false
0001359931
0001359931
2024-12-05
2024-12-05
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
December 5, 2024
Protara Therapeutics, Inc.
(Exact name of registrant as specified in its
charter)
| Delaware |
|
001-36694 |
|
20-4580525 |
(State or other jurisdiction of
incorporation) |
|
(Commission File No.) |
|
(IRS Employer
Identification No.) |
|
345 Park Avenue South
Third Floor
New York, NY |
|
10010 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including
area code: (646) 844-0337
N/A
(Former name or former address, if changed since
last report.)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant
to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to
Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications
pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications
pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b)
of the Act:
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.001 per share |
|
TARA |
|
The Nasdaq Capital Market |
Indicate by check mark whether the registrant
is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the
Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On December 5, 2024, Protara Therapeutics, Inc.
(the “Company” or “Protara”) posted an investor presentation (the “Investor Presentation”) to the
“Investors—Events and Presentations” section of the Company’s website at www.protaratx.com. The Investor Presentation
will be used in connection with a conference call and webcast today, December 5, 2024, at 8:30 am ET, to review the clinical data to be
presented during a poster session at the 25th Annual Meeting of the Society of Urologic Oncology in Dallas, Texas (the “2024 SUO
Conference”) and provides an update on the ongoing Phase 2 ADVANCED-2 trial program. A copy of the Investor Presentation is furnished
herewith as Exhibit 99.1 to this Current Report on Form 8-K.
On December 5, 2024, the Company also issued a
press release reporting new interim clinical data on the ongoing Phase 2 ADVANCED-2 trial program (the “Press Release”). A
copy of the Press Release is furnished herewith as Exhibit 99.2 to this Current Report on Form 8-K.
The information contained in Item 7.01 of this
Current Report on Form 8-K, including Exhibits 99.1 and 99.2 attached hereto, is being furnished and shall not be deemed to be “filed”
for the purposes of Section 18 of the Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities
of that section and shall not be incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange
Act, except as shall be expressly set forth by specific reference in such filing.
Item 8.01 Other Events.
On December 5, 2024, the Company presented interim
results from its ongoing Phase 2 ADVANCED-2 clinical trial of TARA-002 in patients with Non-Muscle Invasive Bladder Cancer (“NMIBC”)
at a poster session at the 2024 SUO Conference. A copy of the poster, which has been published to the “Investors—Events and
Presentations” section of the Company’s website, is filed as Exhibit 99.3 to this Current Report on Form 8-K and is incorporated
herein by reference.
The Company reported data that highlights the
potential of TARA-002 in patients with NMIBC in its Phase 2 ADVANCED-2 clinical trial, which is assessing the safety and anti-tumor activity
of intravesical TARA-002, the Company’s investigational cell-based therapy, in high-risk NMIBC patients with carcinoma in situ (“CIS”)
(± Ta/T1) who are Bacillus Calmette-Guérin (“BCG”)-Unresponsive or BCG-Naïve. The dataset includes 20 patients
who were evaluable at three months, 18 patients who were evaluable at six months and three patients who were evaluable at nine months
with a data cutoff of November 19, 2024. The complete response (“CR”) rate across BCG exposures was 72% (13/18) at six months
and 70% (14/20) at any time, with 100% (9/9) of patients maintaining a CR from three months to six months. In addition, two of three patients
maintained a CR at nine months. In the pivotal cohort of the ADVANCED-2 trial in BCG-Unresponsive patients, the CR rate was 100% (4/4)
at six-months and 80% (4/5) at any time. In the proof-of-concept cohort of BCG-Naïve patients, the CR rate was 64% (9/14) at six
months and 67% (10/15) at any time.
To date, TARA-002 has demonstrated a favorable
safety and tolerability profile with no Grade 2 or greater treatment-related adverse events, and no patients discontinued treatment due
to adverse events. The majority of adverse events were Grade 1 and transient, and the most common adverse events were in line with typical
responses to bacterial immunopotentiation, such as flu-like symptoms. In addition, the most common urinary symptoms reflect urinary tract
instrumentation effects, such as bladder spasm, burning sensation, and urinary tract infection. Most bladder irritations resolved shortly
after administration or within a few hours to a few days.
Forward-Looking Statements
Statements contained in this press release regarding
matters that are not historical facts are “forward looking statements” within the meaning of the Private Securities Litigation
Reform Act of 1995. Protara may, in some cases, use terms such as “predicts,” “believes,” “potential,”
“proposed,” “continue,” “designed,” “estimates,” “anticipates,” “expects,”
“plans,” “intends,” “may,” “could,” “might,” “will,” “should”
or other words or expressions referencing future events, conditions or circumstances that convey uncertainty of future events or outcomes
to identify these forward-looking statements. Such forward-looking statements include but are not limited to, statements regarding Protara’s
intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things: Protara’s business strategy,
including its development plans for its product candidates and plans regarding the timing or outcome of existing or future clinical trials
(including reporting initial data from 12-month evaluable patients in mid-2025); statements related to expectations regarding interactions
with the FDA; Protara’s financial position; statements regarding the anticipated safety or efficacy of Protara’s product candidates;
and Protara’s outlook for the remainder of the year and future periods. Because such statements are subject to risks and uncertainties,
actual results may differ materially from those expressed or implied by such forward-looking statements. Factors that contribute to the
uncertain nature of the forward-looking statements include: risks that Protara’s financial guidance may not be as expected, as well
as risks and uncertainties associated with: Protara’s development programs, including the initiation and completion of non-clinical
studies and clinical trials and the timing of required filings with the FDA and other regulatory agencies; general market conditions;
changes in the competitive landscape; changes in Protara’s strategic and commercial plans; Protara’s ability to obtain sufficient
financing to fund its strategic plans and commercialization efforts; having to use cash in ways or on timing other than expected; the
impact of market volatility on cash reserves; failure to attract and retain management and key personnel; the impact of general U.S. and
foreign, economic, industry, market, regulatory, political or public health conditions; and the risks and uncertainties associated with
Protara’s business and financial condition in general, including the risks and uncertainties described more fully under the caption
“Risk Factors” and elsewhere in Protara’s filings and reports with the United States Securities and Exchange Commission.
All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management's
assumptions and estimates as of such date. Protara undertakes no obligation to update any forward-looking statements, whether as a result
of the receipt of new information, the occurrence of future events or otherwise, except as required by law.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto
duly authorized.
Date: December 5, 2024
| |
Protara Therapeutics, Inc. |
| |
|
|
| |
By: |
/s/ Patrick Fabbio |
| |
|
Patrick Fabbio |
| |
|
Chief Financial Officer |
Exhibit 99.1

ADVANCED - 2 TRIAL INTERIM RESULTS December 2024

FORWARD LOOKING STATEMENTS Statements contained in this presentation regarding matters that are not historical facts are "forward looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 . Protara may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “designed,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words or expressions referencing future events, conditions or circumstances that convey uncertainty of future events or outcomes to identify these forward - looking statements . Such forward - looking statements include but are not limited to, statements regarding Protara’s intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things : Protara’s business strategy, including its development plans for its product candidates and plans regarding the timing or outcome of existing or future clinical trials (including reporting initial data from 12 - month evaluable patients in mid - 2025 ) ; statements related to expectations regarding interactions with the FDA , Protara’s financial footing ; statements regarding the anticipated safety or efficacy of Protara’s product candidates ; and Protara’s outlook for the remainder of the year and future periods . Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward - looking statements . Factors that contribute to the uncertain nature of the forward - looking statements include : risks that Protara’s financial guidance may not be as expected, as well as risks and uncertainties associated with : Protara’s development programs, including the initiation and completion of non - clinical studies and clinical trials and the timing of required filings with the FDA and other regulatory agencies ; general market conditions ; changes in the competitive landscape ; changes in Protara’s strategic and commercial plans ; Protara’s ability to obtain sufficient financing to fund its strategic plans and development and commercialization efforts ; having to use cash in ways or on timing other than expected ; the impact of market volatility on cash reserves ; the loss of key members of management ; the impact of general U . S . and foreign, economic, industry, market, regulatory, political or public health conditions ; and the risks and uncertainties associated with Protara’s business and financial condition in general, including the risks and uncertainties described more fully under the caption “Risk Factors” and elsewhere in Protara's filings and reports with the United States Securities and Exchange Commission . All forward - looking statements contained in this presentation speak only as of the date on which they were made and are based on management's assumptions and estimates as of such date . Protara undertakes no obligation to update any forward - looking statements, whether as a result of the receipt of new information, the occurrence of future events or otherwise, except as required by law . 2 © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute
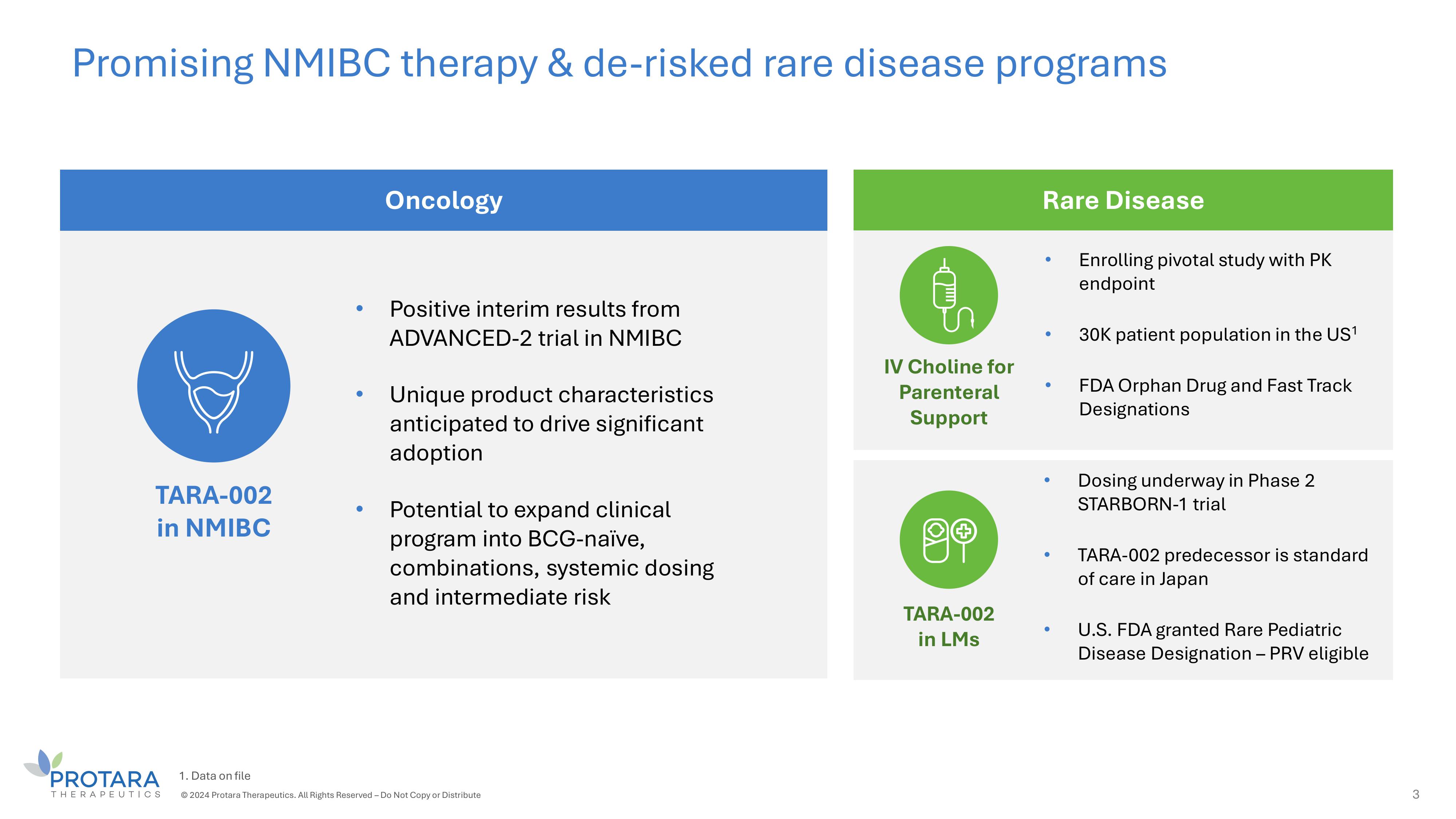
Promising NMIBC therapy C de - risked rare disease programs 3 TARA - 002 in NMIBC • Dosing underway in Phase 2 STARBORN - 1 trial • TARA - 002 predecessor is standard of care in Japan • U.S. FDA granted Rare Pediatric Disease Designation – PRV eligible • Positive interim results from ADVANCED - 2 trial in NMIBC • Unique product characteristics anticipated to drive significant adoption • Potential to expand clinical program into BCG - naïve, combinations, systemic dosing and intermediate risk Oncology Rare Disease TARA - 002 in LMs 1. Data on file © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute IV Choline for Parenteral Support • Enrolling pivotal study with PK endpoint • 30K patient population in the US 1 • FDA Orphan Drug and Fast Track Designations
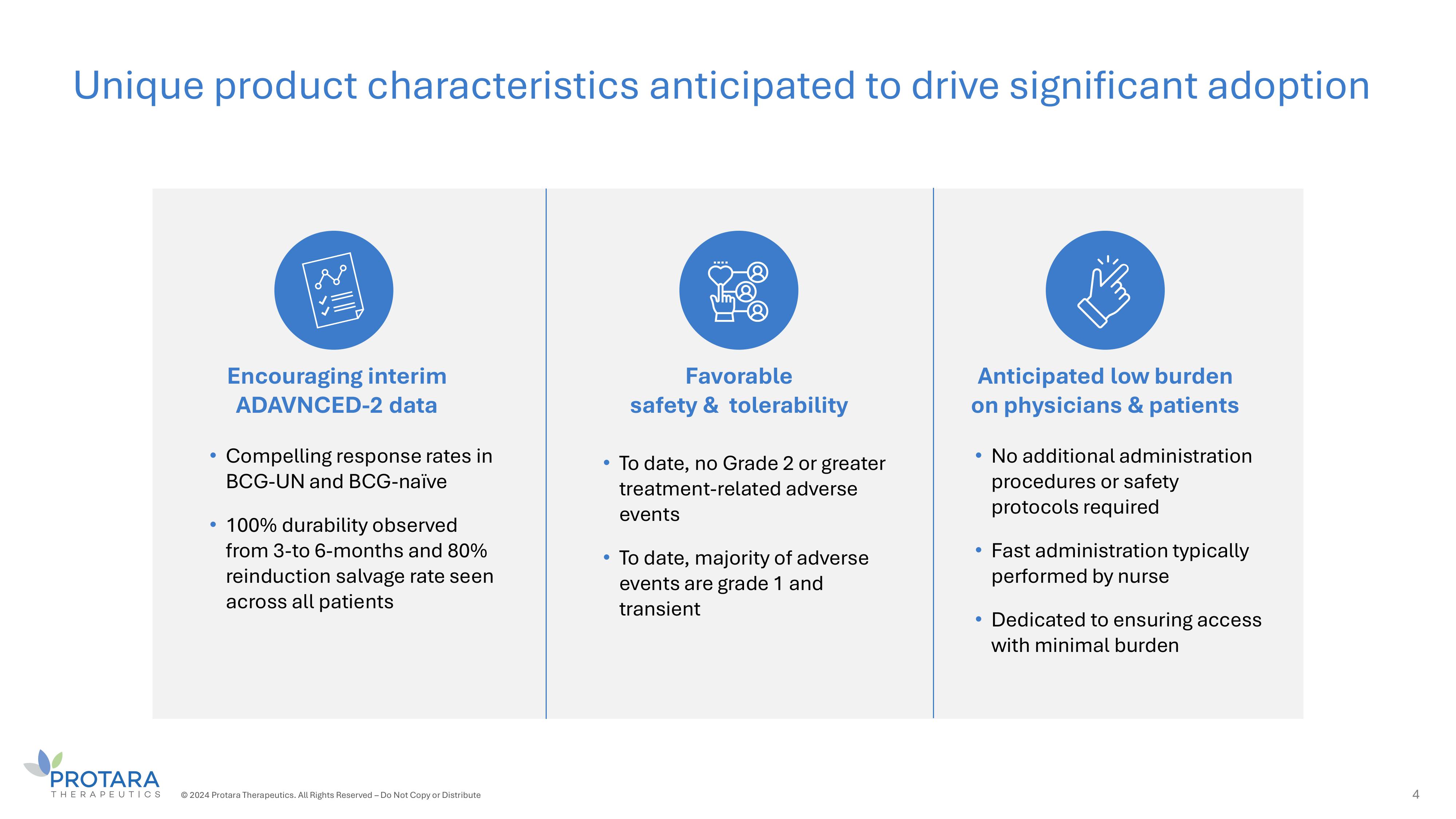
Anticipated low burden on physicians s patients Favorable safety s tolerability Encouraging interim ADAVNCED - 2 data • No additional administration procedures or safety protocols required • Fast administration typically performed by nurse • Dedicated to ensuring access with minimal burden • To date, no Grade 2 or greater treatment - related adverse events • To date, majority of adverse events are grade 1 and transient • Compelling response rates in BCG - UN and BCG - naïve • 100% durability observed from 3 - to 6 - months and 80% reinduction salvage rate seen across all patients Unique product characteristics anticipated to drive significant adoption 4 © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute
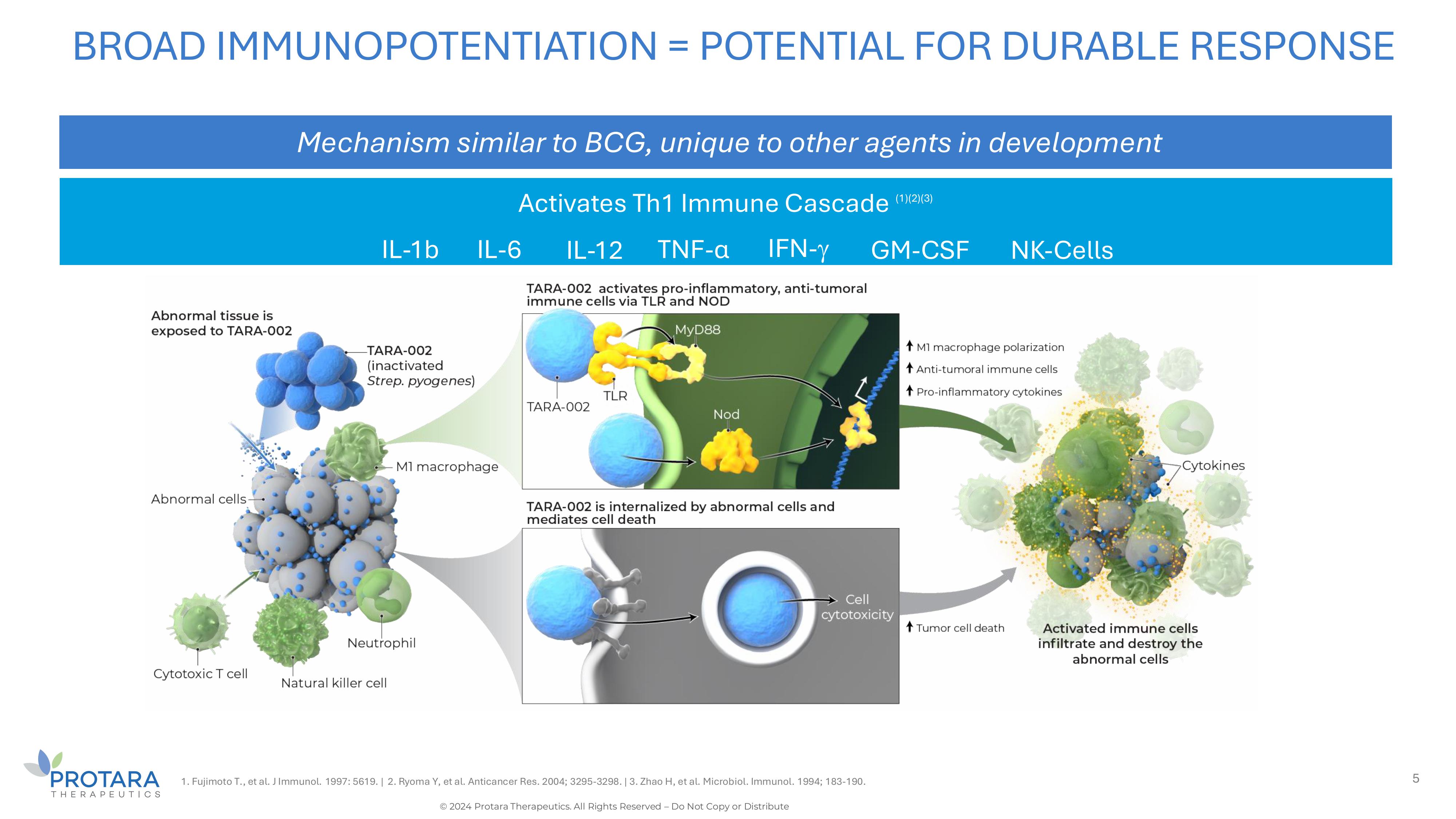
Activates Th1 Immune Cascade (1)(2)(3) BROAD IMMUNOPOTENTIATION = POTENTIAL FOR DURABLE RESPONSE Mechanism similar to BCG, unique to other agents in development 1. Fujimoto T., et al. J Immunol. 1997: 5619. | 2. Ryoma Y, et al. Anticancer Res. 2004; 3295 - 3298. | 3. Zhao H, et al. Microbiol. Immunol. 1994; 183 - 190. 5 IFN - IL - 1b NK - Cells IL - 6 IL - 12 TNF - α GM - CSF © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute
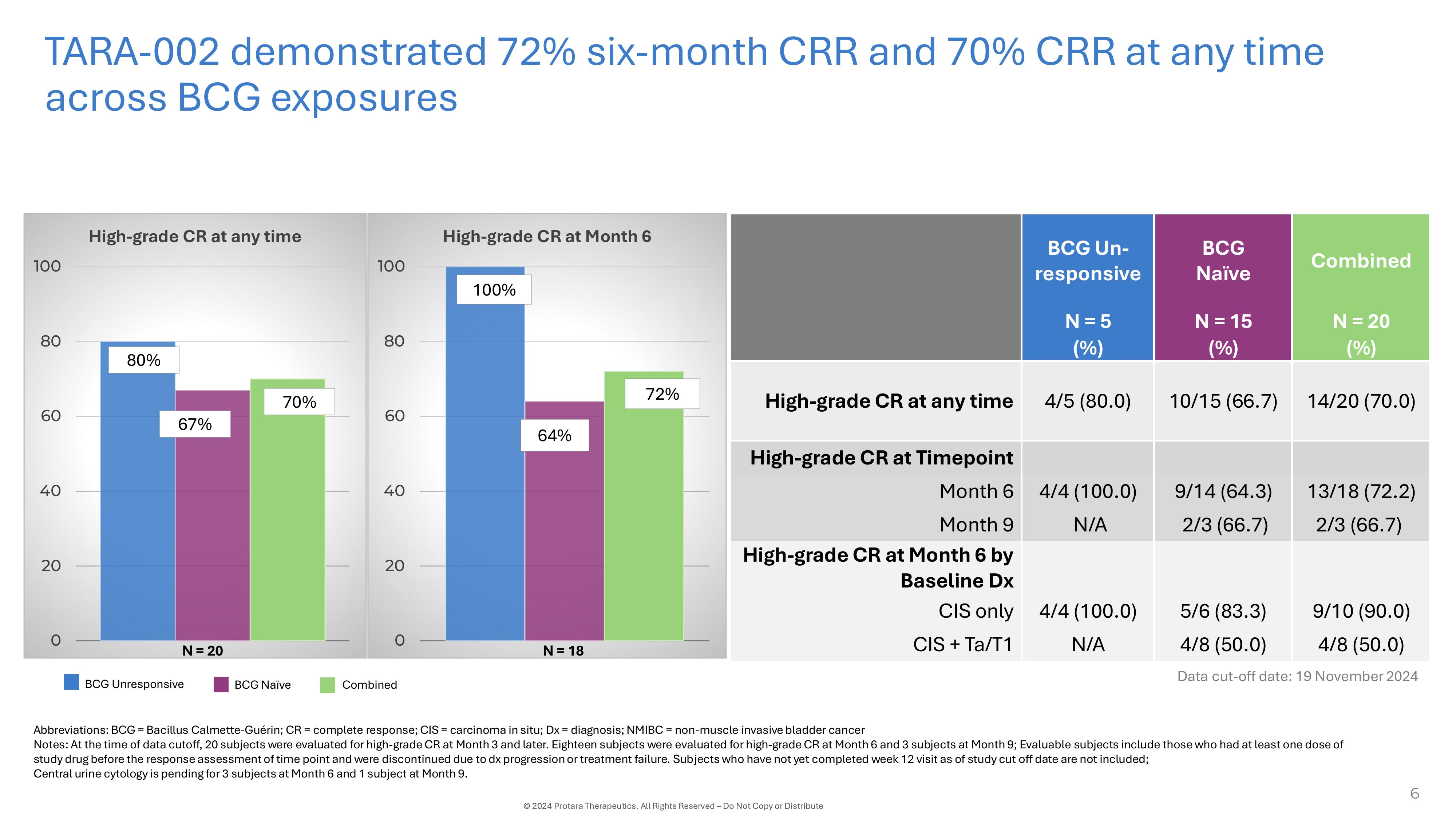
TARA - 002 demonstrated 72% six - month CRR and 70% CRR at any time across BCG exposures Abbreviations: BCG = Bacillus Calmette - Guérin; CR = complete response; CIS = carcinoma in situ; Dx = diagnosis; NMIBC = non - muscle invasive bladder cancer Notes: At the time of data cutoff, 20 subjects were evaluated for high - grade CR at Month 3 and later. Eighteen subjects were evaluated for high - grade CR at Month 6 and 3 subjects at Month 9; Evaluable subjects include those who had at least one dose of study drug before the response assessment of time point and were discontinued due to dx progression or treatment failure. Subjects who have not yet completed week 12 visit as of study cut off date are not included; Central urine cytology is pending for 3 subjects at Month 6 and 1 subject at Month 9. 6 © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute BCG Unresponsive BCG Naïve Combined Combined BCG Naïve BCG Un - responsive High - grade CR at Month 6 100 100% 80 72% 60 64% 40 20 0 N = 18 High - grade CR at any time 100 80 80% 70% 60 67% 40 20 0 N = 20 N = 20 N = 15 N = 5 (%) (%) (%) 14/20 (70.0) 10/15 (66.7) 4/5 (80.0) High - grade CR at any time High - grade CR at Timepoint 13/18 (72.2) 9/14 (64.3) 4/4 (100.0) Month 6 2/3 (66.7) 2/3 (66.7) N/A Month 9 High - grade CR at Month 6 by Baseline Dx 9/10 (90.0) 5/6 (83.3) 4/4 (100.0) CIS only 4/8 (50.0) 4/8 (50.0) N/A CIS + Ta/T1 Data cut - off date: 19 November 2024
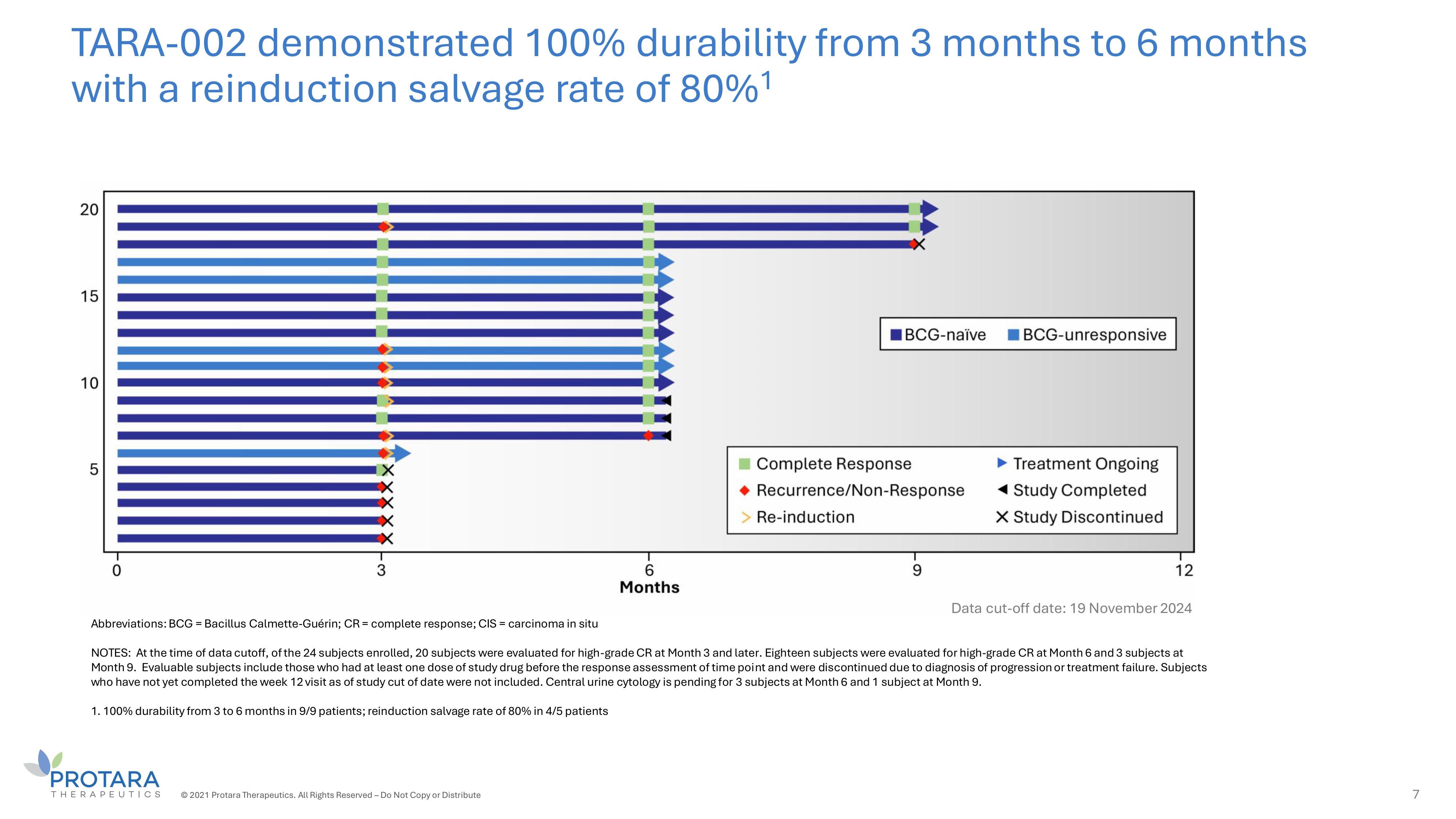
TARA - 002 demonstrated 100% durability from 3 months to 6 months with a reinduction salvage rate of 80% 1 © 2021 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute 7 Abbreviations: BCG = Bacillus Calmette - Guérin; CR = complete response; CIS = carcinoma in situ NOTES: At the time of data cutoff, of the 24 subjects enrolled, 20 subjects were evaluated for high - grade CR at Month 3 and later. Eighteen subjects were evaluated for high - grade CR at Month 6 and 3 subjects at Month 9. Evaluable subjects include those who had at least one dose of study drug before the response assessment of time point and were discontinued due to diagnosis of progression or treatment failure. Subjects who have not yet completed the week 12 visit as of study cut of date were not included. Central urine cytology is pending for 3 subjects at Month 6 and 1 subject at Month 9. 1. 100% durability from 3 to 6 months in 9/9 patients; reinduction salvage rate of 80% in 4/5 patients Data cut - off date: 19 November 2024
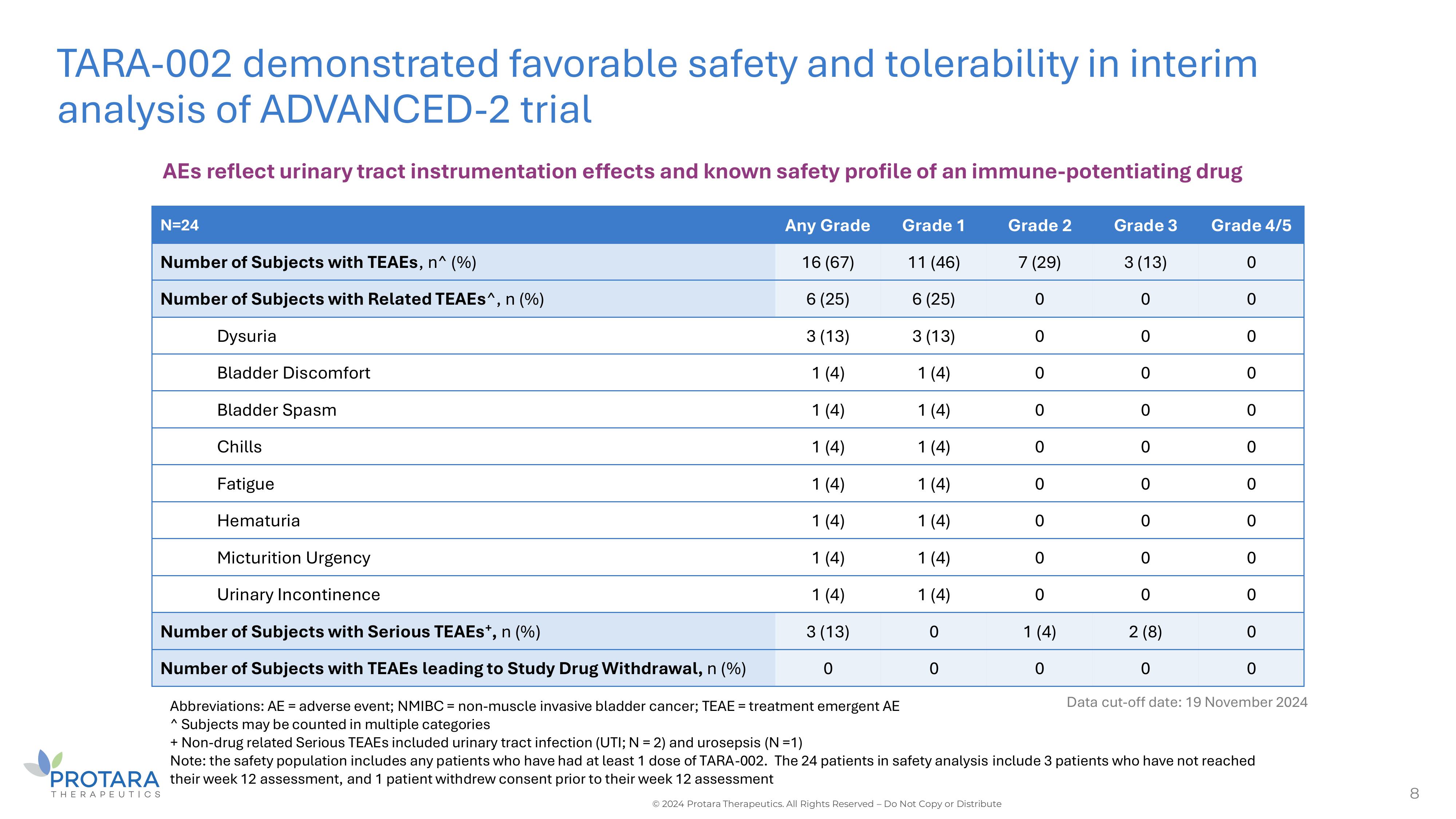
8 TARA - 002 demonstrated favorable safety and tolerability in interim analysis of ADVANCED - 2 trial Data cut - off date: 19 November 2024 AEs reflect urinary tract instrumentation effects and known safety profile of an immune - potentiating drug Abbreviations: AE = adverse event; NMIBC = non - muscle invasive bladder cancer; TEAE = treatment emergent AE ^ Subjects may be counted in multiple categories + Non - drug related Serious TEAEs included urinary tract infection (UTI; N = 2) and urosepsis (N =1) Note: the safety population includes any patients who have had at least 1 dose of TARA - 002. The 24 patients in safety analysis include 3 patients who have not reached their week 12 assessment, and 1 patient withdrew consent prior to their week 12 assessment © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Grade 4/5 Grade 3 Grade 2 Grade 1 Any Grade N=24 0 3 (13) 7 (29) 11 (46) 16 (67) Number of Subjects with TEAEs , n^ (%) 0 0 0 6 (25) 6 (25) Number of Subjects with Related TEAEs ^, n (%) 0 0 0 3 (13) 3 (13) Dysuria 0 0 0 1 (4) 1 (4) Bladder Discomfort 0 0 0 1 (4) 1 (4) Bladder Spasm 0 0 0 1 (4) 1 (4) Chills 0 0 0 1 (4) 1 (4) Fatigue 0 0 0 1 (4) 1 (4) Hematuria 0 0 0 1 (4) 1 (4) Micturition Urgency 0 0 0 1 (4) 1 (4) Urinary Incontinence 0 2 (8) 1 (4) 0 3 (13) Number of Subjects with Serious TEAEs + , n (%) 0 0 0 0 0 Number of Subjects with TEAEs leading to Study Drug Withdrawal, n (%)
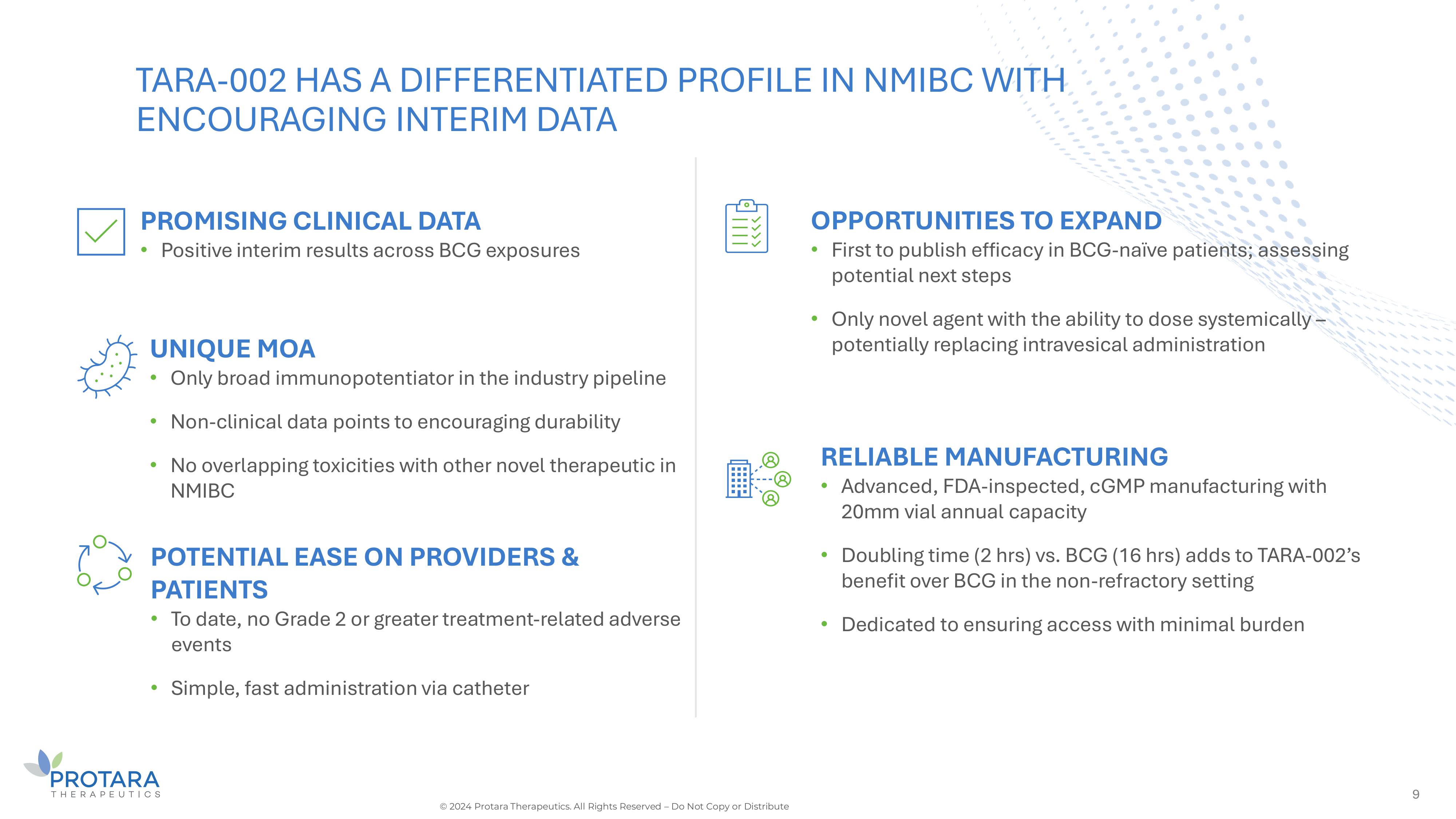
9 UNIQUE MOA • Only broad immunopotentiator in the industry pipeline • Non - clinical data points to encouraging durability • No overlapping toxicities with other novel therapeutic in NMIBC POTENTIAL EASE ON PROVIDERS s PATIENTS • To date, no Grade 2 or greater treatment - related adverse events • Simple, fast administration via catheter OPPORTUNITIES TO EXPAND • First to publish efficacy in BCG - naïve patients; assessing potential next steps • Only novel agent with the ability to dose systemically – potentially replacing intravesical administration TARA - 002 HAS A DIFFERENTIATED PROFILE IN NMIBC WITH ENCOURAGING INTERIM DATA PROMISING CLINICAL DATA • Positive interim results across BCG exposures RELIABLE MANUFACTURING • Advanced, FDA - inspected, cGMP manufacturing with 20mm vial annual capacity • Doubling time (2 hrs) vs. BCG (16 hrs) adds to TARA - 002’s benefit over BCG in the non - refractory setting • Dedicated to ensuring access with minimal burden © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute

QCA © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute 10

APPENDIX
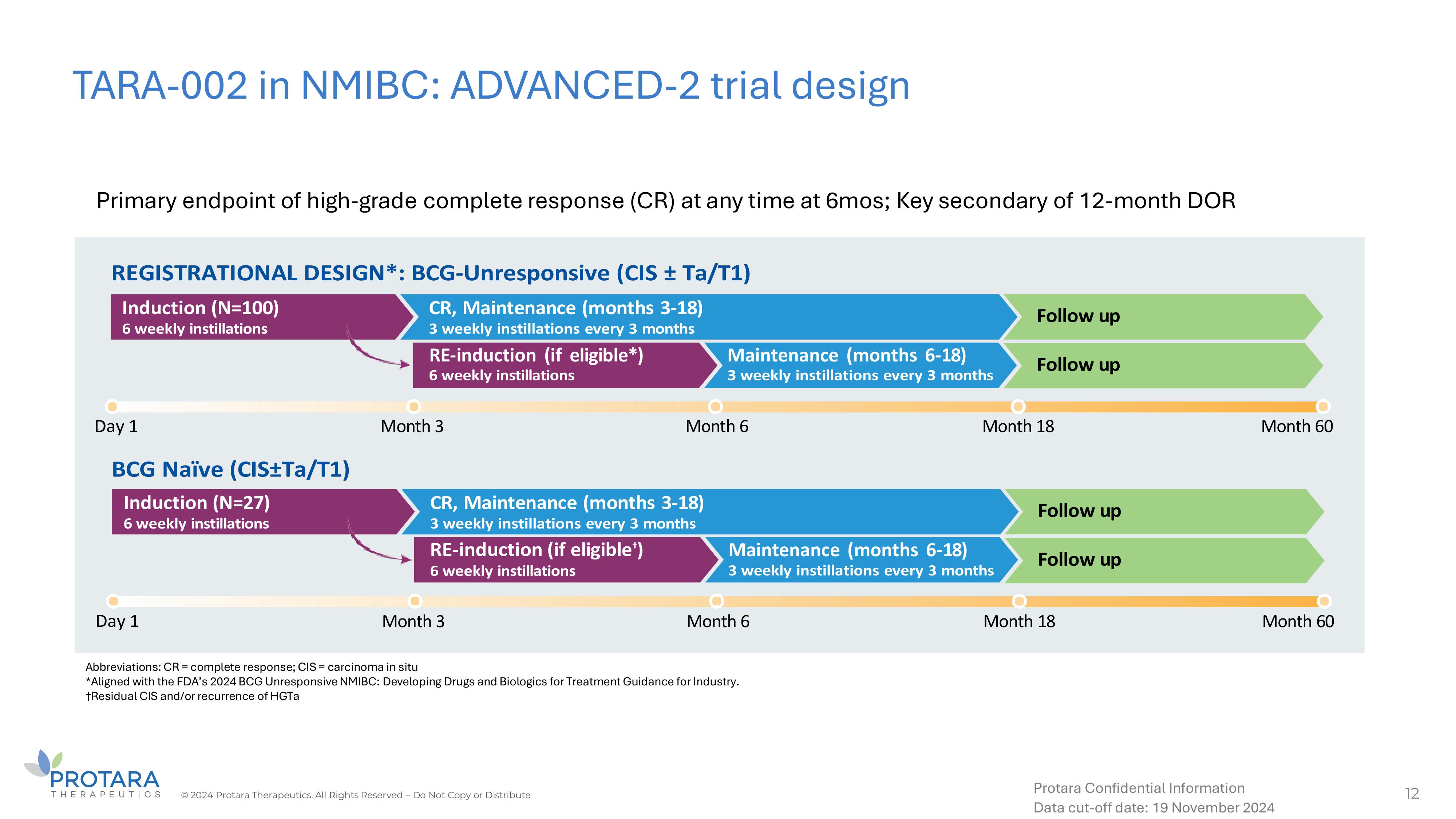
TARA - 002 in NMIBC: ADVANCED - 2 trial design 12 © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Protara Confidential Information Data cut - off date: 19 November 2024 RE - induction (if eligible*) Maintenance (months 6 - 18) Month 3 Month 6 Month 18 Month 60 Maintenance (months 6 - 18) Month 18 Month 60 Month 3 Month 6 Abbreviations: CR = complete response; CIS = carcinoma in situ *Aligned with the FDA’s 2024 BCG Unresponsive NMIBC: Developing Drugs and Biologics for Treatment Guidance for Industry. †Residual CIS and/or recurrence of HGTa Primary endpoint of high - grade complete response (CR) at any time at 6mos; Key secondary of 12 - month DOR
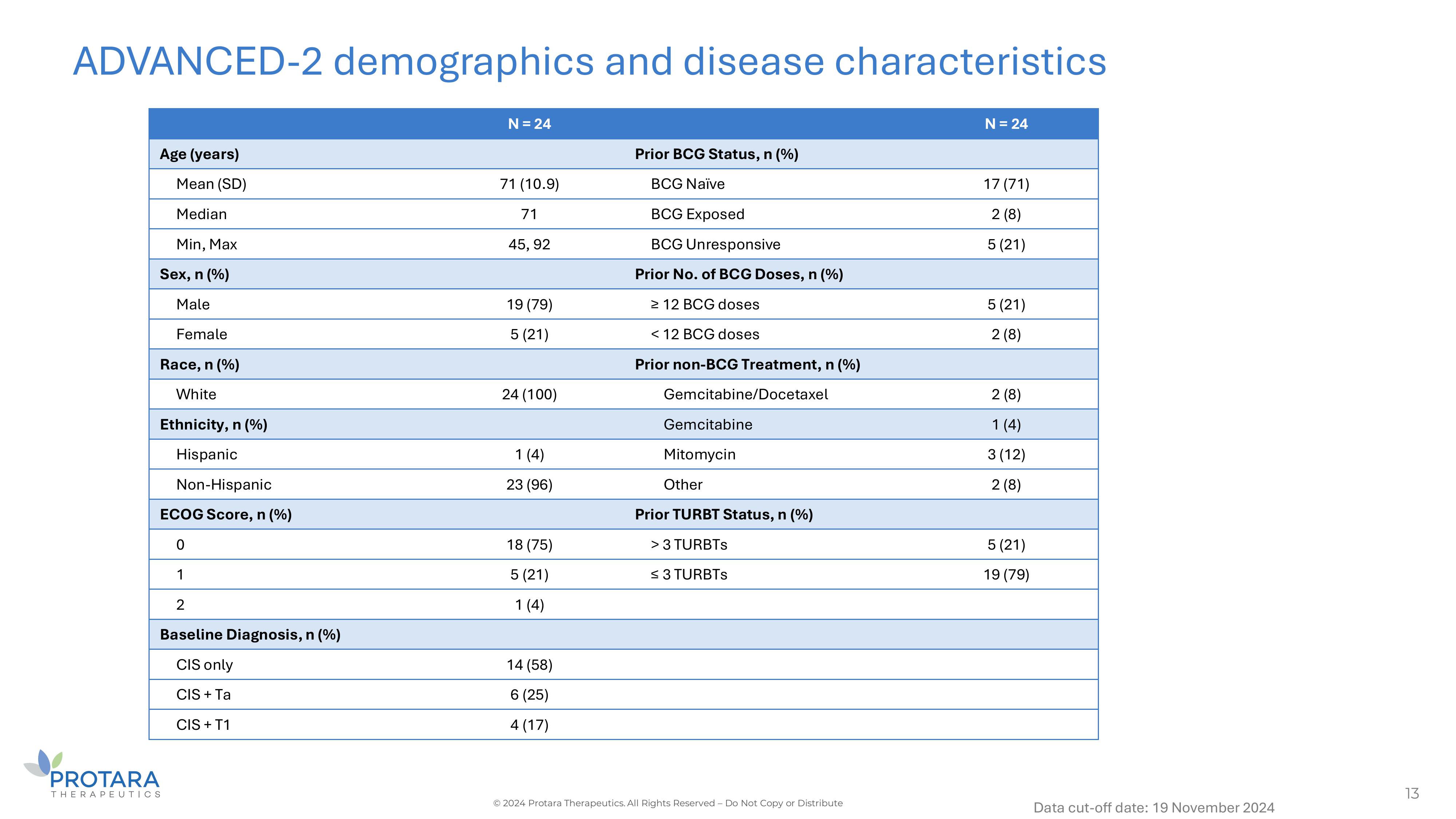
ADVANCED - 2 demographics and disease characteristics 13 © 2024 Protara Therapeutics. All Rights Reserved – Do Not Copy or Distribute Data cut - off date: 19 November 2024 N = 24 N = 24 Prior BCG Status, n (%) Age (years) 17 (71) BCG Naïve 71 (10.9) Mean (SD) 2 (8) BCG Exposed 71 Median 5 (21) BCG Unresponsive 45, 92 Min, Max Prior No. of BCG Doses, n (%) Sex, n (%) 5 (21) ≥ 12 BCG doses 19 (79) Male 2 (8) < 12 BCG doses 5 (21) Female Prior non - BCG Treatment, n (%) Race, n (%) 2 (8) Gemcitabine/Docetaxel 24 (100) White 1 (4) Gemcitabine Ethnicity, n (%) 3 (12) Mitomycin 1 (4) Hispanic 2 (8) Other 23 (96) Non - Hispanic Prior TURBT Status, n (%) ECOG Score, n (%) 5 (21) > 3 TURBTs 18 (75) 0 19 (79) ≤ 3 TURBTs 5 (21) 1 1 (4) 2 Baseline Diagnosis, n (%) 14 (58) CIS only 6 (25) CIS + Ta 4 (17) CIS + T1
Exhibit 99.2

Protara Announces Positive Results
from the Ongoing Phase 2 ADVANCED-2 Trial of TARA-002 in Patients with NMIBC
| ● | TARA-002
demonstrates 72% six-month landmark complete response rate and 70% complete response rate
at any time across BCG exposures |
| ● | 100%
six-month landmark complete response rate and 80% complete response rate at any time observed
in BCG-Unresponsive patients |
| ● | 64%
six-month landmark complete response rate and 67% complete response rate at any time observed
in BCG-Naïve patients |
| ● | 80%
reinduction salvage rate and compelling durability observed with 100% of patients maintaining
a complete response from three months to six months across BCG exposures |
| ● | Favorable
safety and tolerability profile with no Grade 2 or greater treatment-related adverse events |
| ● | Company
to host conference call and webcast today at 8:30 a.m. ET |
NEW YORK, December 5, 2024 (GLOBE NEWSWIRE) --
Protara Therapeutics, Inc. (Nasdaq: TARA), a clinical-stage company developing transformative therapies for the treatment of cancer and
rare diseases, today announced results from its ongoing Phase 2 open-label ADVANCED-2 trial. The trial is assessing intravesical TARA-002,
the Company’s investigational cell-based therapy, in high-risk Non-Muscle Invasive Bladder Cancer (NMIBC) patients with carcinoma
in situ or CIS (± Ta/T1) who are Bacillus Calmette-Guérin (BCG)-Unresponsive or BCG-Naïve. The complete response (CR)
rate across BCG exposures was 72% (13/18) at six months and 70% (14/20) at any time with 100% (9/9) of patients maintaining a CR from
three months to six months. In addition, two of three patients maintained a CR at nine months. These results will be featured today during
a poster session at the 25th Annual Meeting of the Society of Urologic Oncology (SUO) in Dallas, Texas.
The dataset includes 20 patients who were evaluable
at three months, 18 patients who were evaluable at six months and three patients who were evaluable at nine months with a data cutoff
of November 19, 2024. In the pivotal cohort of the ADVANCED-2 trial in BCG-Unresponsive patients, the CR rate was 100% (4/4) at six-months
and 80% (4/5) at any time. In the proof-of-concept cohort of BCG-Naïve patients, the CR rate was 64% (9/14) at six months and 67%
(10/15) at any time. TARA-002 demonstrated a favorable safety and tolerability profile with no Grade 2 or greater treatment-related adverse
events (TRAEs), and no patients discontinued due to adverse events.
“These impressive TARA-002 results demonstrate
meaningful activity in a difficult to treat patient population,” said Brian Mazzarella, MD, Vice President of Research for Urology
America, and ADVANCED-2 study investigator. “The activity of TARA-002 across BCG exposures, coupled with its ease of use and low
procedural burden for physicians, make it an exciting potential treatment option for NMIBC patients.”
“We are thrilled with these positive six-month
data, which reinforce TARA-002’s potential in NMIBC, while offering a compelling product profile for physicians and patients,”
said Jesse Shefferman, Chief Executive Officer of Protara Therapeutics. “We believe these encouraging data together with our international
site expansion will accelerate patient enrollment, and we look forward to reporting initial data from 12-month evaluable patients in mid-2025.”

The majority of adverse events were Grade 1 and
transient with no Grade 2 or greater TRAEs as assessed by study investigators. No patients discontinued treatment due to adverse events.
The most common adverse events were in line with typical responses to bacterial immunopotentiation, such as flu-like symptoms. The most
common urinary symptoms reflect urinary tract instrumentation effects, such as bladder spasm, burning sensation, and urinary tract infection.
Most bladder irritations resolved shortly after administration or within a few hours to a few days.
Conference Call and Webcast
Protara will host a conference call and webcast
to discuss the data today at 8:30 am ET. The live call can be accessed by registering as a participant here.
Upon registration, participants will receive conference call dial-in information. A live webcast of the event can be accessed by
visiting the Events and Presentations section of the Company’s website: https://ir.protaratx.com. The webcast will be archived for
a limited time following the presentation.
About ADVANCED-2
The Phase 2 open-label ADVANCED-2 trial is assessing
intravesical TARA-002 in NMIBC patients with carcinoma in situ or CIS (± Ta/T1) who are Bacillus Calmette-Guérin (BCG)-Unresponsive
(n≈100) and BCG-Naïve (n=27). The BCG-Unresponsive cohort has been designed to be registrational in alignment with the FDA’s
2024 BCG-Unresponsive Non-muscle Invasive Bladder Cancer: Developing Drugs and Biological Products for Treatment Draft Guidance for Industry.
About TARA-002
TARA-002 is an investigational cell therapy in
development for the treatment of NMIBC and of LMs, for which it has been granted Rare Pediatric Disease Designation by the U.S. Food and
Drug Administration. TARA-002 was developed from the same master cell bank of genetically distinct group A Streptococcus pyogenes as OK-432,
a broad immunopotentiator marketed as Picibanil® in Japan and approved in Taiwan by Chugai Pharmaceutical Co., Ltd. Protara has successfully
shown manufacturing comparability between TARA-002 and OK-432.
When TARA-002 is administered, it is hypothesized
that innate and adaptive immune cells within the cyst or tumor are activated and produce a pro-inflammatory response with release of cytokines
such as tumor necrosis factor (TNF)-alpha, interferon (IFN)-gamma, IL-1b, IL-6, IL-12, granulocyte-macrophage colony-stimulating factor
(GM-CSF) and natural killer cells. TARA-002 also directly kills tumor cells and triggers a host immune response by inducing immunogenic
cell death, which further enhances the antitumor immune response.
About Non-Muscle Invasive Bladder Cancer (NMIBC)
Bladder cancer is the sixth most common cancer
in the United States, with NMIBC representing approximately 80% of bladder cancer diagnoses. Approximately 65,000 patients are diagnosed
with NMIBC in the United States each year. NMIBC is cancer found in the tissue that lines the inner surface of the bladder that has not
spread into the bladder muscle.

About Protara Therapeutics, Inc.
Protara is a clinical-stage biotechnology company
committed to advancing transformative therapies for people with cancer and rare diseases. Protara’s portfolio includes its lead
candidate, TARA-002, an investigational cell-based therapy in development for the treatment of non-muscle invasive bladder cancer (NMIBC)
and lymphatic malformations (LMs). The Company is evaluating TARA-002 in an ongoing Phase 2 trial in NMIBC patients with carcinoma in
situ (CIS) who are unresponsive or naïve to treatment with Bacillus Calmette-Guérin (BCG), as well as a Phase 2 trial in pediatric
patients with LMs. Additionally, Protara is developing IV Choline Chloride, an investigational phospholipid substrate replacement for
patients on parenteral nutrition who are otherwise unable to meet their choline needs via oral or enteral routes. For more information,
visit www.protaratx.com.
Forward-Looking Statements
Statements contained in this press release regarding
matters that are not historical facts are "forward looking statements" within the meaning of the Private Securities Litigation
Reform Act of 1995. Protara may, in some cases, use terms such as “predicts,” “believes,” “potential,”
“proposed,” “continue,” “designed,” “estimates,” “anticipates,” “expects,”
“plans,” “intends,” “may,” “could,” “might,” “will,” “should”
or other words or expressions referencing future events, conditions or circumstances that convey uncertainty of future events or outcomes
to identify these forward-looking statements. Such forward-looking statements include but are not limited to, statements regarding Protara’s
intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things: Protara’s business strategy,
including its development plans for its product candidates and plans regarding the timing or outcome of existing or future clinical trials
(including reporting initial data from 12-month evaluable patients in mid-2025); statements related to expectations regarding interactions
with the FDA; Protara’s financial position; statements regarding the anticipated safety or efficacy of Protara’s product candidates;
and Protara’s outlook for the remainder of the year and future periods. Because such statements are subject to risks and uncertainties,
actual results may differ materially from those expressed or implied by such forward-looking statements. Factors that contribute to the
uncertain nature of the forward-looking statements include: risks that Protara’s financial guidance may not be as expected, as well
as risks and uncertainties associated with: Protara’s development programs, including the initiation and completion of non-clinical
studies and clinical trials and the timing of required filings with the FDA and other regulatory agencies; general market conditions;
changes in the competitive landscape; changes in Protara’s strategic and commercial plans; Protara’s ability to obtain sufficient
financing to fund its strategic plans and commercialization efforts; having to use cash in ways or on timing other than expected; the
impact of market volatility on cash reserves; failure to attract and retain management and key personnel; the impact of general U.S. and
foreign, economic, industry, market, regulatory, political or public health conditions; and the risks and uncertainties associated with
Protara’s business and financial condition in general, including the risks and uncertainties described more fully under the caption
“Risk Factors” and elsewhere in Protara's filings and reports with the United States Securities and Exchange Commission. All
forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management's
assumptions and estimates as of such date. Protara undertakes no obligation to update any forward-looking statements, whether as a result
of the receipt of new information, the occurrence of future events or otherwise, except as required by law.
Company Contact:
Justine O'Malley
Protara Therapeutics
Justine.OMalley@protaratx.com
646-817-2836
3
Exhibit 99.3

Presented at the 2024 Annual Meeting of the Society of Urologic Oncology, December 4 - C, 2024; Dallas, Texas ADVANCED - 2: Phase 2 Open - label Study to Evaluate Safety and Anti - tumor Activity of Intravesical Instillation of TARA - 002 in Adults with High - grade Non - muscle Invasive Bladder Cancer Brian Mazzarella 1 , Gautam Jayram 2 , Neal Shore 3 , Jacqueline Zummo 4 , Wei Sun 4 , Khushboo Belani 4 , Eppie Brown 4 , Brian Desch 4 , McKenna Metcalf 4 , Andrea DiFiglia 4 , Chen Ǫuin Lam 5 , Eugene Kramolowsky 6 on behalf of ADVANCED - 2 Investigators 1 Urology Austin, Austin, TX, United States; 2 Urology Associates P.C., Nashville, TN, United States; 3 Carolina Urologic Research Center, Myrtle Beach, SC, United States; 4 Protara Therapeutics, Inc., New York, NY, United States; 5 Pharmapace Inc., San Diego, CA, United States; 6 Virginia Urology, Richmond, VA, United States INTRODUCTION • With the current Bacillus Calmette - Guérin (BCG) shortage and limited effective alternative therapies, there remains a significant unmet need for treatment options for patients with non - muscle invasive bladder cancer (NMIBC). • TARA - 002 is a broad spectrum immune potentiator that elicits a TH1 pro - inflammatory cytokine response. • TARA - 002 is a lyophilized biological preparation for instillation containing cells of Streptococcus pyogenes (Group A, type 3) Su strain treated with benzylpenicillin and is being developed for the treatment of high - grade NMIBC. DISCLOSURE: This study is funded by Protara Therapeutics, Inc. #119 METHODS FIGURE 1. ADVANCED - 2 STUDY SCHEMA Follow up Follow up REGISTRATIONAL DESIGN*: BCG - unresponsive (CIS “ Ta/T1) Induction (N=100) CR, Maintenance (months 3 - 18) 6 weekly instillations 3 weekly instillations every 3 months Re - induction (if eligible*) Maintenance (months 6 - 18) 6 weekly instillations 3 weekly instillations every 3 months Month 3 Month 6 Month 18 Month 60 Day 1 BCG - naïve (CIS “ Ta/T1) Induction (N=27) 6 weekly instillations Follow up Follow up CR, Maintenance (months 3 - 18) 3 weekly instillations every 3 months Re - induction (if eligible † ) 6 weekly instillations Maintenance (months 6 - 18) 3 weekly instillations every 3 months Day 1 Month 3 Month 6 Month 18 Month 60 Abbreviations: BCG = Bacillus Calmette - Guérin; CR = complete response; CIS = carcinoma in situ ; FDA = Food and Drug Administration; HG = high - grade *Aligned with the FDA’s 2024 BCG - Unresponsive NMIBC: Developing Drugs and Biologics for Treatment Guidance for Industry. †Residual CIS and/or recurrence of HGTa • ADVANCED - 2 (NCT05951179) is an ongoing, actively enrolling, Phase 2, open - label study to evaluate the safety and efficacy of intravesical instillation of TARA - 002 (40 KE) in adults ≥ 18 years with BCG - unresponsive and BCG - naïve (never exposed and those who have not received intravesical BCG for at least 24 months prior to the most recent CIS diagnosis) CIS NMIBC ( “ Ta/T1) with active disease ( Figure 1 ). • The primary endpoint is high - grade complete response (CR) at any time up to Month 6 . Key secondary endpoint is durability of response at Month 12 . BCG Unresponsive BCG Naïve N =5 Learn more about our study: NCT05G5117G Contact us at clinicaltrials@protaratx.com for more information. TABLE 1. SUMMARY OF TREATMENT EMERGENT ADVERSE EVENTS IN ALL SUBJECTS Grade 4/5 Grade 3 Grade 2 Grade 1 Any Grade N = 24 0 3 (13) 7 (29) 11 (46) 16 (67) Subjects with TEAEs, n^ (%) 0 0 0 6 (25) 6 (25) Subjects with Related TEAEs^*, n (%) 0 2 (8) 1 (4) 0 3 (13) Subjects with Serious TEAEs † , n (%) 0 0 0 0 0 Subjects with TEAEs leading to Study Drug Withdrawal, n (%) RESULTS FIGURE 2. FIRST RESULTS OF ADVANCED - 2: 70% CR AT ANY TIME IN ALL SUBJECTS TAR FIGURE 3. FIRST RESULTS FROM ADVANCED - 2: MONTHS A - 002 MONOTHERAPY DEMONSTRATED 72% CR AT 6 Swimmers' Plot on Response Assessment High - grade CR High - grade CR BCG - BCG - C C o o m m b b i n i n e e d d High - grade Complete Response (N=20) 100 at any time 100 at Month 6 unresponsive naïve 100% 20 N = 5 N = 15 NN==2200 80 80% 80 High - grade CR at any time, n (%) 4/5 (80.0) 10/15 (66.7) 14/20 (70.0) 67% 70% 72% High - grade CR at Timepoint, n (%) 64% 15 60 60 Month 6 4/4 (100.0) 9/14 (64.3) 13/18 (72.2) Month 9 0 2/3 (66.7) 2/3 (66.7) BCG - naïve BCG - unresponsive High - grade CR at any time by 40 40 Baseline Diagnosis, n (%) 10 CIS only 4/5 (80.0) 6/7 (85.7) 10/12 (83.3) 20 20 CIS + Ta/T1 0 4/8 (50.0) 4/8 (50.0) 5 Complete Response Treatment Ongoing High - grade CR at Month 6 by Recurrence/Non - Response Study Completed 0 N = 20 0 N = 18 Baseline Diagnosis, n (%) Re - induction Study Discontinued BCG - unresponsive BCG - naïve Combined CIS only 4/4 (100.0) 5/6 (83.3) 9/10 (90.0) CIS + Ta/T1 0 4/8 (50.0) 4/8 (50.0) 0 3 6 9 12 Months Safety Abbreviations: BCG = Bacillus Calmette - Guérin; CR = complete response; CIS = carcinoma in situ NOTES: At the time of data cutoff, of the 24 subjects enrolled, 20 subjects were evaluated for high - grade CR at Month 3 and later. Eighteen subjects were evaluated for high - grade CR at Month 6 and 3 subjects at Month 9. Evaluable subjects include those who had at least one dose of study drug before the response assessment of time point and were discontinued due to diagnosis of progression or treatment failure. Subjects who have not yet completed the visit time point were not included. Central urine cytology is pending for 3 subjects at Month 6 and 1 subject at Month 9. Baseline Response Assessment Characteristics • The rates of high - grade CR at any time were Abbreviations: AE = adverse event; TEAE = treatment emergent AE ^ Subjects may be counted in multiple categories; *TEAEs assessed by the Investigator as study drug related included dysuria, urinary incontinence, fatigue, chills, • All 24 enrolled subjects were 70% (14 of 20) overall, 80% (4 of 5) for BCG - bladder discomfort, bladder spasm, hematuria and micturition urgency. All were Grade 1; † Non - drug related Serious TEAEs included urinary tract infection (UTI; N = 2) and urosepsis (N =1). white, and the majority were non - unresponsive subjects, and 67% (10 of 15) Hispanic (96%, 23 of 24) and for BCG - naïve subjects ( Figure 2, Figure 3 ). • Majority of TEAEs were Grade 1 and transient. No subjects experienced drug - male (79%, 19 of 24). The median • The rates of high - grade CR at Month 6 related serious AEs (SAEs) or TEAEs leading to withdrawal or death ( Table 1 ). age was 71 years. were 72% (13 of 18) overall, 100% (4 of 4) • Common AEs reflect urinary tract instrumentation effects, such as bladder • Eastern Cooperative Oncology for BCG - unresponsive subjects, and 64% spasm, burning sensation, and UTI. Group (ECOG) Score was 0 for (9 of 14) for BCG - naïve subjects ( Figure 3 ). • AEs were consistent with the known safety profile of an immune - potentiating 75% (18 of 24) of subjects. • 100% (9 of 9) of subjects who were drug, such as flu - like symptoms. CONCLUSIONS • TARA - 002 appears to be well tolerated with encouraging efficacy and durability of response in subjects with high - risk NMIBC with CIS. • Further study is planned to understand the potential of TARA - 002 in both the BCG - naïve and BCG - unresponsive population. • Majority of patients had a complete responders at Month 3, and baseline diagnosis of CIS continued the study, maintained response only (58%, 14 of 24), and 25% through Month 6. (6 of 24) and 17% (4 of 24) had • Of the 5 subjects who did not achieve initial CIS + Ta and CIS + T1 disease, CR and received re - induction, the CR rate respectively. at Month 6 was 80% (4 of 5).
v3.24.3
Cover
|
Dec. 05, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Dec. 05, 2024
|
| Entity File Number |
001-36694
|
| Entity Registrant Name |
Protara Therapeutics, Inc.
|
| Entity Central Index Key |
0001359931
|
| Entity Tax Identification Number |
20-4580525
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
345 Park Avenue South
|
| Entity Address, Address Line Two |
Third Floor
|
| Entity Address, City or Town |
New York
|
| Entity Address, State or Province |
NY
|
| Entity Address, Postal Zip Code |
10010
|
| City Area Code |
646
|
| Local Phone Number |
844-0337
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.001 per share
|
| Trading Symbol |
TARA
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Protara Therapeutics (NASDAQ:TARA)
Historical Stock Chart
From Nov 2024 to Dec 2024
Protara Therapeutics (NASDAQ:TARA)
Historical Stock Chart
From Dec 2023 to Dec 2024