Filed pursuant to Rule 424(b)(4)
File No. 333-276771
PROSPECTUS

BONE
BIOLOGICS CORPORATION
119,000
Shares of Common Stock
662,251
Prefunded Warrants to purchase 662,251
Shares of Common Stock
662,251 Shares of Common Stock underlying
the Prefunded Warrants
781,251
Common Warrants to purchase 781,251
Shares of Common Stock
781,251
Shares of Common Stock underlying the Common
Warrants
46,875
Placement Agent Warrants to Purchase 46,875
Shares of Common Stock
46,875
Shares of Common Stock Underlying the Placement
Agent Warrants
We are
offering 119,000 shares of common stock, par value $0.001 per share (“common stock”), together with warrants to purchase
781,251 shares of common stock, which we refer to as the “warrants,” at a combined public offering price of
$2.56 per share and accompanying warrant. Each share of our common stock is being sold together with one warrant to purchase
one share of common stock. The warrants will have an exercise price of $2.43 per share and will be exercisable upon issuance and
will expire five years from the date of issuance. This prospectus also relates to the offering of the shares of common stock issuable
upon exercise of warrants.
We
are also offering to those investors, whose purchase of shares of our common stock in this offering would result in such investor, together
with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the investor, 9.99%) of
our outstanding common stock following the consummation of this offering, the opportunity to purchase, in lieu of the common stock that
would otherwise result in the investor’s beneficial ownership exceeding 4.99% (or, at the election of the investor, 9.99%), 662,251
pre-funded warrants to purchase 662,251 shares of common stock, at an exercise price of $0.001 per share, which we refer
to as the “pre-funded warrants.” Each pre-funded warrant will be exercisable upon issuance and may be exercised at any time
until all of the pre-funded warrants are exercised in full. Each pre-funded warrant is being sold together with one warrant to purchase
one share of common stock. The public offering price for each pre-funded warrant and the accompanying warrant is equal to the price per
share of common stock and the accompanying warrant being sold to the public in this offering, minus $0.001. This prospectus also relates
to the offering of the shares of common stock issuable upon exercise of the pre-funded warrants.
The shares of common stock and/or pre-funded warrants and the accompanying warrant can only be purchased together in this offering but
will be issued separately and will be immediately separable upon issuance.
This
offering will terminate on May 13, 2024 unless we decide to terminate the offering (which we may do at any time in our discretion)
prior to that date. We will have one closing for all the securities purchased in this offering. The combined public offering price per
share (or pre-funded warrant) and accompanying warrant will be fixed for the duration of this offering.
We
have engaged H.C. Wainwright & Co., LLC, or the placement agent, to act as our exclusive placement agent in connection with
this offering. The placement agent has agreed to use its reasonable best efforts to arrange for the sale of the securities offered by
this prospectus. The placement agent is not purchasing or selling any of the securities we are offering and the placement agent is not
required to arrange the purchase or sale of any specific number or dollar amount of securities. We have agreed to pay to the placement
agent the placement agent fees set forth in the table below, which assumes that we sell all of the securities offered by this prospectus.
Since we will deliver the securities to be issued in this offering upon our receipt of investor funds, there is no arrangement for funds
to be received in escrow, trust or similar arrangement. There is no minimum offering requirement as a condition of closing of this offering.
Because there is no minimum offering amount required as a condition to closing this offering, we may sell fewer than all of the securities
offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive
a refund in the event that we do not sell an amount of securities sufficient to pursue our business goals described in this prospectus.
In addition, because there is no escrow account and no minimum offering amount, investors could be in a position where they have invested
in our company, but we are unable to fulfill all of our contemplated objectives due to a lack of interest in this offering. Further,
any proceeds from the sale of securities offered by us will be available for our immediate use, despite uncertainty about whether we
would be able to use such funds to effectively implement our business plan. See the section entitled “Risk Factors” for more
information. We will bear all costs associated with the offering. See “Plan of Distribution” on page 19 of this prospectus
for more information regarding these arrangements.
Our
common stock is listed on Nasdaq under the symbol “BBLG.” The last reported sale price of our common stock on Nasdaq on
March 1, 2024 was $2.43. There is no established trading market for the pre-funded warrants or the warrants and we do not expect
an active market to develop. In addition, we do not intend to list the pre-funded warrants or the warrants on any national securities
exchange or other trading market. Without an active trading market, the liquidity of these securities will be limited.
| | |
Per Share and Accompanying Warrant | | |
Per Pre-
Funded
Warrant and
Accompanying
Warrant | | |
Total | |
| Public offering price | |
$ | 2.56 | | |
$ | 2.559 | | |
$ | 1,999,340.31 | |
| Placement Agent fees(1) | |
$ | 0.1792 | | |
$ | 0.1792 | | |
$ | 140,000.18 | |
| Proceeds to us (before expenses)(2) | |
$ | 2.3808 | | |
$ | 2.3798 | | |
$ | 1,859,340.13 | |
| (1) |
We
have agreed to pay the placement agent a cash fee equal to 7.0%. We have also agreed to pay the placement agent a management fee
of 1.0% of the aggregate gross proceeds raised in this offering and to reimburse the placement agent for certain of its offering
related expenses, including reimbursement for non-accountable expenses in an amount up to $10,000, legal fees and expenses
in the amount of up to $100,000, and for its clearing expenses in the amount of $15,950. In addition, we have agreed to issue the
placement agent or its designees warrants to purchase a number of shares of common stock equal to 6.0% of the shares of common stock
sold in this offering (including the shares of common stock issuable upon the exercise of the pre-funded warrants), at an exercise
price of $3.20 per share, which represents 125% of the public offering price per share and accompanying warrant. For a description
of compensation to be received by the placement agent, see “Plan of Distribution” for more information. |
| (2) |
Because
there is no minimum number of securities or amount of proceeds required as a condition to closing in this offering, the actual public
offering amount, placement agent fees, and proceeds to us, if any, are not presently determinable and may be substantially less than
the total maximum offering amounts set forth above. For more information, see “Plan of Distribution.” |
You
should read this prospectus, together with additional information described under the headings “Information Incorporated by
Reference” and “Where You Can Find More Information,” carefully before you invest in any of our securities.
Investing
in our securities involves a high degree of risk. See the section entitled “Risk Factors” beginning on page 9 of this prospectus
and in the documents incorporated by reference into this prospectus for a discussion of risks that should be considered in connection
with an investment in our securities.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Delivery
of the securities offered hereby is expected to be made on or about March 6, 2024, subject to satisfaction of customary closing
conditions.
H.C.
Wainwright & Co.
The
date of this prospectus is March 4, 2024.
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
We incorporate by reference important information
into this prospectus. You may obtain the information incorporated by reference without charge by following the instructions under “Where
You Can Find More Information.” You should carefully read this prospectus as well as additional information described under “Information
Incorporated by Reference,” before deciding to invest in our securities.
We
have not, and the placement agent has not, authorized anyone to provide you with additional information or information different from
that contained in this prospectus or from that contained or incorporated by reference in this prospectus filed with the Securities
and Exchange Commission (the “SEC”). We do not, and the placement agent and its affiliates do not, take any responsibility
for, and can provide no assurance as to the reliability of, any other information that others may give you. We are offering to sell,
and seeking offers to buy, our securities only in jurisdictions where offers and sales are permitted. The information contained in this
prospectus, or any document incorporated by reference in this prospectus, is accurate only as of its date, regardless of the time
of delivery of this prospectus or any sale of our securities. Our business, financial condition, results of operations and prospects
may have changed since that date.
This
prospectus and the information incorporated by reference in this prospectus contain estimates and other statistical data made by independent parties and by us relating to market size and growth
and other data about our industry. We obtained the industry and market data in this prospectus from our own research as well as from
industry and general publications, surveys and studies conducted by third parties. This data involves a number of assumptions and limitations
and contains projections and estimates of the future performance of the industries in which we operate that are subject to a high degree
of uncertainty, including those discussed in “Risk Factors.” We caution you not to give undue weight to such projections,
assumptions and estimates. Further, industry and general publications, studies and surveys generally state that they have been obtained
from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe
that these publications, studies and surveys are reliable, we have not independently verified the data contained in them. In addition,
while we believe that the results and estimates from our internal research are reliable, such results and estimates have not been verified
by any independent source.
Neither
we nor the placement agent have done anything that would permit this offering or the possession or distribution of this prospectus in
any jurisdiction where action for those purposes is required, other than in the United States. Persons outside the United States who
come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities
and the distribution of this prospectus outside of the United States.
CAUTIONARY
NOTE CONCERNING FORWARD-LOOKING STATEMENTS
This
prospectus and the documents incorporated by reference herein contain forward-looking statements that involve risks and uncertainties.
You should not place undue reliance on these forward-looking statements. All statements other than statements of historical fact contained
in this prospectus and the documents incorporated by reference herein are forward-looking statements and are only predictions.
We have based these forward-looking statements largely on our current expectations and projections about future events and financial
trends that we believe may affect our business, financial condition and results of operations. In some cases, you can identify these
forward-looking statements by terms such as “anticipate,” “believe,” “continue,” “could,”
“depend,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,”
“potential,” “predict,” “project,” “should,” “will,” “would”
or the negative of those terms or other similar expressions, although not all forward-looking statements contain those words. We have
based these forward-looking statements on our current expectations and projections about future events and trends that we believe may
affect our financial condition, results of operations, strategy, short- and long-term business operations and objectives, and financial
needs. These forward-looking statements include, but are not limited to, statements concerning the following:
| |
● |
our
ability to maintain compliance with the Nasdaq listing standards and remain listed on Nasdaq; |
| |
● |
our
projected financial position and estimated cash burn rate; |
| |
● |
our
estimates regarding expenses, future revenues and capital requirements; |
| |
● |
our
ability to continue as a going concern; |
| |
● |
our
need to raise substantial additional capital to fund our operations; |
| |
● |
the
success, cost and timing of our clinical trials; |
| |
● |
our
dependence on third parties in the conduct of our clinical trials; |
| |
● |
our
ability to obtain the necessary regulatory approvals to market and commercialize our product candidates; |
| |
● |
the
ultimate impact of health pandemics or epidemics on our business, our clinical trials, our research programs, healthcare systems
or the global economy as a whole; |
| |
● |
the
potential that results of preclinical and clinical trials indicate our current product candidate or any future product candidates
we may seek to develop are unsafe or ineffective; |
| |
● |
the
results of market research conducted by us or others; |
| |
● |
our
ability to obtain and maintain intellectual property protection for our current product candidates; |
| |
● |
our
ability to protect our intellectual property rights and the potential for us to incur substantial costs from lawsuits to enforce
or protect our intellectual property rights; |
| |
● |
the
possibility that a third party may claim we or our third-party licensors have infringed, misappropriated or otherwise violated their
intellectual property rights and that we may incur substantial costs and be required to devote substantial time defending against
claims against us; |
| |
● |
our
reliance on third-party suppliers and manufacturers; |
| |
● |
the
success of competing therapies and products that are or become available; |
| |
● |
our
ability to expand our organization to accommodate potential growth and our ability to retain and attract key personnel; |
| |
● |
the
potential for us to incur substantial costs resulting from product liability lawsuits against us and the potential for these product
liability lawsuits to cause us to limit our commercialization of our product candidate; |
| |
● |
market
acceptance of our product candidate, the size and growth of the potential markets for our current product candidate and any future
product candidates we may seek to develop, and our ability to serve those markets; |
| |
● |
the
successful development of our commercialization capabilities, including sales and marketing capabilities; |
| |
● |
our
expectation regarding the number of shares outstanding after this offering; |
| |
● |
our
intention to use the net proceeds of this offering to fund clinical trials, maintain and extend our patent portfolio, and for working
capital and other general corporate purposes; and |
| |
● |
pending
the intended uses described herein, our intention to invest the net proceeds of this offering in short-term, investment grade, interest-bearing
securities. |
These
forward-looking statements are subject to a number of risks, uncertainties and assumptions, including the successful development and
commercialization of our product candidates, market acceptance of our product candidates, our financial performance, including our ability
to fund operations, our ability to maintain compliance with Nasdaq’s continued listing requirements, regulatory approval and regulation
of our product candidates, our expected use of proceeds from this offering, and other factors and risks identified from time to time
in our filings with the SEC, including this prospectus and those described in “Risk Factors.” Moreover, we operate in a very
competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all
risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may
cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks,
uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results
could differ materially and adversely from those anticipated or implied in the forward-looking statements.
You
should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected
in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events
and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, except as required by law, neither
we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation
to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual
results or to changes in our expectations.
You
should read this prospectus and the documents that we reference and that are incorporated by reference in this prospectus and
have filed with the SEC as exhibits to the registration statement of which this prospectus is a part with the understanding that our
actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.
Risk
Factors Summary
The
following factors are among the principal factors that make in an investment in this offering speculative or risky. For a more detailed
description of the risks material to our business, see “Risk Factors” beginning on page 9. The following summary should not
be considered an exhaustive summary of the material risks we face and should be read in conjunction with the “Risk Factors”
section and the other information in this prospectus and under similar headings in the other documents that are incorporated by reference
into this prospectus including documents that are filed after the date hereof. These factors include:
| ● | Our
recurring operating losses have raised substantial doubt regarding our ability to continue
as a going concern. |
| | ● | Future
sales and issuance of our common stock or equity-linked securities could result in additional
dilution of the percentage ownership of our stockholder and could cause our share price to
fall. |
| ● | There
is not expected to be an active trading market for the warrants in this offering, and warrant
holders have no rights as stockholders. |
| ● | Our
management will have broad discretion over the use of the proceeds we receive in this offering. |
| ● | We
may be unable to comply with the continued listing standards of Nasdaq. |
| ● | Our
Chief Executive Officer and Chief Financial Officer have contractual rights to participate
in future financings. |
| ● | We
have a limited operating history. |
| ● | We
will require substantial capital to complete studies of our product candidates and, if possible,
achieve FDA approval for the products. |
| ● | Our
product candidates are at an early stage of development and may not be successfully developed
or commercialized. |
| ● | FDA
regulation is costly and time consuming, which may delay or prevent us from commercializing
our product candidates. |
| ● | Our
product candidates may cause unacceptable adverse events. |
| ● | Suspensions
or delays in commencing and completing clinical testing could increase our costs and delay
or prevent us from commercializing our product candidates. |
| ● | We
have limited resources to pursue product candidates and indications. |
| ● | We
may have difficulty recruiting patients for our clinical trials. |
| ● | Any
success in preclinical studies and early clinical trials does not predict the success of
later trials; our product candidates may not have favorable results or receive regulatory
approval. |
| ● | We
may be unable to obtain regulatory approval in non-U.S. jurisdictions. |
| ● | We
may be unable to retain or recruit necessary personnel to advance the development of our
product candidates. |
| ● | We
rely on third parties to supply raw materials for our product candidates and to conduct our
preclinical and clinical trials. |
| ● | We
must comply with our obligations under license agreements or risk losing rights that are
important to our business. |
| ● | We
rely on patents and other intellectual property to protect some of our product candidates. |
| ● | We
could be subject to substantial costs in legal proceedings or other actions relating to intellectual
property rights. |
| ● | We
may not be able to obtain patent protection for our product candidates, or our intellectual
property may not be sufficient to protect our product candidates from competition. |
| ● | Our
commercial success depends upon attaining significant market acceptance of our lead product
candidate and future product candidates, if approved, among physicians, patients, healthcare
payors and treatment centers. |
| ● | Our
product candidates, if approved, may not be covered or adequately reimbursed by third-party
payors. |
| ● | Healthcare
legislative measures aimed at reducing healthcare costs may negatively impact our business. |
| ● | Our
future success depends on the performance and continued service of our officers and directors. |
| ● | We
face substantial competition, which may result in others discovering, developing or commercializing
products before or more successfully than we do. |
| ● | Product
liability lawsuits against us could cause us to incur substantial liabilities and to limit
commercialization of any products that we may develop. |
| ● | The
price of our common stock may fluctuate substantially. |
| ● | We
may be at risk of securities class action litigation. |
| ● | Market
and economic conditions may negatively impact our business, financial condition and share
price. |
| ● | We
do not intend to pay cash dividends on our shares of common stock. |
| ● | Our
governing documents and Delaware law have anti-takeover effects that could discourage, delay
or prevent a change in control. |
PROSPECTUS
SUMMARY
The
following summary highlights selected information contained or incorporated by reference in this prospectus and does not contain
all of the information that may be important to you and your investment decision. Before investing in our securities, you should carefully
read this entire prospectus, including our consolidated financial statements and the related notes and other documents incorporated
by reference herein, as well as the information under the caption “Risk Factors” herein and under similar headings
in the other documents that are incorporated by reference into this prospectus including documents that are filed after the date hereof.
Some of the statements in this prospectus constitute forward-looking statements that involve risks and uncertainties. See “Cautionary
Note Concerning Forward-Looking Statements.” In this prospectus, unless context requires otherwise, references to “we,”
“us,” “our,” “BBLG” “Bone Biologics,” or the “Company” refer to Bone Biologics
Corporation and its subsidiary on a consolidated basis.
Company
Overview
We
are a medical device company that is currently focused on bone regeneration in spinal fusion using the recombinant human protein known
as NELL-1. NELL-1 in combination with DBM, demineralized bone matrix, is an osteopromotive recombinant protein that provides target specific
control over bone regeneration. The NELL-1 technology platform has been licensed exclusively for worldwide applications to us through
a technology transfer from the UCLA Technology Development Group on behalf of UC Regents (“UCLA TDG”). UCLA TDG and the Company
received guidance from the Food and Drug Administration (“FDA”) that NELL-1/DBM will be classified as a device/drug combination
product that will require an FDA-approved pre-market approval application (“PMA”) before it can be commercialized in the
United States.
We
were founded by University of California professors in collaboration with an Osaka University professor and a University of Southern
California surgeon in 2004 as a privately-held company with proprietary, patented technology that has been validated in sheep and non-human
primate models to facilitate bone growth. We believe our platform technology has application in delivering improved outcomes in the surgical
specialties of spinal, orthopedic, general orthopedic, plastic reconstruction, neurosurgery, interventional radiology, and sports medicine.
Lead product development and clinical studies are targeted on spinal fusion surgery, one of the larger segments in the orthopedic market.
We
are a development stage entity. The production and marketing of our products and ongoing research and development activities are subject
to extensive regulation by numerous governmental authorities in the United States. Prior to marketing in the United States, any combination
product developed by us must undergo rigorous preclinical (animal) and clinical (human) testing and an extensive regulatory approval
process implemented by the FDA under the Federal Food, Drug, and Cosmetic Act. There can be no assurance that we will not encounter problems
in clinical trials that will cause us or the FDA to delay or suspend the clinical trials.
Our success will depend in part on our ability
to obtain patents and product license rights, maintain trade secrets, and operate without infringing on the proprietary rights of others,
both in the United States and other countries. There can be no assurance that patents issued to or licensed by us will not be challenged,
invalidated, rendered unenforceable, or circumvented, or that the rights granted thereunder will provide proprietary protection or competitive
advantages to us.
Product Candidates
We
have developed a stand-alone platform technology through significant laboratory and small and large animal research over more than ten
years to generate the current applications across broad fields of use.
The platform technology is our recombinant human protein, known as NELL-1, a proprietary skeletal specific growth factor which is a bone
void filler. NELL-1 provides regulation over skeletal tissue formation and stem cell differentiation during bone regeneration. We obtained
the platform technology pursuant to an exclusive license agreement with UCLA TDG which grants us exclusive rights to develop and commercialize
NELL-1 for spinal fusion by local administration, osteoporosis and trauma applications. A major challenge associated with orthopedic
surgery is effective bone regeneration, including challenges related to rapid, uncontrolled bone growth which can cause unsound structure;
cysts and less dense bone formation; unwanted bone formation, and swelling; and intense inflammatory response to current bone regeneration
compounds. We believe NELL-1 will address these unmet clinical challenges for effective bone regeneration, especially in hard healers.
We
are currently focused on bone regeneration in lumbar spinal fusion, in keeping with our exclusive license agreement, using NELL-1 in
combination with DBM, a demineralized bone matrix from Musculoskeletal Transplant Foundation (“MTF”). The NELL-1/DBM medical
device is a combination product which is an osteopromotive recombinant protein that provides target specific control over bone regeneration.
Leveraging the resources of investors and strategic partners, we have successfully surpassed four critical milestones:
| |
● |
Demonstrating
a successful small laboratory scale pilot run for the manufacturing of the recombinant NELL-1 protein in Chinese hamster ovary cells; |
| |
|
|
| |
● |
Validation
of protein dosing and efficacy in established large animal sheep models pilot study; |
| |
|
|
| |
● |
Completed
pivotal animal study; and |
| |
|
|
| |
● |
Initiated
a first-in-man pilot clinical trial in Australia. |
Our
lead product candidate is expected to be purified NELL-1 mixed with 510(k) cleared DBM Demineralized Bone Putty recommended for use in
conjunction with applicable hardware consistent with the indication. The NELL-1/DBM Fusion Device, NB1, will be comprised of a
single dose vial of NELL-1 recombinant protein freeze dried onto DBM. A vial of NELL-1/DBM will be sold in a convenience kit with a diluent
and a syringe of 510(k) cleared demineralized bone (“DBM Putty”) produced by MTF. A delivery device will allow the surgeon
to mix the reconstituted NELL-1 with the appropriate quantity of DBM Putty just prior to implantation.
The
NELL-1/DBM Fusion Device, NB1, is intended for use in lumbar spinal fusion and may have a variety of other spine and orthopedic
applications. While the product is initially targeted at the lumbar spine fusion market, in keeping with our exclusive license agreement,
we believe NELL-1’s novel set of characteristics, target specific mechanism of action, efficacy, safety and affordability position
the product well for application in a variety of procedures including:
| |
Spine
Implants. The global bone graft substitute market presents a $3 billion market opportunity. While use of the patient’s own bone, also referred
to as autograft, to enhance fusion of vertebral segments remains the optimal use for this
type of treatment, complications associated with use of autograft bone including pain, increased
surgical time and infection limit its use.
|
| |
Non-Union
Trauma Cases. While the majority of fractures heal without the need for osteosynthetic products, bone substitutes are used
in complicated breaks where the bone does not mend naturally. Globally an $8 billion market opportunity, management believes that
NELL-1 technology is expected to perform as well as other growth factors in this market. |
| |
|
| |
Osteoporosis.
Globally an $11.2 billion market opportunity, the medical need to find a solution to counter a decrease in bone mass and density
seen in women most frequently after menopause or a similar effect on astronauts in microgravity environments for an extended period
is a major medical challenge. The systemic use of NELL-1 to stimulate bone regeneration throughout the body thereby increasing bone
density could have a very significant impact on the treatment of osteoporosis. |
UCLA’s initial research was funded with
approximately $18 million in resources from UCLA TDG and government grants. Since licensing the exclusive worldwide intellectual property
rights from UCLA TDG, our continued development has been funded through capital raises. Our research and development expenses for the
years ended December 31, 2023 and 2022 were $6,907,824 and $1,579,298, respectively. We anticipate that we will require approximately
$5 million to complete first-in-man studies, and an estimated additional $24 million in scientific expenses to achieve FDA approval,
if possible, for a spine interbody fusion indication. These amounts are estimates based on data currently available to us, and are subject
to many factors including the various risk factors discussed in the section “Risk Factors” included in our Form 10-K
for the fiscal year ended December 31, 2023 (the “2023 Form 10-K”), which is incorporated herein by reference.
NELL-1’s powerful specific bone and cartilage
forming properties are derived from the ability of NELL-1 to only target cells that exhibit an activated “master switch”
to develop into bone or cartilage. NELL-1 is a function specific recombinant human protein that has been proven in laboratory bench models
to recapitulate normal human growth and development to provide control over bone and cartilage regeneration.
NELL-1 was isolated in 1996, and the first NELL-1
patent on bone regeneration was filed in 1999. Subsequent patents and continuations in part describing NELL-1 manufacturing, delivery,
and cartilage regeneration were filed to further strengthen the patent portfolio.
We
have completed two preclinical sheep studies that demonstrated our recombinant NELL-1 (“rhNELL-1”) growth factor effectively
promotes bone formation in a phylogenetically advanced spine model. In addition, rhNELL-1 was shown to be well tolerated and there were
no findings of inflammation. Our pivotal sheep study evaluated the effect of rhNELL-1 combined with DBM on lumbar interbody arthrodesis
in an adult ovine model and demonstrated a 37.5% increased frequency of fusion at 26 weeks from the control.
Our
first-in-man pilot clinical study commenced year-end 2023 and will evaluate the safety and effectiveness of NB1 in adult subjects with
spinal degenerative disc disease at one level from L2-S1, who may also have up to Grade 1 spondylolisthesis or Grade 1 retrolisthesis
at the involved level who undergo transforaminal lumbar interbody fusion. The multi-center, prospective, randomized trial consists of
30 patients in Australia, with the primary end-point being fusion success at 12 months and change from baseline in the Oswestry Disability
Index pain score. We expect completion of the trial 12 months following enrollment of the 30th patient. We intend to use the
pilot clinical trial data from Australia to enable a future larger U.S. pivotal clinical study, prior to submission of a PMA to the FDA.
Our
Business Strategy
Our
business plan is to develop our target-specific growth factor for bone regeneration, based on preclinical and clinical data that has
demonstrated increases in the quantity and quality of bone, and a strong safety profile. Our initial focus on lumbar spinal fusion entails
advancing our target-specific growth factor through clinical studies to achieve FDA approval with comparable efficacy and safety to the
gold standard for spine fusion (autografts). Continued capital funding is critical to facilitate the development of our Nell-1 technology
through the clinical regulatory path.
Intellectual
Property Risks
Our
patent portfolio currently consists of nine patents which expire between 2024 and 2033. We intend to expand our portfolio through
composition of matter, methods of use and methods of production patent applications, as the opportunity arises through the development
of our platform technology. Our success will depend in part on our ability to obtain patents and product license rights, maintain trade
secrets, and operate without infringing on the proprietary rights of others, both in the United States and other countries. There can
be no assurance that patents issued to or licensed by us will not be challenged, invalidated, rendered unenforceable, or circumvented,
or that the rights granted thereunder will provide proprietary protection or competitive advantages to us. The patent positions of medical
device companies are uncertain and involve complex legal and factual questions. We may incur significant expenses in protecting our intellectual
property and defending or assessing claims with respect to intellectual property owned by others. See “Risk Factors” on page
9 and other information included or incorporated by reference in this prospectus for a discussion of intellectual property risks
to consider carefully before deciding to invest in our securities.
Our Management Team
We have two full-time employees. Jeffrey Frelick
has served as our President and Chief Executive Officer since June 2019 and brings more than 25 years of leadership, operational, and
investment experience in the life science industry. Deina Walsh has served as our Chief Financial Officer since November 2014.
Mr. Frelick previously served as our Chief Operating
Officer from 2015 to June 2019. Prior to this Mr. Frelick spent 15 years on Wall Street as a sell-side analyst following the med-tech
industry at investment banks Canaccord Genuity, ThinkEquity and Lazard. He also previously worked at Boston Biomedical Consultants where
he provided strategic planning assistance, market research data and due diligence for diagnostic companies. He began his career at Becton
Dickinson in sales and sales management positions after gaining technical experience as a laboratory technologist with Clinical Pathology
Facility. Mr. Frelick received a B.S. in Biology from University of Pittsburgh and an M.B.A. from Suffolk University’s Sawyer Business
School.
Ms. Walsh is a certified public accountant and
owner/founder of DHW CPA, PLLC, a Public Company Accounting Oversight Board (PCAOB) registered firm since 2014. Prior to forming her
firm, Ms. Walsh spent 13 years at a public accounting firm where, as a partner, she was actively responsible for leading firm audit engagements
of publicly held entities in accordance with PCAOB standards and compliance with SEC regulations, including internal control requirements
under Section 404 of the Sarbanes-Oxley Act. Ms. Walsh had a global client base including entities throughout the United States, Canada
and China. These entities encompass a diverse range of industries including manufacturing, wholesale, life sciences, pharmaceuticals,
and technology. Her experience includes work with start-up companies and well-established operating entities. She has assisted many entities
seeking debt and equity capital. Areas of specialty include mergers, acquisitions, reverse mergers, consolidations, complex equity structures,
foreign currency translations and revenue recognition complexities. Ms. Walsh has an Associates of Science Degree in Business Administration
from Monroe Community College and a Bachelor of Science Degree in Accounting from the State University of New York at Brockport.
We have relied and plan on continuing to rely
on independent organizations, advisors and consultants to perform certain services for us, including handling substantially all aspects
of regulatory approval, clinical management, manufacturing, marketing, and sales. Such services may not always be available to us on
a timely basis or at costs that we can afford. We also have engaged and plan to continue to engage regulatory consultants to advise us
on our dealings with the FDA and other foreign regulatory authorities and have been and will be required to retain additional consultants
and employees.
Our future performance will depend in part on
our ability to successfully integrate newly hired officers into our management team, engage and retain consultants, and to develop an
effective working relationship with our management and consultants. Losing key personnel or failing to recruit necessary additional personnel
would impede our ability to attain our development objectives. Losing key personnel or failing to recruit necessary additional personnel
would impede our ability to attain our development objectives. See “Risk Factors” on page 9 and other information included
or incorporated by reference in this prospectus for a discussion of management risks to consider carefully before deciding to invest
in our securities.
Recent
Developments
November
2023 Offering
On
November 20, 2023 the Company sold and issued, in a registered direct offering (the “Registered Direct Offering”), 142,384
shares of common stock, at an offering price of $5.12 per share to certain institutional investors (the “Purchasers”) pursuant
to a securities purchase agreement (the “Purchase Agreement”). Pursuant to the Purchase Agreement, in a concurrent private
placement (together with the Registered Direct Offering, the “November Offering”), the Company issued to the Purchasers unregistered
warrants (the “November Warrants”) to purchase up to an aggregate of 142,384 shares of common stock, which represent 100%
of the shares of common stock issued and sold in the Registered Direct Offering. The November Warrants are exercisable at an exercise
price of $4.16 per share, were exercisable immediately upon issuance, and will expire five and one-half years from the date of issuance.
In addition, the Company issued the placement agent as compensation in connection with the November Offering, warrants (the “November
Placement Agent Warrants”) to purchase up to an aggregate of 8,543 shares of common stock (equal to 6.0% of the aggregate number
of shares sold in the Registered Direct Offering). The November Placement Agent Warrants have substantially the same terms and conditions
as the November Warrants, except that the November Placement Agent Warrants have a term of five years from the commencement of sales
in the November Offering and an exercise price of $6.40 per share.
Nasdaq
Panel Decision
On
September 27, 2023, the Company received a written notice from the Nasdaq notifying the Company that it was not in compliance with the
$1.00 per share minimum bid price requirement set forth in Nasdaq Listing Rule 5550(a)(2) and that Nasdaq’s staff had determined
to delist the Company’s securities. On December 11, 2023, a Nasdaq Hearings Panel granted the Company’s request for continued
listing on Nasdaq subject to the Company demonstrating compliance with the minimum bid price requirement prior to January 12, 2024. The
Company received notice from Nasdaq on January 9, 2024 that it had regained compliance with the minimum bid price requirement. The Company
will remain under a Nasdaq discretionary panel monitor until June 28, 2024.
Litigation
Update
On
January 10, 2024 we entered into a Settlement Agreement and Mutual General Release (the “Agreement”) with Drs. Bessie
(Chia) Soo and Kang (Eric) Ting, on the one hand (the “plaintiffs”), and the Company and Stephen LaNeve on the other
hand (together with the Company, the “defendants”), in settlement of the claims for breach of contract and tortious
interference with contract against the defendants filed in the United States District Court for the District of Massachusetts (the
“Court”). The Agreement is effective as of January 9, 2024. We had certain indemnification obligations to Mr. LaNeve
arising out of actions taken in connection with his service to the Company. Under the Agreement, the Company agreed to pay the
plaintiffs $750,000, and on February 7, 2024, the Company paid $414,989, and the Company’s insurance carrier paid $335,011 for
the total settlement. The parties to the Agreement filed a joint stipulation to dismiss the action with prejudice with the
Court.
Going
Concern
We
have a history of operating losses since inception and expect to incur additional near-term losses. As discussed further in “Management’s
Discussion and Analysis - Liquidity and Capital Resources,” included in our 2023 Form 10-K, which is incorporated herein
by reference, our independent registered public accounting firm, in its audit report to the financial statements included in our
Annual Report on Form 10-K for the year ended December 31, 2023, expressed substantial doubt about our ability to continue as
a going concern. Our consolidated financial statements do not include any adjustments that may result from the outcome of this uncertainty.
Following this offering, we will need to raise additional capital to fund our operations and continue to support our planned development
and commercialization activities. If we cannot secure the financing needed to continue as a viable business, our stockholders may lose
some or all of their investment in us.
Corporate
Information
We
were incorporated under the laws of the State of Delaware on October 18, 2007 as AFH Acquisition X, Inc. Pursuant to a Merger Agreement,
dated September 19, 2014, by and among the Company, its wholly-owned subsidiary, Bone Biologics Acquisition Corp., a Delaware corporation
(“Merger Sub”), and Bone Biologics, Inc., Merger Sub merged with and into Bone Biologics Inc., with Bone Biologics Inc. remaining
as the surviving corporation in the merger. Upon the consummation of the merger, the separate existence of Merger Sub ceased. On September
22, 2014, the Company officially changed its name to “Bone Biologics Corporation” to more accurately reflect the nature of
its business and Bone Biologics, Inc. became a wholly owned subsidiary of the Company. Bone Biologics, Inc. was incorporated in California
on September 9, 2004.
Our
principal executive offices are located at 2 Burlington Woods Drive, Suite 100, Burlington MA 01803 and our telephone number is (781)
552-4452. Our website address is www.bonebiologics.com. The information contained on our website is not incorporated by reference into
this prospectus, and you should not consider any information contained on, or that can be accessed through, our website as part of this
prospectus or in deciding whether to invest in our securities.
THE
OFFERING
The
following summary contains basic information about this offering. The summary is not intended to be complete. You should read the full
text and more specific details contained elsewhere in this prospectus.
| Common
Stock Offered |
|
119,000
shares. |
| |
|
|
| Pre-Funded
Warrants Offered |
|
We
are also offering to those investors, whose purchase of shares of our common stock in this offering would result in such investor,
together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the investor,
9.99%) of our outstanding common stock following the consummation of this offering, the opportunity to purchase, in lieu of the common
stock that would otherwise result in the investor’s beneficial ownership exceeding 4.99% (or, at the election of the investor,
9.99%), 662,251 pre-funded warrants to purchase 662,251 shares of common stock, at an exercise price of $0.001 per share,
which we refer to as the “pre-funded warrants.” Each pre-funded warrant will be exercisable upon issuance and may
be exercised at any time until all of the pre-funded warrants are exercised in full. Each pre-funded warrant is being sold together
with one warrant to purchase one share of common stock. The public offering price for each pre-funded warrant and the accompanying
warrant is equal to the price per share of common stock and the accompanying warrant being sold to the public in this offering, minus
$0.001. This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the pre-funded warrants. See
“Description of Securities – Pre-Funded Warrants” for additional information. |
| |
|
|
| Warrants
Offered |
|
Each
share of common stock or pre-funded warrant is being offered together with one warrant to
purchase one share of common stock. The warrants will have an exercise price of $2.43
per share and will be exercisable upon issuance (the “Initial Exercise Date”).
The warrants will expire on the five-year anniversary of the Initial Exercise Date. Each
holder of warrants will be prohibited from exercising its warrant for shares of our common
stock if, as a result of such exercise, the holder, together with its affiliates, would own
more than 4.99% of the total number of shares of our common stock then issued and outstanding.
However, any holder may increase such percentage to any other percentage not in excess of
9.99%. This offering also relates to the offering of the shares of common stock issuable
upon the exercise of the warrants. For more information regarding the warrants, you should
carefully read the section titled “Description of Securities — Warrants”
in this prospectus.
|
| Placement
Agent Warrants |
|
We
have agreed to issue to the placement agent or its designees warrants, or the placement agent warrants, to purchase up to 6.0% of
the shares of common stock sold in this offering (including the shares of common stock issuable upon the exercise of the pre-funded
warrants), at an exercise price of $3.20 per share, which represents 125% of the public offering price per share and accompanying
warrant. For a description of the compensation to be received by the placement agent, see “Plan of Distribution” for
more information. |
| |
|
|
Common
Stock Outstanding Prior
to
This Offering |
|
534,238
shares. |
| |
|
|
| Common
Stock to be Outstanding After this Offering |
|
1,315,489
shares (assuming full exercise of the
pre-funded warrants and no exercise of the warrants offered hereby). |
| |
|
|
| Use
of Proceeds |
|
We
estimate that the net proceeds of this offering assuming no exercise of the warrants, after deducting placement agent fees and estimated
offering expenses, will be approximately $1.5 million, assuming full exercise of the pre-funded warrants and
assuming no exercise of the warrants. We intend to use all of the net proceeds we receive from this offering to fund clinical trials,
maintain and extend our patent portfolio, and for working capital and other general corporate purposes. See “Use of Proceeds.” |
| |
|
|
| Lock-up
Agreements |
|
Our
executive officers and directors have agreed with the placement agent not to sell, transfer or dispose of any shares or similar securities
for a period of 60 days after the date of this prospectus. For additional information regarding our arrangement with the
placement agent, please see “Plan of Distribution.” |
| Nasdaq
Trading Symbol |
|
Our
common stock is listed on the Nasdaq Capital Market under the symbol “BBLG.” We do not intend to list the pre-funded
warrants or warrants offered hereunder on any stock exchange. Without an active trading market, the liquidity of the pre-funded warrants
and warrants will be limited. |
| |
|
|
| Risk
Factors |
|
See
“Risk Factors” on page 9 and other information included or incorporated by reference in this prospectus for a discussion of factors to consider carefully before deciding to invest in our securities. |
The
discussion above is based on 534,238 shares of our common stock outstanding as of March 4, 2024, and excludes as of such date
the following:
| |
● |
74,151
shares of common
stock issuable upon exercise of stock options outstanding at a weighted average exercise price of $107.65 per share. |
| |
|
|
| |
● |
197,844
shares of common
stock issuable upon exercise of outstanding common stock warrants with a weighted average exercise price of $127.86
per share. |
| |
|
|
| |
● |
555,338
shares of common
stock reserved for future grants pursuant to the Bone Biologics Corporation 2015 Equity Incentive Plan (the “2015 Equity Incentive
Plan”). |
| |
|
|
| |
● |
781,251 shares of our common
stock issuable upon the exercise of warrants to be issued in this offering; and |
| |
|
|
| |
● |
46,875
shares of common stock issuable upon the exercise
of the placement agent warrants to be issued to the placement agent or its designees as compensation in connection with this offering
and pursuant to this prospectus. |
Unless
expressly indicated or the context requires otherwise, all information in this prospectus assumes (i) we issue no pre-funded warrants
and (ii) no exercise of the warrants offered hereby.
RISK
FACTORS
Investing
in our securities involves a high degree of risk. You should carefully consider all of the information contained in this prospectus
and other information which may be incorporated by reference in this prospectus as provided under “Information Incorporated by
Reference.” In particular, you should carefully consider the risks described below and elsewhere in this prospectus, which
could materially and adversely affect our business, results of operations or financial condition, together with those under the heading
“Risk Factors” in our most recent Annual Report on Form 10-K, which is incorporated by reference into this prospectus, as
those risk factors are amended or supplemented by our subsequent filings with the SEC. These risks and uncertainties are not the
only risks and uncertainties we face. Additional risks and uncertainties not currently known to us, or that we currently view as immaterial,
may also impair our business. If any of the risks or uncertainties described below or in our SEC filings or any additional risks
and uncertainties actually occur, our business, financial condition, results of operations and cash flow could be materially and adversely
affected. As a result, you could lose all or part of your investment.
Risks
Related to This Offering and Ownership of our Securities
Our
recurring operating losses have raised substantial doubt regarding our ability to continue as a going concern.
Our
recurring operating losses raise substantial doubt about our ability to continue as a going concern. During the year ended December 31,
2023, we incurred a net loss of $8.9 million, and used net cash in operating activities of $9.6 million. Our available
cash is expected to fund our operations through the second quarter of 2024. These factors raise substantial doubt about the Company’s
ability to continue as a going concern within a reasonable period of time, which is considered to be one year from the issuance date
of these financial statements. In addition, our independent registered public accounting firm, in its audit report to the financial statements
included in our Annual Report on Form 10-K for the year ended December 31, 2023, expressed substantial doubt about our ability
to continue as a going concern. Our financial statements incorporated by reference into this prospectus do not include any adjustments
that might result if we are unable to continue as a going concern and, therefore, be required to realize our assets and discharge our
liabilities other than in the normal course of business which could cause investors to suffer the loss of all or a substantial portion
of their investment. In order to have sufficient cash and cash equivalents to fund our operations in the future, we will need to raise
additional equity or debt capital and cannot provide any assurance that we will be successful in doing so. The perception of our ability
to continue as a going concern may make it more difficult for us to obtain financing for the continuation of our operations and could
result in the loss of confidence by investors, suppliers and employees.
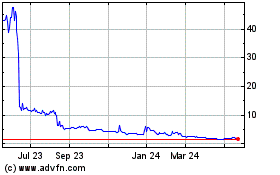
The price of our
common stock and public warrants may fluctuate substantially.
You should consider an
investment in our common stock to be risky. Some factors that may cause the market price of our common stock to fluctuate, in addition
to the other risks mentioned in this “Risk Factors” section are:
| |
● |
our ability to meet
the Nasdaq listing requirements; |
| |
● |
volatility and limitations
in trading volumes of our shares of common stock; |
| |
● |
our ability to obtain
financing to conduct and complete research and development activities including, but not limited to, our clinical trials, and other
business activities; |
| |
● |
the timing and success
of our clinical trials and introduction of products to the market; |
| |
● |
changes in the development
status of our product candidate; |
| |
● |
any delays or adverse
developments or perceived adverse developments with respect to the FDA’s review of our planned preclinical and clinical trials; |
| |
● |
safety concerns related
to the use of our product candidate; |
| |
● |
changes in our capital
structure or dividend policy, future issuances of securities, sales of large blocks of common stock by our stockholders; |
| |
● |
our cash position; |
| |
● |
announcements and events
surrounding financing efforts, including debt and equity securities; |
| |
● |
changes in general economic,
political and market conditions in or any of the regions in which we conduct our business; |
| |
● |
analyst research reports,
recommendation and changes in recommendations, price targets, and withdrawals of coverage; |
| |
● |
departures and additions
of key personnel; |
| |
● |
disputes and litigation; |
| |
● |
changes in applicable
laws, rules, regulations, or accounting practices and other dynamics; and |
| |
● |
other events or factors,
many of which may be out of our control. |
In addition, if the market
for stock in our industry or industries related to our industry, or the stock market in general, experiences a loss of investor confidence,
the trading price of our common stock could decline for reasons unrelated to our business, financial condition and results of operations.
If any of the foregoing occurs, it could cause our stock price to fall and may expose us to lawsuits that, even if unsuccessful, could
be costly to defend and a distraction to management.
Future
sales and issuances of our common stock or equity-linked securities could result in additional dilution of the percentage ownership
of our stockholders and could cause our share price to fall.
Following
this offering we expect that significant additional capital will be needed in the future to continue our planned operations, including
increased marketing, hiring new personnel, commercializing our product, and continuing activities as an operating public company. To
the extent we raise additional capital by issuing common stock, or securities convertible into or exchangeable or exercisable for
shares of common stock, our stockholders, including investors who purchase shares of common stock or pre-funded warrants in this
offering, may experience substantial dilution. We may sell common stock, convertible securities or other equity securities in one
or more transactions at prices and in a manner we determine from time to time. We cannot assure you that we will be able to sell shares
or other securities in any other offering at a price per share that is equal to or greater than the price per share paid by investors
in this offering. If we sell common stock, convertible securities or other equity securities in more than one transaction, investors
may be materially diluted by subsequent sales. Such sales may also result in material dilution to our stockholders, and new investors
could gain rights superior to our existing stockholders, including investors who purchase shares of common stock or pre-funded warrants
in this offering. Moreover, the perceived risk of this potential dilution could cause stockholders to attempt to sell their shares
and investors to short our common stock. These sales also may result in downward pressure on the price of our common stock and
make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable or
appropriate, and may cause you to lose the value of your investment.
The
warrants are speculative in nature.
The
warrants do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends,
but rather merely represent the right to acquire shares of common stock at a fixed price for a limited time. Moreover, following this
offering the market value of the warrants, if any, will be uncertain and there can be no assurance that the market value of the warrants
will equal or exceed their imputed offering price. The warrants will not be listed or quoted for trading on any market or exchange. There
can be no assurance that the market price of our common stock will ever equal or exceed the exercise price of the warrants, and consequently,
the warrants may expire valueless.
There
is no public market for the pre-funded warrants or warrants offered by us.
There
is no established public trading market for the pre-funded warrants or warrants and we do not expect such a market to develop. In addition,
we do not intend to apply to list the pre-funded warrants or warrants on any national securities exchange or other nationally recognized
trading system. Without an active trading market, the liquidity of the pre-funded warrants and warrants will be limited.
Holders
of the warrants and pre-funded warrants will have no rights as common stockholders until they acquire our common stock.
Until
holders of the warrants or pre-funded warrants acquire shares of our common stock upon exercise of the warrants or pre-funded warrants,
the holders will have no rights with respect to shares of our common stock issuable upon exercise of the warrants or pre-funded warrants.
Upon exercise of the warrants or pre-funded warrants, the holder will be entitled to exercise the rights of a common stockholder as to
the security exercised only as to matters for which the record date occurs after the exercise.
Our
management will have broad discretion over the use of the proceeds we receive in this offering and might not apply the proceeds in ways
that increase the value of your investment.
Our
management will have broad discretion in the application of the net proceeds from this public offering, including for any of the currently
intended purposes described in the section entitled “Use of Proceeds.” Because of the number and variability of factors that
will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended
use. Our management may not apply our cash from this offering in ways that ultimately increase the value of any investment in our securities
or enhance stockholder value. The failure by our management to apply these funds effectively could harm our business. Pending their use,
we may invest the net proceeds from this offering in short-term, investment-grade, interest-bearing securities. These investments may
not yield a favorable return to our stockholders. If we do not invest or apply our cash in ways that enhance stockholder value, we may
fail to achieve expected financial results, which may result in a decline in the price of our shares of common stock, and, therefore,
may negatively impact our ability to raise capital, invest in or expand our business, acquire additional products or licenses, commercialize
our product, or continue our operations.
Purchasers
who purchase our securities in this offering pursuant to a securities purchase agreement may have rights not available to purchasers
that purchase without the benefit of a securities purchase agreement.
In
addition to rights and remedies available to all purchasers in this offering under federal securities and state law, the purchasers that
enter into a securities purchase agreement will also be able to bring claims of breach of contract against us. The ability to pursue
a claim for breach of contract provides those investors with the means to enforce the covenants uniquely available to them under the
securities purchase agreement including: (i) timely delivery of shares; (ii) agreement to not enter into variable rate financings for
one year from closing, subject to certain exceptions; (iii) agreement to not enter into any financings for 60 days from closing;
and (iv) indemnification for breach of contract.
This
is a best efforts offering, with no minimum amount of securities is required to be sold, and we may not raise the amount of capital we
believe is required for our business plans, including our near-term business plans.
The
placement agent has agreed to use its reasonable best efforts to solicit offers to purchase the securities in this offering. The placement
agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar
amount of the securities. There is no required minimum number of securities that must be sold as a condition to completion of this offering.
Because there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, placement
agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth above.
We may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds received by us, and
investors in this offering will not receive a refund in the event that we do not sell an amount of securities sufficient to support our
continued operations, including our near-term continued operations. Thus, we may not raise the amount of capital we believe is required
for our operations in the short-term and may need to raise additional funds, which may not be available or available on terms acceptable
to us.
Because
there is no minimum required for the offering to close, investors in this offering will not receive a refund in the event that we do
not sell an amount of securities sufficient to pursue the business goals outlined in this prospectus.
We
have not specified a minimum offering amount nor have or will we establish an escrow account in connection with this offering. Because
there is no escrow account and no minimum offering amount, investors could be in a position where they have invested in our company,
but we are unable to fulfill our objectives due to a lack of interest in this offering. Further, because there is no escrow account in
operation and no minimum investment amount, any proceeds from the sale of securities offered by us will be available for our immediate
use, despite uncertainty about whether we would be able to use such funds to effectively implement our business plan. Investor funds
will not be returned under any circumstances whether during or after the offering.
There
can be no assurance that we will be able to comply with the continued listing standards of Nasdaq, a failure of which could result in
a delisting of our common stock and certain warrants.
Nasdaq
requires that the trading price of listed stock remain above $1.00 in order for the stock to remain listed. If a listed stock trades
below $1.00 for more than 30 consecutive trading days, then it is subject to delisting from the Nasdaq. In addition, to maintain a listing
on Nasdaq, we must satisfy minimum financial and other continued listing standards, including those regarding minimum stockholders’
equity, minimum publicly available shares, director independence and independent committee requirements and other corporate governance
requirements. We recently regained compliance with Nasdaq’s listing standards, and Nasdaq will continue to monitor our compliance
with its requirements including through a Nasdaq discretionary panel monitor until June 28, 2024. If we are unable to satisfy these standards,
we could be subject to delisting, which would have a negative effect on the price of our common stock, impair your ability to sell or
purchase our common stock or warrants when you wish to do so, and potentially cause you to lose the value of your investment in us. In
the event of a delisting, we would expect to take actions to restore our compliance with the listing standards, but we can provide no
assurance that any action we take to restore our compliance would allow our common stock to become listed again, stabilize the market
price or improve the liquidity of our common stock, prevent our common stock from dropping below the minimum bid price requirement, or
prevent future noncompliance with the listing requirements.
If
the Company is delisted from Nasdaq, its common stock may be eligible for trading on an over-the-counter market. If the Company is not
able to obtain a listing on another stock exchange or quotation service for its common stock, it may be extremely difficult or impossible
for stockholders to sell their shares of common stock. Moreover, if the Company is delisted from Nasdaq, but obtains a substitute listing
for its common stock, it will likely be on a market with less liquidity, and therefore experience potentially more price volatility than
experienced on Nasdaq. Stockholders may not be able to sell their shares of common stock on any such substitute market in the quantities,
at the times, or at the prices that could potentially be available on a more liquid trading market. As a result of these factors, if
the Company’s common stock is delisted from Nasdaq, the value and liquidity of the Company’s common stock would likely be
significantly adversely affected. A delisting of the Company’s common stock from Nasdaq could also adversely affect the Company’s
ability to obtain financing for its operations and/or result in a loss of confidence by investors, employees and/or business partners.
We do not intend
to pay cash dividends on our shares of common stock so any returns will be limited to the value of our shares.
We currently anticipate
that we will retain future earnings, if any, for the development, operation and expansion of our business and do not anticipate declaring
or paying any cash dividends for the foreseeable future. Any return to stockholders will therefore be limited to the increase, if any,
of our share price.
The
right of our President and Chief Executive Officer and Chief Financial Officer to participate in future financings of ours could impair
our ability to raise capital.
Jeffrey
Frelick, our President and Chief Executive Officer, and Deina Walsh, our Chief Financial Officer, hold contractual preemptive rights
which allow them to participate, at their option, in all future financings up to an amount necessary to maintain their percentage interest
in our common stock. The existence of such preemptive rights, or the exercise of such rights, may deter potential investors from providing
us needed financing, or may deter investment banks from working with us. This may have a material adverse effect on our ability to raise
capital which, in turn, could have a material adverse effect on our business prospects.
If
our shares of common stock become subject to the penny stock rules, it would become more difficult to trade our shares.
The
SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally
equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized
for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions
in such securities is provided by the exchange or system. If we do not retain a listing on Nasdaq and if the price of our common stock
is less than $5.00, our common stock will be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction
in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information.
In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules,
a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive
(i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions
involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have
the effect of reducing the trading activity in the secondary market for our common stock, and therefore stockholders may have difficulty
selling their shares.
Risks
Relating to Our Financial Position and Capital Needs
Our
limited operating history makes it difficult to evaluate our current business and future prospects.
We
have a limited operating history, and there is a risk that we will be unable to continue as a going concern. We have minimal assets and
no significant financial resources. Our limited operating history makes it difficult to evaluate our current business model and future
prospects. Accordingly, you should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered
by companies in the early stages of development. Potential investors should carefully consider the risks and uncertainties that a company
with a limited operating history will face. In particular, potential investors should consider that there is a significant risk that
we will not be able to, among other things:
| |
● |
implement
or execute our current business plan, which may or may not be sound; |
| |
|
|
| |
● |
maintain
our anticipated management and advisory team; |
| |
|
|
| |
● |
raise
sufficient funds in the capital markets to effectuate our business plan; and |
| |
|
|
| |
● |
utilize
the funds that we do have and/or raise in the future to efficiently execute our business strategy. |
If
we cannot execute any one of the foregoing or similar matters relating to our business, the business may fail, in which case you would
lose the entire amount of your investment in us.
Our
long-term capital requirements are subject to numerous risks.
We
anticipate that we will require approximately $5 million to complete first-in-man studies, and an estimated additional $24 million in
scientific expenses to achieve FDA approval, if possible, for a spine interbody fusion indication. These amounts are estimates
based on data currently available to us, and are subject to many factors, including the risk factors discussed herein. We anticipate
we will need to raise substantial additional funds for the pivotal clinical trial prior to marketing our first product. The above estimates
and our long-term capital requirements will depend on many factors, including, among others:
| |
● |
the
number of potential formulations, products and technologies in development; |
| |
|
|
| |
● |
continued
progress and cost of our research and development programs; |
| |
|
|
| |
● |
progress
with pre-clinical studies and clinical trials; |
| |
|
|
| |
● |
time
and costs involved in obtaining regulatory (including FDA) clearance; |
| |
|
|
| |
● |
costs
involved in preparing, filing, prosecuting, maintaining and enforcing patent claims; |
| |
● |
costs
of developing sales, marketing and distribution channels and our ability to sell our formulations or products; |
| |
|
|
| |
● |
costs
involved in establishing manufacturing capabilities for commercial quantities of our products; |
| |
|
|
| |
● |
competing
technological and market developments; |
| |
|
|
| |
● |
market
acceptance of our device formulations or products; |
| |
|
|
| |
● |
costs
for recruiting and retaining employees and consultants; |
| |
|
|
| |
● |
costs
for training physicians; |
| |
|
|
| |
● |
legal,
accounting and other professional costs; and |
| |
|
|
| |
● |
the
effect of the novel coronavirus will have on our product development, clinical trials, and availability, cost, and type of financing. |
We
may consume available resources more rapidly than currently anticipated, resulting in the need for additional funding. We may seek to
raise any necessary additional funds through equity or debt financings, collaborative arrangements with corporate partners or other sources,
which may be dilutive to existing stockholders or otherwise have a material effect on our current or future business prospects. If adequate
funds are not available, we may be required to significantly reduce or refocus our development and commercialization efforts with regard
to our delivery technologies and our proposed formulations and products.
We
have incurred losses since inception and we expect our operating expenses to increase in the foreseeable future, which may make it more
difficult for us to achieve and maintain profitability.
We
have no significant operating history and since inception to December 31, 2023 have incurred accumulated losses of approximately
$80.9 million. We will continue to incur significant expenses for development activities for our lead product candidate NELL-1/DBM.
We
will continue to attempt to raise additional capital through debt and/or equity financing to provide additional working capital and fund
future operations. However, there is no assurance that such financing will be consummated or obtained in sufficient amounts necessary
to meet our needs. If cash resources are insufficient to satisfy our on-going cash requirements, we will be required to scale back or
discontinue our product development programs, or obtain funds if available (although there can be no certainties) through strategic alliances
that may require us to relinquish rights to our technology, or substantially reduce or discontinue our operations entirely. No assurance
can be given that any future financing will be available or, if available, that it will be on terms that are satisfactory to us. Even
if we are able to obtain additional financing, it may contain undue restrictions on our operations, in the case of debt financing, or
cause substantial dilution for our stockholders, in the case of equity financing. As a result, we can provide no assurance as to whether
or if we will ever be profitable. If we are not able to achieve and maintain profitability, the value of our company and our common stock
could decline significantly.
We
face a number of risks associated with the incurrence of substantial debt which could adversely affect our financial condition.
If
we incur a substantial amount of debt, we may be required to use a significant portion of any cash flow to pay principal and interest
on the debt, which will reduce the amount available to fund working capital, capital expenditures, and other general purposes. Any indebtedness
may negatively impact our ability to operate our business and limit our ability to borrow additional funds by increasing our borrowing
costs, and impact the terms, conditions, and restrictions contained in possible future debt agreements, including the addition of more
restrictive covenants; impact our flexibility in planning for and reacting to changes in our business as covenants and restrictions contained
in possible future debt arrangements may require that we meet certain financial tests and place restrictions on the incurrence of additional
indebtedness and place us at a disadvantage compared to similar companies in our industry that have less debt.
USE
OF PROCEEDS
We
estimate that the net proceeds from this offering will be approximately $1.5 million after deducting estimated placement agent
fees and estimated offering expenses payable by us and assuming full exercise of the pre-funded warrants and no exercise of the warrants.
We intend to use the net proceeds from this offering in the following order of priority: to fund clinical trials, maintain
and extend our patent portfolio, and for working capital and other general corporate purposes. The actual allocation of proceeds realized
from this offering will depend upon how many securities are sold in this best efforts offering and upon our cash position and working
capital requirements. Therefore, as of the date of this prospectus, we cannot specify with certainty all of the particular uses for the
net proceeds to be received upon the completion of this offering. Accordingly, we will have discretion in the application of the net
proceeds, and investors will be relying on our judgment regarding the application of the proceeds of this offering. Pending our use of
the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including
short-term, investment-grade, interest-bearing instruments and U.S. government securities.
CAPITALIZATION
The
following table sets forth our capitalization as of December 31, 2023 as follows:
| |
● |
on
an actual basis; and |
| |
● |
on
a pro forma basis to reflect the issuance and sale by us of 119,000 shares of common stock and accompanying warrants at a public
offering price of $2.56 per share and accompanying warrant and pre-funded warrants to purchase 662,251 shares of common stock at
a public offering price of $2.559 per pre-funded warrant and accompanying warrant in this offering, and after deducting placement
agent fees and estimated offering expenses payable by us, and assuming all of the pre-funded warrants in this offering are
exercised, no exercise of warrants being offered in this offering, that no value is attributed to such warrants and that such warrants
are classified as and accounted for as equity. |
The
information below is illustrative only. You should read this
table in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and
the financial statements and related notes included in the 2023 Form 10-K and subsequent quarterly and annual reports.
| | |
As of December 31, 2023 |
| | |
Actual | | |
Pro Forma | |
| Cash | |
$ | 3,026,569 | | |
$ | 4,557,034 | |
| | |
| | | |
| | |
| Total Liabilities | |
| 831,402 | | |
| 831,402 | |
| | |
| | | |
| | |
| Stockholders’ Equity | |
| | | |
| | |
| Preferred Stock, $0.001 par value per share; 20,000,000 shares authorized; none issued or outstanding
at December 31, 2023 | |
| — | | |
| — | |
| Common stock, $0.001 par value per share; 100,000,000 shares authorized; 534,238
shares issued and outstanding at December 31, 2023; 1,315,489 shares issued and outstanding on a pro forma basis | |
| 534 | | |
| 1,315 | |
| Additional paid in capital | |
| 83,814,785 | | |
| 85,344,469 | |
| Accumulated Deficit | |
| (80,908,958 | ) | |
| (80,908,958 | ) |
| Total stockholders’ equity | |
| 2,906,361 | | |
| 4,436,826 | |
| Total capitalization | |
$ | 3,737,763 | | |
$ | 5,268,228 | |
The
number of shares of our common stock outstanding set forth in the table above excludes, as of December 31, 2023:
| |
● |
34,310
shares of common stock issuable upon exercise of stock options outstanding; |
| |
● |
197,844
shares of common stock issuable upon exercise of outstanding common stock warrants; |
| |
● |
595,179
shares of common stock reserved for future grants pursuant to our 2015 Equity Incentive Plan; |
| |
● |
781,251
shares of our common stock issuable upon the
exercise of the warrants to be issued in this offering; and |
| |
● |
46,875
shares of our common stock issuable upon the
exercise of the placement agent warrants to be issued in this offering. |
DESCRIPTION
OF SECURITIES
The
following description of our capital stock and provisions of our Amended and Restated Certificate of Incorporation and Amended
and Restated Bylaws is only a summary. You should also refer to our Amended and Restated Certificate of Incorporation, a copy
of which is filed as an exhibit to the registration statement of which this prospectus is a part, and our Amended and Restated
Bylaws, a copy of which is filed as an exhibit to the registration statement of which this prospectus is a part.
General
Our
Amended and Restated Certificate of Incorporation, as amended (“Certificate of Incorporation”), authorizes the issuance of
up to 100,000,000 shares of common stock, par value $0.001 per share, and up to 20,000,000 shares of preferred stock, par value $0.001
per share. As of March 4, 2024, there were 534,238 shares of common stock outstanding, which were held by approximately 22 stockholders
of record, and no shares of preferred stock outstanding.
Common
Stock
Each
holder of common stock is entitled to one vote for each share held on all matters submitted to a vote of stockholders and do not have
cumulative voting rights. An election of directors by our stockholders will be determined by a plurality of the votes cast by the stockholders
entitled to vote on the election. All other actions by stockholders will be approved by the majority of the votes cast affirmatively
or negatively (excluding abstentions and broker non-votes) except as otherwise required by law.
Holders
of common stock are entitled to receive proportionately any dividends that may be declared by our Board of Directors, subject to any
preferential dividend rights of any series of preferred stock that we may designate and issue. In the event of our liquidation or dissolution,
the holders of common stock are entitled to receive proportionately our net assets available for distribution to stockholders after the
payment of all debts and other liabilities and subject to the preferential rights of any outstanding preferred stock.
Holders
of our common stock have no preemptive, subscription, redemption, or conversion rights. The rights, preferences, and privileges of holders
of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock
that we may designate and issue.
Preferred
Stock
Under
our Certificate of Incorporation, our Board of Directors has the authority, without further action by stockholders, to designate one
or more series of preferred stock and to fix the voting powers, designations, preferences, limitations, restrictions, and relative rights
granted to or imposed upon the preferred stock, including dividend rights, conversion rights, voting rights, rights and terms of redemption,
liquidation preference, and sinking fund terms, any or all of which may be preferential to or greater than the rights of the common stock.
The
authority possessed by our Board of Directors to issue preferred stock could potentially be used to discourage attempts by third parties
to obtain control of our company through a merger, tender offer, proxy contest, or otherwise by making such attempts more difficult or
more costly. Our Board of Directors may issue preferred stock with voting rights, conversion rights, and other rights that, if exercised,
could adversely affect the voting power of the holders of common stock.
Warrants
The
following summary of certain terms and provisions of the warrants that are being offered hereby is not complete and is subject to, and
qualified in its entirety by, the provisions of warrants, the forms of which are filed as exhibits to the registration statement of which
this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the forms of warrant for a complete
description of the terms and conditions of the warrants.
Duration
and Exercise Price. The warrants will have an exercise price of $2.43 per share and will be exercisable upon issuance (the “Initial Exercise Date”).
The warrants will expire on the five-year anniversary of the Initial Exercise Date. The exercise price and number of shares of common
stock issuable upon exercise of the warrants is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations
or similar events affecting our common stock and the exercise price. The warrants will be issued separately from the common stock and
pre-funded warrants and may be transferred separately immediately thereafter. The warrants will be issued in certificated form only.
Exercise
Limitation. The warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed
exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the
case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of such holder’s
warrants to the extent that the holder would own more than 4.99% of the outstanding common stock immediately after exercise, except that
upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock
after exercising the holder’s warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving
effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants.
Cashless
Exercise. If, at the time a holder exercises its warrants, a registration statement registering the issuance or resale of the shares
of common stock underlying the warrants under the Securities Act is not then effective or available for the issuance of such shares,
then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise
price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock
determined according to a formula set forth in the warrant.
Fractional
Shares. No fractional shares of common stock will be issued upon the exercise of the warrants. Rather, the number of shares of common
stock to be issued will, at our election, either be rounded up to the next whole share or we will pay a cash adjustment in respect of
such final fraction in an amount equal to such fraction multiplied by the exercise price.
Transferability.
Subject to applicable laws, a warrant may be transferred at the option of the holder upon surrender of the warrant to us together
with the appropriate instruments of transfer.
Trading
Market. There is no established trading market for the warrants, and we do not expect such a market to develop. We do not intend
to apply to list the warrants on any securities exchange or other nationally recognized trading system. Without an active trading market,
the liquidity of the warrants will be extremely limited.
Fundamental
Transactions. In the event of a fundamental transaction, as described in the warrants and generally including any reorganization,
recapitalization or reclassification of our shares of common stock, the sale, transfer or other disposition of all or substantially all
of our properties or assets, our consolidation or merger with or into another person, the acquisition of 50% or more of the voting power
represented by our outstanding shares of capital stock, any person or group becoming the beneficial owner of 50% or more of the voting
power represented by our outstanding shares of capital stock, any merger with or into another entity or a tender offer or exchange offer
approved by more than 50% of the voting power represented by our outstanding shares of capital, then upon any subsequent exercise of
a warrant, the holder will have the right to receive as alternative consideration, for each share of our common stock that would have
been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of common
stock of the successor or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration
receivable upon or as a result of such transaction by a holder of the number of shares of our common stock for which the warrant is exercisable
immediately prior to such event. Notwithstanding the foregoing, in the event of a fundamental transaction, the holders of the warrants
have the right to require us or a successor entity to redeem the warrants for cash in the amount of the Black-Scholes Value (as defined
in each warrant) of the unexercised portion of the warrants concurrently with or within 30 days following the consummation of a fundamental
transaction. However, in the event of a fundamental transaction which is not in our control, including a fundamental transaction not
approved by our Board, the holders of the warrants will only be entitled to receive from us or our successor entity, as of the date of
consummation of such fundamental transaction the same type or form of consideration (and in the same proportion), at the Black Scholes
Value of the unexercised portion of the warrant that is being offered and paid to the holders of our common stock in connection with
the fundamental transaction, whether that consideration is in the form of cash, stock or any combination of cash and stock, or whether
the holders of our common stock are given the choice to receive alternative forms of consideration in connection with the fundamental
transaction.
Right
as a Stockholder. Except as otherwise provided in the warrants or by virtue of such holder’s ownership of shares of our common
stock, the holders of the warrants do not have the rights or privileges of holders of our common stock, including any voting rights,
until they exercise their warrants.
Pre-Funded
Warrants
The
following summary of certain terms and provisions of the pre-funded warrants that are being offered hereby is not complete and is subject
to, and qualified in its entirety by, the provisions of the pre-funded warrant, the form of which will be filed as an exhibit to the
registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions
of the form of pre-funded warrant for a complete description of the terms and conditions of the pre-funded warrants.
Duration
and Exercise Price. Each pre-funded warrant offered hereby will have an initial exercise price per share of common stock equal to
$0.001. The pre-funded warrants will be immediately exercisable and may be exercised at any time until all of the pre-funded warrants
are exercised in full. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment
in the event of share dividends, share splits, reorganizations or similar events affecting our shares of common stock and the exercise
price.
Exercise
Limitation. The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a
duly executed exercise notice accompanied by payment in full for the number of shares of common stock purchased upon such exercise (except
in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of the pre-funded
warrant to the extent that the holder would own more than 4.99% of the outstanding shares of common stock immediately after exercise,
except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of beneficial ownership
of outstanding shares after exercising the holder’s pre-funded warrants up to 9.99% of the number of our shares of common stock
outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms
of the pre-funded warrants. Purchasers of pre-funded warrants in this offering may also elect prior to the issuance of the pre-funded
warrants to have the initial exercise limitation set at 9.99% of our outstanding shares of common stock.
Cashless
Exercise. In lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate
exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common
stock determined according to a formula set forth in the pre-funded warrants.
Fractional
Shares. No fractional shares of common stock will be issued upon the exercise of the pre-funded warrants. Rather, at the Company’s
election, the number of shares of common stock to be issued will be rounded up to the next whole share or the Company will pay a cash
adjustment in an amount equal to such fraction multiplied by the exercise price.
Transferability.
Subject to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded
warrants to us together with the appropriate instruments of transfer.
Trading
Market. There is no established trading market for the pre-funded warrants, and we do not expect such a market to develop. We do
not intend to apply to list the pre-funded warrants on any securities exchange or other nationally recognized trading system. Without
an active trading market, the liquidity of the pre-funded warrants will be extremely limited.
Fundamental
Transaction. In the event of a fundamental transaction, as described in the pre-funded warrants and generally including any reorganization,
recapitalization or reclassification of our shares of common stock, the sale, transfer or other disposition of all or substantially all
of our properties or assets, our consolidation or merger with or into another person, the acquisition of 50% or more of the voting power
represented by our outstanding shares of capital stock, any person or group becoming the beneficial owner of 50% or more of the voting
power represented by our outstanding shares of capital stock, any merger with or into another entity or a tender offer or exchange offer
approved by more than 50% of the voting power represented by our outstanding shares of capital, then upon any subsequent exercise of
a pre-funded warrant, the holder will have the right to receive as alternative consideration, for each share of our common stock that
would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares
of common stock of the successor or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration
receivable upon or as a result of such transaction by a holder of the number of shares of our common stock for which the pre-funded warrant
is exercisable immediately prior to such event.
Right
as a Stockholder. Except as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of shares
of common stock, the holders of the pre-funded warrants do not have the rights or privileges of holders of our shares of common stock,
including any voting rights, until they exercise their pre-funded warrants. The pre-funded warrants will provide that the holders of
the pre-funded warrants have the right to participate in distributions or dividends paid on our shares of common stock.
Placement
Agent Warrants
We
have also agreed to issue to the placement agent or its designees as compensation in connection with this offering, the placement agent
warrants to purchase 46,875 shares of common stock at an exercise price of $3.20 per share (representing 125% of the offering
price per share and accompanying warrant). The placement agent warrants will expire five years from the commencement of sales in this
offering. Except as provided above, the placement agent warrants will have substantially the same terms as the warrants described herein.
See “Plan of Distribution–Placement Agent Warrants.”
Anti-Takeover
Effects of Our Certificate of Incorporation and Bylaws
Certain
provisions of our Certificate of Incorporation and Bylaws contain provisions that could have the effect of delaying or discouraging another
party from acquiring control of us. These provisions, which are summarized below, are expected to discourage certain types of coercive
takeover practices and inadequate takeover bids.
Our
Certificate of Incorporation and Bylaws include provisions that:
| ● | authorize
our Board of Directors to issue, without further action by the stockholders, up to 20,000,000
shares of preferred stock in one or more series designated by the Board of Directors; |
| ● | specify
that meetings of our stockholders can be called only by our Board of Directors, or any officer
instructed by the director to call the meeting; and |
| ● | provide
that vacancies on our Board of Directors may be filled only by the vote of a majority of
the remaining directors even though less than a quorum. |
Our
Bylaws also provide that stockholders seeking to bring business before our annual meeting of stockholders, or to nominate candidates
for election as directors at our annual meeting of stockholders, must provide timely notice of their intent in writing. To be timely,
a stockholder’s notice must be delivered to the secretary at our principal executive offices not later than the close of business
on the 90th day nor earlier than the close of business on the 120th day prior to the first anniversary of the preceding
year’s annual meeting; provided, however, that in the event the date of the annual meeting is more than 30 days before or more
than 60 days after such anniversary date, or if no annual meeting was held in the preceding year, notice by the stockholder to be timely
must be so delivered not earlier than the close of business on the 120th day prior to such annual meeting and not later than
the close of business on the later of the 90th day prior to such annual meeting or the 10th day following the day
on which a public announcement of the date of such meeting is first made by us. These provisions may preclude our stockholders from bringing
matters before our annual meeting of stockholders or from making nominations for directors at our annual meeting of stockholders.
Delaware
Anti-Takeover Statute
We
are subject to the provisions of Section 203 of the Delaware General Corporation Law (“DGCL”) regulating corporate takeovers.
In general, Section 203 prohibits a publicly-held Delaware corporation such as Bone Biologics Corp. from engaging in a “business
combination” with an “interested stockholder” for a period of three years following the date the person became an interested
stockholder unless:
| ● | prior
to the date of the transaction, the Board of Directors of the corporation approved either
the business combination or the transaction which resulted in the stockholder becoming an
interested stockholder; |
| ● | upon
completion of the transaction that resulted in the stockholder becoming an interested stockholder,
the interested stockholder owned at least 85% of the voting stock of the corporation outstanding
at the time the transaction commenced, excluding for purposes of determining the voting stock
outstanding, but not for determining the outstanding voting stock owned by the interested
stockholder, (1) shares owned by persons who are directors and also officers of the corporation
and (2) shares owned by employee stock plans in which employee participants do not have the
right to determine confidentially whether shares held subject to the plan will be tendered
in a tender or exchange offer; or |
| ● | at
or subsequent to the date of the transaction, the business combination is approved by the
Board of Directors of the corporation and authorized at an annual or special meeting of stockholders,
and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding
voting stock which is not owned by the interested stockholder. |
In
this context, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial
benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates,
owns or, within three years prior to the determination of interested stockholder status, did own 15% or more of a corporation’s
outstanding voting stock. We expect the existence of this provision to have an anti-takeover effect with respect to transactions our
Board of Directors does not approve in advance. We also anticipate that Section 203 may discourage business combinations or other attempts
that might result in a premium over the market price for the shares of common stock held by our stockholders.
Transfer
Agent and Registrar
The
transfer agent and registrar for our common stock is Equiniti Trust Company, LLC.
Stock
Market Listing
Our
common stock is listed on Nasdaq under the symbol “BBLG.”
PLAN
OF DISTRIBUTION
Pursuant
to an engagement agreement, dated October 30, 2023, we have engaged H.C. Wainwright & Co., LLC, or the placement agent, to act as our exclusive placement
agent to solicit offers to purchase the securities offered pursuant to this prospectus on a best efforts basis. The engagement agreement
does not give rise to any commitment by the placement agent to purchase any of our securities, and the placement agent will have no authority
to bind us by virtue of the engagement agreement. The placement agent is not purchasing or selling any of the securities offered by us
under this prospectus, nor is it required to arrange for the purchase or sale of any specific number or dollar amount of securities.
This is a best efforts offering and there is no minimum offering amount required as a condition to the closing of this offering. The
placement agent has agreed to use reasonable best efforts to arrange for the sale of the securities by us. Therefore, we may not sell
all of the shares of common stock, pre-funded warrants and warrants being offered. The terms of this offering are subject to market conditions
and negotiations between us, the placement agent and prospective investors. The placement agent does not guarantee that it will be able
to raise new capital in any prospective offering. The placement agent may engage sub-agents or selected dealers to assist with the offering.
Investors
purchasing securities offered hereby will have the option to execute a securities purchase agreement with us. In addition to rights
and remedies available to all purchasers in this offering under federal securities and state law, the purchasers which enter into a
securities purchase agreement will also be able to bring claims of breach of contract against us. The ability to pursue a claim for
breach of contract provides those investors with the means to enforce the covenants uniquely available to them under the securities
purchase agreement including: (i) timely delivery of shares; (ii) agreement to not enter into variable rate financings for one
year from closing, subject to certain exceptions; (iii) agreement to not enter into any financings for 60 days from
closing; and (iv) indemnification for breach of contract.. The nature of the representations, warranties and covenants in the
securities purchase agreements shall include:
| ● | standard
issuer representations and warranties on matters such as organization, qualification, authorization,
no conflict, no governmental filings required, current in SEC filings, no litigation, labor
or other compliance issues, environmental, intellectual property and title matters and compliance
with various laws such as the Foreign Corrupt Practices Act; and |
| ● | covenants
regarding matters such as registration of warrant shares, no integration with other offerings,
no stockholder rights plans, no material nonpublic information, use of proceeds, indemnification
of purchasers, reservation and listing of shares of common stock, and no subsequent equity
sales for 60 days. |
We
will deliver the securities being issued to the investors upon receipt of investor funds for the purchase of the securities offered pursuant
to this prospectus. We expect to deliver the securities being offered pursuant to this prospectus on or about March 6, 2024. There
is no minimum number of securities or amount of proceeds that is a condition to closing of this offering.
Fees
and Expenses
We
have agreed to pay the placement agent a total cash fee equal to 7.0% of the aggregate gross proceeds raised in the offering. We have
also agreed to pay the placement agent a management fee of 1.0% of the aggregate gross proceeds raised in this offering and to reimburse
the placement agent a nonaccountable expense allowance of $10,000, its legal fees and expenses in an amount up to $100,000 and
its clearing expense in an amount up to $15,950 in connection with this offering. We estimate the total offering expenses of this offering
that will be payable by us, excluding the placement agent fees and expenses, will be approximately $183,587.60.
Placement
Agent Warrants
In
addition, we have agreed to issue to the placement agent or its designees warrants, or the placement agent warrants, to purchase up to
6.0% of the aggregate number of shares of common stock sold in this offering (including shares underlying any pre-funded warrants), at
an exercise price equal to 125% of the public offering price per share and accompanying warrant to be sold in this offering. The placement
agent warrants will be exercisable upon issuance and will expire five years from the commencement of sales under this offering.
If
at the time of exercise there is no effective registration statement registering, or the prospectus contained therein is not available
for the resale of warrant shares by the holders of the placement agent warrants, then the placement agent warrants may be exercised,
in whole or in part, at such time by means of a “cashless exercise” in which the holders shall be entitled to receive a number
of warrant shares as calculated in the placement agent warrants.
The
placement agent warrants provide for customary anti-dilution provisions (for share dividends, splits and recapitalizations and the like)
consistent with FINRA Rule 5110.
Tail
In
the event that any investors that contacted by the placement agent during the term of our engagement agreement with the placement agent
provide any capital to us in a public or private offering or capital-raising transaction within twelve (12) months following the termination
or expiration of our engagement agreement with the placement agent, we shall pay the placement agent the cash and warrant compensation
provided above on the gross proceeds from such investors. The placement agent will only be entitled to such fee to the extent that the
parties are directly introduced to us by the placement agent, in accordance with FINRA Rule 2010.
Right
of First Refusal
From
the date of the engagement agreement until the 12-month anniversary following consummation of each offering of our securities during
the term of the engagement agreement, subject to compliance with FINRA Rule 5110 (g)(6)(A), if we or any of our subsidiaries
(a) engage in certain transactions, including but not limited to, disposition or acquisition of outstanding securities, tender offers,
mergers, consolidations or other business combinations as set forth in the engagement letter, the placement agent (or any affiliate designated
by the placement agent) shall have the right to act as the Company’s exclusive financial advisor for any such transaction, (b)
decides to finance or refinance any indebtedness, the placement agent (or any affiliates designed by the placement agent), shall have
the right to act as sole book-runner, sole manager, sole placement agent or sole agent with respect to such financing or refinancing
or (c) decides to raise funds by means of a public offering (excluding an at-the-market facility) or a private placement or any other
capital-raising financing of equity, equity-linked or debt securities, the placement agent (or any affiliate designated by the placement
agent) shall have the right to act as sole book-running manager, sole underwriter or sole placement agent for such financing.
Lock-Up
Agreements
Our
officers and directors have agreed with the placement agent to be subject to a lock-up period of 60 days following the
closing of this offering. This means that, during the applicable lock-up period, such persons may not offer for sale, contract to
sell, sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or
indirectly, any shares of our common stock or any securities convertible into, or exercisable or exchangeable for, shares of our
common stock. Certain limited transfers are permitted during the lock-up period if the transferee agrees to these lock-up
restrictions. We have also agreed to similar lock-up restrictions on the issuance and sale of our securities for 60 days
following the closing of this offering, subject to certain exceptions. The placement agent may, in its sole discretion and without
notice, waive the terms of any of these lock-up agreements.
In
addition, subject to certain exceptions, we have agreed to not issue any securities that are subject to a price reset based on the trading
prices of our common stock or upon a specified or contingent event in the future, or enter into any agreement to issue securities at
a future determined price for a period of one year following the closing date of this offering.
Indemnification
We
have agreed to indemnify the placement agent against certain liabilities, including certain liabilities under the Securities Act, or
to contribute to payments that the placement agent may be required to make in respect of those liabilities.
In
addition, we will indemnify the purchasers of securities in this offering against liabilities arising out of or relating to (i) any breach
of any of the representations, warranties, covenants or agreements made by us in the securities purchase agreement or related documents
or (ii) any action instituted against a purchaser by a third party (other than a third party who is affiliated with such purchaser) with
respect to the securities purchase agreement or related documents and the transactions contemplated thereby, subject to certain exceptions
Regulation
M Compliance
The
placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any fees received
by it and any profit realized on the sale of our securities offered hereby by it while acting as principal might be deemed to be underwriting
discounts or commissions under the Securities Act. The placement agent will be required to comply with the requirements of the Securities
Act and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations
may limit the timing of purchases and sales of our securities by the placement agent. Under these rules and regulations, the placement
agent may not (i) engage in any stabilization activity in connection with our securities; and (ii) bid for or purchase any of our securities
or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until they have completed
their participation in the distribution.
Other
Relationships
The
placement agent and its affiliates have engaged, and may in the future engage, in investment banking transactions and other commercial
dealings in the ordinary course of business with us or our affiliates. The placement agent has received, or may in the future receive,
customary fees and commissions for these transactions.
In
addition, in the ordinary course of their business activities, the placement agent and its affiliates may make or hold a broad array
of investments and actively trade debt and equity securities (or related derivative securities) for their own account and for the accounts
of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The
placement agent and its affiliates may also make investment recommendations and/or publish or express independent research views in respect
of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such
securities and instruments.
The placement agent acted as the placement agent in connection with the November Offering and it received compensation
for each such offering.
Electronic
Distribution
A
prospectus in electronic format may be made available on a website maintained by the placement agent and the placement agent may distribute
prospectuses electronically. Other than the prospectus in electronic format, the information on these websites is not part of this prospectus
or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the placement agent
and should not be relied upon by investors.
Transfer
Agent and Registrar
The
transfer agent and registrar for our common stock is Equiniti Trust Company, LLC.
Stock
Market Listing
Our
common stock is listed on Nasdaq under the symbol “BBLG.”
LEGAL
MATTERS
The
validity of the issuance of the common stock offered by us in this offering will be passed upon for us Harter Secrest & Emery LLP,
Rochester, NY. Certain legal matters in connection with this offering will be passed upon for the placement agent by Lowenstein Sandler LLP, New York, NY.
EXPERTS
The consolidated financial statements of Bone
Biologics Corporation appearing in Bone Biologics Corporation’s Annual Report (Form 10-K) for the year ended December 31,
2023, have been audited by Weinberg & Company, P.A., as set forth in their report therein, which includes an explanatory
paragraph as to the Company’s ability to continue as a going concern, and incorporated herein by reference. Such
consolidated financial statements are incorporated herein by reference in reliance upon such report given
on the authority of such firm as experts in accounting and auditing.
INFORMATION INCORPORATED BY REFERENCE
The SEC allows us to “incorporate by reference”
information into this document, which means that we can disclose important information to you by referring you to another document filed
separately with the SEC. The information incorporated by reference is an important part of this prospectus, and information that we file
later with the SEC will automatically update and supersede this information.
We incorporate by reference the documents listed
below and any future filings made with the SEC under Sections 13(a), 14, or 15(d) of the Exchange Act made subsequent to the date of
this prospectus until the termination of the offering of the securities described in this prospectus (other than information in such
filings that was “furnished,” under applicable SEC rules, rather than “filed”). We incorporate by reference the
following documents or information that we have filed with the SEC:
| |
● |
our Annual Report on Form
10-K for the year ended December 31, 2023, filed with the SEC on February 21, 2024; |
| |
|
|
| |
● |
our Current Reports on Form 8-K filed with the SEC on January
2, 2024, January
10, 2024, January
11, 2024, January
12, 2024 and March
1, 2024; and |
| |
|
|
| |
● |
The description of the Common Stock incorporated by reference to
our Registration Statement on Form
8-A that was filed with the SEC on October 8, 2021, Exhibit
4.5 to Amendment No. 1 to our Annual Report for the fiscal year ended December 31, 2022 on Form 10-K/A filed with the SEC on
November 20, 2023, and any amendment or report filed for the purpose of updating such description. |
To obtain copies of these filings, see “Where
You Can Find More Information” in this prospectus. Nothing in this prospectus shall be deemed to incorporate information furnished,
but not filed, with the SEC, including pursuant to Item 2.02 or Item 7.01 of Form 8-K and any corresponding information or exhibit furnished
under Item 9.01 of Form 8-K.
Information in this prospectus supersedes related
information in the documents listed above and information in subsequently filed documents supersedes related information in both this
prospectus and the incorporated documents.
WHERE
YOU CAN FIND MORE INFORMATION
We
are subject to the periodic reporting requirements of the Exchange Act, and we will file periodic reports, proxy statements and other
information with the SEC. These periodic reports, proxy statements and other information are available at www.sec.gov. We maintain a
website at www.bonebiologics.com. We have not incorporated by reference into this prospectus the information contained in, or that can
be accessed through, our website, and you should not consider it to be a part of this prospectus. You may access our Annual Reports on
Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to
Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material
is electronically filed with, or furnished to, the SEC. You may also request a copy of these filings (other than exhibits to these documents
unless the exhibits are specifically incorporated by reference into these documents or referred to in this prospectus), at no cost, by
writing us at 2 Burlington Woods Drive, Suite 100, Burlington, MA 01803 or contacting us at (781) 552-4452.
We
have filed with the SEC a registration statement under the Securities Act relating to the offering of these securities. The registration
statement, including the attached exhibits, contains additional relevant information about us and the securities. This prospectus does
not contain all of the information set forth in the registration statement. You may review a copy of the registration statement and any
documents incorporated by reference herein through the SEC’s website at www.sec.gov.
BONE
BIOLOGICS CORPORATION
119,000
Shares of Common Stock
662,251
Prefunded Warrants to purchase 662,251
Shares of Common Stock
662,251 Shares of Common Stock underlying
the Prefunded Warrants
781,251
Common Warrants to purchase 781,251
Shares of Common Stock
781,251 Shares
of Common Stock underlying the Common Warrants
46,875 Placement
Agent Warrants to Purchase 46,875 Shares of Common Stock
46,875
Shares of Common Stock Underlying the Placement
Agent Warrants
H.C.
Wainwright & Co.
Prospectus
March
4, 2024
Bone Biologics (NASDAQ:BBLG)
Historical Stock Chart
From Apr 2024 to May 2024
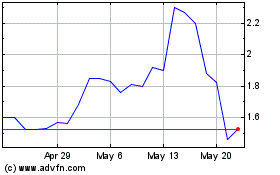
Bone Biologics (NASDAQ:BBLG)
Historical Stock Chart
From May 2023 to May 2024