Statement of Changes in Beneficial Ownership (4)
02 November 2019 - 8:05AM
Edgar (US Regulatory)
|
FORM 4
[ ]
Check this box if no longer subject to Section 16. Form 4 or Form 5 obligations may continue. See Instruction 1(b).
|
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
STATEMENT OF CHANGES IN BENEFICIAL OWNERSHIP OF SECURITIES
|
OMB APPROVAL
OMB Number:
3235-0287
Estimated average burden
hours per response...
0.5
|
|
Filed pursuant to Section 16(a) of the Securities Exchange Act of 1934 or Section 30(h) of the Investment Company Act of 1940
|
|
|
1. Name and Address of Reporting Person
*
Scholefield James H |
2. Issuer Name and Ticker or Trading Symbol
Merck & Co., Inc.
[
MRK
]
|
5. Relationship of Reporting Person(s) to Issuer
(Check all applicable)
_____ Director _____ 10% Owner
__X__ Officer (give title below) _____ Other (specify below)
EVP,Chief Info&Digital Officer
|
|
(Last)
(First)
(Middle)
MERCK & CO., INC., 2000 GALLOPING HILL ROAD |
3. Date of Earliest Transaction
(MM/DD/YYYY)
10/30/2019
|
|
(Street)
KENILWORTH, NJ 07033
(City)
(State)
(Zip)
|
4. If Amendment, Date Original Filed
(MM/DD/YYYY)
|
6. Individual or Joint/Group Filing
(Check Applicable Line)
_X
_ Form filed by One Reporting Person
___ Form filed by More than One Reporting Person
|
Table I - Non-Derivative Securities Acquired, Disposed of, or Beneficially Owned
|
1.Title of Security
(Instr. 3)
|
2. Trans. Date
|
2A. Deemed Execution Date, if any
|
3. Trans. Code
(Instr. 8)
|
4. Securities Acquired (A) or Disposed of (D)
(Instr. 3, 4 and 5)
|
5. Amount of Securities Beneficially Owned Following Reported Transaction(s)
(Instr. 3 and 4)
|
6. Ownership Form: Direct (D) or Indirect (I) (Instr. 4)
|
7. Nature of Indirect Beneficial Ownership (Instr. 4)
|
|
Code
|
V
|
Amount
|
(A) or (D)
|
Price
|
|
Common Stock
|
10/30/2019
|
|
M
|
|
11436.0000
|
A
|
$0.0000
|
11436.0000
|
D
|
|
|
Common Stock
|
10/30/2019
|
|
F
|
|
4294.0000
|
D
|
$85.1000
|
7142.0000
|
D
|
|
Table II - Derivative Securities Beneficially Owned (e.g., puts, calls, warrants, options, convertible securities)
|
1. Title of Derivate Security
(Instr. 3)
|
2. Conversion or Exercise Price of Derivative Security
|
3. Trans. Date
|
3A. Deemed Execution Date, if any
|
4. Trans. Code
(Instr. 8)
|
5. Number of Derivative Securities Acquired (A) or Disposed of (D)
(Instr. 3, 4 and 5)
|
6. Date Exercisable and Expiration Date
|
7. Title and Amount of Securities Underlying Derivative Security
(Instr. 3 and 4)
|
8. Price of Derivative Security
(Instr. 5)
|
9. Number of derivative Securities Beneficially Owned Following Reported Transaction(s) (Instr. 4)
|
10. Ownership Form of Derivative Security: Direct (D) or Indirect (I) (Instr. 4)
|
11. Nature of Indirect Beneficial Ownership (Instr. 4)
|
|
Code
|
V
|
(A)
|
(D)
|
Date Exercisable
|
Expiration Date
|
Title
|
Amount or Number of Shares
|
|
Restricted Stock Unit
|
(1)
|
10/30/2019
|
|
M
|
|
|
11436.0000
|
10/30/2019 (2)
|
10/30/2021
|
Common Stock
|
11436.0000
|
$85.1000
|
22872.0000
|
D
|
|
|
Explanation of Responses:
|
| (1)
|
Each restricted stock unit represents a contingent right to receive one share of Merck & Co., Inc. common stock.
|
| (2)
|
These restricted stock units vest and are distributed as shares of Merck & Co., Inc. common stock in three equal installments on 10/30/2019, 10/30/2020 and 10/30/2021.
|
Reporting Owners
|
|
Reporting Owner Name / Address
|
Relationships
|
|
Director
|
10% Owner
|
Officer
|
Other
|
Scholefield James H
MERCK & CO., INC.
2000 GALLOPING HILL ROAD
KENILWORTH, NJ 07033
|
|
|
EVP,Chief Info&Digital Officer
|
|
Signatures
|
|
Faye C. Brown as Attorney-in-Fact for James H. Scholefield
|
|
11/1/2019
|
|
**Signature of Reporting Person
|
Date
|
|
Reminder: Report on a separate line for each class of securities beneficially owned directly or indirectly.
|
|
*
|
If the form is filed by more than one reporting person, see Instruction 4(b)(v).
|
|
**
|
Intentional misstatements or omissions of facts constitute Federal Criminal Violations. See 18 U.S.C. 1001 and 15 U.S.C. 78ff(a).
|
|
Note:
|
File three copies of this Form, one of which must be manually signed. If space is insufficient, see Instruction 6 for procedure.
|
|
Persons who respond to the collection of information contained in this form are not required to respond unless the form displays a currently valid OMB control number.
|
Merck (NYSE:MRK)
Historical Stock Chart
From Apr 2024 to May 2024
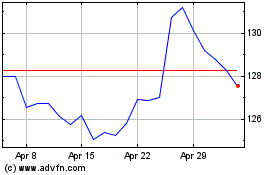
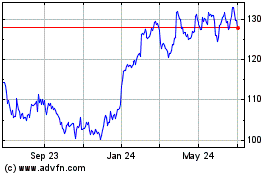
Merck (NYSE:MRK)
Historical Stock Chart
From May 2023 to May 2024
Merck Announces Phase 3 KEYNOTE-811 Trial Met Dual Primary Endpoint of Overall Survival (OS) as First-Line Treatment in Patients With HER2-Positive Advanced Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
Wednesday 1 May 2024 (12 hours ago) • Business Wire |
Merck Announces Positive Data for V116, an Investigational, 21-Valent Pneumococcal Conjugate Vaccine Specifically Designed for Adults
Monday 29 April 2024 (2 days ago) • Business Wire |
U.S. Stocks Climb Well Off Worst Levels But Close Mostly Lower
Friday 26 April 2024 (6 days ago) • IH Market News |
Futures Pointing To Sharply Lower Open On Wall Street
Thursday 25 April 2024 (6 days ago) • IH Market News |
Southwest Shares Tumble 9.6% Post $231 Million 1Q Loss, AstraZeneca Surges on 19% Annual Increase, and More on Earnings
Thursday 25 April 2024 (6 days ago) • IH Market News |
Merck Announces First-Quarter 2024 Financial Results
Thursday 25 April 2024 (7 days ago) • Business Wire |
Health Canada Approves KEYTRUDA® as a first-line treatment for adult patients with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma in combination with fluoropyrimidine- and platinum-conta
Friday 19 April 2024 (2 weeks ago) • PR Newswire (Canada) |
Delta Generates US$37 Million Profit in Q1, Google and Intel Unveil Cutting-Edge AI Chips, and More News
Wednesday 10 April 2024 (3 weeks ago) • IH Market News |
Merck Initiates Phase 3 Clinical Trial of MK-1084, an Investigational Oral KRAS G12C Inhibitor, in Combination with KEYTRUDA® (pembrolizumab) for First-Line Treatment of Certain Patients With Metastatic Non-Small Cell Lung Cancer
Thursday 4 April 2024 (4 weeks ago) • Business Wire |
REJOICE-Ovarian01 Phase 2/3 Trial of Raludotatug Deruxtecan Initiated in Patients with Platinum-Resistant Ovarian Cancer
Wednesday 3 April 2024 (4 weeks ago) • Business Wire |
Merck to Hold First-Quarter 2024 Sales and Earnings Conference Call April 25
Monday 1 April 2024 (1 month ago) • Business Wire |
European Commission Approves Merck’s KEYTRUDA® (pembrolizumab) Plus Chemotherapy as Neoadjuvant Treatment, Then Continued as Monotherapy as Adjuvant Treatment, for Resectable Non-Small Cell Lung Cancer (NSCLC) at High Risk of Recurrence in Adults
Thursday 28 March 2024 (1 month ago) • Business Wire |
More Merck & Co., Inc. News Articles

It looks like you are not logged in. Click the button below to log in and keep track of your recent history.