TScan Gets FDA Clearance of Investigational New Drug Application for Cancer Treatment
29 August 2023 - 10:02PM
Dow Jones News
By Chris Wack
TScan Therapeutics said Tuesday that the U.S. Food and Drug
Administration has cleared its investigational new drug application
for TSC-203-A0201, a TCR-T targeting preferentially expressed
antigen in melanoma.
The clinical-stage biopharmaceutical company said TSC-203-A0201
is the fourth TCR-T cleared for clinical development in the
company's solid tumor program.
TScan said it is on-track to treat the first patient with a
TScan TCR-T and report preliminary data by the end of 2023.
TScan shares were up 8% to $2.37 in premarket trading.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
August 29, 2023 07:47 ET (11:47 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
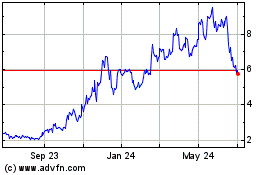
TScan Therapeutics (NASDAQ:TCRX)
Historical Stock Chart
From Apr 2024 to May 2024
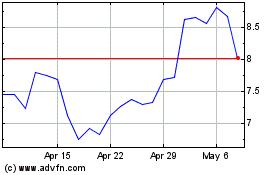
TScan Therapeutics (NASDAQ:TCRX)
Historical Stock Chart
From May 2023 to May 2024