0001599901FALSE00015999012024-03-042024-03-04
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________________________________
FORM 8-K
_________________________________________
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): March 4, 2024
_________________________________________
AVIDITY BIOSCIENCES, INC.
(Exact name of registrant as specified in its charter)
_________________________________________
| | | | | | | | | | | | | | |
| Delaware | | 001-39321 | | 46-1336960 |
(State or other jurisdiction of incorporation or organization) | | (Commission File Number) | | (I.R.S. Employer Identification No.) |
10578 Science Center Drive, Suite 125
San Diego, California 92121 92121
(Address of principal executive offices) (Zip Code)
(858) 401-7900
(Registrant’s telephone number, include area code)
N/A
(Former Name or Former Address, if Changed Since Last Report)
_________________________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| | | | | |
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| | | | | |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| | | | | |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| | | | | |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share | | RNA | | The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| | | | | |
| Item 7.01. | Regulation FD Disclosure. |
On March 4, 2024, Avidity Biosciences, Inc. (“Avidity” or the “Company”) will host an investor and analyst event (the "Event") to discuss new delpacibart etedesiran ("del-desiran") data from the MARINA-OLE™ trial of people living with myotonic dystrophy type 1 ("DM1"). Delpacibart etedesiran is the approved international nonproprietary name of AOC 1001. The Event will begin at 8:00 a.m. Eastern Time and will be available via a live video webcast accessible under the “Events and Presentations” page in the “Investors” section of Avidity’s corporate website, at https://www.aviditybiosciences.com. During the Event, the Company will present the corporate slide presentation attached as Exhibit 99.1 to this Current Report on Form 8-K, which is incorporated herein by reference.
Data from the MARINA-OLE trial is being presented in a poster presentation at the Muscular Dystrophy Association Clinical & Scientific Conference (the "MDA Conference"), being held from March 3, 2024 to March 6, 2024. When this poster presentation is made available at the MDA Conference, it will be made accessible under the “Publications” page in Avidity’s corporate website, at https://www.aviditybiosciences.com.
The information contained in this Item 7.01, including in Exhibit 99.1 hereto and on Avidity’s corporate website, is being “furnished” and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), is not subject to the liabilities of that section and is not deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
On March 4, 2024, the Company announced data from the MARINA-OLE trial of del-desiran showing reversal of disease progression in people living with DM1 across multiple key endpoints, including myotonia, muscle strength and patient reported activities of daily living (together, the "Key Endpoints"). The Key Endpoints correspond to those utilized in the ongoing natural history study called Establishing Biomarkers and Clinical Endpoints in Myotonic Dystrophy Type 1, over one year. The new data from the MARINA-OLE trial demonstrated:
•Long-term efficacy of del-desiran assessed from 12 participants on 4 mg/kg of del-desiran, showing improvements in:
◦Myotonia, as measured by video hand opening time (vHOT).
◦Measures of strength, including hand grip and the Quantitative Muscle Testing (QMT) total score which includes hand grip, elbow extension and elbow flexion, knee extension and knee flexion, and ankle dorsiflexion.
◦DM1-Activ, a patient reported outcome (PRO) that measures activities of daily living, such as taking a shower, visiting family or friends, and walking up stairs.
•Favorable safety and tolerability profile of del-desiran, with all adverse events considered mild or moderate, no study drug related serious adverse events and no discontinuations in the MARINA-OLE trial.
◦Based on over 265 infusions of del-desiran totaling 61.1 patient-years of exposure.
The Company plans to initiate its Phase 3 HARBOR™ trial of del-desiran in the second quarter of 2024. The Key Endpoints are the same endpoints that will be used in the Phase 3 HARBOR trial of del-desiran.
Forward-Looking Statements
Avidity cautions readers that statements contained in this Current Report on Form 8-K regarding matters that are not historical facts are forward-looking statements. These statements are based on the Company's current beliefs and expectations. Such forward-looking statements include, but are not limited to, statements regarding: the characterization of safety, tolerability and long-term efficacy data associated with del-desiran from the MARINA-OLE™ study; the impact of such data on the advancement of del-desiran; and a Phase 3 trial for del-desiran, the timing of its initiation and key endpoints to be used therein. This Current Report on Form 8-K also contains estimates and other statistical data made by independent parties and by us. This data involves a number of assumptions and limitations, and the reader is cautioned not to give undue weight to such estimates. The inclusion of forward-looking statements should not be regarded as a representation by Avidity that any of these plans will be achieved. Actual results may differ from those set forth in this Current Report on Form 8-K due to the risks and uncertainties inherent in Avidity's business, including, without limitation: Avidity may not be able to resolve the partial clinical hold related to the serious adverse event which occurred in the Phase 1/2 MARINA trial, which may result in delays in the clinical development of del-desiran; additional participant data related to del-desiran that continues to become available may be inconsistent with the data produced as of the date hereof, and further analysis of existing data and analysis of new data may lead to conclusions different from those established as of the date hereof; unexpected adverse side effects to, or inadequate efficacy of, Avidity's product candidates that may delay or limit their development, regulatory approval and/or commercialization, or may result in additional clinical holds which may not be timely lifted, recalls or product liability claims; Avidity is early in its development efforts; Avidity's approach to the discovery and development of product candidates based on its AOC platform is unproven, and the Company does not know whether it will be able to develop any products of commercial value; potential delays in the commencement, enrollment, data readouts and completion of preclinical studies or clinical trials; Avidity's dependence on third parties in connection with preclinical and clinical testing and product manufacturing; regulatory developments in the United States and foreign countries; and other risks described in Avidity's Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the Securities and Exchange Commission (SEC) on February 28, 2024, and in subsequent filings with the SEC. Avidity cautions readers not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and the Company undertakes no obligation to update such statements to reflect events that occur or circumstances that arise after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
| | | | | |
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
| | | | | | | | |
Exhibit Number | | Description |
| |
| 99.1 | | |
| |
| 104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | | | | | | | | | | | | | |
| | | | AVIDITY BIOSCIENCES, INC. |
| | | |
| Date: March 4, 2024 | | | | By: | | /s/ Michael F. MacLean |
| | | | | | Michael F. MacLean |
| | | | | | Chief Financial and Chief Business Officer |

Investor & Analyst Event Series – Volume 8 TRANSFORMING MYOTONIC DYSTROPHY Global Phase 3 HARBOR Trial & Long-term MARINA-OLE Data March 4, 2024 NASDAQ: RNA | aviditybio.com

2 Forward-Looking Statements We caution the reader that this presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements other than statements of historical fact contained in this presentation are forward-looking statements. Forward-looking statements include, but are not limited to, statements regarding: our future results of operations; our business strategy; the anticipated timing, design and conduct of our ongoing clinical trials; the timing of release of data from our ongoing clinical programs; the characterization of data and results from clinical trials, and conclusions drawn therefrom; research and development plans; plans and projected timelines for AOC 1001, AOC 1020 and AOC 1044; safety and tolerability profiles of our product candidates; the potential of the AOC platform; the ability of our product candidates to treat rare diseases; timing and likelihood of success; prospective products; product approvals; plans and objectives of management for future operations; and future results of anticipated product development efforts. In some cases, the reader can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The inclusion of forward-looking statements should not be regarded as a representation by Avidity that any of our plans will be achieved. Actual results may differ from those set forth in this presentation due to the risks and uncertainties inherent in our business and beyond our control, including, without limitation: we may not be able to fully resolve the partial clinical hold related to AOC 1001, which may result in delays in the clinical development of AOC 1001; additional requests for data in connection with the partial clinical hold or otherwise may result in significant additional expense and timing delays; data delivered to the FDA in connection with the partial clinical hold may not be satisfactory to the FDA; additional participant data related to AOC 1001 that continues to become available may be inconsistent with the data produced as of the most recent date cutoff, and further analysis of existing data and analysis of new data may lead to conclusions different from those established as of such date cutoff; unexpected adverse side effects or inadequate efficacy of our product candidates may delay or limit their development, regulatory approval and/or commercialization, or may result in additional clinical holds, recalls or product liability claims; we are early in our development efforts; our approach to the discovery and development of product candidates based on our AOC platform is unproven, and we do not know whether we will be able to develop any products of commercial value; the success of our preclinical studies and clinical trials for our product candidates; the results of early clinical trials are not necessarily predictive of future results; potential delays in the commencement, enrollment and completion of clinical trials; our dependence on third parties in connection with preclinical and clinical testing and product manufacturing; we may not realize the expected benefits of our collaborations with third parties, our existing collaborations may terminate earlier than expected or we may not be able to form new collaborations; regulatory developments in the United States and foreign countries, including acceptance of INDs and similar foreign regulatory submissions and our proposed design of future clinical trials; Fast Track Designation by the FDA may not lead to a faster development or regulatory review or approval process; our ability to obtain and maintain intellectual property protection for our product candidates and proprietary technologies; we may exhaust our capital resources sooner than we expect and fail to raise additional needed funds; and other risks described in our filings with the SEC, including under the heading “Risk Factors” in our Form 10-K for the year ended December 31, 2023, filed with the SEC on February 28, 2024, and in subsequent filings with the SEC. The reader is cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and the reader is cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy securities, nor shall there be any sale of securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.

3 OUR VISION To profoundly improve people’s lives by revolutionizing the delivery of RNA therapeutics Luke Living with DM1

4 AOC 1044 in DMD44 ~900 patients in U.S. Delivering in 2024: 3 Data Readouts in 3 Clinical Programs in 3 Rare Diseases AOC 1044 in DMD44 ~900 patients in U.S. 1020 in FSHD ~16,000-38, 00 patients in U.S. Anticipate Phase 1/2 FORTITUDE preliminary data in ~half of participants in Q2 2024 AOC 1001 in DM1 >40,000 patients in U.S. MARINA-OLE data in Q1 2024 On track for initiation of global Phase 3 HARBOR trial Q2 2024 Anticipate Phase 1/2 EXPLORE44 patient data in 2H 2024
5 Goals for the Day Share global Phase 3 HARBORTM trial design • Regulatory agreement on study design • On-track to initiate in Q2 2024 Present long-term data from MARINA-OLETM • Consistent and durable improvement in multiple measures, including vHOT, hand grip, muscle strength and activities of daily living • Favorable long-term safety and tolerability Debut data from END-DM1 natural history study Demonstrate reversal of disease progression compared to natural history delpacibart etedesiran abbreviation: del-desiran (formerly known as AOC 1001)

6 Avidity Management Team John W. Day, M.D., Ph.D., Professor of Neurology and Pediatrics, and Director, Division of Neuromuscular Medicine, Stanford University School of Medicine G U E S T S P E A K E R Steve Hughes, M.D. Chief Medical Officer Sarah Boyce President & CEO W. Michael Flanagan, Ph.D. Chief Scientific & Technical Officer Geoff Grande VP, Investor Relations & Corporate Communications Transforming Myotonic Dystrophy

7 Agenda/Outline Revolutionizing the Delivery of RNA Sarah Boyce, President & CEO Advancing to Phase 3: HARBORTM Trial Steve Hughes, M.D., CMO • MARINA-OLETM Long-term Efficacy and Safety Data John W. Day, MD, PhD, Professor of Neurology and Pediatrics and Director, Division of Neuromuscular Medicine, Stanford University School of Medicine • Delivering For People Living With DM1 Steve Hughes, M.D., CMO Closing Remarks Sarah Boyce, President & CEO Q&A Session Avidity Management & Dr. Day, Stanford Moderator: Geoff Grande, VP of IR/CC
8 0 APPROVED THERAPIES DM1: Significant Patient Burden and Unmet Need >40,000 PEOPLE WITH DM1 IN THE US • Underrecognized, progressive & often fatal neuromuscular disease that primarily affects skeletal, cardiac & smooth muscle • Increases in severity from generation to generation • Significant impact on quality of life • Del-desiran is designed to address root cause of DM1 Loraine, Kristl & Zen Living with DM1
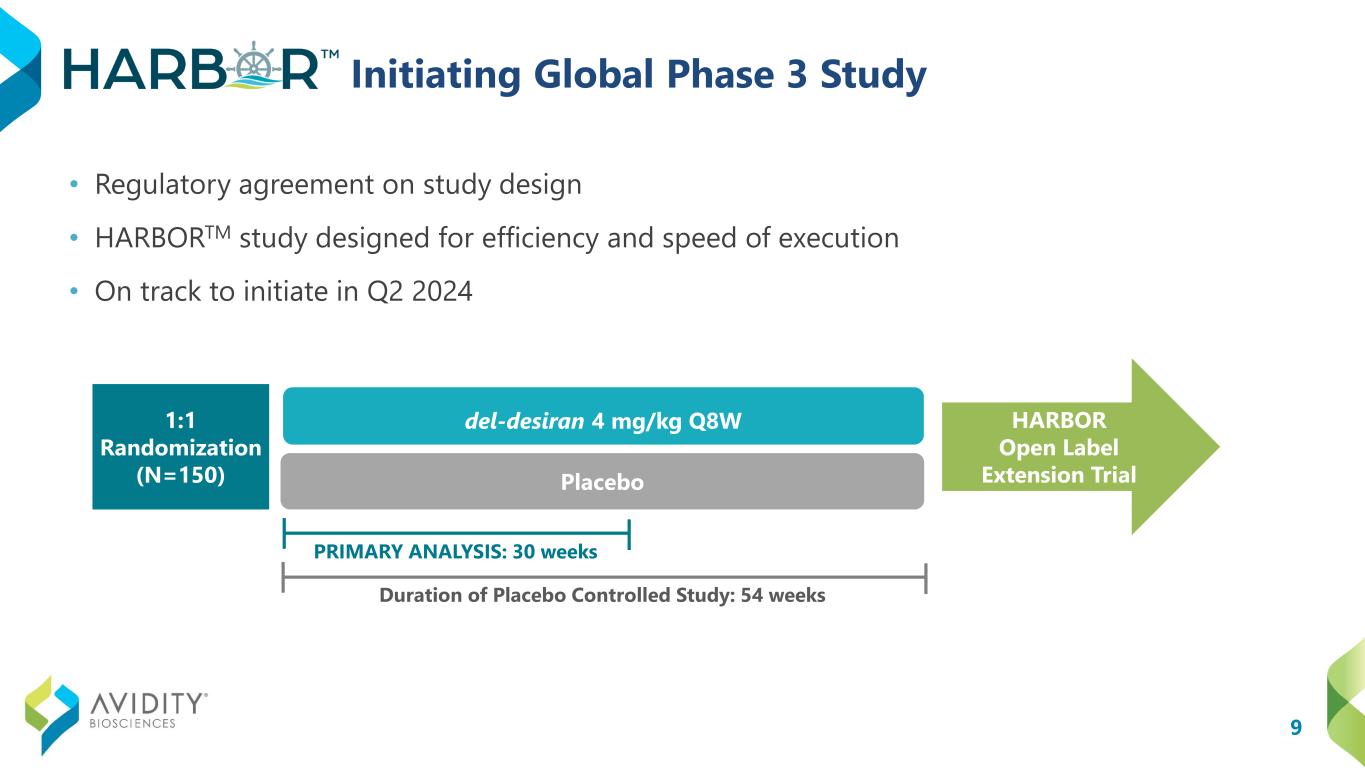
9 Initiating Global Phase 3 Study • Regulatory agreement on study design • HARBORTM study designed for efficiency and speed of execution • On track to initiate in Q2 2024 del-desiran 4 mg/kg Q8W Placebo Duration of Placebo Controlled Study: 54 weeks HARBOR Open Label Extension Trial 1:1 Randomization (N=150) PRIMARY ANALYSIS: 30 weeks
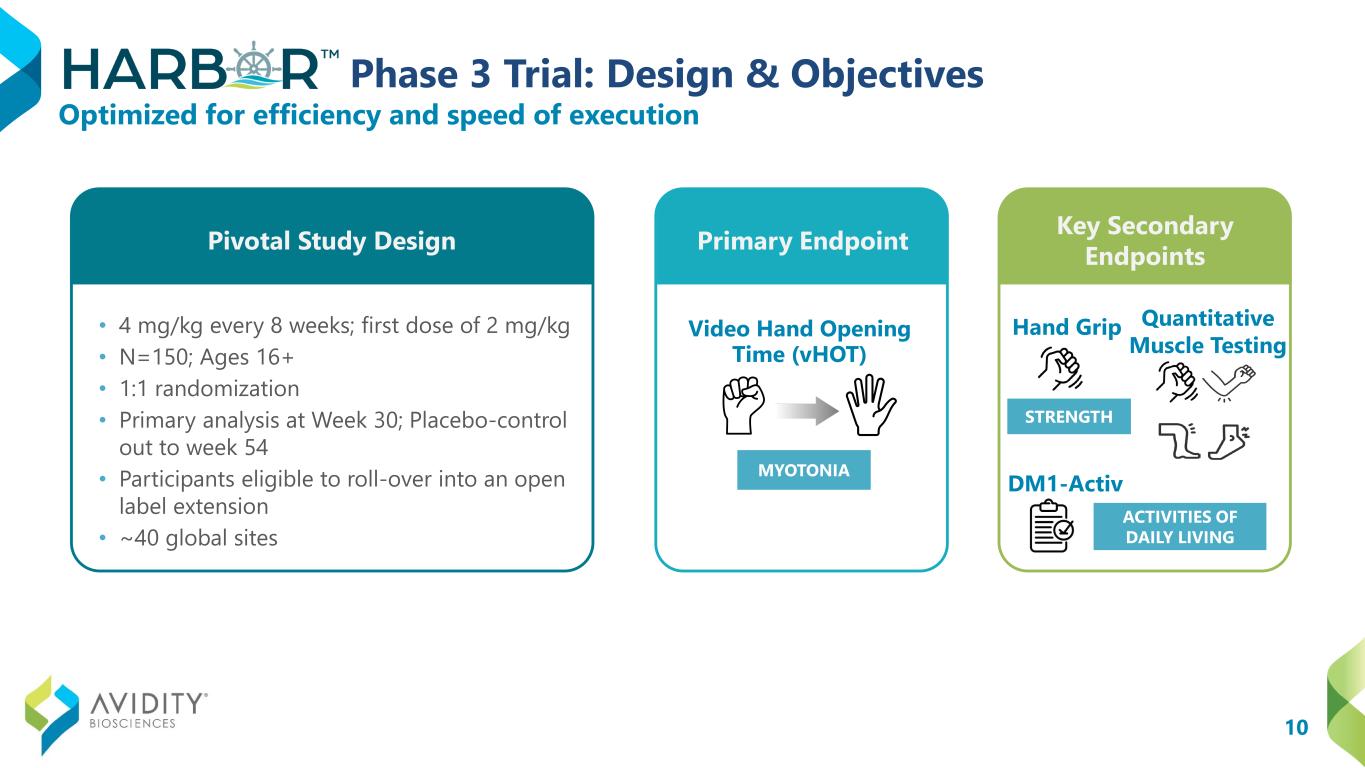
10 Phase 3 Trial: Design & Objectives • 4 mg/kg every 8 weeks; first dose of 2 mg/kg • N=150; Ages 16+ • 1:1 randomization • Primary analysis at Week 30; Placebo-control out to week 54 • Participants eligible to roll-over into an open label extension • ~40 global sites Video Hand Opening Time (vHOT) Hand Grip Quantitative Muscle Testing DM1-Activ Optimized for efficiency and speed of execution STRENGTH MYOTONIA ACTIVITIES OF DAILY LIVING Pivotal Study Design Primary Endpoint Key Secondary Endpoints

11 Agenda/Outline Revolutionizing the Delivery of RNA Sarah Boyce, President & CEO Advancing to Phase 3: HARBORTM Trial Steve Hughes, M.D., CMO • MARINA-OLETM Long-term Efficacy and Safety Data John W. Day, MD, PhD, Professor of Neurology and Pediatrics and Director, Division of Neuromuscular Medicine, Stanford University School of Medicine • Delivering For People Living With DM1 Steve Hughes, M.D., CMO Closing Remarks Sarah Boyce, President & CEO Q&A Session Avidity Management & Dr. Day, Stanford Moderator: Geoff Grande, VP of IR/CC

John W. Day, M.D., Ph.D., Stanford University School of Medicine Professor of Neurology, Pediatrics (Genetics) and Pathology, and Director of the Division of Neuromuscular Medicine John W. Day is Professor of Neurology, Pediatrics (Genetics) and Pathology, and Director of the Division of Neuromuscular Medicine at Stanford University. Dr. Day received his MD from the University of Minnesota, and PhD in Neuroscience from Albert Einstein College of Medicine, where he studied synaptic physiology and plasticity. After completing his neurology and neuromuscular training at UCSF he was recruited to the University of Minnesota, where, as Professor of Neurology, Pediatrics and Genetics, he founded and directed the Paul and Sheila Wellstone Muscular Dystrophy Center. In 2011 he was recruited to Stanford to establish a comprehensive Division of Neuromuscular Medicine. Dr. Day has investigated the genetic causes and multisystemic effects of neuromuscular disorders and has more than 30 years of experience designing and directing clinical trials of novel therapeutics including antisense oligonucleotides and gene replacement therapies. 12

Recognizing the spectrum of DM1 13
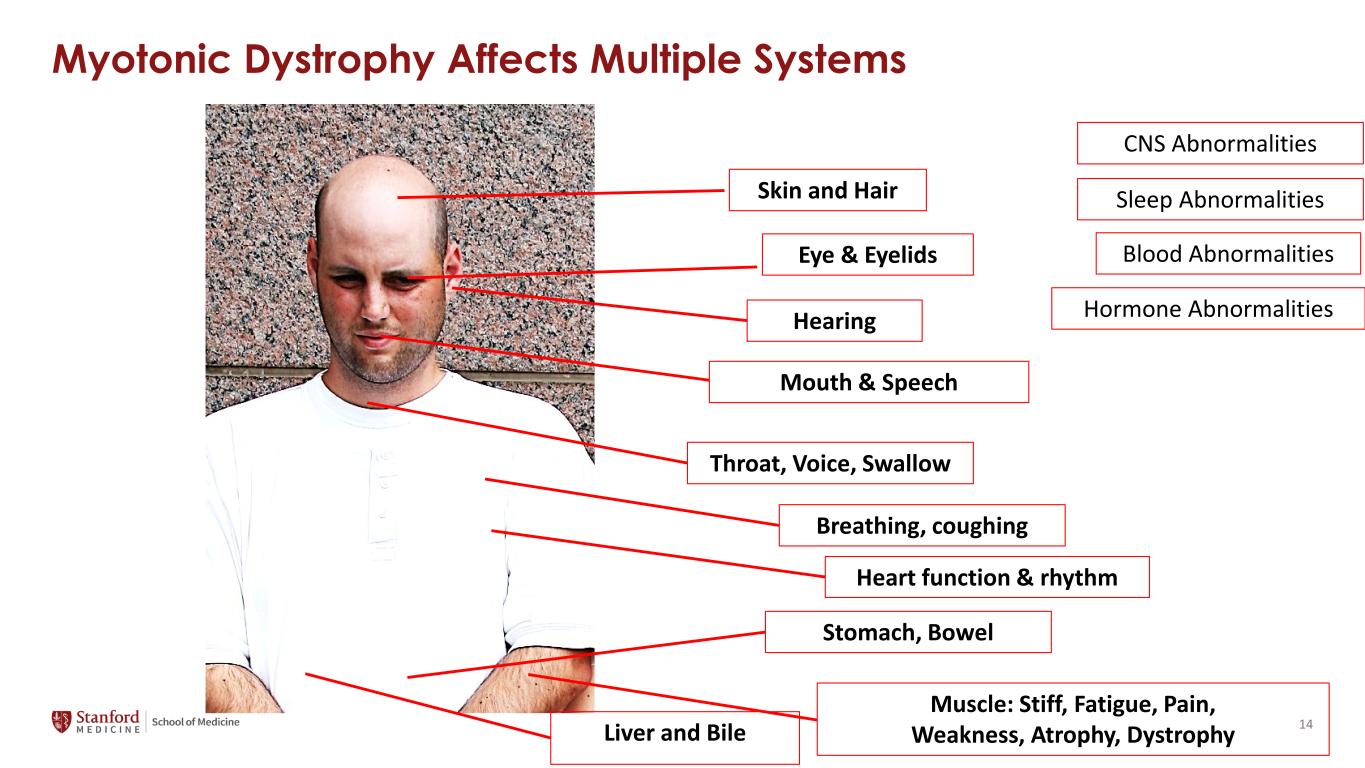
Myotonic Dystrophy Affects Multiple Systems Eye & Eyelids Hearing Mouth & Speech Skin and Hair Throat, Voice, Swallow Breathing, coughing Heart function & rhythm Stomach, Bowel Liver and Bile Muscle: Stiff, Fatigue, Pain, Weakness, Atrophy, Dystrophy Sleep Abnormalities Blood Abnormalities Hormone Abnormalities CNS Abnormalities 14
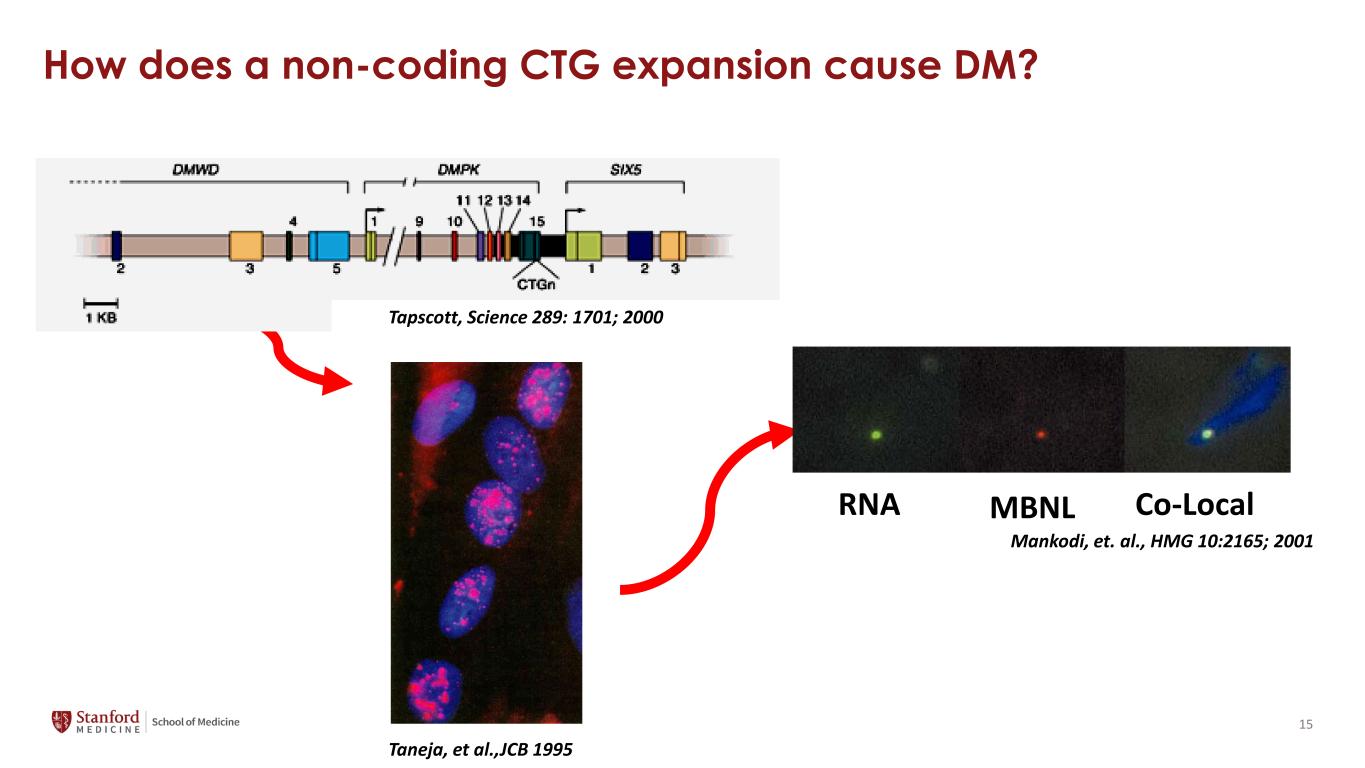
How does a non-coding CTG expansion cause DM? Taneja, et al.,JCB 1995 RNA MBNL Co-Local Mankodi, et. al., HMG 10:2165; 2001 Tapscott, Science 289: 1701; 2000 15
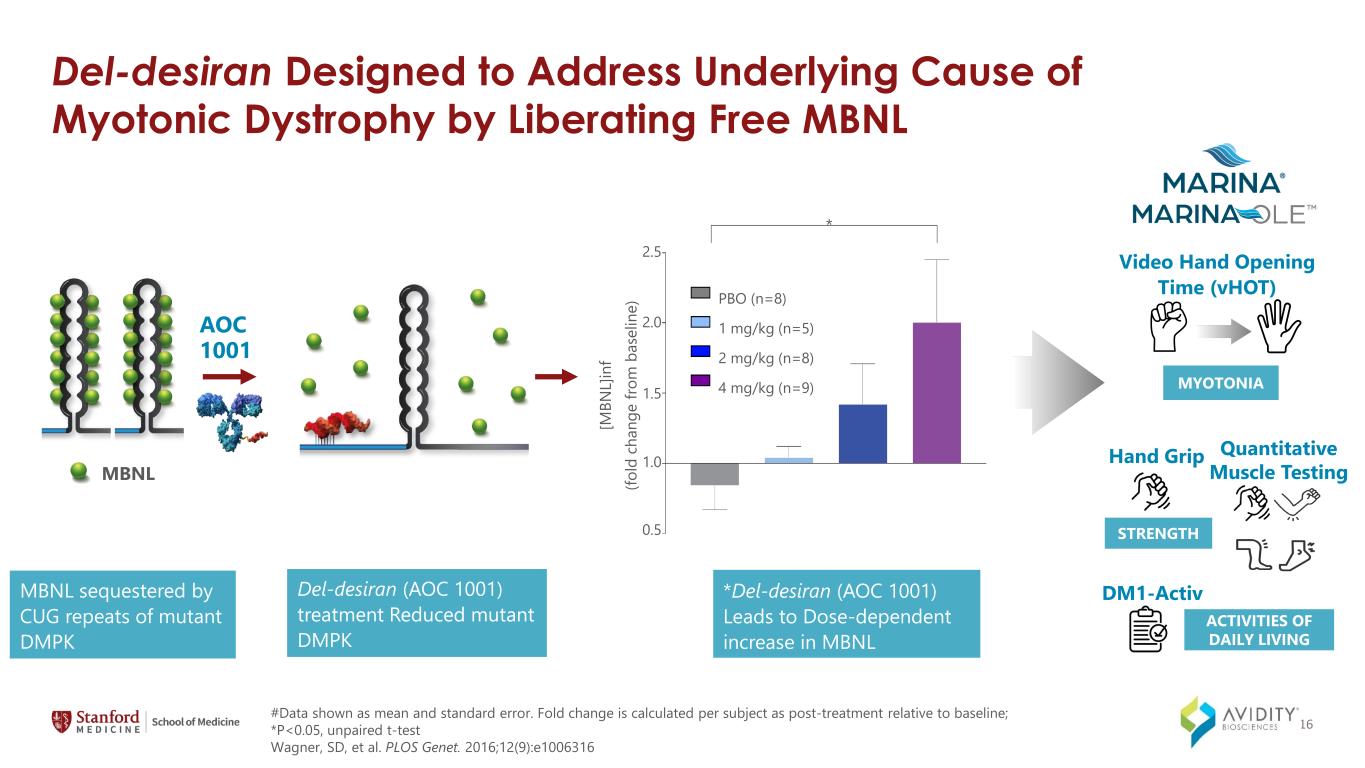
Del-desiran Designed to Address Underlying Cause of Myotonic Dystrophy by Liberating Free MBNL Del-desiran (AOC 1001) treatment Reduced mutant DMPK MBNL sequestered by CUG repeats of mutant DMPK AOC 1001 MBNL [M BN L] in f (fo ld c ha ng e fro m b as el in e) 2.5 2.0 1.5 1.0 0.5 * PBO (n=8) 1 mg/kg (n=5) 2 mg/kg (n=8) 4 mg/kg (n=9) #Data shown as mean and standard error. Fold change is calculated per subject as post-treatment relative to baseline; *P<0.05, unpaired t-test Wagner, SD, et al. PLOS Genet. 2016;12(9):e1006316 *Del-desiran (AOC 1001) Leads to Dose-dependent increase in MBNL Video Hand Opening Time (vHOT) MYOTONIA Hand Grip Quantitative Muscle Testing STRENGTH DM1-Activ ACTIVITIES OF DAILY LIVING 16

Long-term Safety & Efficacy Data from MARINA-OLE Trial in Patients with DM1 17
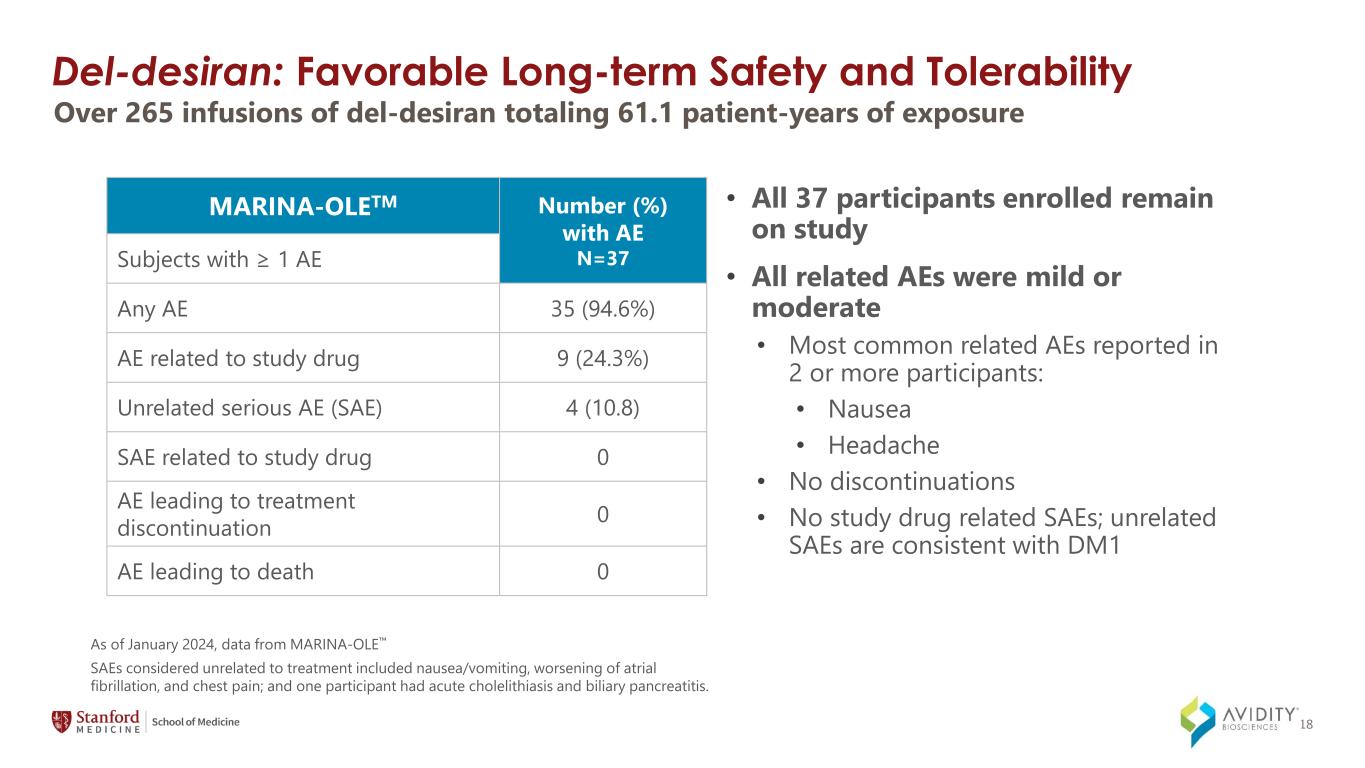
18 Del-desiran: Favorable Long-term Safety and Tolerability As of January 2024, data from MARINA-OLE • All 37 participants enrolled remain on study • All related AEs were mild or moderate • Most common related AEs reported in 2 or more participants: • Nausea • Headache • No discontinuations • No study drug related SAEs; unrelated SAEs are consistent with DM1 SAEs considered unrelated to treatment included nausea/vomiting, worsening of atrial fibrillation, and chest pain; and one participant had acute cholelithiasis and biliary pancreatitis. Over 265 infusions of del-desiran totaling 61.1 patient-years of exposure MARINA-OLETM Number (%) with AE N=37Subjects with ≥ 1 AE Any AE 35 (94.6%) AE related to study drug 9 (24.3%) Unrelated serious AE (SAE) 4 (10.8) SAE related to study drug 0 AE leading to treatment discontinuation 0 AE leading to death 0

19 • Non-interventional NHS aimed to advance the understanding of disease progression in DM1 patients • Focuses on clinical outcome assessments to support development of therapies for DM1 • 700 patient, 2 year study, ~ 20 centers • Designed and run by the Myotonic Dystrophy Clinical Research Network (DMCRN) • Supported by FDA, MDA, MDF; Avidity is one of several sponsoring organizations END-DM1 Natural History Study: Understanding DM1 Disease Progression
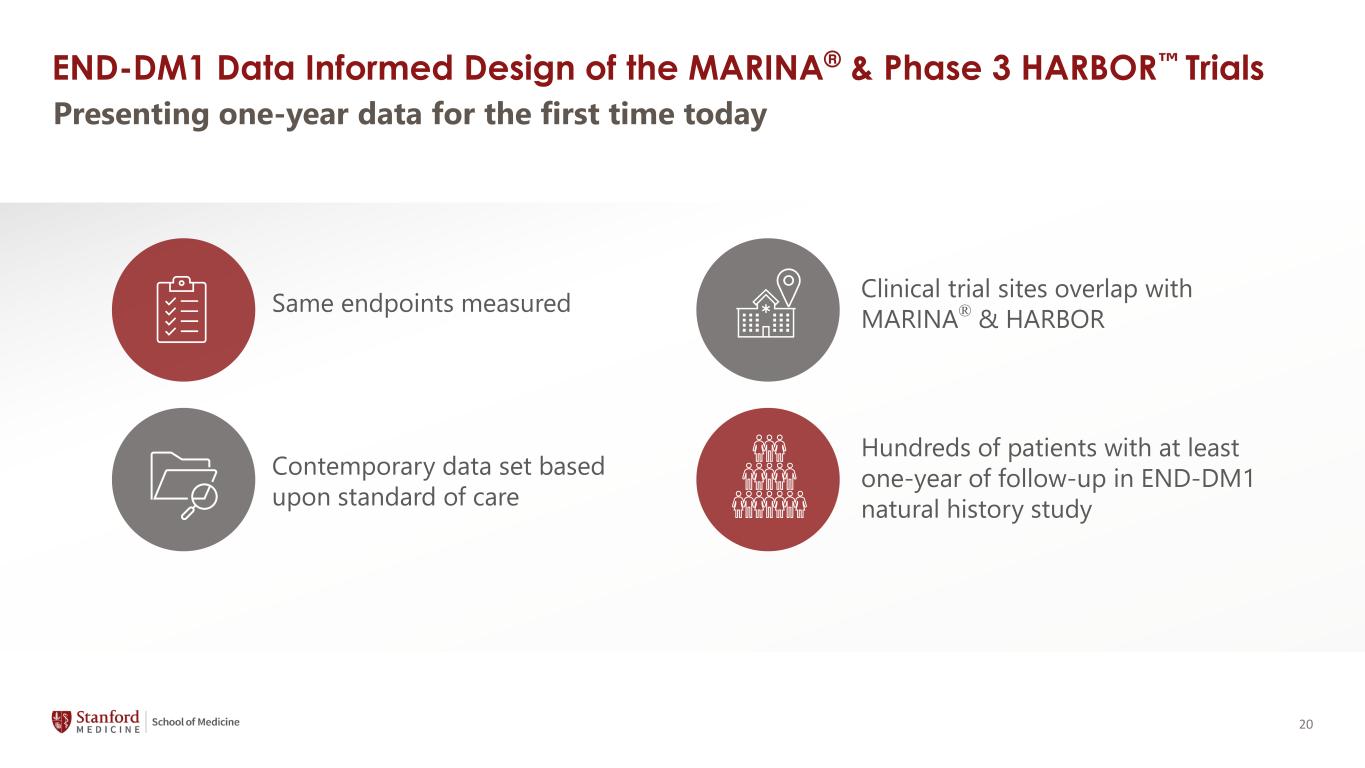
20 END-DM1 Data Informed Design of the MARINA® & Phase 3 HARBOR Trials Same endpoints measured Clinical trial sites overlap with MARINA® & HARBOR Contemporary data set based upon standard of care Hundreds of patients with at least one-year of follow-up in END-DM1 natural history study Presenting one-year data for the first time today
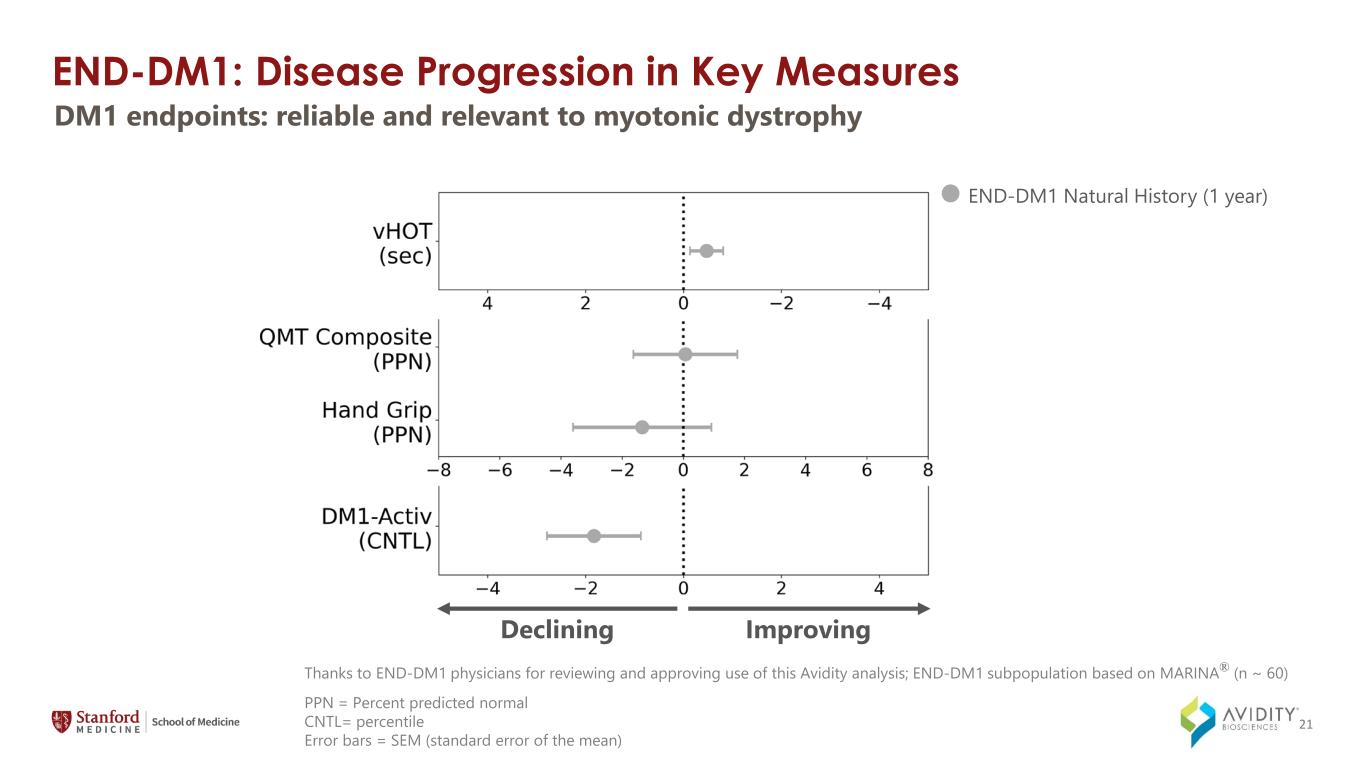
END-DM1: Disease Progression in Key Measures END-DM1 Natural History (1 year) 4 mg/kg Q12W (1 year) ImprovingDeclining DM1 endpoints: reliable and relevant to myotonic dystrophy PPN = Percent predicted normal CNTL= percentile Error bars = SEM (standard error of the mean) Thanks to END-DM1 physicians for reviewing and approving use of this Avidity analysis; END-DM1 subpopulation based on MARINA® (n ~ 60) 21
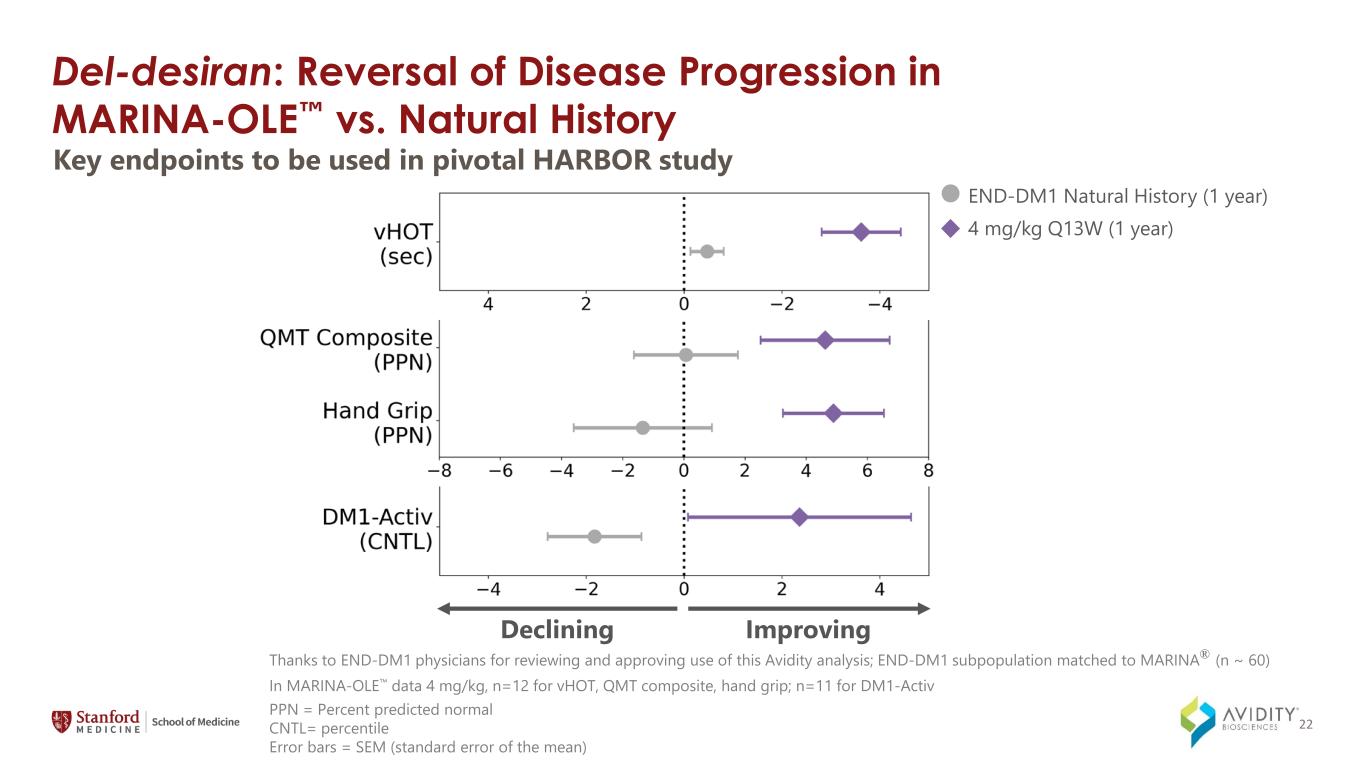
Del-desiran: Reversal of Disease Progression in MARINA-OLE vs. Natural History Key endpoints to be used in pivotal HARBOR study ImprovingDeclining PPN = Percent predicted normal CNTL= percentile Error bars = SEM (standard error of the mean) Thanks to END-DM1 physicians for reviewing and approving use of this Avidity analysis; END-DM1 subpopulation matched to MARINA® (n ~ 60) In MARINA-OLE data 4 mg/kg, n=12 for vHOT, QMT composite, hand grip; n=11 for DM1-Activ END-DM1 Natural History (1 year) 4 mg/kg Q13W (1 year) 22
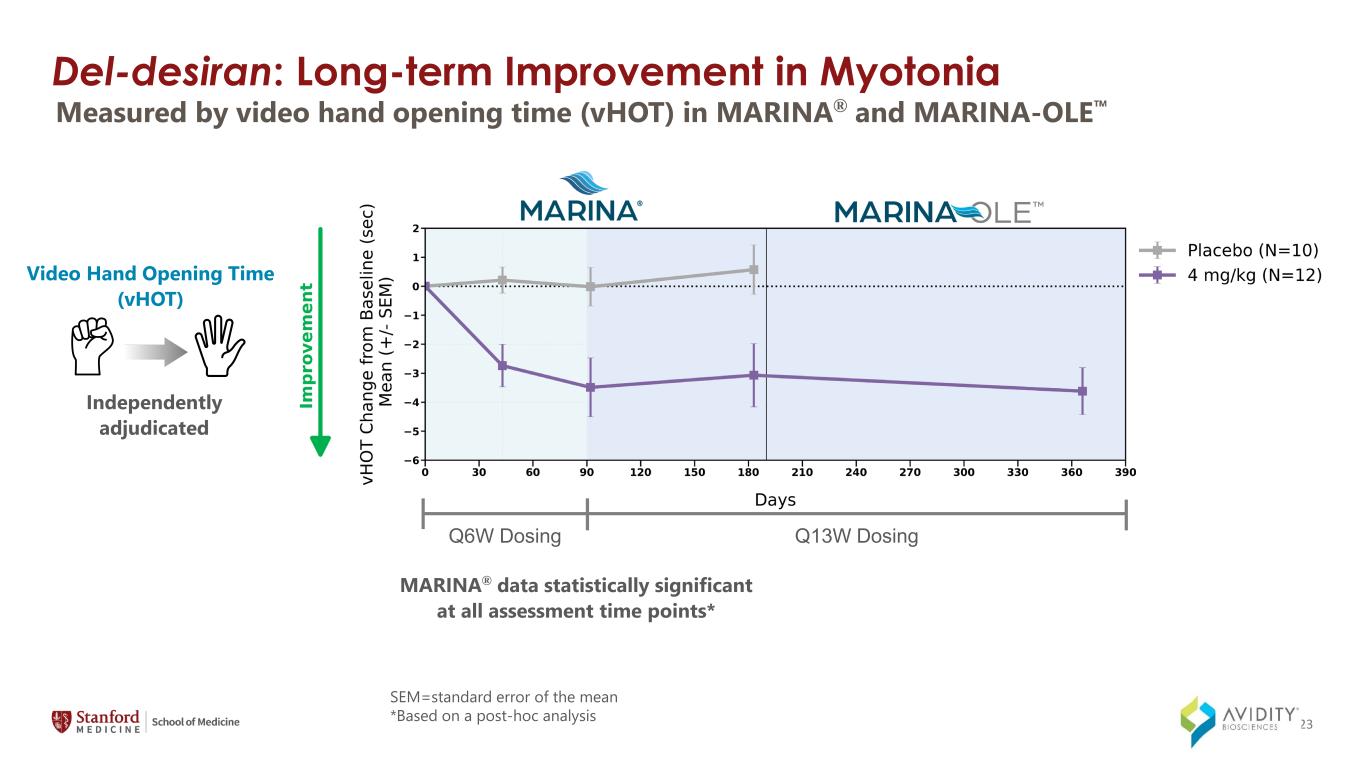
Del-desiran: Long-term Improvement in Myotonia Measured by video hand opening time (vHOT) in MARINA® and MARINA-OLE MARINA® data statistically significant at all assessment time points* Video Hand Opening Time (vHOT) Independently adjudicated Q6W Dosing Q13W Dosing SEM=standard error of the mean *Based on a post-hoc analysis 23
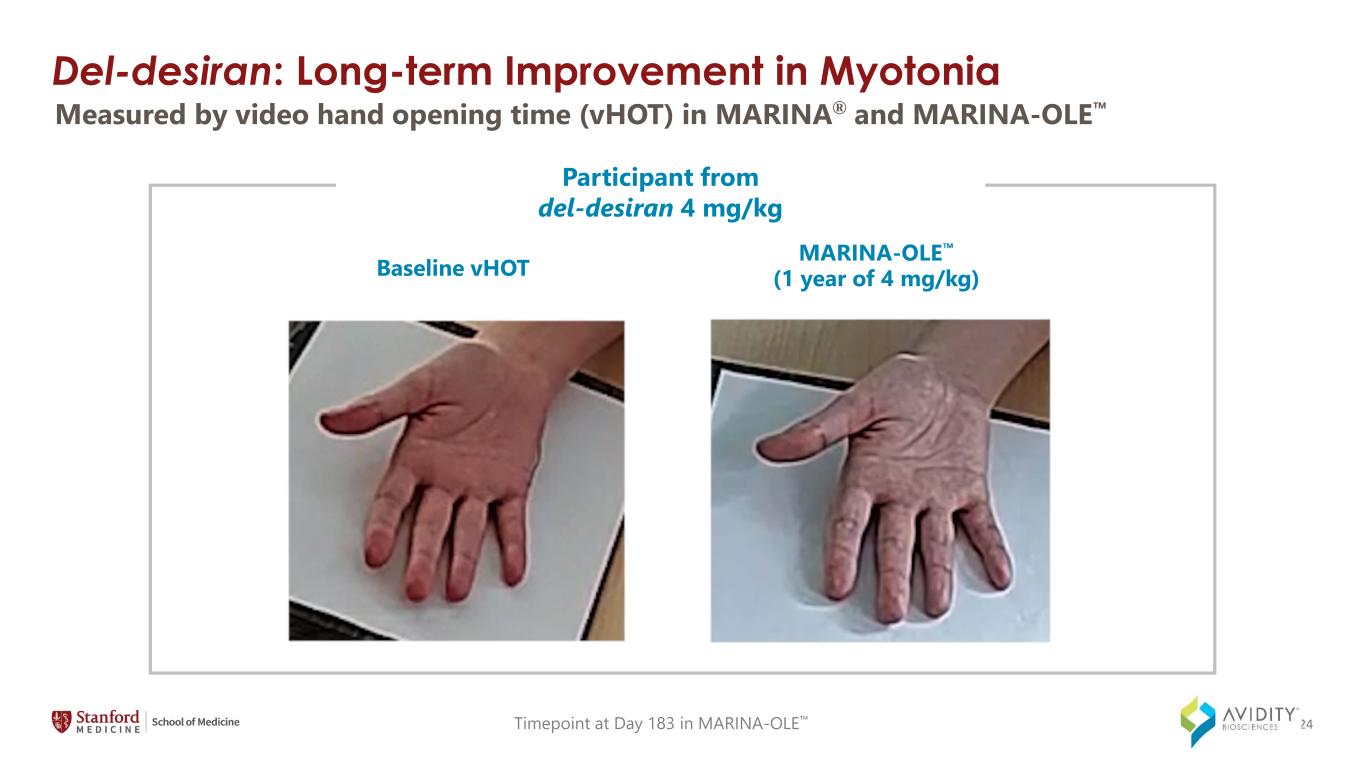
Del-desiran: Long-term Improvement in Myotonia Baseline vHOT MARINA-OLE (1 year of 4 mg/kg) ` Participant from del-desiran 4 mg/kg Timepoint at Day 183 in MARINA-OLE Measured by video hand opening time (vHOT) in MARINA® and MARINA-OLE 24
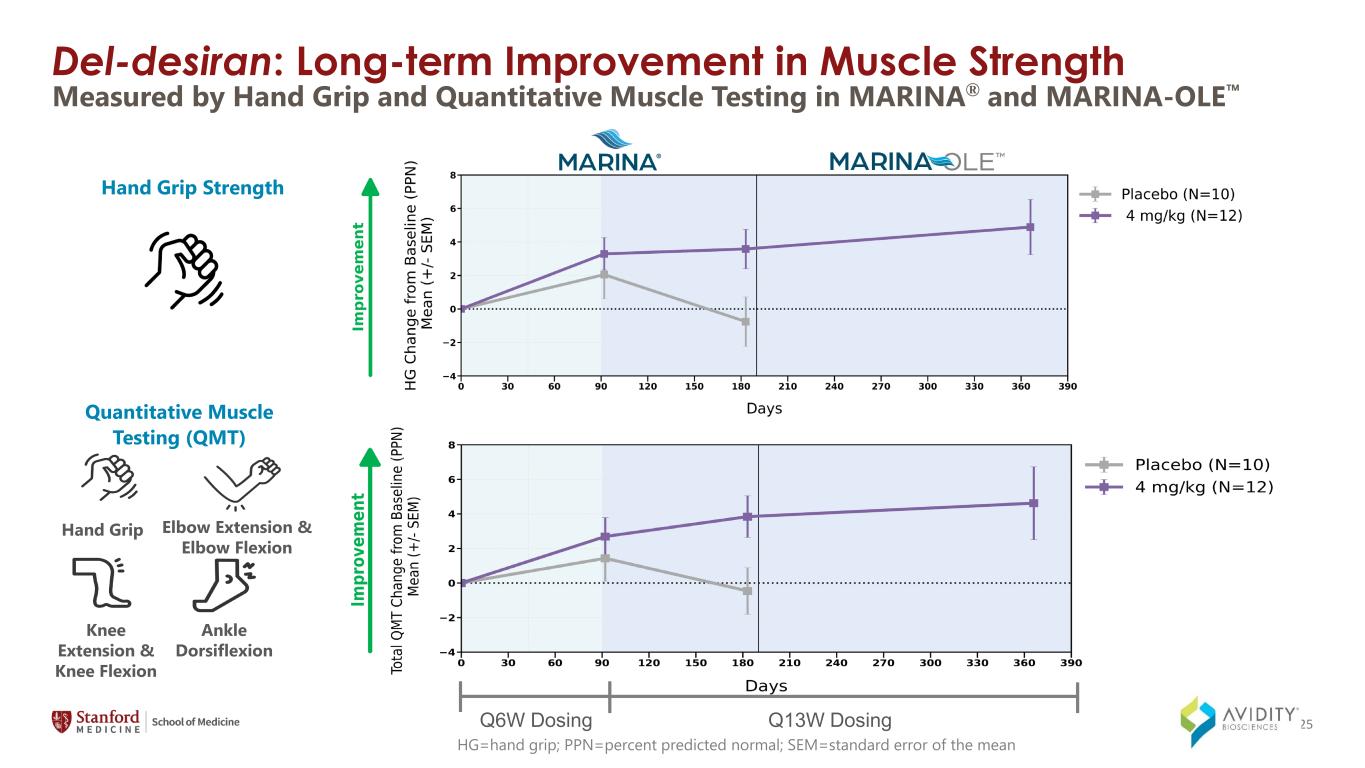
Del-desiran: Long-term Improvement in Muscle Strength HG=hand grip; PPN=percent predicted normal; SEM=standard error of the mean Quantitative Muscle Testing (QMT) Hand Grip Elbow Extension & Elbow Flexion Knee Extension & Knee Flexion Ankle Dorsiflexion Hand Grip Strength Q6W Dosing Q13W Dosing Measured by Hand Grip and Quantitative Muscle Testing in MARINA® and MARINA-OLE 25
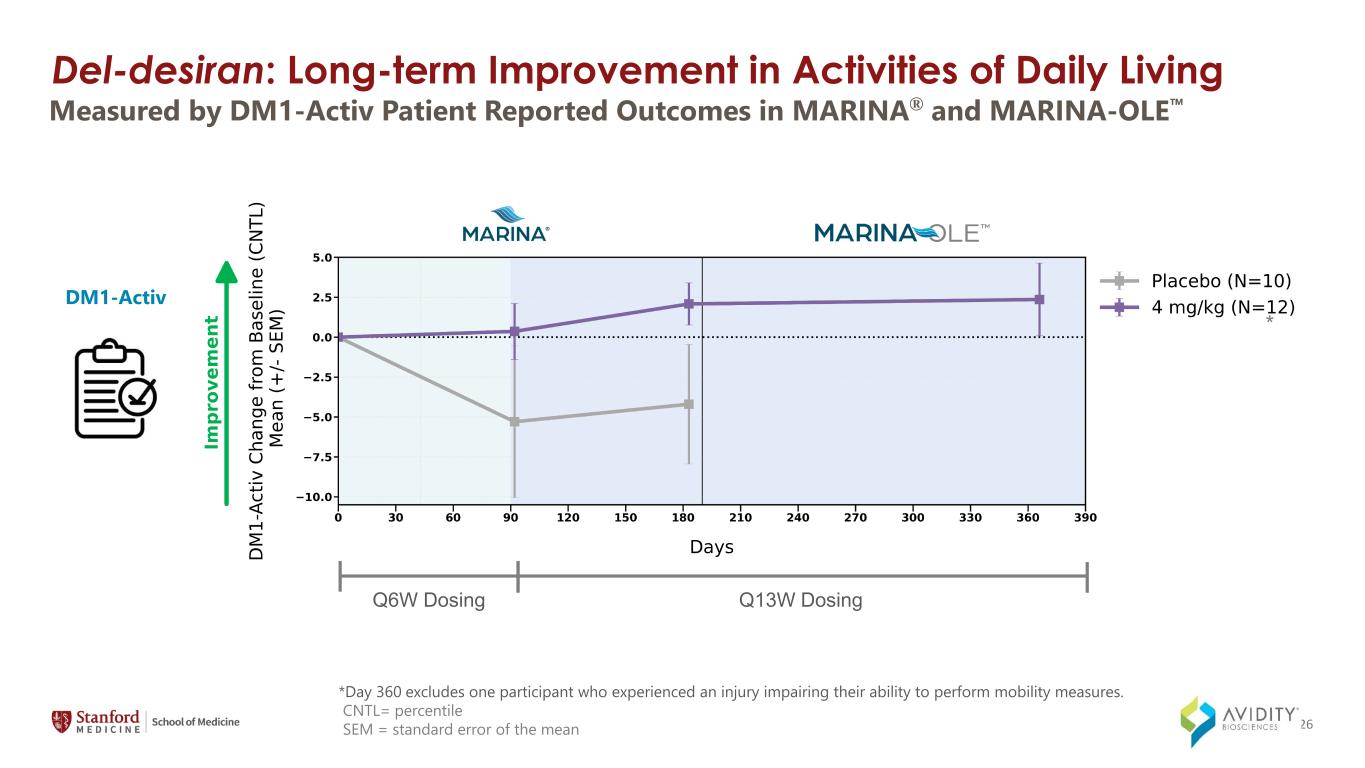
*Day 360 excludes one participant who experienced an injury impairing their ability to perform mobility measures. CNTL= percentile SEM = standard error of the mean Measured by DM1-Activ Patient Reported Outcomes in MARINA® and MARINA-OLE DM1-Activ Q6W Dosing Q13W Dosing * 26 Del-desiran: Long-term Improvement in Activities of Daily Living
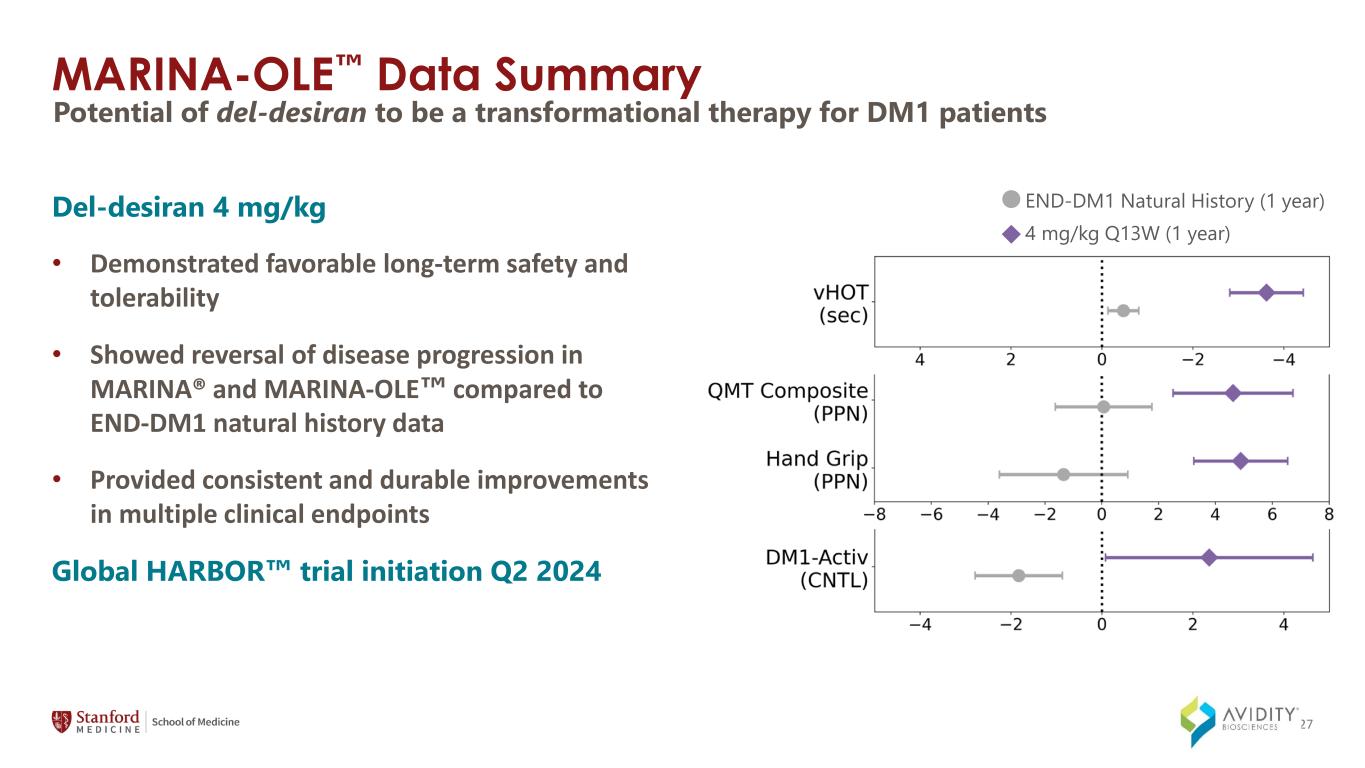
27 Del-desiran 4 mg/kg • Demonstrated favorable long-term safety and tolerability • Showed reversal of disease progression in MARINA® and MARINA-OLE compared to END-DM1 natural history data • Provided consistent and durable improvements in multiple clinical endpoints Global HARBOR trial initiation Q2 2024 MARINA-OLE Data Summary Potential of del-desiran to be a transformational therapy for DM1 patients END-DM1 Natural History (1 year) 4 mg/kg Q13W (1 year)

28 Agenda/Outline Revolutionizing the Delivery of RNA Sarah Boyce, President & CEO Advancing to Phase 3: HARBORTM Trial Steve Hughes, M.D., CMO • MARINA-OLE Long-term Efficacy and Safety Data John W. Day, MD, PhD, Professor of Neurology and Pediatrics and Director, Division of Neuromuscular Medicine, Stanford University School of Medicine • Delivering For People Living With DM1 Steve Hughes, M.D., CMO Closing Remarks Sarah Boyce, President & CEO Q&A Session Avidity Management & Dr. Day, Stanford Moderator: Geoff Grande, VP of IR/CC

29 Patient Experience: Impact of del-desiran on their life I started this drug in June and like, two weeks after I took the first infusion, I went to open up a pop bottle, which I never would've been able to do. It was a twist pop bottle…and it opened right up. My strength was better, my outlook was better, my hands were working. I had more strength, and I could stretch them out. I could open things and I could turn door knobs and all these things that were harder. Like, my upper arm strength was better. I could walk better. I didn't need to wear my neck brace all the time and everything just improved a lot. “ ”

30 Patient Experience: Impact of del-desiran on their life Before the study I couldn't stand on my toes and since I've been going back to working out, I can actually stand on my toes again. So hopefully building up some strength. The myotonia, if I would make a fist, I wouldn't be able to open my hand…I was able to squeeze my fist and open my hand with no problems. My tongue would cramp up when I would speak, and I have not had any signs of that happening since the very first dose. “ ”

31 Patient Experience: Impact of del-desiran on their life I've noticed a really big difference in the fact that I used to be a really active person before I got more symptomatic. After a few rounds of the infusion, I've actually been able to get back to the gym and start working out, working with a trainer. That's all because my mobility has definitely increased. My range of motion has also increased. I think that it's amazing that when I was diagnosed, I was told there's no treatment, no cure. The study has given me a lot of hope. I would love for that to be able to be shared with other people in the community who have DM1. “ ”

32 Agenda/Outline Revolutionizing the Delivery of RNA Sarah Boyce, President & CEO Advancing to Phase 3: HARBORTM Trial Steve Hughes, M.D., CMO • MARINA-OLE Long-term Efficacy and Safety Data John W. Day, MD, PhD, Professor of Neurology and Pediatrics and Director, Division of Neuromuscular Medicine, Stanford University School of Medicine • Delivering For People Living With DM1 Steve Hughes, M.D., CMO Closing Remarks Sarah Boyce, President & CEO Q&A Session Avidity Management & Dr. Day, Stanford Moderator: Geoff Grande, VP of IR/CC

33 OUR VISION To profoundly improve people’s lives by revolutionizing the delivery of RNA therapeutics Luke Living with DM1

Investor & Analyst Event Series – Volume 8 TRANSFORMING MYOTONIC DYSTROPHY Global Phase 3 HARBOR Trial & Long-term MARINA-OLE Data Q&A March 4, 2024 NASDAQ: RNA | aviditybio.com

Investor & Analyst Event Series – Volume 8 TRANSFORMING MYOTONIC DYSTROPHY Global Phase 3 HARBOR Trial & Long-term MARINA-OLE Data March 4, 2024 NASDAQ: RNA | aviditybio.com
v3.24.0.1
Cover
|
Mar. 04, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Document Period End Date |
Mar. 04, 2024
|
| Entity Registrant Name |
AVIDITY BIOSCIENCES, INC.
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity File Number |
001-39321
|
| Entity Tax Identification Number |
46-1336960
|
| Entity Address, Address Line One |
10578 Science Center Drive
|
| Entity Address, Address Line Two |
Suite 125
|
| Entity Address, City or Town |
San Diego
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
92121
|
| City Area Code |
858
|
| Local Phone Number |
401-7900
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
RNA
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| Entity Central Index Key |
0001599901
|
| Amendment Flag |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Avidity Biosciences (NASDAQ:RNA)
Historical Stock Chart
From Mar 2024 to Apr 2024
Avidity Biosciences (NASDAQ:RNA)
Historical Stock Chart
From Apr 2023 to Apr 2024