FDA Expands Emergency Use for Lilly Antibody Combination for Covid-19 to Include Infants
04 December 2021 - 7:33AM
Dow Jones News
By Josh Beckerman
The U.S. Food and Drug Administration expanded its emergency use
authorization for a combination of two Eli Lilly & Co.
monoclonal antibodies for treating mild to moderate Covid-19,
expanding authorization to all ages including newborns for those
who are at high risk for progression to severe Covid-19.
The combination of bamlanivimab and etesevimab was previously
authorized for people 12 years of age and older weighing at least
40 kilograms.
Lilly said it is "working quickly to understand neutralization
activity of our therapies on the Omicron variant of concern."
Write to Josh Beckerman at josh.beckerman@wsj.com
(END) Dow Jones Newswires
December 03, 2021 15:18 ET (20:18 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
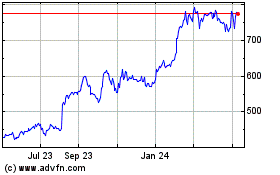
Eli Lilly (NYSE:LLY)
Historical Stock Chart
From Mar 2024 to Apr 2024
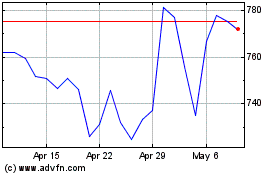
Eli Lilly (NYSE:LLY)
Historical Stock Chart
From Apr 2023 to Apr 2024