false
0001000694
0001000694
2024-01-08
2024-01-08
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported):
January 8, 2024
NOVAVAX, INC.
(Exact name of registrant as specified
in charter)
| Delaware |
|
0-26770 |
|
22-2816046 |
|
(State or Other Jurisdiction
of Incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer
Identification No.) |
700 Quince Orchard Road
Gaithersburg, Maryland 20878
(Address of Principal Executive Offices,
including Zip Code)
(240) 268-2000
(Registrant’s telephone number,
including area code)
(Former name or former address, if changed
since last report.)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General
Instruction A.2. below):
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section
12(b) of the Act:
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which
registered |
| Common Stock, Par Value $0.01 per share |
|
NVAX |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant
is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2
of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ¨
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 2.02 Results of Operations and Financial
Condition
On January 8, 2024, Novavax,
Inc. (the “Company”) provided an update for investors in which it reaffirmed its guidance for full year 2023 combined research
and development and selling, general and administrative expenses, which is anticipated to be between $1.15 billion and $1.25 billion.
The Company is in the
process of finalizing its financial results for the year ended December 31, 2023, and the foregoing preliminary financial data is based
on available information to date. This financial data for the year ended December 31, 2023 is preliminary and may change. This preliminary
financial data has been prepared by, and is the responsibility of, the Company’s management. Ernst & Young LLP, the Company’s
independent registered public accounting firm, has not audited, reviewed, compiled or performed any procedures with respect to this preliminary
financial data, nor have any other independent accountants. Accordingly, Ernst & Young LLP does not express an opinion or any other
form of assurance with respect thereto. The Company’s actual results for this period may differ from the foregoing preliminary financial
data and such changes could be material. In addition, this preliminary financial data should not be viewed as a substitute for full financial
statements for the year ended December 31, 2023 prepared in accordance with U.S. generally accepted accounting standards. Additional information
that will be material to investors will be provided in these financial statements, and, accordingly, investors should not place undue
reliance on the limited preliminary information being provided herein.
This Current Report on
Form 8-K includes forward-looking statements including statements regarding the Company’s current expectations, financial results
and anticipated results of operations, including financial guidance for full year 2023 and 2024. Generally,
forward-looking statements can be identified through the use of words or phrases such as “could,” “will,” “would,”
“can,” “estimate,” “continue,” “ongoing,” “consider,” “anticipate,”
“intend,” “seek,” “plan,” “project,” “expect,” “should,” “prepare”,
or “aim”, the negative of these terms, or other comparable terminology, although not all forward-looking statements contain
these words. Forward-looking statements involve estimates, assumptions, risks, and uncertainties that could cause actual results or outcomes
to differ materially from those expressed or implied in any forward-looking statements, and, therefore, you should not place considerable
reliance on any such forward-looking statements. Such risks and uncertainties include, among others, that the Company’s
full financial statements for the year ended December 31, 2023 prepared in accordance with U.S. generally accepted accounting standards
may differ materially from the preliminary and unaudited amounts reported herein and other risks and uncertainties are identified under
the heading “Risk Factors” in the Company’s most recent Annual Report on Form 10-K, in subsequent Quarterly Reports
on Form 10-Q and in any subsequent filings with the Securities and Exchange Commission. Further,
any forward-looking statement speaks only as of the date when it is made, and the Company undertakes no obligation to update or revise
any forward-looking statements, whether as a result of new information, future events, or otherwise, unless required by law. New factors
emerge from time to time, and it is not possible for the Company to predict which factors will arise. In addition, the Company cannot
assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results
to differ materially from those contained in any forward-looking statements.
| Item 7.01. |
Regulation FD Disclosure. |
On January 8, 2024, the Company provided an update
for investors at the 42nd Annual J.P. Morgan Healthcare Conference in San Francisco, California, presenting information relating to certain
strategic and business updates (the “Investor Presentation”), which is attached as Exhibit 99.1 to this Current Report on
Form 8-K and incorporated into this Item 7.01 by reference. A copy of the Investor Presentation will also be accessible on the Company’s
website at www.novavax.com under “Latest Investor Presentation.”
Cautionary Note Regarding Forward-Looking
Statements. The Investor Presentation contains forward-looking statements that involve certain risks and uncertainties that could
cause actual results to differ materially from those expressed or implied by these statements. Please refer to the cautionary notes in
the Investor Presentation regarding these forward-looking statements.
The information in Item 2.02 and Item 7.01, including
Exhibit 99.1, is being furnished and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange
Act of 1934, as amended, or otherwise subject to the liabilities of that Section and shall not be deemed incorporated by reference into
any registration statement or other document filed pursuant to the Securities Act of 1933, as amended, or the Securities Exchange Act
of 1934, as amended, except as shall be expressly set forth by specific reference in such filing. In addition, the contents of the Company’s
website are not incorporated by reference into this Current Report on Form 8-K and you should not consider information provided on the
Company’s website to be part of this Current Report on Form 8-K.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto
duly authorized.
| |
Novavax, Inc. |
| |
|
|
| Date: January 8, 2024 |
By: |
/s/ Mark Casey |
| |
Name: |
Mark Casey |
| |
Title: |
Executive Vice President, Chief Legal Officer and Corporate Secretary |
Exhibit 99.1

J.P. Morgan 42 nd Annual Healthcare Conference 1 © 2024 NOVAVAX. All rights reserved. NASDAQ: NVAX | JANUARY 2024

This presentation includes forward - looking statements . These forward - looking statements generally can be identified by the use of words such as “anticipate,” “expect,” “plan,” “may,” “will,” “believe,” “estimate,” “forecast,” “goal,” “project,” and other words of similar meaning . These forward - looking statements address various matters including information relating to the future of Novavax, its near term priorities including reducing rate of spend, managing cash flow and evolving its scale and structure, the amount and impact of its global restructuring and cost reduction plans, its operating plans, objectives and prospects, full year 2023 and 2024 financial guidance, its future financial or business performance, conditions or strategies, the potential sale of the CZ manufacturing facility, the Company’s partnerships, including with respect to the launch of R 21 /Matrix - M Malaria vaccine, the ongoing development of its updated COVID - 19 vaccine, COVID - 19 - Influenza Combination (CIC) investigational vaccine candidate and influenza vaccine candidate, the timing of delivery and distribution of its vaccine, the scope, timing and outcome of future and pending regulatory filings and actions, including expected U . S . Biologics License Application (BLA) for its updated COVID - 19 vaccine, the timing for advancing CIC vaccine candidate to Phase 3 and for its potential commercial launch, anticipated label expansion and market access, and the 2024 and future global COVID - 19 , influenza and combination market opportunities, are forward - looking statements . Novavax cautions that these forward - looking statements are subject to numerous risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements . These risks and uncertainties include, without limitation, Novavax’s ability to successfully manufacture, distribute or market its updated COVID - 19 vaccine for the 2023 - 2024 and future vaccination seasons ; challenges satisfying, alone or together with partners, various safety, efficacy, and product characterization requirements, including those related to process qualification, assay validation, and stability testing, necessary to satisfy applicable regulatory authorities ; challenges or delays in conducting clinical trials ; challenges or delays in obtaining regulatory authorization for its product candidates, including future COVID - 19 variant strain changes ; the size of the COVID - 19 , influenza and combination market ; potential developments during the 2023 - 2024 and 2024 - 2025 vaccination season ; manufacturing, distribution or export delays or challenges ; Novavax's exclusive dependence on Serum Institute of India Pvt . Ltd . and Serum Life Sciences Limited for co - formulation and filling and PCI Pharma Services for finishing Novavax’s COVID - 19 vaccines and the impact of any delays or disruptions in their operations on the delivery of customer orders ; difficulty obtaining scarce raw materials and supplies ; resource constraints, including human capital and manufacturing capacity, and constraints on Novavax’s ability to pursue planned regulatory pathways, alone or with partners, in multiple jurisdictions simultaneously, leading to staggered regulatory filings, and potential regulatory actions ; the loss of future funding from the U . S . government ; the potential for an unfavorable outcome in disputes, including the pending arbitration with Gavi ; challenges in implementing its global restructuring and cost reduction plan ; challenges in obtaining commercial adoption, market access and market acceptance for its vaccines or vaccine candidates ; challenges meeting contractual requirements under agreements with multiple commercial, governmental, and other entities, including requirements to deliver doses that may require Novavax to refund portions of upfront and other payments previously received or result in reduced future payments pursuant to such agreements ; challenges related to the seasonality of vaccinations against COVID - 19 , seasonal influenza or other diseases ; and the risks identified under the heading “Risk Factors” in Novavax' most recent Annual Report on Form 10 - K and subsequent Quarterly Reports on Form 10 - Q, as well as subsequent filings with the Securities and Exchange Commission . Novavax cautions investors not to place considerable reliance on the forward - looking statements contained in this presentation . Investors are encouraged to read Novavax’ filings with the Securities and Exchange Commission, available at www . sec . gov and on our website at www . novavax . com, for a discussion of these and other risks and uncertainties . The forward - looking statements in this presentation speak only as of the date of this presentation, and we undertake no obligation to update or revise any of these statements . Our business is subject to substantial risks and uncertainties, including those referenced above . Investors, potential investors, and others should give careful consideration to these risks and uncertainties . Cautionary note regarding forward - looking statements 2 © 2024 NOVAVAX. All rights reserved.

Introduction SE C T I O N 3 © 2024 NOVAVAX. All rights reserved.

4 © 2024 NOVAVAX. All rights reserved. “It’s just another number” … or is it? 3,000,000 1,300 630,000 Sources: WHO.int, CDC.gov, Ourworldindata.org. 41,000,000 710,000 52,000

5 © 2024 NOVAVAX. All rights reserved. 50,000,000 deaths can be prevented through immunization between 2021 - 2030 Est. 150,000,000 – 200,000,000 lives have been saved between 1980 and 2018 from smallpox vaccination alone Sources: CDC.gov, Ourworldindata.org.

6 Novavax is here to make a difference. We’re a biotech company focused solely on developing life - saving vaccines to fight infectious diseases. © 2024 NOVAVAX. All rights reserved. What we do as an industry matters, we have made and will continue to make a positive impact on health for millions of people around the globe “ Next to creating a life, the finest thing a person can do is save one.” Abraham Lincoln “To know even one life has breathed easier because you have lived. This is to have succeeded.” Ralph Waldo Emerson We never rest in our quest to protect the health of people everywhere.

C o m p a ny Overview SE C T I O N 7 © 2024 NOVAVAX. All rights reserved.
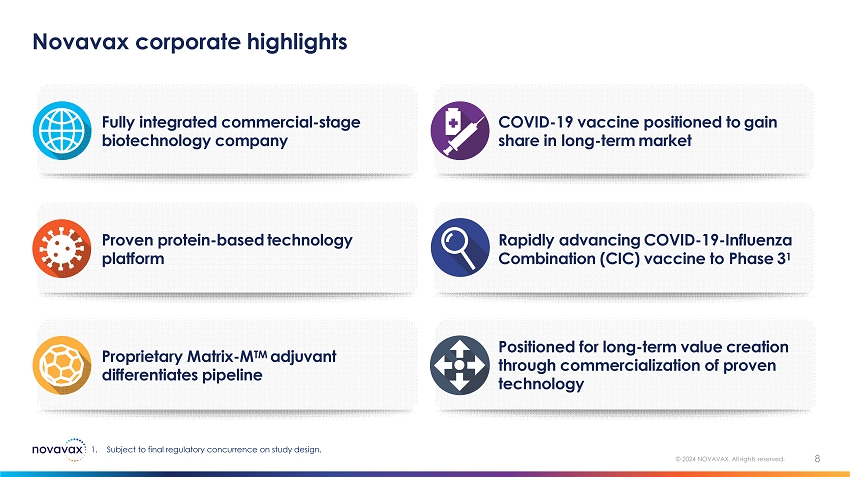
Novavax corporate highlights 8 © 2024 NOVAVAX. All rights reserved. Positioned for long - term value creation through commercialization of proven technology Proprietary Matrix - M TM adjuvant differentiates pipeline Rapidly advancing COVID - 19 - Influenza Combination (CIC) vaccine to Phase 3 1 Proven protein - based technology platform COVID - 19 vaccine positioned to gain share in long - term market Fully integrated commercial - stage biotechnology company 1. Subject to final regulatory concurrence on study design.

Fully integrated commercial - stage biotechnology company with global presence 9 © 2024 NOVAVAX. All rights reserved. Global HQ & Corporate Offices • R&D and discovery laboratories • Manufacturing operations • Commercial Novavax HQ Novavax HQ Gaithersburg, Maryland Novavax AB Uppsala, Sweden Novavax CZ Bohumil, Czech Republic Serum Institute of India SK bioscience Takeda EU Commercial Operations Zurich, Switzerland APAC Commercial Operations Singapore Novavax AB – Matrix - M adjuvant Novavax CZ – Antigen Novavax Ma nuf ac turing North America European Union (EU) Asia - Pacific (APAC) Novavax C o mm e r c ia l Operations Serum Institute of India (SII) SK bioscience Takeda Str a t eg ic Partners North America Commercial Operations Gaithersburg, Maryland
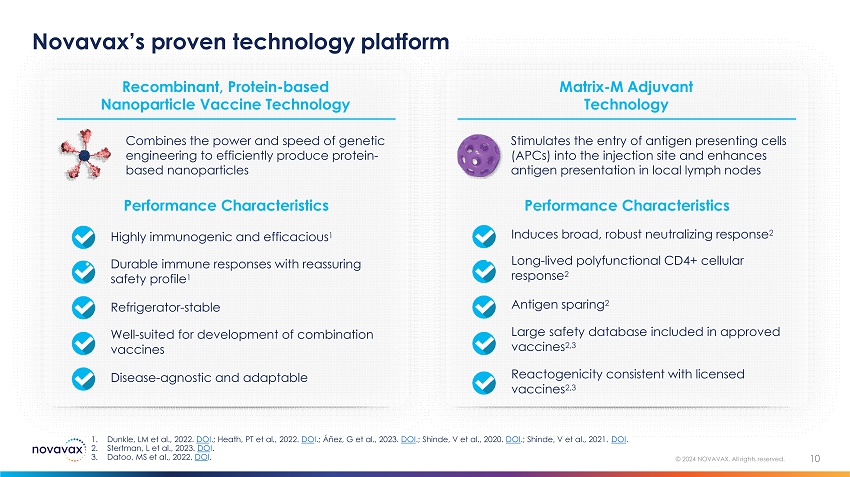
Novavax’s proven technology platform 10 © 2024 NOVAVAX. All rights reserved. • Antigen sparing 2 • Large safety database included in approved vaccines 2,3 • Reactogenicity consistent with licensed vaccines 2,3 Matrix - M Adjuvant Technology Performance Characteristics • Induces broad, robust neutralizing response 2 • Long - lived polyfunctional CD4+ cellular response 2 Stimulates the entry of antigen presenting cells (APCs) into the injection site and enhances antigen presentation in local lymph nodes • Refrigerator - stable • Well - suited for development of combination vaccines • Disease - agnostic and adaptable Recombinant, Protein - based Nanoparticle Vaccine Technology Performance Characteristics • Highly immunogenic and efficacious 1 • Durable immune responses with reassuring safety profile 1 Combines the power and speed of genetic engineering to efficiently produce protein - based nanoparticles 1. Dunkle, LM et al., 2022. DO I .; Heath, PT et al ., 2022. DO I .; Áñez, G et al., 2023. DOI .; Shinde, V et al., 2020. DOI . ; Shinde, V et al., 2021. DOI . 2. Stertman, L et al., 2023. DO I . 3. Datoo, MS et al., 2022. DO I .
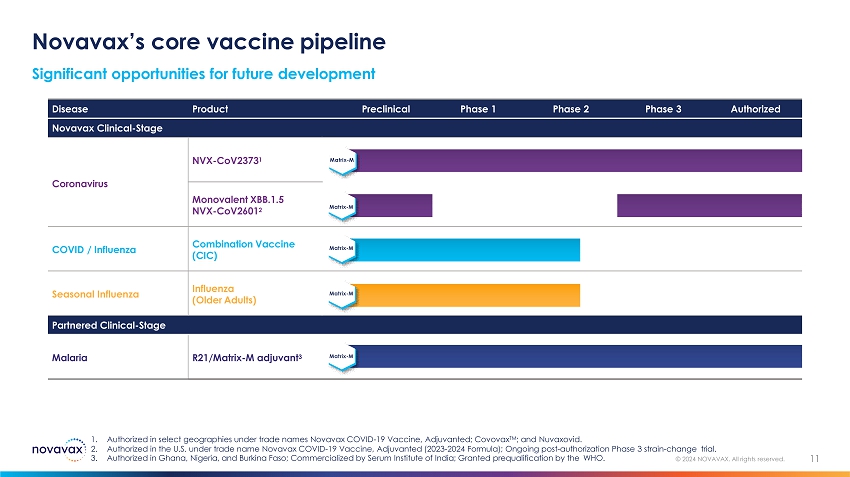
Novavax’s core vaccine pipeline Significant opportunities for future development 11 © 2024 NOVAVAX. All rights reserved. 1. Authorized in select geographies under trade names Novavax COVID - 19 Vaccine, Adjuvanted; Covovax TM ; and Nuvaxovid. 2. Authorized in the U.S. under trade name Novavax COVID - 19 Vaccine, Adjuvanted (2023 - 2024 Formula); Ongoing post - authorization Phase 3 strain - change trial. 3. Authorized in Ghana, Nigeria, and Burkina Faso; Commercialized by Serum Institute of India; Granted prequalification by the WHO. Disease Product Preclinical Phase 1 Phase 2 Phase 3 Authorized Novavax Clinical - Stage Coronavirus NVX - CoV2373 1 Matrix - M Matrix - M Monovalent XBB.1.5 NVX - CoV2601 2 COVID / Influenza Combination Vaccine (CIC) Matrix - M Seasonal Influenza Influenza (Older Adults) Matrix - M Partnered Clinical - Stage Malaria R21/Matrix - M adjuvant 3 Matrix - M

R21/Matrix - M vaccine against malaria approved to help address dire global public health need 12 © 2024 NOVAVAX. All rights reserved. Highly Efficacious Vaccine Demonstrates Promise of Novavax Technology Platform • Formulated with Novavax’s Matrix - M adjuvant 1 • Prequalified by WHO in Dec. 2023; 2024 launch • 75% efficacy 2 ; exceeds current standard of care • Noted as a “vital tool” by WHO Director - General 3 Malaria is One of the Deadliest Diseases in Human History • Kills 630,000 people/year 4 , incl. ~1,300 children/day 4 o Children under the age of five accounted for ~78% of malaria deaths 5 • Significant economic, social and health equity burden 1. Developed by University of Oxford and manufactured and commercialized by Serum Institute of India. 2. Datoo, MS et al., 2023. DO I . 3. WHO press release published 10/2/2023. 4. Ourworldindata.org. 5. WHO.int. Photo credit: University of Oxford “As a malaria researcher, I used to dream of the day we would have a safe and effective vaccine against malaria. Now we have two,” said Dr Tedros Adhanom Ghebreyesus, WHO Director - General. “Demand for the RTS,S vaccine far exceeds supply, so this second vaccine is a vital additional tool to protect more children faster , and to bring us closer to our vision of a malaria - free future .” 4

2023: A Year in Review SE C T I O N 13 © 2024 NOVAVAX. All rights reserved.

14 © 2024 NOVAVAX. All rights reserved. 2023 was a year of significant change for Novavax with a strong focus on building a robust and sustainable business • Delivered updated vaccine in - line with regulatory authority requirements • Delivered on our APA commitments, gained experience in U.S. market • Outlined faster path forward for our Flu/Covid combination vaccine program, with anticipated launch in 2026* Streamlined strain selection and manufacturing process Critical steps taken to advance CIC development program • Reduced current liabilities by ~ $1B as of 9/30/2023 including resolution of pandemic era manufacturing commitments • On tack to reduce SG&A and R&D expenses by over 25% in 2023 and by over 55% in 2024 vs FY 2022 baseline Restructured costs and significantly reduced current liabilities • Created CSO, COO and Corp Affairs positions, appointed new President of R&D, hired new GC and CHRO among other changes • ~ 20% reduction to our headcount in 2023 and prepared to further reduce size and scope of operations in 2024 Restructured management team Significant reductions to scale of global footprint 1. *Subject to final regulatory concurrence on study design and successful completion of our clinical development program. 2. *Intend to launch CIC vaccine in 2026, if approved.

Looking Ahead: Significant Future Opportunity for Novavax SE C T I O N 15 © 2024 NOVAVAX. All rights reserved.

Setting the stage for a stronger U.S. commercial performance in 2024+ 1. IPSOS syndicated Awareness, Trial and Usage tracker (From March 2023 to September 2023), HCP monthly pulse (starting in October 2023). 2. IQVIA NPA Data and Claims Data. 16 © 2024 NOVAVAX. All rights reserved. 2023 - 2024 Vaccination Season 2024 - 2025 Vaccination Season 5 - dose vial Product Presentation Single - dose presentation E UA FDA Authorization Status BLA anticipated Ages 12+ Label Expansion Seeking ages 6+ October Timing of Product Availability Targeting September 2024 46% as of June 2023 1 HCP - Aided Awareness 80% as of January 2024 1 Not on equal footing in all retail chains. Retail channel ~90% of market opportunity 2 Market Access Anticipate broader coverage and access to Novavax’s vaccine

0 20 40 60 80 1 0 0 1 2 0 1 4 0 1 6 0 Flu COVID - 19 Millions of doses 2019 - 2022 Average 130M - 140M Significant future U.S. market opportunity expected for combination respiratory vaccines • High unmet need and preference for a combination Flu & COVID - 19 vaccine o Potential for increased COVID - 19 vaccination rates as a result of combination • Flu represents a significant opportunity o U.S. flu season doses administered 2019 - 2022 average: 130M - 140M 1 o Annual U.S. flu market opportunity: $4B+ 2 30M - 35M 3 17 1. NVAX Internal estimates for season average using two data sources from CDC FluVaxView: a) NIS - Flu data for 6 months – 17 years of age and b) Weekly Cumulative Estimated Influenza Vaccinations Administered for adults 18+ via IQVIA LRx and Dx data. 2. Jefferies, Global Vaccines Deep Dive, Equity Research Report, May 30, 2023. 3. NVAX Internal projected estimate based on current total retail vaccinations as of 12/22 per IQVIA Rx Rapid weekly data. 2023 - 2024 season from September 2023 to March 2024. 4. Illustrative percent market share based on potential peak dose administration based by NVAX Internal Consumer and HCP demand studies conducted in April and June 2023. A combination vaccine dose equals two doses administered. © 2024 NOVAVAX. All rights reserved. Illustrative Future U.S. Market % of Seasonal Doses 4 U.S. Market Seasonal Doses C O VI D - 19 Flu C o m bo Preference for a combination respiratory vaccine creates a significant opportunity for Novavax 4 Potential to exceed 50% 2023 - 2024 Estimate

18 © 2024 NOVAVAX. All rights reserved. Novavax is well - positioned to deliver a differentiated combination vaccine and capture significant market opportunity Leverage Proven Technology Platform 1. Protein Receptivity Study (January 2022). 2. IQVIA Demand Study (2023). 3. IPSOS syndicated Awareness, Trial and Usage tracker (From March 2023 to September 2023), HCP monthly pulse (starting in October 2023). • ~25 - 30% of consumers 1 and healthcare professionals 2 surveyed, prefer a protein - based option Growing Awareness of Novavax in the U.S. 1 0 0% 8 0 % 6 0 % 4 0 % 2 0 % 0% 80% 72% 44% 46% Mar - 23 Jun - 23 Oct - 23 Jan - 24 % HCPs Strong Preference Share for Protein - Based Option • Commercialized COVID - 19 vaccine with demonstrated efficacy and tolerability profile • Matrix - M induces broad antibody response and long - lived cellular response • Quadrivalent influenza vaccine candidate with positive Phase 3 results generated o Potential to be competitive with market - leading, stand - alone influenza vaccine U.S. HCP - Aided Awareness 3

CIC/Flu Phase 2 study summary observations 19 © 2024 NOVAVAX. All rights reserved. S t and - a lo ne Influenza Vaccine CIC Vaccine Combines stand - alone influenza vaccine and NVX - CoV 2373 in single injection Matrix - M a d j uv ant + Quadrivalent vaccine with four influenza Hemagglutinin genes Matrix - M a d j uv ant + CIC Vaccine • Preliminary safety profile reassuring with reactogenicity comparable to Fluzone HD ® and FLUAD ® • Anti - S IgG and neutralization responses achieved levels seen in Nuvaxovid Phase 3 study • HAI 1 geometric mean ratios in line with a licensure criteria Stand - alone Quadrivalent Influenza Vaccine • Preliminary safety profile reassuring with reactogenicity comparable to Fluzone HD and FLUAD • HAI 1 responses 31 - 56% higher for all four strains compared to FLUAD • HAI 1 responses 44 - 89% higher for A strains compared to Fluzone HD FLUAD ® is a registered trademark of Seqirus UK Limited; Fluzone High - Dose Quadrivalent ® is a registered trademark of Sanofi Pasteur Inc. 1. Baseline - adjusted wild - type HAIs for the three strains to be included in future vaccines.

20 © 2024 NOVAVAX. All rights reserved. Novavax is positioned to support the expansion of our product portfolio Product Portfolio Novavax Readiness Posture T oday COVID - 19 Vaccine R21 / M a tr i x - M Malaria Vaccine 1 CIC Vaccine Potential launch in 2026 2 COVID - 19 Vaccine R21 / M a tr i x - M Malaria Vaccine 1 2026 • Cash and APAs support our ability to execute against opportunities globally in non - APA markets (U.S., Europe, low - and middle - income countries) • Significantly lowering our FY 2024 expenses o Targeting 55%+ overall reduction in SG&A + R&D compared to FY 2022 • 2 additional COVID - 19 seasons to further prepare for CIC launch • CIC clinical program supports option for stand - alone influenza late - stage development 1. R21 vaccine developed by Oxford University and SII includes Novavax’s Matrix - M adjuvant as a key component. This vaccine is marketed by SII. 2. Intend to launch CIC vaccine in 2026, if approved.
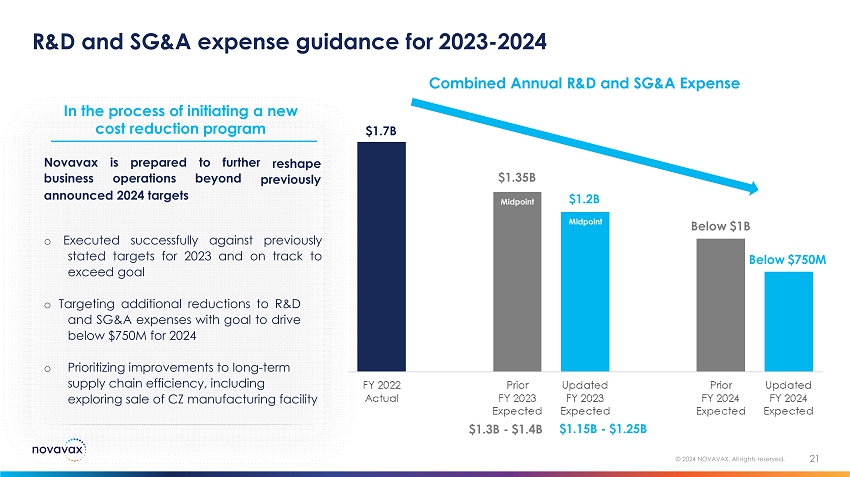
R&D and SG&A expense guidance for 2023 - 2024 21 © 2024 NOVAVAX. All rights reserved. $1 . 7B $1.15B - $1.25B cost reduction program reshape Novavax business is prepared t o fu r t h er operations beyond previously announced 2024 targets o Executed successfully against previously stated targets for 2023 and on track to exceed goal o Targeting additional reductions to R&D and SG&A expenses with goal to drive below $ 750 M for 2024 o Prioritizing improvements to long - term supply chain efficiency, including exploring sale of CZ manufacturing facility Below $1B Combined Annual R&D and SG&A Expense In the process of initiating a new $1.3B - $1.4B Below $750M $1.35B Midpoint $1.2B Midpoint

22 © 2024 NOVAVAX. All rights reserved. Summary: Progress and learnings from 2023 create strong foundation 2024+ • Potential CIC vaccine launch in fall 2026 season 2 • Large market and potential for differentiation as only protein - based option Significant future opportunity for Novavax • Identified a faster potential pathway forward for CIC • Significant improvements in our financial profile better position us to independently advance CIC vaccine to Phase 3 in 2H 2024 1 Well - positioned to advance pipeline with focus on CIC • Restructured management team • Significantly reduced expenses • Evolved corporate culture Created a more lean and focused organization 1. Subject to final regulatory concurrence on study design and successful completion of our clinical development program. 2. Intend to launch CIC vaccine in 2026, if approved.

23 © 2024 NOVAVAX. All rights reserved. We never rest in our quest to protect the health of people everywhere.

Q&A 24 © 2024 NOVAVAX. All rights reserved.
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Novavax (NASDAQ:NVAX)
Historical Stock Chart
From Mar 2024 to Apr 2024
Novavax (NASDAQ:NVAX)
Historical Stock Chart
From Apr 2023 to Apr 2024