
® Proteins by Design® XmAb® Antibody Therapeutics Corporate Overview March 2024

2 Forward-Looking Statements Certain statements contained in this presentation, other than statements of historical fact, may constitute forward- looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements regarding Xencor's development plans and timelines; potential regulatory actions; expected use of cash resources; the timing and results of clinical trials; the plans and objectives of management for future operations; and the potential markets for Xencor's product and development candidates. Forward-looking statements are based on the current expectations of management and upon what management believes to be reasonable assumptions based on information currently available to it, and involve numerous risks and uncertainties, many of which are beyond Xencor's control. These risks and uncertainties could cause future results, performance or achievements to differ significantly from the results, performance or achievements expressed or implied by such forward-looking statements. Such risks include, but are not limited to, potential delays in development timelines or negative preclinical or clinical trial results, reliance on third parties for development efforts and changes in the competitive landscape including changes in the standard of care, as well as other risks described in Xencor's filings with the Securities and Exchange Commission (SEC). Xencor expressly disclaims any duty, obligation or undertaking to update or revise any forward-looking statements contained herein to reflect any change in Xencor's expectations with regard thereto of any subsequent change in events, conditions or circumstances on which any such statements are based, except in accordance with applicable securities laws. For all forward-looking statements, we claim the protection of the safe harbor for forward looking statements contained in the Private Securities Litigation Reform Act of 1995.
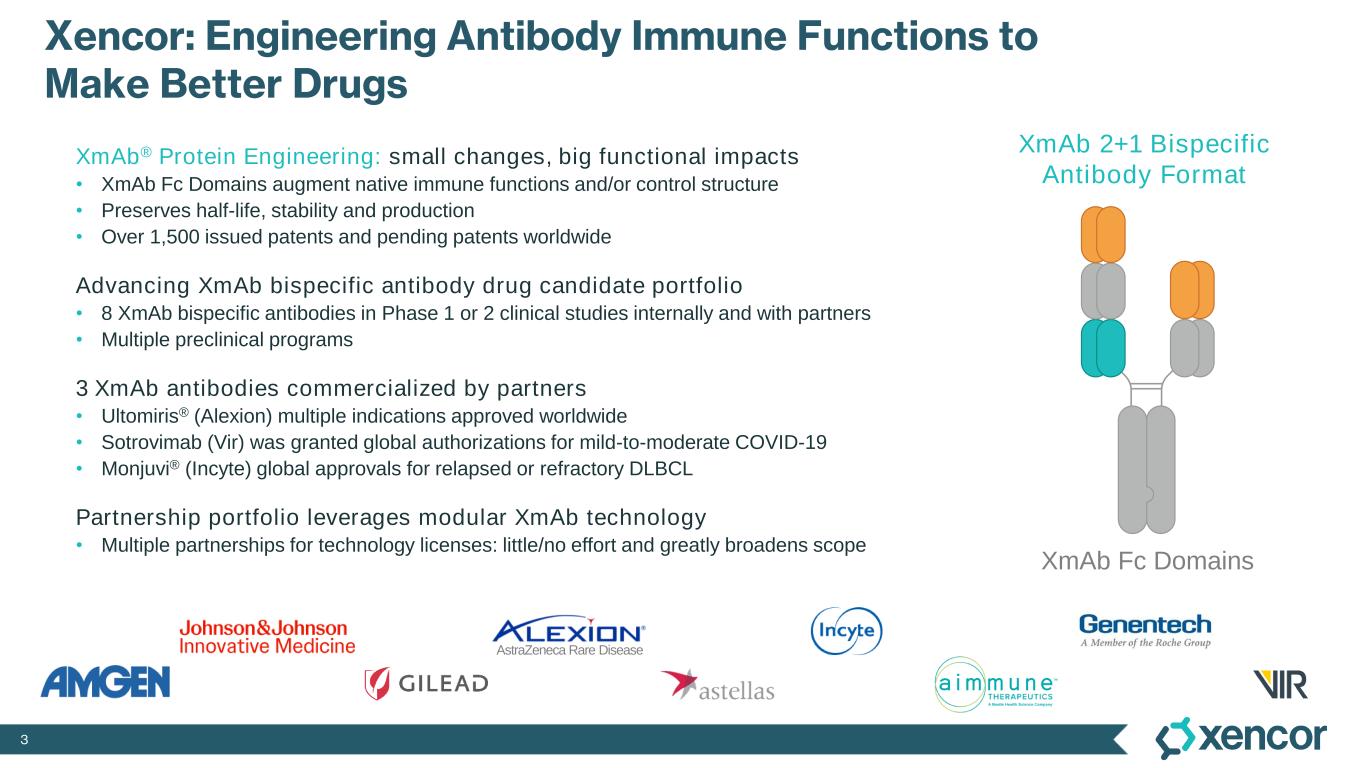
3 Xencor: Engineering Antibody Immune Functions to Make Better Drugs XmAb® Protein Engineering: small changes, big functional impacts • XmAb Fc Domains augment native immune functions and/or control structure • Preserves half-life, stability and production • Over 1,500 issued patents and pending patents worldwide Advancing XmAb bispecific antibody drug candidate portfolio • 8 XmAb bispecific antibodies in Phase 1 or 2 clinical studies internally and with partners • Multiple preclinical programs 3 XmAb antibodies commercialized by partners • Ultomiris® (Alexion) multiple indications approved worldwide • Sotrovimab (Vir) was granted global authorizations for mild-to-moderate COVID-19 • Monjuvi® (Incyte) global approvals for relapsed or refractory DLBCL Partnership portfolio leverages modular XmAb technology • Multiple partnerships for technology licenses: little/no effort and greatly broadens scope XmAb Fc Domains AstraZeneca Rare Disease XmAb 2+1 Bispecific Antibody Format
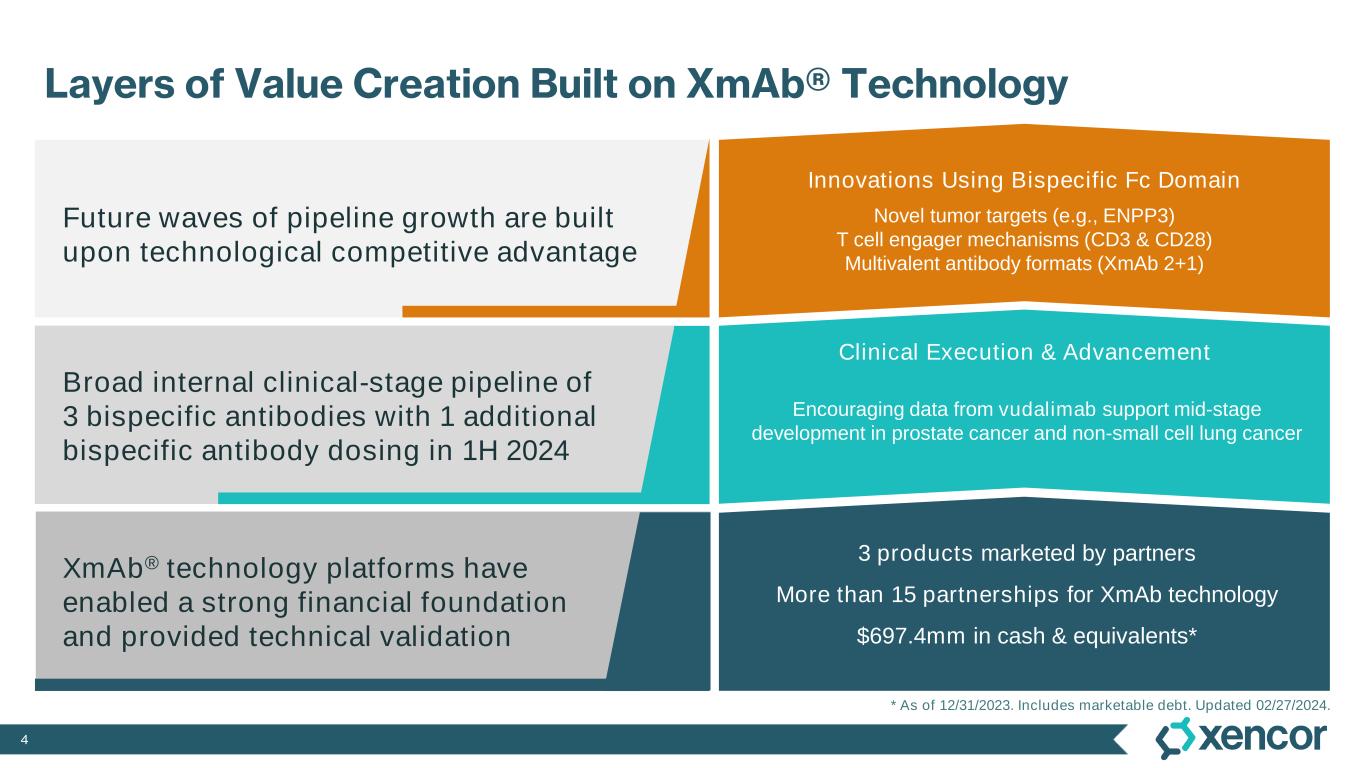
4 Layers of Value Creation Built on XmAb® Technology XmAb® technology platforms have enabled a strong financial foundation and provided technical validation Broad internal clinical-stage pipeline of 3 bispecific antibodies with 1 additional bispecific antibody dosing in 1H 2024 Future waves of pipeline growth are built upon technological competitive advantage Clinical Execution & Advancement Innovations Using Bispecific Fc Domain Novel tumor targets (e.g., ENPP3) T cell engager mechanisms (CD3 & CD28) Multivalent antibody formats (XmAb 2+1) Encouraging data from vudalimab support mid-stage development in prostate cancer and non-small cell lung cancer * As of 12/31/2023. Includes marketable debt. Updated 02/27/2024. 3 products marketed by partners More than 15 partnerships for XmAb technology $697.4mm in cash & equivalents*

5 Efficient XmAb® Platform Builds Differentiated Pipeline & Early Clinical Testing Rigorously Vets Lead Programs XmAb technology Early clinical testing Registration-enabling Continually grows drug pipeline Always renewing edge Clinical data drives decisions • Advance internally to late phase and plan to launch • Partner if necessary: risk/reward, competitive density, resource constraints • Stop if warranted Invest in trials for differentiated product profile Build organization for launch Guide earlier pipeline to align with therapeutic area Xtend → bispecifics → cytokines, 2+1, CD28 Select a program for late-phase commit, if compelling data and competitive landscape Broad platform enables strategy with renewable pipeline and cash flows
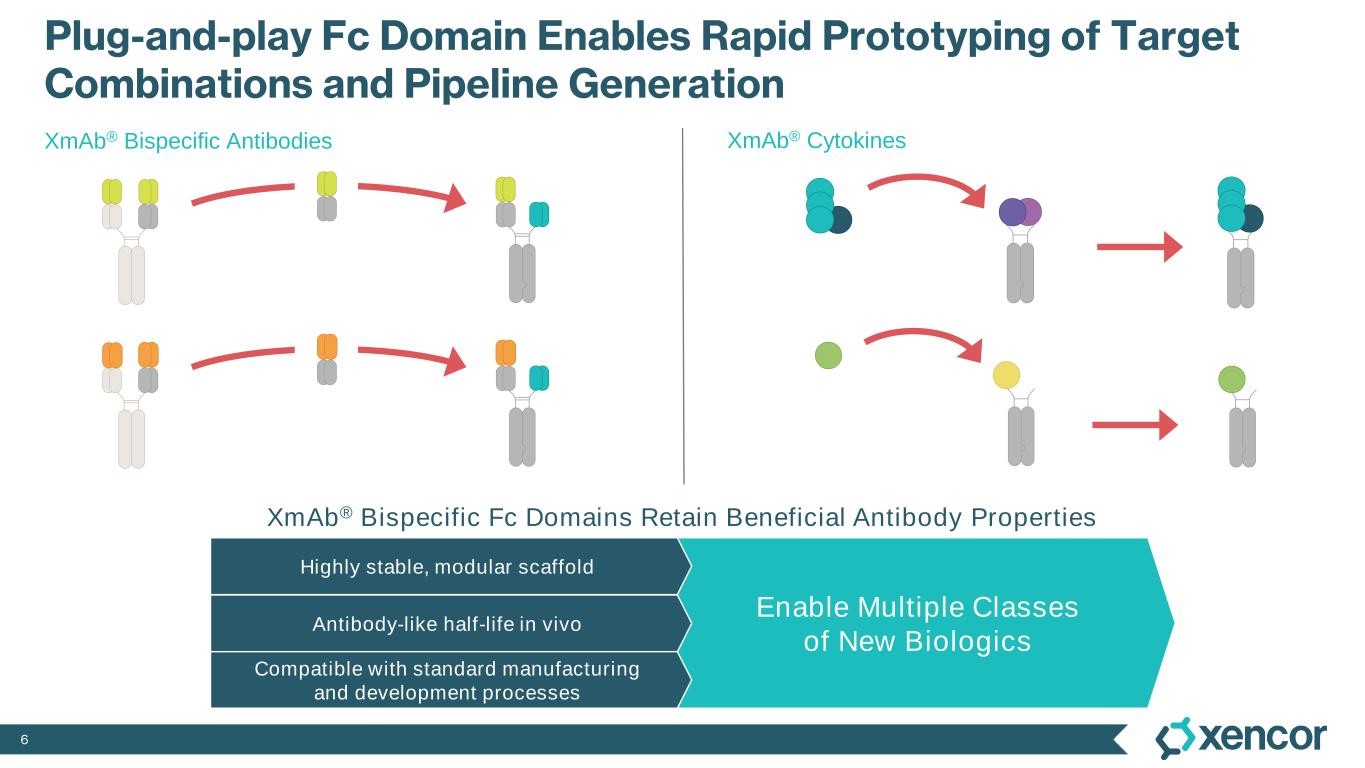
6 Plug-and-play Fc Domain Enables Rapid Prototyping of Target Combinations and Pipeline Generation XmAb® Bispecific Fc Domains Retain Beneficial Antibody Properties XmAb® Cytokines XmAb® Bispecific Antibodies Enable Multiple Classes of New Biologics Highly stable, modular scaffold Antibody-like half-life in vivo Compatible with standard manufacturing and development processes
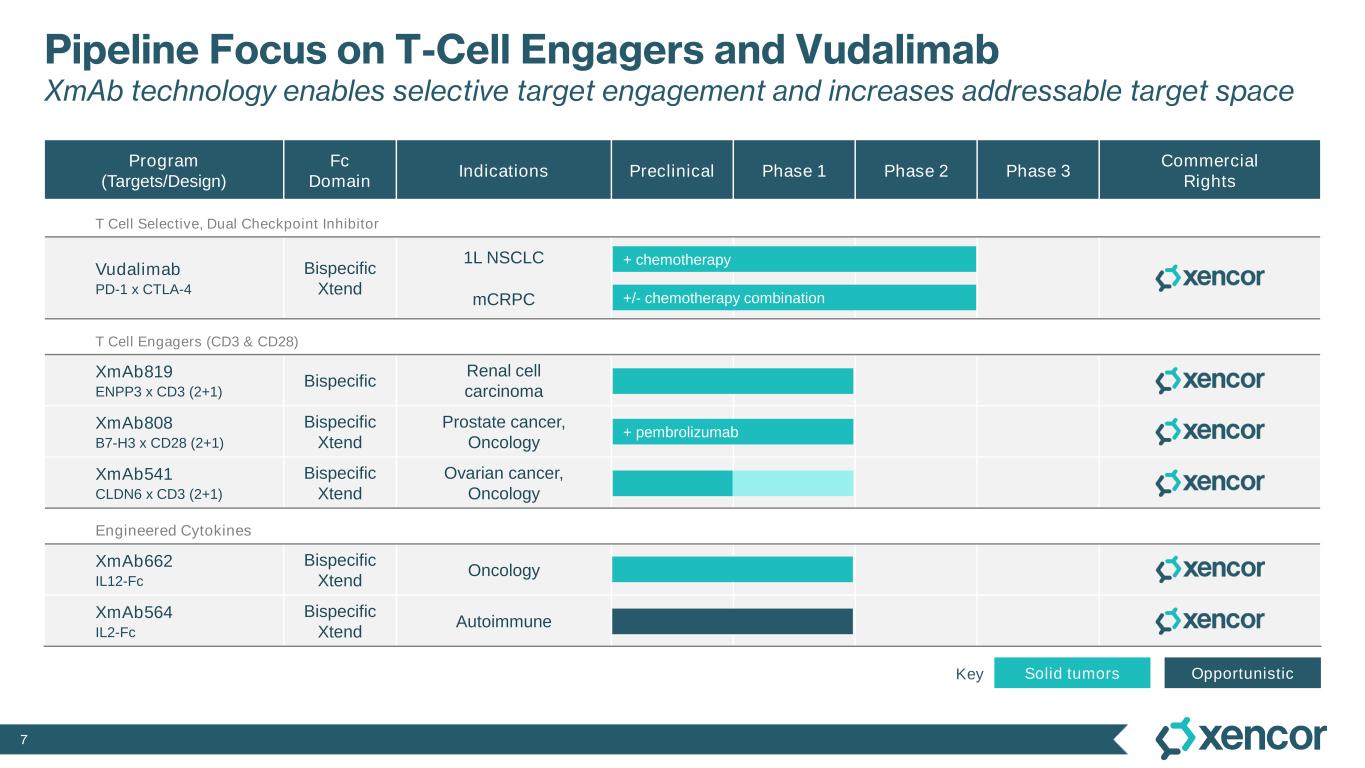
7 Pipeline Focus on T-Cell Engagers and Vudalimab XmAb technology enables selective target engagement and increases addressable target space Program (Targets/Design) Fc Domain Indications Preclinical Phase 1 Phase 2 Phase 3 Commercial Rights T Cell Selective, Dual Checkpoint Inhibitor Vudalimab PD-1 x CTLA-4 Bispecific Xtend 1L NSCLC mCRPC T Cell Engagers (CD3 & CD28) XmAb819 ENPP3 x CD3 (2+1) Bispecific Renal cell carcinoma XmAb808 B7-H3 x CD28 (2+1) Bispecific Xtend Prostate cancer, Oncology XmAb541 CLDN6 x CD3 (2+1) Bispecific Xtend Ovarian cancer, Oncology Engineered Cytokines XmAb662 IL12-Fc Bispecific Xtend Oncology XmAb564 IL2-Fc Bispecific Xtend Autoimmune +/- chemotherapy combination Solid tumors Opportunistic + pembrolizumab + chemotherapy Key
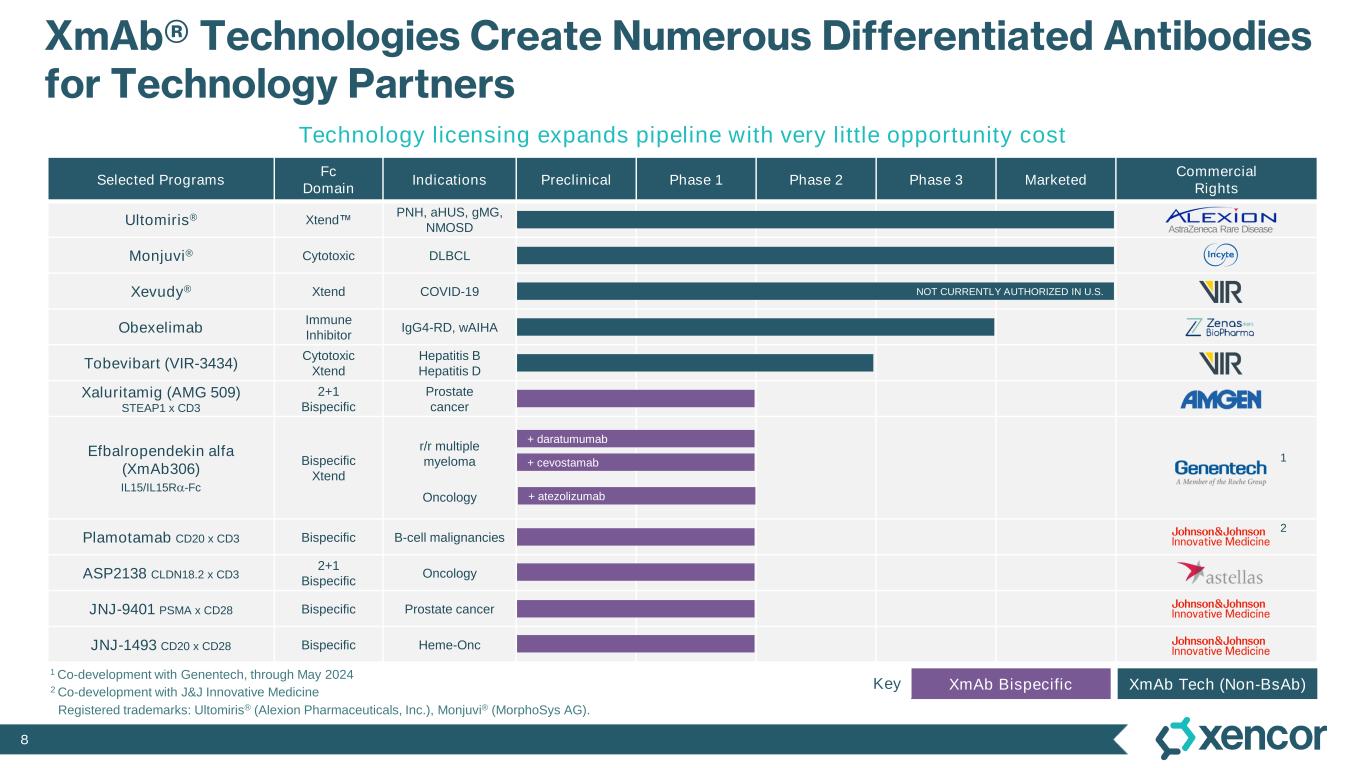
8 XmAb® Technologies Create Numerous Differentiated Antibodies for Technology Partners Selected Programs Fc Domain Indications Preclinical Phase 1 Phase 2 Phase 3 Marketed Commercial Rights Ultomiris® Xtend PNH, aHUS, gMG, NMOSD Monjuvi® Cytotoxic DLBCL Xevudy® Xtend COVID-19 Obexelimab Immune Inhibitor IgG4-RD, wAIHA Tobevibart (VIR-3434) Cytotoxic Xtend Hepatitis B Hepatitis D Xaluritamig (AMG 509) STEAP1 x CD3 2+1 Bispecific Prostate cancer Efbalropendekin alfa (XmAb306) IL15/IL15Ra-Fc Bispecific Xtend r/r multiple myeloma Oncology Plamotamab CD20 x CD3 Bispecific B-cell malignancies ASP2138 CLDN18.2 x CD3 2+1 Bispecific Oncology JNJ-9401 PSMA x CD28 Bispecific Prostate cancer JNJ-1493 CD20 x CD28 Bispecific Heme-Onc Technology licensing expands pipeline with very little opportunity cost NOT CURRENTLY AUTHORIZED IN U.S. Registered trademarks: Ultomiris® (Alexion Pharmaceuticals, Inc.), Monjuvi® (MorphoSys AG). AstraZeneca Rare Disease + cevostamab + daratumumab + atezolizumab 2 Co-development with J&J Innovative Medicine 1 Co-development with Genentech, through May 2024 1 2 XmAb BispecificKey XmAb Tech (Non-BsAb)

9 ® XmAb® Bispecific Fc Domain Enabling New Classes of Biologics and Therapeutic Mechanisms of Action
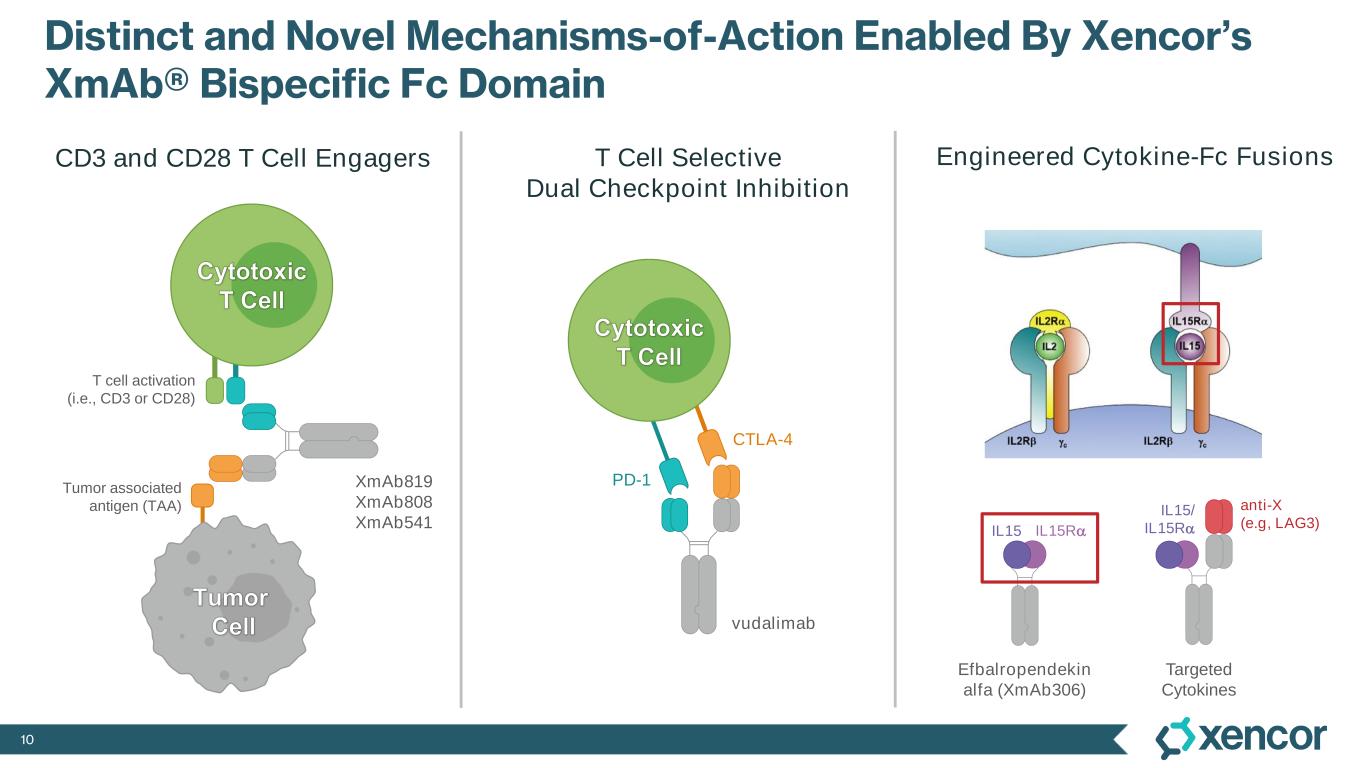
10 Distinct and Novel Mechanisms-of-Action Enabled By Xencor’s XmAb® Bispecific Fc Domain PD-1 CTLA-4 Tumor associated antigen (TAA) vudalimab Efbalropendekin alfa (XmAb306) Targeted Cytokines Tumor Cell Cytotoxic T Cell CD3 and CD28 T Cell Engagers T cell activation (i.e., CD3 or CD28) T Cell Selective Dual Checkpoint Inhibition XmAb819 XmAb808 XmAb541 Cytotoxic T Cell Engineered Cytokine-Fc Fusions anti-X (e.g, LAG3) IL15/ IL15RaIL15 IL15Ra

11 ® XmAb® Bispecific T Cell Engagers XmAb 2+1 Bispecific Antibody Format XmAb819 (ENPP3 x CD3) XmAb541 (CLDN6 x CD3) XmAb808 (B7-H3 x CD28)
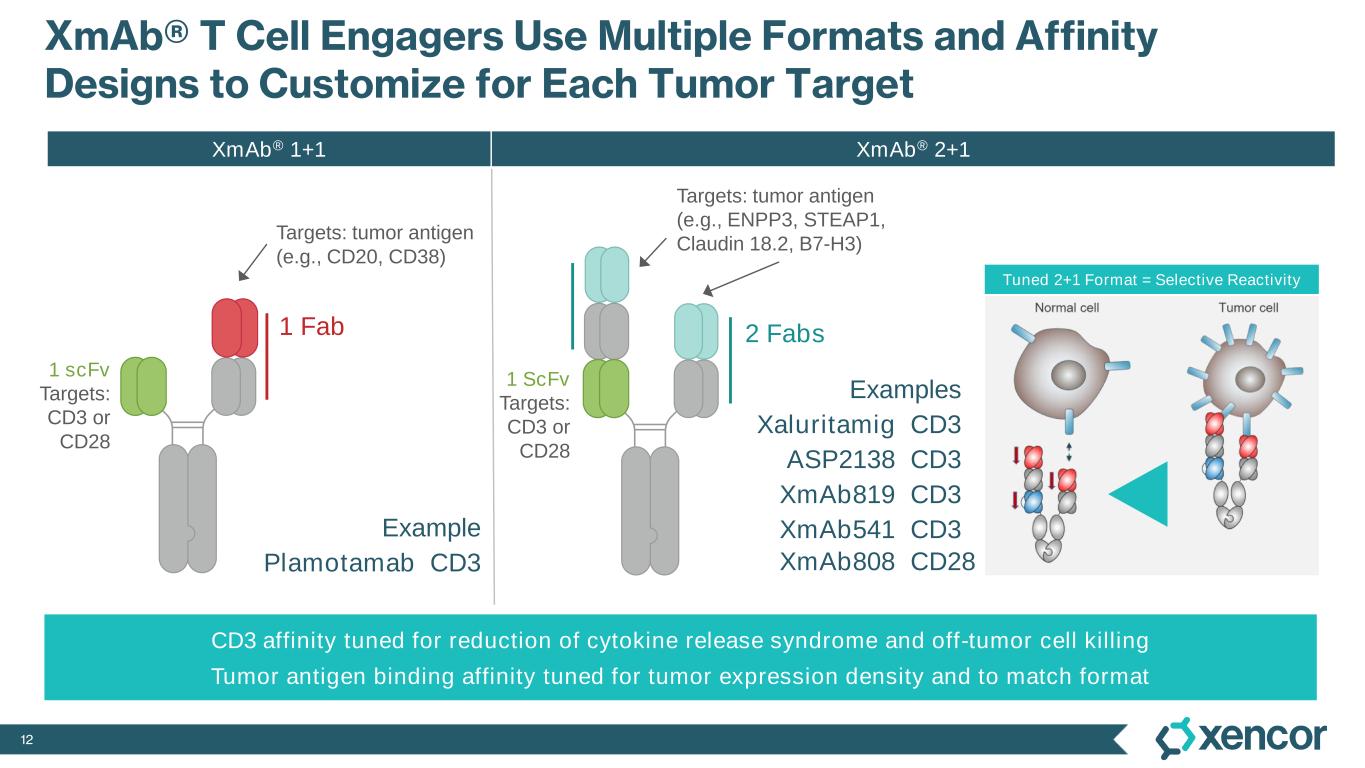
12 XmAb® T Cell Engagers Use Multiple Formats and Affinity Designs to Customize for Each Tumor Target 1 ScFv Targets: CD3 or CD28 Targets: tumor antigen (e.g., ENPP3, STEAP1, Claudin 18.2, B7-H3) Targets: tumor antigen (e.g., CD20, CD38) Examples Xaluritamig CD3 ASP2138 CD3 XmAb819 CD3 XmAb541 CD3 CD3 affinity tuned for reduction of cytokine release syndrome and off-tumor cell killing Tumor antigen binding affinity tuned for tumor expression density and to match format Tuned 2+1 Format = Selective Reactivity Example Plamotamab CD3 1 scFv Targets: CD3 or CD28 1 Fab 2 Fabs XmAb808 CD28 XmAb® 2+1XmAb® 1+1
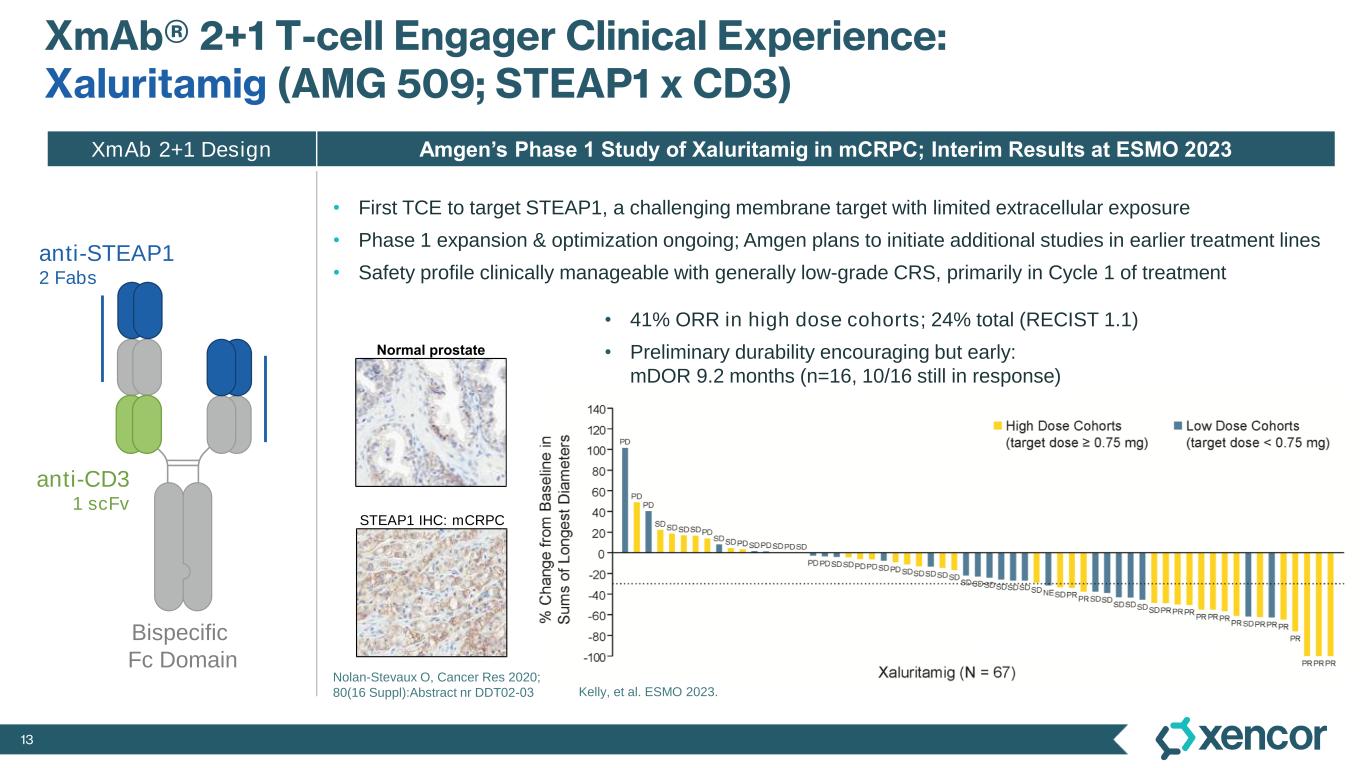
13 XmAb® 2+1 T-cell Engager Clinical Experience: Xaluritamig (AMG 509; STEAP1 x CD3) • First TCE to target STEAP1, a challenging membrane target with limited extracellular exposure • Phase 1 expansion & optimization ongoing; Amgen plans to initiate additional studies in earlier treatment lines • Safety profile clinically manageable with generally low-grade CRS, primarily in Cycle 1 of treatment anti-CD3 1 scFv Bispecific Fc Domain anti-STEAP1 2 Fabs STEAP1 IHC: mCRPC Nolan-Stevaux O, Cancer Res 2020; 80(16 Suppl):Abstract nr DDT02-03 Normal prostate • 41% ORR in high dose cohorts; 24% total (RECIST 1.1) • Preliminary durability encouraging but early: mDOR 9.2 months (n=16, 10/16 still in response) Kelly, et al. ESMO 2023. Amgen’s Phase 1 Study of Xaluritamig in mCRPC; Interim Results at ESMO 2023 XmAb 2+1 Design
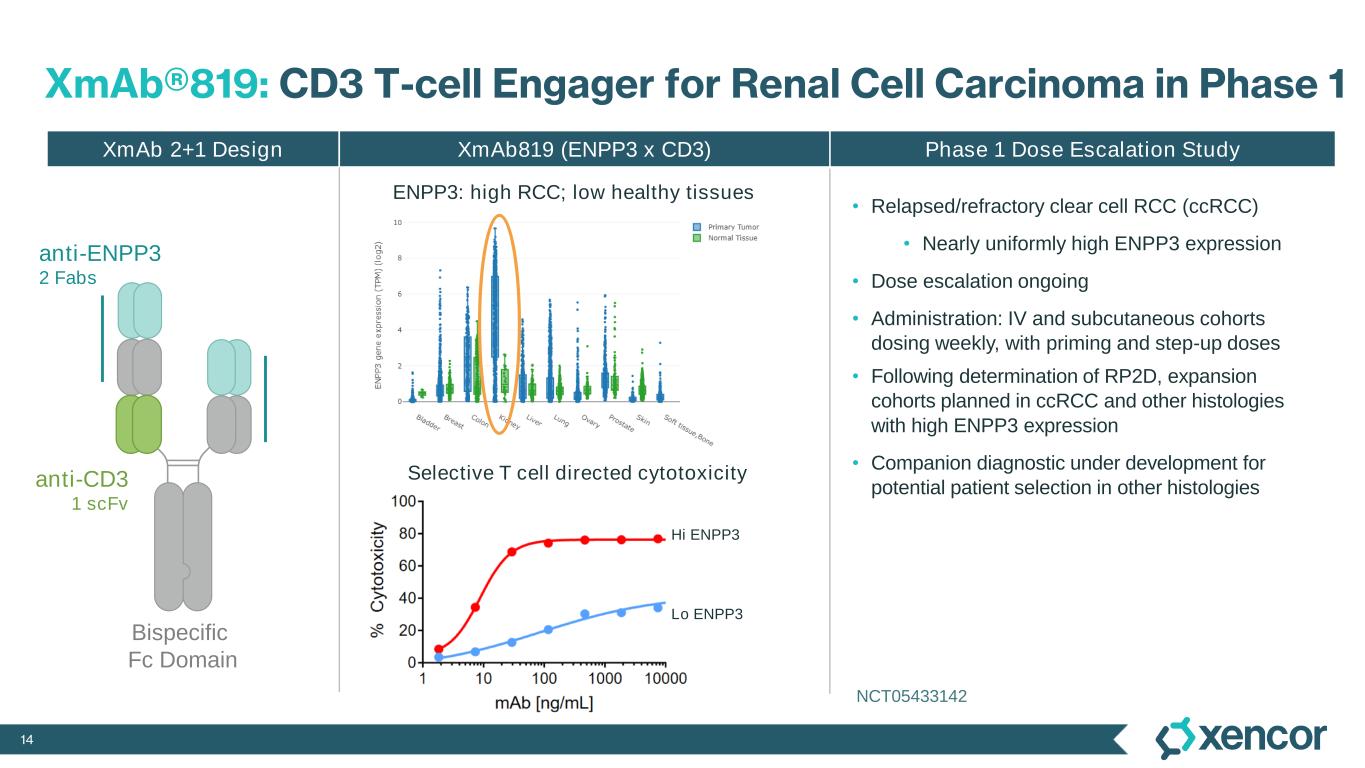
14 XmAb®819: CD3 T-cell Engager for Renal Cell Carcinoma in Phase 1 XmAb819 (ENPP3 x CD3) Phase 1 Dose Escalation Study Selective T cell directed cytotoxicity Hi ENPP3 Lo ENPP3 XmAb 2+1 Design ENPP3: high RCC; low healthy tissues • Relapsed/refractory clear cell RCC (ccRCC) • Nearly uniformly high ENPP3 expression • Dose escalation ongoing • Administration: IV and subcutaneous cohorts dosing weekly, with priming and step-up doses • Following determination of RP2D, expansion cohorts planned in ccRCC and other histologies with high ENPP3 expression • Companion diagnostic under development for potential patient selection in other histologiesanti-CD3 1 scFv Bispecific Fc Domain anti-ENPP3 2 Fabs NCT05433142

15 XmAb®541: CD3 T-cell Engager for Ovarian Cancer & Solid Tumors XmAb541 (CLDN6 x CD3) CLDN6 Avoids Normal TissueXmAb 2+1 Design 0.1 1 10 100 1000 10000 100000 0 20 40 60 80 100 0 5 10 15 20 Ab concentration [ng/ml] % Z o m b ie + o n H u tu 8 0 ( C L D N 6 + ) % Z o m b ie + o n 2 9 3 -T R E X (C L D N 9 + ) EC50 = 5.6 ng/mL XENP37227 CLDN9 CLDN6 0.1 1 10 100 1000 10000 100000 0 20 40 60 80 100 0 5 10 15 20 Ab concentration [ng/ml] % Z o m b ie + o n H u tu 8 0 ( C L D N 6 + ) % Z o m b ie + o n 2 9 3 -T R E X (C L D N 9 + ) CLDN6 CLDN9 EC50 = 22.8 ng/mL XENP35386 (H1L1 - parental) CLDN6 selective over CLDN9 CLDN6 CLDN9 P a n c re a s S m a ll in te s ti n e E s o p h a g u s C e rv ix FDA999w normal tissue staining • Differential expression in cancerous tissue presents CLDN6 as an intriguing target • CLDN family members, which are small membrane proteins, have high sequence identity, complicating antibody design • XmAb541 engineered for CLDN6 selectivity over similar CLDN9, CLDN3 and CLDN4 • Clinical status: IND open; planned dosing 1H24 anti-CD3 1 scFv Bispecific Fc Domain anti-CLDN6 2 Fabs
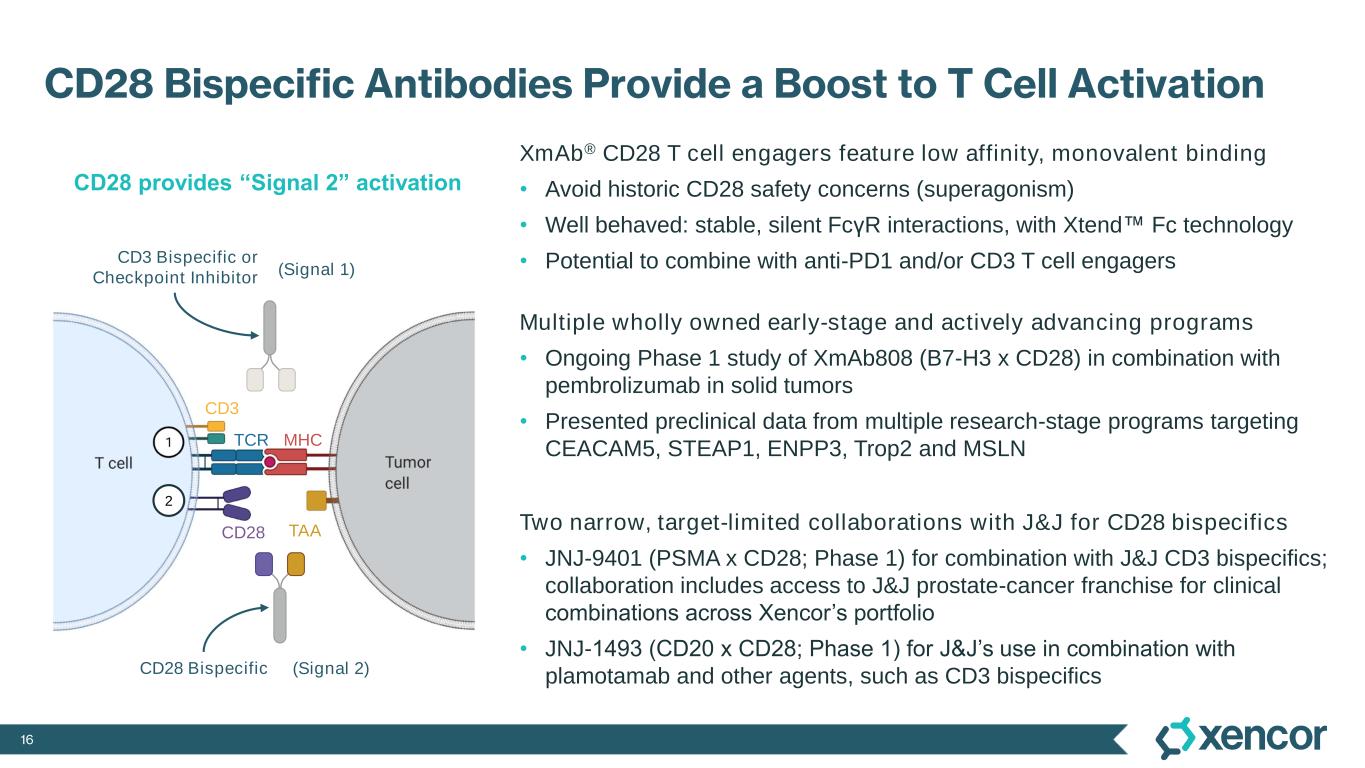
16 CD28 Bispecific Antibodies Provide a Boost to T Cell Activation XmAb® CD28 T cell engagers feature low affinity, monovalent binding • Avoid historic CD28 safety concerns (superagonism) • Well behaved: stable, silent FcγR interactions, with Xtend Fc technology • Potential to combine with anti-PD1 and/or CD3 T cell engagers Multiple wholly owned early-stage and actively advancing programs • Ongoing Phase 1 study of XmAb808 (B7-H3 x CD28) in combination with pembrolizumab in solid tumors • Presented preclinical data from multiple research-stage programs targeting CEACAM5, STEAP1, ENPP3, Trop2 and MSLN Two narrow, target-limited collaborations with J&J for CD28 bispecifics • JNJ-9401 (PSMA x CD28; Phase 1) for combination with J&J CD3 bispecifics; collaboration includes access to J&J prostate-cancer franchise for clinical combinations across Xencor’s portfolio • JNJ-1493 (CD20 x CD28; Phase 1) for J&J’s use in combination with plamotamab and other agents, such as CD3 bispecifics CD28 provides “Signal 2” activation (Signal 1) (Signal 2) TAA 2 CD3 Bispecific or Checkpoint Inhibitor CD28 CD3 TCR MHC CD28 Bispecific
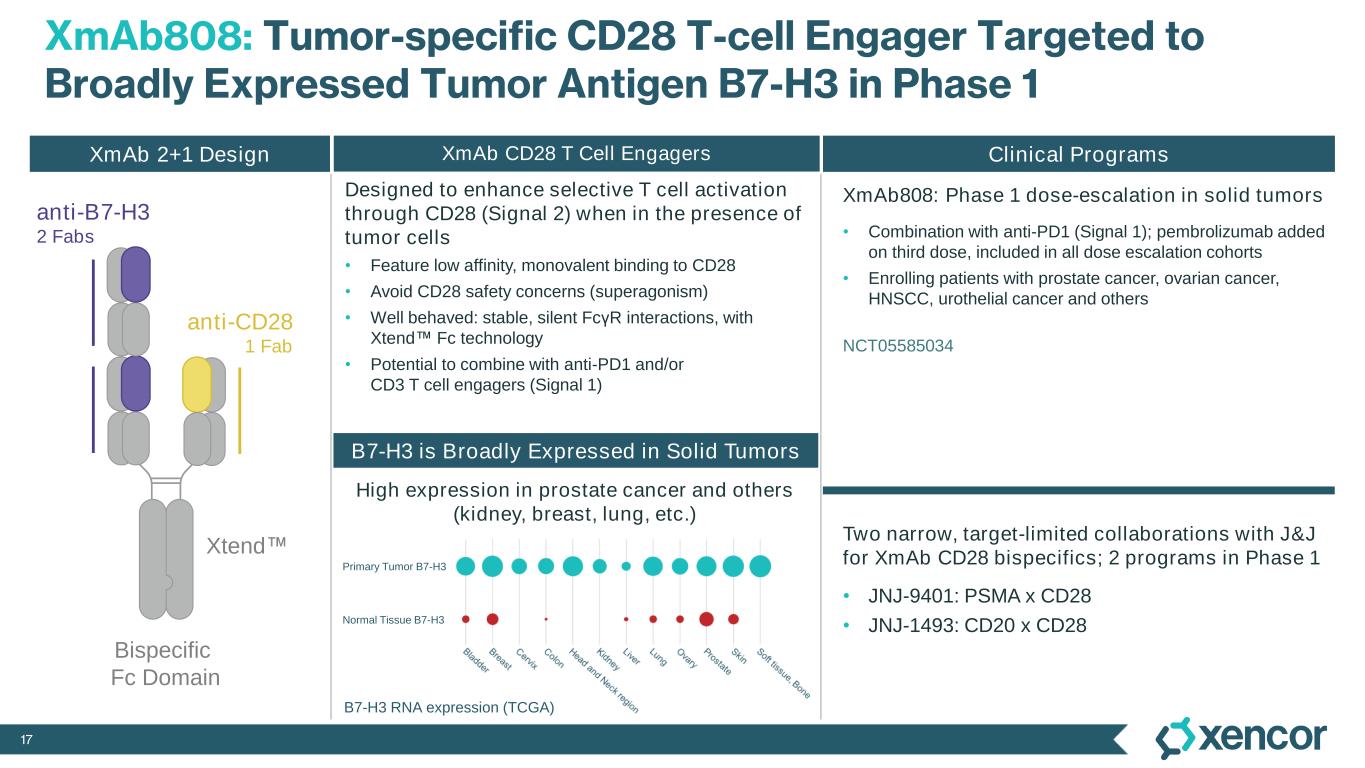
17 XmAb808: Tumor-specific CD28 T-cell Engager Targeted to Broadly Expressed Tumor Antigen B7-H3 in Phase 1 Clinical Programs XmAb808: Phase 1 dose-escalation in solid tumors • Combination with anti-PD1 (Signal 1); pembrolizumab added on third dose, included in all dose escalation cohorts • Enrolling patients with prostate cancer, ovarian cancer, HNSCC, urothelial cancer and others Two narrow, target-limited collaborations with J&J for XmAb CD28 bispecifics; 2 programs in Phase 1 • JNJ-9401: PSMA x CD28 • JNJ-1493: CD20 x CD28 anti-CD28 1 Fab anti-B7-H3 2 Fabs Xtend Bispecific Fc Domain XmAb 2+1 Design B7-H3 is Broadly Expressed in Solid Tumors High expression in prostate cancer and others (kidney, breast, lung, etc.) XmAb CD28 T Cell Engagers B7-H3 RNA expression (TCGA) Primary Tumor B7-H3 Normal Tissue B7-H3 NCT05585034 Designed to enhance selective T cell activation through CD28 (Signal 2) when in the presence of tumor cells • Feature low affinity, monovalent binding to CD28 • Avoid CD28 safety concerns (superagonism) • Well behaved: stable, silent FcγR interactions, with Xtend Fc technology • Potential to combine with anti-PD1 and/or CD3 T cell engagers (Signal 1)

18 ® T Cell Selective, Dual Checkpoint Inhibitor Vudalimab (PD-1 x CTLA-4)
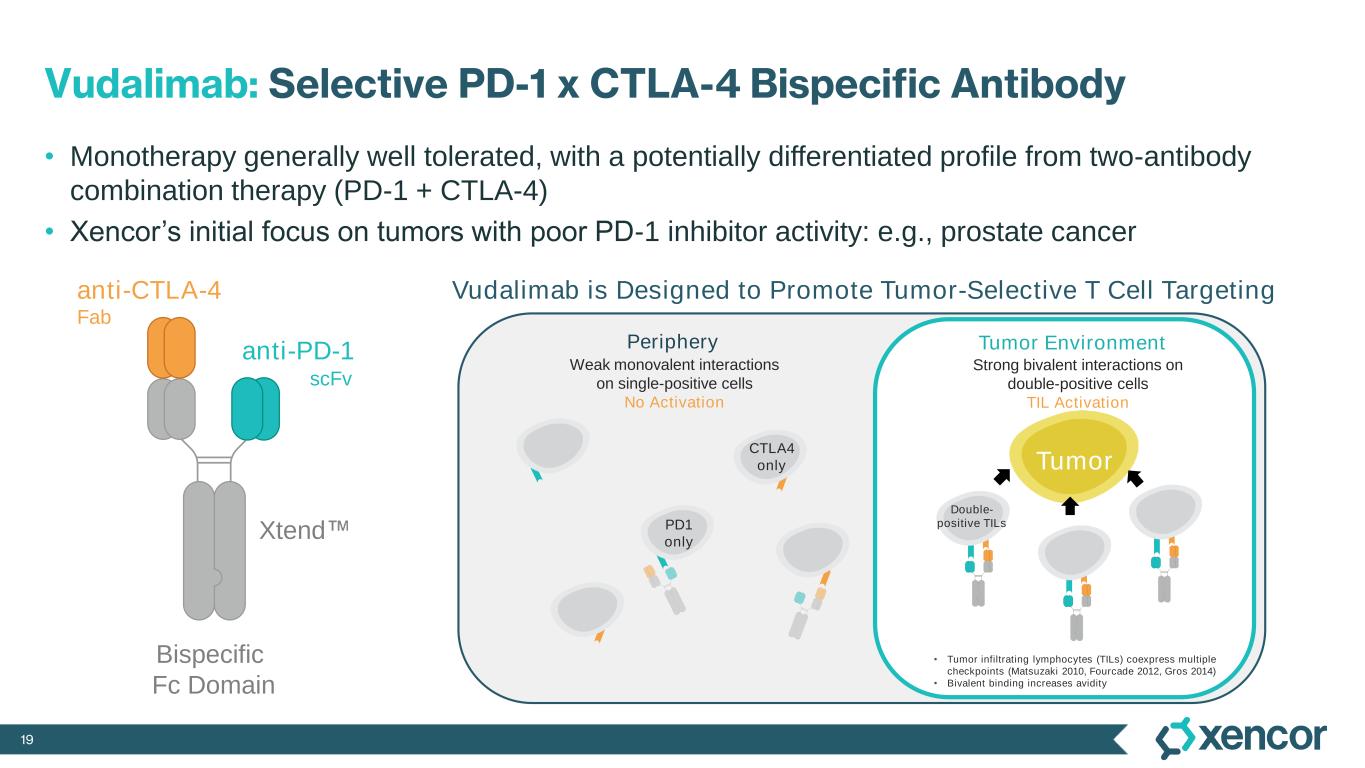
19 Vudalimab: Selective PD-1 x CTLA-4 Bispecific Antibody • Monotherapy generally well tolerated, with a potentially differentiated profile from two-antibody combination therapy (PD-1 + CTLA-4) • Xencor’s initial focus on tumors with poor PD-1 inhibitor activity: e.g., prostate cancer anti-PD-1 scFv anti-CTLA-4 Fab Xtend Bispecific Fc Domain • Tumor infiltrating lymphocytes (TILs) coexpress multiple checkpoints (Matsuzaki 2010, Fourcade 2012, Gros 2014) • Bivalent binding increases avidity Periphery Strong bivalent interactions on double-positive cells TIL Activation Weak monovalent interactions on single-positive cells No Activation Tumor Environment Tumor PD1 only CTLA4 only Double- positive TILs Vudalimab is Designed to Promote Tumor-Selective T Cell Targeting
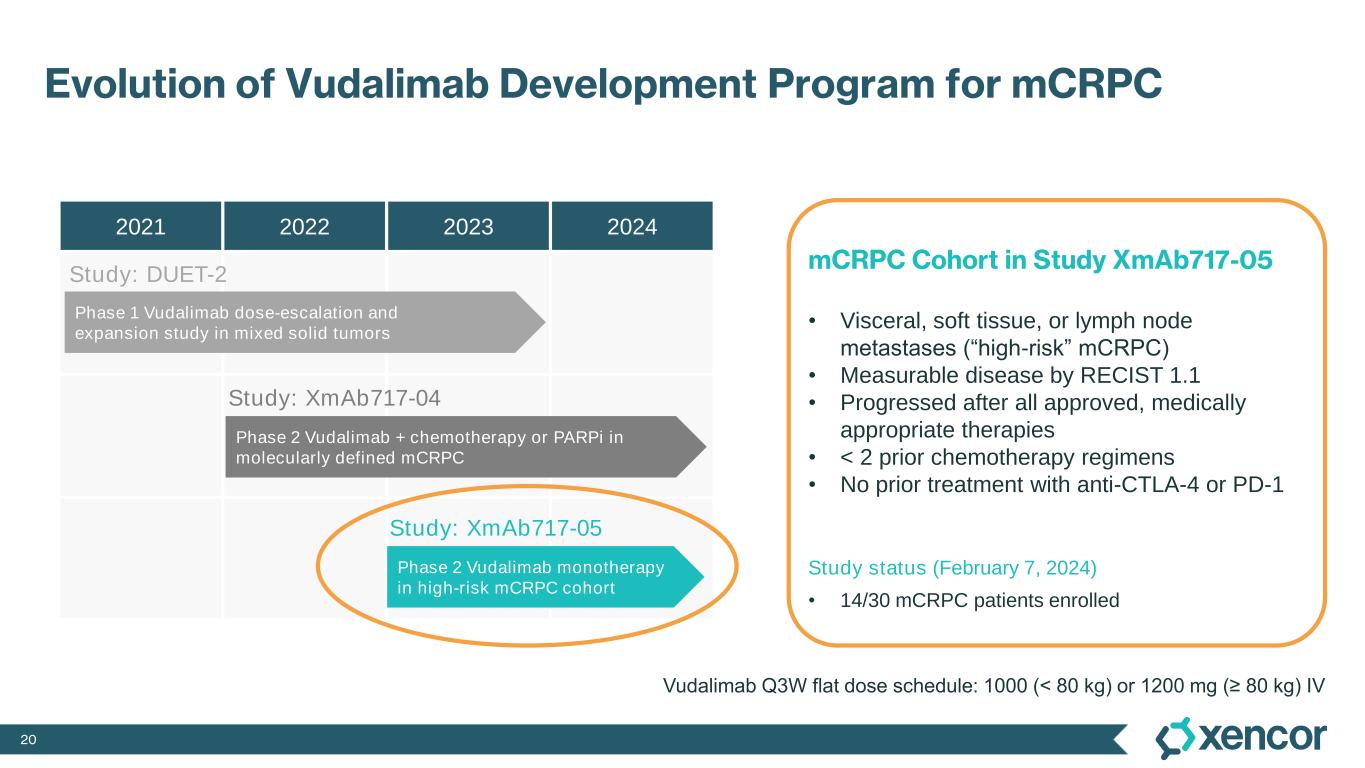
20 Evolution of Vudalimab Development Program for mCRPC 2021 2022 2023 2024 Phase 2 Vudalimab + chemotherapy or PARPi in molecularly defined mCRPC Phase 2 Vudalimab monotherapy in high-risk mCRPC cohort Phase 1 Vudalimab dose-escalation and expansion study in mixed solid tumors mCRPC Cohort in Study XmAb717-05 • Visceral, soft tissue, or lymph node metastases (“high-risk” mCRPC) • Measurable disease by RECIST 1.1 • Progressed after all approved, medically appropriate therapies • < 2 prior chemotherapy regimens • No prior treatment with anti-CTLA-4 or PD-1 Study status (February 7, 2024) • 14/30 mCRPC patients enrolled Study: DUET-2 Study: XmAb717-04 Study: XmAb717-05 Vudalimab Q3W flat dose schedule: 1000 (< 80 kg) or 1200 mg (≥ 80 kg) IV
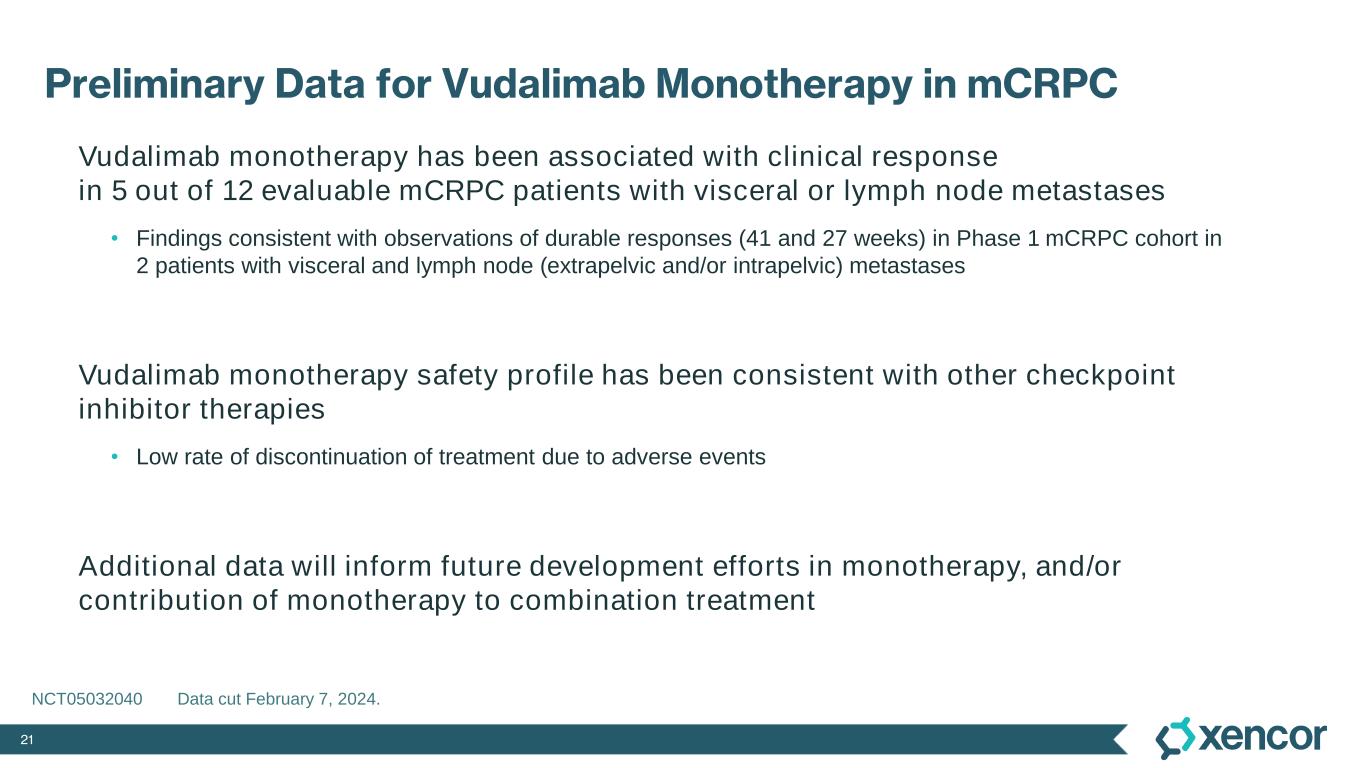
21 Preliminary Data for Vudalimab Monotherapy in mCRPC Vudalimab monotherapy has been associated with clinical response in 5 out of 12 evaluable mCRPC patients with visceral or lymph node metastases • Findings consistent with observations of durable responses (41 and 27 weeks) in Phase 1 mCRPC cohort in 2 patients with visceral and lymph node (extrapelvic and/or intrapelvic) metastases Vudalimab monotherapy safety profile has been consistent with other checkpoint inhibitor therapies • Low rate of discontinuation of treatment due to adverse events Additional data will inform future development efforts in monotherapy, and/or contribution of monotherapy to combination treatment NCT05032040 Data cut February 7, 2024.
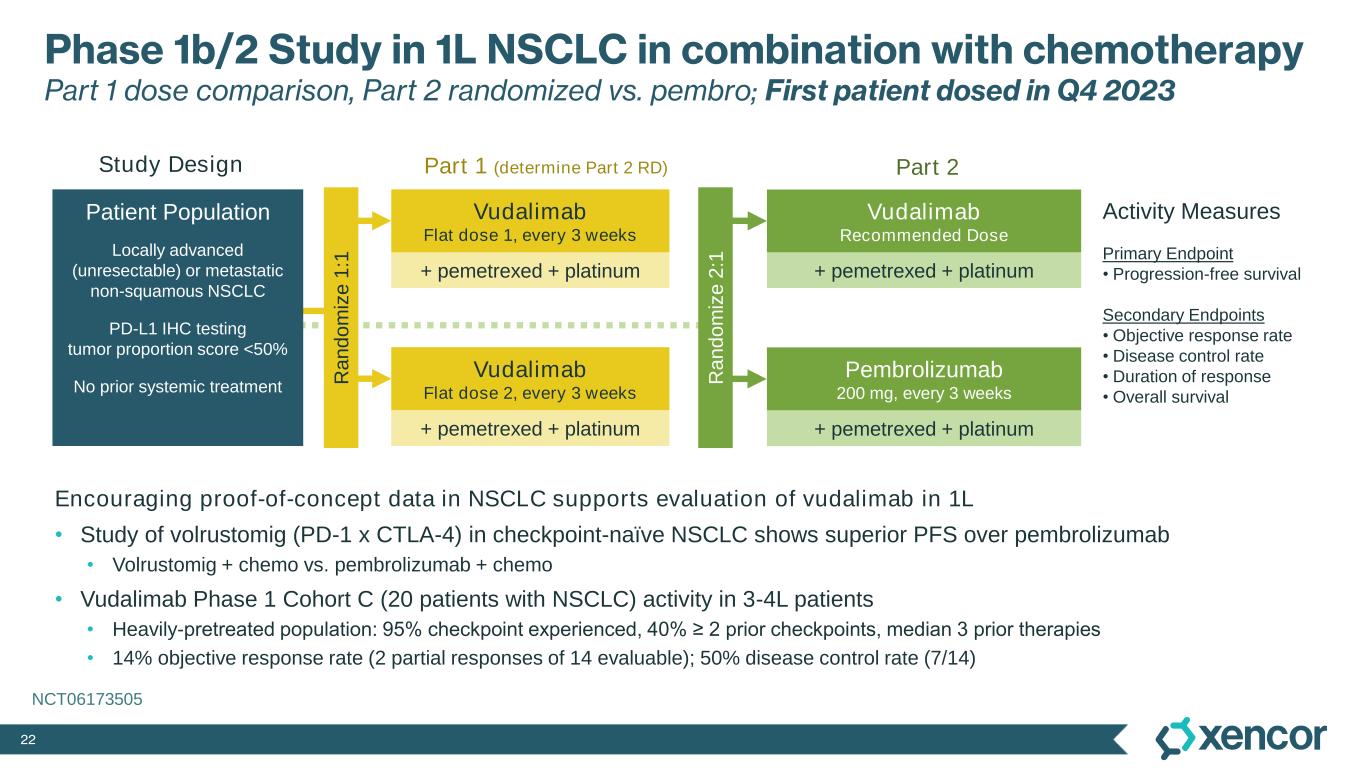
22 Phase 1b/2 Study in 1L NSCLC in combination with chemotherapy Part 1 dose comparison, Part 2 randomized vs. pembro; First patient dosed in Q4 2023 Encouraging proof-of-concept data in NSCLC supports evaluation of vudalimab in 1L • Study of volrustomig (PD-1 x CTLA-4) in checkpoint-naïve NSCLC shows superior PFS over pembrolizumab • Volrustomig + chemo vs. pembrolizumab + chemo • Vudalimab Phase 1 Cohort C (20 patients with NSCLC) activity in 3-4L patients • Heavily-pretreated population: 95% checkpoint experienced, 40% ≥ 2 prior checkpoints, median 3 prior therapies • 14% objective response rate (2 partial responses of 14 evaluable); 50% disease control rate (7/14) Activity Measures Primary Endpoint • Progression-free survival Secondary Endpoints • Objective response rate • Disease control rate • Duration of response • Overall survival Vudalimab Flat dose 2, every 3 weeks Vudalimab Flat dose 1, every 3 weeks + pemetrexed + platinum + pemetrexed + platinum Vudalimab Recommended Dose + pemetrexed + platinum Pembrolizumab 200 mg, every 3 weeks + pemetrexed + platinum Part 1 (determine Part 2 RD) Part 2 Locally advanced (unresectable) or metastatic non-squamous NSCLC PD-L1 IHC testing tumor proportion score <50% No prior systemic treatment Patient Population R a n d o m iz e 1 :1 Study Design R a n d o m iz e 2 :1 NCT06173505
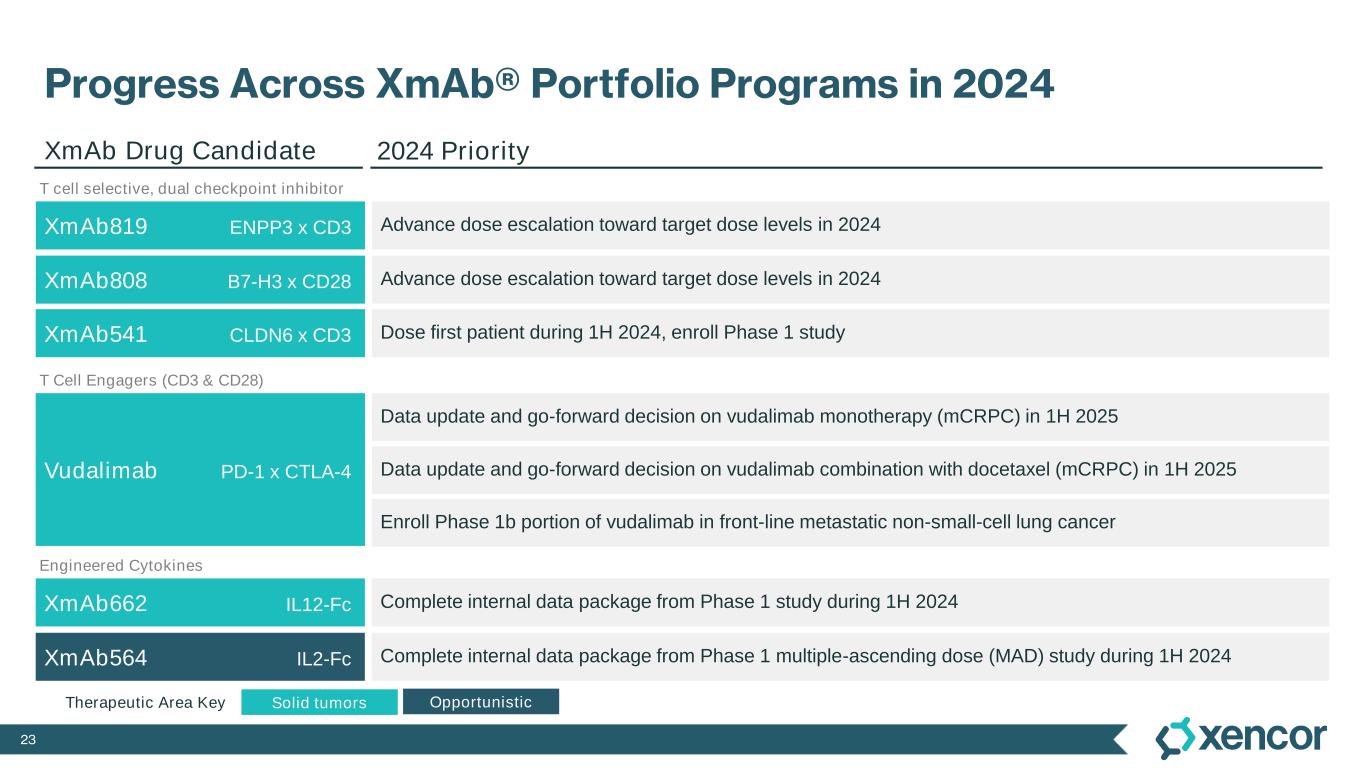
23 Progress Across XmAb® Portfolio Programs in 2024 Data update and go-forward decision on vudalimab monotherapy (mCRPC) in 1H 2025 XmAb808 B7-H3 x CD28 XmAb819 ENPP3 x CD3 XmAb662 IL12-Fc Complete internal data package from Phase 1 study during 1H 2024 XmAb541 CLDN6 x CD3 Dose first patient during 1H 2024, enroll Phase 1 study XmAb564 IL2-Fc Complete internal data package from Phase 1 multiple-ascending dose (MAD) study during 1H 2024 Vudalimab PD-1 x CTLA-4 XmAb Drug Candidate 2024 Priority Solid tumors OpportunisticTherapeutic Area Key Advance dose escalation toward target dose levels in 2024 Advance dose escalation toward target dose levels in 2024 Data update and go-forward decision on vudalimab combination with docetaxel (mCRPC) in 1H 2025 Enroll Phase 1b portion of vudalimab in front-line metastatic non-small-cell lung cancer T Cell Engagers (CD3 & CD28) T cell selective, dual checkpoint inhibitor Engineered Cytokines

® Proteins by Design® XmAb® Antibody Therapeutics Corporate Overview March 2024

®Vudalimab Monotherapy in Patients with mCRPC Study XmAb717-05 Data cut 7 February 2024
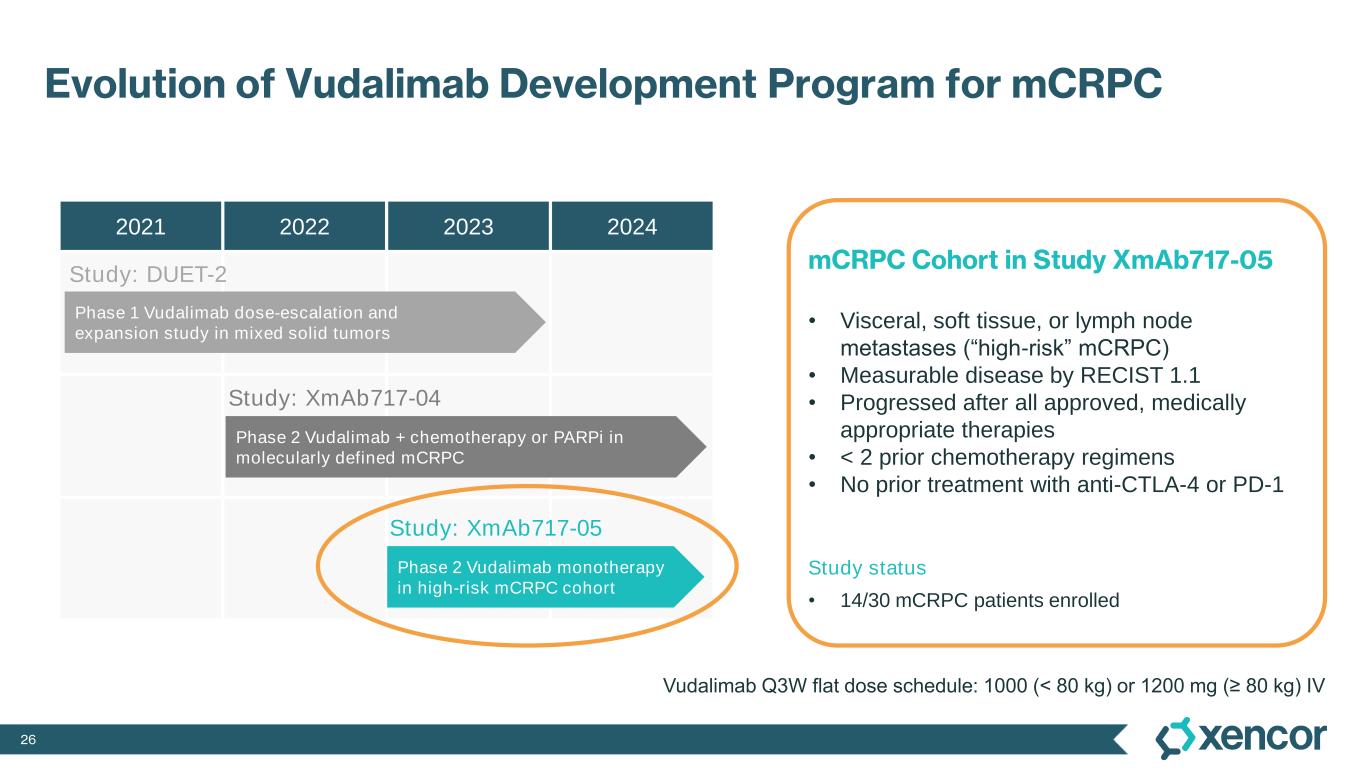
26 Evolution of Vudalimab Development Program for mCRPC 2021 2022 2023 2024 Phase 2 Vudalimab + chemotherapy or PARPi in molecularly defined mCRPC Phase 2 Vudalimab monotherapy in high-risk mCRPC cohort Phase 1 Vudalimab dose-escalation and expansion study in mixed solid tumors mCRPC Cohort in Study XmAb717-05 • Visceral, soft tissue, or lymph node metastases (“high-risk” mCRPC) • Measurable disease by RECIST 1.1 • Progressed after all approved, medically appropriate therapies • < 2 prior chemotherapy regimens • No prior treatment with anti-CTLA-4 or PD-1 Study status • 14/30 mCRPC patients enrolled Study: DUET-2 Study: XmAb717-04 Study: XmAb717-05 Vudalimab Q3W flat dose schedule: 1000 (< 80 kg) or 1200 mg (≥ 80 kg) IV

27 Summary of Preliminary Data Vudalimab monotherapy has been associated with clinical response in 5 out of 12 evaluable mCRPC patients with visceral or lymph node metastases • Findings consistent with observations of durable responses (41 and 27 weeks) in Phase 1 mCRPC cohort in 2 patients with visceral and lymph node (extrapelvic and/or intrapelvic) metastases Vudalimab monotherapy safety profile has been consistent with other checkpoint inhibitor therapies • Low rate of discontinuation of treatment due to adverse events Additional data will inform future development efforts in monotherapy, and/or contribution of monotherapy to combination treatment Data cut 7 February 2024
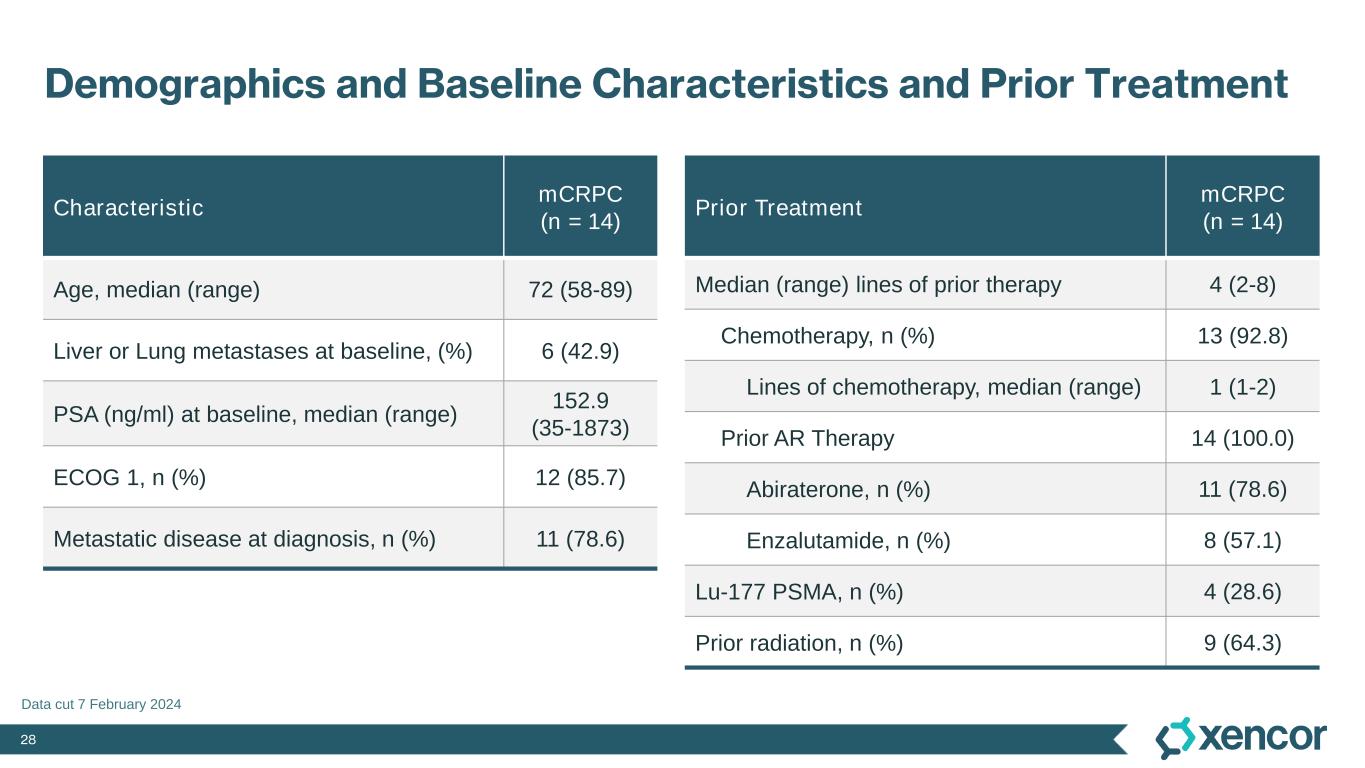
28 Demographics and Baseline Characteristics and Prior Treatment Data cut 7 February 2024 Characteristic mCRPC (n = 14) Age, median (range) 72 (58-89) Liver or Lung metastases at baseline, (%) 6 (42.9) PSA (ng/ml) at baseline, median (range) 152.9 (35-1873) ECOG 1, n (%) 12 (85.7) Metastatic disease at diagnosis, n (%) 11 (78.6) Prior Treatment mCRPC (n = 14) Median (range) lines of prior therapy 4 (2-8) Chemotherapy, n (%) 13 (92.8) Lines of chemotherapy, median (range) 1 (1-2) Prior AR Therapy 14 (100.0) Abiraterone, n (%) 11 (78.6) Enzalutamide, n (%) 8 (57.1) Lu-177 PSMA, n (%) 4 (28.6) Prior radiation, n (%) 9 (64.3)
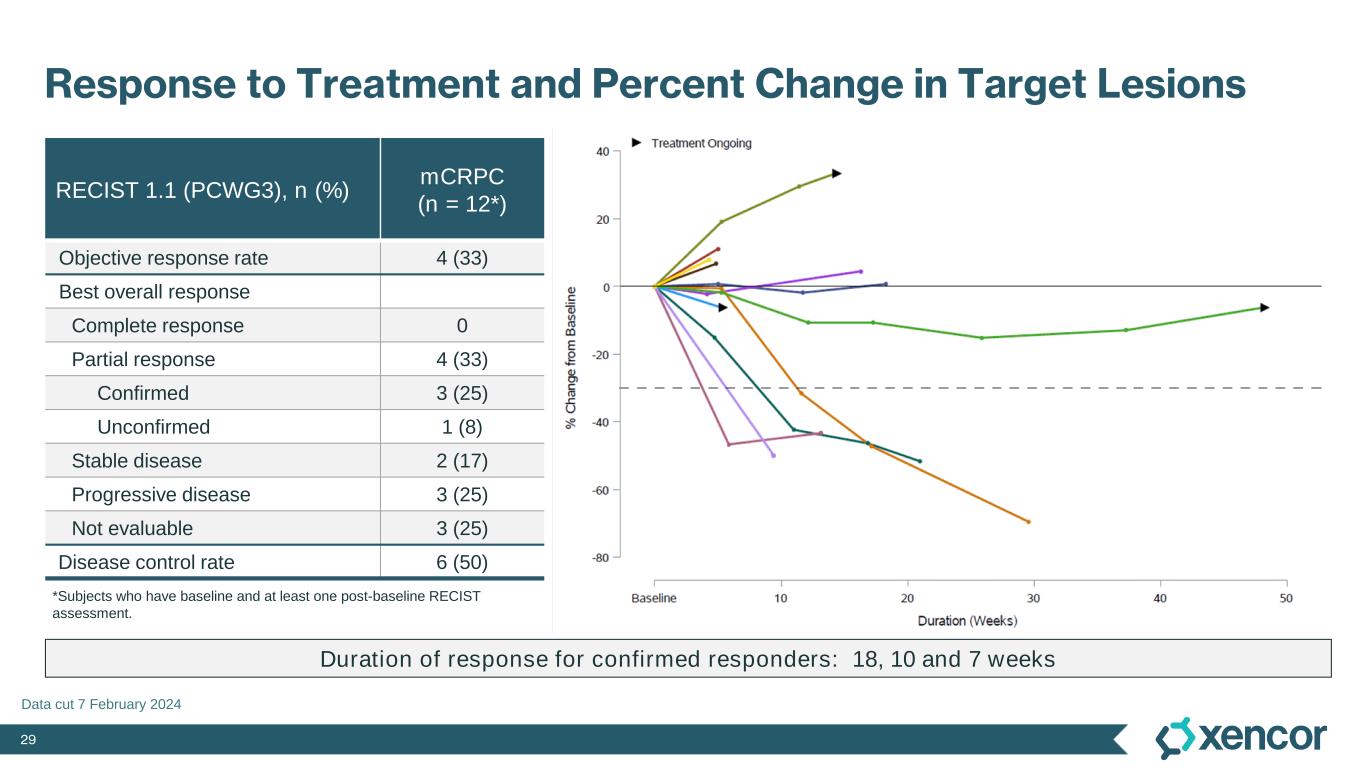
29 Response to Treatment and Percent Change in Target Lesions RECIST 1.1 (PCWG3), n (%) mCRPC (n = 12*) Objective response rate 4 (33) Best overall response Complete response 0 Partial response 4 (33) Confirmed 3 (25) Unconfirmed 1 (8) Stable disease 2 (17) Progressive disease 3 (25) Not evaluable 3 (25) Disease control rate 6 (50) *Subjects who have baseline and at least one post-baseline RECIST assessment. Duration of response for confirmed responders: 18, 10 and 7 weeks Data cut 7 February 2024 Median (range) duration of treatment 16.5 (5.1-51) weeks
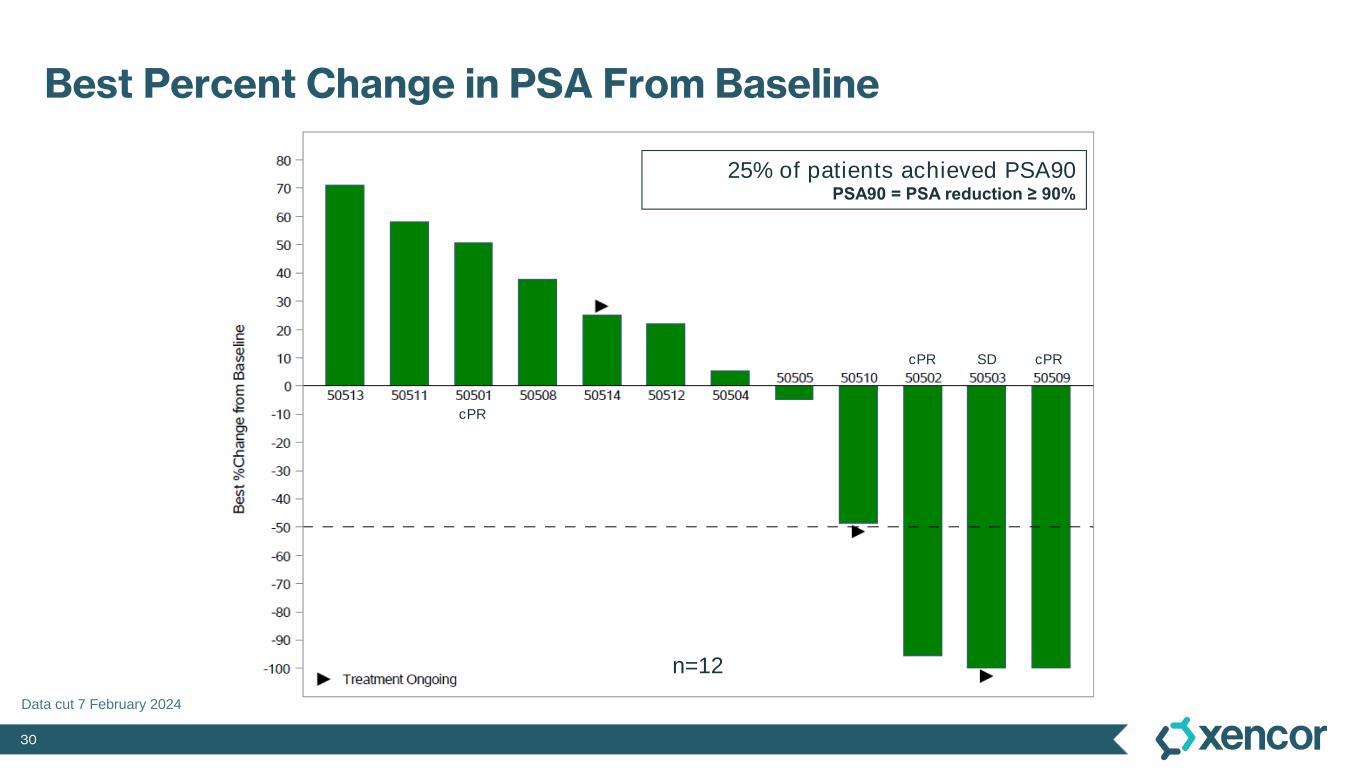
30 Best Percent Change in PSA From Baseline Data cut 7 February 2024 25% of patients achieved PSA90 PSA90 = PSA reduction ≥ 90% n=12 cPR cPR cPRSD
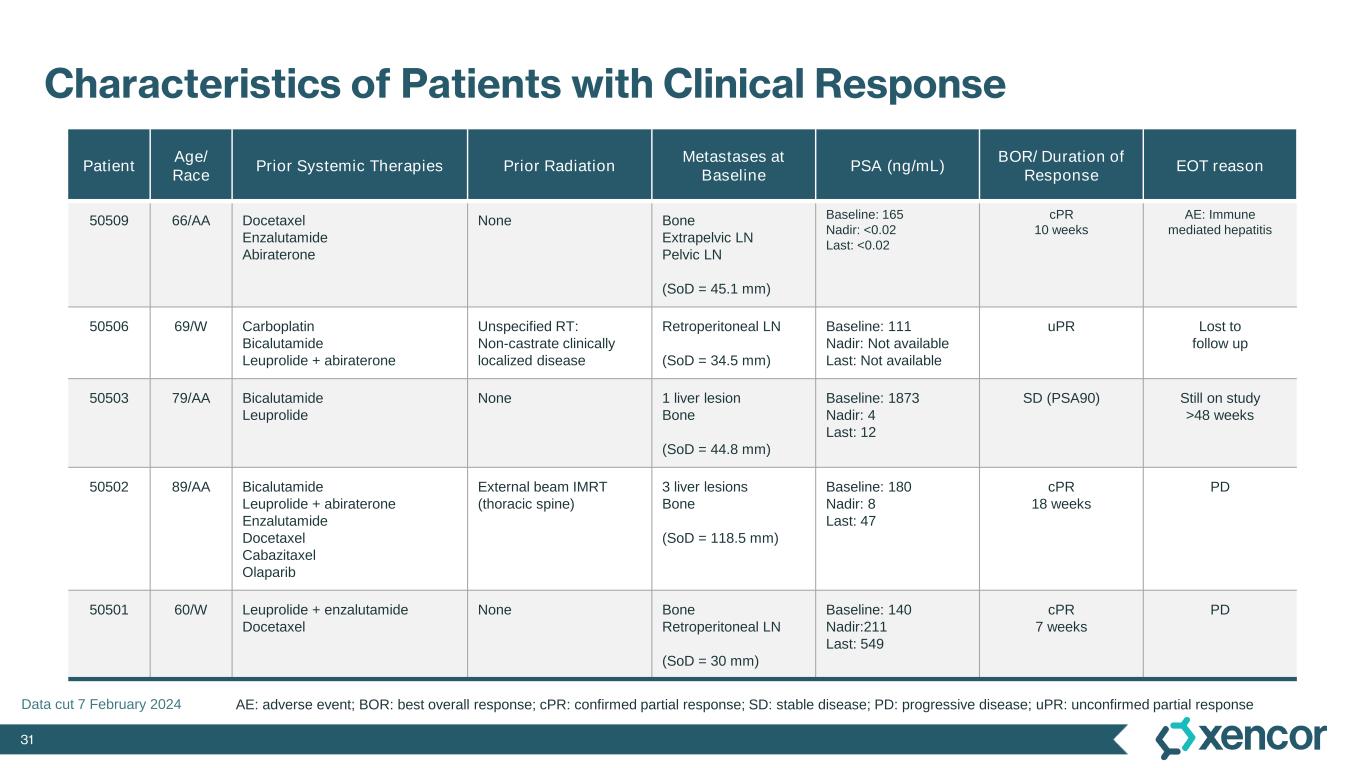
31 Characteristics of Patients with Clinical Response Patient Age/ Race Prior Systemic Therapies Prior Radiation Metastases at Baseline PSA (ng/mL) BOR/ Duration of Response EOT reason 50509 66/AA Docetaxel Enzalutamide Abiraterone None Bone Extrapelvic LN Pelvic LN (SoD = 45.1 mm) Baseline: 165 Nadir: <0.02 Last: <0.02 cPR 10 weeks AE: Immune mediated hepatitis 50506 69/W Carboplatin Bicalutamide Leuprolide + abiraterone Unspecified RT: Non-castrate clinically localized disease Retroperitoneal LN (SoD = 34.5 mm) Baseline: 111 Nadir: Not available Last: Not available uPR Lost to follow up 50503 79/AA Bicalutamide Leuprolide None 1 liver lesion Bone (SoD = 44.8 mm) Baseline: 1873 Nadir: 4 Last: 12 SD (PSA90) Still on study >48 weeks 50502 89/AA Bicalutamide Leuprolide + abiraterone Enzalutamide Docetaxel Cabazitaxel Olaparib External beam IMRT (thoracic spine) 3 liver lesions Bone (SoD = 118.5 mm) Baseline: 180 Nadir: 8 Last: 47 cPR 18 weeks PD 50501 60/W Leuprolide + enzalutamide Docetaxel None Bone Retroperitoneal LN (SoD = 30 mm) Baseline: 140 Nadir:211 Last: 549 cPR 7 weeks PD AE: adverse event; BOR: best overall response; cPR: confirmed partial response; SD: stable disease; PD: progressive disease; uPR: unconfirmed partial response Data cut 7 February 2024
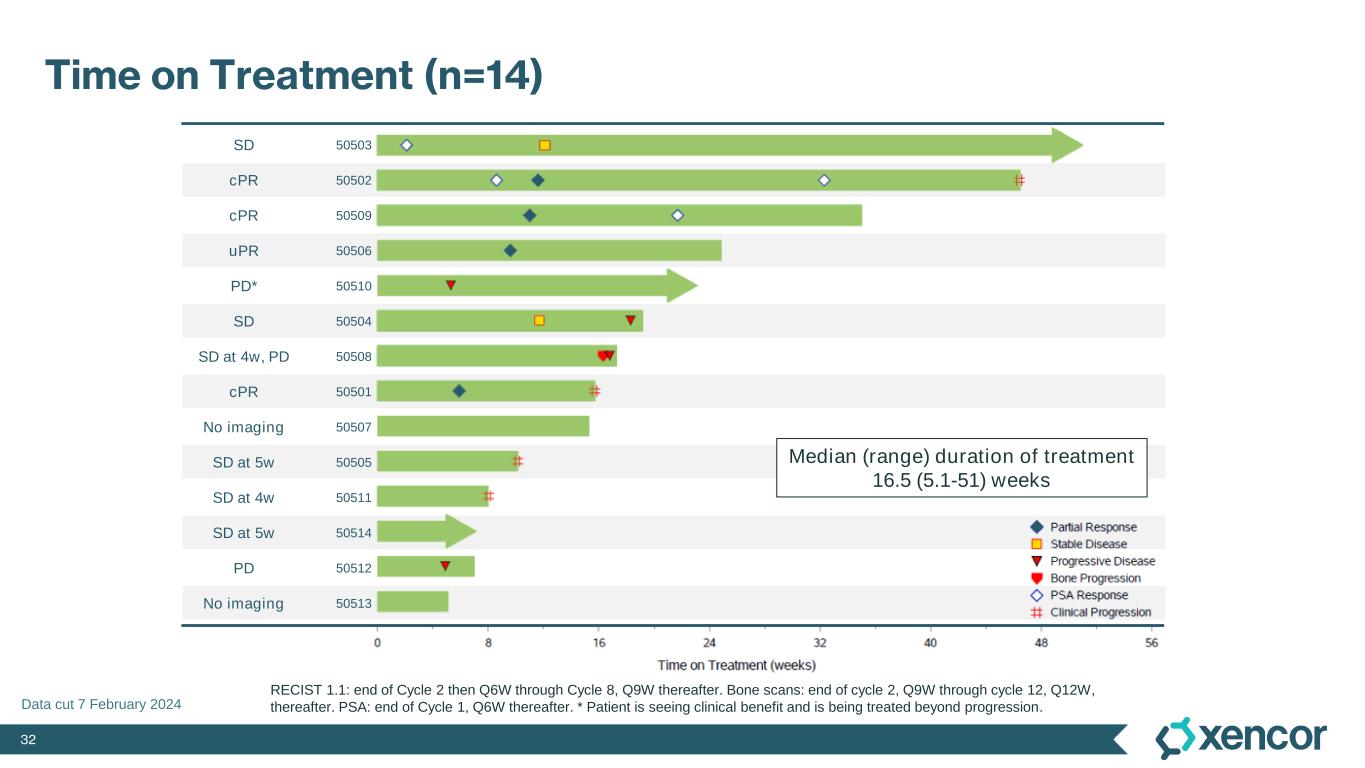
32 SD 50503 cPR 50502 cPR 50509 uPR 50506 PD* 50510 SD 50504 SD at 4w, PD 50508 cPR 50501 No imaging 50507 SD at 5w 50505 SD at 4w 50511 SD at 5w 50514 PD 50512 No imaging 50513 Time on Treatment (n=14) RECIST 1.1: end of Cycle 2 then Q6W through Cycle 8, Q9W thereafter. Bone scans: end of cycle 2, Q9W through cycle 12, Q12W, thereafter. PSA: end of Cycle 1, Q6W thereafter. * Patient is seeing clinical benefit and is being treated beyond progression. Median (range) duration of treatment 16.5 (5.1-51) weeks Data cut 7 February 2024
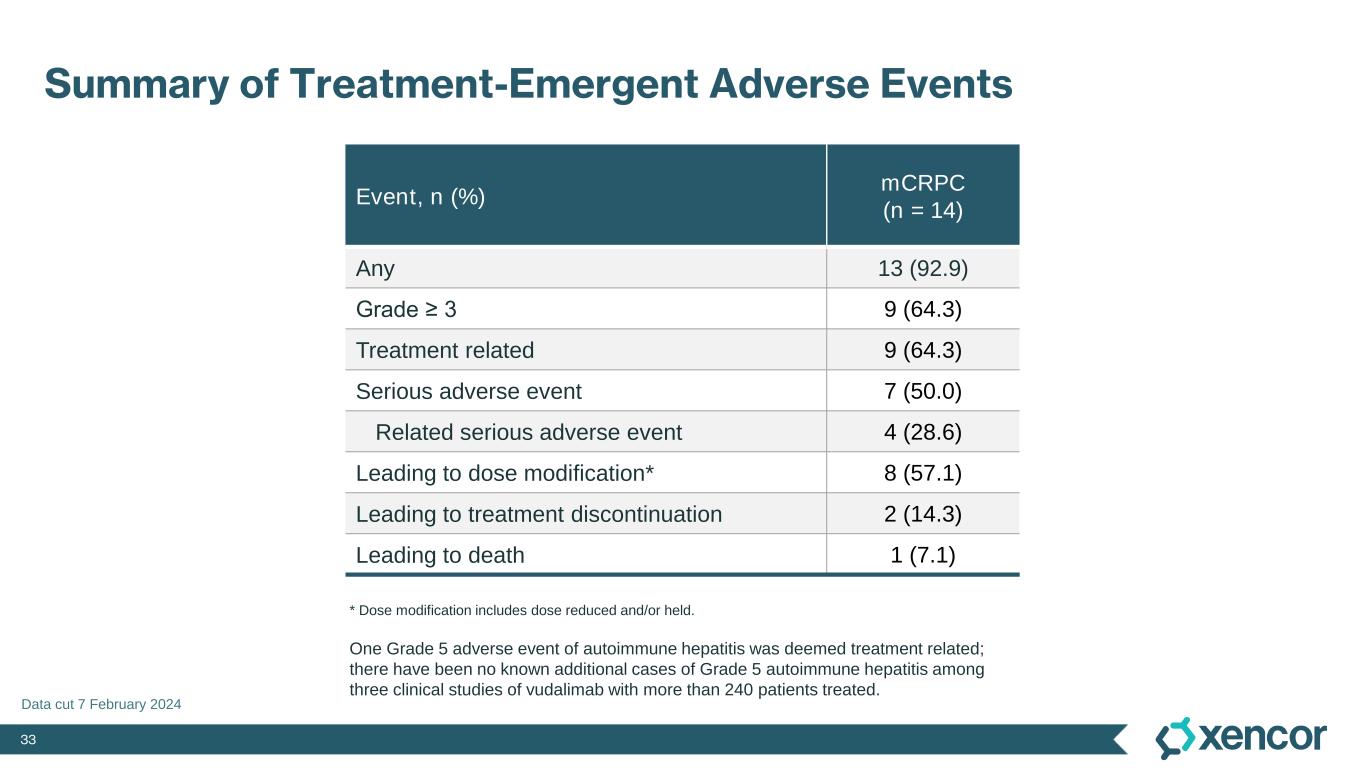
33 Summary of Treatment-Emergent Adverse Events Event, n (%) mCRPC (n = 14) Any 13 (92.9) Grade ≥ 3 9 (64.3) Treatment related 9 (64.3) Serious adverse event 7 (50.0) Related serious adverse event 4 (28.6) Leading to dose modification* 8 (57.1) Leading to treatment discontinuation 2 (14.3) Leading to death 1 (7.1) * Dose modification includes dose reduced and/or held. Data cut 7 February 2024 One Grade 5 adverse event of autoimmune hepatitis was deemed treatment related; there have been no known additional cases of Grade 5 autoimmune hepatitis among three clinical studies of vudalimab with more than 240 patients treated.

34 irAEs of Any Grade in ≥ 2 Patients and Grade ≥ 3 in Any Patients Any Grade, n (%) mCRPC (n = 14) Number of subjects with ≥ 1 event 8 (57.1) Rash maculo-papular 4 (28.6) ALT increased 3 (21.4) Amylase increased 2 (14.3) AST increased 2 (14.3) Blood bilirubin increased 2 (14.3) Hyperthyroidism 2 (14.3) Grade ≥ 3, n (%) mCRPC (n = 14) Number of subjects with ≥ 1 event 3 (21.4) ALT increased 1 (7.1) AST increased 1 (7.1) Blood AP increased 1 (7.1) Blood bilirubin increased 1 (7.1) Diabetic ketoacidosis 1 (7.1) Hyperkalaemia 1 (7.1) Immune-mediated hepatitis 1 (7.1) Lipase increased 1 (7.1) Data cut 7 February 2024

35 Benchmark Rates of Immune-Mediated Hepatitis Drug (Target) Vudalimab (PD-1 x CTLA-4) Ipilimumab (CTLA-4) Ipilimumab (CTLA-4) Ipilimumab (CTLA-4) + Nivolumab (PD-1) Ipilimumab (CTLA-4) + Nivolumab (PD-1) Dosage N=218*, includes 10 mg/kg Q2W & 1000 mg/1200mg Q3W 3 mg/kg 10 mg/kg 1 mg/kg ipilimumab + 3 mg/kg nivolumab for RCC or mCRC 3 mg/kg ipilimumab + 1 mg/kg nivolumab for melanoma or HCC Immune-Mediated Hepatitis†, All Grade 7.3% 4.1% 15.0% 7.0% 15.0% Immune-Mediated Hepatitis†, Grade 3 - 5 5.0% 1.6% 10.8% 6.1% 13.4% † Immune-Mediated Hepatitis defined as: For vudalimab: Treatment-related adverse event (TRAE) immune-mediated hepatitis, hepatitis, autoimmune hepatitis, hepatic cirrhosis, hepatic failure, hepatitis acute, hyperbilirubinemia, immune-mediate cholangitis, or liver injury. For ipilimumab: U.S. FDA label, Yervoy® (ipilimumab) injection, for intravenous use, as revised 2/2023. Yervoy is a registered trademark of Bristol-Myers Squibb Company. * Excludes 27 patients treated at vudalimab doses less than 10 mg/kg. Data cut 7 February 2024
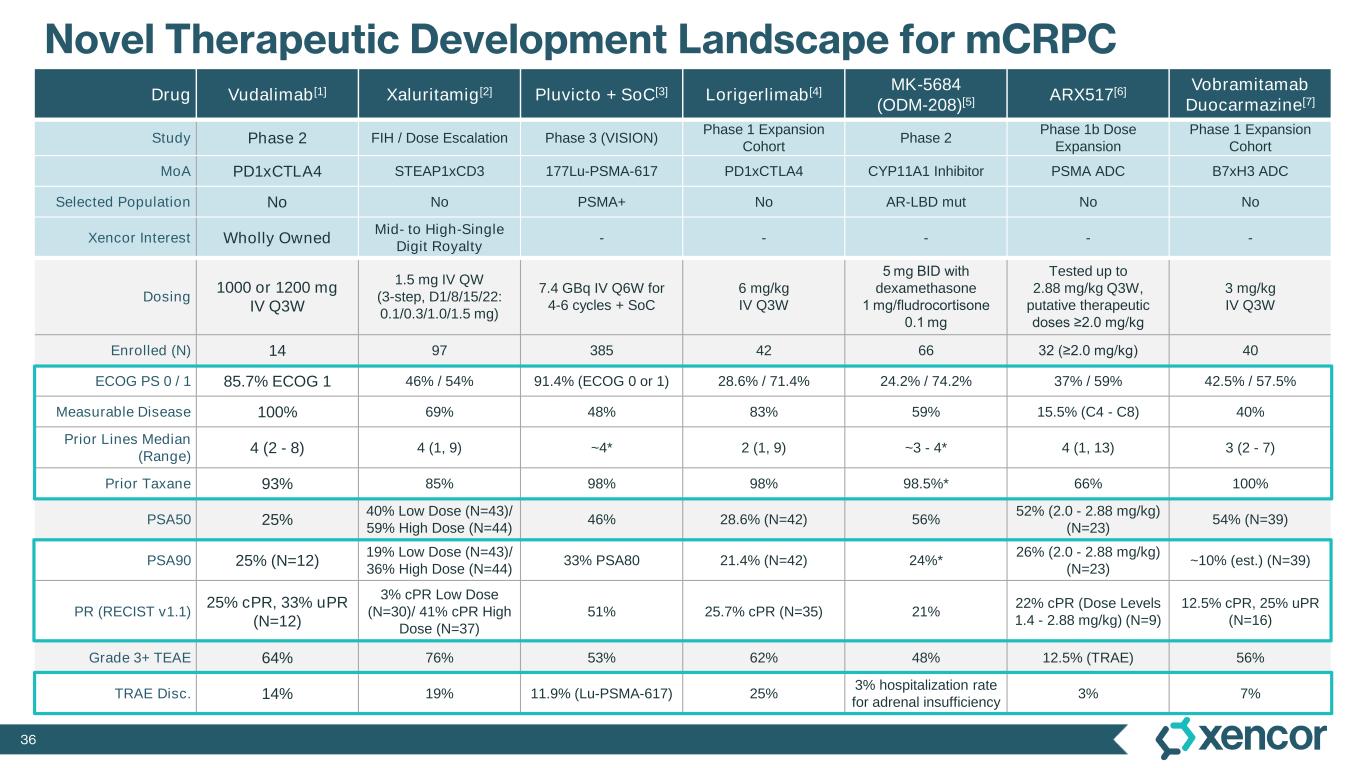
36 Novel Therapeutic Development Landscape for mCRPC Drug Vudalimab[1] Xaluritamig[2] Pluvicto + SoC[3] Lorigerlimab[4] MK-5684 (ODM-208)[5] ARX517[6] Vobramitamab Duocarmazine[7] Study Phase 2 FIH / Dose Escalation Phase 3 (VISION) Phase 1 Expansion Cohort Phase 2 Phase 1b Dose Expansion Phase 1 Expansion Cohort MoA PD1xCTLA4 STEAP1xCD3 177Lu-PSMA-617 PD1xCTLA4 CYP11A1 Inhibitor PSMA ADC B7xH3 ADC Selected Population No No PSMA+ No AR-LBD mut No No Xencor Interest Wholly Owned Mid- to High-Single Digit Royalty - - - - - Dosing 1000 or 1200 mg IV Q3W 1.5 mg IV QW (3-step, D1/8/15/22: 0.1/0.3/1.0/1.5 mg) 7.4 GBq IV Q6W for 4-6 cycles + SoC 6 mg/kg IV Q3W 5 mg BID with dexamethasone 1 mg/fludrocortisone 0.1 mg Tested up to 2.88 mg/kg Q3W, putative therapeutic doses ≥2.0 mg/kg 3 mg/kg IV Q3W Enrolled (N) 14 97 385 42 66 32 (≥2.0 mg/kg) 40 ECOG PS 0 / 1 85.7% ECOG 1 46% / 54% 91.4% (ECOG 0 or 1) 28.6% / 71.4% 24.2% / 74.2% 37% / 59% 42.5% / 57.5% Measurable Disease 100% 69% 48% 83% 59% 15.5% (C4 - C8) 40% Prior Lines Median (Range) 4 (2 - 8) 4 (1, 9) ~4* 2 (1, 9) ~3 - 4* 4 (1, 13) 3 (2 - 7) Prior Taxane 93% 85% 98% 98% 98.5%* 66% 100% PSA50 25% 40% Low Dose (N=43)/ 59% High Dose (N=44) 46% 28.6% (N=42) 56% 52% (2.0 - 2.88 mg/kg) (N=23) 54% (N=39) PSA90 25% (N=12) 19% Low Dose (N=43)/ 36% High Dose (N=44) 33% PSA80 21.4% (N=42) 24%* 26% (2.0 - 2.88 mg/kg) (N=23) ~10% (est.) (N=39) PR (RECIST v1.1) 25% cPR, 33% uPR (N=12) 3% cPR Low Dose (N=30)/ 41% cPR High Dose (N=37) 51% 25.7% cPR (N=35) 21% 22% cPR (Dose Levels 1.4 - 2.88 mg/kg) (N=9) 12.5% cPR, 25% uPR (N=16) Grade 3+ TEAE 64% 76% 53% 62% 48% 12.5% (TRAE) 56% TRAE Disc. 14% 19% 11.9% (Lu-PSMA-617) 25% 3% hospitalization rate for adrenal insufficiency 3% 7%

37 Novel Therapeutic Development Landscape for mCRPC - Sources

®Vudalimab Monotherapy in Patients with mCRPC Study XmAb717-05 Data cut 7 February 2024