Filed Pursuant to Rule 424(b)(5)
Registration No. 333-266589
PROSPECTUS SUPPLEMENT
(To the Prospectus Dated August 16, 2022)
Up to $335,921
Common Stock
We previously entered into an At the Market Offering Agreement dated as of February 5, 2021, or the sales agreement, with Ladenburg Thalmann & Co. Inc., or Ladenburg, relating to shares of our common stock, par value $0.0001 per share, offered by this prospectus supplement. In accordance with the terms of the sales agreement, we may offer and sell shares of our common stock from time to time through Ladenburg, acting as sales agent.
Sales of our common stock, if any, under this prospectus supplement and the accompanying prospectus will be made in sales deemed to be an “at the market offering” as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended, or the Securities Act. Ladenburg is not required to sell any specific amount of securities, but will act as our sales agent using commercially reasonable efforts consistent with its normal trading and sales practices, on mutually agreed terms between Ladenburg and us. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
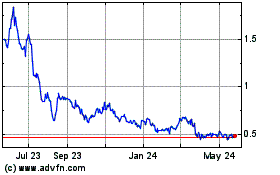
Our common stock is listed on The Nasdaq Stock Market LLC under the symbol “SLRX.” On July 25, 2024, the last reported sale price of our common stock on The Nasdaq Capital Market was $3.50 per share. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell shares pursuant to this prospectus supplement with a value of more than one-third of the aggregate market value of our common stock held by non-affiliates in any 12-month period, so long as the aggregate market value of our common stock held by non-affiliates is less than $75,000,000. As of July 25, 2024, the aggregate market value of our outstanding common stock held by non-affiliates, or the public float, was $5,050,219 which was calculated based on 1,320,664 shares of our outstanding common stock held by non-affiliates at a price of $3.824 per share, the closing price of our common stock on June 3, 2024, a date that is within 60 days of filing this prospectus supplement. As of the date hereof, we have sold $1,347,484 of shares of our common stock pursuant to General Instruction I.B.6 of Form S-3 during the prior 12 calendar month period that ends on and includes the date hereof. As a result of these limitations and the current public float of our common stock, and in accordance with the terms of the sales agreement, we may offer and sell shares of our common stock having an aggregate offering price of up to $335,921 from time to time through Ladenburg.
The compensation to Ladenburg for sales of common stock sold pursuant to the sales agreement will be an amount equal to 3.0% of the gross proceeds of any shares of common stock sold under the sales agreement. In connection with the sale of the common stock on our behalf, Ladenburg may be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of Ladenburg may be deemed to be underwriting commissions or discounts. We have also agreed to provide indemnification and contribution to Ladenburg with respect to certain liabilities, including liabilities under the Securities Act or the Securities Exchange Act of 1934, as amended, or the Exchange Act.
Investing in our securities involves a high degree of risk. See “Risk Factors” beginning on page S-5 of this prospectus supplement and elsewhere in this prospectus supplement, the accompanying base prospectus and the other documents that are incorporated by reference in this prospectus supplement and the accompanying prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Ladenburg Thalmann
The date of this prospectus supplement is July 25, 2024.
TABLE OF CONTENTS
Prospectus Supplement
| | | | | |
ABOUT THIS PROSPECTUS SUPPLEMENT | |
PROSPECTUS SUPPLEMENT SUMMARY | |
THE OFFERING | |
RISK FACTORS | |
NOTE REGARDING FORWARD-LOOKING STATEMENTS | |
USE OF PROCEEDS | |
PLAN OF DISTRIBUTION | |
LEGAL MATTERS | |
EXPERTS | |
WHERE YOU CAN FIND MORE INFORMATION | |
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE | |
Prospectus
| | | | | |
|
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| INCORPORATION BY REFERENCE | |
| |
| EXPERTS | |
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying prospectus are part of a “shelf” registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or SEC, on August 5, 2022 and which was declared effective by the SEC on August 16, 2022. This document is in two parts. The first part is this prospectus supplement, which describes the terms of this offering of our common stock and adds to and updates the information contained in the accompanying prospectus. The second part, the accompanying prospectus, provides more general information, some of which may not apply to this offering. Generally, when we refer to this prospectus, we are referring to both parts of this document combined. To the extent there is a conflict between the information contained in this prospectus supplement and the information contained in the accompanying prospectus, you should rely on the information in this prospectus supplement.
This prospectus supplement and the accompanying prospectus relate to the offering of shares of our common stock. Before buying any of the shares of common stock offered hereby, we urge you to read carefully this prospectus supplement and the accompanying prospectus, together with the information incorporated herein by reference as described below under the heading “Incorporation of Certain Information by Reference.” This prospectus supplement contains information about the common stock offered hereby and may add to, update or change information in the accompanying prospectus.
You should rely only on the information contained in, or incorporated by reference into, this prospectus supplement and the accompanying prospectus. We have not, and Ladenburg has not, authorized anyone to provide you with different or additional information.
We are not making offers to sell or solicitations to buy our common stock in any jurisdiction in which an offer or solicitation is not authorized or in which the person making that offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make an offer or solicitation. You should assume that the information in this prospectus supplement and the accompanying prospectus is accurate only as of the date on the front of the respective document and that any information that we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus supplement or the accompanying prospectus or the time of any sale of our common stock.
This prospectus supplement and the accompanying prospectus contain summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated herein by reference as exhibits to the registration statement, and you may obtain copies of those documents as described below under the section entitled “Where You Can Find More Information.”
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference herein were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
This prospectus supplement and the accompanying prospectus contain and incorporate by reference market data and industry statistics and forecasts that are based on independent industry publications and other publicly-available information. Although we believe these sources are reliable, we do not guarantee the accuracy or completeness of this information and we have not independently verified this information. Although we are not aware of any misstatements regarding the market and industry data presented in this prospectus supplement, the accompanying prospectus or the documents incorporated herein by reference, these estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the headings “Risk Factors” in this prospectus supplement and the accompanying prospectus, and under similar headings in the other documents that are incorporated herein by reference. Accordingly, investors should not place undue reliance on this information.
Unless the context otherwise requires, in this prospectus supplement the “Company,” “we,” “us,” “our” and similar names refer to Salarius Pharmaceuticals, Inc. and its subsidiaries.
“Salarius Pharmaceuticals,” “SLRX” and the Salarius logo are our trademarks. This prospectus supplement and the accompanying prospectus and the documents incorporated by reference herein and therein may also contain trademarks and trade names that are the property of their respective owners. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply relationships with, or endorsements or sponsorship of us by, these other companies.
On June 14, 2024, we filed a Certificate of Amendment to our restated certificate of incorporation, as amended, with the Secretary of State of the State of Delaware to effect a 1-for-8 reverse stock split of our issued and outstanding shares of common stock, par value $0.0001 per share, which became effective on June 14, 2024. All historical share and per share amounts reflected throughout this report have been adjusted to reflect the reverse stock split.
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights selected information about us and this offering and does not contain all of the information that you should consider in making your investment decision. You should carefully read this entire prospectus supplement and the accompanying prospectus, including the risks and uncertainties discussed under the heading “Risk Factors” beginning on page S-5 of this prospectus supplement, and the information incorporated by reference in this prospectus supplement and the accompanying prospectus, including our financial statements, before making an investment decision. If you invest in our securities, you are assuming a high degree of risk.
Company Overview
We are a clinical-stage biopharmaceutical company focused on developing effective treatments for parties with cancer with high, unmet medical need. Specifically, we are concentrated on developing treatments for cancers caused by dysregulated gene expression, i.e., genes which are incorrectly turned on or off. We have two classes of drugs that address gene dysregulation: targeted protein inhibitors and targeted protein degraders. Our technologies have the potential to work in both liquid and solid tumors. Our current pipeline consists of two small molecule drugs: 1) SP-3164, targeted protein degrader, and 2) seclidemstat (SP-2577), a targeted inhibitor. We are located in Houston, Texas.
On August 8, 2023, we announced that we retained Canaccord Genuity, LLC to lead a comprehensive review of strategic alternatives focusing on maximizing stockholder value, including but not limited to, an acquisition, merger, reverse merger, divestiture of assets, licensing, or other strategic transactions involving our company. In connection with the evaluation of strategic alternatives and in order to extend our resources, we implemented multiple cost-savings plans to extend our expected cash runway in the first half of 2025.
For more information about our company, please refer to other documents that we have filed with the SEC and that are incorporated by reference into this prospectus, as listed under the heading “Incorporation of Certain Information by Reference.”
Recent Developments
On July 9, 2024, we were notified by researchers at The University of Texas MD Anderson Cancer Center, or MDACC, that a patient in MDACC’s sponsored clinical trial evaluating seclidemstat (SP-2577) in combination with azacitidine in adult patients with myelodysplastic syndromes and chronic myelomonocytic leukemia experienced a serious and unexpected grade 4 adverse event. Per protocol, the U.S. Food and Drug Administration (FDA) was notified and MDACC subsequently received notification from the FDA placing the clinical trial on partial clinical hold. Under the partial clinical hold, no new patients may be enrolled at this time, but currently enrolled subjects may continue treatment and all study procedures if they are benefiting. We intend to support researchers at MDACC to analyze the available data and respond to questions submitted by the FDA.
On July 19, 2024, we announced we have determined to close our ongoing Phase 1/2 clinical trial evaluating seclidemstat for Ewing sarcoma, including closing the remaining clinical trial sites. We are terminating the ongoing clinical trial in an effort to conserve cash while our Board of Directors continues its exploration of potential strategic alternatives focused on maximizing shareholder value and potential options to continue the clinical development for Ewing sarcoma in the future. We intend to continue supporting The University of Texas MD Anderson Cancer Center (“MDACC”) in MDACC’s sponsored clinical trial evaluating seclidemstat (SP-2577) in combination with azacitidine in adult patients with myelodysplastic syndromes and chronic myelomonocytic leukemia, which remains on partial clinical hold following a serious and unexpected grade 4 adverse event.
Corporate Information
We were incorporated as Flex Pharma, Inc., or Flex Pharma, in Delaware in February 2014. In July 2019, our wholly owned subsidiary, Falcon Acquisition Sub, LLC, merged with and into Salarius Pharmaceuticals, LLC, or Private Salarius, with Private Salarius becoming our wholly owned subsidiary, or the Merger, and we changed our
named to Salarius Pharmaceuticals, Inc. Our principal executive offices are located at 2450 Holcombe Blvd., Suite X, Houston, Texas 77021, and our telephone number is (832) 804-9144. Our website address is www.salariuspharma.com. We do not incorporate the information on, or accessible through, our website into this prospectus, and you should not consider any information on, or accessible through, our website as part of this prospectus.
THE OFFERING
| | | | | |
| Common stock offered by us pursuant to this prospectus supplement | Shares of our common stock having an aggregate offering price of up to $335,921. |
| Common stock to be outstanding after this offering | Up to 1,427,428 shares, assuming sales of 95,977 shares of our common stock in this offering at an offering price of $3.50 per share, which was the last reported sale price of our common stock on the Nasdaq Capital Market on July 25, 2024. The actual number of shares issued will vary depending on the sales price under this offering. |
| Manner of Offering | “At the market offering” as defined in Rule 415(a)(4) under the Securities Act, that may be made from time to time on the Nasdaq Capital Market, the existing trading market for our common stock, through our sales agent, Ladenburg. See “Plan of Distribution” on page S-13 of this prospectus supplement. |
| Use of Proceeds | We intend to use the net proceeds from this offering for general corporate purposes and working capital. We may also use a portion of the net proceeds from this offering to acquire or invest in complementary businesses, technologies, product candidates or other intellectual property, although we have no present commitments or agreements to do so. See “Use of Proceeds” on page S-12 of this prospectus supplement. |
| Risk Factors | An investment in our common stock involves a high degree of risk. See the information contained in or incorporated by reference under “Risk Factors” on page S-5 of this prospectus supplement, page 25 of our Annual Report on Form 10-K for the year ended December 31, 2023, page 21 of our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2024 and under similar headings in the other documents that are incorporated by reference herein, as well as the other information included in or incorporated by reference in this prospectus supplement and the accompanying prospectus. |
| The Nasdaq Capital Market symbol | SLRX |
Outstanding Shares
The number of shares of our common stock to be outstanding after this offering is based on 539,304 shares of our common stock outstanding as of March 31, 2024, less (i) 96 shares that were extinguished in connection with the reverse stock split on June 14, 2024 pursuant to the exchange of fractional shares for cash payments in lieu thereof, plus (ii) 499,243 shares of our common stock issued pursuant to the sales agreement subsequent to March 31, 2024 through July 25, 2024, and (iii) 293,000 shares of our common stock issued pursuant to the exercise of pre-funded warrants subsequent to March 31, 2024 through July 25, 2024. Unless specifically stated otherwise, the information in this prospectus supplement is as of March 31, 2024, and excludes:
•28,990 shares of our common stock issuable upon the exercise of stock options outstanding as of March 31, 2024 at a weighted-average exercise price of $72.32 per share;
•131 shares of our common stock issuable upon the settlement of restricted stock units outstanding as of March 31, 2024;
•1,015,384 shares of our common stock issuable upon the exercise of warrants outstanding as of March 31, 2024 at a weighted average exercise price of $23.92 per share;
•12,407 shares of our common stock available for future issuance under our 2015 Equity Incentive Plan as of March 31, 2024; and
•25,501 shares of our common stock reserved for future issuance under our 2015 Employee Stock Purchase Plan, or the ESPP, as of March 31, 2024.
RISK FACTORS
Investing in our common stock involves a high degree of risk. Before investing in our common stock, you should carefully consider the risks described below, together with all of the other information contained in this prospectus supplement and the accompanying prospectus and incorporated by reference herein and therein, including from our most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q or Current Reports on Form 8-K, as well as any amendments or update to our risk factors thereto reflected in subsequent filings with the SEC. Some of these factors relate principally to our business and the industry in which we operate. Other factors relate principally to your investment in our securities. The risks and uncertainties described therein and below are not the only risks facing us. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also materially and adversely affect our business and operations.
If any of the matters included in the following risks were to occur, our business, financial condition, results of operations, cash flows or prospects could be materially and adversely affected. In such case, you may lose all or part of your investment.
Risks Related to our Financial Position and Capital Needs and Company
We do not currently have sufficient working capital to fund our planned operations for the next twelve months and may not be able to continue as a going concern. There is uncertainty regarding our ability to maintain liquidity sufficient to operate our business, which raises substantial doubt about our ability to continue as a going concern.
We do not currently have adequate financial resources to fund our forecasted operating costs for at least twelve months from the filing of this prospectus. As of March 31, 2024, we had a cash and cash equivalents balance of approximately $4.4 million. As of March 31, 2024, we have incurred an accumulated deficit of $78.1 million. For the year ended December 31, 2023, we reported net losses of $12.5 million. As a result, our existing cash resources are sufficient to meet our anticipated needs into the first half of 2025, even after taking into account our significantly reduced operations, we would need to raise additional capital in the next several months in order to avoid a wind down and dissolution of our company. Our auditor’s report on our financial statements for the year ended December 31, 2023 includes an explanatory paragraph related to the existence of substantial doubt about our ability to continue as a going concern. Our ability to continue as a going concern is dependent upon our ability to obtain additional equity or debt financing, attain further operating efficiencies, reduce expenditures, and, ultimately, to generate revenue. Since inception, we have incurred net losses and negative cash flows from operations. We may not ever obtain additional financing. Our existing cash and cash equivalents will not be sufficient to enable us to continue the clinical development and commercialization of our product candidates for any indications or to in license any other product candidates and develop them. Although we are currently exploring various strategic alternatives, these strategic alternatives may not be successful in the next several months prior to our cash position getting to the point that we will need to pursue the winding down and dissolution of our company. If we do not raise capital in the next several months or engage a strategic partner, we will be forced to cease operations and liquidate our assets and seek bankruptcy protection or engage in a similar process. As such, we cannot conclude that such plans will be effectively implemented within one year after the date of this prospectus and there is uncertainty regarding our ability to maintain liquidity sufficient to operate our business effectively, which raises substantial doubt about our ability to continue as a going concern.
If we do not successfully complete a strategic transaction or raise additional capital, we will need to pursue a dissolution and liquidation of our company. In such an event, the amount of cash available for distribution to our stockholders will depend heavily on the timing of such liquidation as well as the amount of cash that will need to be reserved for commitments and contingent liabilities.
There can be no guarantee that the process to identify a strategic transaction will result in a successfully completed transaction. If no strategic transaction is completed and we are unable to raise additional capital in the next several months, we will be forced to cease operations, liquidate assets and possibly seek bankruptcy protection or engage in a similar process. In that event, the amount of cash available for distribution to our stockholders will depend heavily
on the timing of such decision and, ultimately, such liquidation, since the amount of cash available for distribution continues to decrease as we fund our operations and evaluate our strategic alternatives. In addition, if our board of directors were to approve and recommend, and our stockholders were to approve, a dissolution of our company, we would be required under Delaware corporate law to pay our outstanding obligations, as well as to make reasonable provision for contingent and unknown obligations, prior to making any distributions in liquidation to our stockholders. As a result of this requirement, a portion of our assets may need to be reserved pending the resolution of such obligations. In addition, we may be subject to litigation or other claims related to a dissolution and liquidation of our company. If a dissolution and liquidation were pursued, our board of directors, in consultation with its advisors, would need to evaluate these matters and make a determination about a reasonable amount to reserve. Accordingly, holders of our common stock could lose all or a significant portion of their investment in the event of a dissolution, liquidation or winding up of our company.
Our common stock may be subject to delisting from Nasdaq.
Our common stock is currently listed on the Nasdaq Capital Market, or Nasdaq. To maintain our listing on Nasdaq, we are required to maintain: (i) a minimum bid price of $1.00 per share, (ii) a market value of publicly held securities of $1 million, (iii) a certain number of round lot stockholders and (iv) one of the following: a net income from continuing operations (in the latest fiscal year or two of the three last fiscal years) of at least $500,000, a market value of listed securities of at least $35 million or a stockholders' equity of at least $2.5 million. Nasdaq has the authority to delist our common stock if we fail to maintain these minimum requirements. In addition, Nasdaq may delist us if, based on Nasdaq’s review of our company and pursuant to Nasdaq Listing Rule 5101, Nasdaq believes that we are a “public shell” and that the continued listing of our securities in no longer warranted. We have no current plans to delist our shares of common stock from Nasdaq. However, following the decision to close the clinical development of seclidemstat for Ewing sarcoma, we may be treated as a public shell under Nasdaq rules. Although Nasdaq evaluates whether a listed company is a public shell company based on a facts and circumstances determination, a Nasdaq-listed company with no or nominal operations and either no or nominal assets, assets consisting solely of cash and cash equivalents, or assets consisting of any amount of cash and cash equivalents and nominal other assets is generally considered to be a public shell company. Listed companies determined to be public shell companies by Nasdaq may be subject to delisting proceedings or additional and more stringent listing criteria.
As of July 25, 2025, the market value of our publicly held securities was approximately $4.6 million. Further, as of March 31, 2024, (i) we had a total stockholders’ equity of approximately $3.6 million, (ii) we have not had net income in any period during this fiscal year or either of the two last fiscal years and (iii) the market value of our listed securities is below $35 million. We expect our stockholders’ equity to be approximately $2.5 million as of June 30, 2024. If the market value of our publicly held securities drops below $1 million and/or our total stockholders’ equity drops below $2.5 million, we will be subject to delisting from Nasdaq subject to certain applicable cure periods.
We are actively monitoring the market value of our publicly held securities and our stockholders’ equity and will consider any and all options available to us to maintain compliance. There can be no assurance, however, that we will be able to maintain compliance and meet Nasdaq’s continued listing requirements.
If our common stock is delisted from Nasdaq, whether because Nasdaq determines we are a “public shell” or we fail to maintain compliance with the continued listed requirements, or otherwise, our securities may qualify for trading over-the-counter, or OTC, in the United States on a market colloquially referred to as the “Pink Sheets.” Securities quoted on OTC are generally subject to lesser requirements than securities listed for trading on a U.S. national stock exchange, such as Nasdaq, including reduced corporate governance and public reporting standards. If Nasdaq should delist our common stock from trading, a reduction in some or all of the following may occur, each of which could have a material adverse effect on holders of our common stock: the liquidity of our common stock; the market price of the common stock; the number of institutional and general investors that will consider investing in the common stock; the number of investors in general that will consider investing in the common stock; the number of market makers in our common stock; the availability of information concerning the trading prices and volume of the common stock; and the number of broker-dealers willing to execute trades in our common stock. In addition to the foregoing, there are certain consequences under the Securities Act of being a public shell company, including the
unavailability of Rule 144 thereunder for the resale of restricted securities and the inability to utilize Form S-8 for the registration of employee benefit plan securities.
Actions of an activist stockholder against us could be disruptive and costly, may cause uncertainty about the strategic direction of our business, result in litigation, divert management’s and the board’s attention and resources, and may have an adverse effect on our business.
From time to time, we may be subject to proposals by activist stockholders urging us to take certain corporate actions or to nominate certain individuals to our board of directors. For example, Elvin Lee has provided notice to us that he intends to propose two nominees to stand for election to the our board of directors in opposition to any nominees recommended by our board of directors.
Future activist stockholder matters, including a proxy contest and potential related litigation, could have a material adverse effect on us for the following reasons:
•Such stockholders may attempt to effect changes in our governance and strategic direction or to acquire control over the board of directors or the Company.
•While we welcome the opinions of all stockholders, responding to proxy contests and related litigation by stockholders has been, and could be, costly and time-consuming, and could disrupt our operations, and divert the attention of our board of directors, management team and other employees away from their regular duties and the pursuit of business opportunities to enhance stockholder value.
•Perceived uncertainties as to our future direction, strategy or leadership created as a consequence of activist stockholder initiatives may harm our ability to attract new investors, and could cause our stock price to experience periods of volatility or stagnation based on temporary or speculative market perceptions or other factors that do not necessarily reflect the underlying fundamentals and prospects of our business.
Risks Related to this Offering
A substantial number of shares may be sold in the market following this offering, which may depress the market price for our common stock.
Sales of a substantial number of shares of our common stock in the public market following this offering could cause the market price of our common stock to decline. Although there can be no assurance that any of the $335,921 worth of shares being offered under this prospectus supplement will be sold or the price at which any such shares might be sold, assuming that an aggregate of 95,977 shares of our common stock are sold during the remaining term of the sales agreement with Ladenburg, in each case, for example, at a price of $3.50 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on July 25, 2024, upon completion of this offering, we will have outstanding an aggregate of approximately 1.4 million shares of common stock, assuming no exercise of our outstanding stock options or warrants. A substantial majority of the outstanding shares of our common stock are, and all of the shares sold in this offering upon issuance will be, freely tradable without restriction or further registration under the Securities Act, unless these shares are owned or purchased by “affiliates” as that term is defined in Rule 144 under the Securities Act.
In addition, as of March 31, 2024, there were outstanding (i) options to purchase an aggregate of 28,990 shares of our common stock at a weighted average exercise price of $72.32 per share, of which options to purchase 8,221 shares of our common stock were then exercisable, and (ii) warrants to purchase 1,015,383 shares of our common stock at a weighted average exercise price of $23.92 per share. The shares of our common stock issuable upon exercise of these options and warrants may be immediately eligible for resale in the open market. Such sales, along with any other market transactions, could adversely affect the market price of our common stock. Additional dilution may result from the issuance of shares of our common stock in connection with collaborations or manufacturing arrangements or in connection with other financing efforts.
Moreover, if we issue options, restricted stock units, warrants or other securities to purchase or acquire our common stock in the future and those options, restricted stock units, warrants or other securities are exercised, converted or settled you may experience further dilution. Holders of shares of our common stock have no preemptive rights that entitle them to purchase their pro rata share of any offering of shares of any class or series.
You may experience future dilution as a result of future equity offerings or other equity issuances.
In order to raise additional capital, we may in the future offer and issue additional shares of our common stock or other securities convertible into, exercisable or exchangeable for, or settled in, our common stock. We cannot assure you that we will be able to sell shares or other securities in any other offering at a price per share that is equal to or greater than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock or other securities convertible into, exercisable or exchangeable for, or settled in, our common stock in future transactions may be higher or lower than the price per share in this offering. As of March 31, 2024, we had reserved 28,990 shares of our common stock for issuance upon the exercise of outstanding stock options, 12,407 shares of our common stock for future issuance under the 2015 Equity Incentive Plan, 1,015,383 shares of our common stock for issuance upon exercise of outstanding warrants, and 25,501 shares of our common stock reserved for future issuance under the ESPP. You will incur dilution upon the grant of any shares pursuant to such plan, upon vesting of any stock awards under any such plan, or upon exercise of any such outstanding options or warrants.
We have broad discretion in the use of the net proceeds of this offering and, despite our efforts, we may use the net proceeds in a manner that does not increase the value of your investment.
We intend to use the net proceeds from this offering for general corporate purposes and working capital. We may also use a portion of the net proceeds from this offering to acquire or invest in complementary businesses, technologies, product candidates or other intellectual property, although we have no present commitments or agreements to do so. However, we have not determined the specific allocation of the net proceeds among these potential uses. Our management will have broad discretion over the use and investment of the net proceeds of this offering, and, accordingly, investors in this offering will need to rely upon the judgment of our management with respect to the use of proceeds, with only limited information concerning our specific intentions. These proceeds could be applied in ways that do not improve our operating results or increase the value of your investment. Please see the section entitled “Use of Proceeds” on page S-12 of this prospectus supplement for further information.
We do not anticipate paying any cash dividends on our capital stock in the foreseeable future; accordingly, capital appreciation, if any, will be your sole source of gain and you may never receive a return on your investment.
We have never declared or paid cash dividends on our capital stock, and you should not rely on an investment in our common stock to provide dividend income. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. In addition, our credit agreement prohibits us, and the terms of any future debt agreements may also preclude us, from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
It is not possible to predict the aggregate proceeds resulting from sales made under the sales agreement.
Subject to certain limitations in the sales agreement and compliance with applicable law, we have the discretion to deliver a placement notice to Ladenburg at any time throughout the term of the sales agreement. The number of shares that are sold through Ladenburg after delivering a placement notice will fluctuate based on a number of factors, including the market price of our common stock during the sales period, any limits we may set with Ladenburg in any applicable placement notice and the demand for our common stock. Because this offering can be terminated at any time and the price per share of each share sold pursuant to the sales agreement will fluctuate over
time, it is not currently possible to predict the aggregate proceeds to be raised in connection with sales under the sales agreement.
Sales of common stock offered hereby will be in “at the market offerings,” and investors who buy shares at different times will likely pay different prices.
Investors who purchase shares in this offering at different times will likely pay different prices, and accordingly may experience different levels of dilution and different outcomes in their investment results. We will have discretion, subject to market demand, to vary the timing, prices and number of shares sold in this offering. In addition, subject to the final determination by our board of directors or any restrictions we may place in any applicable placement notice delivered to Ladenburg, there is no minimum or maximum sales price for shares to be sold in this offering. Investors may experience a decline in the value of the shares they purchase in this offering as a result of sales made at prices lower than the prices they paid.
The actual number of shares we will issue under the sales agreement, at any one time or in total, is uncertain.
Subject to certain limitations in the sales agreement and compliance with applicable law, we have the discretion to deliver a sales notice to Ladenburg at any time throughout the term of the sales agreement. The number of shares that are sold by Ladenburg after we deliver a sales notice will fluctuate based on the market price of the common stock during the sales period and limits we set with Ladenburg. Because the price per share of each share sold will fluctuate based on the market price of our common stock during the sales period, it is not possible at this stage to predict the number of shares that will be ultimately issued.
NOTE REGARDING FORWARD-LOOKING STATEMENTS
Various statements made in this prospectus supplement are forward-looking and involve risks and uncertainties. All statements that address activities, events or developments that we intend, expect or believe may occur in the future are forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Such statements give our current expectations or forecasts of future events and are not statements of historical or current facts. These statements include, among others, statements about:
•our ability to continue as a going concern to support our operations in the first half of 2025;
•our expectations regarding the exploration of strategic alternatives;
•our strategy, including significantly reducing our expenditures on operational and research and development activities and taking other cost savings measures in connection with our ongoing review of strategic alternatives;
•our expectations regarding the benefits of our cost-saving measures;
•our ability to preserve capital while we continue to assess potential strategic alternatives;
•the expected timing for incurring costs associated with the cost savings measures;
•our expectations regarding our clinical trials and any investigator-initiated clinical trials, including our assessment of potential options to continue the clinical development of seclidemstat for Ewing sarcoma;
•our liquidity position, the expected sufficiency of such position for anticipated operating and capital requirements;
•our expectations regarding our ability to remain listed on Nasdaq; and
•our use of proceeds.
Forward-looking statements also include statements other than statements of current or historical fact, including, without limitation, all statements related to any expectations of revenues, expenses, cash flows, earnings or losses from operations, cash required to maintain current and planned operations, capital or other financial items; any statements of the plans, strategies and objectives of management for future operations; any plans or expectations with respect to product research, development and commercialization, including regulatory approvals; any other statements of expectations, plans, intentions or beliefs; and any statements of assumptions underlying any of the foregoing. We often, although not always, identify forward-looking statements by using words or phrases such as “likely”, “expect”, “intend”, “anticipate”, “believe”, “estimate”, “plan”, “project”, “forecast” and “outlook”.
The following are some of the factors that could cause actual results to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements:
•the risk that if we do not successfully complete a strategic transaction or obtain financing in the near term, the company will need to pursue a dissolution and liquidation of our company;
•the risk that we are delisted from Nasdaq;
•the imposition of restrictions imposed by the FDA on The University of Texas MD Anderson Cancer Center, or MDACC, investigator-initiated clinical trial evaluating seclidemstat (SP-2577) in combination with azacitidine in adult patients with myelodysplastic syndromes and chronic myelomonocytic leukemia, including the partial clinical hold imposed on or about July 9, 2024;
•uncertainties about the exploration and evaluation of strategic alternatives, including that they may not result in a definitive transaction or enhance stockholder value and may create a distraction or uncertainty that may adversely affect our operating results, business or investor perceptions;
•potential adverse impacts regarding our announcement regarding our implementation of a series of additional cost-savings measures designed to extend our expected cash runway into the first half of 2025, including the cessation of employment of David Arthur, our Chief Executive Officer, who is continuing to serve in such role as a part-time consulting basis;
•the risk that the Company’s cost saving initiatives and exploration of strategic alternatives are not successful and do not increase stockholder value;
•uncertainties related to a potential proxy contest;
•unanticipated difficulties with preserving capital;
•unanticipated charges not currently contemplated that may occur as a result of our cost savings plan;
•uncertainties about the paths of our programs and our ability to evaluate and identify a path forward for those programs, particularly given the constraints we have as a small company with limited financial, personnel and other operating resources;
•the adequacy of our capital to support our future operations;
•fluctuations in our operating results; and
•other factors described in our filings with the SEC.
We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. The risks set forth under Item 1A of our Annual Report on Form 10-K for the fiscal year ended December 31, 2023 and any supplementary risks set forth under Item 1A of our Quarterly Reports on Form 10-Q and other documents we file with the SEC describe major risks to our business, and you should read and interpret any forward-looking statements together with these risks. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. You should read and interpret any forward-looking statements in light of these risks. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. We do not undertake any obligation to publicly update or revise our forward-looking statements even if experience or future changes makes it clear that any projected results expressed or implied in such statements will not be realized.
Before deciding to purchase our securities, you should carefully consider the risk factors discussed and incorporated by reference in this prospectus supplement and the accompanying prospectus and in the registration statement of which this prospectus supplement and the accompanying prospectus form a part.
USE OF PROCEEDS
We may issue and sell shares of common stock having aggregate sales proceeds of up to $335,921 from time to time, before deducting sales agent commissions and expenses. The amount of proceeds from this offering will depend upon the number of shares of our common stock sold and the market price at which they are sold. There can be no assurance that we will be able to sell any shares under or fully utilize the sales agreement with Ladenburg as a source of financing.
We intend to use the net proceeds from this offering for general corporate purposes and working capital. We may also use a portion of the net proceeds from this offering to acquire or invest in complementary businesses, technologies, product candidates or other intellectual property, although we have no present commitments or agreements to do so.
The amounts and timing of our use of the net proceeds from this offering will depend on a number of factors, such as the timing and progress of our strategic alternative evaluation, research and development efforts, the timing and progress of any partnering efforts, technological advances and the competitive environment for our product candidates. As of the date of this prospectus supplement, we cannot specify with certainty all of the particular uses for the net proceeds to us from the sale of the shares of common stock offered by us hereunder. Accordingly, our management will have broad discretion in the timing and application of these proceeds. Pending application of the net proceeds as described above, we intend to temporarily invest the proceeds in short-term, interest-bearing instruments.
PLAN OF DISTRIBUTION
We have entered into an At The Market Offering Agreement, dated February 5, 2021, or the sales agreement, with Ladenburg under which we may issue and sell shares of our common stock having aggregate sales proceeds from time to time through Ladenburg, acting as sales agent, in an amount not to exceed the lesser of such number of shares of our common stock that (a) equals the number or dollar amount of shares of common stock registered on our shelf registration statement, (b) equals the number of authorized but unissued shares of our common stock (less the number of shares of common stock issuable upon exercise, conversion or exchange of any outstanding securities or otherwise reserved from our authorized capital stock), or (c) would cause us to not satisfy the eligibility and transaction requirements for use of Form S-3. Pursuant to this prospectus supplement we may offer and sell shares of our common stock having an aggregate offering price of up to $335,921 under the sales agreement. Any such sales will be made by any method that is deemed to be an “at the market offering” as defined in Rule 415(a)(4) under the Securities Act, including sales made directly on or through the Nasdaq Capital Market or any other existing trading market for our common stock in the United States or to or through a market maker, subject to the limitations imposed by General Instruction I.B.6 to Form S-3, as applicable.
Each time we wish to issue and sell shares of common stock under the sales agreement, we will notify Ladenburg of the number of shares to be issued, the dates on which such sales are anticipated to be made, any limitation on the number of shares to be sold in any one day and any minimum price below which sales may not be made. Once we have so instructed Ladenburg, subject to the terms and conditions of the sales agreement, Ladenburg has agreed to use its commercially reasonable efforts consistent with its normal trading and sales practices to sell such shares up to the amount specified on such terms. The obligations of Ladenburg under the sales agreement to sell our shares of common stock are subject to a number of conditions that we must meet.
Ladenburg will provide written confirmation to us following the close of trading on the Nasdaq Capital Market following each day in which shares of our common stock are sold under the sales agreement. Each confirmation will include the number of shares sold on the day, the aggregate gross sales proceeds, the net proceeds to us, and the compensation payable by us to Ladenburg with respect to the sales. The settlement of sales of shares between us and Ladenburg is generally anticipated to occur on the second trading day following the date on which the sale was made. Sales of our shares of common stock as contemplated in this prospectus supplement will be settled through the facilities of The Depository Trust Company or by such other means as we and Ladenburg may agree. There is no arrangement for funds to be received in an escrow, trust or similar arrangement. We will report at least quarterly the number of shares of common stock sold through Ladenburg under the sales agreement, the net proceeds to us and the compensation paid by us to Ladenburg in connection with the sales of common stock.
We will pay Ladenburg a commission equal to 3.0% of the aggregate gross proceeds we receive from each sale of our shares of common stock. Because there is no minimum offering amount required as a condition of this offering, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. In addition, we agreed to reimburse Ladenburg for the fees and disbursements of its counsel, payable upon execution of the sales agreement, in an amount not to exceed $50,000, in addition to certain ongoing disbursements of its legal counsel up to $2,500 per calendar quarter. We estimate that the total expenses for the offering, excluding any commissions or expense reimbursement payable, or previously paid, to Ladenburg under the terms of the sales agreement, will be approximately $70,000. The remaining sale proceeds, after deducting any other transaction fees, will equal our net proceeds from the sale of such shares.
In connection with the sale of the shares of common stock on our behalf, Ladenburg may be deemed to be an “underwriter” within the meaning of the Securities Act, and the compensation of Ladenburg may be deemed to be underwriting commissions or discounts. We have agreed to indemnify Ladenburg against certain civil liabilities, including liabilities under the Securities Act. We have also agreed to contribute to payments Ladenburg may be required to make in respect of such liabilities.
The offering of our shares of common stock pursuant to the sales agreement will terminate upon the earlier of (i) the sale of all shares of our common stock subject to the sales agreement and (ii) the termination of the sales agreement
as permitted therein. We and Ladenburg may each terminate the sales agreement at any time upon ten days’ prior notice.
This summary of the material provisions of the sales agreement does not purport to be a complete statement of its terms and conditions. A copy of the sales agreement is filed as an exhibit to our Current Report on Form 8-K filed under the Exchange Act on February 5, 2021 and incorporated by reference in this prospectus supplement. See “Where You Can Find More Information” and “Incorporation of Certain Information by Reference” below. Ladenburg and its affiliates have previously and may in the future provide various investment banking, commercial banking, financial advisory and other financial services for us and our affiliates, for which services they have and may in the future receive customary fees. In the course of its business, Ladenburg may actively trade our securities for its own account or for the accounts of customers, and, accordingly, Ladenburg may at any time hold long or short positions in such securities. To the extent required by Regulation M, Ladenburg will not engage in any market making activities involving our common stock while the offering is ongoing under this prospectus supplement.
This prospectus supplement and the accompanying prospectus in electronic format may be made available on a website maintained by Ladenburg, and Ladenburg may distribute this prospectus supplement and the accompanying prospectus electronically.
LEGAL MATTERS
The validity of the securities we are offering will be passed upon by Hogan Lovells US LLP. Ladenburg Thalmann & Co. Inc. is being represented by Ellenoff Grossman & Schole LLP, New York, New York, in connection with this offering.
EXPERTS
Ernst & Young LLP, independent registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2023, as set forth in their report (which contains an explanatory paragraph describing conditions that raise substantial doubt about our ability to continue as a going concern as described in Note 1 to the consolidated financial statements), which is incorporated by reference in this prospectus supplement and elsewhere in the registration statement. Our financial statements are incorporated by reference in reliance on Ernst & Young LLP’s report, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-3 under the Securities Act, of which this prospectus supplement forms a part. The rules and regulations of the SEC allow us to omit from this prospectus supplement certain information included in the registration statement. For further information about us and the securities we are offering under this prospectus, you should refer to the registration statement and the exhibits and schedules filed with the registration statement. With respect to the statements contained in this prospectus supplement regarding the contents of any agreement or any other document, in each instance, the statement is qualified in all respects by the complete text of the agreement or document, a copy of which has been filed as an exhibit to the registration statement.
Because we are subject to the information and reporting requirements of the Exchange Act, we file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at www.sec.gov.
We make available free of charge on our website our annual, quarterly and current reports, including amendments to such reports, as soon as reasonably practicable after we electronically file such material with, or furnish such material to, the SEC. Please note, however, that we have not incorporated any other information by reference from our website, other than the documents listed under the heading “Incorporation of Certain Information by Reference” on page S-15 of this prospectus supplement. In addition, you may request copies of these filings at no cost by writing or telephoning us at the following address or telephone number:
Salarius Pharmaceuticals, Inc.
2450 Holcombe Blvd., Suite X
Houston, TX 77021
Telephone: (832) 804-9144
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” information into this prospectus supplement. This means that we can disclose important information to you by referring you to other documents we have filed separately with the SEC, without actually including the specific information in this prospectus supplement. The information incorporated by reference is considered to be part of this prospectus supplement, and information that we file later with the SEC (and that is deemed to be “filed” with the SEC) will automatically update, and may supersede, information in this prospectus supplement.
•our Current Reports on Form 8-K, filed with the SEC on February 23, 2024, March 6, 2024, June 14, 2024, June 17, 2024, July 11, 2024 , July 19, 2024, and July 23, 2024;and •the description of our common stock contained in our Registration Statement on Form 8-A filed on January 23, 2015, as updated by Exhibit 4.10 to our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, including any amendments or reports filed for the purpose of updating such description. All reports and other documents subsequently filed by us pursuant to Sections 13(a), 13(c), 14 and 15(d) of the Exchange Act after the date of this prospectus supplement and prior to the termination or completion of the offering of securities under this prospectus supplement shall be deemed to be incorporated by reference in this prospectus supplement and to be a part hereof from the date of filing such reports and other documents.
To obtain copies of these filings, see “Where You Can Find More Information” on page S-15 of this prospectus supplement.
PROSPECTUS
$50,000,000
Common Stock
Preferred Stock
Warrants
Debt Securities
We may offer to the public from time to time in one or more series or issuances and on terms that we will determine at the time of the offering:
☐ shares of our common stock;
☐ shares of our preferred stock;
☐ warrants to purchase shares of our common stock, preferred stock and/or debt securities;
☐ debt securities consisting of debentures, notes or other evidences of indebtedness;
☐ units consisting of a combination of the foregoing securities; or
☐ any combination of these securities.
The aggregate initial offering price of all securities sold by us pursuant to this prospectus will not exceed $50,000,000.
This prospectus provides a general description of the securities that we may offer. Each time that we offer securities under this prospectus, we will provide the specific terms of the securities offered, including the public offering price, in a supplement to this prospectus. Any prospectus supplement may add to, update or change information contained in this prospectus.
The securities may be sold by us to or through underwriters or dealers, directly to purchasers or through agents designated from time to time. For additional information on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus and the comparable section of any applicable prospectus supplement. If any underwriters are involved in the sale of the securities with respect to which this prospectus is being delivered, the names of such underwriters and any applicable discounts or commissions and over-allotment options will be set forth in the applicable prospectus supplement.
Our common stock trades on the Nasdaq Capital Market under the ticker symbol “SLRX.” On August 4, 2022, the last reported sale price per share of our common stock was $0.1903. We have not yet determined whether the other securities that may be offered by this prospectus will be listed on any exchange, interdealer quotation system or over-the-counter market. If we decide to seek the listing of any such securities upon issuance, the prospectus supplement relating to those securities will disclose the exchange, quotation system or market on which those securities will be listed.
INVESTING IN OUR SECURITIES INVOLVES A HIGH DEGREE OF RISK. RISKS ASSOCIATED WITH AN INVESTMENT IN OUR SECURITIES WILL BE DESCRIBED IN THE APPLICABLE PROSPECTUS SUPPLEMENT AND CERTAIN OF OUR FILINGS WITH THE SECURITIES AND EXCHANGE COMMISSION INCORPORATED BY REFERENCE INTO THIS PROSPECTUS, AS DESCRIBED UNDER “RISK FACTORS” ON PAGE 5.
You should read this prospectus and any applicable prospectus supplement together with additional information described under the heading “Where You Can Find More Information” before you make your investment decision.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is August 16, 2022.
TABLE OF CONTENTS
| | | | | | | | |
| | Page |
ABOUT THIS PROSPECTUS | | ii |
FORWARD-LOOKING STATEMENTS | | |
MARKET, INDUSTRY AND OTHER DATA | | |
SUMMARY | | |
RISK FACTORS | | |
USE OF PROCEEDS | | |
PLAN OF DISTRIBUTION | | |
GENERAL DESCRIPTION OF OUR SECURITIES | | |
DESCRIPTION OF OUR COMMON STOCK | | |
DESCRIPTION OF OUR PREFERRED STOCK | | |
DESCRIPTION OF OUR WARRANTS | | |
DESCRIPTION OF OUR DEBT SECURITIES | | |
DESCRIPTION OF OUR UNITS | | |
WHERE YOU CAN FIND MORE INFORMATION | | |
INCORPORATION BY REFERENCE | | |
LEGAL MATTERS | | |
EXPERTS | | |
ABOUT THIS PROSPECTUS
This prospectus is a part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or the SEC, using a “shelf” registration process. Under this shelf registration process, we may offer to sell any of the securities, or any combination of the securities, described in this prospectus, in each case in one or more offerings, up to a total dollar amount of $50,000,000.
This prospectus provides you only with a general description of the securities that we may offer. Each time securities are sold under the shelf registration statement, we will provide a prospectus supplement that will contain specific information about the terms of those securities and the terms of that offering. The prospectus supplement may also add, update or change information contained in this prospectus. If there is any inconsistency between the information in this prospectus and any prospectus supplement, you should rely on the information in the prospectus supplement. You should read both this prospectus and any prospectus supplement, including all documents incorporated by reference herein and therein, together with the additional information described under “Where You Can Find More Information” below.
The information contained in this prospectus is not complete and may be changed. You should rely only on the information provided in or incorporated by reference in this prospectus or in any prospectus supplement, or documents to which we otherwise refer you. We have not authorized anyone else to provide you with different information.
We have not authorized any dealer, agent or other person to give any information or to make any representation other than those contained or incorporated by reference in this prospectus and any accompanying prospectus supplement. You must not rely upon any information or representation not contained or incorporated by reference in this prospectus or an accompanying prospectus supplement. This prospectus and the accompanying prospectus supplement, if any, do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus and the accompanying prospectus supplement, if any, constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction. You should not assume that the information contained in this prospectus and the accompanying prospectus supplement, if any, is accurate on any date subsequent to the date set forth on the front of such document or that any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus and any accompanying prospectus supplement is delivered or securities are sold on a later date.
References in this prospectus to the terms “the Company,” “Salarius,” “we,” “our” and “us” or other similar terms mean Salarius Pharmaceuticals, Inc. and our wholly owned subsidiaries, unless we state otherwise or the context indicates otherwise.
“Salarius Pharmaceuticals,” “SLRX” and the Salarius logo are our trademarks. This prospectus and the documents incorporated by reference into this prospectus may also contain trademarks and trade names that are the property of their respective owners. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply relationships with, or endorsements or sponsorship of us by, these other companies.
FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference herein contain, and any prospectus supplement and the documents incorporated therein, may contain forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this prospectus, any prospectus supplement or the documents incorporated herein and therein by reference, including statements regarding our future financial condition, results of operations, business strategy and plans and objectives of management for future operations, industry trends and other future events, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “believe,” “will,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “expect,” “predict,” “project,” “could,” “potentially,” “continue,” “ongoing,” “scheduled” or the negative of these terms or other similar expressions, although not all forward-looking statements contain these identifying terms. The forward-looking statements in this prospectus and the documents incorporated herein by reference include, among other things, statements about:
•future periods;
•our strategy and ongoing development programs;
•our clinical trials, including status, costs, goals, timing and other expectations related thereto;
•our belief as to the potential of our lead compound, SP-2577;
•our strategic collaborations and license agreements, and intellectual property;
•the potential for seclidemstat to target the epigenetic dysregulation underlying Ewing sarcoma and advanced solid tumors;
•expected timing and results of clinical studies;
•the ability of our product candidates to demonstrate drug activity;
•the nature, strategy and focus of our company;
•the development and commercial potential of any product candidates;
•our ability and plan to regain and maintain compliance with Nasdaq’s continued listing standards;
•our expectations as to revenue, cash flow, and expenses;
•the potential impact of the COVID-19 pandemic on our business, operations, cash flow and ability to obtain additional financing;
•our liquidity position, the expected sufficiency of such position for anticipated operating and capital requirements;
•future capital requirements, and need for, and ability to secure, additional financing;
•our ability to access additional financing under the Grant Contract with Cancer Prevention and Research Institute of Texas;
•our operating losses and ability to continue as a going concern;
•our decision to engage in any new collaborations or selectively partnering its technology to improve our ability to continue as a going concern;
•our beliefs regarding our prospects for our business;
•the adequacy of our capital resources, our ability to raise additional financing, and the consequences if we fail to obtain adequate funding; and
•our use of proceeds.
Forward-looking statements also include statements other than statements of current or historical fact, including, without limitation, all statements related to any expectations of revenues, expenses, cash flows, earnings or losses from operations, cash required to maintain current and planned operations, capital or other financial items; any statements of the plans, strategies and objectives of management for future operations; any plans or expectations with respect to product research, development and commercialization, including regulatory approvals; any other statements of expectations, plans, intentions or beliefs; and any statements of assumptions underlying any of the foregoing. We often, although not always, identify forward-looking statements by using words or phrases such as “likely”, “expect”, “intend”, “anticipate”, “believe”, “estimate”, “plan”, “project”, “forecast” and “outlook”.
The following are some of the factors that could cause actual results to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements:
•the effectiveness and timeliness of our preclinical studies and clinical trials, and the usefulness of the data;
•our ability to achieve profitable operations and access to needed capital;
•fluctuations in our operating results;
•the extent to which the COVID-19 pandemic impacts our business, the medical community and the global economy;
•our dependence on contract research organizations, vendors and investigators;
•effects of competition and other developments affecting sales of products;
•market acceptance of our products;
•protection of intellectual property and avoiding intellectual property infringement;
•product liability; and
•other factors described in our filings with the SEC.
We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. The risks set forth under Item 1A of our Annual Report on Form 10-K for the fiscal year ended December 31, 2021, as revised or supplemented by our Quarterly Reports on Form 10-Q and other documents we file with the SEC, describe major risks to our business, and you should read and interpret any forward-looking statements together with these risks. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements.
You should read this prospectus, any prospectus supplement and the documents that we incorporate by reference herein and therein completely and with the understanding that our actual future results may be materially different from what we expect. The forward-looking statements contained in this prospectus are made as of the date of this prospectus and we do not assume any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law.
MARKET, INDUSTRY AND OTHER DATA
This prospectus and any applicable prospectus supplement and the documents incorporated by reference herein and therein contain estimates, projections, market research and other information concerning, among other things, our industry, our business, and markets for our product candidates. Unless otherwise expressly stated, we obtain this information from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources as well as from our own internal estimates and research and from publications, research, surveys and studies conducted by third parties on our behalf. Information that is based on estimates, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances that are reflected in this information. As a result, you are cautioned not to give undue weight to such information.
SUMMARY
This summary highlights selected information from this prospectus and does not contain all of the information that you need to consider in making your investment decision. You should carefully read the entire prospectus, the applicable prospectus supplement and any related free writing prospectus, including the risks of investing in our securities discussed under the heading “Risk Factors” contained in the applicable prospectus supplement and any related free writing prospectus, and under similar headings in the other documents that are incorporated by reference into this prospectus. You should also carefully read the information incorporated by reference into this prospectus, including our financial statements, and the exhibits to the registration statement of which this prospectus is a part.
Company Overview
We are a clinical-stage biopharmaceutical company focused on developing effective treatments for cancers with high, unmet medical need. Specifically, we are developing treatments for cancers caused by dysregulated gene expression, i.e., genes that are incorrectly turned on or off. We are developing two classes of drugs that address gene dysregulation: epigenetic inhibitors and targeted protein degraders. Our technologies have the potential to work in both liquid and solid tumors. Our current pipeline consists of two small molecule drugs: (i) seclidemstat (SP-2577), a targeted protein inhibitor, and (ii) SP-3164, a targeted protein degrader. We are located in Houston, Texas.
For more information about our company, please refer to other documents that we have filed with the SEC and that are incorporated by reference into this prospectus, as listed under the heading “Incorporation of Certain Information by Reference.”
Corporate Information
We were incorporated as Flex Pharma, Inc., or Flex Pharma, in Delaware in February 2014. In July 2019, our wholly owned subsidiary, Falcon Acquisition Sub, LLC, merged with and into Salarius Pharmaceuticals, LLC, or Private Salarius, with Private Salarius becoming our wholly owned subsidiary, or the Merger, and we changed our named to Salarius Pharmaceuticals, Inc. Our principal executive offices are located at 2450 Holcombe Blvd., Suite X, Houston, Texas 77021, and our telephone number is (832) 834-6992. Our website address is www.salariuspharma.com. We do not incorporate the information on, or accessible through, our website into this prospectus, and you should not consider any information on, or accessible through, our website as part of this prospectus.
RISK FACTORS
Investing in our securities involves a high degree of risk. The prospectus supplement applicable to each offering of our securities will contain a discussion of the risks applicable to an investment in our securities. Prior to making a decision about investing in our securities, you should carefully consider the specific factors discussed under the heading “Risk Factors” in the applicable prospectus supplement, together with all of the other information contained or incorporated by reference in the prospectus supplement or appearing or incorporated by reference in this prospectus. You should also consider the risks, uncertainties and assumptions discussed under the heading “Risk Factors” in our most recent Annual Report on Form 10-K and our Quarterly Reports on Form 10-Q and other documents that we file with the SEC, which are incorporated herein by reference as described in this prospectus under the heading “Where You Can Find More Information”. The risks and uncertainties we have described in such documents are not the only risks that we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our operations.
USE OF PROCEEDS
Except as otherwise provided in the applicable prospectus supplement relating to a specific offering, we intend to use the net proceeds from the sale of securities by us under this prospectus for general corporate purposes, which may include working capital, capital expenditures, research and development expenditures, clinical trial expenditures, acquisitions of new technologies, products or businesses, and investments. Additional information on the use of net proceeds from the sale of securities by us under this prospectus may be set forth in the prospectus supplement relating to the specific offering.
PLAN OF DISTRIBUTION
We may sell the securities, from time to time pursuant to public offerings, negotiated transactions, block trades, “At the Market Offerings,” within the meaning of Rule 415(a)(4) of the Securities Act of 1933, as amended, or the Securities Act, into an existing trading market, at prevailing market prices, or a combination of these methods. We may sell the securities to or through underwriters or dealers, through agents or remarketing firms, or directly to one or more purchasers. We may distribute securities from time to time in one or more transactions:
•at a fixed price or prices, which may be changed;
•at market prices prevailing at the time of sale;
•at prices related to such prevailing market prices; or
•at negotiated prices.
A prospectus supplement or supplements (and any related free writing prospectus that we may authorize to be provided to you) will describe the terms of the offering of the securities, including, to the extent applicable:
•the name or names of the underwriters, dealers or agents, if any;
•if the securities are to be offered through the selling efforts of brokers or dealers, the plan of distribution and the terms of any agreement, arrangement, or understanding entered into with broker(s) or dealer(s) prior to the effective date of the registration statement, and, if known, the identity of any broker(s) or dealer(s) who will participate in the offering and the amount to be offered through each;
•the purchase price of the securities or other consideration therefor, and the proceeds, if any, we will receive from the sale;
•if any of the securities being registered are to be offered otherwise than for cash, the general purposes of the distribution, the basis upon which the securities are to be offered, the amount of compensation and other expenses of distribution, and by whom they are to be borne;
•any delayed delivery arrangements;
•any options under which underwriters may purchase additional securities from us;
•any agency fees or underwriting discounts and other items constituting agents’ or underwriters’ compensation;
•any public offering price;
•any discounts, commissions or concessions allowed or reallowed or paid to dealers;
•the identity and relationships of any finders, if applicable; and
•any securities exchange or market on which the securities may be listed.
Only underwriters named in the prospectus supplement will be underwriters of the securities offered by the prospectus supplement.
If underwriters are used in the sale, they will acquire the securities for their own account and may resell the securities from time to time in one or more transactions at a fixed public offering price or at varying prices determined at the time of sale. The obligations of the underwriters to purchase the securities will be subject to the conditions set forth in the applicable underwriting agreement. We may offer the securities to the public through underwriting syndicates represented by managing underwriters or by underwriters without a syndicate. Unless otherwise indicated in the prospectus supplement, subject to certain conditions, the underwriters will be obligated to purchase all of the securities offered by the prospectus supplement, other than securities covered by any over-allotment or other option. Any public offering price and any discounts or concessions allowed or reallowed or paid to dealers may change from time to time. We may use underwriters, dealers or agents with whom we have a material relationship. We will describe in the prospectus supplement, naming the underwriter, dealer or agent, the nature of any such relationship.
We may use a remarketing firm to offer the securities in connection with a remarketing arrangement upon their purchase. Remarketing firms will act as principals for their own account or as agents for us. These remarketing firms will offer or sell the securities pursuant to the terms of the securities. A prospectus supplement will identify any
remarketing firm and the terms of its agreement, if any, with us and will describe the remarketing firm’s compensation. Remarketing firms may be deemed to be underwriters in connection the securities they remarket.
If we offer and sell securities through a dealer, we or an underwriter will sell the securities to the dealer, as principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale. The name of the dealer and the terms of the transaction will be set forth in the applicable prospectus supplement.
We may sell securities directly or through agents we designate from time to time. We will name any agent involved in the offering and sale of securities and we will describe any commissions we will pay to the agent in the prospectus supplement. Unless the prospectus supplement states otherwise, our agent will act on a best-efforts basis for the period of its appointment.
Dealers and agents participating in the distribution of the securities may be deemed to be underwriters, and compensation received by them on resale of the securities may be deemed to be underwriting discounts. If such dealers or agents were deemed to be underwriters, they may be subject to statutory liabilities under the Securities Act.
We may sell securities directly to one or more purchasers without using underwriters or agents. Underwriters, dealers and agents that participate in the distribution of the securities may be underwriters as defined in the Securities Act, and any discounts or commissions they receive from us and any profit on their resale of the securities may be treated as underwriting discounts and commissions under the Securities Act.
We may authorize agents or underwriters to solicit offers by certain types of institutional investors to purchase securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus supplement.
We may provide agents, underwriters and dealers with indemnification against civil liabilities, including liabilities under the Securities Act, or contribution with respect to payments that the agents, underwriters or dealers may make with respect to these liabilities. Agents, underwriters and dealers, or their respective affiliates, may engage in transactions with, or perform services for, us in the ordinary course of business.
All securities we may offer, other than common stock, will be new issues of securities with no established trading market. Any underwriter may make a market in these securities, but will not be obligated to do so and may discontinue any market making at any time without notice. We cannot guarantee the liquidity of the trading markets for any securities.
Any underwriter may engage in over-allotment, stabilizing transactions, short-covering transactions and penalty bids in accordance with Regulation M under the Exchange Act. Over-allotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum price. Syndicate-covering or other short-covering transactions involve purchases of the securities, either through exercise of the over-allotment option or in the open market after the distribution is completed, to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer are purchased in a stabilizing or covering transaction to cover short positions. Those activities may cause the price of the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time.
Any underwriters that are qualified market makers on the Nasdaq Stock Market may engage in passive market making transactions in the common stock on the Nasdaq Stock Market in accordance with Regulation M under the Securities Exchange Act of 1934, as amended, or the Exchange Act, during the business day prior to the pricing of the offering, before the commencement of offers or sales of the common stock. Passive market makers must comply with applicable volume and price limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for such security; if all independent bids are lowered below the passive market maker’s bid, however, the passive market maker’s bid must then be lowered when certain purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
GENERAL DESCRIPTION OF OUR SECURITIES
We may offer and sell, at any time and from time to time:
•shares of our common stock;
•shares of our preferred stock;
•warrants to purchase shares of our common stock, preferred stock and/or debt securities;
•debt securities consisting of debentures, notes or other evidences of indebtedness;
•units consisting of a combination of the foregoing securities; or
•any combination of these securities.
The terms of any securities we offer will be determined at the time of sale. We may issue debt securities that are exchangeable for and/or convertible into common stock or any of the other securities that may be sold under this prospectus. When particular securities are offered by us, a supplement to this prospectus will be filed with the SEC, which will describe the terms of the offering and sale of the offered securities.
DESCRIPTION OF OUR COMMON STOCK
The summary of general terms and provisions of our capital stock set forth below does not purport to be complete and is subject to and qualified by reference to our Amended and Restated Certificate of Incorporation, or the Certificate of Incorporation, and Amended and Restated Bylaws, or the Bylaws, and together with the Certificate of Incorporation, the Charter Documents, each of which is included as an exhibit our most recent Annual Report on Form 10-K filed with the SEC and incorporated by reference herein. For additional information, please read the Charter Documents and the applicable provisions of the Delaware General Corporation Law, or the DGCL.
Authorized Capital Stock
We are authorized to issue up to 110,000,000 shares, of which (i) 100,000,000 have been designated common stock, par value $0.0001 per share, and (ii) 10,000,000 have been designated preferred stock, par value $0.0001 per share. As of August 1, 2022, there were 56,116,243 shares of our common stock outstanding, held by 143 stockholders of record. This figure does not reflect the number of beneficial owners of shares of our common stock as a single stockholder of record often holds shares in nominee name (also referred to as, in “street name”) on behalf of multiple beneficial owners.
Voting Rights
The holders of shares of our common stock have the exclusive power to vote on all matters presented to our stockholders unless Delaware law or the certificate of designation for an outstanding series of our preferred stock gives the holders of that series of our preferred stock the right to vote on certain matters. Each holder of shares of our common stock is entitled to one vote per share.
When a quorum is present at any meeting, the vote of the holders of a majority of the voting power of our common stock entitled to vote and present in person or represented by proxy shall decide any question brought before such meeting, unless the question is one upon which by express provision of the Charter Documents or by law, a different vote is required in which case such express provision shall govern and control the decision of such question. Directors are elected by a plurality of the voting power of the shares present in person or represented by proxy and entitled to vote on the election of directors at a meeting at which a quorum is present, and stockholders are not entitled to cumulate their votes for the election of directors.
Dividend Rights
Subject to any prior rights of any preferred stock then outstanding, the holders of shares of our common stock are entitled to receive dividends ratably out of funds legally available, when and if declared by our board of directors.
No Preemptive or Similar Rights
Our common stock is not entitled to preemptive rights and is not subject to conversion, redemption or sinking fund provisions.
Rights to Receive Liquidation Distributions
If we become subject to a liquidation, dissolution or winding-up, the assets legally available for distribution to our stockholders would be distributable ratably among the holders of our common stock and any participating preferred stock outstanding at that time, subject to prior satisfaction of all outstanding debt and liabilities and the preferential rights and payment of liquidation preferences, if any, on any outstanding shares of our preferred stock.
Outstanding Stock Options
As of June 30, 2022, we had outstanding options to purchase 2,869,972 shares of our common stock at a weighted-average exercise price of $1.74 per share, pursuant to our 2015 Equity Incentive Plan. As of June 30, 2022, there were 1,108,976 shares of our common stock reserved for future issuance under our 2015 Equity Incentive Plan.
2015 Employee Stock Purchase Plan
As of June 30, 2022, there were 163,313 shares of our common stock reserved for future issuance under our 2015 Employee Stock Purchase Plan.
Outstanding Warrants
As of June 30, 2022, there were the following warrants to purchase shares of our common stock outstanding:
•warrants to purchase 42,928 shares of our common stock issued to Wedbush Securities Inc., with an exercise price of $18.90 per share;
•warrants to purchase 3,783,522 shares of our common stock issued in connection with our public offering completed on February 11, 2020, with an exercise price of $1.15 per share;
•warrants to purchase 3,964,065 shares of our common stock issued in a private placement completed on December 11, 2020, with an exercise price of $1.182 per share;
•warrants to purchase 142,711 shares of common stock that were distributed to holders of rights that were granted under that certain merger agreement that we entered into with Salarius Pharmaceuticals, LLC on January 3, 2019, with an exercise price of $15.17 per share; and
•warrants to purchase 7,004,578 shares of our common stock issued in connection with that certain offering completed on April 26, 2022, with an exercise price of $0.3399 per share.
Anti-Takeover Provisions in Charter Documents
Certain provisions of the Charter Documents, which are summarized below, may have the effect of delaying, deferring or preventing another person from acquiring control of our company. These provisions may discourage takeovers, coercive or otherwise, and are also designed, in part, to encourage persons seeking to acquire control of our company to negotiate first with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us because negotiation of these proposals could result in an improvement of their terms. These provisions include the following:
Board of Directors Vacancies. Pursuant to the Charter Documents, our board of directors may fill vacant directorships. In addition, directors may only be removed for cause and only upon the affirmative vote of at least sixty-six and two-thirds percent of the voting power of outstanding voting stock. In addition, the number of directors constituting our board of directors may be set only by a resolution adopted by a majority vote of our board of directors. These provisions may have the effect of deferring, delaying or discouraging hostile takeovers, or changes in control or management of our company and will make it more difficult to change the composition of our board of directors, which will promote continuity of management.
Classified Board. The Charter Documents provide that our board of directors is classified into three classes of directors, with each class serving three-year staggered terms. A third-party may be discouraged from making a tender offer or otherwise attempting to obtain control of our company as it is more difficult and time-consuming for stockholders to replace a majority of the directors on a classified board of directors.
Stockholder Action; Special Meeting of Stockholders. Pursuant to Section 228 of the DGCL, any action required to be taken at any annual or special meeting of the stockholders may be taken without a meeting, without prior notice and without a vote if a consent or consents in writing, setting forth the action so taken, is signed by the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares of stock entitled to vote thereon were present and voted, unless the Certificate of Incorporation provides otherwise. The Certificate of Incorporation provides that stockholders may not take action by written consent but may only take action at annual or special meetings of stockholders. As a result, a holder controlling a majority of our capital stock would not be able to amend the Bylaws or remove directors without holding a meeting of stockholders called in accordance with the Charter Documents. The Bylaws provides that special meetings of the stockholders may be called only upon a resolution approved by a majority of the total number of directors that we would have if there were no vacancies. These provisions might delay the ability of our stockholders to force consideration of a proposal or for stockholders controlling a majority of our capital stock to take any action, including the removal of directors.
Advance Notice Requirements for Stockholder Proposals and Director Nominations. The Bylaws provide advance notice procedures for stockholders seeking to bring business before our annual meeting of stockholders or to nominate candidates for election as directors at our annual meeting of stockholders. The Bylaws specify certain requirements regarding the form and content of a stockholder’s notice and prohibit the conduct of any business at a special meeting other than as specified in the notice for such meeting. These provisions might preclude stockholders from bringing matters before our annual meeting of stockholders or from making nominations for directors at our annual meeting of stockholders if the proper procedures are not followed. These provisions may also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our company.
No Cumulative Voting. The DGCL provides that stockholders are not entitled to cumulate votes in the election of directors unless a corporation’s certificate of incorporation provides otherwise. The Certificate of Incorporation does not provide for cumulative voting.
Amendment of Charter Provisions and Bylaws. The Charter Documents provides that the Bylaws may be adopted, amended, altered or repealed by either (i) a vote of a majority of the total number of directors of our board of directors or (ii) in addition to any other vote otherwise required by law, the affirmative vote of the holders of at least sixty-six and two-thirds percent of the voting power of all of the then outstanding shares of capital stock entitled to vote generally in the election of directors.
Our Charter Documents also provide that the provisions of the Certificate of Incorporation relating to provisions relating to the management of the business, board of directors, director liability, indemnification and forum selection, may only be amended, altered, changed or repealed by the affirmative vote of the holders of at least sixty-six and two-thirds percent of the voting power of all of our outstanding shares of capital stock entitled to vote generally in the election of directors, voting together as a single class.
Issuance of Undesignated Preferred Stock. Our board of directors has the authority, without further action by our stockholders, to designate and issue shares of preferred stock with rights and preferences, including super voting, special approval, dividend or other rights or preferences on a discriminatory basis. The existence of authorized but unissued shares of undesignated preferred stock would enable our board of directors to render more difficult or to discourage an attempt to obtain control of our company by means of a merger, tender offer, proxy contest or other means.
Business Combinations with Interested Stockholders. We are subject to the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a business combination, such as a merger, with an interested stockholder (i.e., subject to certain exceptions, a person or group owning 15% or more of the corporation’s voting stock) for a period of three years following the date the person
became an interested stockholder, unless (with certain exceptions) the business combination or the transaction in which the person became an interested stockholder is approved in a prescribed manner.
Forum Selection. The Charter Documents provide that unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will, to the fullest extent permitted by applicable law, be the sole and exclusive forum for:
•any derivative action or proceeding brought on behalf of our company;
•any action asserting a claim of breach of a fiduciary duty owed by any director, officer, or other employee of our company to us or our stockholders;
•any action asserting a claim of breach of a fiduciary duty owed by any director, officer, or other employee of our company to us or our stockholders; and
•any action asserting a claim against us governed by the internal affairs doctrine,
in each such case, subject to such Court of Chancery of the State of Delaware having personal jurisdiction over the indispensable parties named as defendants therein. The Charter Documents also provides that any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock will be deemed to have notice of, and to have consented to, this forum selection provision.
Although these provisions benefit us by providing increased consistency in the application of Delaware law for the specified types of actions and proceedings, the provisions may have the effect of increasing the costs of and discouraging lawsuits against our directors, officers, employees and agents. The enforceability of similar exclusive forum provisions in other companies’ charters has been challenged in legal proceedings, and it is possible that, in connection with one or more actions or proceedings described above, a court could rule that this provision in the Certificate of Incorporation is inapplicable or unenforceable. For example, the choice of forum provisions summarized above are not intended to, and would not, apply to suits brought to enforce any liability or duty created by the Exchange Act, or other claim for which the federal courts have exclusive jurisdiction. Additionally, there is uncertainty as to whether our choice of forum provisions would be enforceable with respect to suits brought to enforce any liability or duty created by the Securities Act, or other claims for which the federal courts have concurrent jurisdiction, and in any event stockholders will not be deemed to have waived our compliance with federal securities laws and rules and regulations thereunder.
Listing
Our common stock is listed on the Nasdaq Capital Market under the symbol “SLRX.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company, LLC.
DESCRIPTION OF OUR PREFERRED STOCK
We currently have authorized 10,000,000 shares of preferred stock, par value $0.0001 per share, of which no shares have been designated.
Our board of directors may, without further action by our stockholders, from time to time, direct the issuance of shares of preferred stock in series and may, at the time of issuance, determine and fix the number of shares of such series and the designation of such series, the voting powers, if any, of the shares of such series, the preferences and relative, participating, optional or other special rights, if any, and the qualifications, limitations or restrictions thereof, including without limitation thereof, dividend rights, conversion rights, redemption privileges and liquidation preferences, of the shares of such series. Satisfaction of any dividend preferences of outstanding shares of our preferred stock would reduce the amount of funds available for the payment of dividends on shares of our common stock. Holders of shares of our preferred stock may be entitled to receive a preference payment in the event of any liquidation, dissolution or winding-up of our Company before any payment is made to the holders of shares of our common stock. In some circumstances, the issuance of shares of preferred stock may render more difficult or tend to discourage a merger, tender offer or proxy contest, the assumption of control by a holder of a large block of
our securities or the removal of incumbent management. Upon the affirmative vote of our board of directors, without stockholder approval, we may issue shares of preferred stock with voting and conversion rights which could adversely affect the holders of shares of our common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock on the rights of holders of common stock until the board of directors determines the specific rights attached to that preferred stock. We have no current plan to issue any shares of preferred stock.
If we offer a specific series of preferred stock under this prospectus, we will describe the terms of the preferred stock in the prospectus supplement for such offering and will file a copy of the certificate establishing the terms of the preferred stock with the SEC. To the extent required, this description will include:
•the title and stated value;
•the number of shares offered, the liquidation preference per share, and the purchase price;
•the dividend rate(s), period(s), and/or payment date(s), or method(s) of calculation for such dividends;
•whether dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate;
•the procedures for any auction and remarketing, if any;
•the provisions for a sinking fund, if any;
•the provisions for redemption, if applicable;
•any listing of the preferred stock on any securities exchange or market;
•whether the preferred stock will be convertible into our common stock or our other securities and, if applicable, the conversion price (or how it will be calculated), the conversion period and any other terms of conversion (including any anti-dilution provisions, if any);
•whether the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange price (or how it will be calculated), the exchange period and any other terms of exchange (including any anti-dilution provisions, if any);
•voting rights, if any, of the preferred stock;
•a discussion of any material and/or special U.S. federal income tax considerations applicable to the preferred stock;
•the relative ranking and preferences of the preferred stock as to dividend rights and rights upon liquidation, dissolution, or winding up of our affairs;
•any material limitations on issuance of any class or series of preferred stock ranking senior to or on a parity with the series of preferred stock as to dividend rights and rights upon our liquidation, dissolution, or winding up; and
•any other affirmative, negative or other covenants or contractual rights which might be attendant with the specific series of preferred stock.
The preferred stock offered by this prospectus, when issued, will not have, or be subject to, any preemptive or similar rights.
Transfer Agent and Registrar
The transfer agent and registrar for any series of preferred stock will be set forth in each applicable prospectus supplement.
DESCRIPTION OF OUR WARRANTS
We may issue warrants to purchase shares of our common stock, preferred stock and/or debt securities in one or more series together with other securities or separately, as described in each applicable prospectus supplement. Below is a description of certain general terms and provisions of the warrants that we may offer. Particular terms of the warrants will be described in the applicable warrant agreements and the applicable prospectus supplement for the warrants.
The applicable prospectus supplement will contain, where applicable, the following terms of and other information relating to the warrants:
•the specific designation and aggregate number of, and the price at which we will issue, the warrants;
•the currency or currency units in which the offering price, if any, and the exercise price are payable;
•the designation, amount and terms of the securities purchasable upon exercise of the warrants;
•if applicable, the exercise price for shares of our common stock and the number of shares of common stock to be received upon exercise of the warrants;
•if applicable, the exercise price for shares of our preferred stock, the number of shares of preferred stock to be received upon exercise of the warrants, and a description of that series of our preferred stock;
•if applicable, the exercise price for our debt securities, the amount of our debt securities to be received upon exercise of the warrants, and a description of that series of debt securities;
•the date on which the right to exercise the warrants will begin and the date on which that right will expire or, if the warrants may not be continuously exercised throughout that period, the specific date or dates on which the warrants may be exercised;
•whether the warrants will be issued in fully registered form or bearer form, in definitive or global form or in any combination of these forms, although, in any case, the form of a warrant included in a unit will correspond to the form of the unit and of any security included in that unit;
•any applicable material U.S. federal income tax or foreign tax consequences;
•the identity of the warrant agent for the warrants, if any, and of any other depositaries, execution or paying agents, transfer agents, registrars or other agents;
•the proposed listing, if any, of the warrants or any securities purchasable upon exercise of the warrants on any securities exchange or market;
•if applicable, the date from and after which the warrants and the common stock, preferred stock and/or debt securities will be separately transferable;
•if applicable, the minimum or maximum amount of the warrants that may be exercised at any one time;
•information with respect to book-entry procedures, if any
•the anti-dilution provisions of the warrants, if any;
•any redemption, put or call provisions;
•whether the warrants are to be sold separately or with other securities as parts of units; and
•any additional terms of the warrants, including terms, procedures and limitations relating to the exchange and exercise of the warrants.
Transfer Agent and Registrar
The transfer agent and registrar for any warrants will be set forth in the applicable prospectus supplement.
Description of Outstanding Warrants
As of June 30, 2022, there were a total of 14,937,804 warrants to purchase shares of our common stock outstanding. See “Description of Our Capital Stock – Description of Our Common Stock – Outstanding Warrants.”
DESCRIPTION OF OUR DEBT SECURITIES
This section describes the general terms and provisions of the debt securities that we may offer under this prospectus, any of which may be issued as convertible or exchangeable debt securities. We will set forth the particular terms of the debt securities we offer in a prospectus supplement. The extent, if any, to which the following general provisions apply to particular debt securities will be described in the applicable prospectus supplement. The following description of general terms relating to the debt securities and the indenture under which the debt securities will be issued are summaries only and therefore are not complete. You should read the indenture and the prospectus supplement regarding any particular issuance of debt securities.
We will issue the debt securities offered by this prospectus and any accompanying prospectus supplement under an indenture to be entered into between us and the trustee identified in the applicable prospectus supplement. The terms of the debt securities will include those stated in the indenture and those made part of the indenture by reference to the Trust Indenture Act of 1939, as in effect on the date of the indenture. We have filed or will file a copy of the form of indenture as an exhibit to the registration statement in which this prospectus is included. The indenture will be subject to and governed by the terms of the Trust Indenture Act of 1939.
We may offer under this prospectus up to an aggregate principal amount of $50,000,000 in debt securities, or if debt securities are issued at a discount, or in a foreign currency, foreign currency units or composite currency, the principal amount as may be sold for an aggregate initial public offering price of up to $50,000,000. Unless otherwise specified in the applicable prospectus supplement, the debt securities will represent direct, unsecured obligations of our company and will rank equally with all of our other unsecured indebtedness.
The following statements relating to the debt securities and the indenture are summaries, qualified in their entirety by reference to the detailed provisions of the indenture and the final form indenture as may be filed with a future prospectus supplement.
General
We may issue the debt securities in one or more series with the same or various maturities, at par, at a premium, or at a discount. We will describe the particular terms of each series of debt securities in a prospectus supplement relating to that series, which we will file with the SEC.
The prospectus supplement will set forth, to the extent required, the following terms of the debt securities in respect of which the prospectus supplement is delivered:
•the title of the series;
•the aggregate principal amount;
•the issue price or prices, expressed as a percentage of the aggregate principal amount of the debt securities;
•any limit on the aggregate principal amount;
•the date or dates on which principal is payable;
•the interest rate or rates (which may be fixed or variable) or, if applicable, the method used to determine such rate or rates;
•the date or dates from which interest, if any, will be payable and any regular record date for the interest payable;
•the place or places where principal and, if applicable, premium and interest, is payable;
•the terms and conditions upon which we may, or the holders may require us to, redeem or repurchase the debt securities;
•the denominations in which such debt securities may be issuable, if other than denominations of $1,000 or any integral multiple of that number;
•whether the debt securities are to be issuable in the form of certificated debt securities (as described below) or global debt securities (as described below);
•the portion of principal amount that will be payable upon declaration of acceleration of the maturity date if other than the principal amount of the debt securities;
•the currency of denomination;
•the designation of the currency, currencies or currency units in which payment of principal and, if applicable, premium and interest, will be made;
•if payments of principal and, if applicable, premium or interest, on the debt securities are to be made in one or more currencies or currency units other than the currency of denomination, the manner in which the exchange rate with respect to such payments will be determined;
•if amounts of principal and, if applicable, premium and interest may be determined by reference to an index based on a currency or currencies or by reference to a commodity, commodity index, stock exchange index or financial index, then the manner in which such amounts will be determined;
•the provisions, if any, relating to any collateral provided for such debt securities;
•any addition to or change in the covenants and/or the acceleration provisions described in this prospectus or in the indenture;
•any events of default, if not otherwise described below under “Events of Default”;
•the terms and conditions, if any, for conversion into or exchange for shares of our common stock or preferred stock;
•any depositaries, interest rate calculation agents, exchange rate calculation agents or other agents; and
•the terms and conditions, if any, upon which the debt securities shall be subordinated in right of payment to other indebtedness of our company.
We may issue discount debt securities that provide for an amount less than the stated principal amount to be due and payable upon acceleration of the maturity of such debt securities in accordance with the terms of the indenture. We may also issue debt securities in bearer form, with or without coupons. If we issue discount debt securities or debt securities in bearer form, we will describe material U.S. federal income tax considerations and other material special considerations that apply to these debt securities in the applicable prospectus supplement.
We may issue debt securities denominated in or payable in a foreign currency or currencies or a foreign currency unit or units. If we do, we will describe the restrictions, elections, and general tax considerations relating to the debt securities and the foreign currency or currencies or foreign currency unit or units in the applicable prospectus supplement.
Exchange and/or Conversion Rights
We may issue debt securities which can be exchanged for or converted into shares of our common stock or preferred stock. If we do, we will describe the terms of exchange or conversion in the prospectus supplement relating to these debt securities.
Transfer and Exchange
We may issue debt securities that will be represented by either:
•“book-entry securities,” which means that there will be one or more global securities registered in the name of a depositary or a nominee of a depositary; or
•“certificated securities,” which means that they will be represented by a certificate issued in definitive registered form.
We will specify in the prospectus supplement applicable to a particular offering whether the debt securities offered will be book-entry or certificated securities.
Certificated Debt Securities
If you hold certificated debt securities issued under an indenture, you may transfer or exchange such debt securities in accordance with the terms of the indenture. You will not be charged a service charge for any transfer or exchange of certificated debt securities but may be required to pay an amount sufficient to cover any tax or other governmental charge payable in connection with such transfer or exchange.
Global Securities
The debt securities of a series may be issued in the form of one or more global securities that will be deposited with a depositary or its nominees identified in the prospectus supplement relating to the debt securities. In such a case, one or more global securities will be issued in a denomination or aggregate denominations equal to the portion of the aggregate principal amount of outstanding debt securities of the series to be represented by such global security or securities.
Unless and until it is exchanged in whole or in part for debt securities in definitive registered form, a global security may not be registered for transfer or exchange except as a whole by the depositary for such global security to a nominee of the depositary and except in the circumstances described in the prospectus supplement relating to the debt securities. The specific terms of the depositary arrangement with respect to a series of debt securities will be described in the prospectus supplement relating to such series.
Protection in the Event of Change of Control
Any provision in an indenture that governs our debt securities covered by this prospectus that includes any covenant or other provision providing for a put or increased interest or otherwise that would afford holders of our debt securities additional protection in the event of a recapitalization transaction, a change of control of our company, or a highly leveraged transaction will be described in the applicable prospectus supplement.
Covenants
Unless otherwise indicated in this prospectus or the applicable prospectus supplement, our debt securities may not have the benefit of any covenant that limits or restricts our business or operations, the pledging of our assets or the incurrence by us of indebtedness. We will describe in the applicable prospectus supplement any material covenants in respect of a series of debt securities.
Consolidation, Merger and Sale of Assets
We may agree in any indenture that governs the debt securities of any series covered by this prospectus that we will not consolidate with or merge into any other person or convey, transfer, sell or lease our properties and assets substantially as an entirety to any person, unless such person and such proposed transaction meets various criteria, which we will describe in detail in the applicable prospectus supplement.
Defaults and Notice
The debt securities of any series will contain events of default to be specified in the applicable prospectus supplement, which may include, without limitation:
•failure to pay the principal of, or premium or make-whole amount, if any, on any debt security of such series when due and payable (whether at maturity, by call for redemption, through any mandatory sinking fund, by redemption at the option of the holder, by declaration or acceleration or otherwise);
•failure to make a payment of any interest on any debt security of such series when due;
•failure to perform or observe any other covenants or agreements in the indenture with respect to the debt securities of such series;
•certain events relating to our bankruptcy, insolvency or reorganization; and
•certain cross defaults, if and as applicable.
If an event of default with respect to debt securities of any series shall occur and be continuing, we may agree that the trustee or the holders of at least 25% in aggregate principal amount of the then outstanding debt securities of such series may declare the principal amount (or, if the debt securities of such series are issued at an original issue discount, such portion of the principal amount as may be specified in the terms of the debt securities of such series) of all debt securities of such series or such other amount or amounts as the debt securities or supplemental indenture with respect to such series may provide, to be due and payable immediately. Any provisions pertaining to events of default and any remedies associated therewith will be described in the applicable prospectus supplement.
Any indenture that governs our debt securities covered by this prospectus may require that the trustee under such indenture shall, within 90 days after the occurrence of a default, give to holders of debt securities of any series notice of all uncured defaults with respect to such series known to it. However, in the case of a default that results from the failure to make any payment of the principal of, premium or make-whole amount, if any, or interest on the debt securities of any series, or in the payment of any mandatory sinking fund installment with respect to debt securities of such series, if any, the trustee may withhold such notice if it in good faith determines that the withholding of such notice is in the interest of the holders of debt securities of such series. Any terms and provisions relating to the foregoing types of provisions will be described in further detail in the applicable prospectus supplement.
Any indenture that governs our debt securities covered by this prospectus will contain a provision entitling the trustee to be indemnified by holders of debt securities before proceeding to exercise any trust or power under the indenture at the request of such holders. Any such indenture may provide that the holders of at least a majority in
aggregate principal amount of the then outstanding debt securities of any series may direct the time, method and place of conducting any proceedings for any remedy available to the trustee, or of exercising any trust or power conferred upon the trustee with respect to the debt securities of such series. However, the trustee under any such indenture may decline to follow any such direction if, among other reasons, the trustee determines in good faith that the actions or proceedings as directed may not lawfully be taken, would involve the trustee in personal liability or would be unduly prejudicial to the holders of the debt securities of such series not joining in such direction.
Any indenture that governs our debt securities covered by this prospectus may endow the holders of such debt securities to institute a proceeding with respect to such indenture, subject to certain conditions, which will be specified in the applicable prospectus supplement and which may include, that the holders of at least a majority in aggregate principal amount of the debt securities of such series then outstanding make a written request upon the trustee to exercise its power under the indenture, indemnify the trustee and afford the trustee reasonable opportunity to act. Even so, such holders may have an absolute right to receipt of the principal of, premium or make-whole amount, if any, and interest when due, to require conversion or exchange of debt securities if such indenture provides for convertibility or exchangeability at the option of the holder and to institute suit for the enforcement of such rights. Any terms and provisions relating to the foregoing types of provisions will be described in further detail in the applicable prospectus supplement.
Modification of the Indenture
We and the trustee may modify any indenture that governs our debt securities of any series covered by this prospectus with or without the consent of the holders of such debt securities, under certain circumstances to be described in a prospectus supplement.
Defeasance; Satisfaction and Discharge
The prospectus supplement will outline the conditions under which we may elect to have certain of our obligations under the indenture discharged and under which the indenture obligations will be deemed to be satisfied.
Regarding the Trustee
We will identify the trustee and any relationship that we may have with such trustee, with respect to any series of debt securities, in the prospectus supplement relating to the applicable debt securities. You should note that if the trustee becomes a creditor of Salarius, the indenture and the Trust Indenture Act of 1939 limit the rights of the trustee to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim, as security or otherwise. The trustee and its affiliates may engage in, and will be permitted to continue to engage in, other transactions with us and our affiliates. If, however, the trustee acquires any “conflicting interest” within the meaning of the Trust Indenture Act of 1939, it must eliminate such conflict or resign.
Governing Law
The law governing the indenture and the debt securities will be identified in the prospectus supplement relating to the applicable indenture and debt securities.
DESCRIPTION OF OUR UNITS
The following description, together with the additional information we include in any applicable prospectus supplement, summarizes the material terms and provisions of the units that we may offer under this prospectus. Units may be offered independently or together with common stock, preferred stock, debt securities and/or warrants offered by any prospectus supplement, and may be attached to or separate from those securities. While the terms we have summarized below will generally apply to any future units that we may offer under this prospectus, we will describe the particular terms of any series of units that we may offer in more detail in the applicable prospectus supplement. The terms of any units offered under a prospectus supplement may differ from the terms described below.
We will incorporate by reference into the registration statement of which this prospectus forms a part the form of unit agreement, including a form of unit certificate, if any, that describes the terms of the series of units we are offering before the issuance of the related series of units. The following summaries of material provisions of the units, and the unit agreements, are subject to, and qualified in their entirety by reference to, all the provisions of the
unit agreement applicable to a particular series of units. We urge you to read the applicable prospectus supplements related to the units that we sell under this prospectus, as well as the complete unit agreements that contain the terms of the units.
General
We may issue units comprised of one or more shares of our common stock or preferred stock, debt securities and warrants in any combination. Each unit will be issued so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at any time or at any time before a specified date.
We will describe in the applicable prospectus supplement the terms of the series of units, including:
•the designation and terms of the units and of the securities comprising the units, including whether, and under what circumstances, those securities may be held or transferred separately;
•the rights and obligations of the unit agent, if any;
•any provisions of the governing unit agreement that differ from those described below; and
•any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units.
The provisions described in this section, as well as those described under “Description of Our Common Stock,” “Description of our Preferred Stock,” “Description of Our Debt Securities” and “Description of Our Warrants,” will apply to each unit and to any common stock, preferred stock, debt securities or warrants included in each unit, respectively.
Issuance in Series
We may issue units in such amounts and in numerous distinct series as we determine.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. The SEC maintains an Internet website at www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. Our reports on Forms 10-K, 10-Q and 8-K, and amendments to those reports, are also available for download, free of charge, as soon as reasonably practicable after these reports are filed with, or furnished to, the SEC, at our website at www.salariuspharma.com. Information contained on or accessible through our website is not a part of this prospectus or any prospectus supplement, and the inclusion of our website address in this prospectus is an inactive textual reference only.
INCORPORATION BY REFERENCE
The SEC allows us to “incorporate by reference” into this prospectus the information in other documents that we file with it. This means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be a part of this prospectus, and information in documents that we file later with the SEC will automatically update and supersede information contained in documents filed earlier with the SEC or contained in this prospectus. We incorporate by reference in this prospectus (i) the documents listed below, (ii) all documents that we file with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of the initial filing of the registration statement of which this prospectus is included and prior to the effectiveness of such registration statement, and (iii) and any future filings that we may make with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act prior to the termination of the offering under this prospectus; provided, however, that we are not incorporating, in each case, any documents or information deemed to have been furnished and not filed, including any information that we disclose under Items 2.02 or 7.01 of any Current Report on Form 8-K, in accordance with SEC rules:
•our Annual Report on Form 10-K for the fiscal year ended December 31, 2021, filed with the SEC on March 25, 2022; •our Quarterly Reports on Form 10-Q for the quarterly periods ended March 31, 2022 and June 30, 2022, filed with the SEC on May 12, 2022 and August 5, 2022, respectively; •our Current Reports on Form 8-K, filed with the SEC on January 13, 2022 (except Item 7.01 and Exhibit 99.1), April 1, 2022, April 22, 2022, April 26, 2022, June 17, 2022, and August 3, 2022; and •the description of our common stock contained in our Registration Statement on Form 8-A filed on January 23, 2015, as updated by Exhibit 4.6 to our Annual Report on Form 10-K for the fiscal year ended December 31, 2021, including any amendments or reports filed for the purpose of updating such description. You may request, orally or in writing, a copy of any or all of the documents incorporated herein by reference. These documents will be provided to you at no cost, by contacting: Salarius Pharmaceuticals, Inc., Chief Financial Officer, at 2450 Holcombe Blvd., Suite X, Houston, TX 77021. In addition, copies of any or all of the documents incorporated herein by reference may be accessed at our website at www.salariuspharma.com. The information on such website is not incorporated by reference and is not a part of this prospectus.
LEGAL MATTERS
The validity of the issuance of the securities offered hereby will be passed upon for us by Hogan Lovells US LLP, Houston, Texas. As appropriate, legal counsel representing the underwriters, dealers or agents will be named in the accompanying prospectus supplement and may opine to certain legal matters.
EXPERTS
Ernst & Young LLP, independent registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2021, as set forth in their report (which contains an explanatory paragraph describing conditions that raise substantial doubt about our ability to continue as a going concern as described in Note 1 to the consolidated financial statements), which is incorporated by reference in this prospectus supplement and elsewhere in the registration statement. Our financial statements are incorporated by reference in reliance on Ernst & Young LLP’s report, given on their authority as experts in accounting and auditing.
Up to $335,921
Common Stock
Ladenburg Thalmann
July 25, 2024
The prospectus supplement to which this exhibit is attached is a final prospectus for the related offering. The maximum aggregate offering price of that offering is $50,000,000.
Salarius Pharmaceuticals (NASDAQ:SLRX)
Historical Stock Chart
From Nov 2024 to Dec 2024
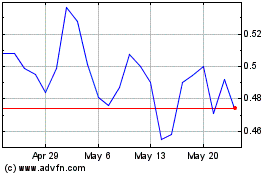
Salarius Pharmaceuticals (NASDAQ:SLRX)
Historical Stock Chart
From Dec 2023 to Dec 2024