false
0001682639
0001682639
2024-01-25
2024-01-25
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE
COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13
or 15(d)
of the Securities Exchange
Act of 1934
Date of Report (Date
of earliest event reported): January 25, 2024
EYENOVIA, INC.
(Exact Name of Registrant
as Specified in its Charter)
| Delaware |
|
001-38365 |
|
47-1178401 |
(State or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
295 Madison Avenue, Suite 2400, New York, NY
10017
(Address of Principal Executive Offices, and
Zip Code)
(833) 393-6684
Registrant’s Telephone Number, Including
Area Code
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
¨ Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
¨ Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
¨ Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
¨ Pre-commencement communications pursuant to Rule 13e-4(c) under
the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section
12(b) of the Act:
| (Title of each class) |
|
(Trading
Symbol) |
|
(Name of each exchange
on which registered) |
| Common stock, par value $0.0001 per share |
|
EYEN |
|
The Nasdaq Stock Market
(Nasdaq Capital Market) |
Indicate by check mark whether the registrant
is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities
Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging
growth company ¨
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 7.01. | Regulation FD Disclosure. |
On January 25, 2024, Eyenovia released an updated investor presentation,
a copy of which is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
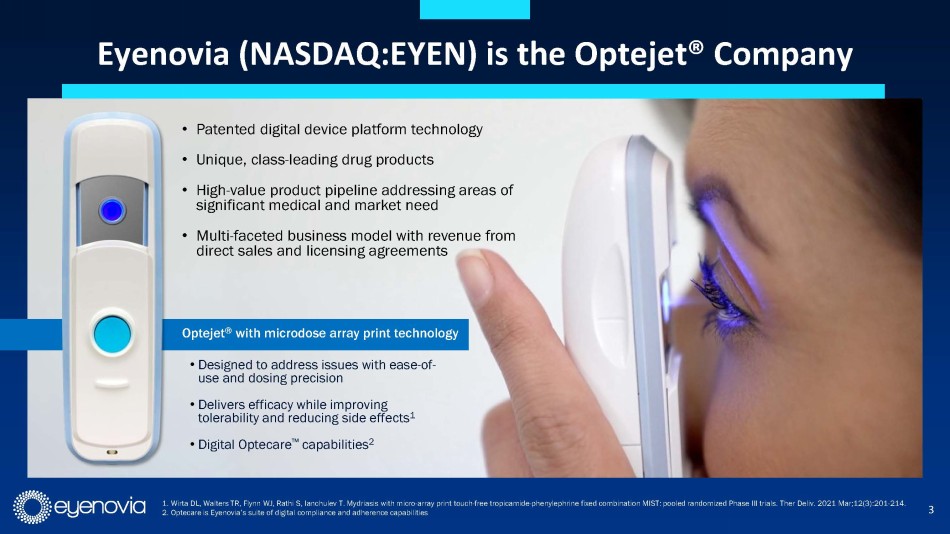
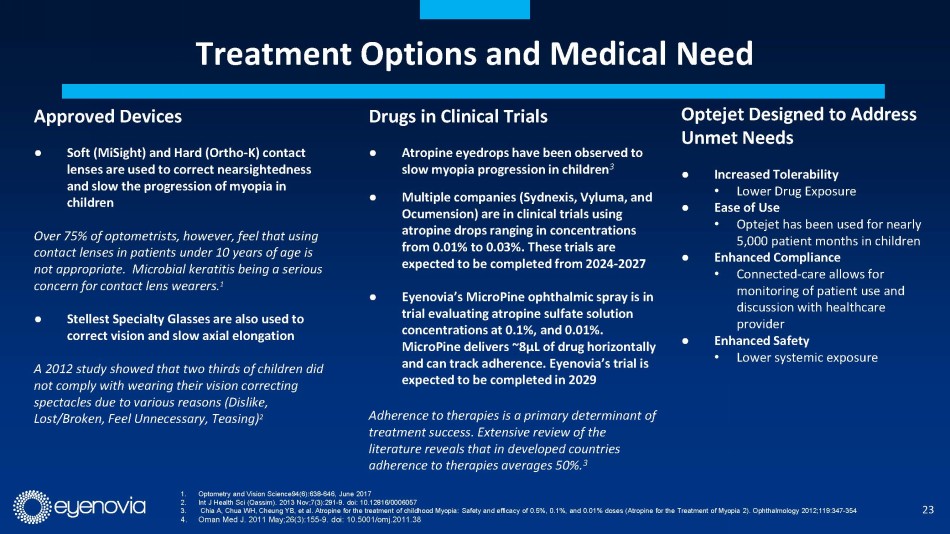
Eyenovia is developing topical ophthalmic medications that utilize
its novel, patented Optejet® drug-device dispensing platform to address large market indications with significant unmet medical needs.
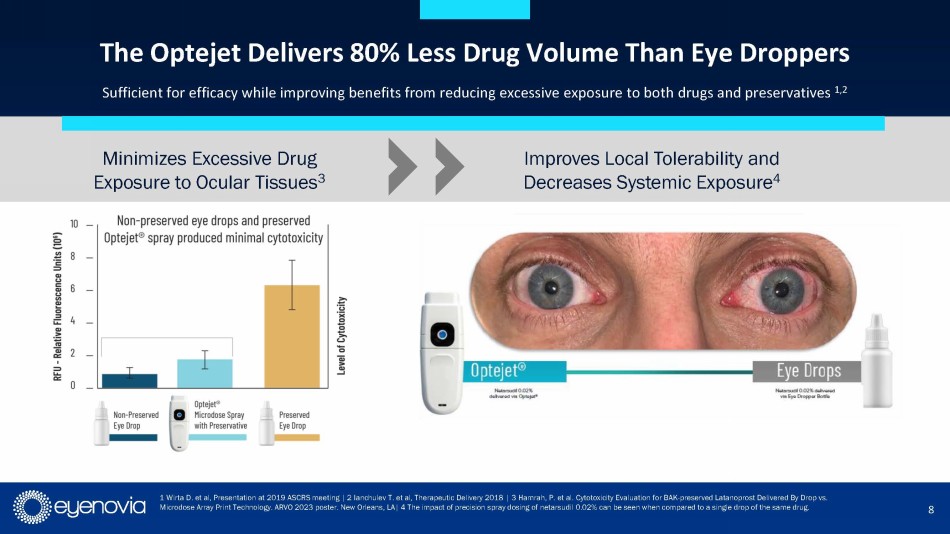
Numerous studies have demonstrated the ability of the Optejet to achieve efficacy with up to 80% less medication than traditional eye
drops, resulting in increased local tolerability and decreased systemic exposure to both drug and preservatives. The Optejet technology
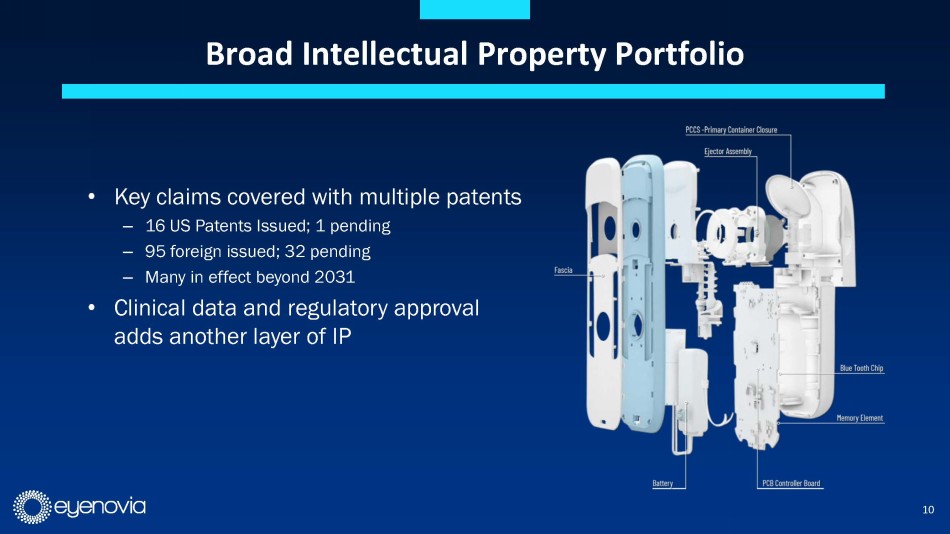
is protected by a comprehensive IP portfolio, with many claims in effect beyond 2031.
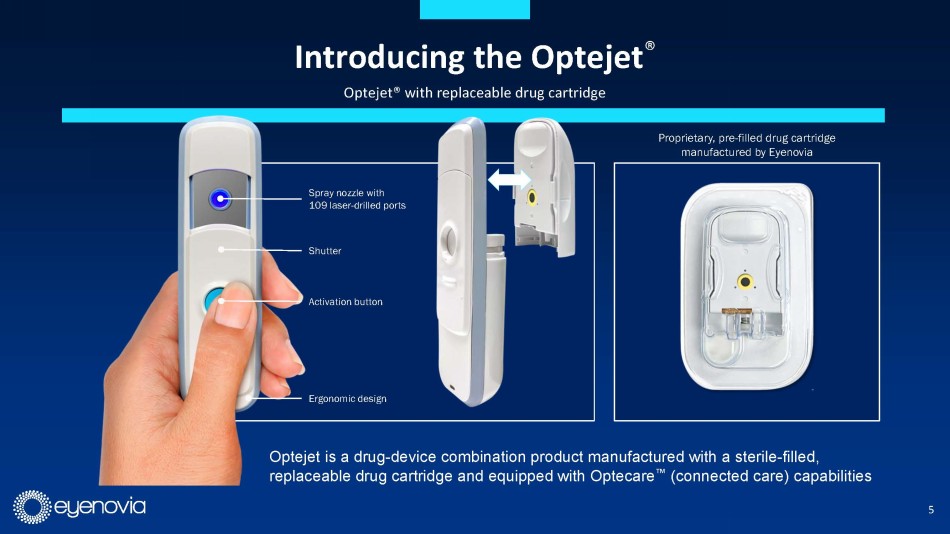
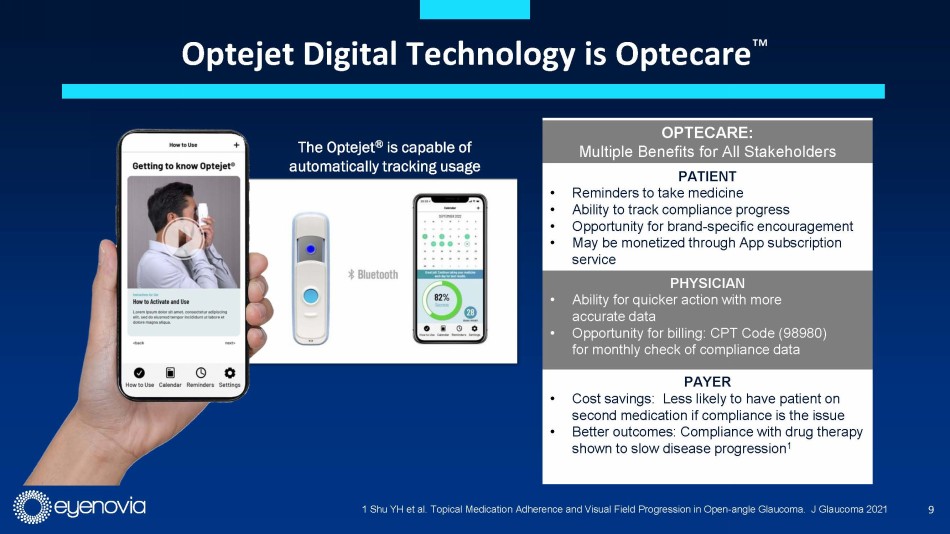
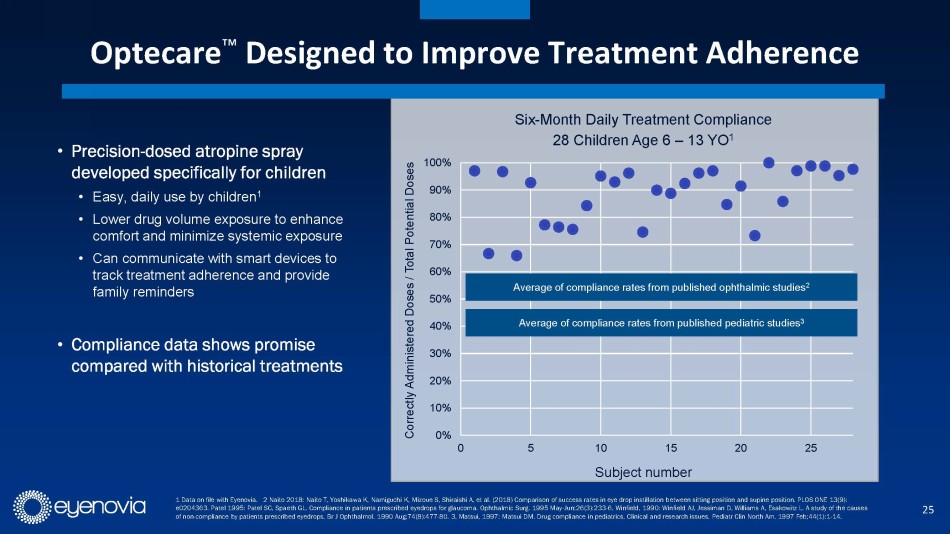
Complementing its Optejet device, Eyenovia is developing its Optecare™
suite of digital applications which leverages the onboard programming and Bluetooth technology in the Optejet to track usage and boost
compliance through reminders sent to the patient, which may result in improved patient outcomes. This also represents a potential additional
revenue stream for eye doctors under a CPT code for “Remote Therapeutic Monitoring Treatment Management Services.”
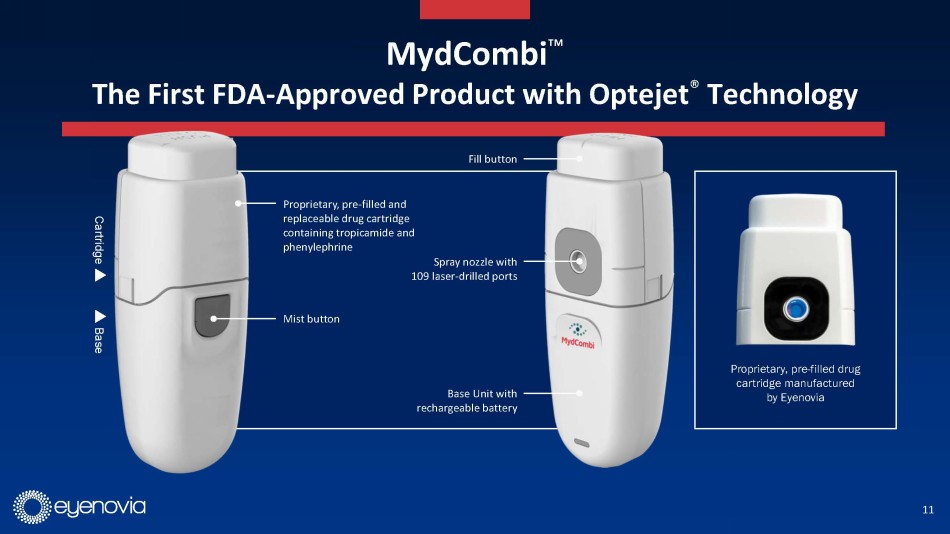
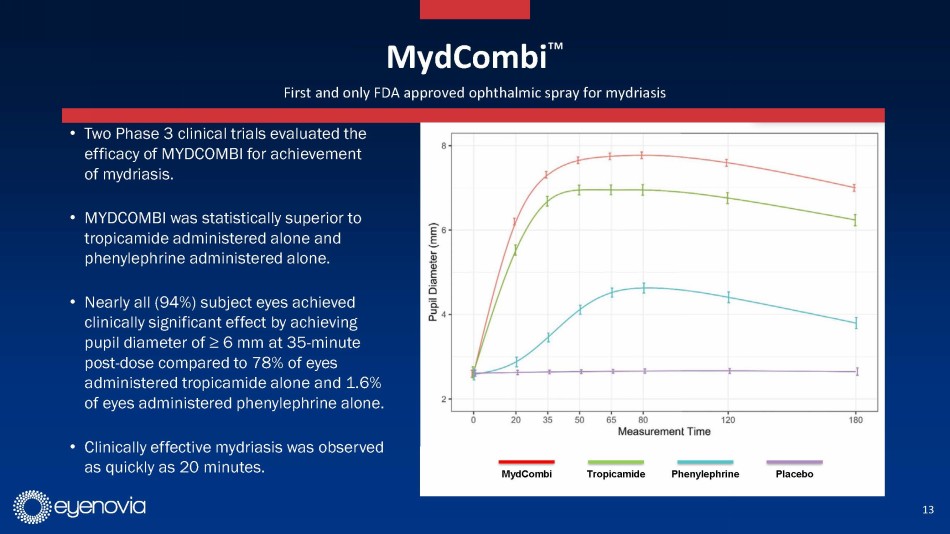
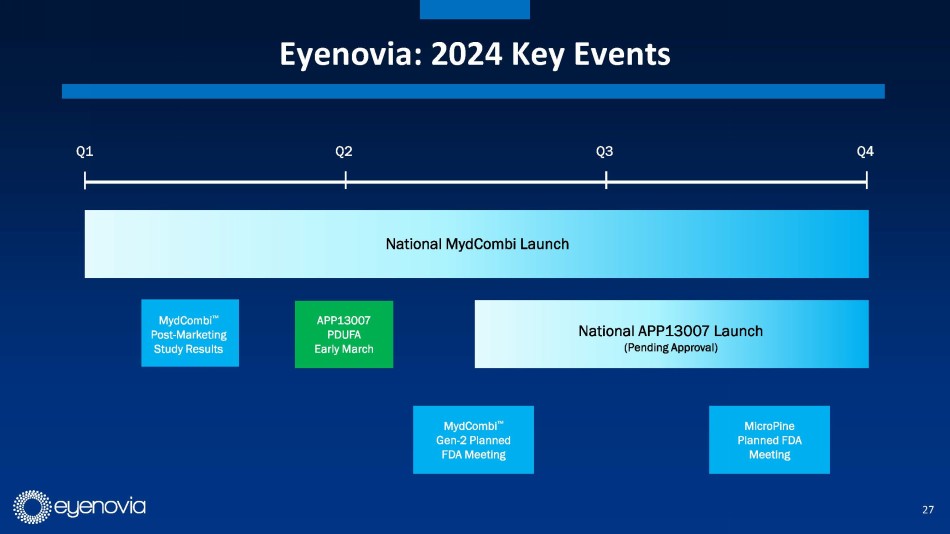
Eyenovia currently has one commercial asset, Mydcombi for mydriasis
(in-office and surgical pupil dilation), which is currently being launched commercially. Eyenovia estimates this to be a $250 million
market annually, and the updated investor presentation contains several testimonials from early adopters of the technology. Mydcombi
represents the first FDA approved drug in the Optejet, providing important validation of the technology.
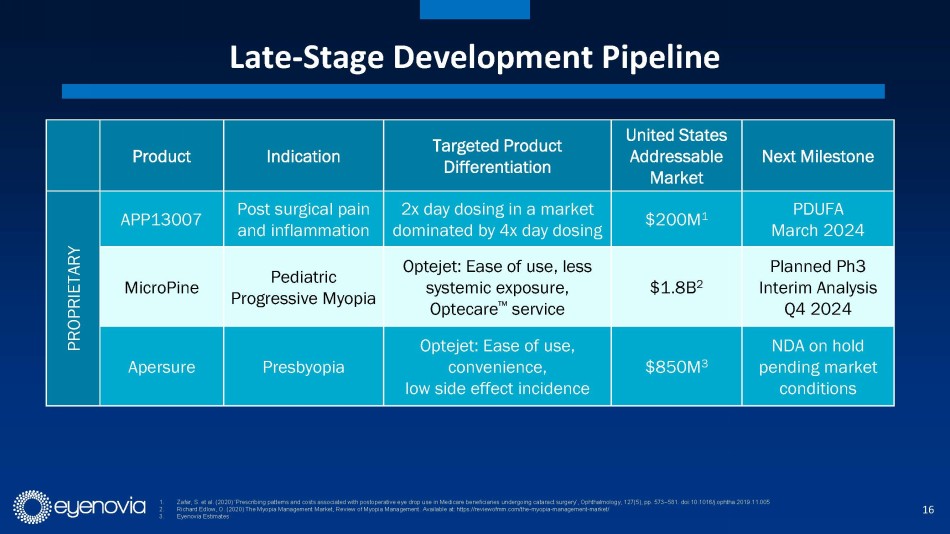
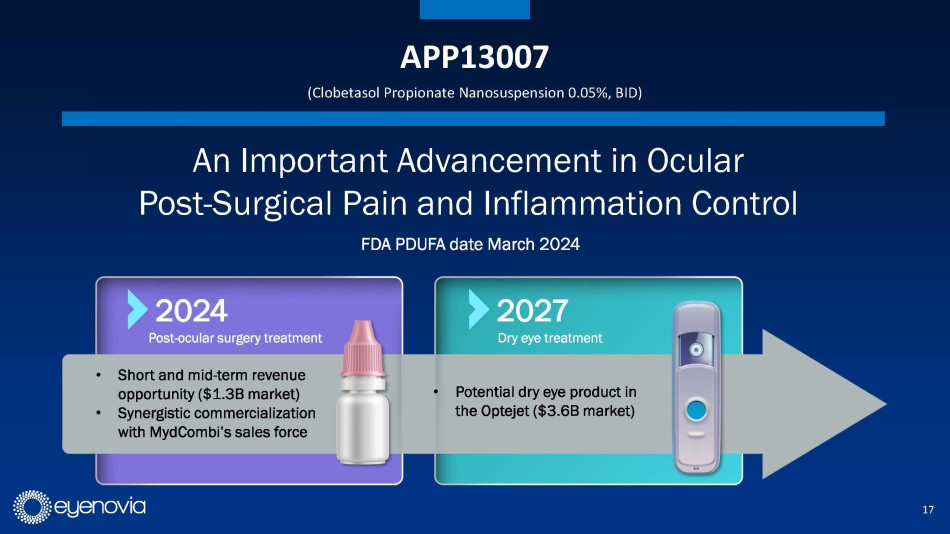
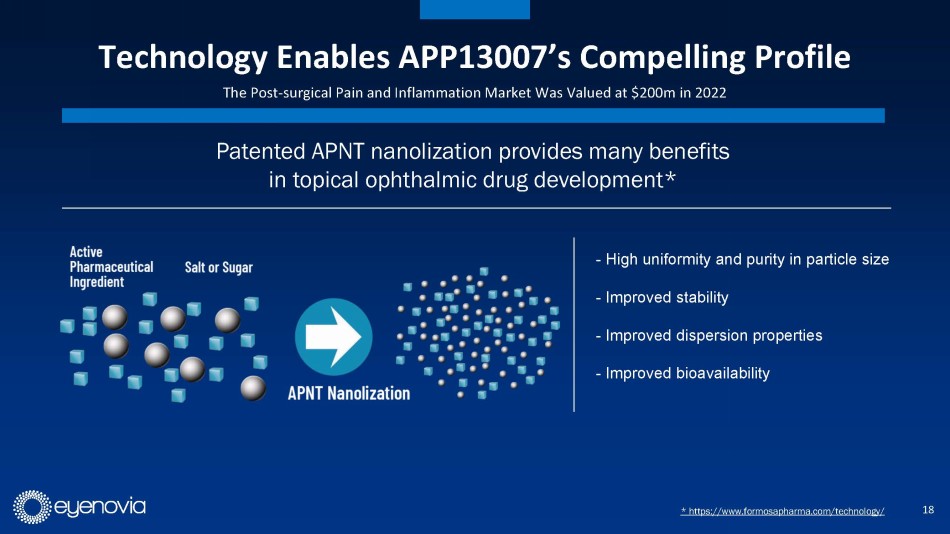
Eyenovia in-licensed its second asset, APP13007 for pain and inflammation
following ocular surgery, from Formosa Pharmaceuticals in August of 2023. APP13007 has an FDA PDUFA date of March 4, 2024. APP13007 utilizes
Formosa’s APNT™ platform which reduces an active pharmaceutical ingredient’s particle size with high uniformity and
purity, ultimately enhancing bioavailability.
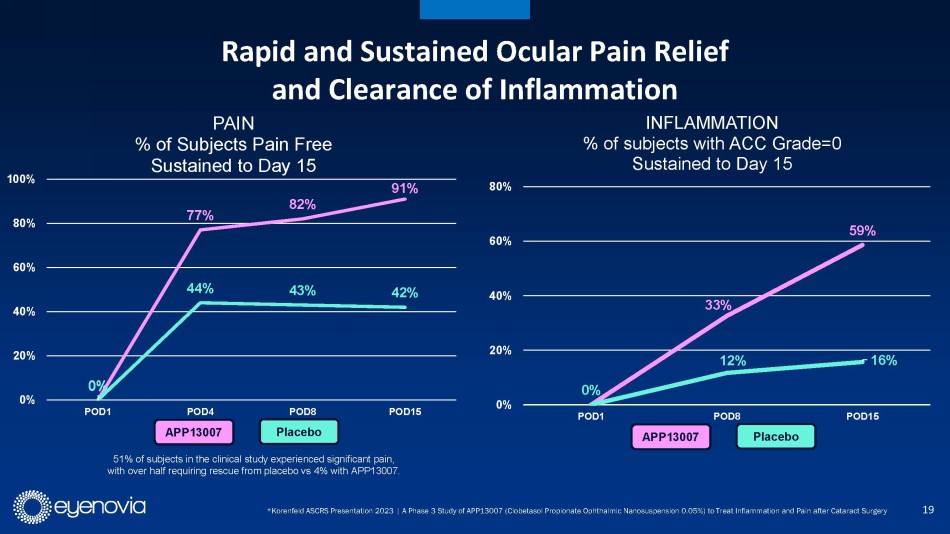
New clinical data in the updated investor presentation demonstrates
that 91% of APP13007-treated patients were pain free through day 15, as compared to 42% for placebo. Similarly, 59% of APP13007-tretaed
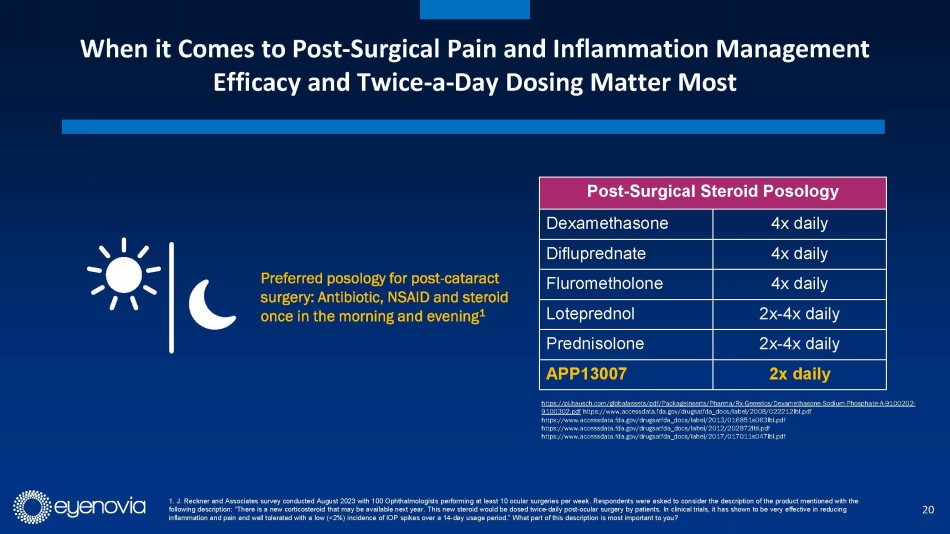
patients were free from inflammation (ACC Grade 0) through day 15, versus 16% for placebo. Importantly, the clinical profile of APP13007
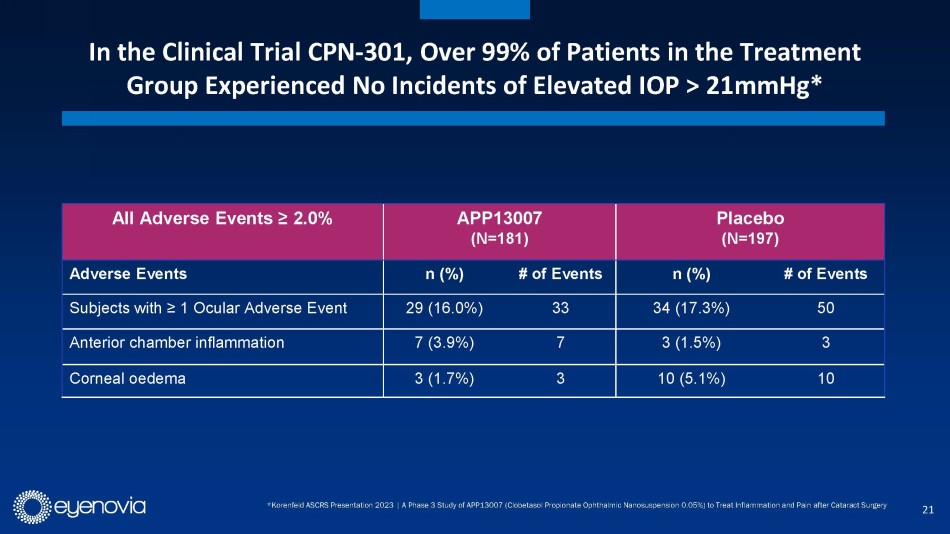
allows for 2x/day dosing in a market where most approved treatments require up to 4x/day dosing. APP13007 was well tolerated in clinical
trials. Eyenovia plans to launch APP13007 in 2H 2024, if approved. This would allow the company to further leverage its planned 10-person
field sales force.
In addition, Eyenovia recently announced that it has re-acquired the
development rights to MicroPine (precision dosed atropine spray) from Bausch+Lomb, which is currently in Phase 3 for pediatric myopia.
Myopia, which typically begins in early childhood, is characterized by an elongation of the eye, resulting in significant vision loss
and even blindness if not treated. It is estimated that myopia affects 25 million children in the U.S. alone, with five million of those
believed to be at high risk. The Review of Myopia Management states this equates to a $1.8 billion annual market opportunity in the U.S.,
with a similar opportunity in China. With myopia, treatment compliance is particularly important to slow disease progression, early indications
from use of Eyenovia’s Optecare remote therapeutic monitoring suggest enhanced dosing compliance as compared to historical treatments
without such monitoring.
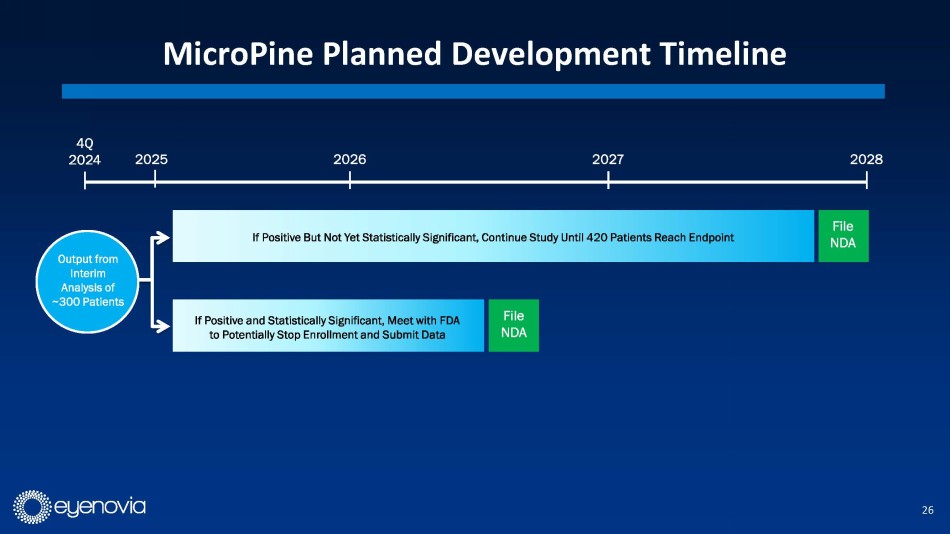
In terms of remaining development steps for MicroPine, Eyenovia is
planning to meet with FDA to discuss possible changes to the Phase 3 CHAPERONE clinical trial protocol to expedite development, including
a possible interim analysis of data from ~300 patients in late 2024. If positive and statistically significant, Eyenovia plans to meet
with FDA again with the goal of submitting an NDA in 2H 2026. If positive but not statistically significant, Eyenovia will continue the
trial until the original enrollment target of 420 patients reaches the study endpoint. Under that scenario, the Company would plan to
file an NDA in 2H 2027.
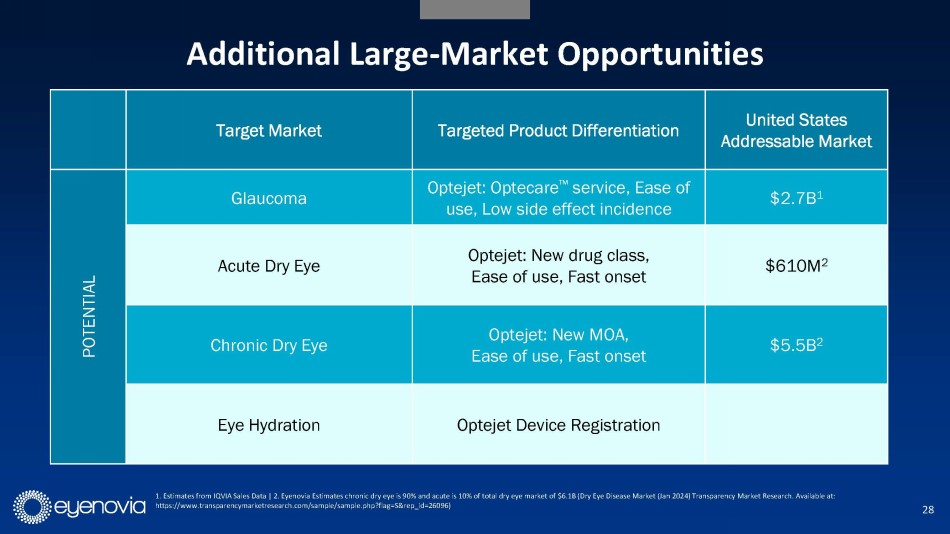
Longer term, the Company sees potential applications for the Optejet
in glaucoma (annual U.S. market opportunity of $2.7 billion), acute dry eye ($610 million), chronic dry eye ($5.5 billion) and eye hydration.
Eyenovia’s updated investor presentation is also available for
download under “Events and Presentations” in the “Investors” section of the Company’s website, www.eyenovia.com.
The information contained
in this Item 7.01, including Exhibit 99.1, is being “furnished” and shall not be deemed “filed” for purposes of
Section 18 of the Exchange Act, or otherwise subject to the liability of that Section or Sections 11 and 12(a)(2) of the Securities Act.
The information contained in this Item 7.01, including Exhibit 99.1, shall not be incorporated by reference into any registration statement
or other document pursuant to the Securities Act or into any filing or other document pursuant to the Exchange Act, except as otherwise
expressly stated in any such filing.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
EYENOVIA, INC. |
| |
|
| Date: January 25, 2024 |
/s/ John Gandolfo |
| |
John Gandolfo |
| |
Chief Financial Officer |
Exhibit 99.1









v3.23.4
Cover
|
Jan. 25, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 25, 2024
|
| Entity File Number |
001-38365
|
| Entity Registrant Name |
EYENOVIA, INC.
|
| Entity Central Index Key |
0001682639
|
| Entity Tax Identification Number |
47-1178401
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
295 Madison Avenue
|
| Entity Address, Address Line Two |
Suite 2400
|
| Entity Address, City or Town |
New York
|
| Entity Address, State or Province |
NY
|
| Entity Address, Postal Zip Code |
10017
|
| City Area Code |
833
|
| Local Phone Number |
393-6684
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common stock, par value $0.0001 per share
|
| Trading Symbol |
EYEN
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
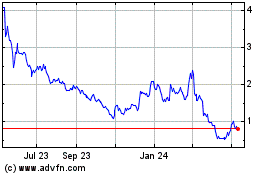
Eyenovia (NASDAQ:EYEN)
Historical Stock Chart
From Mar 2024 to Apr 2024
Eyenovia (NASDAQ:EYEN)
Historical Stock Chart
From Apr 2023 to Apr 2024