OXB’s lentiviral vector manufacturing technology to support Boehringer Ingelheim’s newly initiated Phase I/II trial of first-in-class gene therapy for cystic fibrosis
21 February 2025 - 12:36AM

OXB’s
lentiviral vector manufacturing technology to support Boehringer
Ingelheim’s newly initiated Phase I/II trial of first-in-class gene
therapy for cystic fibrosis
Commencement of
LENTICLAIR™ 1 trial, utilising OXB lentiviral vectors; builds on
long term collaboration with Boehringer Ingelheim
Oxford, UK – 20 February
2025: OXB (LSE: OXB), a global quality and
innovation-led cell and gene therapy CDMO, today announces that its
proprietary lentiviral vector manufacturing technology will be used
in Boehringer Ingelheim's newly initiated LENTICLAIR™ 1 trial. The
Phase I/II study is evaluating BI 3720931, a novel, first-in-class,
inhaled gene therapy for the treatment of cystic fibrosis (CF).
With three decades of expertise in viral vector
development and manufacturing, OXB will support the development of
this potential treatment for CF, a genetic condition caused by
mutations in the CFTR gene that affects more than 100,000 people
worldwide. While CFTR modulators have provided treatment options
for some patients, there remains a significant unmet need for
10-15% of people with CF due to mutation type or modulator
intolerance.
BI 3720931 is a first-in-class, inhaled
lentiviral vector-based gene therapy designed to address this need
through a novel approach of inserting a functional copy of the CFTR
gene in the DNA of airway epithelial cells.
This trial builds on the successful
collaboration initiated in August 2018 with Boehringer Ingelheim.
OXB’s ongoing role in this programme highlights its reputation as a
trusted CDMO and its commitment to advancing pioneering gene
therapies that address significant unmet needs.
Frank Mathias, Chief Executive Officer
of OXB, said: "We are delighted that Boehringer Ingelheim
is using OXB’s proprietary lentiviral vector manufacturing
technology for the clinical development of their CF gene therapy.
This programme has the potential to transform the lives of patients
living with CF and exemplifies OXB’s commitment to supporting
cutting-edge cell and gene therapies. We look forward to playing a
key role in its progression."
-Ends-
Enquiries:
OXB:
Sebastien Ribault, Chief Business
Officer – T: +44 (0) 1865 783 000 / E: partnering@oxb.com
ICR Healthcare:T: +44
(0)20 3709 5700 / E: oxb@icrhealthcare.com
Mary-Jane Elliott / Angela Gray / Davide
Salvi
About OXB
OXB (LSE: OXB) is a
global quality and innovation-led contract development and
manufacturing organisation (CDMO) in cell and gene therapy with a
mission to enable its clients to deliver life-changing therapies to
patients around the world.
One of the original
pioneers in cell and gene therapy, OXB has 30 years of experience
in viral vectors; the driving force behind the majority of cell and
gene therapies. OXB collaborates with some of the world's most
innovative pharmaceutical and biotechnology companies, providing
viral vector development and manufacturing expertise in lentivirus,
adeno-associated virus (AAV), adenovirus and other viral vector
types. OXB's world-class capabilities span from early-stage
development to commercialisation. These capabilities are supported
by robust quality-assurance systems, analytical methods and depth
of regulatory expertise.
OXB offers a vast
number of unique technologies for viral vector manufacturing,
including a 4th generation lentiviral vector system (the
TetraVecta™ system), dual plasmid system for AAV production,
suspension and perfusion process using process enhancers and stable
producer and packaging cell lines.
OXB, a FTSE4Good
constituent, is headquartered in Oxford, UK. It has bioprocessing
and manufacturing facilities across Oxfordshire, UK, Lyon and
Strasbourg, France and near Boston, MA, US. Learn more at
www.oxb.com, and follow us on LinkedIn and YouTube.
About
Boehringer Ingelheim
Boehringer Ingelheim
is a biopharmaceutical company active in both human and animal
health. As one of the industry’s top investors in Research and
Development, the company focuses on developing innovative therapies
in areas of high unmet medical need. Independent since its
foundation in 1885, Boehringer takes a long-term perspective,
embedding sustainability along the entire value chain. More than
53,500 employees serve over 130 markets to build a healthier, more
sustainable, and equitable tomorrow. Learn more at
https://www.boehringer-ingelheim.com.
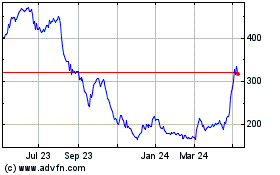
Oxford Biomedica (LSE:OXB)
Historical Stock Chart
From Feb 2025 to Mar 2025
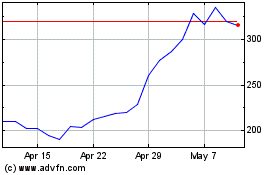
Oxford Biomedica (LSE:OXB)
Historical Stock Chart
From Mar 2024 to Mar 2025