0001635077
false
0001635077
2023-09-28
2023-09-28
0001635077
ACON:CommonStockParValue0.00001PerShareMember
2023-09-28
2023-09-28
0001635077
ACON:WarrantsEachExercisableForOneShareOfCommonStockMember
2023-09-28
2023-09-28
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND
EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities
Exchange Act of 1934
Date of Report (Date of earliest event reported):
September 28, 2023
Aclarion,
Inc.
(Exact name of registrant as specified in its charter)
| Delaware |
001-41358 |
47-3324725 |
| (State or other jurisdiction |
(Commission |
(IRS Employer |
| of incorporation) |
File Number) |
Identification No.) |
| 8181 Arista Place, Suite 100 |
|
| Broomfield, Colorado |
80021 |
| (Address of Principal Executive Offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (833) 275-2266
Not
Applicable
(Former name or former address, if changed since last report)
Check the appropriate box
below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following
provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| |
Trading |
|
| Title of each class |
Symbol(s) |
Name of each exchange on which registered |
| Common Stock |
ACON |
Nasdaq Stock Market |
| Common
Stock Warrants |
ACONW |
Nasdaq Stock Market |
Indicate by check mark whether
the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule
12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company,
indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial
accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure.
On September 25, 2023, Aclarion, Inc. (the “Company”) released
an updated investor presentation.
A copy of the updated investor slide presentation is furnished as Exhibit
99.1 to this Form 8-K and incorporated herein by reference
The information contained in this Item 7.01 of this current report
on Form 8-K and in the accompanying exhibit 99.1 incorporated by reference herein shall not be incorporated by reference into any filing
of the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing, unless
expressly incorporated by specific reference to such filing. This information, including the exhibit 99.1 hereto, shall not be deemed
to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, or otherwise subject to the liabilities of
that section or Sections 11 and 12(a)(2) of the Securities Act of 1933.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
ACLARION, INC. |
| |
|
|
| September 28, 2023 |
By: |
/s/ John Lorbiecki |
| |
Name: |
John Lorbiecki |
| |
Title: |
Chief Financial Officer |
Exhibit 99.1

Better Data Better Care OCTOBER 2023 NASDAQ: ACON

Aclarion 2023 2 Disclaimer The information in this material is provided for general information purposes only and does not take into account the investment objectives, financial situation and particular needs of any individual or entity . This presentation contains forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 , Section 27 A of the Securities Act of 1933 and Section 21 E of the Securities Exchange Act of 1934 about our current expectations about future results, performance, prospects and opportunities of Aclarion, Inc . (“Aclarion” or the “Company”) . Statements that are not historical facts, such as "anticipates," "believes" and "expects" or similar expressions, are forward - looking statements . These forward - looking statements are based on the current plans and expectations of management and are subject to a number of uncertainties and risks that could significantly affect the Company's current plans and expectations, as well as future results of operations and financial condition . These and other risks and uncertainties are discussed more fully in our filings with the Securities and Exchange Commission . Readers are encouraged to review the section titled "Risk Factors" in the Company's Annual Report on Form 10 - K for the year ended December 31 , 2022 , as well as other disclosures contained in the Annual Report and subsequent filings made with the Securities and Exchange Commission . Forward - looking statements contained in this announcement are made as of this date and the Company undertakes no obligation to publicly update or revise any forward - looking statements, whether as a result of new information, future events or otherwise . Unless otherwise indicated, information contained in this presentation concerning Aclarion’s industry and markets in which it operates, including its general expectations and market opportunity and market size, is based upon information from various sources, including independent industry publications in presenting information . Aclarion makes no representations as to the accuracy, timeliness, suitability, completeness, or relevance of any information prepared by any unaffiliated third party and takes no responsibility therefore . The data presented herein were obtained from various third - party sources . While we believe the data to be reliable, no representation is made as to, and no responsibility, warranty or liability is accepted for the accuracy or completeness of such information . Aclarion has also made assumptions based upon such data and other similar sources, and on Aclarion’s knowledge of and its experience to date in the markets for its product candidates . This information involves a number of assumptions and limitations and you are cautioned not to give undue weight to such estimates . The industry in which Aclarion operates is subject to a high degree of uncertainty and risk due to a variety of factors . These and other factors could cause results to differ materially from those expressed in the estimates made by independent parties and by Aclarion . This presentation uses Aclarion’s trademarks and trade names such as “Nocicalc” and “Nocigram . " This presentation also includes trademarks, trade names and service marks that are the property of other organizations . Solely for convenience, trademarks and tradenames referred to in this presentation appear without ® and Œ symbols, but those references, are not intended to indicate that Aclarion will not assert to the fullest extent under applicable law, its rights, or that the applicable owner will not assert its rights to these trademarks and trade names . Aclarion does not intend to use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of Aclarion by any other companies .

Aclarion 2023 3 Problem Patient is in pain but which disc is the cause? High surgical decision variability Expensive Poor Outcomes Low value

Aclarion 2023 4 Solution Technology labels each disc as painful or pain free Personalized treatment path is optimized Lower cost Better outcomes Higher value care

Aclarion 2023 5 Published in the European Spine Journal in 2019 & 2023 Better Surgical Outcomes Gornet et al Gornet study published in European Spine Journal 1 139 cLBP patients with 73 undergoing surgery 85 % of patients improved if all Nociscan positive discs treated 2 63 % of patients improved if a Nociscan positive disc was not treated 2 Durable at 2 year follow up 2 2019 139 85 % 63 % 2 year 1 Gornet et al. Magnetic resonance spectroscopy (MRS) can identify painful lumber discs and may facilitate improved clinical ou tco mes of lumbar surgeries for discogenic pain. European Spine Journal. 2019; 28 674 - 687 2 Gornet et al. Magnetic resonance spectroscopy (MRS) identification of chemically painful lumbar discs leads to improved 6 - 12 - 24 month outcomes for discogenic low back pain surgeries. European Spine Journal. 2023; 32(6) 1973 - 1984

Aclarion 2023 6 EVAL E conomic V alue A nalysis of L ow back pain Objective: Determine if non - invasive diagnostic (Nociscan®) is cost - effective compared to provocative discography • Economic modeling based on the Gornet trial and spine industry economic cost factors • Statistical modeling and analysis performed by independent PhD healthcare economist • Statistically dominant clinical and economic results 1 • Presented at SPINEWEEK, International Society for the Advancement of Spine Surgery (ISASS), American Society of Pain and Neuroscience (ASPN) (min) (min) 1 Wilson et al , Cost - effectiveness of a Novel, Non - Invasive Diagnostic Test for Chronic Low Back Pain Patients, 2023 2 15 - point ODI Change; Sx Success = Surgical Success 3 Min and Max Estimate

Aclarion 2023 7 CLARITY C hronic L ow b A ck pain R andomized I ndependent T rial stud Y Prospective, randomized, controlled trial evaluating the outcomes of surgical patients when the surgeon has access to Nociscan data compared to when the surgeon does not have access to Nociscan data Principal Investigator & Anchor Site • 300 patients estimated • Begins Q4 2023 • 42 months to complete • Anticipated outcome: Demonstrate improved clinical results when using Nociscan Nicholas Theodore, MD, MS Director of Neurosurgical Spine Center, John Hopkins Medicine

Aclarion 2023 8 NIH - BACPAC Back Pain Consortium (BACPAC) Two NIH studies aimed at finding the best diagnostic tools for chronic low back pain and evaluating their effectness via clinical outcomes in four different patient sub - groups. • Sponsored by the National Institutes of Health (NIH) • comeBACK - Up to 300 patients to undergo Nociscan to provide unique, objective, intradiscal biomarker data for enhanced phenotyping - https://comeback.ucsf.edu/research • BEST – Up to 200 patients to undergo Nociscan to evaluate effectiveness in determining optimal treatments including going directly to surgery https://besttrial.org/ • Began Q4 2021 • Complete Q2 2024

Aclarion 2023 9 ACTIVE A utologous mesynchymal stem/stromal C ells for T reatment of workers affected by chronic low back pain due to multilevel I nter VE rtebral disc degeneration • Sponsored by Campus Bio - medico University • Up to 204 scans: T0, T3, T6, and T12 months • Began Q4 2020 • Complete Q1 2024 • Anticipated outcomes: Demonstrate that Nociscan results correlate to improved treatment outcomes • https://clinicaltrials.gov/ct2/show/NCT04759105 ACTIVE will evaluate the efficacy of intradiscal injection of autologous BM - MSCs in workers affected by chronic low back pain (cLBP) unresponsive to conventional therapy

Aclarion 2023 10 Nociscan Product Spectroscopy analyzes the chemical composition of tissue Proprietary signal processing software transforms raw spectral data into clear biomarkers Acidic pain markers 1 • Alanine (LAAL = lactic acid + alanine) • Lactic Acid (LAC) • Propionate (PRO) Structural integrity markers 1 • Carbohydrate/collagen (CARB) • Proteoglycan (PG) 1 Keshari KR, Spine (2008) 33(3):312 – 317

Aclarion 2023 11 Clear Delineation Between Pain & No Pain 1 Freemont AJ, Rheumatology (2009) 48:5 – 10 2 Immke and McCleskey, Nat Neurosci. (2001) Sep;4(9):869 - 70 Six ratios for each disc • Pain markers in the numerator (3) • Structural integrity markers in the denominator (2) • Differentially weighted & correlated to clinically painful discs • Leverage AI for major advancements Painful Disc • Structural degradation with acid & neoinnervation 1 • Acid + Nerves = PAIN 2 • Elevated pain markers relative to normal internal controls

Aclarion 2023 12 Provocative Discogram Nociscan Virtual Discogram Invasive Non - invasive Subjective Objective Severely painful by design No pain Lumbar CT = ~10x radiation of chest x - ray No radiation Additional exam after MRI Can be added to a standard lumbar MRI Half - day procedure with days out of work 25 - 45 min Twice the cost of Nociscan - $2,000 to $5,000 2 Half the cost of discogram - $1,450 list price Competition VS. An invasive procedure in an awake patient that damages healthy discs 1 Discogram – Current Standard of Care Similar to a standard MRI Nociscan 1 Cuellar JM, Spine J (2016),16(3):273 – 280 2 Definitive Healthcare; Does not include Physician Fees

Aclarion 2023 13 Intellectual Property 22 issued U.S. Patents 17 issued International Patents Post processing technologies to more reliably & accurately measure biomarkers from MRS spectra Exclusive worldwide license from UCSF for intervertebral disc biomarkers & ratios Use of proprietary biomarkers & ratios to identify clinically painful and non - painful discs 13 Pending Patent Applications IP broadly covers: Use of internal tissue controls when using MRS AI to correlate raw spectra to clinical outcomes

Aclarion 2023 14 Product Status Current product Spine surgery decisioning tool to replace discography & improve surgical outcomes • Early commercialization in progress • AI process improvements for reproducibility & scalability in progress • MRI integrations to support scalability Next Generation Population management platform for chronic low back pain • Selected for NIH BEST Study • Predictive analytics capability based on clinical outcomes – Surgical interventions – Regenerative therapies – Conservative care – Preventive care 1 1 510(k) pathway required

Aclarion 2023 15 Regulatory Approvals Secured Current product approvals Next generation AI product • NOCICALC – marketed through FDA registration as a Class 1 exempt device & CE Mark • NOCIGRAM – marketing indication established through the 21 st Century Cures Act as clinical decision support software & CE Mark • AI based predicates from HeartFlow & Cleerly via 510(k)
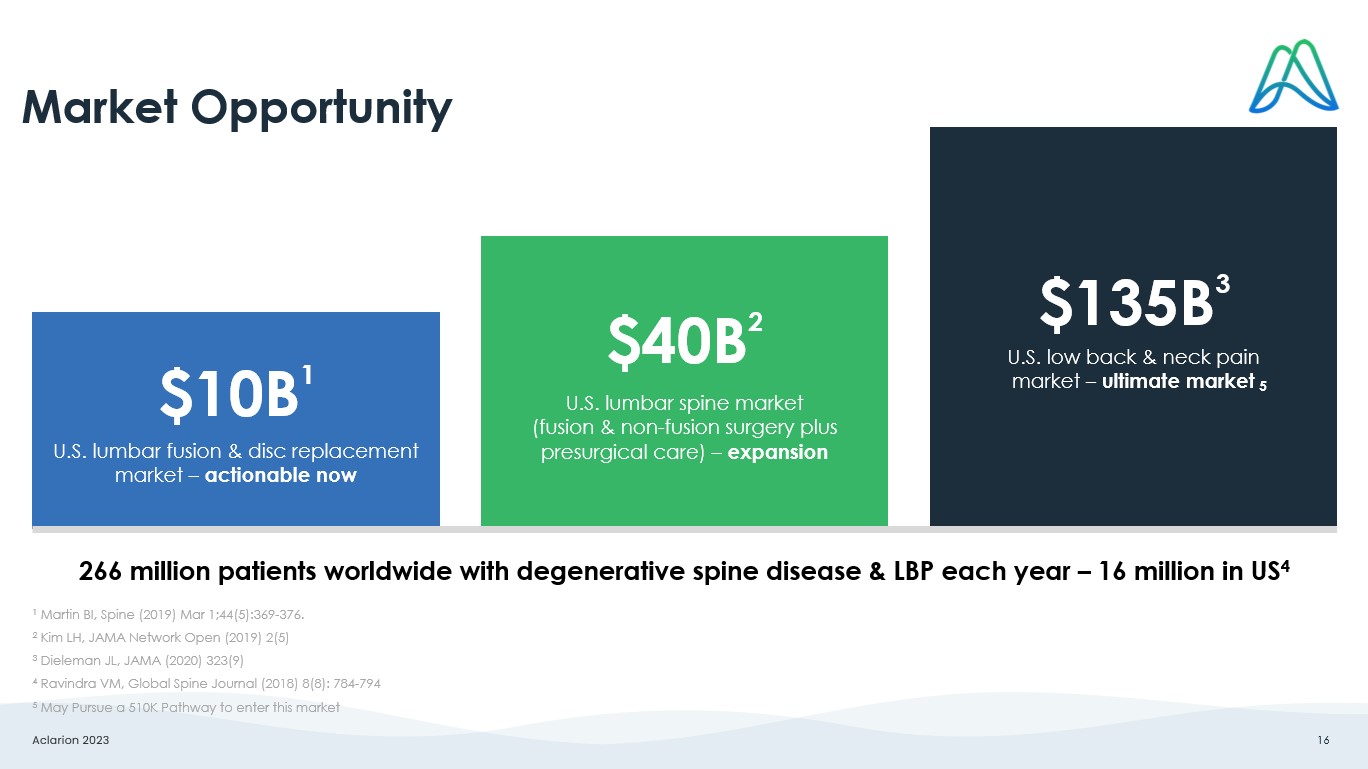
Aclarion 2023 16 $10B 1 U.S. lumbar fusion & disc replacement market – actionable now $40B 2 U.S. lumbar spine market (fusion & non - fusion surgery plus presurgical care) – expansion $135B 3 U.S. low back & neck pain market – ultimate market Market Opportunity 1 Martin BI, Spine (2019) Mar 1;44(5):369 - 376. 2 Kim LH, JAMA Network Open (2019) 2(5) 3 Dieleman JL, JAMA (2020) 323(9) 4 Ravindra VM, Global Spine Journal (2018) 8(8): 784 - 794 5 May Pursue a 510K Pathway to enter this market 266 million patients worldwide with degenerative spine disease & LBP each year – 16 million in US 4 5

Aclarion 2023 17 Augmented Intelligence Landscape • Founded 2016 - stroke • Cloud - based model • Earlier stroke detection • $100M Series D in 2022 • Series D Valuation - $1.3B post • Founded 2016 – heart disease • Cloud - based model • Avoid invasive angiogram • $223M Series C in 2022 • Series C Valuation - undisclosed • Founded 2009 – heart disease • Cloud - based model • Avoid invasive angiogram • $27M Series B in 2022 • Series B Valuation - undisclosed • Founded 2007 – heart disease • Cloud - based model • Avoid invasive angiogram • $240M Series E in 2018 • Series E Valuation - $1.5B post
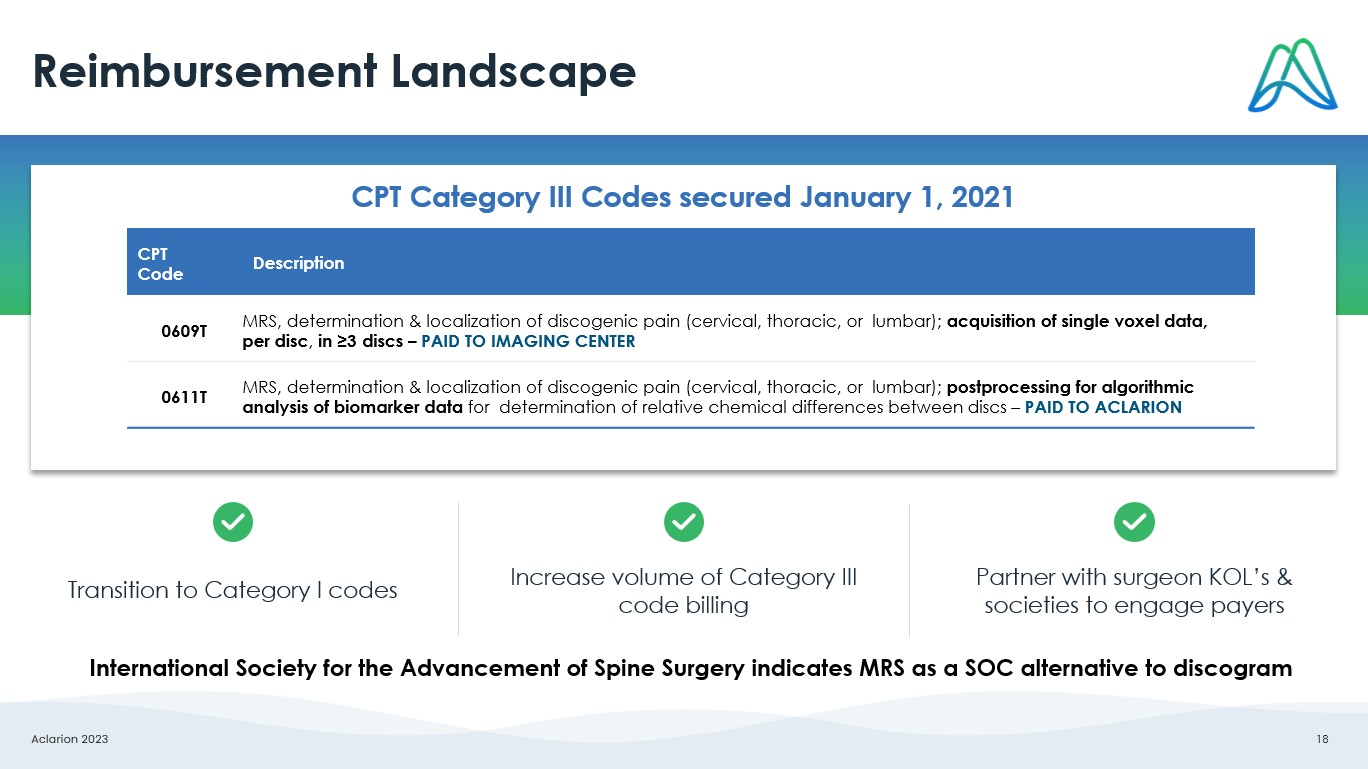
Aclarion 2023 18 Reimbursement Landscape Description CPT Code MRS, determination & localization of discogenic pain (cervical, thoracic, or lumbar); acquisition of single voxel data, per disc , in ≥3 discs – PAID TO IMAGING CENTER 0609T MRS, determination & localization of discogenic pain (cervical, thoracic, or lumbar); postprocessing for algorithmic analysis of biomarker data for determination of relative chemical differences between discs – PAID TO ACLARION 0611T CPT Category III Codes secured January 1, 2021 Transition to Category I codes Increase volume of Category III code billing Partner with surgeon KOL’s & societies to engage payers International Society for the Advancement of Spine Surgery indicates MRS as a SOC alternative to discogram

Aclarion 2023 19 Process to Standard of Care = not yet accomplished = initial steps accomplished = accomplished KOL Development & Advocacy Opportunity Assessment Product Development Broad Physician Adoption Standard of Care Patient Driven Adoption Coverage & Reimbursement Product Refinement Capacity Healthcare Economics Product Adoption Market Access Product Readiness Secure Local Payer Coverage • Bill Category III CPT codes • Appeal denials • Track payment by customer Increase Local Scan Volume • Add local sales coverage • Increase physician customers • Broaden imaging center access Leverage Local Marketing Support • Build customer facing material • Support trade shows • Publish clinical studies Broad Commercial Adoption

Aclarion 2023 20 Local Payer Coverage Decisions – Key Catalyst Key Near Term Catalysts • MRI activations for KOLs • Increased scan volumes • Coverage decision from local payers KOL Rx • Nociscan utilization Evidence • KOL evidence • Pre - existing evidence LCD • Health insurers • Employers • Workers comp

Aclarion 2023 21 Key Opinion Leader Development & Advocacy John Keller MD Juan Uribe MD Sig Berven MD Christopher Ames MD George Frey MD Grand Rapids, MI Phoenix, AZ San Francisco, CA San Francisco, CA Denver, CO Region Dean Karahalios MD Apesh Patel MD Greg Basil MD Roger Hartl MD Eric Potts MD Chicago, IL Evanston, IL Miami, FL New York City, NY Indianapolis, IN Region

Aclarion 2023 22 Management Team Executive Chairman Jeff Thramann, MD CEO & Director Brent Ness CFO John Lorbiecki Chief Strategy Officer Ryan Bond • Serial entrepreneur with multiple exits • Over 100 US & International patents • Public company board experience • Neurosurgeon & spine fellow • U.S. Military Academy @ West Point • Cornell University Medical College • Barrow Neurological Institute • Experienced healthcare leader with highly relevant background • AI experience at HeartFlow & Cleerly • Spine experience at Medtronic, Mighty Oak & ProNerve • Imaging experience at GE • University of North Dakota • University of Colorado MBA • Seasoned financial executive • Divisional CFO at Kyphon & SNT within Medtronic • Crossover operational experience • Early & late stage company exposure • University of St. Thomas magna cum laude • University of Chicago MBA • Leading strategy since 2018 • Instrumental in securing Cat III CPT codes • Coordinated key clinical trials • Spine experience at Nuvasive • Ohio University

Aclarion 2023 23 Independent Board Non - independent Independent Steve Deitsch Audit committee chair Bill Wesemann Lead independent director Compensation committee chair Amanda Williams Nominating committee chair Scott Breidbart, MD Jeff Thramann, MD Executive chairman Brent Ness CEO David Neal

Aclarion 2023 24 Advisory Board Scientific Medical Larry Tanenbaum, MD Radiology Section Chair VP, CTO, Director at RadNet Mount Sinai Associate Professor University of Pennsylvania, BA, Biochemistry UCSD, MD USC, residency Bob Eastlack, MD Surgical Section Chair Head of Spine Surgery, Scripps Clinic Director of clinical research Co - director spine fellowship program President San Diego Ortho Society Stanford, BS, Human Biology Baylor, MD UCSD residency & Mayo spine fellowship Jeff Lotz, PhD Chairman Aclarion co - founder UCSF e ndowed chair in Orthopaedic Surgery Vice chair for research D eputy editor for Spine Principal investigator, BACPAC UC Berkeley, BS, Mechanical Engineering Stanford, MS, Mechanical Engineering MIT/Harvard, PhD, Medical Engineering

Aclarion 2023 25 Key Takeaways Address a leading cause of healthcare expenditures in the U.S. – up to $134.5B market Industry 1st noninvasive diagnostic with strong clinical evidence of value CPT codes issued & regulatory path secured in US, EU & UK – gateway to commercialization Established path to success for AI algorithms in heart disease and stroke Successful management team with relevant industry knowledge 39 issued US & International patents with another 13 pending – supports a platform technology Strong value proposition for patients, doctors, imaging centers and payers 1 Dieleman JL, JAMA (2020) 323(9) 1

www.aclarion.com Investor Relations Kirin Smith, President ksmith@pcgadvisory.com Ph: 646.823.8656
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=ACON_CommonStockParValue0.00001PerShareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=ACON_WarrantsEachExercisableForOneShareOfCommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Aclarion (NASDAQ:ACON)
Historical Stock Chart
From Apr 2024 to May 2024
Aclarion (NASDAQ:ACON)
Historical Stock Chart
From May 2023 to May 2024