mortality, transfer to an intensive care unit setting, prolonged hospitalizations, and hospital readmission6

GEM-AKI and GEM-CKD Programs Gemini For the Prevention of Acute Kidney Injury and Chronic Kidney Disease
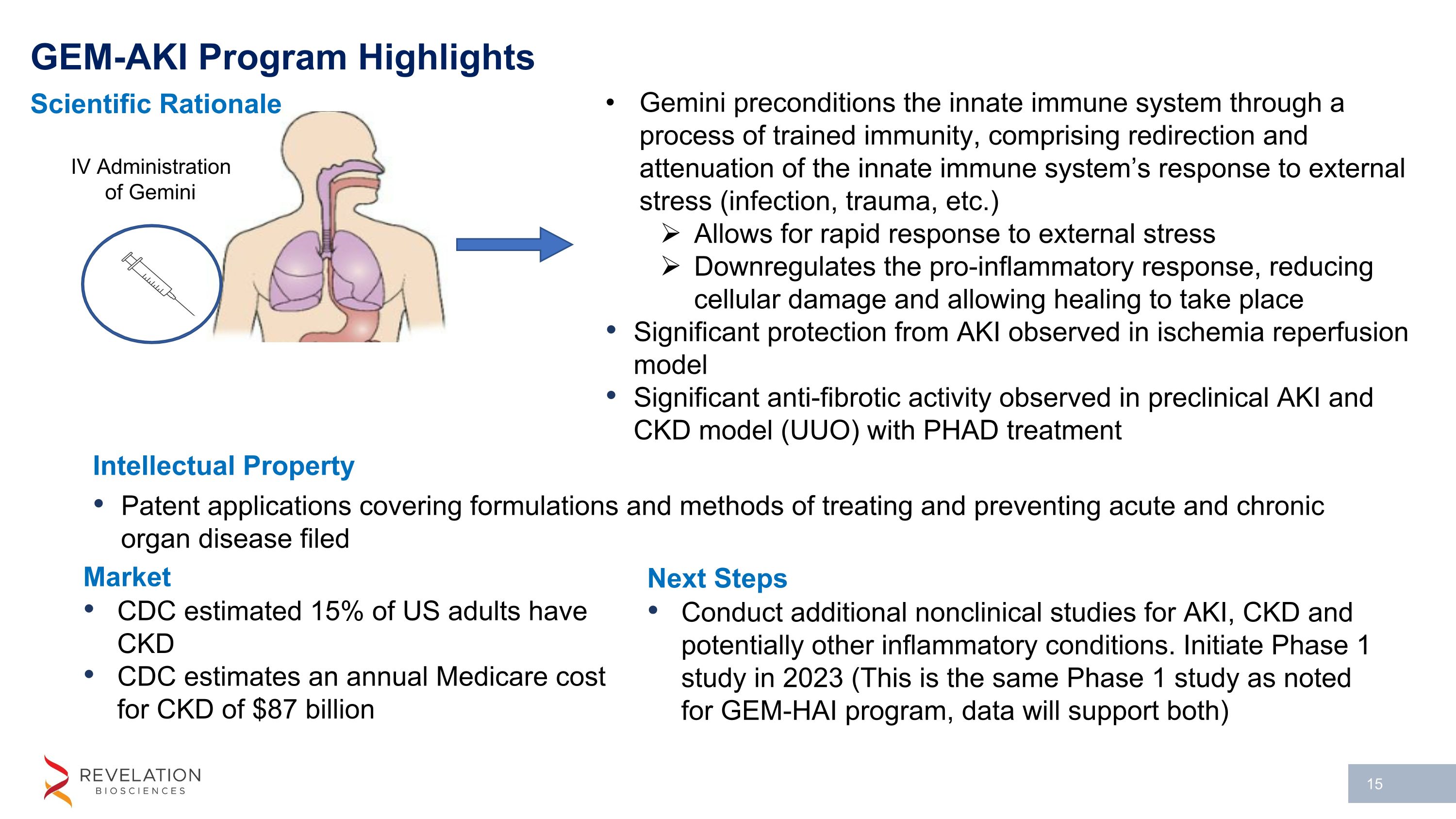
Gemini preconditions the innate immune system through a process of trained immunity, comprising redirection and attenuation of the innate immune system’s response to external stress (infection, trauma, etc.) Allows for rapid response to external stress Downregulates the pro-inflammatory response, reducing cellular damage and allowing healing to take place Significant protection from AKI observed in ischemia reperfusion model Significant anti-fibrotic activity observed in preclinical AKI and CKD model (UUO) with PHAD treatment IV Administration of Gemini GEM-AKI Program Highlights Intellectual Property Patent applications covering formulations and methods of treating and preventing acute and chronic organ disease filed Scientific Rationale Market CDC estimated 15% of US adults have CKD CDC estimates an annual Medicare cost for CKD of $87 billion Next Steps Conduct additional nonclinical studies for AKI, CKD and potentially other inflammatory conditions. Initiate Phase 1 study in 2023 (This is the same Phase 1 study as noted for GEM-HAI program, data will support both)
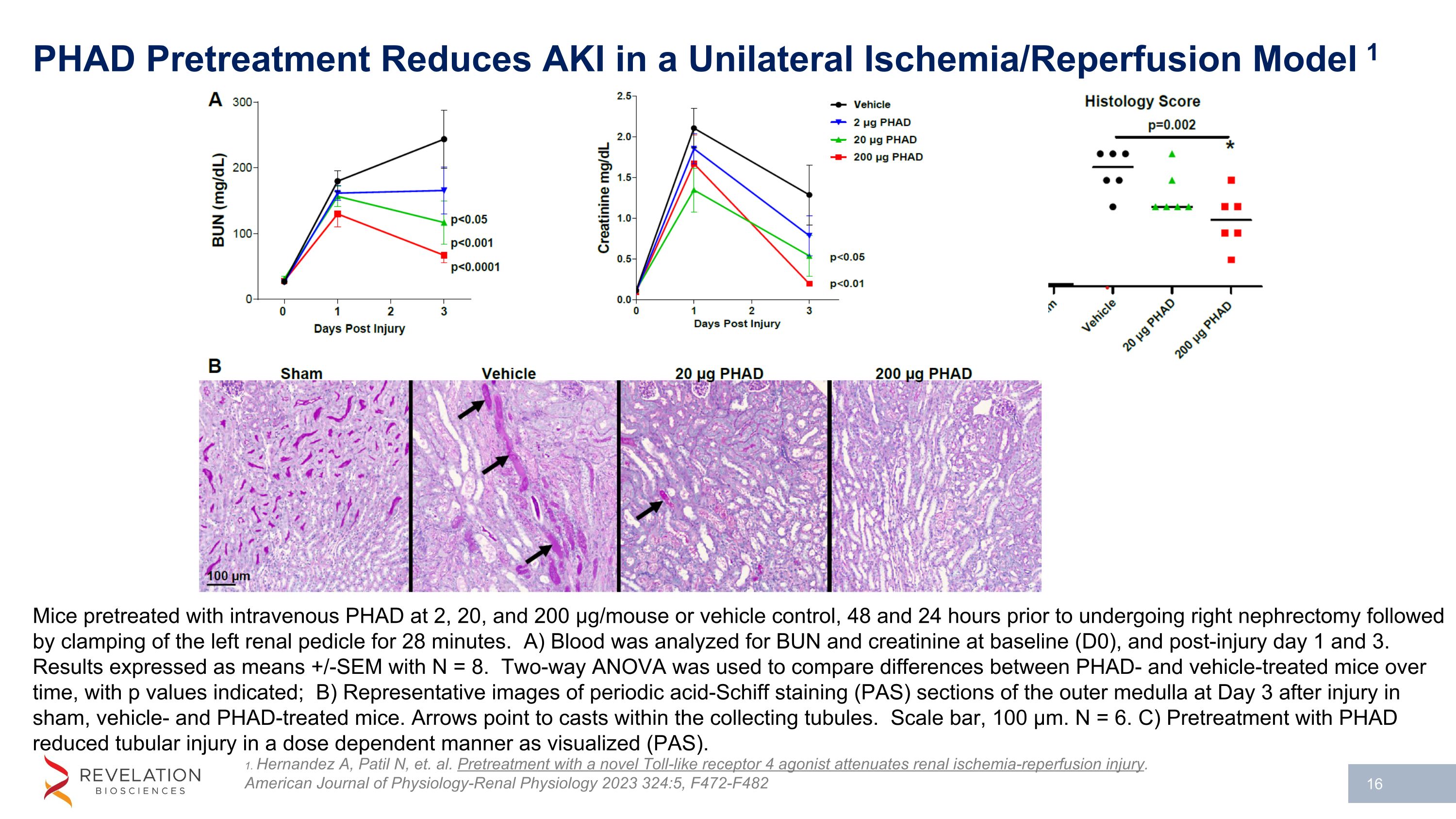
PHAD Pretreatment Reduces AKI in a Unilateral Ischemia/Reperfusion Model 1 1. Hernandez A, Patil N, et. al. Pretreatment with a novel Toll-like receptor 4 agonist attenuates renal ischemia-reperfusion injury. American Journal of Physiology-Renal Physiology 2023 324:5, F472-F482 Mice pretreated with intravenous PHAD at 2, 20, and 200 µg/mouse or vehicle control, 48 and 24 hours prior to undergoing right nephrectomy followed by clamping of the left renal pedicle for 28 minutes. A) Blood was analyzed for BUN and creatinine at baseline (D0), and post-injury day 1 and 3. Results expressed as means +/-SEM with N = 8. Two-way ANOVA was used to compare differences between PHAD- and vehicle-treated mice over time, with p values indicated; B) Representative images of periodic acid-Schiff staining (PAS) sections of the outer medulla at Day 3 after injury in sham, vehicle- and PHAD-treated mice. Arrows point to casts within the collecting tubules. Scale bar, 100 µm. N = 6. C) Pretreatment with PHAD reduced tubular injury in a dose dependent manner as visualized (PAS).
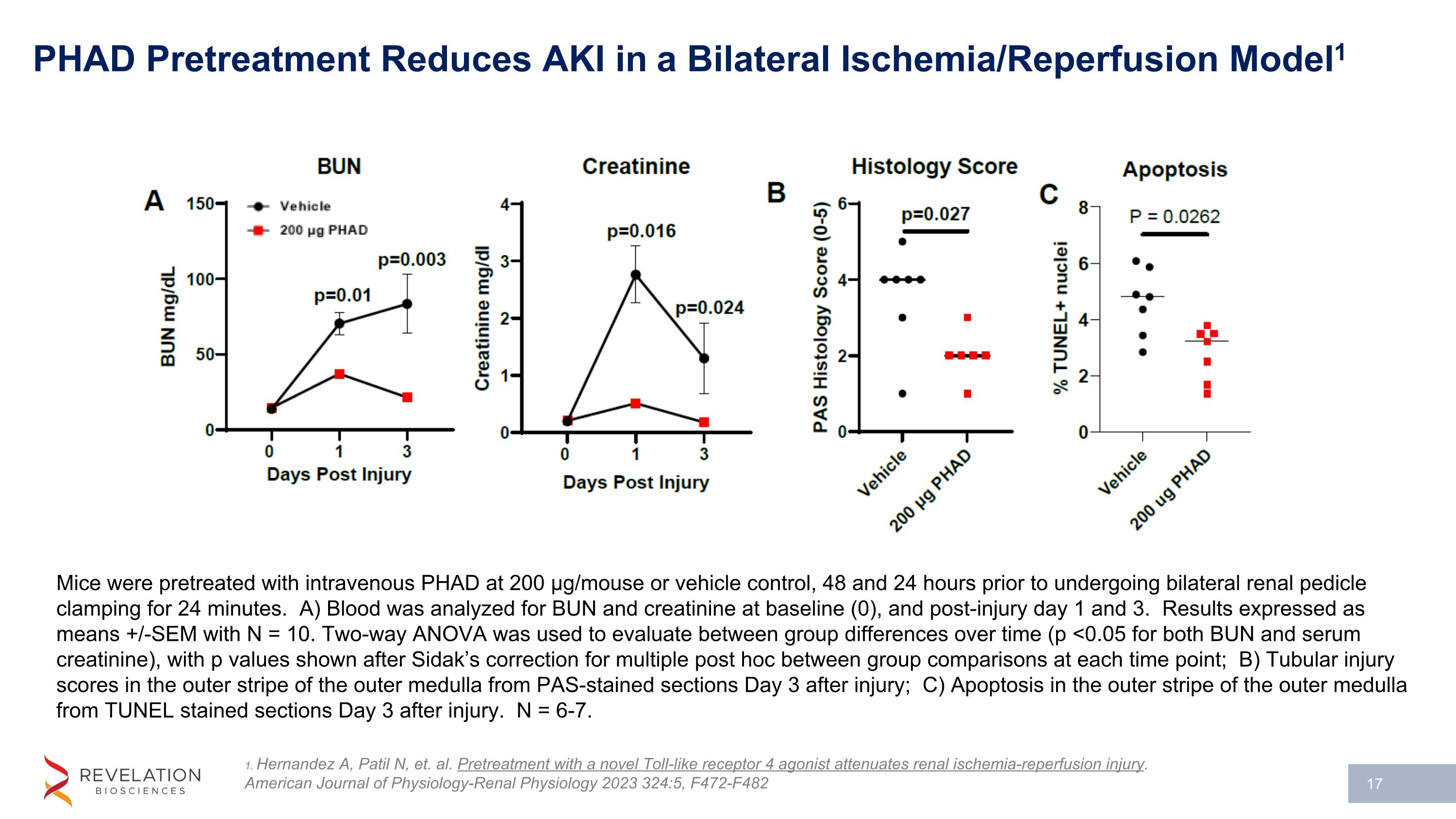
PHAD Pretreatment Reduces AKI in a Bilateral Ischemia/Reperfusion Model1 1. Hernandez A, Patil N, et. al. Pretreatment with a novel Toll-like receptor 4 agonist attenuates renal ischemia-reperfusion injury. American Journal of Physiology-Renal Physiology 2023 324:5, F472-F482 Mice were pretreated with intravenous PHAD at 200 µg/mouse or vehicle control, 48 and 24 hours prior to undergoing bilateral renal pedicle clamping for 24 minutes. A) Blood was analyzed for BUN and creatinine at baseline (0), and post-injury day 1 and 3. Results expressed as means +/-SEM with N = 10. Two-way ANOVA was used to evaluate between group differences over time (p <0.05 for both BUN and serum creatinine), with p values shown after Sidak’s correction for multiple post hoc between group comparisons at each time point; B) Tubular injury scores in the outer stripe of the outer medulla from PAS-stained sections Day 3 after injury; C) Apoptosis in the outer stripe of the outer medulla from TUNEL stained sections Day 3 after injury. N = 6-7.
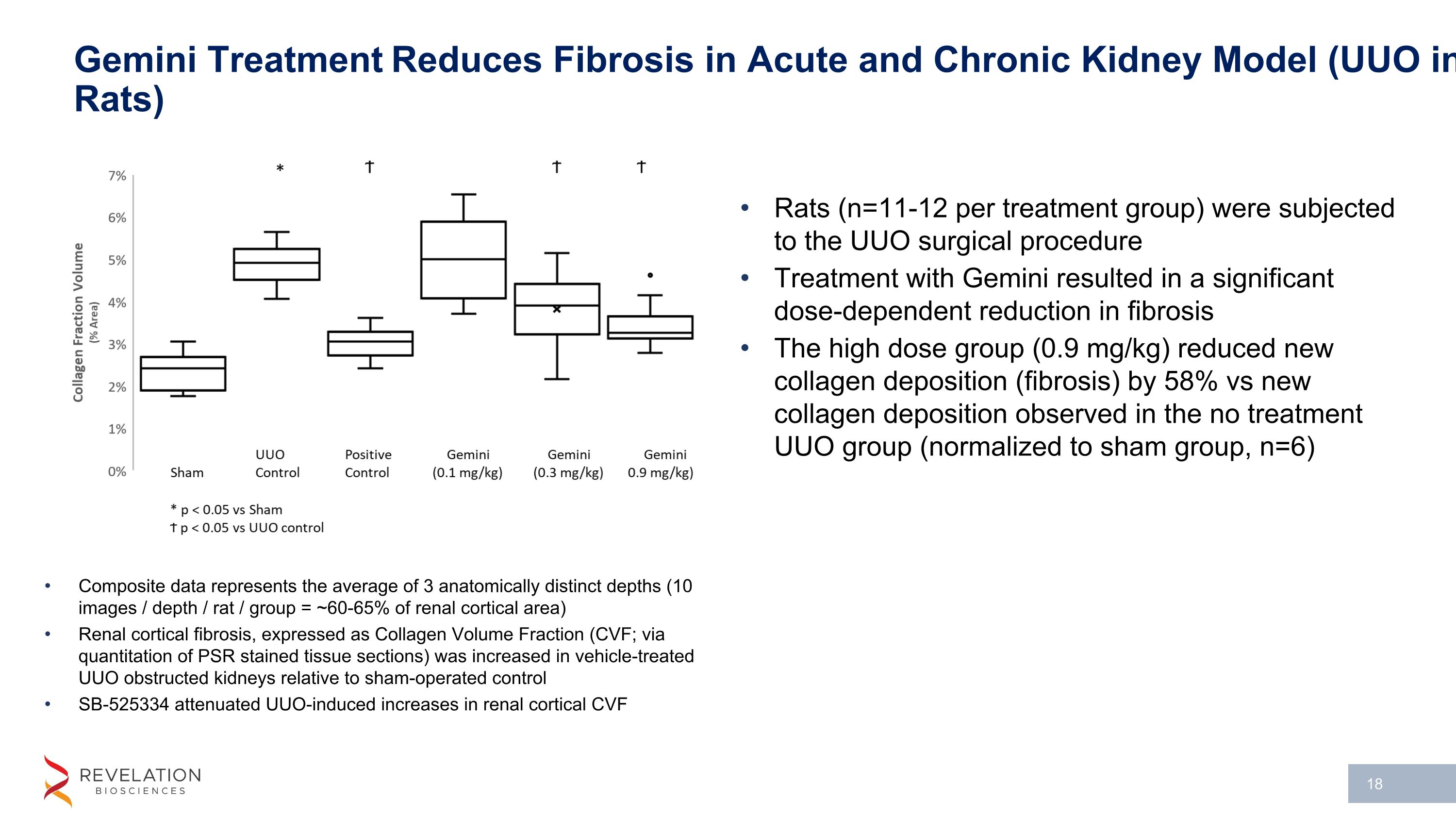
Gemini Treatment Reduces Fibrosis in Acute and Chronic Kidney Model (UUO in Rats) Composite data represents the average of 3 anatomically distinct depths (10 images / depth / rat / group = ~60-65% of renal cortical area) Renal cortical fibrosis, expressed as Collagen Volume Fraction (CVF; via quantitation of PSR stained tissue sections) was increased in vehicle-treated UUO obstructed kidneys relative to sham-operated control SB-525334 attenuated UUO-induced increases in renal cortical CVF Rats (n=11-12 per treatment group) were subjected to the UUO surgical procedure Treatment with Gemini resulted in a significant dose-dependent reduction in fibrosis The high dose group (0.9 mg/kg) reduced new collagen deposition (fibrosis) by 58% vs new collagen deposition observed in the no treatment UUO group (normalized to sham group, n=6)
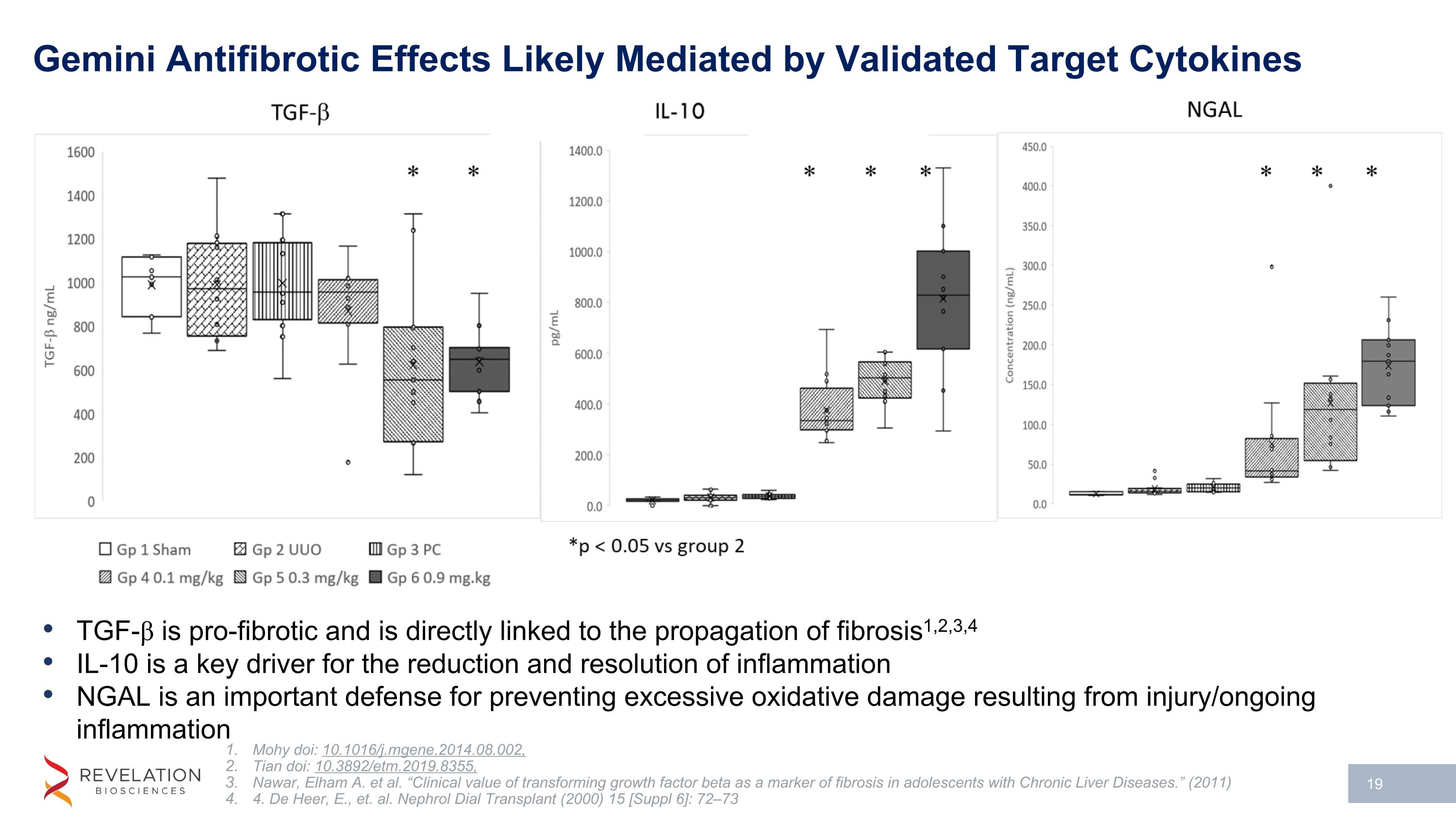
Gemini Antifibrotic Effects Likely Mediated by Validated Target Cytokines TGF-β is pro-fibrotic and is directly linked to the propagation of fibrosis1,2,3,4 IL-10 is a key driver for the reduction and resolution of inflammation NGAL is an important defense for preventing excessive oxidative damage resulting from injury/ongoing inflammation Mohy doi: 10.1016/j.mgene.2014.08.002, Tian doi: 10.3892/etm.2019.8355, Nawar, Elham A. et al. “Clinical value of transforming growth factor beta as a marker of fibrosis in adolescents with Chronic Liver Diseases.” (2011) 4. De Heer, E., et. al. Nephrol Dial Transplant (2000) 15 [Suppl 6]: 72–73
Phase 1 Clinical Study1 8 subjects per cohort randomized 1:4 placebo vs drug Study start anticipated in Q1 2024 Title: A Phase 1, Randomized, Placebo Controlled, Single Blind, Single-Ascending Dose in Healthy Volunteers Part 1 Single ascending doses of Gemini followed for 7 days, 5 cohorts total Readouts: safety, tolerability, PK, and biomarker assessments 1. Phase 1 study will support both GEM-HAI and GEM-AKI programs
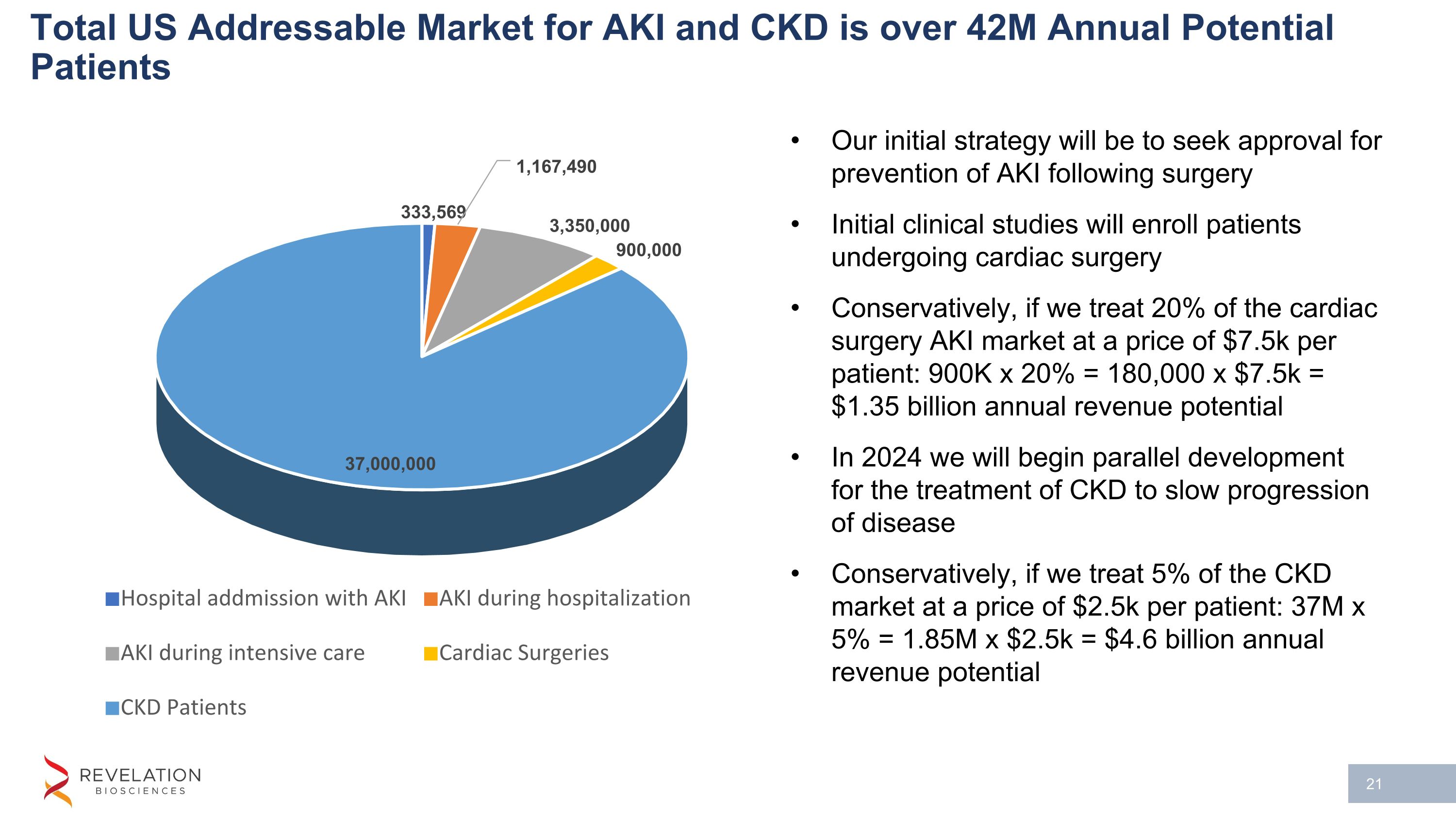
Total US Addressable Market for AKI and CKD is over 42M Annual Potential Patients Our initial strategy will be to seek approval for prevention of AKI following surgery Initial clinical studies will enroll patients undergoing cardiac surgery Conservatively, if we treat 20% of the cardiac surgery AKI market at a price of $7.5k per patient: 900K x 20% = 180,000 x $7.5k = $1.35 billion annual revenue potential In 2024 we will begin parallel development for the treatment of CKD to slow progression of disease Conservatively, if we treat 5% of the CKD market at a price of $2.5k per patient: 37M x 5% = 1.85M x $2.5k = $4.6 billion annual revenue potential
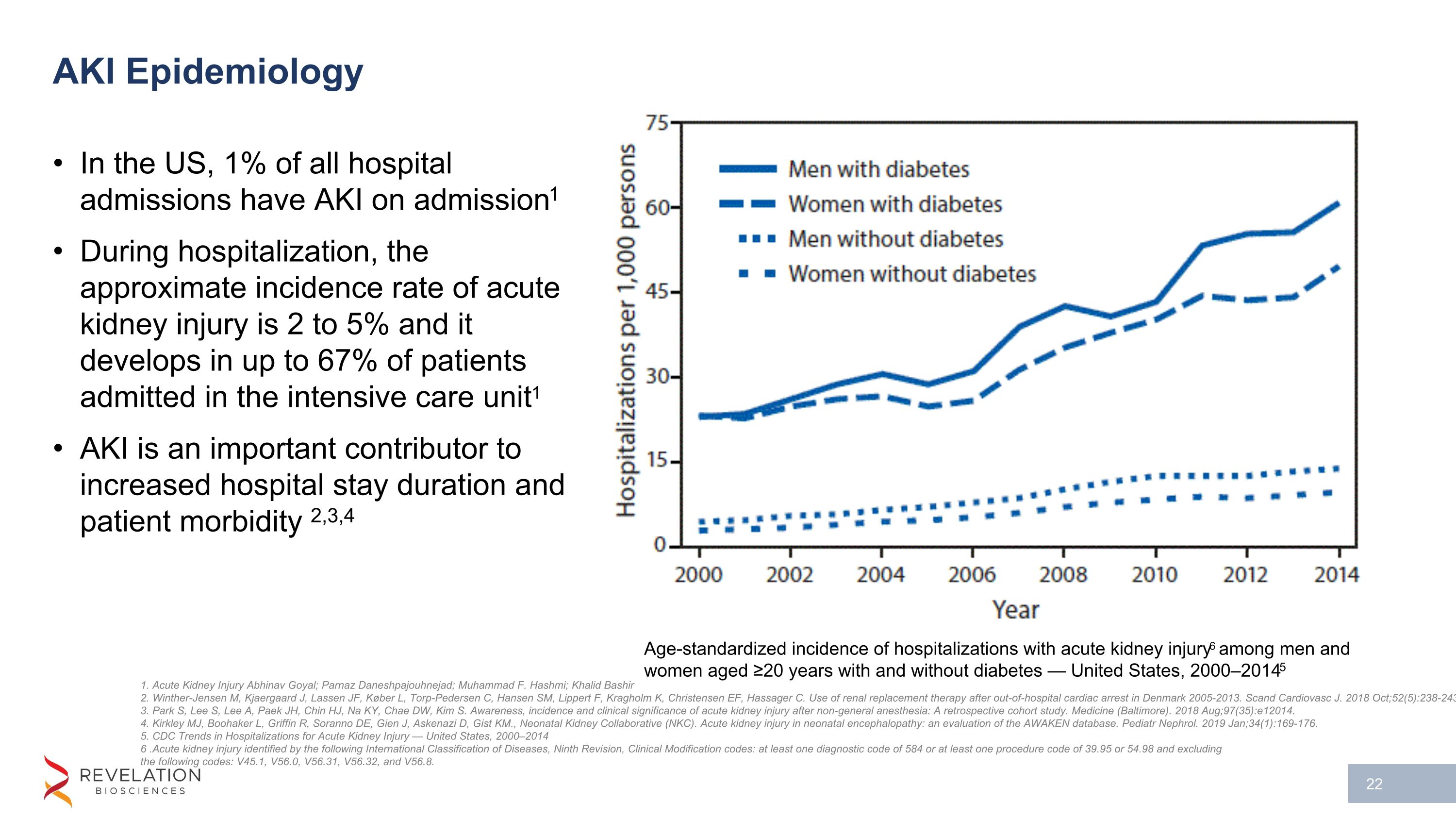
AKI Epidemiology In the US, 1% of all hospital admissions have AKI on admission1 During hospitalization, the approximate incidence rate of acute kidney injury is 2 to 5% and it develops in up to 67% of patients admitted in the intensive care unit1 AKI is an important contributor to increased hospital stay duration and patient morbidity 2,3,4 1. Acute Kidney Injury Abhinav Goyal; Parnaz Daneshpajouhnejad; Muhammad F. Hashmi; Khalid Bashir 2. Winther-Jensen M, Kjaergaard J, Lassen JF, Køber L, Torp-Pedersen C, Hansen SM, Lippert F, Kragholm K, Christensen EF, Hassager C. Use of renal replacement therapy after out-of-hospital cardiac arrest in Denmark 2005-2013. Scand Cardiovasc J. 2018 Oct;52(5):238-243 3. Park S, Lee S, Lee A, Paek JH, Chin HJ, Na KY, Chae DW, Kim S. Awareness, incidence and clinical significance of acute kidney injury after non-general anesthesia: A retrospective cohort study. Medicine (Baltimore). 2018 Aug;97(35):e12014. 4. Kirkley MJ, Boohaker L, Griffin R, Soranno DE, Gien J, Askenazi D, Gist KM., Neonatal Kidney Collaborative (NKC). Acute kidney injury in neonatal encephalopathy: an evaluation of the AWAKEN database. Pediatr Nephrol. 2019 Jan;34(1):169-176. 5. CDC Trends in Hospitalizations for Acute Kidney Injury — United States, 2000–2014 6 .Acute kidney injury identified by the following International Classification of Diseases, Ninth Revision, Clinical Modification codes: at least one diagnostic code of 584 or at least one procedure code of 39.95 or 54.98 and excluding the following codes: V45.1, V56.0, V56.31, V56.32, and V56.8. Age-standardized incidence of hospitalizations with acute kidney injury6 among men and women aged ≥20 years with and without diabetes — United States, 2000–20145
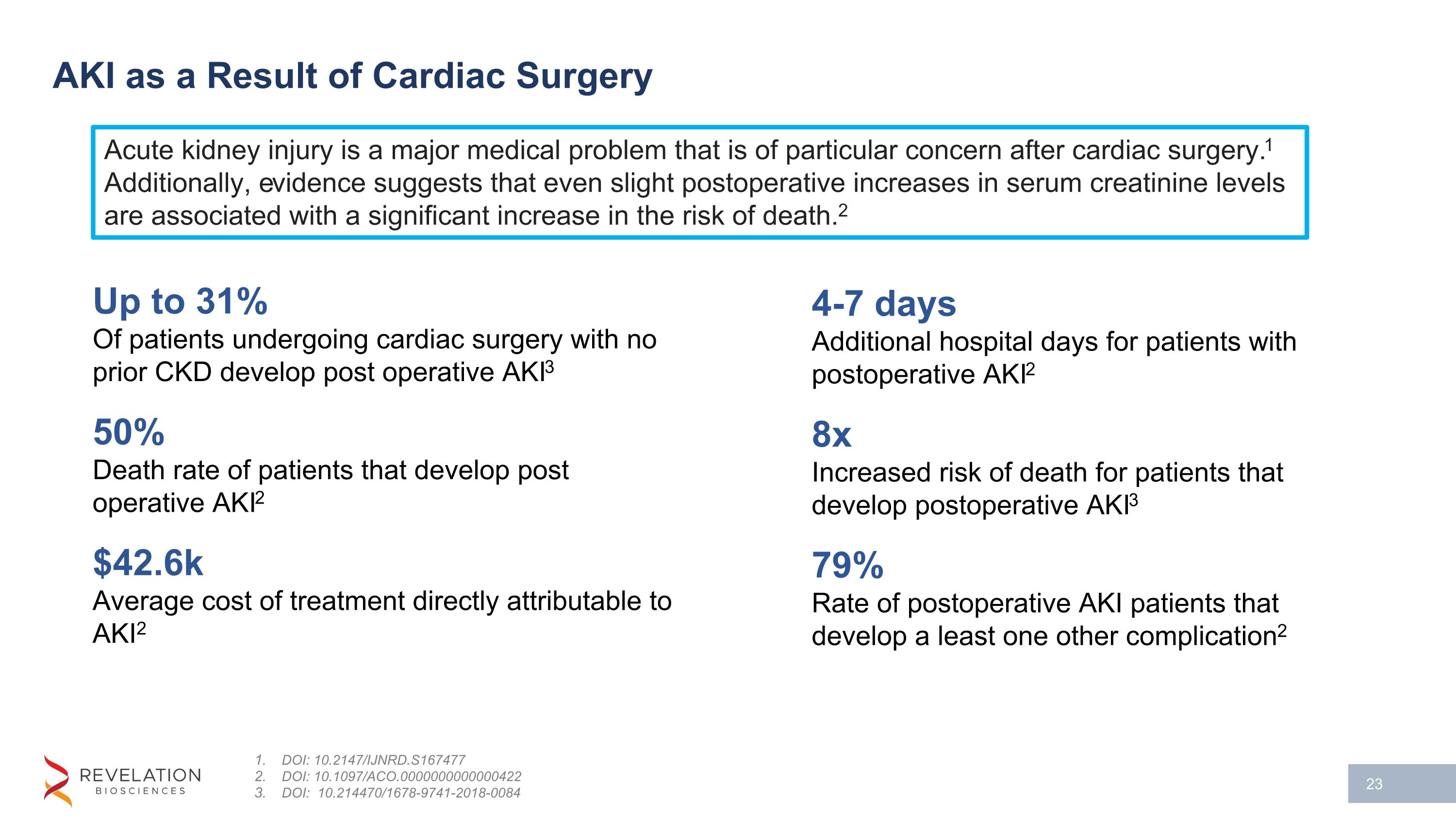
AKI as a Result of Cardiac Surgery DOI: 10.2147/IJNRD.S167477 DOI: 10.1097/ACO.0000000000000422 DOI: 10.214470/1678-9741-2018-0084 Up to 31% Of patients undergoing cardiac surgery with no prior CKD develop post operative AKI3 50% Death rate of patients that develop post operative AKI2 $42.6k Average cost of treatment directly attributable to AKI2 4-7 days Additional hospital days for patients with postoperative AKI2 8x Increased risk of death for patients that develop postoperative AKI3 79% Rate of postoperative AKI patients that develop a least one other complication2 Acute kidney injury is a major medical problem that is of particular concern after cardiac surgery.1 Additionally, evidence suggests that even slight postoperative increases in serum creatinine levels are associated with a significant increase in the risk of death.2

Financial Overview
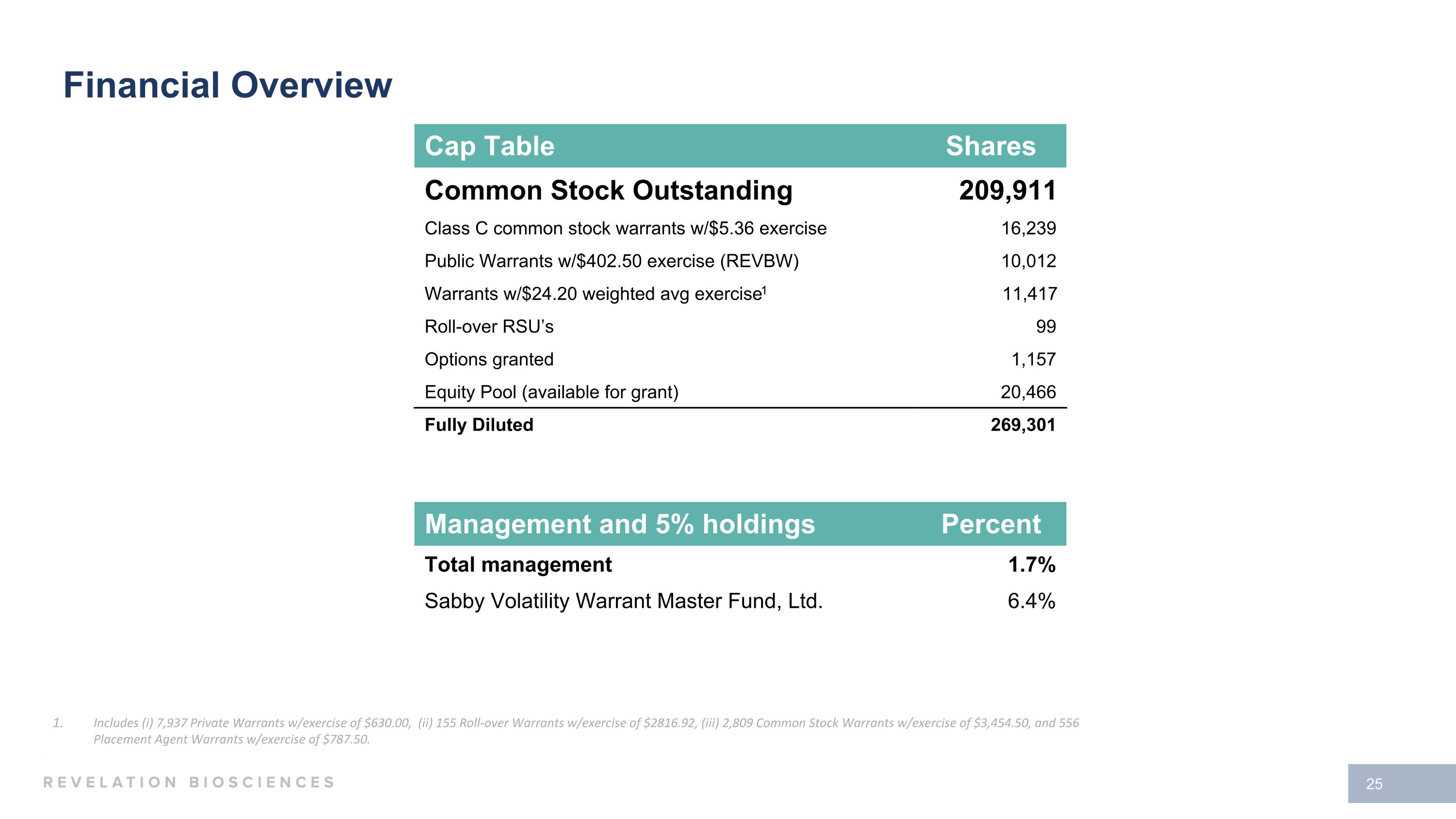
Management and 5% holdings Percent Total management 1.7% Sabby Volatility Warrant Master Fund, Ltd. 6.4% Cap Table Shares Common Stock Outstanding 209,911 Class C common stock warrants w/$5.36 exercise 16,239 Public Warrants w/$402.50 exercise (REVBW) 10,012 Warrants w/$24.20 weighted avg exercise1 11,417 Roll-over RSU’s 99 Options granted 1,157 Equity Pool (available for grant) 20,466 Fully Diluted 269,301 Financial Overview Includes (i) 7,937 Private Warrants w/exercise of $630.00, (ii) 155 Roll-over Warrants w/exercise of $2816.92, (iii) 2,809 Common Stock Warrants w/exercise of $3,454.50, and 556 Placement Agent Warrants w/exercise of $787.50.

For more information please visit www.revbiosciences.com Thank you!
v3.24.0.1
Document And Entity Information
|
Jan. 30, 2024 |
| Document Information [Line Items] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 30, 2024
|
| Entity Registrant Name |
REVELATION BIOSCIENCES, INC.
|
| Entity Central Index Key |
0001810560
|
| Entity Emerging Growth Company |
true
|
| Entity File Number |
001-39603
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
84-3898466
|
| Entity Address, Address Line One |
4660 La Jolla Village Drive
|
| Entity Address, Address Line Two |
Suite 100
|
| Entity Address, City or Town |
San Diego
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
92122
|
| City Area Code |
(650)
|
| Local Phone Number |
800-3717
|
| Entity Information, Former Legal or Registered Name |
Not Applicable
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Ex Transition Period |
false
|
| Redeemable Warrants Each Exercisable for A 1/1,050th Share of Common Stock at An Exercise Price of $12,075.00 per Share [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Redeemable warrants, each exercisable for a 1/1,050th share of common stock at an exercise price of $12,075.00 per share
|
| Trading Symbol |
REVBW
|
| Security Exchange Name |
NASDAQ
|
| Common Stock, Par Value $0.001 Per Share [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Common stock, par value $0.001 per share
|
| Trading Symbol |
REVB
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=revb_RedeemableWarrantsEachExercisableForA11050thShareOfCommonStockAtAnExercisePriceOf1207500PerShareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=revb_CommonStockParValue0001PerShareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Revelation Biosciences (NASDAQ:REVB)
Historical Stock Chart
From Mar 2025 to Apr 2025
Revelation Biosciences (NASDAQ:REVB)
Historical Stock Chart
From Apr 2024 to Apr 2025