Form 8-K - Current report
20 September 2023 - 10:23PM
Edgar (US Regulatory)
0001682639
false
0001682639
2023-09-19
2023-09-19
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE
COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13
or 15(d)
of the Securities Exchange
Act of 1934
Date of Report (Date
of earliest event reported): September 19, 2023
EYENOVIA, INC.
(Exact Name of Registrant
as Specified in its Charter)
| Delaware |
|
001-38365 |
|
47-1178401 |
(State or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
295 Madison Avenue, Suite 2400, New York, NY
10017
(Address of Principal Executive Offices, and
Zip Code)
(833) 393-6684
Registrant’s Telephone Number, Including
Area Code
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
¨ Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
¨ Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
¨ Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
¨ Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section
12(b) of the Act:
| (Title of each class) |
|
(Trading
Symbol) |
|
(Name of each exchange
on which registered) |
| Common stock, par value $0.0001 per share |
|
EYEN |
|
The Nasdaq Stock Market
(Nasdaq Capital Market) |
Indicate by check mark whether the registrant
is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities
Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging
growth company x
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 7.01. | Regulation FD Disclosure. |
On
September 19, 2023, the Company began using an updated corporate presentation with various investors and analysts. A copy
of the presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information contained
in this Item 7.01, including Exhibit 99.1, is being “furnished” and shall not be deemed “filed” for purposes
of Section 18 of the Exchange Act, or otherwise subject to the liability of that Section or Sections 11 and 12(a)(2) of
the Securities Act. The information contained in this Item 7.01, including Exhibit 99.1, shall not be incorporated by reference into
any registration statement or other document pursuant to the Securities Act or into any filing or other document pursuant to the Exchange
Act, except as otherwise expressly stated in any such filing.
| Item 9.01. | Financial Statements and Exhibits. |
| 104 | Cover Page Interactive Data File (embedded within the Inline
XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
EYENOVIA, INC. |
| |
|
| Date: September 20, 2023 |
/s/ John Gandolfo |
| |
John Gandolfo |
| |
Chief Financial Officer |
Exhibit 99.1
| September 2023
EYEN-COM-V2-0012 |

| 1
Forward-looking Statements
Except for historical information, all the statements, expectations and assumptions contained in this presentation are forward-looking
statements. Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations,
strategies, predictions or any other statements relating to our future activities or other future events or conditions, including estimated
market opportunities for our product candidates and platform technology. These statements are based on current expectations, estimates
and projections about our business based, in part, on assumptions made by management. These statements are not guarantees of future
performance and involve risks, uncertainties and assumptions that are difficult to predict. Therefore, actual outcomes and results may,
and in some cases are likely to, differ materially from what is expressed or forecasted in the forward-looking statements due to numerous
factors discussed from time to time in documents which we file with the U.S. Securities and Exchange Commission.
In addition, such statements could be affected by risks and uncertainties related to, among other things: risks of our clinical trials,
including, but not limited to, the costs, design, initiation and enrollment, timing, progress and results of such trials; the timing of, and our
ability to submit applications for, obtaining and maintaining regulatory approvals for our product candidates; the potential advantages of
our product candidates and platform technology and the potential for approval of APP13007; the rate and degree of market acceptance
and clinical utility of our product candidates; our estimates regarding the potential market opportunity for our product candidates; reliance
on third parties to develop and commercialize our product candidates; the ability of us and our partners to timely develop, implement and
maintain manufacturing, commercialization and marketing capabilities and strategies for our product candidates; intellectual property
risks; changes in legal, regulatory, legislative and geopolitical environments in the markets in which we operate and the impact of these
changes on our ability to obtain regulatory approval for our products; and our competitive position.
Any forward-looking statements speak only as of the date on which they are made, and except as may be required under applicable
securities laws, Eyenovia does not undertake any obligation to update any forward-looking statements. |

| 2
• Horizontal delivery
• Precision dose
• Digital compliance capabilities
Eyenovia at a Glance
Eyenovia (NASDAQ | EYEN) is a US based medical device and ocular therapeutics company
Optejet® with microdose array print technology
• Patented digital device platform technology
• Exciting and diverse product pipeline
• Multi-faceted business model that combines
partnerships, licensing agreements, internal
product development and sales |

| 3
Over the past 125 years,
changes in eyedropper design
have done little to improve the
usability of topical ophthalmic
medications
Today’s Eyedropper Bottle
Designed for manufacturing ease, not patient ease
1800’s
Glass Pipette
1900’s
Glass Pipette with Bulb
and Separate Vial
Today
Integrated Bottle with Dropper Tip
1. Survey conducted in January 2023 with 100 people (19 - 65+ Age Range, Mean Age = 51YO) who regularly take eye drop medications. Respondents were asked to rank common drug
forms from easiest to most difficult to administer on a 0-10 scale (0 meaning no difficulty, 10 meaning extremely difficult). Of the 11 medication types ranked, eye drops were the third most
difficult behind suppositories and eye ointments. The topical ointments were ranked the easiest to administer with an average score of 1.1, and suppositories ranked the most difficult with a
score of 6.48. Eye drops received an average score of 4.6.
In a recent survey conducted by J. Reckner and Associates, consumers reported that
taking eye drops was among the most difficult ways to self-administer medication1 |
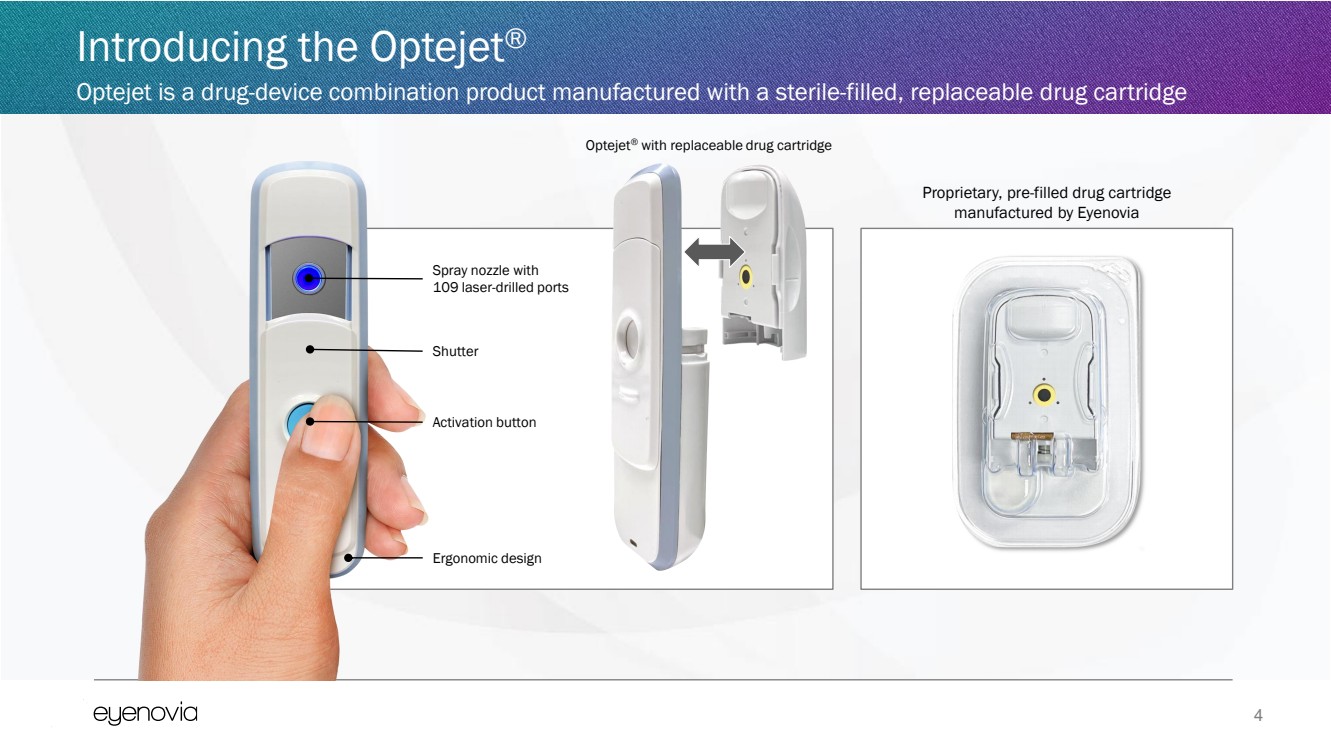
| Introducing the Optejet®
Optejet is a drug-device combination product manufactured with a sterile-filled, replaceable drug cartridge
4
Optejet® with replaceable drug cartridge
Spray nozzle with
109 laser-drilled ports
Shutter
Activation button
Ergonomic design
Proprietary, pre-filled drug cartridge
manufactured by Eyenovia |

| 5
Ergonomic Design to Improve Usability
Horizontal delivery, push button dosing and no protruding tip
Eye Dropper Bottle administration
requires head-tilting, squeezing,
and reliance on gravity
Eye Dropper Bottle tips
can touch the eye surface
Optejet has a recessed nozzle,
protected by a shutter when
not in use to prevent cross-contamination
Optejet administration can be done
horizontally with the push of a button |
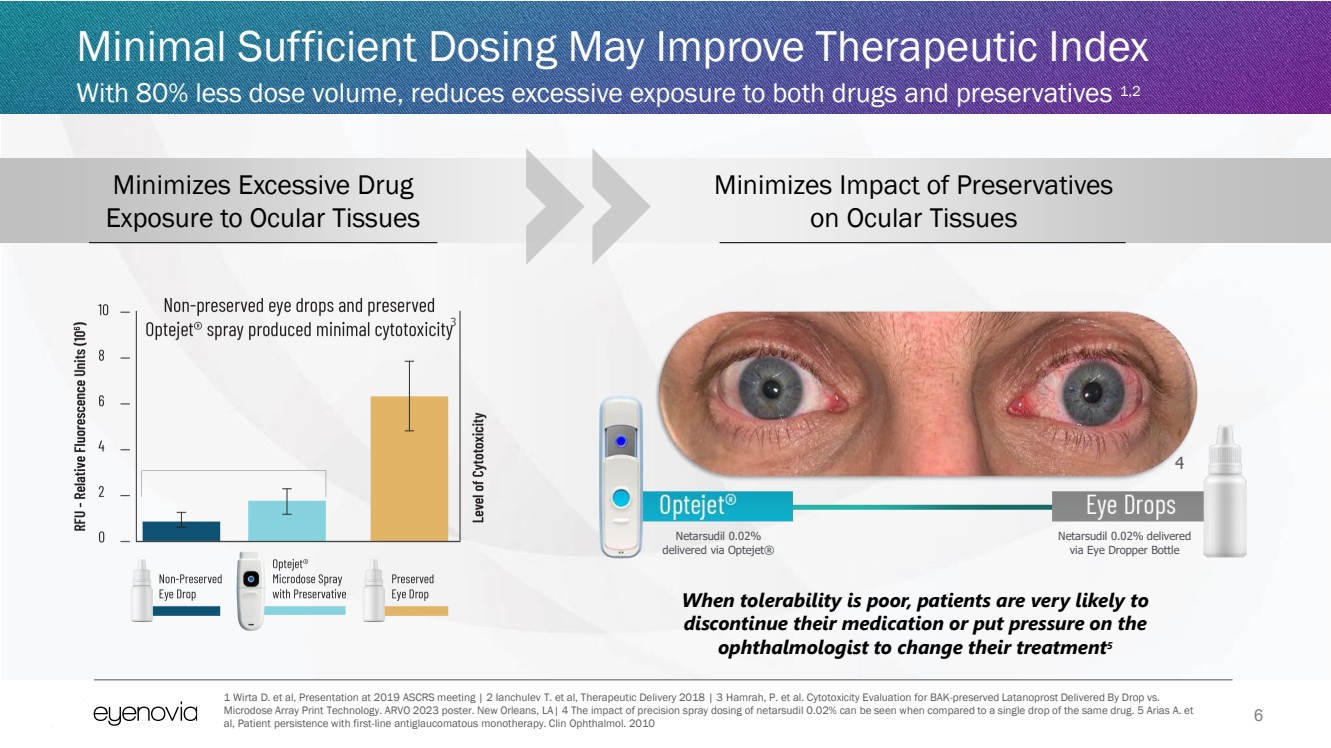
| 6
Minimal Sufficient Dosing May Improve Therapeutic Index
1 Wirta D. et al, Presentation at 2019 ASCRS meeting | 2 Ianchulev T. et al, Therapeutic Delivery 2018 | 3 Hamrah, P. et al. Cytotoxicity Evaluation for BAK-preserved Latanoprost Delivered By Drop vs.
Microdose Array Print Technology. ARVO 2023 poster. New Orleans, LA| 4 The impact of precision spray dosing of netarsudil 0.02% can be seen when compared to a single drop of the same drug. 5 Arias A. et
al, Patient persistence with first-line antiglaucomatous monotherapy. Clin Ophthalmol. 2010
With 80% less dose volume, reduces excessive exposure to both drugs and preservatives 1,2
Netarsudil 0.02%
delivered via Optejet®
Netarsudil 0.02% delivered
via Eye Dropper Bottle
Minimizes Impact of Preservatives
on Ocular Tissues
Minimizes Excessive Drug
Exposure to Ocular Tissues
When tolerability is poor, patients are very likely to
discontinue their medication or put pressure on the
ophthalmologist to change their treatment5
3
4 |
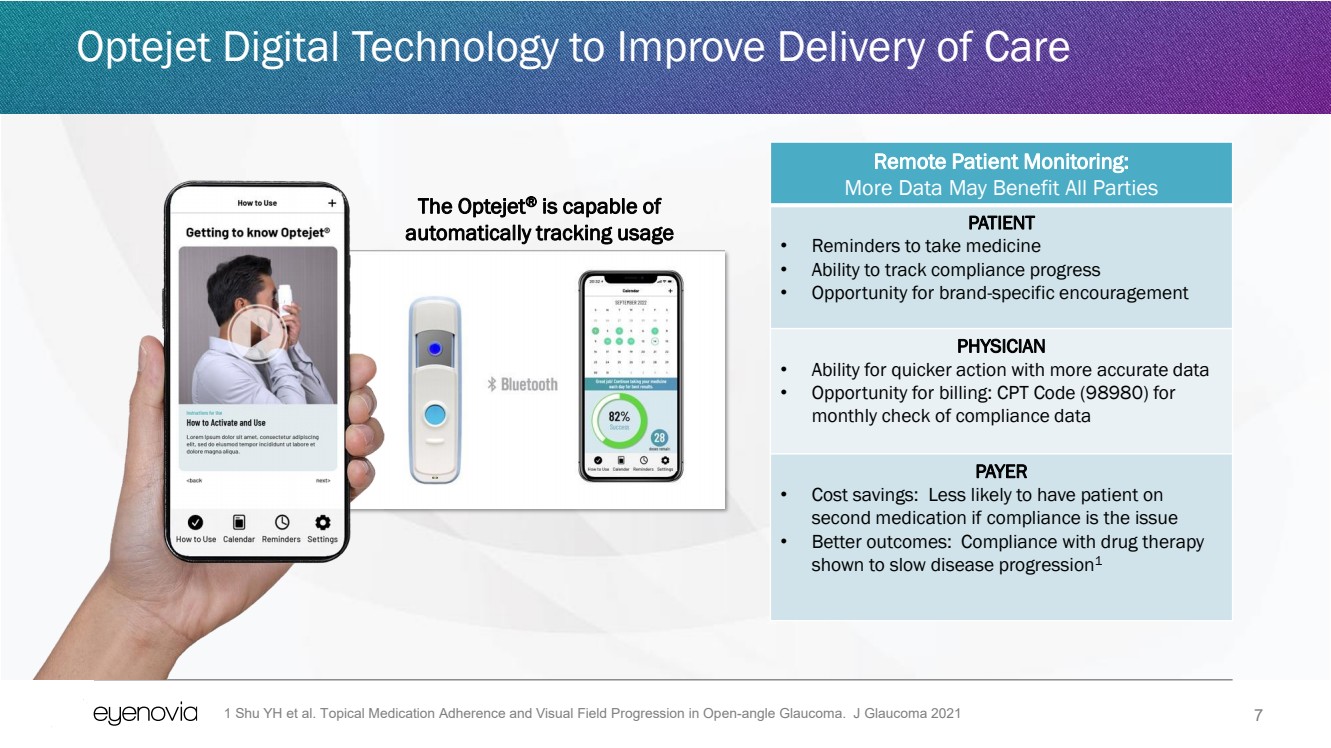
| 7
Optejet Digital Technology to Improve Delivery of Care
The Optejet® is capable of
automatically tracking usage
Remote Patient Monitoring:
More Data May Benefit All Parties
PATIENT
• Reminders to take medicine
• Ability to track compliance progress
• Opportunity for brand-specific encouragement
PHYSICIAN
• Ability for quicker action with more accurate data
• Opportunity for billing: CPT Code (98980) for
monthly check of compliance data
PAYER
• Cost savings: Less likely to have patient on
second medication if compliance is the issue
• Better outcomes: Compliance with drug therapy
shown to slow disease progression1
1 Shu YH et al. Topical Medication Adherence and Visual Field Progression in Open-angle Glaucoma. J Glaucoma 2021 |
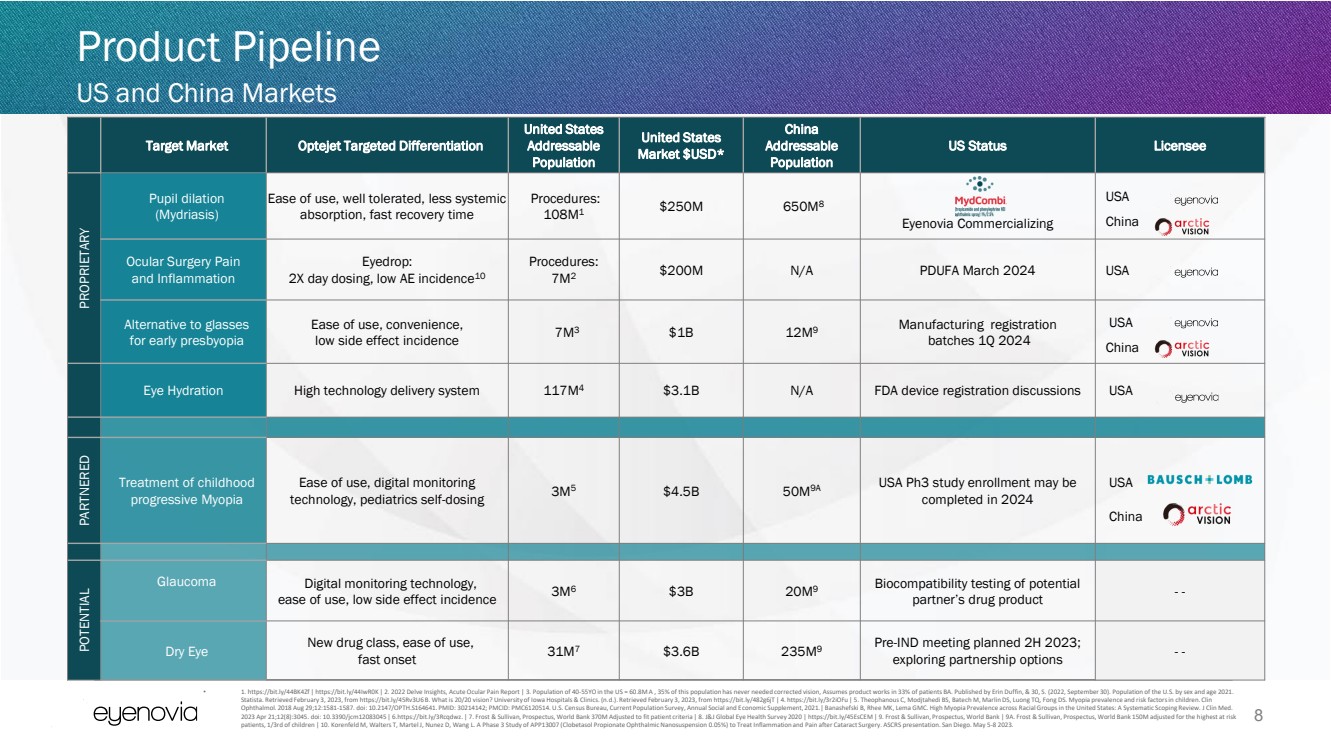
| Target Market Optejet Targeted Differentiation
United States
Addressable
Population
United States
Market $USD*
China
Addressable
Population
US Status Licensee
PROPRIETARY
Pupil dilation
(Mydriasis)
Ease of use, well tolerated, less systemic
absorption, fast recovery time
Procedures:
108M1
$250M 650M8
Eyenovia Commercializing
USA
China
Ocular Surgery Pain
and Inflammation
Eyedrop:
2X day dosing, low AE incidence10
Procedures:
7M2
$200M N/A PDUFA March 2024 USA
Alternative to glasses
for early presbyopia
Ease of use, convenience,
low side effect incidence 7M3 $1B 12M9 Manufacturing registration
batches 1Q 2024
USA
China
Eye Hydration High technology delivery system 117M4 $3.1B N/A FDA device registration discussions USA
PARTNERED
Treatment of childhood
progressive Myopia
Ease of use, digital monitoring
technology, pediatrics self-dosing 3M5 $4.5B 50M9A USA Ph3 study enrollment may be
completed in 2024
USA
China
POTENTIAL
Glaucoma
Digital monitoring technology,
ease of use, low side effect incidence 3M6 $3B 20M9 Biocompatibility testing of potential
partner’s drug product - -
Dry Eye New drug class, ease of use,
fast onset 31M7 $3.6B 235M9
Pre-IND meeting planned 2H 2023;
exploring partnership options - -
8
Product Pipeline
US and China Markets
• 1. https://bit.ly/44BK4Zf | https://bit.ly/44IwR0K | 2. 2022 Delve Insights, Acute Ocular Pain Report | 3. Population of 40-55YO in the US = 60.8M A , 35% of this population has never needed corrected vision, Assumes product works in 33% of patients BA. Published by Erin Duffin, & 30, S. (2022, September 30). Population of the U.S. by sex and age 2021.
Statista. Retrieved February 3, 2023, from https://bit.ly/45Rv3U6 B. What is 20/20 vision? University of Iowa Hospitals & Clinics. (n.d.). Retrieved February 3, 2023, from https://bit.ly/482g6jT | 4. https://bit.ly/3r2iOFu | 5. Theophanous C, Modjtahedi BS, Batech M, Marlin DS, Luong TQ, Fong DS. Myopia prevalence and risk factors in children. Clin
Ophthalmol. 2018 Aug 29;12:1581-1587. doi: 10.2147/OPTH.S164641. PMID: 30214142; PMCID: PMC6120514. U.S. Census Bureau, Current Population Survey, Annual Social and Economic Supplement, 2021.| Banashefski B, Rhee MK, Lema GMC. High Myopia Prevalence across Racial Groups in the United States: A Systematic Scoping Review. J Clin Med.
2023 Apr 21;12(8):3045. doi: 10.3390/jcm12083045 | 6.https://bit.ly/3Rcqdwz. | 7. Frost & Sullivan, Prospectus, World Bank 370M Adjusted to fit patient criteria | 8. J&J Global Eye Health Survey 2020 | https://bit.ly/45EsCEM | 9. Frost & Sullivan, Prospectus, World Bank | 9A. Frost & Sullivan, Prospectus, World Bank 150M adjusted for the highest at risk
patients, 1/3rd of children | 10. Korenfeld M, Walters T, Martel J, Nunez D, Wang L. A Phase 3 Study of APP13007 (Clobetasol Propionate Ophthalmic Nanosuspension 0.05%) to Treat Inflammation and Pain after Cataract Surgery. ASCRS presentation. San Diego. May 5-8 2023. |
| 9
• MydCombi is the first and only FDA-approved fixed-dose
combination ophthalmic spray indicated for inducing mydriasis
for diagnostic procedures and in conditions where short term
pupil dilation is desired
• Pupil dilation (mydriasis) is part of a comprehensive eye exam
and ocular surgery
– Estimated 108 million dilations in US annually
– Estimated $250 million US market opportunity1
• Eyedrops are the current standard of care and ripe for
innovation
– Multiple eyedrops usually needed
– Patient discomfort and avoidance
– Time consuming administration and slow recovery to “normal”
– Cross-contamination risk
1. $200M annual sales of pharmaceutical mydriatic products used during 108M office-based exams ($2 * 100M) + $50M of single bottle mydriatic agents used cataract
replacement surgery ($12.5 x 4M)
MydCombi™
For pupil dilation and mydriasis |

| 10
MydCombi™
Speed and simplicity with each spray
1
Indication: MYDCOMBI (tropicamide 1% and phenylephrine HCl 2.5%) ophthalmic spray is indicated to induce mydriasis for routine diagnostic procedures and in conditions where short term pupil dilation is desired. IMPORTANT SAFETY INFORMATION. CONTRAINDICATIONS: Known
hypersensitivity to any component of the formulation. WARNINGS AND PRECAUTIONS. FOR TOPICAL OPHTHALMIC USE. NOT FOR INJECTION. This preparation may cause CNS disturbances which may be dangerous in pediatric patients. The possibility of psychotic reaction and behavioral disturbance
due to hypersensitivity to anticholinergic drugs should be considered. Mydriatics may produce a transient elevation of intraocular pressure. Significant elevations in blood pressure have been reported. Caution in patients with elevated blood pressure. Rebound miosis has been reported one day
after installation. Remove contact lenses before using. DRUG INTERACTIONS. Atropine-like Drugs: May exaggerate the adrenergic pressor response. Cholinergic Agonists and Ophthalmic Cholinesterase Inhibitors: May interfere with the antihypertensive action of carbachol, pilocarpine, or
ophthalmic cholinesterase inhibitors. Potent Inhalation Anesthetic Agents: May potentiate cardiovascular depressant effects of some inhalation anesthetic agents. ADVERSE REACTIONS. Most common ocular adverse reactions include transient blurred vision, reduced visual acuity, photophobia,
superficial punctate keratitis, and mild eye discomfort. Increased intraocular pressure has been reported following the use of mydriatics. Systemic adverse reactions including dryness of the mouth, tachycardia, headache, allergic reactions, nausea, vomiting, pallor, central nervous system
disturbances and muscle rigidity have been reported with the use of tropicamide. To report SUSPECTED ADVERSE REACTIONS, contact Eyenovia, Inc. At 1-833-393-6684 or FDA at 1-800-FDA-1088 (www.fda.gov/medwatch) www.mydcombi.comfor FULL PRESCRIBING INFORMATION
The only FDA approved fixed-dose combination of the leading
pupil dilating drugs
Reliable time to peak efficacy and dilation resolution
In clinical studies 97% of patients reported zero side effects1
To check on availability in your area, please go to
MydCombi.com
1. Wirta DL, Walters TR, Flynn WJ, Rathi S, Ianchulev T. Mydriasis with micro-array print touch-free tropicamide-phenylephrine fixed combination MIST: pooled randomized Phase III trials.
Ther Deliv. 2021 Mar;12(3):201-214. |
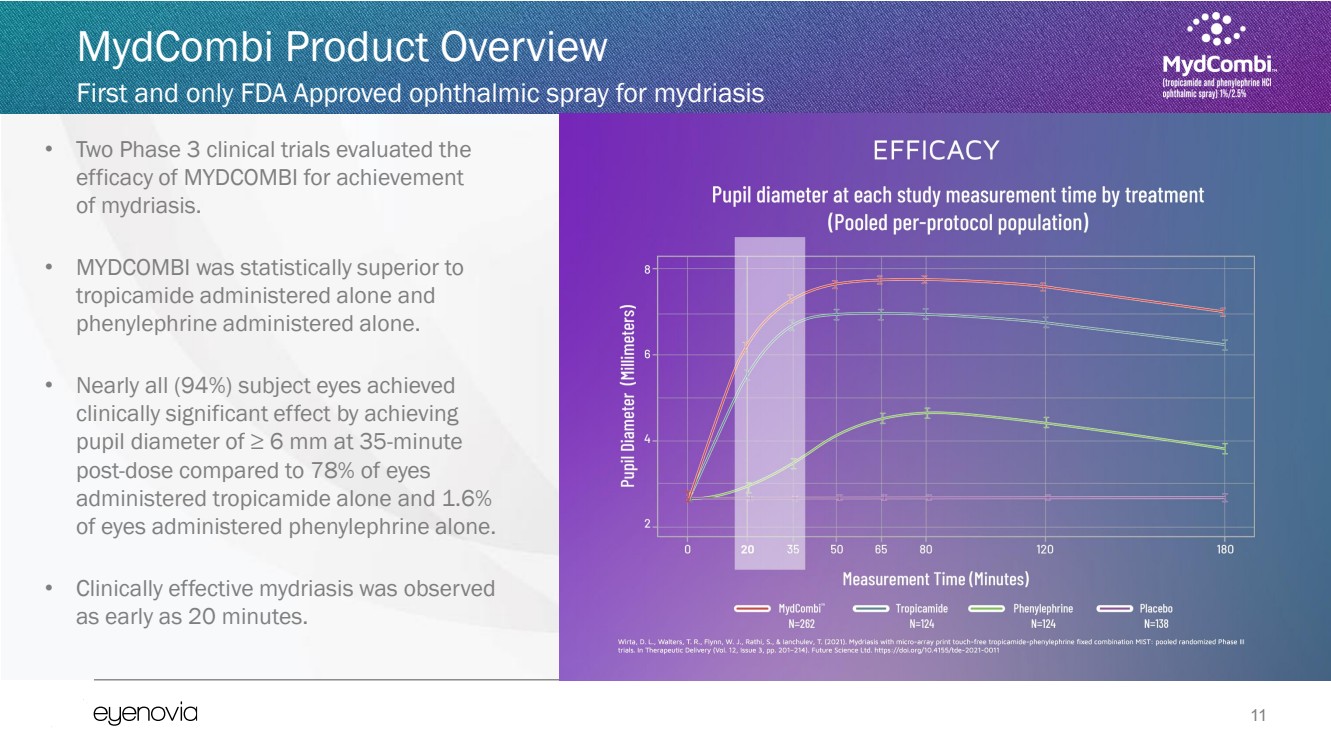
| 11
MydCombi Product Overview
First and only FDA Approved ophthalmic spray for mydriasis
• Two Phase 3 clinical trials evaluated the
efficacy of MYDCOMBI for achievement
of mydriasis.
• MYDCOMBI was statistically superior to
tropicamide administered alone and
phenylephrine administered alone.
• Nearly all (94%) subject eyes achieved
clinically significant effect by achieving
pupil diameter of ≥ 6 mm at 35-minute
post-dose compared to 78% of eyes
administered tropicamide alone and 1.6%
of eyes administered phenylephrine alone.
• Clinically effective mydriasis was observed
as early as 20 minutes. |
| 12
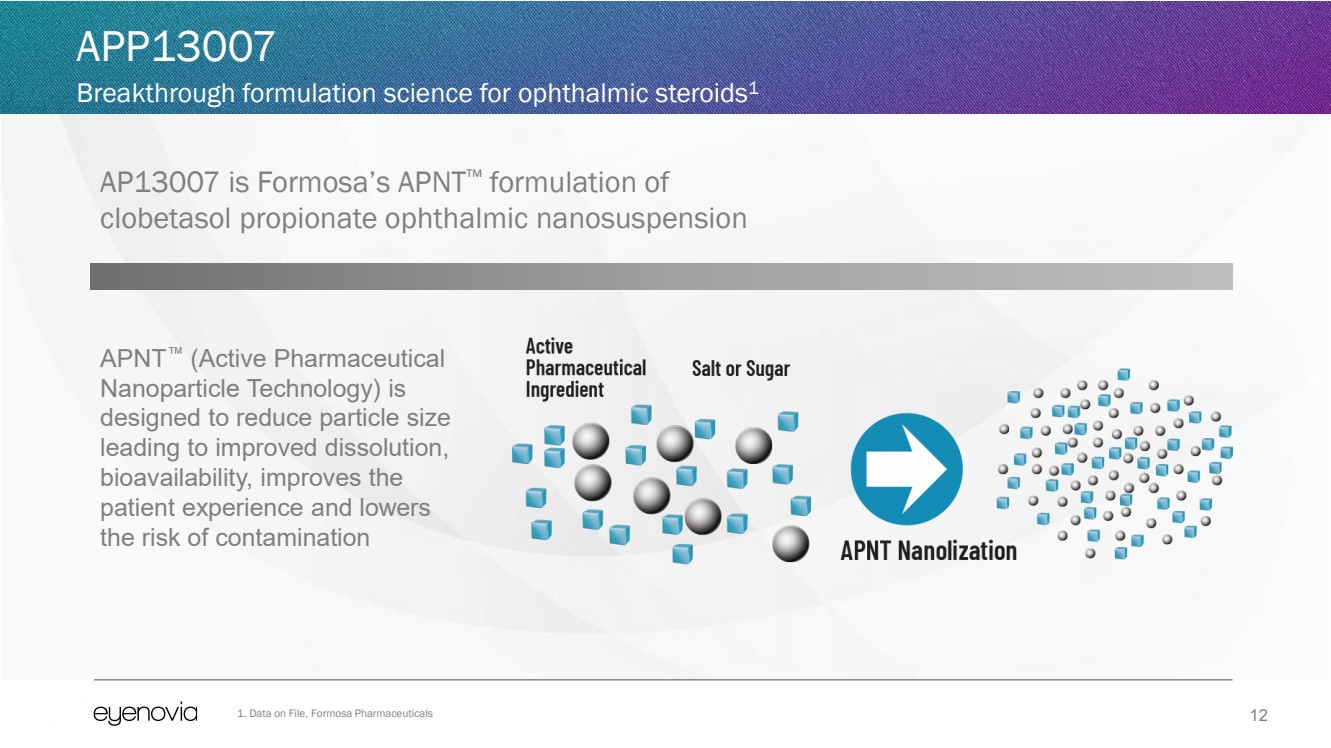
AP13007 is Formosa’s APNT™ formulation of
clobetasol propionate ophthalmic nanosuspension
APP13007
Breakthrough formulation science for ophthalmic steroids1
APNT™ (Active Pharmaceutical
Nanoparticle Technology) is
designed to reduce particle size
leading to improved dissolution,
bioavailability, improves the
patient experience and lowers
the risk of contamination
1. Data on File, Formosa Pharmaceuticals |
| 13
• CPN-301, A Multicenter, Randomized,
Double-Masked, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy and
Safety of APP13007 for the Treatment of
Inflammation and Pain after Cataract Surgery
• 395 subjects of which 180 on active, 197 on
placebo completed the study
– Mean age 68 YO
– ~40% male, ~ 60% female
– Multiple races/ethnicity represented
• 107 subjects (100 on placebo, 7 on active)
rescued during the 14-day study for
insufficient pain or inflammation control
PRIMARY EFFICACY ENDPOINTS
APP13007 was statistically and clinically
significantly superior to placebo
(p<0.001) for both primary efficacy
endpoints:
• Proportion of subjects with anterior
chamber cell (ACC) count = 0 (ACC
Grade=0) at POD8 maintained through
POD15
• Proportion of subjects with Ocular Pain
Grade = 0 at POD4 maintained
through POD15
APP13007
Breakthrough formulation science for ophthalmic steroids1
1. Korenfeld M, Walters T, Martel J, Nunez D, Wang L. A Phase 3 Study of APP13007 (Clobetasol Propionate Ophthalmic Nanosuspension
0.05%) to Treat Inflammation and Pain after Cataract Surgery. ASCRS presentation. San Diego. May 5-8 2023 |
| 14
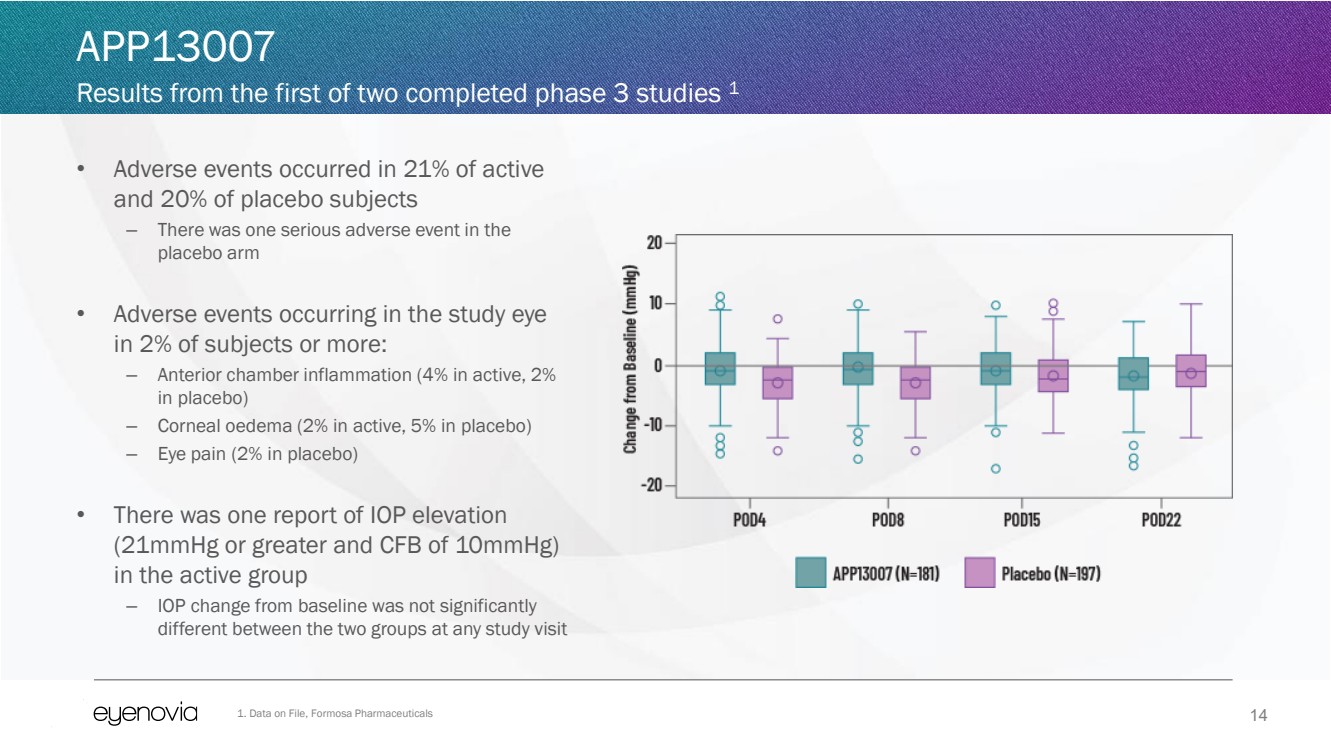
• Adverse events occurred in 21% of active
and 20% of placebo subjects
– There was one serious adverse event in the
placebo arm
• Adverse events occurring in the study eye
in 2% of subjects or more:
– Anterior chamber inflammation (4% in active, 2%
in placebo)
– Corneal oedema (2% in active, 5% in placebo)
– Eye pain (2% in placebo)
• There was one report of IOP elevation
(21mmHg or greater and CFB of 10mmHg)
in the active group
– IOP change from baseline was not significantly
different between the two groups at any study visit
APP13007
Results from the first of two completed phase 3 studies 1
1. Data on File, Formosa Pharmaceuticals |
| 15
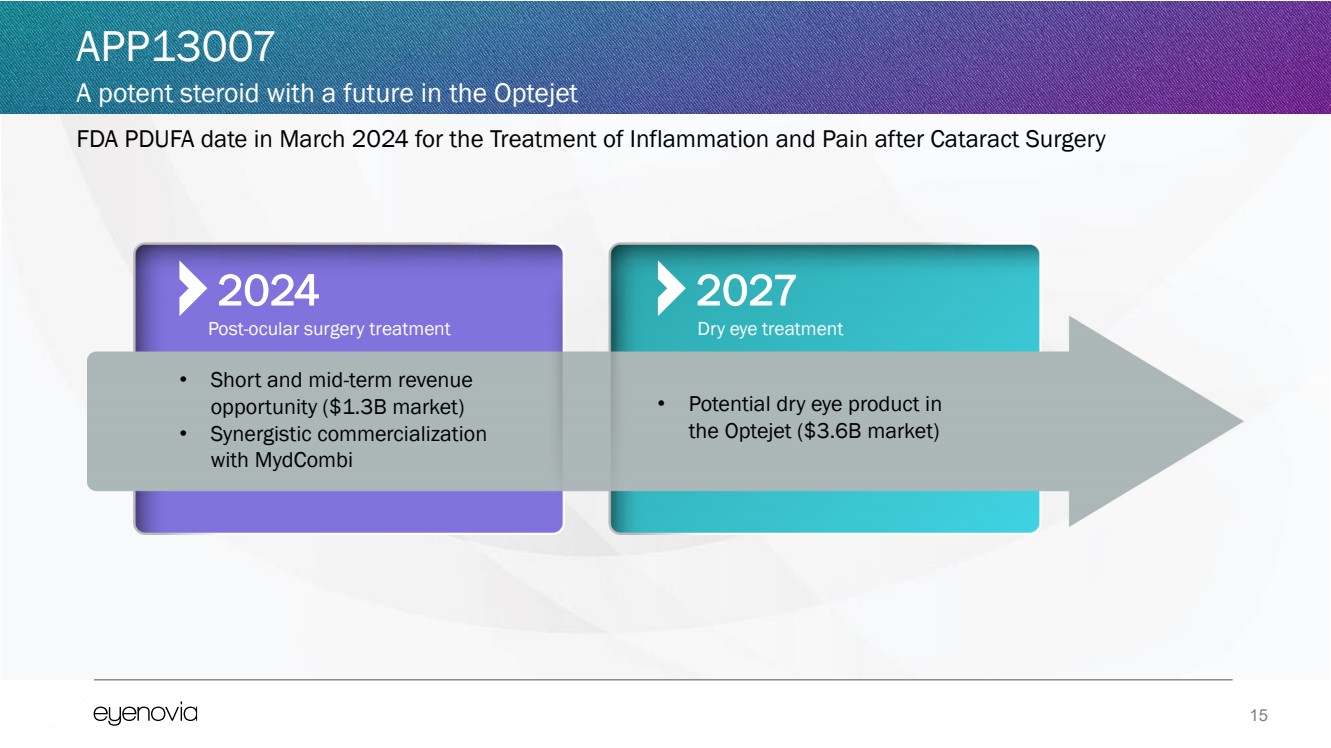
FDA PDUFA date in March 2024 for the Treatment of Inflammation and Pain after Cataract Surgery
APP13007
A potent steroid with a future in the Optejet
• Short and mid-term revenue
opportunity ($1.3B market)
• Synergistic commercialization
with MydCombi
2024
Post-ocular surgery treatment
2027
• Potential dry eye product in
the Optejet ($3.6B market)
Dry eye treatment |
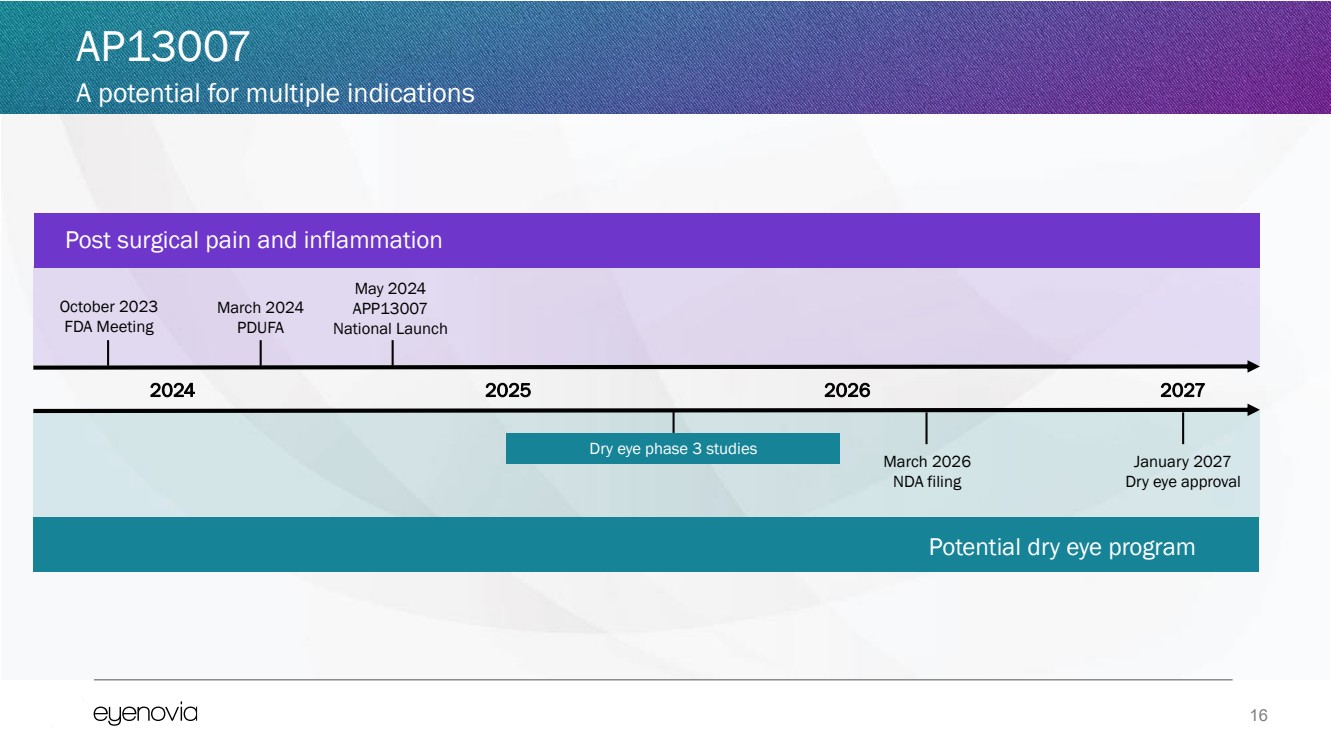
| 16
October 2023
FDA Meeting
2024 2027
March 2026
NDA filing
Dry eye phase 3 studies
March 2024
PDUFA
Potential dry eye program
2025
May 2024
APP13007
National Launch
January 2027
Dry eye approval
Post surgical pain and inflammation
2026
AP13007
A potential for multiple indications |

| 17
Apersure™ for Presbyopia
• Presbyopia is the age-related loss of near vision
that occurs as the lens becomes inelastic
• 18 million people aged 40 – 55 in the
US have presbyopia, with roughly half never
having to use glasses earlier in their lives
• Apersure is a lifestyle product designed to avoid the
appearance and inconvenience of reading glasses
– Use “as needed” with rapid onset
improvement of near vision
– Easy to administer
– Discreet – compatible with modern lifestyle |

| 18
Apersure™
Phase 3 clinical results
• Vision-1
1 and Vision-2
2 clinical studies
– 6.0x more patients achieved ≥ 3-line gain on a
vision chart in the active group vs. placebo3,5
– Well-tolerated with fewer than 2% of patients
reporting moderate hyperemia4
, instillation
discomfort, or brow ache
• People prefer Apersure over eyedrops
– Among 100 presbyopic patients aged 40-55,
80% said they would prefer Apersure over the
traditional eyedrop bottle5
– Price sensitivity tests indicate approximately
$100 for 80 doses would be well accepted
1. https://clinicaltrials.gov/ct2/show/NCT04657172 | 2. https://clinicaltrials.gov/ct2/show/NCT05114486
3. Cohort of subjects with baseline DCNVA < 0.6 logMAR | 4. Resolved by 3-hours post dose | 5. Data on file
Apersure delivered
via Optejet |
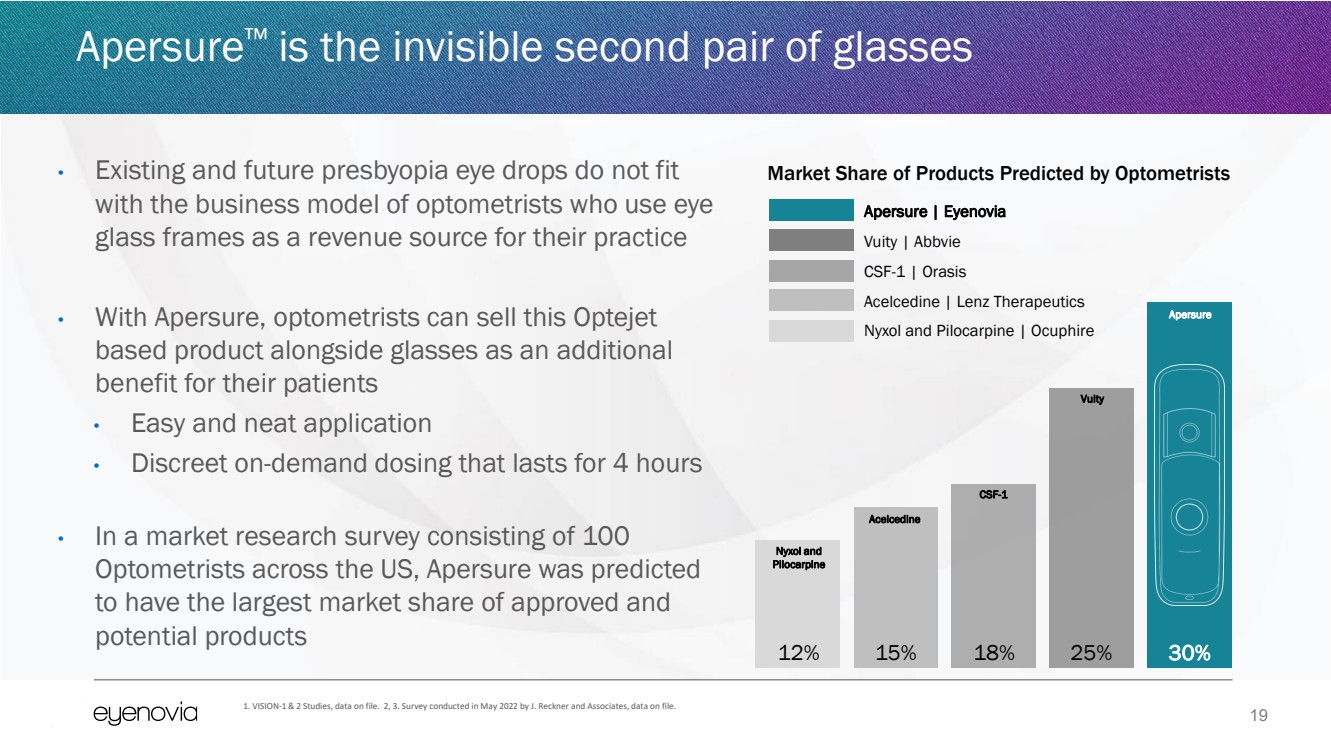
| 19
Apersure™ is the invisible second pair of glasses
• Existing and future presbyopia eye drops do not fit
with the business model of optometrists who use eye
glass frames as a revenue source for their practice
• With Apersure, optometrists can sell this Optejet
based product alongside glasses as an additional
benefit for their patients
• Easy and neat application
• Discreet on-demand dosing that lasts for 4 hours
• In a market research survey consisting of 100
Optometrists across the US, Apersure was predicted
to have the largest market share of approved and
potential products
1. VISION-1 & 2 Studies, data on file. 2, 3. Survey conducted in May 2022 by J. Reckner and Associates, data on file.
12% 15% 18% 25% 30%
Apersure | Eyenovia
Vuity | Abbvie
CSF-1 | Orasis
Acelcedine | Lenz Therapeutics
Nyxol and Pilocarpine | Ocuphire
Market Share of Products Predicted by Optometrists
Apersure
Vuity
CSF-1
Acelcedine
Nyxol and
Pilocarpine |

| 20
Apersure™
1. Population of 40-55YO in the US = 60.8MA , 35% of this population has never needed corrected visionB, assumes product will work for 33% of the remaining population
A. Published by Erin Duffin, & 30, S. (2022, September 30). Population of the U.S. by sex and age 2021. Statista. Retrieved February 3, 2023, from https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age/ | B. What is 20/20 vision? University of Iowa Hospitals & Clinics. (n.d.). Retrieved February 3, 2023, from https://uihc.org/health-topics/what-2020-vision
Market Receptivity
High among optometrists who are intrigued by the
ability to sell the device through their offices; high
among patients who are attracted to the benefits of
the device
Potential Market Size 3.5 million people1 @ $250 per year = $877M
Pricing
Approximately $100 per cartridge (similar to Vuity on
a per-use basis); market research indicates patients
would use 2.5 cartridges/year on average
Reimbursement
Status
Cash-pay cosmeceutical; can be purchased with
HSA/FSA funds
The only presbyopia treatment with the Optejet that may enhance office economics |
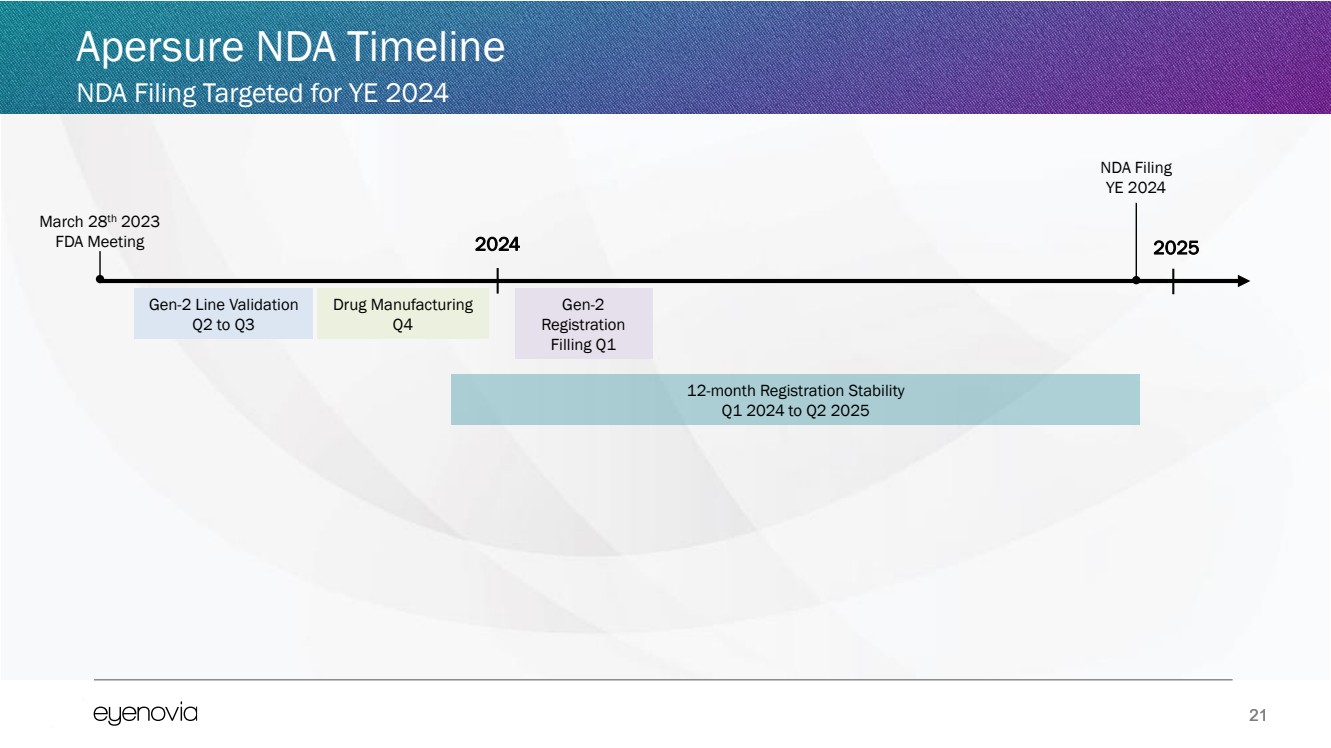
| 21
Apersure NDA Timeline
NDA Filing Targeted for YE 2024
March 28th 2023
FDA Meeting 2024
Gen-2 Line Validation
Q2 to Q3
Gen-2
Registration
Filling Q1
Drug Manufacturing
Q4
2025
NDA Filing
YE 2024
12-month Registration Stability
Q1 2024 to Q2 2025 |
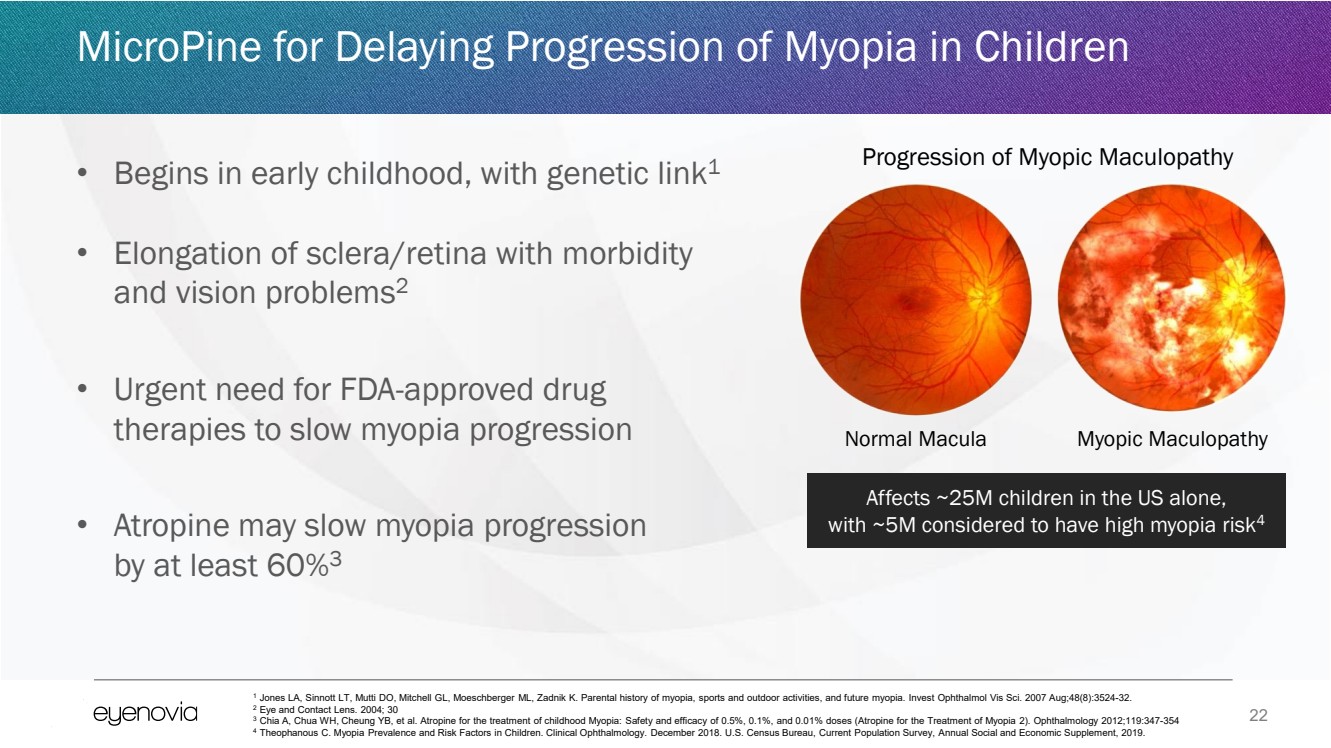
| 22
• Begins in early childhood, with genetic link1
• Elongation of sclera/retina with morbidity
and vision problems2
• Urgent need for FDA-approved drug
therapies to slow myopia progression
• Atropine may slow myopia progression
by at least 60%3
MicroPine for Delaying Progression of Myopia in Children
Progression of Myopic Maculopathy
Normal Macula Myopic Maculopathy
Affects ~25M children in the US alone,
with ~5M considered to have high myopia risk4
1 Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007 Aug;48(8):3524-32.
2 Eye and Contact Lens. 2004; 30
3 Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood Myopia: Safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012;119:347-354
4 Theophanous C. Myopia Prevalence and Risk Factors in Children. Clinical Ophthalmology. December 2018. U.S. Census Bureau, Current Population Survey, Annual Social and Economic Supplement, 2019. |
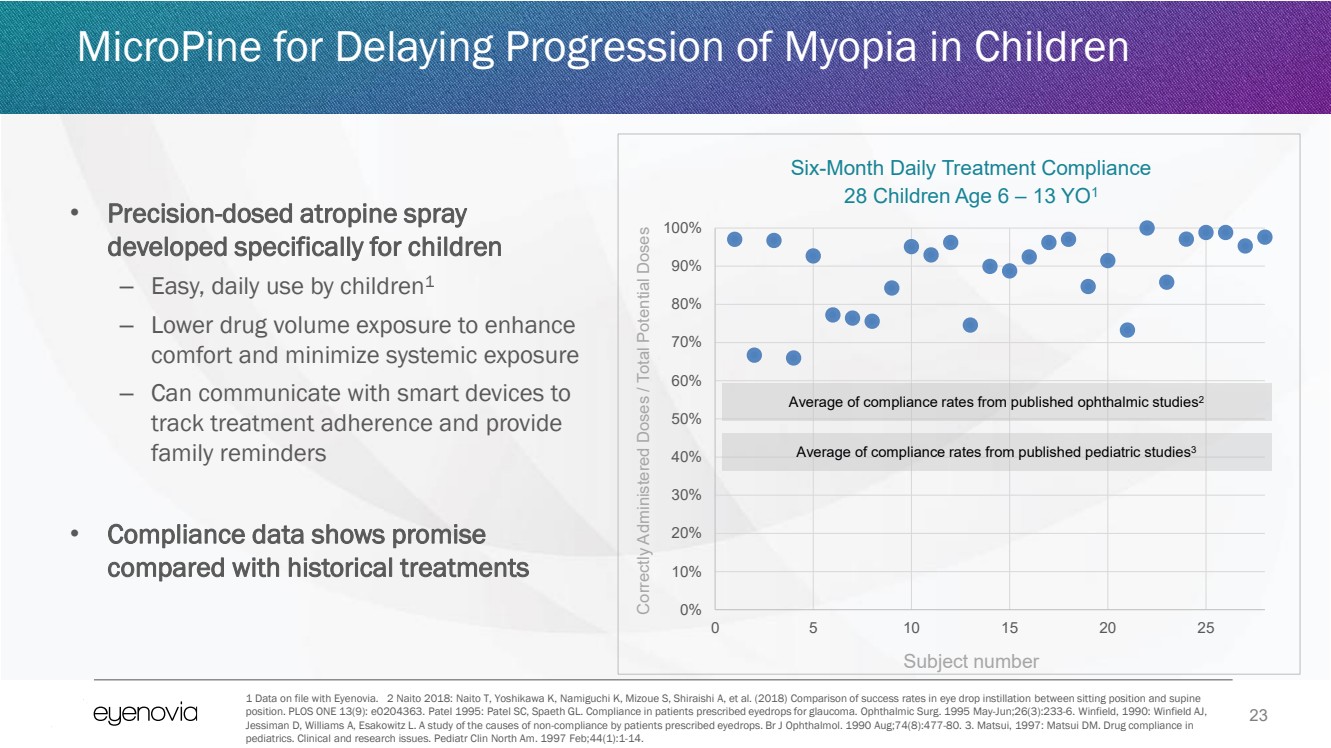
| 23
• Precision-dosed atropine spray
developed specifically for children
– Easy, daily use by children1
– Lower drug volume exposure to enhance
comfort and minimize systemic exposure
– Can communicate with smart devices to
track treatment adherence and provide
family reminders
• Compliance data shows promise
compared with historical treatments
MicroPine for Delaying Progression of Myopia in Children
1 Data on file with Eyenovia. 2 Naito 2018: Naito T, Yoshikawa K, Namiguchi K, Mizoue S, Shiraishi A, et al. (2018) Comparison of success rates in eye drop instillation between sitting position and supine
position. PLOS ONE 13(9): e0204363. Patel 1995: Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg. 1995 May-Jun;26(3):233-6. Winfield, 1990: Winfield AJ,
Jessiman D, Williams A, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol. 1990 Aug;74(8):477-80. 3. Matsui, 1997: Matsui DM. Drug compliance in
pediatrics. Clinical and research issues. Pediatr Clin North Am. 1997 Feb;44(1):1-14.
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
0 5 10 15 20 25
Six-Month Daily Treatment Compliance
28 Children Age 6 – 13 YO1
Average of compliance rates from published ophthalmic studies2
Average of compliance rates from published pediatric studies3
Subject number
Correctly Administered Doses / Total Potential Doses |
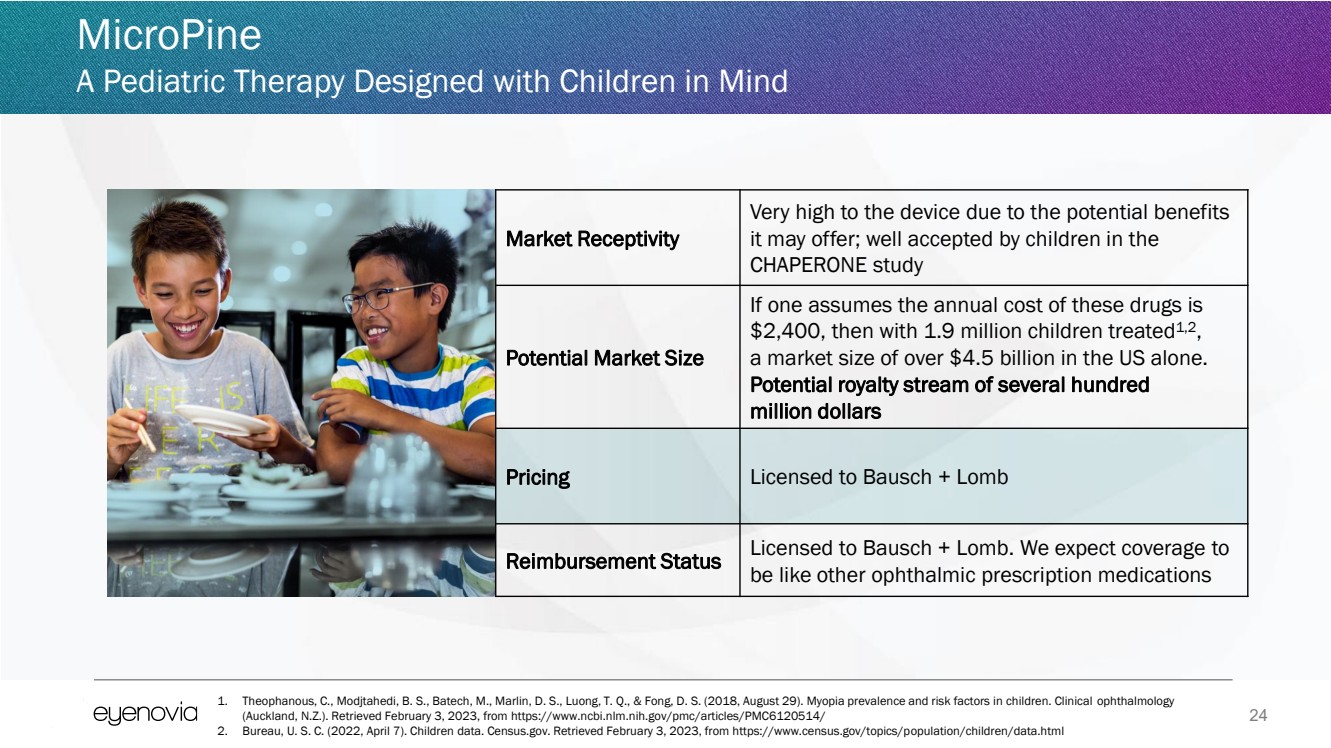
| 24
MicroPine
A Pediatric Therapy Designed with Children in Mind
Market Receptivity
Very high to the device due to the potential benefits
it may offer; well accepted by children in the
CHAPERONE study
Potential Market Size
If one assumes the annual cost of these drugs is
$2,400, then with 1.9 million children treated1,2
,
a market size of over $4.5 billion in the US alone.
Potential royalty stream of several hundred
million dollars
Pricing Licensed to Bausch + Lomb
Reimbursement Status Licensed to Bausch + Lomb. We expect coverage to
be like other ophthalmic prescription medications
1. Theophanous, C., Modjtahedi, B. S., Batech, M., Marlin, D. S., Luong, T. Q., & Fong, D. S. (2018, August 29). Myopia prevalence and risk factors in children. Clinical ophthalmology
(Auckland, N.Z.). Retrieved February 3, 2023, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6120514/
2. Bureau, U. S. C. (2022, April 7). Children data. Census.gov. Retrieved February 3, 2023, from https://www.census.gov/topics/population/children/data.html |
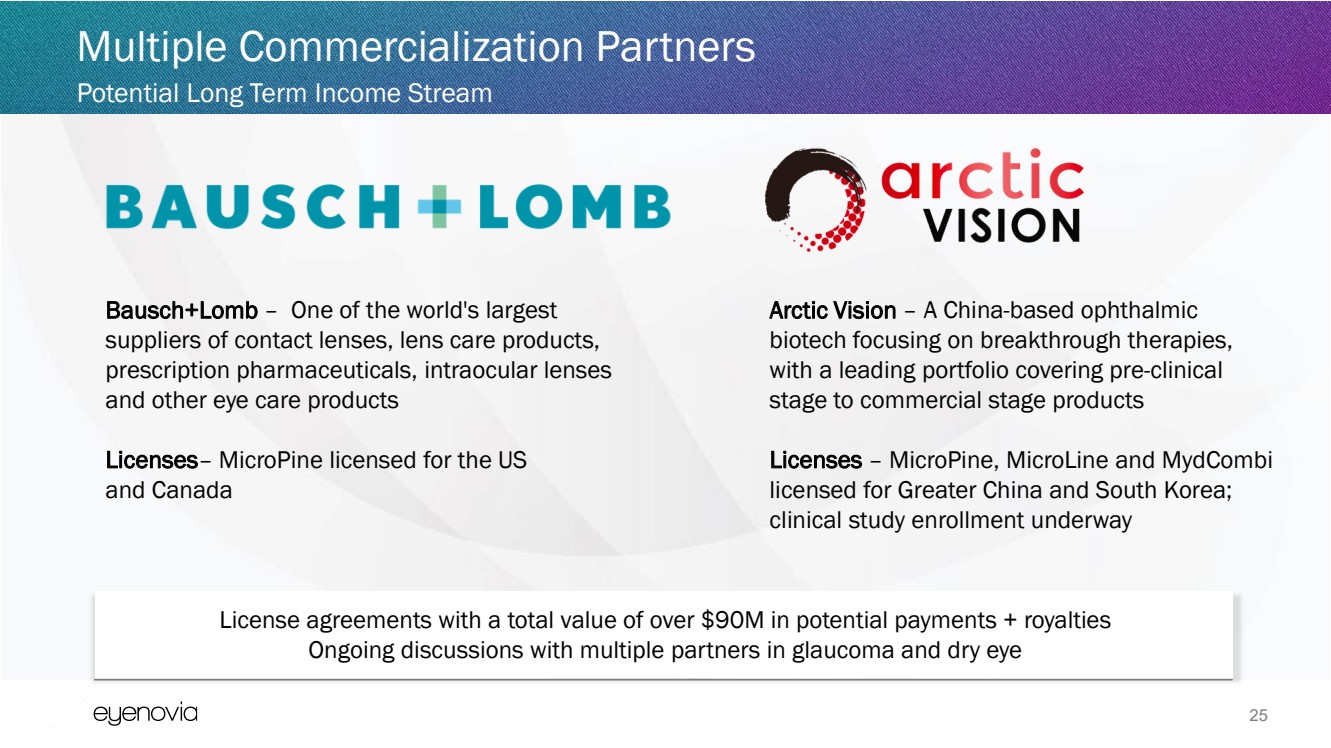
| 25
Multiple Commercialization Partners
Arctic Vision – A China-based ophthalmic
biotech focusing on breakthrough therapies,
with a leading portfolio covering pre-clinical
stage to commercial stage products
Licenses – MicroPine, MicroLine and MydCombi
licensed for Greater China and South Korea;
clinical study enrollment underway
Bausch+Lomb – One of the world's largest
suppliers of contact lenses, lens care products,
prescription pharmaceuticals, intraocular lenses
and other eye care products
Licenses– MicroPine licensed for the US
and Canada
Potential Long Term Income Stream
License agreements with a total value of over $90M in potential payments + royalties
Ongoing discussions with multiple partners in glaucoma and dry eye |
| 26
Broad Intellectual Property Portfolio
• Key claims covered with multiple patents
– 16 US Patents Issued; 1 pending
– 95 foreign issued; 32 pending
– Many in effect beyond 2031
• Clinical data and regulatory approval
adds another layer of IP |
| 27
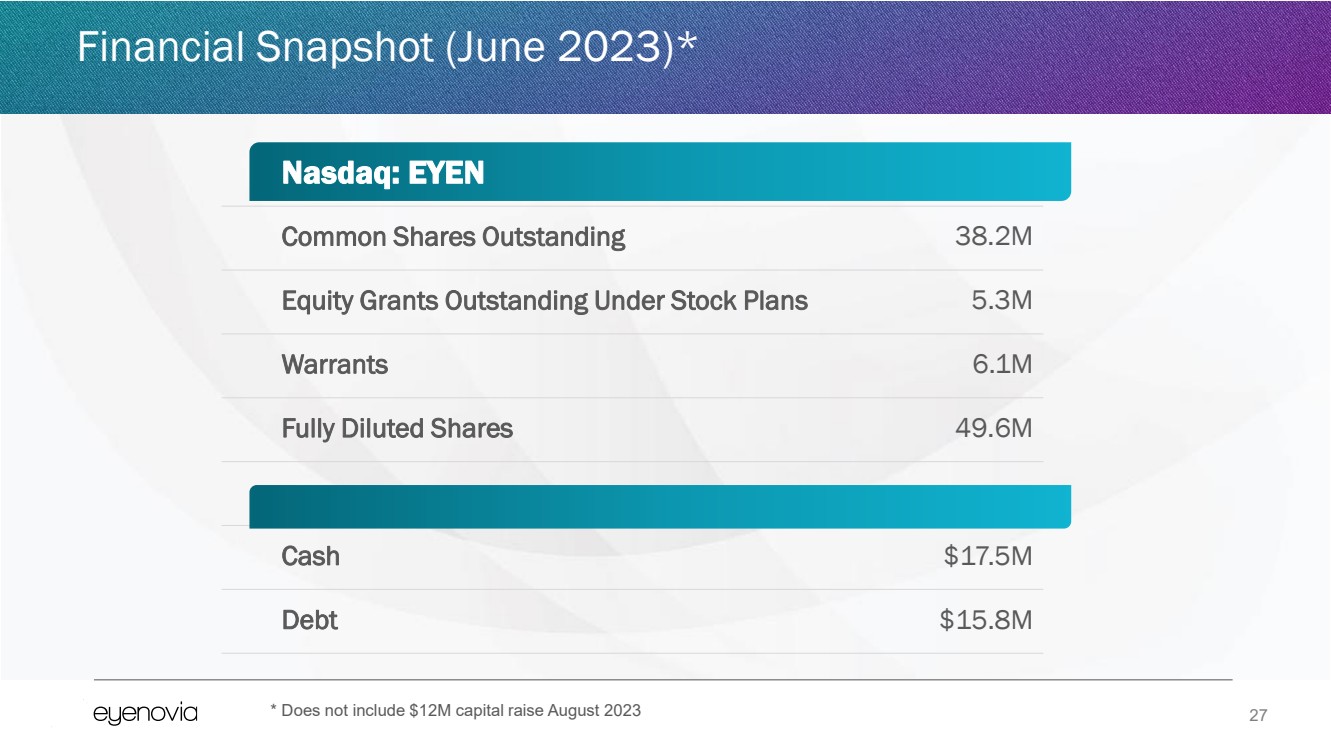
Financial Snapshot (June 2023)*
Nasdaq: EYEN
Common Shares Outstanding 38.2M
Equity Grants Outstanding Under Stock Plans 5.3M
Warrants 6.1M
Fully Diluted Shares 49.6M
Cash $17.5M
Debt $15.8M
* Does not include $12M capital raise August 2023 |
| 28
Experienced Leadership Team
Bren Kern
Chief Operating Officer
Michael Rowe
Chief Executive Officer
John Gandolfo
Chief Financial Officer |
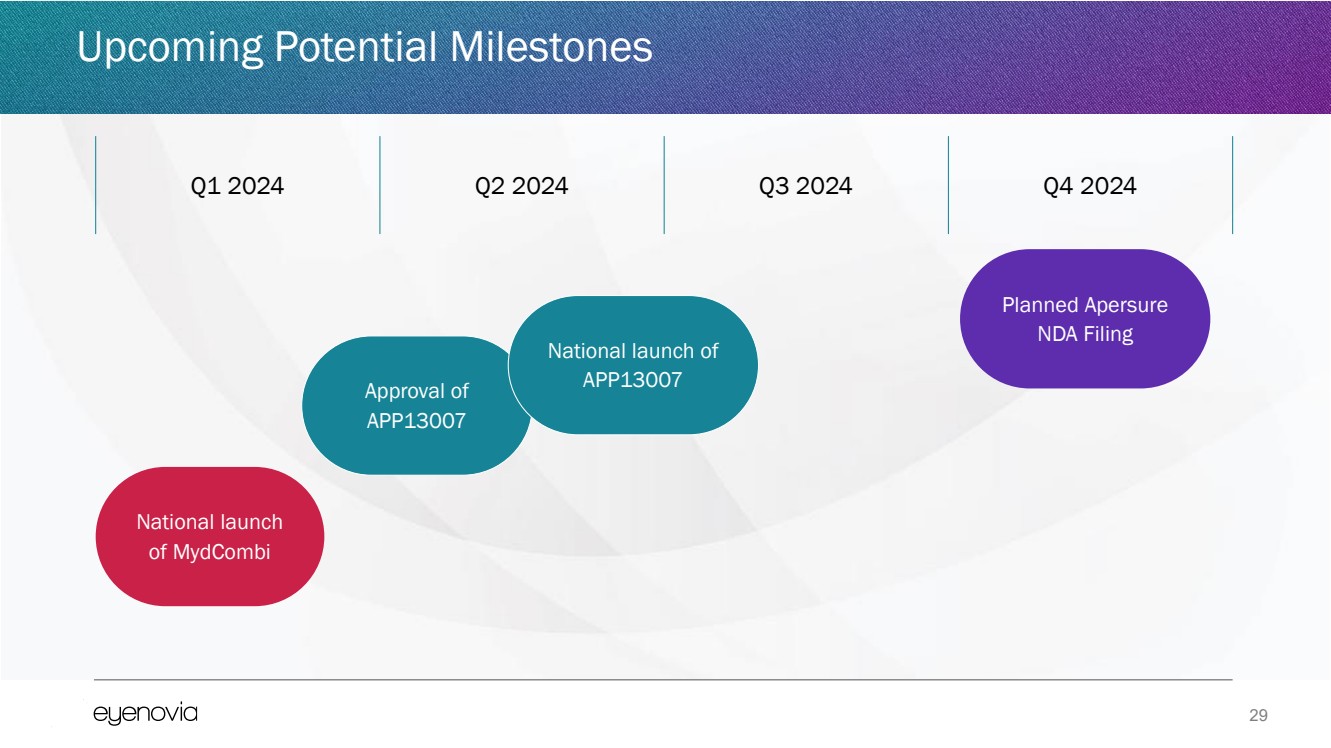
| 29
National launch
of MydCombi
Approval of
APP13007
Planned Apersure
NDA Filing
Q1 2024 Q2 2024 Q3 2024 Q4 2024
National launch of
APP13007
Upcoming Potential Milestones |

| 30
• Optejet platform technology with ergonomic design facilitates ease
of use and delivers precise doses
– Addresses many long-term unmet clinical needs surrounding the use of
conventional eye drops
– Protected with a strong intellectual property portfolio
• Eyenovia owns a pipeline of products in large therapeutic categories
– With multiple commercial partnerships in place and more being developed
• Poised for leadership as a technology partner and therapy provider
in potentially huge markets
• First FDA approved product May 2023
– MydCombi (tropicamide and phenylephrine HCl ophthalmic spray) 1%/2.5%
– Validates the underlying Optejet technology
Investment Summary |
Cover
|
Sep. 19, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Sep. 19, 2023
|
| Entity File Number |
001-38365
|
| Entity Registrant Name |
EYENOVIA, INC.
|
| Entity Central Index Key |
0001682639
|
| Entity Tax Identification Number |
47-1178401
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
295 Madison Avenue
|
| Entity Address, Address Line Two |
Suite 2400
|
| Entity Address, City or Town |
New York
|
| Entity Address, State or Province |
NY
|
| Entity Address, Postal Zip Code |
10017
|
| City Area Code |
833
|
| Local Phone Number |
393-6684
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common stock, par value $0.0001 per share
|
| Trading Symbol |
EYEN
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
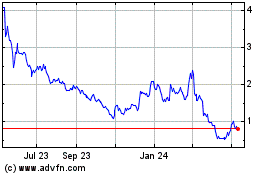
Eyenovia (NASDAQ:EYEN)
Historical Stock Chart
From Mar 2024 to Apr 2024
Eyenovia (NASDAQ:EYEN)
Historical Stock Chart
From Apr 2023 to Apr 2024