false000182246200018224622024-03-072024-03-07
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
| | |
| ________________________________________________________________________________________________ |
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 7, 2024
| | |
| ________________________________________________________________________________________________ |
Foghorn Therapeutics Inc.
(Exact name of registrant as specified in its charter)
| | |
| ________________________________________________________________________________________________ |
| | | | | | | | | | | | | | |
| Delaware | | 001-39634 | | 47-5271393 |
| (State or other jurisdiction of incorporation) | | (Commission File Number) | | (IRS Employer Identification No.) |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
| 500 Technology Square, Ste 700 | | | |
| Cambridge, | MA | | 02139 | |
| (Address of principal executive offices) | | (Zip Code) | |
(Registrant’s telephone number, including area code): (617) 586-3100
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| | | | | |
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| | | | | |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| | | | | |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| | | | | |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
|
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Common Stock, $0.0001 par value per share | | FHTX | | The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On March 7, 2024, Foghorn Therapeutics Inc. (the “Company”) issued a press release announcing certain of the Company’s financial results for the year ended December 31, 2023. A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Item 2.02 (including Exhibit 99.1 attached hereto) is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any filing by the Company under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 7.01 Regulation FD Disclosure.
The Company is furnishing as Exhibit 99.2 to this Current Report on Form 8-K a presentation, dated March 7, 2024, which the Company intends to use in meetings with or presentations to investors.
The information in this Item 7.01 (including Exhibit 99.2 attached hereto) is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any filing by the Company under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | | | | |
| | | |
| FOGHORN THERAPEUTICS INC. |
| By: | | /s/ Michael LaCascia |
| | | Michael J. LaCascia |
| | | Chief Legal Officer |
| | | |
Date: March 7, 2024 Foghorn Therapeutics Provides Financial Update for 2023 and 2024 Strategic Outlook
•Dose escalation in FHD-286 combination study in AML continues to progress; clinical data anticipated in the second half of 2024
•FHD-909, a first-in-class BRM selective inhibitor, selected for clinical development by partner Lilly; preclinical data to be presented at AACR with IND planned for the second quarter, primary target patient population in non-small cell lung cancer
• Selective CBP and EP300 degrader preclinical data to be presented at AACR; IND-enabling studies for CBP degrader program planned to begin by end of 2024
•Foghorn anticipates at least six new INDs targeting significant oncology patient populations over the next four years, reflecting the continued productivity of its precision medicine platform
•Cash, cash equivalents, and marketable securities of $234.1 million, as of December 31, 2023, provide cash runway into the first half of 2026
CAMBRIDGE, Mass. -- (GLOBE NEWSWIRE) – March 7, 2024 -- Foghorn® Therapeutics Inc. (Nasdaq: FHTX), a clinical-stage biotechnology company pioneering a new class of medicines that treat serious diseases by correcting abnormal gene expression, today provided a financial update and corporate outlook in conjunction with the Company’s 10-K filing for the year ending December 31, 2023. With an initial focus in oncology, Foghorn’s Gene Traffic Control® Platform and resulting broad pipeline have the potential to transform the lives of people with a wide spectrum of diseases.
“The research and clinical advances we made in 2023 set the stage for Foghorn to deliver significant value with the potential for several differentiated, high-impact medicines in 2024 and beyond,” said Adrian Gottschalk, President and Chief Executive Officer of Foghorn. “In 2023 we initiated a combination study with FHD-286 in AML, with data anticipated in the second half of 2024. Based on the mutation-agnostic differentiation effect observed in our single-agent escalation study, we believe FHD-286 has the potential to be a first-in-class broad-based differentiation therapeutic in AML. We are also making progress with our selective BRM program with FHD-909, a first-in-class BRM selective inhibitor, selected for clinical development by partner Lilly. The IND is planned for the second quarter of 2024, with an initial focus in non-small cell lung cancer. Finally, we are excited by the preclinical efficacy and safety data for our CBP and EP300 selective degrader programs and target IND-enabling studies for CBP by the end of the year. Our cash position remains strong with runway into the first half of 2026.”
Key Recent Updates and Upcoming Milestones
•FHD-286. FHD-286 is a potent, selective inhibitor of the BRG1 and BRM subunits of the BAF chromatin remodeling complex where dependency on BRG1/BRM is well-established preclinically with multiple tumor types, including acute myelogenous leukemia (AML)/myelodysplastic syndrome (MDS), non-small cell lung cancer (NSCLC) and prostate cancer.
•AML Update. Foghorn commenced a Phase 1 study of FHD-286 in combination with decitabine or low-dose cytarabine (LDAC) in relapsed and/or refractory AML patients, with the first patient dosed during the third quarter of 2023. Dose escalation is ongoing, and the first clinical data are expected in the second half of 2024.
•TKI Resistance. Recently published data, along with Foghorn’s work, suggest that FHD-286 may play an important role in overcoming resistance in EGFR/KRAS tumors. The Company is conducting preclinical work to further explore the opportunity and expects data in the second quarter of 2024.
•Differentiated Pipeline Advancement. Foghorn continues to expand its platform and pipeline. The Company anticipates the potential for six new investigational new drug (IND) applications in the next four years. The Company continues to progress programs for multiple targets that include chromatin remodeling complexes, transcription factors, helicases, and other chromatin-related factors. These targets include selective BRM and wholly owned programs including CBP, EP300, and ARID1B, as well as other undisclosed targets, which combined could address more than 20 tumor types impacting more than 500,000 new patients annually.
•Selective CBP and Selective EP300 programs. Foghorn is presenting new preclinical data for its CBP and EP300 selective degrader programs at the 2024 AACR Annual Meeting, April 5-10, 2024.
•CBP selective degraders have shown significant tumor growth inhibition in a colorectal cancer in vivo model. Antiproliferative effects were also observed for numerous cancer cell lines, including colorectal, gastric and bladder cancers.
•EP300 selective degraders have shown potent cellular antiproliferation and in vivo tumor growth inhibition in an AR+ enzalutamide prostate in vivo model.
•At preclinical efficacious doses, neither the CBP nor the EP300 selective degraders cause thrombocytopenia, a commonly observed safety liability for dual CBP/EP300 inhibitors.
•Lilly Collaboration. Foghorn is engaged in a strategic collaboration with Lilly and continues to advance the BRM selective inhibitor and degrader programs along with other undisclosed programs.
◦In the first quarter of 2024, Lilly selected FHD-909, a first-in-class oral BRM selective inhibitor, for clinical development. Lilly plans to file an IND for
FHD-909 in the second quarter of 2024. The primary target patient population is BRG1-mutated NSCLC.
◦Selective BRM inhibition has been a sought-after objective in cancer research for many years. A variety of tumor types, including NSCLC, are known to have mutations in BRG1, which we believe make them dependent on BRM activity for their survival. Selective blocking of BRM activity is considered a promising strategy for causing tumor cell death while sparing healthy cells.
◦Preclinical data will be presented in 2024, including at AACR with a poster presentation on April 8.
In December 2021, Foghorn announced a strategic collaboration with Lilly to create novel oncology medicines. The collaboration includes a co-development and co-commercialization agreement for Foghorn’s Selective BRM oncology program and an additional undisclosed oncology target. In addition, the collaboration includes three discovery programs using Foghorn’s proprietary Gene Traffic Control platform.
Full Year 2023 Financial Highlights
•Collaboration Revenues. Collaboration revenues were $34.2 million for the year ended December 31, 2023, compared to $19.2 million for the year ended December 31, 2022. The increase year-over-year was primarily driven by revenue recognized under the Merck collaboration due to the termination of the agreement and the subsequent recognition of the remaining deferred revenue.
•Research and Development Expenses. Research and development expenses were $109.7 million for the year ended December 31, 2023, compared to $105.6 million for the year ended December 31, 2022. This increase was primarily due to costs associated with continued investment in R&D personnel, platform, and other early-stage research, partially offset by a decrease in spend on FHD-286 and FHD-609.
•General and Administrative Expenses. General and administrative expenses were $32.4 million for the year ended December 31, 2023, compared to $30.7 million for the year ended December 31, 2022. This increase was primarily due to an increase in investments to support the growing business, which included increases in personnel-related costs and stock-based compensation expense.
•Net Loss. Net loss was $98.4 million for the year ended December 31, 2023, compared to a net loss of $108.9 million for the year ended December 31, 2022.
•Cash, cash equivalents and marketable securities. As of December 31, 2023, the Company had $234.1 million in cash, cash equivalents and marketable securities, providing cash runway into the first half of 2026.
About FHD-286
FHD-286 is a highly potent, selective, allosteric, and orally available small-molecule, enzymatic inhibitor of BRG1 (SMARCA4) and BRM (SMARCA2), two highly similar proteins that are the ATPases, or the catalytic engines, of the BAF complex, one of the key regulators within the chromatin regulatory system. In preclinical studies, FHD-286 has shown anti-tumor activity across a broad range of malignancies including both hematologic and solid tumors.
About AML
Adult acute myeloid leukemia (AML) is a cancer of the blood and bone marrow and the most common type of acute leukemia in adults. AML is a diverse disease associated with multiple genetic mutations. It is diagnosed in about 20,000 people every year in the United States.
About FHD-909
FHD-909 (a.k.a. LY4050784) is a highly potent, allosteric and orally available small molecule that selectively inhibits the ATPase activity of BRM (SMARCA2) over its closely related paralog BRG1 (SMARCA4), two proteins that are the catalytic engines across all forms of the BAF complex, one of the key regulators of the chromatin regulatory system. In preclinical studies, tumors with mutations in BRG1 rely on BRM for BAF function. FHD-909 has shown significant anti-tumor activity across multiple BRG1-mutant lung tumors.
About Foghorn Therapeutics
Foghorn® Therapeutics is discovering and developing a novel class of medicines targeting genetically determined dependencies within the chromatin regulatory system. Through its proprietary scalable Gene Traffic Control® platform, Foghorn is systematically studying, identifying and validating potential drug targets within the chromatin regulatory system. The Company is developing multiple product candidates in oncology. Visit our website at www.foghorntx.com for more information on the Company, and follow us on X (formerly Twitter) and LinkedIn.
Forward-Looking Statements
This press release contains “forward-looking statements.” Forward-looking statements include statements regarding the Company’s clinical trials, product candidates and research efforts and other statements identified by words such as “could,” “may,” “might,” “will,” “likely,” “anticipates,” “intends,” “plans,” “seeks,” “believes,” “estimates,” “expects,” “continues,” “projects” and similar references to future periods. Forward-looking statements are based on our current expectations and assumptions regarding capital market conditions, our business, the economy and other future conditions. Because forward-looking statements relate to the future, by their nature, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict. As a result, actual results may differ materially from those contemplated by the forward-looking statements. Important factors that could cause actual results to differ materially from those in the forward-looking statements include regional, national or global political, economic, business, competitive, market and regulatory conditions, including risks relating to our clinical trials and other factors set forth under the heading “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, as filed with
the Securities and Exchange Commission. Any forward-looking statement made in this press release speaks only as of the date on which it is made.
Condensed Consolidated Balance Sheets
(In thousands)
| | | | | | | | | | | |
| Dec. 31, 2023 | | Dec. 31, 2022 |
| Cash, cash equivalents and marketable securities | $ | 234,057 | | | $ | 345,798 | |
| Collaboration receivable | — | | | — | |
| All other assets | 51,859 | | | 59,085 | |
| Total assets | $ | 285,916 | | | $ | 404,883 | |
| Deferred revenue, total | $ | 302,665 | | | $ | 336,820 | |
| All other liabilities | 60,441 | | | 67,951 | |
| Total liabilities | 363,106 | | | 404,771 | |
| Total stockholders’ equity | (77,190) | | | 112 | |
| Total liabilities and stockholders’ equity | $ | 285,916 | | | $ | 404,883 | |
Condensed Consolidated Statements of Operations
(In thousands, except share and per share amounts)
| | | | | | | | | | | | | | | |
| Twelve Months Ended December 31, | | |
| 2023 | | 2022 | | | | |
| Collaboration revenue | $ | 34,155 | | | $ | 19,228 | | | | | |
| Operating expenses: | | | | | | | |
| Research and development | 109,689 | | | 105,618 | | | | | |
| General and administrative | 32,372 | | | 30,747 | | | | | |
| Total operating expenses | 142,061 | | | 136,365 | | | | | |
| Loss from operations | (107,906) | | | (117,137) | | | | | |
| Total other income, net | 13,706 | | | 8,255 | | | | | |
| Loss before income taxes | (94,200) | | | (108,882) | | | | | |
| Provision for income taxes | (4,226) | | | — | | | | | |
| Net loss | $ | (98,426) | | | $ | (108,882) | | | | | |
| Net loss per share attributable to common stockholders—basic and diluted | $ | (2.34) | | | $ | (2.62) | | | | | |
| Weighted average common shares outstanding—basic and diluted | 41,974,484 | | | 41,591,433 | | | | | |
Contacts:
Greg Dearborn, Foghorn Therapeutics Inc. (Investors)
gdearborn@foghorntx.com
Karin Hellsvik, Foghorn Therapeutics Inc. (Investors and Media)
khellsvik@foghorntx.com
Adam Silverstein, ScientPR (Media)
adam@scientpr.com
Peter Kelleher, LifeSci Advisors (Investors)
pkelleher@lifesciadvisors.com

March 2024 Unique biology Precision therapeutics Broad impact Exhibit 99.2

| Forward Looking Statements 2 This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “could,” “may,” “might,” “will,” “likely,” “anticipates,” “intends,” “plans,” “seeks,” “believes,” “estimates,” “expects,” “continues,” “projects” or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements include, but are not limited to, statements concerning: the potential outcomes from our collaboration agreements with Lilly; the initiation, timing, progress and results of our research and development programs and pre-clinical studies and clinical trials, including with respect to our Phase 1 study of FHD-286 in combination with decitabine or cytarabine in relapsed and/or refractory AML patients and anticipated timing of release of clinical data, and the planned Phase 1 dose escalation study of FHD-909 with Loxo@Lilly; our ability to advance product candidates that we may develop and to successfully complete preclinical and clinical studies; our ability to leverage our initial programs to develop additional product candidates using our Gene Traffic Control Platform; the impact of exogeneous factors, including macroeconomic and geopolitical circumstances, on our and our collaborators’ business operations, including our research and development programs and pre-clinical studies; developments related to our competitors and our industry; our ability to expand the target populations of our programs and the availability of patients for clinical testing; our ability to obtain regulatory approval for FHD-286 and any future product candidates from the FDA and other regulatory authorities; our ability to identify and enter into future license agreements and collaborations; our ability to continue to rely on our CDMOs and CROs for our manufacturing and research needs; regulatory developments in the United States and foreign countries; our ability to attract and retain key scientific and management personnel; the scope of protection we are able to establish, maintain and enforce for intellectual property rights covering FHD-286, our future products and our Gene Traffic Control Platform; and our use of proceeds from capital-raising transactions, estimates of our expenses, capital requirements, and needs for additional financing. Any forward-looking statements represent the Company’s views only as of today and should not be relied upon as representing its views as of any subsequent date. The Company explicitly disclaims any obligation to update any forward-looking statements. The Company’s business is subject to substantial risks and uncertainties.
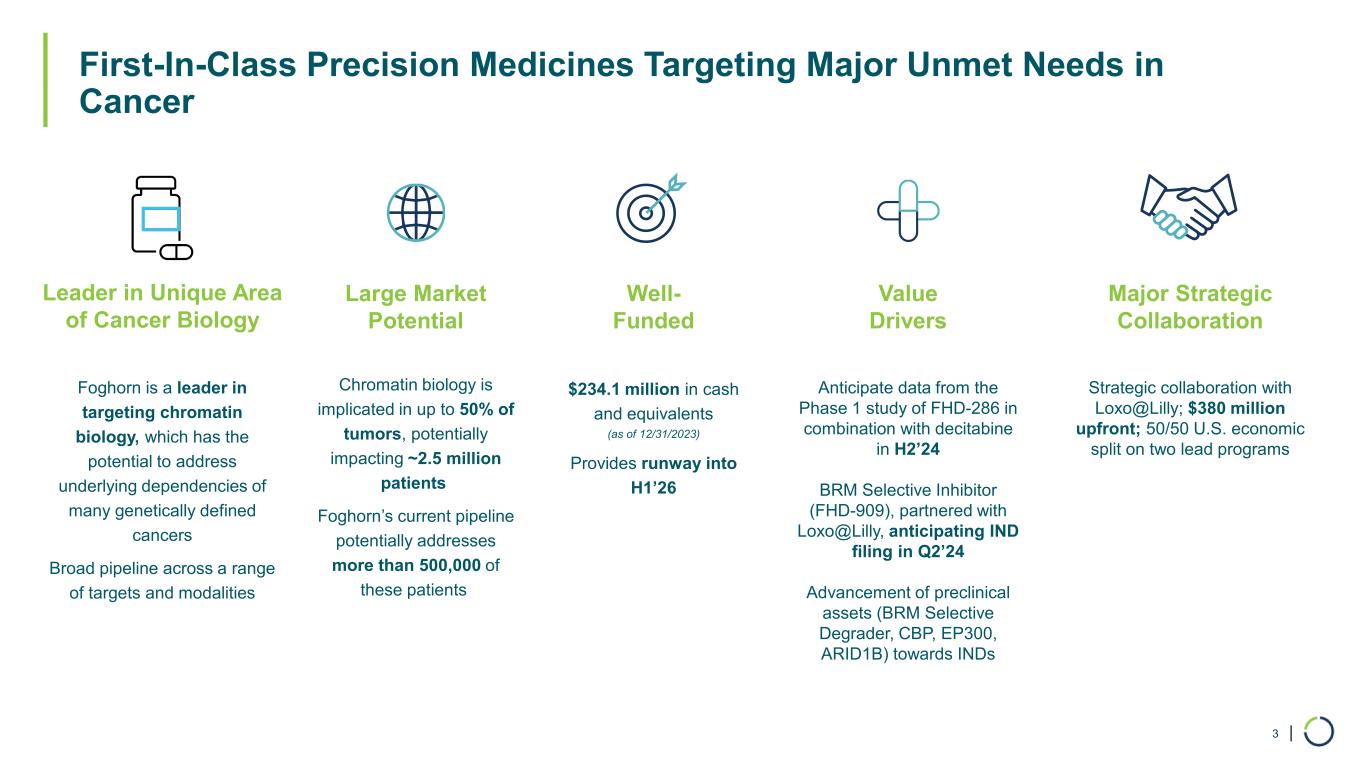
| First-In-Class Precision Medicines Targeting Major Unmet Needs in Cancer 3 Large Market Potential Chromatin biology is implicated in up to 50% of tumors, potentially impacting ~2.5 million patients Foghorn’s current pipeline potentially addresses more than 500,000 of these patients Major Strategic Collaboration Strategic collaboration with Loxo@Lilly; $380 million upfront; 50/50 U.S. economic split on two lead programs Well- Funded $234.1 million in cash and equivalents (as of 12/31/2023) Provides runway into H1’26 Value Drivers Anticipate data from the Phase 1 study of FHD-286 in combination with decitabine in H2’24 BRM Selective Inhibitor (FHD-909), partnered with Loxo@Lilly, anticipating IND filing in Q2’24 Advancement of preclinical assets (BRM Selective Degrader, CBP, EP300, ARID1B) towards INDs Leader in Unique Area of Cancer Biology Foghorn is a leader in targeting chromatin biology, which has the potential to address underlying dependencies of many genetically defined cancers Broad pipeline across a range of targets and modalities

| Unique Insights into Chromatin Biology to Prosecute Untapped Area for Novel Targets and Therapeutics 4 Novel Targets Guided by Genetic Dependencies Tailored Drugging Approaches Transcription Factor Mutations / Overexpression Chromatin Remodeling Complex Mutations / Overexpression Helicases & Other Chromatin Binding Proteins involved in gene expression / function Enzymatic Inhibitors Highly selective and allosteric small molecule inhibitors Transcription Factor Disruptors Disrupt interactions between chromatin remodeling complexes and transcription factors Potential druggable sites ATP ADP Chromatin Regulatory System Critical for Gene Expression Targeted Protein Degradation Molecular glue and bi-functional protein degraders Chromatin – compacted form of DNA inside the nucleus of the cell Chromatin Remodeling Complex – specialized multiprotein machineries that allow access to DNA Transcription Factor – proteins that help turn specific genes "on" or "off" by working in concert with the chromatin remodeling complex to bind to DNA
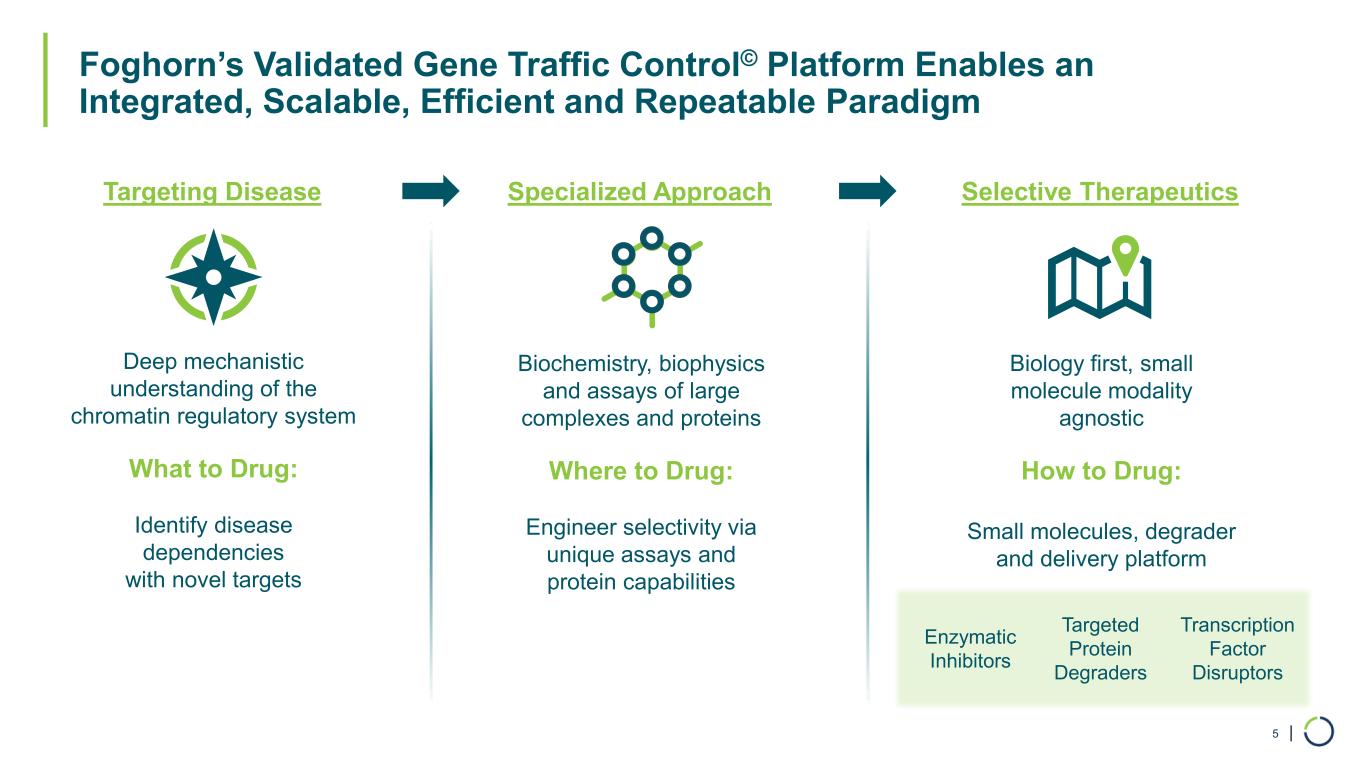
| Foghorn’s Validated Gene Traffic Control© Platform Enables an Integrated, Scalable, Efficient and Repeatable Paradigm 5 Deep mechanistic understanding of the chromatin regulatory system Biology first, small molecule modality agnostic Targeting Disease Selective Therapeutics What to Drug: Identify disease dependencies with novel targets How to Drug: Small molecules, degrader and delivery platform Biochemistry, biophysics and assays of large complexes and proteins Specialized Approach Where to Drug: Engineer selectivity via unique assays and protein capabilities Transcription Factor Disruptors Enzymatic Inhibitors Targeted Protein Degraders

| Broad and Deep Pipeline Across a Range of Targets and Modalities 6 Modality Program Discovery Phase 1 Phase 2 Phase 3 Enzyme Inhibitors Transcription Factor Disruptors Undisclosed Protein Degraders Commercial Rights FHD-286 (BRG1/BRM) FHD-909 (Selective BRM) 3 Discovery Programs Undisclosed Partnered Program Undisclosed Disease BRG1 mutant cancers (e.g., ~8-10% of NSCLC, bladder, endometrial, colorectal) Relapsed/Refractory AML Selective BRM BRG1 mutant cancers (e.g., ~8-10% of NSCLC, bladder, endometrial, colorectal) Selective ARID1B ARID1A mutant cancers (~5% of all solid tumors) Selective CBP EP300 mutant cancers (e.g., ~5-10% of bladder, gastric, breast, NSCLC, colorectal) Selective EP300 EP300 dependent cancers (e.g., prostate, DLBCL), CBP mutant cancers (e.g., ~9-10% of NSCLC, bladder, melanoma) Undisclosed Undisclosed Undisclosed

FHD-286: Dual BRM/BRG1 Inhibition Targeting BAF Dependency in Cancer FHD-609 is a Selective, Potent, Protein Degrader of the BRD9 component of the BAF complex
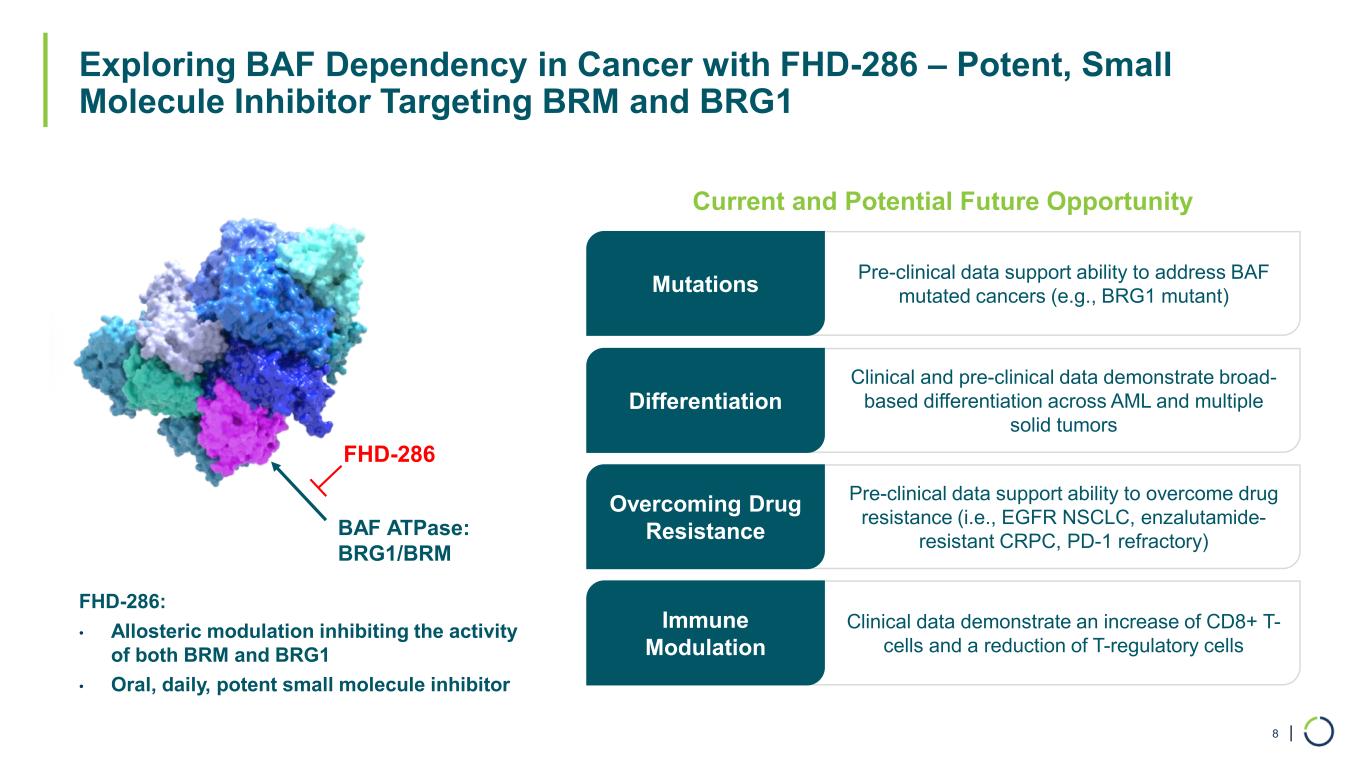
| Exploring BAF Dependency in Cancer with FHD-286 – Potent, Small Molecule Inhibitor Targeting BRM and BRG1 8 Clinical and pre-clinical data demonstrate broad- based differentiation across AML and multiple solid tumors BAF ATPase: BRG1/BRM FHD-286: • Allosteric modulation inhibiting the activity of both BRM and BRG1 • Oral, daily, potent small molecule inhibitor FHD-286 Differentiation Pre-clinical data support ability to overcome drug resistance (i.e., EGFR NSCLC, enzalutamide- resistant CRPC, PD-1 refractory) Overcoming Drug Resistance Clinical data demonstrate an increase of CD8+ T- cells and a reduction of T-regulatory cells Immune Modulation Current and Potential Future Opportunity Pre-clinical data support ability to address BAF mutated cancers (e.g., BRG1 mutant)Mutations
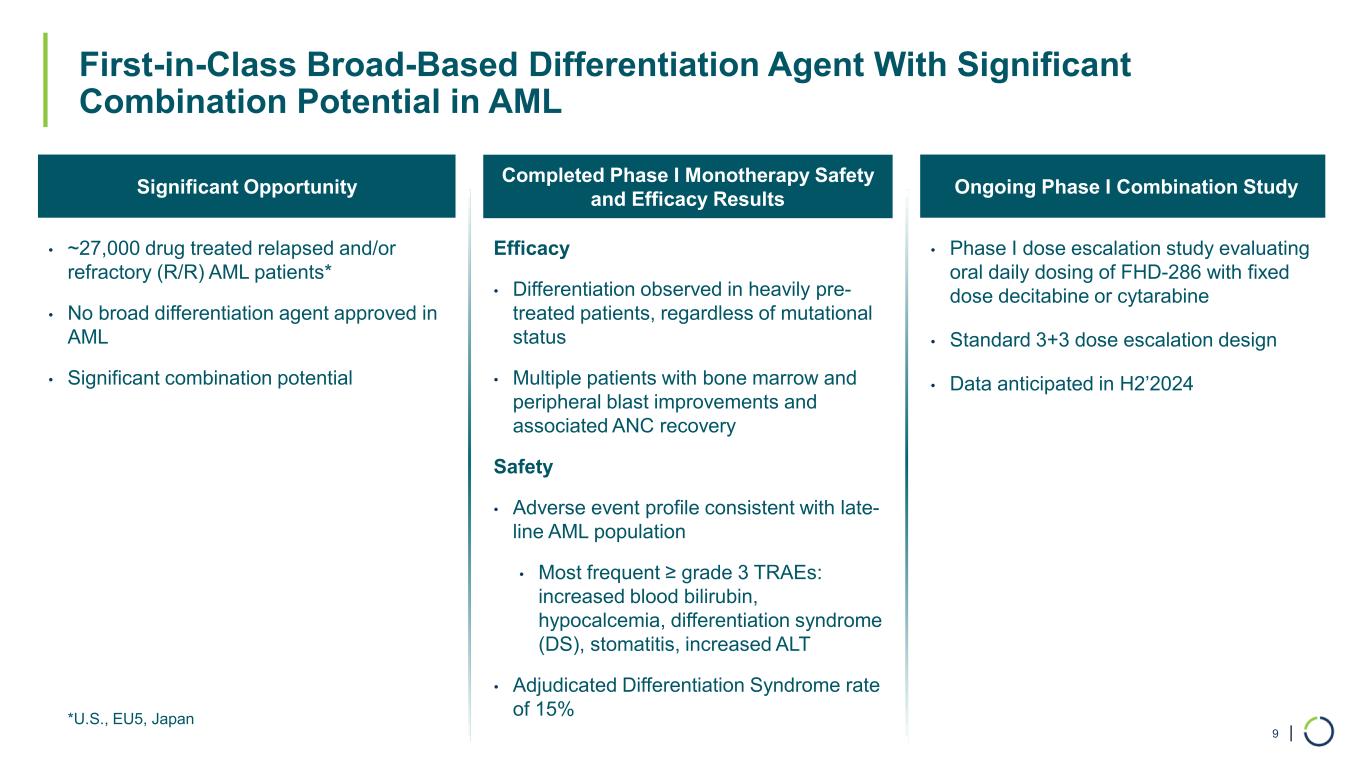
| First-in-Class Broad-Based Differentiation Agent With Significant Combination Potential in AML 9 Completed Phase I Monotherapy Safety and Efficacy Results Ongoing Phase I Combination StudySignificant Opportunity • ~27,000 drug treated relapsed and/or refractory (R/R) AML patients* • No broad differentiation agent approved in AML • Significant combination potential Efficacy • Differentiation observed in heavily pre- treated patients, regardless of mutational status • Multiple patients with bone marrow and peripheral blast improvements and associated ANC recovery Safety • Adverse event profile consistent with late- line AML population • Most frequent ≥ grade 3 TRAEs: increased blood bilirubin, hypocalcemia, differentiation syndrome (DS), stomatitis, increased ALT • Adjudicated Differentiation Syndrome rate of 15% • Phase I dose escalation study evaluating oral daily dosing of FHD-286 with fixed dose decitabine or cytarabine • Standard 3+3 dose escalation design • Data anticipated in H2’2024 *U.S., EU5, Japan
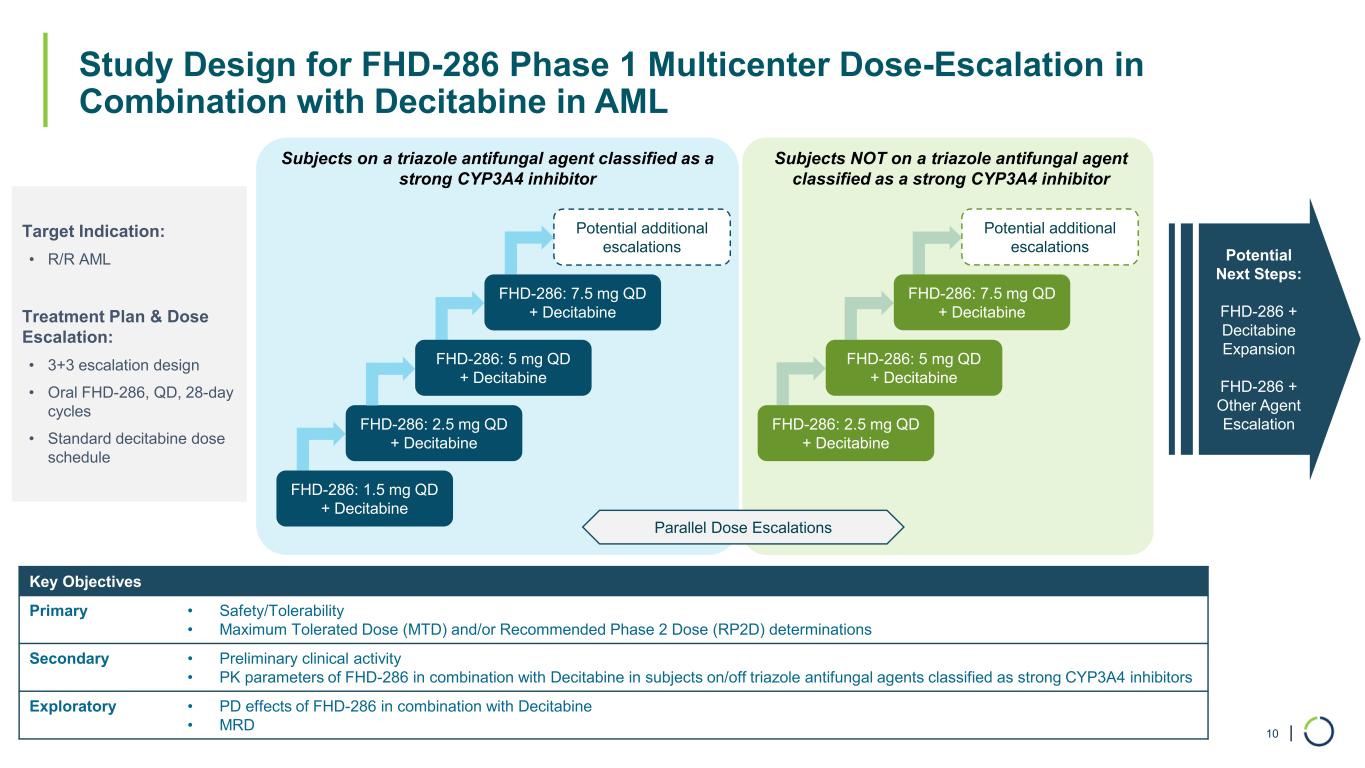
| Study Design for FHD-286 Phase 1 Multicenter Dose-Escalation in Combination with Decitabine in AML 10 Target Indication: • R/R AML Treatment Plan & Dose Escalation: • 3+3 escalation design • Oral FHD-286, QD, 28-day cycles • Standard decitabine dose schedule Key Objectives Primary • Safety/Tolerability • Maximum Tolerated Dose (MTD) and/or Recommended Phase 2 Dose (RP2D) determinations Secondary • Preliminary clinical activity • PK parameters of FHD-286 in combination with Decitabine in subjects on/off triazole antifungal agents classified as strong CYP3A4 inhibitors Exploratory • PD effects of FHD-286 in combination with Decitabine • MRD FHD-286: 1.5 mg QD + Decitabine FHD-286: 2.5 mg QD + Decitabine FHD-286: 5 mg QD + Decitabine FHD-286: 7.5 mg QD + Decitabine FHD-286: 2.5 mg QD + Decitabine FHD-286: 5 mg QD + Decitabine FHD-286: 7.5 mg QD + Decitabine Potential Next Steps: FHD-286 + Decitabine Expansion FHD-286 + Other Agent Escalation Subjects on a triazole antifungal agent classified as a strong CYP3A4 inhibitor Subjects NOT on a triazole antifungal agent classified as a strong CYP3A4 inhibitor Parallel Dose Escalations Potential additional escalations Potential additional escalations

| FHD-286 Demonstrated Differentiation Across a Broad Range of Genetic Backgrounds 11 Dose Level Mutations Cytogenetics Risk Starting CD11b% Max CD11b% CD11b+ Fold Change Starting CD34% Min CD34% CD34+ % Decrease 10mg N/A Adverse 7 62 9.2x 94 27 (71%) 7.5mg CBFB (locus at 16q22) 2 94 59.4x 70 2 (97%) 7.5mg KMT2A rearrangement Adverse 3 58 21.4x 85 9 (90%) 7.5mg RUNX1, KRAS, ASXL1, JAK2, TET2, EZH2, ETNK Adverse 5 73 15x 95 18 (81%) 7.5mg N/A Adverse 8 52 6.3x 94 33 (65%) 7.5mg ASXL1, TP53, U2AF1 Adverse 19 63 3.3x 92 51 (45%) 5mg RUNX1, NRAS, KRAS, SF3B1, ASXL2, CSF3R, GATA2 Adverse 3 74 29x 94 19 (80%) 5mg RUNX1, NRAS, ASLX1 Adverse 4 97 22.8x 98 7 (93%) 5mg N/A Adverse 6 79 13x 93 11 (88%) 5mg TET2, WT1 GATA2 PLCG2 ARHGEF28, BRD4, CDK12, DDX41, KMT20, PARP1, ZRSR2 3 24 8.1x 86 62 (27%) 5mg N/A Adverse 4 28 6.5x 93 66 (29%) 5mg DNMT3a, TET2 21 88 4.1x 30 4 (88%) 2.5mg NRAS, WT1 Adverse 3 13 4.8x 93 89 (4%) CD11b (marker of differentiation) increases CD34 (leukemic stem cell marker) decreases

| Bone Marrow Aspirate: Clear Evidence of Differentiation Bone Marrow Blast Reduction from 40% to 6% Clear Signs of Differentiation in Heavily Pre-Treated, Secondary AML Patient with Abnormal Karyotype 12 Patient Background: • 47-year-old male, secondary AML • Abnormal karyotype: Del (7Q), Inv (3), Der (7;12), -8, ADD(1) Prior AML Treatment: • Progressive disease: 4 lines prior treatment and 2 bone marrow transplants Prior non-AML treatment: • MDS with inv(3) and der(7;12) and ASXL1 mut. Received AZA x 4. Initiation of FHD-286 at 10 MG Dose: • Bone marrow blast from 40% to 6% with clear evidence of differentiation with persistence of cytogenetics abnormalities. ANC recovery.
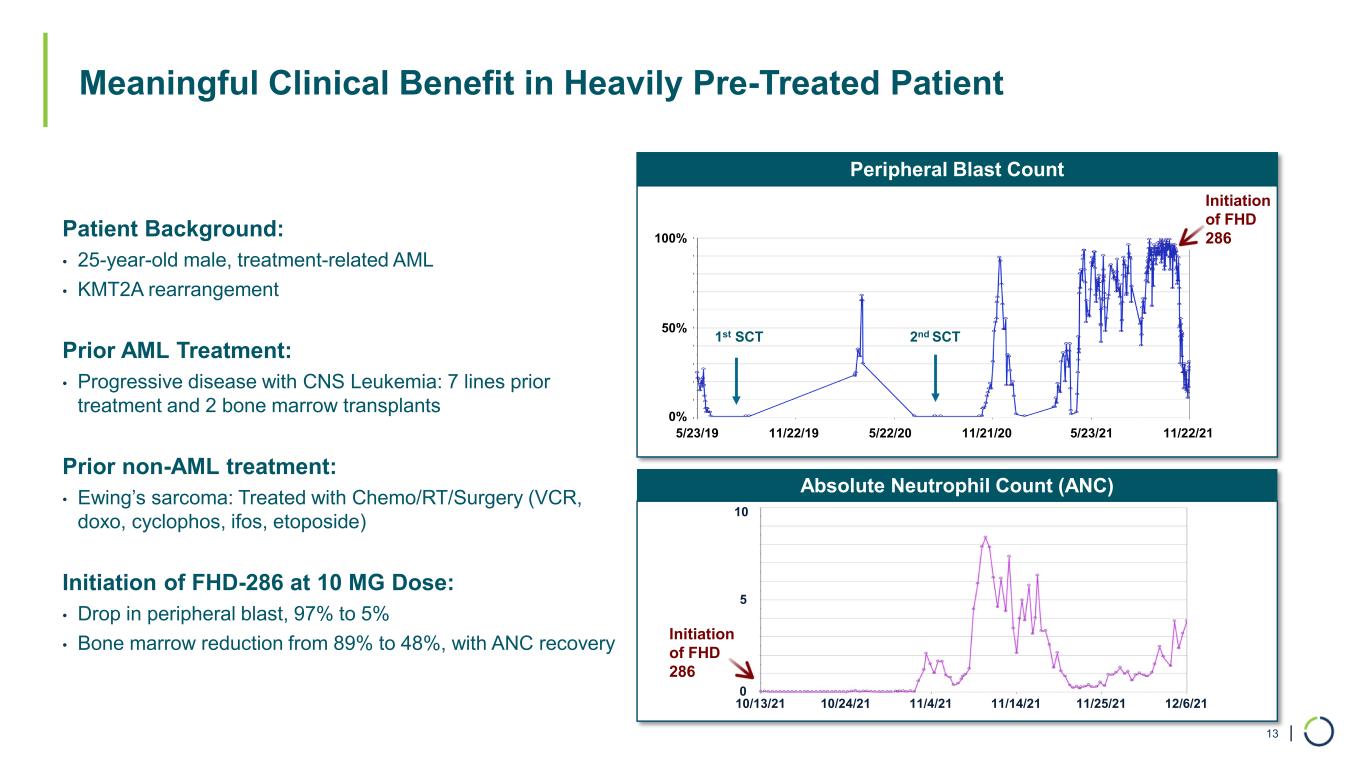
| Absolute Neutrophil Count (ANC) Peripheral Blast Count Meaningful Clinical Benefit in Heavily Pre-Treated Patient 13 100% 0% 50% 10 5 10/13/21 10/24/21 11/4/21 11/14/21 11/25/21 12/6/21 0 Initiation of FHD 286 1st SCT 2nd SCT Patient Background: • 25-year-old male, treatment-related AML • KMT2A rearrangement Prior AML Treatment: • Progressive disease with CNS Leukemia: 7 lines prior treatment and 2 bone marrow transplants Prior non-AML treatment: • Ewing’s sarcoma: Treated with Chemo/RT/Surgery (VCR, doxo, cyclophos, ifos, etoposide) Initiation of FHD-286 at 10 MG Dose: • Drop in peripheral blast, 97% to 5% • Bone marrow reduction from 89% to 48%, with ANC recovery 5/23/19 11/22/19 5/22/20 5/23/2111/21/20 11/22/21 Initiation of FHD 286

| FHD-286 + decitabine (MLL-AFP + FLT3 TKD Luc/GFP PDX) Pre-Clinical Data Demonstrate Significant Combination Potential with Multiple Agents in AML 14 FHD-286 + BET inhibitor (mtNPM1 + FLT3 ITD Luc/GFP AML PDX) FHD-286 + menin inhibitor (mtNPM1 + FLT3 ITD Luc/GFP AML PDX) FHD-286 + cytarabine (MV4, 11 FLT3 ITD CDX) SNDX-5613: Menin inhibitor 0 20 40 60 0 25 50 75 100 Days, post-infusion % S ur vi va l FHD-286 + decitabine (n=8) 1 mg/kg decitabine (n=7) 1.5 mg/kg FHD-286 (n=7) Vehicle (n=7)6 weeks Rx ✱ ✱ ✱ ✱ ✱

Selective BRM Modulators For BRG1 Mutated Cancers FHD-286 is a Potent, Selective, Allosteric, Small Molecule Inhibitor of the BRG1 and BRM subunits of the BAF complex
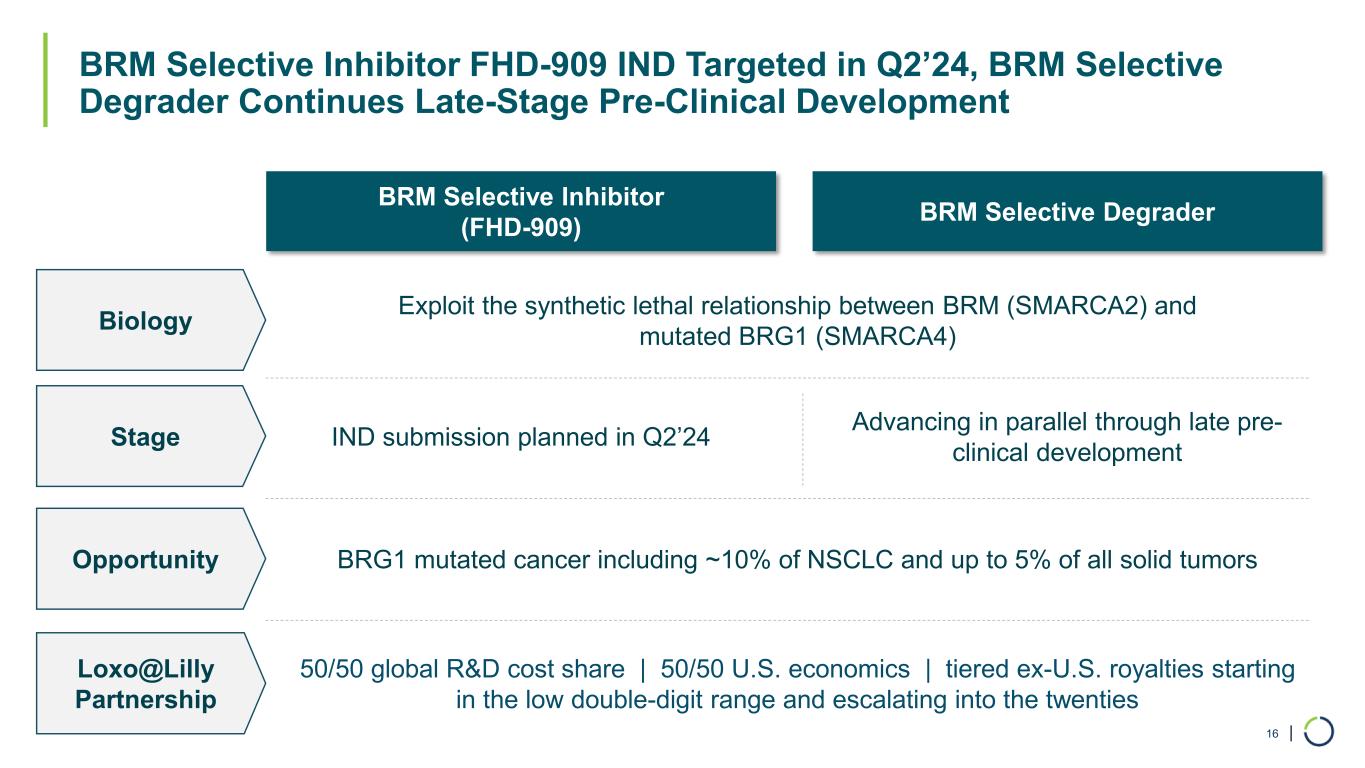
| BRM Selective Inhibitor FHD-909 IND Targeted in Q2’24, BRM Selective Degrader Continues Late-Stage Pre-Clinical Development 16 BRM Selective Inhibitor (FHD-909) BRM Selective Degrader Biology Exploit the synthetic lethal relationship between BRM (SMARCA2) and mutated BRG1 (SMARCA4) Stage IND submission planned in Q2’24 Advancing in parallel through late pre- clinical development Opportunity BRG1 mutated cancer including ~10% of NSCLC and up to 5% of all solid tumors Loxo@Lilly Partnership 50/50 global R&D cost share | 50/50 U.S. economics | tiered ex-U.S. royalties starting in the low double-digit range and escalating into the twenties
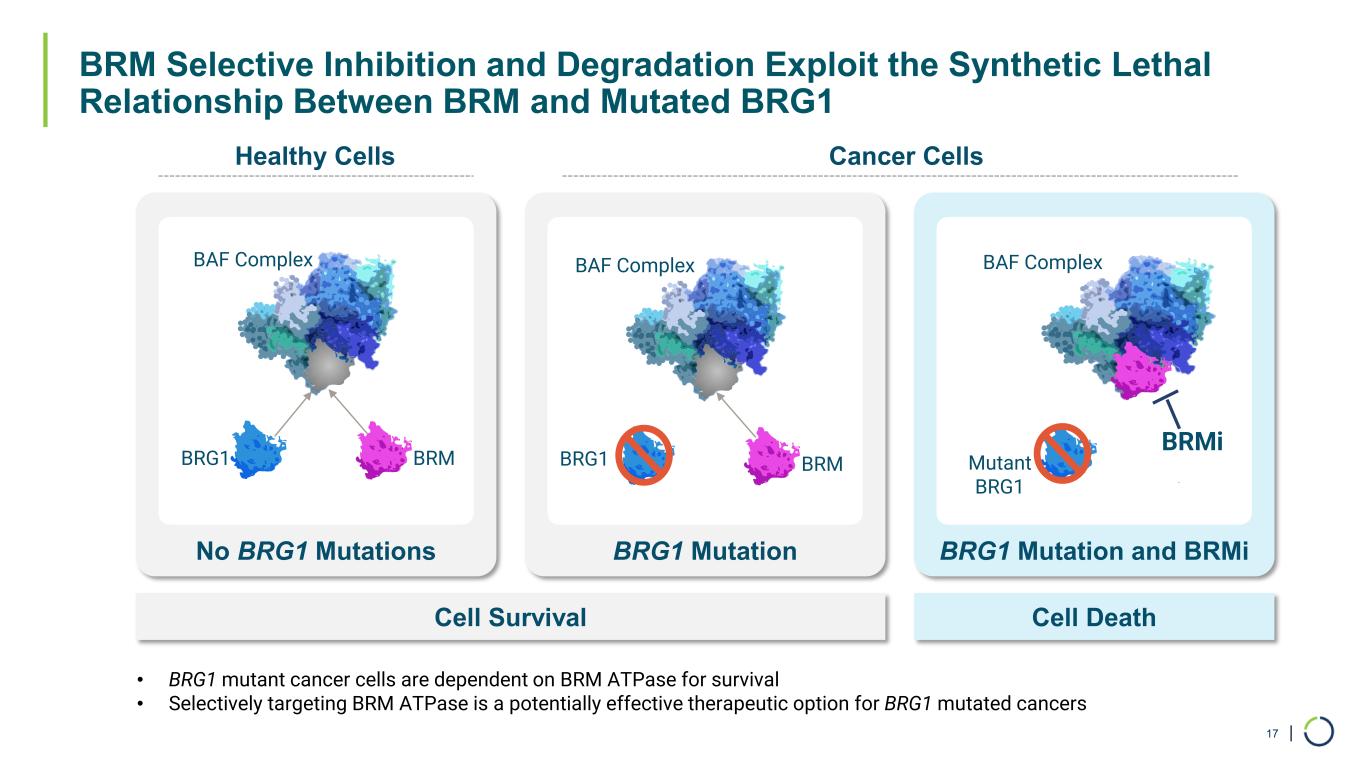
| BRM Selective Inhibition and Degradation Exploit the Synthetic Lethal Relationship Between BRM and Mutated BRG1 17 No BRG1 Mutations BRG1 Mutation BRG1 Mutation and BRMi Cell Survival Cell Death Healthy Cells Cancer Cells BRG1 BRM BAF Complex BRM BAF Complex Mutant BRG1 BAF Complex BRMi • BRG1 mutant cancer cells are dependent on BRM ATPase for survival • Selectively targeting BRM ATPase is a potentially effective therapeutic option for BRG1 mutated cancers BRG1

| BRG1 is Mutated in Up to 10% of NSCLC; Up to 5% of Solid Tumors 18 BRG1 mutated across range of tumors Accounts for ~5% of all tumors BRG1 mutated in up to 10% of NSCLC tumors, minimal overlap with other mutations

| BRM Selective Inhibitor Demonstrates PK/PD and In Vivo Efficacy in a BRG1 Mutant Lung CDX Model 19 PK/PD Body Weight LossA549-BRG1 Mutant NSCLC Model BRG1 cellular IC50 BRM cellular IC50 0 5 10 15 1 10 100 1,000 Time (h) M ea n U nb ou nd Pl as m a C on ce nt ra tio n (n M ) 10 20 30 40 0 500 1,000 1,500 Days After Tumor Inoculation Tu m or V ol um e (m m 3 ± S EM ) Vehicle Control (BID) Cisplatin, 4 mg/kg (IP) FHT-BRMi, 15 mg/kg (BID) FHT-BRMi, 30 mg/kg (BID) 10 20 30 40 80 90 100 110 120 Days After Inoculation B od y W ei gh t (% D ay 0 ) Data as of Q4 2021
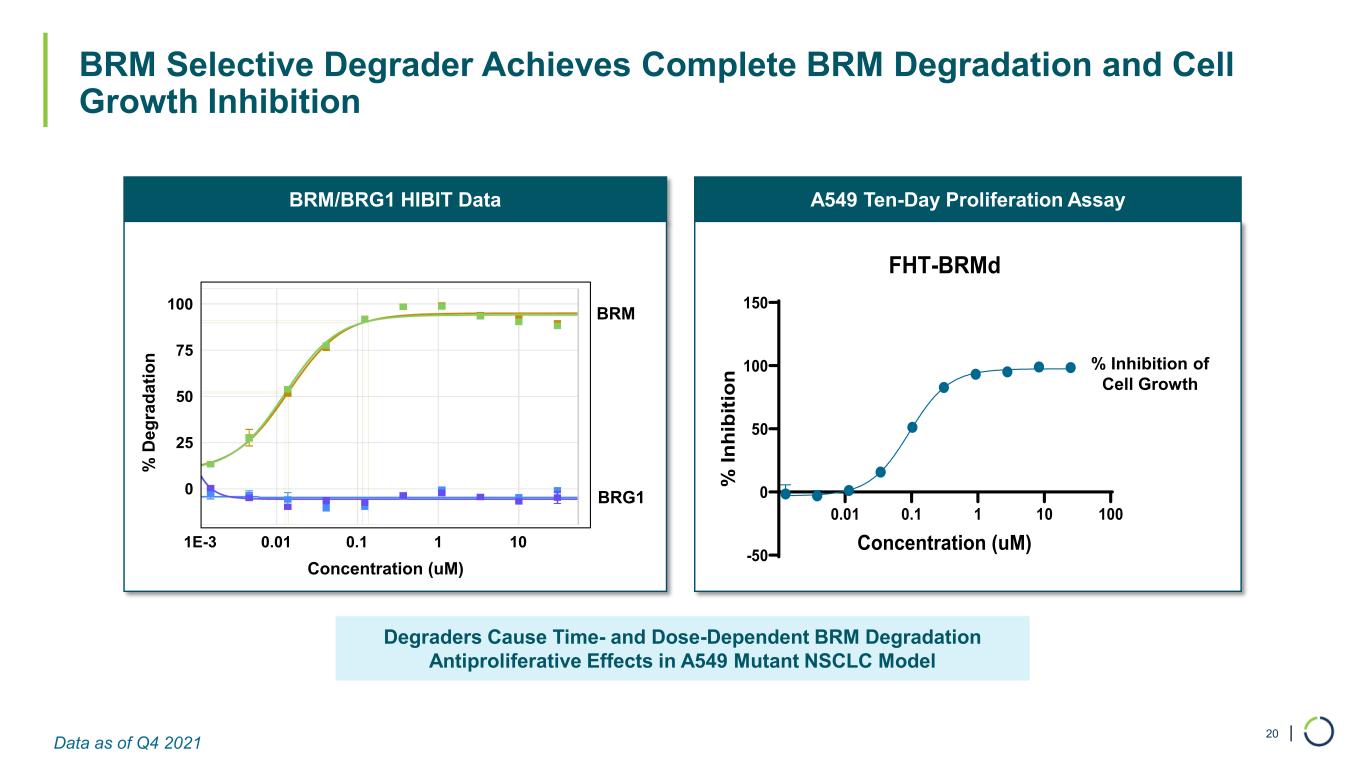
| BRM Selective Degrader Achieves Complete BRM Degradation and Cell Growth Inhibition 20 Degraders Cause Time- and Dose-Dependent BRM Degradation Antiproliferative Effects in A549 Mutant NSCLC Model A549 Ten-Day Proliferation AssayBRM/BRG1 HIBIT Data BRM BRG1 % D eg ra da tio n Concentration (uM) 0.01 0.1 1 10 100 -50 0 50 100 150 FHT-BRMd Concentration (uM) % In hi bi tio n % Inhibition of Cell Growth 0 25 50 75 100 1E-3 0.01 0.1 1 10 Data as of Q4 2021

Selective CBP Protein Degrader For EP300 Mutated Cancers

| Summary: Selective CBP Protein Degrader for EP300 Mutated Cancers 22* Per year incidence in the U.S., EU5, Japan Target / Approach • CREB binding protein (CBP) • Targeted protein degrader Initial Indication • EP300 mutated cancers (e.g., subsets of bladder, colorectal, breast, gastric and lung cancers) Mutation / Aberration • EP300 mutated cancers Stage • Pre-clinical New Patients Impacted / Year* • Over 100,000 10% 10% 8% 7% 6% 6% 5% 5% 3% 0% 5% 10% 15% Melanoma NSCLC Bladder Cancer Endometrial Gastric Breast Pancreatic Cancer Colorectal Cancer Commercial Opportunity SCCHN % of Patients with EP300 Mutation
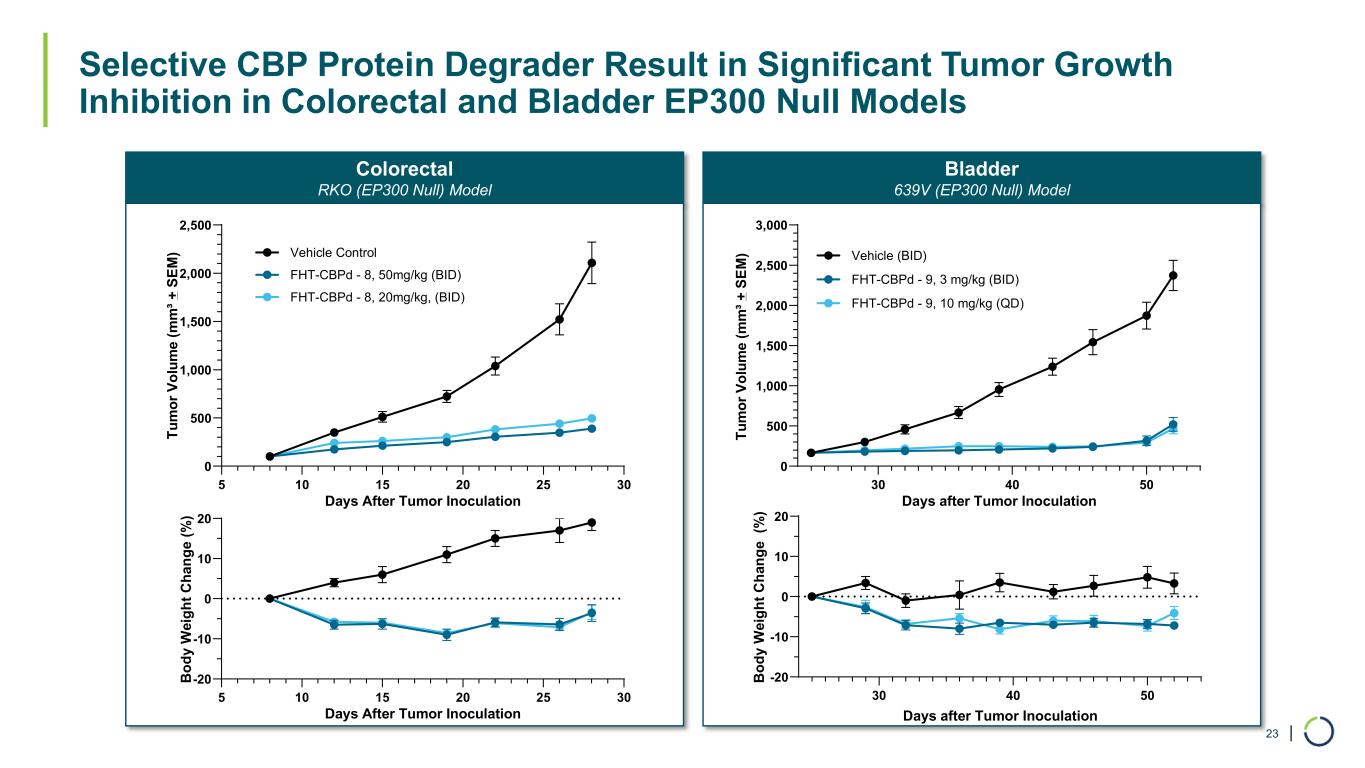
| Colorectal RKO (EP300 Null) Model Selective CBP Protein Degrader Result in Significant Tumor Growth Inhibition in Colorectal and Bladder EP300 Null Models 23 Bladder 639V (EP300 Null) Model 30 40 50 -20 -10 0 10 20 Days after Tumor Inoculation B od y W ei gh t C ha ng e (% ) 30 40 50 0 500 1,000 1,500 2,000 2,500 3,000 Days after Tumor Inoculation Tu m or V ol um e (m m ³+ S EM ) Vehicle (BID) FHT-CBPd - 9, 3 mg/kg (BID) FHT-CBPd - 9, 10 mg/kg (QD) 5 10 15 20 25 30 0 500 1,000 1,500 2,000 2,500 Days After Tumor Inoculation Tu m or V ol um e (m m ³+ S EM ) Vehicle Control FHT-CBPd - 8, 50mg/kg (BID) FHT-CBPd - 8, 20mg/kg, (BID) 5 10 15 20 25 30 -20 -10 0 10 20 Days After Tumor Inoculation B od y W ei gh t C ha ng e (% )
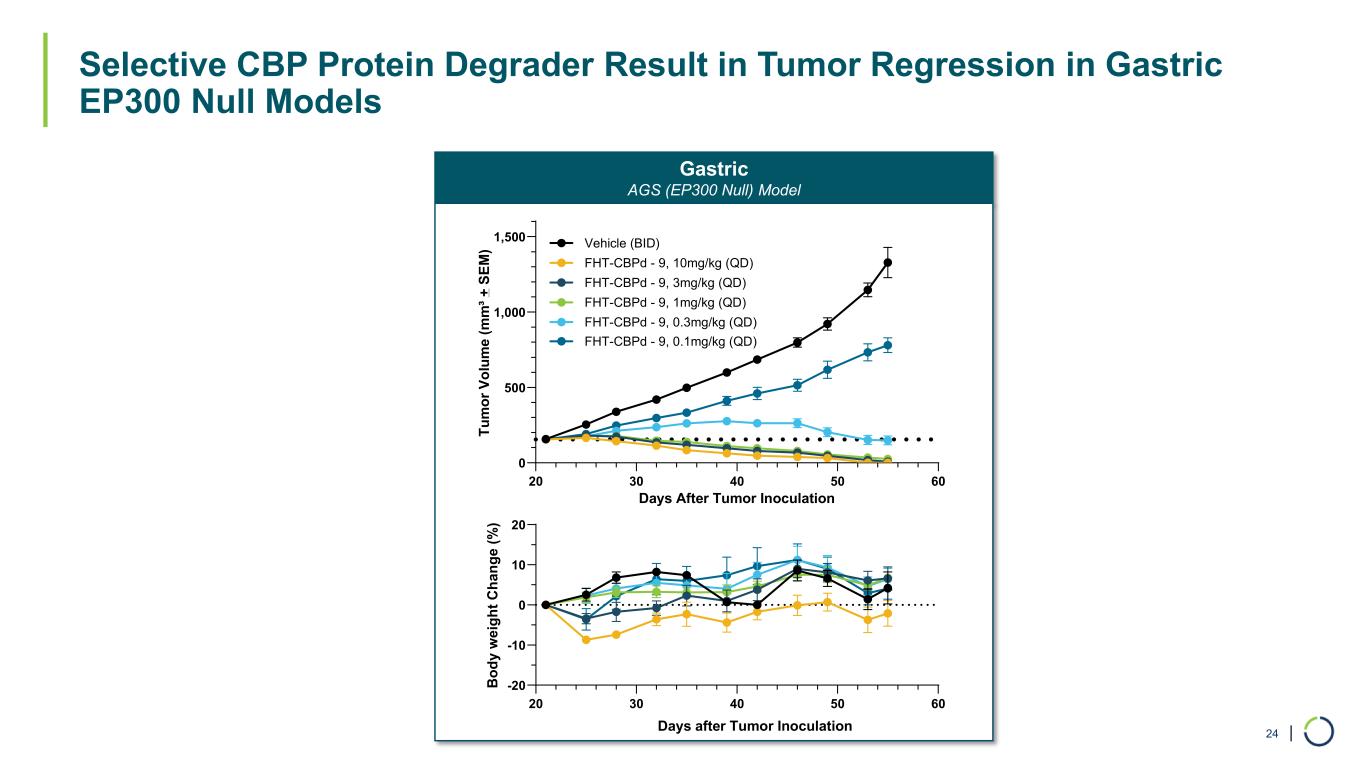
| Selective CBP Protein Degrader Result in Tumor Regression in Gastric EP300 Null Models 24 Gastric AGS (EP300 Null) Model 20 30 40 50 60 -20 -10 0 10 20 B od y w ei gh t C ha ng e (% ) Days after Tumor Inoculation 20 30 40 50 60 0 500 1,000 1,500 Days After Tumor Inoculation Tu m or V ol um e (m m ³+ S EM ) Vehicle (BID) FHT-CBPd - 9, 10mg/kg (QD) FHT-CBPd - 9, 3mg/kg (QD) FHT-CBPd - 9, 1mg/kg (QD) FHT-CBPd - 9, 0.3mg/kg (QD) FHT-CBPd - 9, 0.1mg/kg (QD)

| CBP Degrader Plasma Concentration Long-Acting Injectable Formulations of CBP Degrader Could Enable Once Every 2 Weeks (or Better) Dosing Frequency 25 0 100 200 300 400 0 2,000 4,000 6,000 8,000 Time (hours) C on cn et ra tio n (n g/ m l) CBPd - 9, SC, 150 mg/kg CBPd - 9, IM, 150 mg/kg

Selective EP300 Protein Degrader For CBP Mutated and EP300 Dependent Cancers
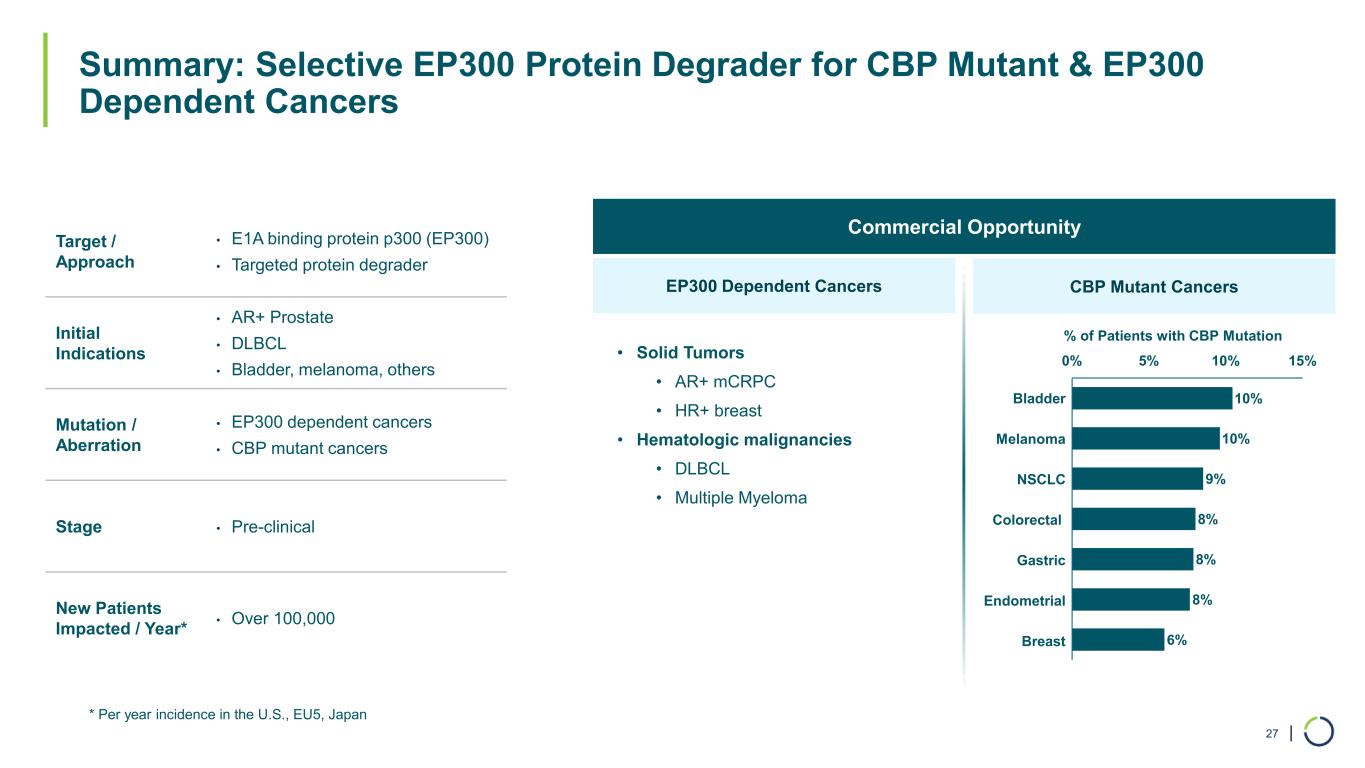
| Summary: Selective EP300 Protein Degrader for CBP Mutant & EP300 Dependent Cancers 27 * Per year incidence in the U.S., EU5, Japan Target / Approach • E1A binding protein p300 (EP300) • Targeted protein degrader Initial Indications • AR+ Prostate • DLBCL • Bladder, melanoma, others Mutation / Aberration • EP300 dependent cancers • CBP mutant cancers Stage • Pre-clinical New Patients Impacted / Year* • Over 100,000 Commercial Opportunity EP300 Dependent Cancers • Solid Tumors • AR+ mCRPC • HR+ breast • Hematologic malignancies • DLBCL • Multiple Myeloma CBP Mutant Cancers 10% 10% 9% 8% 8% 8% 6% 0% 5% 10% 15% Melanoma Bladder NSCLC Endometrial Colorectal Gastric Breast % of Patients with CBP Mutation
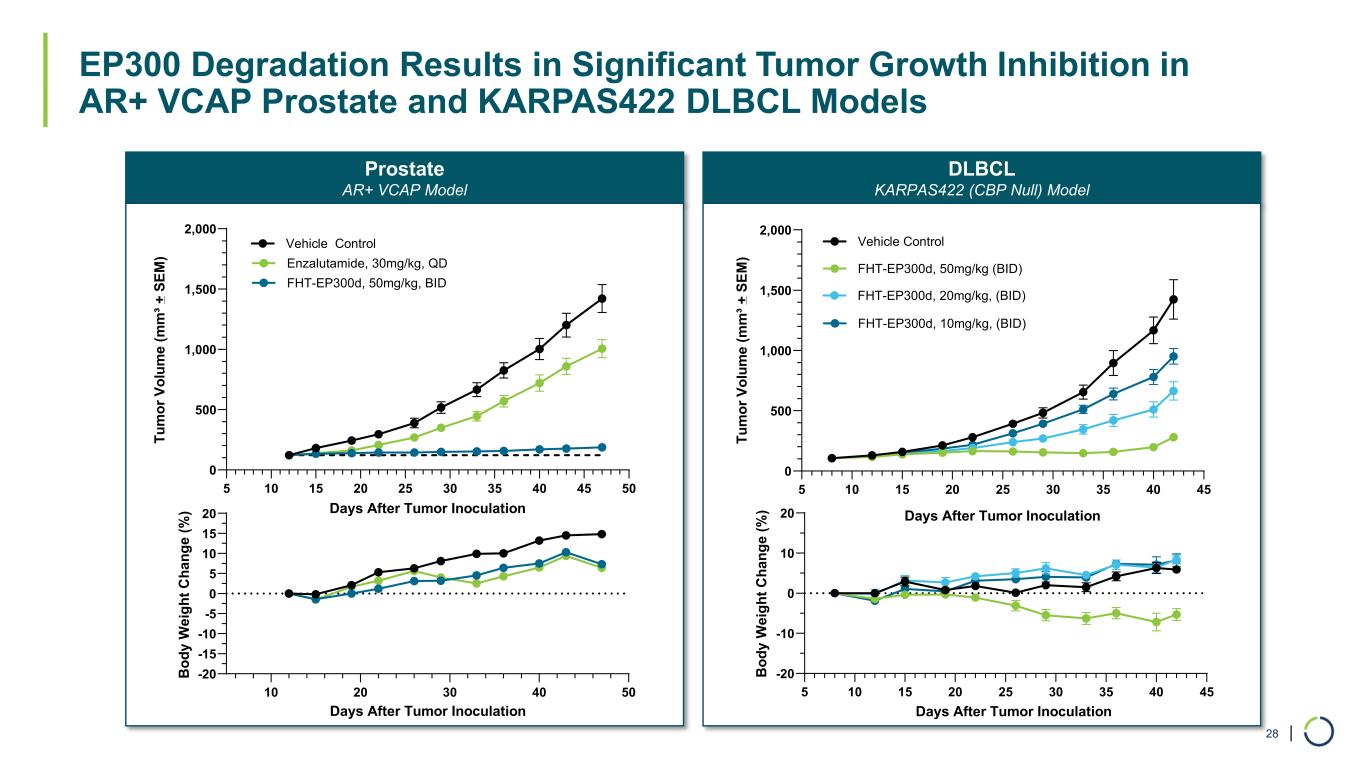
| EP300 Degradation Results in Significant Tumor Growth Inhibition in AR+ VCAP Prostate and KARPAS422 DLBCL Models 28 Prostate AR+ VCAP Model DLBCL KARPAS422 (CBP Null) Model 5 10 15 20 25 30 35 40 45 50 0 500 1,000 1,500 2,000 Days After Tumor Inoculation Tu m or V ol um e (m m ³ + S EM ) Vehicle Control Enzalutamide, 30mg/kg, QD FHT-EP300d, 50mg/kg, BID 10 20 30 40 50 -20 -15 -10 -5 0 5 10 15 20 Days After Tumor Inoculation B od y W ei gh t C ha ng e (% ) 5 10 15 20 25 30 35 40 45 -20 -10 0 10 20 B od y W ei gh t C ha ng e (% ) Days After Tumor Inoculation 5 10 15 20 25 30 35 40 45 0 500 1,000 1,500 2,000 Days After Tumor Inoculation Tu m or V ol um e (m m ³+ S EM ) Vehicle Control FHT-EP300d, 50mg/kg (BID) FHT-EP300d, 20mg/kg, (BID) FHT-EP300d, 10mg/kg, (BID)

| Platelet Counts Post Two Weeks of Dosing (In-Vivo) Selective Degradation of EP300 and CBP Does Not Show Thrombocytopenia in Mice at Relevant Doses 29 PLT (x109cells/L) 0 500 1,000 1,500 2,000 2,500 Study Day 14 Pl at el et s (X 10 3 c el ls /u L) Vehicle FHT-EP300d, 50mg/kg, sc, BID FHT-CBPd, 10mg/kg, sc, QD Dual BD inhibitor, 30mg/kg, p.o, BID FHT-EP300d FHT-CBPd Dual CBP/EP300 Inhibitor Vehicle

Selective ARID1B Protein Degrader For ARID1A Mutated Cancers

| ARID1B is a Major Synthetic Lethal Target Implicated in Up To 5% of All Solid Tumors 31* Per year incidence in the U.S., EU5, Japan Target / Approach • ARID1B • Targeted protein degrader Initial Indication • ARID1A mutated cancers Mutation / Aberration • ARID1A mutations (e.g., ovarian, endometrial, colorectal, bladder and other cancers) Stage • Pre-clinical New Patients Impacted / Year* • > 175,000 ARID1A ARID1B ~5% of all solid tumors harbor ARID1A mutations Uterine Bladder Stomach Cholangiocarcinoma Liver Esophageal Ovarian Colorectal Melanoma 0% 10% 20% 30% 40% Commercial Opportunity % of Patients with ARID1A Mutation

| Targeting ARID1B for ARID1A Mutated Cancers is Enabled by Foghorn’s Unique Biology and Discovery Capabilities 32 Gene Traffic Control Platform Protein Degrader Capabilities Program Status • Platform produces BAF complexes and subcomplexes containing either ARID1A or ARID1B at scale • Enables proprietary screens against ARID1B • Utilize protein degrader toolbox to create ARID1B hetero-bifunctional degraders PROGRAM STATUS • Validated selective chemical binders of ARID1B • In process of expanding binders into novel selective protein degraders • Assessing outcomes of ARID1B degradation and impact on BAF complex formation ARID1B Highly purified ARID1B / BAF complex

Transcription Factors A Novel Approach FHD-609 is a Selective, Potent, Protein Degrader of the BRD9 component of the BAF complex
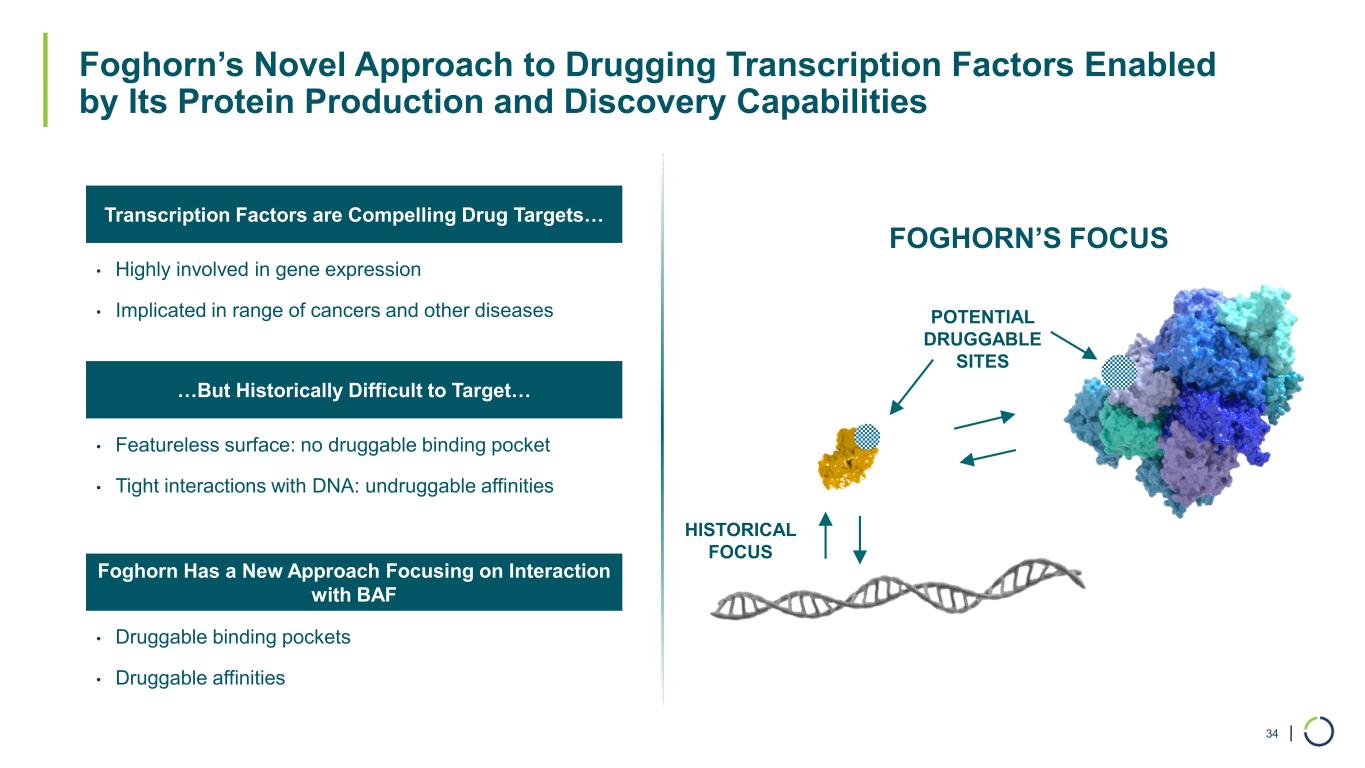
| Foghorn’s Novel Approach to Drugging Transcription Factors Enabled by Its Protein Production and Discovery Capabilities 34 • Highly involved in gene expression • Implicated in range of cancers and other diseases • Featureless surface: no druggable binding pocket • Tight interactions with DNA: undruggable affinities • Druggable binding pockets • Druggable affinities Transcription Factors are Compelling Drug Targets… …But Historically Difficult to Target… Foghorn Has a New Approach Focusing on Interaction with BAF HISTORICAL FOCUS POTENTIAL DRUGGABLE SITES FOGHORN’S FOCUS

| Transcription Factors Bind to BAF Directly with High Degree of Specificity; Unique Insights into Where and How Transcription Factors Bind 35 Mass spec. foot-printing Pull-down assays Foghorn’s collection of BAF sub-complexes and domains Biophysical Biochemical Structural AUC / SPR / ITC TR-FRET / FP Crystal / NMR SPI1 Mapping the TF-BAF Interaction Validating the TF-BAF Interaction SPI1 TF B TF C TF D
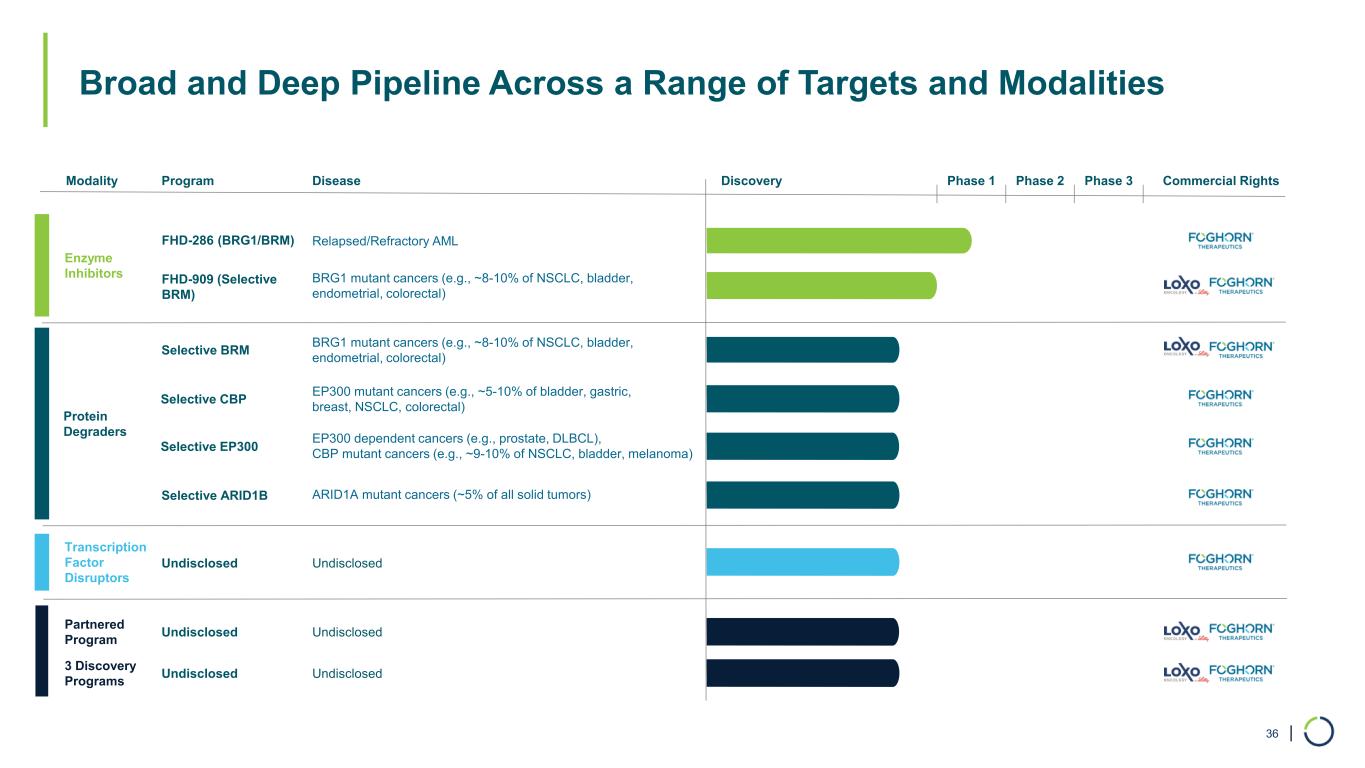
| Broad and Deep Pipeline Across a Range of Targets and Modalities 36 Modality Program Discovery Phase 1 Phase 2 Phase 3 Enzyme Inhibitors Transcription Factor Disruptors Undisclosed Protein Degraders Commercial Rights FHD-286 (BRG1/BRM) FHD-909 (Selective BRM) 3 Discovery Programs Undisclosed Partnered Program Undisclosed Disease BRG1 mutant cancers (e.g., ~8-10% of NSCLC, bladder, endometrial, colorectal) Relapsed/Refractory AML Selective BRM BRG1 mutant cancers (e.g., ~8-10% of NSCLC, bladder, endometrial, colorectal) Selective ARID1B ARID1A mutant cancers (~5% of all solid tumors) Selective CBP EP300 mutant cancers (e.g., ~5-10% of bladder, gastric, breast, NSCLC, colorectal) Selective EP300 EP300 dependent cancers (e.g., prostate, DLBCL), CBP mutant cancers (e.g., ~9-10% of NSCLC, bladder, melanoma) Undisclosed Undisclosed Undisclosed
| First-In-Class Precision Medicines Targeting Major Unmet Needs in Cancer 37 Large Market Potential Chromatin biology is implicated in up to 50% of tumors, potentially impacting ~2.5 million patients Foghorn’s current pipeline potentially addresses more than 500,000 of these patients Major Strategic Collaboration Strategic collaboration with Loxo@Lilly; $380 million upfront; 50/50 U.S. economic split on two lead programs Well- Funded $234.1 million in cash and equivalents (as of 12/31/2023) Provides runway into H1’26 Value Drivers Anticipate data from the Phase 1 study of FHD-286 in combination with decitabine in H2’24 BRM Selective Inhibitor (FHD-909), partnered with Loxo@Lilly, anticipating IND filing in Q2’24 Advancement of preclinical assets (BRM Selective Degrader, CBP, EP300, ARID1B) towards INDs Leader in Unique Area of Cancer Biology Foghorn is a leader in targeting chromatin biology, which has the potential to address underlying dependencies of many genetically defined cancers Broad pipeline across a range of targets and modalities
v3.24.0.1
Cover
|
Mar. 07, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Document Period End Date |
Mar. 07, 2024
|
| Entity Registrant Name |
Foghorn Therapeutics Inc.
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity File Number |
001-39634
|
| Entity Tax Identification Number |
47-5271393
|
| Entity Address, Address Line One |
500 Technology Square, Ste 700
|
| Entity Address, Postal Zip Code |
02139
|
| City Area Code |
617
|
| Local Phone Number |
586-3100
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of each class |
Common Stock, $0.0001 par value per share
|
| Trading Symbol(s) |
FHTX
|
| Name of each exchange on which registered |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Entity Ex Transition Period |
false
|
| Amendment Flag |
false
|
| Entity Central Index Key |
0001822462
|
| Entity Address, City or Town |
MA
|
| Entity Address, City or Town |
Cambridge,
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Foghorn Therapeutics (NASDAQ:FHTX)
Historical Stock Chart
From Mar 2024 to May 2024
Foghorn Therapeutics (NASDAQ:FHTX)
Historical Stock Chart
From May 2023 to May 2024