NASDAQ false 0001636282 0001636282 2023-10-24 2023-10-24
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 24, 2023
AEGLEA BIOTHERAPEUTICS, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-37722 |
|
46-4312787 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
|
|
|
| 221 Crescent Street Building 23 Suite 105 |
|
|
| Waltham, Massachusetts |
|
02453 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: 617 651-5940
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Common Stock, $0.0001 Par Value Per Share |
|
AGLE |
|
The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure. |
On October 24, 2023, the Aeglea BioTherapeutics, Inc. (the “Company”) posted an updated corporate presentation (the “Corporate Presentation”) on its website. The Corporate Presentation includes, without limitation, non-clinical updates for the anti-TL1A antibody research program, SPY002, showing monomer and trimer binding at picomolar potencies. A copy of the Corporate Presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in Item 7.01 of this Current Report on Form 8-K, including the information in the presentation attached as Exhibit 99.1 to this Current Report on Form 8-K, is furnished pursuant to Item 7.01 of Form 8-K and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section. Furthermore, the information in Item 7.01 of this Current Report on Form 8-K, including the information in the presentation attached as Exhibit 99.1 to this Current Report on Form 8-K, shall not be deemed to be incorporated by reference in the filings of the Company under the Securities Act of 1933, as amended.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
AEGLEA BIOTHERAPEUTICS, INC. |
|
|
|
|
| Date: October 24, 2023 |
|
|
|
By: |
|
/s/ Cameron Turtle |
|
|
|
|
|
|
Cameron Turtle |
|
|
|
|
|
|
Chief Operating Officer |

Exhibit 99.1 CORPORATE OVERVIEW 24 October 2023

Disclosures The information contained in this presentation has been
prepared by Aeglea Biotherapeutics, Inc. and its affiliates, including Spyre Therapeutics, Inc. (“Spyre” or the “Company”) and contains information pertaining to the business and operations of the Company. The information
contained in this presentation: (a) is provided as at the date hereof, is subject to change without notice, and is based on publicly available information, internally developed data as well as third party information from other sources; (b) does not
purport to contain all the information that may be necessary or desirable to fully and accurately evaluate an investment in the Company; (c) is not to be considered as a recommendation by the Company that any person make an investment in the
Company; (d) is for information purposes only and shall not constitute an offer to buy, sell, issue or subscribe for, or the solicitation of an offer to buy, sell or issue, or subscribe for any securities of the Company in any jurisdiction in which
such offer, solicitation or sale would be unlawful. Where any opinion or belief is expressed in this presentation, it is based on certain assumptions and limitations and is an expression of present opinion or belief only. This presentation should
not be construed as legal, financial or tax advice to any individual, as each individual’s circumstances are different. This document is for informational purposes only and should not be considered a solicitation or recommendation to purchase,
sell or hold a security. Forward-Looking Information Certain information set forth in this presentation contains “forward-looking statements” within the meaning of applicable United States securities legislation. Except for statements of
historical fact, certain information contained herein constitutes forward-looking statements which include but are not limited to statements regarding: stockholder approval of the conversion rights of the Series A Preferred Stock; the expected
effects, perceived benefits or opportunities and related timing with respect to the June 2023 acquisition of Spyre Therapeutics, Inc. and the concurrent financing; expectations regarding or plans for discovery, preclinical studies, clinical trials
and research and development programs; expectations regarding the use of proceeds and the time periods over which the Company’s capital resources will be sufficient to fund its anticipated operations; the market and potential opportunities for
inflammatory bowel disease therapies; other activities, events or developments that the Company expects or anticipates will or may occur in the future; the Company’s business strategy objectives and goals; and management’s assessment of
future plans and operations which are based on current internal expectations, estimates, projections, assumptions and beliefs, which may prove to be incorrect. Forward-looking statements can often be identified by the use of words such as
“may”, “will”, “could”, “would”, “anticipate”, ‘believe”, expect”, “intend”, “potential”, “estimate”, “scheduled”,
“plans”, “planned”, “forecasts”, “goals” and similar expressions or the negatives thereof. Forward-looking statements are neither historical facts nor assurances of future performance. Forward-looking
statements are based on a number of factors and assumptions made by management and considered reasonable at the time such information is provided, and forward-looking statements involve known and unknown risks, uncertainties and other factors that
may cause the actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements including those uncertainties and factors described under the heading “Risk Factors,”
“Risk Factor Summary” and “Cautionary Information Regarding Forward-Looking Statements” in Aeglea’s Preliminary Proxy Statement on Form 14A filed with the Securities and Exchange Commission (“SEC”) on August
8, 2023, as well as discussions of potential risks, uncertainties, and other filings by Aeglea from time to time, as well as risk factors associated with companies, such as Spyre, that operate in the biopharma industry. All of the forward-looking
statements made in this presentation are qualified by these cautionary statements and other cautionary statements or other factors contained herein. Although management believes that the expectations conveyed by forward-looking statements herein are
reasonable based on information available on the date such forward-looking statements are made, there can be no assurance that forward looking statements will prove to be accurate, as actual results and future events could differ materially from
those anticipated in such statements. The Company undertakes no obligation to update forward-looking statements if circumstances or management’s estimates or opinions should change except as required by applicable securities laws. The
forward-looking statements contained herein are presented for the purposes of assisting readers in understanding the Company’s plan, objectives and goals and may not be appropriate for other purposes. The reader is cautioned not to place undue
reliance on forward- looking statements. Industry Information This presentation also contains or references certain industry data that is based upon information from independent industry publications, market research, and surveys and other publicly
available sources. Although the Company believes these sources to be generally reliable, such information is subject to interpretation and cannot be verified with complete certainty due to limits on the availability and reliability of data, the
voluntary nature of the data gathering process and other inherent limitations and uncertainties. The Company has not independently verified any of the data from third party sources referred to in this presentation and accordingly, the Company makes
no representation or warranty as to the origin, validity, accuracy, completeness, currency or reliability of the information in this presentation. 2

Targeting new heights in the treatment of IBD THREE-PILLAR STRATEGY
PARALLEL LEAD PROGRAMS AGAINST HIGH VALUE TARGETS OPPORTUNITY FOR BEST-IN-CLASS TARGET PROGRAM DISCOVERY IND-ENABLING CLINICAL LONG-ACTING ANTIBODIES SUBCUTANEOUS (SC) Q8-12W DOSING Phase 1 interim data α4β7 SPY001 expected YE24 Phase 1
interim data TL1A SPY002 expected 1H25 IL-23 SPY003 RATIONAL THERAPEUTIC COMBINATIONS Novel MOA SPY004 α4β7 + IL-23 SPY130 α4β7 + TL1A SPY120 PRECISION IMMUNOLOGY TL1A + IL-23 SPY230

Potential best-in-class lead programs SPY001 (α4β7) SPY002
(TL1A) COMBINATIONS ü IDENTICAL EPITOPE TARGETING ü DUAL MONOMER AND TRIMER ü ONLY KNOWN PORTFOLIO WITH AS VEDOLIZUMAB BINDER α4β7, TL1A, AND IL-23 ü COMPARABLE POTENCY AND ü PICOMOLAR POTENCY AGAINST ü
POTENTIAL TO ADDRESS SELECTIVITY AS VEDOLIZUMAB MONOMERS AND TRIMERS ORTHOGONAL BIOLOGY ü SC EXTENDED HALF-LIFE mAb TO ü SC EXTENDED HALF-LIFE mAb TO ü TARGETING UNIFIED SC Q8-12W ENABLE Q8-12W REGIMEN ENABLE Q8-12W REGIMEN DOSING
INTERIM HV PK DATA INTERIM HV PK DATA INITIATION EXPECTED EXPECTED YE 2024 EXPECTED 1H 2025 AFTER PH1 DATA 4 DESIGN ATTRIBUTES

Despite advances, new approaches are needed to break the efficacy
ceiling and improve convenience of biologics UNMET NEED 80 ~1.7 million individuals are currently diagnosed with 1 IBD in the U.S. SPYRE TARGET There remain substantial unmet needs in IBD 60 management, including: 40 Inadequate response or loss of 2
SOC TODAY response to existing therapies vedolizumab mirikizumab Side effects and safety concerns vedolizumab ustekinumab 20 associated with long-term medication use golimumab infliximab adalimumab Adherence to frequent and/or inconvenient dosing
regimens 0 0 2 4 6 8 10 12 14 MAINTENANCE DOSING FREQUENCY (WEEKS) 1 2 Source: Crohn’s and Colitis Foundation. Summary data for select agents that randomize patients post response to induction; data from labels, company reports. Vedolizumab
approved in US as Q8W IV – Q2W SC approval pending following CRL in 2019. TL1A maintenance data not available. Orals not shown (pbo-adj maintenance remission rates): Jaks - tofacitinib (27%), upadacitinib (30% 15mg, 40% 30mg); S1Ps: ozanimod
(19%); etrasimod (25% - P3 treat-through design). 5 UC MAINTENANCE REMISSION RATES (%)

Our strategy is built on three validated approaches to address unmet
needs in IBD BEST-IN-CLASS ANTIBODY RATIONAL THERAPEUTIC PRECISION IMMUNOLOGY ENGINEERING COMBINATIONS APPROACHES GENETIC AND BIOMARKER SELECTION HALF-LIFE EXTENSION TECHNOLOGIES COMBINATION TARGETING TO ENHANCE TO IDENTIFY MOST LIKELY 1 2 TO
IMPROVE CONVENIENCE REMISSION RATES 3 RESPONDERS 1 2 Sources: Several half-life extended antibodies are in clinical development, with at least two commercially approved (Evusheld, Beyfortus), Rosario, M, et. al. (2017); JNJ VEGA Phase 2 study
demonstrated approximately additive efficacy with a TNFα and IL-23 combination (~47% clinical remission at week 12) versus monotherapies (25% and 24% remission rates, respectively, Feagan, B. G. et al. Lancet 3 Gastroenterol. Hepatol. 8,
307–320 (2023); Prometheus Biosciences demonstrated a ~13% increase in clinical remission rates in CDx+ patients versus all-comers, Prometheus corporate materials 6

Spyre is building a potentially best-in-class IBD pipeline with
next-generation mAbs and rational combinations 1 STRATEGY TARGET PROGRAM DISCOVERY IND-ENABLING CLINICAL Phase 1 interim data Potentially α4β7 SPY001 expected YE24 best-in-class mAbs Phase 1 interim data TL1A SPY002 expected 1H25 IL-23
SPY003 Novel MOA SPY004 Rational α4β7 + IL-23 SPY130 combinations α4β7 + TL1A SPY120 TL1A + IL-23 SPY230 1 Spyre exercised its option to license worldwide rights from Paragon Therapeutics, Inc. for SPY001. Spyre continues to hold
an option to license similar rights from Paragon for all other programs. SPY003 license will be restricted to IBD, all other program licenses will be indication agnostic. 7

SPY001 LONG-ACTING Α4Β7 ANTIBODY

SPY001 is designed to be a long-acting anti-α4β7 IDENTICAL
EPITOPE TARGET AS VEDOLIZUMAB ü COMPARABLE POTENCY AND SELECTIVITY AS VEDOLIZUMAB ü SUB-CUTANEOUS, EXTENDED HALF-LIFE mAb PREDICTED TO ENABLE Q8-12W DOSING ü 9

SPY001 potency & selectivity matches vedolizumab SPY001 &
VEDOLIZUMAB EPITOPE POTENT AND SELECTIVE INHIBITION OF CELLULAR ADHESION SPY001 and SPY001 and vedolizumab potently inhibit No inhibition of unwanted VCAM-1- vedolizumab MAdCAM-1-mediated (gut) cellular adhesion mediated (CNS) cellular adhesion bind
the same α4 Subunit epitope (α4β1) β7 Subunit POTENT & SELECTIVE BINDING TO α4β7 1 Antibodyα4β7α4β1αEβ7 2 2 SPY001 K <1 nM NB NB D 2 2 Vedolizumab K <1 nM NB NB D 1 Dissociation
constant (K ) measured by surface plasmon resonance (SPR) D 2 NB = no binding by a particular antibody to a test molecule Source: Data on file. 10
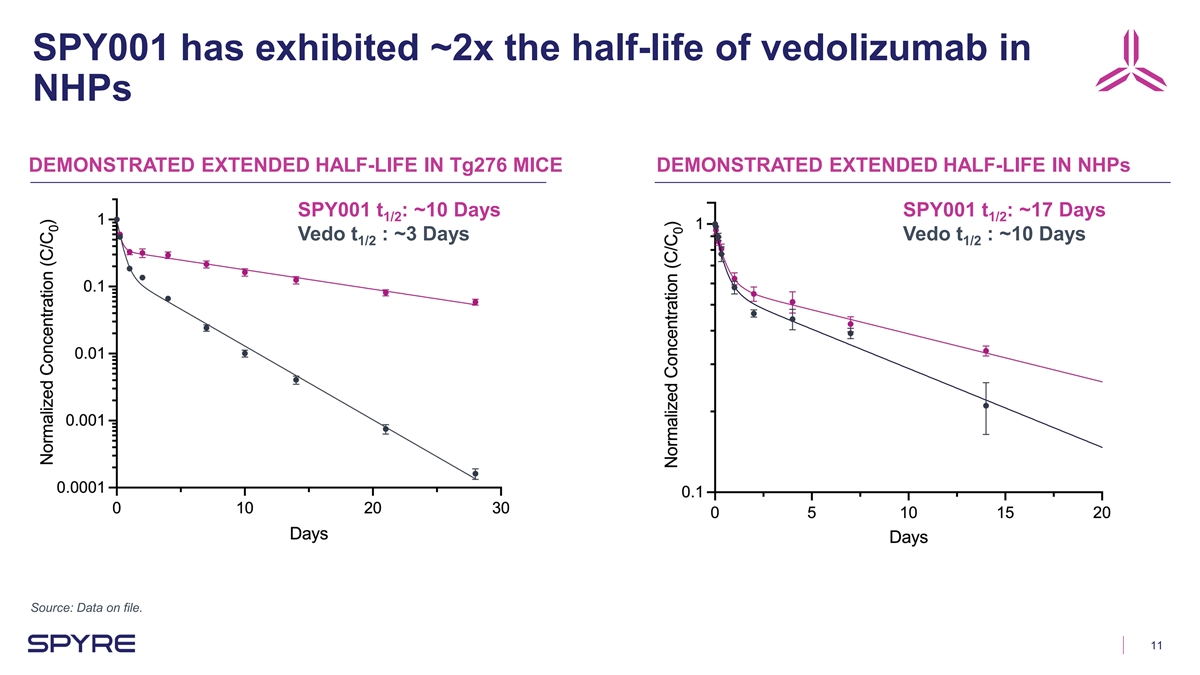
SPY001 has exhibited ~2x the half-life of vedolizumab in NHPs
DEMONSTRATED EXTENDED HALF-LIFE IN Tg276 MICE DEMONSTRATED EXTENDED HALF-LIFE IN NHPs SPY001 t : ~10 Days SPY001 t : ~17 Days 1/2 1/2 Vedo t : ~3 Days Vedo t : ~10 Days 1/2 1/2 Source: Data on file. 11

SPY001 Phase 1 clinical trial design SINGLE-ASCENDING DOSE
MULTIPLE-ASCENDING DOSE SAD 4 IV MAD 2 SC SAD 3 SC SAD 2 SC MAD 1 SC SAD 1 SC Expected interim YE2024 readout to include 2-3 SAD cohorts and is sufficient to answer key objectives with PK modeling Single-ascending dose cohorts Multiple-ascending
dose cohorts • Healthy volunteers • Healthy volunteers • N=8/cohort (3:1 randomization) • N=8/cohort (3:1 randomization) • Two doses 12

We aim to demonstrate the following for SPY001 in the expected interim
Phase 1 readout HALF-LIFE ENABLES Q8-12W SC MAINTENANCE DOSING BASED ON PK MODELING 1 POTENTIAL TO ADDRESS VEDO’S SLOW ONSET OF ACTION WITH HIGHER INDUCTION EXPOSURES 2 ESTABLISH SPY-001 HAS FAVORABLE SAFETY PROFILE AND IS WELL-TOLERATED 3
MINIMAL TO NO IMPACT ON ADA RATES VS VEDOLIZUMAB 4 13

Expected Phase 1 interim PK data informs dose and schedule modeling for
target maintenance profile SPY001 HUMAN HALF-LIFE PREDICTIONS MAINTENANCE PK SIMULATIONS Q12W SPY001 Dosing Simulation based on 50d half-life 1 SPY001 SC Q12W VEDO IV Q8W Half-life (days) Q8W based on PK Q12W based on PK modeling modeling 43-56 days
Humans ~35 ~40 1 Predicted range 17 NHPs SPY001 C trough exceeds vedo by ~1.5x 6 ug/mL Humans 25 C associated trough with maximal 2 efficacy NHPs 10 1 2 Source: Human YTE mAb half-life is on average 3.1x of NHP half-life; Rosario, M, et. al. (2017);
Feagan, et. al. (2013) 14 VEDO SPY001
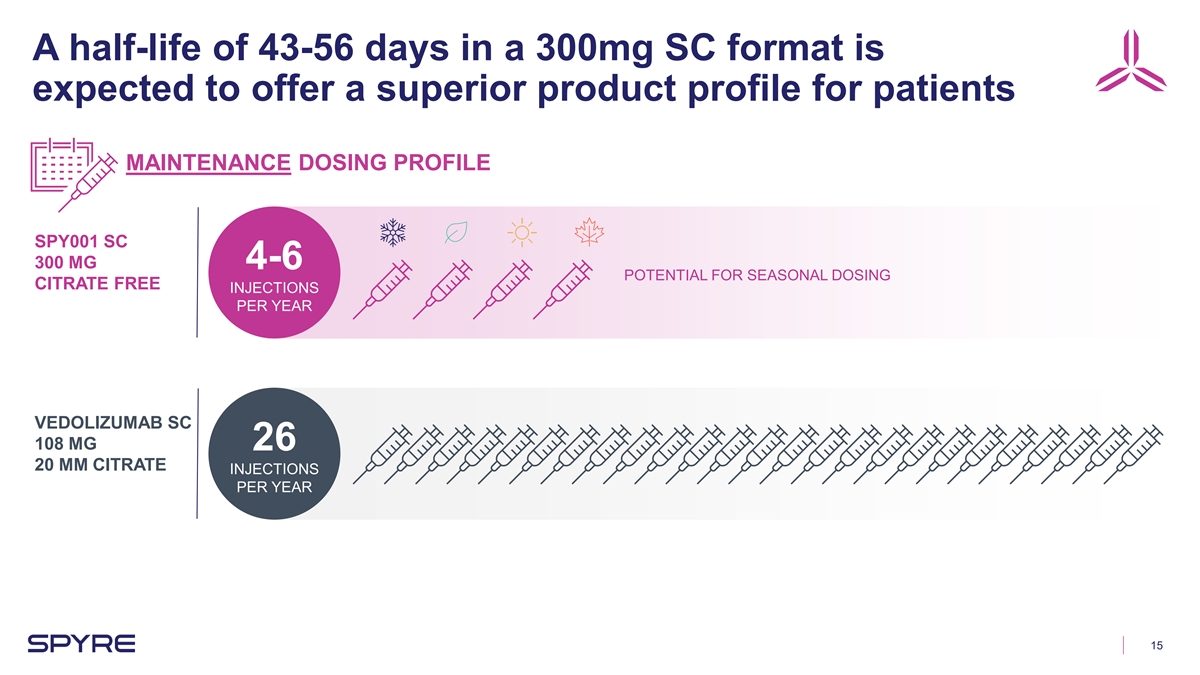
A half-life of 43-56 days in a 300mg SC format is expected to offer a
superior product profile for patients MAINTENANCE DOSING PROFILE SPY001 SC 300 MG 4-6 POTENTIAL FOR SEASONAL DOSING CITRATE FREE INJECTIONS PER YEAR VEDOLIZUMAB SC 108 MG 26 20 MM CITRATE INJECTIONS PER YEAR 15

Expected interim PK data will also inform our ability to test induction
exposure-response relationship in Phase 2 UPSIDE: GREATER REMISSION VIA HIGHER EXPOSURE INDUCTION PK SIMULATIONS SPY001 INDUCTION SIMULATION BASED ON 50-DAY HALF-LIFE GEMINI I: WEEK 6 CLINICAL REMISSION RATES IN UC (%) 2 SPY001: ALL modeled patients
above SPY001 SC vedo Q4 trough concentration VEDO IV (per label) Q4 37% ≥35.7 Q3 22% 25.0-35.7 Q2 13% 17.1-25.0 Q1 7% ≤17.1 Vedo Quartile 4: 36 µg/mL 17% W6 PBO 6% +20% Higher anti-α4β7 mAb exposure may Weeks lead to more
rapid remission 1 Source: Vedolizumab exposure-response data from Rosario, M., et. al. (2017); Vedolizumab FDA Clinical Pharmacology Review 16 1 Vedolizumab trough concentration quartiles (µg/ml) Concentration (µg/mL)
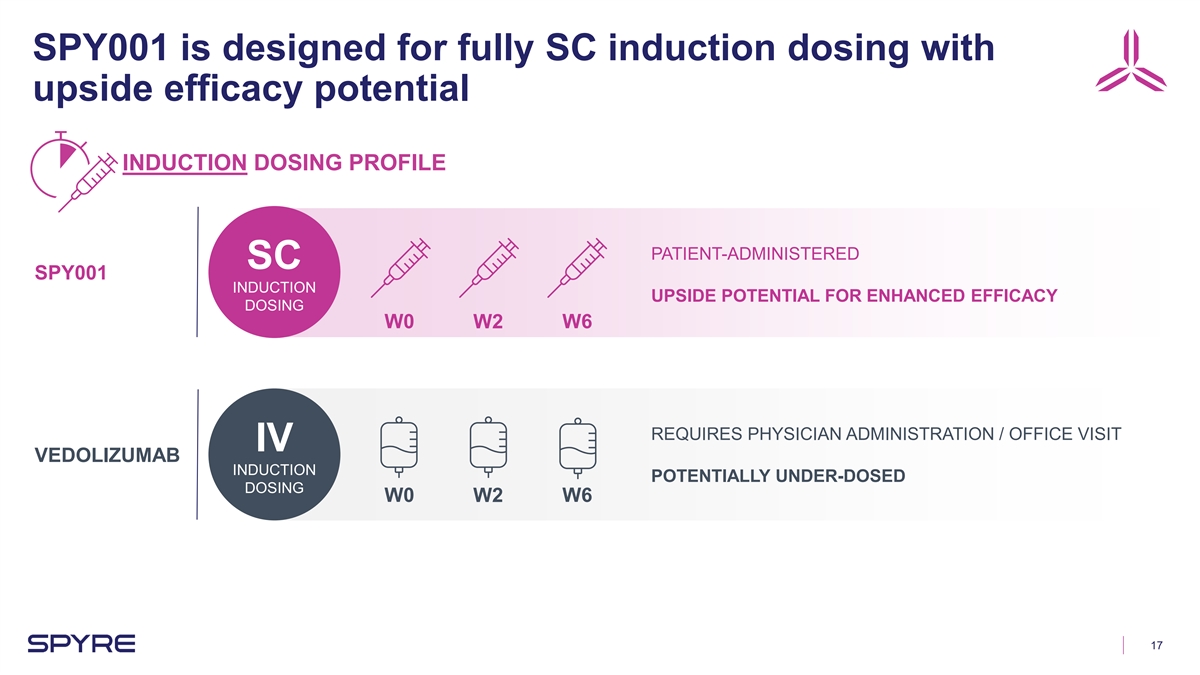
SPY001 is designed for fully SC induction dosing with upside efficacy
potential INDUCTION DOSING PROFILE PATIENT-ADMINISTERED SC SPY001 INDUCTION UPSIDE POTENTIAL FOR ENHANCED EFFICACY DOSING W0 W2 W6 REQUIRES PHYSICIAN ADMINISTRATION / OFFICE VISIT IV VEDOLIZUMAB INDUCTION POTENTIALLY UNDER-DOSED DOSING W0 W2 W6
17
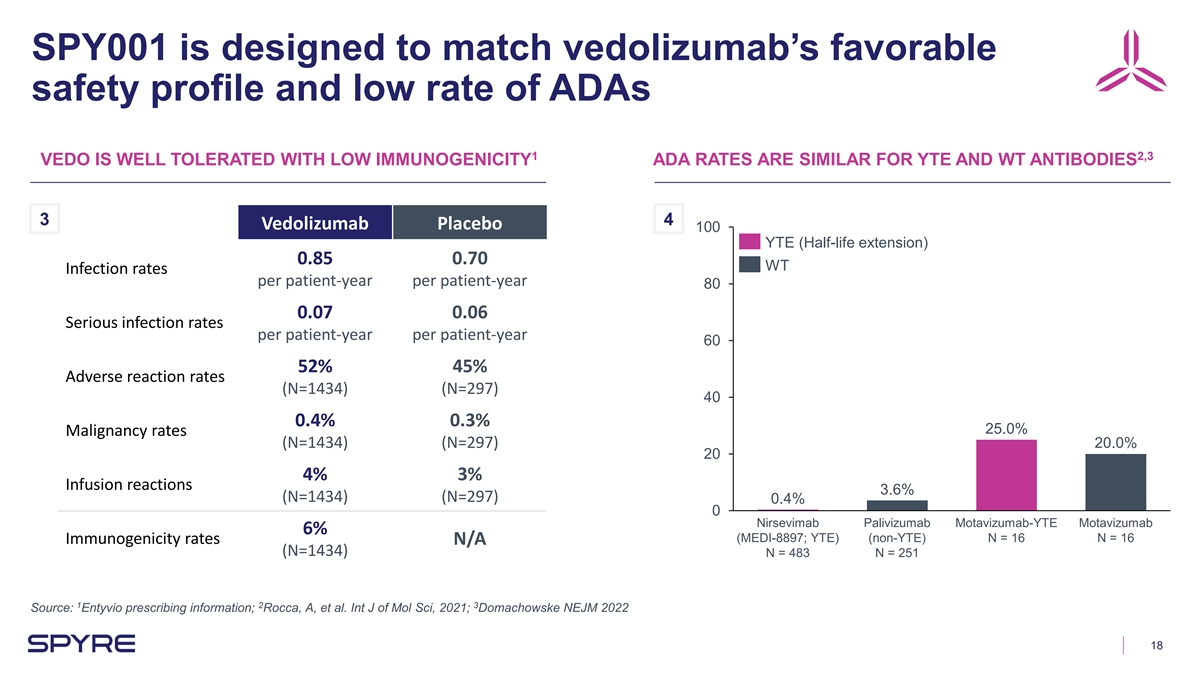
SPY001 is designed to match vedolizumab’s favorable safety
profile and low rate of ADAs 1 2,3 VEDO IS WELL TOLERATED WITH LOW IMMUNOGENICITY ADA RATES ARE SIMILAR FOR YTE AND WT ANTIBODIES 3 4 Vedolizumab Placebo 100 YTE (Half-life extension) 0.85 0.70 WT Infection rates per patient-year per patient-year 80
0.07 0.06 Serious infection rates per patient-year per patient-year 60 52% 45% Adverse reaction rates (N=1434) (N=297) 40 0.4% 0.3% 25.0% Malignancy rates (N=1434) (N=297) 20.0% 20 4% 3% Infusion reactions 3.6% (N=1434) (N=297) 0.4% 0 Nirsevimab
Palivizumab Motavizumab-YTE Motavizumab 6% (MEDI-8897; YTE) (non-YTE) N = 16 N = 16 Immunogenicity rates N/A (N=1434) N = 483 N = 251 1 2 3 Source: Entyvio prescribing information; Rocca, A, et al. Int J of Mol Sci, 2021; Domachowske NEJM 2022
18

SPY001 summary PK MODELLING SUPPORTS COMPARABLE IN VITRO DEVELOPING
PATIENT Q8-12W SC DOSING; UPSIDE PERFORMANCE TO SELECTION APPROACHES TO POTENTIAL FOR GREATER VEDOLIZUMAB IDENTIFY RESPONDERS INDUCTION EFFICACY INTERIM PHASE 1 PK DATA EXPECTED YE2024 19

SPY002 POTENTIAL BEST-IN-CLASS TL1A ANTIBODY
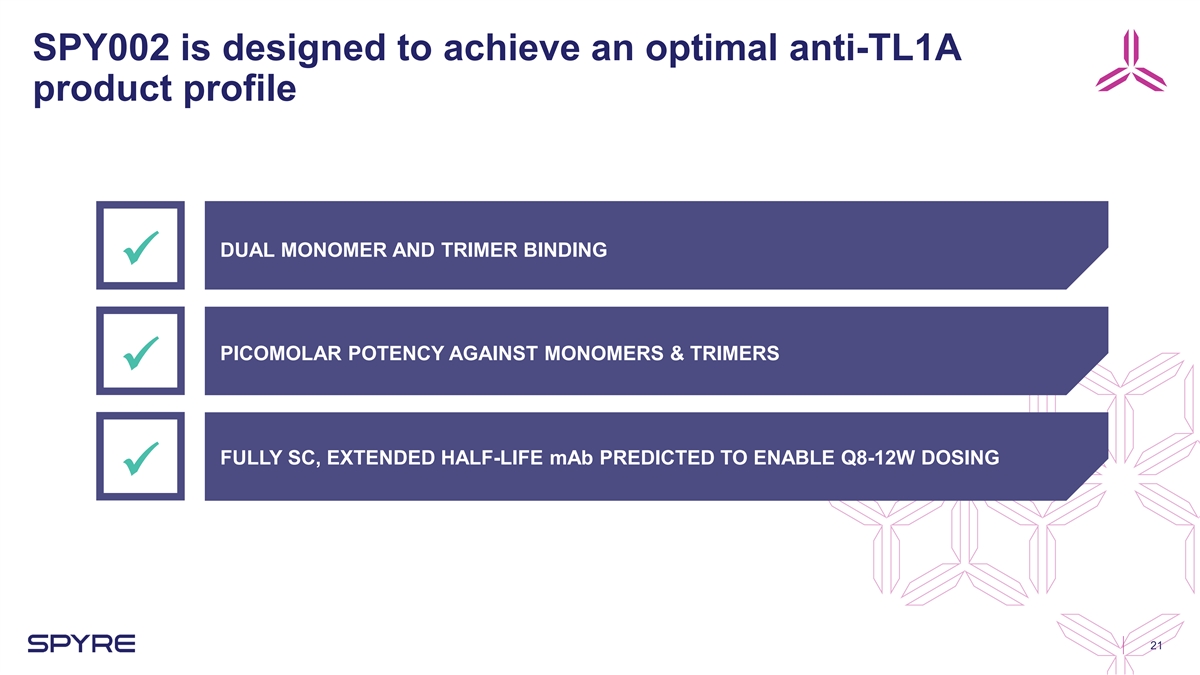
SPY002 is designed to achieve an optimal anti-TL1A product profile DUAL
MONOMER AND TRIMER BINDING ü PICOMOLAR POTENCY AGAINST MONOMERS & TRIMERS ü FULLY SC, EXTENDED HALF-LIFE mAb PREDICTED TO ENABLE Q8-12W DOSING ü 21

Extensive discovery campaign pursued to identify an uncompromising TL1A
antibody Heavily resourced campaign employing multiple strategies and formats enabled discovery of desirable TL1A clones 1000+ CLONES Format 3 Format 4 Format 1 Format 2 MONOMER AND TRIMER BINDER Set A Set G Strategy 1 Set B Set H Strategy 2
PICOMOLAR POTENCY Set C Set E Strategy 3 EXTENDED HALF-LIFE Set D Set F Set I Strategy 4 POTENTIAL BEST-IN-CLASS TL1A 22

SPY002 clones are unique in binding both monomers and trimers with
picomolar potency 1 1 SUPERIOR MONOMER AND TRIMER BINDING SUPERIOR TF-1 APOPTOSIS INHIBITION RVT-3101 NO MONOMER BINDING TEV-48574 TEV-48574 MK-7240 RVT-3101 SELECT SPYRE CLONES MK-7240 SELECT SPYRE CLONES Greater trimer binding (M) 1 Source:
Nonclinical data on file. Compared to TEV-48574, RVT-3101, MK-7240 23 Greater monomer binding (M)

SPY002 is a potential best-in-class TL1A mAb SPY002 MK-7240 RVT-3101
TEV-48574 Complete TL1A blockade üü Monomer and trimer Picomolar trimer üüü potency Half-life extension ü Q8-12W dosing Source: Company materials 24

SPY002 is potentially the most advanced unencumbered TL1A in
development April 16, 2023 Oct 3, 2023 Announcement dates: Oct 23, 2023 $10.8B 50-50 $7.1B acquisition licensing deal acquisition Global rights $0.5B upfront +$1B in milestones +$150M near-term milestone North America, Japan, Asia rights U.S. and
Japan rights Source: Company press releases 25
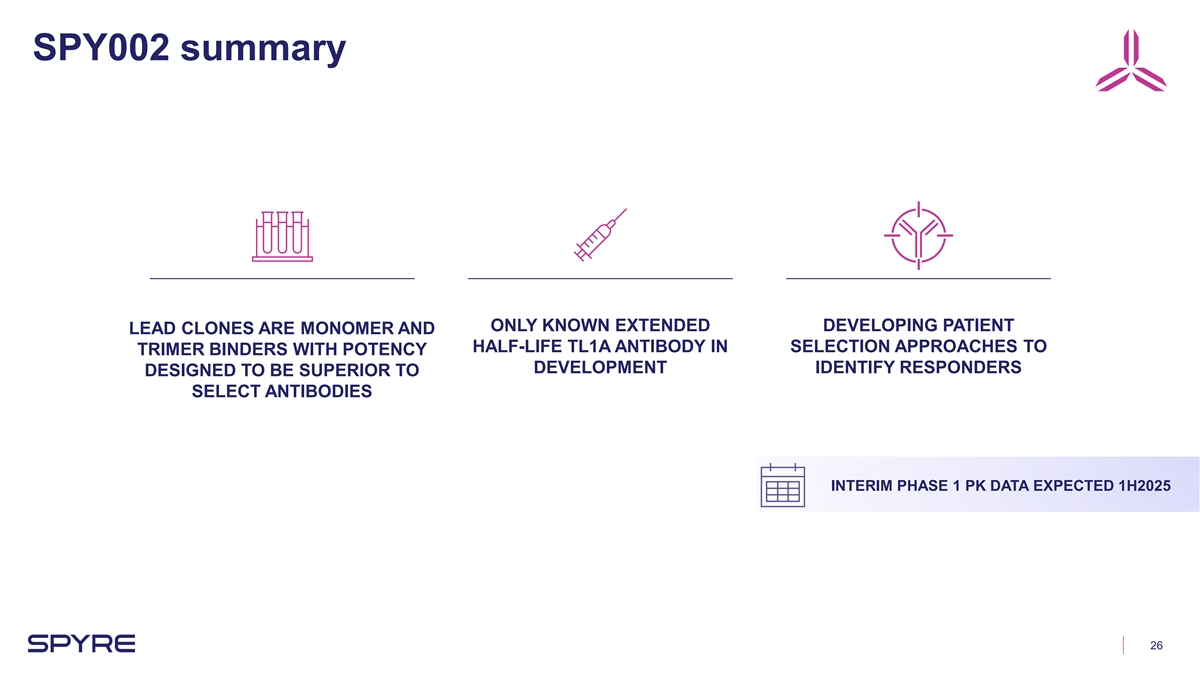
SPY002 summary ONLY KNOWN EXTENDED DEVELOPING PATIENT LEAD CLONES ARE
MONOMER AND HALF-LIFE TL1A ANTIBODY IN SELECTION APPROACHES TO TRIMER BINDERS WITH POTENCY DEVELOPMENT IDENTIFY RESPONDERS DESIGNED TO BE SUPERIOR TO SELECT ANTIBODIES INTERIM PHASE 1 PK DATA EXPECTED 1H2025 26

THERAPEUTIC COMBINATIONS
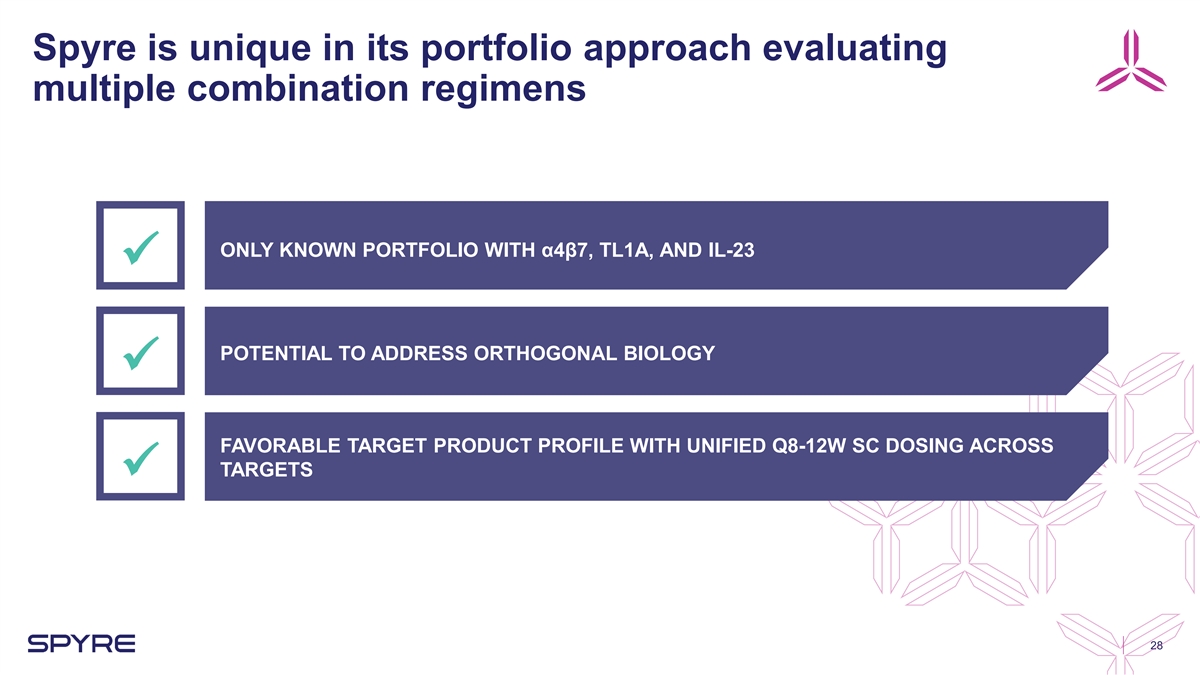
Spyre is unique in its portfolio approach evaluating multiple
combination regimens ONLY KNOWN PORTFOLIO WITH α4β7, TL1A, AND IL-23 ü POTENTIAL TO ADDRESS ORTHOGONAL BIOLOGY ü FAVORABLE TARGET PRODUCT PROFILE WITH UNIFIED Q8-12W SC DOSING ACROSS TARGETS ü 28

Spyre portfolio addresses the diverse pathophysiology of IBD Blockade
of α4β7 prevents circulating T cells Neutralization of TL1A suppresses inflammation within Neutralization of IL-23 inhibits cascade of from entering inflammatory gut tissues gut tissue and blocks immune cell activation various
proinflammatory cytokines Sypre is a pioneer in developing potential best-in-class mAbs against three top targets with the goal of enabling superior combinations for IBD 29 Intestinal epithelium MOA Blood Vessel

JNJ's VEGA study demonstrated power of combination therapy despite
relative weakness of components VEGA COMBINATION STUDY (N=71/ARM) Δ+22% 47% ~additive absolute clinical remission rates (induction) 22% 25% 24% 25% Anti-TNF Anti-IL23 Combination (Golimumab) (Guselkumab) BLACK BOX WARNING PROBABLE BLACK BOX
NOTE: In VEGA, only guselkumab (IL-23) was continued in maintenance for the combo arm in a treat-through design; Spyre plans to use combinations in maintenance. Maintenance remission rates for the anti-TNF arm, anti-IL23 arm, and induction
combination arm were 28%, 31%, and 48%, respectively.; Feagan, B. G. et al. Lancet Gastroenterol. Hepatol. 8, 307–320 (2023). 30

Spyre aims to build on this success with combinations of potentially
best-in-class mAbs from favorable MOAs SPY130 SPY120 SPY230 JNJ-’4804 Targets α4β7 + IL-23 α4β7 + TL1A TL1A + IL-23 TNF + IL-23 Format Co-formulation Co-formulation Co-formulation Co-formulation Monotherapies lack
üüü black box warning Anti-cytokine + anti-lymphocyte üü trafficking Half-life extension Q8-12W dosingüüü Source: Company materials 31

Combination programs summary COMBINATION OF TARGETING UNIFIED Q8-12W
DEVELOPING PATIENT POTENTIALLY BEST-IN-CLASS DOSING FOR OPTIMIZED SELECTION APPROACHES TO MONOTHERAPY BUILDING PATIENT CONVENIENCE IDENTIFY RESPONDERS BLOCKS SPY130 Phase 2 study initiation expected in 2025 32

CORPORATE TEAM AND CASH RUNWAY

Leadership and BOD 34

Cash and anticipated milestones 2024 2025 1 CASH RUNWAY CATALYSTS
EXPECTED EXPECTED 1H - IND/CTN PHASE 1 FULL DATA SPY001 (α4β7) 236.7M as of 6/30/2023 2H - PHASE 1 INTERIM DATA PHASE 2 INITIATION 2H - IND/CTN PHASE 1 INTERIM DATA SPY002 (TL1A) expected to fund pipeline into 2026 through multiple key
value creation events IND/CTN SPY003 (IL-23) PHASE 2 INITIATION COMBINATIONS 1H – PRA023 SSc-ILD P2 2H - TEV-48574 P2b MORF057 P2b EXTERNAL EVENTS 2H – DUET UC/CD P2b 1 Note: As of October 23, 2023 35

Thank you

Aeglea-Spyre Transaction highlights Structure: The acquisition of Spyre
was structured as a stock-for-stock transaction whereby all of Spyre’s outstanding equity interests were exchanged for a combination of shares of Aeglea common stock and a newly created non-voting Series A convertible preferred stock.
Financing: Concurrent with the acquisition of Spyre, Aeglea closed a $210 million private placement with a group of institutional accredited investors led by Fairmount Funds and joined by a robust syndicate of dedicated biotechnology investors as
well as additional undisclosed institutional investors. Primary use of proceeds: The proceeds from the private placement are expected to be primarily used to advance the Spyre pipeline and deliver the following anticipated milestones: two INDs for
SPY001 and SPY002 in 2024, HV PK/PD data for SPY001 in 2H2024, and HV PK/PD data for SPY002 in 1H2025. Proceeds are expected to provide cash runway into 2026. 37

Capitalization * As of 6/20/2023 • Shares of common stock and
preferred stock Shares of common stock outstanding 2.6 million were issued to Spyre Therapeutics Inc. security holders in exchange for all of Spyre’s outstanding Pre-funded warrants 1.2 million equity interests. Merger consideration •
Shares of preferred stock were issued to Spyre Therapeutics Inc. stockholders and to investors in Shares of common 0.5 million the $210 million private placement. Shares of preferred 0.4 million • Each share of preferred stock will
automatically Preferred conversion ratio 1:40 convert into 40 shares of common stock upon Total pre-financing common equivalent 18.9 million stockholder approval, subject to certain beneficial ownership restrictions set by each holder. Concurrent
financing • Please refer to the company’s SEC filings for Shares of preferred 0.7 million additional information. Common equivalent 28.9 million Total capitalization (Common) 47.7 million *Note: All figures have been retroactively
adjusted to reflect the company’s 1-for-25 reverse stock split that will be effective as of 12:01 a.m. Eastern Time on September 8, 2023. 38
v3.23.3
Document and Entity Information
|
Oct. 24, 2023 |
| Cover [Abstract] |
|
| Security Exchange Name |
NASDAQ
|
| Amendment Flag |
false
|
| Entity Central Index Key |
0001636282
|
| Document Type |
8-K
|
| Document Period End Date |
Oct. 24, 2023
|
| Entity Registrant Name |
AEGLEA BIOTHERAPEUTICS, INC.
|
| Entity Incorporation State Country Code |
DE
|
| Entity File Number |
001-37722
|
| Entity Tax Identification Number |
46-4312787
|
| Entity Address, Address Line One |
221 Crescent Street
|
| Entity Address, Address Line Two |
Building 23
|
| Entity Address, Address Line Three |
Suite 105
|
| Entity Address, City or Town |
Waltham
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02453
|
| City Area Code |
617
|
| Local Phone Number |
651-5940
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre Commencement Tender Offer |
false
|
| Pre Commencement Issuer Tender Offer |
false
|
| Security 12b Title |
Common Stock, $0.0001 Par Value Per Share
|
| Trading Symbol |
AGLE
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 3 such as an Office Park
| Name: |
dei_EntityAddressAddressLine3 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Aeglea BioTherapeutics (NASDAQ:AGLE)
Historical Stock Chart
From Apr 2024 to May 2024
Aeglea BioTherapeutics (NASDAQ:AGLE)
Historical Stock Chart
From May 2023 to May 2024