As
filed with the Securities and Exchange Commission on January 8, 2025
Registration
Statement No. 333-
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
ASPIRA
WOMEN’S HEALTH INC.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
2835 |
|
33-0595156 |
(State
or other jurisdiction of
incorporation
or organization) |
|
(Primary
Standard Industrial
Classification
Code Number) |
|
(I.R.S.
Employer
Identification
Number) |
12117
Bee Caves Road, Building III, Suite 100
Austin,
Texas 78738
(512) 519-0400
(Address,
including zip code and telephone number, including area code of registrant’s principal executive offices)
Sandra
Milligan
Interim
Chief Executive Officer
Aspira
Women’s Health Inc.
12117
Bee Caves Road, Building III, Suite 100
Austin,
Texas 78738
(512) 519-0400
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
Jeffrey
J. Fessler, Esq.
Nazia
J. Khan, Esq.
Sheppard,
Mullin, Richter & Hampton LLP
30
Rockefeller Plaza
New
York, NY 10112-0015
Tel.:
(212) 653-8700 |
|
Lawrence
Metelitsa, Esq.
Lucosky
Brookman LLP
101
Wood Avenue South, 5th Floor
Woodbridge,
NJ 08830
Tel.:
(732) 395-4400 |
Approximate
date of commencement of proposed sale to the public:
As
soon as practicable after the date this registration statement becomes effective.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933 check the following box: ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large
accelerated filer ☐ |
Accelerated
filer ☐ |
Non-accelerated
filer ☒ |
Smaller
reporting company ☒ |
| |
|
|
Emerging
growth company ☐ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
The
registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the
registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective
in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective
on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The
information contained in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration
statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities
and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY
PROSPECTUS |
SUBJECT
TO COMPLETION |
DATED
JANUARY 8, 2025 |
Up
to 16,000,000 Shares of Common Stock
Up
to 16,000,000 Pre-Funded Warrants to purchase up to 16,000,000 Shares of Common Stock

Aspira
Women’s Health Inc.
We
are offering up to 16,000,000 shares of our common stock at an assumed public offering price of $0.75 per share, the last
reported sale price of our common stock as reported on The Nasdaq Capital Market on January 7, 2025. The actual public offering
price per share of common stock will be determined between us and the underwriters at the time of pricing and may be at a discount to
this assumed offering price. Therefore, the assumed public offering price used throughout this prospectus may not be indicative of the
final offering price.
We
are also offering up to 16,000,000 pre-funded warrants (each a “Pre-funded Warrant”) to purchase up to 16,000,000
shares of our common stock, exercisable at an exercise price of $0.001 per share, to those purchasers whose purchase of common
stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially
owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock immediately following
the consummation of this offering. The purchase price of each Pre-funded Warrant is equal to the price per share of common stock being
sold to the public in this offering, minus $0.001. The Pre-funded Warrants will be immediately exercisable and may be exercised
at any time until all of the Pre-funded Warrants are exercised in full. For each Pre-funded Warrant we sell, the number of shares
of common stock that we are offering will be decreased on a one-for-one basis.
Our
common stock is listed on The Nasdaq Capital Market under the symbol “AWH”. On January 7, 2025, the closing price of our
common stock on The Nasdaq Capital Market was $0.75. There is no established trading market for the Pre-funded Warrants and we do
not intend to list the Pre-funded Warrants on any securities exchange or nationally recognized trading system.
We
are a “smaller reporting company” as defined in the federal securities laws and will be subject to reduced public company
reporting requirements. See “Prospectus Summary — Implications of Being a Smaller Reporting Company.”
You
should read this prospectus, together with the additional information described under the headings “Where You Can Find More Information”
and “Incorporation of Documents by Reference,” carefully before you invest in any of our securities.
Investing
in our common stock is highly speculative and involves a high degree of risk. See “Risk Factors” beginning on page
13 of this prospectus for a discussion of information that should be considered in connection with an investment in our common
stock.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| | |
Per
Share | | |
Per
Pre-funded Warrant | | |
Total | |
| Public offering price | |
$ | | | |
$ | | | |
$ | | |
| Underwriting discounts and
commissions (1) | |
$ | | | |
$ | | | |
$ | | |
| Proceeds to us, before expenses | |
$ | | | |
$ | | | |
$ | | |
| (1) |
Underwriting
discounts and commissions do not include a non-accountable expense allowance equal to 1.0% of the public offering price payable to
the representative of the underwriters. See “Underwriting” beginning on page 38 for additional information
regarding underwriters’ compensation. |
We
have granted a 45-day option to the underwriters to purchase up to 2,400,000 additional shares of common stock and/or up to 2,400,000 Pre-funded Warrants solely to cover over-allotments, if any.
The
underwriters expect to deliver the securities to purchasers on or about ,
2025.
ThinkEquity
The
date of this prospectus is , 2025
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
We
incorporate by reference important information into this prospectus. You may obtain the information incorporated by reference without
charge by following the instructions under “Where You Can Find More Information.” You should carefully read this prospectus
as well as additional information described under “Incorporation of Documents by Reference” before deciding to invest in
our securities.
Neither
we nor the underwriters have authorized anyone to provide you with additional information or information different from that contained
or incorporated by reference in this prospectus filed with the Securities and Exchange Commission (the “SEC”). We take no
responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. The underwriters
are offering to sell, and seeking offers to buy, our securities only in jurisdictions where offers and sales are permitted. The information
contained in this prospectus, or any document incorporated by reference in this prospectus, is accurate only as of the date of those
respective documents, regardless of the time of delivery of this prospectus or any sale of our securities. Our business, financial condition,
results of operations and prospects may have changed since that date.
The
information incorporated by reference or provided in this prospectus contains estimates and other statistical data made by independent
parties and by us relating to market size and growth and other data about our industry. We obtained the industry and market data in this
prospectus from our own research as well as from industry and general publications, surveys and studies conducted by third parties. This
data involves a number of assumptions and limitations and contains projections and estimates of the future performance of the industry
in which we operate that are subject to a high degree of uncertainty, including those discussed in “Risk Factors.” We caution
you not to give undue weight to such projections, assumptions, and estimates. Further, industry and general publications, studies and
surveys generally state that they have been obtained from sources believed to be reliable, although they do not guarantee the accuracy
or completeness of such information. While we believe that these publications, studies, and surveys are reliable, we have not independently
verified the data contained in them. In addition, while we believe that the results and estimates from our internal research are reliable,
such results and estimates have not been verified by any independent source.
For
investors outside the United States (“U.S.”): We and the underwriters have not done anything that would permit this offering
or the possession or distribution of this prospectus in any jurisdiction where action for those purposes is required, other than in the
U.S. Persons outside the U.S. who come into possession of this prospectus must inform themselves about, and observe any restrictions
relating to, the offering of the securities and the distribution of this prospectus outside of the U.S.
INFORMATION
REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities
Act”), and Section 21E of the Exchange Act of 1934, as amended (the “Exchange Act”) that involve risks and uncertainties.
You should not place undue reliance on these forward-looking statements. All statements other than statements of historical facts contained
in this prospectus are forward-looking statements. The forward-looking statements in this prospectus are only predictions. We have based
these forward-looking statements largely on our current expectations and projections about future events and financial trends that we
believe may affect our business, financial condition, and results of operations. In some cases, you can identify these forward-looking
statements by terms such as “anticipate,” “believe,” “continue,” “could,” “depends,”
“estimate,” “expects,” “intend,” “may,” “ongoing,” “plan,” “potential,”
“predict,” “project,” “should,” “will,” “would” or the negative of those
terms or other similar expressions, although not all forward-looking statements contain those words. We have based these forward-looking
statements on our current expectations and projections about future events and trends that we believe may affect our financial condition,
results of operations, strategy, short- and long-term business operations and objectives, and financial needs. These forward-looking
statements include, but are not limited to, statements concerning the following:
| ● |
projections
or expectations regarding our future test volumes, revenue, average unit price, cost of revenue, operating expenses, research and
development expenses, gross profit margin, cash flow, results of operations and financial condition; |
| ● |
the
ability to maintain the listing of our common stock and public warrants on The Nasdaq Capital Market; |
| ● |
our
plan to broaden our commercial focus from ovarian cancer to differential diagnosis of women with a range of gynecological diseases,
including additional pelvic disease conditions such as endometriosis and benign pelvic mass monitoring; |
| ● |
our
planned business strategy and the anticipated effects thereof, including partnerships such as those based on our Aspira Synergy platform,
specimen or research collaborations, licensing arrangements, commercial collaborations and distribution agreements; |
| ● |
plans
to expand our current or future products to markets outside of the United States through distribution collaborations or out-licensing; |
| ● |
plans
to develop new algorithms, molecular diagnostic tests, products and tools and otherwise expand our product offerings; |
| ● |
plans
to develop, launch and establish payer coverage and secure contracts for current and new products, including ENDOinform (formerly
EndoMDx) and OVAinform (formerly OvaMDx); |
| ● |
expectations
regarding local and/or national coverage under Novitas, our Medicare Administrative Carrier; |
| ● |
anticipated
efficacy of our products, product development activities and product innovations, including our ability to improve sensitivity and
specificity over traditional diagnostics; |
| ● |
expected
competition in the markets in which we operate; |
| ● |
plans
with respect to Aspira Labs, Inc. (“Aspira Labs”), including plans to expand Aspira Labs’ testing capabilities,
specifically molecular lab capabilities; |
| ● |
expectations
regarding continuing future services provided by Quest Diagnostics Incorporated; |
| ● |
expectations
regarding continuing future services provided by BioReference Health, LLC; |
| ● |
plans
to develop informatics products as laboratory developed tests (“LDTs”) and potential Food and Drug Administration (“FDA”)
oversight changes of LDTs; |
| ● |
expectations
regarding existing and future collaborations and partnerships for our products, including plans to enter into decentralized arrangements
for our Aspira Synergy platform and to provide and expand access to our risk assessment tests; |
| ● |
plans
regarding future publications and presentations; |
| ● |
expectations
regarding potential collaborations with governments, legislative bodies and advocacy groups to enhance awareness and drive policies
to provide broader access to our tests; |
| ● |
our
ability to continue to comply with applicable governmental regulations, including regulations applicable to the operation of our
clinical lab, expectations regarding pending regulatory submissions and plans to seek regulatory approvals for our tests within the
United States and internationally, as applicable; |
| ● |
our
continued ability to expand and protect our intellectual property portfolio; |
| ● |
anticipated
liquidity and capital requirements; |
| ● |
anticipated
future losses and our ability to continue as a going concern; |
| ● |
expectations
regarding raising capital and the amount of financing anticipated to be required to fund our planned operations; |
| ● |
expectations
regarding attrition and recruitment of top talent; |
| ● |
expectations
regarding the results of our clinical research studies and our ability to recruit patients to participate in such studies; |
| ● |
our
ability to use our net operating loss carryforwards and anticipated future tax liability under U.S. federal and state income tax
legislation; |
| ● |
expected
market adoption of our current and prospective diagnostic tests, including Ova1, Overa, Ova1Plus, OvaWatch, ENDOinform and OVAinform,
as well as our Aspira Synergy platform; |
| ● |
expectations
regarding our ability to launch new products we develop, license, co-market or acquire; |
| ● |
expectations
regarding the size of the markets for our products; |
| ● |
expectations
regarding reimbursement for our products, and our ability to obtain such reimbursement, from third-party payers such as private insurance
companies and government insurance plans; |
| ● |
potential
plans to pursue clearance designation with the FDA with respect to OvaWatch, ENDOinform and OVAinform; |
| ● |
expected
potential target launch timing for future products; |
| ● |
expectations
regarding compliance with federal and state laws and regulations relating to billing arrangements conducted in coordination with
laboratories; |
| ● |
plans
to advocate for legislation and professional society guidelines to broaden access to our products and services; |
| ● |
ability
to protect and safeguard against cybersecurity risks and breaches; and |
| ● |
expectations
regarding the results of our academic research agreements. |
These
forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk
Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is
not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which
any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements
we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus
may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
You
should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected
in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events
and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, except as required by law, neither
we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation
to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual
results or to changes in our expectations.
You
should read this prospectus and the documents that we reference in this prospectus and have filed with the SEC as exhibits to the registration
statement of which this prospectus is a part with the understanding that our actual future results, levels of activity, performance and
events and circumstances may be materially different from what we expect.
PROSPECTUS
SUMMARY
The
following summary highlights selected information contained elsewhere in this prospectus and is qualified in its entirety by the more
detailed information and financial statements included elsewhere in this prospectus and the information incorporated by reference
herein. It does not contain all the information that may be important to you and your investment decision. You should carefully read
this entire prospectus, including the matters set forth under “Risk Factors,” included elsewhere in this prospectus and our
financial statements and related notes incorporated by reference herein. In this prospectus, unless context requires otherwise, references
to “we,” “us,” “our,” “Aspira,” or “the Company” refer to Aspira Women’s
Health Inc., a Delaware corporation, and its subsidiaries, unless the context otherwise requires.
Corporate
Vision
Aspira
Women’s Health Inc. is dedicated to pioneering the discovery, development, and commercialization of non-invasive diagnostic tests
enabled by artificial intelligence (“AI”) and machine learning algorithms. Our goal is to transform the way gynecologic
diseases, including ovarian cancer and endometriosis, are diagnosed, by providing advanced, AI-enabled tools that improve
accuracy in the diagnosis of gynecologic diseases.
We
plan to broaden our focus to the differential diagnosis of other gynecologic diseases that typically cannot be assessed through traditional
non-invasive clinical procedures. We will continue to increase the market penetration of our existing and new technology through our
direct sales force, channel partners, and our decentralized technology transfer service platform, Aspira Synergy. We also intend to continue
to raise public awareness regarding the higher sensitivity and negative predictive value for ovarian malignancy of Ova 1 as compared
to cancer antigen 125 (“CA-125”) on its own for women with adnexal masses planned for surgery, as well
as the performance of our machine learning algorithms in detecting ovarian cancer risk in different racial and ethnic populations. We
plan to continue to expand payor coverage for commercial and Federal payors, including Medicaid, as part of our corporate mission to
make the best care available to all women. We will also continue to focus on advocacy for legislation and the adoption of our technology
in professional society guidelines to provide broad access to our products and services.
We
continue to focus on three key initiatives: growth, innovation, and operational excellence.
Growth
As a revenue-generating diagnostics company focused exclusively on gynecologic disease, our commercial capabilities are one of our most
important differentiators. We expect our extensive experience with gynecologists and healthcare providers, along with the historical
adoption of our OvaSuite tests, to drive growth as we introduce new products.
During
2023, we conducted a comprehensive review of our commercial programs to identify people, processes, and technology enhancements and to
refine our product messaging for greater impact and reach. As a result of the findings of that review, we implemented a revised commercial
strategy in the second half of 2023. In the first quarter of 2024, we completed the successful implementation of the revised commercial
strategy including eliminating unprofitable territories, enhancing our sales training program, establishing a remote sales team to support
the field sales team, and expanding sales analytics capabilities, and expect to leverage these enhancements as we continue to focus
on growth through the improved profitability, efficiency, and effectiveness of the sales and marketing teams.
The
average number of field sales representatives during the nine months ended September 30, 2024, was 17 (13 as of January 7, 2025),
compared with 20 representatives in the nine months ended September 30, 2023. The average OvaSuite volume per field sales representative
increased from 917 tests per representative in the nine months ended September 30, 2023, to 1,109 tests per representative in the nine
months ended September 30, 2024, nearly a 21% increase.
Innovation
We believe our ability to successfully develop novel AI-enabled assays is superior to others based on our knowledge and extensive experience
in designing and successfully launching FDA-cleared and laboratory developed blood tests to aid in the diagnosis of ovarian cancer. Our
history of successfully collaborating with world-class research and academic institutions allows us to leverage additional specimen collections
to continue our product innovation. Moreover, we own and operate Aspira Labs, Inc., a research and commercial CLIA laboratory in Texas.
We have an extensive biospecimen repository from ovarian malignancy risk patients with samples from nearly 500 ovarian malignancies from
all stages, and representative controls, which permits the development of new algorithms that incorporate unique features, and supports
extensive analytical testing of current and new diagnostic tests.
Our
product pipeline is focused on two areas: ovarian cancer and endometriosis.
For
ovarian cancer, in the second quarter of 2024, we published a study in the journal of the Society of Gynecologic Oncology (“SGO”),
supporting the repeated use of our OvaWatch test for the monitoring of an adnexal mass. This successful expansion of the OvaWatch mass
monitoring feature resulted in a tenfold increase in the addressable market for our tests when compared to the addressable market for
Ova1Plus of approximately 200,000 to 400,000 based on patients identified for surgery.
Further,
our OVAinform development program continues to progress which increases our indication to include Familial and Germline risk for ovarian
cancer which we believe will fulfill a major clinical need and result in an increase of our addressable market from 2 to 4 million. OVAinform
is a multi-marker test that combines serum proteins, clinical data (metadata), and miRNA for assessing the risk of ovarian cancer in
women with an adnexal mass.
In
endometriosis, we are developing and intend to introduce a new non-invasive test to aid in the diagnosis of this debilitating disease
that impacts millions of women worldwide. We completed the design of a protein-based non-invasive blood test to aid the detection of
endometrioma, one of the most common forms of endometriosis. The algorithm was confirmed with three independent cohorts and is an important
input for our ENDOinform program focused on developing a multi-marker test that combines serum proteins, clinical data (metadata) and
miRNA for the identification of endometriosis.
Our
endometriosis portfolio addresses an even larger addressable market. According to the U.S. Department of Health and Human Services, endometriosis
affects more than 6.5 million women in the United States. We believe the proliferation of commercially available and in-development therapeutics
for the treatment of endometriosis will create a significant demand for a non-invasive diagnostic.
In
the second quarter of 2024, we completed a comprehensive analysis of our biobank and identified up to 70,000 serum, plasma, and whole
blood samples that are available for our continued product research and development.
Our
Business and Products
We
currently commercialize the following blood test products and related services:
(1)
the Ova1Plus workflow, which uses Ova1 as the primary test and Overa as a reflex for Ova1 intermediate range results. Ova1 is a qualitative
serum test intended as an aid to further assess the likelihood of malignancy in women with an ovarian adnexal mass for which surgery
is planned when the physician’s independent clinical and radiological evaluation does not indicate malignancy. Overa is a second-generation
biomarker test intended to maintain Ova1’s high sensitivity while improving specificity. The Ova1Plus workflow leverages the strengths
of Ova1’s MIA sensitivity and Overa’s (MIA2G) specificity to increase performance; and
(2)
OvaWatch, which is intended to assist in the initial and periodic clinical assessment of malignancy risk in all women thought to have
an indeterminate or benign adnexal mass.
Our
products are distributed through our own national sales force, including field and inside sales, through our proprietary decentralized
testing platform and cloud service, marketed as Aspira Synergy, and through marketing and distribution agreements with labs, including
BioReference Health, LLC (“BioReference”) and ARUP Laboratories. In November of 2024, we expanded our distribution agreement
with BioReference to include OvaWatch. This timing aligns with our approval from New York State and increases our ability to market OvaWatch
in New York. This important addition will allow providers who currently order Ova1 through BioReference to also order Aspira’s
products for any woman with a mass within their existing BioReference workflows.
Our
Ova1 test received FDA de novo classification in September 2009. Ova1 comprises instruments, assays, reagents, and the
OvaCalc software, which includes a proprietary algorithm that produces a risk score. Our Overa test, which includes an updated version
of OvaCalc, received FDA 510(k) clearance in March 2016. Ova1, Overa and OvaWatch each use the Roche Cobas 4000, 6000 and 8000 platforms
for analysis of proteins.
In
2021, we began entering into decentralized arrangements with large healthcare networks and physician practices for our Aspira Synergy
platform, our decentralized testing platform and cloud service for decentralized global access of protein biomarker testing. Ova1, Overa,
and the Ova1Plus workflow continue to be available through the Aspira Synergy platform. As of December 31, 2024, we had two active Aspira
Synergy contracts, Women’s Care Florida and Hi-Precision Laboratories.
OvaWatch
has been developed and is validated for use in Aspira’s CLIA-certified lab as a non-invasive blood-based risk assessment test for
use in conjunction with clinical assessment and imaging to determine ovarian cancer risk for patients with an adnexal mass whose adnexal
mass has been determined by an initial clinical assessment as indeterminate or benign. OvaWatch is the only commercially available blood
test available for assessment of the risk of ovarian cancer in women diagnosed with an adnexal mass considered indeterminate or benign
by a physician’s preliminary clinical assessment.
We
collected clinical data to support the utility of OvaWatch to aid in surgical referral and as a longitudinal monitoring test, resulting
in two manuscripts that were peer reviewed and published in Frontiers and SGO Journals in May 2024. In addition, an abstract highlighting
data evaluating the use of OvaWatch to assess ovarian cancer risk in pre- and post-menopausal women was accepted for a poster presentation
at the Annual Meeting of The Menopause Society in September 2024.
Outside
of the United States, we sponsored studies in the Philippines aimed at validating Overa and Ova1 in specific populations. In February
2024, we signed an exclusive license agreement with Hi-Precision Laboratories to offer OvaSuite tests in the Philippines. In November
2024, Hi-Precision Laboratories communicated the completion of all laboratory and regulatory processes required for it to offer Ova1Plus
commercially to patients in the Philippines under the terms of our licensing agreement. Accordingly, it began marketing the test to physicians
through its existing sales and marketing channels at that time. We continue to assist Hi-Precision in the design and execution of its
commercialization and physician adoption strategy. Building on the successful launch of Ova1Plus in the Philippines, we have created
a process roadmap for future global expansion efforts utilizing the Aspira Synergy platform.
We
own and operate Aspira Labs, based in Austin, Texas, a Clinical Chemistry and Endocrinology Laboratory accredited by the College of American
Pathologists, which specializes in applying biomarker-based technologies to address critical needs in the management of gynecologic cancers
and disease. Aspira Labs provides expert diagnostic services using a state-of-the-art biomarker-based risk assessment to aid in clinical
decision making and to advance personalized treatment plans. The lab currently performs our Ova1Plus workflow and OvaWatch testing, and
we plan to expand the testing to other gynecologic conditions with high unmet need. Aspira’s Labs holds a CLIA Certificate of Accreditation
and a state laboratory license in California, Maryland, New York, Pennsylvania and Rhode Island. The Centers for Medicare & Medicaid
Services issued a supplier number to Aspira Labs in 2015. Aspira Labs also holds a current ISO 13485 certification which is the most
accepted standard worldwide for medical devices.
In
the United States, revenue for diagnostic tests comes from several sources, including third-party payers such as insurance companies,
government healthcare programs, such as Medicare and Medicaid, client bill accounts and patients. Novitas Solutions, a Medicare Administrative
Carrier, covers and reimburses for Ova1 tests performed in certain states, including Texas. Due to our billed Ova1 tests being performed
exclusively at Aspira Labs in Texas, the Local Coverage Determination (“LCD”) from Novitas Solutions essentially provides
national coverage for patients enrolled in Medicare as well as Medicare Advantage health plans. We have applied for an LCD for OvaWatch,
which is currently under review.
In
November 2016, the American College of Obstetricians and Gynecologists (“ACOG”) issued Practice Bulletin Number 174 which
included Ova1, defined as the “Multivariate Index Assay,” outlining ACOG’s clinical management guidelines for adnexal
mass management. Practice Bulletin Number 174 recommends that obstetricians and gynecologists evaluating women with adnexal masses who
do not meet Level A criteria of a low-risk transvaginal ultrasound should proceed with Level B clinical guidelines. Level B guidelines
state that the physician may use risk-assessment tools such as existing CA-125 technology or Ova1 (“Multivariate Index Assay”)
as listed in the bulletin. Based on this, Ova1 achieved parity with CA-125 as a Level B clinical recommendation for the management of
adnexal masses.
Practice
Bulletins summarize current information on techniques and clinical management issues for the practice of obstetrics and gynecology. Practice
Bulletins are evidence-based documents, and recommendations are based on the evidence. This is also the only clinical management tool
used for adnexal masses. Although there are Practice Bulletins, guidelines do not exist for adnexal masses. ACOG guidelines do exist,
however, for ovarian cancer management.
Product
Pipeline
We
aim to introduce new gynecologic diagnostic products and to expand our product offerings to additional women’s gynecologic health
diseases by adding additional gynecologic bio-analytic solutions involving biomarkers, clinical risk factors and patient data to aid
diagnosis and risk stratification. Future product expansions will be accelerated by the development of lab developed testing in a CLIA
environment, relationships with strategic research and development partners, and access to specimens in our biobank.
| ● | OVAinform
(formerly OvaMDX) is a multi-marker test that combines serum proteins, clinical data
(metadata), and miRNA for assessing the risk of ovarian cancer in women with an adnexal mass.
The test is being developed in collaboration with Harvard’s Dana-Farber Cancer Institute
(providing clinical and trial design expertise), Brigham & Women’s Hospital (providing
miRNA technical expertise), and Medical University of Lodz (providing miRNA biomarker and
bioinformatics analytic support). |
The
miRNAs used in the OVAinform test were the subject of a 2017 paper, “Diagnostic potential for a serum miRNA neural network for
detection of ovarian cancer” published in the peer-reviewed journal Cancer Biology. In October 2023, a poster entitled “Improving
the diagnostic accuracy of an ovarian cancer triage test using a joint miRNA-protein model,” was presented at the AACR Special
Conference in Cancer Research: Ovarian Cancer by senior author, Dr. Kevin Elias M.D., Director, Gynecologic Oncology Laboratory at Brigham
and Women’s Hospital and Assistant Professor of Obstetrics, Gynecology and Reproductive Biology at Harvard Medical School. The
poster highlighted data from a study that combined serum protein and patient clinical information (metadata) from Aspira’s ovarian
cancer registry studies with miRNA determined by the Elias laboratory. The data showed that using miRNA in combination with serum proteins,
provided superior performance over existing ovarian cancer risk assessment blood tests.
We
have tested our entire set of selected miRNA biomarkers and, based on their performance, we are refining the features on our droplet
digital PCR commercial platform. As a next step, we intend to increase our patient sample testing to refine the algorithm.
| ● | ENDOinform
(formerly EndoMDx) is a multi-marker test program that combines serum proteins,
clinical data (metadata), and miRNA for the identification of endometriosis. The test is
being developed in collaboration with a consortium of academic and clinical partners led
by Dana Farber Cancer Institute. We are currently in the process of analyzing the first 100
patient samples to verify protein and miRNA biomarkers for their analytical properties on
our droplet digital PCR commercial platform. This is a critical step in evaluating the strength
of algorithms that incorporate miRNAs. |
Business
Updates
On
May 7, 2024, we announced the publication of two peer-reviewed manuscripts. The first manuscript, entitled “Ovarian Cancer Surgical
Consideration is Markedly Improved by the Neural Network Powered-MIA3G Multivariate Index Assay” was published in the peer-reviewed
journal Frontiers of Medicine on May 2, 2024. The findings of this study demonstrate that use of OvaWatch to stratify risk in patients
with an adnexal mass might help to reduce surgical backlogs and unnecessary surgical referrals. The second manuscript, entitled “Neural
Network-derived Multivariate Index Assay Demonstrates Effective Clinical Performance in Longitudinal Monitoring of Ovarian Cancer Risk”
was published in the journal Gynecologic Oncology on May 3, 2024. The findings of this study demonstrate that OvaWatch could be an effective
tool for the monitoring of ovarian cancer risk over time in women with indeterminate or low risk adnexal masses. Based on common practice
for adnexal mass management and consistent with the study, OvaWatch can be drawn by the provider every three to six months for active
surveillance of an adnexal mass.
A
publication entitled Serum miRNA improves the accuracy of a multivariate index assay for triage of an adnexal mass was
published in the journal Gynecologic Oncology, in August 2024 from the laboratory of our collaborator Dr. Kevin Elias
at the Brigham and Women’s Hospital. The paper describes the novel combination of microRNAs (miRNAs) and serum proteins to
achieve increased performance in the assessment of malignancy risk in patients with an adnexal mass. The miRNAs, discovered by Dr
Elias’ team, in combination with serum proteins from Aspira’s proprietary multivariate index assays Ova1 and Overa showed
increased sensitivity for detection of malignancy and broader detection of diverse ovarian cancer subtypes. This publication establishes
the feasibility of improved tests using multi-omic information. (Webber JW, Wollborn L, Mishra S, Vitonis AF, Cramer DW, Phan RT,
Pappas TC, Stawiski K, Fendler W, Chowdhury D, Elias KM. Serum miRNA improves the accuracy of a multivariate index assay for triage of
an adnexal mass. Gynecol Oncol. 2024 Aug 23;190:124-130.)
An
abstract entitled “Application of a Deep Neural Network-Based Algorithm to Provide Additional Information in the Assessment
of Adnexal Masses Classified as Indeterminate by Imaging” was presented as a poster at the Annual Meeting of The Menopause
Society in September 2024. This presentation highlighted data evaluating the use of OvaWatch to assess ovarian cancer risk in pre- and
post-menopausal women. The data demonstrated that in women with an adnexal mass and an indeterminate ultrasound imaging result, the OvaWatch
result indicated low malignant potential of the mass in more than 70% of patients. The use of OvaWatch could provide additional information
to reduce surgical referrals.
An
EndoCheck-related abstract entitled “Association of the Endometriosis Health Profile-5 (EHP-5) with Non-Invasive Biomarkers
in Patients with Suspected Endometriosis” was presented as a poster at the 27th Annual National Association
of Nurse Practitioner’s in Women’s Health Women’s Healthcare Conference in September 2024. This poster examined the
association of biomarkers for ovarian endometriosis (endometrioma) with quality-of-life survey responses before and after surgical intervention.
There was no association between endometrioma biomarkers and self-reported patient quality of life either prior to or after surgery,
and this was consistent with other research.
An
EndoCheck-related virtual poster entitled “A Proprietary Protein-Based Algorithm May Increase Sensitivity of Endometrioma Detection
When Combined with Imaging” was presented at the annual meeting of the American Association of Gynecologic Laparoscopists in
November 2024. This poster summarizes a preliminary study on the performance of imaging combined with a protein biomarker-based algorithm.
The combination of these diagnostic tools resulted in increased sensitivity of detection of endometrioma and could be effective in risk
assessment and surgical planning for this condition.
On
October 15, 2024, the Company announced that it had received approval from the New York State Department of Health’s Clinical Laboratory
Evaluation Program for OvaWatch. This approval is required for all lab developed tests to ensure compliance with New York State’s
clinical laboratory regulatory standards. The comprehensive review process includes an assessment of procedures for maintaining quality,
traceability, and risk management.
On
October 23, 2024, First Lady Dr. Jill Biden announced that we had been selected by the Advanced Research Projects Agency for Health (“ARPA-H”)
as an awardee of the Sprint for Women’s Health, an initiative to address critical unmet challenges in women’s health, champion
transformative innovations, and tackle health conditions that uniquely or disproportionately affect women. Under this initiative, we
will receive up to $10.0 million in milestone-based funding over two years to develop our multi-marker blood test to aid in the detection
of endometriosis. Our test will rely on a powerful, AI-enabled algorithm that combines protein and microRNA biomarkers and patient data,
and leverage technology that we pioneered for our ovarian cancer risk assessment blood tests.
The
award also provides for access to a team of world-class subject matter experts and advisors to support the successful completion and
commercial launch of the test before the end of the two-year contract term. We will work with an ARPA-H Program Manager and the ARPA-H
Investor Catalyst Hub in the design, development, and commercial launch of this first-of-its kind endometriosis diagnostic test.
In
December 2024, we met our first ARPA-H milestone and received a milestone payment of $2 million.
Market
Access and Reimbursement
We
continue to gain momentum in our activities to increase coverage and reimbursement of OvaSuite products by contracting with payers and
gaining OvaWatch reimbursement. The volume of Northeast Anthem claims, contracted in August 2024, is increasing and expected to improve
the reimbursement per test in upcoming quarters. We further expect additional Anthem regions to complete contracting in the Anthem Central
and Virginia geographic regions in the first quarter of 2025, adding an additional 16 million lives.
In
the third quarter of 2024, we completed a comprehensive analysis of select national, regional and Integrated Delivery Network payers
with the assistance of an experienced third-party consultant. The project provided additional insights regarding evidence requirements
for broad support and reimbursement of OvaWatch. The findings of the analysis are being incorporated into a clinical utility study which
is currently under development, the timing of which will depend on working capital available for its completion. While this project was
focused on OvaWatch, we believe the output will help us advocate for expanded coverage for Ova1 and inform future market access strategies
for future product launches.
Additionally,
we met with numerous Medical Directors from Laboratory Benefit Management organizations and regional payers and have a further understanding
of their requirements for OvaSuite medical necessity.
We
also initiated an audit of our commercial payer contracts to ensure appropriate reimbursement. With this we have engaged in discussions
that could improve the average unit price for OvaSuite tests. Additionally, we continue to monitor the state biomarker laws, which are
increasingly mandating insurance coverage for biomarker testing in oncology. This trend is driven by the growing recognition
of the importance of precision medicine and the need to ensure that patients have access to the most appropriate treatments. While these
laws are a significant step forward, challenges remain, including inconsistencies across states, difficulties in defining “medically
necessary,” and the requirement to demonstrate clinical utility utilizing real world evidence as a benchmark.
Nasdaq
Listing
On
July 1, 2024, we received written notice (the “Notice”) from the Listing Qualifications Staff (the “Staff”) of
the Nasdaq Stock Market, LLC (“Nasdaq”) notifying us that for the 30 consecutive business days preceding the date of the
Notice, our Market Value of Listed Securities was below the minimum of $35 million required for continued listing on The Nasdaq Capital
Market pursuant to Nasdaq Listing Rule 5550(b)(2) (the “MVLS Requirement”). In accordance with Nasdaq Listing Rule 5810(c)(3)(C),
Nasdaq provided us with 180 calendar days, or until December 30, 2024 (the “Compliance Date”), to regain compliance with
the MVLS Requirement. On December 31, 2024, we received written notice from the Staff of Nasdaq notifying us that we failed to regain
compliance with the MVLS Requirement by the Compliance Date. As of the date of this prospectus, we have requested an appeal of Nasdaq’s
determination to delist our common stock and paid Nasdaq a hearing fee of $20,000. The request for an appeal will stay the delisting
of our common stock pending Nasdaq’s decision. Although we have requested an appeal of Nasdaq’s determination to delist our
common stock, no assurance can be provided that we will be successful in our appeal and that our common stock will continue to be listed
on The Nasdaq Capital Market.
Furthermore,
on October 17, 2024, we received written notice from Nasdaq that we were not in compliance with Nasdaq Listing Rule 5550(a)(2), as the
minimum bid price of our common stock had been below $1.00 per share for 30 consecutive business days. In accordance with Nasdaq
Listing Rule 5810, and assuming our common stock is not delisted for our failure to satisfy the MVLS Requirement by the MVLS Compliance
Date, we will have a period of 180 calendar days, or until April 15, 2025, to regain compliance with the minimum bid price requirement.
To regain compliance with the Nasdaq bid price requirement, the closing bid price of our common stock must meet or exceed $1.00 per share
for at least ten consecutive business days during this 180- calendar day period. In the event we do not regain compliance by April 15,
2025, we may be eligible for an additional 180 calendar day grace period.
Corporate
Information
We
were incorporated in 1993. Our principal executive offices are located at 12117 Bee Caves Road, Building III, Suite 100, Austin,
Texas 78738, and our telephone number is (512) 519-0400. We maintain a website at www.aspirawh.com where general information about
us is available. The information contained on our website is not incorporated by reference into this prospectus, and you should not consider
any information contained on, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase
our securities.
Implications
of Being a Smaller Reporting Company
We
are a “smaller reporting company,” meaning that the market value of our shares held by non-affiliates is less than $700 million
and our annual revenue was less than $100 million during the most recently completed fiscal year. We have elected to rely on exemptions
from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company,
we may choose to present only the two most recent fiscal years of audited financial statements in this prospectus and have reduced disclosure
obligations regarding executive compensation.
The
Offering
| Shares
of common stock being offered |
|
Up
to 16,000,000 shares of common stock at an assumed public offering price of $0.75 per share, the last
reported sale price of our common stock as reported on The Nasdaq Capital Market on January 7, 2025. |
| |
|
|
| Pre-funded
Warrants being offered |
|
We
are also offering up to 16,000,000 Pre-funded Warrants to purchase up to 16,000,000
shares of our common stock, exercisable at an exercise price of $0.001
per share, to those purchasers whose purchase of common stock in this offering would otherwise
result in the purchaser, together with its affiliates and certain related parties, beneficially
owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common
stock immediately following the consummation of this offering. The purchase price of each
Pre-funded Warrant is equal to the price per share of common stock being sold to the public
in this offering minus $0.001. The Pre-funded Warrants will be immediately exercisable
and may be exercised at any time until exercised in full.
This
prospectus also relates to the offering of common stock issuable upon exercise of the Pre-funded Warrants.
For
each Pre-funded Warrant we sell, the number of shares of common stock that we are offering will be decreased on a one-for-one basis.
For
additional information regarding the terms of the Pre-funded Warrants, see “Description of Securities We Are Offering.” |
| |
|
|
| Underwriters’
over-allotment option |
|
We
have granted the underwriters a 45 day option from the date of this prospectus, exercisable one or more times in whole or in part,
to purchase up to an additional 2,400,000 shares of common stock and/or up to an additional 2,400,000 Pre-funded Warrants
(15% of the total number of shares of common stock and/or Pre-funded Warrants to be offered by us in the offering), solely
to cover over-allotments, if any. |
| |
|
|
| Number
of shares of common stock to be outstanding after this offering (1) |
|
33,529,793 shares,
(or 35,929,793 shares if the underwriters exercise the option to purchase additional shares in full, assuming no sale
of the Pre-funded Warrants). |
| |
|
|
| Use
of proceeds |
|
We
expect to receive net proceeds, after deducting underwriting discounts and estimated expenses payable by us, of approximately $10.6
million (or approximately $12.3 million if the underwriters exercise their option to purchase additional shares and/or
Pre-funded Warrants in full), assuming a public offering price of $0.75 per share, the last reported sale price of
our common stock on The Nasdaq Capital Market on January 7, 2025, after deducting underwriting discounts and commissions
and other estimated offering expenses payable by us, and assuming no sale of any Pre-funded Warrants. We intend to use the
net proceeds from this offering for working capital and general corporate purposes. We may also use a portion of the net proceeds
to in-license, acquire or invest in complementary businesses, technologies or products, however, we have no current commitments or
obligations to do so. See “Use of Proceeds,” for a more complete description of the intended use of proceeds from
this offering. |
| |
|
|
| Lock
Up Agreements |
|
We
and our executive officers, directors and
certain of our stockholders have agreed with the underwriters not to sell, transfer or dispose of any shares or similar securities
for a period of three months from the date of this prospectus. For additional information regarding our arrangement with the
underwriters, please see “Underwriting.” |
| Risk
factors |
|
Investing
in our securities is highly speculative and involves a high degree of risk. See “Risk Factors” beginning on page
13 of this prospectus, and the other information included in this prospectus for a discussion of factors you should
consider carefully before deciding to invest in our securities. |
| |
|
|
| Nasdaq
Capital Market Listing |
|
Our
common stock is listed on The Nasdaq Capital Market under the symbol “AWH”. There is no established trading market
for the Pre-funded Warrants, and we do not expect a trading market to develop. We do not intend to list the Pre-funded Warrants on
any securities exchange or nationally recognized trading system. |
(1)
The number of shares of our common stock to be outstanding after this offering is based on 17,529,793 shares of our common stock
outstanding as of January 7, 2025, and excludes:
| |
● |
4,475,068
shares of common stock issuable upon exercise of warrants with a weighted average exercise price of $2.90; |
| |
● |
853,862
shares of common stock issuable upon exercise
of options with a weighted average exercise price of $6.10; |
| |
● |
134,060
shares of common stock issuable upon vesting
of outstanding restricted stock units; |
| |
● |
553,000
shares of common stock reserved for future issuance
under our existing stock incentive plan; |
| |
● |
800,000 shares
of common stock (or 920,000 shares if the underwriters exercise their over-allotment option in full) issuable
upon exercise of warrants to be issued to the representative of the underwriters as part of this offering at an exercise price of
$0.9375 (assuming a public offering price of $0.75 per share, the last reported sale price of our common stock as reported
on The Nasdaq Capital Market on January 7, 2025. |
Unless
otherwise indicated, all information in this prospectus assumes:
| ● | no
exercise of the warrants or options described above; and |
| ● | no
exercise by the underwriters of their option to purchase an additional 2,400,000 shares
of common stock and/or Pre-funded Warrants to cover over-allotments, if any. |
RISK
FACTORS
An
investment in our securities involves a high degree of risk. Prior to making a decision about investing in our securities, you should
carefully consider the risk factors described below and the risk factors discussed in the sections entitled “Risk Factors”
contained in our most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, as may be amended, supplemented or superseded
from time to time by other reports we file with the SEC and incorporated by reference in this prospectus, together with all of the other
information contained in this prospectus. Additional risks and uncertainties not presently known to us, or that we currently view as
immaterial, may also impair our business. If any of the risks or uncertainties described in our SEC filings or this prospectus or any
additional risks and uncertainties actually occur, our business, financial condition and results of operations could be materially and
adversely affected. In that case, the trading price of our common stock could decline, and you might lose all or part of your investment.
Risks
Related to this Offering and Our Common Stock
Our
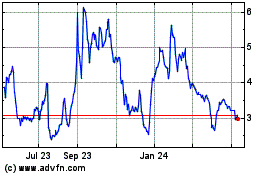
stock price has been, and may continue to be, highly volatile.
The
trading price of our common stock has been highly volatile. During the 12 months ended December 31, 2023, the closing trading price of
our common stock ranged from a high of $8.70 per share to a low of $2.40 per share. Between January 1, 2024, and December 31,
2024, the closing trading price of our common stock ranged from $5.63 to $0.70. The trading price of our common stock could continue
to be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including:
| ● | failure
to significantly increase revenue and volumes of OvaSuite or Aspira Synergy; |
| ● | actual
or anticipated period-to-period fluctuations in financial results; |
| ● | failure
to achieve, or changes in, financial estimates by securities analysts; |
| ● | announcements
or introductions of new products or services or technological innovations by us or our competitors; |
| ● | failure
to complete clinical studies that validate clinical utility sufficiently to increase positive
medical policy among payers at large; |
| ● | publicity
regarding actual or potential discoveries of biomarkers by others; |
| ● | comments
or opinions by securities analysts or stockholders; |
| ● | the
ability to maintain the listing of our common stock and public warrants on The Nasdaq Capital
Market; |
| ● | conditions
or trends in the pharmaceutical, biotechnology or life science industries; |
| ● | announcements
by us of significant acquisitions and divestitures, strategic partnerships, joint ventures
or capital commitments; |
| ● | developments
regarding our patents or other intellectual property or that of our competitors; |
| ● | litigation
or threat of litigation; |
| ● | additions
or departures of key personnel; |
| ● | limited
daily trading volume; |
| ● | our
ability to continue as a going concern; |
| ● | economic
and other external factors, disasters or crises; and |
| ● | our
announcement of future fundraisings. |
In
addition, the stock market in general and the market for diagnostic technology companies, in particular, have experienced significant
price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. These
broad market and industry factors may adversely affect the market price of our common stock, regardless of our operating performance.
In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation
has often been instituted. A securities class action suit against us could result in substantial costs, potential liabilities and the
diversion of our attention and our resources.
Management
will have broad discretion as to the use of the net proceeds from this offering, and we may not use the proceeds effectively.
Our
management will have broad discretion as to the application of the net proceeds from this offering and could use them for purposes other
than those contemplated at the time of this offering. Our stockholders may not agree with the manner in which our management chooses
to allocate and spend the net proceeds. Moreover, our management may use the net proceeds for corporate purposes that may not have a
positive impact on our results of operations or increase the market value of our common stock. Our failure to apply these funds effectively
could have a material adverse effect on our business, delay the development of our products and/or cause the price of our common stock
to decline.
You
may experience immediate and substantial dilution in the net tangible book value per share of the common stock you purchase in the offering.
The
offering price per share in this offering may exceed the net tangible book value per share of our common stock outstanding prior to this
offering. After giving effect to the sale by us of 16,000,000 shares of common stock at an assumed public offering
price of $0.75 per share, the last reported sale price of our common stock as reported on The Nasdaq Capital Market on January 7, 2025 after deducting commissions and estimated offering expenses payable by us and assuming no sale of any Pre-funded Warrants,
you will experience immediate dilution of $0.480 per share, representing the difference between our net tangible book value per
share as of September 30, 2024 after giving effect to this offering and the offering price. The exercise of outstanding warrants and
stock options may also result in further dilution of your investment. See the section entitled “Dilution” on page 17 below for a more detailed illustration of the dilution you may incur if you participate in this offering.
If
we raise additional capital in the future, your ownership in us could be diluted.
In
order to raise additional capital, we may offer additional shares of common stock or other securities convertible into or exchangeable
for our common stock. We may sell shares or other securities in any other offering at a price per share that is less than the price per
share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior
to existing stockholders, including investors who purchase common stock in this offering. The price per share at which we sell additional
shares of common stock or securities convertible into common stock in future transactions may be higher or lower than the price per share
in this offering.
Until
such time, if ever, as we can generate substantial revenue from our operations, we anticipate financing our cash needs through a combination
of equity offerings, debt financings and license agreements. To the extent that we raise additional capital through the further sale
of equity securities or convertible debt securities, your ownership interest will be diluted.
Sales
of a substantial number of our shares of common stock in the public market could cause our stock price to fall.
We
may issue and sell additional shares of common stock in the public markets. Sales of a substantial number of shares of our common stock
in the public markets or the perception that such sales could occur could depress the market price of our common stock and impair our
ability to raise capital through the sale of additional equity securities.
Because
we do not currently intend to declare cash dividends on our shares of common stock in the foreseeable future, stockholders must rely
on appreciation of the value of our common stock for any return on their investment.
We
have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to
finance the operation, development and growth of our business. Furthermore, any future debt agreements may also preclude us from paying
or place restrictions on our ability to pay dividends. As a result, capital appreciation, if any, of our common stock will be your sole
source of gain with respect to your investment for the foreseeable future.
The
exercise of our outstanding options and warrants will dilute stockholders and could decrease our stock price.
The
exercise of our outstanding options and warrants may adversely affect our stock price due to sales of a large number of shares or the
perception that such sales could occur. These factors also could make it more difficult to raise funds through future offerings of our
securities, and could adversely impact the terms under which we could obtain additional equity capital. Exercise of outstanding options
and warrants or any future issuance of additional shares of common stock or other equity securities, including, but not limited to, options,
warrants, restricted stock units or other derivative securities convertible into our common stock, may result in significant dilution
to our stockholders and may decrease our stock price.
If
we fail to comply with the continued listing requirements of The Nasdaq Capital Market, our common stock may be delisted and the price
of our common stock and our ability to access the capital markets could be negatively impacted.
Our
common stock is currently listed on The Nasdaq Capital Market and the continued listing of our common stock on The Nasdaq Capital Market
is contingent on our continued compliance with a number of listing requirements. If we are unable to comply with the continued listing
requirements of The Nasdaq Capital Market, our common stock would be delisted from The Nasdaq Capital Market, which would limit investors’
ability to effect transactions in our common stock and subject us to additional trading restrictions. In order to maintain our listing,
we must maintain certain share prices, financial and share distribution targets, including maintaining a minimum amount of stockholders’
equity and a minimum number of public stockholders, as well as satisfying other listing requirements of The Nasdaq Capital Market. In
addition to these objective standards, The Nasdaq Capital Market may delist the securities of any issuer for other reasons involving
the judgment of The Nasdaq Capital Market.
On
July 1, 2024, we received a deficiency letter (the “Notice”) from the Listing Qualifications Department of The Nasdaq Stock
Market, LLC (“Nasdaq”) stating that for the 30 consecutive business days prior to the date of the Notice, our Market
Value of Listed Securities was below the minimum of $35 million required for continued listing on Nasdaq pursuant to Nasdaq Listing
Rule 5550(b)(2) (the “MVLS Requirement”). To regain compliance with the MVLS Requirement, the market value of our common
stock must have met or exceeded $35.0 million for a minimum of 10 consecutive business days during the 180-day grace period
ending on December 30, 2024 (the “MVLS Compliance Date”), unless the Staff of Nasdaq exercises its discretion to extend this
10 consecutive business day period. As of the date of this prospectus, we were unable to regain compliance by the MVLS Compliance Date.
As such, on December 31, 2024, Nasdaq notified us that our securities are subject to delisting. While we have requested an appeal
of Nasdaq’s delisting determination, no assurance can be provided that we will be successful in appealing such determination
and maintaining the listing of our common stock on The Nasdaq Capital Market.
Furthermore,
on October 17, 2024, we received written notice from Nasdaq that we were not in compliance with Nasdaq Listing Rule 5550(a)(2), as the
minimum bid price of our common stock had been below $1.00 per share for 30 consecutive business days. In accordance with Nasdaq
Listing Rule 5810, and assuming our common stock is not delisted for our failure to satisfy the MVLS Requirement by the MVLS Compliance
Date, we will have a period of 180 calendar days, or until April 15, 2025, to regain compliance with the minimum bid price requirement
and market value of common stock requirement. To regain compliance with the Nasdaq bid price requirement, the closing bid price of our
common stock must meet or exceed $1.00 per share for at least 10 consecutive business days during this 180- calendar day period. In the
event we do not regain compliance by April 15, 2025, we may be eligible for an additional 180 calendar day grace period.
There
is no assurance that we will be able to maintain compliance with The Nasdaq Capital Market continued listing standards and/or continue
our listing on The Nasdaq Capital Market in the future.
If
The Nasdaq Capital Market delists our common stock from trading on its exchange and we are not able to list our securities on another
national securities exchange, we expect our common stock would qualify to be quoted on an over-the-counter market. If this were to occur,
we could face significant material adverse consequences, including:
| ● | a
limited availability of market quotations for our securities; |
| ● | reduced
liquidity for our securities; |
| ● | substantially
impair our ability to raise additional funds; |
| ● | the
loss of institutional investor interest and a decreased ability to issue additional securities
or obtain additional financing in the future; |
| ● | a
determination that our common stock is a “penny stock,” which will require brokers
trading in our common stock to adhere to more stringent rules and possibly result in a reduced
level of trading activity in the secondary trading market for our securities; |
| ● | a
limited amount of news and analyst coverage; and |
| ● | potential
breaches of representations or covenants of our agreements pursuant to which we made representations
or covenants relating to our compliance with applicable listing requirements, which, regardless
of merit, could result in costly litigation, significant liabilities and diversion of our
management’s time and attention and could have a material adverse effect on our financial
condition, business and results of operations. |
There
is no established public trading market for the Pre-funded Warrants being offered in this offering, and we do not expect a market to
develop for the Pre-funded Warrants.
There
is no established public trading market for the Pre-funded Warrants being offered in this offering, and we do not expect a market to
develop. In addition, we do not intend to apply to list the Pre-funded Warrants on any national securities exchange or other nationally
recognized trading system. Without an active market, the liquidity of the Pre-funded Warrants will be limited. Further, the existence
of the Pre-funded Warrants may act to reduce both the trading volume and the trading price of our common stock.
The
Pre-funded Warrants are speculative in nature.
Except
as otherwise provided in the Pre-funded Warrants, until holders of Pre-funded Warrants acquire our common stock upon exercise of the
Pre-funded Warrants, holders of Pre-funded Warrants will have no rights with respect to our common stock underlying such Pre-funded Warrants.
Upon exercise of the Pre-funded Warrants, the holders will be entitled to exercise the rights of a stockholder of our common stock only
as to matters for which the record date occurs after the exercise date.
Moreover,
following this offering, the market value of the Pre-funded Warrants is uncertain. There can be no assurance that the market price of
our common stock will ever equal or exceed the price of the Pre-funded Warrants, and, consequently, whether it will ever be profitable
for investors to exercise their Pre-funded Warrants.
USE
OF PROCEEDS
We
estimate that the net proceeds from our issuance and sale of shares of our common stock and Pre-funded Warrants in this offering will
be approximately $10.6 million, or approximately $12.3 million if the underwriters exercise their option to purchase additional
securities in full, based on an assumed public offering price of $0.75 per share, which was the closing price of shares of
our common stock on The Nasdaq Capital Market on January 7, 2025, after deducting estimated underwriting discounts
and commissions and estimated offering expenses payable by us assuming no sale of the Pre-funded Warrants.
We
intend to use the net proceeds from this offering for working capital and general corporate purposes. We may also use a portion of the
net proceeds to in-license, acquire or invest in complementary businesses, technologies or products, however, we have no current commitments
or obligations to do so.
A
$0.25 increase or decrease in the assumed public offering price of $0.75 per share would increase or decrease the net proceeds
from this offering by approximately $4.0 million, assuming that the number of shares offered by us, as set forth on the cover
page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions. An increase (decrease)
of 5,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease)
the net proceeds from this offering by approximately $3.8 million, assuming no change in the assumed public offering price per
share and after deducting estimated underwriting discounts and commissions.
Although
we have identified some potential uses of the net proceeds to be received from this offering, we cannot specify these uses with certainty.
Our management will have broad discretion in the application of the net proceeds from this offering and could use them for purposes other
than those contemplated at the time of this offering. Our stockholders may not agree with the manner in which our management chooses
to allocate and spend the net proceeds. Moreover, our management may use the net proceeds for corporate purposes that may not positively
impact our results of operations or increase the market value of our common stock.
Pending
any use, as described above, we plan to deposit the net proceeds in money market accounts with our primary bank or otherwise invest the
net proceeds in high-quality, short-term, interest-bearing securities.
DILUTION
If
you invest in our common stock in this offering, your ownership interest will be diluted to the extent of the difference between the
offering price per share of our common stock and the as adjusted net tangible book value per share of our common stock immediately after
the offering. Historical net tangible book value per share represents the amount of our total tangible assets less total liabilities,
divided by the number of shares of our common stock outstanding.
The
historical net tangible book value of our common stock as of September 30, 2024 was approximately ($2.5 million) or ($0.155) per share
based on 16,284,381 shares of common stock outstanding on such date. Historical net tangible book value per share represents the
amount of our total tangible assets reduced by the amount of our total liabilities, divided by the total number of shares of common stock
outstanding.
After
giving effect to the sale of 1,180,722 shares of our common stock pursuant to that certain At The Market Agreement with H.C. Wainwright
& Co., LLC dated as of August 2, 2024 for proceeds of approximately $939,000, and after deducting the estimated sales agent
fees and offering expenses payable by us, from October 23, 2024 to January 7, 2025, our pro forma net tangible book value
at September 30, 2024 would have been ($1.6 million), or ($0.091) per share of common stock.
After
giving further effect to the sale of shares of our common stock and Pre-funded Warrants in this offering at an assumed public offering
price of $0.75 per share, which was the closing price of shares of our common stock on The Nasdaq Capital Market on January
7, 2025 and after deducting the estimated underwriting discounts and commissions and other estimated offering expenses payable
by us and assuming no sale of the Pre-funded Warrants, our pro forma as adjusted net tangible book value at September 30, 2024 would
have been $9.0 million, or $0.270 per share of common stock. This represents an immediate increase in pro forma
as adjusted net tangible book value of $0.360 per share to existing stockholders and immediate dilution of $0.480 per share
to new investors purchasing shares of our common stock in this offering.
The
following table illustrates this dilution on a per share basis:
| Assumed public offering price per share | |
| | | |
$ | 0.750 |
|
| Pro forma net tangible book value per share as of
September 30, 2024 | |
$ | (0.091 | ) | |
| |
|
| Pro forma increase in
net tangible book value per share attributable to new investors in this offering | |
| 0.360 | | |
| |
|
| | |
| | | |
| |
|
| Pro forma as adjusted
net tangible book value per share immediately after this offering | |
| | | |
| 0.270 |
|
| | |
| | | |
| |
|
| Dilution per share to
new investors in this offering | |
| | | |
$ | 0.480 |
|
A
$0.25 increase (decrease) in the assumed public offering price of $0.75 per share, which was the closing price of our common
stock on The Nasdaq Capital Market on January 7, 2025, would increase (decrease) our pro forma as adjusted net tangible
book value after this offering by $0.160 per share and the dilution to new investors purchasing shares of our common
stock in this offering by $0.110 per share, assuming that the number of shares offered by us, as set forth on the cover
page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions. An increase (decrease)
of 5,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase our
pro forma as adjusted net tangible book value after this offering by $0.074 per share and decrease the dilution to new
investors purchasing shares of our common stock in this offering by $0.026 per share, assuming no change in the assumed
public offering price per share and after deducting estimated underwriting discounts and commissions.
If
the underwriters exercise their option to purchase additional shares and/or Pre-funded Warrants in full, the pro forma as adjusted net
tangible book value per share after giving effect to the offering would be $0.298 per share. This represents an increase in net
tangible book value of $0.389 per share to existing stockholders and dilution in net tangible book value of $0.452 per
share to new investors.
To
the extent that stock options or warrants are exercised, new stock options are issued under our equity incentive plan, or we issue additional
common stock in the future, there will be further dilution to investors participating in this offering. In addition, we may choose to
raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for
our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the
issuance of these securities could result in further dilution to our stockholders.
The
number of shares of our common stock to be outstanding after this offering is based on 16,284,381 shares of our common stock outstanding
as of September 30, 2024, assumes no exercise by the underwriters of their over-allotment option or the sale of Pre-funded Warrants and
excludes:
| ● | 4,475,068
shares of common stock issuable upon exercise of warrants with a weighted average exercise
price of $2.90; |
| ● | 839,450
shares of common stock issuable upon exercise of options with a weighted average exercise
price of $8.28; |
| ● | 153,750
shares of common stock issuable upon vesting of outstanding restricted stock units; |
| ● | 642,865
shares of common stock reserved for future issuance under our existing stock incentive plan; |
| ● | 800,000
shares
of common stock (or 920,000 shares if the representative exercises its over-allotment
option in full) issuable upon exercise of warrants to be issued to the representative of
the underwriters as part of this offering at an exercise price of $0.9375 (assuming
a public offering price of $0.75 per share, the last reported sale price of our common
stock as reported on The Nasdaq Capital Market on January 7, 2025. |
MANAGEMENT
Directors
and Executive Officers
The
following table sets forth the name, age and position of each of our executive officers and directors as of January 7, 2025.
| Name |
|
Age |
|
Position
with Aspira |
| Sandra
Milligan, M.D., J.D. |
|
61 |
|
Interim
Chief Executive Officer and President |
| John
Kallassy |
|
60 |
|
Interim
Chief Financial Officer |
| Stefanie
Cavanaugh |
|
60 |
|
Director |
| Celeste
R. Fralick, Ph.D. |
|
69 |
|
Director |
| Jannie
Herchuk |
|
62 |
|
Chair
of the Board of Directors |
| Ellen
O’ Connor Vos |
|
69 |
|
Director |
| Winfred
Parnell, M.D. |
|
72 |
|
Director |
| John
Ragard |
|
70 |
|
Director |
Sandra
Milligan, Chief Executive Officer and President. Sandra Milligan has served as our Interim Chief Executive Officer since
December 16, 2024, and President since April 1, 2024. Dr. Milligan has over twenty-five years of experience in the healthcare
sector. Prior to joining the Company, Dr. Milligan served as Executive Vice President, Research and Development at Organon (NYSE:
OGN). Prior to that, she served in executive roles of increasing responsibility at innovative biopharmaceutical companies such as
Amgen (Nasdaq: AMGN), Genentech and Merck (NYSE: MRK). She has demonstrated success in supporting pipeline and product development
in both oncology and non-oncology disease areas, with recent emphasis in women’s health. Dr. Milligan is a known and respected
leader in women’s health who drives cross-functional strategies to achieve corporate success. Dr. Milligan earned a Doctor of
Medicine from the George Washington University School of Medicine as well as a Juris Doctor from Georgetown University Law Center.
After serving as a General Medical Officer in the US Army Medical Corps, she began her corporate career as an attorney in healthcare
and corporate law prior to joining the pharmaceutical industry. Dr. Milligan also currently serves on the boards of Gossamer Bio
(Nasdaq: GOSS) and Spyre Therapeutics (Nasdaq: SYRE).
John
Kallassy, Interim Chief Financial Officer. John Kallassy has served as Interim Chief Financial Officer of the Company since
August 2024. He is a seasoned Wall Street veteran having served in senior executive positions for over four decades. Mr. Kallassy
served as Chief Financial Officer of Torigen Pharmaceuticals from January 2018 to January 2024; Chief Financial Officer of
Integrated Animal Health from May 2016 to August 2017; Chief Operating and Financial Officer of Jaguar Health (Nasdaq: JAGX) from
November 2013 to April 2016, where he oversaw the company’s Nasdaq IPO. Earlier in his career, Mr. Kallassy held various roles
at Speedus Corp from 2000 to 2011, including Chief Executive Officer of its subsidiary, Zargis Medical and Chief Financial Officer.
Mr. Kallassy co-founded Bactana Corp in 2016, where he continues to serve as its Chief Executive Officer. In addition to his
corporate roles, Mr. Kallassy currently serves as an Executive-in-Residence at the University of Connecticut and Managing Member of
Aimers Venture Partners. Mr. Kallassy holds a Master of Business Administration from Cornell University, completed graduate
coursework in pharmacology at the University of Leeds, and earned a Bachelor of Science in Biology from the State University of New
York.
Stefanie
Cavanaugh, Director. Stefanie Cavanaugh has served as a director of the Company since May 9, 2023. Ms. Cavanaugh has been a senior
financial executive for healthcare companies for over 30 years. Ms. Cavanaugh has served as Chief Financial Officer of Giving Home Health
Care, an at-home healthcare services company, since August 2022. Before her tenure at Giving Home Health Care, Ms. Cavanaugh was the
Chief Financial Officer of Apollo Endosurgery (Nasdaq: APEN), a publicly traded medical technology company, from February 2015 to January
2022. After completing Apollo’s initial public offering via a reverse merger, she worked closely with the board and Chief Executive
Officer to develop guidance and investor communications raising over $170 million in debt and equity financing to support Apollo’s
strategic and organizational objectives. Ms. Cavanaugh began her career as an auditor at Ernst & Young after earning her B.B.A. in
Accounting and Finance at the University of Texas at Austin. Ms. Cavanaugh is a licensed CPA in the state of Texas. Our Board has determined
that based upon Ms. Cavanaugh’s extensive financial expertise and leadership and her extensive experience in the medical industry,
she has the qualifications and skills to serve as a member of our Board.
Celeste
Fralick, Director. Celeste Fralick has served as a director of the Company since February 2022. Dr. Fralick has served as Chief
Technology & Data Officer, Technology Design of Choir Power since February 2023. Additionally, since 2018, Dr. Fralick has served
as Chief Data Officer of Innovatio HealthDesign, a privately held health predictive analytics company. Previously, from 2017 to 2022,
Dr. Fralick served as Chief Data Scientist at McAfee, a cybersecurity company, which was spun-off from Intel Corporation in 2017. Prior
to her position at McAfee, Dr. Fralick served as Principal Engineer and Chief Technology Officer at Intel (Nasdaq: INTC) from 2015 to
2017. She has also served as Managing Principal at Prukinje Science and Technology, LLC, a privately held data analytics consulting business,
from 2019 to 2022. Dr. Fralick earned a Bachelor of Science in Microbiology and Chemistry from Texas Tech University and a Ph.D. in Bioengineering
from Arizona State University. Our Board has determined that based upon Dr. Fralick’s extensive executive-level leadership and
management experience in technology and health sciences, she has the qualifications and skills to serve as a member of our Board.
Jannie Herchuk,
Chair of the Board of Directors. Jannie Herchuk has served as a director of the Company since May 9, 2023 and Chair of the
Company’s Board since December 27, 2023. Ms. Herchuk retired from Deloitte & Touche LLP in 2022 after nearly 40
years of audit experience. Ms. Herchuk served on the Deloitte LLP board of directors and led strategic initiatives and practices. As
the audit partner for some of the firm’s most important healthcare and life sciences companies, Ms. Herchuk oversaw her
client’s preparation of financial statements and led audit engagements for public and private companies. Ms. Herchuk has
routinely attended client board and audit committee meetings throughout her career. Ms. Herchuk received her B.B.A. in Accounting
from Texas A&M University and is a licensed CPA in the state of Texas. Our Board has determined that based upon Ms.
Herchuk’s extensive industry experience and financial accounting, SEC reporting, internal controls, and auditing expertise,
she qualifies to serve as a member of our Board.
Ellen
O’Connor Vos, Director. Ellen O’Connor Vos has served as a director of the Company since May 9, 2023. Ms. Vos has
served as president of VosHealth LLC, a healthcare consultancy firm since November 2022. From August 2021 until February 2022, she served
as chief executive officer of Modular Medical (Nasdaq: MODD), a development state insulin pump company. Previously she served as the
President and Chief Executive Officer of the Muscular Dystrophy Association, the leader of genetic/neuromuscular and amyotrophic lateral
sclerosis research, patient care and advocacy from October 2017 to November 2020. Previously, she spent almost 30 years as Chief Executive
Officer of Greyhealth Group (and its predecessor company), where she built a global healthcare communications firm and worked on launches
for clients across the pharmaceutical, biotech, surgical, and diagnostic sectors. Ms. Vos currently serves as the Board Chair for OptimizeRX
(Nasdaq: OPRX) and also serves on the board for Modular Medical. She previously served on the board of nTelos (Nasdaq: NTLS), prior to
the sale to Shentel, and on the boards of several WPP investment portfolio companies. She has significant nonprofit board experience
with The Jed Foundation, Multiple Myeloma Research Foundation, Healthcare Businesswomen’s Association and a trustee of The Windward
School and the YMCA of the City of New York. Ms. Vos was named the Woman of the Year by the Healthcare Businesswomen’s Association
in 2005. She was also a member of the Kraft Precision Medicine Accelerator at Harvard Business School focused on innovation and improving
outcomes in oncology and rare diseases. She earned a Bachelor of Science in Nursing from Alfred University. Our Board has determined
that based upon Ms. Vos’s extensive experience in the healthcare industry, including relevant experience as a current and prior
director and as an executive officer, she has the qualifications and skills to serve as a member of our Board.
Winfred
Parnell, Director. Winfred Parnell has served as a director of the Company since June 1, 2023. Dr. Parnell is a board-certified
physician in obstetrics and gynecologic care bringing more than 20 years of board experience and expertise in strategic planning, governance,
cultural transformation, regulatory & compliance, quality of care, and crisis management. Dr. Parnell was a founding partner of Carlos
& Parnell, M.D., P.A., an obstetrics and gynecology practice in Dallas, Texas. Since January 2021, Dr. Parnell serves on the board
of private equity-backed SCA Pharm, one of the largest compounding pharmacies in the country. Dr. Parnell is a graduate of Florida A&M
University and received his MD from the University of Florida College of Medicine. He completed his internship and residency training
at Parkland Hospital in Dallas, TX and holds an Executive Certificate in Non-Profit Governance. Our Board has determined that based upon
Dr. Parnell’s extensive experience in gynecologic care and as a current and prior director, he has the qualifications and skills
to serve as a member of our Board.
John
Ragard, Director. John Ragard has served as a director of the Company since July 25, 2024 and was designated by Jack W. Schuler,
a stockholder of the Company and holding the right to designate an individual to be nominated to the Board pursuant to the Stockholders
Agreement dated May 13, 2013 by and among the Company and the purchasers of the Company’s common stock and warrants named therein.
Mr. Ragard has served as a Senior Investment Advisor at Wayve Capital Management (“Wayve”), an SEC-registered investment
firm specializing in strategic public and private market investments for high-net-worth individuals, family offices and institutions,
since June 2024 and has over 46 years as a venture capital investor, public equity buy-side analyst, and portfolio manager. Prior to
joining Wayve, Mr. Ragard was a portfolio manager at Spouting Rock from October 2017 until February 2024, where he co-managed a small-cap
growth portfolio that outperformed the benchmark over the 5-year and since-inception periods. Mr. Ragard received his Bachelor of Science
in Economics cum laude from the Wharton School and is a Chartered Financial Analyst. Our Board has determined that based upon his extensive
experience with financial markets and investors, he has the qualifications and skills to serve as a member of our Board.
Family
Relationships
There
are no family relationships among any of our executive officers or directors.
Director
Independence
As
required under the Nasdaq listing standards, a majority of the members of a listed company’s board of directors must qualify as
“independent,” as affirmatively determined by the Board. The Board has affirmatively determined, after considering all relevant
facts and circumstances, that each of Stefanie Cavanaugh, Celeste R. Fralick, Jannie Herchuk, Ellen O’Connor Vos, Winfred Parnell
and John Ragard is an independent director, as the term is currently defined under Nasdaq Listing Rule 5605(a)(2).
Committees
of Our Board of Directors
Our
board of directors directs the management of our business and affairs, as provided by Delaware law, and conducts its business through
meetings of the board of directors and its standing committees. We will have a standing audit committee, compensation committee and nominating
and corporate governance committee. In addition, from time to time, special committees may be established under the direction of the
board of directors when necessary to address specific issues.
Audit
Committee
The
Audit Committee of the Board was established by the Board to oversee our corporate accounting and financial reporting processes, systems
of internal control over financial reporting and the quality and integrity of our financial statements and reports. In addition, the
Audit Committee oversees the qualification, independence and performance of our independent registered public accounting firm. The Audit
Committee also recommends to the Board the appointment of our independent registered public accounting firm.
The
Audit Committee is currently composed of three directors: Ms. Herchuk (Chair), Ms. Cavanaugh and Ms. O’Connor Vos. The Audit Committee
is governed by a written charter adopted by the Board. The Audit Committee charter can be found in the Investors - Governance section
of our website at www.aspirawh.com. The Board has determined that all members of our Audit Committee are independent pursuant
to applicable Nasdaq and SEC requirements. The Board has also determined that Ms. Herchuk qualifies as an “audit committee financial
expert,” as defined in applicable SEC rules. In making the determination that Ms. Herchuk qualifies as an “audit committee
financial expert,” the Board made a qualitative assessment of Ms. Herchuk’s level of knowledge and experience based on a
number of factors, including her education as well as her experience as an audit partner.
Compensation
Committee
The
Compensation Committee of the Board acts on behalf of the Board to review, adopt and oversee our compensation strategy, policies, plans
and programs. The Compensation Committee approves, or recommends for approval, the compensation (i.e., salary, bonus and stock-based
compensation grants) and other terms of employment or service of our Chief Executive Officer and other executive officers and administers
the 2019 Plan. The Compensation Committee also reviews and recommends for the Board’s approval the compensation for members of
the Board. The Compensation Committee has the authority to retain compensation consultants to assist in its evaluation of executive and
director compensation, including the authority to approve the consultant’s reasonable fees and other retention terms. The Compensation
Committee may form and delegate authority to subcommittees when appropriate. Any such subcommittee, to the extent provided in the resolutions
of the Compensation Committee and to the extent not limited by applicable law, will have and may exercise all the powers and authority
of the Compensation Committee.
Our
executive officers recommend to the Compensation Committee of the Board business performance targets and objectives and provide background
information about the Company’s underlying strategic objectives. Our Chief Executive Officer generally makes recommendations to
the Compensation Committee regarding salary increases for other executive officers during the regular merit increase process. Our executive
officers are not present or involved in deliberations concerning their own compensation.
The
Compensation Committee is currently composed of three directors: Ms. Cavanaugh (Chair), Mr. John Ragard and Dr. Parnell. The Board has
determined that the members of our Compensation Committee are independent pursuant to applicable Nasdaq and SEC requirements. The Compensation
Committee has adopted a written charter that can be found in the Investors - Governance section of our website at www.aspirawh.com.
Nominating
and Governance Committee
The
Nominating and Governance Committee is responsible for identifying individuals qualified to serve as members of the Board, recommending
to the Board nominees for election as our directors, and providing oversight with respect to corporate governance and ethical conduct.
Our
Nominating and Governance Committee currently consists of Dr. Fralick (Chair), Mr. John Ragard and Dr. Parnell. The Board has determined
that the members of our Nominating and Governance Committee are independent pursuant to applicable Nasdaq listing standards. The Nominating
and Governance Committee has adopted a written charter that can be found in the Investors - Governance section of our website at www.aspirawh.com.
Code
of Business Conduct and Ethics
We
have adopted the Aspira Women’s Health Inc. Code of Business Conduct and Ethics (the “Code of Ethics”) that applies
to all of our officers, directors, employees, agents, contractors, and consultants. The Code of Ethics is available in the Investors
- Governance section of our website at www.aspirawh.com. We intend to disclose on our website any waiver of, or amendment
to, the Code of Ethics as required by applicable SEC and Nasdaq requirements.
Clawback
Policy
As
a public company, if we are required to restate our financial results due to our material noncompliance with any financial reporting
requirements under the federal securities laws as a result of misconduct, the Chief Executive Officer and Chief Financial Officer may
be legally required to reimburse our Company for any bonus or other incentive-based or equity-based compensation they receive in accordance
with the provisions of Section 304 of the Sarbanes-Oxley Act of 2002, as amended. Additionally, we have implemented a Dodd-Frank Act-compliant
clawback policy, as required by SEC rules.
EXECUTIVE
AND DIRECTOR COMPENSATION
The
following individuals who served as executive officers of the Company during 2024 were our “Named Executive Officers” for
2024:
| Name |
|
Positions |
| Sandra
Milligan, M.D., J.D.(1) |
|
President
and Interim Chief Executive Officer |
| John
Kallassy (2) |
|
Interim
Chief Financial Officer |
| Torsten
Hombeck, Ph.D.(3) |
|
Former Chief Financial Officer and Senior Vice President |
| Minh
Merchant(4) |
|
Former
General Counsel and Corporate Secretary |
| Nicole
Sandford(5) |
|
Former Chief Executive Officer, President and Director |
| (1) | Dr.
Milligan commenced employment as our President effective April 1, 2024, and the amount reported
reflects the portion of her annual base salary earned in 2024 from such date. Dr. Milligan
was appointed as the Company’s Chief Executive Officer effective December 16, 2024. |
| (2) | Mr.
Kallassy commenced employment as our Interim Chief Financial Officer effective August 15,
2024, and the amount reflects the portion of his contracted salary earned in 2024 from such
date. |
| (3) | Dr.
Hombeck commenced employment as our Chief Financial officer on June 15, 2023, and the amount
reported reflects the portion of his annual base salary earned in 2023 from such date. Dr.
Hombeck’s employment terminated with the Company effective June 27, 2024,
and the amount reported reflects the portion of his annual base salary earned in 2024 through
such date. |
| (4) | Ms.
Merchant’s employment terminated with the Company effective May 6, 2024, and the amount
reported in 2024 reflects the portion of her annual base salary earned in 2024 through such
date. |
| (5) | Ms.
Sandford’s employment terminated with the Company effective December 16, 2024, and
the amount reported in 2024 reflects the portion of her annual base salary earned in 2024
through such date. |
2024
Summary Compensation Table
The
compensation earned by the Named Executive Officers for the years ended December 31, 2024 and December 31, 2023 was as follows:
| Name and Principal Position |
|
Year |
|
Salary |
|
|
Bonus |
|
|
Stock
Awards(1) |
|
|
Option
Awards(1) |
|
|
Non-Equity Incentive Plan(2) |
|
|
All Other
Compensation |
|
|
Total |
|
| Sandra Milligan, M.D., J.D. |
|
2024 |
|
$ |
268,926 |
(3) |
|
$ |
- |
|
|
$ |
18,800 |
|
|
$ |
70,703 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
358,429 |
|
| President, Interim Chief Executive Officer |
|
2023 |
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
| John Kallassy |
|
2024 |
|
$ |
62,500 |
(4) |
|
$ |
- |
|
|
$ |
- |
|
|
$ |
20,355 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
82,855 |
|
| Interim Chief Financial Officer |
|
2023 |
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
| Torsten Hombeck |
|
2024 |
|
$ |
177,404 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
97,800 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
275,204 |
|
| Former Chief Financial Officer |
|
2023 |
|
$ |
177,500 |
|
|
$ |
- |
|
|
$ |
97,500 |
(5) |
|
$ |
42,762 |
(6) |
|
$ |
37,000 |
|
|
$ |
10,900 |
(7) |
|
$ |
365,662 |
|
| Minh Merchant |
|
2024 |
|
$ |
126,250 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
33,220 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
159,470 |
|
| Former General Counsel & Secretary |
|
2023 |
|
$ |
325,000 |
|
|
$ |
50,000 |
(8) |
|
$ |
32,500 |
(5) |
|
$ |
96,595 |
|
|
$ |
48,750 |
|
|
$ |
9,656 |
(9) |
|
$ |
562,501 |
|
| Nicole Sandford |
|
2024 |
|
$ |
466,667 |
|
|
$ |
- |
|
|
$ |
23,500 |
|
|
$ |
224,020 |
|
|
$ |
135,000 |
|
|
$ |
393,093 |
(10) |
|
$ |
849,187 |
|
| Former President, Chief Executive Officer |
|
2023 |
|
$ |
500,000 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
392,074 |
|
|
$ |
112,500 |
|
|
$ |
31,016 |
(9) |
|
$ |
1,035,590 |
|
(1)
Represents restricted stock unit (“RSU”) and option awards
granted to the Named Executive Officers. The amounts reported in this column are valued based on the aggregate grant date fair value computed
in accordance with Accounting Standards Codification (“ASC”) Topic 718. Additional information regarding the assumptions made
in calculating these amounts will be provided in Note 9, Employee Share Based Compensation and Benefit Plans, to the consolidated financial
statements to be included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024.
(2)
Amount represents annual incentive bonus earned for 2024 and 2023. The amount of annual incentive bonuses earned for Dr.
Milligan is not yet calculable. The Company expects that the amount of annual incentive bonus earned for Dr. Milligan
will be calculable on or about February 15, 2025.
(3)
Represents cash paid to Dr. Milligan for consulting services prior to her joining the Company.
(4)
Represents cash paid for consulting under the terms
of the consulting agreement with Mr. Kallassy.
(5)
Represents RSUs awarded to Dr. Hombeck and
Ms. Merchant for retention. 50% of the RSUs vested on March 31, 2024, and the remaining 50% were forfeited upon their respective
termination dates.
(6)
Includes aggregate grant value of $14,281 related
to 6,667 performance options granted to Dr. Hombeck in 2023, which reflected the then-probable achievement of the performance conditions
as of the date of grant. Assuming maximum achievement of the performance goals, the grant date fair value of such options was $14,281.
(7)
Represents cash paid to Mr. Hombeck for consulting
services prior to his joining the Company.
(8)
Represents retention cash bonus paid to Ms. Merchant.
Under the terms of the retention agreement, the Named Executive Officer would have to repay the Company if she resigned prior to December
31, 2023.
(9)
Represents payments to cover certain tax liabilities.
(10)
Represents nine months of severance totaling $375,000
and nine months of health and dental coverage totaling $18,093.
2024
Outstanding Equity Awards at Fiscal Year-End
The
outstanding equity awards held by the Named Executive Officers as of December 31, 2024 were as follows:
| | |
Option
Awards | | |
| | |
| |
Stock
Awards | | |
|
| Name | |
Number
of Securities Underlying Unexercised Options - Exercisable | | |
Number
of Securities Underlying Unexercised Options – Unexercisable | | |
Equity
Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options | | |
Option
Exercise Price | | |
Option
Expiration Date | |
Number
of Shares or Units of Stock That Have Not Vested | | |
Market
Value of Shares or Units of Stock That Have Not Vested | | |
Vest
Commencement Date |
| Sandra
Milligan | |
| — | | |
| 30,000 | | |
| — | | |
$ | 3.21 | | |
5/3/2034(1) | |
| | | |
| | | |
3/25/2025 |
| | |
| 25,000 | | |
| — | | |
| — | | |
$ | 0.84 | | |
11/12/2034 | |
| | | |
| | | |
11/12/2024 |
| | |
| | | |
| | | |
| | | |
| | | |
| |
| 20,000 | | |
$ | 18,800 | | |
10/28/2024(2) |
| John
Kallassy | |
| 20,833 | | |
| 4,167 | | |
| — | | |
$ | 1.10 | | |
8/15/2034(3) | |
| | | |
| | | |
8/15/2024 |
| | |
| 25,000 | | |
| — | | |
| — | | |
$ | 0.84 | | |
11/12/2034 | |
| | | |
| | | |
11/12/2024 |
| Nicole
Sandford | |
| 333 | | |
| — | | |
| — | | |
$ | 15.75 | | |
3/1/2032 | |
| | | |
| | | |
3/1/2022 |
| | |
| 3,334 | | |
| 3,332 | | |
| — | | |
$ | 15.60 | | |
3/31/2032(1) | |
| | | |
| | | |
3/31/2022 |
| | |
| 333 | | |
| — | | |
| — | | |
$ | 15.75 | | |
4/1/2032 | |
| | | |
| | | |
4/1/2022 |
| | |
| 333 | | |
| — | | |
| — | | |
$ | 10.50 | | |
5/2/2032 | |
| | | |
| | | |
5/2/2022 |
| | |
| 333 | | |
| — | | |
| — | | |
$ | 8.40 | | |
6/1/2032 | |
| | | |
| | | |
6/1/2022 |
| | |
| 333 | | |
| — | | |
| — | | |
$ | 10.95 | | |
7/1/2032 | |
| | | |
| | | |
4/1/2022 |
| | |
| 333 | | |
| — | | |
| — | | |
$ | 12.00 | | |
8/1/2032 | |
| | | |
| | | |
8/1/2022 |
| | |
| 333 | | |
| — | | |
| — | | |
$ | 7.95 | | |
9/1/2032 | |
| | | |
| | | |
9/1/2022 |
| | |
| 333 | | |
| — | | |
| — | | |
$ | 5.70 | | |
10/3/2032 | |
| | | |
| | | |
10/1/2022 |
| | |
| 333 | | |
| — | | |
| — | | |
$ | 5.85 | | |
11/1/2032 | |
| | | |
| | | |
11/1/2022 |
| | |
| 333 | | |
| — | | |
| — | | |
$ | 5.70 | | |
12/1/2032 | |
| | | |
| | | |
12/1/2022 |
| | |
| 333 | | |
| — | | |
| — | | |
$ | 4.80 | | |
1/1/12033 | |
| | | |
| | | |
1/1/2023 |
| | |
| 333 | | |
| — | | |
| — | | |
$ | 7.65 | | |
2/1/2033 | |
| | | |
| | | |
2/1/2023 |
| | |
| 11,111 | | |
| 22,221 | | |
| — | | |
$ | 8.55 | | |
2/9/2033(4) | |
| | | |
| | | |
2/9/2023 |
| | |
| 16,666 | | |
| — | | |
| — | | |
$ | 8.55 | | |
2/9/2033 | |
| | | |
| | | |
2/9/2023 |
| | |
| 20,000 | | |
| — | | |
| — | | |
$ | 3.75 | | |
8/29/2033 | |
| | | |
| | | |
8/29/2023 |
| | |
| 13,333 | | |
| 26,667 | | |
| — | | |
$ | 3.25 | | |
11/17/2033(4) | |
| | | |
| | | |
11/17/2023 |
| | |
| — | | |
| 30,000 | | |
| — | | |
$ | 4.73 | | |
2/15/2034(4) | |
| | | |
| | | |
2/15/2024 |
| | |
| — | | |
| 80,000 | | |
| — | | |
$ | 2.88 | | |
5/13/2034(1) | |
| | | |
| | | |
5/13/2024 |
| | |
| | | |
| | | |
| | | |
| | | |
| |
| 25,000 | | |
$ | 23,500 | | |
10/28/2024(2) |
(1)
Stock options vest in four equal annual installments beginning one year following the vesting commencement date indicated in the chart
above.
(2)
Represents RSUs which vested on December 31, 2024.
(3)
Stock options vest in six equal monthly installments beginning on August 15, 2024.
(4)
Stock options vest in three equal annual installments
beginning one year following the vesting commencement date indicated in the chart above.
Narrative
Disclosure to Summary Compensation Table
Compensation
Program Overview
Base
Salaries. Executive salaries are determined based on the data from our comparator group, an evaluation of each officer’s
individual performance throughout the year, level of responsibility, overall salary structure, budget guidelines and assessment of our
financial condition. We believe that this approach allows us to remain competitive in the market. The Compensation Committee normally
reviews and adjusts as appropriate the base salaries for the Named Executive Officers in the first half of each calendar year.
Effective
September 1, 2024, the Company entered into a second amendment (the “Sandford Amendment”) to its employment agreement with
Nicole Sandford, the Company’s former Chief Executive Officer. Pursuant to the terms of the Sandford Amendment, Ms. Sandford’s
annual base salary was decreased from $500,000 to $400,000 and she was eligible for a cash bonus of up to $375,000 for the achievement
of goals defined by the Company’s board of directors. The Company also granted Ms. Sandford an RSU award for 25,000 shares
with a grant date fair value of $23,500 on September 1, 2024, which vested in full on December 31, 2024.
Effective
September 1, 2024, the Company entered into an amendment (the “Milligan Amendment”) to its employment agreement with Sandra
Milligan, M.D., J.D., the Company’s President. Pursuant to the terms of the Milligan Amendment, Dr. Milligan’s annual base
salary was decreased from $400,000 to $320,000 and she is eligible for a cash bonus of up to $200,000, prorated for partial years for
the achievement of goals defined by the Company’s board of directors. The Company also granted Dr. Milligan an RSU award
for 20,000 shares with a grant date fair value of $18,800 on September 1, 2024, which vested in full on December 31, 2024.
Effective December 16, 2024, the Company’s Board of Directors approved the reinstatement of Dr. Milligan’s full salary to
$400,000.
| | |
Annual
Base Salary | |
| Sandra Milligan, M.D., J.D. | |
$ | 400,000 | |
| John Kallassy | |
$ | 150,000 | |
Annual
Incentive Bonuses. Consistent with our objectives to tie a significant portion of the Named Executive Officers’ total compensation
to our performance, all Named Executive Officers have a target bonus of a fixed percentage of their base salary. At the beginning of
each fiscal year, the Compensation Committee establishes performance measures and goals. These are formulated into specific metrics on
which to measure performance and attainment of goals during the year. The Compensation Committee generally establishes the individual
payout targets for each Named Executive Officer based on the executive’s position, level of responsibility and a review of the
peer group. The Compensation Committee typically assigns weightings to the various performance goals to provide a balanced approach to
the various factors applied to determining bonus amounts. For 2024, these measures and goals were designed to be challenging yet achievable
with strong management performance. The Compensation Committee has not yet met to determine the achievement of the 2024 performance goals
for the Named Executive Officers at the time of this filing. The Company expects to determine the achievement of the 2024 performance
goals on or about February 15, 2025. Effective September 1, 2024, the employment agreements for Nicole Sandford and Sandra Milligan
were amended to reduce each of their annual salaries. The amounts eligible for bonuses and severance remained a factor of the unadjusted
salaries. Ms. Sanford’s 2024 bonus was determined by the Board of Directors as part of the negotiation of her separation from
the Company. In addition, Mr. Kallassy’s bonus entitlements are described in his consulting agreement, as further described below.
Equity
Incentive Compensation. The equity component of our executive compensation program is designed to fulfill our performance alignment
and retention objectives. In general, Named Executive Officers receive incentive stock option grants at the time of hire. Annually thereafter,
they receive additional equity-based compensation as recommended by the Compensation Committee. Equity-based compensation is based on
individual performance and contributions toward the achievement of our business objectives, as well as overall Company performance. The
number of underlying shares that may be purchased pursuant to the stock options granted to each Named Executive Officer varies based
on the executive’s position and responsibilities. The Compensation Committee granted equity-based compensation to the Named Executive
Officers during the year ended December 31, 2024 in the form of stock options and RSUs, as described above in the Summary Compensation
Table.
Executive
Employment Agreements
Sandra
Milligan, M.D., J.D.
Effective
September 1, 2024, the Company and Dr. Milligan entered into an amendment (the “Milligan Amendment”)
to the employment agreement by and between the Company and Sandra Milligan dated March 16, 2024 (as amended, the “Milligan
Agreement”), pursuant to which the Company would pay Dr. Milligan an annual base salary of $320,000. In addition,
Dr. Milligan is eligible for a bonus of up to $200,000, based on the achievement of reasonable Company goals defined by the Board.
Under the terms of the Milligan Amendment, the Company also granted an RSU award of 20,000 shares, which vested in
full on December 31, 2024. On January 5, 2025, the Board of Directors of the Company approved an annual base salary for Dr.
Milligan of $400,000 retrospectively effective as of December 16, 2024.
If
Dr. Milligan is terminated without cause or resigns for good reason after twelve months of employment (as these terms are defined in
the Milligan Agreement)
and provided that she complies with certain requirements (including signing a standard separation agreement release), under the Milligan
Agreement: (i) she will be entitled to continued payment of her base salary as then in effect for a period of six months following the
date of termination; and (ii) she will be entitled to continued health and dental benefits through COBRA premiums paid by the Company
(“COBRA Coverage”) until the earlier of six months after termination or the time that she obtains employment with reasonably
comparable or greater health and dental benefits. Additionally, the Milligan Agreement provides that if Dr. Milligan’s employment
is terminated without cause or if she resigns for good reason within the 12-month period following a change of control (as such term
is defined in the Milligan Agreement), then, in addition to the benefits above, 100% of any then-unvested options to purchase Company
common stock previously granted by the Company will vest upon the date of such termination (the “Acceleration Benefit”).
The Milligan Agreement also contains a non-solicitation provision that applies until 12 months after the termination of the Milligan
Agreement.
Prior
to the effectiveness of the Milligan Agreement, Dr. Milligan was party to an employment agreement with the Company effective as of April
1, 2024. Pursuant to such prior agreement. Dr. Milligan was paid a base salary of $400,000 and was eligible to earn a bonus of up to
$200,000. She was also granted a stock option to purchase 30,000 shares of the Company’s common stock, vesting in substantially
equal installments following the completion of each of four years of continuous service following Dr. Milligan’s employment commencement
date.
John
Kallassy
Effective
August 15, 2024, the Company and Mr. Kallassy entered into a consulting agreement (the “Kallassy Agreement”). Pursuant to
the Kallassy Agreement, the Company would pay Mr. Kallassy a monthly retainer of $12,500 for up to six months. In addition, Mr. Kallassy
is eligible for a discretionary bonus of up to 50% of his retainer amount, based on the achievement of reasonable Company goals
defined by the Board. Under the terms of the Kallassy Agreement, the Company also granted a stock option to purchase 25,000 shares of
the Company’s common stock, vesting in six substantially equal installments over six months of continuous service following Mr.
Kallassy’s employment commencement date, and he will have a period of twelve months after the termination of his contract to exercise
the option award.
Mr.
Kallassy submitted notice of his resignation to the Company on January 6, 2025, which will be effective January 10, 2025.
Nicole
Sandford
Effective
March 1, 2023, the Company and Ms. Sandford entered into an amended and restated employment agreement (as amended, the “Sandford
Agreement”). Pursuant to the Sandford Agreement, the Company paid Ms. Sandford an annual base salary of $500,000. In addition,
Ms. Sandford was eligible for a bonus of up to 75% of her salary, based on the achievement of reasonable Company goals to be defined
by the Board. Effective September 1, 2024, the Company entered into the Sandford Amendment pursuant to which Ms. Sandford’s annual
base salary was decreased from $500,000 to $400,000 and she was eligible for a cash bonus of up to $375,000 for the achievement of goals
defined by the Company’s board of directors. The Company also granted Ms. Sandford an RSU award for 25,000 shares which vested in full
on December 31, 2024.
If
Ms. Sandford was terminated without cause or she resigned for good reason (as these terms are defined in the Sandford Agreement),
and provided that she complies with certain requirements (including signing a standard separation agreement release), under the Sandford
Agreement: (i) she was entitled to continued payment of her base salary as then in effect for a period of nine months following
the date of termination; and (ii) she was entitled to COBRA Coverage until the earlier of nine months after termination or the
time that she obtains employment with reasonably comparable or greater health and dental benefits. Additionally, she was also eligible
for the Acceleration Benefit, on substantially the same terms and conditions as Dr. Milligan. The Sandford Agreement also contains
a non-solicitation provision that applies until 12 months after the termination of the Sandford Agreement.
Prior
to the effectiveness of the Sandford Agreement, Ms. Sandford was party to an employment agreement with the Company effective as of March
1, 2022, for a term concluding on February 28, 2023. Pursuant to such prior agreement. Ms. Sandford was paid a base salary of $500,000
and was eligible to earn a discretionary bonus upon completion of the term. She was also eligible to receive a stock option grant to
purchase 333 shares of the Company’s common stock, as adjusted for the reverse stock split, on the first trading day of each calendar
month during the term, with each such option vesting in full on the last day of the calendar month in which it is granted.
Ms.
Sandford’s agreement was terminated on December 16, 2024. Pursuant to the Sandford Agreement, Ms. Sandford will be paid $375,000
in equal biweekly installments over a period of nine months as well as be paid a bonus of $135,000 to be paid upon the earlier of a fund
raising or September 30, 2025. In conjunction with her termination, the Company entered into a consulting agreement with Ms. Sandford
(the “Sandford Consulting Agreement”). Pursuant to the Sandford Consulting Agreement, the Company will pay Ms. Sandford at
a rate of $500 per hour for consulting services provided.
Torsten
Hombeck, Ph.D.
Effective
March 13, 2024, the Company and Dr. Hombeck entered into an amended and restated employment agreement (the “Hombeck Agreement”),
pursuant to which the Company paid Dr. Hombeck an annual base salary of $325,000. In addition, Dr. Hombeck was
eligible for a bonus of up to 50% of his salary based on the Company’s performance, with the exact payment terms, if any, to be
determined by the Compensation Committee of the Board in its sole and absolute discretion. Under the terms of such agreement, Dr. Hombeck
was also eligible for a bonus of up to $50,000 upon the closing of one or more equity or debt financing transactions that result
in a total aggregate net proceeds to the Company of $3,000,000 or more prior to March 31, 2025. The bonus will increase to $100,000 upon
the aggregates closing of one or more equity, debt or other financing transactions that resulted in total net proceeds to the
Company of $5,000,000 or more prior to March 31, 2025. Furthermore, Dr. Hombeck was granted a stock option to purchase 13,334
shares of the Company’s common stock, vesting in substantially equal installments following the completion of each of three
years of continuous service following Dr. Hombeck’s employment commencement date. Dr. Hombeck was also granted a stock option
to purchase 6,667 shares of the Company’s common stock, based on individual performance goals for the 12 months ending on the first
anniversary of his employment commencement date.
If
Dr. Hombeck was terminated without cause
or he resigned for good reason (as these terms are defined in the Hombeck Agreement), and provided that he complies
with certain requirements (including signing a standard separation agreement release), under the Hombeck Agreement: (i) he was entitled
to continued payment of his base salary as then in effect for a period of six months following the date of termination; and (ii)
he was entitled to COBRA Coverage until the earlier of six months after termination or the time that he obtained employment
with reasonably comparable or greater health and dental benefits. Further, he was also eligible for the Acceleration Benefit,
on substantially the same terms and conditions as Dr. Milligan. The Hombeck Agreement also contains a non-solicitation provision that
applies until 12 months after the termination of the Hombeck Agreement.
In
addition, if Dr. Hombeck was terminated by the Company without cause or resigns for good reason (each term as defined in the Hombeck
Agreement), any then-vested stock options granted to him would remain exercisable for up to 12 months following his employment
termination date. The Hombeck Agreement also contains a non-solicitation provision that applies until 12 months after the termination
of the Hombeck Agreement.
The
Hombeck Agreement was terminated effective June 27, 2024.
Minh
Merchant
Effective
March 28, 2023, the Company and Ms. Merchant entered into an amended and restated employment agreement (the “Merchant Agreement”),
pursuant to which the Company paid Ms. Merchant an annual base salary of $325,000. In addition, Ms. Merchant was
eligible for a bonus of up to 50% of her salary, based on the achievement of reasonable Company and individual performance related
goals to be mutually agreed upon by Ms. Merchant and the Company’s Chief Executive Officer and recommend by the Compensation Committee
for the Board’s approval. Ms. Merchant was granted a stock option to purchase 10,000 shares of the Company’s common stock,
as adjusted for the reverse stock split, vesting in substantially equal installments following the completion of each of four
years of continuous service following Ms. Merchant’s employment commencement date. In addition, if Ms. Merchant was terminated
by the Company with cause or resigned for good reason (each term as defined in the Merchant Agreement), any then-vested stock
options granted to her would remain exercisable for up to 12 months following her employment termination date.
Ms.
Merchant was eligible for continued payment of her base salary for nine months and nine months of COBRA Coverage on substantially
the same terms and conditions as Dr. Milligan in the event that she was terminated without cause or resigns for good reason
(as these terms are defined in the Merchant Agreement). Further, she was also eligible for the Acceleration Benefit, on substantially
the same terms and conditions as Dr. Milligan. The Merchant Agreement also contains a non-solicitation provision that applies
until 12 months after the termination of the Merchant Agreement.
The
Merchant Agreement was terminated effective May 6, 2024.
Severance
and Change in Control Arrangements
Under
the terms of each Named Executive Officer’s employment agreement, each is eligible to receive severance benefits upon a termination
by the Company without cause or by the executive officer due to good reason. The Compensation Committee believes that these arrangements
are important in order to attract and retain executive officer talent.
In
addition, the Compensation Committee believes that executive officers have a greater risk of job loss or modification as a result of
a change in control transaction than other employees. Accordingly, the employment agreements include change in control provisions under
which Named Executive Officers currently employed by the Company will receive certain payments and benefits upon qualifying terminations
that follow a change in control. The principal purpose of the change in control provisions is to provide executive officers with appropriate
incentives to remain with us before, during and after any change in control transaction by providing the executive officers with security
in the event their employment is terminated or materially changed following a change in control. By providing this type of security,
the employment agreements help ensure that the executive officers support any potential change in control transaction that may be considered
in the best interests of our stockholders, even while the transaction may create uncertainty in the executive officer’s personal
employment situation. The Compensation Committee believes that salary and benefits for nine months for our President and Chief Executive
Officer and six months for our Chief Financial Officer and General Counsel are reasonable and appropriate to achieve the desired objectives
of the agreements.
The
following table sets forth amounts payable to the Named Executive Officers who were providing service to the Company on December
31, 2024, if such officer had been terminated as of December 31, 2024. Neither Ms. Merchant nor Dr. Hombeck was eligible
for any such benefits as of December 31, 2024.
| Name | |
Qualifying
Termination
Scenario | |
Continued Payment of
Base Salary | | |
Immediate Vesting
of Stock Options(2) | | |
Health
and
Dental
Insurance
Benefits(3) | |
| Sandra Milligan | |
Qualifying
Termination(1) | |
$ | 200,000 | | |
$ | — | | |
$ | 865 | (4) |
| | |
Qualifying Termination Within 12 Months
After Change-in Control | |
| 200,000 | | |
| — | | |
| — | |
| | |
For cause | |
| — | | |
| — | | |
| — | |
| John Kallassy | |
Qualifying Termination(1) | |
$ | — | | |
$ | — | | |
$ | — | |
| | |
Qualifying Termination Within 12 Months After
Change-in Control | |
| — | | |
| — | | |
| — | |
| | |
For cause | |
| — | | |
| — | | |
| — | |
(1)
A Qualifying Termination means an involuntary termination by the Company other than for cause or a voluntary resignation by the executive
for good reason (in all cases, assuming the executive is not entering into competitive or other activity detrimental to us, and subject
to the executive’s execution of an effective release of claims and compliance with certain other conditions). Mr. Kallassy is
not entitled to receive any benefits under this arrangement, as he is not an employee of the Company.
(2)
Reflects the difference between the exercise price of all options that would have vested upon such a termination and $0.75 (the
January 7, 2025 closing price of our common stock).
(3)
Assumes each executive does not obtain employment with reasonably comparable or better health and dental benefits within the time period
specified in the respective employment agreements.
(4)
Dr. Milligan does not avail herself of the Company’s health insurance benefits, only its dental insurance benefits.
Other
Benefits
Except
as set forth herein, our Named Executive Officers participate in our standard employee benefits programs, including medical, dental,
vision, life, short-term and long-term disability insurance, 401(k) Plan and flexible spending accounts.
Board
Compensation
Our
director compensation program is designed both to attract qualified non-employee directors and to fairly compensate them for their substantial
responsibilities and time commitment. Periodically, the Compensation Committee reviews and determines the adequacy of the compensation
program for non-employee directors and, based upon the results of its review, the Compensation Committee may make recommendations regarding
the compensation program for non-employee directors to the Board. Directors are eligible to elect a mix of RSUs, stock option awards
and cash awards. For 2024, the Compensation Committee engaged Pearl Meyer and Partners, LLC to assist in the review of director compensation.
Based on the independent review, and after considering the recommendation of the Compensation Committee, the Board changed the director
compensation program for the second half of 2024. Under the updated director compensation program, the program will run on annual
meeting to annual meeting cycles, rather than calendar year cycles. The Compensation Committee decreased the annual equity
retainer from $175,000 to $115,000 for the Chair of the Board and decreased the annual equity retainer for the other outside directors
from $140,000 to $70,000. In addition, the Board approved a cash retainer for each of the outside directors of $40,000. Such changes
were effective as of July 2024.
Historically
and during the first half of the 2024 non-employee director compensation program consisted of the following (as annualized):
| | |
Director Retainer | | |
| |
| | |
Equity
Awards(1) | | |
Cash | |
| Chairperson | |
$ | 175,000 | | |
$ | 35,000 | |
| Other Outside Directors | |
| 140,000 | | |
$ | 28,000 | |
| Chair of the Audit Committee | |
| 15,000 | | |
| | |
| Other Audit Committee Members | |
| 7,500 | | |
| | |
| Chair of the Compensation Committee | |
| 12,000 | | |
| | |
| Other Compensation Committee Members | |
| 6,000 | | |
| | |
| Chair of the Nominating and Governance
Committee | |
| 6,000 | | |
| | |
| Other Nominating and Governance Committee
Members | |
| 4,000 | | |
| | |
(1)
One half of the annualized RSUs were granted to non-employee directors in conjunction with their service during the first half of 2024.
The RSUs vested 100% on August 12, 2024.
The
new non-employee director compensation program, which was applied in the second half of 2024, consisted of the following (as annualized):
| | |
Director Retainer | | |
| |
| | |
Equity Awards
(1) | | |
Cash | |
| Chairperson | |
$ | 115,000 | | |
$ | 40,000 | |
| Other Outside Directors | |
| 70,000 | | |
| 40,000 | |
| Chair of the Audit Committee | |
| 20,000 | | |
| - | |
| Other Audit Committee Members | |
| 10,000 | | |
| - | |
| Chair of the Compensation Committee | |
| 15,000 | | |
| - | |
| Other Compensation Committee Members | |
| 7,500 | | |
| - | |
| Chair of the Nominating and Governance
Committee | |
| 10,000 | | |
| - | |
| Other Nominating and Governance Committee
Members | |
| 5,000 | | |
| - | |
(1)
RSUs granted to non-employee directors vest over a one-year period, 25% on September 30, 2024, 25% on December 31, 2024, 25% on
March 31, 2025 and 25% at the time of the annual meeting.
2024
Director Compensation Table
The
table below presents the compensation earned by our non-employee directors for the year ended December 31, 2024. Ms. Sandford did not
receive any additional compensation for her service on the Board during 2024. Please see the “2024 Summary Compensation Table”
for a summary of the compensation received by Ms. Sandford for her service as our President and Chief Executive Officer during
2024.
| Name | |
Fees
Earned or Paid in Cash | | |
Stock
Awards (1)(2) | | |
Total | |
| Stefanie Cavanaugh | |
$ | 19,000 | | |
$ | 44,164 | | |
$ | 63,164 | |
| Celeste Fralick, Ph.D. | |
$ | 19,000 | | |
$ | 38,581 | | |
$ | 57,581 | |
| Jannie Herchuk | |
$ | 22,500 | | |
$ | 58,394 | | |
$ | 80,894 | |
| Ellen O’Connor-Vos | |
$ | 19,000 | | |
$ | 41,275 | | |
$ | 60,275 | |
| Winfred Parnell, M.D. | |
$ | 19,000 | | |
$ | 39,720 | | |
$ | 58,720 | |
| John Ragard(3) | |
$ | 5,000 | | |
$ | 20,538 | | |
$ | 25,538 | |
(1)
Reflects the grant date fair value, calculated in accordance with Financial Accounting Standards Board ASC Topic 718, Compensation-Stock
Compensation, of RSUs and options granted in fiscal year 2024 by the Company under its 2019 Stock Incentive Plan. The number of RSUs
granted on August 12, 2024 was determined by dividing the targeted grant value by a five day trailing average price of our common stock
for the week prior to the date of grant of the RSUs. The price and target value was fixed as of February 2024 when the trailing average
price per share of our common stock was $4.746 per share. The number of RSUs granted on August 22, 2024 was determined by dividing the
targeted grant value by the higher of $4.00 or a twenty day trailing average price of our common stock for the week prior to the
date of grant of the RSUs. The price and target value was fixed in August 2024 using $4.00. Additional information regarding the
assumptions made in calculating these amounts will be provided in Note 9, Employee Share Based Compensation and Benefit Plans, to the
consolidated financial statements to be included in the Company’s Annual Report on Form 10-K for the year ended December
31, 2024.
(2)
As of December 31, 2024, each individual who served as a non-employee director during 2024 held outstanding RSUs as follows: Ms. Cavanaugh
- 17,812; Dr. Fralick - 15,000; Ms. Herchuk - 25,312; Ms. O’Connor Vos - 15,937; Dr. Parnell - 15,469; and Mr. Ragard - 14,531.
The awards vest in four substantially equal installments on September 30, 2024, December 31, 2024, March 31, 2025 and the date of the
annual shareholders’ meeting, subject to such person’s continued service with the Company through the applicable vesting
dates.
(3)
Mr. Ragard was appointed to the Board, effective as of July 25, 2024. Mr. Ragard was designated by Jack W. Schuler, a stockholder
of the Company and holding the right to designate an individual to be nominated to the Board pursuant to the Stockholders Agreement dated
May 13, 2013 by and among the Company and the purchasers of the Company’s common stock and warrants named therein.
Company Policies and Practices Related to the
Grant of Certain Equity Awards Close in Time to the Release of Material Nonpublic Information
The
Compensation Committee last granted a stock option in January 2025. The Company does not grant stock options or similar awards to
Section 16 Insiders, most SVPs, and other Vice Presidents and above who directly report to the CEO in anticipation of the release of
material nonpublic information that is likely to result in changes to the price of the Company’s stock, such as a significant
positive or negative earnings announcement, or time the public release of such information based on stock option grant dates. In
addition, the Company does not grant stock options or similar awards during the four business days prior to or the one business day
following the filing of our periodic reports or the filing or furnishing of a Current Report on Form 8-K that discloses material
nonpublic information. These restrictions do not apply to RSUs or other types of equity awards
that do not include an exercise price related to the market price of the Company’s stock on the date of grant. The Company
grants annual retention stock options to all of its employees during the February Compensation Committee meeting. The Company
also grants stock options to new hires on the first day of the month following the employee start date.
The
Company’s executive officers would not be permitted to choose the grant date for any stock option grants.
During
fiscal 2024, none of the Company’s Named Executive Officers were awarded stock options, and the Company did not time the disclosure
of material nonpublic information for the purpose of affecting the value of executive compensation.
CERTAIN
RELATIONSHIPS AND RELATED PERSON TRANSACTIONS
The
following is a summary of transactions since January 1, 2022, to which we have been a participant in which the amount involved exceeded
or will exceed the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years, and
in which any of our directors, executive officers, or holders of more than five percent of our capital stock, or any member of the immediate
family of the foregoing persons, had or will have a direct or indirect material interest, other than compensation arrangements described
in the section titled “Executive and Director Compensation” included elsewhere in this prospectus.
Relationships
Resulting from 2013 Private Placement
In
connection with a May 8, 2013 private placement, on May 13, 2013, the Company entered into a Stockholders Agreement (the “Stockholders
Agreement”) with certain of the purchasers in the private placement (the “2013 Purchasers”). Among other things, the
Stockholders Agreement provides certain of the 2013 Purchasers with rights to participate in any future equity offerings by the Company
on the same price and terms as other investors. Certain of the 2013 Purchasers were also offered the opportunity to participate in the
underwritten public offering of our common stock that we completed on February 8, 2021, and certain trusts and other entities affiliated
with H. George Schuler and Tanya Schuler Sharman, as well as Seamark Capital, L.P., purchased an aggregate of 40,555 shares of our common
stock (as adjusted for the reverse stock split) for aggregate gross proceeds of $4,562,497.50. In addition, certain of the 2013 Purchasers
were also offered the opportunity to participate in the underwritten public offering of our common stock that we completed on August
22, 2022, and certain trusts and other entities affiliated with H. George Schuler and Tanya Schuler Sharman purchased an aggregate of
8,888 shares of our common stock (as adjusted for the reverse stock split) for aggregate gross proceeds of $117,333.04. Also, certain
of the 2013 Purchasers were also offered the opportunity to participate in the underwritten public offering of our common stock that
we completed on July 24, 2023, and certain trusts and other entities affiliated with H. George Schuler and Tanya Schuler Sharman purchased
an aggregate of 181,800 shares of our common stock for aggregate gross proceeds of $499,950.00. Further, certain of the 2013 Purchasers
were also offered the opportunity to participate in the underwritten public offering of our common stock that we completed on January
26, 2024, and certain trusts and other entities affiliated with H. George Schuler and Tanya Schuler Sharman purchased an aggregate of
28,500 shares of our common stock for aggregate gross proceeds of $99,750.00.
In
addition, in connection with the May 8, 2013 private placement, Oracle Partners, LP and Oracle Ten Fund Master LP, (together, “Oracle”),
and Jack W. Schuler (“Schuler” and together with Oracle, the “Principal Purchasers”) received rights to prohibit
the Company from taking any of the following actions unless agreed to by at least one of the Principal Purchasers:
| |
● |
Making
any acquisition with a value greater than $2 million; |
| |
|
|
| |
● |
Entering
into, or amending the terms of our agreements with Quest Diagnostics, which consent shall not be unreasonably withheld, conditioned
or delayed following good faith consultation with the Company; |
| |
|
|
| |
● |
Submitting
any resolution at a meeting of stockholders or in any other manner changing or authorizing a change in the size of our Board; |
| |
|
|
| |
● |
Offering,
selling or issuing any securities senior to the Company’s common stock or any securities that are convertible into or exchangeable
or exercisable for securities ranking senior to our common stock; |
| |
|
|
| |
● |
Amending
our Certificate of Incorporation or Bylaws in any manner that effects the rights, privileges or economics of the Company’s
common stock; |
| |
|
|
| |
● |
Making
any action that would result in a change in control of the Company or an insolvency event; |
| |
|
|
| |
● |
Paying
or declaring dividends on any securities of the Company or distributing the assets of the Company other than in the ordinary course
of business or repurchasing any outstanding securities of the Company; or |
| |
|
|
| |
● |
Adopting
or amending any shareholder rights plan. |
In
addition, the Principal Purchasers each received the right to nominate a member to serve on our Board. Dr. Eric Varma was designated
as a Board nominee by Oracle pursuant to the Stockholders Agreement and was appointed as a director on September 12, 2013. Dr. Varma
did not stand for re-election at the 2018 annual meeting of the stockholders of the Company. Oracle did not notify the Company of the
person who would succeed Dr. Varma as the Oracle designee on the Board. James T. LaFrance was designated as a Board nominee by Schuler.
The Board appointed Mr. LaFrance as a director and Chair of the Board on December 12, 2013, and effective from March 2022 to December
2022 he served as Lead Independent Director of the Board. Mr. LaFrance resigned from the Board effective December 15, 2022. Subsequently,
John Ragard was designated as a Board nominee by Schuler and was appointed to the Company’s Board effective as of July 25, 2024.
The
rights and prohibitions of the 2013 Purchasers under the Stockholders Agreement terminate for each 2013 Purchaser when it ceases to own
or hold less than 50% of the shares, warrants or warrant shares that were purchased at the closing of the 2013 private placement. We
believe that the rights and prohibitions of the Principal Purchasers under the Stockholders Agreement terminated for Oracle.
Directors
and Officers
In
the underwritten public offering of our common stock that we completed on August 22, 2022, certain of our directors and officers purchased
an aggregate of 113,333 shares of our common stock (as adjusted for the reverse stock split) for aggregate gross proceeds of $170,000.00.
Director and officer purchasers included former officers Minh Merchant, Ryan Phan and Nicole Sanford, former directors Robert Auerbach
and Ruby Sharma, as well as current director Celeste Fralick. Also, certain of our directors and officers participated in the underwritten
public offering of our common stock that we completed on July 24, 2023, and purchased an aggregate of 44,347 shares of our common stock
for aggregate gross proceeds of $176,501.06. Director and officer purchasers included former officers Torsten Hombeck, Minh Merchant,
Ryan Phan and Nicole Sanford, former director Veronica Jordan, as well as current directors Stefanie Cavanaugh, Celeste Fralick, Jannie
Herchuk, Ellen O’Connor Vos and Winfred Parnell. Further, certain of our directors and officers participated in the underwritten
public offering of our common stock that we completed on January 26, 2024, and purchased an aggregate of 2,400 shares of our common stock
for aggregate gross proceeds of $10,212. Director and officer purchasers included former officer Nicole Sanford.
PRINCIPAL
STOCKHOLDERS
The
following table sets forth certain information regarding the beneficial ownership of our common stock as of January 7, 2025 by:
| ● | each
of our named executive officers; |
| ● | each
of our directors; |
| ● | all
of our current directors and named executive officers as a group; and |
| ● | each
stockholder known by us to own beneficially more than 5% of our common stock. |
Beneficial
ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities.
Shares of common stock that may be acquired by an individual or group within 60 days of January 7, 2025, pursuant to the exercise of
options or warrants, vesting of common stock or conversion of convertible debt, are deemed to be outstanding for the purpose of computing
the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage
ownership of any other person shown in the table. Percentage of ownership is based on 17,529,793 shares of common stock issued and outstanding
as of January 7, 2025 and prior to the offering. Percentage of ownership is based on 33,529,793 shares of common stock issued and outstanding
after the completion of this offering, assuming the sale of 16,000,000 shares of common stock.
Unless
noted otherwise, the address of all listed stockholders is c/o Aspira Women’s Health Inc., 12117 Bee Caves Road, Building III,
Suite 100, Austin, Texas 78738.
Except
as indicated by the footnotes below, we believe, based on information furnished to us, that each of the stockholders listed has sole
voting and investment power with respect to the shares beneficially owned by the stockholder unless noted otherwise, subject to community
property laws where applicable.
| Name and Address of Beneficial Owner | |
Number of
Shares
Beneficially Owned | | |
Percentage of
Outstanding
Shares
Beneficially Owned Prior to Offering | | |
Percentage of
Outstanding
Shares
Beneficially After Offering | |
| Directors and Named Executive Officers: | |
| | | |
| | | |
| | |
| Sandra Milligan(1) | |
| 45,000 | | |
| * | | |
| * | |
| John Kallassy(2) | |
| 50,000 | | |
| * | | |
| * | |
| Jannie Herchuk(3) | |
| 94,022 | | |
| * | | |
| * | |
| Stefanie Cavanaugh(4) | |
| 70,119 | | |
| * | | |
| * | |
| Celeste Fralick(5) | |
| 51,109 | | |
| * | | |
| * | |
| Ellen O’Connor Vos(6) | |
| 66,632 | | |
| * | | |
| * | |
| Winfred Parnell(7) | |
| 64,165 | | |
| * | | |
| * | |
| John Ragard(8) | |
| 190,406 | | |
| 1.1 | % | |
| * | |
| All Directors and Executive Officers as a Group (8 persons) | |
| 631,453 | | |
| 3.6 | % | |
| 1.9 | % |
| Beneficial Owners of More Than 5%: | |
| | | |
| | | |
| | |
| Jack Schuler(9) | |
| 1,918,692 | | |
| 10.9 | % | |
| 5.8 | % |
| Robert Drysdale(10) | |
| 1,354,975 | | |
| 7.7 | % | |
| 4.0 | % |
| Armistice Capital, LLC(11) | |
| 1,256,000 | | |
| 7.2 | % | |
| 3.7 | % |
| (1) | Includes
(i) 20,000 RSUs and (ii) 25,000 shares issuable upon the exercise of stock options. |
| (2) | Includes
50,000 shares issuable upon the exercise of stock options. |
| (3) | Includes
(i) 8,437 RSUs, (ii) 4,902 common stock warrants and (iii) 23,493 shares issuable upon the
exercise of stock options. |
| (4) | Includes
(i) 5,937 RSUs and (ii) 3,268 common stock warrants. |
| (5) | Includes
444 common stock warrants. |
| (6) | Includes
(i) 5,312 RSUs and (ii) 3,268 common stock warrants. |
| (7) | Includes
3,268 common stock warrants. |
| (8) | Includes
65,359 common stock warrants. |
| (9) | Based
on the information provided in Amendment No. 16 to Schedule 13D filed with the SEC on July
11, 2024 by Jack W. Schuler with respect to himself and the Jack W. Schuler Living Trust).
Includes (i) 6,536 shares of common stock held
by Jack Schuler and (ii) 1,912,156 shares held by
Jack W. Schuler Living Trust. The number of shares beneficially owned excludes (i) 37,388
shares issuable upon the exercise of warrants owned by Jack W. Schuler Living Trust and (ii)
6,536 shares issuable upon the exercise of warrants owned by Jack W. Schuler, all of which
are not currently exercisable due to beneficial ownership limitations. The address of the
Jack W. Schuler is P.O. Box 531, Lake Bluff, IL 60044. |
| (10) | Based
on the information provided in Schedule 13G filed with the SEC on February 14, 2022 by Robert
H. Drysdale and Form 3 filed on December 8, 2023. Mr. Drysdale reported that as of November
28, 2023, he had sole voting and dispositive power with respect to 1,310,531 shares of our
common stock. The number of shares beneficially owned includes 44,444 shares issuable upon
the exercise of stock warrants. The address of Robert H. Drysdale is 132A Royal Circle, Honolulu,
Hawaii 96816. |
| (11) | Based
on the information provided in Schedule 13G filed with the SEC on November 14, 2024 by Armistice
Capital, LLC and Steven Boyd. Armistice reported that as of September 30, 2024, it held 1,256,000
shares of our common stock. Armistice Capital, LLC (“Armistice Capital”) is the
investment manager of Armistice Capital Master Fund Ltd. (the “Master Fund”), the
direct holder of the shares, and pursuant to an Investment Management Agreement, Armistice
Capital exercises voting and investment power over the securities of the Company held by
the Master Fund and thus may be deemed to beneficially own the securities of the Company
held by the Master Fund. Mr. Boyd, as the managing member of Armistice Capital, may be deemed
to beneficially own the securities of the Company held by the Master Fund. The Master Fund
specifically disclaims beneficial ownership of the securities of the Company directly held
by it by virtue of its inability to vote or dispose of such securities as a result of its
Investment Management Agreement with Armistice Capital. The address of Armistice Capital
and Mr. Boyd is 510 Madison Avenue, 7th Floor, New York, New York 10022. |
DESCRIPTION
OF SECURITIES WE ARE OFFERING
Common
Stock
We
are authorized to issue up to a total of 200,000,000 shares of common stock, par value $0.001 per share. As of January 7, 2025, 17,529,793
shares of our common stock were outstanding, 553,000 shares of our common stock were reserved for future issuance to employees,
directors and consultants pursuant to our employee stock plans, which excludes 853,862 shares of our common stock that were subject
to outstanding options and 134,060 restricted stock units. As of January 7, 2025, we had 46 registered holders of
record of our common stock. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely
affected by, the rights of the holders of shares of any series of preferred stock which we may designate in the future.
Voting
Rights
Each
holder of common stock is entitled to one vote for each share on all matters to be voted upon by the stockholders, and there are no cumulative
voting rights. In all matters other than the election of directors, stockholder approval requires the affirmative vote of the majority
of the holders of our common stock entitled to vote on the subject matter unless the matter is one upon which, by express provision of
law, our Certificate of Incorporation or our Bylaws, a different vote is required. Directors are elected by a plurality of the votes
of the shares present in person or represented by proxy and entitled to vote on the election of directors.
Dividend
Rights
Subject
to preferences to which holders of preferred stock may be entitled and the rights of certain of our stockholders set forth in the Stockholder’s
Agreement (as defined below), holders of common stock are entitled to receive ratably such dividends, if any, as may be declared from
time to time by our Board of Directors out of funds legally available therefor. We have never paid or declared any dividend on our common
stock, and we do not anticipate paying cash dividends on our common stock in the foreseeable future. We intend to retain all available
funds and any future earnings to fund the development and expansion of our business.
No
Preemptive or Similar Rights
Holders
of our common stock do not have preemptive rights, and our common stock is not convertible or redeemable. As described under “Stockholder’s
Agreement,” certain holders of our common stock have the right to purchase shares in connection with most equity offerings made
by the Company.
Right
to Receive Liquidation Distributions
In
the event of our liquidation, dissolution or winding up, holders of common stock would be entitled to share in our assets remaining after
the payment of liabilities and the satisfaction of any liquidation preference granted the holders of any outstanding shares of any senior
class of securities. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely affected
by, the rights of the holders of shares of any series of preferred stock which we may designate in the future.
Pre-funded
Warrants
The
following is a brief summary of certain terms and conditions of the Pre-funded Warrants being offered in this offering. The following
description is subject in all respects to the provisions contained in the Pre-funded Warrants, a form of which is an exhibit to this
Registration Statement.
Form
The
Pre-funded Warrants will be issued as individual warrant agreements to the purchasers.
Term
The
Pre-funded Warrants will not expire until they are fully exercised.
Exercisability
The
Pre-funded Warrants are exercisable at any time until they are fully exercised. The Pre-funded Warrants will be exercisable, at the option
of each holder, in whole or in part by delivering to us a duly executed exercise notice and payment of the exercise price. No fractional
shares of common stock will be issued in connection with the exercise of a Pre-funded Warrant. The holder of the Pre-funded Warrant may
also satisfy its obligation to pay the exercise price through a “cashless exercise,” in which the holder receives the net
value of the Pre-funded Warrants in shares of common stock determined according to the formula set forth in the Pre-funded Warrant.
Exercise
Limitations
Under
the terms of the Pre-funded Warrants, we may not effect the exercise of any such warrant, and a holder will not be entitled to exercise
any portion of any such warrant, if, upon giving effect to such exercise, the aggregate number of shares of common stock beneficially
owned by the holder (together with its affiliates, any other persons acting as a group together with the holder or any of the holder’s
affiliates, and any other persons whose beneficial ownership of common stock would or could be aggregated with the holder’s for
purposes of Section 13(d) or Section 16 of the Exchange Act) would exceed 4.99% of the number of shares of common stock outstanding immediately
after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of such warrant, which percentage
may be increased or decreased at the holder’s election upon 61 days’ notice to us subject to the terms of such warrants,
provided that such percentage may in no event exceed 9.99%.
Exercise
Price
The
exercise price of our shares of common stock purchasable upon the exercise of the Pre-funded Warrants is $0.001 per share.
The exercise price of the Pre-funded Warrants and the number of shares of common stock issuable upon exercise of the Pre-funded Warrants
is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications
or similar events affecting our shares of common stock, as well as upon any distribution of assets, including cash, stock or other property,
to our stockholders.
Transferability
Subject
to applicable laws, the Pre-funded Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange
Listing
We
do not intend to list the Pre-funded Warrants on The Nasdaq Capital Market, any other national securities exchange or any other nationally
recognized trading system.
Fundamental
Transactions
Upon
the consummation of a fundamental transaction (as described in the Pre-funded Warrants, and generally including any reorganization, recapitalization
or reclassification of our shares of common stock, the sale, transfer or other disposition of all or substantially all of our properties
or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding shares of common
stock, or any person or group becoming the beneficial owner of 50% of the voting power of our outstanding shares of common stock), the
holders of the Pre-funded Warrants will be entitled to receive, upon exercise of the Pre-funded Warrants, the kind and amount of securities,
cash or other property that such holders would have received had they exercised the Pre-funded Warrants immediately prior to such fundamental
transaction, without regard to any limitations on exercise contained in the Pre-funded Warrants. Notwithstanding the foregoing, in the
event of a fundamental transaction where the consideration consists solely of cash, solely of marketable securities or a combination
of cash and marketable securities, then each Pre-funded Warrant shall automatically be deemed to be exercised in full in a cashless exercise
effective immediately prior to and contingent upon the consummation of such fundamental transaction.
No
Rights as a Stockholder
Except
by virtue of such holder’s ownership of shares of common stock, the holder of a Pre-funded Warrant does not have the rights or
privileges of a holder of our shares of common stock, including any voting rights, until such holder exercises the Pre-funded Warrant.
Stockholder’s
Agreement
In
connection with a private placement in May 2013, we entered into a Stockholder’s Agreement with the purchasers named therein. Pursuant to and subject to the terms of the Stockholder’s Agreement, certain
of the investors received rights to participate in any future equity offerings on the same price and terms as other investors. These
rights terminate for each investor when that investor ceases to beneficially own at least 50% of the shares and warrants (taking into
account shares issued upon exercise of the warrants), in the aggregate, that such investor purchased at the closing of our May 2013 private
placement. We believe that the rights of one of the primary investors have terminated. As a result, the remaining investor, whose rights
have yet to be terminated, may participate in future equity offerings made pursuant to this prospectus.
In
addition, the Stockholder’s Agreement prohibits the Company from taking material actions without the consent of Schuler as the only investor still holding these rights. These material
actions include:
| ● | making
any acquisition with value greater than $2 million; |
| ● | entering
into, or amending the terms of agreements with Quest Diagnostics, provided that such investors’
consent shall not be unreasonably withheld, conditioned or delayed following good faith consultation
with the Company; |
| ● | submitting
any resolution at a meeting of stockholders or in any other manner changing or authorizing
a change in the size of our Board of Directors; |
| ● | offering,
selling or issuing any securities senior to our common stock or any securities that are convertible
into or exchangeable or exercisable for securities ranking senior to our common stock; |
| ● | amending
our Certificate of Incorporation or our Bylaws in any manner that affects the rights, privileges
or economics of our common stock; |
| ● | taking
any action that would result in a change in control of Aspira or an insolvency event; |
| ● | paying
or declaring dividends on any securities of the Company or distributing any assets of the
Company other than in the ordinary course of business or repurchasing any outstanding securities
of the Company; or |
| ● | adopting
or amending any stockholder rights plan. |
In
addition, the primary investor received the right to designate a person to serve on our Board of Directors. The investor notified the
Company of its intention to exercise this right in 2024. These rights terminate for such investor when that investor ceases to beneficially
own less than 50% of the shares and warrants (taking into account shares issued upon exercise of the warrants), in the aggregate, that
such investor purchased at the closing of our May 2013 private placement.
Section
203 of the Delaware Corporation Law
We
are subject to Section 203 of the DGCL, which prevents an “interested stockholder” (defined in Section 203 of the DGCL, generally,
as a person owning 15% or more of a corporation’s outstanding voting stock), from engaging in a “business combination”
(as defined in Section 203 of the DGCL) with a publicly-held Delaware corporation for three years following the date such person became
an interested stockholder, subject to exceptions, unless:
| ● | before
such person became an interested stockholder, the board of directors of the corporation approved
the transaction in which the interested stockholder became an interested stockholder or approved
the business combination; |
| ● | upon
consummation of the transaction that resulted in the interested stockholder becoming an interested
stockholder, the interested stockholder owns at least 85% of the voting stock of the corporation
outstanding at the time the transaction commenced (excluding stock held by directors who
are also officers of the corporation and by employee stock plans that do not provide employees
with the rights to determine confidentially whether shares held subject to the plan will
be tendered in a tender or exchange offer); or |
| ● | following
the transaction in which such person became an interested stockholder, the business combination
is approved by the board of directors of the corporation and authorized at a meeting of stockholders
by the affirmative vote of the holders of two-thirds of the outstanding voting stock of the
corporation not owned by the interested stockholder. |
The
provisions of Section 203 of the DGCL could make a takeover of the Company difficult.
Effect
of Certain Provisions of Our Certificate of Incorporation and Bylaws
Certain
provisions of our Certificate of Incorporation and Bylaws may also have the effect of making it more difficult for a third party to acquire,
or of discouraging a third party from attempting to acquire, control of us. Such provisions could limit the price that certain investors
might be willing to pay in the future for our securities. Our Certificate of Incorporation eliminates the right of stockholders to call
special meetings of stockholders or to act by written consent without a meeting, and our Bylaws require advance notice for stockholder
proposals and director nominations, which may preclude stockholders from bringing matters before an annual meeting of stockholders or
from making nominations for directors at an annual meeting of stockholders. Our Certificate of Incorporation authorizes undesignated
preferred stock, which makes it possible for our Board of Directors to issue preferred stock with voting or other rights or preferences
that could impede the success of any attempt to change control of us.
These
and other provisions may have the effect of deferring hostile takeovers or delaying changes in control or management of us. The amendment
of any of the provisions of our Certificate of Incorporation described in the immediately preceding paragraph would require approval
by our Board of Directors and the affirmative vote of at least 66 2/3% of our then outstanding voting securities, and the amendment of
any of the provisions of our Bylaws described in the immediately preceding paragraph would require approval by our Board of Directors
or the affirmative vote of at least 66 2/3% of our then outstanding voting securities.
Transfer
Agent
The
transfer agent and registrar for our common stock is Broadridge Financial Solutions, Inc.
Listing
Our
common stock is listed on Nasdaq under the symbol “AWH.”
UNDERWRITING
We
have entered into an underwriting agreement dated , 2025 with
ThinkEquity LLC, as the representative of the underwriters (the “Representative”), with respect to the securities
sold in this offering. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters
named below, and the underwriters have agreed, severally and not jointly, to purchase from us the securities set
forth opposite the underwriter’s name in the following table at the public offering price less the underwriting discounts set
forth in the cover page of this prospectus:
| Underwriter | |
Number of Shares | | |
Number of Pre-funded
Warrants | |
| ThinkEquity LLC | |
| | | |
| | |
| Total | |
| | | |
| | |
The
underwriters have committed to purchase all of the securities offered by us other than those covered by the over-allotment option
described below, if they purchase any securities. The obligations of the underwriters may be terminated upon the occurrence of
certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriters’
obligations are subject to customary conditions, representations and warranties contained in the underwriting agreement,
such as receipt by the underwriters of officers’ certificates and legal opinions.
The
underwriters are offering the securities, subject
to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel and other conditions
contained in the underwriting agreement. The underwriters reserve the right to withdraw, cancel or modify offers to the public
and to reject orders in whole or in part.
We
have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, or to
contribute to payments the underwriters may be required to make in respect of those liabilities.
Over-Allotment
Option
We
have granted a 45-day option to the underwriters to purchase up to 2,400,000 additional shares of our common stock and/or Pre-funded
Warrants at an assumed public offering price of $0.75 per share (or $0.749 per
Pre-funded Warrant), the last reported sale price of our common stock as reported on The Nasdaq Capital Market on January 7, 2025,
less underwriting discounts and commissions, solely to cover over-allotments, if any. The underwriters may exercise this option
for 45 days from the closing date of this offering. If any of these additional securities are purchased, the underwriters will
offer the additional shares and/or Pre-funded Warrants on the same terms as those on which they are being offered.
Underwriting
Discount, Commissions and Expenses
The
underwriters propose initially to offer the shares of common stock and/or Pre-funded Warrants to the public at the public offering price
set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $
per share of common stock and $ per Pre-funded Warrant. If all of the shares
of common stock and/or Pre-funded Warrants offered by us are not sold at the public offering price, the underwriters may change the offering
price and other selling terms by means of a supplement to this prospectus.
The
following table shows the offering price, underwriting discounts and proceeds, before expenses, to us. The information assumes either
no exercise or full exercise by the underwriters of their over-allotment option.
| | |
Per Share | | |
Per
Pre-funded
Warrant | | |
Total
Assuming No Exercise of Over- Allotment Option | | |
Total
Assuming Full Exercise of Over- Allotment Option | |
| Offering price | |
$ | | | |
$ | | | |
$ | | | |
$ | | |
| Underwriting discount and commissions (7.0%) | |
$ | | | |
$ | | | |
$ | | | |
$ | | |
| Proceeds, before expense, to us | |
$ | | | |
$ | | | |
$ | | | |
$ | | |
We
have agreed to pay all of the expenses relating to the offering, including, but not limited to the following: (a) all filing fees
and communication expenses relating to the registration of the securities to be sold in the offering with the SEC; (b) all filing
fees and expenses associated with the review of the offering by FINRA; (c) all fees and expenses relating to the listing of the
shares of common stock being offered on Nasdaq and on such other stock exchanges we and the Representative shall together determine,
including any fees charged by The Depository Trust Company for new securities; (d) all fees, expenses and disbursements relating to
background checks of the Company’s officers, directors and entities in an amount not to exceed $15,000 in the aggregate; (e)
all fees, expenses and disbursements relating to the registration or qualification of the securities offered hereby under the
“blue sky” securities laws of such states, if applicable, and other jurisdictions as the Representative may reasonably
designate; (f) all fees, expenses and disbursements relating to the registration, qualification or exemption of the securities
offered hereby under the securities laws of such foreign jurisdictions as the Representative may reasonably designate; (g) the costs
of all mailing and printing of the underwriting documents (including, without limitation, the Underwriting Agreement, any Blue Sky
Surveys and, if appropriate, any Agreement Among Underwriters, Selected Dealers’ Agreement, Underwriters’ Questionnaire
and Power of Attorney), Registration Statements, Prospectuses and all amendments, supplements and exhibits thereto and as many
preliminary and final Prospectuses as the Representative may reasonably deem necessary; (h) the costs and expenses of our public
relations firm; (i) the costs of preparing, printing and delivering certificates representing the securities offered hereby; (j)
fees and expenses of the transfer agent for our common stock; (k) stock transfer and/or stamp taxes, if any, payable upon the
transfer of securities from us to the underwriters; (l) the costs associated with post-closing advertising the offering in the
national editions of the Wall Street Journal and New York Times; (m) the costs associated with bound volumes of the public offering
materials as well as commemorative mementos and lucite tombstones in such quantities as the Representative may reasonably request,
in an amount not to exceed $3,000; (n) the fees and expenses of our accountants; (o) the fees and expenses of our legal counsel and
other agents and representatives; (p) the fees and expenses of counsel to the underwriters, not to exceed $125,000; (q) the $29,500
cost associated with the use of Ipreo’s book building, prospectus tracking and compliance software for the offering; (r)
$10,000 for data services and communications expenses; (s) up to $10,000 of actual accountable “road show” expenses; and
(t) up to $30,000 of market making and trading, and clearing firm settlement expenses for the offering.
We
have paid an expense deposit of $35,000 to the Representative, which will be applied against the out-of-pocket accountable
expenses that will be paid by us to the underwriters in connection with this offering and will be reimbursed to us to the extent not
actually incurred in compliance with FINRA Rule 5110(g)(4)(A).
We
have agreed to pay a non-accountable expense allowance to the underwriters, equal to 1.0% of the gross proceeds received in this offering
at the closing of the offering. The Representative may deduct from the net proceeds of the offering payable to us at the closing of the
offering the expenses to be paid by us to the underwriters less the expense allowance.
Our
total estimated expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses,
but excluding underwriting discounts and commissions and excluding the non-accountable expense allowance, are approximately $ .
Representative’s
Warrants
We
have also agreed to issue to the Representative or its designees at the closing of this offering Representative’s Warrants to purchase
an aggregate of 800,000 shares of common stock (or 920,000 shares of common stock if the underwriters exercise their over-allotment option
in full) (5% of the number of shares sold in the offering). We are registering the Representative’s Warrants and the shares of
common stock issuable upon exercise of such warrants. The Representative’s Warrants will be exercisable at any time and from time
to time, in whole or in part, during a period commencing six months from the commencement of sales in this offering and expiring five
years thereafter. The Representative’s Warrants will be exercisable at a price equal to 125% of the public offering price per share
of common stock and such warrants shall be exercisable on a cash basis, provided that if a registration statement registering the common
stock underlying the Representative’s Warrants is not effective, the Representative’s Warrants may be exercised on a cashless
basis. The Representative’s Warrants have been deemed compensation by FINRA and are, therefore, subject to a 180-day lock-up pursuant
to Rule 5110(e)(1) of FINRA. The Representative or its permitted assignees under Rule 5110(e)(1) shall not sell, transfer, assign, pledge
or hypothecate the Representative’s Warrants, nor engage in any hedging, short sale, derivative, put or call transaction that would
result in the effective economic disposition of the Representative’s Warrants, for a period of 180 days from the commencement of
sales in the offering, except that they may be assigned, in whole or in part, as specifically set forth in the underwriting agreement.
The Representative’s Warrants will provide for customary anti-dilution provisions (for stock dividends, splits and recapitalizations
and the like) consistent with FINRA Rule 5110(g)(8). Further, the Representative’s Warrants will provide for a one-time demand
registration right at our expense, and unlimited piggyback registration rights, in each case for period of up to five years from the
commencement of sales in this offering in compliance with FINRA Rule 5110(g)(8)(C) and (D), respectively.
Lock-Up Agreements
We
have agreed that, for a period of three months from the closing of the offering, we will not (a) offer, pledge, sell, contract to sell,
sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend,
or otherwise transfer or dispose of, directly or indirectly, any shares of capital stock of our Company or any securities convertible
into or exercisable or exchangeable for shares of capital stock of our Company; (b) file or caused to be filed any registration statement
with the SEC relating to the offering of any shares of capital stock of our Company or any securities convertible into or exercisable
or exchangeable for shares of capital stock of our Company; (c) complete any offering of debt securities of our Company, other than entering
into a line of credit with a traditional bank or (d) enter into any swap or other arrangement that transfers to another, in whole or
in part, any of the economic consequences of ownership of capital stock of our Company, whether any such transaction described in clause
(a), (b), (c) or (d) above is to be settled by delivery of shares of capital stock of our Company or such other securities, in cash or
otherwise.
We
have also agreed that, for a period of six months from the closing of the offering, we will not directly or indirectly offer to sell,
sell, contract to sell, grant any option to sell or otherwise dispose of shares of capital stock of our Company or any securities convertible
into or exercisable or exchangeable for shares of capital stock of our Company in any “at-the-market”, continuous equity
or variable rate transaction, without the prior written consent of the Representative.
Moreover, pursuant
to “lock-up” agreements, our executive officers, directors and holders of 5% or more of the issued and outstanding shares
of our common stock have agreed for a period of three months from the date of this prospectus, subject to customary exceptions, without
the prior written consent of the Representative, not to, directly or indirectly (a) offer, sell, agree to offer or sell, solicit offers
to purchase, grant any call option or purchase any put option with respect to, pledge, encumber, assign, borrow or otherwise dispose
of any shares of common stock, any warrant or option to purchase such shares or any other of our securities or of any other entity that
is convertible into, or exercisable or exchangeable for, shares of our common stock or any other of our equity securities (each a “Relevant
Security” and collectively, “Relevant Securities”), in each case owned beneficially owned by them or otherwise publicly
disclose the intention to do so, or (b) establish or increase any “put equivalent position” or liquidate or decrease any
“call equivalent position” with respect to any Relevant Security (in each case within the meaning of Section 16 of the Exchange
Act with respect to any Relevant Security or otherwise enter into any swap, derivative or other transaction or arrangement that transfers
to another, in whole or in part, any economic consequence of ownership of a Relevant Security, whether or not such transaction is to
be settled by the delivery of Relevant Securities, other securities, cash or other consideration, or otherwise publicly disclose the
intention to do so.
Right
of First Refusal
We
have granted the representative a right of first refusal, for a period of 12 months from the closing of the offering, to act as sole
investment banker, sole book-runner, and/or sole placement agent, at the representative’s sole and exclusive discretion, for each
and every future public and private equity and debt offering, including all of our equity linked financings (each, a “Subject Transaction”),
or any successor (or any of our subsidiaries), on terms and conditions customary to the representative for such Subject Transactions.
Discretionary
Accounts
The
underwriters do not intend to confirm sales of the shares of common stock and/or Pre-funded Warrants offered hereby to any accounts over
which they have discretionary authority.
Electronic
Distribution
This
prospectus in electronic format may be made available on websites or through other online services maintained by one or more of the underwriters,
or by their affiliates. Other than this prospectus in electronic format, the information on any underwriter’s website and any information
contained in any other website maintained by an underwriter is not part of this prospectus, has not been approved and/or endorsed by
us or any underwriter in its capacity as underwriter, and should not be relied upon by investors.
Nasdaq
Capital Market Listing
Our
common stock is listed on The Nasdaq Capital Market under the symbol “AWH”. There is no established trading market for the
Pre-funded Warrants and we do not intend to list the Pre-funded Warrants on any securities exchange or nationally recognized trading
system.
Stabilization
In
connection with this offering, the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate-covering
transactions, penalty bids, and purchases to cover positions created by short sales.
| |
● |
Stabilizing
transactions permit bids to purchase securities so long as the stabilizing bids do not exceed a specified maximum and are engaged
in for the purpose of preventing or retarding a decline in the market price of the securities while the offering is in progress. |
| |
|
|
| |
● |
Over-allotment
transactions involve sales by the underwriter of securities in excess of the number of securities the underwriter is obligated to
purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered
short position, the number of securities over-allotted by the underwriter is not greater than the number of securities that they
may purchase in the over-allotment option. In a naked short position, the number of securities involved is greater than the number
of securities in the over-allotment option. The underwriter may close out any short position by exercising their over-allotment option
and/or purchasing securities in the open market. |
| |
|
|
| |
● |
Syndicate
covering transactions involves purchases of securities in the open market after the distribution has been completed in order to cover
syndicate short positions. In determining the source of securities to close out the short position, the underwriter will consider,
among other things, the price of securities available for purchase in the open market as compared with the price at which they may
purchase securities through exercise of the over-allotment option. If the underwriter sells more securities than could be covered
by exercise of the over-allotment option and, therefore, have a naked short position, the position can be closed out only by buying
securities in the open market. A naked short position is more likely to be created if the underwriter is concerned that after pricing
there could be downward pressure on the price of the securities in the open market that could adversely affect investors who purchase
in the offering. |
| |
|
|
| |
● |
Penalty
bids permit the underwriter to reclaim a selling concession from a syndicate member when the securities originally sold by that syndicate
member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions. |
These
stabilizing transactions, over-allotment transactions, syndicate covering transactions, and penalty bids may have the effect of raising
or maintaining the market price of our securities or preventing or retarding a decline in the market price of our securities. As a result,
the price of our securities in the open market may be higher than it would otherwise be in the absence of these transactions. Neither
we nor the underwriter make any representation or prediction as to the effect that the transactions described above may have on the price
of our securities. These transactions may be affected on The Nasdaq Capital Market, in the over-the-counter market or otherwise and,
if commenced, may be discontinued at any time.
Passive
Market Making
In
connection with this offering, underwriter, and selling group members may engage in passive market making transactions in our securities
on The Nasdaq Capital Market in accordance with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement
of offers or sales of the shares and extending through the completion of the distribution. A passive market maker must display its bid
at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive
market maker’s bid, then that bid must then be lowered when specified purchase limits are exceeded.
Other
Relationships
The
underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include
sales and trading, commercial and investment banking, advisory, investment management, investment research, principal investment, hedging,
market making, brokerage and other financial and non-financial activities and services. Certain of the underwriters and their respective
affiliates have provided, and may in the future provide, a variety of these services to us and to persons and entities with relationships
with us, for which they received or will receive customary fees and expenses.
Selling
Restrictions
Other
than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered
by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be
offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with
the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result
in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are
advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus.
This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in
any jurisdiction in which such an offer or a solicitation is unlawful.
European
Economic Area
In
relation to each member state of the European Economic Area that has implemented the Prospectus Directive (each, a relevant member state),
with effect from and including the date on which the Prospectus Directive is implemented in that relevant member state (the relevant
implementation date), an offer of securities described in this prospectus may not be made to the public in that relevant member state
other than:
| |
● |
to
any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| |
|
|
| |
● |
to
fewer than 100 or, if the relevant member state has implemented the relevant provision of the 2010 PD Amending Directive, 150 natural
or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive,
subject to obtaining the prior consent of the relevant Dealer or Dealers nominated by us for any such offer; or |
| |
|
|
| |
● |
in
any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall
require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive. |
For
purposes of this provision, the expression an “offer of securities to the public” in any relevant member state means the
communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as
to enable an investor to decide to purchase or subscribe for the securities, as the expression may be varied in that member state by
any measure implementing the Prospectus Directive in that member state, and the expression “Prospectus Directive” means Directive
2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the relevant member state)
and includes any relevant implementing measure in the relevant member state. The expression 2010 PD Amending Directive means Directive
2010/73/EU.
The
sellers of the securities have not authorized and do not authorize the making of any offer of securities through any financial intermediary
on their behalf, other than offers made by the underwriters with a view to the final placement of the securities as contemplated in this
prospectus. Accordingly, no purchaser of the securities, other than the underwriters, is authorized to make any further offer of the
securities on behalf of the sellers or the underwriters.
United
Kingdom
This
prospectus is only being distributed to, and is only directed at, persons in the United Kingdom that are qualified investors within the
meaning of Article 2(1)(e) of the Prospectus Directive that are also (i) investment professionals falling within Article 19(5) of the
Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”) or (ii) high net worth entities, and
other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (each such person being referred
to as a “relevant person”). This prospectus and its contents are confidential and should not be distributed, published or
reproduced (in whole or in part) or disclosed by recipients to any other persons in the United Kingdom. Any person in the United Kingdom
that is not a relevant person should not act or rely on this document or any of its contents.
Switzerland
The
securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (the “SIX”) or on
any other stock exchange or regulated trading facility in Switzerland. This document does not constitute a prospectus within the meaning
of and has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss
Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules
of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material
relating to the securities or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither
this document nor any other offering or marketing material relating to the offering, the Company, the securities have been or will be
filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities
will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (the “FINMA”), and the offer of securities
has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (the “CISA”). The investor
protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of securities.
Canada
The
securities may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined
in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients,
as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the
securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable
securities laws.
Securities
legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus
(including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by
the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser
should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars
of these rights or consult with a legal advisor.
Israel
The
securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority (the “ISA”),
nor have such securities been registered for sale in Israel. The securities may not be offered or sold, directly or indirectly,
to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection
with the offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or
completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly,
to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in
compliance with the Israeli securities laws and regulations.
United
Arab Emirates
Neither
this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates
or any other governmental authority in the United Arab Emirates, nor has the Company received authorization or licensing from the Central
Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within
the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services
relating to the securities, including the receipt of applications and/or the allotment or redemption of such securities, may be rendered
within the United Arab Emirates by the Company.
No
offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
Singapore
This
prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other
document or material in connection with the offer or sale, or invitation for subscription or purchase, of our securities may not be circulated
or distributed, nor may the securities be offered or sold, or be made the subject of an invitation for subscription or purchase, whether
directly or indirectly, to persons in Singapore other than (i) to an institutional investor (as defined under Section 4A of the Securities
and Futures Act, Chapter 289 of Singapore (the “SFA”) ) pursuant to Section 274 of the SFA, (ii) to a relevant person (as
defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A), and in accordance
with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any
other applicable provision of the SFA, in each case subject to conditions set forth in the SFA.
Where
our securities are subscribed or purchased under Section 275 by a relevant person which is a corporation (which is not an accredited
investor as defined in Section 4A of the SFA) the sole business of which is to hold investments and the entire share capital of which
is owned by one or more individuals, each of whom is an accredited investor, the securities or securities-based derivatives contracts
(each as defined in Section 2(1) of the SFA) of that corporation shall not be transferable for six months after that corporation has
acquired our securities under Section 275 except: (a) to an institutional investor under Section 274 of the SFA or to a relevant person,
(b) where such transfer arises from an offer in that corporation’s securities pursuant to Section 275(1A) of the SFA, and in accordance
with the conditions, specified in Section 275 of the SFA; (c) where no consideration is or will be given for the transfer; (d) where
such transfer is by operation of law; or (e) as specified in Section 276(7) of the SFA.
Where
the securities are subscribed or purchased under Section 275 of the SFA by a relevant person which is a trust (where the trustee is not
an accredited investor (as defined in Section 4A of the SFA)) whose sole purpose is to hold investments and each beneficiary of the trust
is an accredited investor, the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferable
for six months after that trust has acquired the securities under Section 275 of the SFA except: (1) to an institutional investor under
Section 274 of the SFA or to a relevant person, (2) where such transfer arises from an offer that is made on terms that such rights or
interest are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction (whether
such amount is to be paid for in cash or by exchange of securities or other assets), (3) where no consideration is or will be given for
the transfer, (4) where the transfer is by operation of law, or (5) as specified in Section 276(7) of the SFA.
Hong
Kong
Our
securities may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the
public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), (ii) to “professional investors” within
the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder or (iii) in other circumstances
which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong
Kong), and no advertisement, invitation or document relating to the securities may be issued or may be in the possession of any person
for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely
to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect
to the securities which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors”
within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder.
Australia
This
prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian
Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter
6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to
whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions
set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons
as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the
offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations
Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer
to the offeree under this prospectus.
Japan
The
securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan
(Law No. 25 of 1948), as amended (the “FIEL”), pursuant to an exemption from the registration requirements applicable to
a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of
the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly,
in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional
Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition
by any such person of securities is conditional upon the execution of an agreement to that effect.
People’s
Republic of China
The
information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s
Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region
and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly
to “qualified domestic institutional investors.”
LEGAL
MATTERS
The
validity of the issuance of the shares of common stock offered by us in this offering will be passed upon for us by Sheppard, Mullin,
Richter & Hampton LLP, New York, New York. Lucosky Brookman LLP, New York, New York has acted as counsel for the underwriters
in connection with certain legal matters related to this offering.
EXPERTS
The
consolidated financial statements of Aspira Women’s Health Inc. as of December 31, 2023 and 2022 and for the years then ended incorporated
by reference in this prospectus and in the registration statement have been so incorporated in reliance on the report of BDO USA, P.C.,
an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting. The report
on the consolidated financial statements contains an explanatory paragraph regarding the Company’s ability to continue as a going
concern.
WHERE
YOU CAN FIND MORE INFORMATION
This
prospectus is part of a registration statement we filed with the SEC. This prospectus does not contain all of the information set forth
in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities
we are offering under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the
registration statement. You should rely only on the information contained in this prospectus or incorporated by reference into this prospectus.
We have not authorized anyone else to provide you with different information. We are not making an offer of these securities in any jurisdiction
where the offer is not permitted. You should assume that the information contained in this prospectus, or any document incorporated by
reference in this prospectus, is accurate only as of the date of those respective documents, regardless of the time of delivery of this
prospectus or any sale of our securities.
We
file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the
public from commercial document retrieval services and over the Internet at the SEC’s website at http://www.sec.gov.
We
maintain a website at www.aspirawh.com. You may access our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current
Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the
SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to,
the SEC. The information contained in, or that can be accessed through, our website is not incorporated by reference into, and is not
part of, this prospectus.
INCORPORATION
OF DOCUMENTS BY REFERENCE
This
prospectus is part of the registration statement, but the registration statement includes and incorporates by reference additional information
and exhibits. The SEC permits us to “incorporate by reference” the information contained in documents we file with the SEC,
which means that we can disclose important information to you by referring you to those documents rather than by including them in this
prospectus. Information that is incorporated by reference is considered to be part of this prospectus and you should read it with the
same care that you read this prospectus. Information that we file later with the SEC will automatically update and supersede the information
that is either contained, or incorporated by reference, in this prospectus, and will be considered to be a part of this prospectus from
the date those documents are filed.
We
incorporate by reference the documents listed below, all filings filed by us pursuant to the Exchange Act after the date of the registration
statement of which this prospectus form a part, and any future filings we make with the SEC under Sections 13(a), 13(c), 14 or 15(d)
of the Exchange Act prior to the time that all securities covered by this prospectus have been sold; provided, however, that we are not
incorporating any information furnished under either Item 2.02 or Item 7.01 of any Current Report on Form 8-K and exhibits furnished
on such form that relate to such items:
| |
● |
our
Annual Report on Form
10-K for the year ended December 31, 2023 filed with the SEC on April 1, 2024; |
| |
|
|
| |
● |
our
Quarterly Reports on Form 10-Q for the quarters ended March
31, 2024, June
30, 2024 and September
30, 2024 filed with the SEC on May 15, 2024, August 13, 2024 and November 19, 2024, respectively; |
| |
|
|
| |
● |
our
Current Reports on Forms 8-K and 8-K/A filed with the SEC on January
25, 2024, January
26, 2024, March
21, 2024, March
25, 2024, April
26, 2024, April
26, 2024, May
14, 2024, June
7, 2024, June
27, 2024, July
2, 2024, July
5, 2024, July
29, 2024, July
31, 2024, August
2, 2024, October
18, 2024, October
23, 2024, October
24, 2024, December 16, 2024, December
31, 2024, and January 7, 2025; |
| |
|
|
| |
● |
those
portions of our Definitive Proxy Statement on Schedule 14A filed on April 1, 2024, that are deemed “filed” with the SEC;
and |
| |
|
|
| |
● |
the
description of our common stock set forth in the Registration Statement on Form
8-A filed with the SEC on July 6, 2010 (File No. 001-34810), and any amendment or report filed with the SEC for the purpose of
updating such description, including Exhibit
4.7 of our Annual Report on Form 10-K for the year ended December 31, 2023. |
Any
statements made in a document incorporated by reference in this prospectus are deemed to be modified or superseded for purposes of this
prospectus to the extent that a statement in this prospectus or in any other subsequently filed document, which is also incorporated
by reference, modifies or supersedes the statement. Any statement made in this prospectus is deemed to be modified or superseded to the
extent a statement in any subsequently filed document, which is incorporated by reference in this prospectus, modifies or supersedes
such statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part
of this prospectus.
The
information relating to us contained in this prospectus should be read together with the information in the documents incorporated by
reference. In addition, certain information, including financial information, contained in this prospectus or incorporated by reference
in this prospectus should be read in conjunction with documents we have filed with the SEC.
We
will provide to each person, including any beneficial holder, to whom a prospectus is delivered, at no cost, upon written or oral request,
a copy of any or all of the information that has been incorporated by reference in the prospectus but not delivered with the prospectus.
Requests for documents should be by writing to or telephoning us at the following address: Aspira Women’s Health Inc., 12117 Bee
Caves Road, Building III, Suite 100, Austin, Texas 78738, (512) 519-0400. Exhibits to these filings will not be sent unless those
exhibits have been specifically incorporated by reference in such filings.
Up
to 16,000,000 Shares of Common Stock
Up
to 16,000,000 Pre-Funded Warrants to Purchase up to 16,000,000 Shares of Common Stock

Aspira
Women’s Health Inc.
PRELIMINARY PROSPECTUS
ThinkEquity
,
2025
PART
II—INFORMATION NOT REQUIRED IN PROSPECTUS
Item
13. Other Expenses of Issuance and Distribution
The
following table sets forth all expenses, other than the underwriting discounts and commissions, payable by the registrant in connection
with the sale of the securities being registered. All the amounts shown are estimates except the SEC registration fee and the FINRA filing
fee.
| | |
Amount to be paid | |
| SEC registration fee | |
$ | 2,245 | |
| FINRA filing fee | |
$ | 2,700 | |
| Accounting fees and expenses | |
$ | 51,000 | |
| Legal fees and expenses | |
$ | 125,000 | |
| Miscellaneous | |
$ | 22,055 | |
| | |
| | |
| Total | |
$ | 203,000 | |
Item
14. Indemnification of Directors and Officer
Sections
145 and 102(b)(7) of the DGCL provide that a corporation may indemnify any person made a party to an action by reason of the fact that
he or she was a director, executive officer, employee or agent of the corporation or is or was serving at the request of the corporation
against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred
by him or her in connection with such action if he or she acted in good faith and in a manner he or she reasonably believed to be in,
or not opposed to, the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause
to believe his or her conduct was unlawful, except that, in the case of an action by or in right of the corporation, no indemnification
may generally be made in respect of any claim as to which such person is adjudged to be liable to the corporation.
Certificate
of Incorporation and Bylaws. Article VII of the Company’s Certificate of Incorporation and Article VI of the Registrant’s
Amended and Restated Bylaws provide in substance that, to the fullest extent permitted by the DGCL, each director and officer shall be
indemnified against reasonable costs and expenses, including attorney’s fees, and any liabilities which the person may incur in
connection with any action to which the person may be made a party by reason of his or her being or having been a director or officer
of the Registrant, a predecessor of the Registrant, or serves or served as a director, officer or employee of another enterprise at the
request of the Registrant or any predecessor of the Registrant. The indemnification provided by the Company’s Certificate of Incorporation
is not deemed exclusive of or intended in any way to limit any other rights to which any person seeking indemnification may be entitled.
D&O
Insurance. The Company maintains standard policies of insurance under which coverage is provided to the Company’s directors
and officers against loss arising from claims made by reason of breach of duty or other wrongful act, and to the Company with respect
to payments which may be made by the Company to such officers and directors pursuant to the above indemnification provision or otherwise
as a matter of law. Any underwriting agreement that we may enter into (Exhibit 1.1) may provide for indemnification by any underwriters,
of us, our directors, our officers who sign the registration statement and our controlling persons for some liabilities, including liabilities
arising under the Securities Act.
Item
15. Recent Sales of Unregistered Securities
Set
forth below is information regarding securities issued by us, since during the prior three years that were not registered under
the Securities Act.
On
January 24, 2024, we entered into a placement agency agreement (the “Placement Agency Agreement”) with A.G.P./Alliance Global
Partners (“AGP”) and a securities purchase agreement (the “Securities Purchase Agreement”) with a single healthcare
focused institutional investor alongside participation from Nicole Sandford, our former CEO, as well as certain existing shareholders
of the Company (collectively, the “Purchasers”). Pursuant to the Placement Agency Agreement, we paid AGP as the placement
agent a cash fee equal to 7.0% of the aggregate gross proceeds generated from the offering, except that, with respect to proceeds raised
in the offering from certain designated persons, AGP’s cash fee was reduced to 3.5% of such proceeds, and to reimburse certain
fees and expenses of the placement agent in connection with the offering.
Pursuant
to the Securities Purchase Agreement, among other transactions, we issued the Purchase Warrants to purchase 1,571,000 shares of the Company’s
common stock. The Purchase Warrants have an exercise price of $4.13 per share, and are exercisable any time on or after July 26, 2024,
which is the six month anniversary date after issuance, and on or prior to 5:00p.m. (New York City time) on July 25, 2029.
We
believe each of these transactions was exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities
Act (and Regulation D promulgated thereunder) as transactions by an issuer not involving any public offering. The recipients of the securities
in each of these transactions represented their intentions to acquire the securities for investment only and not with a view to or for
sale in connection with any distribution thereof, and appropriate legends were placed on the share certificates issued in these transactions.
All recipients had adequate access, through their relationships with us, to information about us. The sales of these securities were
made without any general solicitation or advertising.
Item
16. Exhibits and Financial Statement Schedules
EXHIBIT
INDEX
EXHIBIT
NUMBER | |
DESCRIPTION OF EXHIBIT |
| 1.1** | |
Form of Underwriting Agreement |
| 1.2 | |
At
The Market Offering Agreement between Aspira Women’s Health Inc. and H.C. Wainwright & Co., LLC dated August 2, 2024 (incorporated
by reference to Exhibit 1.1 of the Registrant’s Current Report on Form 8-K, filed with the Commission on August 2, 2024). |
| 3.1 | |
Fourth
Amended and Restated Certificate of Incorporation of Vermillion, Inc. dated January 22, 2010 (incorporated by reference to Exhibit
3.1 of the Company’s Current Report on Form 8-K, filed with the Commission on January 25, 2010). |
| 3.2 | |
Certificate
of Amendment of Fourth Amended and Restated Certificate of Incorporation, effective June 19, 2014 (incorporated by reference
to Exhibit 3.2 of the Company’s Quarterly Report on Form 10-Q, filed with the Commission on August 14, 2014). |
| 3.3 | |
Certificate
of Amendment to Fourth Amended and Restated Certificate of Incorporation of Vermillion, Inc., dated June 11, 2020 (incorporated by
reference to Exhibit 3.1 of the Company’s Current Report on Form 8-K, filed with the Commission on June 11, 2020). |
| 3.4 | |
Certificate
of Amendment to Fourth Amended and Restated Certificate of Incorporation of Aspira Women’s Health Inc, dated February 7, 2023 (incorporated
by reference to Exhibit 3.1 of the Company’s Current Report on Form 8-K, filed with the Commission on February 7, 2023). |
| 3.5 | |
Certificate
of Amendment to Fourth Amended and Restated Certificate of Incorporation of Aspira Women’s Health Inc., dated May 11, 2023 (incorporated
by reference to Exhibit 3.1 of the Company’s Current Report on Form 8-K, filed with the Commission on May 11, 2023). |
| 3.6 | |
Amended
and Restated Bylaws of Aspira Women’s Health Inc., effective February 23, 2022 (incorporated by reference to Exhibit 3.1 of
the Company’s Current Report on Form 8-K, filed with the Commission on February 28, 2022). |
| 4.1 | |
Form
of Aspira Women’s Health Inc.’s (formerly Ciphergen Biosystems, Inc.) Common Stock Certificate (incorporated by reference
to Exhibit 4.1 of the Registrant’s Registration Statement on Form S-1/A, filed with the Commission on August 24, 2000). |
| 4.2 | |
Stockholder’s
Agreement dated May 13, 2013, by and among Vermillion, Inc., Oracle Partners, LP, Oracle Ten Fund Master, LP, Jack W. Schuler and
other purchasers named therein (incorporated by reference to Exhibit 10.2 of the Registrant’s Current Report on Form 8-K,
filed with the Commission on May 14, 2013). |
| 4.3 | |
Form
of Warrant 2022 (incorporated by reference to Exhibit 4.1 of the Registrant’s Current Report on Form 8-K, filed with the Commission
on August 24, 2022). |
| 4.4 | |
Form
of Warrant Amendment to Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.3 of the Registrant’s Current
Report on Form 8-K, filed with the Commission on January 26, 2024). |
| 4.5 | |
Form
of Pre-Funded Warrant 2024 (incorporated by reference to Exhibit 4.1 of the Registrant’s Current Report on Form 8-K, filed
with the Commission on January 26, 2024). |
| 4.6 | |
Form
of Warrant 2024 (incorporated by reference to Exhibit 4.2 of the Registrant’s Current Report on Form 8-K, filed with the
Commission on January 26, 2024). |
| 4.7 | |
Registration
Rights Agreement, dated as of March 28, 2023, by and between Aspira Women’s Health Inc. and Lincoln Park Capital Fund, LLC.
(incorporated by reference to Exhibit 10.2 of the Registrant’s Current Report on Form 8-K, filed with the Commission on March
30, 2023). |
| 4.8 | |
Form
of Common Warrant (incorporated by reference to Exhibit 4.1 of the Registrant’s Current Report on Form 8-K, filed with the
Commission on July 2, 2024). |
| 4.9 | |
Form of Warrant (incorporated by reference to Exhibit 4.1 of the Registrant’s Current Report on Form 8-K, filed with the Commission on July 31, 2024). |
| 4.10** | |
Form of Representative’s Warrant |
| 4.11** | |
Form of Pre-funded Warrant |
| 5.1* | |
Opinion of Sheppard, Mullin, Richter & Hampton, LLP |
| 10.1# | |
Form of Proprietary Information Agreement between Vermillion, Inc. (formerly Ciphergen Biosystems, Inc.) and certain of its employees (incorporated by reference to Exhibit 10.9 of the Registrant’s Registration Statement on Form S-1/A, filed with the Commission on August 24, 2000). |
| 10.2# | |
Vermillion,
Inc. Second Amended and Restated 2010 Stock Incentive Plan (as amended effective June 21, 2018) (incorporated by reference to Exhibit
10.1 of the Registrant’s Current Report on Form 8-K, filed with the Commission on June 27, 2018). |
| 10.3# | |
Form
of Vermillion, Inc. Stock Option Award Agreement through December 31, 2021 (incorporated by reference to Exhibit 10.7 of the Registrant’s
Annual Report on Form 10-K, filed with the Commission on March 28, 2019). |
| 10.4# | |
Form
of Vermillion, Inc. Stock Option Award Agreement after January 1, 2022 (incorporated by reference to Exhibit 10.1 of the Registrant’s
Quarterly Report on Form 10-Q, filed with the Commission on August 10, 2022). |
| 10.5# | |
Form
of Aspira Women’s Health Inc Stock Option Award Agreement (incorporated by reference to Exhibit 10.4 of the Registrant’s
Quarterly Report on Form 10-Q, filed with the Commission on August 10, 2022). |
| 10.6# | |
Form
of Vermillion, Inc. Restricted Stock Award Agreement through December 31, 2021 (incorporated by reference to Exhibit 10.8 of the
Registrant’s Annual Report on Form 10-K, filed with the Commission on March 28, 2019). |
| 10.7# | |
Form
of Vermillion, Inc. Restricted Stock Award Agreement after January 1, 2022 (incorporated by reference to Exhibit 10.2 of the Registrant’s
Quarterly Report on Form 10-Q, filed with the Commission on August 10, 2022). |
| 10.8# | |
Form
of Aspira Women’s Health Inc Restricted Stock Award Agreement (incorporated by reference to Exhibit 10.5 of the Registrant’s
Quarterly Report on Form 10-Q, filed with the Commission on August 10, 2022). |
| 10.9# | |
Aspira
Women’s Health Inc. 2019 Stock Incentive Plan (incorporated by reference to Exhibit 10.3 of the Registrant’s Quarterly
Report on Form 10-Q, filed with the Commission on August 10, 2022). |
| 10.10# | |
Form
of Vermillion, Inc. Stock Option Award Agreement (non-employee) (incorporated by reference to Exhibit 10.6 of the Registrant’s
Quarterly Report on Form 10-Q, filed with the Commission on August 10, 2022). |
| 10.11# | |
Form
of Aspira Women’s Health Inc. Stock Option Award Agreement (non-employee) (incorporated by reference to Exhibit 10.7
of the Registrant’s Quarterly Report on Form 10-Q, filed with the Commission on August 10, 2022). |
| 10.12 | |
Testing
and Services Agreement between Vermillion, Inc., Aspira Labs, Inc. and Quest Diagnostics Incorporated, dated as of March 11, 2015
(incorporated by reference to Exhibit 10.5 of the Registrant’s Quarterly Report on Form 10-Q, filed with the Commission on
May 12, 2015). |
| 10.13 | |
Amendment
No. 1 to the Testing and Services Agreement between Vermillion, Inc., Aspira Labs, Inc. and Quest Diagnostics Incorporated dated
April 10, 2015 (incorporated by reference to Exhibit 10.6 of the Registrant’s Quarterly Report on Form 10-Q, filed with the
Commission on May 12, 2015). |
| 10.14 | |
Amendment
No. 2 to Testing and Services Agreement, executed as of March 7, 2017 and effective as of March 11, 2017, by and among Vermillion,
Inc., Aspira Labs, Inc. and Quest Diagnostics Incorporated (incorporated by reference to Exhibit 10.1 of the Registrant’s Current
Report on Form 8-K, filed with the Commission on March 13, 2017). |
| 10.15 | |
Amendment
No. 3 to Testing and Services Agreement, executed as of March 1, 2018 by and among Vermillion, Inc., Aspira Labs, Inc. and Quest
Diagnostics Incorporated (incorporated by reference to Exhibit 10.1 of the Registrant’s Current Report on Form 8-K, filed
with the Commission on March 6, 2018). |
| 10.16 | |
Amendment
No. 4 to Testing and Services Agreement, executed as of March 11, 2020 by and among Vermillion, Inc., Aspira Labs, Inc. and Quest
Diagnostics Incorporated (incorporated by reference to Exhibit 10.1 of the Registrant’s Current Report on Form 8-K, filed with
the Commission on March 17, 2020). |
| 10.17 | |
Amendment
No. 5 to Testing and Services Agreement, executed as of December 6, 2022 by and among Aspira Women’s Health Inc., Aspira Labs,
Inc. and Quest Diagnostics Incorporated (incorporated by reference to Exhibit 10.18 of the Registrant’s Annual Report on Form
10-K, filed with the Commission on April 1, 2024). |
| 10.18 | |
Assistance
Agreement by and between the State of Connecticut, acting by and through the Department of Economic and Community Development and
Vermillion, Inc. effective March 22, 2016 (incorporated by reference to Exhibit 10.1 of the Registrant’s Quarterly Report on
Form 10-Q, filed with the Commission on May 16, 2016). |
| 10.19 | |
Patent
Security Agreement by Vermillion, Inc. in favor of the State of Connecticut, acting by and through the Department of Economic and
Community Development, effective March 22, 2016 (incorporated by reference to Exhibit 10.3 of the Registrant’s Quarterly Report
on Form 10-Q, filed with the Commission on May 16, 2016). |
| 10.20 | |
Security
Agreement by Vermillion, Inc. in favor of the State of Connecticut, acting by and through the Department of Economic and Community
Development, effective March 22, 2016 (incorporated by reference to Exhibit 10.4 of the Registrant’s Quarterly Report on Form
10-Q, filed with the Commission on May 16, 2016). |
| 10.21 | |
First
Amendment to the Assistance Agreement by and between the State of Connecticut, acting by and through the Department of Economic and
Community Development and Vermillion, Inc. dated March 7, 2018 (incorporated by reference to Exhibit 10.21 of the Registrant’s
Annual Report on Form 10-K, filed with the Commission on March 13, 2018). |
| 10.22 | |
Second
Amendment to the Assistance Agreement by and between the State of Connecticut, acting by and through the Department of Economic and
Community Development and Vermillion, Inc. dated April 3, 2020 (incorporated by reference to Exhibit 10.22 of the Registrant’s
Annual Report on Form 10-K, filed with the Commission on April 7, 2020). |
| 10.23#† | |
Amended
and Restated Employment Agreement between Aspira Women’s Health Inc. and Nicole Sandford effective March 1, 2023 (incorporated
by reference to Exhibit 10.1 of the Registrant’s Current Report on Form 8-K, filed with the Commission on March 2, 2023). |
| 10.24#† | |
Employment
Agreement between Aspira Women’s Health Inc. and Sandra Milligan effective April 1, 2024 (incorporated by reference to Exhibit
10.1 of the Registrant’s Current Report on Form 8-K, filed with the Commission on March 21, 2024). |
| 10.25 | |
License
Agreement between Aspira Women’s Health Inc. and Dana-Farber Cancer Institute, Inc. effective March 20, 2023 (incorporated
by reference to Exhibit 10.28 of the Registrant’s Annual Report on Form 10-K, filed with the Commission on April 1, 2024). |
| 10.26 | |
Form
of Securities Purchase Agreement (incorporated by reference to Exhibit 10.1 of the Registrant’s Current Report on Form 8-K,
filed with the Commission on July 2, 2024). |
| 10.27 | |
Form
of Warrant Inducement Agreement (incorporated by reference to Exhibit 10.1 of the Registrant’s Current Report on Form
8-K, filed with the Commission on July 31, 2024). |
| 10.28 | |
Second
Amended Employment Agreement between Aspira Women’s Health Inc. and Nicole Sandford, effective September 1, 2024 (incorporated
by reference to Exhibit 10.3 of the Registrant’s Quarterly Report on Form 10-Q, filed with the Commission on August 13, 2024). |
| 10.29 | |
Amended
Employment Agreement between Aspira Women’s Health Inc. and Sandra Milligan, M.D., J.D., effective September 1, 2024 (incorporated
by reference to Exhibit 10.4 of the Registrant’s Quarterly Report on Form 10-Q, filed with the Commission on August 13, 2024). |
| 10.30 | |
Consulting
Agreement between Aspira Women’s Health Inc. and John Kallassy (incorporated by reference to Exhibit 10.5 of the Registrant’s
Quarterly Report on Form 10-Q, filed with the Commission on August 13, 2024). |
| 21.1 | |
Subsidiaries
of the Company (incorporated by reference to Exhibit 21.0 of the Registrant’s Annual Report on Form 10-K, filed with the Commission
on March 31, 2022) |
| 23.1* | |
Consent of BDO USA, P.C., independent registered public accounting firm. |
| 23.2* | |
Consent of Sheppard, Mullin, Richter & Hampton, LLP (included in Exhibit 5.1). |
| 24.1* | |
Power of Attorney (included
on signature page of this Form S-1). |
| 107* | |
Filing Fee Table. |
*
Filed herewith.
**
Filed by amendment.
#
Management contract or compensatory plan or arrangement.
†
Confidential treatment has been granted with respect to certain provisions of this agreement. Omitted portions have been filed separately
with the SEC.
Item
17. Undertakings
The
undersigned registrant hereby undertakes:
| |
(1) |
To
file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| |
|
|
| |
(i) |
To
include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
| |
|
|
| |
(ii) |
To
reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set
forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if
the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end
of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b)
if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set
forth in the “Calculation of Registration Fee” table in the effective registration statement. |
| |
|
|
| |
(iii) |
To
include any material information with respect to the plan of distribution not previously
disclosed in the registration statement or any material change to such information in the
registration statement;
provided,
however, that paragraphs (i), (ii) and (iii) do not apply if the registration statement is on Form S-1 and the information required
to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission
by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference
in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration
statement; |
| |
|
|
| |
(2) |
That,
for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed
to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof. |
| |
|
|
| |
(3) |
To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering. |
| |
|
|
| |
(4) |
That,
for the purpose of determining liability under the Securities Act of 1933 to any purchaser: each prospectus filed pursuant to Rule
424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other
than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of
the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus
that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration
statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior
to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the
registration statement or made in any such document immediately prior to such date of first use; and |
| |
(5) |
That,
for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution
of the securities: the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant
to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities
are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller
to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| |
|
|
| |
(i) |
Any
preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule
424; |
| |
|
|
| |
(ii) |
Any
free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by
the undersigned registrant; |
| |
|
|
| |
(iii) |
The
portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant
or its securities provided by or on behalf of the undersigned registrant; and |
| |
|
|
| |
(iv) |
Any
other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| |
|
|
| |
|
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling
persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion
of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933
and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment
by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense
of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities
being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent,
submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed
in the Act and will be governed by the final adjudication of such issue. |
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this Registration Statement on Form S-1
to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Austin, State of Texas, on the 8th day
of January 2025.
| |
ASPIRA
WOMEN’S HEALTH INC. |
| |
|
|
| |
By: |
/s/
Sandra Milligan |
| |
|
Sandra
Milligan |
| |
|
Interim
Chief Executive Officer (Principal Executive Officer) and President |
POWER
OF ATTORNEY
KNOW
ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Sandra Milligan his/her true and lawful
attorney-in-fact, with full power of substitution and resubstitution for him/her and in his/her name, place and stead, in any and all
capacities to sign any and all amendments to this prospectus, and to file the same, with all exhibits thereto, and other documents in
connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority
to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and
purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorney-in-fact or his/her substitute,
each acting alone, may lawfully do or cause to be done by virtue thereof.
Pursuant
to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed below by the following persons
in the capacities and on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/
Sandra Milligan |
|
Interim
Chief Executive Officer and President |
|
January
8, 2025 |
| Sandra
Milligan |
|
(Principal
Executive Officer) |
|
|
| |
|
|
|
|
| /s/
John Kallassy |
|
Interim
Chief Financial Officer |
|
January
8, 2025 |
| John
Kallassy |
|
(Principal
Accounting and Financial Officer) |
|
|
| |
|
|
|
|
| /s/
Jannie Herchuk |
|
Chair
of the Board of Directors |
|
January
8, 2025 |
| Jannie
Herchuk |
|
|
|
|
| |
|
|
|
|
| /s/
Stefanie Cavanaugh |
|
Director |
|
January
8, 2025 |
| Stefanie
Cavanaugh |
|
|
|
|
| |
|
|
|
|
| /s/
Celeste Fralick |
|
Director |
|
January
8, 2025 |
| Celeste
Fralick |
|
|
|
|
| |
|
|
|
|
| /s/
Ellen O’Connor Vos |
|
Director |
|
January
8, 2025 |
| Ellen
O’Connor Vos |
|
|
|
|
| |
|
|
|
|
| /s/
Winfred Parnell |
|
Director |
|
January
8, 2025 |
| Winfred
Parnell |
|
|
|
|
| |
|
|
|
|
| /s/
John Ragard |
|
Director |
|
January
8, 2025 |
| John
Ragard |
|
|
|
|
Exhibit 5.1
 |
Sheppard, Mullin, Richter & Hampton LLP
30 Rockefeller Plaza
New York, New York 10112-0015
212.653.8700 main
212.653.8701 fax
www.sheppardmullin.com |
January
8, 2025
VIA
ELECTRONIC MAIL
Aspira
Women’s Health Inc.
12117
Bee Caves Road, Building III, Suite 100
Austin,
Texas 78738
| Re: |
Registration
Statement on Form S-1 |
Ladies
and Gentlemen:
We
are acting as counsel to Aspira Women’s Health Inc. (the “Company”) in connection with its registration statement on
Form S-1 (the “Registration, Statement”) filed with the Securities and Exchange Commission (the “Commission”)
under the Securities Act of 1933, as amended (the “Act”), relating to the proposed public offering of (a) $13,800,000 of
shares (the “Shares”) of common stock of the Company, par value $0.001 per share (the “Common Stock”); (b) to
each purchaser whose purchase of shares of Common Stock in such offering would otherwise result in the purchaser, together with its affiliates
and certain related parties, beneficially owning more than 4.99% (or, at the election of the holder, 9.99%) of the Company’s outstanding
Common Stock immediately following the consummation of such offering, the opportunity to purchase, if the purchaser so chooses, pre-funded
warrants (the “Pre-Funded Warrants”) to purchase shares (the “Pre-Funded Warrant Shares” and together with the
Shares and the Pre-Funded Warrants, the “Securities”) of the Company’s Common Stock, in lieu of shares of Common Stock
and (c) warrants to be issued by the Company to the representative of the underwriter of the Company named in the Registration Statement
to purchase up to 5% of the number of shares of Common Stock and Pre-Funded Warrants sold in the offering (the “Representative’s
Warrants” and together with the Pre-Funded Warrants, the “Warrants”) upon the closing of the public offering pursuant
to which the Registration Statement relate. The Securities will be sold by the Company pursuant to an underwriting agreement (the “Underwriting
Agreement”) to be entered into by and between the Company and ThinkEquity LLC as the representative of the several underwriters
to be named therein. This opinion letter is furnished to you at your request to enable you to fulfill
the requirements of Item 601(b)(5) of Regulation S-K in connection with the Registration Statement.
In
connection with this opinion, we have reviewed and relied upon the following:
| |
● |
the
Registration Statement and the related prospectus included therein; |
| |
● |
the
Fourth Amended and Restated Certificate of Incorporation of the Company, as amended and in effect on the date hereof; |
| |
● |
the
Amended and Restated Bylaws of the Company in effect on the date hereof; |
| |
● |
the
form of Underwriting Agreement; |
| |
● |
the
form of Pre-Funded Warrant; |
| |
● |
The
form of the Representative’s Warrant; |
| |
● |
the
resolutions of the Board of Directors of the Company authorizing/ratifying the issuance and sale of the Securities, the Representative’s
Warrants and the shares of Common Stock issuable upon exercise thereof, the preparation and filing of the Registration Statement,
and other actions with regard thereto; and |
| |
● |
such
other documents, records, certificates, memoranda and other instruments as we deem necessary as a basis for this opinion. |
In
our examination, we have assumed the genuineness of all signatures, including endorsements, the legal capacity and competency of all
natural persons, the authenticity of all documents submitted to us as originals, the conformity to original documents of all documents
submitted to us as facsimile, electronic, certified or photocopy, and the authenticity of the originals of such copies. As to any facts
relevant to the opinions stated herein that we did not independently establish or verify, we have relied upon statements and representations
of officers and other representatives of the Company and others and of public officials.

Page
2
Based
upon, subject to and limited by the foregoing, we are of the opinion that:
| |
1. |
Following
(i) execution and delivery by the Company of the Underwriting Agreement, (ii) effectiveness of the Registration Statement, (iii)
issuance of the Shares pursuant to the terms of the Underwriting Agreement, and (iv) receipt by the Company of the consideration
for the Shares specified in the resolutions, the Shares will be duly authorized for issuance and, when issued, delivered and paid
for in accordance with the terms of the Underwriting Agreement, will be validly issued, fully paid and non-assessable. |
| |
2. |
The
Warrants have been duly authorized by all requisite corporate action on the part of the Company under the General
Corporation Law of the State of Delaware (“DGCL”) and the laws of the State
of New York and, provided that the Warrants have been duly executed and delivered by the Company
and duly delivered to the purchasers thereof against payment therefor, the Warrants, when issued and sold as contemplated in the
Registration Statement will be valid and legally binding obligations of the Company, enforceable against the Company in accordance
with their terms, except as enforcement thereof may be limited by bankruptcy, insolvency, reorganization, moratorium or other similar
laws relating to or affecting creditors’ rights generally and by general equitable principles (regardless of whether such enforceability
is considered in a proceeding at law or in equity). |
| |
3. |
The
shares of Common Stock issuable upon exercise of the Warrants (the “Warrant Shares”) have been duly authorized by all
requisite corporate action on the part of the Company under the DGCL and the laws of the State of New York and, when the Warrant
Shares are delivered to and paid for in accordance with the terms of the Warrants and when evidence of the issuance thereof is duly
recorded in the Company’s books and records, the Warrant Shares will be validly issued, fully paid and non-assessable. |
We
also hereby consent to the filing of this opinion as an exhibit to the Registration Statement,
and to the reference to our firm under the caption “Legal Matters” in the prospectus which forms part of the Registration
Statement. In giving this consent, we do not admit that we are within the category of persons whose consent is required under Section
7 of the Act, the rules and regulations of the Commission promulgated thereunder or Item 509 of Regulation S-K.
We
express no opinion as to matters governed by any laws other than the DGCL and the laws of the State
of New York. No opinion is expressed herein with respect to the qualification of the Securities under the securities or blue sky
laws of any state or any foreign jurisdiction.
This
opinion letter is rendered as of the date first written above and we disclaim any obligation to advise you of facts, circumstances, events
or developments which hereafter may be brought to our attention and which may alter, affect or modify the opinion expressed herein. Our
opinion is expressly limited to the matters set forth above and we render no opinion, whether by implication or otherwise, as to any
other matters relating to the Company or the Securities, or any other agreements or transactions that may be related thereto or contemplated
thereby. We are expressing no opinion as to any obligations that parties other than the Company may have under or in respect of the Securities
or as to the effect that their performance of such obligations may have upon any of the matters referred to above. No opinion may be
implied or inferred beyond the opinion expressly stated above.
| Very
truly yours, |
|
| |
|
| /s/
Sheppard, Mullin, Richter & Hampton LLP |
|
| |
|
| SHEPPARD,
MULLIN, RICHTER & HAMPTON LLP |
|
Exhibit
23.1
Consent
of Independent Registered Public Accounting Firm
We
hereby consent to the incorporation by reference in the Prospectus constituting a part of this Registration Statement of our report dated
March 29, 2024, relating to the consolidated financial statements of Aspira Women’s Health Inc, (the Company) appearing in the
Company’s Annual Report on Form 10-K for the year ended December 31, 2023. Our report contains an explanatory paragraph regarding
the Company’s ability to continue as a going concern.
We
also consent to the reference to us under the caption “Experts” in the Prospectus.
/s/
BDO USA, P.C
Boston,
Massachusetts
January
8, 2025
Exhibit
107
Calculation
of Filing Fee Tables
FORM
S-1
(Form
Type)
ASPIRA
WOMEN’S HEALTH INC.
(Exact
Name of Registrant as Specified in its Charter)
Table
1: Newly Registered Securities
| |
|
Security
Type |
|
Security
Class Title |
|
Fee
Calculation Rule |
|
|
Amount
Registered |
|
|
Proposed
Maximum Offering Price Per Share |
|
|
Maximum
Aggregate Offering
Price(1)(3) |
|
|
Fee
Rate |
|
|
Amount
of Registration Fee |
|
| Fees
to Be Paid |
|
Equity |
|
Common
Stock, $0.001 par value (2) |
|
|
457(o) |
|
|
|
|
|
|
|
|
|
|
$ |
13,800,000 |
|
|
$ |
0.00015310 |
|
|
$ |
2,112.78 |
|
| Fees
to Be Paid |
|
Equity |
|
Pre-Funded
Warrants to Purchase Common Stock (4) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
(4 |
) |
| Fees
to Be Paid |
|
Equity |
|
Common
Stock Underlying Pre-Funded Warrants (4) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
(4 |
) |
| Fees
to Be Paid |
|
Equity |
|
Representative’s
Warrants to Purchase Common Stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
(5 |
) |
| Fees
to Be Paid |
|
Equity |
|
Common
Stock Underlying Representative’s Warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
862,500 |
(6)
|
|
$ |
0.00015310 |
|
|
$ |
132.05 |
|
| |
|
Total
Offering Amounts |
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
2,244.83 |
|
| |
|
Total
Fees Previously Paid |
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
- |
|
| |
|
Total
Fee Offsets |
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
- |
|
| |
|
Net
Fee Due |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
2,244.83 |
|
| (1) |
This
registration statement also includes an indeterminate number of securities that may become offered, issuable or sold to prevent dilution
resulting from stock splits, stock dividends and similar transactions, which are included pursuant to Rule 416 under the Securities
Act of 1933, as amended (the “Securities Act”). |
| |
|
| (2) |
Estimated
solely for the purpose of computing the registration fee in accordance with Rule 457(o) under the Securities Act. |
| |
|
| (3) |
Includes
the offering price of additional shares that the underwriters have the option to purchase to cover over-allotments, if any. |
| |
|
| (4) |
The
proposed maximum aggregate offering price of the common stock will be reduced on a dollar-for-dollar basis based on the offering
price of any pre-funded warrants issued in the offering, and the proposed maximum aggregate offering price of the pre-funded warrants
to be issued in the offering will be reduced on a dollar-for-dollar basis based on the offering price of any common stock issued
in the offering. Accordingly, the proposed maximum aggregate offering price of the common stock and pre-funded warrants (including
the common stock issuable upon exercise of the pre-funded warrants), if any, is $13,800,000. |
| |
|
| (5) |
No
separate registration fee is payable pursuant to Rule 457(g) under the Securities Act. |
| |
|
| (6) |
Estimated
solely for the purpose of calculating the registration fee pursuant to Rule 457(g) under the Securities Act. The warrants have an
exercise price equal to 125% of the public offering price. As estimated solely for the purpose of calculating the registration fee
pursuant to Rule 457(g) under the Securities Act, the proposed maximum aggregate offering price of the shares underlying the Representative’s
Warrants is equal to $862,500 (which is equal to 5% of the proposed maximum aggregate offering price for the common stock of $13,800,000
multiplied by 125%). |
Aspira Womans Health (NASDAQ:AWH)
Historical Stock Chart
From Dec 2024 to Jan 2025
Aspira Womans Health (NASDAQ:AWH)
Historical Stock Chart
From Jan 2024 to Jan 2025