Pfizer, BioNTech Get Positive CHMP Opinion for Covid-19 Vaccine Booster in EU
13 September 2022 - 2:14AM
Dow Jones News
By Stephen Nakrosis
Pfizer Inc. and BioNTech SE said Monday the European Medicines
Agency's Committee for Medicinal Products for Human Use, or CHMP,
recommended a conditional marketing authorization for their Omicron
BA.4/BA.5 bivalent-adapted Covid-19 vaccine.
The CHMP recommended the booster for individuals ages 12 years
and older, the companies said. They also said the vaccine is ready
to ship, pending approval from the European Commission.
The companies also said "the European Commission will review the
CHMP recommendation and is expected to make a final decision
soon."
According to the companies, the recommendation follows guidance
from the European Medicines Agency, the World Health Organization
and International Coalition of Medicines Regulatory Authorities "to
advance bivalent vaccine candidates with the goal of making an
Omicron-adapted vaccine available to European Union member states
as soon as possible."
Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
September 12, 2022 11:59 ET (15:59 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
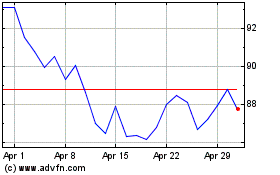
BioNTech (NASDAQ:BNTX)
Historical Stock Chart
From Mar 2024 to Apr 2024
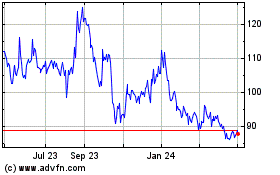
BioNTech (NASDAQ:BNTX)
Historical Stock Chart
From Apr 2023 to Apr 2024