Health Canada Authorizes Omicron Subvariant Booster Vaccine
08 October 2022 - 4:23AM
Dow Jones News
By Adriano Marchese
Canada's health regulator on Friday said it authorized a second
dose of Pfizer-BioNTech's vaccine that targets two Omicron
subvariants of Covid-19.
Health Canada said the booster vaccine targets Omicron BA.4 and
BA.5 subvariants and it has authorized shots for individuals 12
years of age or older.
The regulator said that the authorization follows a thorough and
independent scientific review of the evidence and determined that
it is safe and effective, with similar safety profile as its
original Pfizer-BioNTech Comirnaty vaccine.
Pfizer-BioNTech is still required to continue to provide
information to Health Canada on the safety and efficacy of the
bivalent vaccine, the regulator said.
Health Canada said that the continuous flow of data and studies
ensures that the benefits of the vaccine continue to outweigh the
risks.
Write to Adriano Marchese at adriano.marchese@wsj.com
(END) Dow Jones Newswires
October 07, 2022 13:08 ET (17:08 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
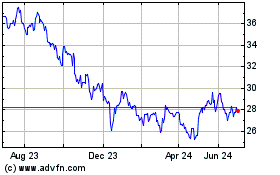
Pfizer (NYSE:PFE)
Historical Stock Chart
From Mar 2024 to Apr 2024
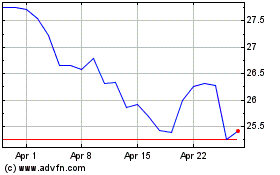
Pfizer (NYSE:PFE)
Historical Stock Chart
From Apr 2023 to Apr 2024