false
0000727207
0000727207
2023-12-11
2023-12-11
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE
COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT
REPORT
Pursuant to Section 13
or 15(d) of the Securities Exchange Act of 1934
| Date of Report (Date of earliest event reported) |
December 11, 2023 |
Accelerate
Diagnostics, Inc.
(Exact name of registrant
as specified in its charter)
Delaware
(State
or other jurisdiction of incorporation)
| 001-31822 |
|
84-1072256 |
| (Commission File Number) |
|
(IRS Employer Identification No.) |
| 3950
South Country Club Road, Suite 470,
Tucson, Arizona |
|
85714 |
| (Address of principal executive offices) |
|
(Zip Code) |
(520)
365-3100
(Registrant’s
telephone number, including area code)
Not Applicable
(Former
name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b)
of the Act:
| Title
of each class |
Trading
Symbol |
Name
of each exchange on which
registered |
| Common
Stock, $0.001 par value per share |
AXDX |
The
Nasdaq Stock Market LLC
(The Nasdaq Capital Market) |
Indicate by check mark whether the registrant is
an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the
Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 1.01 Entry into a Material Definitive Agreement.
On
December 12, 2023, Accelerate Diagnostics, Inc. (the “Company”) entered into the First Amendment to
Securities Purchase Agreement (the “SPA Amendment”), with the Jack W. Schuler Living Trust (the “Investor”).
The SPA Amendment amends the Securities Purchase Agreement between the Company and the Investor, dated as of June 9, 2023 (the “SPA”). The SPA
Amendment, among other things, extends the date by which the Investor is required to backstop an underwritten offering by the
Company from December 15, 2023 to February 15, 2024. In addition, the SPA Amendment provides that if the Company elects to
conduct a public offering of common stock and other investors purchase at least $10.0 million of shares of common stock by
February 15, 2024, the Investor has agreed to purchase $2.0 million shares of common stock at the public offering price in the
backstopped offering. The previous backstop commitment of $10.0 million has not changed.
Additionally, on December 11,
2023, the Company entered into the First Amendment to the Note Purchase Agreement (the “NPA Amendment”), with the investors
party thereto constituting Majority Investors (as defined in the NPA Amendment). The NPA Amendment amends the Note Purchase Agreement,
dated as of June 9, 2023. Pursuant to the NPA Amendment, the date by which the Company is to consummate the transactions contemplated
by the SPA was extended from December 15, 2023 to February 15, 2024.
Furthermore, on December 11,
2023, the Company entered into the First Amendment to the Note Exchange Agreement (the “NEA Amendment”), with the investors
party thereto constituting Majority Investors (as defined in the NEA Amendment). The NEA Amendment amends the Note Exchange Agreement,
dated as of June 9, 2023. Pursuant to the NEA Amendment, the date by which the Company is to consummate the transactions contemplated
by the SPA was extended from December 15, 2023 to February 15, 2024.
A copy of each of the SPA
Amendment, the NPA Amendment and the NEA Amendment is filed with this Current Report on Form 8-K as Exhibits 10.1, 10.2 and 10.3,
respectively, and is incorporated herein by reference, and the foregoing descriptions of the SPA Amendment, the NPA Amendment and the
NEA Amendment are qualified in their entirety by reference thereto.
Item 7.01. Regulation FD Disclosure.
The Company is providing an
updated corporate presentation to include information regarding the Company’s Accelerate Wave™ system, a copy of which is
attached to this Current Report on Form 8-K as Exhibit 99.1.
This information contained
in this Item 7.01 of this Current Report on Form 8-K and the presentation attached hereto as Exhibit 99.1 are being furnished
to the Securities and Exchange Commission and shall not be deemed to be “filed” for purposes of Section 18 of the Securities
Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and such information
shall not be deemed to be incorporated by reference into any of the Company’s filings under the Securities Act or the Exchange Act,
except as expressly set forth by specific reference in such filing.
Item 8.01. Other Events.
The Company is providing the following update on
the Accelerate Wave™ system:
While we will continue to
seek ways to improve the utility of our existing products, the primary focus of our current research and development efforts is our next
generation antibiotic susceptibility testing (“AST”) platform, Accelerate Wave, which is being developed with the goal to
have lower cost, higher throughput, and the capability to test a broader set of sample types when compared to our Accelerate Pheno®
system.
The Accelerate Wave
system, currently in development, performs AST directly from positive blood culture (“PBC”) bottles and bacterial
isolate colonies (“Isolates”) to report minimum inhibitory concentrations (“MICs”) with a goal of delivering
results within 4.5 hours. The fully automated system is based on the principle of digital holographic microscopy, which allows for
simultaneous volumetric imaging of a sample suspension at a high spatial resolution, enabling direct observation of phenotypic
responses of individual bacterial cells in relatively short time periods versus more traditional AST methods. The Accelerate Wave
system uses a time series of holograms to provide microbial quantitation under antimicrobial stress, enabling rapid determination of
minimum inhibitory concentration. Additionally, Accelerate Wave holograms provide morphological information at the level of
individual cells. For some bug-drug combinations morphology is a leading indicator of future MIC.
A key differentiator of the
Accelerate Wave system to current on-market and emerging AST competitors is the system’s ability to process PBC as well as Isolates
AST diagnostics results. Our initial launch of menu items will include a PBC assay that can be run on the Accelerate Wave system. This
will be followed by development of an Isolate assay. Our ability to process Isolates with the Wave system expands our addressable market
today with our Accelerate Pheno system and Accelerate ArcTM Products with PBC samples to include a much larger sample volumes
segment of the market, Isolates within the microbiology testing market. Based on our on-market experience with our Accelerate Pheno
system, we see significant workflow benefits to microbiology labs by offering consolidated PBC and Isolate susceptibility testing on
to a single, rapid, AST diagnostic. Further, recent voice-of-customer interviews highlight the value that Accelerate Wave brings to the
global marketplace with a high percentage of interviewed customers expressing interest in evaluating Wave once available. The Accelerate
Wave system AST modules will be able to test five-to-ten assays per module and can scale to have 5 modules per system which will address
the vast majority of microbiology lab volumes and workflows. Additionally, the cost to produce Accelerate Wave assays will be significantly
lower than our standard costing for the Accelerate PhenoTestTM BC Kit which could improve the Company’s margin profile
as we seek FDA regulatory clearance for the Accelerate Wave system and assays.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
Exhibit
Number |
|
Description |
| |
|
|
| 10.1 |
|
First Amendment to Securities Purchase Agreement, dated December 12, 2023, between the Company and the Jack W. Schuler Living Trust. |
| 10.2 |
|
First Amendment to Note Purchase Agreement, dated December 11, 2023, between the Company and certain investors named therein. |
| 10.3 |
|
First Amendment to Note Exchange Agreement, dated December 11, 2023, between the Company and certain investors named therein. |
| 99.1 |
|
Accelerate Diagnostics, Inc., Investor Presentation, dated December 2023. |
| 104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto
duly authorized.
| |
ACCELERATE DIAGNOSTICS, INC. |
| |
(Registrant) |
| Date: December 13, 2023 |
|
| |
/s/ David Patience |
| |
David Patience |
| |
Chief Financial Officer |
Exhibit 10.1
FIRST AMENDMENT TO
SECURITIES PURCHASE AGREEMENT
Accelerate Diagnostics, Inc. and Jack W.
Schuler Living Trust
This amendment (this “Amendment”)
to the Securities Purchase Agreement by and among Accelerate Diagnostics, Inc., a Delaware corporation (the “Company”),
and the Jack W. Schuler Living Trust (including its successors and assigns, the “Investor”) dated as of June 9,
2023 (the “Agreement”), is dated as of December 12, 2023.
WHEREAS, pursuant to Section 6.4
of the Agreement, any provision of the Agreement may be waived or amended if, and only if, such waiver or amendment is in writing and
signed by the Company and the Investor;
WHEREAS the Investor and the
Company wish to extend the Closing Date of the Agreement.
NOW, THEREFORE, IN CONSIDERATION
of the mutual covenants contained in this Agreement, and for other good and valuable consideration the receipt and adequacy of which are
hereby acknowledged, the Company and the Investor agree as follows:
1. Closing
Date. The definition of Closing Date set forth in Section 1.1 of the Agreement is
hereby amended and restated in its entirety as follows:
“Closing Date” means
a date on or prior to February 15, 2024, that is (i) in the case of the purchase of the Securities pursuant to the Fixed Commitment,
the closing date specified by the Company in its request to the Investor pursuant to Section 2.1(a) (which request shall
be made no less than two nor more than 10 Business Days prior to such specified closing date, but no later than 5:00 p.m. Central
Standard Time on February 12, 2024), or (ii) in the case of the purchase of the Securities pursuant to the Backstopped Commitment,
the closing date of the Backstopped Offering.
2. Backstopped
Offering. Sections 2.1(b) and (c) of the Agreement are hereby amended and restated
in its entirety as follows:
(b) Provided
that the Company has not requested the Investor to purchase shares of Common Stock pursuant to the Fixed Commitment set forth in Section 2.1(a),
the Investor shall backstop the underwritten public offering (the “Backstopped Offering”) by the Company of Common
Stock for gross proceeds of a maximum of $10 million in accordance with the following provisions (the “Backstop Commitment”):
(i) If
the Company shall obtain purchase commitments in such Backstopped Offering and the aggregate amount of such commitments total to at
least $10 million by February 15, 2024, then, regardless of the aggregate amount of purchase commitments in such Backstopped
Offering, the Investor shall, pursuant to the Backstop Commitment, be required to purchase, at a price per share equal to the public offering
price for the Backstopped Offering, a number of shares of Common Stock of the Company equal to the quotient obtained by dividing
(x) $2 million, by (y) the public offering price for the Backstopped Offering.
(ii) If
Company shall obtain purchase commitments in such Backstopped Offering and the aggregate of such commitments total to less than $10
million by February 15, 2024, then the Investor shall, pursuant to the Backstop Commitment, be required to purchase, at a price
per share equal to the public offering price for the Backstopped Offering, a number of shares of Common Stock of the Company equal to
the quotient obtained by dividing (x) the difference between $10 million and the aggregate dollar amount of the purchase commitments
that the Company shall have received from investors pursuant to the Backstopped Offering, by (y) the public offering price
for the Backstopped Offering.
(iii) If
the Company shall fail to obtain any purchase commitments pursuant to the Backstopped Offering by February 15, 2024, then the Investor
shall, pursuant to the Backstop Commitment, purchase such shares of Common Stock equal to the quotient obtained by dividing (x) $10
million, by (y) the public offering price for the Backstopped Offering.
The aggregate purchase price paid by
the Investor for shares of Common Stock pursuant to the Backstop Commitment is herein referred to as the “Backstopped Amount.”
(c) In
the event that the Company shall engage in a Backstopped Offering, the Investor shall have the right, but not the obligation, to purchase,
on the Closing Date, at a price per share equal to the public offering price, a number of shares of Common Stock equal to the quotient
obtained by dividing (x) the difference between $10 million and the Backstopped Amount, by (y) the public offering
price for the Backstopped Offering; provided that if the Backstopped Amount is $10 million, then the Investor shall not have any right
to purchase additional shares pursuant to this Section 2.1(c).
3. Termination.
Section 6.5 of the Agreement is hereby amended and restated in its entirety as follows:
Termination. This Agreement may
be terminated prior to the Closing Date by written agreement of the Investor and the Company. In addition, this Agreement shall terminate
effective as of February 13, 2024, if by 5:00 p.m. Central Standard Time on February 12, 2024 the Company shall not have
requested the Investor to purchase Securities or to fulfill its Backstop Commitment in accordance with the provisions of Section 2.1
hereof. Upon a termination in accordance with this Section 6.5, the Company and the Investor shall not have any further obligation
or liability (including as arising from such termination) to the other.
4. Capitalized
terms not defined herein shall have the meeting set forth in the Agreement. Except as expressly amended by this Amendment, the Agreement
shall remain in full force and effect and be enforceable against the parties in accordance with its terms. This Amendment may be executed
in any number of counterparts, each of which shall be an original but all of which together shall constitute one and the same instrument,
binding on all of the parties notwithstanding that all such parties have not signed the same counterpart. Delivery of an executed counterpart
of a signature page to this Agreement by electronic mail transmission of a “.pdf” or other similar data file shall be
effective as delivery of a manually executed counterpart to this Amendment. For clarity, this Amendment shall be governed by all provisions
of the Agreement (other than as the Agreement is specifically amended herein), unless the context otherwise requires, including (but not
limited to) all provisions concerning construction, notices, governing law, jurisdiction, waiver of jury trial and confidentiality, mutatis
mutandis.
(Signature Page Follows)
IN WITNESS WHEREOF, the parties hereto have caused
this First Amendment to be duly executed by their respective authorized signatories as of December 11, 2023.
| |
|
| |
COMPANY: |
| |
|
| |
ACCELERATE DIAGNOSTICS, INC., |
| |
a Delaware corporation |
| |
|
| |
/s/
Jack Phillips |
| |
Name: |
Jack Phillips |
| |
Title: |
President & CEO |
| |
|
| |
INVESTOR: |
| |
|
| |
Jack W. Schuler Living Trust |
| |
|
| |
/s/
Jack W. Schuler |
| |
Name: Jack W. Schuler |
Exhibit 10.2
FIRST AMENDMENT TO
NOTE
Purchase AGREEMENT
This First Amendment to Note
Purchase Agreement (this “Amendment”), dated as of December 11, 2023, is entered into by and between Accelerate
Diagnostics, Inc., a Delaware corporation (the “Company”), and the undersigned investors constituting Majority
Investors.
WHEREAS, reference is made
to that certain Note Purchase Agreement, dated as of June 9, 2023 (as may have been amended, supplemented or otherwise modified and
as may be amended, supplemented or otherwise modified from time to time, the “Note Purchase Agreement”), by and between
the Company and the other parties thereto;
WHEREAS, all capitalized terms
used by not otherwise defined herein shall have the respective meanings ascribed to such terms in the Note Purchase Agreement;
WHEREAS, pursuant to Section 7.8
of the Note Purchase Agreement, any provision of the Note Purchase Agreement may be waived or amended if, and only if, such waiver or
amendment is in writing and signed by the Company and the Majority Investors; and
WHEREAS, the parties desire
to amend the Note Purchase Agreement as set forth herein.
NOW, THEREFORE, in consideration
of the foregoing premises and for other good and valuable consideration, the sufficiency of which is hereby acknowledged, the parties
hereto hereby agree to the following:
1. Defined
Terms. As used in this Amendment, the following terms shall have the respective meanings set forth below.
| (a) | “New Securities Purchase Agreement Amendment” shall mean an amendment to the New Securities
Purchase Agreement (as defined in the RSA), which will (i) change the “Closing Date” under the New Securities Purchase
Agreement to February 15, 2024 and (ii) amend the terms of the Backstopped Offering (as defined in the New Securities Purchase
Agreement) in a manner that is acceptable to the Majority Investors. |
2. New
Securities Purchase Agreement. Section 5.13 of the Note Purchase Agreement is hereby amended and restated as follows:
“New Securities Purchase Agreement.
The Company shall consummate the transactions envisioned by the New Securities Purchase Agreement (as defined in the RSA) with the Jack
W. Schuler Living Trust no later than February 15, 2024, unless such date is amended by the written consent of the Majority Investors.”
3. Conditions
Precedent to Effectiveness. This Amendment shall be effective on the date (the “Amendment Effective Date”) on which
the following conditions shall have been satisfied or waived by the Majority Investors:
| (a) | This Amendment is duly executed by the Company and the Majority Investors. |
| (b) | The Company shall have entered into the New Securities Purchase Agreement Amendment with the Jack W. Schuler
Living Trust in a form and substance acceptable to the Majority Investors. |
4. Effect
of Amendment. Except as expressly amended by this Amendment, the Note Purchase Agreement shall remain in full force and effect and
be enforceable against the Parties in accordance with its terms. This Amendment may be executed in any number of counterparts, each of
which shall be an original but all of which together shall constitute one and the same instrument, binding on all of the Parties notwithstanding
that all such Parties have not signed the same counterpart. Delivery of an executed counterpart of a signature page to this Agreement
by electronic mail transmission of a “.pdf” or other similar data file shall be effective as delivery of a manually executed
counterpart to this Amendment. For clarity, this Amendment shall be governed by all provisions of the Purchase Agreement (other than as
the Note Purchase Agreement is specifically amended herein), unless the context otherwise requires, including (but not limited to) all
provisions concerning construction, notices, governing law, jurisdiction, waiver of jury trial and confidentiality, mutatis mutandis.
(Remainder of page intentionally left blank)
IN WITNESS WHEREOF, each of
undersigned has caused this Amendment to be duly executed as of the date written above.
| |
THE COMPANY: |
| |
|
| |
Accelerate Diagnostics, Inc. |
| |
|
| |
By: |
/s/ Jack Phillips |
| |
|
Jack Phillips |
| |
|
President and Chief Executive Officer |
| |
|
| |
MAJORITY INVESTORS: |
| |
|
| |
Streeterville Capital, LLC |
| |
|
| |
By: |
/s/ John Fife |
| |
|
John Fife |
| |
|
President, Manager, and Secretary |
| |
|
| |
Indaba
Capital Management, L.P. |
| |
|
| |
By: |
/s/ Derek Schrier |
| |
|
Derek Schrier |
| |
|
CIO and Partner |
Signature Page to First Amendment to Note
Purchase Agreement
Exhibit 10.3
FIRST AMENDMENT TO
Note
Exchange AGREEMENT
This First Amendment to Note
Exchange Agreement (this “Amendment”), dated as of December 11, 2023, is entered into by and between Accelerate
Diagnostics, Inc., a Delaware corporation (the “Company”), and the undersigned investors constituting Majority
Investors.
WHEREAS, reference is made
to that certain Note Exchange Agreement, dated as of June 9, 2023 (as may have been amended, supplemented or otherwise modified and
as may be amended, supplemented or otherwise modified from time to time, the “Note Exchange Agreement”), by and between
the Company and the other parties thereto;
WHEREAS, all capitalized terms
used by not otherwise defined herein shall have the respective meanings ascribed to such terms in the Note Exchange Agreement;
WHEREAS, pursuant to Section 7.8
of the Note Exchange Agreement, any provision of the Note Exchange Agreement may be waived or amended if, and only if, such waiver or
amendment is in writing and signed by the Company and the Majority Investors; and
WHEREAS, the parties desire
to amend the Note Exchange Agreement as set forth herein.
NOW, THEREFORE, in consideration
of the foregoing premises and for other good and valuable consideration, the sufficiency of which is hereby acknowledged, the parties
hereto hereby agree to the following:
1. Defined
Terms. As used in this Amendment, the following terms shall have the respective meanings set forth below.
| (a) | “New Securities Purchase Agreement Amendment” shall mean an amendment to the New Securities
Purchase Agreement (as defined in the RSA), which will (i) change the “Closing Date” under the New Securities Purchase
Agreement to February 15, 2024 and (ii) amend the terms of the Backstopped Offering (as defined in the New Securities Purchase
Agreement) in a manner that is acceptable to the Majority Investors. |
2. New
Securities Purchase Agreement. Section 5.13 of the Note Exchange Agreement is hereby amended and restated as follows:
“New Securities Purchase Agreement.
The Company shall consummate the transactions envisioned by the New Securities Purchase Agreement (as defined in the RSA) with the Jack
W. Schuler Living Trust no later than February 15, 2024, unless such date is amended by the written consent of the Majority Investors.”
3. Conditions
Precedent to Effectiveness. This Amendment shall be effective on the date (the “Amendment Effective Date”) on which
the following conditions shall have been satisfied or waived by the Majority Investors:
| (a) | This Amendment is duly executed by the Company and the Majority Investors. |
| (b) | The Company shall have entered into the New Securities Purchase Agreement Amendment with the Jack W. Schuler
Living Trust in a form and substance acceptable to the Majority Investors. |
4. Effect
of Amendment. Except as expressly amended by this Amendment, the Note Exchange Agreement shall remain in full force and effect and
be enforceable against the Parties in accordance with its terms. This Amendment may be executed in any number of counterparts, each of
which shall be an original but all of which together shall constitute one and the same instrument, binding on all of the Parties notwithstanding
that all such Parties have not signed the same counterpart. Delivery of an executed counterpart of a signature page to this Agreement
by electronic mail transmission of a “.pdf” or other similar data file shall be effective as delivery of a manually executed
counterpart to this Amendment. For clarity, this Amendment shall be governed by all provisions of the Note Exchange Agreement (other than
as the Note Exchange Agreement is specifically amended herein), unless the context otherwise requires, including (but not limited to)
all provisions concerning construction, notices, governing law, jurisdiction, waiver of jury trial and confidentiality, mutatis mutandis.
(Remainder of page intentionally left blank)
IN WITNESS WHEREOF, each of
undersigned has caused this Amendment to be duly executed as of the date written above.
| |
THE COMPANY: |
| |
|
| |
Accelerate Diagnostics, Inc. |
| |
|
| |
By: |
/s/
Jack Phillips |
| |
|
Jack Phillips |
| |
|
President and Chief Executive Officer |
| |
|
| |
MAJORITY INVESTORS: |
| |
|
| |
Streeterville Capital, LLC |
| |
|
| |
By: |
/s/ John Fife |
| |
|
John Fife |
| |
|
President, Manager, and Secretary |
| |
|
| |
Indaba
Capital Management, L.P. |
| |
|
| |
By: |
/s/ Derek Schrier |
| |
|
Derek Schrier |
| |
|
CIO and Partner |
Signature Page to First Amendment to Note
Exchange Agreement
Exhibit 99.1
| December 2023
Company Presentation |

| 1
Legal Disclaimer
Forward-Looking Statements
This presentation contains, and the Company’s responses to various questions from investors may include “forward-looking statements” within the meaning of Section 27A of the Securities
Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the Company intends that such forward-looking
statements be subject to the safe harbors created thereby. These forward-looking statements, which can be identified by the use of words such as “may,” “will,” “expect,” “believe,”
“anticipate,” “estimate,” or “continue,” or variations thereon or comparable terminology, include but are not limited to, statements about the Company’s future development plans and
growth strategy, including plans and objectives relating to its future operations, products and performance; financial projections, including the Company’s projected long-term financial model
and forecast; projections as to when certain key business milestones may be achieved; expectations regarding the potential or benefits of the Company’s products and technologies;
projections of future demand for the Company’s products and the growth of the market for its products; the Company’s estimates as to the size of its market opportunity; the Company’s
competitive position and estimates of time reduction to results; the Company’s continued investment in new product development to both enhance its existing products and bring new ones
to market; the Company’s expectations relating to current supply chain impacts and inflationary pressures, including its belief that it currently has sufficient inventory of Accelerate Pheno®
system instruments to limit the impact of cost increases on such devices; the Company’s expectations regarding its commercial partnership with Becton, Dickinson and Company (“BD”),
including anticipated benefits from such collaboration; the Company’s expectations and plans relating to regulatory approvals and submissions, including with respect to the U.S. Food and
Drug Administration (“FDA”) and its Accelerate ArcTM product, WaveTM instrument and Positive Blood Culture (PBC) Gram Negative assay; the Company’s liquidity and capital requirements,
including, without limitation, as to its ability to continue as a going concern; the Company’s plans and expectations relating to the terms and consummation of the restructuring transactions
contemplated by its restructuring support agreement, including, but not limited to, the anticipated issuance of significant amounts of common stock and securities convertible into significant
amounts of common stock and the resulting impact to its capital structure; and the Company's ability to achieve expected future financial performance and results. In addition, all statements
other than statements of historical facts that address activities, events, or developments the Company expects, believes, or anticipates will or may occur in the future, and other such matters,
are forward-looking statements.
The following factors, among others, could cause actual results and financial position and timing of certain events to differ materially from those described in the forward-looking statements:
interest rate movements; local, regional, national and global economic performance and geopolitical factors such as the conflict in Ukraine and instability in Israel and the Middle East;
competitive factors; government policy changes; disruptions in the Company’s supply chain, shipping, logistics or manufacturing processes; the demand for Rapid ID/AST products; the
Company’s ability to drive conversion of the market to rapid/digital testing; quality and performance of the Company’s current and future products; the Company’s ability to complete
development, obtain regulatory approval, and successfully launch the commercialization of its future products; the Company’s ability to convert commercial momentum into sales and
implementations; the Company’s ability to realize the benefits contemplated by its commercial partnership with BD; risks related to the Company’s technology, intellectual property and
infrastructure; any material market changes and trends that could affect the Company's business strategy; and difficulties in resolving the Company’s continuing financial condition and ability
to obtain additional capital to meet its financial obligations, including, without limitation, difficulties in obtaining adequate capital resources to fund its operations and whether it will be
successful in consummating the restructuring transactions contemplated by its restructuring support agreement. For further discussion of factors that could materially affect the outcome of
the Company’s forward-looking statements and its future results and financial condition, see the section entitled “Risk Factors” in the Company’s various filings with the U.S. Securities and
Exchange Commission (the “SEC”), including its most recent Annual Report on Form 10-K. The Company cautions you not to place undue reliance on any forward-looking statements, which
are made as of the date of this investor presentation. The Company undertakes no obligation to update publicly any of these forward-looking statements to reflect actual results, new
information or future events, changes in assumptions or changes in other factors affecting forward-looking statements, except to the extent required by applicable laws. If the Company
updates one or more forward-looking statements, no inference should be drawn that it will make additional updates with respect to those or other forward-looking statements. |

| 2
Executive Summary
The Accelerate Pheno® is the first rapid Antimicrobial Susceptibility Testing (AST) platform to receive FDA
approval and has strong IP, thought-leading customers, and dozens of publications proving the high clinical
impact from rapid diagnostic results on patient outcomes
We believe the commercial partnership with Becton Dickinson (BD) has generated increased market
opportunities and shown Pheno® to be the leading Rapid AST platform in the market
Wave™ is designed to address a growing and attractive $2B total estimated market opportunity in AST and we
expect to begin clinical trials in early Q2 2024, followed by platform and GN FDA submission in Q3 2024
Wave™ + Arc™ is designed to bring a novel opportunity for cost effective rapid PBC Identification (ID) + AST,
and the Company has developed a plan of attack for transitioning its existing customer base and increasing
revenues by consolidating PBC and Isolate testing onto the same platform
Wave™ is designed to bring improved consumable platform economics with gross margins of ~90% and ~60%
for PBC and Isolates, respectively, which could result in a material contribution to profitability |
| 3
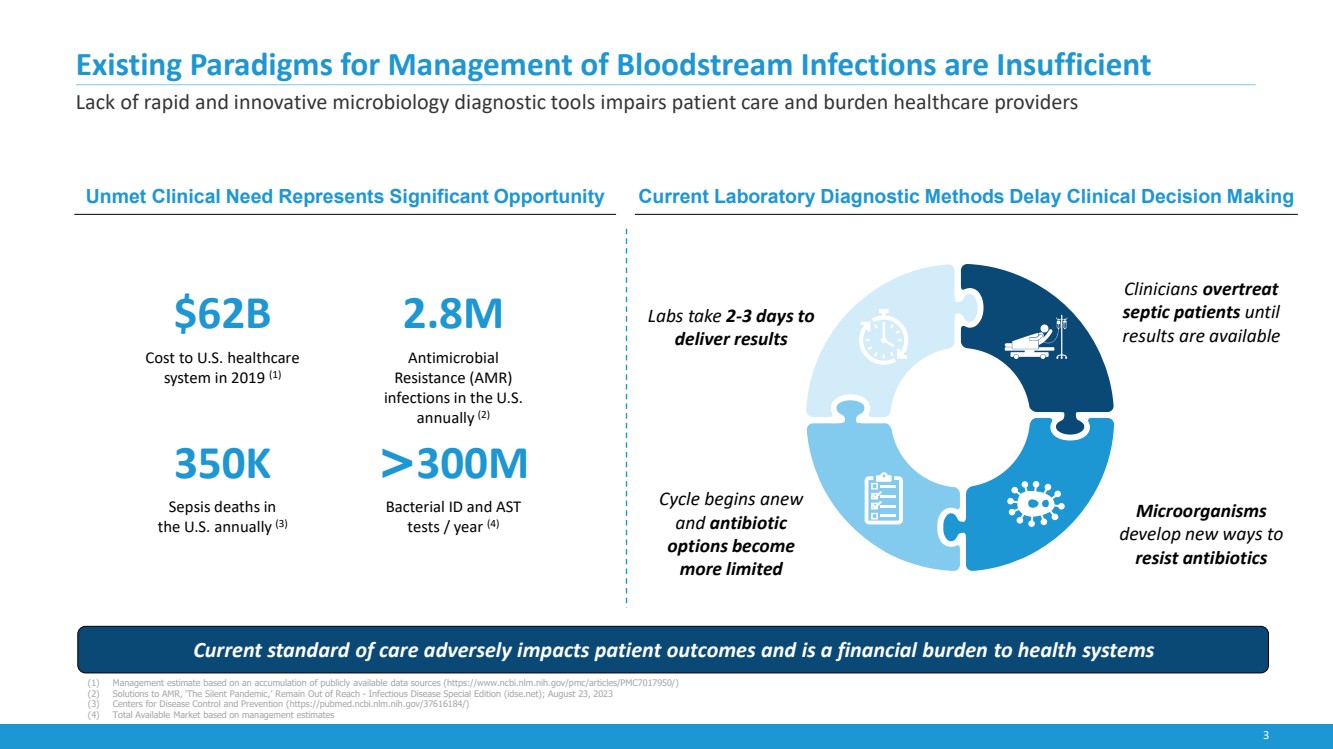
Existing Paradigms for Management of Bloodstream Infections are Insufficient
Lack of rapid and innovative microbiology diagnostic tools impairs patient care and burden healthcare providers
Cycle begins anew
and antibiotic
options become
more limited
Clinicians overtreat
septic patients until
results are available
Labs take 2-3 days to
deliver results
Microorganisms
develop new ways to
resist antibiotics
Current standard of care adversely impacts patient outcomes and is a financial burden to health systems
$62B
Cost to U.S. healthcare
system in 2019 (1)
2.8M
Antimicrobial
Resistance (AMR)
infections in the U.S.
annually (2)
350K
Sepsis deaths in
the U.S. annually (3)
>300M
Bacterial ID and AST
tests / year (4)
Unmet Clinical Need Represents Significant Opportunity Current Laboratory Diagnostic Methods Delay Clinical Decision Making
(1) Management estimate based on an accumulation of publicly available data sources (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7017950/)
(2) Solutions to AMR, ‘The Silent Pandemic,’ Remain Out of Reach - Infectious Disease Special Edition (idse.net); August 23, 2023
(3) Centers for Disease Control and Prevention (https://pubmed.ncbi.nlm.nih.gov/37616184/)
(4) Total Available Market based on management estimates |
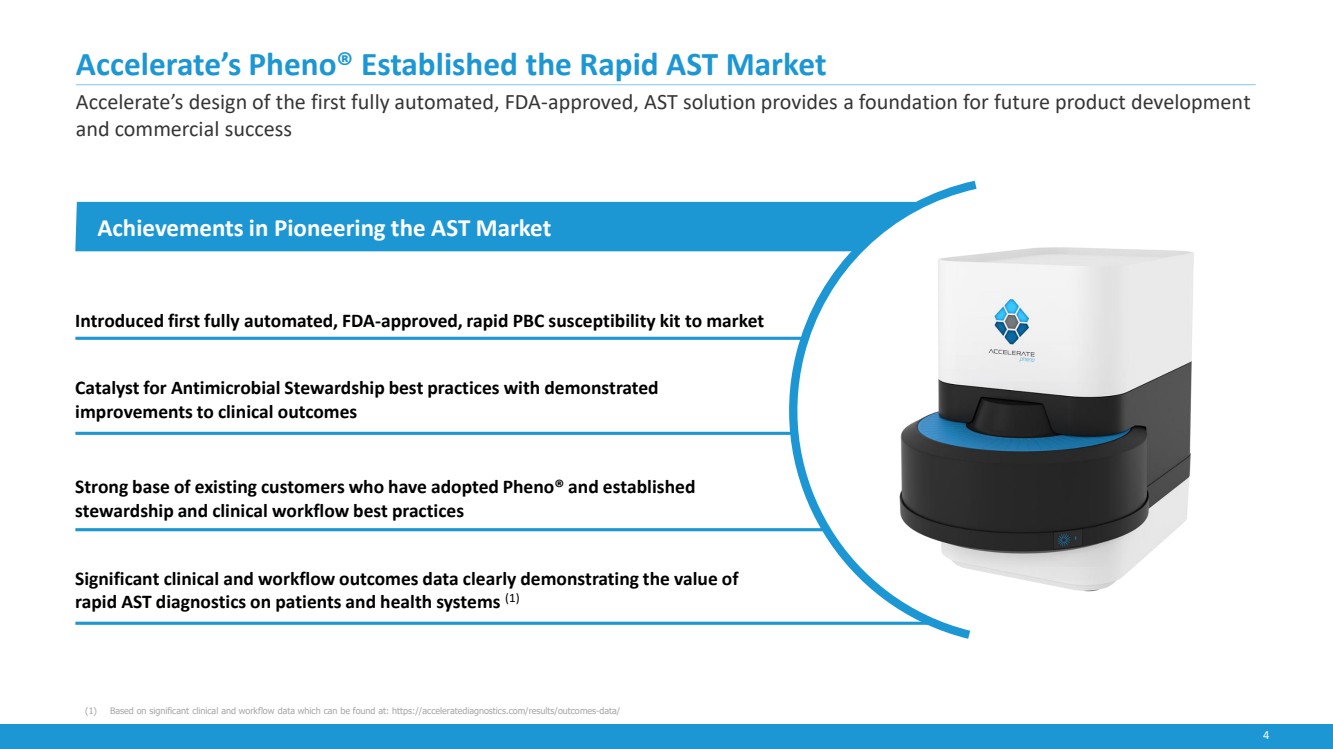
| 4
Accelerate’s Pheno® Established the Rapid AST Market
Accelerate’s design of the first fully automated, FDA-approved, AST solution provides a foundation for future product development
and commercial success
Achievements in Pioneering the AST Market
Introduced first fully automated, FDA-approved, rapid PBC susceptibility kit to market
Catalyst for Antimicrobial Stewardship best practices with demonstrated
improvements to clinical outcomes
Strong base of existing customers who have adopted Pheno® and established
stewardship and clinical workflow best practices
Significant clinical and workflow outcomes data clearly demonstrating the value of
rapid AST diagnostics on patients and health systems (1)
(1) Based on significant clinical and workflow data which can be found at: https://acceleratediagnostics.com/results/outcomes-data/ |
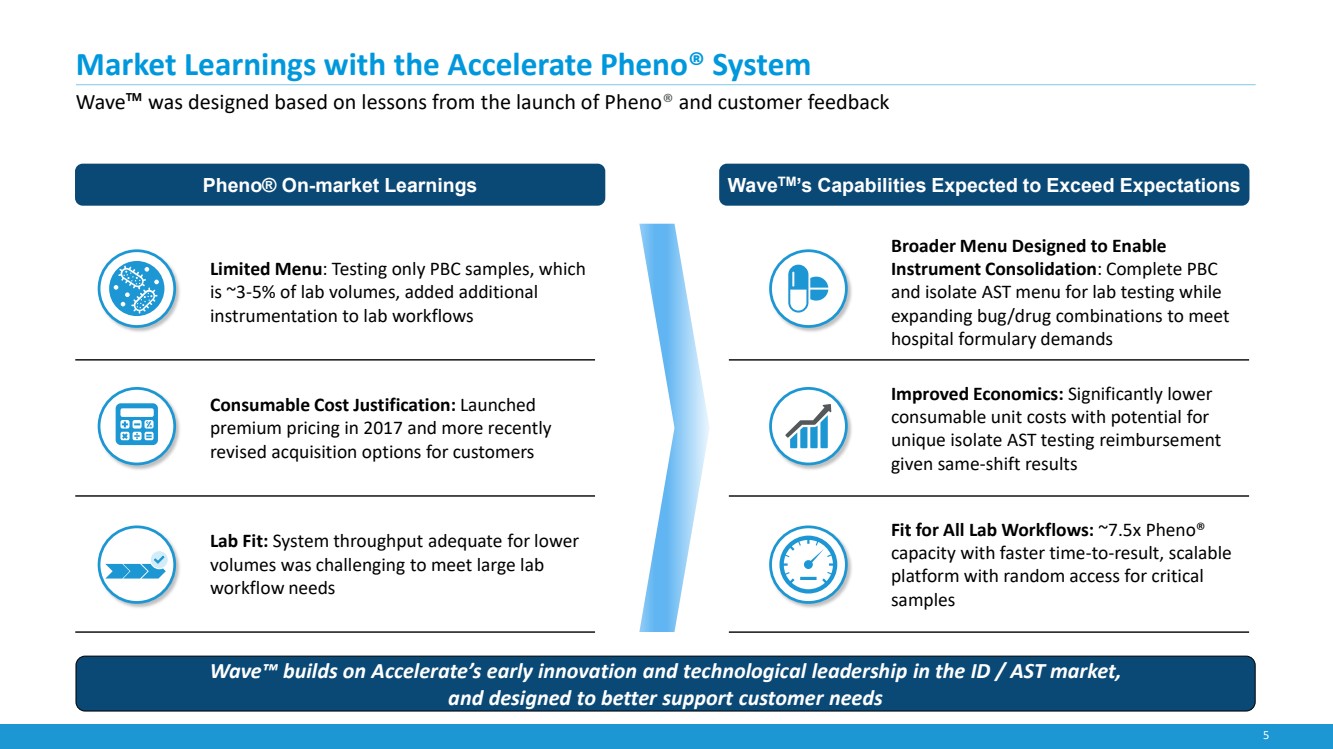
| 5
Market Learnings with the Accelerate Pheno® System
Wave™ was designed based on lessons from the launch of Pheno® and customer feedback
Limited Menu: Testing only PBC samples, which
is ~3-5% of lab volumes, added additional
instrumentation to lab workflows
Consumable Cost Justification: Launched
premium pricing in 2017 and more recently
revised acquisition options for customers
Lab Fit: System throughput adequate for lower
volumes was challenging to meet large lab
workflow needs
Broader Menu Designed to Enable
Instrument Consolidation: Complete PBC
and isolate AST menu for lab testing while
expanding bug/drug combinations to meet
hospital formulary demands
Improved Economics: Significantly lower
consumable unit costs with potential for
unique isolate AST testing reimbursement
given same-shift results
Fit for All Lab Workflows: ~7.5x Pheno®
capacity with faster time-to-result, scalable
platform with random access for critical
samples
Pheno® On-market Learnings
Wave™ builds on Accelerate’s early innovation and technological leadership in the ID / AST market,
and designed to better support customer needs
WaveTM’s Capabilities Expected to Exceed Expectations |
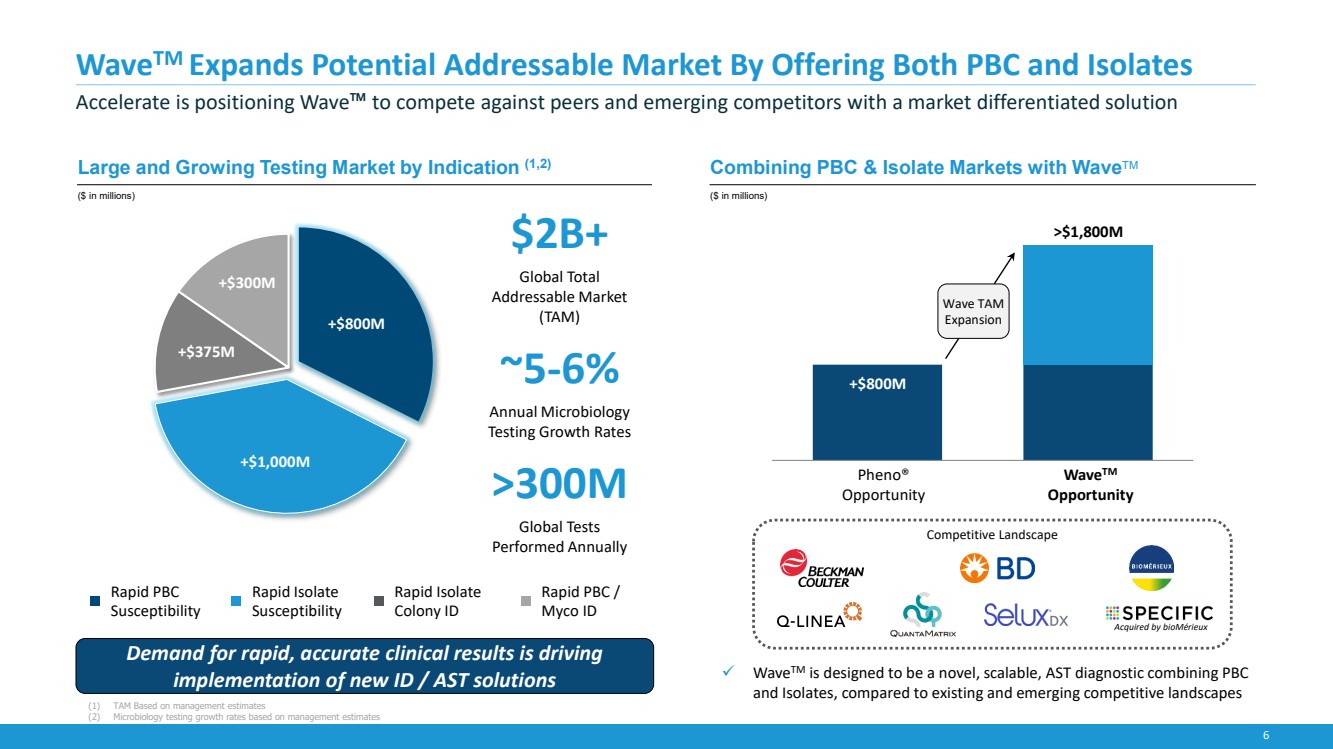
| 6
WaveTM Expands Potential Addressable Market By Offering Both PBC and Isolates
Accelerate is positioning Wave™ to compete against peers and emerging competitors with a market differentiated solution
+$800M
+$1,000M
+$375M
+$300M
Rapid PBC
Susceptibility
Rapid Isolate
Colony ID
Rapid Isolate
Susceptibility
Rapid PBC /
Myco ID
✓ WaveTM is designed to be a novel, scalable, AST diagnostic combining PBC
and Isolates, compared to existing and emerging competitive landscapes
Competitive Landscape
Large and Growing Testing Market by Indication (1,2)
($ in millions)
Combining PBC & Isolate Markets with WaveTM
($ in millions)
$2B+
Global Total
Addressable Market
(TAM)
~5-6%
Annual Microbiology
Testing Growth Rates
>300M
Global Tests
Performed Annually
Pheno®
Opportunity
WaveTM
Opportunity
+$800M
>$1,800M
Acquired by bioMérieux
Demand for rapid, accurate clinical results is driving
implementation of new ID / AST solutions
Wave TAM
Expansion
(1) TAM Based on management estimates
(2) Microbiology testing growth rates based on management estimates |
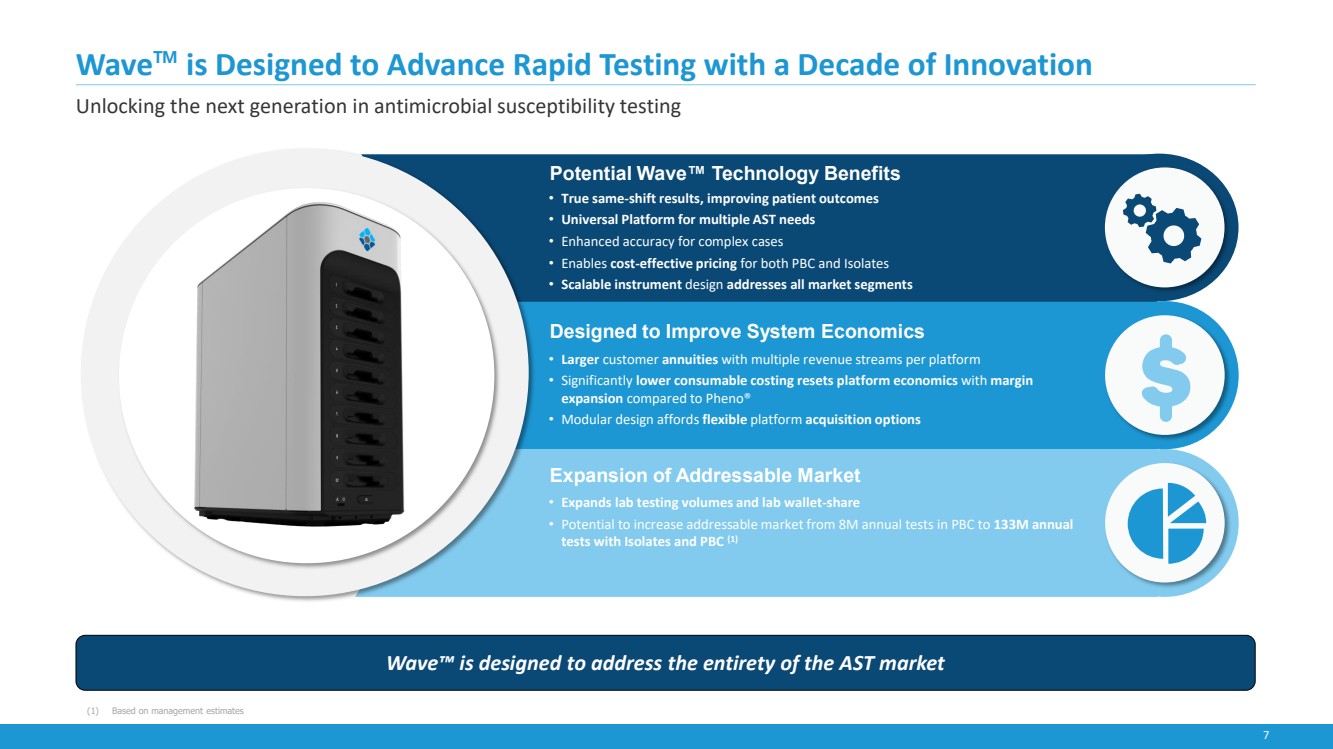
| 7
Wave™ is Designed to Advance Rapid Testing with a Decade of Innovation
Unlocking the next generation in antimicrobial susceptibility testing
Wave™ is designed to address the entirety of the AST market
• True same-shift results, improving patient outcomes
• Universal Platform for multiple AST needs
• Enhanced accuracy for complex cases
• Enables cost-effective pricing for both PBC and Isolates
• Scalable instrument design addresses all market segments
Potential Wave™ Technology Benefits
(1) Based on management estimates
Expansion of Addressable Market
• Expands lab testing volumes and lab wallet-share
• Potential to increase addressable market from 8M annual tests in PBC to 133M annual
tests with Isolates and PBC (1)
Designed to Improve System Economics
• Larger customer annuities with multiple revenue streams per platform
• Significantly lower consumable costing resets platform economics with margin
expansion compared to Pheno®
• Modular design affords flexible platform acquisition options |
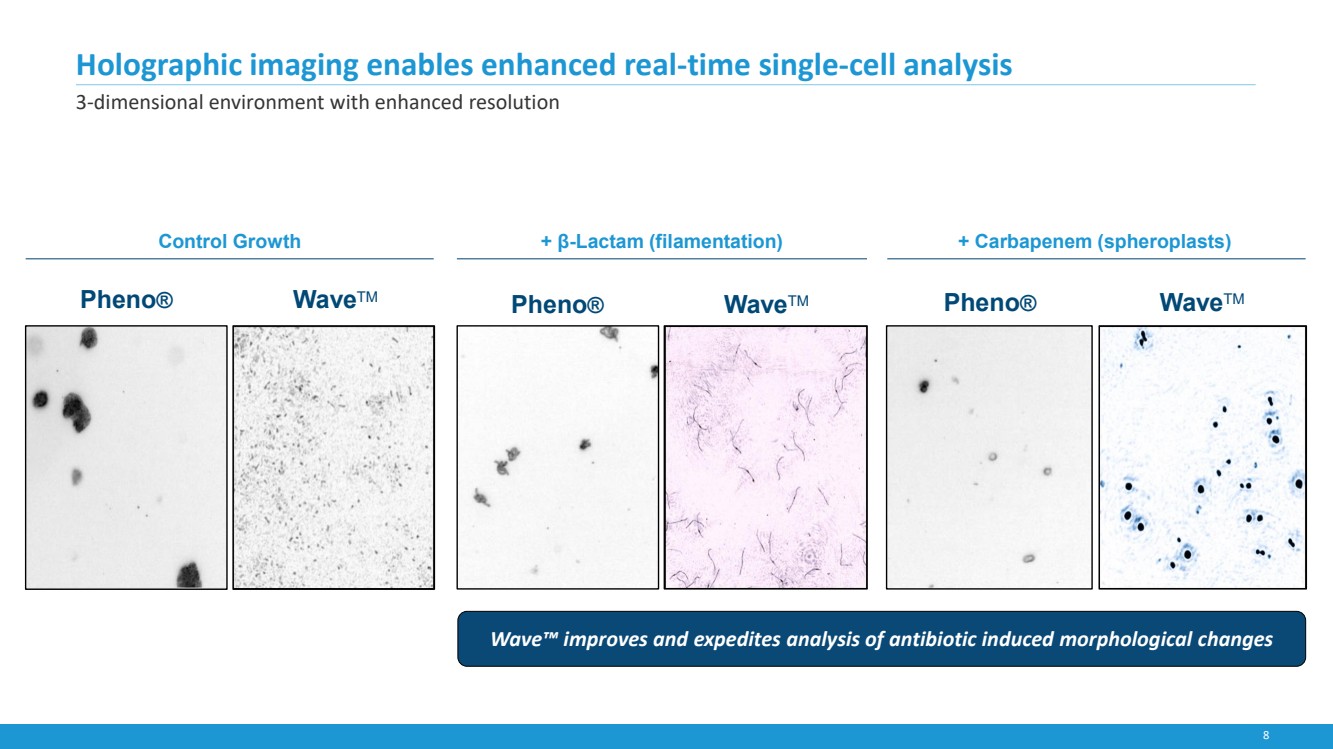
| 8
+ β-Lactam (filamentation) + Carbapenem (spheroplasts)
Holographic imaging enables enhanced real-time single-cell analysis
3-dimensional environment with enhanced resolution
Pheno® WaveTM Pheno® WaveTM Pheno® WaveTM
Wave™ improves and expedites analysis of antibiotic induced morphological changes
Control Growth |
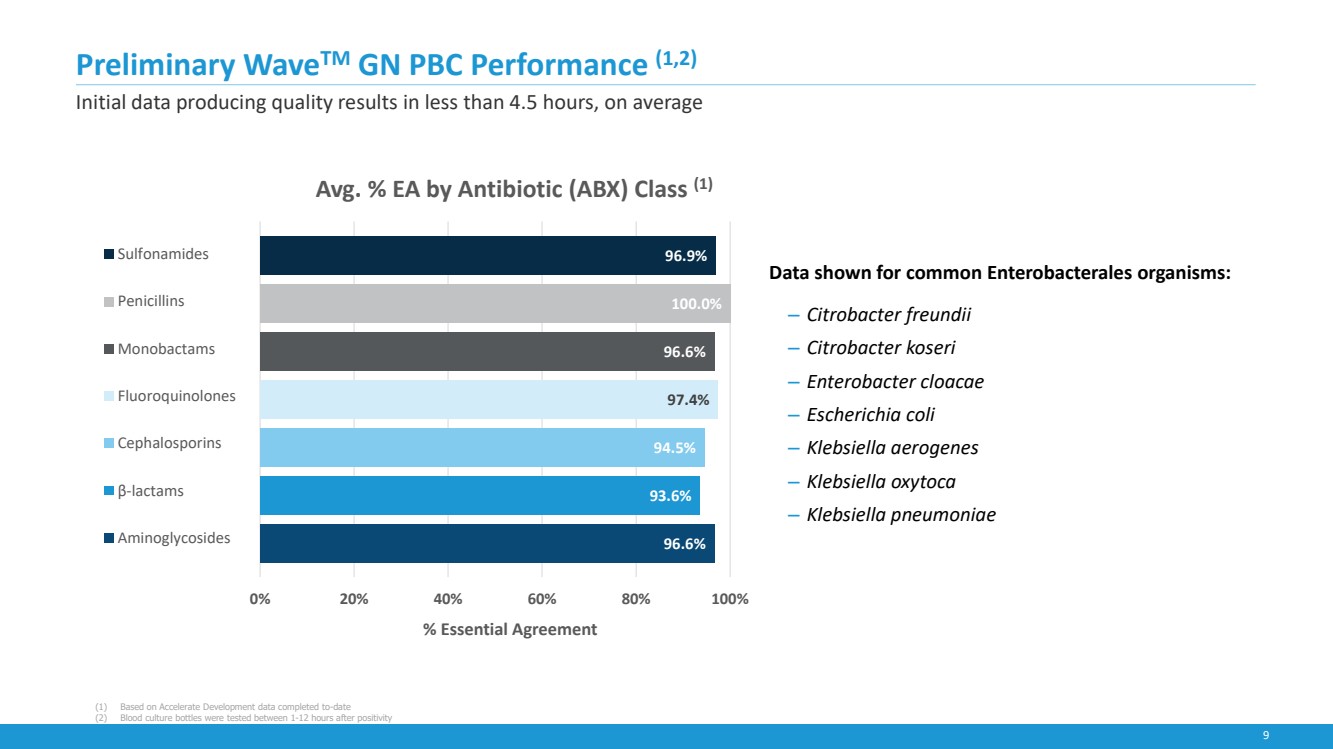
| 9
Preliminary WaveTM GN PBC Performance (1,2)
Initial data producing quality results in less than 4.5 hours, on average
Data shown for common Enterobacterales organisms:
‒ Citrobacter freundii
‒ Citrobacter koseri
‒ Enterobacter cloacae
‒ Escherichia coli
‒ Klebsiella aerogenes
‒ Klebsiella oxytoca
‒ Klebsiella pneumoniae
96.6%
93.6%
94.5%
97.4%
96.6%
100.0%
96.9%
0% 20% 40% 60% 80% 100%
% Essential Agreement
Avg. % EA by Antibiotic (ABX) Class (1)
Sulfonamides
Penicillins
Monobactams
Fluoroquinolones
Cephalosporins
β-lactams
Aminoglycosides
(1) Based on Accelerate Development data completed to-date
(2) Blood culture bottles were tested between 1-12 hours after positivity |

| 10
Customer Feedback on WaveTM
~90% of existing customers indicated interest in evaluating WaveTM
Universal AST Platform
Ability to run PBC & Isolates together
Fastest AST Results
Delivering same-shift results in less than 4.5 hrs
Budget-Friendly
Cost-effective pricing for PBC & Isolates
Leveraging Existing Pheno® Customers for WaveTM Launch
Existing customer base could kickstart Wave™ launch with ~150 customers
already implementing Rapid PBC results
Pheno® Customer Feedback & Early Marketing Strategy for WaveTM
Review of existing customer feedback and marketing strategy building on WaveTM features and potential benefits for clinical and
laboratory stakeholders
✓ Pheno® customers’ clinical workflows are already setup to utilize rapid AST
✓ ~65% of U.S. customers secured for rapid PBC susceptibility testing through
year-end 2025
✓ Accelerate has established clinical, laboratory, and administrative
relationships at ~500 accounts worldwide
✓ Securing a partnership with a multi-national partner provides potential
access to a global salesforce with well-established market-share in
microbiology
#1
#2
#3
“The size and random-access capability is a real advantage for the laboratory"
“Impressed with < 4.5-hr calls from Enterobacterales training runs”
“Systems that run over ~6 hrs are not rapid since they lose same shift results”
“Ability to price isolate testing competitively with incumbent providers is real
differentiator to emerging competitors”
Top Customer Identified Potential Benefits:
Customer Comments:
~150 Current Pheno® Customers
Multi-national Commercial Partner
further expands new opportunities
Targeting ~500+ Accounts Ready for
Rapid AST at Launch |
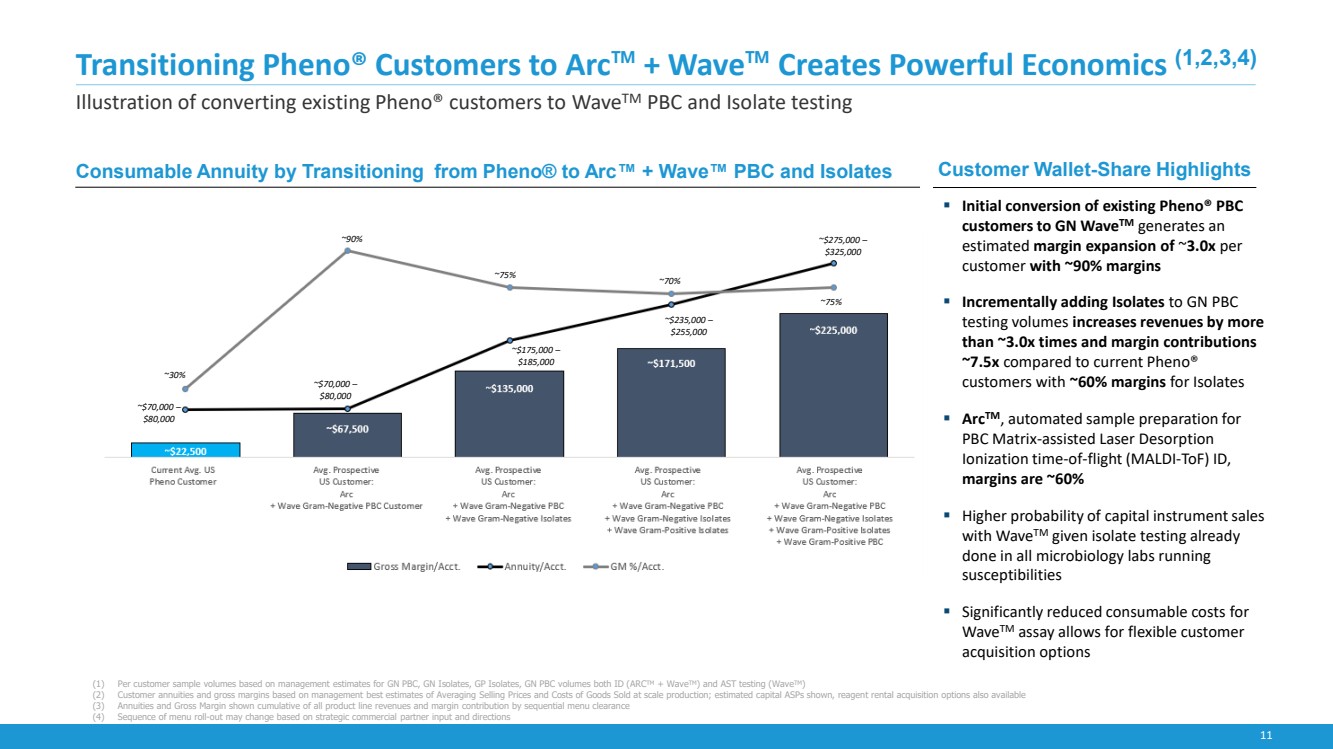
| 11
Transitioning Pheno® Customers to Arc™ + Wave™ Creates Powerful Economics (1,2,3,4)
Illustration of converting existing Pheno® customers to WaveTM PBC and Isolate testing
Customer Wallet-Share Highlights
▪ Initial conversion of existing Pheno® PBC
customers to GN WaveTM generates an
estimated margin expansion of ~3.0x per
customer with ~90% margins
▪ Incrementally adding Isolates to GN PBC
testing volumes increases revenues by more
than ~3.0x times and margin contributions
~7.5x compared to current Pheno®
customers with ~60% margins for Isolates
▪ ArcTM, automated sample preparation for
PBC Matrix-assisted Laser Desorption
Ionization time-of-flight (MALDI-ToF) ID,
margins are ~60%
▪ Higher probability of capital instrument sales
with WaveTM given isolate testing already
done in all microbiology labs running
susceptibilities
▪ Significantly reduced consumable costs for
WaveTM assay allows for flexible customer
acquisition options
(1) Per customer sample volumes based on management estimates for GN PBC, GN Isolates, GP Isolates, GN PBC volumes both ID (ARCTM + WaveTM) and AST testing (WaveTM)
(2) Customer annuities and gross margins based on management best estimates of Averaging Selling Prices and Costs of Goods Sold at scale production; estimated capital ASPs shown, reagent rental acquisition options also available
(3) Annuities and Gross Margin shown cumulative of all product line revenues and margin contribution by sequential menu clearance
(4) Sequence of menu roll-out may change based on strategic commercial partner input and directions
Consumable Annuity by Transitioning from Pheno® to Arc™ + Wave™ PBC and Isolates
~$70,000 –
$80,000
~30%
~90%
~75% ~70%
~75%
~$70,000 –
$80,000
~$175,000 –
$185,000
~$235,000 –
$255,000
~$275,000 –
$325,000 |
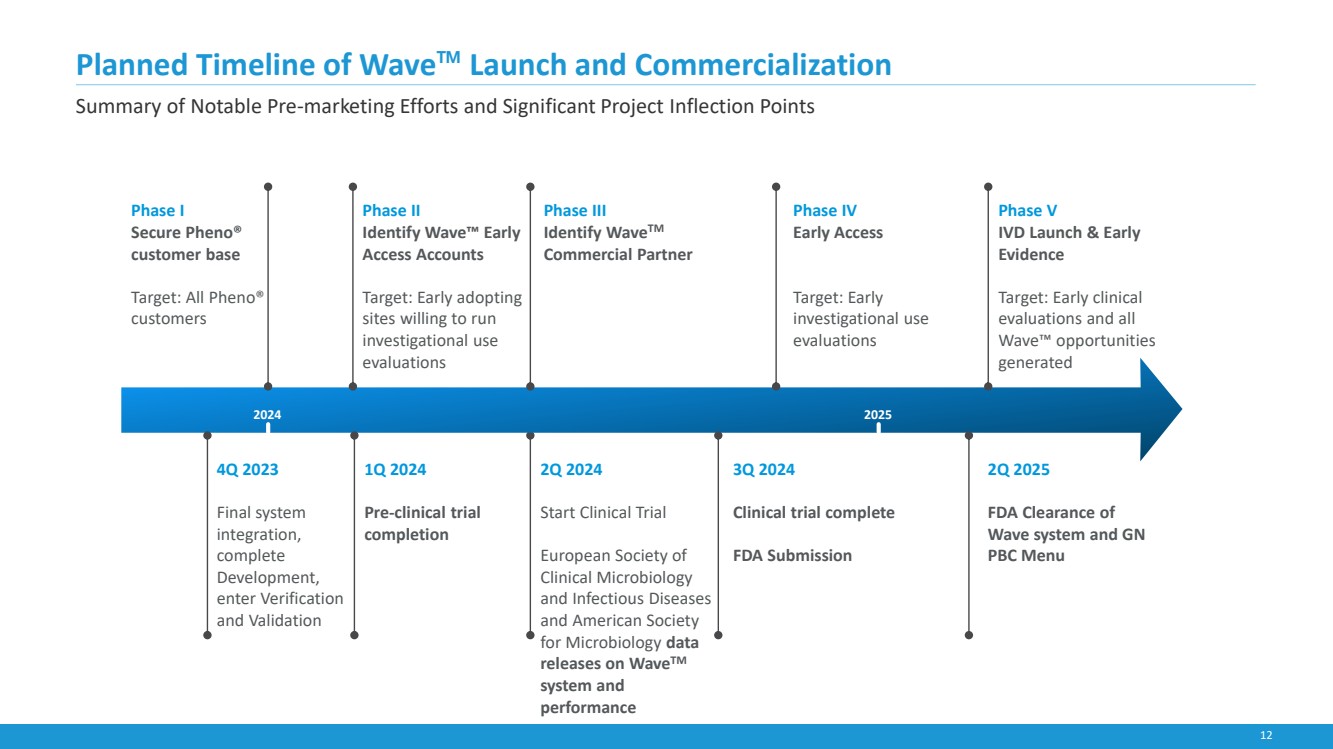
| 12
4Q 2023
Final system
integration,
complete
Development,
enter Verification
and Validation
2Q 2024
Start Clinical Trial
European Society of
Clinical Microbiology
and Infectious Diseases
and American Society
for Microbiology data
releases on WaveTM
system and
performance
1Q 2024
Pre-clinical trial
completion
3Q 2024
Clinical trial complete
FDA Submission
2Q 2025
FDA Clearance of
Wave system and GN
PBC Menu
Phase II
Identify Wave™ Early
Access Accounts
Target: Early adopting
sites willing to run
investigational use
evaluations
Planned Timeline of Wave™ Launch and Commercialization
Phase III
Identify WaveTM
Commercial Partner
Phase V
IVD Launch & Early
Evidence
Target: Early clinical
evaluations and all
Wave™ opportunities
generated
Phase I
Secure Pheno®
customer base
Target: All Pheno®
customers
2024 2025
Phase IV
Early Access
Target: Early
investigational use
evaluations
Summary of Notable Pre-marketing Efforts and Significant Project Inflection Points |
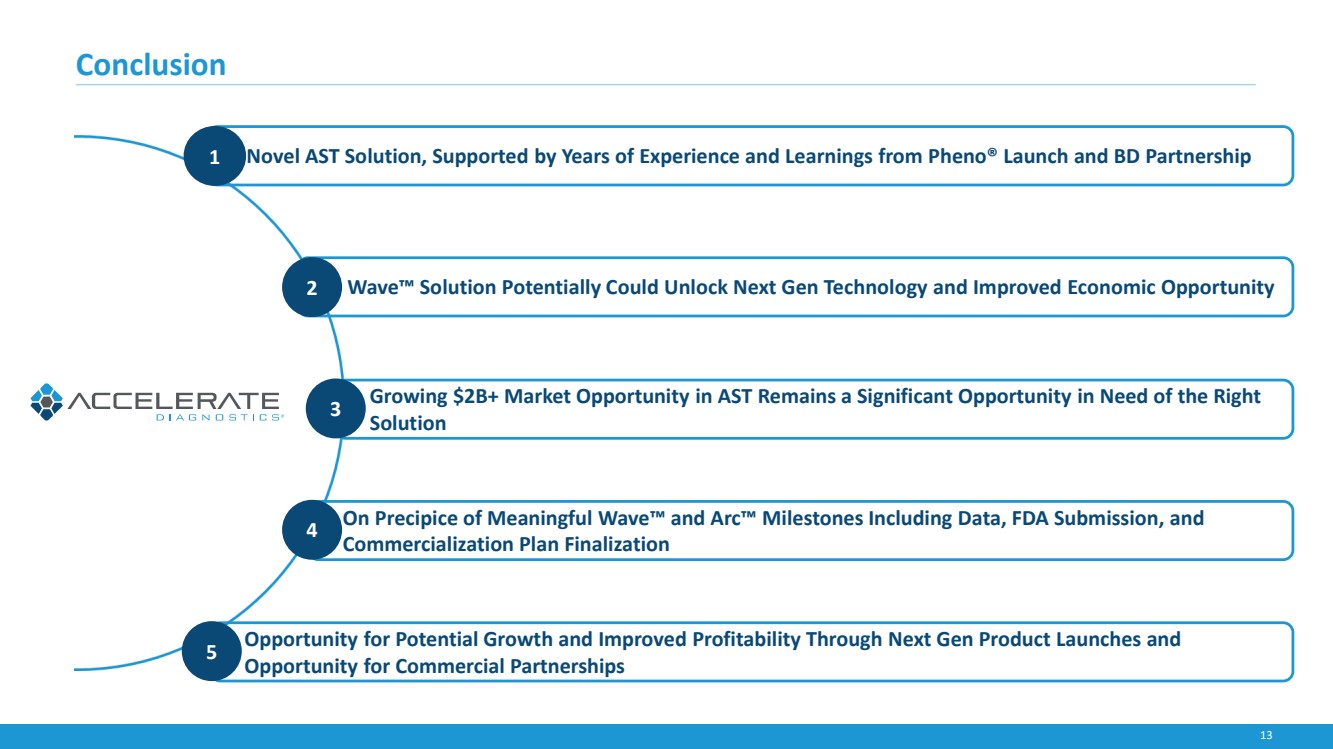
| 13
Conclusion
1• Novel AST Solution, Supported by Years of Experience and Learnings from Pheno® Launch and BD Partnership
•2 Wave™ Solution Potentially Could Unlock Next Gen Technology and Improved Economic Opportunity
• Growing $2B+ Market Opportunity in AST Remains a Significant Opportunity in Need of the Right
Solution 3
• On Precipice of Meaningful Wave™ and Arc™ Milestones Including Data, FDA Submission, and
Commercialization Plan Finalization 4
• Opportunity for Potential Growth and Improved Profitability Through Next Gen Product Launches and
Opportunity for Commercial Partnerships 5 |
v3.23.3
Cover
|
Dec. 11, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Dec. 11, 2023
|
| Entity File Number |
001-31822
|
| Entity Registrant Name |
Accelerate
Diagnostics, Inc.
|
| Entity Central Index Key |
0000727207
|
| Entity Tax Identification Number |
84-1072256
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
3950
South Country Club Road
|
| Entity Address, Address Line Two |
Suite 470
|
| Entity Address, City or Town |
Tucson
|
| Entity Address, State or Province |
AZ
|
| Entity Address, Postal Zip Code |
85714
|
| City Area Code |
520
|
| Local Phone Number |
365-3100
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common
Stock, $0.001 par value per share
|
| Trading Symbol |
AXDX
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
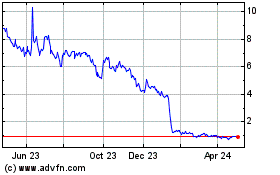
Accelerate Diagnostics (NASDAQ:AXDX)
Historical Stock Chart
From Apr 2024 to May 2024
Accelerate Diagnostics (NASDAQ:AXDX)
Historical Stock Chart
From May 2023 to May 2024