Kymera Therapeutics Gets Orphan-Drug Status for Leukemia Treatment
22 June 2023 - 11:21PM
Dow Jones News
By Dean Seal
Kymera Therapeutics said it has received orphan drug designation
from regulators for its acute myeloid leukemia treatment.
The U.S. Food and Drug Administration has granted the
designation for KT-253, which targets a crucial regulator of the
most common tumor suppressor and has been shown to rapidly induce
cancer cell death.
Orphan-drug designation is a special status given to drugs that
show promise for potentially treating rare, or orphan, diseases
that have fewer than 200,000 cases a year in the U.S.
Shares slipped 2% to $24.15 in premarket trading.
Write to Dean Seal at dean.seal@wsj.com
(END) Dow Jones Newswires
June 22, 2023 09:06 ET (13:06 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
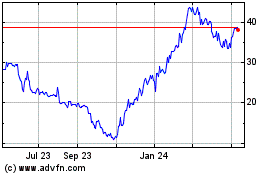
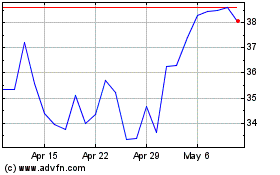
Kymera Therapeutics (NASDAQ:KYMR)
Historical Stock Chart
From Apr 2024 to May 2024
Kymera Therapeutics (NASDAQ:KYMR)
Historical Stock Chart
From May 2023 to May 2024