Filed Pursuant to Rule
253(g)(1)
File Number: 024-12505
OFFERING CIRCULAR SUPPLEMENT
NO. 1
Date of Qualification
of the Offering Circular: December 10, 2024
Supplement dated February
24, 2025
This document (the “Supplement”) supplements
the Offering Statement filed on Form 1-A of Regen Biopharma, Inc. (the “Company”), originally filed on September 16, 2024
and amended on November 21,2024 (the “Offering Circular”) relating to the offer and sale by us of 10,000,000 shares of our
common stock . Any inconsistent information made by us in a previous offering circular supplement or post-qualification amendment is
modified or superseded by the information contained in this Supplement. Unless otherwise defined in this Supplement, capitalized terms
used herein shall have the same meanings as set forth in the Offering Circular, including the disclosures incorporated by reference therein.
The purpose of this supplement is to provide
a list of the States where the Offering has been registered:
Colorado registered as of December 10, 2024
Delaware registered as of January 3, 2025
New York registered as of January 8, 2025
SEC Disclaimer
An offering statement regarding the offering described
in the Offering Circular has been filed with the Commission. The Commission has qualified that offering statement, which
only means that the Company may make sales of the securities described by that offering statement. It does not mean that the Commission
has approved, passed upon the merits, or passed upon the accuracy or completeness of the information in the offering statement. You should
read the Offering Circular before making any investment.
This Supplement to the Offering Circular does
not otherwise modify or update disclosure in, or exhibits to, the original Offering Circular. This Supplement to the Offering Circular
should be read in conjunction with the Offering Circular and is qualified by reference to the Offering Circular except to the extent
that the information contained herein supplements the information contained in the Offering Circular.
This Offering Circular shall not constitute
an offer to sell or the solicitation of an offer to buy nor there would any sales of these securities in any state in which such
offer, solicitation or sale be unlawful before registration or qualification under the laws of any such state.
| OFFERING
CIRCULAR |
ACCREDITED
INVESTORS ONLY |
REGEN
BIOPHARMA, INC.
Up
to 10,000,000 Shares of Common Stock
Offering
under Tier I of Regulation A+ of the Securities and Exchange Commission
10,000,000
Shares of Common Stock, $0.0001 Par Value,
Offering
Amount: A Maximum of $400,000
Price:
$0.04 per share of Common Stock
Minimum
Purchase Amount: 0
Sales
will be made to Accredited Investors Only
You
may only rely on the information contained in this Offering Circular or that we have referred you to. We have not authorized anyone to
provide you with different information. This Offering Circular does not constitute an offer to sell or a solicitation of an offer to
buy any securities other than the common stock offered by this Offering Circular. This Offering Circular does not constitute an offer
to sell or a solicitation of an offer to buy any common stock in any circumstances in which such offer or solicitation is unlawful. Neither
the delivery of this Offering Circular nor any sale made in connection with this Offering Circular shall, under any circumstances, create
any implication that there has been no change in our affairs since the date of this Offering Circular is correct as of any time after
its date.
There is no minimum proceeds threshold for the
offering. The offering will commence within two days of qualification by the United States Securities and Exchange Commission and will
terminate 90 days after qualification.
The Company will retain all proceeds received from the shares sold in this offering. The Company has not made any arrangements to place
the proceeds in an escrow or trust account. Any proceeds received in this offering may be immediately used by the Company in its sole
discretion. There are no minimum purchase requirements for each investor. All proceeds retained by the Company may not be sufficient
to continue operations.
THIS
INVESTMENT INVOLVES A HIGH DEGREE OF RISK. BEFORE INVESTING, YOU SHOULD CAREFULLY READ THIS PROSPECTUS AND, PARTICULARLY, THE RISK FACTORS
SECTION, BEGINNING ON PAGE 7.
NEITHER
THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED
IF THIS OFFERING CIRCULAR IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
| | |
Price
to Public | | |
Underwriting
Discount and
Commissions | | |
Proceeds
to
Issuer | |
| Per
Share | |
$ | 0.04 | | |
| 0 | | |
$ | 0.04 | |
| Total
Minimum | |
$ | 0.00 | | |
| 0 | | |
$ | 0.00 | |
| Total
Maximum | |
$ | 400,000 | | |
| 0 | | |
$ | 400,000 | |
The
date of this Offering Circular is November 21, 2024.
THIS
OFFERING CIRCULAR FOLLOWS THE OFFERING CIRCULAR FORMAT DESCRIBED IN PART II OF SEC FORM 1-A.
Contents
In
this Offering Circular, the terms “Regen Biopharma, Inc.. “, “Regen”, “Company”, “we”,
or “our”, unless the context otherwise requires, mean Regen Biopharma, Inc., a Nevada corporation and its wholly owned subsidiary
KCL, Therapeutics, Inc., a Nevada corporation.
CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This
Offering Circular contains statements that are considered forward-looking statements. Forward-looking statements give the Company’s
current expectations, plans, objectives, assumptions or forecasts of future events. All statements other than statements of current or
historical fact contained in this Offering Circular, including statements regarding the Company’s future financial position, business
strategy, budgets, projected costs and plans and objectives of management for future operations, are forward-looking statements. In some
cases, you can identify forward-looking statements by terminology such as “anticipate,” “estimate,” “plans,”
“potential,” “projects,” “ongoing,” “expects,” “management believes,” “we
believe,” “we intend,” and similar expressions. These statements are based on the Company’s current plans and
are subject to risks and uncertainties, and as such the Company’s actual future activities and results of operations may be materially
different from those set forth in the forward looking statements. Any or all of the forward-looking statements in this Offering Circular
may turn out to be inaccurate and as such, you should not place undue reliance on these forward-looking statements. The Company has based
these forward-looking statements largely on its current expectations and projections about future events and financial trends that it
believes may affect its financial condition, results of operations, business strategy and financial needs. The forward-looking statements
can be affected by inaccurate assumptions or by known or unknown risks, uncertainties and assumptions due to a number of factors, including:
| |
● |
dependence
on key personnel; |
| |
● |
degree
of success of research and development programs |
| |
● |
the
operation of our business; and |
| |
● |
general
economic conditions |
These
forward-looking statements speak only as of the date on which they are made, and except to the extent required by federal securities
laws, we undertake no obligation to update any forward-looking statements to reflect events or circumstances after the date on which
the statement is made or to reflect the occurrence of unanticipated events. In addition, we cannot assess the impact of each factor on
our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained
in any forward-looking statements. All subsequent written and oral forward-looking statements attributable to the Company or persons
acting on its behalf are expressly qualified in their entirety by the cautionary statements contained in this Offering Circular.
SUMMARY
ABOUT
US
We
were incorporated April 24, 2012 under the laws of the State of Nevada. We intend to engage primarily in the development of regenerative
medical applications which we intend to license, develop internally or acquire outright from other entities up to the point of successful
completion of Phase I and or Phase II clinical trials after which we would either attempt to sell or license those developed applications
or, alternatively, advance the application further to Phase III clinical trials. The primary factor to be considered by us in arriving
at a decision to advance an application further to Phase III clinical trials would be a greater than anticipated indication of efficacy
seen in Phase I trials.
The
Company has the following therapies in development:
HemaXellarate
: HemaXellarate is a cellular composition of autologous stromal vascular fraction derived from adipose tissue. HemaXellarate contains
endothelial progenitor cells as well as mesenchymal stem cells. It is believed by the Company that once re-infused into the patient,
the patient’s bone marrow will regenerate and begin to function normally.
dCellVax:
dCellVax is comprised of autologous dendritic cells which have been treated with an siRNA inhibitor of indoleamine-2,3-dioxygenase (IDO),
an immunosuppressive enzyme. The Company believes that by inhibiting this enzyme in these dendritic cells, the patient’s cells
can now attack cancers, particularly breast cancer.
tCellVax:
Immune cells are removed from the patient, treated with siRNA to inhibit NR2F6 and the cells re-infused to the patient. The Company believes
that once the inhibitor protein is blocked, the immune system will be very activated and kill tumors. siRNA is a double-stranded RNA
molecule that is non-coding and is a powerful tool in drug targeting and therapeutics development as it is used to modulate gene expression
through transcriptional or translational repression. The NR2F6 nuclear receptor has been identified as a potentially very important immune
cell inhibitor (an immune checkpoint) and cancer stem cell differentiator.
DiffronC:
This drug is intended to use our proprietary siRNA in vivo to inhibit cancer growth and activate T cells. The siRNA targets NR2F6. T
cells are part of the immune system and develop from stem cells in the bone marrow.
DuraCar:
DuraCar is comprised of CAR-T cells which have been treated with an shRNA targeting the gene NR2F6. By inhibiting NR2F6, we expect our
DuraCar cells to have greater efficacy and persistence than conventional CAR-T cells and create a new, optimal way to manufacture CAR-T
cells. We are currently in pre-clinical testing of this drug. Chimeric antigen receptor T cells ( CAR-T cells) are T cells that have
been genetically engineered to produce an artificial T cell receptor for use in immunotherapy. Chimeric antigen receptors are receptor
proteins that have been engineered to give T cells the new ability to target a specific antigen.
Small
molecule: We have identified and patented a series of small molecules which can both activate and inhibit NR2F6. We are currently in
pre-clinical testing of these drugs.
None
of the abovementioned statements regarding any of our products in development are intended to be a prediction or conclusion of efficacy.
No clinical trials on our product candidates have commenced so no conclusions of efficacy can be made.
As
of November 5, 2024 we have not licensed any existing therapies which may be marketed.
The
Company has entered into license agreements with Zander Therapeutics, Inc. ( an entity under common control) and Oncology Pharma Inc.
( an unrelated entity).
Both
Zander and Oncology Pharma, Inc. will be required to obtain approval from the United States Food and Drug Administration (“FDA”)
in order to market any Licensed Product which may be developed within the United States and no assurance may be given that such approval
would be granted.
As
a Tier I issuer under Regulation A+ of the Securities and Exchange Commission (the “SEC”), the Company will be required to
file with the SEC a Form 1-Z (Exit Report Under Regulation A+) upon termination of this Offering. The Company is also required to file
periodic reports with the SEC pursuant to the Securities Act of 1934.
Selected
Financial Data
The
stockholders’ equity section of the Company contains the following classes of capital stock :
As
of November 20, 2024
Common
stock, $ 0.0001 par value; 5, 800,000,000 shares authorized: 21,554,705 shares issued and outstanding.
Preferred
Stock, $0.0001 par value, 800,000,000 shares authorized of which 600,000 is designated as Series AA Preferred Stock: 34 shares issued
and outstanding as of November 20, 2024, 540,000,000 is designated Series A Preferred Stock of which 10,123,771 shares are outstanding
as of November 20, 2024, 60,000,000 is designated Series M Preferred Stock of which 29,338 shares are outstanding as of November
20, 2024 and 20,000 is designated Series NC Preferred Stock of which 15,007 shares are outstanding as of November 20, 2024.
Our
common stock is traded on the OTC Pink Market under the symbol “RGBP” and our Series A Preferred stock is traded on the OTC
Pink Market under the symbol “RGBPP”. No public market currently exists for any other equity securities of the Company.
| | |
At September 30, 2024 (unaudited) | |
| Selected Balance Sheet Information: | |
| | |
| Cash | |
$ | 716 | |
| Current assets | |
| 159,878 | |
| Total assets | |
$ | 177,611 | |
| Current liabilities | |
$ | 5,371,640 | |
| Total liabilities | |
| 5,371,640 | |
| Total stockholders’ equity (deficit) | |
$ | (5,194,029 | ) |
Retroactively
adjusted to reflect a 1 for 1500 reverse stock split of all issued series of stock effective as of March 6, 2023
| | |
For
the year ended September 30, 2023 | | |
For
the year ended September 30, 2024 | |
| | |
| | |
(unaudited) | |
| | |
| | |
| |
| Selected Statement of Operations Information: | |
| | | |
| | |
| | |
| | | |
| | |
| Revenues | |
$ | 236,560 | | |
$ | 236,560 | |
| Total operating expenses | |
| 923,509 | | |
| 654,749 | |
| Operating income (loss) | |
| (686,950 | ) | |
| (418,189 | ) |
| Net income (loss) to common shareholders | |
$ | 1,023,508 | | |
$ | (867,252 | ) |
| Basis and diluted earnings (loss) per common share | |
$ | 0.29 | | |
$ | (0.21 | ) |
| Weighted average common shares outstanding basic and diluted | |
| 3,536,963 | | |
| 4,110,265 | |
All
stock amounts have been retroactively adjusted to reflect a 1 for 1500 reverse stock split of all issued series of stock effective as
of March 6, 2023.
DILUTION
The
following unaudited table illustrates the dilution on a per share of common stock basis under the scenarios of the Company achieving
the sale of 10%, 25%, 50%, 75% and 100% of this offering*:
| | |
If 10% of | | |
If 25% of | | |
If 50% of | | |
If 75% of | | |
If 100% of | |
| | |
shares sold | | |
shares sold | | |
shares sold | | |
shares sold | | |
shares sold | |
| Book value per share before offering | |
$ | (0.16 | ) | |
$ | (0.16 | ) | |
$ | (0.16 | ) | |
$ | (0.16 | ) | |
$ | (0.16 | ) |
| Book value per share after offering | |
$ | (0.16 | ) | |
$ | (0.15 | ) | |
$ | (0.14 | ) | |
$ | (0.12 | ) | |
$ | (0.12 | ) |
| Net increase to original shareholders | |
$ | 0.00 | | |
$ | 0.01 | | |
$ | 0.02 | | |
$ | 0.04 | | |
$ | 0.04 | |
| Decrease in investment to new shareholders | |
$ | 0.20 | | |
$ | 0.19 | | |
$ | 0.18 | | |
$ | 0.16 | | |
$ | 0.16 | |
| Dilution percentage to new shareholders | |
| 500 | % | |
| 475 | % | |
| 450 | % | |
| 400 | % | |
| 400 | % |
*
Based on book value as of 9/30/2024, based upon $0.04 offering price, includes
15,426,385 common distributed as a dividend to all shareholders of record as of October 17, 2024 (“Record Date”) paid to shareholders
on or about November 1, 2024
RISK
FACTORS
An
investment in our common stock involves a high degree of risk. You should carefully consider the risks described below as well as other
information provided to you in this Offering Circular, including information in the section of this document entitled “CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS”. If any of the following risks actually occur, our business, financial condition
or results of operations could be materially adversely affected, the value of our common stock could decline, and you may lose all or
part of your investment. The following discussion and analysis should be read in conjunction with the other financial information and
consolidated financial statements and related notes appearing in this Offering Circular.
Risks
Related to our Business:
THERE
IS SUBSTANTIAL DOUBT ABOUT THE COMPANY’S ABILITY TO CONTINUE AS A GOING CONCERN.
The
Company generated net losses of $20,616,114 during the period from April 24, 2012 (inception) through September 30, 2024.
This condition raises substantial doubt about the Company’s ability to continue as a going concern. The Company’s continuation
as a going concern is dependent on its ability to meet its obligations, to obtain additional financing as may be required and ultimately
to attain profitability. Because obtaining investment capital is not certain, we may not have the funds necessary to continue our operations.
Our ability to meet our operating needs depends in large part on our ability to secure third party financing. We cannot provide any assurances
that we will be able to obtain sufficient financing.
THE
COMPANY DOES NOT CURRENTLY OWN OR OPERATE ANY LABORATORY OR MANUFACTURING FACILITIES, THE COMPANY CAN PROVIDE NO ASSURANCE THAT THE USAGE
OF SUCH FACILITIES CAN BE OBTAINED ON TERMS FAVORABLE TO THE COMPANY
The
Company does not currently own or operate any laboratory or manufacturing facilities. As a result, we plan to outsource certain functions,
tests and services to Contract Research Organizations (“CROs”) and collaborators as well as outsourcing manufacturing to
collaborators and/or contract manufacturers. We also plan to engage CROs to run all aspects of preclinical studies and clinical trials
on our behalf. There is no assurance that such individuals or organizations will be able to provide the functions, tests, or services
as agreed upon or in a quality fashion or on terms favorable to the Company. Any failure to do so could cause us to suffer significant
delays in the development of our products.
WE
ARE IN THE EARLY STAGES OF DEVELOPING OUR PRODUCTS, THE EFFECTIVENESS OF WHICH ARE UNPROVEN.
The
Company is currently in the early stage of developing its products. No assurance can be given that the Company’s products will
prove effective for their intended purpose or otherwise that any of our work will result in any commercially viable product.
COMPETITORS
WITH MORE RESOURCES MAY FORCE US OUT OF BUSINESS.
In
the event that we have sufficient financial resources, we anticipate that we will compete with many large and well-established companies.
Aggressive pricing by our competitors or the entrance of new competitors into our markets could reduce our revenue and profit margins
and otherwise result in significant financial losses that could result in insolvency or bankruptcy.
WE
MAY NOT BE ABLE TO ATTAIN PROFITABILITY WITHOUT SIGNIFICANT ADDITIONAL FINANCING WHICH MAY BE UNAVAILABLE.
To
date we have funded our operations with minimal financial resources, and we have not generated sufficient cash from operations to be
profitable. Unless we are successful in generating sufficient revenues to finance operations as a going concern while also achieving
profitability and positive cash flow, we may experience liquidity and solvency problems. Such liquidity and solvency problems may force
us to cease operations if additional financing is not available.
WE
MAY NOT BE ABLE TO RAISE ADDITIONAL CAPITAL ON ACCEPTABLE TERMS.
We
are aware that our business may require significant capital in the future each year and for many years even if we can implement our business
plans. Even if we are successful in implementing our business plan, any person who acquires our Common Stock or our Preferred Stock will
likely suffer significant and immediate dilution or otherwise become subordinate to the rights and claims of creditors. In addition,
any financing that we obtain may not be available on terms favorable to us, or at all. Our ability to obtain additional funding will
be subject to various factors, including market conditions, our operating performance, lender and investor sentiment and our ability
to incur additional debt or equity financing in compliance with other contractual restrictions which may arise. These factors may make
the timing, amount, terms and conditions of additional financings unattractive. Our inability to raise capital could impede our growth.
Any person who acquires our securities should be prepared to lose all of their investment.
WE
RELY ON HIGHLY SKILLED PERSONNEL AND, IF WE ARE UNABLE TO RETAIN OR MOTIVATE KEY PERSONNEL OR HIRE QUALIFIED PERSONNEL, WE MAY NOT BE
ABLE TO GROW EFFECTIVELY.
Our
performance largely depends on the talents and efforts of highly skilled individuals. Competition in our industry for qualified employees
is intense. In addition, our compensation arrangements may not always be successful in attracting new employees and retaining and motivating
our existing employees. Our continued ability to compete effectively depends on our ability to attract new employees and to retain and
motivate our existing employees.
THE
COMPANY DOES NOT MAINTAIN CERTAIN INSURANCE, INCLUDING ERRORS AND OMISSIONS INSURANCE.
The
Company has limited capital and, therefore, does not currently have a policy of insurance against liabilities arising out of the negligence
of its officers and directors and/or deficiencies in any of its business operations. Even assuming that the Company obtained insurance,
there is no assurance that such insurance coverage would be adequate to satisfy any potential claims made against the Company, its officers
and directors, or its business operations or products. Any such liability which might arise could be substantial and may exceed the assets
of the Company.
WE
MAY HAVE DIFFICULTY IN ATTRACTING AND RETAINING MANAGEMENT AND OUTSIDE INDEPENDENT MEMBERS TO OUR BOARD OF DIRECTORS AS A RESULT OF THEIR
CONCERNS RELATING TO THEIR INCREASED PERSONAL EXPOSURE TO LAWSUITS AND STOCKHOLDER CLAIMS BY VIRTUE OF HOLDING THESE POSITIONS IN A PUBLICLY-HELD
COMPANY.
We
are aware that directors and management of publicly-traded corporations are increasingly concerned with the extent of their personal
exposure to lawsuits and stockholder claims, as well as governmental and creditor claims which may be made against them, particularly
in view of recent changes in securities laws imposing additional duties, obligations and liabilities on management and directors. Due
to these perceived risks, directors and management are also becoming increasingly concerned with the availability of directors’
and officers’ liability insurance to pay on a timely basis the costs incurred in defending such claims. We currently do not carry
directors’ and officers’ liability insurance. Directors’ and officers’ liability insurance has recently become
much more expensive and difficult to obtain. If we are unable to provide directors’ and officers’ liability insurance at
affordable rates or at all, it may become increasingly more difficult to attract and retain qualified outside directors to serve on our
board of directors. We may lose potential independent board members and management candidates to other companies that have greater directors’
and officers’ liability insurance to insure them from liability or to companies that have revenues or have received greater funding
to date which can offer more lucrative compensation packages. The fees of directors are also rising in response to their increased duties,
obligations and liabilities as well as increased exposure to such risks. As a company that is in the early stages of development and
which has limited resources, we will have a more difficult time attracting and retaining management and outside independent directors
than a more established company due to these enhanced duties, obligations and liabilities.
IN
THE FUTURE WE MAY BE SUBJECT TO INTELLECTUAL PROPERTY RIGHTS CLAIMS, WHICH ARE COSTLY TO DEFEND, COULD REQUIRE US TO PAY DAMAGES AND
COULD LIMIT OUR ABILITY TO SELL SOME OF OUR PRODUCTS.
Although
we have not been subject to any intellectual property litigation or infringement claims, we may be in the future, which could cause us
to incur significant expenses to defend such claims, divert management’s attention or prevent us from manufacturing, selling or
using some aspect of our products. If we chose or are forced to settle such claims, we may be required to pay for a license to certain
rights, paying royalties on both a retrospective and prospective basis, and/or cease our manufacturing and sale of certain products that
are alleged to be infringing. Future infringement claims against us by third parties may adversely impact our business, financial condition
and results of operations.
WE
MAY BE SUBJECT TO VARIOUS FORMS OF LITIGATION INCLUDING, BUT NOT LIMITED TO, CLASS ACTION LAWSUITS, WHICH ARE COSTLY TO DEFEND, COULD
REQUIRE US TO PAY DAMAGES AND COULD LIMIT OUR ABILITY TO SELL SOME OF OUR PRODUCTS.
Companies
have been the target of class action lawsuits and other proceedings alleging, among other things, violations of federal and state workplace
and employment laws. Proceedings of this nature, if successful, could result in our payment of substantial damages.
Our
results of operations may be adversely affected by legal or governmental proceedings brought by or on behalf of employees or consumers.
In recent years, a number of companies, have been subject to lawsuits, including class action lawsuits, alleging violations of federal
and state law. A number of these lawsuits have resulted in the payment of substantial awards by the defendants. Although we are not currently
a party to any class action lawsuits, we could incur substantial damages and expenses resulting from lawsuits, which would increase the
cost of operating the business and decrease the cash available for other uses.
WE
ARE SUBJECT TO NUMEROUS LAWS AND REGULATIONS, FAILURE TO COMPLY WITH THOSE LAWS AND REGULATIONS MAY ADVERSELY IMPACT OUR BUSINESS.
Products
we are currently developing and which may be developed by us would be highly regulated. We currently have no products approved for sale
and we cannot guarantee that we will ever have marketable products. The development of a product candidate and issues relating to its
approval and marketing are subject to extensive regulation by the Food and Drug Administration (FDA) in the United States and regulatory
authorities in other countries, with regulations differing from country to country. We are not permitted to market our product candidates
in the United States until we receive approval of a New Drug Application (NDA) or a Biologic License Application (BLA), as applicable,
from the FDA.
In
the United States, NDAs and BLAs must include extensive preclinical and clinical data and supporting information to establish the product
candidate’s safety and effectiveness for each desired indication. NDAs and BLAs must also include significant information regarding
the chemistry, manufacturing and controls for the product. Obtaining approval of a NDA or BLA is a lengthy, expensive and uncertain process,
and we may not be successful in obtaining approval. Regulators of other jurisdictions, such as the European Medicines Agency (EMA),
a European Union agency for the evaluation of medicinal products, have their own procedures for approval of product candidates. Even
in the event that a product is approved, the FDA or the EMA, as the case may be, may limit the indications for which the product may
be marketed, require extensive warnings on the product labeling or require expensive and time-consuming clinical trials or reporting
as conditions of approval. Regulatory authorities in countries outside of the United States and Europe also have requirements for approval
of drug candidates with which we must comply prior to marketing in those countries. Obtaining regulatory approval for marketing of a
product candidate in one country does not ensure that we will be able to obtain regulatory approval in any other country.
NO
ASSURANCE CAN BE GIVEN THAT ANY PRODUCT IN DEVELOPMENT OR WHICH MAY BE PUT INTO DEVELOPMENT WILL SUCCESSFULLY COMPLETE ANY CLINICAL TRIALS.
Clinical
trials involving new drugs and biologics are commonly classified into three phases. Each phase of the drug approval process is treated
as a separate clinical trial and the drug-development process usually advances through all four phases over many years. Each phase exposes
greater number of subjects to the drug and each phase builds on existing safety and efficacy information. Phase 1 trials are designed
to assess the safety and tolerability of a drug or biologic. Phase II trials are designed to assess how well the drug or biologic works,
as well as to continue Phase I safety assessments in a larger group of volunteers and patients. Phase III trials are aimed at being the
definitive assessment of how effective the drug or biologic is, in comparison with current treatment and to provide an adequate basis
for physician labeling. If the drug or biologic successfully passes through Phases I, II, and III, it will usually be approved by the
national regulatory authority for use in the general population.
The
Company’s plan is to engage primarily in the development of regenerative medical applications up to the point of successful completion
of Phase I and or Phase II clinical trials after which we would either attempt to sell or license those developed applications or, alternatively,
advance the application further to Phase III clinical trials.
We
have yet to complete a successful clinical trial of any product under development and no assurance can be made that any product under
development will successfully complete a clinical trial.
THE
COMPANY CAN PROVIDE NO ASSURANCE THAT IT WILL BE ABLE TO SELL OR LICENSE ANY PRODUCT UNDER DEVELOPMENT OR WHICH WE MAY DEVELOPIN THE
FUTURE.
The
Company’s current plans include the development of regenerative medical applications up to the point of successful completion of
Phase I and/ or Phase II clinical trials after which we would either attempt to sell or license those developed applications or, alternatively,
advance the application further to Phase III clinical trials. We can provide no assurance that the Company will be able to sell or license
any product or that, if such product is sold or licensed, such sale or license will be on terms favorable to the Company.
WE
HAVE NOT OBTAINED PATENT PROTECTION FOR MUCH OF OUR INTELLECTUAL PROPERTY.
The
Company has not obtained patent protection on much of its intellectual property. Although the Company plans on attempting to obtain patents
on its products and services, there can be no assurance that the Company can obtain effective protection against unauthorized duplication
or the introduction of substantially similar products.
LIABILITY
OF DIRECTORS FOR BREACH OF DUTY OF CARE IS LIMITED. OUR BYLAWS INDEMNIFY MEMBERS OF OUR BOARD OF DIRECTORS, OUR OFFICERS, EMPLOYEES,
AND AGENTS AND PERSONS WHO FORMERLY HELD SUCH POSITIONS, AND THE LEGAL REPRESENTATIVES OF ANY OF THEM, TO THE FULLEST EXTENT LEGALLY
PERMISSIBLE UNDER THE GENERAL CORPORATION LAW OF THE STATE OF NEVADA AGAINST ANY OR ALL EXPENSE, LIABILITY AND LOSS REASONABLY INCURRED
IN DEFENDING A CIVIL OR CRIMINAL ACTION, SUIT OR PROCEEDING TO WHICH ANY SUCH PERSON SHALL HAVE BECOME SUBJECT BY REASON OF HIS HAVING
HELD SUCH A POSITION OR HAVING ALLEGEDLY TAKEN OR OMITTED TO TAKE ANY ACTION IN CONNECTION WITH SUCH POSITION.
According
to Nevada law (NRS 78.138(7)), all Nevada corporations limit the liability of directors and officers, including acts not in good faith.
Our stockholders’ ability to recover damages for fiduciary breaches may be reduced by this statute. In addition our Bylaws indemnify
members of the board of directors, our officers, employees, and agents and persons who formerly held such positions, and the legal representatives
of any of them, to the fullest extent legally permissible under the general corporation law of the state of Nevada against any or all
expense, liability and loss reasonably incurred in defending a civil or criminal action, suit or proceeding to which any such person
shall have become subject by reason of his having held such a position or having allegedly taken or omitted to take any action in connection
with such position.
DEPENDENCE
ON DAVID R. KOOS, WITHOUT WHOSE SERVICES COMPANY BUSINESS OPERATIONS COULD CEASE.
At
this time, the sole officer and director of the Company is David R. Koos, who is wholly responsible for the development and execution
of our business. Mr. Koos is not party to an employment agreement with us. If Mr. Koos should choose to leave us for any reason before
we have hired additional personnel our operations may fail. Even if we are able to find additional personnel, it is uncertain whether
we could find qualified management who could develop our business along the lines described herein or would be willing to work for compensation
the Company could afford. Without such management, the Company could be forced to cease operations and investors in our common stock
or other securities could lose their entire investment. David Koos is not party to an employment agreement with the Company.
LIABILITY
OF DIRECTORS FOR BREACH OF DUTY OF CARE IS LIMITED.
According
to Nevada law (NRS 78.138(7)), all Nevada corporations limit the liability of directors and officers, including acts not in good faith.
Our stockholders’ ability to recover damages for fiduciary breaches may be reduced by this statute.
EVENTS
OUTSIDE OF OUR CONTROL, INCLUDING PUBLIC HEALTH CRISES SUCH AS THE COVID-19 PANDEMIC, COULD NEGATIVELY AFFECT OUR BUSINESS AND OUR OPERATING
RESULTS.
A
public health crisis such as the COVID-19 pandemic may cause us to experience disruptions that could severely impact our business including
interruptions in preclinical studies due to restricted or limited operations at laboratory facilities, interruption or delays in the
operations of the FDA or other regulatory authorities, which may impact review and approval timelines and interruption of, or delays
in receiving, supplies for productions of our product candidates from our third party suppliers due to staffing shortages, production
slowdowns or stoppages and disruptions in delivery system.
While
we are not currently conducting any clinical trials in the event of a public health crisis during a time when we are in the process of
conducting one or more clinical trials such trials may be adversely impacted due to:
| ● |
delays or difficulties in enrolling patients in our clinical
trials; |
| ● |
delays or difficulties in clinical trial site activities, including
difficulties in recruiting clinical trial staff; |
| ● |
diversion of healthcare resources away from the conduct of
clinical trials, including the diversion of hospitals serving as our clinical trial sites and hospital staff supporting the conduct of
our clinical trials; |
| ● |
interruption of key clinical trial activities, such as clinical
trial site data monitoring, due to limitations on travel imposed or recommended by federal or state governments, employers and others
or interruption of clinical trial subject visits and study procedures (i.e., those that are deemed non-essential), which may impact the
integrity of subject data and clinical study endpoints. |
Risks
Related to an Investment in Our Common Stock
WE
DO NOT PLANT TO PAY CASH DIVIDENDS IN THE FORESEEABLE FUTURE.
We
currently intend to retain all future earnings for use in the operation and expansion of our business. We do not intend to pay any cash
dividends in the foreseeable future. Unless we pay dividends, our stockholders will not be able to receive a return on their shares unless
they sell them. There is no assurance that stockholders will be able to sell shares when desired or that any continuous and liquid trading
market will develop or, if it does develop, that it will be sustained for any period of time and at a level that will allow a stockholder
an opportunity to sell any shares of our common stock in any amount at any time.
OUR
COMMON STOCK IS QUOTED ON THE OTC PINK MARKET WHICH MAY HAVE AN UNFAVORABLE IMPACT ON OUR STOCK PRICE AND LIQUIDITY.
Our
common stock is quoted on the OTC Pink Market. The OTC Pink Market is a significantly more limited market than the New York Stock Exchange
or NASDAQ system. The quotation of our shares on the OTC Pink Market may result in a less liquid market available for existing and potential
stockholders to trade shares of our common stock, could depress the trading price of our common stock and could have a long-term adverse
impact on our ability to raise capital in the future.
PENNY
STOCK” RULES MAY MAKE BUYING OR SELLING OUR COMMON STOCK DIFFICULT.
Trading
in our securities is subject to the “penny stock” rules. The SEC has adopted regulations that generally define a penny stock
to be any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. These rules require that
any broker-dealer who recommends our securities to persons other than prior customers and accredited investors, must, prior to the sale,
make a special written suitability determination for the purchaser and receive the purchaser’s written agreement to execute the
transaction. Unless an exception is available, the regulations require the delivery, prior to any transaction involving a penny stock,
of a disclosure schedule explaining the penny stock market and the risks associated with trading in the penny stock market. In addition,
broker-dealers must disclose commissions payable to both the broker-dealer and the registered representative and current quotations for
the securities they offer. The additional burdens imposed upon broker-dealers by such requirements may discourage broker-dealers from
effecting transactions in our securities, which could severely limit the market price and liquidity of our securities. Broker-dealers
who sell penny stocks to certain types of investors are required to comply with the Commission’s regulations concerning the transfer
of penny stocks. These regulations require broker- dealers to:
| |
● |
Make
a suitability determination prior to selling a penny stock to the purchaser; |
| |
● |
Receive
the purchaser’s written consent to the transaction; and |
| |
● |
Provide
certain written disclosures to the purchaser. |
These
requirements may restrict the ability of broker-dealers to sell our common stock and may affect your ability to resell our common stock.
CONCENTRATED
CONTROL RISKS; SHAREHOLDERS COULD BE UNABLE TO CONTROL OR INFLUENCE KEY CORPORATE ACTIONS OR EFFECT CHANGES IN THE COMPANY’S BOARD
OF DIRECTORS OR MANAGEMENT
Our
sole officer and director, David R. Koos, has voting power over 436,997 shares of our common stock, 413,281 of our Series A Preferred
stock, 34 shares of our Series AA Preferred Stock, 7,667 shares of our Series M Preferred Stock and 15,007 shares of our Series NC Preferred
stock representing approximately 14% of the voting control of the Company as of November 5, 2024 Mr. Koos therefore has
significant influence with regard to many major decisions regarding our affairs. In addition, due to Mr. Koos voting power, investors
in this offering will have limited control over matters requiring approval by our security holders, including the election of directors,
whether or not to sell all or substantially all of our assets and for what consideration and whether or not to authorize more stock for
issuance or otherwise amend our charter or bylaws.
BECAUSE
WE HAVE ELECTED TO DEFER COMPLIANCE WITH NEW OR REVISED ACCOUNTING STANDARDS PURSUANT TO SECTION 102(b)(1) OF THE JOBS ACT OUR FINANCIAL
STATEMENT DISCLOSURE MAY NOT BE COMPARABLE TO SIMILAR COMPANIES.
We
have elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(1) of
the JOBS Act. This allows us to delay the adoption of new or revised accounting standards that have different effective dates for public
and private companies until those standards apply to private companies. As a result of our election, our financial statements may not
be comparable to companies that comply with public company effective dates.
LIKELIHOOD
OF IMMEDIATE AND SUBSTANTIAL DILUTION.
We
anticipate that we may need to raise additional capital to implement our business plan. At present we have not had any definitive discussions
with any venture capital, angel investors, FINRA-registered broker dealers, or other persons regarding the extent of their interest in
investing into the Company. Since we are an early-stage company with no track record of generating revenues, positive cash flow, or profitability,
there can be no guarantee that we will raise the additional capital that we anticipate that we will need to raise or, if we are successful
in raising any such additional capital that we can do so on a reasonable and timely basis, in sufficient amounts and on terms that are
reasonable in light of our present circumstances. For these and other reasons, any person who acquires our Common Stock is likely to
incur immediate and substantial dilution with respect to the book value of the Company’s common stock offered hereby.
FUTURE
ISSUANCE OF COMMON STOCK RELATED TO CONVERTIBLE NOTES PAYABLE AND ACCRUED INTEREST ON CONVERTIBLE NOTES PAYABLE MAY HAVE A DILUTING FACTOR
ON EXISTING AND FUTURE SHAREHOLDERS.
As
of November 4, 2024 the Company has outstanding an aggregate of $800,217 of convertible debt and accrued interest on
convertible debt. Of that aggregate amount approximately $581,799 is convertible into common or Series A preferred shares of the
Company at various discounts from the market price of the Company’s publicly traded shares. It is the Company’s belief that
shares issuable to the holders of $800,217 of combined convertible debt and accrued interest on convertible debt may be resold
pursuant to the safe harbor provisions of Rule 144.
WE
DO NOT CURRENTLY INTEND TO REGISTER OUR COMMON SHARES UNDER THE SECURITIES AND EXCHANGE ACT OF 1934 (“EXCHANGE ACT”). OUR
REPORTING OBLIGATIONS UNDER SECTION 15(D) OF THE EXCHANGE ACT MAY BE SUSPENDED AUTOMATICALLY IF WE HAVE FEWER THAN 300 HOLDERS OF RECORD
ON THE FIRST DAY OF OUR FISCAL YEAR AFTER THE YEAR OF EFFECTIVENESS OF THE REGISTRATION STATEMENT FILED PURSUANT TO THE SECURITIES ACT
OF 1933 OF WHICH THIS PROSPECTUS CONSTITUTES PART.
We
became subject to the Exchange Act reporting requirements under Section 15(d) on September 20,2023 upon effectiveness of
a registration statement and will be for at least one year after effectiveness. Our obligation to file reports under Section
15(d) of the Exchange Act will be automatically suspended if, on the first day of any fiscal year, other than a fiscal year in which
a registration statement under the Securities Act has gone effective, we have fewer than 300 holders of record. In such an event, we
may cease providing periodic reports and current or periodic information, including operational and financial information
WE
DO NOT CURRENTLY INTEND TO REGISTER OUR COMMON SHARES UNDER THE SECURITIES AND EXCHANGE ACT OF 1934 (“EXCHANGE ACT”). UNLESS
WE REGISTER A CLASS OF OUR SECURITIES PURSUANT TO SECTION 12 OF THE EXCHANGE ACT, WE WILL ONLY BE SUBJECT TO THE PERIODIC REPORTING OBLIGATIONS
IMPOSED BY SECTION 15(D) OF THE EXCHANGE ACT WHICH MAY LIMIT THE INFORMATION ON THE COMPANY AVAILABLE TO SHAREHOLDERS.
We
do not currently intend to register our common shares under the Securities and Exchange act of 1934 (“Exchange Act”). Unless
we register a class of our securities pursuant to Section 12 of the Exchange Act, we will only be subject to the periodic reporting obligations
imposed by Section 15(d) of the Exchange Act. Accordingly, we will not be subject to the proxy rules, short-swing profit provisions,
going-private regulation, beneficial ownership reporting, and the majority of the tender offer rules and the reporting requirements of
the Exchange Act. Accordingly, shareholders may have access to less information regarding the activities of the Company and its officers
and directors than they otherwise may have if a class of the Company’s securities was registered under the Exchange Act.
ABOUT
THE OFFERING
WHO
MAY INVEST IN THE OFFERING
The
Company is offering for sale in this Regulation A+ offering (the “Offering”) up to 10,000,000 shares of its common stock,
$0.0001 par value, for a maximum purchase price of $0.04 per share, for a total offering amount of $400,000. The shares
are being offered for sale by this Offering Circular only to accredited investors, as such term is defined in Regulation D of the Securities
and Exchange Commission (the “SEC”) under the Securities Act of 1933, as amended (the “Act”).
Accredited
Investor
To
be an “accredited investor,” an investor must come within any of the following categories, or be a person who the issuer
reasonably believes comes within any of the following categories at the time of the sale of the shares to that investor:
| |
● |
Any
bank as defined in Section 3(a)(2) of the Act, or any savings and loan association or other institution as defined in Section 3(a)(5)(A)
of the act whether acting in its individual or fiduciary capacity; any broker or dealer registered pursuant to Section 15 of the
Securities Exchange Act of 1934; any insurance company registered under the Investment Company Act of 1940 or a business development
company as defined in Section 2(a)(48) of that Act; any Small Business Investment Company licensed by the U.S. Small Business Administration
under Section 301(c) or (d) of the Small Business Investment Act of 1958; any plan established and maintained by a state, its political
subdivisions, or any agency or instrumentality of a state or political subdivisions, for the benefit of its employees, if such plan
has total assets in excess of $5,000,000; any employee benefit plan within the meaning of the Employee Retirement Income Security
Act of 1974, if the investment decision is made by a plan fiduciary, as defined in Section 3(21) of such Act, which is either a bank,
savings and loan association, insurance company, or registered investment advisor, or if the employee benefit plan has total assets
in excess of $5,000,000, or, if a self-directed plan, with investment decisions made solely by persons that are accredited investors; |
| |
● |
Any
private business development company as defined in Section 202(a)(22) of the Investment Advisers Act of 1940; |
| |
● |
Any
organization described in Section 501(c)(3) of the Internal Revenue Code, or corporation, Massachusetts or similar business trust,
or partnership, not formed for the specific purpose of acquiring the shares offered, with total assets in excess of $5,000,000; |
| |
● |
Any
director, executive officer, or general partner of the issuer of the securities being offered or sold, or any director, executive
officer, or general partner of a general partner of that issuer; |
| |
● |
Any
natural person whose individual net worth, or joint net worth with that person’s spouse or spousal equivalent, at the time
of his purchase (excluding the value of the person’s primary residence) exceeds $1,000,000; |
| |
● |
Any
natural person who had an individual income in excess of $200,000 in each of the two most recent years, or joint income with that
person’s spouse or spousal equivalent in excess of $300,000 in each of those years and has a reasonable expectation of reaching
the same income level in the current year; |
| |
● |
Any
trust, with total assets in excess of $5,000,000, not formed for the specific purpose of acquiring the shares offered, whose purchase
is directed by a sophisticated person as described in Rule 506(b)(2)(ii) of Regulation D; |
| |
● |
Any
entity in which all of the equity owners are accredited investors (as defined above). |
| |
● |
Any
entity, of a type not listed above, not formed for the specific purpose of acquiring the securities offered, owning investments in
excess of $5,000,000: |
| |
● |
Any
natural person holding in good standing one or more professional certifications or designations or credentials from an accredited
educational institution that the SEC has designated as qualifying an individual for accredited investor status; |
| |
● |
Any
natural person who is a “knowledgeable employee,” as defined in Rule 3c-5(a)(4) under the Investment Company Act of 1940,
of the issuer of the securities being offered or sold where the issuer would be an investment company, as defined in Section 3 of
such Act, but for the exclusion provided by either section 3(c)(1) or section 3(c)(7) of such Act; |
| |
● |
Any
“family office” as defined in Rule 202(a)(11)(G)-1 under the Investment Advisers Act of 1940 with assets under management
in excess of $5,000,000, that is not formed for the specific purpose of acquiring the securities offered, and whose prospective investment
is directed by a person who has such knowledge and experience in financial and business matters that such family office is capable
of evaluating the merits and risks of the prospective investment; and |
| |
● |
Any
“family client” as defined in Rule 202(a)(G)-1 under the Investment Advisers Act of 1940, of a family office meeting
the requirements of a family office and whose prospective investment in the issuer is directed by such family office. |
Each
subscriber will represent and warrant to the Company in such subscriber’s subscription agreement that such subscriber is an accredited
investor and shall designate in the subscription agreement the specific section or sections of the above description of the definition
of accredited investor which applies to the subscriber.
USE
OF PROCEEDS
If
the Offering is consummated and all 10,000,000 Shares offered hereby are sold, the gross proceeds from the sale of those Shares at the price of $0.04 per share would be $400,000 and the net proceeds would be approximately $392,000
after giving effect to estimated expenses in connection with the Offering of approximately $8,000, including, but not limited
to, expenses of filing on the SEC’s EDGAR system, printing and copying costs, legal fees, accounting fees, filing fees, postage,
and other miscellaneous costs and expenses, including meeting expenses. Notwithstanding the foregoing, the Company can provide no assurances
as to the total number of Shares that may be sold or the amount of expenses to be paid. The abovementioned offering expenses are estimates
only and the actual offering expenses may be higher or lower than anticipated.
The
net proceeds from the Offering of $392,000 will be used by the Company as working capital to support the operational and research
and development expenses of the Company. The Company has significant discretion over the net proceeds of the Offering. As is the case
with any business, it should be expected that certain expenses unforeseeable to management at this juncture will arise in the future.
There can be no assurance that management’s use of proceeds generated through this Offering will prove optimal or translate into
revenue or profitability for the Company.
ABOUT
THIS OFFERING
| Offering
Entity |
|
Regan
Biopharma, Inc. |
| |
|
|
| Address
and Telephone Number |
|
4700
Spring Street, Suite 304, La Mesa, California, 91942 (619) 722-5505 |
| |
|
|
| OTC
Pink Trading Symbol |
|
RGBP |
| |
|
|
| Securities
Offered |
|
Up
to 10,000,000 shares of the common stock of the Company (the “Shares”) |
| |
|
|
| Offering
Price Per Share |
|
$0.04
per share |
| |
|
|
| Minimum
Subscription Total |
|
There
is no minimum number of Shares that must be sold. |
| |
|
|
| Minimum
Subscription Per Subscriber |
|
No
Minimum |
| |
|
|
| Maximum
Offering Amount |
|
$400,000
assuming all 10,000,000 Shares are purchased
in the Offering. at $0.04 per share |
| |
|
|
| Shares
of Common Stock Outstanding after the Offering |
|
As
of the date of this Offering Circular, the Company has 21,554,705 shares of common stock outstanding. If all Shares
are sold in the Offering, the Company will have an aggregate of 31,554,745 shares of common stock issued and outstanding,
assuming no conversion of any outstanding convertible instruments. |
| |
|
|
| How
to Subscribe |
|
To
subscribe for Shares in the Offering, complete a subscription agreement (the form is included with this Offering Circular), and deliver
it, together with the total subscription price for all the Shares you wish to purchase, on or before the Closing Date, as defined
below, to the Company |
The offering will commence within two days of qualification by the
United States Securities and Exchange Commission and will terminate 90 days after qualification. The Company will retain all proceeds
received from the shares sold in this offering. The Company has not made any arrangements to place the proceeds in an escrow or trust
account. Any proceeds received in this offering may be immediately used by the Company in its sole discretion. There are no minimum purchase
requirements for each investor. All proceeds retained by the Company may not be sufficient to continue operations. Subscription proceeds
will not be escrowed and will be immediately available to the Company.
*
includes 15,426,386 common shares to be distributed as a dividend to all shareholders of record as of October 17, 2024 (“Record
Date”) to be paid to shareholders on or about November 1, 2024
DISQUALIFYING
EVENTS AND BAD ACTOR DISCLOSURE
Regulation
A+ promulgated under the Securities Act prohibit an issuer from claiming an exemption from registration of its securities under such
rule if the issuer, or any of its predecessors, any affiliated issuer, any director, executive officer, other officer participating in
the offering of the interests, general partner or managing member of the issuer, any beneficial owner of 20% or more of the voting power
of the issuer’s outstanding voting securities, any promoter connected with the issuer in any capacity as of the date hereof, an
investment manager of the issuer any person that has been or will be paid (directly or indirectly) remuneration for solicitation of purchasers
in connection with such sale of the issuer’s interests, any general partner or managing member of any such investment manager or
solicitor, or any director, executive officer or other officer participating in the offering of any such investment manager or solicitor
or general partner or managing member of such investment manager or solicitor has been subject to certain “Disqualifying Events”
described in 17 CFR 230.262(a), subject to certain limited exceptions. The Company is required to exercise reasonable care in conducting
an inquiry to determine whether any such persons have been subject to such Disqualifying Events and is required to disclose any Disqualifying
Events that occurred prior to November 2014 to investors in the Company.
The
Company believes that it has exercised reasonable care in conducting an inquiry into Disqualifying Events by the foregoing persons and
is aware of no such Disqualifying Events. Under 17 CFR 230.262(d), the Company is also required to include in this Offering Circular
a description of any matters that would have triggered disqualification that occurred before June 19, 2015.
On
June 26 - 28 of 2001 the NYSE held an administrative hearing panel regarding Mr. David Koos’ ( the Company’s sole officer
and director) handling of a client’s account while he was a Registered Representative at Everen Securities. The panel found Mr.
Koos had engaged in excessive, unsuitable and discretionary trading in a client’s account. The NYSE found Mr. Koos guilty of the
aforementioned and suspended him from association with the NYSE and its affiliates for a period of 9 months. On appeal, the Enforcement
Division requested the suspension be 18 months, which was upheld by the Appeal Board. The final disposition by the Appeal Board was not
further appealed due to legal costs., Mr. Koos agreed to accept the suspension even though he maintained his innocence in any wrongdoing.
MARKET
FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS
The
Company’s common stock is a “penny stock,” as defined in Rule 3a51-1 under the Exchange Act. The penny stock rules
require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk
disclosure document that provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer
also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its
sales person in the transaction, and monthly account statements showing the market value of each penny stock held in the customer’s
account. In addition, the penny stock rules require that the broker-dealer, not otherwise exempt from such rules, must make a special
written determination that the penny stock is suitable for the purchaser and receive the purchaser’s written agreement to the transaction.
These disclosure rules have the effect of reducing the level of trading activity in the secondary market for a stock that becomes subject
to the penny stock rules. So long as the common stock of the Company is subject to the penny stock rules, it may be more difficult to
sell common stock of the Company.
The
stockholders’ equity section of the Company contains the following classes of capital stock as of November 20, 2024:
Common
stock, $ 0.0001 par value; 5,800,000,000 shares authorized: 21,554,705 shares issued and outstanding.
With
respect to each matter submitted to a vote of stockholders of the Corporation, each holder of Common Stock shall be entitled to cast
that number of votes which is equivalent to the number of shares of Common Stock owned by such holder times one (1).
On
any voluntary or involuntary liquidation, dissolution or winding up of the Corporation, the holders of the Common Stock shall receive,
out of assets legally available for distribution to the Company’s stockholders, a ratable share in the assets of the Corporation.
Preferred
Stock, $0.0001 par value, 800,000,000 shares authorized of which 600,000 is designated as Series AA Preferred Stock: 34 shares issued
and outstanding as of November 20, 2024, 739,000,000 is designated Series A Preferred Stock of which 10,123,771 shares are outstanding
as of November 20, 2024, 60,000,000 is designated Series M Preferred Stock of which 29,338 shares are outstanding as of November
20, 2024, and 20,000 is designated Series NC stock of which 15,007 shares are outstanding as of November 20, 2024.
The
abovementioned shares authorized pursuant to the Company’s certificate of incorporation may be issued from time to time without
prior approval of the shareholders. The Board of Directors of the Company shall have the full authority permitted by law to establish
one or more series and the number of shares constituting each such series and to fix by resolution full or limited, multiple or fractional,
or no voting rights, and such designations, preferences, qualifications, restrictions, options, conversion rights and other special or
relative rights of any series of the Stock that may be desired.
Series
AA Preferred Stock
On
September 15, 2014 the Company filed a CERTIFICATE OF DESIGNATION (“Certificate of Designations”) with the Nevada Secretary
of State setting forth the preferences rights and limitations of a newly authorized series of preferred stock designated and known as
“Series AA Preferred Stock” (hereinafter referred to as “Series AA Preferred Stock”).
The
Board of Directors of the Company have authorized 600,000 shares of the Series AA Preferred Stock, par value $0.0001. With respect to
each matter submitted to a vote of stockholders of the Corporation, each holder of Series AA Preferred Stock shall be entitled to cast
that number of votes which is equivalent to the number of shares of Series AA Preferred Stock owned by such holder times seven (7). Except
as otherwise required by law holders of Common Stock, other series of Preferred issued by the Corporation, and Series AA Preferred Stock
shall vote as a single class on all matters submitted to the stockholders.
Series
A Preferred Stock
On
January 15, 2015 the Company filed a CERTIFICATE OF DESIGNATION (“Certificate of Designations”) with the Nevada Secretary
of State setting forth the preferences rights and limitations of a newly authorized series of preferred stock designated and known as
“Series A Preferred Stock” (hereinafter referred to as “Series A Preferred Stock”).
The
Board of Directors of the Company have authorized 739,000,000 shares of the Series A Preferred Stock, par value $0.0001. With respect
to each matter submitted to a vote of stockholders of the Corporation, each holder of Series A Preferred Stock shall be entitled to cast
that number of votes which is equivalent to the number of shares of Series A Preferred Stock owned by such holder times one . Except
as otherwise required by law holders of Common Stock, other series of Preferred issued by the Corporation, and Series A Preferred Stock
shall vote as a single class on all matters submitted to the stockholders.
Holders
of the Series A Preferred Stock will be entitled to receive, when, as and if declared by the board of directors of the Company (the “Board”)
out of funds legally available therefore, non-cumulative cash dividends of $0.01 per quarter. In the event any dividends are declared
or paid or any other distribution is made on or with respect to the Common Stock, the holders of Series A Preferred Stock as of the
record date established by the Board for such dividend or distribution on the Common Stock shall be entitled to receive, as additional
dividends (the “Additional Dividends”) an amount (whether in the form of cash, securities or other property) equal to the
amount (and in the form) of the dividends or distribution that such holder would have received had each share of the Series A Preferred
Stock been one share of the Common Stock, such Additional Dividends to be payable on the same payment date as the payment date for the
Common Stock.
Upon
any liquidation, dissolution, or winding up of the Company, whether voluntary or involuntary (collectively, a “Liquidation”),
before any distribution or payment shall be made to any of the holders of Common Stock or any other series of preferred stock, the holders
of Series A Preferred Stock shall be entitled to receive out of the assets of the Company, whether such assets are capital, surplus or
earnings, an amount equal to $0.01 per share of Series A Preferred (the “Liquidation Amount”) plus all declared and unpaid
dividends thereon, for each share of Series A Preferred held by them.
If,
upon any Liquidation, the assets of the Company shall be insufficient to pay the Liquidation Amount, together with declared and unpaid
dividends thereon, in full to all holders of Series A Preferred, then the entire net assets of the Company shall be distributed among
the holders of the Series A Preferred, ratably in proportion to the full amounts to which they would otherwise be respectively entitled
and such distributions may be made in cash or in property taken at its fair value (as determined in good faith by the Board), or both,
at the election of the Board.
On
January 10, 2017 Regen Biopharma, Inc. (“Regen”) filed a CERTIFICATE OF DESIGNATION (“Certificate of Designations”)
with the Nevada Secretary of State setting forth the preferences rights and limitations of a newly authorized series of preferred stock
designated and known as “Series M Preferred Stock” (hereinafter referred to as “Series M Preferred Stock”).
The
Board of Directors of Regen have authorized 60,000,000 shares of the Series M Preferred Stock, par value $0.0001. With respect to each
matter submitted to a vote of stockholders of Regen, each holder of Series M Preferred Stock shall be entitled to cast that number of
votes which is equivalent to the number of shares of Series M Preferred Stock owned by such holder times one. Except as otherwise required
by law holders of Common Stock, other series of Preferred issued by Regen, and Series M Preferred Stock shall vote as a single class
on all matters submitted to the stockholders.
The
holders of Series M Preferred Stock shall be entitled receive dividends, when, as and if declared by the Board of Directors in accordance
with Nevada Law, in its discretion, from funds legally available therefore
On
any voluntary or involuntary liquidation, dissolution or winding up of Regen, the holders of the Series M Preferred Stock shall receive,
out of assets legally available for distribution to Regen’s stockholders, a ratable share in the assets of Regen.
On
March 26, 2021 Regen Biopharma, Inc. ( “Regen”) filed a CERTIFICATE OF DESIGNATION (“Certificate of Designations”)
with the Nevada Secretary of State setting forth the preferences rights and limitations of a newly authorized series of preferred stock
designated and known as Nonconvertible Series NC Preferred Stock (hereinafter referred to as “Series NC Preferred Stock”).
The
Board of Directors of Regen have authorized 20,000 shares of the Series NC Preferred Stock, par value $0.0001. With respect to each matter
submitted to a vote of stockholders of Regen, each holder of Series NC Preferred Stock shall be entitled to cast that number of votes
which is equivalent to the number of shares of Series NC Preferred Stock owned by such holder times 334. Except as otherwise required
by law holders of Common Stock, other series of Preferred issued by Regen, and Series NC Preferred Stock shall vote as a single class
on all matters submitted to the stockholders.
The
holders of Series NC Preferred Stock shall be entitled receive dividends, when, as and if declared by the Board of Directors in accordance
with Nevada Law, in its discretion, from funds legally available therefore
On
any voluntary or involuntary liquidation, dissolution or winding up of Regen, the holders of the Series NC Preferred Stock shall receive,
out of assets legally available for distribution to Regen’s stockholders, a ratable share in the assets of Regen.
On
May 20, 2024 Regen Biopharma, Inc. amended its Certificate of Incorporation adding the following Article 8 which is and reads as follows:
Shares
of one class or series of stock may be issued as a share dividend in respect of another class or series.
On
May 21, 2024 the Board of Directors of Regen Biopharma, Inc declared a dividend to all shareholders of record as of June 20, 2024 (“Record
Date”) to be paid to shareholders on or about July 1, 2024 such dividend to be payable in shares of the Regen’s authorized
but unissued Series A Preferred Stock and to consist of two share of Series A Preferred Stock for every one share of Regen Biopharma,
Inc. Common Stock owned as of the Record Date, every one share of Regen Biopharma, Inc. Series A Preferred Stock owned as of the Record
Date, every one share of Series AA Preferred Stock owned as of the Record Date, every one share of Series M Preferred Stock owned as
of the Record Date and every one share of Series NC Preferred Stock owned as of the Record Date
We
have never paid any cash dividends on our common stock. We currently anticipate that we will retain all future earnings for use in our
business. Consequently, we do not anticipate paying any cash dividends in the foreseeable future. The payment of dividends in the future
will depend upon our results of operations, as well as our short term and long-term cash availability, working capital, working capital
needs, and other factors as determined by our Board of Directors. Currently, except as may be provided by applicable laws, there are
no contractual or other restrictions on our ability to pay dividends if we were to decide to declare and pay them.
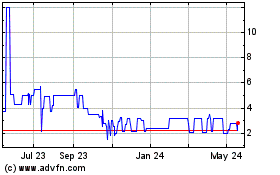
Below
is the range of high and low bid information for our common equity for each quarter within the last two fiscal years. These quotations
reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions.
All
stock prices have been retroactively adjusted to reflect a 1 for 1500 reverse stock split of all issued series of stock effective as
of March 6, 2023.
| October 1, 2023 to September 30, 2024 | |
HIGH | | |
LOW | |
| First Quarter | |
$ | 1.6279 | | |
$ | 0.4337 | |
| Second Quarter | |
$ | 0.9302 | | |
$ | 0.4983 | |
| Third Quarter | |
$ | 0.9966 | | |
$ | 0.333 | |
| Fourth Quarter | |
$ | 0.6645 | | |
$ | -.1152 | |
| October 1, 2022 to December 31, 2022 | |
HIGH | | |
LOW | |
| First Quarter | |
$ | 10.89 | | |
$ | 5.89 | |
| January 1, 2023 to March 31, 2023 | |
HIGH | | |
LOW | |
| Second Quarter | |
$ | 7.16 | | |
$ | 1.25 | |
| April 1, 2023 to June 30, 2023 | |
HIGH | | |
LOW | |
| Third Quarter | |
$ | 2.28 | | |
$ | 1.50 | |
| July 1, 2023 to September 30, 2023 | |
HIGH | | |
LOW | |
| Fourth Quarter | |
$ | 2.00 | | |
$ | 1.46 | |
As
of November 5, 2024 there were approximately 482 holders of our Common Stock.
As of November 5, 2024 there were approximately
479 holders of our Series A Preferred Stock.
As of November 5, 2024 there was 1 holder
of our Series AA Preferred Stock.
As of November 5, 2024 there were approximately
7 holders of our Series M Preferred Stock
As of November 5, 2024 there was one holder
of our Series NC Preferred Stock.
Dividends
No
cash dividends were paid during the fiscal year ending September 30, 2024. We do not expect to declare cash dividends in the immediate
future.
Director
Independence
Audit
Committee and Audit Committee Financial Expert
The
members of the Company’s board of Directors may not be considered independent. The Company is not a “listed company”
under Securities and Exchange Commission (“SEC”) rules and is therefore not required to have an audit committee comprised
of independent directors. The Company does not currently have an audit committee, however, for certain purposes of the rules and regulations
of the SEC and in accordance with the Sarbanes-Oxley Act of 2002, the Company’s Board of Directors is deemed to be its audit committee
and as such functions as an audit committee and performs some of the same functions as an audit committee including: (1) selection and
oversight of our independent accountant; (2) establishing procedures for the receipt, retention and treatment of complaints regarding
accounting, internal controls and auditing matters; and (3) engaging outside advisors. The Board of Directors has determined that its
member is able to read and understand fundamental financial statements and has substantial business experience that results in that member’s
financial sophistication. Accordingly, the Board of Directors believes that its member has the sufficient knowledge and experience necessary
to fulfill the duties and obligations that an audit committee would have.
Nominating
and Compensation Committees
The
Company does not have standing nominating or compensation committees, or committees performing similar functions. The board of directors
believes that it is not necessary to have a compensation committee at this time because the functions of such committee are adequately
performed by the board of directors. The board of directors also is of the view that it is appropriate for the Company not to have a
standing nominating committee because the board of directors has performed and will perform adequately the functions of a nominating
committee. The Company is not a “listed company” under SEC rules and is therefore not required to have a compensation committee
or a nominating committee.
Shareholder
Communications
There
has not been any defined policy or procedure requirements for stockholders to submit recommendations or nomination for directors. There
are no specific, minimum qualifications that the board of directors believes must be met by a candidate recommended by the board of directors.
Currently, the entire board of directors decides on nominees, on the recommendation of any member of the board of directors followed
by the board’s review of the candidates’ resumes and interview of candidates. Based on the information gathered, the board
of directors then makes a decision on whether to recommend the candidates as nominees for director. The Company does not pay any fee
to any third party or parties to identify or evaluate or assist in identifying or evaluating potential nominee.
Because
the Chief Executive Officer of the Company is also the Chairman of the Board of Directors of the Company, the Board of Directors has
determined not to adopt a formal methodology for communications from shareholders on the belief that any communication would be brought
to the Board of Directors’ attention by virtue of the co-extensive capacities of the Chairman of the Board of Directors.
MANAGEMENT
AND DIRECTORS
David
R. Koos:
David
R. Koos has served as Chairman of the Board of Directors, Chief Executive Officer, Secretary, and Treasurer since April 24, 2012 until
his resignation in January 22, 2020.
David
R. Koos has served as Acting Chief Financial Officer of the Company for the period beginning April 24, 2012 and ending February 11, 2015.
On
March 23, 2021 David R. Koos was appointed Chairman and Sole Director of Regen Biopharma, Inc. On March 23, 2021 David R. Koos was appointed
Chief Executive Officer, President, Secretary and Treasurer of Regen Biopharma, Inc.
On
March 23, 2021 David R. Koos was appointed Chairman and Sole Director of KCL Therapeutics, Inc. On March 23, 2021 David R. Koos was appointed
Chief Executive Officer, President, Secretary and Treasurer of KCL Therapeutics, Inc.
KCL
Therapeutics, Inc. is a wholly owned subsidiary of Regen Biopharma, Inc.
Education:
DBA
- Finance (December 2003)
Atlantic
International University
Ph.D.
- Sociology (September 2003)
Atlantic
International University
MA
- Sociology (June 1983)
University
of California - Riverside, California
Employment
History:
David
R. Koos, 62 has served as Chairman of the Board of Directors, Chief Executive Officer, President, Secretary and Treasurer of SYBLEU INC.,
a biotechnology company, from June 12, 2020 to December 13, 2022. David R. Koos served as Chief Financial Officer of SYBLEU INC. from
June 12, 2020 to July 21, 2020. On March 23, 2021 David R. Koos assumed the position of sole officer and director of Zander Therapeutics,
Inc., a biotechnology company.
| Position: |
|
Company
Name: |
|
Employment
Dates: |
| Chairman,
President, Chief Executive Officer, Secretary, Acting Chief Financial Officer, Principal Accounting Officer |
|
Entest
Group, Inc. |
|
June
19, 2009 to November 28, 2018 |
| Chairman,
President, Chief Executive Officer, Secretary, Chief Financial Officer, Principal Accounting Officer |
|
Entest
BioMedical, Inc.(a California corporation) |
|
August
22,2008 to the Present |
| Chairman
and CEO |
|
Regen
BioPharma, Inc. |
|
April
24, 2012 to January 22,2020 |
| Acting
CFO |
|
Regen
BioPharma, Inc. |
|
April
24, 2012 to February 11, 2015 |
| President |
|
Regen
BioPharma, Inc. |
|
May
29, 2013 to October 9, 2013 |
| Chairman,
CEO |
|
Zander
Therapeutics, Inc. |
|
February
2017 to January 22,2020 |
| Sole
Officer and Director |
|
Cell
Source Research, Inc. |
|
March
24, 2003 to the Present |
| Chairman,
President, CEO and Acting CFO |
|
Bio-Matrix
Scientific Group, Inc. |
|
June
14, 2006 (Chairman) to July 31;2019 June 19, 2006 (President, CEO and Acting CFO); June 19, 2006 (Secretary) to July 31, 2019 |
| Chairman
& CEO |
|
BST
Partners Inc. (A California Corporation) |
|
November
30, 2018 to the Present |
| Chairman
& CEO |
|
BST
Partners Inc. (A Wyoming Corporation) |
|
March
17, to 2017 to the Present |
EXECUTIVE
COMPENSATION
| Name and Principal Position | |
Year | |
Salary
($) | |
Bonus
($) | |
Option
Awards
($) | |
Non Equity
Incentive
Plan
Compensation
($) | |
Nonqualified Total
Deferred
Compensation
Earnings
($) |
David Koos
Chairman, and CEO | |
| From October 1, 2023 to September 30, 2024 | | |
$ | 0 | | |
| 0 | | |
| 0 | | |
| 0 | | |
| 0 | |
| Name and Principal Position | |
Year | |
Salary
($) | | |
Bonus
($) | | |
Option
Awards
($) | | |
Non Equity
Incentive
Plan
Compensation
($) | | |
Nonqualified Total
Deferred
Compensation
Earnings
($) | |
| David Koos Chairman, and CEO | |
From October 1, 2022 to September 30, 2023 | |
$ | 10,050 | * | |
| 0 | | |
| 0 | | |
| 0 | | |
| 0 | |
| Name and Principal Position | |
Year | |
Salary
($) | | |
Bonus
($) | | |
Option
Awards
($) | | |
Non Equity
Incentive
Plan
Compensation
($) | | |
Nonqualified Total
Deferred
Compensation
Earnings
($) | |
David Koos
Chairman, and CEO | |
From October 1, 2021 to September 30, 2022 | |
$ | 0 | | |
| 0 | | |
| 0 | | |
| 0 | | |
| 0 | |
There
is a balance of $457,111 of salary accrued but unpaid due to David Koos.
*On
March 17, 2023 David Koos was issued 15,000 Series NC Preferred shares in satisfaction of $10,050 of salaries accrued yet unpaid.
Employment
Agreements
Currently
neither the Company nor the Company’s wholly owned subsidiary is party to any employment agreement.
BUSINESS
We
were incorporated April 24, 2012 under the laws of the State of Nevada. We intend to engage primarily in the development of regenerative
medical applications which we intend to license, develop internally or acquire outright from other entities up to the point of successful
completion of Phase I and or Phase II clinical trials after which we would either attempt to sell or license those developed applications
or, alternatively, advance the application further to Phase III clinical trials. The primary factor to be considered by us in arriving
at a decision to advance an application further to Phase III clinical trials would be a greater than anticipated indication of efficacy
seen in Phase I trials.
As
of September 15, 2023 we have not licensed any existing therapies which may be marketed.
Patents
and Patent Applications:
The
following is a list of intellectual property (“IP”) controlled by either Regen Biopharma, Inc. (the “Company”)
or KCL Therapeutics (“KCL”). KCL is a wholly owned subsidiary of the Company.
IP
which has been granted patent protection by the United States Patent and Trademark Office (“USPTO”)
GENE
SILENCING OF THE BROTHER OF THE REGULATOR OF IMPRINTED SITES (BORIS)
Provides
methods and compositions useful for inhibiting expression of the gene encoding the transcription factor, Brother of the Regulatory of
Imprinted Sites (BORIS) by RNA interference. Methods of the present invention can be used to silence BORIS in cancer cells, which results
in apoptosis and may be useful as for treating cancer in mammals. The methods of the invention directed to cancer therapy can be used
alone or in combination with standard cancer treatments such as surgery, radiation, chemotherapy, and immunotherapy.
Patent
No: 8263571
METHODS
AND MEANS OF GENERATING IL-17 ASSOCIATED ANTITUMOR EFFECTOR CELLS BY INHIBITION OF NR2F6 INHIBITION
Means,
methods, and compositions of matter useful for generation of cancer inhibitory effector cells producing interleukin-17 (IL-17). In one
embodiment a cellular population is obtained, said cellular population is exposed to agents capable of inhibiting NR2F6, whereby said
inhibition of NR2F6 results in upregulation of IL-17 production, said upregulation of IL-17 production associated with acquisition of
anti-tumor activity.
Patent
No : 11,053,503
METHODS
OF SCREENING COMPOUNDS THAT CAN MODULATE NR2F6 BY DISPLACEMENT OF A REFERENCE LIGAND
Compositions
of matter, protocols and methods of screening test compounds to identifying agonists and antagonists of the orphan nuclear receptor NR2F6
by measuring the ability of a test compound to occupy the active site of NR2F6, in the presence of a reference compound.
Patent
No: 10,088,485
MODULATION
OF NR2F6 AND METHODS AND USES THEREOF
The
application provides methods of modulating NR2F6 in a cell or animal in need thereof by administering an effective amount of a NR2F6
modulator
Patent
No: 9091696
“UNIVERSAL
DONOR CHECKPOINT INHIBITOR SILENCED/GENE EDITED CORD BLOOD KILLER CELLS”
The
invention encompasses compositions of matters, cells, and treatment protocols useful for induction of anticancer responses in a patient
suffering from cancer. In one embodiment the invention provides the use of NR2F6 silencing or gene editing in cord blood cells possessing
anti-tumor activity in order to induce potentiated killer cells suitable for therapeutic use. In one embodiment said allogeneic cord
blood killer cells are administered to initiate a cascade of antitumor immune responses, with initially responses mediated by allogeneic
killer cells, and followed by endogenous immune responses.
Patent
No: 11,141,471 B2
ANTIGEN
SPECIFIC MRNA CELLULAR CANCER VACCINES
Antigen
specific cancer vaccines in which immunogenic epitopes are produced intracellularly by administration of modified mRNA encoding said
immunogenic epitopes. In one embodiment of the invention, said modified mRNA encodes peptides derived from the protein survivin. By directly
inducing gene expression of the antigens to which an immune response is desired, immunogenic peptides are generated intracellularly,
thus allowing for a wider repertoire of epitopes to be presented to the adaptive immune system, which augments likelihood of successful
induction of immunity.
Patent
No. 11,090,332
METHOD
OF CANCER TREATMENT USING SIRNA SILENCING
Comprises
administering to a subject one or more siRNA constructs capable of inhibiting the expression of an immunosuppressive molecule. The invention
also provides siRNA constructs and compositions.
Patent
No: 8389708
SMALL
MOLECULE AGONISTS AND ANTAGONISTS OF NR2F6 ACTIVITY IN HUMANS.
Patent
No. 11,324,719
The
invention relates to compounds useful to alteration of NR2F6 activity.
Patent
No. 11,712,474
Means
of stimulating systemic immunity and reduction of post-surgery tumor metastasis through the concurrent intralymphatic inhibition of NR2F6
and treatment with cannabidiol. Through the combination of immunogenic cell death and immune stimulation, the invention provides a means
of enhancing the abscopal effect and in some embodiments to cause immunological mediated destruction primary and secondary neoplasia.
Patent
No. 11,241,427
Compounds
useful for alteration of NR2F6 activity.
Patent
no. 11,655,474
Means,
methods and compositions of matter useful for suppressing pathological production of new blood vessels in conditions such as cancer and
wet macular degeneration. In one embodiment the invention provides silencing of NR2F6 using nucleic acid based approaches such as RNA
interference, antisense oligonucleotides, or DICER. In another embodiment, the invention teaches the administration of small molecule
NR2F6 inhibitors as means of selectively inhibiting pathological but not healthy angiogenesis.
Active
Patent Applications:
| Title | |
Application Number | |
| Enhancement Of Chimeric Antigen Receptor T Cell Efficacy By Dedifferentiation | |
| 18447150 | |
| Enhanced Dendritic Cell Immune Activation By Combined Inhibition Of NR2F6 With Cannibidiol | |
| 62882931 | |
| Immune Modulation By TLR Activation For Treatment Of Filovirus Infections Including Ebola | |
| 14954902 | |
| Nr2f6 Silenced Autologous Immunotherapeutics | |
| 15299400 | |
| Treatment Of Liver Cancer Through Embolization Depot Delivery Of BORIS Gene Silencing Agents | |
| 15250877 | |
| Acceleration Of Hematopoietic Reconstitution By Placental, Endothelial And Endothelial Progenitor Cells | |
| 13 /897,735 | |
| Cells, Compositions, And Treatment Methods For Stimulation Of Hematopoiesis | |
| 13 /957,427 | |
| Cancer Therapy By Ex Vivo Activated Autologous Immune Cells | |
| 13 /957,431 | |
| Nr2f6 Inhibited Chimeric Antigen Receptor Cells | |
| 62254330 | |
| Personalized T Cell Immunotherapy Utilizing Nr2f6 Gene Silencing | |
| 15402151 | |
| Reduction Of Post-Surgery Cancer Metastasis By Combination Of Cannabidiol And NR2F6 Inhibition | |
| 62885740 | |
| Stimulation Of Immunity To Tumor Specific And Endothelial Specific Proteins By In Vivo DC Attractio | |
| 62 /050,418 | |
| Augmentation Of Survivin Modified Mrna Vaccine Efficacy Using Dendritic Cells | |
| 18 /358,432 | |
| Enhancement Of T Cell Homing To Tumors Through Augmentation Of Chemokine Responsiveness And Activation Dependent Chemokine Secretion | |
| 63 /410,205 | |
| Combination Therapy Of Solid Tumors Using Chimeric Antigen Receptor Cells Representing Adaptive And Innate Immunity | |
| 18 /455,544 | |
| Dual Checkpoint Inhibitor Aptamer Based Therapeutics | |
| 63 /406,160 | |
| Modulation Of Tumor Microenvironment To Augment Efficacy Of Immunotherapy | |
| 63 /384,754 | |
| Generation Of Tolerance Promoting CAR-T Cells By Enhancement Of NR2F6 | |
| 63 /520,062 | |
| Stimulation Of T Regulatory Cells By Cannabidiol As A Means Of Treating Arthritis And Autoimmunity | |
| 17 /010,720 | |
| Activation Of Survivin-Specific Immune Responses Using Dendritic Cell Derived Exosomes Alone And/Or From Sirna / Gene Edited Dendritic Cells | |
| 63 /439,526 | |
License
Agreements:
On
June 23, 2015 Regen Biopharma, Inc. (“Regen”) entered into an agreement (“Agreement”) with Zander Therapeutics,
Inc. (“Zander”) whereby Regen granted to Zander an exclusive worldwide right and license for the development and commercialization
of certain intellectual property controlled by Regen (“ License IP”) for non-human veterinary therapeutic use for a term
of fifteen years. Zander is under common control with the Company.
Pursuant
to the Agreement, Zander shall pay to Regen one-time, non-refundable, upfront payment of one hundred thousand US dollars ($100,000) as
a license initiation fee which must be paid within 90 days of June 23, 2015 and an annual non-refundable payment of one hundred thousand
US dollars ($100,000) on July 15th, 2016 and each subsequent anniversary of the effective date of the Agreement.
The
abovementioned payments may be made, at Zander’s discretion, in cash or newly issued common stock of Zander or in common stock
of Entest BioMedical Inc. valued as of the lowest closing price on the principal exchange upon which said common stock trades publicly
within the 14 trading days prior to issuance.
Pursuant
to the Agreement, Zander shall pay to Regen royalties equal to four percent (4%) of the Net Sales, as such term is defined in the Agreement,
of any Licensed Products, as such term is defined in the Agreement, in a Quarter.
Pursuant
to the Agreement, Zander will pay Regen ten percent (10%) of all consideration (in the case of in-kind consideration, at fair market
value as monetary consideration) received by Zander from sublicensees (excluding royalties from sublicensees based on Net Sales of any
Licensed Products for which Regen receives payment pursuant to the terms and conditions of the Agreement).
Zander
is obligated pay to Regen minimum annual royalties of ten thousand US dollars ($10,000) payable per year on each anniversary of the Effective
Date of this Agreement, commencing on the second anniversary of June 23, 2015. This minimum annual royalty is only payable to the extent
that royalty payments made during the preceding 12-month period do not exceed ten thousand US dollars ($10,000).
The
Agreement may be terminated by Regen:
If
Zander has not sold any Licensed Product by ten years of the effective date of the Agreement or Zander has not sold any Licensed Product
for any twelve (12) month period after Zander’s first commercial sale of a Licensed Product.
The
Agreement may be terminated by Zander with regard to any of the License IP if by five years from the date of execution of the Agreement
a patent has not been granted by the United States patent and Trademark Office to Regen with regard to that License IP.
The
Agreement may be terminated by Zander with regard to any of the License IP if a patent that has been granted by the United States patent
and Trademark Office to Regen with regard to that License IP is terminated.
The
Agreement may be terminated by either party in the event of a material breach by the other party.
On
December 17, 2018 Regen Biopharma, Inc.(“Licensor”), KCL Therapeutics, Inc. (“Assignee”) and Zander Therapeutics,
Inc. (“Licensee”) entered into a LICENSE ASSIGNMENT AND CONSENT AGREEMENT whereby, with regards to certain intellectual property
which was assigned by Regen Biopharma, Inc.(“Assigned Properties”) to its wholly owned subsidiary KCL Therapeutics, Inc.,
Licensor hereby transfers and assigns to Assignee all rights, duties, and obligations of Licensor under the Agreement with respect to
the Assigned Properties, and Assignee agrees to assume such duties and obligations thereunder and be bound to the terms of the Agreement
with respect thereto.
On
April 7, 2021 Regen Biopharma, Inc. (“Regen”) entered into an agreement (“Agreement”) with Oncology Pharma, Inc.
(“Licensee”) whereby Regen granted to Licensee an exclusive right and license for the development and commercialization of
certain intellectual property (“License IP”) for the treatment in humans of pancreatic cancer for a term of fifteen years
from April 7, 2021.
The
License IP consists of antigen specific cancer vaccines in which modified mRNA is administered to produce epitopes able to produce an
immune response which augments likelihood of successful induction of immunity. An epitope is the part of an antigen that is recognized
by the immune system.
As
consideration to Regen for the rights and license granted pursuant to the Agreement Licensee shall:
(a)
pay to Regen a nonrefundable fee of $55,000 no later than April 20,2021
(b)
pay to Regen royalties equal to five percent (5%) of the Net Sales as Net Sales are defined in the Agreement of any Licensed Products
in a quarter.
(c)
pay to Regen ten percent (10%) of all consideration (in the case of in-kind consideration, at fair market value as monetary consideration)
received by Licensee from sublicensees, excluding royalties from sublicensees based on Net Sales of any Licensed Products for which Regen
receives payment.
Licensed
Product is defined in the Agreement as (a) any method, procedure, service or process that incorporates, uses, used, is covered by, infringes
or would infringe any of the License IP in the U.S. or foreign jurisdictions; and (b) any apparatus, material, equipment, machine or
other product that incorporates, uses, used, is covered by, infringes or would infringe any of the License IP in the U.S. or foreign
jurisdictions but for the rights granted pursuant to the Agreement.
In
the event that development of the License IP by the Licensee is not commenced as of the date that is nine months from the effective date
of the Agreement the rights and license granted pursuant to the Agreement shall become nonexclusive.
The
foregoing description of the Agreement is not complete and is qualified in its entirety by reference to the text of the Agreement, which
is attached to this Current Report on Form 8-K as Exhibit 10.1 and incorporated in this Item 1.01 by reference.
On
April 7, 2021 KCL Therapeutics, Inc. (“KCL”) entered into an agreement (“Agreement”) with Oncology Pharma, Inc.
(“Licensee”) whereby KCL granted to Licensee an exclusive right and license for the development and commercialization of
certain intellectual property (“License IP”) for the treatment in humans of colon cancer for a term of fifteen years from
April 7, 2021.
As
consideration to KCL for the rights and license granted pursuant to the Agreement Licensee shall:
(a)
pay to KCL a nonrefundable fee of Fifty Thousand common shares of Oncology Pharma, Inc. no later than April 20,2021
(b)
pay to KCL royalties equal to five percent (5%) of the Net Sales as Net Sales are defined in the Agreement of any Licensed Products in
a quarter.
(c)
pay to KCL ten percent (10%) of all consideration (in the case of in-kind consideration, at fair market value as monetary consideration)
received by Licensee from sublicensees, excluding royalties from sublicensees based on Net Sales of any Licensed Products for which KCL
receives payment.
Licensed
Product is defined in the Agreement as (a) any method, procedure, service or process that incorporates, uses, used, is covered by, infringes
or would infringe any of the License IP in the U.S. or foreign jurisdictions; and (b) any apparatus, material, equipment, machine or
other product that incorporates, uses, used, is covered by, infringes or would infringe any of the License IP in the U.S. or foreign
jurisdictions but for the rights granted pursuant to the Agreement.
In
the event that development of the License IP by the Licensee is not commenced as of the date that is nine months from the effective date
of the Agreement the rights and license granted pursuant to the Agreement shall become nonexclusive.
Zander
and Regen are under common control. David Koos serves as sole officer and director of both Regen BioPharma, Inc. and Zander Therapeutics
Inc.
Both
Zander and Oncology Pharma, Inc. will be required to obtain approval from the United States Food and Drug Administration (“FDA”)
in order to market any Licensed Product which may be developed within the United States and no assurance may be given that such approval
would be granted.
Principal
Products and Services
The
Company has begun development of HemaXellerate, a cellular therapy designed to heal damaged bone marrow. HemaXellerate is a patient-specific
composition of cells that have been demonstrated to repair damaged bone marrow and stimulate production of blood cells based in previous
animal studies. The initial application of HemaXellerate will be the treatment of severe aplastic anemia which is characterized by immune-mediated
bone marrow hypoplasia (underdevelopment or incomplete development of a tissue) and pancytopenia (reduction in the number of blood cells
and platelets).
Adipose
tissue is collected from the patient and processed in order to separate, extract and isolate Stromal Vascular Fraction (SVF), a mix of
various cell types including mesenchymal stem cells and endothelial cells. Mesenchymal stem cells are connective tissue cells that can
differentiate into a variety of cell types and endothelial cells are the cells that line the interior surface of blood vessels and lymphatic
vessels and which play a vital role in angiogenesis (the physiological process through which new blood vessels form from pre-existing
vessels).
The
isolated SVF is then intravenously administered to the patient. The Company believes that the isolated SVF will generate growth factors
with the ability to repair damaged hematopoietic stem cells. Hematopoietic stem cells are immature cells that can develop into all types
of blood cells, including white blood cells, red blood cells, and platelets. Hematopoietic stem cells are found in the peripheral blood
and the bone marrow.
On
February 5, 2013 Regen filed an Investigational New Drug (IND) application with the United States Food and Drug Administration (“FDA”)
to initiate a Phase I clinical trial assessing HemaXellerate in patients with drug-refractory aplastic anemia. The Phase I clinical trial
is intended to determine safety and potential efficacy of intravenously administered autologous SVF cells in patients with severe, immune
suppressive refractory aplastic anemia with the primary endpoints of safety and feasibility and secondary endpoints of efficacy as determined
by patients having complete response, partial response or relapse.
Under
the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a previously unapproved drug or biologic intended to
treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals annually in the
United States. Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication
for which it has such designation, the product is entitled to a seven year period of marketing exclusivity, which precludes the FDA from
approving another marketing application for the same drug for that time period. The sponsor of the product would also be entitled to
a United States federal tax credit equal to 50% of clinical investigation expenses as well as exemptions from certain fees.
The
Company believes that this application of HemaXellerate qualifies for Orphan designation under the Orphan Drug Act due to the fact that
aplastic anemia is a rare disease with prevalence in the United States of less than 200,000 and intends to apply to the FDA for Orphan
designation for HemaXellerate.
On
December 10, 2015 Regen was informed by the United States Food and Drug Administration that Regen has satisfactorily addressed all clinical
hold issues related to Regen’s Investigational New Drug Application for HemaXellerate and may initiate a Phase I clinical trial
assessing HemaXellerate in patients with drug-refractory aplastic anemia. The Phase I clinical trial is intended to determine safety
and potential efficacy of intravenously administered autologous stromal vascular fraction (SVF) cells in patients with severe, immune
suppressive refractory aplastic anemia with the primary endpoints of safety and feasibility and secondary endpoints of efficacy as determined
by patients having complete response, partial response or relapse.
dCellVax
is intended to be a therapy whereby dendritic cells of the cancer patient are harvested from the body, treated with siRNA that has the
ability to block the dendritic cell from expressing indoleamine 2,3-dioxygenase (“IDO”) and subsequently reimplanted in the
cancer patient.
The
dendritic cells that are treated with the IDO-blocking RNA become resistant to the influence of tumor cells which produce factors which
cause the dendritic cell to express the IDO. Expression of IDO in the dendritic cell halts the dendritic cell from activating T cells
and causes the dendritic cell to suppress T cells. T lymphocytes (‘T cells”) are a lymphocyte that play a central role in
the human immune system’s attempt to eradicate tumors. The Company has filed an Investigational New Drug (IND) application with
the United States Food and Drug Administration (“FDA”) to initiate a Phase I/II clinical trial assessing safety with signals
of efficacy of the dCellVax gene-silenced dendritic cell immunotherapy for treating breast cancer. The proposed trial will recruit 10
patients with metastatic breast cancer and will involve 4 monthly injections of the dCellVax gene-silenced dendritic cell therapy. The
trial is anticipated to last one year, with tumor assessment before therapy and at 6 and 12 months.
On
May 12, 2021 the “Company executed a consulting agreement with Biotech Research Group Corporation, an FDA Specialist Group and
Global Regulatory and Scientific Experts, for the purpose of review and guidance with regard to the planned reinstatement of the Company’s
inactive Investigational New Drug applications (INDs) #15376 (HemaXellerate) and #16200 (dCellVax) filed with the United States Food
and Drug Administration (“FDA”). The securing of the services to be provided to the Company pursuant to this consulting agreement
marks the first step taken by the Company with regard to activating the Company’s currently inactive applications to initiate clinical
trials.
tCellVax
is intended to be a therapy where immune cells are removed from the cancer patient, treated with siRNA which inhibits NR2F6 and the cells
re-infused to the patient. NR2F6 normally acts as a brake on the ability of various immune cells from being activated. The immune cells
that are treated with the NR2F6-blocking siRNA become highly activated and can efficiently kill tumors. The Company has filed an Investigational
New Drug (IND) application with the United States Food and Drug Administration (“FDA”) to initiate a Phase I clinical trial
assessing safety and feasibility of the dCellVax gene-silenced immune cell immunotherapy for treating patients with solid tumors that
are metastatic or not able to be removed surgically. The proposed trial will recruit 25 patients with metastatic cancer and will involve
3 monthly injections of the dCellVax gene-silenced dendritic cell therapy. The trial is anticipated to last one year, with tumor assessment
before therapy and at 6 and 12 months.
DiffronC:
NR2F6 is a transcription factor that is present in many cells in the body, including immune cells but also highly expressed in certain
solid tumors. NR2F6 normally acts as a brake on the ability of various immune cells from being activated and also allows tumor cells
to keep growing. The Company has developed a proprietary drug that is based on shRNA technology, which prevents NR2F6 from being expressed.
By inhibiting the expression of NR2F6, immune cells that are treated with the NR2F6-blocking shRNA become highly activated and can efficiently
kill tumors and tumors that have NR2F6 suppressed begin to differentiate. We are currently in pre-clinical testing of this drug to optimize
its delivery in vivo.
DuraCar:
DuraCar is a new cellular therapy being developed by the Company. It is comprised of CAR-T cells which contain an shRNA targeting the
gene NR2F6. CAR-T cells are T cells (the lymphoid cells of the body that kill tumors) isolated from a cancer patient that have been modified
by expressing a chimeric antigen receptor (CAR) which is specific for the patient’s tumor. These CAR-T cells are then re-infused
back into the patient. The CAR-T cells then home in directly on the tumor because they have been given the tumor-specific address via
the CAR. While CAR-T cells are very effective in treating leukemias, they are not effective at treating most solid tumors. The reason
for this is believed to be that the CAR-T cells are “turned-off” by the physical environment surround solid tumors. By inhibiting
NR2F6, we expect our DuraCar cells to have greater efficacy and persistence than conventional CAR-T cells and create a new, optimal way
to manufacture CAR-T cells. We are currently in pre-clinical testing of this drug.
Experiments
performed on behalf of the Company by two unrelated contract research organizations (CROs) found that T cells which express the chimeric
antigen receptor (CAR) construct targeting CD19 and expressing siRNA for NR2F6 had high expression levels of NR2F6 mRNA. NR2F6 is considered
an immune checkpoint and thus increasing its activity is likely to lead to immune suppression which may be utilized in the development
of therapies for the treatment of autoimmune disorders.
Small
molecule: We have identified and patented a series of small molecules which can both activate and inhibit NR2F6. NR2F6 normally acts
as a brake on the ability of various immune cells from being activated and also allows tumor cells to keep growing. By inhibiting the
function of NR2F6 using small molecules, immune cells that are treated with the NR2F6-blocking agents, similar to using the shRNA approach,
should become highly activated and efficiently kill tumors. In addition, tumors that have NR2F6 blocked by using these small molecules
should begin to differentiate. Conversely, activating NR2F6 is expected to suppress the immune system. This ability to suppress the immune
system can be very useful for treating autoimmune disorders. We are currently in pre-clinical testing of these drugs.
None
of the abovementioned statements regarding any of our products in development are intended to be a prediction or conclusion of efficacy.
No clinical trials on our product candidates have commenced so no conclusions of efficacy can be made.
Research
Conducted
The
Company has begun development of HemaXellerate, a cellular therapy designed to heal damaged bone marrow. HemaXellerate is a patient-specific
composition of cells that have been demonstrated to repair damaged bone marrow and stimulate production of blood cells based in previous
animal studies. The initial application of HemaXellerate will be the treatment of severe aplastic anemia which is characterized by immune-mediated
bone marrow hypoplasia (underdevelopment or incomplete development of a tissue) and pancytopenia (reduction in the number of blood cells
and platelets).
Adipose
tissue is collected from the patient and processed in order to separate, extract and isolate Stromal Vascular Fraction (SVF), a mix of
various cell types including mesenchymal stem cells and endothelial cells. Mesenchymal stem cells are connective tissue cells that can
differentiate into a variety of cell types and endothelial cells are the cells that line the interior surface of blood vessels and lymphatic
vessels and which play a vital role in angiogenesis (the physiological process through which new blood vessels form from pre-existing
vessels).
The
isolated SVF is then intravenously administered to the patient. The Company believes that the isolated SVF will generate growth factors
with the ability to repair damaged hematopoietic stem cells. Hematopoietic stem cells are immature cells that can develop into all types
of blood cells, including white blood cells, red blood cells, and platelets. Hematopoietic stem cells are found in the peripheral blood
and the bone marrow.
On
February 5, 2013 Regen filed an Investigational New Drug (IND) application with the United States Food and Drug Administration (“FDA”)
to initiate a Phase I clinical trial assessing HemaXellerate in patients with drug-refractory aplastic anemia. The Phase I clinical trial
is intended to determine safety and potential efficacy of intravenously administered autologous SVF cells in patients with severe, immune
suppressive refractory aplastic anemia with the primary endpoints of safety and feasibility and secondary endpoints of efficacy as determined
by patients having complete response, partial response or relapse.
Under
the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a previously unapproved drug or biologic intended to
treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals annually in the
United States. Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication
for which it has such designation, the product is entitled to a seven year period of marketing exclusivity, which precludes the FDA from
approving another marketing application for the same drug for that time period. The sponsor of the product would also be entitled to
a United States federal tax credit equal to 50% of clinical investigation expenses as well as exemptions from certain fees.
The
Company believes that this application of HemaXellerate qualifies for Orphan designation under the Orphan Drug Act due to the fact that
aplastic anemia is a rare disease with prevalence in the United States of less than 200,000 and intends to apply to the FDA for Orphan
designation for HemaXellerate.
On
December 10, 2015 Regen was informed by the United States Food and Drug Administration that Regen has satisfactorily addressed all clinical
hold issues related to Regen’s Investigational New Drug Application for HemaXellerate and may initiate a Phase I clinical trial
assessing HemaXellerate in patients with drug-refractory aplastic anemia. The Phase I clinical trial is intended to determine safety
and potential efficacy of intravenously administered autologous stromal vascular fraction (SVF) cells in patients with severe, immune
suppressive refractory aplastic anemia with the primary endpoints of safety and feasibility and secondary endpoints of efficacy as determined
by patients having complete response, partial response or relapse.
The
costs to perform this Phase I clinical trial is estimated to be approximately $5,000,000 and it is estimated to take 1 year to complete.
The
company is developing another cell therapy product termed dCellVax. dCellVax is intended to be a therapy whereby dendritic cells of the
cancer patient are harvested from the body, treated with siRNA that has the ability to block the dendritic cell from expressing indoleamine
2,3-dioxygenase (“IDO”) and subsequently reimplanted in the cancer patient.
The
dendritic cells that are treated with the IDO-blocking RNA become resistant to the influence of tumor cells which produce factors which
cause the dendritic cell to express the IDO. Expression of IDO in the dendritic cell halts the dendritic cell from activating T cells
and causes the dendritic cell to suppress T cells. T lymphocytes (‘T cells”) are a lymphocyte that play a central role in
the human immune system’s attempt to eradicate tumors. The Company has filed an Investigational New Drug (IND) application with
the United States Food and Drug Administration (“FDA”) to initiate a Phase I/II clinical trial assessing safety with signals
of efficacy of the dCellVax gene-silenced dendritic cell immunotherapy for treating breast cancer. The proposed trial will recruit 10
patients with metastatic breast cancer and will involve 4 monthly injections of the dCellVax gene-silenced dendritic cell therapy. The
trial is anticipated to cost $5,000,000 and last one year, with tumor assessment before therapy and at 6 and 12 months.
On
May 12, 2021 the “Company executed a consulting agreement with Biotech Research Group Corporation, an FDA Specialist Group and
Global Regulatory and Scientific Experts, for the purpose of review and guidance with regard to the planned reinstatement of the Company’s
inactive Investigational New Drug applications (INDs) #15376 (HemaXellerate) and #16200 (dCellVax) filed with the United States Food
and Drug Administration (“FDA”). The securing of the services to be provided to the Company pursuant to this consulting agreement
marks the first step taken by the Company with regard to activating the Company’s currently inactive applications to initiate clinical
trials.
Another
cell therapy that focuses on a different mechanism of action than dCellVax is tCellVax. tCellVax is intended to be a therapy in which
immune cells are removed from the cancer patient, treated with siRNA which inhibits NR2F6 and the cells re-infused to the patient. NR2F6
normally acts as a brake on the ability of various immune cells from being activated. The immune cells that are treated with the NR2F6-blocking
siRNA become highly activated and can efficiently kill tumors. The Company has filed an Investigational New Drug (IND) application with
the United States Food and Drug Administration (“FDA”) to initiate a Phase I clinical trial assessing safety and feasibility
of the dCellVax gene-silenced immune cell immunotherapy for treating patients with solid tumors that are metastatic or not able to be
removed surgically. The proposed trial will recruit 25 patients with metastatic cancer and will involve 3 monthly injections of the dCellVax
gene-silenced dendritic cell therapy. The trial is anticipated to cost $5,000,000 and last one year, with tumor assessment before therapy
and at 6 and 12 months.
DiffronC:
NR2F6 is a transcription factor that is present in many cells in the body, including immune cells but also highly expressed in certain
solid tumors. NR2F6 normally acts as a brake on the ability of various immune cells from being activated and also allows tumor cells
to keep growing. The Company has developed a proprietary drug that is based on shRNA technology, which prevents NR2F6 from being expressed.
By inhibiting the expression of NR2F6, immune cells that are treated with the NR2F6-blocking shRNA become highly activated and can efficiently
kill tumors and tumors that have NR2F6 suppressed begin to differentiate. We are currently in pre-clinical testing of this drug to optimize
its delivery in vivo. The two main risks associated with this drug development plan is that the NR2F6 siRNA is not effective at inhibiting
NR2F6 expression or that this inhibition will not result in immune cells with enhanced tumoricidal activity.
DuraCar:
DuraCar is a new cellular therapy being developed by the Company. It is comprised of CAR-T cells which contain an shRNA targeting the
gene NR2F6. CAR-T cells are T cells (the lymphoid cells of the body that kill tumors) isolated from a cancer patient that have been modified
by expressing a chimeric antigen receptor (CAR) which is specific for the patient’s tumor. These CAR-T cells are then re-infused
back into the patient. The CAR-T cells then home in directly on the tumor because they have been given the tumor-specific address via
the CAR. While CAR-T cells are very effective in treating leukemias, they are not effective at treating most solid tumors. The reason
for this is believed to be that the CAR-T cells are “turned-off” by the physical environment surround solid tumors. By inhibiting
NR2F6, we expect our DuraCar cells to have greater efficacy and persistence than conventional CAR-T cells and create a new, optimal way
to manufacture CAR-T cells. We have engaged two contract research organizations to advance our pre-clinical testing of this drug. Pre-clinical
testing includes design and construction of the relevant plasmids, efficient transfection of T cells, assessment of the expression levels
of the siRNA directed at NR2F6 and measurement of its effectiveness at inhibition of NR2F6 expression. Then, these cells will be analyzed
for enhanced tumor-killing activity. The two main risks associated with this drug development plan is that the NR2F6 siRNA is not effective
at inhibiting NR2F6 expression or that this inhibition will not result in a T cell with enhanced tumoricidal activity. Successful completion
of these pre-clinical experiments will significantly de-risk the project.
Experiments
performed on behalf of the Company by two unrelated contract research organizations (CROs) found that T cells which express the chimeric
antigen receptor (CAR) construct targeting CD19 and expressing siRNA for NR2F6 had high expression levels of NR2F6 mRNA. NR2F6 is considered
an immune checkpoint and thus increasing its activity is likely to lead to immune suppression which may be utilized in the development
of therapies for the treatment of autoimmune disorders
Small
Molecule Drugs: We have identified and patented a series of small molecules which can both activate and inhibit NR2F6. NR2F6 normally
acts as a brake on the ability of various immune cells from being activated and also allows tumor cells to keep growing. By inhibiting
the function of NR2F6 using small molecules, immune cells that are treated with the NR2F6-blocking agents, similar to using the shRNA
approach, should become highly activated and efficiently kill tumors. In addition, tumors that have NR2F6 blocked by using these small
molecules should begin to differentiate. Conversely, activating NR2F6 is expected to suppress the immune system. This ability to suppress
the immune system can be very useful for treating autoimmune disorders. We are currently in pre-clinical testing of these drugs.
Distribution
methods of the products or services:
It
is anticipated that Regen and /or KCL will enter into licensing and/or sublicensing agreements with outside entities in order that Regen
and/or KCL may obtain royalty income on the products and services which it may develop and commercialize.
Competitive
business conditions and Regen’s competitive position in the industry and methods of competition
We
have yet to achieve significant revenues or profits. The pharmaceutical and biologics industries in which we intend to compete are highly
competitive and characterized by rapid technological advancement. Many of our competitors have greater resources than we do.
We
intend to be competitive by utilizing the services and advice of individuals that we believe have expertise in their field in order that
we can concentrate our resources on projects in which products and services in which we have the greatest potential to secure a competitive
advantage may be developed and commercialized. The Company’s intent is to enter into nonemployee consulting agreements with individuals
who we believe have a high level of expertise in their professional fields and who have agreed to provide counsel and assistance to us
in (a) determining the viability of proposed projects (b) obtaining financing for projects and (c) obtaining the resources required to
initiate and complete a project in the most cost effective and rapid manner.
Sources
and availability of raw materials and the names of principal suppliers
The
supplies and materials required to conduct our operations are available through a wide variety of sources and may be obtained through
a wide variety of sources.
Need
for any government approval of principal products or services, effect of existing or probable governmental regulations on the business.
The
US Food and Drug Administration (“FDA”) and foreign regulatory authorities will regulate our proposed products as drugs or
biologics, depending upon such factors as the use to which the product will be put, the chemical composition, and the interaction of
the product on the human body. In the United States, products that are intended to be introduced into the body will generally be regulated
as drugs, while tissues and cells intended for transplant into the human body will be generally be regulated as biologics.
Our
domestic human drug and biological products will be subject to rigorous FDA review and approval procedures. After testing in animals,
an Investigational New Drug Application (“IND”) must be filed with the FDA to obtain authorization for human testing. Extensive
clinical testing, which is generally done in three phases, must then be undertaken at a hospital or medical center to demonstrate optimal
use, safety, and efficacy of each product in humans.
Phase
I
Phase
1 trials are designed to assess the safety (pharmacovigilance), tolerability, pharmacokinetics, and pharmacodynamics of a drug. These
trials are often conducted in an inpatient clinic, where the subject can be observed by full-time staff. The subject who receives the
drug is usually observed until several half-lives of the drug have passed. Phase I trials normally include dose-ranging, also called
dose escalation, studies so that the appropriate dose for therapeutic use can be found. The tested range of doses usually are a fraction
of the dose that causes harm in animal testing and involve a small group of healthy volunteers. However, there are some circumstances
when real patients are used, such as patients who have end-stage disease and lack other treatment options.
Phase
II
Phase
II trials are designed to assess how well the drug or biologic works, as well as to continue Phase I safety assessments in a larger group
of volunteers and patients. Phase II trials are performed on larger groups.
Phase
III
Phase
III trials are aimed at being the definitive assessment of how effective the product is in comparison with current best standard treatment
and to provide an adequate basis for physician labeling. Phase III trials may also be conducted for the purposes of (i) “label
expansion” (to show the product works for additional types of patients/diseases beyond the original use for which the drug was
approved for marketing or (ii) to obtain additional safety data, or to support marketing claims for the product.
On
occasion Phase IV (Post Approval) trials may be required by the FDA. Phase IV trials involve the safety surveillance (pharmacovigilance)
and ongoing technical support of a drug after it receives permission to be sold.The safety surveillance is designed to detect any rare
or long-term adverse effects over a much larger patient population and longer time period than was possible during the Phase I-III clinical
trials.
All
phases, must be undertaken at a hospital or medical center to demonstrate optimal use, safety, and efficacy of each product in humans.
Each clinical study is conducted under the auspices of an independent Institutional Review Board (“IRB”). The IRB will consider,
among other things, ethical factors, the safety of human subjects, and the possible liability of the institution. The time and expense
required to perform this clinical testing can far exceed the time and expense of the research and development initially required to create
the product. No action can be taken to market any therapeutic product in the United States until an appropriate New Drug Application
(“NDA”) or Biologic License Application (“BLA”) or has been approved by the FDA. FDA regulations also restrict
the export of therapeutic products for clinical use prior to NDA or BLA approval.
Even
after initial FDA approval has been obtained, further studies may be required to provide additional data on safety or to gain approval
for the use of a product as a treatment for clinical indications other than those initially targeted. In addition, use of these products
during testing and after marketing could reveal side effects that could delay, impede, or prevent FDA marketing approval, resulting in
FDA-ordered product recall, or in FDA-imposed limitations on permissible.
The
FDA regulates the manufacturing process of pharmaceutical products, and human tissue and cell products, requiring that they be produced
in compliance with Current Good Manufacturing Practices (“cGMP”). The FDA also regulates the content of advertisements used
to market pharmaceutical products. Generally, claims made in advertisements concerning the safety and efficacy of a product, or any advantages
of a product over another product, must be supported by clinical data filed as part of an NDA or an amendment to an NDA, and statements
regarding the use of a product must be consistent with the FDA approved labeling and dosage information for that product.
Sales
of drugs and biologics outside the United States are subject to foreign regulatory requirements that vary widely from country to country.
Even if FDA approval has been obtained, approval of a product by comparable regulatory authorities of foreign countries must be obtained
prior to the commencement of marketing the product in those countries. The time required to obtain such approval may be longer or shorter
than that required for FDA approval
Amount
spent during the fiscal year ended September 30, 2023 $212,297 on research and development activities
During
the fiscal year ended September 30, 2022 we expended $275,388 on research and development activities.
Costs
and effects of compliance with environmental laws (federal, state and local)
Regen
has not incurred any unusual or significant costs to remain in compliance with any environmental laws and does not expect to incur any
unusual or significant costs to remain in compliance with any environmental laws in the foreseeable future.
Number
of total employees and number of full-time employees
As
of November 4, 2024 the Company has 1 full time employee.
PROPERTIES
The
Company currently occupies 2,320 square feet of office space at 4700 Spring Street, Suite 304, La Mesa, California 91942. The property
is utilized as office space. We believe that the foregoing properties are adequate to meet our current needs for office space.
On
January 13, 2022 Regen Biopharma, Inc. entered into a sublease agreement with BST Partners (“BST”) whereby Regen Biopharma,
Inc. would sublet the aforementioned office space located at 4700 Spring Street, Suite 304, La Mesa, California 91942 from BST on a month
to month basis for $5,000 per month beginning January 14, 2022. BST Partners is controlled by David Koos who serves as the sole officer
and director of Regen Biopharma, Inc.
On
April 26, 2024 the Company and BST Partners ( Sublessor) agreed to amend that sublease agreement (“Sublease Agreement”) entered
into between the parties as follows:
The
Company agreed that in addition to the base rent of $5,000 per month to be paid by the Company to Sublessor the Company shall also reimburse
Sublessor for any and all shared expenses as such term is defined within the Sublease Agreement.
LEGAL
PROCEEDINGS.
None
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
As
of September 30, 2023 we had Cash of $ 121,037 and as of September 30, 2024 we had cash of $716. The decrease in cash of approximately
99% is primarily attributable to cash expensed in the operation of the Company’s business.:
As
of September 30, 2023 we had Accounts Receivable, Related Party of $0 and as of September 30, 2024 we had Accounts Receivable, Related
Party of $ 94,873. The increase in Accounts Receivable, Related Party is attributable to the accrual by Zander Therapeutics, Inc. of
licensing fees due and payable to the Company.
As
of September 30, 2023 we had Prepaid Expenses of $0 and as of September 30, 2024 we had prepaid expenses of $59,289 attributable to an
overpayment of $200 paid to a patent attorney , the payment of 20,068 shares of the Company’s Series A Preferred stock paid to
an independent consultant for services to be provided for a period of one year commencing on April 26, 2024 and 249,915 common shares
issued to a consultant for services to be provided over a six month period beginning September 20, 2024.
As
of September 30, 2024 we had Notes Payable of $293,819 ( net of Discount) and as of September 30, 2023 we had Notes Payable of $245,324(
Net of Discount) . The increase of 19% is primarily attributable to the issuance on September 4, 2024 of a Promissory Note in the face
amount of $250,000 as well as issuance of promissory Notes in the aggregate principal amount of $40,000 to Zander Therapeutics Inc. during
the quarter ended June 30, 2024 offset by the satisfaction of $226,500 in principal indebtedness during the year ended September 20,
2024. .
As
of September 30, 2024 we had Accrued Interest Payable of $363,533 and as of September 30, 2023 we had Accrued Interest Payable of $342,588.
The increase in Accrued Interest Payable of approximately 5.8% is attributable to interest accrued but unpaid during the year ended September
30, 2024 on Notes Payable and Convertible Notes Payable.
As
of September 30, 2023 we had Unearned Income of $1,591,731 and as of September 30, 2024 we had Unearned Income of $1,465,171. Unearned
Income represents that portion of $1,905,000 of license fees paid during the quarter ended June 30, 2021 to be recognized as revenue
over the 15 year term of the licenses granted in accordance with ASC 606. The decrease of 7.9% is attributable to the recognition by
the Company of $126,560 of licensing revenue over the year ended September 30, 2024.
As
of September 30, 2023 we had Unearned Income ( Related Party) of $15,126 and as of September 30, 2024 we had Unearned Income( Related
Party) of $0. Unearned Income (Related Party) as of September 30, 2023 consisted solely of licensing fees paid to the Company by Zander
Therapeutics, Inc. but not yet earned. Zander Therapeutics, Inc. and the Company are under common control.
As
of September 30, 2024 we had a Derivative Liability of $1,397,274 and as of September 30, 2023 we had a Derivative Liability of $1,400,000.
The decrease in Derivative Liability of approximately 19% is attributable to the recognition by the Company of embedded derivatives on
Convertible Notes Payable with an aggregate face value of $350,000 outstanding as of September 30, 2024 as opposed to recognition by
the Company of embedded derivatives on Convertible Notes Payable with an aggregate face value of $350,000 outstanding as of September
30, 2023 .
Revenues
from continuing operations were $236,560 for the twelve months ended September 30, 2023 and $236,560 for the same period ended 2024.
$110,000 of revenue from related parties recognized during the twelve months ended September 30, 2023 and September 30, 2024 consisted
of anniversary expense receivable pursuant to a license granted by the Company to Zander Therapeutics, Inc. and minimum royalties recognized
during the twelve months ended September 30, 2023 and 2022 respectively pursuant to the same license. $126,560 of revenue recognized
during the twelve months ended September 30, 2023 and September 30, 2024 were recognized pursuant to licenses granted to Oncology Pharma,Inc.
The
Company recognized an Operating Loss of $689,650 during the twelve months ended September 30, 2023 whereas the Company recognized an
Operating Loss of 418,189 for the same period ended 2024. The large disparity in Operating Losses is primarily attributable to $606,237
in Consulting and Professional fees expensed during the period ended 2023. The Company recognized Net Income of $1,156,507 for the twelve
months ended September 30, 2023 as opposed to a Net Loss of $867,252 for the same period ended 2034 the difference primarily attributable
to the recognition by the Company of Derivative Income of $2,151,755 during the twelve months ended September 30, 2023.
As
of September 30, 2024 we had $716 in cash on hand and current liabilities of $5,371,640 such liabilities materially consisting of Accounts
Payable, Notes Payable, Convertible Notes Payable, Derivative Liability Recognized, Unearned Income and Accrued Expenses. We feel we
will not be able to satisfy our cash requirements over the next twelve months and shall be required to seek additional financing.
On
September 12, 2023 the Company entered into a common stock purchase agreement (the “Equity Line Agreement”) with Coventry
Enterprises LLC ( “Coventry”) providing for an equity financing facility (the “Equity Line”). The Equity Line
Agreement provides that upon the terms and subject to the conditions in the Equity Line Agreement, Coventry is committed to purchase
up to Ten Million Dollars ($10,000,000) of shares of common stock, $0.0001 par value per share (the “Common Stock”), over
the 36-month term of the Equity Line Agreement (the “Total Commitment”).
Under
the terms of the Equity Line Agreement, Coventry will not be obligated to purchase shares of Common Stock unless and until certain conditions
are met, including but not limited to a Registration Statement on Form S-1 (the “Registration Statement”) becoming effective
which registers Coventry’s resale of any Common Stock purchased by Coventry under the Equity Line.
From
time to time over the 36-month term of the Commitment Period ( as such term is defined in the Equity Line Agreement) the Company, in
its sole discretion, may provide Coventry with a draw down notice (each, a “Draw Down Notice”), to purchase a specified number
of shares of Common Stock (each, a “Draw Down Amount Requested”), subject to the limitations discussed below. The actual
amount of proceeds the Company will receive pursuant to each Draw Down Notice (each, a “Draw Down Amount”) is to be determined
by multiplying the Draw Down Amount Requested by the applicable purchase price. The purchase price of each share of Common Stock equals
80% of the lowest trading price of the Common Stock during the ten business days prior to the Draw Down Notice date (the “Pricing
Period”).
The
maximum number of shares of Common Stock requested to be purchased pursuant to any single Draw Down Notice cannot exceed the lesser of
(i) 200% of the Average Daily Traded Value ( as such term is defined in the Equity Line Agreement) during the ten business days immediately
preceding the Drawdown Notice Date or (ii) $250,000. The Company is prohibited from delivering a Draw Down Notice if the sale of shares
of Common Stock pursuant to the Draw Down Notice would cause the Company to issue and sell to Coventry or Coventry to acquire or purchase
an aggregate number of shares of Common Stock that would result in Coventry beneficially owning more than 4.99% of the issued and outstanding
shares of Common Stock of the Company.
During
the quarter ended December 31, 2023 the Company sold 244,199 common shares to Coventry pursuant to the terms and conditions of the Equity
Line Agreement for total cash consideration of $212,297.
During
the quarter ended March 31, 2024 the Company sold 364,057 common shares to Coventry pursuant to the terms and conditions of the Equity
Line Agreement for total cash consideration of $187,973.
During
the quarter ended June 30, 2024 the Company sold 258,456 common shares to Coventry pursuant to the terms and conditions of the Equity
Line Agreement for total cash consideration of $135,327.
On
July 12, 2024 the Company sold 135,242 common shares to Coventry pursuant to the terms and conditions of the Equity Line Agreement for
total cash consideration of $28,125.
As
of September 30, 2024 the Company was not party to any binding agreements which would commit Regen to any material capital expenditures.
FINANCIAL
STATEMENTS
INDEPENDENT
AUDITOR’S REPORT
To
the Board of Directors and Shareholders
Regen
Biopharma, Inc.
Opinion
on the Financial Statements
We
have audited the accompanying consolidated balance sheets of Regen Biopharma, Inc. (the “Company”) as of September 30, 2023
and the related consolidated statements of operations, shareholders’ equity, and cash flows for the year in the period ended September
30, 2023, and the related notes and schedules (collectively referred to as the financial statements). In our opinion, the financial statements
present fairly, in all material respects, the financial position of the Company as of September 30, 2023, and the results of its operations
and its cash flows for the year in the period ended September 30, 2023, in conformity with accounting principles generally accepted in
the United States of America.
Going
Concern
The
accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note
3 to the financial statements, the Company has suffered recurring losses from operations, has a net capital deficiency, and has stated
that substantial doubt exists about the Company’s ability to continue as a going concern. Management’s evaluation of the
events and conditions and management’s plans regarding these matters are also described in Note 3. The financial statements do
not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to this
matter.
Basis
for Opinion
We
conducted our audit in accordance with auditing standards generally accepted in the United States of America (GAAS). Our responsibilities
under those standards are further described in the Auditor’s Responsibilities for the Audit of the Financial Statements section
of our report. We are required to be independent of Regen Biopharma, Inc. and to meet our other ethical responsibilities, in accordance
with the relevant ethical requirements relating to our audit. We believe that the audit evidence we have obtained is sufficient and appropriate
to provide a basis for our audit opinion.
Responsibilities
of Management for the Financial Statements
Management
is responsible for the preparation and fair presentation of the financial statements in accordance with accounting principles generally
accepted in the United States of America, and for the design, implementation, and maintenance of internal control relevant to the preparation
and fair presentation of financial statements that are free from material misstatement, whether due to fraud or error.
In
preparing the financial statements, management is required to evaluate whether there are conditions or events, considered in the aggregate,
that raise substantial doubt about Regen Biopharma’s ability to continue as a going concern.
Auditor’s
Responsibilities for the Audit of the Financial Statements
Our
objectives are to obtain reasonable assurance about whether the financial statements as a whole are free from material misstatement,
whether due to fraud or error, and to issue an auditor’s report that includes our opinion. Reasonable assurance is a high level
of assurance but is not absolute assurance and therefore is not a guarantee that an audit conducted in accordance with GAAS will always
detect a material misstatement when it exists. The risk of not detecting a material misstatement resulting from fraud is higher than
for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of
internal control. Misstatements are considered material if there is a substantial likelihood that, individually or in the aggregate,
they would influence the judgment made by a reasonable user based on the financial statements.
In
performing an audit in accordance with GAAS, we:
| ● | Exercise
professional judgment and maintain professional skepticism throughout the audit. |
| ● | Identify
and assess the risks of material misstatement of the financial statements, whether due to
fraud or error, and design and perform audit procedures responsive to those risks. Such procedures
include examining, on a test basis, evidence regarding the amounts and disclosures in the
financial statements. |
| ● | Obtain
an understanding of internal control relevant to the audit in order to design audit procedures
that are appropriate in the circumstances, but not for the purpose of expressing an opinion
on the effectiveness of Regen Biopharma, Inc’s internal control. Accordingly, no such
opinion is expressed. |
| ● | Evaluate
the appropriateness of accounting policies used and the reasonableness of significant accounting
estimates made by management, as well as evaluate the overall presentation of the financial
statements. |
| ● | Conclude
whether, in our judgment, there are conditions or events, considered in the aggregate, that
raise substantial doubt about Regen Biopharma, Inc’s ability to continue as a going
concern for a reasonable period of time. |
We
are required to communicate with those charged with governance regarding, among other matters, the planned scope and timing of the audit,
significant audit findings, and certain internal control-related matters that we identified during the audit.
Critical
Audit Matter
Critical
audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be
communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and
(2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
Audit
Scope and Procedures:
We
conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (PCAOB) and U.S. Generally
Accepted Accounting Principles (GAAP). Those standards require that we plan and perform the audit to obtain reasonable assurance about
whether the financial statements are free of material misstatement, whether due to error or fraud.
An
audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also
includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial
statement presentation.
As
part of our audit, we focused on the following key areas:
1.
Balance Sheet
Assets:
Accounts
Receivable: A detailed review of accounts receivable was performed. We confirm that no material misstatements exist and the receivables
are appropriately stated. No allowance for doubtful accounts was deemed necessary based on management’s assessment.
Prepaid
Expenses and Investment Securities were audited, and the company’s treatment is compliant with U.S. GAAP. Prepaid expenses
are correctly amortized, and investment securities are valued appropriately at fair value.
Liabilities:
Convertible
Debt: The audit confirms that the convertible debt is accurately bifurcated into its debt and equity components, per ASC 470-20.
Accrued
Liabilities and Unearned Income: The balances related to accrued expenses and unearned income were traced to supporting documents.
All accruals were found to be accurate and properly classified.
Equity:
Stock-Based
Compensation: Common and preferred stock issued for debt, compensation, and interest were fairly valued at the grant date. These
transactions are properly reflected in equity, and stock-based compensation is reported in line with ASC 718.
2.
Profit and Loss (Statement of Operations)
Revenue
Recognition (ASC 606):
We
reviewed the Company’s revenue recognition policy and tested a sample of sales transactions. The revenue is recognized in accordance
with ASC 606, and the related-party transactions were found to be at arm’s length.
Cost
and Expenses:
The
treatment of Research & Development (R&D) and Consulting & Professional Fees complies with U.S. GAAP. R&D
costs are appropriately expensed as incurred, and consulting fees were traced to supporting agreements and services provided.
Other
Income (Expense):
Interest
Expense and Derivative Expenses were properly accounted for. The derivative instruments were terminated correctly, and gains
or losses on extinguishment were recognized accurately per ASC 815.
3.
Cash Flow Statement
Operating
Activities:
Adjustments
to net income, including stock-based compensation, changes in accounts receivable, accounts payable, and other operating items, were
tested for accuracy. The net cash used in operating activities was found to be properly reconciled.
Investing
Activities:
The
sale of investment securities for $25,000 was correctly recorded and reflected in both the cash flow and balance sheet, complying with
ASC 320.
Financing
Activities:
Proceeds
from notes payable ($243,750) and the issuance of convertible notes were confirmed to be correctly recorded. Stock issued
for interest payments was valued appropriately.
Noncash
Transactions:
The
company issued shares as compensation for debt and interest. These transactions were properly valued and disclosed, ensuring compliance
with ASC 470-50.
Audit
Conclusion:
Based
on the audit procedures performed, we are confident that:
The
consolidated balance sheet, income statement, and cash flow statement of Regen BioPharma, Inc. for the year ended September 30,
2023, are fairly presented in all material respects in conformity with U.S. GAAP.
The
financial statements reflect an accurate and fair representation of the financial condition, results of operations, and cash flows of
the company.
Key
transactions, including stock-based compensation, convertible debt, and related-party transactions, were properly valued, disclosed,
and presented.
All
material misstatements, if any, have been adjusted, and no further modifications are required.
Audit
Opinion:
In
our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position
of Regen BioPharma, Inc. as of September 30, 2023, and the results of its operations and its cash flows for the year then ended,
in conformity with U.S. Generally Accepted Accounting Principles (GAAP).
/s/Hardik
Joshi
CUBIXFIN,
LLC
HARDIK
JOSHI, CPA
LICENSE
NO. 56351
OCTOBER 24, 2024
REGEN
BIOPHARMA , INC.
CONDENSED
CONSOLIDATED BALANCE SHEETS
| | |
September
30, 2024 | | |
September
30, 2023 | |
| | |
(unaudited) | | |
| |
| ASSETS | |
| | |
| |
| CURRENT
ASSETS | |
| | | |
| | |
| Cash | |
$ | 716 | | |
$ | 121,037 | |
| Accounts
Recievable, Related Party | |
| 94,873 | | |
| 0 | |
| Note
Receivable, Related Party | |
| | | |
| 0 | |
| Prepaid
Expenses | |
| 59,289 | | |
| 0 | |
| Prepaid
Rent | |
| 5,000 | | |
| 10,000 | |
| Total
Current Assets | |
| 159,878 | | |
| 131,037 | |
| | |
| | | |
| | |
| OTHER
ASSETS | |
| | | |
| | |
| Investment
Securities, Related Party | |
| 17,733 | | |
| 222,580 | |
| Total
Other Assets | |
| 17,733 | | |
| 222,580 | |
| | |
| | | |
| | |
| TOTAL
ASSETS | |
$ | 177,611 | | |
$ | 353,617 | |
| | |
| | | |
| | |
| LIABILITIES
AND STOCKHOLDERS’ EQUITY | |
| | | |
| | |
| Current
Liabilities: | |
| | | |
| | |
| Accounts
payable | |
| 29,669 | | |
| 29,674 | |
| Notes
Payable | |
| 293,819 | | |
| 95,710 | |
| Accrued
payroll taxes | |
| 4,241 | | |
| 4,241 | |
| Accrued
Interest | |
| 362,533 | | |
| 342,588 | |
| Accrued
Payroll | |
| 1,256,630 | | |
| 1,256,630 | |
| Other
Accrued Expenses | |
| 41,423 | | |
| 41,423 | |
| Bank
Overdraft | |
| 1,000 | | |
| 1,000 | |
| Due
to Investor | |
| 20,000 | | |
| 20,000 | |
| Unearned
Income | |
| 1,465,171 | | |
| 1,591,731 | |
| Unearned
Income ( Related Party) | |
| 0 | | |
| 15,126 | |
| Derivative
Liability | |
| 1,397,274 | | |
| 1,400,000 | |
| Convertible
Notes Payable Less unamortized discount | |
| 499,880 | | |
| 499,880 | |
| Convertible
Notes Payable, Related Parties Less unamortized discount | |
| 0 | | |
| 10,000 | |
| Total
Current Liabilities | |
| 5,371,640 | | |
| 5,308,003 | |
| Long
Term Liabilities: | |
| | | |
| | |
| Convertible
Notes Payable, Related Parties Less unamortized discount | |
| | | |
| | |
| Notes
Payable | |
| 0 | | |
| 149,614 | |
| Total
Long Term Liabilities | |
| 0 | | |
| 149,614 | |
| Total
Liabilities | |
| 5,371,640 | | |
| 5,457,617 | |
| | |
| | | |
| | |
| STOCKHOLDERS’
EQUITY (DEFICIT) | |
| | | |
| | |
| |
| | | |
| | |
| Common Stock ($.0001 par value) 500,000,000 shares authorized;
5,800,000,000 authorized and 5,258,235 issued and outstanding
as of September 30, 2024 and 3,506,366 shares issuedand outstanding as of September 30,2023. | |
| 527 | | |
| 352 | |
| Preferred
Stock, 0.0001 par value, 800,000,000 authorized as of September 30, 2024 and September 30, 2023 respectively | |
| | | |
| | |
| Series
A Preferred 739,000,000 authorized as of September 30, 2023 and September 30,2024 10,123,771 outstanding as of September 30, 2024
and 409,551 outstanding as of September 30, 2023 | |
| 1,011 | | |
| 40 | |
| Series
AA Preferred $0.0001 par value 600,000 authorized and 34 and 34 outstanding as of September 30, 2024 and September 30,2023 respectively | |
| 0 | | |
| 0 | |
| Series
M Preferred $0.0001 par value 60,000,000 authorized and 29,338 outstanding as of September 30, 2023 and 60,000,000 authorized
and 29,338 outstanding as of September 30, 2024 | |
| 3 | | |
| 3 | |
| Series
NC Preferred $0.0001 par value 20,000 authorized and 15,007 outstanding as of September 30, 2023 and September 30, 2024 | |
| 2 | | |
| 2 | |
| Additional
Paid in capital | |
| 14,684,216 | | |
| 13,908,141 | |
| Contributed
Capital | |
| 736,326 | | |
| 736,326 | |
| Retained
Earnings (Deficit) | |
| (20,616,114 | ) | |
| (19,748,863 | ) |
| | |
| | | |
| | |
| Total
Stockholders’ Equity (Deficit) | |
| (5,194,029 | ) | |
| (5,104,000 | ) |
| | |
| | | |
| | |
| TOTAL
LIABILITIES & STOCKHOLDERS’ EQUITY (DEFICIT) | |
$ | 177,611 | | |
$ | 353,617 | |
The
Accompanying Notes are an Integral Part of These Financial Statements
All
stock amounts have been retroactively adjusted to reflect a 1 for 1500 reverse stock split of all issued series of stock effective as
of March 6, 2023
REGEN
BIOPHARMA , INC.
CONDENSED
CONSOLIDATED STATEMENTS OF OPERATIONS
| | |
Year
Ended
September 30, 2024 | | |
Year
Ended
September 30, 2023 | |
| | |
(unaudited) | | |
| |
| REVENUES | |
| | |
| |
| Revenues | |
$ | 126,560 | | |
$ | 126,560 | |
| Revenues,
Related Party | |
| 110,000 | | |
| 110,000 | |
| TOTAL
REVENUES | |
$ | 236,560 | | |
$ | 236,560 | |
| | |
| | | |
| | |
| COST
AND EXPENSES | |
| | | |
| | |
| Research
and Development | |
| 153,685 | | |
| 212,297 | |
| General
and Administrative | |
| 58,922 | | |
| 44,975 | |
| Consulting
and Professional Fees | |
| 364,927 | | |
| 606,237 | |
| Rent | |
| 77,215 | | |
| 60,000 | |
| Total
Costs and Expenses | |
| 654,749 | | |
| 923,509 | |
| | |
| | | |
| | |
| OPERATING
INCOME (LOSS) | |
$ | (418,189 | ) | |
$ | (686,950 | ) |
| | |
| | | |
| | |
| OTHER
INCOME & (EXPENSES) | |
| | | |
| | |
| Interest
Expense | |
| (72,445 | ) | |
| (58,584 | ) |
| Interest
Expense attributable to | |
| | | |
| | |
| Amortrization
of Discount | |
| (28,998 | ) | |
| (864 | ) |
| Unrealized
Gain ( Loss) on sale of Investment Securites | |
| (204,847 | ) | |
| 0 | |
| Derivative
Income (Expense) | |
| 2,726 | | |
| 2,151,755 | |
| Financing
Fees | |
| (145,500 | ) | |
| (250,000 | ) |
| Gain
(Loss) on Extinguishment Convertible Debt | |
| | | |
| 1150 | |
| TOTAL
OTHER INCOME (EXPENSE) | |
| (449,063 | ) | |
| 1,843,457 | |
| | |
| | | |
| | |
| NET
INCOME (LOSS) | |
$ | (867,252 | ) | |
$ | 1,156,507 | |
| NET
INCOME (LOSS) attributable to common shareholders | |
$ | (867,252 | ) | |
$ | 1,023,508 | |
| | |
| | | |
| | |
| BASIC
AND FULLY DILUTED EARNINGS (LOSS) PER SHARE | |
$ | (0.21 | ) | |
$ | 0.29 | |
| | |
| | | |
| | |
| WEIGHTED
AVERAGE NUMBER OF COMMON | |
| | | |
| | |
| SHARES
OUTSTANDING | |
| 4110265 | | |
| 3536963 | |
The
Accompanying Notes are an Integral Part of These Financial Statements
All
stock amounts have been retroactively adjusted to reflect a 1 for 1500 reverse stock split of all issued series of stock effective as
of March 6, 2023
REGEN
BIOPHARMA, INC.
CONDENSED
CONSOLIDATED STATEMENT OF SHAREHOLDERS’ EQUITY ( DEFICIT)
Years
Ended September 30, 2023 ( audited) and September 30, 2024 (unaudited)
| |
|
|
Series
A Preferred | | |
Series
AA Preferred | | |
Series
NC Preferred | | |
Common | | |
Series
M Preferred | | |
Additional Paid-in | | |
Retained | | |
Contributed | | |
Accumulated
Other
Comprehensive
| |
| |
| |
|
|
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Earnings | | |
Capital | | |
Income(Loss) | |
Total | |
| Balance
September 30,2022 |
|
|
| 293,053 | | |
$ | 28 | | |
| 34 | | |
$ | 0 | | |
| 7 | | |
$ | - | | |
| 3,354,886 | | |
$ | 335 | | |
| 29,338 | | |
$ | 3 | | |
$ | 12,132,620 | | |
$ | (20,905,369 | ) | |
$ | 736,326 | | |
| |
$ | (8,036,059 | ) |
| 10/25/2022 |
Preferred
Shares Issued for Nonemployee Services |
|
| 6,667 | | |
$ | 1 | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 299,999 | | |
| | | |
| | | |
| |
$ | 300,000 | |
| 11/11/2022 |
Preferred
Shares Issued for Debt |
|
| 70,114 | | |
$ | 7 | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 761,493 | | |
| | | |
| | | |
| |
$ | 761,500 | |
| 11/11/2022 |
Preferred
Shares Issued for Interest |
|
| 35,012 | | |
$ | 4 | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 380,258 | | |
| | | |
| | | |
| |
$ | 380,262 | |
| 11/11/2022 |
Common
Shares Issued For Interest |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 11,279 | | |
$ | 1 | | |
| | | |
| | | |
| 25,368 | | |
| | | |
| | | |
| |
| 25,369 | |
| 12/5/2022 |
Preferred
Shares Issued for Non employee Services |
|
| 1,112 | | |
$ | 0 | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 48,372 | | |
| | | |
| | | |
| |
$ | 48,372 | |
| |
Net
Income for the Quarter ended December 31, 2022 |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 1,635,730 | | |
| | | |
| |
| 1,635,730 | |
| Balance
December 31,2022 |
|
|
| 405,958 | | |
$ | 40 | | |
| 34 | | |
$ | 0 | | |
| 7 | | |
$ | - | | |
| 3,366,165 | | |
$ | 337 | | |
| 29,338 | | |
| 3 | | |
$ | 13,648,107 | | |
$ | (19,269,640 | ) | |
$ | 736,326 | | |
| |
$ | (4,884,827 | ) |
3/13/2023 |
Common
Shares issued pursuant to round up provision |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | |
| |
March
6,2023 reverse stock split |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 15,201 | | |
$ | 2 | | |
| | | |
| | | |
| (2 | ) | |
| | | |
| | | |
| |
| 0 | |
3/13/2023 |
Preferred
Shares issued pursuant to round up provision |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | |
| |
March
6,2023 reverse stock split |
|
| 3,593 | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | |
3/17/2023 |
Preferred
Shares issued for accrued salaries |
|
| | | |
| | | |
| | | |
| | | |
| 15,000 | | |
| 2 | | |
| | | |
| | | |
| | | |
| | | |
| 10,048 | | |
| | | |
| | | |
| |
| 10,050 | |
| Net
Income (Loss) for the Quarter Ended March 31, 2023 |
|
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (54,978 | ) | |
| | | |
| |
| (54,978 | ) |
| Balance
March 31, 2023 |
|
|
| 409,551 | | |
$ | 40 | | |
| 34 | | |
$ | 0 | | |
| 15,007 | | |
$ | 2 | | |
| 3,381,366 | | |
$ | 339 | | |
| 29,338 | | |
| 3 | | |
$ | 13,658,153 | | |
$ | (19,324,617 | ) | |
$ | 736,326 | | |
| |
$ | (4,929,755 | ) |
| |
|
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | |
| Net
Income (Loss) for the Quarter Ended June 30, 2023 |
|
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (99,218 | ) | |
| | | |
| |
| (99,218 | ) |
| Balance
June 30, 2023 |
|
|
| 409,551 | | |
$ | 40 | | |
| 34 | | |
$ | 0 | | |
| 15,007 | | |
$ | 2 | | |
| 3,381,366 | | |
$ | 339 | | |
| 29,338 | | |
| 3 | | |
$ | 13,658,153 | | |
$ | (19,423,836 | ) | |
$ | 736,326 | | |
| |
$ | (5,028,973 | ) |
| 9/12/2023 |
Common
shares issued for financing expenses |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 125,000 | | |
$ | 13 | | |
| | | |
| | | |
| 249,987.50 | | |
| | | |
| | | |
| |
| 250,000 | |
| Net
Income (Loss) for the Quarter Ended September 30, 2023 |
|
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (325,027.13 | ) | |
| | | |
| |
| (325,027 | ) |
| Balance
September 30, 2023 |
|
|
| 409,551 | | |
$ | 40 | | |
| 34 | | |
$ | 0 | | |
| 15,007 | | |
$ | 2 | | |
| 3,506,366 | | |
$ | 352 | | |
| 29,338 | | |
| 3 | | |
$ | 13,908,141 | | |
$ | (19,748,863 | ) | |
$ | 736,326 | | |
| |
$ | (5,104,000 | ) |
| 10/13/2023 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 16,710 | | |
| 2 | | |
| | | |
| | | |
| 22,724 | | |
| | | |
| | | |
| |
| 22,726 | |
| 10/27/2023 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 35,785 | | |
| 4 | | |
| | | |
| | | |
| 46,088 | | |
| | | |
| | | |
| |
| 46,091 | |
11/10/2023 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 31,732 | | |
| 3 | | |
| | | |
| | | |
| 38,202 | | |
| | | |
| | | |
| |
| 38,205 | |
| 11/27/2023 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 33,989 | | |
| 3 | | |
| | | |
| | | |
| 32,626 | | |
| | | |
| | | |
| |
| 32,629 | |
12/11/2023 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 43,297 | | |
| 4 | | |
| | | |
| | | |
| 38,097 | | |
| | | |
| | | |
| |
| 38,101 | |
12/20/2023 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 82,686 | | |
| 8 | | |
| | | |
| | | |
| 34,535 | | |
| | | |
| | | |
| |
| 34,543 | |
| Net
Income (Loss) Quarter Ended December 31, 2023 |
|
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (349,760 | ) | |
| | | |
| |
| (349,760 | ) |
| Balance
December 31, 2023 |
|
|
| 409,551 | | |
$ | 40 | | |
| 34 | | |
$ | 0 | | |
| 15,007 | | |
| | | |
| 3,750,565 | | |
| 376 | | |
| 29,338 | | |
| 3 | | |
| 14,120,412 | | |
| (20,098,623 | ) | |
$ | 736,326 | | |
| |
| (5,241,463 | ) |
| 1/3/2024 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 94,883 | | |
| 9 | | |
| | | |
| | | |
| 39,629 | | |
| | | |
| | | |
| |
| 39,638 | |
| 1/10/2024 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 82,643 | | |
| 8 | | |
| | | |
| | | |
| 44,288 | | |
| | | |
| | | |
| |
| 44,297 | |
| 2/2/2024 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 40,229 | | |
| 4 | | |
| | | |
| | | |
| 19,609 | | |
| | | |
| | | |
| |
| 19,614 | |
| 2/21/2024 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 52,569 | | |
| 5 | | |
| | | |
| | | |
| 32,356 | | |
| | | |
| | | |
| |
| 32,362 | |
| 3/6/2023 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 44,503 | | |
| 4 | | |
| | | |
| | | |
| 25,278 | | |
| | | |
| | | |
| |
| 25,282 | |
3/20/2024 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 49,230 | | |
| 5 | | |
| | | |
| | | |
| 26,776 | | |
| | | |
| | | |
| |
| 26,781 | |
| Net
Income (Loss) Quarter Ended March 31, 2024 |
|
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (122,473 | ) | |
| | | |
| |
| (122,473 | ) |
| Balance
March 31, 2024 |
|
|
| 409,551 | | |
$ | 40 | | |
| 34 | | |
$ | 0 | | |
| 15,007 | | |
| | | |
| 4,114,622 | | |
| 413 | | |
| 29,338 | | |
| 3 | | |
| 14,308,349 | | |
| (20,221,096 | ) | |
| 736,326 | | |
| |
| (5,175,963 | ) |
| 4/3/2024 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 52763 | | |
| 5.2763 | | |
| | | |
| | | |
| 25321.0437 | | |
| | | |
| | | |
| |
| 25326.32 | |
| 5/2/2024 |
Preferred
Shares Issued for Services |
|
| 20,068 | | |
| 2 | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 13,042 | | |
| | | |
| | | |
| |
| 13,044 | |
| 5/29/2024 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 68,185 | | |
| 7 | | |
| | | |
| | | |
| 29,993 | | |
| | | |
| | | |
| |
| 30,000 | |
| 6/7/2024 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 62,207 | | |
| 6 | | |
| | | |
| | | |
| 29,994 | | |
| | | |
| | | |
| |
| 30,000 | |
6/20/2024 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 75,301 | | |
| 8 | | |
| | | |
| | | |
| 49,992 | | |
| | | |
| | | |
| |
| 50,000 | |
| Net
Income (Loss) Quarter Ended June 30, 2024 |
|
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (130,120 | ) | |
| | | |
| |
| (130,120 | ) |
| Balance
June 30, 2024 |
|
|
| 429,619 | | |
$ | 42 | | |
| 34 | | |
$ | 0 | | |
| 15,007 | | |
| | | |
| 4,373,078 | | |
| 439 | | |
| 29,338 | | |
| 3 | | |
| 14,456,692 | | |
| (20,351,216 | ) | |
| 736,326 | | |
| |
| (5,157,712 | ) |
7/3/2024 |
Preferred
Shares Distributed as dividend |
|
| 9,694,152 | | |
$ | 969 | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (969 | ) | |
| | | |
| | | |
| |
| 0 | |
7/12/2024 |
Common
Shares issued for Cash |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 135,242 | | |
| 14 | | |
| | | |
| | | |
| 28,112 | | |
| | | |
| | | |
| |
| 28,126 | |
9/4/2024 |
Common
Shares issued for Financing Expenses |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 500,000 | | |
| 50 | | |
| | | |
| | | |
| 145,450 | | |
| | | |
| | | |
| |
| 145,500 | |
9/26/2024 |
Common
Shares issued for services |
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| 249,915 | | |
| 25 | | |
| | | |
| | | |
| 54,931 | | |
| | | |
| | | |
| |
| 54,956 | |
| |
|
|
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| (264,899 | ) | |
| | | |
| |
| (264,899 | ) |
| Net
Income (Loss) for the Quarter Ended September 30, 2024 |
|
|
| 10,123,771 | | |
$ | 1,011 | | |
| 34 | | |
$ | 0 | | |
| 15,007 | | |
| | | |
| 5,258,235 | | |
| 527 | | |
| 29,338 | | |
| | | |
| 14,684,216 | | |
| (20,616,114 | ) | |
| 736,326 | | |
| |
| (5,194,029 | ) |
The
Accompanying Notes are an Integral Part of These Financial Statements
All
stock amounts have been retroactively adjusted to reflect a 1 for 1500 reverse stock split of all issued series of stock effective as
of March 6, 2023
REGEN
BIOPHARMA , INC.
CONDENSED
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | |
Year
Ended | | |
Year
Ended | |
| | |
September
30, 2024 | | |
September
30, 2023 | |
| |
(unaudited) | | |
| |
| CASH
FLOWS FROM OPERATING ACTIVITIES | |
| | |
| |
| Net
Income (loss) | |
$ | (867,252 | ) | |
$ | 1,156,507 | |
| Adjustments
to reconcile net Income to net cash | |
| 0 | | |
| 348,372 | |
| Preferred
Stock issued as compensation | |
| 5,876 | | |
| | |
| Common
Stock issued for Compensation | |
| 3,036 | | |
| | |
| Increase
(Decrease) in Interest expense attributable to | |
| | | |
| | |
| amortization
of Discount | |
| 28,998 | | |
| 864 | |
| Common
Stock issued for Expenses | |
| 145,500 | | |
| 250000 | |
| Increase
(Decrease) in Accounts Payable | |
| (5 | ) | |
| 875 | |
| (Increase)
Decrease in Accounts Receivable | |
| (94,874 | ) | |
| 254272 | |
| Increase
(Decrease) in accrued Expenses | |
| 19,946 | | |
| 58,583 | |
| (Increase)
Decrease in Prepaid Expenses | |
| 5,200 | | |
| 20,947 | |
| Increase(Decrease)
in Contributed Capital | |
| 0 | | |
| | |
| Increase
( Decrease) in Derivative Expense | |
| (2,726 | ) | |
| (2,151,755 | ) |
| Increase
( Decrease) in Unearned Income | |
| (141,687 | ) | |
| (111,433 | ) |
| (Gain)Loss
on forgiveness of Debt | |
| 0 | | |
| (1,150 | ) |
| Unrealized
Loss(Gain) on Investment Securities | |
| 204,847 | | |
| 0 | |
| Net
Cash Provided by (Used in) Operating Activities | |
$ | (693,141 | ) | |
$ | (173,917 | ) |
| | |
| | | |
| | |
| | |
| | | |
| | |
| CASH
FLOWS FROM FINANCING ACTIVITIES | |
| | | |
| | |
| | |
| | | |
| | |
| | |
| | | |
| | |
| Increase
(Decrease)in Convertible Notes Payable | |
| (10,000 | ) | |
| 0 | |
| Increase
(Decrease)in Notes Payable | |
| 19,133 | | |
| 243,750 | |
| Increase
( Decrease) in Common Stock issued for cash | |
| 563,686 | | |
| 0 | |
| Net
Cash Provided by (Used in) Financing Activities | |
| 572,819 | | |
| 243,750 | |
| | |
| | | |
| | |
| | |
| | | |
| | |
| Net
Increase (Decrease) in Cash | |
$ | (120,322 | ) | |
$ | 69,833 | |
| | |
| | | |
| | |
| Cash
at Beginning of Period | |
$ | 121,037 | | |
| 51204 | |
| | |
| | | |
| | |
| Cash
at End of Period | |
$ | 715 | | |
$ | 121,037 | |
| | |
| | | |
| | |
| Supplemental
Disclosure of Noncash investing and financing activities: | |
| | | |
| | |
| Common
shares Issued for Debt | |
| | | |
| | |
| Preferred
Shares Issued for Debt | |
| | | |
$ | 761,500 | |
| Cash
Paid for Interest | |
$ | 17,500 | | |
| | |
| Common
shares Issued for Interest | |
| | | |
$ | 25,369 | |
| Preferred
Shares issued for Interest | |
| | | |
$ | 380,262 | |
The
Accompanying Notes are an Integral Part of These Financial Statements
All
stock amounts have been retroactively adjusted to reflect a 1 for 1500 reverse stock split of all issued series of stock effective as
of March 6, 2023
REGEN
BIOPHARMA, INC.
Notes
to Condensed Consolidated Financial Statements
As
of September 30, 2024
These
Notes have been retroactively adjusted to reflect a 1 for 1500 reverse stock split of all issued series of stock effective as of March
6, 2023
NOTE
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The
Company was organized April 24, 2012 under the laws of the State of Nevada
The
Company intends to engage primarily in the development of regenerative medical applications which we intend to license from other entities
up to the point of successful completion of Phase I and or Phase II clinical trials after which we would either attempt to sell or license
those developed applications or, alternatively, advance the application further to Phase III clinical trials.
The
Company is currently engaged in actively identifying small molecules that inhibit or express NR2F6 leading to immune cell activation
for oncology applications and immune cell suppression for autoimmune disease.
The
Company is in the early stages of development of its proposed products and therapies. The Company will be required to obtain approval
from the FDA in order to market any of The Company’s products or therapies. No approval has been granted by the FDA for the marketing
and sale of any of the Company’s products and therapies and no assurance may be given that any of the Company’s products
or therapies will be granted such approval. The Company’s current plans include the development of regenerative medical applications
up to the point of successful completion of Phase I and/ or Phase II clinical trials after which the Company would either attempt to
sell or license those developed applications or, alternatively, advance the application further to Phase III clinical trials. The Company
can provide no assurance that the Company will be able to sell or license any product or that, if such product is sold or licensed, such
sale or license will be on terms favorable to the Company.
A.
BASIS OF ACCOUNTING
The
financial statements have been prepared using the basis of accounting generally accepted in the United States of America. Under this
basis of accounting, revenues are recorded as earned and expenses are recorded at the time liabilities are incurred. The Company has
adopted a September 30 year-end.
B.
PRINCIPLES OF CONSOLIDATION
The
consolidated financial statements include the accounts of KCL Therapeutics, Inc., a Nevada corporation and wholly owned subsidiary of
Regen. Significant inter-company transactions have been eliminated.
The
Company analyzes the conversion feature of Convertible Notes for derivative accounting consideration under ASC 815-15 “Derivatives
and Hedging. ASC 815-15 requires that the conversion features are bifurcated and separately accounted for as an embedded derivative contained
in the Company’s convertible debt. The embedded derivative is carried on the balance sheet at fair value. Any unrealized change
in fair value, as determined at each measurement period, is recorded as a component of the income statement and the associated carrying
amount on the balance sheet is adjusted by the change. The Company values the embedded derivative using the Black-Scholes pricing model.
The
Black Scholes pricing model used to determine the Derivative Liability on convertible notes issued by the Company in which an embedded
derivative is recognized as of September 30, 2024 utilized the following inputs:
| Schedule of Derivative liability | |
| |
| Risk Free Interest Rate | |
| 4.74 | % |
| Expected Term | |
| (3.78)
– (4.41) Yrs | |
| Expected Volatility | |
| 1142.86 | % |
| Expected Dividends | |
| | |
H.
INCOME TAXES
The
Company accounts for income taxes using the liability method prescribed by ASC 740, “Income Taxes.” Under this method, deferred
tax assets and liabilities are determined based on the difference between the financial reporting and tax bases of assets and liabilities
using enacted tax rates that will be in effect in the year in which the differences are expected to reverse. The Company records a valuation
allowance to offset deferred tax assets if based on the weight of available evidence, it is more-likely-than-not that some portion, or
all, of the deferred tax assets will not be realized. The effect on deferred taxes of a change in tax rates is recognized as income or
loss in the period that includes the enactment date.
The
Company applied the provisions of ASC 740-10-50, “Accounting For Uncertainty In Income Taxes”, which provides clarification
related to the process associated with accounting for uncertain tax positions recognized in our financial statements. Audit periods remain
open for review until the statute of limitations has passed. The completion of review or the expiration of the statute of limitations
for a given audit period could result in an adjustment to the Company’s liability for income taxes. Any such adjustment could be
material to the Company’s results of operations for any given quarterly or annual period based, in part, upon the results of operations
for the given period. As of September 30, 2024 the Company had no uncertain tax positions, and will continue to evaluate for uncertain
positions in the future.
The
Company generated a deferred tax credit through net operating loss carry forward. However, a valuation allowance of 100% has been established.
Interest
and penalties on tax deficiencies recognized in accordance with ACS accounting standards are classified as income taxes in accordance
with ASC Topic 740-10-50-19.
I.
BASIC EARNINGS (LOSS) PER SHARE
The
Financial Accounting Standards Board (FASB) issued Accounting Standards Codification (ASC) 260, “Earnings Per Share”, which
specifies the computation, presentation and disclosure requirements for earnings (loss) per share for entities with publicly held common
stock. ASC 260 requires the presentation of basic earnings (loss) per share and diluted earnings (loss) per share. The Company has adopted
the provisions of ASC 260 effective from inception.
Basic
net loss per share amounts is computed by dividing the net income by the weighted average number of common shares outstanding.
J. ADVERTISING
Costs
associated with advertising are charged to expense as incurred. Advertising expenses were $0 for the years ended September 30 , 2023
and 2024
K.
NOTES RECEIVABLE
Notes
receivable are stated at cost, less impairment, if any.
L.
REVENUE RECOGNITION
Sales
of products and related costs of products sold are recognized when: (i) persuasive evidence of an arrangement exists; (ii) delivery has
occurred; (iii) the price is fixed or determinable; and (iv) collectability is reasonably assured. These terms are typically met upon
the prepayment or invoicing and shipment of products.
The
Company determines the amount and timing of royalty revenue based on its contractual agreements with intellectual property licensees.
The Company recognizes royalty revenue when earned under the terms of the agreements and when the Company considers realization of payment
to be probable. Where royalties are based on a percentage of licensee sales of royalty-bearing products, the Company recognizes royalty
revenue by applying this percentage to the Company’s estimate of applicable licensee sales. The Company bases this estimate on
an analysis of each licensee’s sales results. Where warranted, revenue from licensees for contractual obligations such as License
Initiation Fees are recognized upon satisfaction of all conditions required to be satisfied in order for that revenue to have been earned
by the Company.
M.
INTEREST RECEIVABLE
Interest
receivable is stated at cost, less impairment, if any.
NOTE
2. RECENT ACCOUNTING PRONOUNCEMENTS
In
June 2014, the Financial Accounting Standards Board issued Accounting Standards Update No. 2014-10, which eliminated certain financial
reporting requirements of companies previously identified as “Development Stage Entities” (Topic 915). The amendments in
this ASU simplify accounting guidance by removing all incremental financial reporting requirements for development stage entities. The
amendments also reduce data maintenance and, for those entities subject to audit, audit costs by eliminating the requirement for development
stage entities to present inception-to-date information in the statements of income, cash flows, and shareholder equity. Early application
of each of the amendments is permitted for any annual reporting period or interim period for which the entity’s financial statements
have not yet been issued (public business entities) or made available for issuance (other entities). Upon adoption, entities will no
longer present or disclose any information required by Topic 915. The Company has adopted this standard.
As
of the fiscal year ending September 30, 2019 the Company has adopted Accounting Standards Update 2014-09, Revenue from Contracts with
Customers (Topic 606). The guidance in this Update supersedes the revenue recognition requirements in Topic 605, Revenue Recognition,
and most industry-specific guidance throughout the Industry Topics of the Codification.
The
core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers
in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve
that core principle, an entity should apply the following steps: Step 1: Identify the contract(s) with a customer. Step 2: Identify the
performance obligations in the contract. Step 3: Determine the transaction price. Step 4: Allocate the transaction price to the performance
obligations in the contract. Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation.
In
June 2014, FASB issued Accounting Standards Update (ASU) No. 2014-12 Compensation — Stock Compensation (Topic 718), Accounting
for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period.
A performance target in a share-based payment that affects vesting and that could be achieved after the requisite service period should
be accounted for as a performance condition under Accounting Standards Codification (ASC) 718, Compensation — Stock Compensation.
As a result, the target is not reflected in the estimation of the award’s grant date fair value. Compensation cost would be recognized
over the required service period, if it is probable that the performance condition will be achieved. The guidance is effective for annual
periods beginning after 15 December 2015 and interim periods within those annual periods. Early adoption is permitted. The Company has
reviewed the applicable ASU and has not, at the current time, quantified the effects of this pronouncement, however it believes that
there will be no material effect on the consolidated financial statements.
In
August 2014, FASB issued Accounting Standards Update (ASU) No. 2014-15 Preparation of Financial Statements – Going Concern (Subtopic
205-40), Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. Under generally accepted accounting
principles (GAAP), continuation of a reporting entity as a going concern is presumed as the basis for preparing financial statements
unless and until the entity’s liquidation becomes imminent. Preparation of financial statements under this presumption is commonly
referred to as the going concern basis of accounting. If and when an entity’s liquidation becomes imminent, financial statements
should be prepared under the liquidation basis of accounting in accordance with Subtopic 205-30, Presentation of Financial Statements—Liquidation
Basis of Accounting. Even when an entity’s liquidation is not imminent, there may be conditions or events that raise substantial
doubt about the entity’s ability to continue as a going concern. In those situations, financial statements should continue to be
prepared under the going concern basis of accounting, but the amendments in this Update should be followed to determine whether to disclose
information about the relevant conditions and events. The amendments in this Update are effective for the annual period ending after
December 15, 2016, and for annual periods and interim periods thereafter. Early application is permitted. The Company will evaluate the
going concern considerations in this ASU, however, at the current period, management does not believe that it has met the conditions
which would subject these financial statements for additional disclosure.
On
January 31, 2013, the FASB issued Accounting Standards Update [ASU] 2013-01, entitled Clarifying the Scope of Disclosures about Offsetting
Assets and Liabilities. The guidance in ASU 2013-01 amends the requirements in the FASB Accounting Standards Codification [FASB ASC]
Topic 210, entitled Balance Sheet. The ASU 2013-01 amendments to FASB ASC 210 clarify that ordinary trade receivables and receivables
in general are not within the scope of ASU 2011-11, entitled Disclosure about Offsetting Assets and Liabilities, where that ASU amended
the guidance in FASB ASC 210. As those disclosures now are modified with the ASU 2013-01 amendments, the FASB ASC 210 balance sheet offsetting
disclosures now clearly are applicable only where reporting entities are involved with bifurcated embedded derivatives, repurchase agreements,
reverse repurchase agreements, and securities borrowing and lending transactions that either are offset using the FASB ASC 210 or 815
requirements, or that are subject to enforceable master netting arrangements or similar agreements. ASU 2013-01 is effective for annual
reporting periods beginning on or after January 1, 2013, and interim periods within those annual periods. The adoption of this ASU is
not expected to have a material impact on our financial statements.
On
February 28, 2013, the FASB issued Accounting Standards Update [ASU] 2013-04, entitled Obligations Resulting from Joint and Several Liability
Arrangements for Which the Total Amount of the Obligation Is Fixed at the Reporting Date. The ASU 2013-04 amendments add to the guidance
in FASB Accounting Standards Codification [FASB ASC] Topic 405, entitled Liabilities and require reporting entities to measure obligations
resulting from certain joint and several liability arrangements where the total amount of the obligation is fixed as of the reporting
date, as the sum of the following:
The
amount the reporting entity agreed to pay on the basis of its arrangement among co-obligors.
Any
additional amounts the reporting entity expects to pay on behalf of its co-obligors.
While
early adoption of the amended guidance is permitted, for public companies, the guidance is required to be implemented in fiscal years,
and interim periods within those years, beginning after December 15, 2013. The amendments need to be implemented retrospectively to all
prior periods presented for obligations resulting from joint and several liability arrangements that exist at the beginning of the year
of adoption. The adoption of ASU 2013-04 is not expected to have a material effect on the Company’s operating results or financial
position.
On
April 22, 2013, the FASB issued Accounting Standards Update [ASU] 2013-07, entitled Liquidation Basis of Accounting. With ASU 2013-07,
the FASB amends the guidance in the FASB Accounting Standards Codification [FASB ASC] Topic 205, entitled Presentation of Financial Statements.
The amendments serve to clarify when and how reporting entities should apply the liquidation basis of accounting. The guidance is applicable
to all reporting entities, whether they are public or private companies or not-for-profit entities. The guidance also provides principles
for the recognition of assets and liabilities and disclosures, as well as related financial statement presentation requirements. The
requirements in ASU 2013-07 are effective for annual reporting periods beginning after December 15, 2013, and interim reporting periods
within those annual periods. Reporting entities are required to apply the requirements in ASU 2013-07 prospectively from the day that
liquidation becomes imminent. Early adoption is permitted. The adoption of ASU 2013-07 is not expected to have a material effect on the
Company’s operating results or financial position.
In
January 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (ASU) 2016-01, which amends
the guidance in U.S. GAAP on the classification and measurement of financial instruments. Changes to the current guidance primarily affect
the accounting for equity investments, financial liabilities under the fair value option, and the presentation and disclosure requirements
for financial instruments. In addition, the ASU clarifies guidance related to the valuation allowance assessment when recognizing deferred
tax assets resulting from unrealized losses on available-for-sale debt securities. The new standard is effective for fiscal years and
interim periods beginning after December 15, 2017, and upon adoption, an entity should apply the amendments by means of a cumulative-effect
adjustment to the balance sheet at the beginning of the first reporting period in which the guidance is effective. Early adoption is
not permitted except for the provision to record fair value changes for financial liabilities under the fair value option resulting from
instrument-specific credit risk in other comprehensive income. The Company adopted ASU 2016-01 as of the fiscal year ending September
30, 2019.
In
August 2020, FASB issued ASU 2020-06, Accounting for Convertible Instruments and Contracts in an Entity; Own Equity (“ASU 2020-06”),
as part of its overall simplification initiative to reduce costs and complexity of applying accounting standards while maintaining or
improving the usefulness of the information provided to users of financial statements. Among other changes, the new guidance removes
from GAAP separation models for convertible debt that require the convertible debt to be separated into a debt and equity component,
unless the conversion feature is required to be bifurcated and accounted for as a derivative or the debt is issued at a substantial premium.
As a result, after adopting the guidance, entities will no longer separately present such embedded conversion features in equity, and
will instead account for the convertible debt wholly as debt. The new guidance also requires use of the “if-converted” method
when calculating the dilutive impact of convertible debt on earnings per share, which is consistent with the Company’s current
accounting treatment under the current guidance. The guidance is effective for financial statements issued for fiscal years beginning
after December 15, 2021, and interim periods within those fiscal years, with early adoption permitted, but only at the beginning of the
fiscal year. The Company has adopted ASU 2020-06 as of the Fiscal Year ending September 30, 2022.
A
variety of proposed or otherwise potential accounting standards are currently under study by standard setting organizations and various
regulatory agencies. Due to the tentative and preliminary nature of those proposed standards, the Company’s management has not
determined whether implementation of such standards would be material to its financial statements.
NOTE
3. GOING CONCERN
The
accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The Company generated
net losses of $20,616,114 during the period from April 24, 2012 (inception) through September 30, 2024. This condition raises substantial
doubt about the Company’s ability to continue as a going concern. The Company’s continuation as a going concern is dependent
on its ability to meet its obligations, to obtain additional financing as may be required and ultimately to attain profitability. The
financial statements do not include any adjustments that might result from the outcome of this uncertainty.
On
September 12, 2023 the Company entered into a common stock purchase agreement (the “Equity Line Agreement”) with Coventry
Enterprises LLC ( “Coventry”) providing for an equity financing facility (the “Equity Line”). The Equity Line
Agreement provides that upon the terms and subject to the conditions in the Equity Line Agreement, Coventry is committed to purchase
up to Ten Million Dollars ($10,000,000) of shares of common stock, $0.0001 par value per share (the “Common Stock”), over
the 36-month term of the Equity Line Agreement (the “Total Commitment”).
Under
the terms of the Equity Line Agreement, Coventry will not be obligated to purchase shares of Common Stock unless and until certain conditions
are met, including but not limited to a Registration Statement on Form S-1 (the “Registration Statement”) becoming effective
which registers Coventry’s resale of any Common Stock purchased by Coventry under the Equity Line.
From
time to time over the 36-month term of the Commitment Period ( as such term is defined in the Equity Line Agreement) the Company, in
its sole discretion, may provide Coventry with a draw down notice (each, a “Draw Down Notice”), to purchase a specified number
of shares of Common Stock (each, a “Draw Down Amount Requested”), subject to the limitations discussed below. The actual
amount of proceeds the Company will receive pursuant to each Draw Down Notice (each, a “Draw Down Amount”) is to be determined
by multiplying the Draw Down Amount Requested by the applicable purchase price. The purchase price of each share of Common Stock equals
80% of the lowest trading price of the Common Stock during the ten business days prior to the Draw Down Notice date (the “Pricing
Period”).
The
maximum number of shares of Common Stock requested to be purchased pursuant to any single Draw Down Notice cannot exceed the lesser of
(i) 200% of the Average Daily Traded Value ( as such term is defined in the Equity Line Agreement) during the ten business days immediately
preceding the Drawdown Notice Date or (ii) $250,000. The Company is prohibited from delivering a Draw Down Notice if the sale of shares
of Common Stock pursuant to the Draw Down Notice would cause the Company to issue and sell to Coventry or Coventry to acquire or purchase
an aggregate number of shares of Common Stock that would result in Coventry beneficially owning more than 4.99% of the issued and outstanding
shares of Common Stock of the Company.
The
Company issued Coventry 125,000 shares of its Common Stock in connection with the Equity Line Agreement.
Coventry
has agreed that:
| |
(a) |
for
so long as the market price of the Company’s common stock is above $1.25 per share and |
| |
|
|
| |
(b) |
the
Company is in full compliance with all agreements entered into with Coventry and |
| |
|
|
| |
(c) |
and
the Company has not issued any common shares at a per share price below $1.50, Coventry will agree to a leak out provision and will
not sell more than 10,000 shares of the Commitment shares without permission from the Issuer. |
In
connection with the Equity Line Agreement the Company also entered into a Registration Rights Agreement, dated September 12, 2023 with
Coventry (the “Registration Rights Agreement”), pursuant to which the Company agreed to register for resale under the Securities
Act of 1933 shares issuable in accordance with the Equity Line Agreement as well as the aforementioned 125,000 common shares issued in
connection with the Equity Line Agreement in a Registration Statement to be filed with the Securities and Exchange Commission. Up to
1,126,954 Shares of Common Stock were registered for resale under the Securities Act of 1933 pursuant to the Registration Rights Agreement.
During
the quarter ended December 31, 2023 the Company issued 244,199 common shares pursuant to the Equity Line Agreement for aggregate cash
consideration of $212,296. During the Quarter ended March 31, 2024 the Company issued 364,057 common shares pursuant to the Equity Line
Agreement for aggregate cash consideration of $187,937. During the Quarter ended June 30, 2024 the Company issued 258,456 common shares
pursuant to the Equity Line Agreement for aggregate cash consideration of $135,326. During the Quarter ended September 30, 2024 the Company
issued 135,242 common shares pursuant to the Equity Line Agreement for aggregate cash consideration of $28,126
NOTE
4. NOTES PAYABLE
(a)
RELATED PARTY
| | |
As of September 30, 2024 | |
| David Koos | |
$ | 1,708 | |
| Zander Therapeutics, Inc. | |
$ | 40,000 | |
| Total: | |
| $
41,708 | |
$1,708
lent to the Company by David Koos is due and payable at the demand of the holder and bears simple interest at a rate of 15% per annum.
$15,000
lent to the Company by Zander Therapeutics, Inc. is due and payable on May 3. 2025 and bears simple interest at a rate of 10% per annum.
$25,000
lent to the Company by Zander Therapeutics, Inc. is due and payable on June 5. 2025 and bears simple interest at a rate of 10% per annum.
Zander
Therapeutics, Inc. and the Company are under common control.
(b)
NON RELATED PARTY As of September 30, 2024
| Bostonia Partners, Inc. | |
$ | 48,500 | |
| Coventry Enterprises LLC | |
$ | 250,000 | |
| Total: | |
$ | 298,500 | |
$48,500
lent to the Company by Bostonia Partners, Inc is due and payable on March 10, 2024 and bears simple interest at a rate of 10% per annum.
Effective
September 4, 2024 the Company entered into a securities purchase agreement (the “Purchase Agreement”) with Coventry Enterprises,
LLC (“Coventry”), pursuant to which Coventry Enterprises purchased a 10% unsecured promissory Note (the “Note”)
from the Company in the principal amount of $250,000 for consideration of $200,000.
The
Note carries “Guaranteed Interest” on the principal amount at the rate of 10% per annum for the ten month term of the Note
for an aggregate Guaranteed Interest $25,000. The Principal Amount and the Guaranteed Interest shall be due and payable in ten equal
monthly payments $27,500 commencing on November 4, 2024, and continuing on the fourth day of each month thereafter (each, a “Monthly
Payment Date”) until paid in full not later than September 4, 2025.
Upon
an Event of Default (as such term is defined in the Note) the Note shall become convertible, in whole or in part, into shares of Common
Stock at the option of the Holder at price per share equivalent to 90% of the lowest per-share trading price for the 20 Trading Days
preceding a Conversion Date.
$152,000
of the proceeds received has been utilized to repay an aggregate of $152,000 of outstanding principal indedebteness and interest due
to Coventry by the Company resulting from a $175,000 Note issued to Coventry on September 12, 2023.
In
Connection with the Purchase Agreement the Company issued to Coventry 500,000 common shares (“Commitment Shares”). If The
Company has satisfied all the terms of the Note without default the Coventry shall, within 10 calendar days thereafter, return to the
Company’s treasury for cancellation 350,000 of the Commitment Shares.
NOTE
5. CONVERTIBLE NOTES PAYABLE
On
March 8, 2016 (“Issue date”) the Company issued a Convertible Note (“Note”) in the face amount of $100,000 for
consideration consisting of $100,000 cash. The Note pays simple interest in the amount of 8% per annum. The maturity of the Note is three
years from the issue date.
The
Lender shall have the right from time to time to convert all or a part of the outstanding and unpaid principal amount of this Note into
fully paid and non- assessable shares of Common Stock, as such Common Stock exists on the Issue Date, or any shares of capital stock
or other securities of the Company into which such Common Stock shall hereafter be changed or reclassified pursuant to the following
terms and conditions:
(a)
For the period beginning on the Issue Date and ending 365 days subsequent to the Issue Date (“Year 1”) a 50% discount to
the lowest Trading Price (as defined below) for the Common Stock during the ten (10) Trading Day (as defined below) period ending on
the latest complete Trading Day prior to the Conversion Date or $150 per share (whichever is greater).
(b)
For the period beginning one day subsequent to the final day of Year One and ending 365 days subsequent to Year One (“Year 2”)
a 35% discount to the lowest Trading Price (as defined below) for the Common Stock during the ten (10) Trading Day (as defined below)
period ending on the latest complete Trading Day prior to the Conversion Date or $150 per share (whichever is greater).
(c)
For the period beginning one day subsequent to the final day of Year 2 and ending 365 days subsequent to Year 2 (“Year 3”)
a 25% discount to the lowest Trading Price (as defined below) for the Common Stock during the ten (10) Trading Day (as defined below)
period ending on the latest complete Trading Day prior to the Conversion Date or $150 per share (whichever is greater).
(d)
“Trading Price” means the closing bid price on the Over-the-Counter Bulletin Board, or applicable trading market (the “OTCQB”)
as reported by a reliable reporting service (“Reporting Service”) designated by the Lender (i.e. Bloomberg) or, if the OTCQB
is not the principal trading market for such security, the closing bid price of such security on the principal securities exchange or
trading market where such security is listed or traded or, if no closing bid price of such security is available in any of the foregoing
manners, the average of the closing bid prices of any market makers for such security that are listed in the “pink sheets”
by the National Quotation Bureau, Inc. If the Trading Price cannot be calculated for such security on such date in the manner provided
above, the Trading Price shall be the fair market value as mutually determined by the Company and the Lender. “Trading Day”
shall mean any day on which the Common Stock is tradable for any period on the OTCQB, or on the principal securities exchange or other
securities market on which the Common Stock is then being traded. “Trading Volume” shall mean the number of shares traded
on such Trading Day as reported by such Reporting Service. The Conversion Price shall be equitably adjusted for stock splits, stock dividends,
rights offerings, combinations, recapitalization, reclassifications, extraordinary distributions and similar events by the Company relating
to the Lender’s securities.
The
Company shall have the right, exercisable on not less than five (5) Trading Days prior written notice to the Lender, to prepay the outstanding
Note in part or in full, including outstanding principal and accrued interest.
Upon
closing of a Transaction Event the Lender shall receive 0.10% (one tenth of one percent)of the consideration actually received by the
Company from an unaffiliated third party as a result of the closing of a Transaction Event.
“Transaction
Event” shall mean either of:
(a)
The sale by the Company of the Company’s proprietary NR2F6 intellectual property to an unaffiliated third party
(b)
The granting of a license by the Company to an unaffiliated third party granting that unaffiliated third party the right to develop and/or
commercialize the Company’s proprietary NR2F6 intellectual property
As
of September 30, 2024 $100,000 of the principal amount of the Note remains outstanding.
On
April 6, 2016 (“Issue date”) the Company issued a Convertible Note (“Note”) in the face amount of $50,000 for
consideration consisting of $50,000 cash. The Note pays simple interest in the amount of 8% per annum. The maturity of the Note is three
years from the issue date.
The
Lender shall have the right from time to time to convert all or a part of the outstanding and unpaid principal amount of this Note into
fully paid and non- assessable shares of Common Stock, as such Common Stock exists on the Issue Date, or any shares of capital stock
or other securities of the Company into which such Common Stock shall hereafter be changed or reclassified pursuant to the following
terms and conditions:
(a)
For the period beginning on the Issue Date and ending 365 days subsequent to the Issue Date (“Year 1”) a 50% discount to
the lowest Trading Price (as defined below) for the Common Stock during the ten (10) Trading Day (as defined below) period ending on
the latest complete Trading Day prior to the Conversion Date or$150 per share (whichever is greater).
(b)
For the period beginning one day subsequent to the final day of Year One and ending 365 days subsequent to Year One (“Year 2”)
a 35% discount to the lowest Trading Price (as defined below) for the Common Stock during the ten (10) Trading Day (as defined below)
period ending on the latest complete Trading Day prior to the Conversion Date or $150 per share (whichever is greater).
(c)
For the period beginning one day subsequent to the final day of Year 2 and ending 365 days subsequent to Year 2 (“Year 3”)
a 25% discount to the lowest Trading Price (as defined below) for the Common Stock during the ten (10) Trading Day (as defined below)
period ending on the latest complete Trading Day prior to the Conversion Date or $150 per share (whichever is greater).
(d)
“Trading Price” means the closing bid price on the Over-the-Counter Bulletin Board, or applicable trading market (the “OTCQB”)
as reported by a reliable reporting service (“Reporting Service”) designated by the Lender (i.e. Bloomberg) or, if the OTCQB
is not the principal trading market for such security, the closing bid price of such security on the principal securities exchange or
trading market where such security is listed or traded or, if no closing bid price of such security is available in any of the foregoing
manners, the average of the closing bid prices of any market makers for such security that are listed in the “pink sheets”
by the National Quotation Bureau, Inc. If the Trading Price cannot be calculated for such security on such date in the manner provided
above, the Trading Price shall be the fair market value as mutually determined by the Company and the Lender. “Trading Day”
shall mean any day on which the Common Stock is tradable for any period on the OTCQB, or on the principal securities exchange or other
securities market on which the Common Stock is then being traded. “Trading Volume” shall mean the number of shares traded
on such Trading Day as reported by such Reporting Service. The Conversion Price shall be equitably adjusted for stock splits, stock dividends,
rights offerings, combinations, recapitalization, reclassifications, extraordinary distributions and similar events by the Company relating
to the Lender’s securities.
The
Company shall have the right, exercisable on not less than five (5) Trading Days prior written notice to the Lender, to prepay the outstanding
Note in part or in full, including outstanding principal and accrued interest.
Upon
closing of a Transaction Event the Lender shall receive 0.10% (one tenth of one percent) of the consideration actually received by the
Company from an unaffiliated third party as a result of the closing of a Transaction Event.
“Transaction
Event” shall mean either of:
(a)
The sale by the Company of the Company’s proprietary NR2F6 intellectual property to an unaffiliated third party
(b)
The granting of a license by the Company to an unaffiliated third party granting that unaffiliated third party the right to develop and/or
commercialize the Company’s proprietary NR2F6 intellectual property
As
of September 30, 2024 $50,000 of the principal amount of the Note remains outstanding.
On
October 31, 2016 (“Issue date”) the Company issued a Convertible Note (“Note”) in the face amount of $50,000
for consideration consisting of $50,000 cash. The Note pays simple interest in the amount of 10% per annum. The maturity of the Note
is two years from the issue date.
The
Lender shall have the right from time to time to convert all or a part of the outstanding and unpaid principal amount of this Note into
fully paid and non- assessable shares of Common Stock and/or Series A Preferred Stock, as such Stock exists on the Issue Date, or any
shares of capital stock or other securities of the Company into which such Stock shall hereafter be changed or reclassified at a conversion
price of $18.75 per share.
The
Company shall have the right, exercisable on not less than ten (10) Trading Days prior written notice to the Lender, to prepay the outstanding
Note in part or in full, including outstanding principal and accrued interest.
As
of September 30, 2024 $50,000 of the principal amount of the Note remains outstanding.
On
May 5, 2017 (“Issue date”) the Company issued a Convertible Note (“Note”) in the face amount of $200,000 for
consideration consisting of $200,000 cash. The Note pays simple interest in the amount of 10% per annum. The maturity of the Note is
May 5, 2020. The Note is convertible into the Common Shares of Regen at a price per share (“Conversion Price”) equivalent
to the lower of (a) a 75% discount to the closing price of the common stock of the Company on the trading day immediately prior to the
date a conversion notice is given by the Lender to Regen or (b) $375 per common share as of the date which is the earlier of:
(i)
One day subsequent to the execution of an agreement to a transaction whose completion would result in a “Change of Control”
of the Company. For purposes of this Note, a Change of Control shall be defined as any transaction or series of transactions, whether
by merger, sale of substantially all of the assets, or sale or transfer of more than fifty percent (50%) of the outstanding stock of
the relevant entity in which the members of the Board of Directors immediately preceding the closing of the Change of Control transaction
no longer constitute a majority of the Board of Directors of the surviving entity following the closing of such transaction.
ii)
One day subsequent to the commencement, in compliance with applicable law, of a broad solicitation by a third party to purchase a majority
percentage of the Company’s outstanding equity securities for a limited period of time contingent on shareholders of the Company
tendering a fixed number of their equity securities (“Tender Offer”).
(iii)
That date which is twenty four (24) months subsequent to the date of execution of this Note.
The
Company shall have the right, exercisable on not less than ten (10) Trading Days prior written notice to the Lender, to prepay the outstanding
Note in part or in full, including outstanding principal and accrued interest.
In
the event that that the Company exercises its right to prepay the note, or if the Lender chooses not to convert the remaining amount
of the note into Common Shares of the company, the Lender shall receive warrants equal to 10% of the Common shares it would have received
had the Lender converted the remaining amount of the Note into Common shares of the Company. The warrants shall have a strike price of
$75 per share.
The
warrants shall be exercisable:
In
the event that the Company exercises its right to Prepay the Note on or prior to the close of business on the three (3) month anniversary
of the date that the Note shall have been prepaid by the Company(“Prepayment Date”)
In
the event , part of the outstanding and unpaid principal amount of this Note and any Accrued Interest remains outstanding on the Maturity
Date of the Note, or prior to the close of business on the three (3) month anniversary of the Maturity Date of the Note
As
of September 30, 2024 $200,000 of the principal amount of the Note remains outstanding.
The
Company analyzed the conversion feature of the Note for derivative accounting consideration under ASC 815-15 “Derivatives and Hedging”
and determined that the embedded conversion feature should be classified as a liability due to their being no explicit limit to the number
of shares to be delivered upon settlement of the above conversion features. ASC 815-15 requires that the conversion features are bifurcated
and separately accounted for as an embedded derivative contained in the Company’s convertible debt. The embedded derivative is
carried on the balance sheet at fair value. Any unrealized change in fair value, as determined at each measurement period, is recorded
as a component of the income statement and the associated carrying amount on the balance sheet is adjusted by the change.
The
Company values the embedded derivative using the Black-Scholes pricing model and a derivative liability of $798,442 was recognized by
the Company as of September 30, 2024.
On
December 20, 2017 (“Issue date”) the Company issued a Convertible Note (“Note”) in the face amount of $100,000
for consideration consisting of $100,000 cash. The Note pays simple interest in the amount of 10% per annum. The maturity of the Note
is December 20, 2020. The Note may be converted into the Common Shares of Regen at a price per share (“Conversion Price”)
equivalent to the lower of (a) a 75% discount to the closing price of the common stock of the Company on the trading day immediately
prior to the date a conversion notice is given by the Lender to Regen or (b) $37.50 per common share as of the date which is the earlier
of:
(i)
One day subsequent to the execution of an agreement to a transaction whose completion would result in a “Change of Control”
of the Company or KCL Therapeutics. For purposes of this Note, a Change of Control shall be defined as any transaction or series of transactions,
whether by merger, sale of substantially all of the assets, or sale or transfer of more than fifty percent (50%) of the outstanding stock
of the relevant entity in which the members of the Board of Directors immediately preceding the closing of the Change of Control transaction
no longer constitute a majority of the Board of Directors of the surviving entity following the closing of such transaction.
(ii)
One day subsequent to the commencement, in compliance with applicable law, of a broad solicitation by a third party to purchase a majority
percentage of the Company’s outstanding equity securities for a limited period of time contingent on shareholders of the Company
tendering a fixed number of their equity securities (“Tender Offer”).
(iv)
One day subsequent to a “Transaction Event”)
Transaction
Event” shall mean either of:
(a)
The sale by the Company or by KCL Therapeutics , Inc. of the Company’s proprietary NR2F6 intellectual property to an unaffiliated
third party
(b)
The granting of a license by the Company or by KCL Therapeutics , Inc to an unaffiliated third party granting that unaffiliated third
party the right to develop and/or commercialize the Company’s proprietary NR2F6 intellectual property
(v)
That date which is twenty four (24) months subsequent to the date of execution of this Note.
The
Company shall have the right, exercisable on not less than ten (10) Trading Days prior written notice to the Lender, to prepay the outstanding
Note in part or in full, including outstanding principal and accrued interest.
In
the event that that the Company exercises its right to prepay the note, or if the Lender chooses not to convert the remaining amount
of the note into Common Shares of the company, the Lender shall receive warrants equal to 10% of the Common shares it would have received
had the Lender converted the remaining amount of the Note into Common shares of the Company. The warrants shall have a strike price of
$37.5 per share.
The
warrants shall be exercisable:
In
the event that the Company exercises its right to Prepay the Note on or prior to the close of business on the three (3) month anniversary
of the date that the Note shall have been prepaid by the Company (“Prepayment Date”)
In
the event , part of the outstanding and unpaid principal amount of this Note and any Accrued Interest remains outstanding on the Maturity
Date of the Note, or prior to the close of business on the three (3) month anniversary of the Maturity Date of the Note
As
of September 30, 2024 $100,000 of the principal amount of the Note remains outstanding.
The
Company analyzed the conversion feature of the Note for derivative accounting consideration under ASC 815-15 “Derivatives and Hedging”
and determined that the embedded conversion feature should be classified as a liability due to their being no explicit limit to the number
of shares to be delivered upon settlement of the above conversion features. ASC 815-15 requires that the conversion features are bifurcated
and separately accounted for as an embedded derivative contained in the Company’s convertible debt. The embedded derivative is
carried on the balance sheet at fair value. Any unrealized change in fair value, as determined at each measurement period, is recorded
as a component of the income statement and the associated carrying amount on the balance sheet is adjusted by the change.
The
Company values the embedded derivative using the Black-Scholes pricing model and a derivative liability of $399,221 was recognized by
the Company as of September 30, 2024.
On
October 3, 2017 (“Issue date”) the Company issued a Convertible Note (“Note”) in the face amount of $50,000 for
consideration consisting of $50,000 cash. The Note pays simple interest in the amount of 10% per annum. The maturity of the Note is October
3, 2020. The Note may be converted into the Common Shares of Regen at a price per share (“Conversion Price”) equivalent to
the lower of (a) a 75% discount to the closing price of the common stock of the Company on the trading day immediately prior to the date
a conversion notice is given by the Lender to Regen or (b) $37.5 per common share as of the date which is the earlier of:
(i)
One day subsequent to the execution of an agreement to a transaction whose completion would result in a “Change of Control”
of the Company or KCL Therapeutics. For purposes of this Note, a Change of Control shall be defined as any transaction or series of transactions,
whether by merger, sale of substantially all of the assets, or sale or transfer of more than fifty percent (50%) of the outstanding stock
of the relevant entity in which the members of the Board of Directors immediately preceding the closing of the Change of Control transaction
no longer constitute a majority of the Board of Directors of the surviving entity following the closing of such transaction.
(ii)
One day subsequent to the commencement, in compliance with applicable law, of a broad solicitation by a third party to purchase a majority
percentage of the Company’s outstanding equity securities for a limited period of time contingent on shareholders of the Company
tendering a fixed number of their equity securities (“Tender Offer”).
(iv)
One day subsequent to a “Transaction Event”)
Transaction
Event” shall mean either of:
(a)
The sale by the Company or by KCL Therapeutics , Inc. of the Company’s proprietary NR2F6 intellectual property to an unaffiliated
third party
(b)
The granting of a license by the Company or by KCL Therapeutics , Inc to an unaffiliated third party granting that unaffiliated third
party the right to develop and/or commercialize the Company’s proprietary NR2F6 intellectual property
(v)
That date which is twenty four (24) months subsequent to the date of execution of this Note.
The
Company shall have the right, exercisable on not less than ten (10) Trading Days prior written notice to the Lender, to prepay the outstanding
Note in part or in full, including outstanding principal and accrued interest.
In
the event that that the Company exercises its right to prepay the note, or if the Lender chooses not to convert the remaining amount
of the note into Common Shares of the company, the Lender shall receive warrants equal to 10% of the Common shares it would have received
had the Lender converted the remaining amount of the Note into Common shares of the Company. The warrants shall have a strike price of
$37.5 per share.
The
warrants shall be exercisable:
In
the event that the Company exercises its right to Prepay the Note on or prior to the close of business on the three (3) month anniversary
of the date that the Note shall have been prepaid by the Company (“Prepayment Date”)
In
the event , part of the outstanding and unpaid principal amount of this Note and any Accrued Interest remains outstanding on the Maturity
Date of the Note, or prior to the close of business on the three (3) month anniversary of the Maturity Date of the Note
As
of September 30, 2024, $50,000 of the principal amount of the Note remains outstanding.
The
Company analyzed the conversion feature of the Note for derivative accounting consideration under ASC 815-15 “Derivatives and Hedging”
and determined that the embedded conversion feature should be classified as a liability due to their being no explicit limit to the number
of shares to be delivered upon settlement of the above conversion features. ASC 815-15 requires that the conversion features are bifurcated
and separately accounted for as an embedded derivative contained in the Company’s convertible debt. The embedded derivative is
carried on the balance sheet at fair value. Any unrealized change in fair value, as determined at each measurement period, is recorded
as a component of the income statement and the associated carrying amount on the balance sheet is adjusted by the change.
The
Company values the embedded derivative using the Black-Scholes pricing model and a derivative liability of $199,611 was recognized by
the Company as of September 30, 2024.
NOTE
6. RELATED PARTY TRANSACTIONS
On
June 23, 2015 the Company entered into an agreement (“Agreement”) with Zander Therapeutics, Inc. (“Zander”) whereby
The Company granted to Zander an exclusive worldwide right and license for the development and commercialization of certain intellectual
property controlled by The Company (“ License IP”) for non-human veterinary therapeutic use for a term of fifteen years.
Zander is under common control with the Company.
Pursuant
to the Agreement, Zander shall pay to The Company one-time, non-refundable, upfront payment of one hundred thousand US dollars ($100,000)
as a license initiation fee which must be paid within 90 days of June 23, 2015 and an annual non-refundable payment of one hundred thousand
US dollars ($100,000) on July 15th, 2016 and each subsequent anniversary of the effective date of the Agreement.
The
abovementioned payments may be made, at Zander’s discretion, in cash or newly issued common stock of Zander.
Pursuant
to the Agreement, Zander shall pay to The Company royalties equal to four percent (4%) of the Net Sales , as such term is defined in
the Agreement, of any Licensed Products, as such term is defined in the Agreement, in a Quarter.
Pursuant
to the Agreement, Zander will pay The Company ten percent (10%) of all consideration (in the case of in-kind consideration, at fair market
value as monetary consideration) received by Zander from sublicensees (excluding royalties from sublicensees based on Net Sales of any
Licensed Products for which The Company receives payment pursuant to the terms and conditions of the Agreement).
Zander
is obligated pay to The Company minimum annual royalties of ten thousand US dollars ($10,000) payable per year on each anniversary of
the Effective Date of this Agreement, commencing on the second anniversary of June 23, 2015. This minimum annual royalty is only payable
to the extent that royalty payments made during the preceding 12-month period do not exceed ten thousand US dollars ($10,000).
The
Agreement may be terminated by The Company:
If
Zander has not sold any Licensed Product by ten years of the effective date of the Agreement or Zander has not sold any Licensed Product
for any twelve (12) month period after Zander’s first commercial sale of a Licensed Product.
The
Agreement may be terminated by Zander with regard to any of the License IP if by five years from the date of execution of the Agreement
a patent has not been granted by the United States patent and Trademark Office to The Company with regard to that License IP.
The
Agreement may be terminated by Zander with regard to any of the License IP if a patent that has been granted by the United States patent
and Trademark Office to The Company with regard to that License IP is terminated.
The
Agreement may be terminated by either party in the event of a material breach by the other party.
On
December 17, 2018 Regen Biopharma, Inc.(“Licensor”) , KCL Therapeutics, Inc. (“Assignee”) and Zander Therapeutics,
Inc. (“Licensee”) entered into a LICENSE ASSIGNMENT AND CONSENT AGREEMENT whereby, with regards to certain intellectual property
which was assigned by Regen Biopharma, Inc.(“Assigned Properties”) to its wholly owned subsidiary KCL Therapeutics, Inc.,
Licensor hereby transfers and assigns to Assignee all rights, duties, and obligations of Licensor under the Agreement with respect to
the Assigned Properties , and Assignee agrees to assume such duties and obligations thereunder and be bound to the terms of the Agreement
with respect thereto.
On
December 16, 2019 Zander Therapeutics, Inc. (“Zander”), KCL Therapeutics, Inc. (“KCL”) and Regen Biopharma, Inc.
(“Regen”) entered into an agreement (“Agreement”) whereby:
1)
Zander shall return for cancellation 194,285,714 shares of the Series A Preferred stock of Regen (“Conversion Shares”) acquired
by Zander through conversion of $340,000 of principal indebtedness of a $350,000 convertible note payable issued by Regen to Zander.
Subsequent to this event the principal amount due to Zander by Regen pursuant to the Convertible Note shall be $350,000 which shall be
applied pursuant to the Agreement.
2)
A $35,000 one time charge due to Zander by Regen (“One Time Charge”) shall be applied pursuant to the Agreement.
3)
$75,900 of principal indebtedness due to Regen by Zander and $4,328 of accrued but unpaid interest due by Regen to Zander shall be applied
pursuant to the Agreement.
No
actions were taken by any of the parties to enforce the terms of the Agreement.
On
April 15, 2021 the Agreement was amended as follows so that the material terms and conditions shall be:
a)
Zander shall not return the Conversion shares for cancellation and the principal indebtedness of the aforementioned convertible note
shall not reflect such return
b)
As of December 16, 2019 all principal and accrued interest payable by Regen to Zander on that date resulting from Promissory Notes issued
by Regen to Zander shall be credited towards amounts due by Zander pursuant to that agreement, as amended, entered into by and between
Zander and Regen on June 23, 2015 (“License Agreement”) whereby Regen granted to Zander an exclusive worldwide right and
license for the development and commercialization of certain intellectual property controlled by Regen for non-human veterinary therapeutic
use for a term of fifteen years and that License Assignment And Consent agreement entered into by and between Regen, KCL and Zander on
December 17, 2018 whereby Regen transferred and assigned to KCL all rights, duties, and obligations of Regen under the License Agreement
and KCL agreed to assume such duties and obligations thereunder and be bound to the terms of the License Agreement with respect thereto.
Zander
and Regen are under common control.
On
September 30, 2018 Regen Biopharma, Inc. (“Regen”) issued a convertible promissory note in the principal amount of $350,000
(“Note”) to Zander Therapeutics, Inc. (“Zander”). Consideration for the Note consisted of $350,000. A onetime
interest charge of 10% of the principal amount shall be applied to the principal amount of the Note. The Note is due and payable 24 months
from the effective date.
Zander
has the right, at any time after the September 30, 2018, at its election, to convert all or part of the outstanding and unpaid Principal
Sum and accrued interest (and any other fees) into shares of fully paid and non-assessable shares of Series A Preferred stock of Regen
as per this conversion formula: Number of shares receivable upon conversion equals the dollar conversion amount divided by the Conversion
Price. The Conversion Price is the greater of $0.0001 or 60% of the lowest trade price in the 25 trading days previous to the conversion.
Zander, at any time prior to selling all of the shares from a conversion, may, for any reason, rescind any portion, in whole or in part,
of that particular conversion attributable to the unsold shares and have the rescinded conversion amount returned to the Principal Sum
with the rescinded conversion shares returned to Regen.
As
of June 30, 2024, $0 of the principal amount of the Note remains outstanding and all accrued interest has been paid..
On
January 13, 2022 Regen Biopharma, Inc. entered into a sublease agreement with BST Partners (“BST”) whereby Regen Biopharma,
Inc. would sublet office space located at 4700 Spring Street, Suite 304, La Mesa, California 91942 from BST on a month to month basis
for $5,000 per month beginning January 14, 2022. On April 26, 2024 the Company and BST agreed to amend that sublease agreement as follows:
The
Company agreed that in addition to the base rent of $5,000 per month to be paid by the Company to BST the Company shall also reimburse
BST for any and all shared expenses as such term is defined within the original lease agreement by and between BST and CIF LaMesa LLP
beginning January 1, 2024..
BST
Partners is controlled by David Koos who serves as the sole officer and director of Regen Biopharma, Inc.
$1,708
lent to the Company by David Koos, the Company’s sole Board Member and Officer, is due and payable at the demand of the holder
and bears simple interest at a rate of 15% per annum.
$15,000
lent to the Company by Zander Therapeutics, Inc. is due and payable on May 3. 2025 and bears simple interest at a rate of 10% per annum.
$25,000
lent to the Company by Zander Therapeutics, Inc. is due and payable on June 5. 2025 and bears simple interest at a rate of 10% per annum.
Zander
Therapeutics, Inc. and the Company are under common control.
NOTE
7. STOCKHOLDERS’ EQUITY
The
stockholders’ equity section of the Company contains the following classes of capital stock as of September 30 30, 2024:
Common
stock, $ 0.0001 par value; 5,800,000,000 shares authorized: 5,258,235 shares issued and outstanding.
With
respect to each matter submitted to a vote of stockholders of the Corporation, each holder of Common Stock shall be entitled to cast
that number of votes which is equivalent to the number of shares of Common Stock owned by such holder times one (1).
On
any voluntary or involuntary liquidation, dissolution or winding up of the Corporation, the holders of the Common Stock shall receive,
out of assets legally available for distribution to the Company’s stockholders, a ratable share in the assets of the Corporation.
Preferred
Stock, $0.0001 par value, 800,000,000 shares authorized of which 600,000 is designated as Series AA Preferred Stock: 34 shares issued
and outstanding as of September 30, 2024, 739,000,000 is designated Series A Preferred Stock of which 10,123,771 shares are outstanding
as of September 30, 2024, 60,000,000 is designated Series M Preferred Stock of which 29,338 shares are outstanding as of September 30,
2024, and 20,000 is designated Series NC stock of which 15,007 shares are outstanding as of September 30, 2024..
The
abovementioned shares authorized pursuant to the Company’s certificate of incorporation may be issued from time to time without
prior approval of the shareholders. The Board of Directors of the Company shall have the full authority permitted by law to establish
one or more series and the number of shares constituting each such series and to fix by resolution full or limited, multiple or fractional,
or no voting rights, and such designations, preferences, qualifications, restrictions, options, conversion rights and other special or
relative rights of any series of the Stock that may be desired.
Series
AA Preferred Stock
On
September 15, 2014 the Company filed a CERTIFICATE OF DESIGNATION (“Certificate of Designations”) with the Nevada Secretary
of State setting forth the preferences rights and limitations of a newly authorized series of preferred stock designated and known as
“Series AA Preferred Stock” (hereinafter referred to as “Series AA Preferred Stock”).
The
Board of Directors of the Company have authorized 600,000 shares of the Series AA Preferred Stock, par value $0.0001. With respect to
each matter submitted to a vote of stockholders of the Corporation, each holder of Series AA Preferred Stock shall be entitled to cast
that number of votes which is equivalent to the number of shares of Series AA Preferred Stock owned by such holder times seven (7). Except
as otherwise required by law holders of Common Stock, other series of Preferred issued by the Corporation, and Series AA Preferred Stock
shall vote as a single class on all matters submitted to the stockholders.
Series
A Preferred Stock
On
January 15, 2015 the Company filed a CERTIFICATE OF DESIGNATION (“Certificate of Designations”) with the Nevada Secretary
of State setting forth the preferences rights and limitations of a newly authorized series of preferred stock designated and known as
“Series A Preferred Stock” (hereinafter referred to as “Series A Preferred Stock”).
The
Board of Directors of the Company have authorized 739,000,000 shares of the Series A Preferred Stock, par value $0.0001. With respect
to each matter submitted to a vote of stockholders of the Corporation, each holder of Series A Preferred Stock shall be entitled to cast
that number of votes which is equivalent to the number of shares of Series A Preferred Stock owned by such holder times one. Except as
otherwise required by law holders of Common Stock, other series of Preferred issued by the Corporation, and Series A Preferred Stock
shall vote as a single class on all matters submitted to the stockholders.
Holders
of the Series A Preferred Stock will be entitled to receive, when, as and if declared by the board of directors of the Company (the “Board”)
out of funds legally available therefore, non-cumulative cash dividends of $0.01 per quarter. In the event any dividends are declared
or paid or any other distribution is made on or with respect to the Common Stock , the holders of Series A Preferred Stock as of the
record date established by the Board for such dividend or distribution on the Common Stock shall be entitled to receive, as additional
dividends (the “Additional Dividends”) an amount (whether in the form of cash, securities or other property) equal to the
amount (and in the form) of the dividends or distribution that such holder would have received had each share of the Series A Preferred
Stock been one share of the Common Stock, such Additional Dividends to be payable on the same payment date as the payment date for the
Common Stock.
Upon
any liquidation, dissolution, or winding up of the Company, whether voluntary or involuntary (collectively, a “Liquidation”),
before any distribution or payment shall be made to any of the holders of Common Stock or any other series of preferred stock, the holders
of Series A Preferred Stock shall be entitled to receive out of the assets of the Company, whether such assets are capital, surplus or
earnings, an amount equal to $0.01 per share of Series A Preferred (the “Liquidation Amount”) plus all declared and unpaid
dividends thereon, for each share of Series A Preferred held by them.
If,
upon any Liquidation, the assets of the Company shall be insufficient to pay the Liquidation Amount, together with declared and unpaid
dividends thereon, in full to all holders of Series A Preferred, then the entire net assets of the Company shall be distributed among
the holders of the Series A Preferred, ratably in proportion to the full amounts to which they would otherwise be respectively entitled
and such distributions may be made in cash or in property taken at its fair value (as determined in good faith by the Board), or both,
at the election of the Board.
On
January 10, 2017 Regen Biopharma, Inc. (“Regen”) filed a CERTIFICATE OF DESIGNATION (“Certificate of Designations”)
with the Nevada Secretary of State setting forth the preferences rights and limitations of a newly authorized series of preferred stock
designated and known as “Series M Preferred Stock” (hereinafter referred to as “Series M Preferred Stock”).
The
Board of Directors of Regen have authorized 60,000,000 shares of the Series M Preferred Stock, par value $0.0001. With respect to each
matter submitted to a vote of stockholders of Regen, each holder of Series M Preferred Stock shall be entitled to cast that number of
votes which is equivalent to the number of shares of Series M Preferred Stock owned by such holder times one. Except as otherwise required
by law holders of Common Stock, other series of Preferred issued by Regen, and Series M Preferred Stock shall vote as a single class
on all matters submitted to the stockholders.
The
holders of Series M Preferred Stock shall be entitled receive dividends, when, as and if declared by the Board of Directors in accordance
with Nevada Law, in its discretion, from funds legally available therefore
On
any voluntary or involuntary liquidation, dissolution or winding up of Regen, the holders of the Series M Preferred Stock shall receive,
out of assets legally available for distribution to Regen’s stockholders, a ratable share in the assets of Regen.
On
March 26, 2021 Regen Biopharma, Inc. (“Regen”) filed a CERTIFICATE OF DESIGNATION (“Certificate of Designations”)
with the Nevada Secretary of State setting forth the preferences rights and limitations of a newly authorized series of preferred stock
designated and known as Nonconvertible Series NC Preferred Stock (hereinafter referred to as “Series NC Preferred Stock”).
The
Board of Directors of Regen have authorized 20,000 shares of the Series NC Preferred Stock, par value $0.0001. With respect to each matter
submitted to a vote of stockholders of Regen, each holder of Series NC Preferred Stock shall be entitled to cast that number of votes
which is equivalent to the number of shares of Series NC Preferred Stock owned by such holder times 334. Except as otherwise required
by law holders of Common Stock, other series of Preferred issued by Regen, and Series NC Preferred Stock shall vote as a single class
on all matters submitted to the stockholders.
The
holders of Series NC Preferred Stock shall be entitled receive dividends, when, as and if declared by the Board of Directors in accordance
with Nevada Law, in its discretion, from funds legally available therefore
On
any voluntary or involuntary liquidation, dissolution or winding up of Regen, the holders of the Series NC Preferred Stock shall receive,
out of assets legally available for distribution to Regen’s stockholders, a ratable share in the assets of Regen.
On
May 20, 2024 Regen Biopharma, Inc. amended its Certificate of Incorporation adding the following Article 8 which is and reads as follows:
Shares
of one class or series of stock may be issued as a share dividend in respect of another class or series.
On
May 21 , 2024 the Board of Directors of Regen Biopharma, Inc declared a dividend to all shareholders of record as of June 20,2024 (“Record
Date”) to be paid to shareholders on or about July 1, 2024 such dividend to be payable in shares of the Regen’s authorized
but unissued Series A Preferred Stock and to consist of two share of Series A Preferred Stock for every one share of Regen Biopharma,
Inc. Common Stock owned as of the Record Date, every one share of Regen Biopharma, Inc. Series A Preferred Stock owned as of the Record
Date, every one share of Series AA Preferred Stock owned as of the Record Date, every one share of Series M Preferred Stock owned as
of the Record Date and every one share of Series NC Preferred Stock owned as of the Record Date.
On
July 3, 2024 9,694,152 Series A Preferred Shares were issued as a dividend to the Shareholders of Record.
On
September 18, 2024 the Board of Directors of Regen Biopharma, Inc.(“Regen”) declared a dividend to all shareholders of record
as of October 17,2024 (“Record Date”) to be paid to shareholders on or about November 1, 2024 such dividend to be payable
in shares of the Regen’s authorized but unissued Common Stock and to consist of one share of Common Stock for every one share of
Regen Biopharma, Inc. Common Stock owned as of the Record Date, every one share of Regen Biopharma, Inc. Series A Preferred Stock owned
as of the Record Date, every one share of Series AA Preferred Stock owned as of the Record Date, every one share of Series M Preferred
Stock owned as of the Record Date and every one share of Series NC Preferred Stock owned as of the Record Date
NOTE
8. INVESTMENT SECURITIES, RELATED PARTY
On
June 11, 2018 Regen Biopharma, Inc. was paid a property dividend consisting of 470,588 of the common shares of Zander Therapeutics, Inc.
On
November 29, 2018 the Company accepted 725,000 shares of the Series M Preferred stock of Zander Therapeutics, Inc. in satisfaction of
prepaid rent and accrued interest owed to the Company collectively amounting to $13,124.
On
September 30, 2024 the Company revalued 470,588 of the common shares of Zander Therapeutics, Inc. and 725,000 shares of the Series M
Preferred stock of Zander Therapeutics, Inc. based on the following inputs:
| Fair Value of Intellectual Property | |
$ | 300,000 | |
| Prepaid Expenses | |
| 65,661 | |
| Due from Employee | |
| 0 | |
| Note Receivable | |
| 40000 | |
| Accrued Interest Receivable | |
| 35,000 | |
| Investment Securities | |
| 258,255 | |
| Convertible Note Receivable | |
| 10,000 | |
| Accounts Payable | |
| 30,563 | |
| Notes Payable | |
| 400,000 | |
| Accrued Expenses Related Parties | |
| 162,011 | |
| Notes Payable Related Party | |
| 0 | |
| Accrued Expenses | |
| 647,072 | |
| Enterprise Value | |
| 1,948,562 | |
| Less: Total Debt | |
| (1,239,646 | ) |
| Portion of Enterprise Value Attributable to Shareholders | |
$ | 708,916 | |
| Fair Value per Shares | |
$ | 0.0155 | |
The
abovementioned constitute the Company’s sole related party investment securities as of September 30, 2024.
As
of September 30, 2024:
470,588
Common Shares of Zander Therapeutics, Inc.
| Basis | | |
Fair Value | | |
Total
Unrealized Gains | | |
Net
Unrealized Gain or
(Loss)
realized during
the
quarter ended
September
30, 2024 | |
| $ | 5,741 | | |
$ | 6,496 | | |
$ | 755 | | |
$ | (0 | ) |
725,000
Series M Preferred of Zander Therapeutics, Inc.
| Basis | | |
Fair Value | | |
Total
Unrealized
Loss | | |
Net
Unrealized Gain or
(Loss)
realized during
the
quarter ended
September
30, 2024 | |
| $ | 13,124 | | |
$ | 11,238 | | |
$ | (1,866 | ) | |
$ | (0 | ) |
NOTE
9. STOCK TRANSACTIONS
On
October 13 2023 the Company issued 16,710 common shares for cash consideration of $22,726.
On
October 27 2023 the Company issued 35,785 common shares for cash consideration of $46,091.
On
November 10, 2023 the Company issued 31,732 common shares for cash consideration of $38,205.
On
November 27, 2023 the Company issued 33,989 common shares for cash consideration of $32,629.
On
December 11 2023 the Company issued 43,297 common shares for cash consideration of $38,101.
On
December 20, 2023 the Company issued 82,686 common shares for cash consideration of $34,543.
On
January 3, 2024 the Company issued 94,883 common shares for cash consideration of $39,638.
On
January 10, 2024 the Company issued 82,643 common shares for cash consideration of $44,297.
On
February 2, 2024 the Company issued 40,229 common shares for cash consideration of $19,614.
On
February 21, 2024 the Company issued 52,569 common shares for cash consideration of $32,362.
On
March 6, 2024 the Company issued 44,503 common shares for cash consideration of $25,282.
On
March 20, 2024 the Company issued 49,230 common shares for cash consideration of $26,781.
On
April 3, 2024 the Company issued 52,763 common shares for cash consideration of $25,326.
On
May 2, 2024 the Company issued 20,068 Series A Preferred shares for nonemployee services .
On
May 29, 2024 the Company issued 66185 common shares for cash consideration of $30,000.
On
June 7, 2024 the Company issued 62,207 common shares for cash consideration of $30,000.
On
June 20, 2024 the Company issued 75,301 common shares for cash consideration of $50,000.
On
July 3, 2024 9,694,152 Series A Preferred Shares were distributed as a dividend to shareholders.
On
July 12, 2024 the Company issued 135,242 common shares for cash consideration of $28,126
On
September 4, 2024 , 2024 the Company issued 500,000 common shares as a commitment fee in connection with the issuance of a promissory
note in the face amount of $250,000
On
September 26,2024 the Company issued 249,915 shares as consideration for nonemployee services.
NOTE
10 INCOME TAXES
As
of September 30, 2024
| Deferred tax assets: | |
| |
| Net operating tax carry forwards | |
$ | 4,329,384 | |
| Other | |
| (0 | ) |
| Gross deferred tax assets | |
| 4,329,384 | |
| Valuation allowance | |
| (4,329,384 | ) |
| Net deferred tax assets | |
$ | (0 | ) |
As
of September 30 2024 the Company has a Deferred Tax Asset of $4,329,384 completely attributable to net operating loss carry forwards
of approximately $20,616,114. The amount and availability of any net operating loss carryforward will be subject to the limitations set
forth in the Internal Revenue Code. Such factors as the number of shares ultimately issued within a three-year look-back period; whether
there is a deemed more than 50% change in control; the applicable long-term tax exempt bond rate; continuity of historical business;
and subsequent income of the Company all enter into the annual computation of allowable annual utilization of any net operating loss
carryforward.
Realization
of deferred tax assets is dependent upon sufficient future taxable income during the period that deductible temporary differences and
carry forwards are expected to be available to reduce taxable income. The achievement of required future taxable income is uncertain.
A
corporation is considered to undergo “an ownership change” if, as a result of changes in the stock ownership by “5-percent
shareholders” or as a result of certain reorganizations, the percentage of the corporation’s stock owned by those 5-percent
shareholders increases by more than 50 percentage points over the lowest percentage of stock owned by those shareholders at any time
during the prior three-year testing period. Five-percent shareholders are persons who hold 5% or more of the stock of a corporation at
any time during the testing period as well as certain groups of shareholders (based typically on whether they acquired their shares in
a single offering or exchange transaction) who are not individually 5-percent shareholders.
As
the Company will require cash infusions in order to implement its business plan, and as it is probable, although not guaranteed, that
such funding needs may be met through the sale of equity securities to “5-percent shareholders”, the Company recognized a
valuation allowance equal to the deferred Tax Asset and the Company recorded a valuation allowance reducing all deferred tax assets to
0.
NOTE
11. SUBSEQUENT EVENTS
On
September 18, 2024 the Board of Directors of Regen declared a dividend to all shareholders of record as of October 17,2024 (“Record
Date”) to be paid to shareholders on or about November 1, 2024 such dividend to be payable in shares of the Regen’s authorized
but unissued Common Stock and to consist of one share of Common Stock for every one share of Regen Biopharma, Inc. Common Stock owned
as of the Record Date, every one share of Regen Biopharma, Inc. Series A Preferred Stock owned as of the Record Date, every one share
of Series AA Preferred Stock owned as of the Record Date, every one share of Series M Preferred Stock owned as of the Record Date and
every one share of Series NC Preferred Stock owned as of the Record Date
Security
Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The
following table sets forth information known to the Company with respect to the beneficial ownership of each class of the Company’s
capital stock as of November 20, 2024 for (1) each person known by the Company to beneficially own more than 10% of each class
of the Company’s voting securities, (2) each executive officer, (3) each of the Company’s directors and (4) all of the Company’s
executive officers and directors as a group.
Based
on 21,554,705 shares issued and outstanding
| Title of Class | |
Name and Address of Beneficial Owner | |
Amount and Nature of Beneficial Ownership | | |
Percentage | |
| Common | |
David R. Koos | |
| 436,799 | * | |
| 2 | % |
| | |
c/o Regen Biopharma, Inc. | |
| | | |
| | |
| | |
4700 Spring Street St 304 | |
| | | |
| | |
| | |
La Mesa CA 91942* | |
| | | |
| | |
| Common | |
All Officers and Directors as a Group | |
| 436,799 | * | |
| 2 | % |
*includes
19 shares held by BMXP Holdings Shareholder’s Business Trust and 11 shares held by the AFN Trust and 366,651 shares
held by Zander Therapeutics, Inc.
Based
on 10,123,771 shares issued and outstanding
| Title of Class | |
Name and Address of Beneficial Owner | |
Amount and Nature of Beneficial Ownership | | |
Percentage | |
| Series A Preferred | |
David R. Koos | |
| | | |
| 4.08 | % |
| | |
c/o Regen Biopharma, Inc. | |
| 413,288 | | |
| | |
| | |
4700 Spring Street St 304 | |
| | | |
| | |
| | |
| |
| | | |
| | |
| Series A Preferred | |
All Officers and Directors as a Group | |
| 413,288 | | |
| 4.08 | % |
*
Includes 11 share held by BMXP Holdings Shareholder’s Business Trust,, 366,651 shares held by Zander Therapeutics, Inc. and 7
share held by the AFN Trust.
Based
on 29,338 shares outstanding
| Title of Class | |
Name and Address of Beneficial Owner | |
Amount and Nature of Beneficial Ownership | | |
Percentage | |
| Series M Preferred | |
David R. Koos | |
| 7,667 | | |
| 26.14 | % |
| | |
c/o Regen Biopharma, Inc | |
| | | |
| | |
| | |
4700 Spring Street, Suite 304, | |
| | | |
| | |
| | |
La Mesa, California 91942 | |
| | | |
| | |
| Series M Preferred | |
Todd S. Caven | |
| 6,667 | | |
| 22.73 | % |
| | |
8578 TERRACEVIEW LANE NORTH | |
| | | |
| | |
| | |
MAPLE GROVE, MN 55311 | |
| | | |
| | |
| Series M Preferred | |
Roger Formisano | |
| 2,001 | | |
| 6.82 | % |
| | |
4124 N. 64th Street | |
| | | |
| | |
| | |
Scottsdale, AZ 85251 | |
| | | |
| | |
| Series M Preferred | |
Robert D. Hopkins | |
| 2,001 | | |
| 6.82 | % |
| | |
11642 N. 40th Place | |
| | | |
| | |
| | |
Phoenix, AZ 85028 | |
| | | |
| | |
| Series M Preferred | |
Harry Lander | |
| 6,667 | | |
| 22.73 | % |
| | |
50 SUTTON PLACE SOUTH | |
| | | |
| | |
| | |
APT. 6A | |
| | | |
| | |
| | |
NEW YORK, NY 10022 | |
| | | |
| | |
| Series M Preferred | |
Jean-Pierre Millon | |
| 4,001 | | |
| 13.64 | % |
| | |
3908 E. San Miguel Ave | |
| | | |
| | |
| | |
Paradise Valley, AZ 85253 | |
| | | |
| | |
| Series M Preferred | |
All Officers and Directors as a Group | |
| 7,667 | | |
| 26.14 | % |
based
on 334 shares outstanding
| Title of Class | |
Name and Address of Beneficial Owner | |
Amount and Nature of Beneficial Ownership | | |
Percentage | |
| Series AA Preferred | |
David R. Koos | |
| | | |
| | |
| | |
c/o Regen Biopharma, Inc. | |
| 334 | | |
| 100 | % |
| | |
4700 Spring Street St 304 | |
| | | |
| | |
| | |
La Mesa CA 91942 | |
| | | |
| | |
| Series AA Preferred | |
All Officers and Directors as a Group | |
| 334 | | |
| 100 | % |
based
on 15,007 shares outstanding
| Title of Class | |
Name and Address of Beneficial Owner | |
Amount and Nature of Beneficial Ownership | | |
Percentage | |
| Series NC Preferred | |
David R. Koos | |
| | | |
| | |
| | |
c/o Regen Biopharma, Inc. | |
| 15,007 | | |
| 100 | % |
| | |
4700 Spring Street St 304 | |
| | | |
| | |
| | |
La Mesa CA 91942 | |
| | | |
| | |
| Series NC Preferred | |
All Officers and Directors as a Group | |
| 15,007 | | |
| 100 | % |
SALES
OF UNREGISTERED SECURITIES
October 1, 2024 to November 20, 2024
On November 4, 2024
the Company issued 500,000 common shares in settlement of $20,000 of outstanding principal indebtedness. .
The
abovementioned securities were issued pursuant to Section 4(a) (2) of the securities Act of 1933, as amended (the “Act”).
No underwriters were retained to serve as placement agents for the sale. The securities were sold directly through our management. No
commission or other consideration was paid in connection with the sale of the securities. There was no advertisement or general solicitation
made in connection with this Offer and Sale of securities.
On
November 13, 2024 the Company issued 370,084 common shares as consideration for nonemployee services.
The
abovementioned securities were issued pursuant to Section 4(a) (2) of the securities Act of 1933, as amended (the “Act”).
No underwriters were retained to serve as placement agents for the sale. The securities were sold directly through our management. No
commission or other consideration was paid in connection with the sale of the securities. There was no advertisement or general solicitation
made in connection with this Offer and Sale of securities.
Quarter
Ended September 30, 2024
Effective
September 4, 2024 the Company entered into a securities purchase agreement (the “Purchase Agreement”) with Coventry Enterprises,
LLC (“Coventry”), pursuant to which Coventry Enterprises purchased a 10% unsecured promissory Note (the “Note”)
from the Company in the principal amount of $250,000 of which $25,000 was retained by Coventry through an Original Issue Discount.
The
Note carries “Guaranteed Interest” on the principal amount at the rate of 10% per annum for the ten month term of the Note
for an aggregate Guaranteed Interest $25,000. The Principal Amount and the Guaranteed Interest shall be due and payable in ten equal
monthly payments $27,500 commencing on November 4, 2024, and continuing on the fourth day of each month thereafter (each, a “Monthly
Payment Date”) until paid in full not later than September 4, 2025.
Upon
an Event of Default (as such term is defined in the Note) the Note shall become convertible, in whole or in part, into shares of Common
Stock at the option of the Holder at price per share equivalent to 90% of the lowest per-share trading price for the 20 Trading Days
preceding a Conversion Date.
$152,000
of the proceeds received has been utilized to repay an aggregate of $152,000 of outstanding principal indedebteness and interest due
to Coventry by the Company resulting from a $175,000 Note issued to Coventry on September 12, 2023.
In
Connection with the Purchase Agreement the Company issued to Coventry 500,000 common shares (“Commitment Shares”) on September
4 .2024. If The Company has satisfied all the terms of the Note without default the Coventry shall, within 10 calendar days thereafter,
return to the Company’s treasury for cancellation 350,000 of the Commitment Shares.
The
abovementioned securities were issued pursuant to Section 4(a) (2) of the securities Act of 1933, as amended (the “Act”).
No underwriters were retained to serve as placement agents for the sale. The securities were sold directly through our management. No
commission or other consideration was paid in connection with the sale of the securities. There was no advertisement or general solicitation
made in connection with this Offer and Sale of securities.
On
September 26, 2024 the Company issued 249,915 shares as consideration for nonemployee services.
The
abovementioned securities were issued pursuant to Section 4(a) (2) of the securities Act of 1933, as amended (the “Act”).
No underwriters were retained to serve as placement agents for the sale. The securities were sold directly through our management. No
commission or other consideration was paid in connection with the sale of the securities. There was no advertisement or general solicitation
made in connection with this Offer and Sale of securities.
Quarter
Ended June 30, 2024
On
April 2, 2024 the Company issued 20,068 of its Series A Preferred Shares to an independent consultant as consideration for services.
The
abovementioned securities were issued pursuant to Section 4(a) (2) of the securities Act of 1933, as amended (the “Act”).
No underwriters were retained to serve as placement agents for the sale. The securities were sold directly through our management. No
commission or other consideration was paid in connection with the sale of the securities. There was no advertisement or general solicitation
made in connection with this Offer and Sale of securities.
Quarter
Ended September 30, 2023
On
September 12, 2023 the Company issued 125,000 common shares to Coventry Enterprises LLC pursuant to the terms and conditions of the Investment
Agreement by and between the Company and Coventry Enterprises LLC. These common shares were registered for resale pursuant to the Securities
of 1933 on Form S-1 on September 29, 2023.
On
September 12, 2023 the Company entered into a common stock purchase agreement (the “Investment Agreement”) with Coventry
providing for an equity financing facility (the “Equity Line”). The Investment Agreement provides that upon the terms and
subject to the conditions in the Investment Agreement, Coventry is committed to purchase up to Ten Million Dollars ($10,000,000) of shares
of common stock, $0.0001 par value per share (the “Common Stock”), over the 36-month term of the Investment Agreement (the
“Total Commitment”).
Under
the terms of the Investment Agreement, Coventry will not be obligated to purchase shares of Common Stock unless and until certain conditions
are met, including but not limited to a Registration Statement on Form S-1 (the “Registration Statement”) becoming effective
which registers Coventry’s resale of any Common Stock purchased by Coventry under the Equity Line.
From
time to time over the 36-month term of the Commitment Period (as such term is defined in the Investment Agreement) the Company, in its
sole discretion, may provide Coventry with a draw down notice (each, a “Draw Down Notice”), to purchase a specified number
of shares of Common Stock (each, a “Draw Down Amount Requested”), subject to the limitations discussed below. The actual
amount of proceeds the Company will receive pursuant to each Draw Down Notice (each, a “Draw Down Amount”) is to be determined
by multiplying the Draw Down Amount Requested by the applicable purchase price. The purchase price of each share of Common Stock equals
80% of the lowest trading price of the Common Stock during the ten business days prior to the Draw Down Notice date (the “Pricing
Period”).
The
maximum number of shares of Common Stock requested to be purchased pursuant to any single Draw Down Notice cannot exceed the lesser of
(i) 200% of the Average Daily Traded Value (as such term is defined in the Investment Agreement) during the ten business days immediately
preceding the Drawdown Notice Date or (ii) $250,000. The Company is prohibited from delivering a Draw Down Notice if the sale of shares
of Common Stock pursuant to the Draw Down Notice would cause the Company to issue and sell to Coventry or Coventry to acquire or purchase
an aggregate number of shares of Common Stock that would result in Coventry beneficially owning more than 4.99% of the issued and outstanding
shares of Common Stock of the Company.
Average
Daily Traded Value is defined in the Investment Agreement as a per share price that shall be equal to the lowest trading price of the
Company’s common stock on OTC Pink during the during the ten business days immediately preceding the respective Drawdown Notice
Delivery Date multiplied by the Average Daily Trading Volume.
Average
Daily Trading Volume. is defined in the Investment Agreement as the average trading volume of the Company’s common stock for the
ten business days immediately preceding the respective Drawdown Notice Date.
Drawdown
Notice Date is defined in the Investment Agreement as the business day a Drawdown Notice is received by Coventry.
Pursuant
to the Investment Agreement the Company issued to Coventry as a commitment fee 125,000 shares of its common stock (“Commitment
Shares”) in reliance upon the exemptions from the registration requirements of the Securities Act of 1933, as amended, afforded
the Company under Section 4(a)(2) promulgated thereunder.
Coventry
has agreed that:
(a)
for so long as the market price of the Company’s common stock is above $1.25 per share and
(b)
the Company is in full compliance with all agreements entered into with Coventry and
(c)
and the Company has not issued any common shares at a per share price below $1.50,
Coventry
will agree to a leak out provision and will not sell more than 10,000 shares of the Commitment shares without permission from the Issuer.
Quarter
ended December 31, 2022
On
October 25, 2022 the Company issued 6,667 Series A preferred shares as consideration for nonemployee services
On
November 11, 2022 the Company issued 105126 Series A preferred shares in satisfaction of $761,500 of convertible indebtedness and $380,262
of accrued interest on convertible indebtedness.
On
November 11, 2022 the Company issued 11,279 common shares in satisfaction of $25,639 of accrued interest on convertible indebtedness.
On
December 5, 2022 the Company issued 1,112 Series A preferred shares as consideration for nonemployee services.
All
the abovementioned securities were issued pursuant to Section 4(a) (2) of the securities Act of 1933, as amended (the “Act”).
No underwriters were retained to serve as placement agents for the sale. The securities were sold directly through our management. No
commission or other consideration was paid in connection with the sale of the securities. There was no advertisement or general solicitation
made in connection with this Offer and Sale of securities.
FORM
OF SUBSCRIPTION AGREEMENT
Subscription
Agreement
REGEN
BIOPHARMA, INC.
10,000,000
Shares of Common Stock, $0.0001 Par Value, $0.04 per Share
Offering
Amount: Up to $400,000
Minimum
Purchase Amount: No Minimum
Sales
will be made to Accredited Investors Only
Under
Tier 1 of Regulation A+ of the Securities and Exchange Commission
Subscriber
Full Name:
________________________________________
Number
of Shares Subscribed For:
________________________________________
INSTRUCTIONS
To
purchase shares of the common stock (the “Shares”) of Regen Biopharma, Inc., a Nevada corporation (the “Company”)
in the offering described above (the “Offering”), pursuant to the Regulation A+ Offering Circular dated November 21, 2024
(the “Offering Circular”), please:
(i) review this Subscription Agreement (this “Subscription Agreement” or this “Agreement”); (ii) complete Paragraph
D under Representations and Warranties of Subscribers of this Subscription Agreement regarding accredited investor status; and (iii)
complete, sign and date the appropriate signature pages (individual subscribers should complete, sign and date the individual signature
page; entity subscribers should complete, sign and date the entity signature page.
Please
make your uncertified check, certified check, or cashier’s check payable to “Regen Biopharma, Inc.” and mail your check,
with the completed Subscription Agreement, to :
REGEN
BIOPHARMA, INC
Attention
David R. Koos, CEO
4700
Spring Street, Suite 304, La Mesa, California, 91942
Instructions
for wire transfer may be obtained upon request from David R. Koos at (619) 722-5505
THE
COMPANY WILL NOT ACCEPT ANY SUBSCRIPTION AGREEMENT THAT IS NOT FULLY AND ACCURATELY COMPLETED, DATED AND SIGNED.
THIS
IS AN IMPORTANT LEGAL DOCUMENT. READ EACH PART OF IT CAREFULLY.
This
subscription, submitted as of the date set forth on the signature page, is between the Company and the undersigned subscriber (the “Subscriber”).
Offer
to Purchase. The Subscriber hereby irrevocably offers to purchase that number of Shares of the Company set forth on the signature
page and hereby tenders the Subscriber’s check payable to “Regen Biopharma, Inc.” in the aggregate dollar amount set
forth on the signature page at a per Share purchase price of $0.04, or hereby confirms that a wire for that amount has been sent
to the Company.
The
Subscriber understands that a subscription for the Shares may be rejected for any reason and that, in the event that this subscription
is rejected, the funds delivered herewith will be returned as soon as practicable without interest thereon or deduction therefrom.
Representations
and Warranties of Subscribers. By execution below, the Subscriber acknowledges that the Company is relying upon the accuracy
and completeness of the representations contained herein to comply with its obligations under applicable securities laws. The Subscriber
hereby represents and warrants to the Company and its officers, directors, managers, members, employees and agents as follows:
A.
Information About the Company and the Bank. The Subscriber has received and reviewed the Offering Circular, and has obtained
all information about the Company as the Subscriber believes relevant to the decision to purchase the Shares. The Subscriber has read
the Offering Circular and has also had the opportunity to ask questions of, and to receive answers from, the Company concerning the terms
and conditions of the investment and the business and affairs of the Company and to obtain any additional information necessary to verify
such information, and the Subscriber has received such information concerning the Company as the Subscriber considers necessary or advisable
in order to from a decision concerning an investment in the Company. The Subscriber is not relying on any representation regarding the
Company except as set forth in the Offering Circular. The Subscriber has engaged such advisors as the Subscriber deems appropriate to
evaluate the merits of an investment in the Company.
B.
Forward-Looking Statements. The Subscriber acknowledges and understands that any information provided about the Company’s
future plans and prospects is uncertain and subject to all of the uncertainties inherent in the future predictions, and that the Company, and its officers and directors shall not be liable for the accuracy thereof.
C.
Regulation A+ Offering. The Shares are being sold by the Company in an offering under an exemption from registration under
Tier 1 of Regulation A+ of the Securities and Exchange Commission (the “SEC”) under the Securities Act of 1933 (the “Act”)
D.
Accredited Investor Status. To be an “accredited investor,” an investor must come within any one of the following
categories, or be a person who the issuer reasonably believes comes within any one of the following categories at the time of the sale
of the shares to that investor:
| |
1. |
Any
bank as defined in Section 3(a)(2) of the Act, or any savings and loan association or other institution as defined in Section 3(a)(5)(A)
of the act whether acting in its individual or fiduciary capacity; any broker or dealer registered pursuant to Section 15 of the
Securities Exchange Act of 1934; any insurance company registered under the Investment Company Act of 1940 or a business development
company as defined in Section 2(a)(48) of that Act; any Small Business Investment Company licensed by the U.S. Small Business Administration
under Section 301(c) or (d) of the Small Business Investment Act of 1958; any plan established and maintained by a state, its political
subdivisions, or any agency or instrumentality of a state or political subdivisions, for the benefit of its employees, if such plan
has total assets in excess of $5,000,000; any employee benefit plan within the meaning of the Employee Retirement Income Security
Act of 1974, if the investment decision is made by a plan fiduciary, as defined in Section 3(21) of such Act, which is either a bank,
savings and loan association, insurance company, or registered investment advisor, or if the employee benefit plan has total assets
in excess of $5,000,000, or, if a self-directed plan, with investment decisions made solely by persons that are accredited investors; |
| |
|
|
| |
2. |
Any
private business development company as defined in Section 202(a)(22) of the Investment Advisers Act of 1940; |
| |
|
|
| |
3. |
Any
organization described in Section 501(c)(3) of the Internal Revenue Code, or corporation, Massachusetts or similar business trust,
or partnership, not formed for the specific purpose of acquiring the shares offered, with total assets in excess of $5,000,000; |
| |
|
|
| |
4. |
Any
director, executive officer, or general partner of the issuer of the securities being offered or sold, or any director, executive
officer, or general partner of a general partner of that issuer; |
| |
|
|
| |
5. |
Any
natural person whose individual net worth, or joint net worth with that person’s spouse or spousal equivalent, at the time
of his purchase (excluding the value of the person’s primary residence) exceeds $1,000,000; |
| |
|
|
| |
6. |
Any
natural person who had an individual income in excess of $200,000 in each of the two most recent years, or joint income with that
person’s spouse or spousal equivalent in excess of $300,000 in each of those years and has a reasonable expectation of reaching
the same income level in the current year; |
| |
|
|
| |
7. |
Any
trust, with total assets in excess of $5,000,000, not formed for the specific purpose of acquiring the shares offered, whose purchase
is directed by a sophisticated person as described in Rule 506(b)(2)(ii) of Regulation D; |
| |
|
|
| |
8. |
Any
entity in which all of the equity owners are accredited investors (as defined above). |
| |
|
|
| |
9. |
Any
entity, of a type not listed above, not formed for the specific purpose of acquiring the securities offered, owning investments in
excess of $5,000,000: |
| |
|
|
| |
10. |
Any
natural person holding in good standing one or more professional certifications or designations or credentials from an accredited
educational institution that the SEC has designated as qualifying an individual for accredited investor status; |
| |
|
|
| |
11. |
Any
natural person who is a “knowledgeable employee,” as defined in Rule 3c-5(a)(4) under the Investment Company Act of 1940,
of the issuer of the securities being offered or sold where the issuer would be an investment company, as defined in Section 3 of
such Act, but for the exclusion provided by either section 3(c)(1) or section 3(c)(7) of such Act; |
| |
|
|
| |
12. |
Any
“family office” as defined in Rule 202(a)(11)(G)-1 under the Investment Advisers Act of 1940 with assets under management
in excess of $5,000,000, that is not formed for the specific purpose of acquiring the securities offered, and whose prospective investment
is directed by a person who has such knowledge and experience in financial and business matters that such family office is capable
of evaluating the merits and risks of the prospective investment; and |
| |
|
|
| |
13. |
Any
“family client” as defined in Rule 202(a)(G)-1 under the Investment Advisers Act of 1940, of a family office meeting
the requirements of a family office and whose prospective investment in the issuer is directed by such family office. |
INSTRUCTION:
SUBSCRIBER REPRESENTS AND WARRANTS THAT SUBSCRIBER QUALIFIES AS AN ACCREDITED INVESTOR UNDER THE FOLLOWING PARAGRAPH NUMBER(S) FROM THE
LIST ABOVE:__________
If
the Subscriber is an entity, the individual(s) signing on behalf of the Subscriber and the Subscriber, jointly and severally, agree and
certify that this Agreement has been duly authorized by all necessary action on the part of the Subscriber, has been duly executed by
an authorized representative of the Subscriber, and is a legal, valid, and binding obligation of the Subscriber enforceable in accordance
with its terms.
Entities.
A REPRESENTATIVE OF AN ENTITY SUBSCRIBER MUST INITIAL HERE.
Delivery
of Certificate for Shares. Following the successful closing of the Offering, a book-entry registration of the Shares purchased
will be made in the records of the Transfer Agent for the Company.
Governing
Law; Venue. This Agreement shall be governed by, and construed in accordance with, the substantive laws of the State of California
without reference to California conflict or choice of law provisions. Actions or proceedings litigated in connection with this Agreement,
if any, shall have venue exclusively in the state and federal courts located in San Diego, California.
Additional
Information. Subscriber shall supply such additional information and documentation relating to Subscriber and any persons who
have any rights or interest in Subscriber as may be requested by the Company in order to ensure compliance by the Company with applicable
laws. If at any time prior to the Company’s acceptance of this Agreement, an adverse change occurs with respect to the Subscriber
such that the information, representations and warranties of the Subscriber set forth in this Agreement are no longer accurate, the Subscriber
shall immediately notify the Company of the inaccuracy in writing and shall deliver the updated, accurate information to the Company.
Successors
and Assigns. The representations and warranties made by the Subscriber in this Agreement are binding on the Subscriber’s
permitted successors and assigns and are made for the benefit of the Company and any other person who may become liable for violations
of applicable securities laws as a result of the inaccuracy or falsity of any of the Subscriber’s representations or warranties.
Subscriber shall not assign Subscriber’s obligations hereunder without the consent of the Company, which consent shall be granted,
if at all, in the sole discretion of the Board of Directors of the Company.
Counterparts.
This Agreement may be executed by the Company and by the Subscriber in separate counterparts, each of which shall be deemed an original.
Acceptance.
This Agreement is not binding on the Company until accepted in writing by the Company.
Severability.
Each provision of this Subscription Agreement shall be separate and severable and if for any reason any provision hereunder is found
invalid or unenforceable under applicable law, such invalidity or unenforceability shall not affect the operation of the remaining provisions
of this Subscription Agreement.
INDIVIDUAL
SIGNATURE PAGE
All
individual Subscribers must complete and sign this page. Where the Shares are to be held in joint tenancy or tenancy in common, both
parties must sign and both Social Security numbers should be indicated.
THIS
AGREEMENT SHALL NOT BIND THE COMPANY UNTIL IT HAS COUNTERSIGNED THIS PAGE.
INDIVIDUAL
SIGNATURE PAGE
All
individual Subscribers must complete and sign this page. Where the Shares are to be held in joint tenancy or tenancy in common, both
parties must sign and both Social Security numbers should be indicated.
| Subscriber’s
Name |
|
Social
Security Number |
| |
|
|
| _______________________________ |
|
____________________________ |
| |
|
|
| Subscriber’s
Address |
|
|
| Residence: |
|
|
| |
|
|
| ________________________________ |
|
|
| |
|
|
| ________________________________ |
|
|
| |
|
|
| Mailing: |
|
|
| |
|
|
| ________________________________ |
|
|
| |
|
|
| ________________________________ |
|
|
| |
|
|
| Form
of Ownership |
|
|
| |
|
|
| ________________________________ |
|
|
|
|
|
| Number
of Shares Subscribed for:____________________ |
|
|
| |
|
|
| The
Shares subscribed for herein should be registered as follows. |
|
|
| |
|
|
| Subscriber
Signature |
|
|
| |
|
|
| ________________________________ |
|
|
| |
|
|
| ________________________________ |
|
|
| |
|
|
| Date: |
|
|
The
Company hereby accepts the Subscriber’s offer to purchase ______ Shares for a total purchase price of $__________.
ENTITY
SIGNATURE PAGE
All
entity investors must complete and sign this page.
THIS
AGREEMENT SHALL NOT BIND THE COMPANY UNTIL IT HAS COUNTERSIGNED THIS PAGE.
| Entity
Name |
|
EIN |
| |
|
|
| _______________________________ |
|
____________________________ |
| |
|
|
| Entity
Address |
|
Social
Security Numbers ( if Partnership or Trust) |
| Business |
|
|
| ________________________________ |
|
|
| |
|
|
| ________________________________ |
|
|
| |
|
|
| Mailing: |
|
|
| |
|
|
| ________________________________ |
|
|
| |
|
|
| ________________________________ |
|
|
| |
|
|
| Form
of Ownership |
|
|
| |
|
|
| ________________________________ |
|
|
| |
|
|
| Number
of Shares Subscribed for:____________________________ |
| |
|
|
| The
Shares subscribed for herein should be registered as follows. |
SIGNATURE
| |
By:__________________________ |
|
| |
|
|
| ________________________________________________________ |
| (print name) |
|
| |
|
| |
Its: |
|
| |
|
|
| |
Dated: |
|
The
Company hereby accepts the Subscriber’s offer to purchase ______Shares for a total purchase price of $__________.
EXHIBITS
SIGNATURES
Pursuant
to the requirements of Regulation A+, Regen Biopharma, Inc. certifies that it has reasonable grounds to believe that it meets all of
the requirements for filing on Form 1-A and has duly caused this supplement to the offering statement to be signed on its
behalf by the undersigned, thereunto duly authorized in the City of La Mesa State of California, on February 24, 2025.
REGEN
BIOPHARMA, INC.
| By: |
/s/
David Koos |
|
| |
David Koos
Chairman of the Board of Directors
Chief Executive Officer
President
|
|
This
Supplement to the Offering Statement has been signed by the following persons in the capacities and on the dates
indicated:
| /s/ David
Koos |
|
| |
|
David
Koos
Chairman
of the Board of Directors
Chief
Executive Officer
President
February
24, 2025 |
|
Regen Biopharma (PK) (USOTC:RGBPP)
Historical Stock Chart
From Feb 2025 to Mar 2025
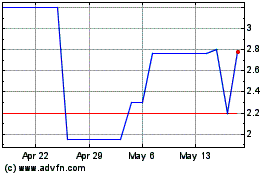
Regen Biopharma (PK) (USOTC:RGBPP)
Historical Stock Chart
From Mar 2024 to Mar 2025