ARS Pharmaceuticals Shares Sink to New Lows on FDA Neffy Setback
21 September 2023 - 12:20AM
Dow Jones News
By Colin Kellaher
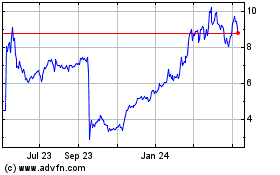
ARS Pharmaceuticals shares lost more than half of their value
and hit an all-time low on Wednesday after the U.S. Food and Drug
Administration turned away its proposed epinephrine nasal spray
"neffy" and called on the company to conduct another study.
Shares of the San Diego company were recently changing hands at
$3.04, down 54%, after touching an all-time low of $2.55 early in
the session.
Following a positive vote by one of its advisory committees in
May, the FDA had been expected to approve neffy as the first
needle-free epinephrine product for severe allergic reactions.
However, ARS late Tuesday said the agency issued a so-called
complete response letter, indicating that it wouldn't approve the
application in its current form and calling on ARS to complete a
repeat-dose study prior to approval.
ARS said had previously reached alignment with the FDA on
conducting the repeat-dose study after a green light for neffy.
ARS said it plans to dispute the FDA's ruling. The company said
it also plans to complete the new trial as quickly as possible, and
that it aims to resubmit neffy to the FDA in the first half of
2024, setting the stage for a decision in the second half of the
year.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
September 20, 2023 10:05 ET (14:05 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
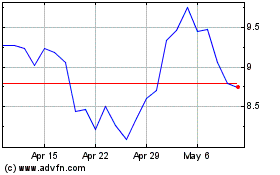
ARS Pharmaceuticals (NASDAQ:SPRY)
Historical Stock Chart
From Apr 2024 to May 2024
ARS Pharmaceuticals (NASDAQ:SPRY)
Historical Stock Chart
From May 2023 to May 2024