UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the Month of February 2024
Commission File Number: 001-39997
Adagene Inc.
(Exact Name of Registrant as Specified in Its
Charter)
4F, Building C14, No. 218
Xinghu Street, Suzhou Industrial Park
Suzhou, Jiangsu Province, 215123
People’s Republic of China
+86-512-8777-3632
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form 20-F
x Form 40-F o
EXPLANTATORY NOTE
On February 9, 2024, Adagene Inc. (the “Company”)
updated information reflected in a press release and an investor presentation, which is attached as Exhibit 99.1 and Exhibit 99.2 to
this Current Report on Form 6-K, respectively. Representatives of the Company intend to use the updated presentation and information contained in
the press release in meetings with investors from time to time.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
Adagene Inc. |
| |
|
|
| |
By: |
/s/ Peter Luo |
| |
Name: Peter Luo |
| |
Title: Chief Executive Officer |
| |
|
| Date: February 9, 2024 |
|
EXHIBIT INDEX
Exhibit 99.1

Adagene Announces
Progress and Expansion of Clinical Collaboration Program for Masked, Anti-CTLA-4 SAFEbody® ADG126 (muzastotug) in Combination
with KEYTRUDA® (pembrolizumab) to Demonstrate Further Efficacy in Patients with Metastatic Microsatellite-stable
(MSS) Colorectal Cancer (CRC)
- Interim data from additional MSS CRC patients
dosed at 10 mg/kg every three weeks (Q3W) in combination with pembrolizumab anticipated in 2024 at a medical conference –
-
Initiated evaluation of 20 mg/kg loading doses of ADG126 in combination with pembrolizumab to explore enhanced efficacy
given superior therapeutic index of ADG126 –
- Received clearance from China’s Center
for Drug Evaluation (CDE) to evaluate ADG126 in combination with pembrolizumab –
SAN DIEGO and SUZHOU, China, February 9,
2024 – Adagene Inc. (“Adagene”) (Nasdaq: ADAG), a company transforming the discovery and development of novel antibody-based
therapies, today announced progress and expansion of the clinical collaboration development program for its masked, anti-CTLA-4 SAFEbody,
ADG126 in combination with Merck & Co., Inc., Rahway, NJ, USA’s anti-PD-1 therapy, KEYTRUDA® (pembrolizumab),
in patients with metastatic microsatellite-stable (MSS) colorectal cancer (CRC).
“Following completion of enrollment
of 12 additional patients at the end of last year, together with our ongoing expansion plans, we are on track to deliver data in 2024
that support the findings released at the recent ASCO-GI Symposium demonstrating the safety and efficacy profile of ADG126 in combination
with pembrolizumab in MSS CRC,” said Peter Luo, Ph.D., Chairman, CEO
and President of R&D at Adagene.
He continued, “To address the requirements for Project Optimus
by FDA, we have initiated evaluation of ADG126 20 mg/kg loading doses in combination with pembrolizumab, which we believe can unlock even
greater efficacy for MSS CRC in planned cohort expansion, while still maintaining a robust safety profile. Additionally, we are now cleared
to evaluate ADG126 in combination with pembrolizumab in China, strengthening our efficacy evaluation with additional patients enrolled
at unprecedented dosing regimens for anti-CTLA-4 therapy.”
The updates, which increase the ongoing phase 2 dose expansion in MSS
CRC to over 50 patients, include the following:
| · | The company announced it completed enrollment of 12 additional patients in the fourth quarter of 2023 in the ongoing phase 2 dose
expansion cohort evaluating ADG126 10 mg/kg Q3W in combination with pembrolizumab in MSS CRC. These Part 2 results are expected
to support data from Part 1 of the dose expansion in MSS CRC that was recently presented at the 2024 ASCO-GI Symposium. |
| · | Given the safety profile of ADG126, Adagene has also initiated evaluation of 20 mg/kg loading doses in combination with pembrolizumab
in patients with advanced/metastatic cancer. Following the ongoing safety evaluation, the company plans to study efficacy of the loading
doses followed by a maintenance regimen of ADG126 10 mg/kg Q3W in combination with pembrolizumab. The company plans dose expansion with
this regimen in patients with MSS CRC in the US and Asia Pacific. |

| · | Adagene has also received clearance from the CDE in China to initiate clinical evaluation of ADG126 in combination with pembrolizumab.
This enables the company to broaden its dose expansion cohorts for MSS CRC at selected dosing regimens, and potentially in other tumor
types. |
2024 Milestones
Data from the ongoing phase 1b/2 clinical trial of ADG126 in combination
with pembrolizumab, including dose expansion cohorts, are anticipated throughout 2024:
| · | Follow up of Part 1 evaluable patients at 10 mg/kg Q3W (n=12) and 10 mg/kg Q6W (n=10) |
| · | Data from Part 2 patients at 10 mg/kg Q3W (n=12) |
| · | Evaluation of 20 mg/kg loading doses for Project Optimus requirements: |
| o | Safety data with repeat doses |
| o | Dose expansion in MSS CRC (n~10) |
| · | Additional patients in China (n≥10) |
About Adagene
Adagene Inc. (Nasdaq:
ADAG) is a platform-driven, clinical-stage biotechnology company committed to transforming the discovery and development of novel antibody-based
cancer immunotherapies. Adagene combines computational biology and artificial intelligence to design novel antibodies that address
unmet patient needs. Powered by its proprietary Dynamic Precision Library (DPL) platform, composed of NEObody™, SAFEbody®,
and POWERbody™ technologies, Adagene’s highly differentiated pipeline features novel immunotherapy programs. Adagene has
forged strategic collaborations with reputable global partners that leverage its technology in multiple approaches at the vanguard of
science.
For
more information, please visit: https://investor.adagene.com. Follow Adagene on WeChat, LinkedIn and Twitter.
SAFEbody® is
a registered trademark in the United States, China, Australia, Japan, Singapore, and the European Union.
KEYTRUDA® is a registered trademark of Merck Sharp & Dohme
LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.

Safe Harbor Statement
This press release
contains forward-looking statements, including statements regarding certain clinical results of ADG126, the potential implications of
clinical data for patients, and Adagene’s advancement of, and anticipated preclinical activities, clinical development, regulatory
milestones, and commercialization of its product candidates. Actual results may differ materially from those indicated in the forward-looking
statements as a result of various important factors, including but not limited to Adagene’s ability to demonstrate the safety and
efficacy of its drug candidates; the clinical results for its drug candidates, which may not support further development or regulatory
approval; the content and timing of decisions made by the relevant regulatory authorities regarding regulatory approval of Adagene’s
drug candidates; Adagene’s ability to achieve commercial success for its drug candidates, if approved; Adagene’s ability to
obtain and maintain protection of intellectual property for its technology and drugs; Adagene’s reliance on third parties to conduct
drug development, manufacturing and other services; Adagene’s limited operating history and Adagene’s ability to obtain additional
funding for operations and to complete the development and commercialization of its drug candidates; Adagene’s ability to enter
into additional collaboration agreements beyond its existing strategic partnerships or collaborations, and the impact of the COVID-19
pandemic on Adagene’s clinical development, commercial and other operations, as well as those risks more fully discussed in the
“Risk Factors” section in Adagene’s filings with the U.S. Securities and Exchange Commission. All forward-looking
statements are based on information currently available to Adagene, and Adagene undertakes no obligation to publicly update
or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required
by law.
Investor & Media Contact:
Ami Knoefler
Adagene
650-739-9952
ir@adagene.com
Exhibit 99.2

| Company Overview &
ADG126 MSS CRC Clinical Results
February 2024 |

| Disclaimer and Cautionary Note on Forward-Looking Statements
The following presentation has been prepared by Adagene Inc. (“Adagene” or the “Company”) solely for informational purposes and should not be construed to be, directly or indirectly, in whole or in part,
an offer to buy or sell and/or an invitation and/or a recommendation and/or a solicitation of an offer to buy or sell any security or instrument or to participate in any investment or trading strategy, nor shall
any part of it form the basis of, or be relied on in connection with, any contract or investment decision in relation to any securities or otherwise. This presentation does not contain all relevant information
relating to the Company or its securities, particularly with respect to the risks and special considerations involved with an investment in the securities of the Company. Nothing contained in this document
shall be relied upon as a promise or representation as to the past or future performance of the Company. Past performance does not guarantee or predict future performance. You acknowledge that any
assessment of the Company that may be made by you will be independent of this document and that you will be solely responsible for your own assessment of the market and the market position of the
Company and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of the business of the Company.
This document contains certain statements that constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1953, as amended, and Section 21E of the Securities
Exchange Act of 1934, as amended, with respect to the Company’s future financial or business performance, anticipated clinical activities and development, strategies or expectations. These statements
typically contain words such as “believe,” “may,” “will,” “could,” “expects” and “anticipates” and words of similar import. Any statement in this document that is not a statement of historical fact is a
forward-looking statement and involves known and unknown risks, uncertainties and other factors which may cause the Company's actual results, performance or achievements to be materially different
from any future results, performances or achievements expressed or implied by such forward-looking statements. Such forward-looking statements including statements regarding the potential implications
of clinical data for patients, and Adagene’s advancement of, and anticipated clinical activities, clinical development, regulatory milestones, and commercialization of its product candidates. Actual results
may differ materially from those indicated in the forward-looking statements as a result of various important factors, including but not limited to Adagene’s ability to demonstrate the safety and efficacy of
its drug candidates; the clinical results for its drug candidates, which may not support further development or regulatory approval; the content and timing of decisions made by the relevant regulatory
authorities regarding regulatory approval of Adagene’s drug candidates; Adagene’s ability to achieve commercial success for its drug candidates, if approved; Adagene’s ability to obtain and maintain
protection of intellectual property for its technology and drugs; Adagene’s reliance on third parties to conduct drug development, manufacturing and other services; Adagene’s limited operating history and
Adagene’s ability to obtain additional funding for operations and to complete the development and commercialization of its drug candidates; Adagene’s ability to enter into additional collaboration
agreements beyond its existing strategic partnerships or collaborations, and the impact of health epidemic, other outbreaks or natural disasters on Adagene’s clinical development, commercial and other
operations, as well as those risks more fully discussed in the “Risk Factors” section in Adagene’s filings with the U.S. Securities and Exchange Commission. There can be no assurance that the results and
events contemplated by the forward-looking statements contained herein will in fact occur. None of the future projections, expectations, estimates or prospects in this document should be taken as
forecasts or promises nor should they be taken as implying any indication, assurance or guarantee that the assumptions on which such future projections, expectations, estimates or prospects have been
prepared are correct or exhaustive or, in the case of assumptions, fully stated in the document. The Company also cautions that forward-looking statements are subject to numerous assumptions, risks and
uncertainties, which change over time and which may be beyond the Company’s control.
This presentation concerns product candidates that are or have been under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration, European
Medicines Agency, The China National Medical Products Administration, or other foreign regulatory authorities. These product candidates are currently limited by U.S. Federal law to investigational use, and
no representations are made as to their safety or effectiveness for the purposes for which they are being investigated.
This presentation contains certain comparison based on publicly available information and represents certain non-head-to-head summary comparison. The Company cautions that results of a head-to-head comparison may different significantly.
The information that can be accessed through the hyperlinks included in this presentation is not incorporated by reference into this presentation or any Adagene’s filings with the U.S. Securities and
Exchange Commission, and you should not consider such information to be part of this presentation.
This document speaks as of February 7, 2024. Neither the delivery of this document nor any further discussions of the Company with any of the recipients shall, under any circumstances, create any
implication that there has been no change in the affairs of the Company since that date. Adagene undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result
of new information, future events or otherwise, except as may be required by law.
2 |
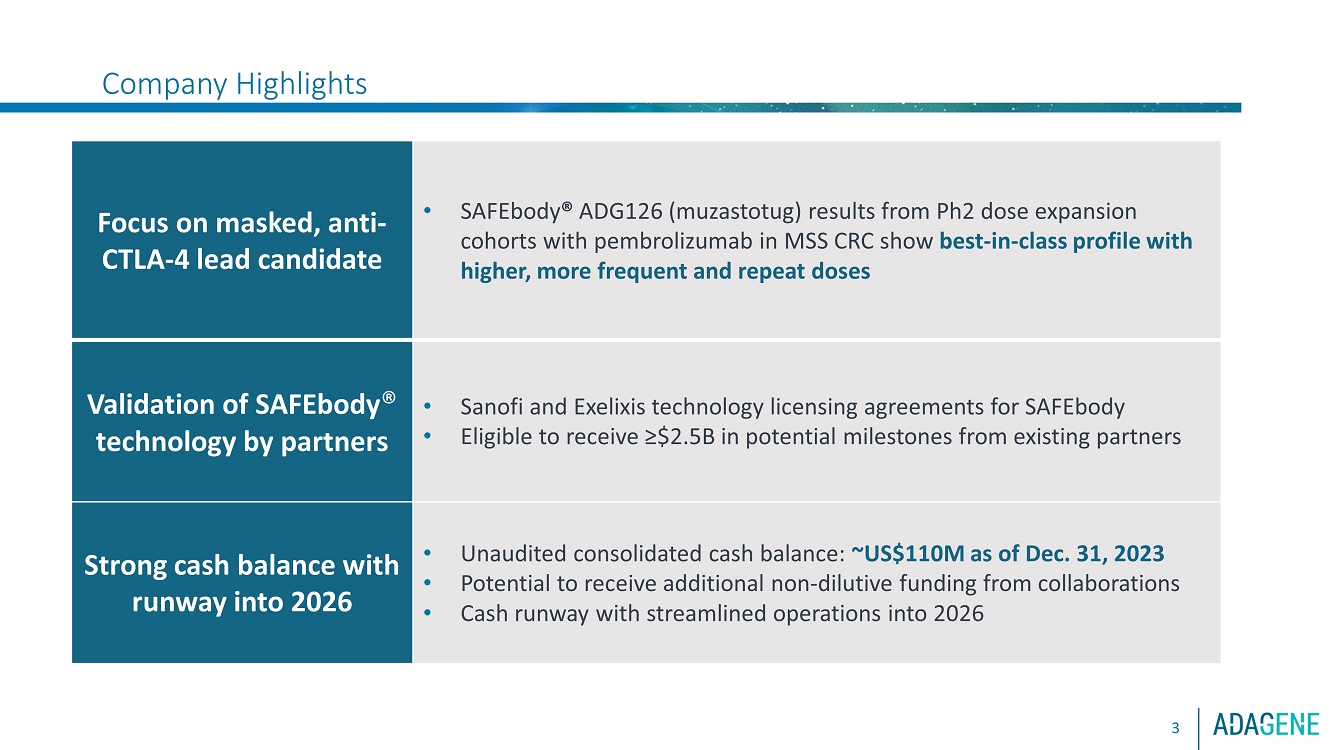
| Company HighlightsFocus on masked, antiCTLA 4 lead candidateoSAFEbody ADG126 ( muzastotug ) results from Ph2 dose expansion cohortwith pembrolizumab in MSS CRC show best in class profile with higher,more frequent and repeat dosesoRoche sponsoring conducting randomized clinical trial of ADG126 intriple combination (with atezo bev ) in 1L liver cancerValidation ofSAFEbody technology by partnersoSanofi andExelixis technology licensing agreements for SAFEbodyoEligible to receive ?$2.5B in potential milestones from existing partnersAdditional pipelinecandidatesoClinical candidates targeting CD137 (one masked, one unmasked)oINDready masked, anti CD47 (IgG1) and HER2xCD3 T cell engageroAdditional discovery programs, including CD28 Tcell engagersStrong cash balance withrunway into 2026oUnaudited consolidated cash balance:~US$110M as of Dec. 31, 2023oPotential to receive additionalnon dilutive funding from collaborationsoCash runwaywith streamlined operations into 20263 |
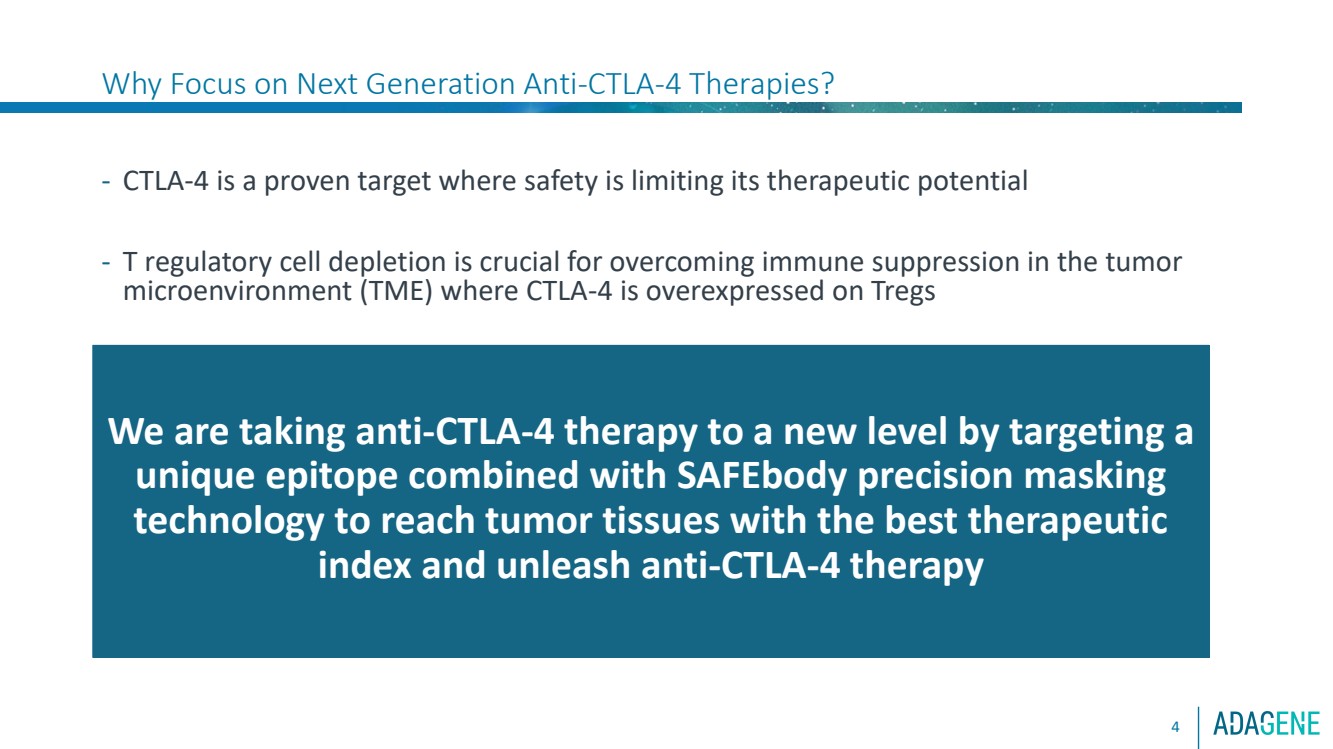
| Why Focus on Next Generation Anti-CTLA-4 Therapies?
- CTLA-4 is a proven target where safety is limiting its therapeutic potential
- T regulatory cell depletion is crucial for overcoming immune suppression in the tumor
microenvironment (TME) where CTLA-4 is overexpressed on Tregs
4
We are taking anti-CTLA-4 therapy to a new level by targeting a
unique epitope combined with SAFEbody precision masking
technology to reach tumor tissues with the best therapeutic
index and unleash anti-CTLA-4 therapy |
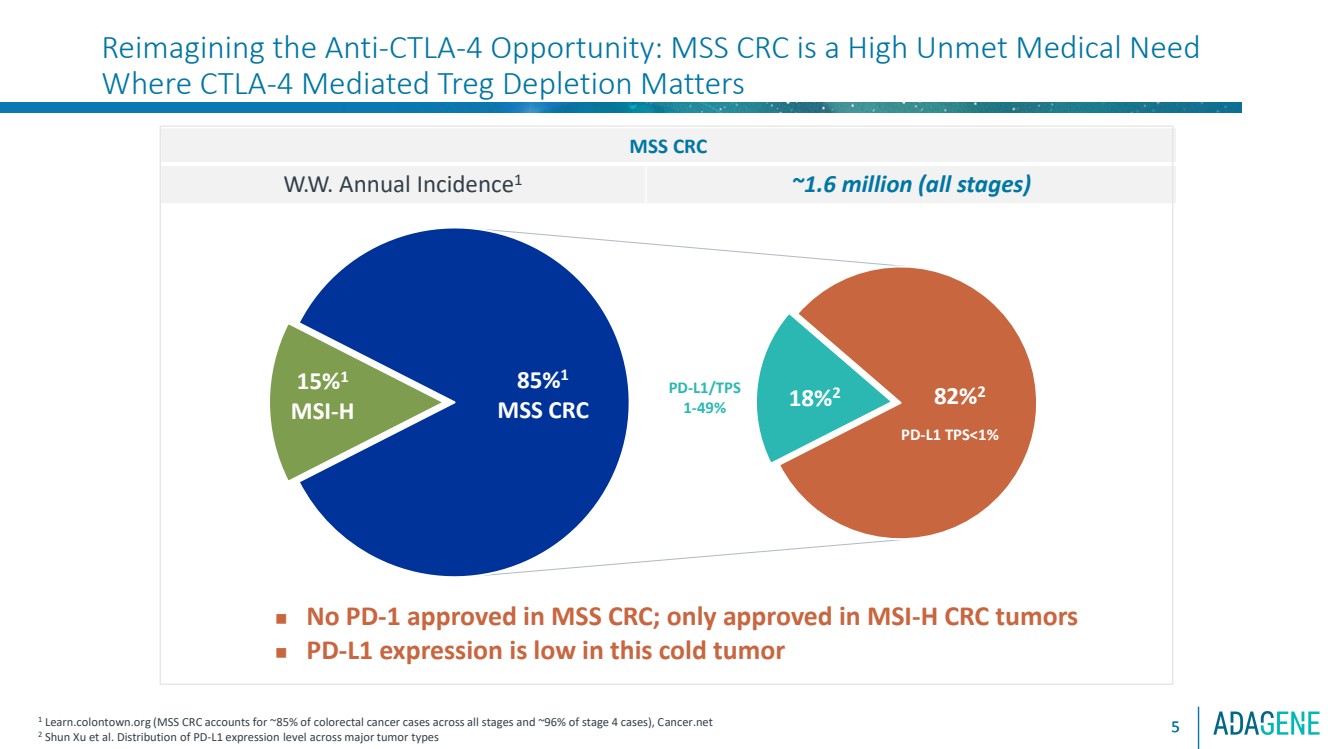
| 82%2 18%2
85%1
MSS CRC
15%1
MSI-H
Reimagining the Anti-CTLA-4 Opportunity: MSS CRC is a High Unmet Medical Need
Where CTLA-4 Mediated Treg Depletion Matters
5
MSS CRC
W.W. Annual Incidence1 ~1.6 million (all stages)
1 Learn.colontown.org (MSS CRC accounts for ~85% of colorectal cancer cases across all stages and ~96% of stage 4 cases), Cancer.net
2 Shun Xu et al. Distribution of PD-L1 expression level across major tumor types
◼ No PD-1 approved in MSS CRC; only approved in MSI-H CRC tumors
◼ PD-L1 expression is low in this cold tumor
PD-L1 TPS<1%
PD-L1/TPS
1-49%
5 |
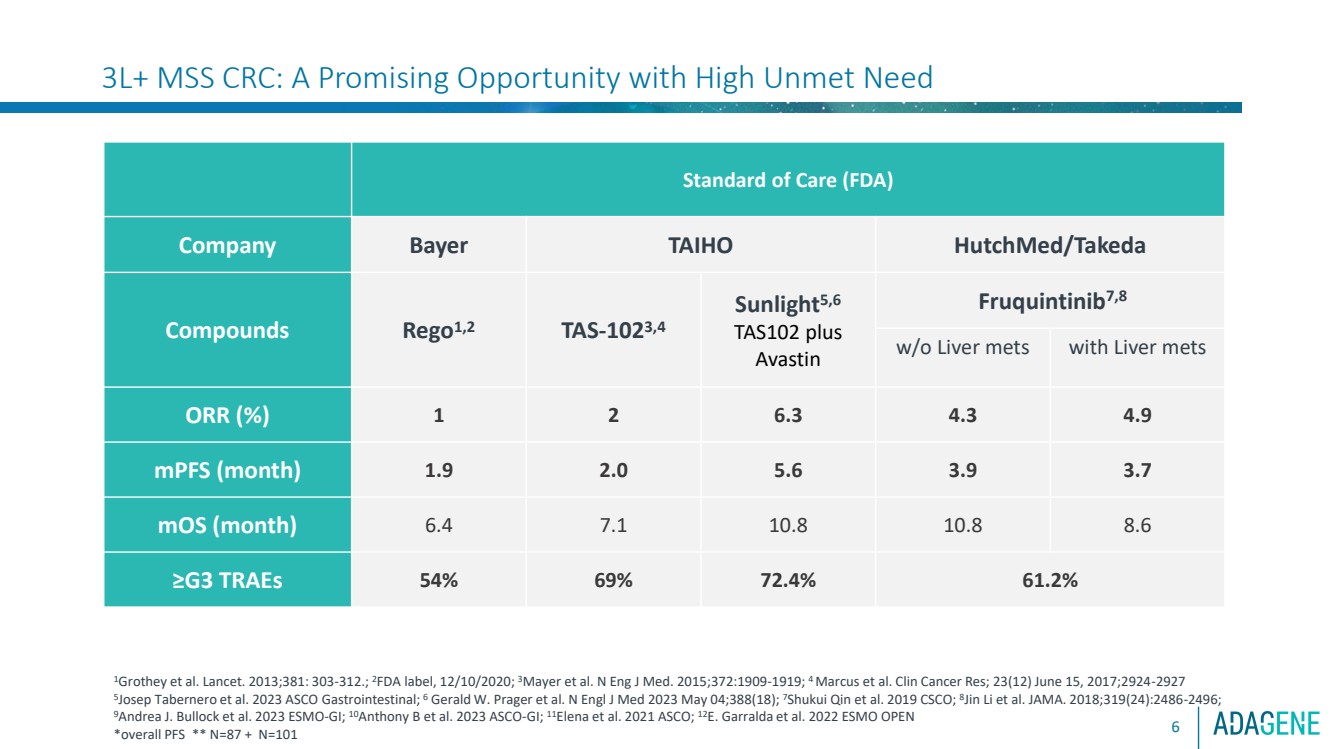
| 3L+ MSS CRC: A Promising Opportunity with High Unmet Need
6
Standard of Care (FDA)
Company Bayer TAIHO HutchMed/Takeda
Compounds Rego1,2 TAS-1023,4
Sunlight5,6
TAS102 plus
Avastin
Fruquintinib7,8
w/o Liver mets with Liver mets
ORR (%) 1 2 6.3 4.3 4.9
mPFS (month) 1.9 2.0 5.6 3.9 3.7
mOS (month) 6.4 7.1 10.8 10.8 8.6
≥G3 TRAEs 54% 69% 72.4% 61.2%
1Grothey et al. Lancet. 2013;381: 303-312.; 2FDA label, 12/10/2020; 3Mayer et al. N Eng J Med. 2015;372:1909-1919; 4 Marcus et al. Clin Cancer Res; 23(12) June 15, 2017;2924-2927
5
Josep Tabernero et al. 2023 ASCO Gastrointestinal; 6 Gerald W. Prager et al. N Engl J Med 2023 May 04;388(18); 7Shukui Qin et al. 2019 CSCO; 8
Jin Li et al. JAMA. 2018;319(24):2486-2496;
9Andrea J. Bullock et al. 2023 ESMO-GI; 10Anthony B et al. 2023 ASCO-GI; 11Elena et al. 2021 ASCO; 12E. Garralda et al. 2022 ESMO OPEN
*overall PFS ** N=87 + N=101 |

| Failures of Immunotherapy and Their Combinations in MSS CRC
7 |
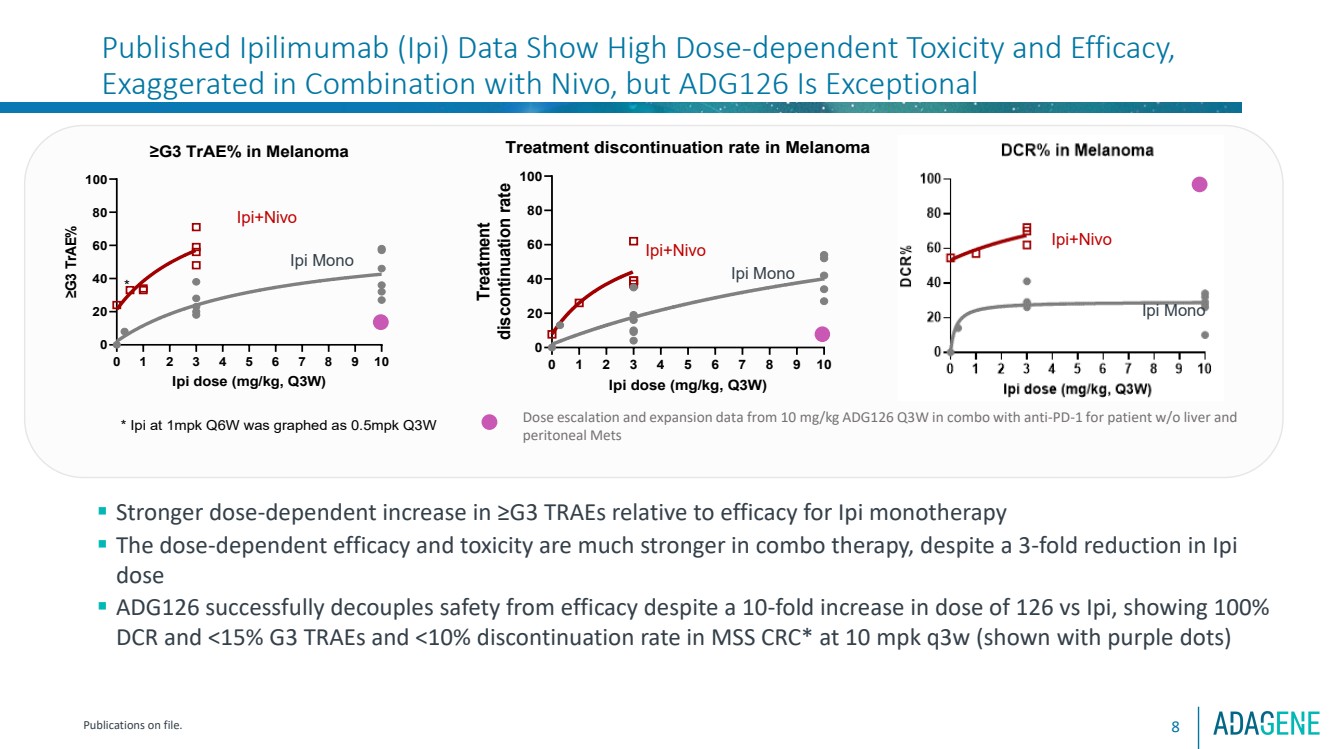
| Published Ipilimumab (Ipi) Data Show High Dose-dependent Toxicity and Efficacy,
Exaggerated in Combination with Nivo, but ADG126 Is Exceptional
8
0 1 2 3 4 5 6 7 8 9 10
0
20
40
60
80
100 ≥G3 TrAE% in Melanoma
Ipi dose (mg/kg, Q3W)
≥G3 TrAE%
* Ipi at 1mpk Q6W was graphed as 0.5mpk Q3W
*
Ipi+Nivo
Ipi Mono
▪ Stronger dose-dependent increase in ≥G3 TRAEs relative to efficacy for Ipi monotherapy
▪ The dose-dependent efficacy and toxicity are much stronger in combo therapy, despite a 3-fold reduction in Ipi
dose
▪ ADG126 successfully decouples safety from efficacy despite a 10-fold increase in dose of 126 vs Ipi, showing 100%
DCR and <15% G3 TRAEs and <10% discontinuation rate in MSS CRC* at 10 mpk q3w (shown with purple dots)
Ipi+Nivo
Ipi Mono
Publications on file.
Dose escalation and expansion data from 10 mg/kg ADG126 Q3W in combo with anti-PD-1 for patient w/o liver and
peritoneal Mets
0 1 2 3 4 5 6 7 8 9 10
0
20
40
60
80
100 Treatment discontinuation rate in Melanoma
Ipi dose (mg/kg, Q3W)
Treatment discontinuation rate
Ipi+Nivo
Ipi Mono |
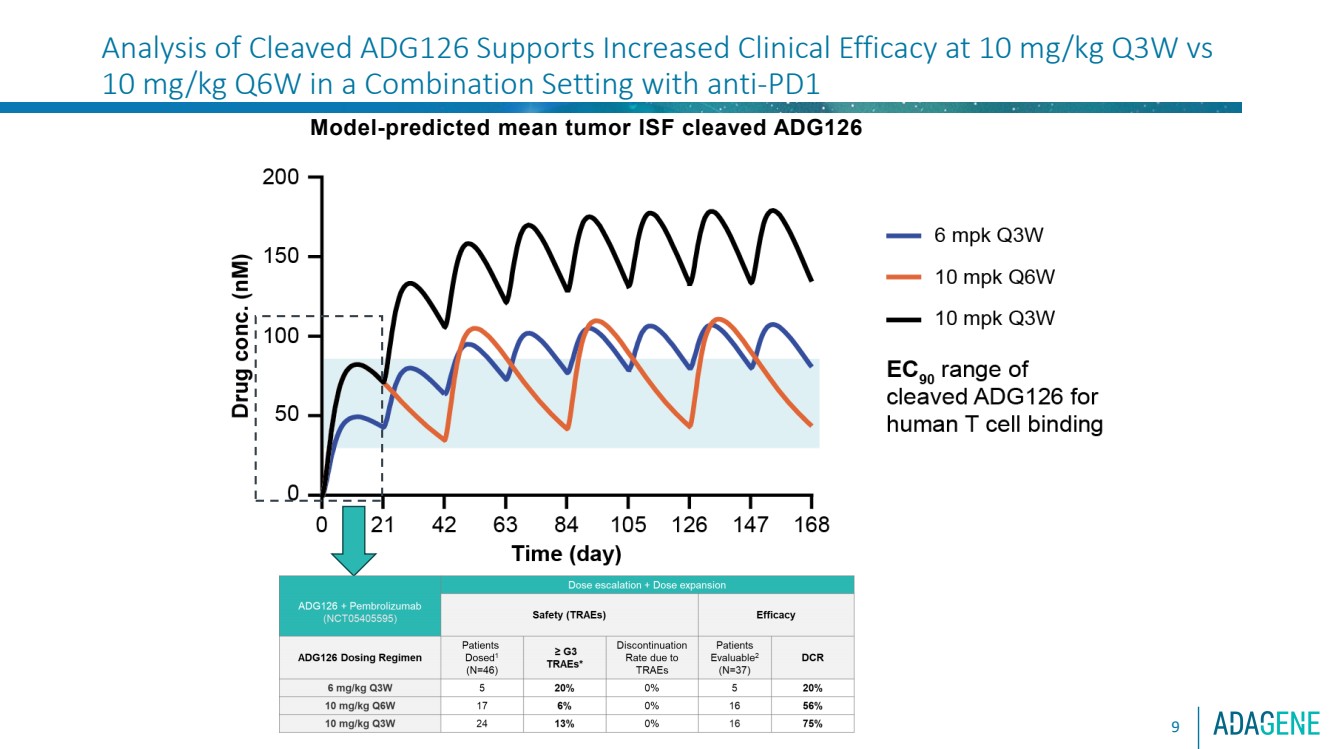
| 9
Model-predicted mean tumor ISF cleaved ADG126
Analysis of Cleaved ADG126 Supports Increased Clinical Efficacy at 10 mg/kg Q3W vs
10 mg/kg Q6W in a Combination Setting with anti-PD1 |
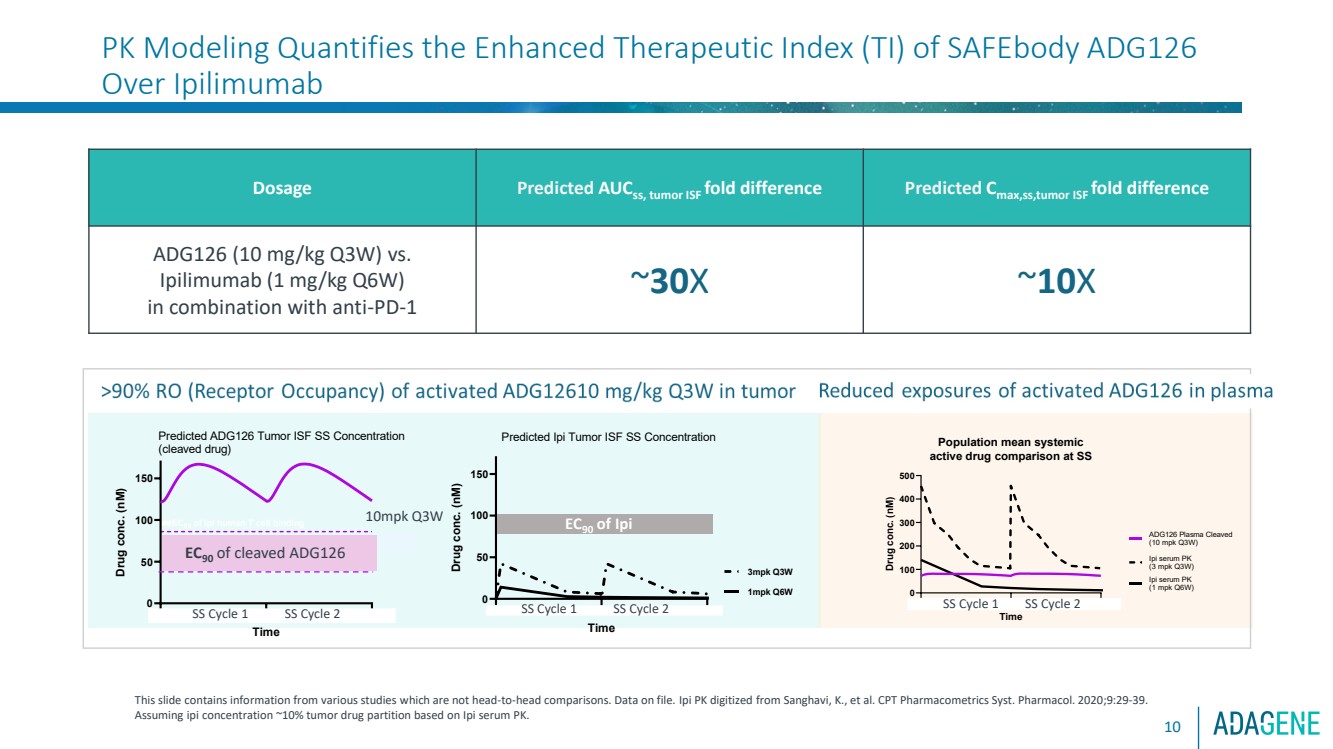
| PK Modeling Quantifies the Enhanced Therapeutic Index (TI) of SAFEbody ADG126
Over Ipilimumab
10
Dosage Predicted AUCss, tumor ISF fold difference Predicted Cmax,ss,tumor ISF fold difference
ADG126 (10 mg/kg Q3W) vs.
Ipilimumab (1 mg/kg Q6W)
in combination with anti-PD-1
~30X ~10X
This slide contains information from various studies which are not head-to-head comparisons. Data on file. Ipi PK digitized from Sanghavi, K., et al. CPT Pharmacometrics Syst. Pharmacol. 2020;9:29-39.
Assuming ipi concentration ~10% tumor drug partition based on Ipi serum PK.
3 0 2 4 3 5 2 8 4 0 3 2 0
50
100
150
Time
Drug conc. (nM)
1mpk Q6W
3mpk Q3W
≈EC90 of Ipi human T cell binding
Predicted Ipi Tumor ISF SS Concentration
SS Cycle 1 SS Cycle 2 3 0 2 4 3 5 2 8 4 0 3 2 0
100
200
300
400
500 Population mean systemic
active drug comparison at SS
Time Drug conc. (nM)
Ipi serum PK
(1 mpk Q6W)
ADG126 Plasma Cleaved
(10 mpk Q3W)
Ipi serum PK
(3 mpk Q3W)
SS Cycle 1 SS Cycle 2
10mpk Q3W
Steady-State (SS) drug exposure of activated ADG126 over Ipilimumab in tumor vs. blood
3 0 2 4 3 5 2 8 4 0 3 2 0
50
100
150
Time
Drug conc. (nM) EC90,upper bound of MMP-9 cleaved ADG126 human T cell binding ≈EC90 of Ipi human T cell binding
Predicted ADG126 Tumor ISF SS Concentration
(cleaved drug)
SS Cycle 1 SS Cycle 2
EC90 of cleaved ADG126
EC90 of Ipi |
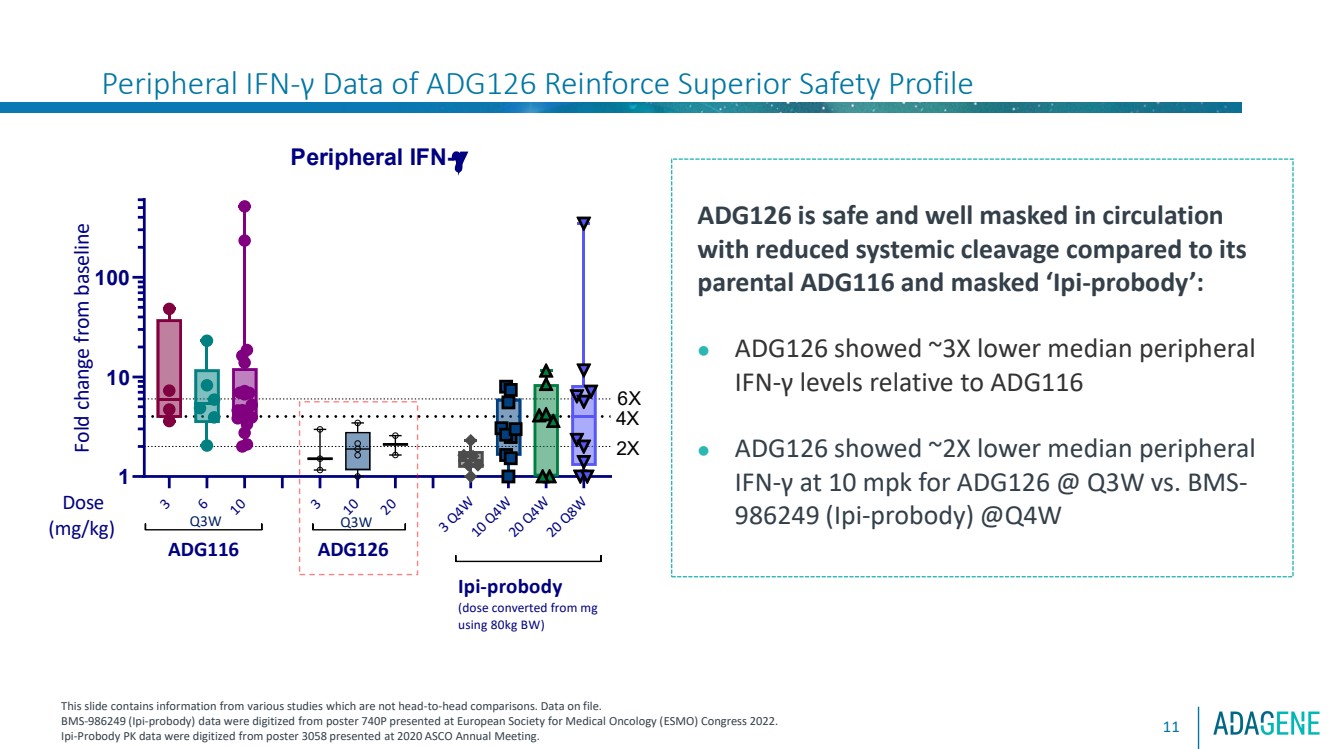
| 11
ADG126 is safe and well masked in circulation
with reduced systemic cleavage compared to its
parental ADG116 and masked ‘Ipi-probody’:
⚫ ADG126 showed ~3X lower median peripheral
IFN-γ levels relative to ADG116
⚫ ADG126 showed ~2X lower median peripheral
IFN-γ at 10 mpk for ADG126 @ Q3W vs. BMS-986249 (Ipi-probody) @Q4W
This slide contains information from various studies which are not head-to-head comparisons. Data on file.
BMS-986249 (Ipi-probody) data were digitized from poster 740P presented at European Society for Medical Oncology (ESMO) Congress 2022.
Ipi-Probody PK data were digitized from poster 3058 presented at 2020 ASCO Annual Meeting.
3
6
10
3
10
20
3 Q4W
10 Q4W
20 Q4W
20 Q8W
1
10
100
Peripheral IFN-g
Fold change from baseline Dose
(mg/kg) ADG126
2X
ADG116
6X
Ipi-probody
(dose converted from mg using 80kg BW)
4X
Q3W Q3W
Peripheral IFN-γ Data of ADG126 Reinforce Superior Safety Profile |
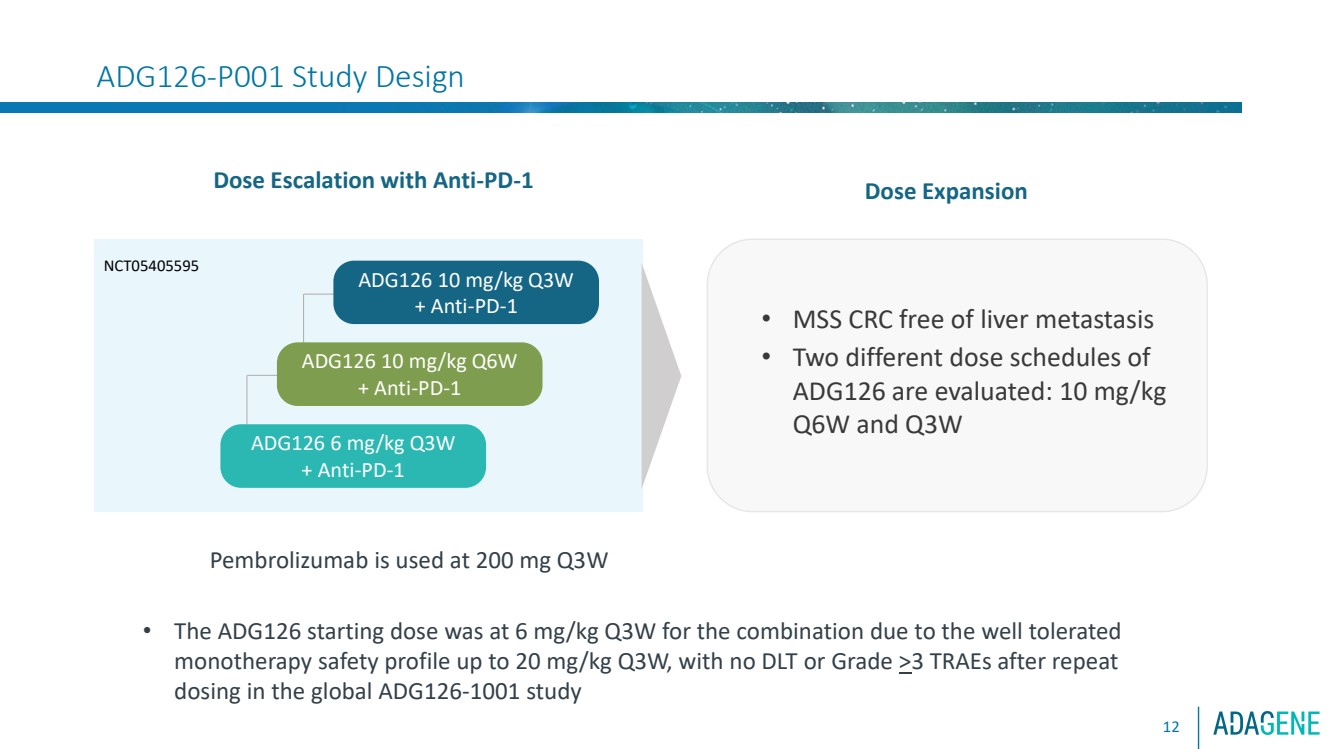
| ADG126-P001 Study Design
Dose Escalation with Anti-PD-1
ADG126 10 mg/kg Q3W
+ Anti-PD-1
ADG126 10 mg/kg Q6W
+ Anti-PD-1
ADG126 6 mg/kg Q3W
+ Anti-PD-1
• MSS CRC free of liver metastasis
• Two different dose schedules of
ADG126 are evaluated: 10 mg/kg
Q6W and Q3W
NCT05405595
Pembrolizumab is used at 200 mg Q3W
Dose Expansion
• The ADG126 starting dose was at 6 mg/kg Q3W for the combination due to the well tolerated
monotherapy safety profile up to 20 mg/kg Q3W, with no DLT or Grade >3 TRAEs after repeat
dosing in the global ADG126-1001 study
12 |
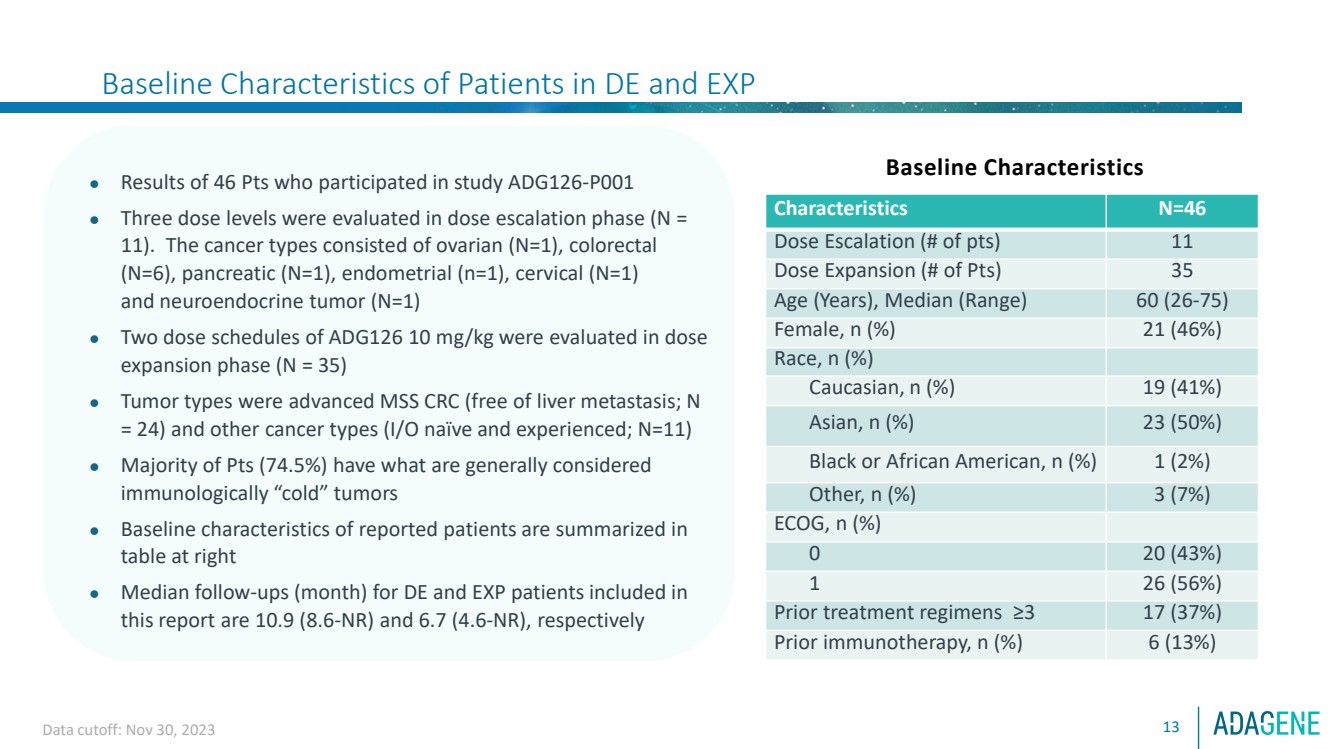
| Baseline Characteristics of Patients in DE and EXP
13
⚫ Results of 46 Pts who participated in study ADG126-P001
⚫ Three dose levels were evaluated in dose escalation phase (N =
11). The cancer types consisted of ovarian (N=1), colorectal
(N=6), pancreatic (N=1), endometrial (n=1), cervical (N=1)
and neuroendocrine tumor (N=1)
⚫ Two dose schedules of ADG126 10 mg/kg were evaluated in dose
expansion phase (N = 35)
⚫ Tumor types were advanced MSS CRC (free of liver metastasis; N
= 24) and other cancer types (I/O naïve and experienced; N=11)
⚫ Majority of Pts (74.5%) have what are generally considered
immunologically “cold” tumors
⚫ Baseline characteristics of reported patients are summarized in
table at right
⚫ Median follow-ups (month) for DE and EXP patients included in
this report are 10.9 (8.6-NR) and 6.7 (4.6-NR), respectively
Baseline Characteristics
Characteristics N=46
Dose Escalation (# of pts) 11
Dose Expansion (# of Pts) 35
Age (Years), Median (Range) 60 (26-75)
Female, n (%) 21 (46%)
Race, n (%)
Caucasian, n (%) 19 (41%)
Asian, n (%) 23 (50%)
Black or African American, n (%) 1 (2%)
Other, n (%) 3 (7%)
ECOG, n (%)
0 20 (43%)
1 26 (56%)
Prior treatment regimens ≥3 17 (37%)
Prior immunotherapy, n (%) 6 (13%)
Data cutoff: Nov 30, 2023 |
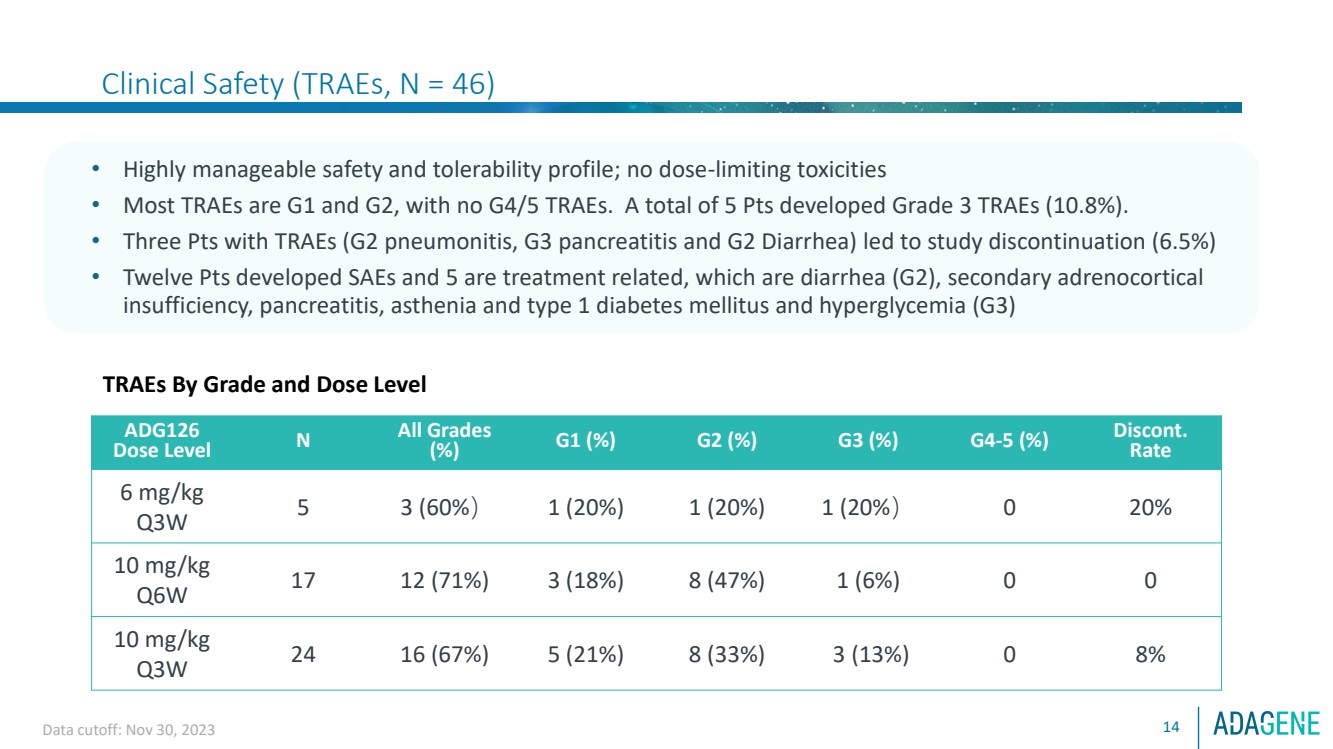
| Clinical Safety (TRAEs, N = 46)
14
• Highly manageable safety and tolerability profile; no dose-limiting toxicities
• Most TRAEs are G1 and G2, with no G4/5 TRAEs. A total of 5 Pts developed Grade 3 TRAEs (10.8%).
• Three Pts with TRAEs (G2 pneumonitis, G3 pancreatitis and G2 Diarrhea) led to study discontinuation (6.5%)
• Twelve Pts developed SAEs and 5 are treatment related, which are diarrhea (G2), secondary adrenocortical
insufficiency, pancreatitis, asthenia and type 1 diabetes mellitus and hyperglycemia (G3)
TRAEs By Grade and Dose Level
ADG126
Dose Level N
All Grades
(%) G1 (%) G2 (%) G3 (%) G4-5 (%) Discont.
Rate
6 mg/kg
Q3W 5 3 (60%) 1 (20%) 1 (20%) 1 (20%) 0 20%
10 mg/kg
Q6W 17 12 (71%) 3 (18%) 8 (47%) 1 (6%) 0 0
10 mg/kg
Q3W 24 16 (67%) 5 (21%) 8 (33%) 3 (13%) 0 8%
Data cutoff: Nov 30, 2023 |
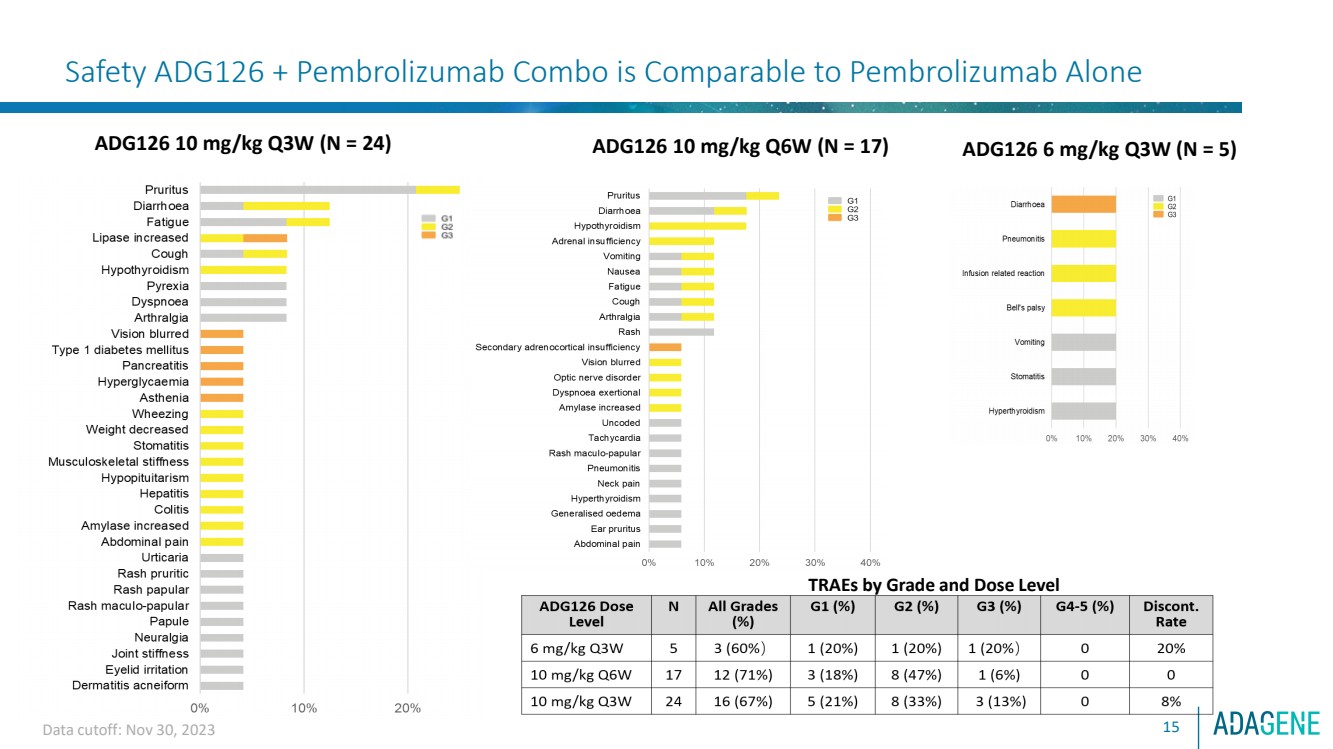
| Safety ADG126 + Pembrolizumab Combo is Comparable to Pembrolizumab Alone
ADG126 6 mg/kg Q3W (N = 5) ADG126 10 mg/kg Q3W (N = 24)
TRAEs by Grade and Dose Level
15
ADG126 10 mg/kg Q6W (N = 17)
Data cutoff: Nov 30, 2023 |
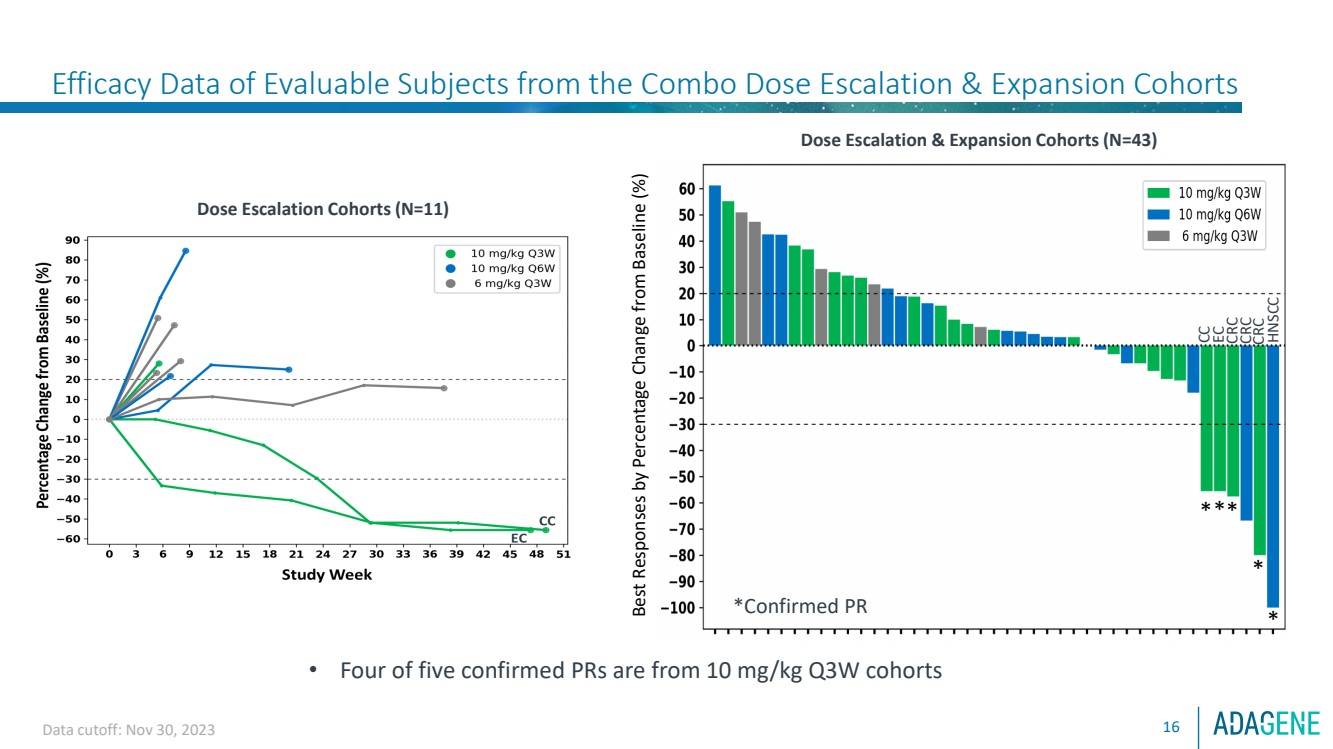
| Best Responses by Percentage Change from Baseline (%)
***
*
*
*Confirmed PR
Dose Escalation & Expansion Cohorts (N=43)
CC
EC
CRC
CRC
HNSCC
Efficacy Data of Evaluable Subjects from the Combo Dose Escalation & Expansion Cohorts
Data cutoff: Nov 30, 2023
Dose Escalation Cohorts (N=11)
• Four of five confirmed PRs are from 10 mg/kg Q3W cohorts
CRC
16 |
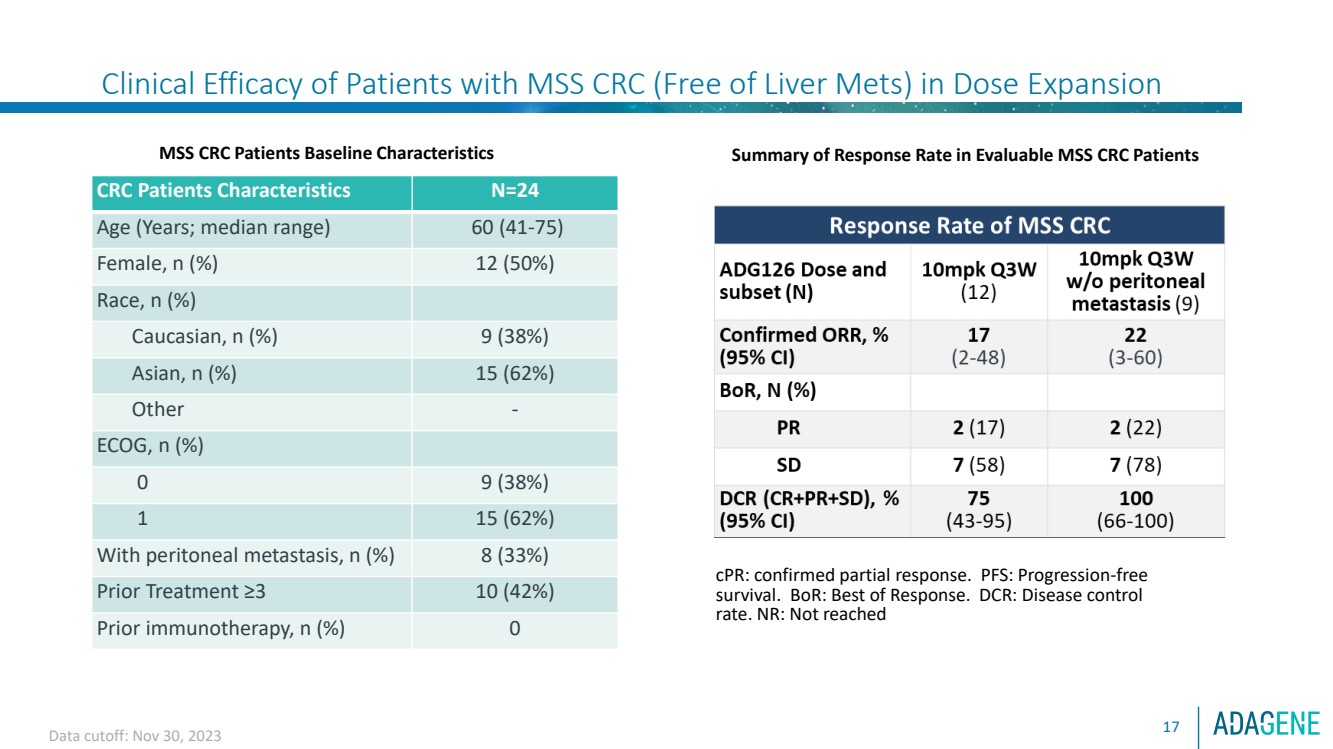
| Clinical Efficacy of Patients with MSS CRC (Free of Liver Mets) in Dose Expansion
17
MSS CRC Patients Baseline Characteristics
CRC Patients Characteristics N=24
Age (Years; median range) 60 (41-75)
Female, n (%) 12 (50%)
Race, n (%)
Caucasian, n (%) 9 (38%)
Asian, n (%) 15 (62%)
Other -
ECOG, n (%)
0 9 (38%)
1 15 (62%)
With peritoneal metastasis, n (%) 8 (33%)
Prior Treatment ≥3 10 (42%)
Prior immunotherapy, n (%) 0
Summary of Response Rate in Evaluable MSS CRC Patients
cPR: confirmed partial response. PFS: Progression-free
survival. BoR: Best of Response. DCR: Disease control
rate. NR: Not reached
Data cutoff: Nov 30, 2023 |
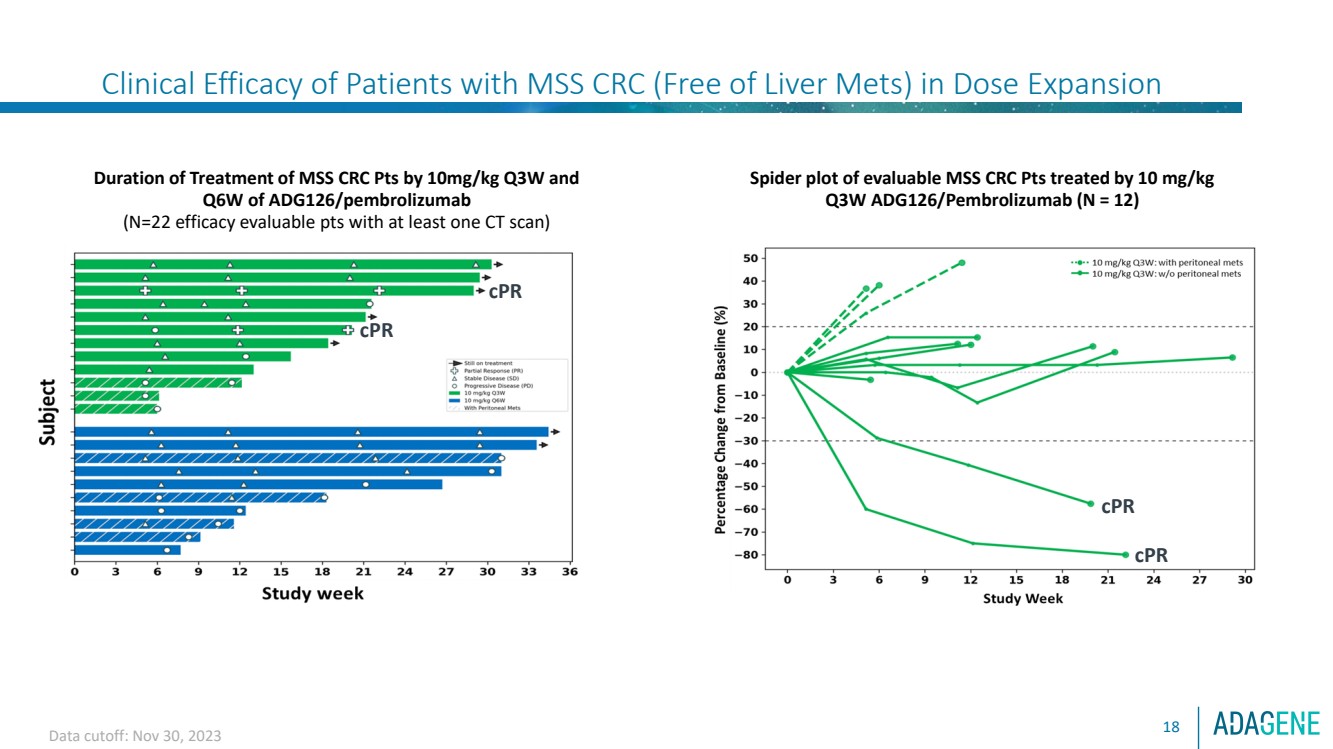
| Clinical Efficacy of Patients with MSS CRC (Free of Liver Mets) in Dose Expansion
18
Spider plot of evaluable MSS CRC Pts treated by 10 mg/kg
Q3W ADG126/Pembrolizumab (N = 12)
Duration of Treatment of MSS CRC Pts by 10mg/kg Q3W and
Q6W of ADG126/pembrolizumab
(N=22 efficacy evaluable pts with at least one CT scan)
cPR
cPR
cPR
cPR
Data cutoff: Nov 30, 2023 |
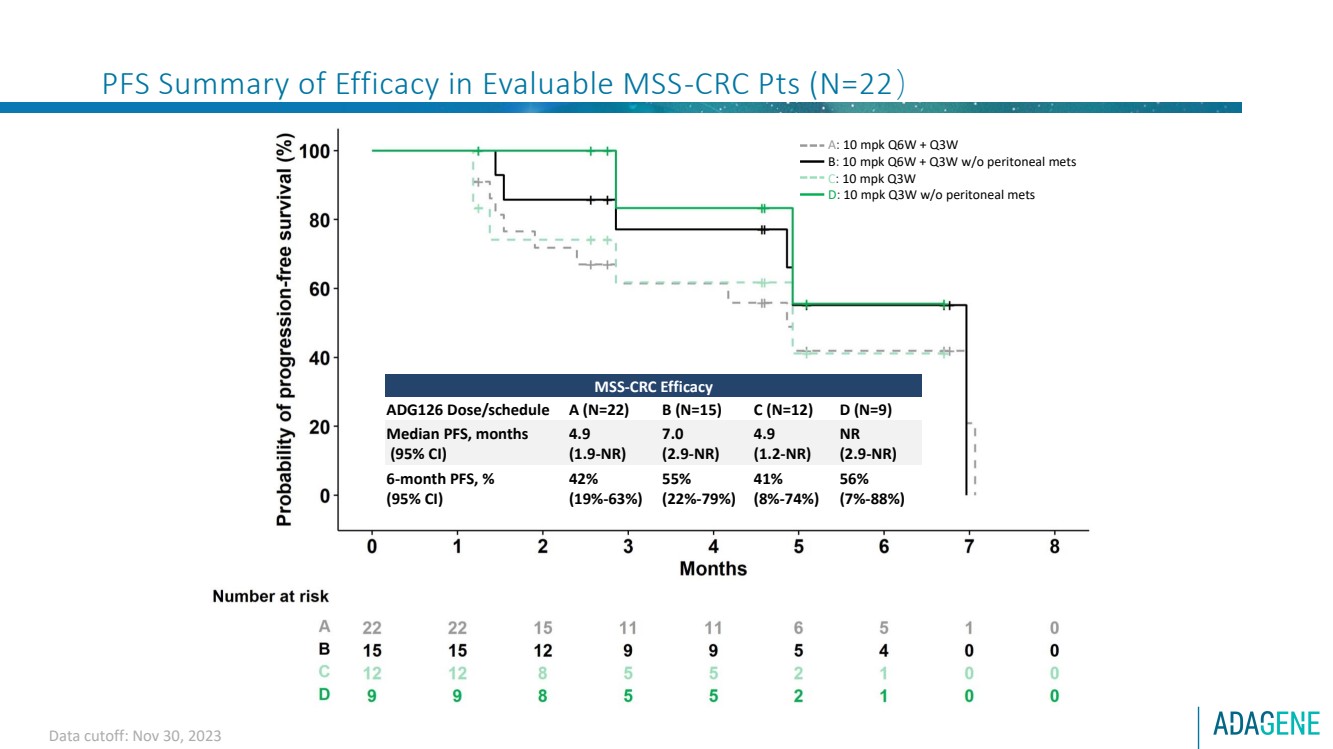
| PFS Summary of Efficacy in Evaluable MSS-CRC Pts (N=22)
MSS-CRC Efficacy
ADG126 Dose/schedule A (N=22) B (N=15) C (N=12) D (N=9)
Median PFS, months
(95% CI)
4.9
(1.9-NR)
7.0
(2.9-NR)
4.9
(1.2-NR)
NR
(2.9-NR)
6-month PFS, %
(95% CI)
42%
(19%-63%)
55%
(22%-79%)
41%
(8%-74%)
56%
(7%-88%)
A: 10 mpk Q6W + Q3W
B: 10 mpk Q6W + Q3W w/o peritoneal mets
C: 10 mpk Q3W
D: 10 mpk Q3W w/o peritoneal mets
Data cutoff: Nov 30, 2023 |
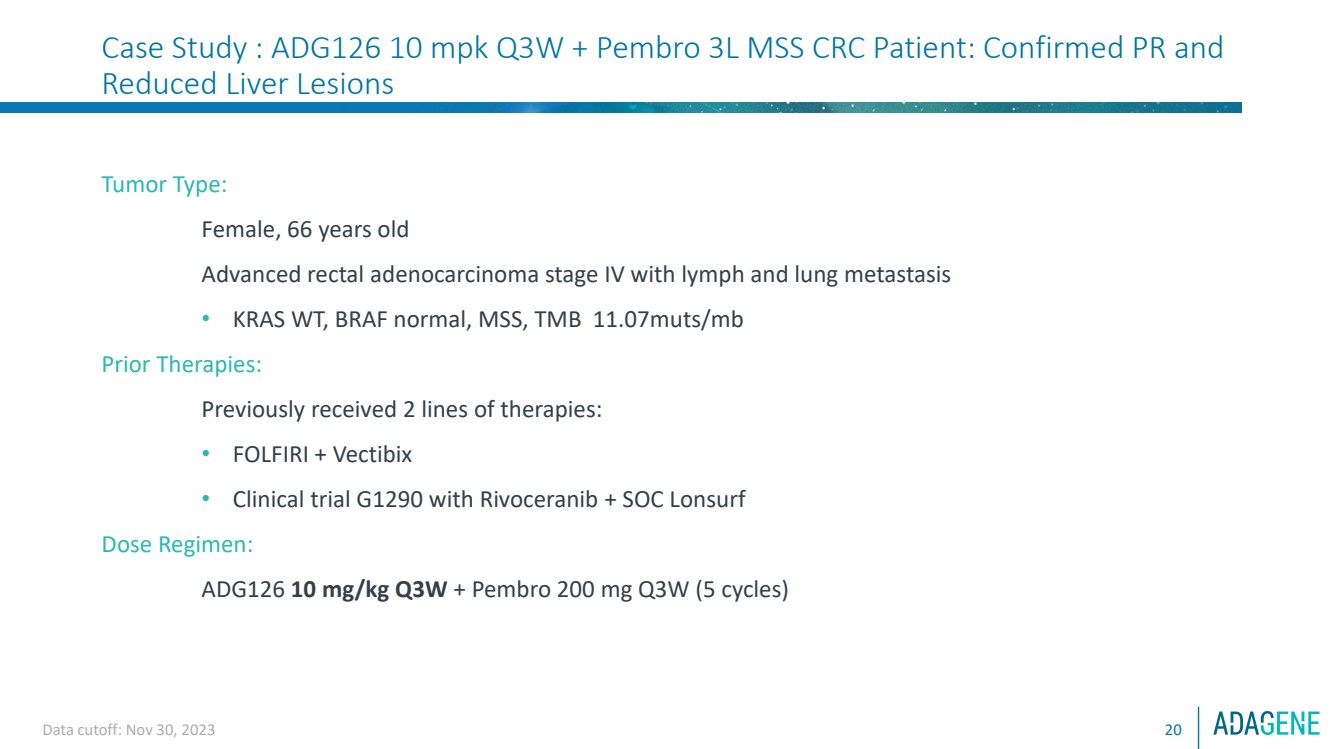
| Data cutoff: Nov 30, 2023 20
Case Study : ADG126 10 mpk Q3W + Pembro 3L MSS CRC Patient: Confirmed PR and
Reduced Liver Lesions
Tumor Type:
Female, 66 years old
Advanced rectal adenocarcinoma stage IV with lymph and lung metastasis
• KRAS WT, BRAF normal, MSS, TMB 11.07muts/mb
Prior Therapies:
Previously received 2 lines of therapies:
• FOLFIRI + Vectibix
• Clinical trial G1290 with Rivoceranib + SOC Lonsurf
Dose Regimen:
ADG126 10 mg/kg Q3W + Pembro 200 mg Q3W (5 cycles) |
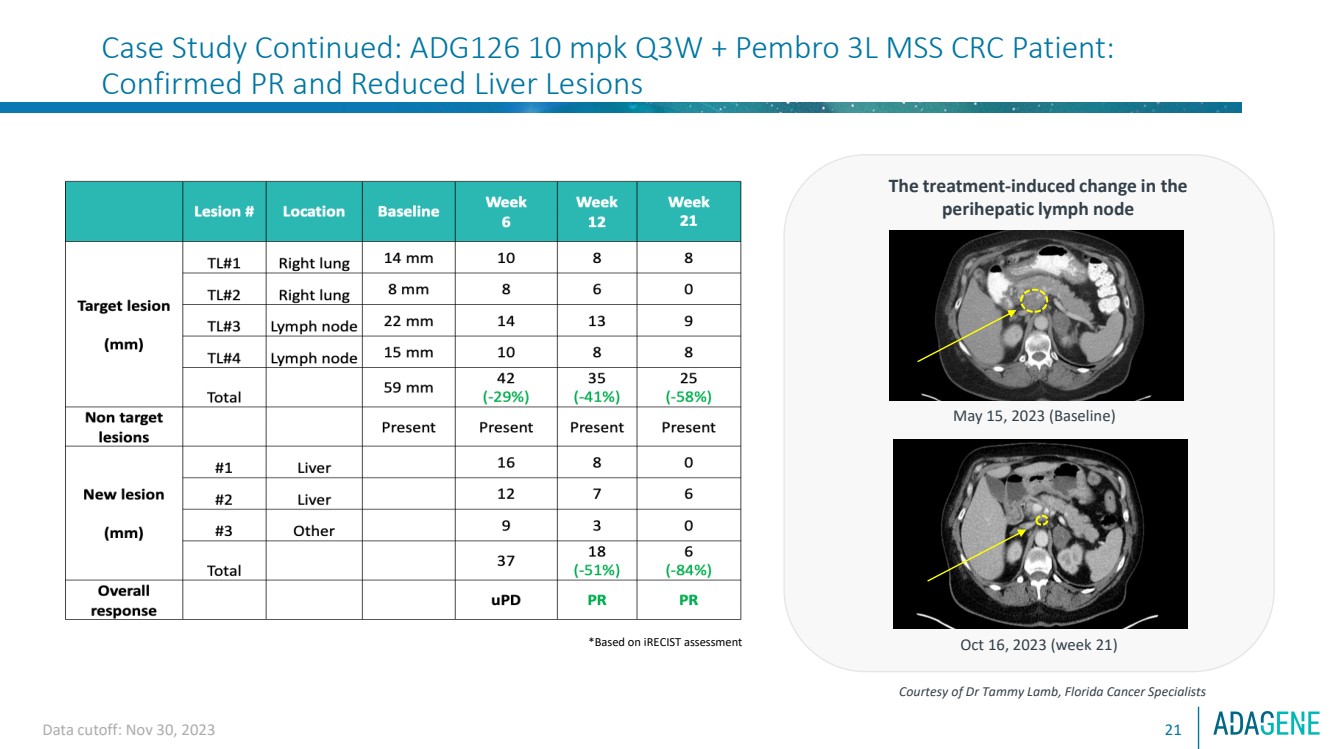
| Data cutoff: Nov 30, 2023 21
Case Study Continued: ADG126 10 mpk Q3W + Pembro 3L MSS CRC Patient:
Confirmed PR and Reduced Liver Lesions
The treatment-induced change in the
perihepatic lymph node
Courtesy of Dr Tammy Lamb, Florida Cancer Specialists
May 15, 2023 (Baseline)
Oct 16, 2023 (week 21) *Based on iRECIST assessment |
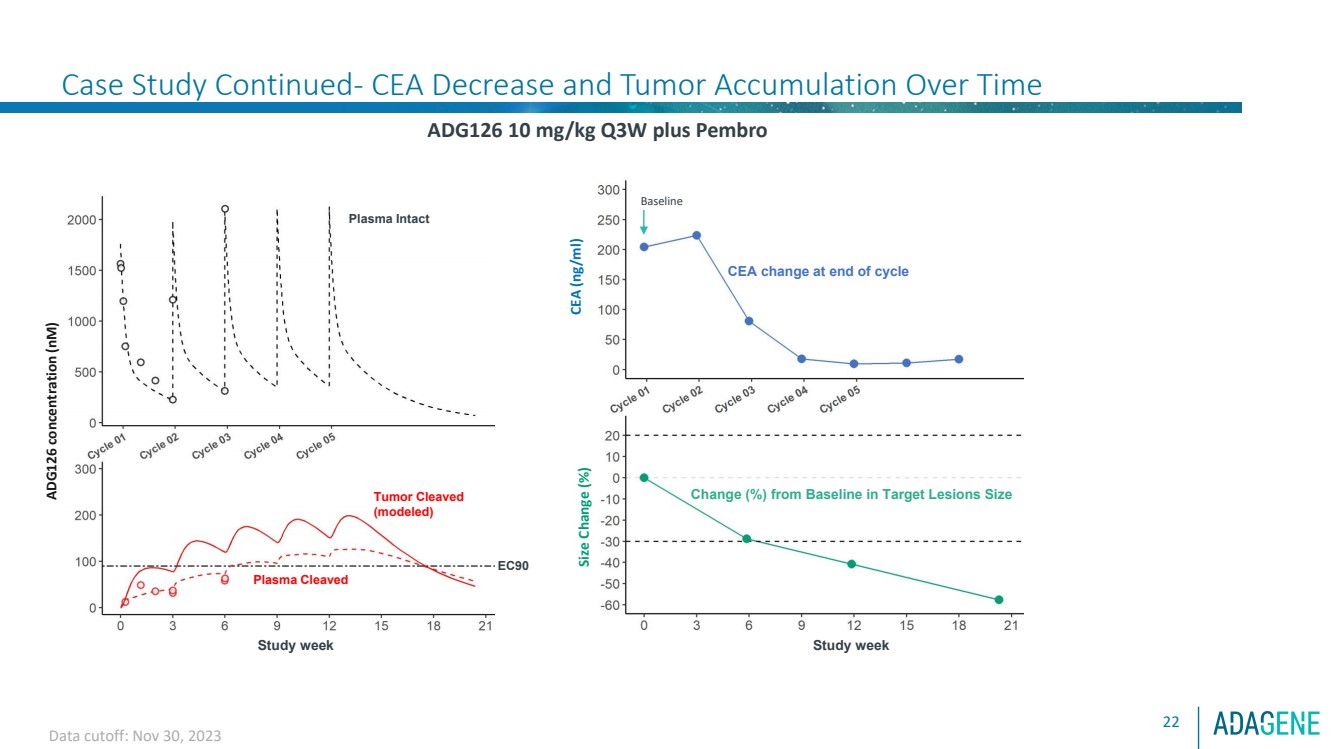
| Confidential
Case Study Continued- CEA Decrease and Tumor Accumulation Over Time
22
ADG126 10 mg/kg Q3W plus Pembro Size Change (%)
Change (%) from Baseline in Target Lesions Size
Study week
CEA change at end of cycle
CEA (ng/ml)
Tumor Cleaved
(modeled)
ADG126 concentration (nM)
Plasma Cleaved
Plasma Intact
EC90
Study week
Tumor Cleaved
(modeled)
Data cutoff: Nov 30, 2023
Baseline |
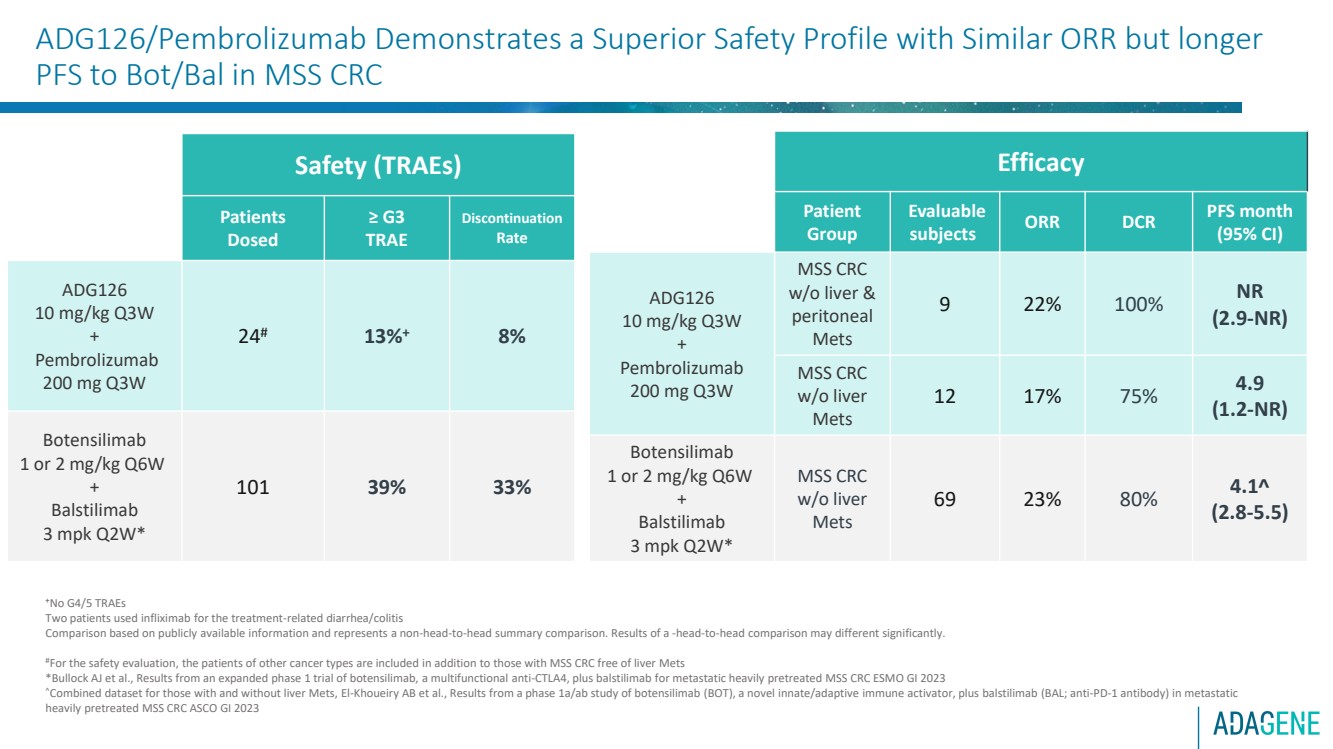
| ADG126/Pembrolizumab Demonstrates a Superior Safety Profile with Similar ORR but longer
PFS to Bot/Bal in MSS CRC
+No G4/5 TRAEs
Two patients used infliximab for the treatment-related diarrhea/colitis
Comparison based on publicly available information and represents a non-head-to-head summary comparison. Results of a -head-to-head comparison may different significantly.
#For the safety evaluation, the patients of other cancer types are included in addition to those with MSS CRC free of liver Mets
*Bullock AJ et al., Results from an expanded phase 1 trial of botensilimab, a multifunctional anti-CTLA4, plus balstilimab for metastatic heavily pretreated MSS CRC ESMO GI 2023
Combined dataset for those with and without liver Mets, El-Khoueiry AB et al., Results from a phase 1a/ab study of botensilimab (BOT), a novel innate/adaptive immune activator, plus balstilimab (BAL; anti-PD-1 antibody) in metastatic
heavily pretreated MSS CRC ASCO GI 2023
Safety (TRAEs)
Patients
Dosed
≥ G3
TRAE
Discontinuation
Rate
ADG126
10 mg/kg Q3W
+
Pembrolizumab
200 mg Q3W
24# 13%+ 8%
Botensilimab
1 or 2 mg/kg Q6W
+
Balstilimab
3 mpk Q2W*
101 39% 33%
Efficacy
Patient
Group
Evaluable
subjects ORR DCR PFS month
(95% CI)
ADG126
10 mg/kg Q3W
+
Pembrolizumab
200 mg Q3W
MSS CRC
w/o liver &
peritoneal
Mets
9 22% 100% NR
(2.9-NR)
MSS CRC
w/o liver
Mets
12 17% 75% 4.9
(1.2-NR)
Botensilimab
1 or 2 mg/kg Q6W
+
Balstilimab
3 mpk Q2W*
MSS CRC
w/o liver
Mets
69 23% 80% 4.1
(2.8-5.5) |
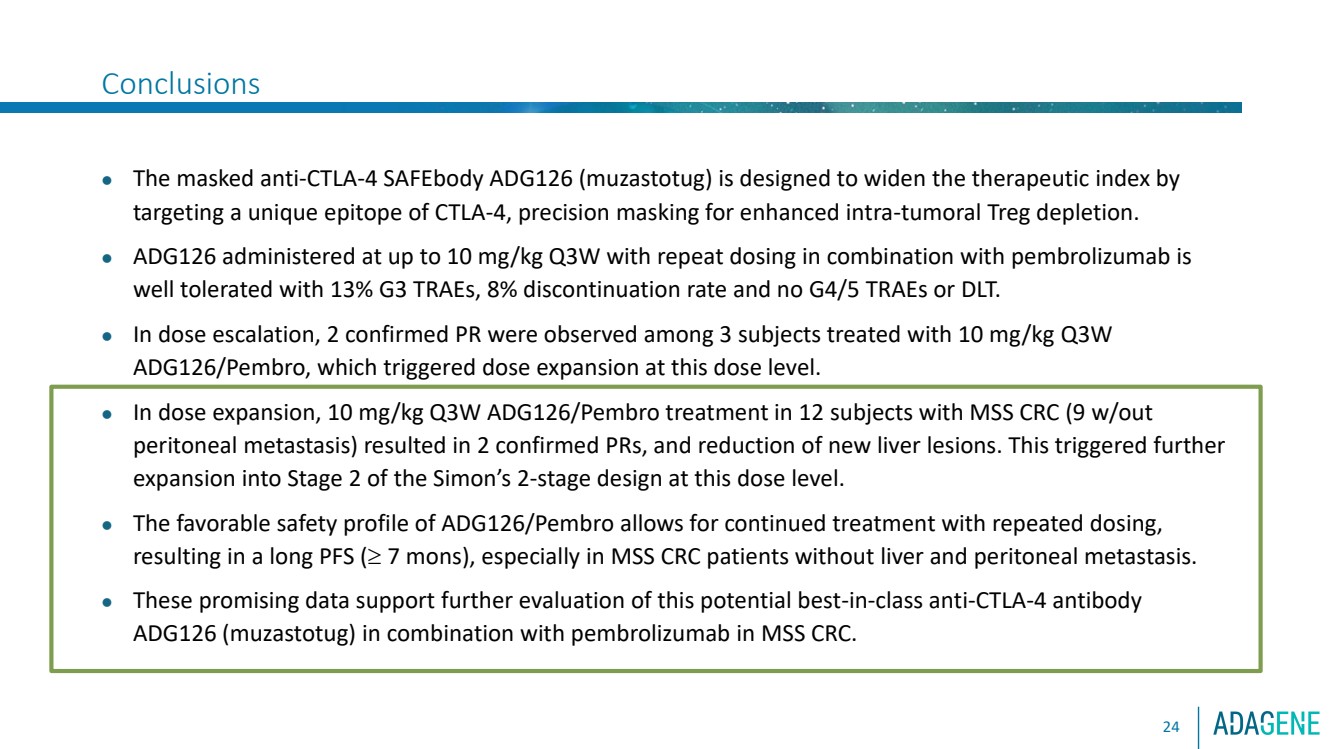
| Conclusions
24
⚫ The masked anti-CTLA-4 SAFEbody ADG126 (muzastotug) is designed to widen the therapeutic index by
targeting a unique epitope of CTLA-4, precision masking for enhanced intra-tumoral Treg depletion.
⚫ ADG126 administered at up to 10 mg/kg Q3W with repeat dosing in combination with pembrolizumab is
well tolerated with 13% G3 TRAEs, 8% discontinuation rate and no G4/5 TRAEs or DLT.
⚫ In dose escalation, 2 confirmed PR were observed among 3 subjects treated with 10 mg/kg Q3W
ADG126/Pembro, which triggered dose expansion at this dose level.
⚫ In dose expansion, 10 mg/kg Q3W ADG126/Pembro treatment in 12 subjects with MSS CRC (9 w/out
peritoneal metastasis) resulted in 2 confirmed PRs, and reduction of new liver lesions. This triggered further
expansion into Stage 2 of the Simon’s 2-stage design at this dose level.
⚫ The favorable safety profile of ADG126/Pembro allows for continued treatment with repeated dosing,
resulting in a long PFS ( 7 mons), especially in MSS CRC patients without liver and peritoneal metastasis.
⚫ These promising data support further evaluation of this potential best-in-class anti-CTLA-4 antibody
ADG126 (muzastotug) in combination with pembrolizumab in MSS CRC. |
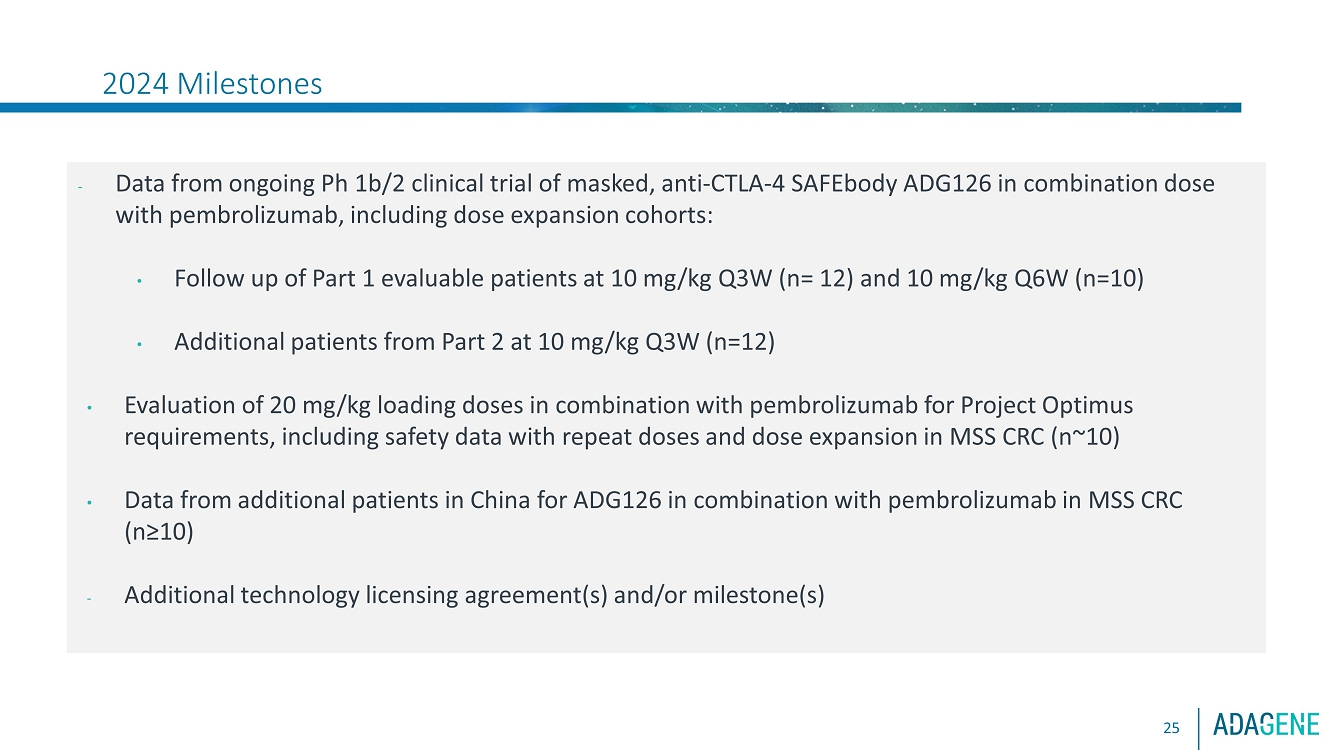
| 2024 Milestones25-Data from masked, antiCTLA 4 SAFEbody ADG126 ongoing Ph2 combination dose expansion in MSSCRC with pembrolizumab:oFollow up of Part 1 evaluable patients at 10 mg/kg Q3W (n= 12) and 10 mg/kg Q6W (n=10)oAdditional patientsfrom Part 2 at 10 mg/kg Q3W (n=12)-Evaluation of 20 mg/kg loading doses in combination with pembrolizumab for Project Optimusrequirements, including dose expansion in MSS CRC-Data from additional patients in China for ADG126 in combination with pembrolizumab in MSS CRC-Additional technology licensing agreement(s) and/or milestone(s)-Advance masked, antiCD137 SAFEbody with enhanced Fc (ADG206) phase 1 and IND enablingSAFEbody programs, as resources allow |
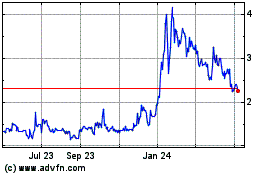
Adagene (NASDAQ:ADAG)
Historical Stock Chart
From Feb 2025 to Mar 2025
Adagene (NASDAQ:ADAG)
Historical Stock Chart
From Mar 2024 to Mar 2025