UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
SCHEDULE 14A
(Amendment No. 1)
Proxy Statement Pursuant to Section 14(a)
of the Securities Exchange Act of 1934
Filed
by the Registrant x
Filed
by a Party other than the Registrant ¨
Check the appropriate box:
| x |
|
Preliminary Proxy Statement |
| ¨ |
|
Confidential, for Use of the SEC Only (as permitted by Rule 14a-6(e)(2)) |
| ¨ |
|
Definitive Proxy
Statement |
| ¨ |
|
Definitive Additional Materials |
| ¨ |
|
Soliciting Material Pursuant to 14a-12 |
ENTERO THERAPEUTICS, INC.
(Name of Registrant as Specified in Its Charter)
(Name of Person(s) Filing Proxy Statement, if other
than the Registrant)
Payment of Filing Fee (Check all boxes that apply):
| x |
|
No fee required. |
| ¨ |
|
Fee paid previously with preliminary materials |
| ¨ |
|
Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11. |
PRELIMINARY PROXY MATERIALS –
SUBJECT TO COMPLETION, DATED JUNE 17, 2024

Entero Therapeutics, Inc.
777 Yamato Road, Suite 502
Boca Raton, Florida 33431
(561) 589-7020
| Dear Fellow Stockholder, |
|
|
[●], 2024 |
On behalf of the Board of Directors and management
of Entero Therapeutics, Inc. (“we”, “us” and “our”), a Delaware corporation, you are invited to attend
our 2024 Annual Meeting of Stockholders including any adjournment or postponement thereof (the “Annual Meeting”) to be held
on [●], 2024 at 9:00 A.M. Eastern Time at our corporate headquarters at 777 Yamato Rd., Suite 502, Boca Raton, FL 33431.
Details of the business to be conducted at the
Annual Meeting are described in this proxy statement. We have also made available a copy of our Annual Report on Form 10-K for the year
ended December 31, 2023 (filed with the Securities Exchange Commission on March 29, 2024) (the “Annual Report”) with this
proxy statement. We encourage you to read our Annual Report. It includes our audited financial statements and provides information about
our business and services.
Your
vote is important. Regardless of whether you plan to attend the Annual Meeting in person, please read the accompanying proxy
statement and then submit your proxy to vote by Internet, telephone or mail as promptly as possible. Returning your proxy will help
us assure that a quorum will be present at the Annual Meeting and avoid the additional expense of duplicate proxy solicitations. Any stockholder
attending the Annual Meeting may vote in person, even if he or she has previously voted. Please refer to your proxy card for voting instructions.
Submitting your proxy promptly may save us additional expense in soliciting proxies and will ensure that your shares are represented at
the Annual Meeting.
Our Board of Directors has approved the proposals
set forth in the proxy statement and recommends that you vote in favor of each such proposal.
| |
Sincerely, |
| |
|
| |
|
| |
JAMES SAPIRSTEIN |
| |
Chief Executive Officer and
Chairman of the Board of Directors |
If you have any questions or require any assistance
in voting your shares, please call:
Alliance Advisors LLC
200 Broadacres Drive, 3rd Floor, Bloomfield, NJ
07003
866-407-1875
NOTICE OF THE ENTERO THERAPEUTICS, INC.
ANNUAL MEETING OF STOCKHOLDERS
| Date and Time |
|
|
[●], 2024 at [●], Eastern Time. |
| |
|
|
|
|
|
|
| Place |
|
|
The offices
of Entero Therapeutics, Inc. (“we”, “us”, “our”, “Entero”,
or the “Company”) at 777 Yamato Rd., Suite 502, Boca Raton, FL 33431. |
| |
|
|
|
|
|
|
| Items of Business |
|
|
1. |
|
|
Election of seven director nominees named in this proxy statement, each for a term of one year expiring at our 2025 annual meeting of stockholders or until their respective successors are duly elected and qualified (the "Director Election Proposal”); |
| |
|
|
|
|
|
|
| |
|
|
2. |
|
|
Approval of an amendment to our 2020 Omnibus Equity Incentive Plan to: (1) increase the number of shares of Common Stock authorized for issuance thereunder from 58,374 shares to 1,500,000 shares and to increase the number of shares that otherwise become available under the plan for grants as incentive stock options (“ISOs”) from 250,000 to 750,000, and (2) to increase the number of shares that may be granted to any one non-employee director of the Board from 5 to 250,000 (the “Equity Plan Amendment Proposal”); |
| |
|
|
|
|
|
|
| |
|
|
3. |
|
|
To
approve, in accordance with Nasdaq Listing Rule 5635, the issuance of: (i) up to 12,373,226 shares of Common Stock, subject to adjustment,
upon conversion of the Company’s Series G Non-Voting Convertible Preferred Stock, par value $0.0001 per share, (ii) the issuance
of shares upon exercise of certain assumed ImmunogenX, Inc. (“ImmunogenX” or “IMGX”) stock options exercisable
for an aggregate of 200,652 shares of Common Stock (the “Assumed Options”), (iii) the issuance of shares upon exercise
of certain assumed ImmunogenX warrants exercisable for an aggregate of 127,680 shares of Common Stock (the “Assumed Warrants”),
and (iv) the prior issuance of 36,830 shares of Common Stock to the stockholders of ImmunogenX (the “Nasdaq Proposal”).
Entero agreed to all of such issuances in connection with its acquisition of ImmunogenX (the “IMGX Transaction”) pursuant
to that certain Agreement and Plan of Merger, dated March 13, 2024 (the “Merger Agreement”), by and among Entero, IMMUNO
Merger Sub I, Inc., IMMUNO Merger Sub II, LLC, and ImmunogenX, all as described in further detail herein; |
| |
|
|
|
|
|
|
| |
|
|
4. |
|
|
Adoption and approval of an amendment to our Amended and Restated Certificate of Incorporation, as amended (the “Charter”), to effect a reverse stock split of our issued and outstanding shares of Common Stock, as a specific ratio, ranging from [●] to [●], at any time prior to the one-year anniversary date of the Annual Meeting, with the exact ratio to be determined by the Board without further approval or authorization of our stockholders (the “Reverse Split Proposal”); |
| |
|
|
|
|
|
|
| |
|
|
5. |
|
|
Approval, on an advisory basis, of the executive compensation of the Company’s named executive officers as described in this proxy statement (the “Say on Pay Proposal”); |
| |
|
|
|
|
|
|
| |
|
|
6. |
|
|
Ratification
of Forvis Mazars, LLP, as our independent registered public accounting firm for the fiscal year ending December 31, 2024 (the “Auditor
Ratification Proposal”); and |
| |
|
|
|
|
|
|
| |
|
|
7. |
|
|
Approval of the adjournment of the annual meeting to the extent there are insufficient proxies at the annual meeting to approve any one or more of the foregoing proposals (the “Adjournment Proposal”). |
| |
|
|
|
|
|
|
| Adjournments and Postponements |
|
|
Any action on the items of business described above may be considered at the 2024 Annual Meeting of Stockholders (the “Annual Meeting”) at the time and on the date specified above or at any time and date to which the Annual Meeting may be properly adjourned or postponed. |
| |
|
|
|
|
|
|
| Record Date |
|
|
[●] 2024 (the “Record Date”). Only stockholders of record holding shares of our Common Stock, par value $0.0001 per share (the “Common Stock”) as of the close of business on the Record Date are entitled to notice of and to vote at the Annual Meeting. |
| |
|
|
|
|
|
|
| Meeting Admission |
|
|
You are invited to attend the Annual Meeting if you are a stockholder of record or a beneficial owner of shares of our Common Stock as of the Record Date. |
| |
|
|
|
|
|
|
| Availability of Proxy
Materials |
|
|
This
proxy statement is dated [●], 2024 and is first being mailed to the stockholders of Entero on or about [●], 2024. Our proxy
materials and the Annual Report for the year ended December 31, 2023 are also available on the internet at: www.viewproxy.com/ENTO/2024. |
| |
|
|
|
|
|
|
| Voting |
|
|
If your shares are held in the name of a bank, broker or other fiduciary, please follow the instructions on the proxy card. Whether or not you expect to attend the Annual Meeting, we urge you to submit your proxy to vote your shares as promptly as possible by following the instructions on your proxy card so that your shares may be represented and voted at the Annual Meeting. Your vote is very important. |
The
Proposals are described in the accompanying proxy statement, which we encourage you to read in its entirety before voting.
Only holders of record of Common Stock at the close of business on [●], 2024 are entitled
to notice of the Annual Meeting and to vote and have their votes counted at the Annual Meeting and any adjournments or postponements
of the Annual Meeting. A complete list of Entero stockholders of record entitled to vote at the Annual Meeting will be available for
ten days before the Annual Meeting at the principal executive offices of Entero for inspection by stockholders during ordinary business hours
for any purpose germane to the Annual Meeting.
The Entero Board unanimously recommends
that Entero stockholders vote “FOR” each of the foregoing proposals.
The existence of any financial and personal
interests of one or more of Entero’s directors may be argued to result in a conflict of interest on the part of such director(s) between
what he, she or they may believe is in the best interests of Entero and its stockholders and what he, she or they may believe is best
for himself, herself or themselves in determining to recommend that stockholders vote for the proposals. See the section entitled “Interests
of Entero’s Directors and Executive Officers in the Proposals” in the accompanying proxy statement for a further discussion
of this issue.
Assuming a quorum is present at the Annual
Meeting, the proposals require the affirmative vote of the majority of the votes cast by stockholders present or represented by proxy
and entitled to vote on the matter at the Annual Meeting. Please vote by proxy over the internet or telephone using the instructions
included with the accompanying proxy card, or promptly complete your proxy card and return it in the enclosed postage-paid envelope,
in order to authorize the individuals named on your proxy card to vote your shares of Entero common stock at the Annual Meeting. If you
hold your shares through a broker, bank or other nominee in “street name” (instead of as a registered holder) please follow
the instructions on the voting instruction form provided by your bank, broker or nominee to vote your shares. The list of Entero stockholders
entitled to vote at the Annual meeting will be available at Entero’s headquarters during regular business hours for examination
by any Entero stockholder for any purpose germane to the Annual Meeting for a period of at least ten days prior to the Annual Meeting.
PLEASE
VOTE AS PROMPTLY AS POSSIBLE, WHETHER OR NOT YOU PLAN TO ATTEND THE ANNUAL MEETING. IF YOU LATER DESIRE TO REVOKE OR CHANGE YOUR PROXY
FOR ANY REASON, YOU MAY DO SO IN THE MANNER DESCRIBED IN THE ACCOMPANYING PROXY STATEMENT. FOR FURTHER INFORMATION CONCERNING THE
PROPOSALS BEING VOTED UPON, THE MERGER AGREEMENT, THE IMGX TRANSACTION, USE OF THE PROXY AND OTHER RELATED MATTERS, YOU ARE URGED TO READ
THE ACCOMPANYING PROXY STATEMENT.
| |
BY ORDER OF THE BOARD OF DIRECTORS, |
| |
|
| |
|
| Boca Raton, Florida |
JAMES SAPIRSTEIN |
| [●], 2024 |
President, Chief Executive Officer and
Chairman of the Board of Directors |
IF YOU RETURN YOUR PROXY CARD WITHOUT AN INDICATION
OF HOW YOU WISH TO VOTE, YOUR SHARES WILL BE VOTED IN FAVOR OF EACH OF THE PROPOSALS.
This proxy statement is dated [●], 2024
and is first being mailed to the stockholders of Entero on or about [●], 2024.
TABLE OF CONTENTS
REFERENCES TO ADDITIONAL INFORMATION
The
accompanying proxy statement incorporates important business and financial information about Entero Therapeutics, Inc. (“we”,
“us”, “our”, “Entero”, or the “Company”) from other documents
that Entero has filed with the U.S. Securities and Exchange Commission (“SEC”) and that are not contained in and are
instead incorporated by reference in the accompanying proxy statement. For a list of documents incorporated by reference in the accompanying
proxy statement, see “Where You Can Find More Information.” This information is available for you, without charge,
to review through the SEC’s website at www.sec.gov.
You may request a copy of the accompanying
proxy statement, any of the documents incorporated by reference in the accompanying proxy statement or other information filed with the
SEC by Entero, without charge, by written request directed to the following contact:
Entero Therapeutics, Inc.
Attention: Chief Financial Officer
777 Yamato Road, Suite 502
Boca Raton, FL 33431
(561) 589-7020
In order for you to receive timely delivery
of the documents in advance of the annual meeting of Entero stockholders to be held on [●], 2024, which is referred to as the “Annual
Meeting,” you must request the information no later than [●], 2024.
If you have any questions about the Annual
Meeting or need to obtain a proxy card or other information, please contact Entero’s proxy solicitor at:
Alliance Advisors LLC
200 Broadacres Drive, 3rd Floor, Bloomfield,
NJ 07003
866-407-1875
The
contents of the websites of the SEC, Entero, ImmunogenX or any other entity are not incorporated in the accompanying proxy statement.
The information about how you can obtain certain documents that are incorporated by reference in the accompanying proxy statement
at these websites is being provided only for your convenience.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING
STATEMENTS
This proxy statement, and the documents incorporated
by reference into this proxy statement, includes certain “forward-looking statements” within the meaning of, and subject
to the safe harbor created by, Section 27A of the Securities Act, Section 21E of the Exchange Act and the Private Securities
Litigation Reform Act of 1995, which are referred to as the “safe harbor provisions.” Statements contained or incorporated
by reference in this proxy statement that are not historical facts are forward-looking statements, including statements regarding Entero’s
or ImmunogenX’s business and future financial and operating results, and other aspects of Entero’s or ImmunogenX’s
operations or operating results. Words such as “may,” “should,” “will,” “believe,” “expect,”
“anticipate,” “target,” “project,” and similar phrases that denote future expectations or intent
regarding Entero’s or ImmunogenX’s financial results, operations, and other matters are intended to identify forward-looking
statements that are intended to be covered by the safe harbor provisions. Investors are cautioned not to rely upon forward-looking statements
as predictions of future events. The outcome of the events described in these forward-looking statements is subject to known and unknown
risks, uncertainties, and other factors that may cause future events to differ materially from the forward-looking statements in this
proxy statement, including:
| |
● |
risks relating to fluctuations
of the market value of Entero Common Stock, including as a result of uncertainty as to the long-term
value of the common stock of Entero or as a result of broader stock market movements; |
| |
● |
Entero stockholders who receive
shares of Entero Common Stock as a result of the conversion of any Series G Preferred Stock will
have rights as Fist Wave common stockholders that differ from their current rights as preferred stockholders; |
| |
● |
our ability to commercialize Latiglutenase and integrate the assets and commercial operations
acquired into Entero’s business; |
| |
● |
failure to attract, motivate and retain executives and other key employees; |
| |
● |
disruptions in the business of Entero or ImmunogenX, which could have an adverse effect on their
respective businesses and financial results; and |
| |
● |
the unaudited pro forma condensed combined financial information in this proxy statement is presented
for illustrative purposes only and may not be reflective of the operating results and financial condition of the combination of Entero
and ImmunogenX. |
The forward-looking statements contained in
this proxy statement are also subject to additional risks, uncertainties, and factors, including those described in financial statements
of Fist Wave included in this proxy statement, as well as Entero’s most recent Annual Report on Form 10-K and Quarterly Reports
on Form 10-Q and other documents filed by either of them from time to time with the SEC. See the section titled “Where
You Can Find More Information.”
The forward-looking statements included in proxy statement are
made only as of the date hereof. Entero does not undertake to update, alter or revise any forward-looking statements made in this proxy
statement to reflect events or circumstances after the date of this proxy statement or to reflect new information or the occurrence of
unanticipated events, except as required by law.
SUMMARY TERM SHEET
For your convenience, provided below is
a brief summary of certain information contained in this proxy statement. This summary highlights selected information from this proxy
statement and does not contain all of the information that may be important to you as an Entero stockholder. To understand the IMGX Transaction
fully and for a more complete description of the terms of the IMGX Transaction, you should read carefully this entire proxy statement,
its annexes and the other documents to which you are referred. You may obtain the information incorporated by reference in this proxy
statement, without charge, by following the instructions under “Where You Can Find More Information.”
The IMGX Transaction
On March 13, 2024, we
acquired ImmunogenX, Inc., a Delaware corporation (“IMGX” or “ImmunogenX”), in accordance with
the terms of an Agreement and Plan of Merger, dated March 13, 2024 (the “Merger Agreement”), by and among the Company, IMMUNO
Merger Sub I, Inc., a Delaware corporation (“First Merger Sub”), IMMUNO Merger Sub II, LLC, a Delaware limited liability
company (“Second Merger Sub”), and ImmunogenX. Pursuant to the Merger Agreement, On March 13, 2024, First Merger Sub
merged with and into ImmunogenX, pursuant to which ImmunogenX was the surviving corporation (the “First Merger”).
Immediately following the First Merger, ImmunogenX merged with and into Second Merger Sub, pursuant to which Second Merger Sub was the
surviving entity and a wholly owned subsidiary of the Company (the “Second Merger” and together with the First Merger,
the “Merger”). The Second Merger Sub amended its certificate of formation concurrently with the Second Merger to change
its company name to ImmunogenX, LLC. The Merger closed on March 13, 2024.
For a more fulsome description
of the Merger, please see the section titled “Description of the IMGX Transaction”.
The Parties to the IMGX Transaction
| · | Entero
Therapeutics, Inc. |
Entero is engaged in the
research and development of targeted, non-systemic therapies for the treatment of patients with gastrointestinal (“GI”)
diseases. Non-systemic therapies are non-absorbable drugs that act locally, i.e., in the intestinal lumen, skin or mucosa, without reaching
an individual’s systemic circulation.
Entero is currently focused
on developing a therapeutic pipeline with multiple late-stage clinical programs built around four proprietary technologies: Latiglutenase
(which was acquired in the merger with ImmunogenX), a targeted oral biotherapeutic for celiac disease designed to breakdown gluten into
non-immunogenic peptides; the biologic Adrulipase, a recombinant lipase enzyme designed to enable the digestion of fats and other nutrients
in cystic fibrosis and chronic pancreatitis patients with exocrine pancreatic insufficiency; Capeserod, a selective 5-HT4 receptor partial
agonist which we are developing as a gastroparesis therapeutic; and Niclosamide, an oral small molecule with anti-inflammatory properties
for patients with inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease.
Entero shares are listed
for trading on The Nasdaq Capital Market under the symbol “ENTO.” For more information, please visit Entero’s website
at http://www.enterothera.com. Entero’s principal executive offices are located at 777 Yamato Road, Suite 502, Boca Raton,
FL 33431, and its telephone number is (561) 589-7020.
| · | ImmunogenX,
Inc. (IMGX) |
Founded in 2013 as a California
LLC, ImmunogenX was a private, clinical stage, biotherapeutics company, headquartered in Newport Beach, California, with a research facility
in North Carolina, formed to address critical needs for individuals with suspected or diagnosed celiac disease. On January 1, 2021, ImmunogenX
converted to a corporation and incorporated in the state of Delaware. Research efforts are focused on therapy, disease management, and
food safety. ImmunogenX has patented formulations related to the use of Latiglutenase, a targeted oral biotherapeutic for celiac disease
designed to breakdown gluten into non-immunogenic peptides. Another key area of research involves a method of monitoring intestinal villi
atrophy by measuring the level of a drug metabolite that is strongly dependent on the villi heath.
ImmunogenX has been primarily
engaged in developing its biopharmaceutical products, research and development, and raising capital through equity and debt offerings.
ImmunogenX has not commenced principal revenue producing operations and has incurred significant expenditures for the research and development
of ImmunogenX’s biopharmaceutical products.
| · | IMMUNO
Merger Sub I, Inc. |
IMMUNO Merger Sub I, Inc. was formed by Entero
solely for the purpose of engaging in the transactions contemplated by the Merger Agreement and did not engage in any business activities
other than in connection with the transactions contemplated by the Merger Agreement.
| · | IMMUNO
Merger Sub II, LLC |
IMMUNO Merger Sub II,
Inc. was formed by Entero solely for the purpose of engaging in the transactions contemplated by the Merger Agreement and did not engage
in any business activities other than in connection with the transactions contemplated by the Merger Agreement.
The Merger Agreement
On March 13, 2024, Entero,
ImmunogenX, First Merger Sub, and Second Merger Sub entered into the Merger Agreement, a copy of which is attached as Annex B to this
proxy statement. The Entero Board of Directors has unanimously approved the Merger Agreement and declared the Merger Agreement advisable
and in the best interests of Entero. Entero encourages you to carefully read the Merger Agreement in its entirety because it is the primary
legal document governing the Merger. For a more detailed description of the Merger Agreement, please see the section titled “Description
of the IMGX Transaction”.
Merger Consideration
Pursuant to the Merger
Agreement, in exchange for the outstanding shares of capital stock of ImmunogenX immediately prior to the effective time of the First
Merger, on March 13, 2024 Entero issued to the stockholders of ImmunogenX an aggregate of (A) 36,830 shares of common stock of Entero,
par value $0.0001 per share (the “Common Stock”), and (B) 11,777.418 shares of Series G Preferred Stock (as defined
and described below), each share of which is convertible into 1,000 shares of Common Stock, subject to certain conditions described below.
In addition, Entero assumed (i) all ImmunogenX stock options immediately outstanding prior to the First Merger, each becoming an option
to purchase Common Stock subject to adjustment pursuant to the terms of the Merger Agreement (the “Assumed Options”)
and (ii) all ImmunogenX warrants immediately outstanding prior to the First Merger, each becoming a warrant to purchase Common Stock
subject to adjustment pursuant to the terms of the Merger Agreement (the “Assumed Warrants”). The Assumed Options
are exercisable for an aggregate of 200,652 shares of Common Stock, have an exercise price of $0.81 and expire between February 1, 2031
and June 6, 2033. The Assumed Warrants are exercisable for and aggregate of 127,682 shares of Common Stock, have exercise prices ranging
from $3.02 to $3.92 and expire between September 30, 2032 and September 6, 2033. For more information regarding the consideration paid
in the Merger, please see the sections titled “Description of the IMGX Transaction” and “Proposal 3: Nasdaq
Proposal”.
Closing Date
The Merger closed on March
13, 2024.
Entero’s Reasons for the IMGX
Transaction and Recommendation of the Entero Board
After careful consideration,
on March 13, 2024, the Entero Board of Directors deemed it advisable and in the best interests of Entero to approve and adopt the Merger
Agreement.
For a description of factors
considered by the Entero Board in reaching its decision to approve the Merger Agreement and the transactions contemplated thereby, including
the IMGX Transaction, and additional information on the recommendation of the Entero Board, see the section titled “Nasdaq Proposal
- Background of the IMGX Transaction” and “Nasdaq Proposal - Board Reasons for the Approval of the IMGX
Transaction”.
Risks Relating to the IMGX Transaction
You should carefully consider
all of the risk factors together with all of the other information in this proxy statement before deciding how to vote. The risks relating
to the IMGX Transaction and the Contemplated Transactions are described under the caption “Risk Factors” in this proxy
statement. The principal risks relating to the IMGX Transaction and the Contemplated Transactions include the following:
| |
● |
Company stockholders
may not realize a benefit from the Merger commensurate with the ownership dilution they will experience in connection with the transactions. |
| |
|
|
| |
● |
Pursuant to the terms
of the Merger Agreement, we are required to recommend that our stockholders approve the conversion of shares of our Series G Preferred
Stock into shares of our Common Stock. We cannot guarantee that our stockholders will approve this matter, and if they fail to do
so we may be required to settle such shares in cash and our operations may be materially harmed. |
| |
|
|
| |
● |
The failure to successfully
integrate the businesses of the Company and IMGX in the expected timeframe would adversely affect our future results. |
| |
|
|
| |
● |
The failure to successfully
integrate the businesses of the Company and IMGX in the expected timeframe would adversely affect our future results. |
| |
|
|
| |
● |
The issuance or conversion
of securities would result in significant dilution in the equity interest of existing stockholders and adversely affect the marketplace
of the securities. |
| |
|
|
| |
● |
In connection with the
Merger, we assumed significantly more indebtedness. Our level of indebtedness and our ability to make payments on or service our
indebtedness could adversely affect our business, financial condition, results of operations, cash flow and liquidity. |
Stockholders Meeting
In
addition to the other matters to be considered at the Annual Meeting, pursuant to the terms of the Merger Agreement and in accordance
with Nasdaq Listing Rule 5635, we are recommending to our stockholders that they approve the issuance of: (i) up to 12,373,226
shares of Common Stock, subject to adjustment, upon conversion of the Company’s Series G Non-Voting Convertible Preferred Stock,
par value $0.0001 per share, (ii) the issuance of shares upon exercise of the Assumed Options, (iii) the issuance of shares upon exercise
of the Assumed Warrants, and (iv) the prior issuance of 36,830 shares of Common Stock to the stockholders of ImmunogenX (the “Nasdaq
Proposal”). See the section titled “Proposal 3: Nasdaq Proposal” for more information regarding the purpose
of the Nasdaq Propsal, a description of the Series G Preferred Stock and background of the IMGX Transaction.
| · | Stockholders
Entitled to Vote |
All holders of record
of shares of Entero Common Stock who held shares at the close of business on [●], 2024, the record date, are entitled to receive
notice of, and to vote at, the Annual Meeting. Attendance at the Annual Meeting is not required to vote. As of the record date, there
were [●] shares of Entero Common Stock issued and outstanding and entitled to vote at the Annual Meeting. Each share of Entero
Common Stock as of the record date is entitled to one vote on the Nasdaq Proposal.
At the Annual Meeting,
a quorum requires the presence of both (i) the holders of one-third of the voting power of the shares of the capital stock of the Company
issued and outstanding and entitled to vote at the Annual Meeting, and (ii) the holders of at least one-third of the shares of Common
Stock issued and outstanding and entitled to vote at the Annual Meeting must be represented at the Annual Meeting, either in person,
by means of remote communication in a manner, if any, authorized by the Board in its sole discretion, or represented by proxy. Abstentions
and broker non-votes, if any, will be included in determining whether a quorum is present at the Annual Meeting.
The approval of the Nasdaq
Proposal requires the affirmative vote of the majority of the votes cast by stockholders present or represented by proxy and entitled
to vote on the matter at the Annual Meeting.
See the section titled
“The Annual Meeting — Methods of Voting” for instructions on how to vote without attending the
Annual Meeting.
Interests of Entero’s Officers
and Directors in the IMGX Transaction
As of the date of this
proxy statement, Entero’s directors and executive officers do not have interests in the proposals that are different from, or in
addition to, the interests of other Entero stockholders generally, except that:
| |
● |
Dr. Jack Syage, our
President, Chief Scientific Officer, and Director, is a holder of 15,400 shares of Common Stock and 4,920.037 shares of Series G Preferred
Stock. |
| |
● |
Dr. Chaitan Khosla, a Director,
is a holder of 440 shares of Common Stock and 140.70 shares of Series G Preferred Stock. |
Stockholder Appraisal Rights
Our stockholders have no dissenter’s
or appraisal rights in connection with any of the proposals described herein.
Third Party Consents and Regulatory
Approvals
No third-party consents
or regulatory approvals were required prior to the consummation of the Merger other than the consent of Mattress Liquidators, Inc., which
was required under the Credit Agreement, dated October 3, 2022 (as amended by that certain Modification of Loan Documents, dated as of
September 6, 2023, the “Credit Agreement”), by and between ImmunogenX, Inc. and Mattress Liquidators, Inc. Such consent
was provided under the Second Modification of Loan Documents, dated March 13, 2024. See the section entitled “Description of
the IMGX Transaction – Debt of ImmunogenX” for information regarding the Credit Agreement.
Management of the Combined Company
Except for the change
in President as described herein, the officers and directors of Entero immediately prior to the Merger continued to be officers and directors
of the combined company immediately after the Merger, each to serve until the earlier of his or her resignation or removal or the due
election and qualification of his or her successor, in each case in accordance with the Entero Amended and Restated Certificate of Incorporation
and Amended and Restated Bylaws. On March 13, 2024, Jack Syage, former CEO of ImmunogenX, was appointed as Chief Operating Officer, President,
and Director of the combined company and Dr. Chaitan Khosla was appointed as a Director of the combined company. James
Sapirstein, the Company’s Chief Executive Officer and Chairman, had served as the Company’s President until the appointment
of Dr. Syage.
Material Federal Income Tax Consequences
of the Merger
The Merger is intended
to qualify as a tax-free reorganization for U.S. federal income tax purposes.
Opinion of Entero’s Financial
Advisor
Ladenburg Thalmann & Co. Inc. (“Ladenburg”) was retained by Entero
to render a fairness opinion (the “Opinion”) regarding the IMGX Transaction to the Entero Board of Directors. The
full text of the Opinion is attached to this proxy statement as Annex C. For more information regarding the Opinion of Ladenburg, including
details regarding the financial analyses Ladenburg performed, please see the section titled “Proposal 3: Nasdaq Proposal –
Opinion of Entero’s Financial Advisor”).
FREQUENTLY ASKED QUESTIONS
The following questions and answers briefly
address some questions that you, as an Entero stockholder, may have regarding the matters being considered at the Annual Meeting. You
are urged to carefully read this proxy statement and the other documents referred to in this proxy statement in their entirety because
this section may not provide all the information that is important to you regarding these matters. See “Summary Term Sheet”
for a summary of important information regarding the Annual Meeting. Additional important information is contained in the annexes to,
and the documents incorporated by reference in, this proxy statement. You may obtain the information incorporated by reference in this
proxy statement, without charge, by following the instructions in the section titled “Where You Can Find More Information.”
Why am I receiving this proxy statement?
We sent you this proxy statement because our
Board is soliciting your proxy to vote at the Annual Meeting that Entero is holding to seek stockholder approval on certain matters described
in further detail herein. This proxy statement summarizes the information you need to vote at the Annual Meeting. You do not need to
attend the Annual Meeting to vote your shares.
What is being voted on?
You are being asked to vote on seven proposals:
| 1. |
Election
of seven director nominees named in this proxy statement, each for a term of one year expiring at our 2025 annual meeting of stockholders
or until their respective successors are duly elected and qualified (the “Director Election Proposal”); |
| |
|
| 2. |
Approval
of an amendment to our 2020 Omnibus Equity Incentive Plan to: (1) increase the number of shares of Common Stock authorized for issuance
thereunder from 58,374 shares to 1,500,000 shares and to increase the number of shares that otherwise become available under the
plan for grants as incentive stock options (“ISOs”) from 250,000 to 750,000, and (2) to increase the number of shares
that may be granted to any one non-employee director of the Board from 5 to 250,000 (the “Equity Plan Amendment Proposal”);
|
| |
|
| 3. |
To
approve, in accordance with Nasdaq Listing Rule 5635, the issuance of: (i) up to 12,373,226 shares of Common Stock, subject to adjustment,
upon conversion of the Company’s Series G Non-Voting Convertible Preferred Stock, par value $0.0001 per share, (ii) the issuance
of shares upon exercise of certain assumed ImmunogenX, Inc. (“ImmunogenX” or “IMGX”) stock options exercisable
for an aggregate of 200,652 shares of Common Stock (the “Assumed Options”), (iii) the issuance of shares upon exercise
of certain assumed ImmunogenX warrants exercisable for an aggregate of 127,680 shares of Common Stock (the “Assumed Warrants”),
and (iv) the prior issuance of 36,830 shares of Common Stock to the stockholders of ImmunogenX (the “Nasdaq Proposal”).
Entero agreed to all of such issuances in connection with its acquisition of ImmunogenX (the “IMGX Transaction”) pursuant
to that certain Agreement and Plan of Merger, dated March 13, 2024 (the “Merger Agreement”), by and among Entero, IMMUNO
Merger Sub I, Inc., IMMUNO Merger Sub II, LLC, and ImmunogenX, all as described in further detail herein; |
| |
|
| 4. |
Adoption
and approval of an amendment to our Amended and Restated Certificate of Incorporation, as amended (the “Charter”), to
effect a reverse stock split of our issued and outstanding shares of Common Stock, as a specific ratio, ranging from [●] to
[●], at any time prior to the one-year anniversary date of the Annual Meeting, with the exact ratio to be determined by the
Board without further approval or authorization of our stockholders (the “Reverse Split Proposal”); |
| |
|
| 5. |
Approval,
on an advisory basis, of the executive compensation of the Company’s named executive officers as described in this proxy statement
(the “Say on Pay Proposal”); |
| |
|
| 6. |
Ratification
of Forvis Mazars, LLP, as our independent registered public accounting firm for the fiscal year ending December 31, 2024 (the “Auditor
Ratification Proposal”); and |
| |
|
| 7. |
Approval
of the adjournment of the annual meeting to the extent there are insufficient proxies at the annual meeting to approve any one or
more of the foregoing proposals (the “Adjournment Proposal”). |
When are this proxy statement and the accompanying
materials scheduled to be sent to stockholders?
On or about [●], 2024, we will begin mailing
our proxy materials, including the Notice of the Annual Meeting, this proxy statement, and the accompanying proxy card or, for shares
held in street name (i.e., shares held for your account by a broker or other nominee), a voting instruction form.
When and where will the Annual Meeting take
place?
The Annual Meeting will be held on [●],
2024 at [●] Eastern Time at our corporate headquarters at 777 Yamato Rd., Suite 502, Boca Raton, FL 33431.
When is the record date for the Annual Meeting?
The record date for determination of stockholders
entitled to vote at the Annual Meeting is the close of business on [●], 2024, which we refer to as the “record date.”
Who is entitled to vote at the Annual Meeting?
All holders of record of shares of Entero
Common Stock who held shares at the close of business on [●], 2024, the record date, are entitled to receive notice of, and to
vote at, the Annual Meeting. Attendance at the Annual Meeting is not required to vote. See below and the section titled “The Annual
Meeting — Methods of Voting” for instructions on how to vote without attending the Annual Meeting.
Does my vote matter?
Yes, your vote is very important, regardless of
the number of shares that you own.
How does the Entero Board recommend that I
vote at the Annual Meeting?
The Entero Board unanimously recommends that
Entero stockholders vote “FOR” each of the proposals.
Why should I vote for the Nasdaq Proposal?
We are subject to the Nasdaq Rules because our
Common Stock is currently listed on the Nasdaq Capital Market.
Pursuant to Nasdaq Rule 5635(a), stockholder approval
is required prior to the issuance by the Company of Common Stock (or securities convertible into or exercisable for Common Stock) in connection
with the acquisition of the stock or assets of another company if, due to the present or potential issuance of common stock, including
shares issued pursuant to an earn-out provision or similar type of provision, or securities convertible into or exercisable for common
stock, other than a public offering for cash: (A) the common stock has or will have upon issuance voting power equal to or in excess of
20% of the voting power outstanding before the issuance of stock or securities convertible into or exercisable for common stock; or (B)
the number of shares of common stock to be issued is or will be equal to or in excess of 20% of the number of shares of common stock outstanding
before the issuance of the stock or securities. Additionally, pursuant to Nasdaq Rule 5635(b), stockholder approval is required prior
to certain issuances with respect to Common Stock or securities convertible into Common Stock which could result in a change of control
of the issuer. Although Nasdaq has not adopted any rule on what constitutes a “change of control” for purposes of Rule 5635(b),
Nasdaq has previously indicated that the acquisition of, or right to acquire, by a single investor or affiliated investor group, as little
as 20% of the common stock (or securities convertible into or exercisable for common stock) or voting power of an issuer could constitute
a change of control.
Combined with the shares issued in the IMGX Transaction,
the shares issuable upon (i) conversion of the Series G Preferred Stock, (ii) exercise of the Assumed Warrants, and (iii) exercise of
the Assumed Options would result in the issuance of more than 20% of the voting power and the number of shares of Common Stock outstanding
as of the issuance of the Series G Preferred Stock. As a result of the foregoing, in accordance with Nasdaq Rule 5635(a) and (b), the
Series G Certificate of Designation provides that the Series G Preferred Stock will not be convertible into Common Stock until such time
as we obtain stockholder approval for their removal, as discussed in “Proposal 3: Nasdaq Proposal.”
If stockholders do not approve the Nasdaq Proposal,
the Company will not be able to honor any conversions of Series G Preferred Stock held by the former shareholders of ImmunogenX (see “Description
of the IMGX Transaction”).
Why should I vote for the Reverse Split Proposal?
Our Board has determined that it is advisable
and in the best interests of the Company and its stockholders, for us to amend our Charter to authorize our Board to effect a reverse
stock split (the “Charter Amendment”) of our issued and outstanding shares of Common Stock at a specific ratio, ranging from
[●] to [●] (the “Approved Split Ratios”), to be determined by the Board (the “Reverse Split”). A vote
for this Reverse Split Proposal will constitute approval of the Reverse Split that, once authorized by the Board and effected by filing
the Charter Amendment with the Secretary of State of the State of Delaware, will combine between [●] and [●] shares of our
Common Stock into one share of our Common Stock. If implemented, the Reverse Split will have the effect of decreasing the number of shares
of our Common Stock issued and outstanding.
Accordingly, stockholders are asked to adopt and
approve the Charter Amendment set forth in Annex D to effect the Reverse Split as set forth in the Charter Amendment, subject to the Board’s
determination, in its sole discretion, whether or not to implement the Reverse Split, as well as the specific ratio within the range of
the Approved Split Ratios, and provided that the Reverse Split must be effected on or prior to the one-year anniversary date of the Annual
Meeting. The text of Annex D remains subject to modification to include such changes as may be required by the Secretary of State of the
State of Delaware and as our Board deems necessary or advisable to implement the Reverse Split.
If adopted and approved by the holders of our
outstanding voting securities, the Reverse Split would be applied at an Approved Split Ratio approved by the Board prior to the one-year
anniversary date of the Annual Meeting. The Board reserves the right to elect to abandon the Reverse Split if it determines, in its sole
discretion, that the Reverse Split is no longer in the best interests of the Company and its stockholders.
Why should I vote for the Equity Plan Amendment
Proposal?
The general purpose of our 2020 Omnibus Equity
Incentive Plan (the “Plan”), is to benefit our Company and its shareholders by assisting the Company and its subsidiaries
to attract, retain and provide incentives to key management employees, officers, directors, and consultants of the Company and its affiliates,
and to align the interests of such service providers with those of the Company’s shareholders.
Our Board believes that the granting of stock
options, restricted stock awards, restricted stock units, stock appreciation rights and similar kinds of cash-based and equity-based compensation
promotes continuity of management and provides a critical incentive to align the interests of those who are primarily responsible for
shaping and carrying out our long range plans and securing our growth and financial success with the interests of our shareholders.
If the Company’s stockholders do not approve
the increase in the number of shares available for issuance under the Plan, the Company will continue to operate the Plan under its current
provisions, but will be limited in its ability to make future grants and incentives under the Plan to individuals we believe are, and
in the future, will be critical to the Company’s success.
Why should I vote for the Say on Pay Proposal?
The Board believes that the Company’s compensation
policies and practices are effective in achieving our goals of motivating and retaining executives by (i) rewarding excellence in leadership
and sustained financial performance, and (ii) aligning our executives’ interests with those of our stockholders to create long-term
value.
Why should I vote for the Auditor Ratification
Proposal?
Mazars USA LLP (“Mazars”)
has served as the Company’s independent registered public accounting firm since July, 2022. On June 1, 2024, Mazars entered into
a transaction with FORVIS, LLP (“FORVIS”) whereby substantially all of the partners and employees of Mazars joined
FORVIS. As a result, on the effective date of June 1, 2024, FORVIS changed its name to Forvis Mazars, LLP (“Forvis Mazars”)
and Mazars resigned as the Company’s independent public accounting firm. Our Audit Committee has appointed Forvis Mazars to serve
as the Company’s independent registered public accounting firm effective June 1, 2024, subject to ratification by the Company’s
stockholders. Our Audit Committee and Board believe that stability and continuity in the Company’s auditor is important as we advance
our business plan.
Why should I vote for the Adjournment Proposal?
If the Adjournment Proposal is not approved,
the Entero Board may not be able to adjourn the Annual Meeting to another time and place if necessary or appropriate to permit the solicitation
of additional proxies if there are insufficient votes at the time of the Annual Meeting to approve the Director Election Proposal, the
Equity Plan Amendment Proposal, the Nasdaq Proposal, the Reverse Split Proposal, the Say on Pay Proposal, or the Auditor Ratification
Proposal.
Will stockholders have the ability to unwind
the IMGX Transaction if they do not approve the Nasdaq Proposal?
No, the IMGX Transaction closed on March 13,
2024, and stockholder approval of the Nasdaq Proposal was not a pre-condition to closing the IMGX Transaction. If stockholders have not
approved the conversion of the Series G Preferred Stock into Common Stock by September 13, 2024, at
the request of the holder setting forth such holder’s request to cash settle a number of shares of Series G Preferred Stock, the
Company shall pay to such holder an amount in cash equal to (i) the Fair Value (as defined below) of the shares of Common Stock underlying
the shares of Series G Preferred Stock set forth in such request multiplied by (ii) the Conversion Ratio (as defined in the Certificate
of Designation of the Series G Preferred Stock) in effect on the trading day on which the
request is delivered to Entero. The “Fair Value” of shares shall be fixed with
reference to the last reported closing stock price on the principal trading market of the Common Stock.
What is a proxy?
A proxy is a stockholder’s legal designation
of another person to vote shares owned by such stockholder on their behalf. If you are a stockholder of record, you can vote by proxy
over the internet or by mail by following the instructions provided in the enclosed proxy card, or, by telephone if you are an Entero
stockholder of record. If you hold shares beneficially through a broker, bank or other nominee in “street name,” you should
follow the voting instructions provided by your broker, bank or other nominee.
How many votes do I have at the Annual
Meeting?
Each Entero stockholder is entitled to one
vote on each proposal for each share of Common Stock held of record at the close of business on the record date. At the close of business
on the record date, there were [●] shares of Common Stock outstanding.
What constitutes a quorum for the Annual Meeting?
A quorum is the minimum number of shares required
to be represented, either through virtual attendance or through representation by proxy, to hold a valid meeting.
In order for any business to be conducted at the
Annual Meeting, both (i) the holders of one-third of the voting power of the shares of the capital stock of the Company issued and outstanding
and entitled to vote at the Annual Meeting, and (ii) the holders of at least one-third of the shares of Common Stock issued and outstanding
and entitled to vote at the Annual Meeting must be represented at the Annual Meeting, either in person, by means of remote communication
in a manner, if any, authorized by the Board in its sole discretion, or represented by proxy. If a quorum is not present at the scheduled
time of the Annual Meeting, the Board, the chairman of the meeting or, if directed to be voted on by the chairman of the meeting, the
stockholders present or represented at the Annual Meeting and entitled to vote thereon, although less than a quorum, may adjourn the Annual
Meeting until a quorum is present. The date, time and place and the means of remote communication, if any, of the adjourned Annual Meeting
will be announced at the time the adjournment is taken, and no other notice will be given unless the adjournment is for more than 30 days,
in which case a notice of the adjourned meeting will be given to each stockholder of record entitled to vote at the Annual Meeting. An
adjournment will have no effect on the business that may be conducted at the Annual Meeting.
Since the Auditor Ratification Proposal is considered
a routine matter, shares held in “street name” through a broker, bank or other nominee will be counted as present for the
purpose of determining the existence of a quorum if such broker, bank or other nominee does not have instructions to vote on such proposal.
How can I vote my shares at the Annual
Meeting?
Shares held directly in your name as an Entero
stockholder of record may be voted at the Annual Meeting.
If
you hold your shares through a stockbroker, nominee, fiduciary or other custodian you may also be able to vote through a program provided
through Alliance Advisors LLC (“Alliance”) that offers Internet voting options. If your shares are held in an account at a
brokerage firm or bank participating in the Alliance program, you are offered the opportunity to elect to vote via the Internet. Votes
submitted via the Internet through the Alliance program must be received by 11:59 p.m. the day before the Annual Meeting.
Even if you plan to attend the Annual Meeting,
Entero recommends that you vote by proxy in advance as described below so that your vote will be counted if you later decide not to or
become unable to attend the Annual Meeting.
For additional information on attending the Annual
Meeting, see the section titled “The Annual Meeting.”
How can I vote my shares without attending
the Annual Meeting?
Whether you hold your shares directly as a
stockholder of record of Entero or beneficially in “street name,” you may direct your vote by proxy without attending the
Annual Meeting.
If you are a stockholder of record, you can vote
by proxy:
| |
● |
by Internet 24 hours a day, seven days a week, until 11:59 p.m. Eastern Time on the day before the Annual Meeting (have your proxy card in hand when you visit the website); |
| |
● |
by telephone in accordance with the instructions on your proxy card, until 11:59 p.m. Eastern Time on the day before the Annual Meeting (have your proxy card in hand when you call); or |
| |
● |
by completing and mailing your proxy card in accordance with the instructions provided on the proxy card. |
If you hold shares beneficially in “street
name,” you should follow the voting instructions provided by your bank, broker, or other nominee. If you hold your shares through
a stockbroker, nominee, fiduciary or other custodian you may also be able to vote through a program provided through Alliance that offers
Internet voting options. If your shares are held in an account at a brokerage firm or bank participating in the Alliance program, you
are offered the opportunity to elect to vote via the Internet. Votes submitted via the Internet through the Alliance program must be received
by 11:59 p.m. Eastern Time on the day before the Annual Meeting.
For additional information on voting procedures,
see the section titled “The Annual Meeting.”
What stockholder vote is required for the approval
of each proposal at the Annual Meeting?
The Equity Plan Amendment Proposal, Nasdaq Proposal,
Reverse Split Proposal, the Say on Pay Proposal, the Auditor Ratification Proposal and the Adjournment Proposal require the affirmative
vote of the majority of the votes cast by stockholders present or represented by proxy and entitled to vote on the matter at the Annual
Meeting.
The Director Election Proposal requires the affirmative
vote of a plurality of votes cast by stockholders present or represented by proxy and entitled to vote on the matter at the Annual Meeting.
What is a “broker non-vote?”
Under Nasdaq rules, banks, brokers and other nominees
may use their discretion to vote “uninstructed” shares (i.e., shares of record held by banks, brokers or other nominees, but
with respect to which the beneficial owner of such shares has not provided instructions on how to vote on a particular proposal) with
respect to matters that are considered to be “routine,” but not with respect to “non-routine” matters. All proposals
other than the Auditor Ratification Proposal are “non-routine” matters.
A “broker non-vote” occurs on
a proposal when (i) a broker, bank or other nominee has discretionary authority to vote on one or more proposals to be voted on
at a meeting of stockholders, but is not permitted to vote on other proposals without instructions from the beneficial owner of the shares,
and (ii) the beneficial owner fails to provide the broker, bank or other nominee with such instructions. The Auditor Ratification
Proposal is the only matter for which Entero expects there to be broker non-votes.
What will happen if I fail to vote or
abstain from voting on each proposal at the Annual Meeting?
An abstention represents a stockholder’s
affirmative choice to decline to vote on a proposal. If a stockholder indicates on its proxy card that it wishes to abstain from voting
its shares, or if a broker, bank or other nominee holding its customers’ shares of record causes abstentions to be recorded for
shares, these shares will be considered present and entitled to vote at the annual meeting. Under Delaware law and our Amended and Restated
Bylaws (our “Bylaws”), abstentions, if any, with respect to the Director Election Proposal, the Equity Plan Amendment Proposal,
the Nasdaq Proposal, the Reverse Split Proposal, the Say on Pay Proposal, the Auditor Ratification Proposal, and the Adjournment Proposal
are not counted as votes cast on the matter and therefore will not affect the outcome of such proposal. Abstentions will be counted for
purposes of determining the presence or absence of a quorum at the Annual Meeting.
What is the difference between holding shares
as a stockholder of record and as a beneficial owner of shares held in “street name”?
If your shares of Common Stock are registered
directly in your name with the transfer agent of Entero, you are considered the stockholder of record with respect to those shares. As
the stockholder of record, you have the right to vote directly at the Annual Meeting. You may also grant a proxy directly to Entero,
or to a third party to vote your shares at the Annual Meeting.
If your shares of Common Stock are held by brokerage
firm, bank, dealer or other similar organization, trustee, or nominee, you are considered the beneficial owner of shares held in “street
name.” Your brokerage firm, bank, dealer or other similar organization, trustee, or nominee will send you, as the beneficial owner,
a package describing the procedures for voting your shares. You should follow the instructions provided by your brokerage firm, bank,
dealer or other similar organization, trustee, or nominee to vote your shares.
If you hold your shares of Common Stock through
a stockbroker, nominee, fiduciary or other custodian you may also be able to vote through a program provided through Alliance that offers
Internet voting options. If your shares of Common Stock are held in an account at a brokerage firm or bank participating in the Alliance
program, you are offered the opportunity to elect to vote via the Internet. Votes submitted via the Internet through the Alliance program
must be received by 11:59 p.m. Eastern Time on the day before the Annual Meeting.
If my shares of Common Stock are held in “street
name” by my brokerage firm, bank, dealer or other similar organization, trustee, or nominee, will my brokerage firm, bank, dealer
or other similar organization, trustee, or nominee automatically vote those shares for me?
No. Your bank, broker or other nominee will only
be permitted to vote your shares of Common Stock at the Annual Meeting if you instruct your bank, broker or other nominee. You should
follow the procedures provided by your bank, broker or other nominee regarding the voting of your shares. Banks, brokers and other nominees
who hold shares of Common Stock in “street name” for their customers have authority to vote on “routine” proposals
when they have not received instructions from beneficial owners. However, banks, brokers and other nominees are prohibited from exercising
their voting discretion with respect to non-routine matters, which includes all proposals other than the Auditor Ratification Proposal.
As a result, absent specific instructions from the beneficial owner of such shares, banks, brokers and other nominees are not empowered
to vote such shares on such proposals.
What should I do if I receive more
than one set of voting materials for the Annual Meeting?
If you hold shares of Common Stock in “street
name” and also directly in your name as a stockholder of record or otherwise, or if you hold shares of Common Stock in more than
one brokerage account, you may receive more than one set of voting materials relating to the Annual Meeting.
Record
Holders. For shares held directly, please vote by proxy over the internet or by telephone, using the instructions included
with the accompanying proxy card, or promptly complete your proxy card and return it in the enclosed postage-paid envelope, in order to
ensure that all of your shares of Common Stock are voted.
Shares
Held in “Street Name.” For shares held in “street name” through a bank, broker or other nominee,
you should follow the procedures provided by bank, broker or other nominee to submit a proxy or vote your shares.
If a stockholder gives a proxy, how are the
shares of Common Stock voted?
Regardless of the method you choose to vote, the
individuals named on the enclosed proxy card will vote your shares of Common Stock in the way that you indicate. For each item before
the Annual Meeting, you may specify whether your shares of Common Stock should be voted “for” or “against,” or
abstain from voting.
For more information regarding how your shares
will be voted if you properly sign, date and return a proxy card, but do not indicate how your Common Stock should be voted, see below
“— How will my shares be voted if I return a blank proxy?”
How will my shares be voted if I return
a blank proxy?
If you sign, date and return your proxy and
do not indicate how you want your shares of Common Stock to be voted, then your shares of Common Stock will be voted in accordance with
the recommendation of the Entero Board, “FOR” each of the proposals.
Can I change my vote after I have
submitted my proxy?
Any Entero stockholder giving a proxy has
the right to revoke the proxy and change their vote before the proxy is voted at the Annual Meeting by doing any of the following:
| |
● |
subsequently submitting a new proxy for the Annual Meeting that is received by the deadline specified on the accompanying proxy card; |
| |
● |
giving written notice
of your revocation to Entero’s Chief Financial Officer or |
| |
● |
attending and voting at the Annual Meeting. Note that a proxy will not be revoked if you attend, but do not vote at, the Annual Meeting. |
Execution or revocation of a proxy will not in
any way affect your right to attend and vote at the Annual Meeting. See the section titled “The Annual Meeting — Revocability
of Proxies.”
If I hold my shares in “street name,”
can I change my voting instructions after I have submitted voting instructions to my bank, broker or other nominee?
If your shares are held in the name of a bank,
broker or other nominee and you previously provided voting instructions to your bank, broker or other nominee, you should follow the instructions
provided by your bank, broker or other nominee to revoke or change your voting instructions.
Where can I find the voting results of
the Annual Meeting?
The preliminary voting results for the Annual
Meeting are expected to be announced at the Annual Meeting. In addition, within four Business Days following certification of the
final voting results, Entero will file the final voting results of the Annual Meeting (or, if the final voting results have not yet been
certified, the preliminary results) with the SEC on a Current Report on Form 8-K.
Do Entero stockholders have dissenters’
or appraisal rights?
The stockholders of Entero are not entitled
to appraisal rights in connection with the proposals at the Annual Meeting under Delaware law.
What happens if I sell my shares of Common
Stock after the record date but before the Annual Meeting?
The record date is earlier than the date of the
Annual Meeting. If you sell or otherwise transfer your shares of Common Stock after the record date but before the Annual Meeting, you
will, unless special arrangements are made, retain your right to vote at the Annual Meeting.
Who will solicit and pay the cost of soliciting
proxies?
Entero has engaged Alliance Advisors LLC,
which is referred to as “Alliance,” to assist in the solicitation of proxies for the Annual Meeting. Entero estimates that
it will pay Alliance a fee of approximately $32,400, plus reimbursement for certain out-of-pocket fees and expenses. Entero has agreed
to indemnify Alliance against various liabilities and expenses that relate to or arise out of its solicitation of proxies (subject to
certain exceptions).
Entero also may reimburse banks, brokers and
other custodians, nominees and fiduciaries or their respective agents for their expenses in forwarding proxy materials to beneficial
owners of Common Stock. Entero directors, officers and employees also may solicit proxies by telephone, by electronic means or in person.
They will not be paid any additional amounts for soliciting proxies.
What should I do now?
You should read this proxy statement carefully
and in its entirety, including the annexes. Then, you may vote by proxy over the internet or by telephone, using the instructions included
with the accompanying proxy card, or promptly complete your proxy card and return it in the enclosed postage-paid envelope, so that your
shares will be voted in accordance with your instructions.
How can I find more information about
Entero?
You can find more information about Entero
from various sources described in the section titled “Where You Can Find More Information.”
Whom do I call if I have questions
about the Annual Meeting?
If you have questions about the Annual Meeting,
or desire additional copies of this proxy statement or additional proxies, you may contact Entero’s proxy solicitor:
Alliance Advisors LLC
200 Broadacres Drive, 3rd Floor, Bloomfield,
NJ 07003
866-407-1875
The Annual Meeting
The Annual Meeting will be
held on [●], 2024, beginning at [●] a.m., Eastern Time at our corporate headquarters at 777 Yamato Rd., Suite 502, Boca Raton,
FL 33431.
The purposes of the Annual
Meeting are as follows:
| 1. |
Election of seven director nominees named in this proxy statement, each for a term of one year expiring at our 2025 annual meeting of stockholders or until their respective successors are duly elected and qualified; |
| |
|
| 2. |
Approval of an amendment to our 2020 Omnibus Equity Incentive Plan to: (1) increase the number of shares of Common Stock authorized for issuance thereunder from 58,374 shares to 1,500,000 shares and to increase the number of shares that otherwise become available under the plan for grants as incentive stock options (“ISOs”) from 250,000 to 750,000, and (2) to increase the number of shares that may be granted to any one non-employee director of the Board from 5 to 250,000 (the “Equity Plan Amendment Proposal”); |
| |
|
| 3. |
To
approve, in accordance with Nasdaq Listing Rule 5635, the issuance of: (i) up to 12,373,226 shares of Common Stock, subject to adjustment,
upon conversion of the Company’s Series G Non-Voting Convertible Preferred Stock, par value $0.0001 per share, (ii) the issuance
of shares upon exercise of certain assumed ImmunogenX, Inc. (“ImmunogenX” or “IMGX”) stock options exercisable
for an aggregate of 200,652 shares of Common Stock (the “Assumed Options”), (iii) the issuance of shares upon exercise
of certain assumed ImmunogenX warrants exercisable for an aggregate of 127,680 shares of Common Stock (the “Assumed Warrants”),
and (iv) the prior issuance of 36,830 shares of Common Stock to the stockholders of ImmunogenX (the “Nasdaq Proposal”).
Entero agreed to all of such issuances in connection with its acquisition of ImmunogenX (the “IMGX Transaction”) pursuant
to that certain Agreement and Plan of Merger, dated March 13, 2024 (the “Merger Agreement”), by and among Entero, IMMUNO
Merger Sub I, Inc., IMMUNO Merger Sub II, LLC, and ImmunogenX, all as described in further detail herein; |
| |
|
| 4. |
Adoption and approval of an amendment to our Amended and Restated Certificate of Incorporation, as amended (the “Charter”), to effect a reverse stock split of our issued and outstanding shares of Common Stock, as a specific ratio, ranging from [●] to [●] at any time prior to the one-year anniversary date of the Annual Meeting, with the exact ratio to be determined by the Board without further approval or authorization of our stockholders (the “Reverse Split Proposal”); |
| |
|
| 5. |
Approval, on an advisory basis, of the executive compensation of the Company’s named executive officers as described in this proxy statement (the “Say on Pay Proposal”); |
| |
|
| 6. |
Ratification
of Forvis Mazars, LLP, as our independent registered public accounting firm for the fiscal year ending December 31, 2024 (the
“Auditor Ratification Proposal”); and |
| |
|
| 7. |
Approval of the adjournment of the annual meeting to the extent there are insufficient proxies at the annual meeting to approve any one or more of the foregoing proposals (the “Adjournment Proposal”). |
A
quorum of Entero stockholders is necessary to conduct business at the Annual Meeting. The presence in person or by proxy of both (i)
the holders of one-third of the voting power of the shares of the capital stock of the Company issued and outstanding and entitled to
vote at the Annual Meeting, and (ii) the holders of at least one-third of the shares of Common Stock issued and outstanding and entitled
to vote at the Annual Meeting will constitute a quorum. Abstentions will count as votes present and entitled to vote for the purpose
of determining the presence of a quorum for the transaction of business at the Annual Meeting. Since the Auditor Ratification Proposal
is considered a routine matter, shares held in “street name” through a broker, bank or other nominee will be counted as present
for the purpose of determining the existence of a quorum if such broker, bank or other nominee does not have instructions to vote on
such proposal.
The Nasdaq Proposal, the Reverse
Split Proposal, the Equity Plan Amendment Proposal, the Say on Pay Proposal, the Auditor Ratification Proposal and the Adjournment Proposal
require the affirmative vote of the majority of the votes cast by stockholders present or represented by proxy and entitled to vote on
the matter at the Annual Meeting. The Director Election Proposal requires the affirmative vote of the plurality of the votes cast by stockholders
present or represented by proxy and entitled to vote on the matter at the Annual Meeting.
An abstention represents a
stockholder’s affirmative choice to decline to vote on a proposal. If a stockholder indicates on its proxy card that it wishes to
abstain from voting its shares, or if a broker, bank or other nominee holding its customers’ shares of record causes abstentions
to be recorded for shares, these shares will be considered present and entitled to vote at the annual meeting. Under Delaware law and
our Bylaws, abstentions, if any, with respect to the Director Election Proposal, the Equity Plan Amendment Proposal, the Nasdaq Proposal,
the Reverse Split Proposal, the Say on Pay Proposal, the Auditor Ratification Proposal, and the Adjournment Proposal are not counted as
votes cast on the matter and therefore will not affect the outcome of such proposal. Abstentions will be counted for purposes of determining
the presence or absence of a quorum at the Annual Meeting.
Interests of Entero’s Directors and
Executive Officers in the IMGX Transaction
As of the date of this
proxy statement, Entero’s directors and executive officers do not have interests in the proposals that are different from, or in
addition to, the interests of other Entero stockholders generally, except that:
| |
● |
Dr. Jack Syage, our President, Chief Scientific Officer, and Director, is a holder of 15,400 shares of Common Stock and 4,920.037 shares of Series G Preferred Stock. |
| |
● |
Dr. Chaitan Khosla,
a Director, is a holder of 440 shares of Common Stock and 140.70 shares of Series G Preferred Stock. |
Certain Beneficial Owners of Entero Common
Stock
At the close of business
on June 12, 2024, the latest practicable date prior to the date of this proxy statement, Entero directors and executive officers and
their affiliates, as a group, owned and were entitled to vote approximately 2% of the shares of Entero common stock.
DESCRIPTION OF THE IMGX TRANSACTION
On March 13, 2024, we
acquired ImmunogenX, Inc., a Delaware corporation, in accordance with the terms of the Merger Agreement, by and among us, IMMUNO Merger
Sub I, Inc., a Delaware corporation (“First Merger Sub”), IMMUNO Merger Sub II, LLC, a Delaware limited liability
company (“Second Merger Sub”), and IMGX. Pursuant to the Merger Agreement, First Merger Sub merged with and into IMGX,
pursuant to which IMGX was the surviving corporation (the “First Merger”). Immediately following the First Merger,
IMGX merged with and into Second Merger Sub, pursuant to which Second Merger Sub was the surviving entity and a wholly owned subsidiary
of the Company (the “Second Merger” and, together with the First Merger, the “Merger”). The Second
Merger Sub amended its certificate of formation concurrently with the Second Merger to change its company name to ImmunogenX, LLC. The
Merger is intended to qualify as a tax-free reorganization for U.S. federal income tax purposes.
Under the terms of the
Merger Agreement, following the consummation of the Merger on March 13, 2024 (the “Closing”), in exchange for the
outstanding shares of capital stock of IMGX immediately prior to the effective time of the First Merger, we issued to the stockholders
of IMGX an aggregate of (A) 36,830 shares of Common Stock, and (B) 11,777.418 shares of Series G Preferred Stock (as defined and described
below), each share of which is convertible into 1,000 shares of Common Stock, subject to certain conditions described below. In addition,
we assumed (i) all IMGX stock options immediately outstanding prior to the First Merger, each becoming an option to purchase Common Stock
subject to adjustment pursuant to the terms of the Merger Agreement (the “Assumed Options”) and (ii) all IMGX warrants
immediately outstanding prior to the First Merger, each becoming a warrant to purchase Common Stock subject to adjustment pursuant to
the terms of the Merger Agreement (the “Assumed Warrants”). The Assumed Options are exercisable for an aggregate of
200,652 shares of Common Stock, have an exercise price of $0.81 and expire between February 1, 2031 and June 6, 2033. The Assumed Warrants
are exercisable for an aggregate of 127,682 shares of Common Stock, have exercise prices ranging from $3.02 to $3.92 and expire between
September 30, 2032 and September 6, 2033.
The foregoing description
of the Assumed Warrants does not purport to be complete and is qualified in its entirety by reference to the form of Assumed Warrant,
which is incorporated by reference to this proxy statement as Exhibit 4.43 to the Company’s Annual Report on Form 10-K for the fiscal
year ended December 31, 2023, filed with the SEC on March 29, 2024.
Tungsten Partners LLC (“Tungsten”)
acted as our financial advisor in connection with the Merger. As partial compensation for services rendered by Tungsten, we issued to
Tungsten or its affiliates or designees an aggregate of 18,475 shares of Common Stock and 595.808 shares of Series G Preferred Stock.
Pursuant to the Merger
Agreement, we have agreed to hold a stockholders’ meeting to submit the following matters to its stockholders for their consideration:
(i) the approval of the conversion of shares of Series G Preferred Stock into shares of Common Stock in accordance with the rules of
the Nasdaq Stock Market LLC (the “Conversion Proposal”) and (ii) if deemed necessary or appropriate by us or as otherwise
required by applicable law or contract, the approval of an amendment to our Charter, to authorize sufficient shares of Common Stock for
conversion of Series G Preferred Stock issued pursuant to the Merger Agreement (the “Share Increase Proposal” and
together with the Conversion Proposal, the “Meeting Proposals”). In connection with these matters, we have agreed
to file a proxy statement with the Securities and Exchange Commission (the “SEC”).
The Board of Directors of
Company (the “Board”) and the holders of a majority of our Series B Convertible Preferred Stock approved the Merger
Agreement and the related transactions, and the consummation of the Merger was not subject to approval of our stockholders.
The foregoing description
of the Merger and the Merger Agreement does not purport to be complete and is qualified in its entirety by reference to the full text
of the Merger Agreement, which is incorporated by reference to this proxy statement as Exhibit 2.2 to the Company’s Annual Report
on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March 29, 2024.
The Merger Agreement contains
representations, warranties and covenants that we and IMGX made to each other as of specific dates. The assertions embodied in those representations,
warranties and covenants were made solely for purposes of the Merger Agreement between us and IMGX and may be subject to important qualifications
and limitations agreed to by us and IMGX in connection with negotiating its terms, including being qualified by confidential disclosures
exchanged between the parties in connection with the execution of the Merger Agreement. Moreover, the representations and warranties may
be subject to a contractual standard of materiality that may be different from what may be viewed as material to investors or securityholders,
or may have been used for the purpose of allocating risk between us and IMGX rather than establishing matters as facts. Moreover, information
concerning the subject matter of the representations and warranties may change after the date of the Merger Agreement, which subsequent
information may or may not be fully reflected in our public disclosures. For the foregoing reasons, no person should rely on the representations
and warranties as statements of factual information at the time they were made or otherwise.
Voting and Support Agreements
In connection with the execution
of the Merger Agreement, together with IMGX, we entered into stockholder voting agreements (the “IMGX Voting Agreements”)
with certain of IMGX’s stockholders. Pursuant to the IMGX Voting Agreements, among other things, each of the IMGX stockholder parties
thereto has agreed to vote or cause to be voted all of the shares of Common Stock owned by such stockholder in favor of the Share Increase
Proposal (if such proposal is brought before our stockholders).
The foregoing description
of the IMGX Voting Agreements does not purport to be complete and is qualified in its entirety by reference to the full text of the form
of the IMGX Voting Agreement, a copy of which is included as Exhibit A to the Merger Agreement, which is incorporated herein by reference
to Exhibit 2.2 of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March
29, 2024.
In connection with the execution
of the Merger Agreement, together with IMGX, we entered into stockholder support agreements (the “Company Support Agreements”)
with certain of our officers (solely in their capacity as our stockholders). Pursuant to the Support Agreements, among other things, each
of our stockholder parties thereto has agreed to vote or cause to be voted all of the shares of Common Stock owned by such stockholder
in favor of the Meeting Proposals.
The foregoing description
of the Company Support Agreements does not purport to be complete and is qualified in its entirety by reference to the full text of the
form of the Company Support Agreement, a copy of which is included as Exhibit B to the Merger Agreement, which is incorporated herein
by reference to Exhibit 2.2 of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with
the SEC on March 29, 2024.
Lock-up Agreements
Concurrently and in connection with the execution
of the Merger Agreement, certain IMGX stockholders as of immediately prior to the Closing, and certain of our officers as of immediately
prior to the Closing, entered into lock-up agreements with us and ImmunogenX, pursuant to which each such stockholder agreed to be subject
to a 180-day lockup on the sale or transfer of our shares held by each such stockholder at the Closing, including those shares of Common
Stock and Series G Preferred Stock (including the shares of Common Stock into which such Series G Preferred Stock is convertible) received
by each such stockholder in the Merger (the “Lock-up Agreements”).
The foregoing description of the Lock-up Agreements
does not purport to be complete and is qualified in its entirety by reference to the full text of the form of the Lock-up Agreement, a
copy of which is included as Exhibit C to the Merger Agreement, which is incorporated herein by reference to Exhibit 2.2 of the Company’s
Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March 29, 2024.
Restrictive Covenants Agreements
Concurrently and in connection
with the execution of the Merger Agreement, certain IMGX officers as of immediately prior to the Closing, entered into restrictive covenants
agreements with us and IMGX, pursuant to which such individuals and each of their affiliates agreed not to compete with IMGX during the
three-year period following the Closing and, during such three-year restricted period, not to solicit Restricted Employees (as defined
in the form of Restrictive Covenants Agreement) (the “Restrictive Covenants Agreements”). The Restrictive Covenants
Agreements also contain customary non-disparagement and confidentiality provisions.
The foregoing description
of the Restrictive Covenants Agreement does not purport to be complete and is qualified in its entirety by reference to the full text
of the form of the Restrictive Covenants Agreement, a copy of which is included as Exhibit D to the Merger Agreement, which is incorporated
herein by reference to Exhibit 2.2 of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed
with the SEC on March 29, 2024.
Debt of ImmunogenX
IMGX is party to that certain
Credit Agreement, dated as of October 3, 2022 (as amended by that certain Modification of Loan Documents, dated as of September 6, 2023,
the “Credit Agreement”), with Mattress Liquidators, Inc. (“Lender”), pursuant to which Lender provided
IMGX with a secured revolving credit facility with a total commitment of $7,500,000. The loans under the Credit Agreement bore interest
at a per annum floating rate equal to the prime rate plus 4.50% and had a maturity date of October 3, 2024. In connection with the Merger,
IMGX entered into a Second Modification of Loan Documents, dated March 13, 2024 (the “Second Amendment,” and the Credit
Agreement as amended by the Second Amendment, the “Amended Credit Agreement”), which provides for, among other things:
(i) Lender’s consent for the Merger, (ii) Second Merger Sub’s assumption all of IMGX obligations, rights and liabilities as
borrower under the Amended Credit Agreement, (iii) an amendment to the interest rate to a per annum floating rate equal to the prime rate
plus 6.00%, (iv) an extension of the maturity date to September 13, 2025, (v) a prohibition on the borrower’s ability to request
additional loans under the revolving credit facility, subject to certain limited exceptions, (vi) the pledge by the stockholders of IMGX
(the “Pledgors”) of their equity interests of the Company as part of the collateral securing the obligations under
the Amended Credit Agreement (the “Pledged Equity”), and (vii) an amendment to the total commitment under the Amended
Credit Agreement to $8,212,345.17, which takes into account a one-time prepayment by IMGX to Lender in the amount of $1,000,000 (the “One-Time
Prepayment”), accrued interest and fees for the remaining term of the facility and certain other fees to be paid under the Amended
Credit Agreement. The Pledged Equity shall be subject to a call right, which shall give Lender the right to, at its option, require the
Pledgors to sell to Lender (on a pro rata basis) the number of shares of Pledged Equity that, when multiplied by the per share valuation
of the Common Stock at Closing, satisfies all or a portion of the then outstanding amount of obligations under the Amended Credit Agreement.
The borrowings under
the Amended Credit Agreement are by evidenced by that certain Second Amended and Restated Revolving Loan Promissory Note, dated March
13, 2024 (the “Note”), and are secured by substantially all of the assets of IMGX pursuant to that certain Security
Agreement, dated as of October 3, 2022, as amended (the “Security Agreement”), by and between IMGX and Lender.
On March 13, 2024, IMGX and
Lender entered into a lender support letter (the “Support Letter”), pursuant to which, among other things, IMGX and
Lender agreed that IMGX is permitted to license or transfer rights to any intellectual property which may constitute collateral under
the Amended Credit Agreement.
The Amended Credit Agreement
contains customary representations and warranties, affirmative covenants and negative covenants, including covenants that limit or restrict
the IMGX’s ability to, among other things, incur additional indebtedness, merge or consolidate, pay dividends or other distributions,
repurchase equity, dispose of assets and enter into certain transactions with affiliates, in each case subject to certain exceptions.
The Amended Credit Agreement also includes customary events of default, and upon the occurrence and during the continuation of an event
of default, Lender may declare the outstanding advances and all other obligations under the Amended Credit Agreement to be immediately
due and payable. Payments owed under the Credit Agreement are also personally guaranteed by Jack Syage (the “Guarantee”).
The foregoing descriptions
of the Amended Credit Agreement, the Note, the Security Agreement and the Support Letter do not purport to be complete and are qualified
in their entirety by reference to the Amended Credit Agreement, the Note, the Security Agreement and the Support Letter, which are incorporated
herein by reference to Exhibits 10.54, 10.55, 10.56 and 10.57 of the Company’s Annual Report on Form 10-K for the fiscal year ended
December 31, 2023, filed with the SEC on March 29, 2024.
Stockholder Notes
In order to fund the One-Time
Prepayment, on March 13, 2024, IMGX entered into (i) a secured promissory note in favor of Jack Syage, in an aggregate principal amount
of $500,000 (the “Syage Note”), and (ii) a secured promissory note in favor of Peter Felker, in an aggregate principal
amount of $500,000 (the “Felker Note” and collectively with the Syage Note, the “Shareholder Notes”).
The Shareholder Notes will bear interest at a per annum floating rate equal to the prime rate plus 4.50% and mature on October 13, 2025.
Each of Jack Syage and Peter Felker, who were shareholders of IMGX prior to the Merger, have entered into a patent and trademark security
agreement (the “Shareholder Security Agreements”), each dated March 13, 2024, pursuant to which the obligations under
each of the Shareholder Notes are secured by, among other things, the patents and trademarks owned by IMGX.
The foregoing descriptions
of the Shareholder Notes and the Shareholder Security Agreements do not purport to be complete and are qualified in their entirety by
reference to the Form of Shareholder Note and Form of Shareholder Security Agreement, which are incorporated herein by reference to Exhibits
10.58 and 10.59 of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March
29, 2024.
Regulatory Approvals
No federal or state regulatory
approvals were required prior to the consummation of the Merger.
THE PARTIES TO THE
TRANSACTION
Entero Therapeutics, Inc.
Entero (formerly known
as First Wave BioPharma, Inc. prior to its corporate name change on May 17, 2024) is engaged in the research and development of targeted,
non-systemic therapies for the treatment of patients with gastrointestinal (“GI”) diseases. Non-systemic therapies
are non-absorbable drugs that act locally, i.e., in the intestinal lumen, skin or mucosa, without reaching an individual’s systemic
circulation.
Entero is currently focused
on developing a therapeutic pipeline with multiple late-stage clinical programs built around four proprietary technologies: Latiglutenase,
a targeted oral biotherapeutic for celiac disease (“CeD”) designed to breakdown gluten into non-immunogenic peptides;
the biologic Adrulipase, a recombinant lipase enzyme designed to enable the digestion of fats and other nutrients in cystic fibrosis
and chronic pancreatitis patients with exocrine pancreatic insufficiency; Capeserod, a selective 5-HT4 receptor partial agonist which
we are developing as a gastroparesis therapeutic; and Niclosamide, an oral small molecule with anti-inflammatory properties for patients
with inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease.
Entero shares are listed
for trading on The Nasdaq Capital Market under the symbol “ENTO.” For more information, please visit Entero’s website
at http://www.enterothera.com. Entero’s principal executive offices are located at 777 Yamato Road, Suite 502, Boca Raton,
FL 33431, and its telephone number is (561) 589-7020.
ImmunogenX Inc.
Founded in 2013 as a California
LLC, ImmunogenX was a private, clinical stage, biotherapeutics company, headquartered in Newport Beach, California, with a research facility
in North Carolina, formed to address critical needs for individuals with suspected or diagnosed celiac disease. On January 1, 2021, ImmunogenX
converted to a corporation and incorporated in the state of Delaware. Research efforts are focused on therapy, disease management, and
food safety. ImmunogenX has patented formulations related to the use of Latiglutenase a targeted oral biotherapeutic for celiac disease
designed to breakdown gluten into non-immunogenic peptides. Another key area of research involves a method of monitoring intestinal villi
atrophy by measuring the level of a drug metabolite that is strongly dependent on the villi heath.
ImmunogenX has been primarily
engaged in developing its biopharmaceutical products, research and development, and raising capital through equity and debt offerings.
ImmunogenX has not commenced principal revenue producing operations and has incurred significant expenditures for the research and development
of ImmunogenX’s biopharmaceutical products.
IMMUNO Merger Sub I, Inc.
IMMUNO Merger Sub I, Inc. was formed by Entero
solely for the purpose of engaging in the transactions contemplated by the Merger Agreement and did not engage in any business activities
other than in connection with the transactions contemplated by the Merger Agreement.
IMMUNO Merger Sub II, LLC
IMMUNO Merger Sub II,
Inc. was formed by Entero solely for the purpose of engaging in the transactions contemplated by the Merger Agreement and did not engage
in any business activities other than in connection with the transactions contemplated by the Merger Agreement.
INFORMATION ABOUT THE BUSINESS OF THE COMBINED
COMPANY
Overview
We are engaged in the
research and development of targeted, non-systemic therapies for the treatment of patients with gastrointestinal diseases. Non-systemic
therapies are non-absorbable drugs that act locally, i.e., in the intestinal lumen, skin or mucosa, without reaching an individual’s
systemic circulation.
We are currently focused on
developing a therapeutic pipeline with multiple late-stage clinical programs built around four proprietary technologies: Latiglutenase,
a targeted oral biotherapeutic for celiac disease designed to breakdown gluten into non-immunogenic peptides; the biologic Adrulipase,
a recombinant lipase enzyme designed to enable the digestion of fats and other nutrients in cystic fibrosis and chronic pancreatitis patients
with exocrine pancreatic insufficiency; Capeserod, a selective 5-HT4 receptor partial agonist which we are developing as a gastroparesis
therapeutic; and Niclosamide, an oral small molecule with anti-inflammatory properties for patients with inflammatory bowel diseases such
as ulcerative colitis and Crohn’s disease.
In March 2024, we announced
the closing of an acquisition, referred to as a merger (the “Merger”) with ImmunogenX, Inc. (“IMGX”),
a private, clinical-stage biopharmaceutical company founded in 2013, which is developing the biologic, Latiglutenase, for celiac disease.
IMGX is also developing CypCel, a metabolic marker compound that can measure the state of small-intestinal recovery of celiac patients
undergoing gluten-free diets (“GFDs”).
In December 2023, we announced
that we entered into a non-binding term (the “Niclosamide LOI”) sheet to sell our Niclosamide program (the “Niclosamide
Sale”). The Niclosamide LOI includes a low seven-figure upfront payment to us for rights to Niclosamide, as well as economics
related to future milestones and royalties.
The Niclosamide LOI only represents
a mutual indication of interest regarding the Niclosamide Sale and the terms of the Niclosamide Sale are subject to a number of contingencies,
including the completion of customary due diligence and the negotiation and execution of definitive agreements. Upon execution of the
definitive agreement, the completion of the transaction will be subject to, among other matters, satisfaction of the conditions negotiated
therein, the buyer having secured adequate financing, and receipt of all third party (including governmental) approvals, licenses, consents,
and clearances, as and when applicable.
There can be no assurance
that the Niclosamide Sale will be completed on the terms contemplated in the Niclosamide LOI or otherwise. In particular, the terms of
the potential transaction, the timing of closing, and the aggregate consideration that we may receive may materially differ from that
currently contemplated by the Niclosamide LOI.
Our Product Candidates
Our Latiglutenase program
is focused on the development of an orally administered, minimally-absorbed, biologic for improving multiple gluten-induced symptoms
and consequent quality of life (”QOL”) due to inadvertent gluten consumption in patients with celiac disease. Latiglutenase’s
mechanism of action involves the use of recombinant proteases to break down the gluten protein into non-immunogenic peptides.
Our Adrulipase programs
are focused on the development of an oral, non-systemic, biologic capsule for the treatment of exocrine pancreatic insufficiency (“EPI”)
in patients with cystic fibrosis (“CF”) and chronic pancreatitis (“CP”). Our goal is to provide
CF and CP patients with a safe and effective therapy to control EPI that is non-animal derived and offers the potential to dramatically
reduce their daily pill burden. In July 2023, we announced topline results from our Phase 2b monotherapy bridging study using a new enteric
microgranule formulation of Adrulipase. While the initial data from the study indicated that the enhanced Adrulipase formulation was
safe and well tolerated and demonstrated an improvement over prior formulations of Adrulipase, it also indicated that it is likely that
the primary efficacy endpoint was not achieved. We are continuing to assess this data, along with secondary efficacy endpoint data, and
plan to schedule a Type C meeting with the FDA in the second half of 2024 to discuss next steps for the Adrulipase program.
Our Capeserod program
was in-licensed from Sanofi in September 2023. Sanofi conducted Phase 1 and Phase 2 Central Nervous System (“CNS”)
trials with over 600 patients. In Sanofi’s CNS trials, Capeserod appeared safe and well-tolerated. Research on Capeserod and subsequent
artificial intelligence (“AI”) empowered analyses suggest that the drug possesses a unique mechanism of action that
is applicable to several GI indications underserved by currently available therapeutics. We plan to repurpose Capeserod for GI indications
and expect to initiate a Phase 2 clinical development program in gastroparesis in the second half of 2025. Sanofi retains the right of
first refusal to develop and commercialize Capeserod.
Our Niclosamide programs
leverage proprietary oral and topical formulations to address multiple potential GI conditions, including inflammatory bowel diseases
(“IBD”) indications. In 2022 we advanced four separate Phase 2 clinical programs of our Niclosamide formulations,
including FW-COV for Severe Acute Respiratory Syndrome Coronavirus 2 (“COVID-19”) GI infections, FW-UP for ulcerative
proctitis (“UP”) and ulcerative proctosigmoiditis (“UPS”), FW ICI AC for Immune Checkpoint Inhibitor
associated colitis (“ICI AC”), and FW CD for Crohn’s disease. We are no longer actively pursuing these programs
and intend to try and outlicense Niclosamide to a third party.
Each of our primary drug candidates
and clinical programs is described below.
Latiglutenase
Latiglutenase, which was formerly
identified as ALV003, is composed of two recombinant peptidases, IMGX001 and IMGX002, designed to work in tandem to breakdown gluten inadvertently
consumed into non-immunogenic peptides. IMGX001 is a modified recombinant version of cysteine endoprotease B, isoform 2 (EP-B2), which
naturally occurs from germinating barley (Hordeum vulgare). IMGX001 is a monomer with three intra-molecular disulfide bonds with an apparent
molecular weight of 27 kDa. IMGX002 is a modified recombinant version of a prolyl endopeptidase from the bacterium Sphingomonas capsulata
(SC-PEP) and is a monomer without cysteine residues with an apparent molecular weight of 67 kDa.
We hold the worldwide rights
to Latiglutenase.
Background
Celiac disease is a systemic
autoimmune disease triggered by gluten consumption in genetically susceptible individuals (Green and Cellier 2007). Currently approximately
1 – 1.4% of the world’s population is affected by celiac disease. There are no mechanisms to prevent the disease since little
is known about potential environmental triggers of the disease.
Gluten, the antigen responsible
for celiac disease, is the main protein present in some of the most common cereals (wheat, barley, rye). Modern diets are increasingly
enriched with gluten, and it is also used as an additive in processed foods, spices, cosmetics, and oral medications. Gluten is also present
in trace amounts in foods labelled as “gluten-free,” as a tableting excipient in drugs, and in products such as toothpaste
and lipstick. As little as 50 mg/day of gluten may trigger the disease. A normal diet contains >10 g/day (Hoppe 2017), 200 times the
amount that can cause damage and intestinal abnormalities in susceptible individuals. As such, patients with celiac disease face enormous
challenges in following a strict GFD due to cross-contamination of food and hidden sources of gluten in unexpected places.
Pre-Clinical Program
Pre-clinical toxicology is
complete. In these studies, Latiglutenase has been shown to efficiently degrade intact gluten containing disease-relevant gluten peptides.
In vitro, concentrations of latiglutenase from 0.25 to 0.9 mg/mL degrade > 90% of immunostimulatory peptides in the context of baked
wheat bread gluten (0.5 to 12 mg/mL) within 30 to 60 minutes. Latiglutenase activity on its substrate gluten is dependent on enzyme concentration
and time.
At pH values typical of a
postprandial stomach (3.5 to 5), IMGX001 has been shown to be active and stable. IMGX002 contributes to gluten digestion above pH 4. Therefore,
in these studies, ALV003 is active in the stomach following a meal.
Overall, in GLP toxicology
studies, ALV003 was well tolerated when administered for up to 6 months at oral doses as high as 360 mg/kg, with no adverse effects observed.
Developmental and Reproductive
Toxicology (“DART”) program is anticipated to begin in 2024 and take approximately 2 years to complete. As discussed
at the End-of Phase 2 meeting with the FDA, a waiver will be submitted for carcinogenicity on the basis that latiglutenase is composed
of two minimally absorbed enzymes that act and are degraded in the GI tract.
Clinical Program
Nine clinical studies with
ALV003 have been completed. These include single doses of ALV003 (100, 300, 900, and 1800 mg) (ALV003-0811) including an additional assessment
of gluten digestion (ALV003-0812). A six-week multiple dose study (ALV003-0921) was conducted in patients with 300 mg TID with 3 levels
of gluten challenge (1.5, 3 and 6 grams) in a decreasing fashion. A second 6-week study (ALV003-1021) assessed 900 mg TID with gluten
challenges at 1.5 and 2.1 g of gluten. These early studies demonstrated clinical activity down to 100 mg and doses up to 1800 mg were
well-tolerated. ALV003 was protective against gluten challenge. Most AEs were mild or moderate in severity.
ALV003 was also assessed as
a food processing agent (ALV003-0801). Sixteen (16) grams of gluten predigested with ALV003 was compared to 16 g gluten in 20 celiac patients.
Pre-digestion was protective against gluten injury. Two powder formulations were also tested for gluten degradation which was demonstrated
(ALV003-1111).
Three
PRO instruments (CDSD©, ICDSQ© and IGFDQ©)
were assessed in a non-treatment study, ALV003-1121. in newly diagnosed celiac patients receiving
sham gluten, 0.5 or 2.0 grams of gluten, or ALV003 digested gluten and evaluated for 8 weeks and showed good convergent validity with
reference to other related questionnaire measures, such as the GSRS and concept-specific and global PGI measures.
A Phase 2b dose ranging
study, ALV003-1221, was conducted evaluating histological changes including Vh:Cd (Villus
height; Crypt depth) and IELs (intraepithelial lymphocytes) and change in symptoms based upon CDSD© (celiac disease symptom diary).
Four hundred ninety-four (494) patients were randomized and entered the treatment phase of the study and received: placebo, 100 mg, 300
mg, 450 mg, 600 mg or 900 mg Latiglutenase. The primary analyses for histology failed to demonstrate superiority over placebo; all arms
improved due to a Hawthorne trial effect. However, statistically and clinically significant reductions in abdominal pain, bloating, tiredness,
and constipation, all components of the CDSD, were observed for the highest doses (600 mg and 900 mg) for patients who were baseline
seropositive.
IMGX003-NCCIH-1721 was a double-blind,
placebo-controlled, gluten-challenge trial to assess the efficacy, safety, and tolerability of 6 weeks of treatment with latiglutenase
or placebo in patients with well-controlled CeD. Latiglutenase protected the mucosa from deliberate gluten challenge (2g/day for 42 days)
compared to PBO. The PBO group had a clinically greater worsening in Vh:Cd (p = 0.057) and IEL count (p = 0.017) compared with the IMGX003.
Gluten Immunogenic Peptides (“GIP”) results demonstrated the MOA of latiglutenase and its ability to degrade gluten.
GIP results over the 42-day treatment period highly favored IMGX003 (between-group difference was strongly statistically significant,
p < 0.001). The AE profile was similar for each treatment group.
Over 500 patients have been
exposed to latiglutenase in doses up to 1,800 mg. In a phase 2b study (ALV003-1221) patients were given daily doses of ALV003 (100, 300,
450, 600 and 900 mg TID) for up to 24 weeks. The drug appeared to be well-tolerated and no dose-limiting toxicities were observed. GI
disorders and infections by system organ class were the most frequently reported AEs. The majority of these events were mild to moderate
in severity and felt by the investigator to be unrelated to ALV003. Thirty-six patients withdrew from the study due to AEs. GI disorders
were the most frequent AEs leading to study discontinuation across treatment groups and most of these GI events were moderate to severe
in severity and deemed to be related to the study medication. There were 5 unrelated serious adverse events in patients treated with latiglutenase,
2 in placebo patients, 1 in 300 mg and 2 in 900 mg dose arms.
Two additional studies, IMGX003-NIAID-1821
and IMGX003-NIDDK-1921, were terminated due to enrollment and other issues resulting from complications and societal restrictions of the
COVID-19 pandemic. In the former study, 34 patients were completed and the study is in the process of being closed. In
the latter study, no subjects were randomized and the study is closed.
Latiglutenase is expected
to enter Phase 3 development with two world-wide clinical trials in the first half of 2025.
Adrulipase
Adrulipase is the active pharmaceutical
ingredient (“API”) derived from Yarrowia lipolytica, an aerobic yeast naturally found in various foods such
as cheese and olive oil that is widely used as a biocatalyst in several industrial processes. Adrulipase is a secreted lipase naturally
produced by Yarrowia lipolytica, known as LIP2, that we are developing through recombinant DNA technology for the treatment of
EPI associated with CF and CP. Lipases are enzymes that help with the digestion of lipids and fat.
We previously held the exclusive
right to commercialize Adrulipase in the U.S., Canada, South America (excluding Brazil), Asia (excluding China and Japan), Australia,
New Zealand and Israel pursuant to a sublicense from Laboratories Mayoly Spindler SAS (“Mayoly”) under the Joint Research
and Development Agreement (“JDLA”), which also granted us joint commercialization rights for Brazil, Italy, China and
Japan. In March 2019, we purchased all rights, title and interest in and to Adrulipase from Mayoly pursuant to the Mayoly Asset Purchase
Agreement (“APA”), provided, however, Mayoly retained exclusive commercial rights in France and Russia.
Background
The pancreas is both an endocrine
gland that produces several important hormones, including insulin, glucagon, and pancreatic polypeptide, as well as a digestive organ
that secretes pancreatic juice containing digestive enzymes that assist with the absorption of nutrients and digestion in the small intestine.
The targeted indication of
Adrulipase is the treatment of EPI, which is observed when the exocrine functions of the pancreas are below 10% of normal. The symptomatology
of EPI is essentially due to the deficiency of pancreatic lipase, an enzyme that hydrolyses triglycerides into monoglycerides and free
fatty acids. The pancreatic lipase enzymatic activity is hardly compensated by extra-pancreatic mechanisms, because gastric lipase has
nearly no lipolytic activity in the pH range of the intestine. On the other hand, when they are impaired, the pancreatic amylase and protease
(enzymes that break up carbohydrates (starches) and proteins, respectively) activities can be compensated by the salivary amylase, the
intestinal glycosidase, the gastric pepsin, and the intestinal peptidases, all of which are components of the gastric juice secreted by
the stomach walls. Lipid maldigestion due to lipase deficiency is responsible for weight loss, steatorrhea featured by greasy diarrhea,
and fat-soluble vitamin deficiencies (i.e. A, D, E and K vitamins).
CP, the most common cause
of EPI, is a long-standing inflammation of the pancreas that alters its normal structure and functions. In the U.S., its prevalence rate
is of 42 cases per 100,000 inhabitants, resulting in approximately 132,000 cases. Approximately 60% of patients affected with CP display
EPI, resulting in approximately 90,000 patients requiring substitution therapy in the U.S. In Western societies, CP is caused by chronic
alcoholic consumption in approximately 55-80% of cases. Other relatively frequent etiologies include the genetic form of the disease that
is inherited as an autosomal dominant condition with variable penetrance, pancreatic trauma and idiopathic causes.
CF, another dominant etiology
of EPI, is a severe genetic disease associated with chronic morbidity and life-span decrease of most affected individuals. In most Caucasian
populations, CF prevalence is of 7-8 cases per 100,000 inhabitants, but is less common in other populations, resulting in more than 30,000
affected individuals in the U.S. and more than 70,000 affected individuals worldwide. CF is inherited as monogenic autosomal recessive
disease due to the defect at a single gene locus that encodes the Cystic Fibrosis Transmembrane Regulator protein, or CFTR, a regulated
chloride channel. Mutation of both alleles of this chloride channel gene results in the production of thick mucus, which causes a multisystem
disease of the upper and lower respiratory tracts, digestive system, and the reproductive tract. The progressive destruction of the pancreas
results in EPI that is responsible for malnutrition and contributes to significant morbidity and mortality. About 80-90% of patients with
CF develop EPI, resulting in approximately 25,000-27,000 patients in the U.S. that require substitution therapy.
Current treatments for EPI
stemming from CP and CF rely on porcine (pig derived) pancreatic enzyme replacement therapies (“PERTs”), which have
been on the market since the late 1800s. PERTs are typically comprised of three digestive enzymes; lipases, proteases, and amylases. The
PERT market is well established with estimated sales of approximately $1.4 billion in 2019 in the U.S. and has been growing for the past
five years at a compound annual growth rate of approximately 20%. In spite of their long-term use, however, PERTs suffer from poor stability,
formulation problems, possible transmission of conventional and non-conventional infectious agents due to their animal origins, and possible
adverse events at high doses in patients with CF and limited effectiveness.
Pre-Clinical Program
The efficacy of Adrulipase
has been investigated in normal minipigs, which are generally considered to be a relevant model for digestive drug development because
of their physiological similarities with humans and their omnivorous diet. Experimental pancreatitis was induced by pancreatic duct ligation,
resulting in severe EPI with baseline CFA around 60% post-ligature. CFA is a measurement obtained by quantifying the amount of fat ingested
orally over a defined time period and subtracting the amount eliminated in the stool to ascertain the amount of fat absorbed by the body.
Pigs were treated with either Adrulipase or enteric-coated PERTs, both administered as a single-daily dose.
At doses ranging from 10.5
to 211 mg, Adrulipase increased the CFA by +25 to +29% in comparison to baseline (p<0.05 at all doses), whereas the 2.5 mg dose had
milder activity. Similar efficacy was observed in pigs receiving 100,000 U lipase of enteric-coated porcine pancreatic extract. These
findings demonstrate the in vivo activity of Adrulipase in a relevant in vivo model at a level similar to the PERTs at dosages
of 10.5mg or greater.
To date, two non-clinical
toxicology studies have been conducted. Both show that Adrulipase lipase is clinically well tolerated at levels up to 1000mg/kg in rats
and 250 mg/kg in minipigs up to 13 weeks. Adrulipase is therefore considered non-toxic in both rodent and non-rodent species up to a maximum
feasible dose of 1,000 mg/kg/day in the rats over six months of administration.
Clinical Program
We are developing Adrulipase
for two principal therapeutic indications: (i) children and adults affected by CF, and (ii) adult patients with CP. We have determined
to initially pursue the adult indication in CF.
Chronic Pancreatitis
During 2010 and 2011, a phase
1/2a clinical trial of Adrulipase was conducted in conjunction with Mayoly in a single center in France. The study was an exploratory
study mainly designed to investigate the safety of Adrulipase and was a randomized, double blind, placebo controlled, parallel clinical
trial in 12 patients affected with CP or pancreatectomy and severe EPI. The primary efficacy endpoint of the study was defined as the
relative change in steatorrhea (an established surrogate biomarker of EPI correction) in comparison to baseline. The study found that
Adrulipase was well tolerated with no serious adverse events. Only two adverse events were observed: constipation (two patients out of
eight with Adrulipase) and hypoglycemia (two patients out of eight with Adrulipase, and one patient out of four with placebo). A non-statistically
significant difference of the primary endpoint, possibly due to the small group size, was found between the two groups both in intention-to-treat,
a group that included three patients who received the in-patient facility study diet but did not fulfill the protocol’s inclusion
criteria, and per-protocol analysis. This study was not designed, nor did it aim to demonstrate statistically significant changes of CFA
or steatorrhea under Adrulipase.
We received regulatory approval
in Australia and New Zealand in 2016, with the addition of a 2018 regulatory approval in France, to conduct a Phase 2 multi-center dose
escalation study of Adrulipase in CP and pancreatectomy. The primary endpoint of this study was to evaluate the safety of escalating doses
of Adrulipase in 11 CP patients. The secondary endpoint was to investigate the efficacy of Adrulipase in these patients by analysis of
the CFA and its change from baseline. In September 2018, we announced that in pre-planned analyses, both the study’s primary and
secondary endpoints were reached with a statistically significant (p=0.002) improvement in the CFA of 21.8%, in a per protocol analysis,
with the highest evaluated dose of 2,240 mg/day of Adrulipase. The statistical significance of the trial results is typically based on
widely used, conventional statistical methods that establish the p-value of the results. A p-value of 0.05 or less is required to demonstrate
statistical significance. As such, these CFA levels are considered to be statistically significant.
Cystic Fibrosis Monotherapy
In October 2018, the FDA cleared
our IND application for Adrulipase in patients with EPI due to CF. In December 2018, we initiated the Phase 2 OPTION Bridging Dose Study
to investigate Adrulipase in CF patients with EPI and in February 2019, we dosed the first patients. The Phase 2 OPTION Bridging Dose
Study investigated the safety, tolerability and efficacy of Adrulipase in a head-to-head comparison against the current PERT standard
of care. The OPTION Bridging Dose Study employed a six-week non-inferiority CFA primary efficacy endpoint comparing Adrulipase to PERTs.
In September 2019, we announced
positive results from the OPTION Bridging Dose Study. Results showed that the primary efficacy endpoint of CFA was comparable to the CFA
in a prior Phase 2 study in patients with CP, while using the same dosage of Adrulipase. The dosage used in the OPTION Bridging Dose Study
was 2.2 grams per day, which was determined in agreement with the FDA as a bridging dose from the highest safe dose used in the Phase
2 CP dose escalation study. Although the study was not powered for statistical significance, the data demonstrated meaningful efficacy
results, with approximately 50% of the patients showing CFAs high enough to reach non-inferiority with standard PERTs. Additionally, the
CNA was comparable between the Adrulipase and PERT arms, 93% vs. 97%, respectively, in the OPTION Bridging Dose Study. This important
finding confirms that protease supplementation is not likely to be required with Adrulipase treatment. A total of 32 patients, ages 18
or older, completed the OPTION Bridging Dose Study.
In October 2019, the CFF DSMB
completed its review of our final results of the OPTION Bridging Dose Study and found no safety concerns for Adrulipase and supported
our plan to proceed to the Phase 2b OPTION 2 Trial. In December 2019, we submitted the clinical trial protocol to the existing IND at
the FDA. In April 2020, we received approval to conduct the OPTION 2 Trial in Therapeutics Development Network clinical sites in the U.S.
The OPTION 2 Trial was designed
to investigate the safety, tolerability and efficacy of Adrulipase (2.2 gram and 4.4 gram doses in enteric capsules) head-to-head versus
the current standard of care, PERT pills. The OPTION 2 Trial was an open-label, crossover study, conducted in 15 sites in the U.S. and
Europe. Enrollment included a total of 30 CF patients 18 years or older. Adrulipase was administered in enteric capsules to provide gastric
protection and test for optimal delivery of enzyme to the duodenum. Patients were first randomized into two cohorts: the Adrulipase arm,
where they received a 2.2 gram daily oral dose of Adrulipase for three weeks; or the PERT arm, where they received their pre-study dose
of PERT pills for three weeks. After three weeks, stools were collected for analysis of CFA. Patients were then crossed over for another
three weeks of the alternative treatment. After three weeks of cross-over therapy, stools were again collected for analysis of CFA. A
parallel group of patients was randomized and studied in the same fashion using a 4.4 gram daily dose of Adrulipase. All patients were
followed for an additional two weeks after completing both crossover treatments for post study safety observation. Patients were assessed
using descriptive methods for efficacy, comparing CFA between Adrulipase and PERT arms, and for safety.
In January 2021, we announced
an additional study arm in OPTION 2 Trial using an immediate release Adrulipase capsules in order to identify the optimal dose and delivery
method of Adrulipase. This extension phase tested patients 18 years or older, who have already completed the crossover phase, at higher
doses relative to the previously conducted OPTION Bridging Dose Study. This allowed us to compare data from the existing crossover arm
using enteric (delayed release) capsules with data from the new immediate release extension arm.
In March 2021 we announced
topline OPTION 2 data. The trial demonstrated that Adrulipase was safe and well-tolerated and data from OPTION 2, and the other Adrulipase
Phase 2 clinical trials, demonstrated drug activity. However, OPTION 2 did not consistently meet the primary efficacy endpoint. Some patients
were able to achieve CFA at levels beyond what is required to demonstrate non-inferiority with PERT therapies, but the majority did not.
We believe that the underlying
cause of the drug’s uneven efficacy performance in the OPTION 2 trial was the enteric capsule formulation. While the enteric coating
protected the capsule from breaking down in the stomach acid, it also appeared to dissolve too slowly in the small intestine to release
the lipase enzyme in time to aid with proper digestion and nutrient absorption.
In August 2021 we announced
that we would begin development of a new enteric microgranule formulation of Adrulipase. The new formulation is planned to be administered
with food as an oral capsule that dissolves in the stomach and disperses acid-resistant micro-granules that thoroughly mix with food during
the digestion process. The resultant mixture then passes to the small intestine where the lipase enzyme breaks up fat molecules so that
they can be absorbed. We completed the reformulation work in the second half of 2022.
In November 2022 we announced
that we had filed an IND amendment with the FDA for a Phase 2b bridging study with the new enteric microgranulation formulation of Adrulipase.
The new trial is designed to investigate the safety, tolerability and efficacy of the new formulation of Adrulipase. It is an open-label
study that will be conducted at three sites in the U.S. A total of 12 cystic fibrosis patients, 18 years or older are expected to be enrolled.
The trial design employs a dose titration strategy. Patients will be screened at baseline to ensure that they have a coefficient of fat
absorption (CFA) of at least 80%. Eligible patients will then be switched from their commercial enzyme product to Adrulipase. Each patient
will be started on a low dose of Adrulipase. If the patient is not clinically controlled, the patient will be switched to a medium dose,
and if not controlled on this dose, the patient will be advanced to a high dose. The titrations will be carried out over a three-week
period, after which a CFA will be obtained. End of study CFAs will be compared to the baseline CFAs in a descriptive fashion. A post treatment
safety visit will be conducted one week after completing the treatment period.
Following FDA review of
the IND amendment, we initiated the Phase 2b pilot monotherapy trial during the first quarter of 2023 and in July 2023, announced topline
results from our Phase 2b study. While the initial data from the study indicated that the enhanced Adrulipase formulation was safe and
well tolerated and demonstrated an improvement over prior formulations of Adrulipase, it also indicated that it is likely that the primary
efficacy endpoint was not achieved. We are continuing to assess this data, along with secondary efficacy endpoint data, and plan to schedule
a Type C meeting with the FDA in the second half of 2024 to discuss next steps for the Adrulipase program.
Combination Therapy
We launched the Phase 2 Combination
Trial in Hungary in July 2019 to investigate Adrulipase, in combination with PERT, in CF patients who suffer from severe EPI but continue
to experience clinical symptoms of fat malabsorption despite taking the maximum daily dose of PERTs. The Combination Trial is designed
to investigate the safety, tolerability and efficacy of escalating doses of Adrulipase (700 mg, 1120 mg and 2240 mg per day, respectively),
in conjunction with a stable dose of PERTs, in order to increase CFA and relieve abdominal symptoms. In October 2020, we opened a total
of five clinical sites in Turkey and dosed the first patients in November 2020. In March 2021, we reached targeted minimum enrollment
of 18 patents.
We announced positive interim
data on the first five patients in the Combination Trial in August 2020. The primary efficacy endpoint was met, with CFAs greater than
80% for all patients across all visits. For secondary efficacy endpoints, we observed that stool weight decreased, the number of stools
per day decreased, steatorrhea improved, and body weight increased. Additionally, no serious adverse events were reported.
In August 2021, we announced
topline data collected from the 20 patients enrolled in the study. The data indicated that Adrulipase in combination with PERT led to
clinically meaningful improvements in CFA, the primary efficacy endpoint. Patients showed an average gain of more than six percentage
points from baseline, compared to the five-point improvement in CFA cited by the clinical literature as clinically significant. The study
also demonstrated positive improvements in weight gain and other secondary endpoints.
We believe a combination
therapy of PERT and Adrulipase has the potential to: (i) correct macronutrient and micronutrient maldigestion; (ii) eliminate abdominal
symptoms attributable to maldigestion; and (iii) sustain optimal nutritional status on a normal diet in CF patients with severe EPI.
We developed a new enteric microgranule formulation and initiated and completed a Ph2b monotherapy trial in 2023. We may elect to conduct
further combination therapy trials using the new formulation following the results of a Type C meeting with the FDA which we plan to
schedule in the second half of 2024.
Capeserod
Capeserod is a selective 5-HT4
receptor partial agonist, which we are repurposing and developing for multiple GI conditions, including gastroparesis. Gastroparesis is
a GI disease in which the movement of food from the stomach to the small intestine is delayed, often resulting in severe constipation.
The most common cause of gastroparesis is diabetes.
Gastroparesis can have a variety
of serious complications for patients including repeated vomiting leading to dehydration, malnutrition, difficulty controlling blood glucose,
low calorie intake, and more, leading to an overall decreased quality of life.
Based on the biological evidence
of expression of 5-HT4b receptor subtype in human GI tract, we expect that Capeserod may directly or indirectly initiate the peristaltic
or secretory reflex by releasing neurotransmitters that can decrease colonic transit time and improve bowel movement.
Background
In September 2023, we announced
an agreement with Sanofi (NASDAQ: SNY) to license Capeserod and repurpose and develop the drug for GI indications.
Under the terms of the agreement,
we will receive from Sanofi an exclusive, global license for Capeserod and will assume responsibility for all future clinical development.
The licensing agreement, which includes a modest upfront payment, backend milestone payments and single digit royalties on net sales,
provides a right of first refusal for Sanofi to reacquire Capeserod following certain stages of clinical development and to commercialize
the product.
Sanofi’s research
on Capeserod and the subsequent artificial intelligence empowered analyses suggest that the drug’s mechanism of action has potential
applications for several gastrointestinal disorders in multibillion-dollar markets where there are significant unmet clinical needs.
Sanofi previously conducted seven Phase 1 and two Phase 2 trials investigating the potential of Capeserod for neurological disorders.
In these trials, involving over 600 patients, Capeserod appeared safe and well-tolerated. We plan to request a meeting with the U.S.
Food and Drug Administration (“FDA”) to establish a development and regulatory pathway for Capeserod in GI diseases
with the intent to initiate clinical trials in 2025.
Niclosamide
Niclosamide, a pro-inflammatory
pathway inhibitor, is a prescription small molecule drug that has been safely used on millions of patients. Niclosamide is listed as
an essential medicine by the World Health Organization (“WHO”). In the U.S., Niclosamide was approved by the FDA in
1982 for the treatment of intestinal tapeworm infections. Niclosamide’s activity as an antihelminthic results from direct action
in the intestinal lumen where it disrupts a parasitic metabolic function called oxidative phosphorylation, killing parasites. Niclosamide
has been commercially available worldwide for more than 50 years as 500mg single-dose tablets intended for use in pediatric and adult
populations, at a dose rate of 2g per adult or child over six years of age. No safety issues have ever been identified. In addition to
its antihelminthic activity, Niclosamide has demonstrated novel anti-inflammatory properties.
We believe Niclosamide, and
more specifically our proprietary and patent-pending micronized Niclosamide formulation, has the potential to be an ideal therapeutic
to treat multiple GI indications due to the following favorable properties:
| (i) |
it has a reduced particle size (D(90) between 5 and 9 µM) as compared to regular non-micronized Niclosamide (approximately D(90) ≥ 60µM) with greater surface to solvent ratio; |
| (ii) |
low oral bio-availability with minimal systemic absorption / exposure; |
| (iii) |
improved dissolution with broader distribution allowing for higher local GI concentrations (up to approximately 200 times based on preclinical study results); and |
| (iv) |
it exhibits anti-inflammatory effects while avoiding steroid-related complications and adverse events. |
In December 2023, we announced
that we entered into a non-binding term sheet to sell our Niclosamide program. The non-binding term sheet includes a low seven-figure
upfront payment to us for rights to Niclosamide, as well as economics related to future milestones and royalties. If successful, the transaction
is expected to close in the second half of 2024. Additional details of the transaction will be disclosed upon finalization and execution
of the definitive agreement. Upon execution of the definitive agreement, the completion of the transaction will be subject to, among other
matters, satisfaction of the conditions negotiated therein, the buyer having secured adequate financing, and receipt of all third party
(including governmental) approvals, licenses, consents, and clearances, as and when applicable.
Scientific Background
Recent discoveries in immune
cell metabolism suggest that it may be possible to selectively target disease-causing immune cells to treat inflammatory diseases without
unwanted side effects associated with broad-based immunosuppression. Research indicates that IBD, including ulcerative colitis, ulcerative
proctitis/proctosigmoiditis and Crohn’s disease, is driven by pathogenic Th17 cells, which release a cascade of local cytokines
that in turn cause inflammation in bowel wall tissues.
Th17 cells rely on a cellular
process called oxidative phosphorylation to survive. Niclosamide is known to disrupt the oxidative phosphorylation in the mitochondria
of pathogenic Th17 cells in a manner that selectively induces apoptosis of pathogenic Th17 cells, overcoming their inherent resistance
to cell death. This effect is mild enough that it does not interfere with normal cells. By killing Th17 cells, Niclosamide may reduce
inflammation and calm the gut, selectively killing pathogenic, inflammatory cells while leaving healthy cells untouched.
Niclosamide has demonstrated
beneficial effects in numerous cell culture studies using cells obtained by biopsy of inflamed bowel tissues from IBD patients, and also
in animal models of IBD.
Our suite of proprietary,
gut-restricted Niclosamide product candidates are designed to target the metabolism of disease-causing Th17 cells to potentially halt
or delay the progression of disease, stop flare-ups, and address patient needs at all stages of IBD, from mild to severe, and for cancer
patients with ICI-AC.
Inflammatory Bowel Disease Background
IBD is an umbrella term
used to describe disorders that involve chronic inflammation of the digestive track. IBD affects approximately 3 million people in the
U.S. annually. IBD is divided into two main classes of gastrointestinal inflammatory diseases: (i) ulcerative colitis (“UC”),
including ulcerative proctitis and ulcerative proctosigmoiditis, and (ii) Crohn’s Disease (“CD”). There are
similarities between UC and CD, such as immunopathology, and equal distribution between males and females. However, there are also notable
differences. UC is generally limited to the colon, while CD may occur anywhere in the small or large intestine. UC usually affects continuous
mucosal surfaces, while CD is patchier, with areas of normal bowel mucosa separating the inflammatory patches. Importantly, CD often
involves deep bowel tissues and can lead to fistulas into the abdominal cavity or out to the skin surface. CD requires surgical intervention
more often than UC. While medical treatments for UC and CD are generally similar, CD is much less responsive.
FW-COV Program for COVID-19 GI Infections
The COVID-19 pandemic was
a global public health emergency caused by the SARS-CoV-2 virus. An increasing volume of convergent evidence indicates that GI infection
and fecal-oral transmission of SARS-CoV-2 are important factors in the clinical presentation, virology and epidemiology of COVID-19. There
is currently no etiological treatment for COVID-19 GI effects. As a result, we believe there is an unmet therapeutic need for safe and
effective treatment of these effects.
An IND for FW-COV micronized
Niclosamide for COVID-19 GI infections was cleared by the FDA in September 2020. In April 2021, we initiated our Phase 2 RESERVOIR clinical
trial for the treatment of COVID-19 related GI infections. The Phase 2 RESERVOIR clinical trial was a two-part, two-arm, randomized, placebo-controlled
study examining the safety and efficacy of micronized oral Niclosamide tablets in patients with COVID-19 GI infection. The two primary
objectives of the RESERVOIR trial are to confirm the safety of Niclosamide in the treatment of patients with COVID-19 GI infection and
to demonstrate efficacy in clearing the SARS-CoV-2 virus from the GI tract.
In April 2022 we announced
that top-line data results from the FW-COV trial were mixed. FW-COV was demonstrated to be very safe with no drug-related serious adverse
events (“SAEs”) reported by the more than 150 patients who participated in the trial. However, the efficacy endpoint
in this trial - the ability of FW-COV to remove the SARS-CoV-2 virus from the digestive tract as measured by viral presence in the patient’s
stool - did not demonstrate statistical significance when compared to placebo. As a result, we decided not to further pursue Niclosamide
clinical studies for the anti-viral treatment of COVID-GI.
FW-UC for Ulcerative Colitis (UC)
UC is an IBD that causes inflammation
and ulcers (sores) in the digestive tract. UC generally affects the innermost lining of the large intestine (colon). UC affects approximately
830,000 patients in the U.S. annually and approximately 84% or 700,000 have mild to moderate disease. The immunopathology of UC is complex
and is generally considered to be caused by a dysregulated immune system. There is evidence that a hereditary trait is involved. While
the cause is not known, many researchers believe that invasive bacteria or virus in the bowel wall sets off an abnormal response by local
T lymphocytes. Normally, a subgroup of lymphocytes called Th17 cells protect the bowel wall from microbial invaders. However, in patients
with UC, these Th17 cells become pathogenic and release a cascade of local cytokines, which in turn cause inflammation in bowel wall tissues.
This persistent inflammation causes tissue damage, and clinical symptoms. Clinical symptoms include abdominal pain, diarrhea, which is
sometimes bloody, intermittent fever, anemia, and weight loss, and symptoms usually develop over time, rather than suddenly.
Our initial clinical trial
in UC involved patients with UP and UPS, who were being treated with a topical rectal formulation in a Phase 1b/2a clinical trial. We
are no longer actively pursuing clinical development for FW-UC.
FW-UP Program for Ulcerative Proctitis (UP) and Ulcerative Proctosigmoiditis
(UPS)
UP and UPS are two types of
UC, a chronic inflammatory bowel disease consisting of fine ulcerations in the inner mucosal lining of the large intestine that do not
penetrate the bowel muscle wall. UPS causes inflammation in the colon and rectum, while UP is confined only to the rectum. Symptoms include
weight loss, fatigue, abdominal pain and cramps, rectal pain and bleeding, and diarrhea, although constipation can also develop as the
body struggles to maintain normal bowel function. UP and UPS affect approximately 200,000 patients in the U.S. annually.
We were developing FW-UP,
a Niclosamide-based, small molecule anti-inflammatory inhibitor therapy in enema formulation for the potential treatment of UP and UPS.
FW-UP was being investigated in a three-stage Phase 1b/2a clinical trial in Europe studying the safety and potential efficacy of Niclosamide
in patients with UP and UPS. We are no longer actively pursuing clinical development for FW-UP.
FW-ICI-AC for Immune Checkpoint Inhibitor Colitis (ICI-AC)
Immune checkpoint inhibitors
(“ICIs”) are monoclonal antibodies that target down-regulators of the anti-cancer immune response and have significantly
affected the treatment of a variety of malignancies. However, many immune-related adverse events, especially diarrhea and colitis, limit
their use. A 2019 study titled, “Immune checkpoint inhibitor-induced colitis: A comprehensive review,” published in World
Journal of Clinical Cases (Sol et.al, 2019) estimated the incidence of IMC ranges from 1% to 25% depending on the type of ICI
and whether they are used in combination. A 2017 study titled “Incidence of immune checkpoint inhibitor-related colitis in solid
tumor patients: a systematic review and meta-analysis” published in Oncoimmunology (Wang et.al, 2017) estimated that
approximately 44%, or 260,000 patients with advanced and metastatic tumors were eligible to receive ICIs. Further, approximately 30% of
ICI patients develop diarrhea, which can progress to colitis. The onset of diarrhea in ICI-AC patients occurs within six to seven weeks
and progressively worsens, and the progression to colitis is rapid and unpredictable.
In October 2021, we received
FDA IND clearance to commence our Phase 1b/2a PASSPORT ICI-AC clinical trial using an oral immediate-release tablet formulation of Niclosamide
for Grade 1 and Grade 2 colitis and diarrhea in oncology patients receiving treatment with ICIs. The Phase 2a PASSPORT clinical trial
was designed as a double-blind, placebo-controlled study to determine the safety, tolerability, and preliminary efficacy of FW-ICI-AC
in the treatment of immune checkpoint inhibitor-associated colitis (“ICI-AC”) and diarrhea in advanced cancer patients.
We are no longer actively pursuing clinical development for FW-ICI-AC.
FW-CD for Crohn’s Disease (CD)
While the immunopathology
of CD resembles that of UC, the location of disease, the response to treatment and the overall morbidity are different, as CD is more
difficult to manage. Patient response to standard therapy is more variable than in UC, thus making clinical management more challenging.
In UC, a reasonably clear course of disease from mild to moderate severity can be predicted based upon response to first line treatment.
In CD, first line treatment often includes more immunosuppressive agents, such as steroids, immunomodulators, and anti-TNF agents as compared
to the 5-ASAs used for first line in UC. CD affects approximately 660,000 patients in the U.S. annually and approximately 76% or 500,000
have mild to moderate disease. We believe FW-CD, an oral Niclosamide-based small molecule anti-inflammatory inhibitor therapy can be an
important therapeutic in the treatment of mild to moderate CD, with the goal of reducing steroid and immunomodulators treatments. We are
no longer actively pursuing clinical development for FW-CD.
CypCel
CypCel is a disease management
tool for monitoring the intestinal health of recovering celiac disease patients. It is a minimally-invasive metabolic marker compound
that can measure the state of small-intestinal recovery of celiac patients undergoing gluten-free diets. It utilizes a drug biomarker
simvastatin, a cholesterol reducing medication, that has the unusual property of being highly metabolized in the small intestine by the
enzyme CYP3A4, which is expressed on the villi. The patient ingests a simvastatin tablet and then provides a blood sample at two later
time points. The concentration of the simvastatin in the blood samples is directly related to the villous health of the patient; in patients
with healthy villi a high rate of metabolism leads to reduced concentration of simvastin in the blood samples whereas in patients with
damaged villi, the converse is true. Simvastatin levels measured in individuals at periodic intervals therefore can monitor progressive
changes that are indicative of whether a treatment, such as a gluten-free diet, is effective.
In December 2018 IMGX announced
the completion of a study with the Mayo Clinic on newly diagnosed and long-term healed celiac disease patients as well as non-celiac healthy
control subjects. The results showed a trend toward systematic improvement in villous health in newly diagnosed patients adhering to a
gluten-free diet whereas the other cohort groups representing healthy villi showed negligible further improvement as expected.
Recent Developments
Merger with ImmunogenX
On March 13, 2024, we
acquired ImmunogenX, Inc., a Delaware corporation, in accordance with the terms of the Merger Agreement, by and among us, First Merger
Sub, Second Merger Sub, and IMGX. Pursuant to the Merger Agreement, First Merger Sub merged with and into IMGX, pursuant to which IMGX
was the surviving corporation. Immediately following the First Merger, IMGX merged with and into Second Merger Sub, pursuant to which
Second Merger Sub was the surviving entity and a wholly owned subsidiary of the Company. The Second Merger Sub amended its certificate
of formation concurrently with the Second Merger to change its company name to ImmunogenX, LLC. The Merger is intended to qualify as
a tax-free reorganization for U.S. federal income tax purposes.
Under the terms of the
Merger Agreement, following the Closing, in exchange for the outstanding shares of capital stock of IMGX immediately prior to the effective
time of the First Merger, we issued to the stockholders of IMGX an aggregate of (A) 36,830 shares of Common Stock, and (B) 11,777.418
shares of Series G Preferred Stock (as defined and described below), each share of which is convertible into 1,000 shares of Common Stock,
subject to certain conditions described below. In addition, we assumed (i) all IMGX stock options immediately outstanding prior to the
First Merger, each becoming an option to purchase Common Stock subject to adjustment pursuant to the terms of the Merger Agreement and
(ii) all IMGX warrants immediately outstanding prior to the First Merger, each becoming a warrant to purchase Common Stock subject to
adjustment pursuant to the terms of the Merger Agreement. The Assumed Options are exercisable for an aggregate of 200,652 shares of Common
Stock, have an exercise price of $0.81 and expire between February 1, 2031 and June 6, 2033. The Assumed Warrants are exercisable for
an aggregate of 127,682 shares of Common Stock, have exercise prices ranging from $3.02 to $3.92 and expire between September 30, 2032
and September 6, 2033.
The foregoing description
of the Assumed Warrants does not purport to be complete and is qualified in its entirety by reference to the form of Assumed Warrant,
which is incorporated by reference to this proxy statement as Exhibit 4.43 to the Company’s Annual Report on Form 10-K for the fiscal
year ended December 31, 2023, filed with the SEC on March 29, 2024.
Tungsten acted as our
financial advisor in connection with the Merger. As partial compensation for services rendered by Tungsten, we issued to Tungsten or
its affiliates or designees an aggregate of 18,475 shares of Common Stock and 595.808 shares of Series G Preferred Stock.
Pursuant to the Merger
Agreement, we have agreed to hold a stockholders’ meeting to submit the following matters to its stockholders for their consideration:
(i) the approval of the Conversion Proposal and (ii) if deemed necessary or appropriate by us or as otherwise required by applicable
law or contract, the approval of an amendment to our Charter, to authorize sufficient shares of Common Stock for conversion of Series
G Preferred Stock issued pursuant to the Merger Agreement. In connection with these matters, we have agreed to file a proxy statement
with the Securities and Exchange Commission.
The Board of Directors
of Company and the holders of a majority of our Series B Convertible Preferred Stock approved the Merger Agreement and the related transactions,
and the consummation of the Merger was not subject to approval of our stockholders.
The foregoing description
of the Merger and the Merger Agreement does not purport to be complete and is qualified in its entirety by reference to the full text
of the Merger Agreement, which is incorporated by reference to this proxy statement as Exhibit 2.2 to the Company’s Annual Report
on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March 29, 2024.
The Merger Agreement contains
representations, warranties and covenants that we and IMGX made to each other as of specific dates. The assertions embodied in those representations,
warranties and covenants were made solely for purposes of the Merger Agreement between us and IMGX and may be subject to important qualifications
and limitations agreed to by us and IMGX in connection with negotiating its terms, including being qualified by confidential disclosures
exchanged between the parties in connection with the execution of the Merger Agreement. Moreover, the representations and warranties may
be subject to a contractual standard of materiality that may be different from what may be viewed as material to investors or securityholders,
or may have been used for the purpose of allocating risk between us and IMGX rather than establishing matters as facts. Moreover, information
concerning the subject matter of the representations and warranties may change after the date of the Merger Agreement, which subsequent
information may or may not be fully reflected in our public disclosures. For the foregoing reasons, no person should rely on the representations
and warranties as statements of factual information at the time they were made or otherwise.
Voting and Support Agreements
In connection with the
execution of the Merger Agreement, together with IMGX, we entered into stockholder voting agreements with certain of IMGX’s stockholders.
Pursuant to the IMGX Voting Agreements, among other things, each of the IMGX stockholder parties thereto has agreed to vote or cause
to be voted all of the shares of Common Stock owned by such stockholder in favor of the Share Increase Proposal (if such proposal is
brought before our stockholders).
The foregoing description
of the IMGX Voting Agreements does not purport to be complete and is qualified in its entirety by reference to the full text of the form
of the IMGX Voting Agreement, a copy of which is included as Exhibit A to the Merger Agreement, which is incorporated herein by reference
to Exhibit 2.2 of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March
29, 2024.
In connection with the
execution of the Merger Agreement, together with IMGX, we entered into the Company Support Agreements with certain of our officers (solely
in their capacity as our stockholders). Pursuant to the Support Agreements, among other things, each of our stockholder parties thereto
has agreed to vote or cause to be voted all of the shares of Common Stock owned by such stockholder in favor of the Meeting Proposals.
The foregoing description
of the Company Support Agreements does not purport to be complete and is qualified in its entirety by reference to the full text of the
form of the Company Support Agreement, a copy of which is included as Exhibit B to the Merger Agreement, which is incorporated herein
by reference to Exhibit 2.2 of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with
the SEC on March 29, 2024.
Lock-up Agreements
Concurrently and in connection
with the execution of the Merger Agreement, certain IMGX stockholders as of immediately prior to the Closing, and certain of our officers
as of immediately prior to the Closing, entered into lock-up agreements with us and ImmunogenX, pursuant to which each such stockholder
agreed to be subject to a 180-day lockup on the sale or transfer of our shares held by each such stockholder at the Closing, including
those shares of Common Stock and Series G Preferred Stock (including the shares of Common Stock into which such Series G Preferred Stock
is convertible) received by each such stockholder in the Merger.
The foregoing description of the Lock-up Agreements
does not purport to be complete and is qualified in its entirety by reference to the full text of the form of the Lock-up Agreement, a
copy of which is included as Exhibit C to the Merger Agreement, which is incorporated herein by reference to Exhibit 2.2 of the Company’s
Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March 29, 2024.
Restrictive Covenants Agreements
Concurrently and in connection
with the execution of the Merger Agreement, certain IMGX officers as of immediately prior to the Closing, entered into restrictive covenants
agreements with us and IMGX, pursuant to which such individuals and each of their affiliates agreed not to compete with IMGX during the
three-year period following the Closing and, during such three-year restricted period, not to solicit Restricted Employees (as defined
in the form of Restrictive Covenants Agreement). The Restrictive Covenants Agreements also contain customary non-disparagement and confidentiality
provisions.
The foregoing description
of the Restrictive Covenants Agreement does not purport to be complete and is qualified in its entirety by reference to the full text
of the form of the Restrictive Covenants Agreement, a copy of which is included as Exhibit D to the Merger Agreement, which is incorporated
herein by reference to Exhibit 2.2 of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed
with the SEC on March 29, 2024.
Debt of ImmunogenX
IMGX is party to the Credit
Agreement, with Lender, pursuant to which Lender provided IMGX with a secured revolving credit facility with a total commitment of $7,500,000.
The loans under the Credit Agreement bore interest at a per annum floating rate equal to the prime rate plus 4.50% and had a maturity
date of October 3, 2024. In connection with the Merger, IMGX entered into the Second Amendment, which provides for, among other things:
(i) Lender’s consent for the Merger, (ii) Second Merger Sub’s assumption all of IMGX obligations, rights and liabilities
as borrower under the Amended Credit Agreement, (iii) an amendment to the interest rate to a per annum floating rate equal to the prime
rate plus 6.00%, (iv) an extension of the maturity date to September 13, 2025, (v) a prohibition on the borrower’s ability to request
additional loans under the revolving credit facility, subject to certain limited exceptions, (vi) the pledge by the Pledgors of their
Pledged Equity as part of the collateral securing the obligations under the Amended Credit Agreement, and (vii) an amendment to the total
commitment under the Amended Credit Agreement to $8,212,345.17, which takes into account the One-Time Payment, accrued interest and fees
for the remaining term of the facility and certain other fees to be paid under the Amended Credit Agreement. The Pledged Equity shall
be subject to a call right, which shall give Lender the right to, at its option, require the Pledgors to sell to Lender (on a pro rata
basis) the number of shares of Pledged Equity that, when multiplied by the per share valuation of the Common Stock at Closing, satisfies
all or a portion of the then outstanding amount of obligations under the Amended Credit Agreement.
The borrowings under the
Amended Credit Agreement are by evidenced by the Note, and are secured by the Security Agreement, by and between IMGX and Lender.
On March 13, 2024, IMGX
and Lender entered into the Support Letter, pursuant to which, among other things, IMGX and Lender agreed that IMGX is permitted to license
or transfer rights to any intellectual property which may constitute collateral under the Amended Credit Agreement.
The Amended Credit Agreement
contains customary representations and warranties, affirmative covenants and negative covenants, including covenants that limit or restrict
the IMGX’s ability to, among other things, incur additional indebtedness, merge or consolidate, pay dividends or other distributions,
repurchase equity, dispose of assets and enter into certain transactions with affiliates, in each case subject to certain exceptions.
The Amended Credit Agreement also includes customary events of default, and upon the occurrence and during the continuation of an event
of default, Lender may declare the outstanding advances and all other obligations under the Amended Credit Agreement to be immediately
due and payable. Payments owed under the Credit Agreement are also guaranteed by the Guarantee.
The foregoing descriptions
of the Amended Credit Agreement, the Note, the Security Agreement and the Support Letter do not purport to be complete and are qualified
in their entirety by reference to the Amended Credit Agreement, the Note, the Security Agreement and the Support Letter, which are incorporated
herein by reference to Exhibits 10.54, 10.55, 10.56 and 10.57 of the Company’s Annual Report on Form 10-K for the fiscal year ended
December 31, 2023, filed with the SEC on March 29, 2024.
Stockholder Notes
In order to fund the One-Time
Prepayment, on March 13, 2024, IMGX entered into (i) the Syage Note, and (ii) the Felker Note. The Shareholder Notes will bear interest
at a per annum floating rate equal to the prime rate plus 4.50% and mature on October 13, 2025. Each of Jack Syage and Peter Felker,
who were shareholders of IMGX prior to the Merger, have entered into the Shareholder Security Agreements, each dated March 13, 2024,
pursuant to which the obligations under each of the Shareholder Notes are secured by, among other things, the patents and trademarks
owned by IMGX.
The foregoing descriptions
of the Shareholder Notes and the Shareholder Security Agreements do not purport to be complete and are qualified in their entirety by
reference to the Form of Shareholder Note and Form of Shareholder Security Agreement, which are incorporated herein by reference to Exhibits
10.58 and 10.59 of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March
29, 2024.
Registered Direct Offering
On March 6, 2024, we closed
on a Registered Direct Offering with an institutional investor for the purchase and sale of 525,625 shares of our Common Stock (or Common
Stock equivalents) at a price of $7.61 per share. In addition, we issued to the investor warrants to purchase up to 525,625 shares of
Common Stock. The warrants have an exercise price of $7.48 per share, are exercisable immediately following the date of issuance, and
have a term of five years following the date of issuance. Roth Capital Partners acted as the exclusive placement agent for the offering.
The gross proceeds to us from this offering were approximately $4.0 million, before deducting the placement agent's fees and other offering
expenses payable by us.
Issuance of Restricted Stock Units
On January 2, 2024, we issued
to employees, consultants and the board of directors ten-year restricted stock units consisting of 120,532 shares of Common Stock, subject
to service-based milestones vesting quarterly over one year under the 2020 Plan as payment for services rendered. Such issuance was exempt
from registration under 4(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”).
Non-Binding Letter of Intent for Potential Sale of Niclosamide
On December 27, 2023, we announced
that we have entered into a non-binding letter of intent (the “Niclosamide LOI”) for a proposed sale of our niclosamide
program, which is designed to treat inflammatory bowel diseases such as ulcerative colitis and related conditions, to an undisclosed biopharmaceutical
company (the “Niclosamide Sale”). The Niclosamide LOI contemplates a low seven-figure upfront payment to the Company
for the rights to Niclosamide, as well as economics to the Company related to future milestones and royalties.
The Niclosamide LOI only represents
a mutual indication of interest regarding the Niclosamide Sale and the terms of the Niclosamide Sale are subject to a number of contingencies,
including the completion of customary due diligence and the negotiation and execution of definitive agreements. Upon execution of the
definitive agreement, the completion of the transaction will be subject to, among other matters, satisfaction of the conditions negotiated
therein, the buyer having secured adequate financing, and receipt of all third party (including governmental) approvals, licenses, consents,
and clearances, as and when applicable.
There can be no assurance
that the Niclosamide Sale will be completed on the terms contemplated in the Niclosamide LOI or otherwise. In particular, the terms of
the potential transaction, the timing of closing, and the aggregate consideration that we may receive may materially differ from that
currently contemplated by the Niclosamide LOI.
Reverse Stock Split
On December 12, 2023, we held
a special meeting of stockholders (the “Special Meeting”) where our stockholders approved a proposal granting our Board of
Directors the discretion to effect a reverse stock split of our issued and outstanding common stock through an amendment (the “Amendment”)
to our Charter, at a ratio of not less than 1-for-10 and not more than 1-for-20, with such ratio to be determined by the Board of Directors.
On December 13, 2023, we filed
the Amendment to our Charter with the Secretary of State of the State of Delaware to effect a reverse stock split of our Common Stock
at a ratio of 1-for-20. The Reverse Stock Split became effective in accordance with the terms of the Amendment at 12:01 AM Eastern Time
on December 18, 2023, and began trading on a split-adjusted basis when the market opened on Monday, December 18, 2023. There was no corresponding
reduction in the number of authorized shares of common stock and no change in the par value per share.
Nasdaq Listing Compliance
On October 26, 2023, we received
written notice (the “Notification Letter”) from the Listing Qualifications Department (the “Staff”) of The Nasdaq
Stock Market LLC (“Nasdaq”) notifying the Company that it was not in compliance with the shareholder approval requirement
set forth in Nasdaq Listing Rule 5635(d), which requires prior shareholder approval for transactions, other than public offerings, involving
the issuance of 20% or more of an issuer’s pre-transaction shares outstanding at less than the applicable Minimum Price (as defined
in Listing Rule 5635(d)(1)(A)).
The Staff’s determination
relates to the offering and issuance by us (the “Offering”) of an aggregate of: (i) 30,500 shares (the “Shares”)
of our common stock, par value $0.0001 per share, (ii) prefunded warrants to purchase up to an aggregate of 133,750 shares of common stock
and (iii) common warrants to purchase up to an aggregate of 328,500 shares of Common Stock (together with the prefunded warrant shares,
the “Warrant Shares”). The public offering price for each Share and accompanying Warrants, each to purchase one share of common
stock, was $12.80, and the public offering price for each prefunded warrant and accompanying warrants, each to purchase one share of Common
Stock, was $12.798. The Offering was previously disclosed in our Current Report on Form 8-K filed with the Securities and Exchange Commission
on July 21, 2023.
The Staff determined that
the Offering was not a “public offering” under Nasdaq Listing Rule 5635(d) due to the type of offering, a best efforts offering
pursuant to a placement agency agreement, and the fact that one investor purchased a predominant portion of the Offering. As a result,
because the Offering represented greater than 20% of the common stock outstanding and was priced below the Minimum Price, the Staff determined
that we were required to obtain shareholder approval prior to the issuance of common stock in the Offering under Listing Rule 5635(d).
On December 12, 2023, during
the Special Meeting, our stockholders’ ratified our entry into the Offering as we received the affirmative vote of the majority
of the votes cast by shares of our common stock present or represented by proxy and entitled to vote at the Special Meeting.
On March 19, 2024, we received
a Letter of Reprimand from the Nasdaq Listing Qualifications Staff (the “Letter of Reprimand”) stating that, while
we failed to comply with Nasdaq’s continued listing requirements, our violation of listing rule 5635(d) does not appear to have
been the result of a deliberate intent to avoid compliance, and as such, the Staff does not believe that delisting our securities is an
appropriate sanction and that it is appropriate to close these matters by issuing the Letter of Reprimand.
Warrant Reload and Repricing
On December 27, 2023 we entered
into a warrant exercise inducement offer letter with a holder of warrants to purchase shares of common stock pursuant to which the holder
agreed to exercise for cash their existing warrants to purchase 881,337 shares of common stock, in the aggregate, at a reduced exercise
price of $5.50 per shares, in exchange for new warrants on substantially the same terms as the existing warrants, to purchase up to 1,762,674
shares of common stock and a cash payment of $0.125 per warrant share which was paid in full upon the exercise of the existing warrants.
We received aggregate gross proceeds of approximately $4.8 million from the exercise of the existing warrants and sale of the inducement
warrants.
Corporate History
We were incorporated on January
30, 2014 in the State of Delaware under the name AzurRx BioPharma, Inc. In May 2014, we entered into a stock purchase agreement with Protea
Biosciences Group, Inc. (“Protea Group”) and its wholly-owned subsidiary, Protea Biosciences, Inc. (“Protea
Sub” and, together with Protea Group, “Protea”), to acquire 100% of the outstanding capital stock of AzurRx
SAS (formerly ProteaBio Europe SAS), a wholly-owned subsidiary of Protea Sub, which was completed in June 2014. In October 2016, we completed
an initial public offering, which allowed us to list our shares of Common Stock on the Nasdaq Capital Market.
On September 13, 2021, we
completed the acquisition of First Wave Bio, Inc. (“FWB”), which became our wholly-owned subsidiary. In connection
with the acquisition, AzurRx BioPharma, Inc. changed its name to First Wave BioPharma, Inc.
Effective October 26, 2022,
the AzurRx SAS subsidiary was dissolved.
On March 13, 2024, we completed
a merger with ImmunogenX, Inc., which became our wholly-owned subsidiary.
On May 17, 2024, First
Wave BioPharma, Inc. changes its name to Entero Therapeutics, Inc.
Intellectual Property
Our goal is to obtain, maintain
and enforce patent protection for our product candidates, formulations, processes, methods and any other proprietary technologies, preserve
our trade secrets, and operate without infringing on the proprietary rights of other parties, both in the United States and in other countries.
Our policy is to actively seek to obtain, where appropriate, the broadest intellectual property protection possible for our current product
candidates and any future product candidates, proprietary information and proprietary technology through a combination of contractual
arrangements and patents, both in the United States and abroad. However, patent protection may not afford us with complete protection
against competitors who seek to circumvent our patents.
We also depend upon the skills,
knowledge, experience and know-how of our management and research and development personnel, as well as that of our advisors, consultants
and other contractors. To help protect our proprietary know-how, which is not patentable, and for inventions for which patents may be
difficult to enforce, we currently rely and will in the future rely on trade secret protection and confidentiality agreements to protect
our interests. To this end, we require all of our employees, consultants, advisors and other contractors to enter into confidentiality
agreements that prohibit the disclosure of confidential information and, where applicable, require disclosure and assignment to us of
the ideas, developments, discoveries and inventions important to our business.
Latiglutenase
The Latiglutenase program
is protected by U.S. Patent 10,434,150 that expires July 3, 2035, U.S. Patent 8,980,254 that expires April 10, 2030, and U.S. Patent 9,993,531
that expires Sept. 9, 2029. We also expect to receive 12-year biologic exclusivity in the United States under the Affordable Care Act
and 10-year data exclusivity in the European Union for Latiglutenase.
All three patents for
Latiglutenase are composition of matter patents and are owned by Entero.
Adrulipase
The Adrulipase program is
protected by the following issued patents that we had originally licensed under the Mayoly Agreement and now own:
| |
· |
PCT/FR2006/001352 patent
family (including the patent EP2035556 and patent US8,334,130 and US8,834,867) “Method for producing lipase, transformed Yarrowia
lipolytica cell capable of producing said lipase and their uses” describes a method for producing Yarrowia lipolytica
acid-resistant recombinant lipase utilizing a culture medium without any products of animal origin or non-characterized mixtures
such as tryptone, peptone or lactoserum, in addition to its uses. These patents cover composition of matter and process. The European
patents expire June 15, 2026, U.S. patent 8,334,130 expires September 11, 2028, and U.S. patent 8,834,867 expires July 17, 2026. |
In addition, a PCT International
application was filed in 2021 directed to our proprietary formulation of Adrulipase covering composition of matter, method of treatment,
and process has been nationalized in the United States, Canada, Mexico, Europe, Brazil, Chile, Columbia, Australia. New Zealand, China,
India, Japan, Singapore and South Korea. Any patents issuing from these filings will have an expected expiration in 2041.
Two U.S. Utility Applications
and corresponding PCT International applications were filed in 2022 directed to stable lipase formulations, composition of matter, methods
of treatment, and process. Any patents issuing from these filings will have an expected expiration in 2042.
A PCT International application
was filed in 2023 directed to Adrulipase formulations covering composition of matter, method of treatment and process. Any patents issuing
from this filing will have an expected expiration in 2043.
We also expect to receive
12-year biologic exclusivity in the United States under the Affordable Care Act and 10-year data exclusivity in the European Union for
Adrulipase.
Capeserod
We obtained proprietary know-how
from Sanofi for the Capeserod program. Currently, Capeserod does not have any patent protection. We will actively seek to obtain, where
appropriate, the broadest intellectual property protection possible based on the future research and development in this program.
Niclosamide
Our FW-ICI-AC, FW-UP, FW-UC
and FW-CD Niclosamide programs are protected by patent filings that include the following:
| |
· |
US10,912,746; US10,905,666; US10,292,951; US10,772,854; US10,744,103; US10,799,468; US10,849,867; and related continuation applications as well as corresponding worldwide patent filings all entitled “Methods and Compositions for Treating Conditions Associated with an Abnormal Inflammatory Process.” The expiration date of the issued patents is September 1, 2036; and |
| |
· |
A PCT International application filed in 2021 that has been nationalized in the United States directed to compositions and methods for treating conditions characterized by an abnormal inflammatory response such as an autoimmune disorder, colitis, autoimmune colitis, an inflammatory bowel disease, Crohn’s disease and ulcerative colitis. Any national designated patent application from this filing upon issuance will have an expected expiration in 2041. |
Our FW-COV Niclosamide programs
are protected by patent filings that include the following:
| |
· |
US10,980,756 and US11,564,896 and corresponding continuation applications directed to the use of Niclosamide for the treatment of COVID-19 gastrointestinal infections. The expiration of the issued patents is March 31, 2040. |
| |
· |
In addition, a PCT International application and a corresponding U.S. application was filed in 2022 directed to methods of treating Long Covid with Niclosamide. Any patents issuing from these filings will have an expected expiration in 2042. |
All of the above Niclosamide patent filings are owned
by Entero.
Manufacturing
We currently outsource all
manufacturing, and we intend to use our collaborators and contract development and manufacturing organizations (“CDMOs”)
for the foreseeable future.
Latiglutenase
Latiglutenase API, including
drug substance is currently manufactured by ACS Dobfar SpA, at a contract facility located in Tribiano (MI), Italy. Charles River Laboratories
based in Malvern, Pennsylvania is responsible for maintaining our master cell bank. We believe there are alternative contract manufacturers
capable of producing the product we need for clinical trials; however, there is no guarantee that the processes are reproducible and transferable.
We currently outsource all manufacturing, and we currently intend to use our collaborators and CDMOs for the foreseeable future.
Adrulipase
Adrulipase API, including
drug substance and drug product, is currently manufactured by Asymchem, Inc. at a contract facility located in Tianjin, China. Charles
River Laboratories based in Malvern, Pennsylvania is responsible for maintaining our master and working cell banks. We believe there are
multiple alternative contract manufacturers capable of producing the product we need for clinical trials; however, there is no guarantee
that the processes are easily reproducible and transferrable.
Capeserod
Capeserod API is obtained
by chemical synthesis and is currently under development to be manufactured by Asymchem Inc. at a facility in Tianjin, China. We believe
there are multiple alternative contract manufacturers capable of producing the product we need for clinical trials; however, there is
no guarantee that the processes are reproducible and transferrable.
Niclosamide
Niclosamide API is obtained
by chemical synthesis and is currently manufactured by Olon SpA at a facility in Murcia, Spain. Niclosamide drug product is currently
manufactured at a contract facility located in Milan, Italy owned by Monteresearch s.r.l. and at a contract facility located in Tianjin,
China owned by Asymchem Inc. We believe there are multiple alternative contract manufacturers capable of producing the product we need
for clinical trials; however, there is no guarantee that the processes are easily reproducible and transferrable.
Competition
The pharmaceutical and biotechnology
industries are characterized by rapidly evolving technology and intense competition. Many companies of all sizes, including major pharmaceutical
companies and specialized biotechnology companies, are engaged in the development and commercialization of therapeutic agents designed
for the treatment of the same diseases and disorders that we target. Many of our competitors have substantially greater financial and
other resources, larger research and development staff and more experience in the regulatory approval process. Moreover, potential competitors
have or may have patents or other rights that conflict with patents covering our technologies.
Latiglutenase
With respect to Latiglutenase,
the only current treatment for celiac disease is the gluten free diet. There are no pharmaceuticals approved for the treatment of celiac
disease. Latiglutenase would be the first product approved for the symptomatic treatment of celiac disease as an adjunct to the gluten
free diet.
Adrulipase
With respect to Adrulipase,
if approved, we would compete with porcine pancreatic enzyme replacement therapies (PERTs) - pancrelipase, a well-established market
that is currently dominated by a few large pharmaceutical companies, including CREON® marketed by AbbVie Inc., ZENPEP® marketed
by Nestlé S.A., PANCREAZE® marketed by VIVUS, Inc. and PERTZYE® marketed by Digestive Care, Inc. There are
currently six PERT products that have been approved by the FDA for sale in the U.S. We believe our ability to compete in this market,
if we are successful in developing and obtaining regulatory approval to market Adrulipase, will depend on our ability (or that of a future
corporate partner) to convince patients, their physicians, healthcare payors and the medical community of the benefits of using a non-animal-based
product to treat EPI, as well as by addressing other shortcomings associated with PERTs, including black box warnings and a large pill
burden.
Capeserod
With respect to Capeserod, the current commercially
available treatments for gastroparesis motility all have safety issues, including black box warnings for serious adverse events, or have
restricted usage labels.
Niclosamide
With respect to FW-ICI-AC,
our oral micronized formulation and Niclosamide for ICI-AC, if approved, would compete with both oral and intravenous administered steroids
as well as hospital-based infusions of biologics, including infliximab and vedolizumab. We believe our ability to compete in this market,
if we are successful in developing and obtaining regulatory approval to market FW- ICI-AC, will depend on our ability (or that of future
corporate partners) to convince patients, their physicians, healthcare agencies and payors and the medical community of the benefits of
using a non-steroidal, non-biologic therapeutic option for the treatment of ICI-AC.
With respect to FW-UP and
FW-UC, our topical formulation of Niclosamide for UP, if approved, would compete with sulfasalazines and 5-ASAs, for the treatment of
mild disease, steroids, including budesonide and prednisone, azathioprine, 6-mercaptopurine, and methotrexate for the treatment of moderate
disease, and Anti-TNFs, Entyvio® (vedolizumab), Xeljanz® (Tofacitinib); Stelara® (Ustekinumab) for the treatment of severe
disease. We believe our ability to compete in this market, if we are successful in developing and obtaining regulatory approval to market
FW-UP and FW-UC, will depend on our ability (or that of future corporate partners) to convince patients, their physicians, healthcare
agencies and payors and the medical community of the benefits of using a non-steroidal, non-biologic therapeutic option to prevent the
advancement disease requiring more toxic immunosuppressive therapeutic option for the treatment of mild and moderate UP and UC.
Government Regulation and Product Approval
Government authorities in
the United States, at the federal, state and local level, and other countries extensively regulate, among other things, the research,
development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution,
post-approval monitoring and reporting, marketing and export and import of products such as those we are developing. To date, our internal
research and development efforts have been conducted in France. We expect to conduct late-stage development work, including clinical trials
for Latiglutenase, Adrulipase, Capeserod, and Niclosamide in both the United States and Europe, as North America is our principal target
market for our product candidates that we may successfully develop.
U.S. Government Regulation
In the United States, the
FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and its implementing regulations, and biologics under the
FDCA and the Public Health Service Act, or PHSA, and its implementing regulations. FDA approval is required before any new unapproved
drug or dosage form, including a new use of a previously approved drug, can be marketed in the United States. Drugs and biologics are
also subject to other federal, state and local statutes and regulations. If we fail to comply with applicable FDA or other requirements
at any time during the drug development process, clinical testing, the approval process or after approval, we may become subject to administrative
or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, license suspension or revocation,
withdrawal of an approval, warning letters, product recalls, product seizures, placement on Import Alerts, debarment of personnel, employees
or officers, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution.
The process required by the
FDA before product candidates may be marketed in the United States generally involves the following:
| |
· |
completion of extensive preclinical laboratory tests, preclinical animal studies, and toxicity data, all performed in accordance with the good laboratory practices, or GLP, regulations; |
| |
· |
submission to the FDA of an IND, which must become effective before human clinical studies may begin; |
| |
· |
approval by an independent IRB or ethics committee representing each clinical site before each clinical study may be initiated; |
| |
· |
performance of adequate and well-controlled human clinical studies to establish the safety and efficacy, or in the case of a biologic, the safety, purity and potency, of the drug candidate for each proposed indication; |
| |
· |
preparation of and submission to the FDA of a new drug application, or NDA, or biologics license application, or BLA, which must include data from required pre-clinical studies and all pivotal clinical studies and information showing that the product can be manufactured in a controlled manner; |
| |
· |
a determination by the FDA within 60 days of its receipt of an NDA or BLA to file the application for review; |
| |
· |
review of the product application by an FDA advisory committee, where appropriate and if applicable; |
| |
· |
satisfactory completion of an FDA pre-approval inspection of the manufacturing facilities where the drug candidate is produced to assess compliance with cGMP; and |
| |
· |
FDA review and approval of an NDA or BLA prior to any commercial marketing or sale of the drug or biologic in the United States. |
An IND is a request for authorization
from the FDA to administer an investigational new drug product to humans. The central focus of an IND submission is on the general investigational
plan and the protocol(s) for human studies. The IND also includes results of animal and in vitro studies assessing the toxicology, pharmacokinetics,
pharmacology and pharmacodynamic characteristics of the product; chemistry, manufacturing and controls information; and any available
human data or literature to support the use of the investigational new drug. An IND must become effective before human clinical studies
may begin. An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns
or questions related to the proposed clinical studies. In such a case, the IND may be placed on clinical hold and the IND sponsor and
the FDA must resolve any outstanding concerns or questions before clinical studies can begin. Accordingly, submission of an IND may or
may not result in the FDA allowing clinical studies to commence.
Clinical Studies
Clinical studies involve the
administration of the investigational new drug to human subjects under the supervision of qualified investigators in accordance with GCPs,
which include the requirement that all research subjects provide their informed consent for their participation in any clinical study.
Clinical studies are conducted under protocols detailing, among other things, the objectives of the study, and the parameters to be used
in monitoring safety and the efficacy criteria to be evaluated. A protocol for each clinical study and any subsequent protocol amendments
must be submitted to the FDA as part of the IND. Additionally, approval must also be obtained from each clinical study site’s IRB
before the studies may be initiated, and the IRB must monitor the study until completed. There are also requirements governing the reporting
of ongoing clinical studies and clinical study results to public registries, such as ClinicalTrials.gov.
The clinical investigation
of a drug or biologic is generally divided into three or four phases. Although the phases are usually conducted sequentially, they may
overlap or be combined.
| |
· |
Phase 1. The drug or biologic is initially introduced into healthy human subjects or patients with the target disease or condition. These studies are designed to evaluate the safety, dosage tolerance, metabolism and pharmacologic actions of the investigational new drug in humans, the side effects associated with increasing doses, and if possible, to gain early evidence on effectiveness. |
| |
· |
Phase 2. The drug or biologic is administered to a limited patient population to evaluate dosage tolerance and optimal dosage, identify possible adverse side effects and safety risks and preliminarily evaluate efficacy. |
| |
· |
Phase 3. The drug or biologic is administered to an expanded patient population, generally at geographically dispersed clinical study sites to generate enough data to statistically evaluate dosage, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the investigational product and to provide an adequate basis for product approval. |
| |
· |
Phase 4. In some cases, the FDA may condition approval of an NDA or BLA for a drug candidate on the sponsor’s agreement to conduct additional clinical studies after approval. In other cases, a sponsor may commit to conducting or voluntarily conduct additional clinical studies after approval to gain more information about the drug. Such post-approval studies are typically referred to as Phase 4 clinical studies. |
A confirmatory or pivotal
study is a clinical study that adequately meets regulatory agency requirements for the evaluation of a drug candidate’s efficacy
and safety such that it can be used to justify the approval of the product. Generally, pivotal studies are Phase 3 studies, but the FDA
may accept results from Phase 2 studies if the study design provides a well-controlled and reliable assessment of clinical benefit, particularly
in situations where there is an unmet medical need and the results are sufficiently robust. In such cases, FDA may require post-market
studies for safety and efficacy to be conducted for the drug candidate. The FDA may withdraw the approval if the results indicate that
the approved drug is not safe or effective.
The FDA, the IRB or the clinical
study sponsor may suspend or terminate a clinical study at any time on various grounds, including a finding that the research subjects
are being exposed to an unacceptable health risk. Additionally, some clinical studies are overseen by an independent group of qualified
experts organized by the clinical study sponsor, known as a data safety monitoring board or committee. This group provides authorization
for whether or not a study may move forward at designated check points based on access to certain data from the study. We may also suspend
or terminate a clinical study based on evolving business objectives and/or competitive climate.
Chemistry, Manufacturing and Control Information
Companies seeking FDA approval
of drugs must also develop data and information about the physical characteristics of the components of a product as well as finalize
processes for manufacturing the components in commercial quantities in accordance with GMP requirements. The manufacturing process must
be capable of consistently producing quality batches of the product candidate and, among other things, the sponsor must develop methods
for testing the identity, strength, quality, potency and purity of the final product. Additionally, appropriate packaging must be selected
and tested, and stability studies must be conducted to demonstrate that the components of a product candidate do not undergo unacceptable
deterioration over their shelf life.
Submission of an NDA or BLA to the FDA
Assuming successful completion
of all required testing in accordance with all applicable regulatory requirements, detailed investigational new drug product information
is submitted to the FDA in the form of an NDA or BLA requesting approval to market the product for one or more indications. Under federal
law, the submission of most NDAs and BLAs is subject to a substantial application user fee. Applications for orphan drug products are
exempted from the NDA and BLA application user fees.
An NDA or BLA must include
all relevant data available from pertinent preclinical and clinical studies, including negative or ambiguous results as well as positive
findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling,
among other things. Data can come from company-sponsored clinical studies intended to test the safety and effectiveness of a use of a
product, or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data
submitted must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational product to the satisfaction
of the FDA.
Once an NDA or BLA has been
submitted, the FDA’s goal is to review the application within ten months after it accepts the application for filing, or, if the
application relates to an unmet medical need in a serious or life-threatening indication, six months after the FDA accepts the application
for filing. The review process is often significantly extended by FDA requests for additional information or clarification.
Before approving an NDA or
BLA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application
unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent
production of the product within required specifications. Additionally, before approving an NDA or BLA, the FDA will typically inspect
one or more clinical sites to assure compliance with GCP.
The FDA is required to refer
an application for an investigational drug or biologic to an advisory committee or explain why such referral was not made. An advisory
committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a
recommendation as to whether the investigational product application should be approved and under what conditions. The FDA is not bound
by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions and typically follows
such recommendations.
The FDA’s Decision on an NDA or BLA
After the FDA evaluates the
NDA or BLA and conducts inspections of manufacturing facilities, it may issue an approval letter or a Complete Response Letter. An approval
letter authorizes commercial marketing of the drug or biologic with specific prescribing information for specific indications. A Complete
Response Letter indicates that the review cycle of the application is complete, and the application is not ready for approval. A Complete
Response Letter may require additional clinical data and/or an additional pivotal Phase 3 clinical study(ies), and/or other significant,
expensive and time-consuming requirements related to clinical studies, preclinical studies or manufacturing. Even if such additional information
is submitted, the FDA may ultimately decide that the NDA or BLA does not satisfy the criteria for approval. The FDA could also approve
the NDA or BLA with a REMS to mitigate risks, which could include medication guides, physician communication plans or elements to assure
safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA also may condition approval
on, among other things, changes to proposed labeling, development of adequate controls and specifications or a commitment to conduct one
or more post-market studies or clinical studies. Such post-market testing may include Phase 4 clinical studies and surveillance to further
assess and monitor the product’s safety and effectiveness after commercialization. The FDA may have the authority to withdraw its
approval if post-market testing fails to verify the approved drug’s clinical benefit, if the applicant does not perform the required
testing with due diligence, or if the any other evidence demonstrates the approved drug is not safe or effective, among other reasons.
Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may
change, which could delay or prevent regulatory approval of our products under development.
Expedited Review and Accelerated Approval Programs
The FDA has various programs,
including fast track designation, breakthrough therapy designation, accelerated approval, regenerative medicine advanced therapy and priority
review, that are intended to expedite the development and approval of new drugs and biologics that address unmet medical needs in the
treatment of serious or life-threatening diseases and conditions. To be eligible for a fast-track designation, the FDA must determine,
based on the request of a sponsor, that a product is intended to treat a serious or life-threatening disease or condition and demonstrates
the potential to address an unmet medical need. The FDA may review sections of the NDA for a fast-track product on a rolling basis before
the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA, the FDA agrees
to accept sections of the NDA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission
of the first section of the NDA.
The FDA may give a priority
review designation to drugs that offer major advances in treatment or provide a treatment where no adequate therapy exists. A priority
review means that the goal for the FDA to review an application is six months, rather than the standard review of ten months under current.
These six and ten-month review periods are measured from the “filing” date rather than the receipt date for NDAs for new molecular
entities, which typically adds approximately two months to the timeline for review and decision from the date of submission. Most products
that are eligible for fast-track designation are also likely to be considered appropriate to receive a priority review.
In addition, products studied
for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over
existing treatments may be eligible for accelerated approval and may be approved on the basis of adequate and well-controlled clinical
studies establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or
on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an
effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity or prevalence of the
condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require a sponsor of a drug
receiving accelerated approval to perform post-marketing studies to verify and describe the predicted effect on irreversible morbidity
or mortality or other clinical endpoint, and the drug may be subject to accelerated withdrawal procedures.
Moreover, under the provisions
of the FDA Safety and Innovation Act passed in July 2012, a sponsor can request designation of a drug candidate as a “breakthrough
therapy.” A breakthrough therapy is defined as a drug or biologic that is intended, alone or in combination with one or more other
drugs or biologics, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the
product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial
treatment effects observed early in clinical development. Drugs designated as breakthrough therapies are also eligible for the other expedited
review and approval programs, including accelerated approval, priority review, regenerative medicine advanced therapy, and fast-track
designation. The FDA must take certain actions, such as holding timely meetings and providing advice, intended to expedite the development
and review of an application for approval of a breakthrough therapy.
In addition, the 21st Century
Cures Act in 2016 made the Regenerative Medicine Advanced Therapy, or RMAT, designation available for investigational drugs that are intended
to treat, modify, reverse, or cure a serious condition, with preliminary clinical evidence indicating that the drug has the potential
for addressing unmet medical needs for such condition. The RMAT designation is available for cell therapy, therapeutic tissue engineering
products, human cell and tissue products, and combination products that use such therapies or products. The advantages of RMAT designation
include those of breakthrough and fast track designations, such as early interactions with FDA and rolling review of applications, and
the drug candidate with the RMAT designation may be eligible for accelerated approval. Requests for RMAT designations should be made with
the IND application (if preliminary clinical evidence is available), but no later than the end-of-phase-2 meeting.
Even if a product qualifies
for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide
that the time period for FDA review or approval will not be shortened.
Post-Approval Requirements
Drugs and biologics marketed
pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating
to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences
with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims are subject
to prior FDA review and approval. There also are continuing, annual user fee requirements.
Manufacturers are subject
to periodic unannounced inspections by the FDA and state agencies for compliance with cGMP requirements. Changes to the manufacturing
process are strictly regulated and, depending on the significance of the change, may require prior FDA approval before being implemented.
FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements
upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money and
effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
Discovery of previously unknown
problems with a product or the failure to comply with applicable requirements may result in restrictions on a product, manufacturer or
holder of an approved NDA or BLA, including withdrawal or recall of the product from the market or other voluntary, FDA-initiated or judicial
action that could delay or prohibit further marketing. Also, new government requirements, including those resulting from new legislation,
may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our products under development.
The FDA may withdraw approval
if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market.
Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with
manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new
safety information; imposition of post-market studies or clinical studies to assess new safety risks; or imposition of distribution restrictions
or other restrictions under a REMS program. Other potential consequences include, among other things.
| |
· |
restrictions on the marketing or manufacturing of the product; |
| |
· |
complete withdrawal of the product from the market or product recalls; |
| |
· |
fines, warning letters or holds on post-approval clinical studies; |
| |
· |
refusal of the FDA to approve pending NDAs or BLAs or supplements to approved NDAs or BLAs, or suspension or revocation of product licenses or approvals; |
| |
· |
product seizure or detention, or refusal to permit the import or export of products; or |
| |
· |
injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates
marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved
indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations
prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to
significant liability.
Orphan Designation and Exclusivity
Under the Orphan Drug Act,
the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, defined as a disease or condition
with a patient population of fewer than 200,000 individuals in the United States, or a patient population greater than 200,000 individuals
in the United States and when there is no reasonable expectation that the cost of developing and making available the drug or biologic
in the United States will be recovered from sales in the United States for that drug or biologic. Orphan drug designation must be requested
before submitting a BLA or NDA. After the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential
orphan use are disclosed publicly by the FDA.
If a product that has orphan
drug designation subsequently receives the first FDA approval for a particular active ingredient for the disease for which it has such
designation, the product is entitled to orphan product marketing exclusivity, which means that the FDA may not approve any other applications,
including a full BLA, to market the same biologic for the same use or indication for seven years, except in limited circumstances, such
as a showing of clinical superiority to the product with orphan drug exclusivity or if FDA finds that the holder of the orphan drug exclusivity
has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease
or condition for which the drug was designated. Orphan drug exclusivity does not prevent the FDA from approving a different drug or biologic
for the same disease or condition, or the same drug or biologic for a different disease or condition. Among the other benefits of orphan
drug designation are tax credits for certain research and a waiver of the BLA or NDA application user fee.
A designated orphan drug may
not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation.
In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for
designation was materially defective or, as noted above, if the second applicant demonstrates that its product is clinically superior
to the approved product with orphan exclusivity or the manufacturer of the approved product is unable to assure sufficient quantities
of the product to meet the needs of patients with the rare disease or condition.
Biosimilars and Exclusivity
The Affordable Care Act, signed
into law in 2010, includes a subtitle called the Biologics Price Competition and Innovation Act of 2009, or BPCIA, which created an abbreviated
approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product.
To date, only a handful of biosimilars have been licensed under the BPCIA, although numerous biosimilars have been approved in Europe.
The FDA has issued several guidance documents outlining an approach to review and approval of biosimilars.
Biosimilarity, which requires
that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity
and potency, can be shown through analytical studies, human PK and PD studies, clinical immunogenicity assessments, animal studies and
a clinical study or studies. Interchangeability requires that a product is biosimilar to the reference product and the product must demonstrate
that it can be expected to produce the same clinical results as the reference product in any given patient and, for products that are
administered multiple times to an individual, the biologic and the reference biologic may be alternated or switched after one has been
previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic.
However, complexities associated with the larger, and often more complex, structures of biological products, as well as the processes
by which such products are manufactured, pose significant hurdles to implementation of the abbreviated approval pathway that are still
being worked out by the FDA.
Under the BPCIA, an application
for a biosimilar product may not be submitted to the FDA until four years following the date that the reference product was first licensed
by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which
the reference product was first licensed. During this 12-year period of exclusivity, another company may still market a competing version
of the reference product if the FDA approves a full BLA for the competing product containing that applicant’s own preclinical data
and data from adequate and well-controlled clinical studies to demonstrate the safety, purity and potency of its product. The BPCIA also
created certain exclusivity periods for biosimilars approved as interchangeable products. At this juncture, it is unclear whether products
deemed “interchangeable” by the FDA will, in fact, be readily substituted by pharmacies, which are governed by state pharmacy
law.
A biological product can also
obtain pediatric market exclusivity in the United States. Pediatric exclusivity, if granted, adds six months to existing exclusivity periods
and patent terms. This six-month exclusivity, which runs from the end of other exclusivity protection or patent term, may be granted based
on the voluntary completion of a pediatric study in accordance with an FDA-issued “Written Request” for such a study.
The BPCIA is complex and continues
to be interpreted and implemented by the FDA. In addition, recent government proposals have sought to reduce the 12-year reference product
exclusivity period. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject
of recent litigation. As a result, the ultimate impact, implementation, and impact of the BPCIA is subject to significant uncertainty.
Hatch-Waxman Amendments and Exclusivity
Section 505 of the FDCA describes
three types of marketing applications that may be submitted to the FDA to request marketing authorization for a new drug. Section 505(b)(1)
NDA is an application that contains full reports of investigations of safety and efficacy. A 505(b)(2) NDA is an application that contains
full reports of investigations of safety and efficacy but where at least some of the information required for approval comes from investigations
that were not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use from the person
by or for whom the investigations were conducted. This regulatory pathway enables the applicant to rely, in part, on the FDA’s prior
findings of safety and efficacy for an existing product, or published literature, in support of its application. Section 505(j) establishes
an abbreviated approval process for a generic version of approved drug products through the submission of an ANDA. An ANDA provides for
marketing of a generic drug product that has the same active ingredients, dosage form, strength, route of administration, labeling, performance
characteristics and intended use, among other things, to a previously approved product. ANDAs are termed “abbreviated” because
they are generally not required to include preclinical (animal) and clinical (human) data to establish safety and efficacy. Instead, generic
applicants must scientifically demonstrate that their product is bioequivalent to, or performs in the same manner as, the innovator drug
through in vitro, in vivo or other testing. The generic version must deliver the same amount of active ingredients into a subject’s
bloodstream in the same amount of time as the innovator drug and can often be substituted by pharmacists under prescriptions written for
the reference listed drug. In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent with
claims that cover the applicant’s drug or a method of using the drug. Upon approval of a drug, each of the patents listed in the
application for the drug is then published in the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential competitors
in support of approval of an ANDA or 505(b)(2) NDA.
Upon submission of an ANDA
or a 505(b)(2) NDA, an applicant must certify to the FDA that (1) no patent information on the drug product that is the subject of the
application has been submitted to the FDA; (2) such patent has expired; (3) the date on which such patent expires; or (4) such patent
is invalid or will not be infringed upon by the manufacture, use or sale of the drug product for which the application is submitted. Generally,
the ANDA or 505(b)(2) NDA cannot be approved until all listed patents have expired, except where the ANDA or 505(b)(2) NDA applicant challenges
a listed patent through the last type of certification, also known as a paragraph IV certification. If the applicant does not challenge
the listed patents or indicates that it is not seeking approval of a patented method of use, the ANDA or 505(b)(2) NDA application will
not be approved until all of the listed patents claiming the referenced product have expired.
If the ANDA or 505(b)(2) NDA
applicant has provided a Paragraph IV certification to the FDA, the applicant must send notice of the Paragraph IV certification to the
NDA and patent holders once the application has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent
infringement lawsuit in response to the notice of the paragraph IV certification. If the paragraph IV certification is challenged by an
NDA holder or the patent owner(s) asserts a patent challenge to the paragraph IV certification, the FDA may not approve that application
until the earlier of 30 months from the receipt of the notice of the paragraph IV certification, the expiration of the patent, when the
infringement case concerning each such patent was favorably decided in the applicant’s favor or settled, or such shorter or longer
period as may be ordered by a court. This prohibition is generally referred to as the 30-month stay. In instances where an ANDA or 505(b)(2)
NDA applicant files a paragraph IV certification, the NDA holder or patent owner(s) regularly take action to trigger the 30-month stay,
recognizing that the related patent litigation may take many months or years to resolve.
The FDA also cannot approve
an ANDA or 505(b)(2) application until all applicable non-patent exclusivities listed in the Orange Book for the branded reference drug
have expired. For example, a pharmaceutical manufacturer may obtain five years of non-patent exclusivity upon NDA approval of a new chemical
entity, or NCE, which is a drug containing an active moiety that has not been approved by FDA in any other NDA. An “active moiety”
is defined as the molecule responsible for the drug substance’s physiological or pharmacologic action. During that five-year exclusivity
period, the FDA cannot accept for filing (and therefore cannot approve) any ANDA seeking approval of a generic version of that drug or
any 505(b)(2) NDA that relies on the FDA’s approval of the drug, provided that that the FDA may accept an ANDA four years into the
NCE exclusivity period if the ANDA applicant also files a Paragraph IV certification.
A drug, including one approved
under Section 505(b)(2), may obtain a three-year period of exclusivity for a particular condition of approval, or change to a marketed
product, such as a new formulation for a previously approved product, if one or more new clinical studies (other than bioavailability
or bioequivalence studies) was essential to the approval of the application and was conducted/sponsored by the applicant. Should this
occur, the FDA would be precluded from approving any ANDA or 505(b)(2) application for the protected modification until after that three-year
exclusivity period has run. However, unlike NCE exclusivity, the FDA can accept an application and begin the review process during the
exclusivity period.
Other Healthcare Laws and Compliance Requirements
Pharmaceutical companies are
subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions
in which they conduct their business. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims,
privacy and security and physician sunshine laws and regulations. If their operations are found to be in violation of any of such laws
or any other governmental regulations that apply, they may be subject to penalties, including, without limitation, civil and criminal
penalties, damages, fines, the curtailment or restructuring of operations, exclusion from participation in federal and state healthcare
programs and individual imprisonment.
Coverage and Reimbursement
Sales of any product depend,
in part, on the extent to which such product will be covered by third-party payors, such as federal, state and foreign government healthcare
programs, commercial insurance and managed healthcare organizations and the level of reimbursement for such product by third-party payors.
Decisions regarding the extent of coverage and amount of reimbursement to be provided are made on a plan-by-plan basis. These third-party
payors are increasingly reducing reimbursements for medical products, drugs and services. In addition, the U.S. government, state legislatures
and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on coverage and
reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption
of more restrictive policies in jurisdictions with existing controls and measures, could further limit sales of any product. Decreases
in third-party reimbursement for any product or a decision by a third-party payor not to cover a product could reduce physician usage
and patient demand for the product and also have a material adverse effect on sales. Even after the FDA approves a product, failure to
have the product covered by third-party payors may have material adverse effect on sales. Federal and state governments continue to promulgate
new policies and regulations; such policies and regulations may have material adverse effect on sales. These laws and regulations may
restrict, prohibit, or preventing us from implementing a wide range of pricing, discounting, marketing, promotion, sales commission, incentive
programs, and other business activities. No uniform policy of coverage and reimbursement among third-party payors exists in the United
States. Such payors often rely upon Medicare coverage policy establishing their coverage and reimbursement policies. However, each payor
makes independent and separate decisions regarding the extent of coverage and amount of reimbursement to be provided.
Legislative and Regulatory Changes, Including Health Care Reform
The laws and regulations that
affect our business are subject to change from time to time, and entirely new laws and regulations are sometimes adopted. In particular,
healthcare reforms that have been adopted, and that may be adopted in the future, could result in further reductions in coverage and levels
of reimbursement for pharmaceutical products, increases in rebates payable under U.S. government rebate programs and additional downward
pressure on pharmaceutical product prices. On September 9, 2021, the Biden administration published a wide-ranging list of policy proposals,
most of which would need to be carried out by Congress, to reduce drug prices and drug payment. The Department of Health and Human Services,
or HHS, plan includes, among other reform measures, proposals to lower prescription drug prices, including by allowing Medicare to negotiate
prices and disincentivizing price increases, and to support market changes that strengthen supply chains, promote biosimilars and generic
drugs, and increase price transparency. Many similar proposals, including the plans to give Medicare Part D authority to negotiate drug
prices, require drug manufacturers to pay rebates on drugs whose prices increase greater than the rate of inflation, and cap out-of-pocket
costs, have already been included in policy statements and legislation currently being considered by Congress. Individual states in the
United States have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including
price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency
measures, and, in some cases, proposing to encourage importation from other countries and bulk purchasing. It is unclear to what extent
these and other statutory, regulatory, and administrative initiatives will be enacted and implemented.
Foreign Corrupt Practices Act
Our business activities may
be subject to the Foreign Corrupt Practices Act, or FCPA, and similar anti-bribery or anti-corruption laws, regulations or rules of other
countries in which we operate. The FCPA generally prohibits offering, promising, giving, or authorizing others to give anything of value,
either directly or indirectly, to a non-U.S. government official in order to influence official action, or otherwise obtain or retain
business. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions
of the corporation and to devise and maintain an adequate system of internal accounting controls. Our business is heavily regulated and
therefore involves significant interaction with public officials, including officials of non-U.S. governments. Additionally, in many other
countries, the health care providers who prescribe pharmaceuticals are employed by their government, and the purchasers of pharmaceuticals
are government entities; therefore, our dealings with these prescribers and purchasers are subject to regulation under the FCPA. There
is no certainty that all of our employees, agents, suppliers, manufacturers, contractors, or collaborators, or those of our affiliates,
will comply with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these
laws and regulations could result in fines, criminal sanctions against us, our officers, or our employees, the closing down of facilities,
including those of our suppliers and manufacturers, requirements to obtain export licenses, cessation of business activities in sanctioned
countries, implementation of compliance programs, and prohibitions on the conduct of our business. Any such violations could include prohibitions
on our ability to offer our products in one or more countries as well as difficulties in manufacturing or continuing to develop our products,
and could materially damage our reputation, our brand, our international expansion efforts, our ability to attract and retain employees,
and our business, prospects, operating results, and financial condition.
European Union Drug Development
In the European Union, our
product candidates may also be subject to extensive regulatory requirements. As in the United States, medicinal products can only be marketed
if a marketing authorization from the competent regulatory agencies has been obtained.
Similar to the United States,
the various phases of preclinical and clinical research in the European Union are subject to significant regulatory controls. Clinical
trials of medicinal products in the European Union must be conducted in accordance with European Union, national regulations and international
standards for good clinical practice, or GCP.
Clinical trials are currently
governed by EU Clinical Trials Directive 2001/20/EC that set out common rules for the control and authorization of clinical trials in
the European Union.
To improve the current system,
Regulation (EU) No 536/2014 on clinical trials on medicinal products for human use was adopted in 2014. The Regulation aims at harmonizing
and streamlining the clinical trials authorization process, simplifying adverse event reporting procedures, improving the supervision
of clinical trials, and increasing their transparency, notably via a clinical trial information system set up by the EMA. The new Regulation
expressly provides that it will not be applied before six months after the publication of a notice delivered by the European Commission
on the European Union clinical trial portal and database. As such notice requires a successful (partial) audit of the database and as
that database is still under development, there is no scheduled application date yet. Pursuant to the transitory provisions of the new
regulation, the Clinical Trials Directive 2001/20/EC will still apply for three years after the implementation of the European Union clinical
trial portal and database. Thus, the sponsor has the possibility to choose between the requirements of the directive and the regulation
for a period of three years from the entry into force of the regulation.
European Union Drug Review and Approval
In the EEA (which is comprised
of the 28 Member States of the European Union plus Norway, Iceland and Liechtenstein), medicinal products can only be commercialized after
obtaining a Marketing Authorization (“MA”). MAs may be granted either centrally (Community MA) or nationally (National
MA).
The Community MA is issued
centrally by the European Commission through the Centralized Procedure, based on the opinion of the CHMP of the EMA and is valid throughout
the entire territory of the EEA. The Centralized Procedure is mandatory for certain types of products such as orphan medicinal products
and medicinal products containing a new active substance indicated for the treatment of neurodegenerative disorders. The Centralized Procedure
is optional for products containing a new active substance not yet authorized in the EEA, or for products that constitute a significant
therapeutic, scientific or technical innovation or which are in the interest of public health in the European Union.
National MAs are issued nationally
by the competent authorities of the Member States of the EEA and only cover their respective territory. National MAs are available for
products not falling within the mandatory scope of the Centralized Procedure. We do not foresee that any of our current product candidates
will be suitable for a National MA as they fall within the mandatory criteria for the Centralized Procedure. Therefore, our product candidates
will be approved through Community MAs.
Under the above-described
procedures, before granting the MA, the EMA or the competent authorities of the Member States of the EEA make an assessment of the risk-benefit
balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy.
The pediatric use marketing
authorization, or PUMA, is a dedicated marketing authorization for medicinal products indicated exclusively for use in the pediatric population,
with, if necessary, an age-appropriate formulation. Pursuant to Regulation (EC) No. 1901/2006 (The “Pediatric Regulation”),
all PUMA applications for marketing authorization for new medicines must include to be valid, in addition to the particulars and documents
referred to in Directive 2001/83/EC, the results of all studies performed and details of all information collected in compliance with
a pediatric investigation plan agreed between regulatory authorities and the applicant, unless the medicine is exempt because of a deferral
or waiver of the EMA.
Before the EMA is able to
begin its assessment of a Community MA application, it will validate that the applicant has complied with the agreed pediatric investigation
plan. The applicant and the EMA may, where such a step is adequately justified, agree to modify a pediatric investigation plan to assist
validation. Modifications are not always possible; may take longer to agree than the period of validation permits; and may still require
the applicant to withdraw its marketing authorization application and to conduct additional non-clinical and clinical studies. Products
that are granted a MA on the basis of the pediatric clinical trials conducted in accordance with the Pediatric Investigation Plan, or
PIP, are eligible for a six-month extension of the protection under a supplementary protection certificate (if any is in effect at the
time of approval) or, in the case of orphan medicinal products, a two-year extension of the orphan market exclusivity. This pediatric
reward is subject to specific conditions and is not automatically available when data in compliance with the PIP are developed and submitted.
Orphan Drugs
In the European Union, Regulation
(EC) No 141/2000 of the European Parliament and of the Council of December 16, 1999 on orphan medicinal products, as amended, states that
a drug shall be designated as an orphan drug if its sponsor can establish that the three following cumulative conditions are met:
| |
· |
the product is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition; |
| |
· |
the prevalence of the conditions is not more than five in ten thousand persons in the European Union when the application is made, or that it is intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition in the European Union and that without incentives it is unlikely that the marketing of the drug in the European Union would generate sufficient return to justify the necessary investment; and |
| |
· |
that there is no satisfactory method of diagnosis, prevention or treatment of the condition in question that has been authorized in the European Union or, if such method exists, that the drug will be of significant benefit to those affected by that condition. |
Pursuant to Regulation (EC)
No. 847/2000 of April 27, 2000 laying down the provisions for implementation of the criteria for designation of a medicinal product as
an orphan medicinal product and definitions of the concepts “similar medicinal product” and “clinical superiority”,
an application for the designation of a drug as an orphan drug must be submitted at any stage of development of the drug before filing
of a MA application.
The European Union offers
incentives to encourage the development of designated orphan medicines (protocol assistance, fee reductions, etc.) and provides opportunities
for market exclusivity. Pursuant to the abovementioned Regulation (EC) No. 141/2000, products receiving orphan designation in the European
Union can obtain market exclusivity for a certain number of years in the European Union following the marketing approval.
If a Community MA in respect
of an orphan drug is granted, regulatory authorities will not, for a period of usually ten years, accept another application for a MA,
or grant a MA or accept an application to extend an existing MA, for the same therapeutic indication, in respect of a similar drug. This
period may however be reduced to six years if, at the end of the fifth year, it is established, in respect of the drug concerned, that
the above-mentioned criteria for orphan drug designation are no longer met, in other words, when it is shown on the basis of available
evidence that the product is sufficiently profitable not to justify maintenance of market exclusivity.
Pursuant to Regulation No.
1901/2006, for orphan medicinal products, instead of an extension of the supplementary protection certificate, the ten-year period of
orphan market exclusivity should be extended to 12 years if the requirement for data on use in the pediatric population is fully met (i.e.
when the request contains the results of all studies carried out under the approved PIP and when the declaration attesting the conformity
of the request to this PIP is included in the MA).
Notwithstanding the foregoing,
a MA may be granted, for the same therapeutic indication, to a similar drug if:
| |
· |
the holder of the MA for the original orphan drug has given its consent to the second applicant; |
| |
· |
the holder of the MA for the original orphan drug is unable to supply sufficient quantities of the drug; or |
| |
· |
the second applicant can establish in the application that the second drug, although similar to the orphan drug already authorized, is safer, more effective or otherwise clinically superior. |
The abovementioned Regulation
(EC) No. 141/2000 provides for other incentives regarding orphan medicinal products.
Post-Approval Controls
The holder of a MA must comply
with EU requirements applicable to manufacturing, marketing, promotion and sale of medicinal products. In particular, the holder of the
MA must establish and maintain a pharmacovigilance system and appoint an individual qualified person for pharmacovigilance, or QPPV, who
is responsible for oversight of that system and who will reside and operate in the EU. Key obligations include safety expedited reporting
of suspected serious adverse reactions and submission of periodic safety update reports, or PSURs.
All new MAs must include a
risk management plan, or RMP, to submit to the EMA, describing the risk management system that the company will put in place and documenting
measures to prevent or minimize the risks associated with the product. The regulatory authorities may also impose specific obligations
as a condition of the MA. Such risk-minimization measures or post-authorization obligations may include additional safety monitoring,
more frequent submission of PSURs, or the conduct of additional clinical trials or post-authorization safety studies. RMPs and PSURs are
routinely available to third parties requesting access, subject to limited redactions. All advertising and promotional activities for
the product must be consistent with the approved summary of product characteristics, and therefore all off-label promotion is prohibited.
Direct-to-consumer advertising of prescription medicines is also prohibited in the European Union. Although general requirements for advertising
and promotion of medicinal products are established under EU directives, the details are governed by regulations in each EU Member State
and can differ from one country to another.
Reimbursement
The European Union provides
options for its Member States to restrict the range of medicinal products for which their national health insurance systems provide reimbursement
and to control the prices of medicinal products for human use. A Member State may approve a specific price for the medicinal product,
or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the
market. For example, in France, effective market access will be supported by agreements with hospitals and products may be reimbursed
by the Social Security Fund. The price of medicines covered by national health insurance is negotiated with the Economic Committee for
Health Products, or CEPS. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical
products will allow favorable reimbursement and pricing arrangements for any of our product candidates. Historically, products launched
in the European Union do not follow price structures of the United States and generally prices tend to be significantly lower.
Other European Regulatory Matters
French Regulatory Framework for Clinical Development
In France, Directive No. 2001/20/EC
has been implemented in French national law, establishing a system of prior authorization and requiring a prior favorable opinion from
an ethics committee.
Parties to a clinical trial
agreement, or CTA, must use a CTA template (“unique agreement” or “convention unique”) to organize the conduct
of interventional clinical trials with commercial purpose, as well as specific template exhibits to this agreement. Once concluded, the
CTA is communicated for information by the sponsor to the French national board of physicians (Ordre national des médecins) without
delay.
The processing of personal
data collected during clinical trials has to comply with the Regulation (EU) 2016/679 of the European Parliament and of the Council of
April 27, 2016 and Law No 2018-493 of June 20, 2018 on the protection of personal data, implementing the Regulation (EU) 2016/679 requirements.
Regarding automatic processing operations for the purpose of research or clinical studies, formalities have to be completed before the
French data protection authority, the Commission Nationale de l’Informatique et des Libertés, or CNIL, so as to obtain the
authorization to process personal data. However, there are simplified standards.
Law No. 2011-2012 of December
29, 2011, or Loi Bertrand, aimed at strengthening the health safety of medicinal and health products, as amended (and its implementing
decrees), introduced into French law provisions regarding transparency of fees received by some healthcare professionals from health product
industries, i.e. companies manufacturing or marketing health products (Article L.1453-1 of the French Public Health Code). These provisions
have been recently extended and redefined by Decree No. 2016-1939 of December 28, 2016, which clarified French “Sunshine”
regulations. The decree notably provides that companies manufacturing or marketing health care products (medicinal products, medical devices,
etc.) in France shall publicly disclose (mainly on a specific public website available at: https://www.entreprises-transparence.sante.gouv.fr)
the advantages and fees paid to healthcare professionals amounting to €10 or above, as well as the agreements concluded with the
latter, along with detailed information about each agreement (the precise subject matter of the agreement, the date of signature of the
agreement, its end date, the total amount paid to the healthcare professional, etc.). Another declaration must also be filed to the competent
healthcare professional body. Law No. 2011-2012 also reinforced the French anti-gift rules and Order No. 2017-49 of January 19, 2017 amended
the law and expanded the scope of the general prohibition of payments from pharmaceutical and device manufacturers to healthcare professionals
to broadly cover any company manufacturing or marketing health products, regardless of whether or not payment for the products is reimbursed
under the French social security system (new Articles L. 1453-3 et seq. of the French Public Health Code). It also changed the procedure
related to the prior submission to the national or departmental board of the relevant healthcare professional body. Moreover, the penalties
incurred for non-compliance with the requirements of the Anti-Gift Law will be doubled to a fine of up to €750,000. The changes of
the anti-gift rules will only enter into force after the publication of implementing measures.
Environmental Matters
Our operations, properties
and products are subject to a variety of U.S. and foreign environmental laws and regulations governing, among other things, air emissions,
wastewater discharges, management and disposal of hazardous and non-hazardous materials and waste and remediation of releases of hazardous
materials. We believe, based on current information, that we are in material compliance with environmental laws and regulations applicable
to us. However, our failure to comply with present and future requirements under these laws and regulations, or environmental contamination
or releases of hazardous materials on our leased premises, as well as through disposal of our products, could cause us to incur substantial
costs, including clean-up costs, personal injury and property damage claims, fines and penalties, costs to redesign our products or upgrade
our facilities and legal costs, or require us to curtail our operations, any of which could seriously harm our business.
Employees
As of December 31, 2023, we had 9 full-time employees,
all of whom are located in the United States. As of March 27, 2024, we had 15 full-time employees following our acquisition of IMGX.
Available Information
As a public company, we
are required to file our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statements on
Schedule 14A and other information (including any amendments) with the Securities and Exchange Commission. The SEC maintains an Internet
site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the
SEC. You can find our SEC filings at the SEC’s website at www.sec.gov.
Our Internet address is
www.enterothera.com. Information contained on our website is not part of this Annual Report. Our SEC filings (including any amendments)
will be made available free of charge on www.enterothera.com, as soon as reasonably practicable after we electronically file such material
with, or furnish it to, the SEC.
ENTERO’s
Management’s Discussion and Analysis of Financial Condition and Results of OperationS
You should read the following discussion
and analysis in conjunction with Entero’s consolidated financial statements, including the notes thereto contained in this Proxy
Statement. This discussion contains forward-looking statements that involve risks, uncertainties and assumptions. Entero’s actual
results may differ materially from those anticipated in these forward-looking statements as a result of a variety of certain factors,
including those set forth under “Risk Factors Associated with Our Business” and elsewhere in this Proxy Statement. Unless
otherwise indicated, reference in this section to “we,” “our” and the “Company” refers to Entero.
Overview
We are engaged in the
research and development of targeted, non-systemic therapies for the treatment of patients with gastrointestinal diseases. Non-systemic
therapies are non-absorbable drugs that act locally, i.e., in the intestinal lumen, skin or mucosa, without reaching an individual’s
systemic circulation.
We are currently focused on
developing a therapeutic pipeline with multiple late-stage clinical programs built around four proprietary technologies - Latiglutenase,
a targeted oral minimally absorbed recombinant enzyme designed to breakdown gluten into non-immunogenic peptides for the treatment of
symptomatic celiac disease as an adjunct to the gluten free diet; the biologic Adrulipase, a recombinant lipase enzyme designed to enable
the digestion of fats and other nutrients in cystic fibrosis and chronic pancreatitis patients with exocrine pancreatic insufficiency;
Capeserod, a selective 5-HT4 receptor partial agonist which we are developing for the treatment of gastroparesis; and Niclosamide, an
oral small molecule with anti-inflammatory properties for patients with inflammatory bowel diseases such as ulcerative colitis and Crohn’s
disease.
In March 2024, we announced
the closing of a merger with ImmunogenX, Inc., a private, clinical-stage biopharmaceutical company founded in 2013, which is developing
the biologic, Latiglutenase, for celiac disease. IMGX is also developing CypCel, a metabolic marker compound that can measure the state
of small-intestinal recovery of celiac patients undergoing gluten-free diets.
In December 2023, we announced
that we have entered into a non-binding term sheet to sell our Niclosamide program. The non-binding term sheet includes a low seven-figure
upfront payment to us for rights to Niclosamide, as well as economics related to future milestones and royalties. The transaction is expected
to close in the first half of 2024.
Our Product Candidates
Our Latiglutenase program
is focused on the development of oral, minimally-absorbed, recombinant enzymes, which will be delivered as powders and combined with flavoring
and water to be taken with the three main gluten-free meals. After a successful End-of-Phase 2 meeting with the FDA, we expect to enter
Phase 3 clinical trials in the first half of 2025.
Our Adrulipase programs
are focused on the development of an oral, non-systemic, biologic capsule for the treatment of exocrine pancreatic insufficiency in patients
with cystic fibrosis and chronic pancreatitis. Our goal is to provide CF and CP patients with a safe and effective therapy to control
EPI that is non-animal derived and offers the potential to dramatically reduce their daily pill burden. In July 2023, we announced topline
results from our Phase 2b monotherapy bridging study using a new enteric microgranule formulation of Adrulipase While the initial data
from the study indicated that the enhanced Adrulipase formulation was safe and well tolerated and demonstrated an improvement over prior
formulations of Adrulipase, it also indicated that it is likely that the primary efficacy endpoint was not achieved. We are continuing
to assess this data, along with secondary efficacy endpoint data, and plan to schedule a Type C meeting with the FDA in the second half
of 2024 to discuss next steps for the Adrulipase program.
Our Capeserod program
was in-licensed from Sanofi in September 2023. Sanofi conducted Phase 1 and Phase 2 Central Nervous System trials with over 600 patients.
In Sanofi’s Phase 1 CNS trial, Capeserod appeared safe and well-tolerated. Research on Capeserod and subsequent artificial intelligence
empowered analyses suggest that the drug possesses a unique mechanism of action that is applicable to several GI indications underserved
by currently available therapeutics. We plan to repurpose Capeserod for GI indications and expect to initiate a Phase 2 clinical development
program in either pediatric ulcerative colitis or gastroparesis. Sanofi retains the right of first refusal to develop and commercialize
Capeserod.
Our Niclosamide programs
leverage proprietary oral and topical formulations to address multiple GI conditions, including inflammatory bowel diseases indications.
In 2022 we advanced two separate Phase 2 clinical programs of our Niclosamide formulations, including FW-COV for COVID-19 GI infections,
and FW-UP for ulcerative proctitis and ulcerative proctosigmoiditis. We are no longer actively pursuing these programs.
Merger with ImmunogenX
On March 13, 2024, we
acquired ImmunogenX, Inc., a Delaware corporation, in accordance with the terms of the Merger Agreement, by and among the Company, First
Merger Sub, Second Merger Sub, and IMGX. Pursuant to the Merger Agreement, First Merger Sub merged with and into IMGX, pursuant to which
IMGX was the surviving corporation. Immediately following the First Merger, IMGX merged with and into Second Merger Sub, pursuant to
which Second Merger Sub was the surviving entity and a wholly owned subsidiary of the Company. The Second Merger Sub amended its certificate
of formation concurrently with the Second Merger to change its company name to ImmunogenX, LLC. The Merger is intended to qualify as
a tax-free reorganization for U.S. federal income tax purposes.
Under the terms of the
Merger Agreement, following the Closing, in exchange for the outstanding shares of capital stock of IMGX immediately prior to the effective
time of the First Merger, we issued to the stockholders of IMGX an aggregate of (A) 36,830 shares of Common Stock, and (B) 11,777.418
shares of Series G Preferred Stock, each share of which is convertible into 1,000 shares of Common Stock, subject to certain conditions
described below. In addition, we assumed (i) all IMGX stock options immediately outstanding prior to the First Merger, each becoming
an option to purchase Common Stock subject to adjustment pursuant to the terms of the Merger Agreement and (ii) all IMGX warrants immediately
outstanding prior to the First Merger, each becoming a warrant to purchase Common Stock subject to adjustment pursuant to the terms of
the Merger Agreement. The Assumed Options are exercisable for an aggregate of 200,652 shares of Common Stock, have an exercise price
of $0.81 and expire between February 1, 2031 and June 6, 2033. The Assumed Warrants are exercisable for an aggregate of 127,682 shares
of Common Stock, have exercise prices ranging from $3.02 to $3.92 and expire between September 30, 2032 and September 6, 2033.
The foregoing description
of the Assumed Warrants does not purport to be complete and is qualified in its entirety by reference to the form of Assumed Warrant,
which is incorporated herein by reference to Exhibit 4.43 of the Company’s Annual Report on Form 10-K for the fiscal year end December
31, 2023, filed with the SEC on March 29, 2024.
Tungsten acted as financial
advisor to the Company in connection with the Merger. As partial compensation for services rendered by Tungsten, the Company issued to
Tungsten or its affiliates or designees an aggregate of 18,475 shares of Common Stock and 595.808 shares of Series G Preferred Stock.
Pursuant to the Merger
Agreement, we have agreed to hold a stockholders’ meeting to submit the following matters to its stockholders for their consideration:
(i) the approval of the Conversion Proposal and (ii) if deemed necessary or appropriate by the Company or as otherwise required by applicable
law or contract, the approval of an amendment to the Company’s Charter, to authorize sufficient shares of Common Stock for the
conversion of all shares of Series G Preferred Stock issued pursuant to the Merger Agreement. In connection with these matters, the Company
has agreed to file a proxy statement with the SEC, and which matters are reflected in this Proxy Statement.
The Board and the holders
of a majority of the Company’s Series B Convertible Preferred Stock approved the Merger Agreement and the related transactions,
and the consummation of the Merger was not subject to approval of Company common stockholders.
The foregoing description
of the Merger and the Merger Agreement does not purport to be complete and is qualified in its entirety by reference to the full text
of the Merger Agreement, which is incorporated herein by reference to Exhibit 2.2 of the Company’s Annual Report on Form 10-K for
the fiscal year ended December 31, 2023, filed with the SEC on March 29, 2024.
The Merger Agreement contains
representations, warranties and covenants that the Company and IMGX made to each other as of specific dates. The assertions embodied in
those representations, warranties and covenants were made solely for purposes of the Merger Agreement between the Company and IMGX and
may be subject to important qualifications and limitations agreed to by the Company and IMGX in connection with negotiating its terms,
including being qualified by confidential disclosures exchanged between the parties in connection with the execution of the Merger Agreement.
Moreover, the representations and warranties may be subject to a contractual standard of materiality that may be different from what may
be viewed as material to investors or securityholders, or may have been used for the purpose of allocating risk between the Company and
IMGX rather than establishing matters as facts. Moreover, information concerning the subject matter of the representations and warranties
may change after the date of the Merger Agreement, which subsequent information may or may not be fully reflected in the Company’s
public disclosures. For the foregoing reasons, no person should rely on the representations and warranties as statements of factual information
at the time they were made or otherwise.
Voting and Support Agreements
In connection with the
execution of the Merger Agreement, the Company and IMGX entered into stockholder voting agreements with certain of IMGX’s stockholders.
Pursuant to the IMGX Voting Agreements, among other things, each of the IMGX stockholder parties thereto has agreed to vote or cause
to be voted all of the shares of Common Stock owned by such stockholder in favor of the Share Increase Proposal (if such proposal is
brought before the Company’s stockholders).
The foregoing description
of the IMGX Voting Agreements does not purport to be complete and is qualified in its entirety by reference to the full text of the form
of the IMGX Voting Agreement, a copy of which is included as Exhibit A to the Merger Agreement, which is incorporated herein by reference
to Exhibit 2.2 of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March
29, 2024.
In connection with the
execution of the Merger Agreement, the Company and IMGX entered into the Company Support Agreements with certain of the Company’s
officers (solely in their capacity as stockholders of the Company). Pursuant to the Support Agreements, among other things, each of the
Company stockholder parties thereto has agreed to vote or cause to be voted all of the shares of Common Stock owned by such stockholder
in favor of the Meeting Proposals.
The foregoing description
of the Company Support Agreements does not purport to be complete and is qualified in its entirety by reference to the full text of the
form of the Company Support Agreement, a copy of which is included as Exhibit B to the Merger Agreement, which is incorporated herein
by reference to Exhibit 2.2 of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with
the SEC on March 29, 2024.
Lock-up Agreements
Concurrently and in connection
with the execution of the Merger Agreement, certain IMGX stockholders as of immediately prior to the Closing, and certain officers of
the Company as of immediately prior to the Closing entered into lock-up agreements with the Company and IMGX, pursuant to which each
such stockholder agreed to be subject to a 180-day lockup on the sale or transfer of shares of the Company held by each such stockholder
at the Closing, including those shares of Common Stock and Series G Preferred Stock (including the shares of Common Stock into which
such Series G Preferred Stock is convertible) received by each such stockholder in the Merger.
The foregoing description
of the Lock-up Agreements does not purport to be complete and is qualified in its entirety by reference to the full text of the form of
the Lock-up Agreement, a copy of which is included as Exhibit C to the Merger Agreement, which is incorporated herein by reference to
Exhibit 2.2 of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March
29, 2024.
Restrictive Covenants Agreements
Concurrently and in connection
with the execution of the Merger Agreement, certain IMGX officers as of immediately prior to the Closing, entered into restrictive covenants
agreements with the Company and IMGX, pursuant to which such individuals and each of their affiliates agreed not to compete with IMGX
during the three-year period following the Closing and, during such three-year restricted period, not to solicit Restricted Employees
(as defined in the form of Restrictive Covenants Agreement). The Restrictive Covenants Agreements also contain customary non-disparagement
and confidentiality provisions.
The foregoing description
of the Restrictive Covenants Agreement does not purport to be complete and is qualified in its entirety by reference to the full text
of the form of the Restrictive Covenants Agreement, a copy of which is included as Exhibit D to the Merger Agreement, which is incorporated
herein by reference to Exhibit 2.2 of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed
with the SEC on March 29, 2024.
Debt of ImmunogenX
IMGX is party to the Credit
Agreement, with Lender, pursuant to which Lender provided IMGX with a secured revolving credit facility with a total commitment of $7,500,000.
The loans under the Credit Agreement bore interest at a per annum floating rate equal to the prime rate plus 4.50% and had a maturity
date of October 3, 2024. In connection with the Merger, IMGX entered into the Second Amendment, which provides for, among other things:
(i) Lender’s consent for the Merger, (ii) Second Merger Sub’s assumption all of IMGX obligations, rights and liabilities
as borrower under the Amended Credit Agreement, (iii) an amendment to the interest rate to a per annum floating rate equal to the prime
rate plus 6.00%, (iv) an extension of the maturity date to September 13, 2025, (v) a prohibition on the borrower’s ability to request
additional loans under the revolving credit facility, subject to certain limited exceptions, (vi) the pledge by the Pledgors of their
Pledged Equity as part of the collateral securing the obligations under the Amended Credit Agreement, and (vii) an amendment to the total
commitment under the Amended Credit Agreement to $8,212,345.17, which takes into account the One-Time Prepayment, accrued interest and
fees for the remaining term of the facility and certain other fees to be paid under the Amended Credit Agreement. The Pledged Equity
shall be subject to a call right, which shall give Lender the right to, at its option, require the Pledgors to sell to Lender (on a pro
rata basis) the number of shares of Pledged Equity that, when multiplied by the per share valuation of the Common Stock at Closing, satisfies
all or a portion of the then outstanding amount of obligations under the Amended Credit Agreement.
The borrowings under the
Amended Credit Agreement are by evidenced by the Note, and are secured by the Security Agreement, by and between IMGX and Lender.
On March 13, 2024, IMGX
and Lender entered into the Support Letter, pursuant to which, among other things, IMGX and Lender agreed that IMGX is permitted
to license or transfer rights to any intellectual property which may constitute collateral under the Amended Credit Agreement.
The Amended Credit Agreement
contains customary representations and warranties, affirmative covenants and negative covenants, including covenants that limit or restrict
the IMGX’s ability to, among other things, incur additional indebtedness, merge or consolidate, pay dividends or other distributions,
repurchase equity, dispose of assets and enter into certain transactions with affiliates, in each case subject to certain exceptions.
The Amended Credit Agreement also includes customary events of default, and upon the occurrence and during the continuation of an event
of default, Lender may declare the outstanding advances and all other obligations under the Amended Credit Agreement to be immediately
due and payable. Payments owed under the Credit Agreement are also guaranteed by the Guarantee.
The foregoing descriptions
of the Amended Credit Agreement, the Note, the Security Agreement and the Support Letter do not purport to be complete and are qualified
in their entirety by reference to the Amended Credit Agreement, the Note, the Security Agreement and the Support Letter, which are incorporated
herein by reference to Exhibits 10.54, 10.55, 10.56 and 10.57 of the Company’s Annual Report on Form 10-K for the fiscal year ended
December 31, 2023, filed with the SEC on March 29, 2024.
Stockholder Notes
In order to fund the One-Time
Prepayment, on March 13, 2024, IMGX entered into (i) the Syage Note, and (ii) the Felker Note. The Shareholder Notes will bear interest
at a per annum floating rate equal to the prime rate plus 4.50% and mature on October 13, 2025. Each of Jack Syage and Peter Felker,
who were shareholders of IMGX prior to the Merger, have entered into the Shareholder Security Agreements, each dated March 13, 2024,
pursuant to which the obligations under each of the Shareholder Notes are secured by, among other things, the patents and trademarks
owned by IMGX.
The foregoing descriptions
of the Shareholder Notes and the Shareholder Security Agreements do not purport to be complete and are qualified in their entirety by
reference to the Form of Shareholder Note and Form of Shareholder Security Agreement, which are incorporated herein by reference to Exhibits
10.58 and 10.59 of the Company’ Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March
29, 2024.
Nasdaq Listing Requirements
On August 17, 2023, we
received a letter from the Staff of Nasdaq indicating that we were not in compliance with the $2.5 million minimum stockholders’
equity requirement for continued listing of our common stock on Nasdaq as set forth in Nasdaq Listing Rule 5550(b)(1) (the “Minimum
Stockholder’s Equity Rule”). In that regard, we reported stockholders’ equity of $881,960 in our Quarterly Report
on Form 10-Q for the period ended June 30, 2023 (we did not then, and do not now, meet the alternative compliance standards relating
to the market value of listed securities of $35 million or net income from continuing operations of $500,000 in the most recently completed
fiscal year or in two of the last three most recently completed fiscal years). On October 2, 2023, we submitted a plan to the Staff to
regain compliance with the Minimum Stockholders’ Equity Rule. On November 13, 2023, we filed our Quarterly Report on Form 10-Q
for the period ended September 30, 2023, reporting total stockholders’ equity of $3,278,805 as of September 30, 2023. On April
1, 2024, we received a letter from the Staff stating that we had regained compliance with the Minimum Stockholder’s Equity Rule.
On August 24, 2023, we received
notice from the Staff indicating that, based upon the closing bid price of our common stock for the prior 30 consecutive business days,
we were not currently in compliance with the requirement to maintain a minimum bid price of $1.00 per share for continued listing on Nasdaq
as set forth in Nasdaq Listing Rule 5550(a)(2) (the “Bid Price Rule”). We had 180 days from August 24, 2023, or through
February 20, 2024, to regain compliance with the Bid Price Rule. On January 4, 2024, we received notice from the Staff that we are in
compliance with the Bid Price Rule.
On October 26, 2023, we
received a notice from the Staff of Nasdaq indicating that in connection with the July 2023 Offering, the Company was not in compliance
with Nasdaq’s shareholder approval requirements set forth in listing rule 5635(d), which requires prior shareholder approval for
transactions, other than public offerings, involving the issuance of 20% or more of the pre-transaction shares outstanding at less than
the Minimum Price, defined as a price that is the lower of: (i) the Nasdaq official closing Price (as reflected on Nasdaq.com) immediately
preceding the signing of the binding agreement; or (ii) the average Nasdaq official Closing Price of the common stock (as reflected on
Nasdaq.com) for the five trading days immediately preceding the signing of the binding agreement. On December 12, 2023, during the Special
Meeting, our stockholders ratified our entry into the July Offering as we received the affirmative vote of the majority of the votes
cast by shares of our common stock present or represented by proxy and entitled to vote at the Special Meeting. On March 19, 2024, we
received a Letter of Reprimand from the Nasdaq Listing Qualifications Staff (the "Letter of Reprimand") stating that,
while we failed to comply with Nasdaq's continued listing requirements, our violation of listing rule 5635 (d) does not appear to have
been the result of a deliberate intent to avoid compliance, and as such, the Staff does not believe that delisting our securities is
an appropriate sanction and that it is appropriate to close these matters by issuing the Letter of Reprimand.
Registered Direct Offerings
On March 6, 2024, we closed
on a Registered Direct Offering with an institutional investor for the purchase and sale of 525,625 shares of our Common Stock (or Common
Stock equivalents) at a price of $7.61 per share. In addition, we issued to the investor warrants to purchase up to 525,625 shares of
Common Stock. The warrants have an exercise price of $7.48 per share, are exercisable immediately following the date of issuance, and
have a term of five years following the date of issuance. Roth Capital Partners acted as the exclusive placement agent for the offering.
The gross proceeds to us from this offering were approximately $4.0 million, before deducting the placement agent's fees and other offering
expenses payable by us.
On
May 10, 2024, we announced that we entered into a Registered Direct Offering (the “May 2024 Offering”) likewise priced
at market under Nasdaq rules. The May 2024 Offering was for an aggregate of (i) 275,000 shares of Common Stock, (ii) pre-funded
warrants (the “May 2024 Pre-Funded Warrants”) to purchase up to an aggregate of 91,000 shares of Common Stock and
(iii) common warrants (the “May 2024 Warrants”) to purchase up to an aggregate of 732,000 shares of Common Stock.
The May 2024 Pre-Funded Warrants had the same exercise price and exercise terms as the March 2024 Pre-Funded Warrants. The
public offering price for each share of Common Stock in the May 2024 Offering was $2.95, and the public offering price for each May 2024
Pre-Funded Warrant, was $2.9499. The May 2024 Warrants have an exercise price of $2.70 per share, are exercisable immediately and will
expire six years from the initial exercise date. In this offering, we received gross proceeds of approximately $1.1 million less
placement agent’s fees and other offering expenses.
In
connection with the May 2024 Offering, we were obligated to file a registration statement with the SEC to register the shares underlying
the warrants sold in the offering under the Securities Act, within 30 days of the closing of the May 2024 Offering and have such registration
statement declared effective by the SEC within 60 days of such closing, or in the case of full review of the applicable registration
statement by the SEC, within 120 days of such closing. If such registration statement is not so filed or declared effective, on
each applicable monthly anniversary for which such registration even is not achieved or cured, we are required to pay to the holder an
amount in cash, as partial liquidated damages and not as a penalty, equal to the product of 1.0% multiplied by the product of the most
recent closing price of our Common Stock on the applicable event date, and the number of shares underlying such warrants, until the shares
underlying such warrants are freely tradeable under Rule 144 of the Securities Act or we regain compliance with the registration rights.
If we fail to pay partial liquidated damages required thereby within seven (7) days after the date payable, we are required to
pay interest thereon at a rate of 12% per annum (or such lesser maximum amount that is permitted to be paid by applicable law) to the
holder, accruing daily from the date such partial liquidated damages are due until such amounts, plus all such interest thereon, are
paid in full. The partial liquidated damages provisions apply on a daily pro rata basis for any applicable portion of a month.
Issuance of Restricted Stock Units
On January 2, 2024, we
issued to employees, consultants and the board of directors ten-year restricted stock units consisting of 120,532 shares of Common Stock,
subject to service-based milestones vesting quarterly over one year under the 2020 Plan as payment for services rendered. Such issuance
was exempt from registration under 4(a)(2) of the Securities Act.
Warrant Reload and Repricing
On December 27, 2023 we entered
into a warrant exercise inducement offer letter with a holder of warrants to purchase shares of common stock pursuant to which the holder
agreed to exercise for cash their existing warrants to purchase 881,337 shares of common stock, in the aggregate, at a reduced exercise
price of $5.50 per shares, in exchange for new warrants on substantially the same terms as the existing warrants, to purchase up to 1,762,674
shares of common stock and a cash payment of $0.125 per warrant share which was paid in full upon the exercise of the existing warrants.
We received aggregate gross proceeds of approximately $4.8 million from the exercise of the existing warrants and sale of the inducement
warrants.
Series B Exchange Right Waivers
Between February 1, 2022 and
February 7, 2022, we entered into waiver agreements (the “Temporary Waiver”) with certain holders of our Series B Convertible
Preferred Stock, par value $0.0001 per share (the “Series B Preferred Stock”), pursuant to which we agreed to pay a
cash waiver fee equal to ten percent of the stated value of the shares of Series B Preferred Stock held by such holder (other than holders
who are insiders who did not receive a cash waiver fee) and such holder agreed to irrevocably waive its Series B Exchange Right (as defined
below) with respect to any Subsequent Financing (as defined below) that occurs from and after the date of the Temporary Waiver until December
31, 2022.
Pursuant to the Series B Preferred
Stock Certificate of Designations (the “Series B Certificate of Designations”), in the event of any issuance by us
or any of our subsidiaries of our common stock or common stock equivalents for cash consideration or a combination of units thereof (a
“Subsequent Financing”), each holder of our Series B Preferred Stock has the right, subject to certain exceptions set
forth in the Series B Certificate of Designations, at its option, to exchange (in lieu of cash subscription payments) all or some of the
Series B Preferred Stock then held (with a value per share of Series B Preferred Stock equal to the stated value of each share of Series
B Preferred Stock, or $7,700.00, plus accrued and unpaid dividends thereon, of the Series B Preferred Stock) for any securities or units
issued in a Subsequent Financing on a dollar-for-dollar basis (the “Series B Exchange Right”).
We entered into Temporary
Waivers with holders of approximately $2.88 million of stated value of our Series B Preferred Stock, including with Company insiders holding
approximately $474,000 of stated value of our Series B Preferred Stock for which we did not pay a waiver fee.
Effective May 12, 2022, the
holders of 81.3% of the outstanding shares of the Series B Preferred Stock permanently waived for themselves and all other holders of
the Series B Preferred Stock the Series B Exchange Right with respect to any Subsequent Financing (as defined below) occurring on or after
January 1, 2022 (the “Permanent Waiver”). Holders of Series B Preferred Stock as of the April 27, 2022 record date
were entitled to notice of and to consent to the Permanent Waiver (the “Record Holders”).
Pursuant to the terms of the
Series B Certificate of Designations, the written consent of the holders of at least a majority of the Series B Preferred Stock outstanding
was required to consent to the Permanent Waiver (the “Required Consent”). We requested that the Record Holders consent
to the Permanent Waiver by executing and delivering a joinder to the Waiver Agreement (as defined below). The execution and delivery of
the joinder to the Waiver Agreement was deemed, for purposes of Section 228 of the General Corporation Law of the State of Delaware, to
be an action by written consent in lieu of a meeting to approve the Permanent Waiver. Our solicitation of consents to the Permanent Waiver
terminated in accordance with its terms at 5:00 p.m., Eastern Time, on May 12, 2022 (the “Expiration Date”). The Record
Holders who consented to the Permanent Waiver prior to the Expiration Date are referred to herein as the “Consenting Holders”.
The Required Consent was obtained
from the Consenting Holders and the solicitation terminated in accordance with its terms as of the Expiration Date. The Permanent Waiver
was effective immediately upon the Expiration Date and is binding on all holders of the Series B Preferred Stock, including those holders
that did not timely consent to the Permanent Waiver prior to the Expiration Date. The Permanent Waiver will also be applicable to any
future holder of Series B Preferred Stock. A notation of the Permanent Waiver was made on the books and records of the Company’s
transfer agent and a legend reflecting the Permanent Waiver will be placed on any physical share certificate representing shares of Series
B Preferred Stock.
Pursuant to the terms of a
Waiver Agreement entered into by us and the Consenting Holders (the “Waiver Agreement”), we have permanently reduced
the exercise price of the Series B Warrants originally issued on July 16, 2020 (the “Series B Warrants”) held by the
Consenting Holders to $1,050.00 per share or, in the case of Consenting Holders who are officers and directors of the Company, $1,383.40
(the “Exercise Price Reduction”). Only Consenting Holders are entitled to the Exercise Price Reduction. Series B Warrants
to purchase an aggregate of approximately 58 shares of our common stock received the Exercise Price Reduction which was effective as of
the Expiration Date.
Financial Operations Overview
Revenue
To date, we have not generated
any revenue from the sale of our product candidates or otherwise. In the future, we expect that we will seek to generate revenue primarily
from product sales, but we may also generate non-product revenue from sources including, but not limited to, research funding, development
and milestone payments, and royalties on future product sales in connection with any out-license or other strategic relationships and/or
government grants we may establish. Our product candidates are at an early stage of development and may never be successfully developed
or commercialized.
Research and Development Expense
Conducting research and development
is central to our business. Historically, the majority of our research and development expenses have been focused on the development of
Adrulipase and the acquisition and development of Niclosamide. Research and development expenses consist primarily of internal and external
costs incurred for our development activities, which include, among other things:
| |
· |
personnel-related costs, which include salaries, benefits, and stock-based compensation expense; |
| |
· |
fees paid to third parties for services directly related to our drug development and regulatory efforts; |
| |
· |
Expenses incurred under agreements with clinical research organizations (“CROs”), investigative sites and consultants and contractors that conduct or provide other services relating to our clinical trials and research activities; |
| |
· |
the
cost of acquiring drug product, drug supply and clinical trial materials from contract development and manufacturing organization
and third-party contractors; |
| |
· |
costs associated with preclinical and non-clinical activities; |
| |
· |
payments and other costs in connection with the acquisition our product candidates under licensing agreements; and |
| |
· |
amortization of intangible assets, including patents, in-process research and development and license agreements. |
| |
· |
Costs incurred in connection with research and development activities are expensed as incurred. |
We expect our research and
development expenses to increase for the foreseeable future as we focus our efforts on the clinical development of our product candidates,
including Latiglutenase, Adrulipase, Capeserod and Niclosamide through late-stage clinical trials, as well as chemistry, manufacturing
and controls (“CMC”) efforts. The process of conducting non-clinical studies and clinical trials necessary to obtain
regulatory approval is costly and time-consuming. It is difficult to determine with certainty the duration and costs of any non-clinical
study or clinical trial that we may conduct. In addition, if our product development efforts are successful, we expect to incur substantial
costs to prepare for potential commercialization of any late-stage product candidates and, in the event any of our product candidates
receives regulatory approval, to potentially fund the launch and sales and marketing efforts of the product.
The probability of success
for any of our current or future product candidates will depend on numerous factors, including competition, manufacturing capability and
commercial viability. We will determine which programs to pursue and how much to fund each program in response to the scientific and clinical
success of each drug candidate, as well as an assessment of each drug candidate’s commercial potential.
We do not record or maintain
information regarding costs incurred in research and development on a program or project specific basis. Our research and development
staff, outside consultants, contractors, CROs, and CDMOs are deployed across several programs and/or indications. Additionally, many of
our costs are not attributable to individual programs and/or indications. Therefore, we believe that allocating costs on the basis of
time incurred by our personnel does not accurately reflect the actual costs of a project.
General and Administrative Expense
General and administrative
expenses consist primarily of personnel-related expenses, including salaries, benefits and stock-based compensation, related to our executive,
finance, business development and support functions, legal fees relating to both intellectual property and corporate matters, insurance,
costs associated with operating as a public company, including corporate communications and investor relations expense, information technology,
professional fees for accounting, auditing and other professional services, and facility-related costs.
We anticipate our general
and administrative expenses to increase for the foreseeable future to support of our expanded research and development activities, intellectual
property, patent and corporate legal expense, insurance, and costs associated with operating as a public company, including corporate
communications and investor relations expense. Additional increases in general and administrative expenses are expected in connection
with increased business development efforts, including potential partnership and/or collaboration agreements and financing activities,
expanding infrastructure, including information technology administration, and the hiring of additional personnel and consultants, among
other expenses.
Consolidated
Results of Operations for the Three Months Ended March 31, 2024 and 2023
The following
table summarizes our consolidated results of operations for the periods indicated:
| | |
Three Months
Ended | | |
| |
| | |
March
31, | | |
Increase | |
| | |
2024 | | |
2023 | | |
(Decrease) | |
| Operating expenses: | |
| | | |
| | | |
| | |
| Research and
development expenses | |
$ | 565,962 | | |
$ | 1,361,683 | | |
$ | (795,721 | ) |
| General
and administrative expenses | |
| 8,659,692 | | |
| 2,700,742 | | |
| 5,958,950 | |
| Total operating expenses | |
| 9,225,654 | | |
| 4,062,425 | | |
| 5,163,229 | |
| Other
expenses | |
| 66,627 | | |
| 8,228 | | |
| 58,399 | |
| Loss
before income tax benefit | |
$ | (9,292,281 | ) | |
$ | (4,070,653 | ) | |
$ | 5,221,628 | |
| Income
tax benefit | |
| 14,859,887 | | |
| - | | |
| 14,859,887 | |
| Net
income (loss) | |
$ | 5,567,606 | | |
$ | (4,070,653 | ) | |
$ | 9,638,259 | |
Research and Development Expense
Research and
development expenses include expenses primarily relating to the development of our Latiglutenase, Capeserod, Adrulipase and Niclosamide
drug candidates.
Research and
development expenses for the three months ended March 31, 2024 totaled approximately $0.6 million, a decrease of approximately $0.8 million,
or 58%, over the approximately $1.4 million recorded for the three months ended March 31, 2023.
The approximately
$0.8 million decrease in total research and development expenses was primarily attributable to decreases of approximately $0.9 million
in clinical related expenses in connection with our Phase 2b Adrulipase SPAN clinical trial which occurred during the three months ended
March 31, 2023.
We expect research
and development expenses to increase during the remainder of this fiscal year as we begin work on our Latiglutenase asset.
General and Administrative Expense
General and
administrative expenses include expenses primarily relating to our overall operations and being a public company, including personnel,
legal and financial professional services, insurance, corporate communications and investor relations, listing and compliance related
costs, rent, and expenses associated with obtaining and maintaining intellectual property and patents, among others.
General and
administrative expenses for the three months ended March 31, 2024 totaled approximately $8.6 million, an increase of approximately $ 5.9
million, or 220% over the approximately $2.7 million recorded for the three months ended March 31, 2023.
The approximately
$5.9 million increase in total general and administrative expenses was primarily attributable to $4.0 million of non-cash expense recorded
for the Tungsten financial advisor fees and as well as increases of approximately $0.8 million in legal fees, $0.8 million in share-based
compensation for consultants, $0.2 million in professional fees, and $0.1 million in personnel related costs.
We expect general
and administrative expenses to decrease during the remainder of this fiscal year.
Other Expenses
Other expenses
for the three months ended March 31, 2024 increased approximately $0.06 million over other expenses recorded for the three months ended
March 31, 2023. The increase in other expenses was mainly due to interest expense related to the revolving line of credit and EIDL loan
assumed with the IMGX acquisition.
Income Tax Benefit
A tax benefit
of approximately $14.9 million was recorded in the three months ended March 31, 2024 due to the release of a portion of our valuation
allowance that had previously been recorded against our deferred tax assets in connection with the IMGX Merger. The release of the valuation
allowance was due to the acquisition of intangible assets which are considered definite lived for tax purposes.
Net Income (Loss)
As a result
of the factors above, our net income for the three months ended March 31, 2024 totaled approximately $5.6 million, an increase of approximately
$9.6 million, or 237%, over the net loss of approximately $4.1 million recorded for the three months ended March 31, 2023.
Consolidated Results of Operations for the Years Ended December
31, 2023 and 2022
The following table summarizes
our consolidated results of operations for the periods indicated:
| | |
| | |
| | |
| |
| | |
Years Ended December 31, | | |
Increase | |
| | |
2023 | | |
2022 | | |
(decrease) | |
| Operating expenses: | |
| | | |
| | | |
| | |
| Research and development expenses | |
$ | 5,033,218 | | |
$ | 8,776,302 | | |
$ | (3,743,084 | ) |
| Research and development recovery - intellectual property acquired | |
| - | | |
| (8,085,045 | ) | |
| 8,085,045 | |
| General and administrative expenses | |
| 10,737,609 | | |
| 11,986,809 | | |
| (1,249,200 | ) |
| Total operating expenses | |
| 15,770,827 | | |
| 12,678,066 | | |
| 3,092,761 | |
| Other expenses | |
| 24,156 | | |
| 240,205 | | |
| (216,049 | ) |
| Loss on dissolution of foreign subsidiary | |
| - | | |
| 1,711,371 | | |
| (1,711,371 | ) |
| Net loss | |
$ | 15,794,983 | | |
$ | 14,629,642 | | |
$ | 1,165,341 | |
Revenues
We have not yet achieved revenue-generating
status from any of our product candidates. Since inception, we have devoted substantially all of our time and efforts to acquiring and
developing our product candidates, including Latiglutenase, Adrulipase, Capeserod and Niclosamide. As a result, we did not have any revenue
during the years ended December 31, 2023 and 2022, respectively.
Research and Development Expenses
Research and development expenses
for the year ended December 31, 2023 totaled approximately $5.0 million, a decrease of approximately $3.8 million, or 43%, over the approximately
$8.8 million recorded for the year ended December 31, 2022. Research and development recovery for intellectual property acquired in connection
with the acquisition of FWB, totaled approximately $8.1 million for the year ended December 31, 2022.
The decrease of approximately
$3.8 million in research and development expenses, excluding recovery related to the acquired intellectual property, was primarily attributable
to decreases of approximately $2.8 million in clinical trial related expenses in connection with the Adrulipase Phase 2b clinical trial
in 2023 compared to the Niclosamide FW - COV clinical trial in 2022, approximately $1.2 million in CMC related costs, and approximately
$0.8 million in personnel related costs; partially offset by an increase of $0.5 million for the exclusivity payment that was paid in
connection with December 2023 execution of the non - binding letter of intent with IMGX, as well as an increase of $0.5 million for the
payment in connection with the license agreement for Capeserod.
The approximately $8.1 million
in research and development recovery for acquired intellectual property recorded during the year ended December 31, 2022 was a result
of a term sheet entered into on July 29, 2022 related to the acquisition of the Niclosamide drug candidates.
General and Administrative Expense
General and administrative
expenses for the year ended December 31, 2023 totaled approximately $10.7 million, a decrease of approximately $1.3 million, or 10%, over
the approximately $12.0 million recorded for the year ended December 31, 2022.
The decrease in total general
and administrative expenses was due primarily to decreases in costs associated with being a publicly reporting company, including investor
relations and corporate communications, of approximately $0.9 million and legal and professional fees of approximately $0.9 million, partially
offset by increases in personnel related costs of approximately $0.6 million.
Other Expenses and Loss on Dissolution of Foreign
Entity
Other expenses for the year
ended December 31, 2023 totaled approximately $0.02 million compared to approximately $0.2 million for the year ended December 31, 2022.
During the year ended December 31, 2023, we recorded interest expense of $0.02 million related to the financing of our corporate insurance
policies. During the year ended December 31, 2022, we recorded an expense of approximately $0.2 million for Waiver fees paid related to
the Series B preferred stock. Additionally, during the year ended December 31, 2022, we recorded a loss of approximately $1.7 million
due to the dissolution of our foreign subsidiary.
Net Loss
As a result of the factors
above, our net loss for the year ended December 31, 2023 totaled approximately $15.8 million, an increase of approximately $1.2 million,
or 8%, over the net loss of approximately $14.6 million recorded for the year ended December 31, 2022.
Liquidity and Capital Resources
To date, we have not generated
any revenues and have experienced net losses and negative cash flows from our activities.
As of March 31, 2024,
we had cash and cash equivalents of approximately $3.4 million, and had sustained cumulative losses attributable to common stockholders
of approximately $178.8 million. Subsequent to March 31, 2024, we have raised aggregate gross proceeds of approximately $1.1 million
from a May 2024 Registered Direct Offering. Based on our cash on hand at June 11, 2024, we anticipate having sufficient cash to fund
planned operations into July 2024, however, the acceleration or reduction of cash outflows by management can significantly impact the
timing for the need to raise additional capital to complete development of our products. We have not yet achieved profitability and anticipate
that we will continue to incur net losses for the foreseeable future. We expect that our expenses will continue to grow and, as a result,
we will need to generate significant product revenues to achieve profitability. We may never achieve profitability. As such, we are dependent
on obtaining, and are continuing to pursue, the necessary funding from outside sources, including obtaining additional funding from the
sale of securities in order to continue our operations. Without adequate funding, we may not be able to meet our obligations. We believe
these conditions may raise substantial doubt about our ability to continue as a going concern.
We have funded our operations
to date primarily through the issuance of debt, convertible debt securities, preferred stock, as well as the issuance of Common Stock
in various public offerings and private placement transactions. We expect to incur substantial expenditures in the foreseeable future
for the development of adrulipase, niclosamide and any other drug candidates. We will require additional financing to develop our drug
candidates, run clinical trials, prepare regulatory filings and obtain regulatory approvals, fund operating losses, and, if deemed appropriate,
establish manufacturing, sales and marketing capabilities. Our current financial condition raises substantial doubt about our ability
to continue as a going concern. Our failure to raise capital as and when needed would have a material adverse impact on our financial
condition, our ability to meet our obligations, and our ability to pursue our business strategies. We will seek funds through additional
equity and/or debt financings, collaborative or other arrangements with corporate sources, or through other sources of financing.
Although, we are primarily
focused on the development of our drug candidates, including adrulipase and niclosamide, we are also opportunely focused on expanding
our product pipeline of clinical assets through collaborations, and also through acquisitions of products and companies. We are continually
evaluating potential asset acquisitions business combinations, and other partnership opportunities. To finance such acquisitions, we
might raise additional equity capital, incur additional debt, or both.
We are able to sell securities
on a shelf registration statement pursuant to the ATM Agreement with H.C. Wainwright & Co., LLC. Under current Securities and Exchange
Commission regulations, because our public float was less than $75 million at the relevant measurement period pursuant to General Instruction
I.B.6. to Form S-3, the amount we can raise through primary public offerings of securities in any subsequent twelve-month period under
our shelf registration statement is limited to an aggregate of one-third of our public float until such time, if any, as our public float
is $75 million or more.
Our ability to issue securities
is subject to market conditions. Each issuance under the shelf registration statements will require the filing of a prospectus supplement
identifying the amount and terms of the securities to be issued.
Cash Flows for the Three Months Ended March
31, 2024 and 2023
The following table summarizes our cash flows
for the periods indicated:
| | |
Three Months Ended | |
| | |
March
31, | |
| | |
2024 | | |
2023 | |
| Net cash (used in) provided by: | |
| | | |
| | |
| Operating activities | |
$ | (3,746,956 | ) | |
$ | (2,845,578 | ) |
| Investing activities | |
| 88,169 | | |
| - | |
| Financing activities | |
| 3,378,930 | | |
| 3,547,108 | |
| Net (decrease) increase in cash,
cash equivalents and restricted cash | |
$ | (279,857 | ) | |
$ | 701,530 | |
Operating Activities
Net cash used in operating activities during
the three months ended March 31, 2024 of approximately $3.7 million was primarily attributable to our non-cash change in deferred tax
valuation allowance of $14.9 million, partially offset by net income of $5.6 million and other non-cash expenses totaling approximately
$5.5 million. Other non-cash expenses include stock issued to our financial advisors in connection with the IMGX acquisition of $4.0
million, common stock granted to consultants of $0.8 million, and stock-based compensation of $0.3 million; a decrease in prepaid expenses
of $0.3 million and a net decrease in accounts payable and accrued expenses of $0.1 million.
Net cash used in operating activities during
the three months ended March 31, 2023 of approximately $2.8 million was primarily attributable to our net loss of approximately $4.1
million, partially offset by non-cash expenses of approximately $0.4 million, mainly related to stock-based compensation, as well as
a decrease in prepaid expenses of $0.5 million and increases in accounts payable and accrued expense of approximately $0.3 million.
Investing Activities
Net cash provided by investing activities
during the three months ended March 31, 2024 of approximately $0.1 million was due to the net cash acquired in the acquisition of IMGX.
Financing Activities
Net cash provided by financing activities
of approximately $3.4 million for the three months ended March 31, 2024 was primarily due to net proceeds of approximately $3.6 million
from the March 2024 registered direct offering, partially offset by approximately $0.2 million of cash repayments of the note payable
financing for corporate insurances.
Net cash provided by financing activities
of approximately $3.5 million for the three months ended March 31, 2023 was primarily due to net proceeds of approximately $3.8 million
from the March 2023 private placement offering, partially offset by approximately $0.2 million of cash repayments of the note payable
financing for corporate insurances.
Cash Flows for the Years Ended December 31, 2023 and 2022
The following table summarizes
our cash flows for the periods indicated:
| | |
| | |
| |
| | |
| |
| | |
Years Ended December 31, | |
| | |
2023 | | |
2022 | |
| Net cash provided by (used in): | |
| | | |
| | |
| Operating activities | |
$ | (12,377,852 | ) | |
$ | (22,344,079 | ) |
| Investing activities | |
| (500,000 | ) | |
| - | |
| Financing activities | |
| 15,226,721 | | |
| 15,739,531 | |
| Net increase (decrease) in cash and cash equivalents | |
$ | 2,348,869 | | |
$ | (6,604,548 | ) |
Operating Activities
Net cash used in operating
activities during the year ended December 31, 2023 of approximately $12.4 million was primarily attributable to our net loss of approximately
$15.8 million. This decrease was partially offset by addbacks for non-cash expenses for stock-based compensation expense of approximately
$1.0 million, an increase in accounts payable and accrued expenses of approximately $1.5 million, and an increase in prepaid expenses
of approximately $0.7 million.
Net cash used in operating
activities during the year ended December 31, 2022 of approximately $22.3 million was primarily attributable to our net loss of approximately
$14.6 million, a decrease in the payable related to the FWB acquisition of approximately $8.1 million, and decreases in accounts payable
and accrued expenses of approximately $2.1 million. These were partially offset by addbacks for non-cash expenses of approximately $2.8
million mainly related to the dissolution of our foreign subsidiary of approximately $1.7 million, stock-based compensation expense of
approximately $0.8 million, and Common Stock granted to consultants of approximately $0.2 million.
Investing Activities
Net cash used in investing
activities during the year ended December 31, 2023 was approximately $0.5 million consisting of the up-front payment related to our license
agreement for Capeserod.
Financing Activities
Net cash provided by financing
activities of approximately $15.2 million for the year ended December 31, 2023 was primarily due to net proceeds received from the exercise
of warrants of approximately $10.3 million; the issuance of Common Stock, pre-funded warrants and warrants for net proceeds of approximately
$5.5 million; partially offset by approximately $0.6 million of cash paid related to the repayment of a note payable.
Net cash provided by financing
activities of approximately $15.7 million for the year ended December 31, 2022 was primarily due to the issuance of common stock under
our ATM Agreement for net proceeds of approximately $7.7 million and the issuance of Common Stock, pre-funded warrants and warrants for
net proceeds of approximately $15.4 million, partially offset by approximately $6.9 million in cash payments related to the FWB Acquisition
under the November 2022 Settlement Agreement.
Critical Accounting Policies and Significant Judgements and Estimates
Our management’s discussion
and analysis of financial condition and results of operations is based on our consolidated financial statements, which have been prepared
in accordance with accounting principles generally accepted in the United States (“GAAP”). The preparation of our consolidated
financial statements and related disclosures requires us to make estimates and assumptions that affect the reported amounts of assets
and liabilities, costs and expenses and related disclosures during the reporting periods. On an ongoing basis, we evaluate our estimates
and judgments, including those described below. We base our estimates on historical experience and on various other factors that we believe
are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and
liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions
or conditions.
While our significant accounting
policies are more fully described in Note 2 of the notes to our consolidated financial statements appearing elsewhere in this document,
management has identified the following as “Critical Accounting Policies and Estimates”: Stock-Based Compensation, Debt and
Equity Instruments, Intangible Assets and Goodwill. We believe that the estimates and assumptions involved in these accounting policies
may have the greatest potential impact on our financial statements.
Stock-Based Compensation
We account for share-based
payment awards issued to employees and members of our Board by measuring the fair value of the award on the date of grant and recognizing
this fair value as stock-based compensation using a straight-line basis over the requisite service period, generally the vesting period.
For awards issued to non-employees, the measurement date is the date when the performance is complete or when the award vests, whichever
is the earliest. Accordingly, non-employee awards are remeasured at each reporting period until the final measurement date. The fair value
of the award is recognized as stock-based compensation over the requisite service period, generally the vesting period.
Goodwill
Goodwill relates to the acquisition
of ProteaBio Europe SAS during 2014 and represents the excess of the total purchase consideration over the fair value of acquired assets
and assumed liabilities, using the purchase method of accounting. Goodwill is not amortized but is subject to periodic review for impairment.
As a result, the amount of goodwill is directly impacted by the estimates of the fair values of the assets acquired and liabilities assumed.
In addition, goodwill will
be reviewed annually, and whenever events or changes in circumstances indicate that the carrying amount of the goodwill might not be recoverable.
Judgment is used in determining when these events and circumstances arise. We perform our review of goodwill on our one reporting unit.
If we determine that the carrying value of the assets may not be recoverable, judgment and estimates are used to assess the fair value
of the assets and to determine the amount of any impairment loss.
The carrying value of goodwill
was approximately $1.7 million at both December 31, 2023 and 2022. Historically, goodwill was denominated in a foreign currency and translated
to U.S. dollars at period end exchange rates. Effective with the dissolution of AzurRx SAS in October 2022, goodwill is carried on the
U.S. books denominated in U.S. dollars. During the year ended December 31, 2022, we recognized a loss of approximately $1.7 million related
to foreign translation adjustments associated with the dissolution. If actual results are not consistent with our estimates or assumptions,
we may be exposed to an impairment charge that could be material.
Immunogenx
Management’s Discussion and Analysis of Financial Condition and Results of OperationS
You should read the following discussion
in conjunction with ImmunogenX’s financial statements and related notes included elsewhere in this proxy statement. This discussion
contains forward-looking statements that involve risks and uncertainties. ImmunogenX’s actual results and the timing of selected
events could differ materially from those anticipated in these forward-looking statements as a result of various factors, including,
but not limited to, those set forth under “Risk Factors” and elsewhere in this proxy statement. Unless otherwise indicated,
references in this section to “we,” “our” and the “Company” refer to ImmunogenX prior to the consummation
of the Merger.
Company Overview
We are a private, clinical-stage,
biotherapeutics company, headquartered in Newport Beach, California, with a research facility in North Carolina. On January 1, 2021, we
converted to a corporation and incorporated in the state of Delaware. Our research efforts are focused on therapy, disease management,
and food safety. We have patented formulations related to the use of Latiglutenase, a digestive enzyme that degrades unintended ingestion
of gluten in the body. Another key area of research involves a method of monitoring intestinal villi atrophy by measuring the level of
a drug metabolite that is strongly dependent on the villi heath.
Through December 31, 2023,
we have been primarily engaged in developing our biopharmaceutical products, research and development, and raising capital through equity
and debt offerings. We have not commenced principal revenue producing operations and have incurred significant expenditures for the research
and development of our biopharmaceutical products. Once our planned principal operations commence, our focus will be on the manufacturing,
refining, licensing, and marketing of our biopharmaceuticals and the continued research and development of new associated biopharmaceutical
products.
Company Updates
In April 2023, we completed our end-of-phase 2
meeting with the FDA discussing the study plan regarding endpoints, measurement methods, and outcomes related to our Latiglutenase drug.
Based on this feedback, we are implementing the Phase 3 trial plan.
In December 2023, we signed
a non-binding term sheet for a business combination with Entero, a publicly traded company. Pursuant to the Merger Agreement, Entero
acquired us in an all-stock transaction on March 13, 2024. For additional information on the IMGX Transaction, see the section titled
“Entero’s Management’s Discussion and Analysis of Financial Condition – Merger with ImmunogenX.”
Our accumulated deficit through December 31, 2023
is $18.8 million. To date, we have generated no revenues and expect to incur additional losses to perform further research and development
activities.
Our drug development efforts are in their early
stages, and we cannot make estimates of the costs or the time that our development efforts will take to complete, or the timing and amount
of revenues related to the sale of our drug. The risk of completion of any program is high because of the many uncertainties involved
in developing new drug candidates to market, including the long duration of clinical testing, the specific performance of proposed products
under stringent clinical trial protocols, extended regulatory approval and review cycles, our ability to raise additional capital, the
nature and timing of research and development expenses, and competing technologies being developed by organizations with significantly
greater resources.
Financial Operations Overview
Revenue
To date, we have not generated
any revenue from our product candidates or otherwise. We do not expect to generate revenue from product sales for at least the next several
years.
Research and Development Expense
Our research and development
expenses primarily consist of clinical trial costs, laboratory supplies, outside services, and licenses and patent costs acquired to be
used in research and development. Historically, the majority of our research and development expenses have been focused on the development
of Latiglutenase.
We expense research and development
costs as incurred. Conducting a significant amount of research and development is central to our business model. Product candidates in
clinical development generally have higher development costs than those in earlier stages of development, primarily due to the size, duration
and cost of clinical trials. The successful development of our clinical and preclinical product candidates is highly uncertain. Clinical
development timelines, the probability of success and development costs can differ materially from our expectations. For example, if the
FDA or another regulatory authority were to require us to conduct clinical trials beyond those which we currently expect will be required
for the completion of clinical development of a product candidate, or if we experience significant delays in enrollment in any of our
clinical trials, we could be required to expend significant additional financial resources and time to complete any clinical development.
General and Administrative Expense
Our general and administrative
expenses primarily consist of outside service and professional fees, personnel costs, including stock-based compensation, and facility
costs.
Grant Income
Our
grant income consists primarily of grant revenue from contracts with the National Institute of Allergy and Infectious Diseases
and other government-sponsored organizations for research and development related activities that provide for payments for reimbursed
costs, which may include overhead, materials and supplies, and other indirect costs.
Change in Fair Value of Convertible Notes
The change in fair value of
our convertible promissory notes is related to the determination of their fair values by a third-party valuation specialist.
Interest Expense
Interest expense consists
primarily of interest expense incurred on our revolving line of credit. For additional information, see the section titled “Entero’s
Management’s Discussion and Analysis of Financial Condition and Results of Operations – Debt of ImmunogenX.”
Other Income, Net
Other income, net consists
primarily of income from licensing agreements.
Results of Operations for the Years Ended December 31, 2023 and
2022
The
following table summarizes our consolidated results of operations for the periods indicated:
| | |
For the years ended December 31, | |
| (in thousands) | |
2023 | | |
2022 | | |
Increase
(decrease) | |
| Operating expenses: | |
| | | |
| | | |
| | |
| Research and development expenses | |
$ | 2,828 | | |
$ | 2,268 | | |
$ | 560 | |
| General & administrative expenses | |
| 2,309 | | |
| 1,932 | | |
| 377 | |
| Total operating expenses | |
| 5,137 | | |
| 4,200 | | |
| 937 | |
| Other expense (income): | |
| | | |
| | | |
| | |
| Grant income | |
| (910 | ) | |
| (1,091 | ) | |
| 181 | |
| Change in fair value of convertible notes | |
| 3,615 | | |
| 190 | | |
| 3,425 | |
| Interest expense | |
| 1,083 | | |
| 375 | | |
| 708 | |
| Other income, net | |
| (68 | ) | |
| (52 | ) | |
| (16 | ) |
| Total other expense (income) | |
| 3,720 | | |
| (578 | ) | |
| 4,298 | |
| Loss before tax provision | |
| (8,857 | ) | |
| (3,622 | ) | |
| 5,235 | |
| Provision for state income taxes | |
| 1 | | |
| 1 | | |
| - | |
| Net loss | |
$ | (8,858 | ) | |
$ | (3,623 | ) | |
$ | 5,235 | |
Research and development expenses
increased by $0.5 million to $2.8 million for the year ended December 31, 2023 from $2.3 million for the year ended December 31, 2022.
The overall increase in expenses was primarily due to costs associated with clinical programs and outside service costs related to the
development of our lead drug candidate, Latiglutenase.
General and administrative
expenses increased by $0.4 million to $2.3 million for the year ended December 31, 2023, from $1.9 million for the year ended December
31, 2022. This was primarily due to an increase in outside services and professional fees due to an increase in consulting costs.
Other
expense (income) increased by $4.3 million to $3.7 million for the year ended December 31, 2023 from $(0.6) million for the year
ended December 31, 2022. This was primarily due to recording the change in fair value of convertible notes of $3.4 million, an increase
in interest expense related to the revolving line of credit of $0.7 million, and lower grant income from license agreements of $0.2 million.
Liquidity and Capital Resources
As of December 31, 2023 and
2022, we had negative working capital of $5.9 million and $2.3 million, respectively. We have incurred net losses since our inception
and have negative operating cash flows. As of December 31, 2023, we had $0.3 million in cash, cash equivalents and short-term investments
and we anticipate the need to raise additional capital to complete development of our products.
Our drug development efforts
are in their early stages, and we cannot make estimates of the costs or the time that our development efforts will take to complete, or
the timing and amount of revenues related to the sale of our drug candidates. The risk of completion of any program is high because of
the many uncertainties involved in developing new drug candidates to market, including the long duration of clinical testing, the specific
performance of proposed products under stringent clinical trial protocols, extended regulatory approval and review cycles, our ability
to raise additional capital, the nature and timing of research and development expenses, and competing technologies being developed by
organizations with significantly greater resources.
For the foreseeable future,
we expect to continue to incur losses and require additional capital to further advance our clinical trial programs and support our other
operations. We cannot be certain that additional funding will be available on acceptable terms, or at all. To the extent that we can raise
additional funds by issuing equity securities, our stockholders may experience additional dilution.
Cash Flows for the Years Ended December 31, 2023 and 2022
The following table summarizes our consolidated
results of operations for the periods indicated:
| | |
For the years ended December 31, | |
| (in thousands) | |
2023 | | |
2022 | |
| Net cash provided by (used in): | |
| | | |
| | |
| Operating activities | |
$ | (4,870 | ) | |
$ | (4,322 | ) |
| Investing activities | |
| (4 | ) | |
| - | |
| Financing activities | |
| 5,030 | | |
| 2,930 | |
| Net increase (decrease) in cash, cash equivalents and restricted cash | |
$ | 156 | | |
$ | (1,392 | ) |
Operating Activities
Net cash used in operating
activities for the year ended December 31, 2023 was $4.9 million, compared to $4.3 million for the year ended December 31, 2022.
The use of cash in operating
activities was primarily a result of the net loss of $8.9 million for the year ended December 31, 2023, adjusted for non-cash items including
the change in fair value of convertible notes of $3.6 million and amortization of patents of $0.1 million. The net change in our operating
assets and liabilities was $0.1 million, decreasing cash used in operations.
The use of cash in operating
activities was primarily a result of the net loss of $3.6 million for the year ended December 31, 2022, adjusted for non-cash items including
the change in fair value of convertible notes of $0.2 million and amortization of patents of $0.1 million. The net change in our operating
assets and liabilities was $1.4 million, increasing cash used in operations.
Investing Activities
Net cash used in investing activities for the year
ended December 31, 2023, was less than $0.1 million and is primarily related to purchases of property and equipment. There was no cash
used in investing activities for the year ended December 31, 2022.
Financing Activities
Net cash provided by financing
activities was $5.0 million during the year ended December 31, 2023, compared to net cash provided by financing activities of $2.9 million
for the same period in 2022. The increase of $2.1 million is primarily due to an increase in proceeds from convertible notes of $0.9 million,
an increase in proceeds from the line of credit of $0.8 million, an increase in related party promissory notes of $0.2 million, and a
decrease of repayments on promissory notes of $0.3 million.
Critical Accounting Estimates
Our accounting policies are
described in Note 2 Summary of Significant Accounting Policies in our audited financial statements. The preparation of financial
statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates
and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the
date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those
estimates. We believe that the following discussion represents our critical accounting estimates.
Stock-Based Compensation
We account for stock-based
compensation in accordance with FASB ASC Topic 718, Compensation – Stock Compensation, which requires all stock-based compensation
to employees and non-employees, including grants of non-employee stock awards to be recognized in the statement of operations based on
the fair value of those awards calculated using a valuation model on the grant date. In accordance with ASC 718, compensation expense
related to stock-based payments are recorded in a straight-line basis over the requisite service period based on the grant date fair value
of the awards. In addition, we have elected to account for forfeitures when they occur in accordance with ASC 718.
RISK FACTORS
Risks related to Entero
We are subject to various risks that could have
a material adverse effect on our business, our financial condition and our results of operations. These risks could cause actual operating
results to differ from those expressed in certain “forward looking statements” contained in this Annual Report as well as
in other communications.
Summary of Risk Factors
| |
● |
Company stockholders may not realize a benefit from the Merger commensurate with the ownership dilution they will experience in connection with the transactions. |
| |
● |
Pursuant to the terms of the Merger Agreement, we are required to recommend that our stockholders approve the conversion of shares of our Series G Preferred Stock into shares of our Common Stock. We cannot guarantee that our stockholders will approve this matter, and if they fail to do so we may be required to settle such shares in cash and our operations may be materially harmed. |
| |
● |
The failure to successfully integrate the businesses of the Company and IMGX in the expected timeframe would adversely affect our future results. |
| |
● |
The issuance or conversion of securities would result in significant dilution in the equity interest of existing stockholders and adversely affect the marketplace of the securities. |
| |
● |
In connection with the Merger, we assumed significantly more indebtedness. Our level of indebtedness and our ability to make payments on or service our indebtedness could adversely affect our business, financial condition, results of operations, cash flow and liquidity. |
| |
● |
Defaults under the Amended Credit Agreement or the Shareholders Notes could result in a substantial loss of our assets. |
| |
● |
We may not complete the Niclosamide Sale on existing terms, if at all. |
| |
● |
We have a limited operating history and have never generated any product revenues. |
| |
● |
We expect to incur significant losses for the foreseeable future and may never achieve or maintain profitability. |
| |
● |
We may incur substantial product liability or indemnification claims relating to the use of our product candidates. |
| |
● |
There
is substantial doubt about our ability to continue as a “going concern,” and we will require substantial additional funding
to finance our long-term operations. If we are unable to raise additional capital when needed, we could be forced to delay, reduce
or terminate certain of our products or other operations. |
| |
● |
Our product candidates are at an early stage of development and may not be successfully developed or commercialized. |
| |
● |
To date, most of our development activities have been focused on our Adrulipase and Niclosamide product candidates, which are still under clinical development, and more recently our Latiglutenase and Capeserod product candidates. If Latiglutenase, Adrulipase, Capeserod and Niclosamide do not receive regulatory approval or is not successfully commercialized, our business will be harmed. |
| |
● |
Geopolitical events, including the war in Ukraine and the Israel-Hamas war could adversely impact our business, including our clinical trials. |
| |
● |
Significant disruptions of information technology systems or breaches of data security could materially adversely affect our business, results of operations and financial condition. |
| |
● |
We will require additional capital to fund our operations, and if we fail to obtain necessary financing, we may not be able to complete the development and commercialization of our product candidates. |
| |
|
|
| |
● |
Clinical trials are very expensive, time-consuming, difficult to design and implement and involve an uncertain outcome. |
| |
● |
We face significant competition from other biotechnology and pharmaceutical companies, and our operating results will suffer if we fail to compete effectively. |
| |
● |
We do not have our own manufacturing capabilities and will rely on third parties to produce clinical and commercial supplies of our product candidates. |
| |
● |
We currently have no commercial organization. If we are unable to establish satisfactory sales and marketing capabilities or secure a sales and marketing partner, we may not successfully commercialize any of our product candidates. |
| |
● |
If we breach our license or other intellectual property-related agreements for our product candidates, we could lose the ability to continue development and commercialization of our product candidates. |
| |
● |
We intend to rely on third parties to conduct, supervise and monitor our clinical trials, and if those third parties perform in an unsatisfactory manner, it may harm our business. |
| |
● |
If we are unable to obtain and maintain patent protection for our technology and products or if the scope of the patent protection obtained is not sufficiently broad, we may not be able to compete effectively in our markets. |
| |
● |
We do not currently intend to pay dividends on our Common Stock in the foreseeable future, and consequently, any gains from an investment in our Common Stock will likely depend on appreciation in the price of our Common Stock. |
Risks Related to Our Business, Financial Position and Capital Requirements
Company stockholders may not realize a benefit
from the Merger commensurate with the ownership dilution they will experience in connection with the transactions.
If the Company is unable to
realize the full strategic and financial benefits currently anticipated from the Merger, our stockholders may experience a dilution of
their ownership interests our Company without receiving any commensurate benefit, or only receiving part of the commensurate benefit to
the extent the Company is able to realize only part of the strategic and financial benefits currently anticipated from the transactions.
The Merger may pose integration challenges which could result in management and business disruptions, any of which could harm our results
of operation, business prospects, and impair the value of the Merger to our stockholders.
Pursuant to the terms of the Merger Agreement,
we are required to recommend that our stockholders approve the conversion of shares of our Series G Preferred Stock into shares of our
Common Stock. We cannot guarantee that our stockholders will approve this matter, and if they fail to do so we may be required to settle
such shares in cash and our operations may be materially harmed.
Under
the terms of the Merger Agreement, we agreed to call and hold a meeting of our stockholders to obtain the requisite approvals for the
conversion of shares of Series G Preferred Stock into shares of our Common Stock. Additionally, beginning on the date that is six months
from the date of the Closing, the holders of our then-outstanding shares of Series G Preferred Stock will be entitled to elect to have
such shares of Series G Preferred Stock redeemed for cash at a price per share equal to the then-current fair value of the Series G Preferred
Stock, as described in the Certificate of Designation of Preferences, Rights and Limitations of the Series G Non-Voting Preferred Stock
(the “Certificate of Designation”). For example, if every share of Series G Preferred Stock were to have been redeemed
for cash on June 13, 2024, each holder of Series G Preferred Stock would be entitled to $2,250.00 per share of Series G Preferred Stock
redeemed, or $27,839,758.50 in the aggregate. Unless we operate profitably, our ability to redeem the Series G Preferred Stock would
require the availability of adequate “surplus,” which is defined as the excess, if any, of our net assets (total assets less
total liabilities) over our capital. If we do have sufficient “surplus” to effect any requested redemption of the Series
G Preferred Stock, our available cash will be negatively impacted and such reduction in our available cash could decrease the trading
price of our Common Stock. In addition, if we are required to redeem the Series G Preferred Stock for cash and we do not have a sufficient
“surplus,” our lack of available cash could negatively impact our ability to continue our operations. Please see the risk
factor entitled “There is substantial doubt about our ability to continue as a “going concern,” and we will
require substantial additional funding to finance our long-term operations. If we are unable to raise additional capital when needed,
we could be forced to delay, reduce or terminate certain of our products or other operations” below.
There is substantial doubt about our ability to continue
as a “going concern,” and we will require substantial additional funding to finance our long-term operations. If we are unable
to raise additional capital when needed, we could be forced to delay, reduce or terminate certain of our products or other operations.
We have incurred substantial
operating losses since inception and expect to continue to incur significant operating losses for the foreseeable future. As of March
31, 2024, we had cash and cash equivalents of approximately $3.4 million, and had sustained cumulative losses attributable to common
stockholders of approximately $178.8 million. As of December 31, 2023, we had cash and cash equivalents of approximately $3.7 million,
working capital of approximately $1.8 million, and had sustained cumulative losses attributable to stockholders of approximately $184.3
million.
Based
on our cash on hand at June 11, 2024, we anticipate having sufficient cash to fund planned operations into July 2024, however, the acceleration
or reduction of cash outflows by management can significantly impact the timing for the need to raise additional capital to complete
development of our products. We have not yet achieved profitability and anticipate that we will continue to incur net losses for the
foreseeable future. We expect that our expenses will continue to grow and, as a result, we will need to generate significant product
revenues to achieve profitability. We may never achieve profitability. Therefore, we are dependent on obtaining, and are continuing
to pursue, the necessary funding from outside sources, including obtaining additional funding from the sale of securities in order to
continue our operations. We are actively working to obtain additional funding. We cannot make any assurances that additional financings
will be available to us and, if available, completed on a timely basis, on acceptable terms or at all. We believe these conditions may
raise substantial doubt about our ability to continue as a going concern. If we are unable to complete an equity and/or debt offering,
or otherwise obtain sufficient financing when and if needed, it would negatively impact our business and operations, which would likely
cause the price of our Common Stock to decline or ultimately force us to cease our operations.
The failure to successfully integrate the
businesses of the Company and IMGX in the expected timeframe would adversely affect our future results.
Our ability to successfully
integrate the operations of the Company and IMGX will depend, in part, on our ability to realize the anticipated benefits from the Merger.
If we are not able to achieve these objectives, the anticipated benefits and cost savings of the Merger may not be realized fully, or
at all, or may take longer to realize than expected, and the value of our Common Stock may be adversely affected. In addition, the integration
of the Company’s and IMGX’s respective businesses will be a time-consuming and expensive process. Proper planning and effective
and timely implementation will be critical to avoid any significant disruption to our operations. It is possible that the integration
process could result in the loss of key employees, the disruption of our business or the identification of inconsistencies in standards,
controls, procedures and policies that adversely affect our ability to maintain relationships with customers, suppliers, distributors,
creditors, lessors, clinical trial investigators or managers or to achieve the anticipated benefits of the Merger. Delays encountered
in the integration process could have a material adverse effect on our operating results and financial condition, including the value
of its Common Stock.
The issuance or conversion of securities
would result in significant dilution in the equity interest of existing stockholders and adversely affect the marketplace of the securities.
The issuance or conversion
of common shares or other securities convertible into common shares would result in significant dilution in the equity interest of existing
stockholders and adversely affect the market price of the common shares. We have issued 11,777.418 shares of Series G Preferred Stock
to former shareholders of ImmunogenX which are initially convertible, in the aggregate, into 11,777,418 shares of the Company’s
common stock, subject to adjustment and certain shareholder approval limitations specified in the Certificate of Designations. These and
other future issuances of or conversions of securities may result in significant dilution to existing stockholders, which could adversely
impact your investment.
In connection with the Merger, we assumed
significantly more indebtedness. Our level of indebtedness and our ability to make payments on or service our indebtedness could adversely
affect our business, financial condition, results of operations, cash flow and liquidity.
Prior to the Merger, IMGX
was party to the Credit Agreement, with Lender. In connection with the Merger, IMGX entered into the Second Amendment, which provides
for, among other things: (i) Lender’s consent for the Merger, (ii) Second Merger Sub’s assumption all of IMGX’s obligations,
rights and liabilities as borrower under the Amended Credit Agreement, (iii) an amendment to the interest rate to a per annum floating
rate equal to the prime rate plus 6.00%, (iv) an extension of the maturity date to September 13, 2025, (v) a prohibition on the borrower’s
ability to request additional loans under the revolving credit facility, subject to certain limited exceptions, (vi) the pledge by the
Pledgors of their Pledged Equity as part of the collateral securing the obligations under the Amended Credit Agreement, and (vii) an
amendment to the total commitment under the Amended Credit Agreement to $8,212,345.17, which takes into account the One-Time Prepayment,
accrued interest and fees for the remaining term of the facility and certain other fees to be paid under the Amended Credit Agreement.
In order to fund the One-Time
Prepayment, on March 13, 2024, IMGX entered into (i) the Syage Note, and (ii) the Felker Note. The Shareholder Notes will bear interest
at a per annum floating rate equal to the prime rate plus 4.50% and mature on October 13, 2025.
If we are not able to repay
or refinance our debt as it becomes due, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt
or obtaining additional debt or equity on terms that may be onerous or highly dilutive, if we can obtain it at all. If we raise equity
through the issuance of preferred stock, the terms of the preferred stock may give the holders rights, preferences and privileges senior
to those of holders of our Common Stock, particularly in the event of liquidation. Our ability to arrange financing or refinancing will
depend on, among other factors, our financial position and performance, as well as prevailing market conditions and other factors beyond
our control. We cannot assure you that we will be able to obtain financing or refinancing on terms acceptable to us or at all.
If funds are not available
when needed, or available on acceptable terms, we may be required to delay, scale back or eliminate some of our obligations. In addition,
we may not be able to grow market share, take advantage of future opportunities or respond to competitive pressures or unanticipated requirements,
which could negatively impact our business, operating results and financial condition.
Defaults under the Amended Credit Agreement
or the Shareholders Notes could result in a substantial loss of our assets.
The borrowings under the Amended
Credit Agreement are secured by substantially all of the assets of IMGX and the borrowings under the Shareholder Notes are secured by,
among other things, the patents and trademarks owned by IMGX. If an event of default under any of such agreements could enable the lenders
or creditors thereunder to declare all borrowings outstanding on such debt, together with accrued and unpaid interest and fees, to be
due and payable. The lenders could also elect to foreclose on our assets securing such debt. In such an event, the Company may not be
able to refinance or repay all of its indebtedness, have sufficient liquidity to meet operating and capital expenditure requirements,
or hold the necessary intellectual property rights to conduct its business. Any such acceleration could cause us to lose a substantial
portion of our assets and will substantially adversely affect our ability to continue our operations.
We will require substantial additional financing
to achieve our goals, and a failure to obtain this necessary capital when needed and on acceptable terms, or at all, could force us to
delay, limit, reduce or terminate our product candidate development programs, testing efforts or other operations, including.
We expect our expenses to
increase in connection with our ongoing activities. We also expect to incur significant expenses related to the development, testing,
and manufacturing of our product candidates. We cannot reasonably estimate the actual amounts necessary to successfully complete the development
and commercialization of our products. Furthermore, following the completion of the Merger, we will incur the additional costs of assuming
IMGX’s indebtedness and the development, testing, and manufacturing of latiglutenase and CypCel. Accordingly, we will need to obtain
substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive
terms, we could be forced to delay, reduce or eliminate our research and development programs or any potential future commercialization
efforts.
We have based our estimates
on assumptions that may prove to be wrong, and we could use our capital resources sooner than we currently expect. Our operating plans
and other demands on our cash resources may change as a result of many factors, including costs required to advance latiglutenase and
CypCel, as well as factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private
equity or debt financings or other capital sources, including potentially government funding, collaborations, licenses and other similar
arrangements. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe
we have sufficient funds for our current or future operating plans. Attempting to secure additional financing may divert our management
from its day-to-day activities, which may adversely affect our ability to develop our product candidates.
Our future capital requirements will depend on
many factors, including:
| |
● |
the costs and timing of manufacturing for our product candidates; |
| |
● |
the costs associated with hiring additional personnel and consultants as our research and development activities increase; |
| |
● |
the costs associated with advancing product candidates obtained through the Merger; and |
| |
● |
the terms and timing of establishing and maintaining collaborations, licenses and other similar arrangements. |
While we have entered into the non-binding
Niclosamide LOI for the Niclosamide Sale, there is no assurance that the Niclosamide Sale will be completed on the terms contained in
the Niclosamide LOI or otherwise.
On December 27, 2023, we announced
that we entered into the Niclosamide LOI. The amount and structure of the consideration could change as a result of subsequent negotiations,
due diligence, or other factors. Any definitive agreement with respect to the Niclosamide Sale would be subject to approval by the respective
parties to the Niclosamide LOI, including approval by our Board of Directors, and would likely include a number of customary provisions,
including without limitation, representations and warranties of the buyer and us, restrictive covenants, and indemnification provisions,
as well as various closing conditions, including without limitation receipt of applicable third party approvals and satisfactory financing
arrangements. There can be no assurance that the Niclosamide LOI parties will ultimately negotiate and enter into definitive transaction
agreements on the terms contemplated by the Niclosamide LOI or otherwise. In particular, the timing and closing of any such transaction
and the aggregate consideration that we may receive may materially differ from that currently contemplated by the Niclosamide LOI. In
addition, in the event the Niclosamide Sale does not occur, we may, in the future, enter into other non-binding or binding letters of
intent, as well as definitive documentation relating to the sale of niclosamide, however there can be no assurance that we will do so.
If we do not complete the sale of Niclosamide pursuant to the Niclosamide LOI, other letters of intent, and any related transaction documentation,
we will have incurred expenses without our stockholders realizing any benefit therefrom. Additionally, if we fail to consummate such anticipated
Niclosamide Sale, such failure could result in fluctuations to the market price of our Common Stock and may have a material adverse impact
on our financial condition and results of operations.
We are a clinical stage biopharmaceutical
company and have a limited operating history upon which to base an investment decision.
We are a clinical stage biopharmaceutical
company. Since inception, we have engaged primarily in research and development activities of Adrulipase and Niclosamide, and more recently
Latiglutenase and Capeserod. We have not generated any revenue from product sales and have incurred significant net losses. We have not
demonstrated our ability to perform the functions necessary for the successful commercialization of any product candidates. The successful
commercialization of any of our products will require us to perform a variety of functions, including:
| ● |
continuing to undertake pre-clinical development and clinical trials; |
| ● |
participating in regulatory approval processes; |
| ● |
formulating and manufacturing products; and |
| ● |
conducting sales and marketing activities. |
Our operations to date have
been limited to organizing and staffing, acquiring, developing and securing the proprietary rights for, and undertaking pre-clinical development,
manufacturing and clinical trials of Latiglutenase, Adrulipase, Capeserod and Niclosamide. These operations provide a limited basis for
our stockholders and prospective investors to assess our ability to complete development of or commercialize Latiglutenase, Adrulipase,
Capeserod, Niclosamide, or any other product candidates and the advisability of investing in our securities.
We have incurred significant
losses and negative cash flows from our operations since inception. As of December 31, 2023, we had accumulated deficit of approximately
$184.3 million and working capital of approximately $1.8 million. Based on our historical and anticipated rate of cash expenditures, we
do not anticipate our existing working capital will be sufficient to sustain our business through the commercialization of our product
candidates. Therefore, we are dependent on obtaining, and are continuing to pursue, the necessary funding from outside sources, including
obtaining additional funding from the sale of securities in order to continue our operations. We are actively working to obtain additional
funding. We cannot make any assurances that additional financings will be available to us and, if available, completed on a timely basis,
on acceptable terms or at all. If we are unable to complete an equity and/or debt offering, or otherwise obtain sufficient financing when
and if needed, it would negatively impact our business and operations, which would likely cause the price of our Common Stock to decline
or ultimately force us to cease our operations.
We will face intense competition and may
not be able to compete successfully.
We operate in highly competitive
segments of the biotechnology and biopharmaceutical markets. We face competition from many different sources, including commercial pharmaceutical
and biotechnology enterprises, academic institutions, government agencies, and private and public research institutions. Latiglutenase,
Adrulipase, Capeserod and Niclosamide, if successfully developed and approved, will compete with established therapies, as well as new
treatments that may be introduced by our competitors. Many of our competitors have significantly greater financial, product development,
manufacturing and marketing resources than us. Large pharmaceutical companies have extensive experience in clinical testing and obtaining
regulatory approval for drugs. We also may compete with these organizations to recruit management, scientists and clinical development
personnel. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements
with large and established companies. New developments, including the development of other biological and pharmaceutical technologies
and methods of treating disease, occur in the pharmaceutical and life sciences industries at a rapid pace. Developments by competitors
may render our product candidates obsolete or noncompetitive. We will also face competition from these third parties in recruiting and
retaining qualified personnel, establishing clinical trial sites and patient registration for clinical trials and in identifying and in-licensing
new product candidates. In the case of Niclosamide, we may also face competition from other companies developing different formulation
of Niclosamide for the same indications for which we intend to develop Niclosamide, or from off-label uses of Niclosamide approved for
other indications.
We may incur substantial product liability
or indemnification claims relating to the use of our product candidates.
We face an inherent risk of
product liability exposure based on the use of Latiglutenase, Adrulipase, Capeserod and Niclosamide in human clinical trials, or, if obtained,
following marketing approval and commercialization. Claims could be brought against us if use or misuse of one of our product candidates
causes, or merely appears to have caused, personal injury or death. Although we have and intend to maintain product liability insurance
relating to our clinical trials, our coverage may not be sufficient to cover claims that may be made against us and we may be unable to
maintain such insurance. Any claims against us, regardless of their merit, could severely harm our financial condition, strain our management
and other resources or destroy the prospects for commercialization of the product which is the subject of any such claim. We are unable
to predict if we will be able to obtain or maintain product liability insurance for any products that may be approved for marketing. Additionally,
we have entered into various agreements where we indemnify third parties for certain claims relating to the testing and use of our product
candidates. These indemnification obligations may require us to pay significant sums of money for claims that are covered by these indemnifications.
We cannot predict all of the
possible harms or side effects that may result from the use of our products and, therefore, the amount of insurance coverage we currently
hold, or that we or our collaborators may obtain, may not be adequate to protect us from any claims arising from the use of our products
that are beyond the limit of our insurance coverage. If we cannot protect against potential liability claims, we or our collaborators
may find it difficult or impossible to commercialize our products, and we may not be able to renew or increase our insurance coverage
on reasonable terms, if at all. The marketing, sale and use of our products and our planned future products could lead to the filing of
product liability claims against us if someone alleges that our products failed to perform as designed. A product liability or professional
liability claim could result in substantial damages and be costly and time-consuming for us to defend.
Any product liability or professional
liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage.
Additionally, any product liability lawsuit could damage our reputation, result in the recall of products, or cause current partners to
terminate existing agreements and potential partners to seek other partners, any of which could impact our results of operations.
We use biological materials and may use
hazardous materials, and any claims relating to improper handling, storage or disposal of these materials could be time consuming or costly.
We may use hazardous materials,
including chemicals and biological agents and compounds, that could be dangerous to human health and safety or the environment. Our operations
also produce hazardous waste products. Federal, state and local laws and regulations govern the use, generation, manufacture, storage,
handling and disposal of these materials and wastes. Compliance with applicable environmental laws and regulations may be expensive, and
current or future environmental laws and regulations may impair our product development efforts. In addition, we cannot entirely eliminate
the risk of accidental injury or contamination from these materials or wastes. We do not carry specific biological or hazardous waste
insurance coverage and our property and casualty, and general liability insurance policies specifically exclude coverage for damages and
fines arising from biological or hazardous waste exposure or contamination. Accordingly, in the event of contamination or injury, we could
be held liable for damages or penalized with fines in an amount exceeding our resources, and our clinical trials or regulatory approvals
could be suspended.
Although we maintain workers’
compensation insurance to cover us for costs and expenses, we may incur due to injuries to our employees resulting from the use of hazardous
materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental
liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological or hazardous materials.
In addition, we may incur
substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future
laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also
may result in substantial fines, penalties or other sanctions.
We will need to grow the size of our organization,
and we may experience difficulties in managing this growth.
As of December 31, 2023, we
had 9 employees, which increased to 15 employees in March 2024 following the Merger. As our development and commercialization plans and
strategies develop, we expect to need additional managerial, operational, research and development, sales, marketing, financial and other
personnel. Future growth would impose significant added responsibilities on members of management, including:
| |
● |
identifying, recruiting, integrating, maintaining and motivating additional employees; |
| |
● |
managing our internal development efforts effectively, including the clinical, FDA and international regulatory review process for our product candidates, while complying with our contractual obligations to contractors and other third parties; and |
| |
● |
improving our operational, financial and management controls, reporting systems and procedures. |
Our future financial performance
and our ability to commercialize our product candidates will depend, in part, on our ability to effectively manage any future growth,
and our management may also have to divert a disproportionate amount of its attention away from day-to-day activities in order to devote
a substantial amount of time to managing these growth activities.
We currently rely, and for
the foreseeable future will continue to rely, in substantial part on certain independent organizations, advisors and consultants to provide
certain services, including certain aspects of regulatory approval, clinical management and manufacturing. There can be no assurance that
the services of independent organizations, advisors and consultants will continue to be available to us on a timely basis when needed,
or that we can find qualified replacements. In addition, if we are unable to effectively manage our outsourced activities or if the quality
or accuracy of the services provided by consultants is compromised for any reason, our clinical trials may be extended, delayed or terminated,
and we may not be able to obtain regulatory approval of our product candidates or otherwise advance our business. There can be no assurance
that we will be able to manage our existing consultants and contractors or find other competent outside contractors and consultants on
economically reasonable terms, or at all.
If we are not able to effectively
expand our organization by hiring new employees and expanding our groups of consultants and contractors, we may not be able to successfully
implement the tasks necessary to further develop and commercialize our product candidates and, accordingly, may not achieve our research,
development and commercialization goals.
Disruptions in the global economy and supply
chains may have a material adverse effect on our business, financial condition and results of operations.
The disruptions to the global
economy in 2020 and into 2022 have impeded global supply chains, resulting in longer lead times and also increased critical component
costs and freight expenses. We have taken and may have to take steps to minimize the impact of these disruptions in lead times and increased
costs by working closely with our suppliers and other third parties on whom we rely for the conduct of our business. Despite the actions
we have undertaken or may have to undertake to minimize the impacts from disruptions to the global economy, there can be no assurances
that unforeseen future events in the global supply chain will not have a material adverse effect on our business, financial condition
and results of operations.
Furthermore, inflation can
adversely affect us by increasing the costs of clinical trials, the research and development of our product candidates, as well as administration
and other costs of doing business. We may experience increases in the prices of labor and other costs of doing business. In an inflationary
environment, cost increases may outpace our expectations, causing us to use our cash and other liquid assets faster than forecasted. If
this happens, we may need to raise additional capital to fund our operations, which may not be available in sufficient amounts or on reasonable
terms, if at all, sooner than expected.
Adverse global conditions, including economic
uncertainty, may negatively impact our financial results.
Global conditions, dislocations
in the financial markets, any negative financial impacts affecting United States as a result of tax reform or changes to existing trade
agreements or tax conventions, may adversely impact our business.
In addition, the global macroeconomic
environment could be negatively affected by, among other things, pandemics or epidemics, instability in global economic markets, increased
U.S. trade tariffs and trade disputes with other countries, instability in the global credit markets, supply chain weaknesses, instability
in the geopolitical environment as a result of the Russian invasion of Ukraine, the war between Israel and Hamas and other political tensions,
and foreign governmental debt concerns. Such challenges have caused, and may continue to cause, uncertainty and instability in local economies
and in global financial markets.
Geopolitical risks associated with Russia’s
invasion of Ukraine and Israel’s war with Hamas could result in increased market volatility and uncertainty, which could negatively
impact our business, financial condition, and results of operations.
The uncertain nature, scope,
magnitude, and duration of hostilities stemming from Russia’s military invasion of Ukraine and Israel’s war with Hamas, including
the potential effects of such hostilities as well as sanctions, embargoes, asset freezes, cyber-attacks and other actions taken in response
to such hostilities on the world economy and markets, have disrupted global markets and contributed to increased market volatility and
uncertainty, which could have an adverse impact on macroeconomic and other factors that affect our business and supply chain. There can
be no certainty regarding the impacts stemming from the invasion, including the imposition of additional sanctions, embargoes, asset freezes
or other economic or military measures resulting from the invasion. The impact of these developments, and additional events that may occur
as a result, is currently unknown and could adversely affect our business, supply chain, suppliers and customers and potential customers.
It is not possible to predict the broader consequences of this conflict, which could include further sanctions, embargoes, regional instability,
geopolitical shifts and adverse effects on macroeconomic conditions, the availability and cost of materials, supplies, labor, currency
exchange rates and financial markets, all of which could negatively impact our business, financial condition and results of operations.
Significant disruptions of information technology
systems or breaches of data security could materially adversely affect our business, results of operations and financial condition.
We collect and maintain information
in digital form that is necessary to conduct our business, and we are increasingly dependent on information technology systems and infrastructure
to operate our business. In the ordinary course of our business, we collect, store and transmit large amounts of confidential information,
including intellectual property, proprietary business information and personal information. It is critical that we do so in a secure manner
to maintain the confidentiality and integrity of such confidential information. We have established physical, electronic and organizational
measures to safeguard and secure our systems to prevent a data compromise, and rely on commercially available systems, software, tools,
and monitoring to provide security for our information technology systems and the processing, transmission and storage of digital information.
We have also outsourced elements of our information technology infrastructure, and as a result a number of third-party vendors may or
could have access to our confidential information. Our internal information technology systems and infrastructure, and those of our current
and any future collaborators, contractors and consultants and other third parties on which we rely, are vulnerable to damage from computer
viruses, malware, natural disasters, terrorism, war, telecommunication and electrical failures, cyber-attacks or cyber-intrusions over
the Internet, attachments to emails, persons inside our organization, or persons with access to systems inside our organization.
The risk of a security breach
or disruption, particularly through cyber-attacks or cyber-intrusion, including by computer hackers, foreign governments and cyber-terrorists,
has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased.
In addition, the prevalent use of mobile devices that access confidential information increases the risk of data security breaches, which
could lead to the loss of confidential information or other intellectual property. The costs to us to mitigate network security problems,
bugs, viruses, worms, malicious software programs and security vulnerabilities could be significant, and while we have implemented security
measures to protect our data security and information technology systems, our efforts to address these problems may not be successful,
and these problems could result in unexpected interruptions, delays, cessation of service and other harm to our business and our competitive
position. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our product
development programs. For example, the loss of clinical trial data from completed or ongoing or planned clinical trials could result in
delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Moreover, if a computer
security breach affects our systems or results in the unauthorized release of personally identifiable information, our reputation could
be materially damaged.
In addition, such a breach
may require notification to governmental agencies, the media or individuals pursuant to various federal and state privacy and security
laws, if applicable, including the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology
for Clinical Health Act of 2009, and its implementing rules and regulations, as well as regulations promulgated by the Federal Trade Commission
and state breach notification laws.
Under the EU regulation and
notably the General Data Protection Regulation, or GDPR, No. 2016/679, which entered into force on May 25, 2018 and is applicable personal
data that we process in relation to our presence in the EU, the offering of products or services to individuals in the EU or the monitoring
of the behavior of individuals in the EU, we have also a legal responsibility to report personal data breaches to the competent supervisory
authority. The EU data protection regulation includes a broad definition and a short deadline for the notification of personal data breaches,
which may be difficult to implement in practice and requires that we implement robust internal processes. Under this regulation, we have
to report personal data breaches to the competent supervisory authority within 72 hours of the time we become aware of a breach “unless
the personal data breach is unlikely to result in a risk to the right and freedoms of natural persons” (Article 33 of the GDPR).
In addition, the GDPR requires that we communicate the breach to the Data Subject if the breach is “likely to result in a high risk
to the rights and freedoms of natural persons” (Article 34 of the GDPR). In order to fulfil these requirements, we have to implement
specific internal processes to be followed in case of a personal data breach, which will allow us to (a) contain and recover the breach,
(b) assess the risk to the data subjects, (c) notify, and possibly communicate the breach to the data subjects, (d) investigate and respond
to the breach. The performance of these processes implies substantial costs in resources and time.
Moreover, as we may rely on
third parties that will also process as processor the data for which we are a data controller-for example, in the context of the manufacturing
of our product candidates or for the conduct of clinical trials, we must contractually ensure that strict security measures, as well as
appropriate obligations including an obligation to report in due delay any security incident are implemented, in order to allow us fulfilling
our own regulatory requirements.
We would also be exposed to
a risk of loss or litigation and potential liability for any security breach on personal data for which we are data controller. The costs
of above-mentioned processes together with legal penalties, possible compensation for damages and any resulting lawsuits arising from
a breach may be extensive and may have a negative impact on reputation and materially adversely affect our business, results of operations
and financial condition.
In August 2019, management
was advised that it was a victim of a cyber-related fraud whereby a hacker impersonated one of our key vendors to redirect payments, totaling
approximately $420,000. Management and our Audit Committee completed our investigation and is reviewing all available avenues of recovery,
including from our financial institution to recover the payments. As of December 31, 2021, we recovered approximately $50,000 from our
financial institution and we do not expect to recover anything further from the cyber-related fraud. As a result of the cyber-related
fraud, we have instituted additional controls and procedures and all employees now undergone cybersecurity training.
Requirements associated with being a public
company will increase our costs significantly and will divert significant company resources and management attention.
Since we are no longer an
“emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, we are no longer able to take advantage
of certain exemptions from various reporting requirements that were previously available to us, but which were not available to other
public companies that are not emerging growth companies. Accordingly, we will be required to comply with increased disclosure obligations
regarding executive compensation in our periodic reports and proxy statements and the requirements of holding a nonbinding advisory vote
on executive compensation and stockholder approval of any golden parachute payments not previously approved. As a result, we will incur
greater expenses associated with such reporting requirements. These expenses would further increase if we ceased to be a “smaller
reporting company.” In addition, if we are deemed an accelerated filer or large accelerated filer in the future, we expect to incur
additional management time and cost to comply with the more stringent reporting requirements applicable to companies that are deemed accelerated
filers or large accelerated filers, including complying with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley
Act. We have not yet completed the process of compiling the system and processing documentation needed to comply with such requirements.
We may not be able to complete our evaluation, testing and any required remediation in a timely fashion when required to do so. In that
regard, we currently do not have an internal audit function, and we will need to hire or contract additional accounting and financial
staff with appropriate public company experience and technical accounting knowledge.
Our management and other personnel
need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal
and financial compliance costs and will make some activities more time-consuming and costly. We cannot predict or estimate the amount
of additional costs we may incur as a result of this.
Risks Related to Clinical Development, Regulatory Approval and Commercialization
Our product candidates are at an early stage
of development and may not be successfully developed or commercialized.
We have no products approved
for sale. Latiglutenase, Adrulipase, Capeserod and Niclosamide are in the early stages of clinical development. Our product candidates
will require substantial capital expenditures, development, testing, and regulatory clearances prior to commercialization. The development
and regulatory approval process take several years, and it is not likely that any such products, even if successfully developed and approved
by the FDA or any comparable foreign regulatory authority, would be commercially available for a significant period of time. Many promising
drug candidates fail at some stage of their clinical development. Accordingly, even if we are able to obtain the requisite financing to
fund our development programs, we cannot assure you that our product candidates will be successfully developed, receive required regulatory
approvals and successfully commercialized. Our failure to develop, manufacture or receive regulatory approval for or successfully commercialize
any of our product candidates, could result in the failure of our business and a loss of all of your investment in our company.
Any product candidates we advance into and
through clinical development are subject to extensive regulation, which can be costly and time consuming, cause unanticipated delays or
prevent the receipt of the required approvals to commercialize our product candidates.
The clinical development,
manufacturing, labeling, storage, record-keeping, advertising, promotion, import, export, marketing and distribution of our product candidates
are subject to extensive regulation by the FDA in the United States and by comparable health authorities in foreign markets, including
Health Canada’s Therapeutic Products Directorate, or the TPD, and the European Medicines Agency, or the EMA. In the United States,
we are not permitted to market our product candidates until we receive approval of an NDA (New Drug Application) or BLA (Biologic License
Application) from the FDA. The process of obtaining such approval is expensive, often takes many years and can vary substantially based
upon the type, complexity and novelty of the products involved. In addition to the significant clinical testing requirements, our ability
to obtain marketing approval for these product candidates depends on obtaining the final results of required non-clinical testing, including
characterization of the manufactured components of our product candidates and validation of our manufacturing processes. The FDA may determine
that our product manufacturing processes, testing procedures or facilities are insufficient to justify approval. Approval policies or
regulations may change, and the FDA has substantial discretion in the pharmaceutical approval process, including the ability to delay,
limit or deny approval of a product candidate for many reasons. Despite the time and expense invested in clinical development of product
candidates, regulatory approval is never guaranteed.
The FDA, the TPD and/or the
EMA can delay, limit or deny approval of a product candidate for many reasons, including, but not limited to:
| |
● |
disagreement with the design or implementation of our clinical trials; |
| |
● |
failure to demonstrate to their satisfaction that a product candidate is safe and effective for any indication; |
| |
● |
failure to accept clinical data from trials which are conducted outside their jurisdiction; |
| |
● |
the results of clinical trials may not meet the level of statistical significance required for approval; |
| |
● |
we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| |
● |
such agencies may disagree with our interpretation of data from preclinical studies or clinical trials; |
| |
● |
failure to approve the manufacturing processes or facilities of third-party manufacturers with which we or our collaborators contract for clinical and commercial supplies; or |
| |
● |
changes in the approval policies or regulations of such agencies may significantly change in a manner rendering our clinical data insufficient for approval. |
Any delay in obtaining, or
inability to obtain, applicable regulatory approvals would prevent us from commercializing our product candidates.
If we encounter difficulties enrolling patients
in our clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
We may experience difficulties
in patient enrollment in our clinical trials for a variety of reasons. The timely completion of clinical trials in accordance with their
protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the trial until its conclusion.
The enrollment of patients depends on many factors, including:
| |
● |
the patient eligibility criteria defined in the protocol; |
| |
● |
the size of the patient population; |
| |
● |
the proximity and availability of clinical trial sites for prospective patients; |
| |
● |
the design of the trial; |
| |
● |
our ability to recruit clinical trial investigators with the appropriate competencies and experience; |
| |
● |
the availability of other clinical trials and competition for eligible patients; |
| |
● |
our ability to obtain and maintain patient consents; and |
| |
● |
the risk that patients enrolled in clinical trials will drop out of the trials before completion. |
Our clinical trials will compete
with other clinical trials for product candidates that are in the same therapeutic areas as our product candidates. This competition will
reduce the number and types of patients and qualified clinical investigators available to us, because some patients who might have opted
to enroll in our trials may instead opt to enroll in a trial being conducted by one of our competitors or clinical trial sites may not
allow us to conduct our clinical trial at such site if competing trials are already being conducted there. Since the number of qualified
clinical investigators is limited, we expect to conduct some of our clinical trials at the same clinical trial sites that some of our
competitors use, which will reduce the number of patients who are available for our clinical trials in such clinical trial site. We may
also encounter difficulties finding a clinical trial site at which to conduct our trials.
Delays in patient enrollment
may result in increased costs or may affect the timing or outcome of our planned clinical trials, which could prevent completion of these
clinical trials and adversely affect our ability to advance the development of our product candidates.
Because the results of preclinical studies
and early clinical trials are not necessarily predictive of future results, any product candidate we advance into clinical trials may
not have favorable results in later clinical trials, if any, or receive regulatory approval.
Pharmaceutical development
has inherent risk. We will be required to demonstrate through well-controlled clinical trials that our product candidates are safe and
effective with a favorable benefit-risk profile for use in their target indications before we can seek regulatory approvals for their
commercial sale. Latiglutenase has completed three Phase 2 trials: Alvine Pharmaceuticals conducted Phase 2a and Phase 2b studies and
ImmunogenX completed a Phase 2b trial. Adrulipase has completed four Phase 2 clinical trials in two separate indications (three Phase
2 in CF patients and one Phase 2 in CP patients). Capeserod was previously studied by Sanofi in seven Phase 1 and two Phase 2 trials
investigating the potential of Capeserod for neurological disorders. Niclosamide has completed a Phase 1b/2a study, conducted by Entero,
in patients with mild-to-moderate ulcerative colitis. Success in pre-clinical studies or early clinical trials does not mean that later
clinical trials will be successful, as product candidates in later-stage clinical trials may fail to demonstrate sufficient safety or
efficacy despite having progressed through initial clinical testing. We also may need to conduct additional clinical trials that are
not currently anticipated. Drug developers frequently suffer significant setbacks in advanced clinical trials, even after earlier clinical
trials have shown promising results.
Any product candidate we advance into and
through clinical trials may cause unacceptable adverse events or have other properties that may delay or prevent their regulatory approval
or commercialization or limit their commercial potential.
Unacceptable adverse events
caused by Latiglutenase, Adrulipase, Capeserod and Niclosamide in clinical trials could cause us or regulatory authorities to interrupt,
delay or halt clinical trials and could result in the denial of regulatory approval by the FDA or other regulatory authorities for any
or all targeted indications and markets. This, in turn, could prevent us from commercializing the affected product candidate and generating
revenues from its sale. We have not yet completed testing of any of our product candidates for the treatment of the indications for which
we intend to seek product approval in humans, and we currently do not know the extent of adverse events, if any, that will be observed
in patients who receive any of our product candidates. If any of our product candidates cause unacceptable adverse events in clinical
trials, we may not be able to obtain regulatory approval or commercialize such product or, if such product candidate is approved for marketing,
future adverse events could cause us to withdraw such product from the market.
Delays in the commencement or completion
of our clinical trials could result in increased costs and delay our ability to pursue regulatory approval and commercialization of our
product candidates.
The commencement and completion of clinical
trials can be delayed for a variety of reasons, including delays in:
| |
· |
obtaining regulatory clearance to commence a clinical trial; |
| |
· |
identifying, recruiting and training suitable clinical investigators; |
| |
· |
reaching agreement
on acceptable terms with prospective clinical research organizations and trial sites, the terms of which can be subject to extensive
negotiation, may be subject to modification from time to time and may vary significantly among different CROs and trial sites; |
| |
· |
obtaining sufficient quantities of investigational product (“IP”) for our product candidates for use in clinical trials; |
| |
· |
obtaining Institutional Review Board (“IRB”) or ethics committee approval to conduct a clinical trial at a prospective site; |
| |
· |
identifying, recruiting and enrolling patients to participate in a clinical trial, including delays and/or interruptions resulting from geo-political actions, such as the war in Ukraine, disease or public health epidemics, such as the coronavirus, or natural disasters; |
| |
· |
retaining patients who have initiated a clinical trial but may withdraw due to adverse events from the therapy, insufficient efficacy, changing clinical protocols, fatigue with the clinical trial process, or personal issues; |
| |
· |
retaining patients who may not follow the clinical trial protocols due to factors including the coronavirus epidemic; and |
Any delays in the commencement
of our clinical trials will delay our ability to pursue regulatory approval for our product candidates. In addition, many of the factors
that cause, or lead to, a delay in the commencement of clinical trials may also ultimately lead to the denial of regulatory approval of
a product candidate.
If we encounter difficulties enrolling patients
in our clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
The timely completion of clinical
trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain
in the trial until its conclusion. We may experience difficulties in patient enrollment in our clinical trials for a variety of reasons,
including the size and nature of the patient population and the patient eligibility criteria defined in the protocol, competition from
competing companies, natural disasters, geo-political events, such as the war in Ukraine or public health epidemics, such as the coronavirus
impacting the U.S., Europe and elsewhere.
We may be required to suspend, repeat or
terminate our clinical trials if they are not conducted in accordance with regulatory requirements, the results are negative or inconclusive
or the trials are not well designed.
Regulatory agencies, IRBs
or data safety monitoring boards may at any time recommend the temporary or permanent discontinuation of our clinical trials or request
that we cease using investigators in the clinical trials if they believe that the clinical trials are not being conducted in accordance
with applicable regulatory requirements, or that they present an unacceptable safety risk to participants. Clinical trials must be conducted
in accordance with current cGCPs or other applicable foreign government guidelines governing the design, safety monitoring, quality assurance
and ethical considerations associated with clinical studies. Clinical trials are subject to oversight by the FDA, other foreign governmental
agencies and IRBs at the study sites where the clinical trials are conducted. In addition, clinical trials must be conducted with product
candidates produced in accordance with applicable cGMPs, which are the FDA’s regulations governing the design, monitoring and control
of manufacturing processes and facilities. Clinical trials may be suspended by the FDA, other foreign governmental agencies, or us for
various reasons, including:
| |
· |
deficiencies in the conduct of the clinical trials, including failure to conduct the clinical trial in accordance with regulatory requirements or clinical protocols; |
| |
· |
deficiencies in the clinical trial operations or trial sites; |
| |
· |
the product candidate may have unforeseen adverse side effects; |
| |
· |
deficiencies in the trial design necessary to demonstrate efficacy; |
| |
· |
fatalities or other adverse events arising during a clinical trial due to medical problems that may not be related to clinical trial treatments; |
| |
· |
the product candidate may not appear to be more effective than current therapies; or |
| |
· |
the quality or stability of the product candidate may fall below acceptable standards. |
If we elect or are forced
to suspend or terminate a clinical trial for Latiglutenase, Adrulipase, Capeserod and Niclosamide the commercial prospects for that product
candidate will be harmed and our ability to generate product revenue from that product candidate may be delayed or eliminated. Furthermore,
any of these events could prevent us or our partners from achieving or maintaining market acceptance of the affected product candidate
and could substantially increase the costs of commercializing our product candidates and impair our ability to generate revenue from the
commercialization of these product candidates either by us or by our collaboration partners.
The approval processes of regulatory authorities
are lengthy, time consuming, expensive and inherently unpredictable. If we are unable to obtain approval for our product candidates from
applicable regulatory authorities, we will not be able to market and sell those product candidates in those countries or regions and our
business will be substantially harmed.
The time required to obtain
approval by the FDA and comparable foreign authorities is unpredictable, but typically takes many years following the commencement of
clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. We have not submitted
an NDA or similar filing or obtained regulatory approval for any drug candidate in any jurisdiction and it is possible that none of our
existing product candidates or any product candidates we may seek to develop in the future will ever obtain regulatory approval.
Latiglutenase, Adrulipase,
Capeserod and Niclosamide could fail to receive regulatory approval for many reasons, including any one or more of the following:
| |
· |
the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; |
| |
· |
we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for its proposed indication; |
| |
· |
the results of clinical trials may not meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities for approval; |
| |
· |
we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| |
· |
the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| |
· |
the data collected from clinical trials of our product candidates may not be sufficient to support the submission of an NDA, BLA or other submission or to obtain regulatory approval in the United States or elsewhere; |
| |
· |
the FDA or comparable foreign regulatory authorities may fail to hold to previous agreements or commitments; |
| |
· |
the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; |
| |
· |
the FDA or comparable foreign regulatory authorities may fail to approve our product candidates; |
| |
· |
invest significant additional cash in each of the above activities; and |
| |
· |
the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
The time and expense of the
approval process, as well as the unpredictability of clinical trial results and other contributing factors, may result in our failure
to obtain regulatory approval to market, in one or more jurisdictions, Latiglutenase, Adrulipase, Capeserod, Niclosamide, or future product
candidates, which would significantly harm our business, results of operations and prospects.
In addition, even if we were
to obtain regulatory approval in one or more jurisdictions, regulatory authorities may approve Latiglutenase, Adrulipase, Capeserod and
Niclosamide for fewer or more limited indications than we request, may not approve the prices we may propose to charge for our products,
may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve a product candidate with labeling
that does not include the claims necessary or desirable for the successful commercialization of that product candidate. Any of the foregoing
circumstances could materially harm the commercial prospects for Latiglutenase, Adrulipase, Capeserod and Niclosamide.
Our product candidates may cause undesirable
side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of the approved
labeling, or result in significant negative consequences following marketing approval, if any.
Results of current and future
clinical trials of Latiglutenase, Adrulipase, Capeserod and Niclosamide could reveal a high and/or unacceptable severity and frequency
of these or other side effects. In such an event, our trials could be suspended or terminated, and the FDA or comparable foreign regulatory
authorities could order us to cease further development of, or deny approval of, our product candidates for any or all targeted indications.
Further, any observed drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial
or result in potential product liability claims. Any of these occurrences could materially harm our business, financial condition and
prospects.
Additionally, if Latiglutenase,
Adrulipase, Capeserod and Niclosamide receive marketing approval, and we or others later identify undesirable side effects caused by our
products, a number of potentially significant negative consequences could result, including:
| |
· |
regulatory authorities may withdraw approvals of such product; |
| |
· |
regulatory authorities may require additional warnings in the product’s labeling; |
| |
· |
we may be required to create a medication guide for distribution to patients that outlines the risks of such side effects; |
| |
· |
we could be sued and held liable for harm caused to patients; and |
| |
· |
our reputation may suffer. |
Any of these events could
prevent us from achieving or maintaining market acceptance of the particular product, if approved, and could significantly harm our business,
results of operations and prospects.
If we are unable to execute our sales and
marketing strategy for our products and are unable to gain market acceptance, we may be unable to generate sufficient revenue to sustain
our business.
We are a clinical-stage biopharmaceutical
company and have yet to begin to generate revenue from Latiglutenase, Adrulipase, Capeserod and Niclosamide. Our product candidates are
in an early stage of clinical development, and, if we obtain marketing approval for any of products in the future, which we anticipate
would not occur for several years, if at all.
We may never gain significant
market acceptance for our product candidates and therefore may never generate substantial revenue or profits for us. We will need to establish
a market for any of our product candidates that receive regulatory approval through physician education, sales and marketing efforts,
awareness programs and the publication of clinical data. Gaining acceptance in medical communities requires, among other things, publication
in leading peer-reviewed journals of results from our studies. The process of publication in leading medical journals is subject to a
peer review process and peer reviewers may not consider the results of our studies sufficiently novel or worthy of publication. Failure
to have our studies published in peer-reviewed journals could limit the adoption of Latiglutenase, Adrulipase, Capeserod and Niclosamide.
Our ability to successfully market our product candidates that we may develop will depend on numerous factors, including:
| |
· |
the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; |
| |
· |
the efficacy and safety as demonstrated in clinical trials; |
| |
· |
the clinical indications for which the product is approved; |
| |
· |
the inability to demonstrate effectively that the clinical and other benefits of a product candidate outweigh any safety or other perceived risks; |
| |
· |
the inability to demonstrate effectively that the efficacy of a product candidate is superior to a competing treatment; |
| |
· |
conducting clinical utility studies of our product candidates to demonstrate economic usefulness to providers and payers; |
| |
· |
relative convenience and ease of administration; |
| |
· |
whether our current or future partners, support our offerings; |
| |
· |
the success of the sales force and marketing effort; |
| |
· |
unfavorable publicity relating to the product; |
| |
· |
whether healthcare providers believe our product candidates provide clinical utility relative to their cost; and |
| |
· |
whether private health insurers, government health programs and other third-party payers will cover our product candidates. |
We currently have no commercial organization.
If we are unable to establish satisfactory sales and marketing capabilities or secure a sales and marketing partner, we may not successfully
commercialize any of our product candidates.
We have no commercial infrastructure.
In order to commercialize products that are approved for marketing, we must either establish our own sales and marketing infrastructure
or collaborate with third parties that have such commercial infrastructure.
We may not be able to enter
into collaboration agreements on terms acceptable to us or at all. In addition, even if we enter into such relationships, we may have
limited or no control over the sales, marketing and distribution activities of these third parties. Our future revenues may depend heavily
on the success of the efforts of these third parties. If we elect to establish a sales and marketing infrastructure, we may not realize
a positive return on this investment. In addition, we will have to compete with established and well-funded pharmaceutical and biotechnology
companies to recruit, hire, train and retain sales and marketing personnel. Factors that may inhibit our efforts to commercialize our
product candidates without strategic partners or licensees include:
| |
· |
our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| |
· |
the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to prescribe our future products; |
| |
· |
the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| |
· |
unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
If we are not successful in
recruiting sales and marketing personnel or in building a sales and marketing infrastructure, or if we do not successfully enter into
appropriate collaboration arrangements, we will have difficulty successfully commercializing our product candidates and any we may develop
or acquire, which would adversely affect our business, operating results and financial condition. Outside the United States, we may commercialize
our product candidates by entering into collaboration agreements with pharmaceutical partners. We may not be able to enter into such agreements
on terms acceptable to us or at all. In addition, even if we enter into such relationships, we may have limited or no control over the
sales, marketing and distribution activities of these third parties. Our future revenues may depend heavily on the success of the efforts
of these third parties.
We may form or seek strategic alliances
or enter into additional licensing arrangements in the future, and we may not realize the benefits of such alliances or licensing arrangements.
From time to time, we may
form or seek strategic alliances, create joint ventures or collaborations or enter into additional licensing arrangements with third parties
that we believe will complement or augment our development and commercialization efforts with respect to Latiglutenase, Adrulipase, Capeserod
and Niclosamide and any future product candidates that we may develop. Any of these relationships may require us to incur non-recurring
and other charges, increase our near and long-term expenditures, issue securities that dilute our existing stockholders or disrupt our
management and business. These relationships also may result in a delay in the development of Latiglutenase, Adrulipase, Capeserod and
Niclosamide if we become dependent upon the other party and such other party does not prioritize the development of our product candidates
relative to its other development activities. In addition, we face significant competition in seeking appropriate strategic partners and
the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership
or other alternative arrangements for our product candidates because they may be deemed to be at too early of a stage of development for
collaborative effort and third parties may not view our product candidates as having the requisite potential to demonstrate safety and
efficacy. If we license products or businesses, we may not be able to realize the benefit of such transactions if we are unable to successfully
integrate them with our existing operations and company culture. We cannot be certain that, following a strategic transaction or license,
we will achieve the revenue or specific net income that justifies such transaction. We rely completely on third parties to manufacture
our preclinical and clinical pharmaceutical supplies and expect to continue to rely on third parties to produce commercial supplies of
our product candidates, and our dependence on third party suppliers could adversely impact our business.
We rely completely on third parties to manufacture
our preclinical and clinical pharmaceutical supplies and expect to continue to rely on third parties to produce commercial supplies of
any approved product candidate, and our dependence on third party suppliers could adversely impact our business.
We rely on third parties to
manufacture our product candidates, including Latiglutenase, Adrulipase, Capeserod, and Niclosamide.
The proprietary E. Coli cell
line from which the Latiglutenase API is derived is kept at a storage facility maintained by Charles River Laboratories Inc. Latiglutenase
drug substance is currently manufactured at a contract facility located in Tribiano (MI), Italy owned by ACS Dobfar SpA, which is not
currently GMP certified. While we believe that the GMP certification of the facility owned by ACS Dobfar SpA is achievable, there can
be no assurances that such certification will occur. We believe there are multiple alternative contract manufacturers capable of producing
the Latiglutenase API that we need for clinical trials. There is no guarantee that the processes are reproducible and transferrable.
Capeserod API is obtained
by chemical synthesis and is currently under development to be manufactured by Asymchem Inc. at a facility in Tianjin, China. We believe
there are multiple alternative contract manufacturers capable of producing the product we need for clinical trials; however, there is
no guarantee that the processes are reproducible and transferrable.
The proprietary yeast cell
line from which the Adrulipase API is derived is kept at a storage facility maintained by Charles River Laboratories Inc. Adrulipase drug
substance and drug product are currently manufactured at a contract facility located in Tianjin, China owned by Asymchem Life Science
Co., Ltd. We believe there are multiple alternative contract manufacturers capable of producing the Adrulipase product we need for clinical
trials. There is no guarantee that the processes are easily reproducible and transferrable.
Niclosamide API is obtained
by chemical synthesis and is currently manufactured by Olon SpA at a facility in Murcia, Spain. The drug product manufacturing for Niclosamide
is currently conducted at a contract facility located in Milan, Italy and Tianjin, China owned by Monteresearch s.r.l. and Asymchem Life
Science Co., Ltd, respectively.
We are completely dependent
on these third parties for product supply and our Latiglutenase, Adrulipase, Capeserod and Niclosamide development programs would be adversely
affected by a significant interruption in our ability to receive such materials. We have not yet entered into long-term manufacturing
or supply agreements with any third parties. Furthermore, our third-party suppliers will be required to obtain and maintain compliance
with cGMPs and will be subject to inspections by the FDA or comparable regulatory authorities in other jurisdictions to confirm such compliance.
In the event that the FDA or such other authorities determine that our third-party suppliers have not complied with cGMP, our clinical
trials could be terminated or subjected to a clinical hold until such time as we are able to obtain appropriate replacement material.
Any delay, interruption or other issues that arise in the manufacture, packaging, or storage of our products, including Latiglutenase,
as a result of a failure of the facilities or operations of our third-party suppliers to pass any regulatory agency inspection could significantly
impair our ability to develop and commercialize our products.
We do not expect to have the
resources or capacity to commercially manufacture any of our proposed products, if approved, and will likely continue to be dependent
upon third party manufacturers. Our dependence on third parties to manufacture and supply us with clinical trial materials and any approved
products may adversely affect our ability to develop and commercialize our products on a timely basis or at all.
We rely on third parties to conduct our
clinical trials. If these third parties do not meet our deadlines or otherwise conduct the trials as required, our clinical development
programs could be delayed or unsuccessful and we may not be able to obtain regulatory approval for or commercialize our product candidates
when expected or at all.
We do not have the ability
to conduct all aspects of our preclinical testing or clinical trials ourselves. We use contract research organizations (CROs) to conduct
our clinical trials and will rely upon such CROs, as well as medical institutions, clinical investigators and consultants, to conduct
our trials in accordance with our clinical protocols. Our CROs, investigators and other third parties will play a significant role in
the conduct of these trials and the subsequent collection and analysis of data from the clinical trials.
There is no guarantee that
any CROs, investigators and other third parties upon which we rely for administration and conduct of our clinical trials will devote adequate
time and resources to such trials or perform as contractually required. If any of these third parties fail to meet expected deadlines,
fail to adhere to our clinical protocols or otherwise perform in a substandard manner, our clinical trials may be extended, delayed or
terminated. If any of our clinical trial sites terminate for any reason, we may experience the loss of follow-up information on patients
enrolled in our ongoing clinical trials unless we are able to transfer the care of those patients to another qualified clinical trial
site. In addition, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to
time and receive cash or equity compensation in connection with such services. If these relationships and any related compensation result
in perceived or actual conflicts of interest, the integrity of the data generated at the applicable clinical trial site may be jeopardized.
We intend to rely on market exclusivity periods that may not
be or remain available to us.
We intend to rely on our ability
to obtain and maintain a regulatory period of market exclusivity for any of our product candidates, including Latiglutenase, Adrulipase,
Capeserod and Niclosamide that are successfully developed and approved for commercialization. Although this period in the United States
is currently 12 years from the date of marketing approval, reductions to this period have been proposed. This exclusivity period in Europe
is currently 10 years from the date of marketing approval by the EMA. Once any regulatory period of exclusivity expires, depending on
the status of our patent coverage and the nature of the product, we may not be able to prevent others from marketing products that are
biosimilar to or interchangeable with our products, which would materially adversely affect us.
Because Niclosamide is a small
molecule it would be subject either to three or five year exclusivity, depending on the regulatory pathway of any clinical trials. Niclosamide
is not entitled to the same 12-year exclusivity as our biologic product candidates.
Due to the significant resources required
for the development of our product candidates, we must prioritize development of certain product candidates and/or certain disease indications.
We may expend our limited resources on candidates or indications that do not yield a successful product and fail to capitalize on product
candidates or indications that may be more profitable or for which there is a greater likelihood of success.
We intend to develop a pipeline
of product candidates to treat GI and other diseases. Due to the significant resources required for the development of product candidates,
we must focus our attention and resources on specific diseases and/or indications and decide which product candidates to pursue and the
amount of resources to allocate to each. We are currently focusing our resources on the development of our product candidates, Latiglutenase,
Adrulipase, Capeserod and Niclosamide.
Our decisions concerning the
allocation of research, development, collaboration, management and financial resources toward particular product candidates or therapeutic
areas may not lead to the development of any viable commercial product and may divert resources away from better opportunities. Similarly,
any decision to delay, terminate or collaborate with third parties in respect of certain programs or product candidates may subsequently
prove to be suboptimal and could cause us to miss valuable opportunities. If we make incorrect determinations regarding the viability
or market potential of any of our programs or product candidates or misread trends in the GI, CF, CP, COVID-19, ICI-AC or biotechnology
industry, our business, financial condition and results of operations could be materially adversely affected. As a result, we may fail
to capitalize on viable commercial products or profitable market opportunities, be required to forego or delay pursuit of opportunities
with other product candidates or other diseases and indications that may later prove to have greater commercial potential than those we
choose to pursue, or relinquish valuable rights to such product candidates through collaboration, licensing or other royalty arrangements
in cases in which it would have been advantageous for us to invest additional resources to retain development and commercialization rights.
If we fail to attract and retain key management
and clinical development personnel, we may be unable to successfully develop or commercialize our product candidates.
We are dependent on our management
team and clinical development personnel and our success will depend on their continued service, as well as our ability to attract and
retain highly qualified personnel. In particular, the continued employment of our senior management team, which includes James Sapirstein,
our Chief Executive Officer and Sarah Romano, our Chief Financial Officer, is critical to our success. The market for the services of
qualified personnel in the biotechnology and pharmaceutical industries are highly competitive. The loss of service of any member of our
senior management team or key personnel could prevent, impair or delay the implementation of our business plan, the successful conduct
and completion of our planned clinical trials and the commercialization of any product candidates that we may successfully develop. We
do not carry key man insurance for any member of our senior management team.
Healthcare reform and restrictions on reimbursements may limit
our financial returns.
Our ability or the ability
of our collaborators to commercialize any of our product candidates that we successfully develop may depend, in part, on the extent to
which government health administration authorities, private health insurers and other organizations will reimburse consumers for the cost
of these products. These third parties are increasingly challenging both the need for and the price of new drug products. Significant
uncertainty exists as to the reimbursement status of newly approved therapeutics. Adequate third-party reimbursement may not be available
for our product candidates to enable us or our collaborators to maintain price levels sufficient to realize an appropriate return on their
and our investments in research and product development.
Changes in healthcare law and implementing
regulations, including government restrictions on pricing and reimbursement, as well as healthcare policy and other healthcare payor cost-containment
initiatives, may negatively impact our ability to generate revenues.
The potential pricing and
reimbursement environment for Latiglutenase, Adrulipase, Capeserod Niclosamide, and any future drug products may change in the future
and become more challenging due to, among other reasons, policies advanced by the current or any new presidential administration, federal
agencies, healthcare legislation passed by Congress, or fiscal challenges faced by all levels of government health administration authorities.
If we or any of our independent contractors,
consultants, collaborators, manufacturers, vendors or service providers fail to comply with healthcare laws and regulations, we or they
could be subject to enforcement actions, which could result in penalties and affect our ability to develop, market and sell our product
candidates and may harm our reputation.
We are subject to federal,
state, and foreign healthcare laws and regulations pertaining to fraud and abuse and patients’ rights. These laws and regulations
include:
| |
· |
the U.S. federal healthcare program anti-kickback law, which prohibits, among other things, persons and entities from soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual for a healthcare item or service, or the purchasing or ordering of an item or service, for which payment may be made under a federal healthcare program such as Medicare or Medicaid; |
| |
· |
the U.S. federal false claims and civil monetary penalties laws, which prohibit, among other things, individuals or entities from knowingly presenting or causing to be presented, claims for payment by government funded programs such as Medicare or Medicaid that are false or fraudulent, and which may apply to us by virtue of statements and representations made to customers or third parties; |
| |
· |
the U.S. federal Health Insurance Portability and Accountability Act (“HIPAA”), which prohibits, among other things, executing a scheme to defraud healthcare programs; |
| |
· |
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, imposes requirements relating to the privacy, security, and transmission of individually identifiable health information, and requires notification to affected individuals and regulatory authorities of certain breaches of security of individually identifiable health information; |
| |
· |
the federal Physician Payment Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies to report annually to the Centers for Medicare & Medicaid Services, or CMS, information related to payments and other transfers of value to physicians, other healthcare providers and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members, which is published in a searchable form on an annual basis; and |
| |
· |
state laws comparable to each of the above federal laws, such as, for example, anti-kickback and false claims laws that may be broader in scope and also apply to commercial insurers and other non-federal payors, requirements for mandatory corporate regulatory compliance programs, and laws relating to patient data privacy and security. |
If our operations are found
to be in violation of any such health care laws and regulations, we may be subject to penalties, including administrative, civil and criminal
penalties, monetary damages, disgorgement, imprisonment, the curtailment or restructuring of our operations, loss of eligibility to obtain
approvals from the FDA, or exclusion from participation in government contracting, healthcare reimbursement or other government programs,
including Medicare and Medicaid, any of which could adversely affect our financial results. Although effective compliance programs can
mitigate the risk of investigation and prosecution for violations of these laws, these risks cannot be entirely eliminated. Any action
against us for an alleged or suspected violation could cause us to incur significant legal expenses and could divert our management’s
attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with
applicable laws and regulations may be costly to us in terms of money, time and resources.
Our employees and independent contractors,
including principal investigators, consultants, commercial collaborators, service providers and other vendors may engage in misconduct
or other improper activities, including noncompliance with regulatory standards and requirements, which could have an adverse effect on
our results of operations.
We are exposed to the risk
that our employees and independent contractors, including principal investigators, consultants, any future commercial collaborators, service
providers and other vendors may engage in misconduct or other illegal activity. Misconduct by these parties could include intentional,
reckless and/or negligent conduct or other unauthorized activities that violate the laws and regulations of the FDA, EMA and other similar
regulatory bodies, including those laws that require the reporting of true, complete and accurate information to such regulatory bodies;
manufacturing standards; healthcare fraud and abuse, data privacy laws and other similar laws; or laws that require the true, complete
and accurate reporting of financial information or data. Activities subject to these laws also involve the improper use or misrepresentation
of information obtained in the course of clinical trials, the creation of fraudulent data in our preclinical studies or clinical trials,
or illegal misappropriation of product, which could result in regulatory sanctions and cause serious harm to our reputation. It is not
always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent
this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations
or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. In addition, we are subject to
the risk that a person or government could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted
against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on
our business and financial results, including, without limitation, the imposition of significant civil, criminal and administrative penalties,
damages, monetary fines, disgorgements, possible exclusion from participation in governmental healthcare programs, individual imprisonment,
other sanctions, contractual damages, reputational harm, diminished profits and future earnings and curtailment of our operations, any
of which could adversely affect our ability to operate our business and our results of operations.
Risks Related to our Intellectual Property
Our success will depend upon intellectual
property, proprietary technologies and regulatory market exclusivity periods, and we may be unable to protect our intellectual property.
Our success will depend, in
large part, on obtaining and maintaining patent protection and trade secret protection for Latiglutenase, Adrulipase, Capeserod and Niclosamide
and their formulations and uses, as well as successfully defending these patents against third-party challenges. If we or our licensors
fail to appropriately prosecute and maintain patent protection for our product candidates, our ability to develop and commercialize these
product candidates may be adversely affected and we may not be able to prevent competitors from making, using and selling competing products.
This failure to properly protect the intellectual property rights relating to these product candidates could have a material adverse effect
on our financial condition and results of operations.
The patent application process
is subject to numerous risks and uncertainties, and there can be no assurance that we or our partners will be successful in protecting
our product candidates by obtaining and defending patents. These risks and uncertainties include the following:
| |
· |
patent applications may not result in any patents being issued or any issued patents may not be of sufficient scope to cover our products; |
| |
· |
patents that may be issued or in-licensed may be challenged, invalidated, modified, revoked, circumvented, found to be unenforceable, or otherwise may not provide any competitive advantage; |
| |
· |
our competitors, many of which have substantially greater resources than we or our partners and many of which have made significant investments in competing technologies, may seek, or may already have obtained, patents that will limit, interfere with, or eliminate our ability to make, use, and sell our potential products; |
| |
· |
there may be significant pressure on the United States government and other international governmental bodies to limit the scope of patent protection both inside and outside the United States for disease treatments that prove successful as a matter of public policy regarding worldwide health concerns; |
| |
· |
countries other than the United States may have patent laws less favorable to patentees than those upheld by United States courts, allowing foreign competitors a better opportunity to create, develop, and market competing products; |
| |
|
|
| |
· |
others may allege ownership of our patents or patent applications or those of our licensors, and defense of such allegation or potential proceeding could be expensive, time consuming and unsuccessful; and |
| |
· |
we may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time consuming and unsuccessful. |
In addition to patents, we
and our partners also rely on trade secrets and proprietary know-how. Although we have taken steps to protect our trade secrets and unpatented
know-how, including entering into confidentiality agreements with third parties, and confidential information and inventions agreements
with employees, consultants and advisors, third parties may still obtain this information or come upon this same or similar information
independently. We may become subject to claims that we or consultants, advisors or independent contractors that we may engage to assist
us in developing Latiglutenase, Adrulipase, Capeserod and Niclosamide have wrongfully or inadvertently disclosed to us or used trade secrets
or other proprietary information of their former employers or their other clients.
If we or our partners are sued for infringing
intellectual property rights of third parties, it will be costly and time consuming, and an unfavorable outcome in that litigation would
have a material adverse effect on our business.
Our success also depends upon
our ability and the ability of any of our future collaborators to develop, manufacture, market and sell our product candidates without
infringing the proprietary rights of third parties. Numerous United States and foreign issued patents and pending patent applications,
which are owned by third parties, exist in the fields in which we are developing products, some of which may be directed at claims that
overlap with the subject matter of our intellectual property. Because patent applications do not publish for 18 months from their priority
date, can be filed with a non-publication request in the United States, and can take many years to issue, there may be currently pending
applications, unknown to us, which may later result in issued patents that our product candidates or proprietary technologies may infringe.
Similarly, there may be issued patents relevant to our product candidates of which we are not aware.
There is a substantial amount
of litigation involving patent and other intellectual property rights in the biotechnology and biopharmaceutical industries generally.
If a third-party claims that we or any of our licensors, suppliers or collaborators infringe the third party’s intellectual property
rights, we may have to:
| |
· |
obtain licenses, which may not be available on commercially reasonable terms, if at all; |
| |
· |
abandon an infringing product candidate or redesign our products or processes to avoid infringement; |
| |
· |
pay substantial damages, including the possibility of treble damages and attorneys’ fees, if a court decides that the product or proprietary technology at issue infringes on or violates the third party’s rights; |
| |
· |
pay substantial royalties, fees and/or grant cross licenses to our technology; and/or |
| |
· |
defend litigation or administrative proceedings which may be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources. |
Our ability to compete may decline if we do not adequately protect
our proprietary rights.
Our success depends on obtaining
and maintaining proprietary rights to our product candidates for the treatment of age-related diseases, as well as successfully defending
these rights against third-party challenges. We will only be able to protect our product candidates, and their uses from unauthorized
use by third parties to the extent that valid and enforceable patents, or effectively protected trade secrets, cover them. Our ability
to obtain patent protection for our product candidates is uncertain due to a number of factors, including:
| |
· |
we may not have been the first to make the inventions covered by pending patent applications or issued patent; |
| |
· |
we may not have been the first to file patent applications for our product candidates or the compositions we developed or for their uses; |
| |
|
|
| |
· |
others may independently develop identical, similar or alternative products or compositions and uses thereof; |
| |
· |
our disclosures in patent applications may not be sufficient to meet the statutory requirements for patentability; |
| |
· |
any or all of our pending patent applications may not result in issued patents; |
| |
· |
we may not seek or obtain patent protection in countries that may eventually provide us a significant business opportunity; |
| |
· |
any patents issued to us may not provide a basis for commercially viable products, may not provide any competitive advantages, or may be successfully challenged by third parties; |
| |
· |
our compositions and methods may not be patentable; |
| |
· |
others may design around our patent claims to produce competitive products which fall outside of the scope of our patents; |
| |
· |
others may identify prior art or other bases which could invalidate our patents. |
Even if we have or obtain
patents covering our product candidates or compositions, we may still be barred from making, using and selling our product candidates
or technologies because of the patent rights of others. Others may have filed, and in the future may file, patent applications covering
compositions or products that are similar or identical to ours. There are many issued U.S. and foreign patents relating to chemical compounds
and therapeutic products, and some of these relate to compounds we intend to commercialize. These could materially affect our ability
to develop our product candidates or sell our products if approved. Because patent applications do not publish for 18 months from their
priority date, can be filed with a non-publication request in the United States, and can take many years to issue, there may be currently
pending applications unknown to us that may later result in issued patents that our product candidates or compositions may infringe. These
patent applications may have priority over patent applications filed by us.
Obtaining and maintaining
a patent portfolio entails significant expense and resources. Part of the expense includes periodic maintenance fees, renewal fees, annuity
fees, various other governmental fees on patents and/or applications due in several stages over the lifetime of patents and/or applications,
as well as the cost associated with complying with numerous procedural provisions during the patent application process. We may or may
not choose to pursue or maintain protection for particular inventions. In addition, there are situations in which failure to make certain
payments or noncompliance with certain requirements in the patent process can result in abandonment or lapse of a patent or patent application,
resulting in partial or complete loss of patent rights in the relevant jurisdiction. If we choose to forgo patent protection or allow
a patent application or patent to lapse purposefully or inadvertently, our competitive position could suffer.
Legal actions to enforce our
proprietary rights (including patents and trademarks) can be expensive and may involve the diversion of significant management time. In
addition, these legal actions could be unsuccessful and could also result in the invalidation of our patents or trademarks or a finding
that they are unenforceable. We may or may not choose to pursue litigation or other actions against those that have infringed on our patents
or trademarks, or used them without authorization, due to the associated expense and time commitment of monitoring these activities. If
we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could harm
our results of operations.
If we breach our license or other intellectual
property-related agreements for our product candidates or otherwise experience disruptions to our business relationships with our licensor,
we could lose the ability to continue the development and commercialization of our product candidates.
On September 13, 2023, we
entered into a license agreement with Sanofi (the “Sanofi License Agreement”), pursuant to which we received a license
to obtain certain exclusive worldwide rights to develop and commercialize Capeserod, a selective 5-HT4 receptor partial agonist which
we intend to repurpose and develop for gastrointestinal indications. Our license may not cover all intellectual property rights owned
or controlled by our licensor and relevant to our product candidates. If we have not obtained a license to all intellectual property rights
owned or controlled by our licensor that are relevant to our product candidates, we may need to obtain additional licenses to such intellectual
property rights which may not be available on an exclusive basis, on commercially reasonable terms or at all. In addition, if our licensor
breaches such agreement, we may not be able to enforce such agreements against our licensor or their parent entity or affiliates. Under
the Sanofi License Agreement, in exchange for licensing to us the right to develop and commercialize the applicable product candidate,
Sanofi will be eligible to receive from us milestone payments, tiered royalties from commercial sales of such product candidates, assuming
relevant approvals from government authorities are obtained, or other payments.
If we fail to meet any of
our obligations under our license and intellectual property-related agreements, our licensors may have the right to terminate our licenses
and sublicenses and, upon the effective date of such termination, have the right to re-obtain the licensed and sublicensed technology
and intellectual property. If any of our licensors terminate any of our licenses or sublicenses, we will lose the right to develop and
commercialize our applicable product candidates and other third parties may be able to market product candidates similar or identical
to ours. In such case, we may be required to provide a grant back license to the licensors under our own intellectual property with respect
to the terminated products. While we would expect to exercise all rights and remedies available to us, including seeking to cure any breach
by us, and otherwise seek to preserve the intellectual property rights licensed and sublicensed to us, we may not be able to do so in
a timely manner, at an acceptable cost or at all. In particular, some of the milestone payments are payable upon our product candidates
reaching development milestones before we have commercialized, or received any revenue from, sales of such product candidate, and we cannot
guarantee that we will have sufficient resources to make such milestone payments. Any uncured, material breach under the license agreements
could result in our loss of exclusive rights and may lead to a complete termination of our rights to the applicable product candidate.
Any of the foregoing could have a material adverse effect on our business, financial conditions, results of operations and prospects.
In addition, disputes may
further arise regarding intellectual property subject to a license agreement, including, but not limited to:
| |
● |
the scope of rights granted under the license agreement and other interpretation-related issues; |
| |
● |
the extent to which our technology and processes infringe, misappropriate or otherwise violate intellectual property of the licensor that is not subject to the licensing agreement; |
| |
● |
the sublicensing of patent and other rights under our collaborative development relationships; |
| |
● |
our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
| |
● |
the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
| |
● |
the priority of invention of patented technology. |
In addition, the agreements
under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements
may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow
what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our
financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial
condition, results of operations, and prospects. Moreover, if disputes over intellectual property that we have licensed or sublicensed
prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully
develop and commercialize the affected product candidates, which could have a material adverse effect on our business, financial conditions,
results of operations and prospects.
If we experience disruptions
to our business relationships with our licensors, we could lose the ability to continue to source, develop and commercialize our product
candidates, including ultimately losing our rights to such product candidates.
Risks Related to our Securities
Our failure to maintain compliance with
applicable Nasdaq’s continued listing requirements could result in the delisting of our Common Stock.
Our common stock is currently
listed for trading on The Nasdaq Stock Market LLC. We must satisfy applicable listing requirements of Nasdaq, to maintain the listing
of our common stock on The Nasdaq Stock Market LLC.
On August 17, 2023, we
received notice from the Staff of Nasdaq indicating that we were not in compliance with the $2.5 million minimum stockholders’
equity requirement for continued listing of the Common Stock on Nasdaq, as set forth in Nasdaq Listing Rule 5550(b)(1). In that regard,
we reported a stockholders’ deficit of $(881,960) in our Quarterly Report on Form 10-Q for the period ended June 30, 2023 (we did
not then, and do not now, meet the alternative compliance standards relating to the market value of listed securities of $35 million
or net income from continuing operations of $500,000 in the most recently completed fiscal year or in two of the last three most recently
completed fiscal years). On October 2, 2023, we submitted a plan to the Staff to regain compliance with the Minimum Stockholders’
Equity Rule. On November 13, 2023, we filed our Quarterly Report on Form 10-Q for the period ended September 30, 2023, reporting total
stockholders’ equity of $3,278,805 as of September 30, 2023. On March 29, 2024, we filed our Annual Report on Form 10-K for the
year ended December 31, 2023, reporting total stockholders’ equity of $3,602,929 as of December 31, 2023. On April 1, 2024, we
received a letter from Nasdaq Staff stating that, based on our Annual Report on Form 10-K for the fiscal year ended December 31, 2024,
which evidenced stockholders’ equity of $3,602,929, the Staff had determined that we were in compliance with the Minimum Stockholders’
Equity Rule and that the matter was now closed.
As we have previously reported,
on August 24, 2023, we received a notice (the “Minimum Bid Price Notice”) from the Staff indicating that, based upon
the closing bid price of our common stock for the last 30 consecutive business days, we were not in compliance with the requirement to
maintain a minimum bid price of $1.00 per share for continued listing on The Nasdaq Capital Market, as set forth in Nasdaq Listing Rule
5550(a)(2) (the “Minimum Bid Price Rule”). We were provided a compliance period of 180 calendar days from the date
of the Notice, or until February 20, 2024, to regain compliance with the Minimum Bid Price Rule, pursuant to Nasdaq Listing Rule 5810(c)(3)(A).
If at any time before February 20, 2024, the closing bid price of the Common Stock closes at or above $1.00 per share for a minimum of
10 consecutive business days, subject to Nasdaq’s discretion to extend this period pursuant to Nasdaq Listing Rule 5810(c)(3)(G)
to 20 consecutive business days, Nasdaq will provide written notification that we have achieved compliance with the minimum bid price
requirement, and the matter would be resolved. On January 4, 2024, we received notice from Nasdaq Listing Qualifications stating that
the Staff had determined that for the prior eleven consecutive business days, from December 18, 2023, to January 3, 2024, the closing
bid price of our Common Stock had been at $1.00 per share or greater, and accordingly, we had regained compliance with the Bid Price Rule.
On October 26, 2023, we received
notice from the Staff of Nasdaq indicating that, in connection with our July 2023 Offering, we were not in compliance with Nasdaq’s
shareholder approval requirements set forth in Listing Rule 5635(d), which requires prior shareholder approval for transactions, other
than public offerings, involving the issuance of 20% or more of the pre-transaction shares outstanding at less than the Minimum Price,
defined as a price that is the lower of: (i) the Nasdaq official closing Price (as reflected on Nasdaq.com) immediately preceding the
signing of the binding agreement; or (ii) the average Nasdaq official Closing Price of the common stock (as reflected on Nasdaq.com) for
the five trading days immediately preceding the signing of the binding agreement. On December 12, 2023, during the Special Meeting, our
stockholders ratified our entry into the Offering as we received the affirmative vote of the majority of the votes cast by shares of our
common stock present or represented by proxy and entitled to vote at the Special Meeting. On March 19, 2024, we received the Letter of
Reprimand from the Nasdaq Listing Qualifications Staff stating that, while we failed to comply with Nasdaq’s continued listing requirements,
our violation of listing rule 5635(d) does not appear to have been the result of a deliberate intent to avoid compliance, and as such,
the Staff does not believe that delisting our securities is an appropriate sanction and that it is appropriate to close these matters
by issuing the Letter of Reprimand.
There can be no assurance
that we will be able to ultimately sustain compliance with all applicable requirements for continued listing on The Nasdaq Stock Market
LLC. In 2020, the SEC approved a previously proposed Nasdaq rule change to expedite delisting of securities with a closing bid price at
or below $0.10 for 10 consecutive trading days during any bid price compliance period and that have had one or more reverse stock splits
with a cumulative ratio of one for 250 or more shares over the prior two-year period. In addition, if a company falls out of compliance
with the $1.00 minimum bid price after completing reverse stock splits over the immediately preceding two years that cumulatively result
in a ratio one for 250 shares, the company will not be able to avail itself of any bid price compliance periods under Rule 5810(c)(3)(A),
and Nasdaq will instead require the issuance of a Staff delisting determination. We could appeal the determination to a hearings panel,
which could grant us a 180-day exception to remain listed if it believes we would be able to achieve and maintain compliance with the
bid price requirement. Following the exception, the company would be subject to the procedures applicable to a company with recurring
deficiencies (Nasdaq Rule 5815(d)(4)(B)).
Additionally, on March
28, 2024, we received a letter from the Staff stating that the Staff had determined Entero’s acquisition of ImmunogenX constitutes
a business combination that results in a “Change of Control” pursuant to Nasdaq Listing Rule 5110(a), and that, as a result,
we will be required to satisfy all of Nasdaq’s initial listing criteria and to complete Nasdaq’s initial listing process
prior to shareholder approval of the conversion of the Series G Preferred Stock, or other material changes triggering a change of control.
In the event that we are unable
to sustain compliance with all applicable requirements to remain listed on the Nasdaq, our common stock may be delisted from Nasdaq.
If our common stock were delisted from Nasdaq, trading of our common stock would most likely take place on an over-the-counter market
established for unlisted securities, such as the OTCQB or the Pink Market maintained by OTC Markets Group Inc. An investor would likely
find it less convenient to sell, or to obtain accurate quotations in seeking to buy, our common stock on an over-the-counter market, and
many investors would likely not buy or sell our common stock due to difficulty in accessing over-the-counter markets, policies preventing
them from trading in securities not listed on a national exchange or other reasons. In addition, as a delisted security, our common stock
would be subject to SEC rules as a “penny stock,” which impose additional disclosure requirements on broker-dealers. The regulations
relating to penny stocks, coupled with the typically higher cost per trade to the investor of penny stocks due to factors such as broker
commissions generally representing a higher percentage of the price of a penny stock than of a higher-priced stock, would further limit
the ability of investors to trade in our common stock. In addition, delisting would materially and adversely affect our ability to raise
capital on terms acceptable to us, or at all, and may result in the potential loss of confidence by investors, suppliers, customers and
employees and fewer business development opportunities. For these reasons and others, delisting would adversely affect the liquidity,
trading volume and price of our common stock, causing the value of an investment in us to decrease and having an adverse effect on our
business, financial condition and results of operations, including our ability to attract and retain qualified employees and to raise
capital.
The limited public market for our securities
may adversely affect an investor’s ability to liquidate an investment in us.
Although our Common Stock
is currently listed on the Nasdaq Capital Market, there is limited trading activity. We can give no assurance that an active market will
develop, or if developed, that it will be sustained. If an investor acquires shares of our Common Stock, the investor may not be able
to liquidate our shares should there be a need or desire to do so.
The market price of our Common Stock may
be volatile which could subject us to securities class action litigation and prevent you from being able to sell your shares at or above
the offering price.
The market price for our Common
Stock has been and may continue to be volatile and subject to wide fluctuations in response to factors including the following:
| |
· |
sales or potential sales of substantial amounts of our Common Stock, including sales required for us to regain and maintain compliance with Nasdaq’s continued listing requirements; |
| |
· |
delay or failure in initiating or completing pre-clinical or clinical trials or unsatisfactory results of these trials; |
| |
· |
announcements about us or about our competitors, including clinical trial results, regulatory approvals or new product introductions; |
| |
· |
developments concerning our licensors or product manufacturers; |
| |
· |
litigation and other developments relating to our patents or other proprietary rights or those of our competitors; |
| |
· |
conditions in the pharmaceutical or biotechnology industries; |
| |
· |
governmental regulation and legislation; |
| |
· |
variations in our anticipated or actual operating results; |
| |
· |
change in securities analysts’ estimates of our performance, or our failure to meet analysts’ expectations; foreign currency values and fluctuations; and |
| |
· |
overall economic conditions. |
Many of these factors are
beyond our control. The stock markets in general, and the market for pharmaceutical and biotechnological companies in particular, have
historically experienced extreme price and volume fluctuations. These fluctuations often have been unrelated or disproportionate to the
operating performance of these companies. These broad market and industry factors could reduce the market price of our Common Stock, regardless
of our actual operating performance.
We have never paid and do not intend to
pay cash dividends on our Common Stock. As a result, capital appreciation, if any, will be your sole source of gain.
We have never paid cash dividends
on any of our capital stock and we currently intend to retain future earnings, if any, to fund the development and growth of our business.
Our Series B Preferred Stock carries a dividend rate of 9.0% per year, which is cumulative and continues to accrue on a daily basis whether
or not declared and whether or not we have assets legally available therefor. We may pay such dividends at our option either in cash or
in kind in additional shares of preferred stock. We do not expect to pay any dividends in cash and have paid accrued dividends in kind
in additional shares of preferred stock to date. In addition, the terms of future debt agreements may preclude us from paying dividends.
As a result, capital appreciation, if any, of our Common Stock will be your sole source of gain for the foreseeable future.
Provisions in our Charter, our amended and
restated by-laws and Delaware law might discourage, delay or prevent a change in control of our company or changes in our management and,
therefore, depress the trading price of our Common Stock.
Provisions of our Charter,
our amended and restated by-laws and Delaware law may have the effect of deterring unsolicited takeovers or delaying or preventing a change
in control of our company or changes in our management, including transactions in which our stockholders might otherwise receive a premium
for their shares over then current market prices. In addition, these provisions may limit the ability of stockholders to approve transactions
that they may deem to be in their best interests. These provisions include:
| |
· |
the inability of stockholders to call special meetings; |
| |
· |
the ability of our board of directors to designate the terms of and issue new series of preferred stock without stockholder approval, which could include the right to approve an acquisition or other change in our control or could be used to institute a rights plan, also known as a poison pill, that would work to dilute the stock ownership of a potential hostile acquirer, likely preventing acquisitions that have not been approved by our board of directors; |
| |
· |
advance notice required for any nomination or other business to be properly brought before an annual meeting of stockholders which requires notice to be delivered to our secretary not later than the close of business on the 90th day, nor earlier than the close of business on the 120th day prior to the first anniversary of the preceding year’s annual meeting, subject to certain exceptions; |
| |
· |
any vacancies on our board of directors that results from the death, disability, resignation, disqualification or removal of any director or from any other cause shall be filled solely by the affirmative vote of a majority of the total number of directors then in office, even if less than a quorum, or by a sole remaining director and shall not be filled by the stockholders; provided that a vacancy created by the removal of a director by the stockholders may be filled by the stockholders; and |
| |
|
|
| |
· |
forum selection provisions that state that unless we consent in writing to the selection of an alternative forum, (A) (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty owed by any current or former director, officer, other employee or stockholder of the Company to us or our stockholders, (iii) any action asserting a claim arising pursuant to any provision of the General Corporation Law of the State of Delaware (“DGCL”), the Charter or the by-laws as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware or (iv) any action asserting a claim governed by the internal affairs doctrine of the law of the State of Delaware shall, to the fullest extent permitted by law, be exclusively brought in the Court of Chancery of the State of Delaware or, if such court does not have subject matter jurisdiction thereof, the federal district court of the State of Delaware; and (B) the federal district courts of the United States shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act of 1933, as amended. |
In addition, Section 203 of
the DGCL prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder, generally
a person which together with its affiliates owns, or within the last three years, has owned 15% of our voting stock, for a period of three
years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved
in a prescribed manner.
The existence of the foregoing
provisions and anti-takeover measures could limit the price that investors might be willing to pay in the future for shares of our Common
Stock. They could also deter potential acquirers of our company, thereby reducing the likelihood that you could receive a premium for
your Common Stock in an acquisition.
If securities or industry analysts do not
publish research or reports about our business, if they adversely change their recommendations regarding our shares or if our results
of operations do not meet their expectations, our share price and trading volume could decline.
The trading market for our
shares is influenced by the research and reports that industry or securities analysts publish about us or our business. We do not have
any control over these analysts. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly,
we could lose visibility in the financial markets, which in turn could cause our share price or trading volume to decline. Moreover, if
one or more of the analysts who cover us downgrade our stock, or if our results of operations do not meet their expectations, our share
price could decline.
We currently have Series B Preferred Stock
and Series G Preferred Stock outstanding. Our certificate of incorporation authorizes our Board to create new series of preferred stock
without further approval by our stockholders, which could adversely affect the rights of the holders of our Common Stock.
Our Board has the authority
to fix and determine the relative rights and preferences of preferred stock. Our Board also has the authority to issue preferred stock
without further stockholder approval.
As of March 27, 2024, we have
approximately 514.96 shares of Series B Preferred Stock outstanding with a stated value of $7,700 per share, which are currently convertible
at the holder’s option at any time, together with any accrued but unpaid dividends thereon, into shares of Common Stock at a conversion
price of $32,340.00, subject to certain adjustments.
Our Series B Preferred Stock
gives its holders the preferred right to our assets upon liquidation and the right to receive dividend payments at 9.00% per annum before
dividends are distributed to the holders of Common Stock, among other things. The holders of the Series B Preferred Stock, voting as a
separate class, also have customary consent rights with respect to certain corporate actions, including the issuance of an increased number
of shares of Series B Preferred Stock, the establishment of any capital stock ranking senior to or on parity the Series B Preferred Stock
as to dividends or upon liquidation, the incurrence of indebtedness, and certain changes to our Charter or Bylaws including other actions.
As of March 27, 2024, we have
12,373.226 shares of Series G Preferred Stock, par value $0.0001, outstanding. If the Conversion proposal is approved by our stockholders,
each share of Series G Preferred Stock will automatically convert into 1,000 shares of Common Stock, subject to certain limitations, including
that a holder of Series A Preferred Stock is prohibited from converting shares of Series G Preferred Stock into shares of Common Stock
if, as a result of such conversion, such holder, together with its affiliates, would beneficially own more than a specified percentage
(to be established by the holder between 4.9% and 19.9%) of the total number of shares of Common Stock issued and outstanding immediately
after giving effect to such conversion. Alternatively, the Series G Preferred Stock is redeemable for cash at the option of the holder
thereof at any time following the date that is six months after the initial issuance of the Series G Preferred Stock (without regard to
the lack of obtaining the requisite stockholder approval to convert the Series G Preferred Stock into Common Stock), at a price per share
equal to the then-current fair value of the Series G Preferred Stock.
Holders of Series G Preferred
Stock are entitled to receive dividends equal to, on an as-if-converted basis, and in the same form as dividends actually paid on shares
of the Common Stock. Our Series G Preferred Stock does not have voting rights, except as required by law. The holders of the Series G
Preferred Stock, voting as a separate class, have customary consent rights with respect to certain corporate actions, including the issuance
of an increased number of shares of Series G Preferred Stock, the establishment of any capital stock, the incurrence of indebtedness,
and certain changes to our Charter or Bylaws including other actions. Additionally, the Company shall not prior to the earlier of stockholder
approval of the Conversion Proposal or the six-month anniversary of the Closing, without the affirmative vote of the holders of a majority
of the then-outstanding Series G Preferred Stock, consummate or enter into any agreement with respect to any fundamental transactions
or any stock sale to, or any merger, consolidation or other business combination of the Company with or into, another entity in which
the stockholders of the Company immediately before such transaction do not hold at least a majority of the capital stock of the Company
immediately after such transaction.
Our Board also created the
following series of preferred stock: (i) Series C Preferred Stock (“Series C Preferred Stock”), of which 75,000 shares
are authorized for issuance, none of which are currently outstanding; (ii) Series D Preferred Stock (“Series D Preferred Stock”),
of which 150 shares are authorized for issuance, none of which are currently outstanding; (iii) Series E Preferred Stock (“Series
E Preferred Stock”), of which 150 shares are authorized for issuance, none of which are currently outstanding; and (iv) Series
F Preferred Stock (“Series F Preferred Stock”), of which 7,000 shares are authorized for issuance, none of which are
currently outstanding. Pursuant to the Series B Exchange Right, we may be required to issue shares of Series C Preferred Stock in certain
circumstances.
Our obligations to the holders
of the Series B Preferred Stock, Series G Preferred Stock and any future holders of any additional series of preferred stock we may issue
could limit our ability to obtain additional financing or increase our borrowing costs, which could have an adverse effect on our financial
condition and hinder the accomplishment of our corporate goals.
In addition to the above-referenced
preferred stock, our Board could authorize the issuance of additional series of preferred stock with such rights preferential to the rights
of our Common Stock, including the issuance of a series of preferred stock that has greater voting power than our Common Stock or that
is convertible into our Common Stock, which could decrease the relative voting power of our Common Stock or result in dilution to our
existing stockholders.
As a result of the Series B Exchange Right
in the Certificate of Designations, Powers, Preferences and Rights of the Series B Preferred Stock (the “Series B Certificate of
Designations”), we may be required to issue additional securities to the investors who purchased shares of our Series B Preferred
Stock and related warrants to purchase shares of our Common Stock in a private placement in July 2020.
On July 16, 2020, we consummated
a private placement offering (the “Series B Private Placement”) in which we issued an aggregate of approximately 2,912.58
shares of Series B Preferred Stock, at a price of $7,700 per share, initially convertible into an aggregate of 693 shares of Common Stock
at $32,340 per share, together with warrants to purchase an aggregate of 346 shares of Common Stock at an exercise price of $35,700 per
share. The Series B Preferred Stock carries a cumulative dividend at a rate of 9.0% per annum, payable at our option either in cash or
in kind in additional shares of Series B Preferred Stock.
As a result of previous conversions
and exchanges, as of December 31, 2023, 514.96 shares of Series B Preferred Stock were outstanding, with an aggregate stated value of
approximately $4.0 million, plus accrued and unpaid dividends through such date of approximately $1.1 million, and a conversion price
of $32,340.00 per share.
Under the Series B Certificate
of Designations, in the event we effect any issuance of Common Stock or Common Stock equivalents for cash consideration, or a combination
of units thereof (a “Subsequent Financing”), each holder of the Series B Preferred Stock has the right to exchange
the stated value, plus accrued and unpaid dividends, of the Series B Preferred Stock for any securities issued in the Subsequent Financing,
in lieu of any cash subscription payments therefor (the “Series B Exchange Right”). As a result of sales of additional
shares of common stock made on November 30, 2021, at a price of $10,985.94 per share, pursuant to our At The Market Agreement dated May
26, 2021 (the “ATM Agreement”) (such price being the lowest price per share sold under the ATM Agreement to date),
as of March 27, 2024, we may be required to issue in aggregate up to 449 shares of common stock, with no warrants, to any holders of Series
B Preferred Stock who elect to exercise their Series B Exchange Right into shares of common stock. In any event, we anticipate that we
would convert any shares of Series C Preferred Stock to be issued pursuant to the Series B Exchange Right into underlying shares of common
stock immediately upon issuance.
In February 2022, we entered
into waiver agreements (the “February 2022 Waiver”) with certain holders of Series B Preferred Stock, pursuant to which
we agreed to pay a cash waiver fee equal to ten percent of the stated value of the shares of Series B Preferred Stock held by such holder
(other than holders who are company insiders who did not receive a cash waiver fee), and such holder agreed to irrevocably waive its Series
B Exchange Right with respect to any Subsequent Financing that occurs from and after the date of the Waiver until December 31, 2022. Effective
May 12, 2022, the holders of 81.3% of the outstanding shares of the Series B Preferred Stock permanently waived for themselves and all
other holders of the Series B Preferred Stock the Series B Exchange Right with respect to any Subsequent Financing occurring on or after
January 1, 2022 (the “Permanent Waiver”).
If the holders of our Series B Preferred Stock
exercise their Series B Exchange Rights, it will result in certain dilution to our stockholders, and would afford our stockholders a smaller
percentage interest in our voting power, liquidation value and aggregate book value. The sale or resale of the Common Stock issued upon
conversion of the preferred stock could cause the market price of our Common Stock to decline. In addition, the issuance of Common Stock
upon the exercise of the Investor Warrants will result in similar dilution to our stockholders. This dilution, or the possibility that
it may occur, may make it more difficult for us to sell equity securities in the future at a time and a price that we deem appropriate.
UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL INFORMATION
The following unaudited
pro forma combined financial information has been prepared in accordance with Article 11 of Regulation S-X under the Securities Act and
presents the combined historical financial position and results of operations of Entero and the historical financial position and results
of operations of ImmunogenX, adjusted to give effect to (i) the March 13, 2024 (“Closing Date”) acquisition of ImmunogenX
as further described below (the “Transaction”) and (ii) the pro forma effects of certain assumptions and adjustments described
in “Notes to the Pro Forma Combined Financial Information” below.
The following unaudited
pro forma combined financial information is presented to illustrate the estimated effects of the Transaction, based on the historical
financial statements and accounting records of Entero and ImmunogenX after giving effect to these transactions and the related pro forma
adjustments as described in the notes included below.
The unaudited pro forma
combined balance sheet as of December 31, 2023 gives effect to the Transaction as if it took place on December 31, 2023 and combines
the historical balance sheets of Entero and ImmunogenX as of such date. The unaudited pro forma combined statement of operations for
the year ended December 31, 2023, combines the historical statements of operations of Entero and ImmunogenX, giving effect to the Transaction
as if it had occurred on January 1, 2023.
The unaudited pro forma
combined statement of operations for the three-months ended March 31, 2024, combines the historical statements of operations of Entero
and ImmunogenX, giving effect to the Transaction as if it had occurred on January 1, 2024.
Entero was preliminarily
determined to be the accounting acquirer based upon the terms of the Transaction and other factors including Entero’s security
holders retaining voting control. The historical financial statements of Entero and ImmunogenX have been adjusted to give pro forma effect
to events that are (1) directly attributable to the Transaction, (2) factually supportable, and (3) with respect to the unaudited pro
forma combined statements of operations, expected to have a continuing impact on the combined results of operations of the combined company.
The unaudited pro forma combined financial statements should be read in conjunction with the accompanying notes to the unaudited pro
forma combined financial statements.
The unaudited pro forma
combined financial information, including the notes thereto, should be read in conjunction with the separate Entero and ImmunogenX historical
financial statements referenced or included as Annexes to this proxy statement.
These unaudited pro forma
combined financial statements are for informational purposes only. They do not purport to indicate the results that would have been obtained
had the Business Combination and related transactions actually been completed on the assumed date or for the period presented, or which
may be realized in the future. The pro forma adjustments are based on the information currently available and the assumptions and estimates
underlying the pro forma adjustments are described in the accompanying notes. Actual results may differ materially from the assumptions
within the accompanying unaudited pro forma condensed consolidated combined financial information.
Description of the Business Combination
On March 13, 2024,
Entero acquired ImmunogenX in accordance with the Merger Agreement, by and among the Company, First Merger Sub, Second Merger Sub,
and ImmunogenX. Pursuant to the Merger Agreement, First Merger Sub merged with and into ImmunogenX, pursuant to which ImmunogenX was
the surviving corporation. Immediately following the First Merger, ImmunogenX merged with and into Second Merger Sub, pursuant to
which Second Merger Sub was the surviving entity and a wholly owned subsidiary of the Company. The Second Merger Sub amended its
certificate of formation concurrently with the Second Merger to change its company name to ImmunogenX, LLC. The Merger is intended
to qualify as a tax-free reorganization for U.S. federal income tax purposes.
Under the terms of the
Merger Agreement, upon the Closing, in exchange for the outstanding shares of capital stock of ImmunogenX immediately prior to the effective
time of the First Merger, the Company issued to the stockholders of ImmunogenX an aggregate of (A) 36,830 shares of common stock of the
Company, par value $0.0001 per share, and (B) 11,777.418 shares of Series G Preferred Stock (as defined and described below), each share
of which is convertible into 1,000 shares of Common Stock, subject to certain conditions described below. In addition, the Company assumed
(i) all ImmunogenX stock options immediately outstanding prior to the First Merger, each becoming an option to purchase Common Stock
subject to adjustment pursuant to the terms of the Merger Agreement and (ii) all ImmunogenX warrants immediately outstanding prior to
the First Merger, each becoming a warrant to purchase Common Stock subject to adjustment pursuant to the terms of the Merger Agreement.
The Assumed Options are exercisable for an aggregate of 200,652 shares of Common Stock, have an exercise price of $0.81 and expire between
February 1, 2031 and June 6, 2033. The Assumed Warrants are exercisable for and aggregate of 127,680 shares of Common Stock, have exercise
prices ranging from $3.02 to $3.92 and expire between September 30, 2032 and September 6, 2033.
Pursuant to the Merger
Agreement, the Company has agreed to hold a stockholders’ meeting to submit the following matters to its stockholders for their
consideration: (i) the approval of the Conversion Proposal and (ii) if deemed necessary or appropriate by the Company or as otherwise
required by applicable law or contract, the approval of an amendment to the Company’s Charter, to authorize sufficient shares of
Common Stock for the conversion of Series G Preferred Stock issued pursuant to the Merger Agreement.
Unaudited Pro Forma Condensed
Combined Statement of Operations
The following unaudited pro forma combined financial
information has been prepared in accordance with Article 11 of Regulation S-X under the Securities Act of 1933, as amended (the “Securities
Act”) and presents the combined historical results of operations of Entero Therapeutics, Inc. (formerly known as First Wave BioPharma,
Inc., “Entero” or the “Company”) and the historical results of operations of ImmunogenX, Inc. (“ImmunogenX”),
adjusted to give effect to (i) the March 13, 2024 (“Closing Date”) acquisition of ImmunogenX as further described below (the
“Transaction”) and (ii) the pro forma effects of certain assumptions and adjustments described in “Notes to the Pro
Forma Combined Financial Information” below.
The following unaudited pro forma combined financial
information is presented to illustrate the estimated effects of the Transaction, based on the historical financial statements and accounting
records of Entero and ImmunogenX after giving effect to these transactions and the related pro forma adjustments as described in the notes
included below.
The unaudited pro forma combined statement of operations for the three-months
ended March 31, 2024, combines the historical statements of operations of Entero and ImmunogenX, giving effect to the Transaction as if
it had occurred on January 1, 2024.
Entero was preliminarily determined to be the accounting acquirer based
upon the terms of the Transaction and other factors including Entero’s security holders retaining voting control. The historical
financial statements of Entero and ImmunogenX have been adjusted to give pro forma effect to events that are (1) directly attributable
to the Transaction, (2) factually supportable, and (3) with respect to the unaudited pro forma combined statements of operations, expected
to have a continuing impact on the combined results of operations of the combined company. The unaudited pro forma combined financial
statements should be read in conjunction with the accompanying notes to the unaudited pro forma combined financial statements.
The unaudited pro forma combined financial information, including the
notes thereto, should be read in conjunction with the separate Entero and ImmunogenX historical financial statements referenced or included
as exhibits to Form 8-K/A issued on May 8, 2024.
These unaudited pro forma combined financial statements
are for informational purposes only. They do not purport to indicate the results that would have been obtained had the Business Combination
and related transactions actually been completed on the assumed date or for the period presented, or which may be realized in the future.
The pro forma adjustments are based on the information currently available and the assumptions and estimates underlying the pro forma
adjustments are described in the accompanying notes. Actual results may differ materially from the assumptions within the accompanying
unaudited pro forma condensed consolidated combined financial information.
Description of the Business Combination
On March 13, 2024, Entero acquired ImmunogenX
in accordance with the terms of an Agreement and Plan of Merger (the “Merger Agreement”), by and among the Company, IMMUNO
Merger Sub I, Inc., a Delaware corporation (“First Merger Sub”), IMMUNO Merger Sub II, LLC, a Delaware limited liability company
(“Second Merger Sub”), and ImmunogenX. Pursuant to the Merger Agreement, First Merger Sub merged with and into ImmunogenX,
pursuant to which ImmunogenX was the surviving corporation (the “First Merger”). Immediately following the First Merger, ImmunogenX
merged with and into Second Merger Sub, pursuant to which Second Merger Sub was the surviving entity and a wholly owned subsidiary of
the Company (the “Second Merger” and together with the First Merger, the “Merger”). The Merger is intended to
qualify as a tax-free reorganization for U.S. federal income tax purposes.
Under the terms of the Merger Agreement, upon
the consummation of the Merger on March 13, 2024 (the “Closing”), in exchange for the outstanding shares of capital stock
of ImmunogenX immediately prior to the effective time of the First Merger, the Company issued to the stockholders of ImmunogenX an aggregate
of (A) 36,830 shares of common stock of the Company, par value $0.0001 per share (the “Common Stock”), and (B) 11,777.418
shares of Series G Preferred Stock (as defined and described below), each share of which is convertible into 1,000 shares of Common Stock,
subject to certain conditions described below. In addition, the Company assumed (i) all ImmunogenX stock options immediately outstanding
prior to the First Merger, each becoming an option to purchase Common Stock subject to adjustment pursuant to the terms of the Merger
Agreement (the “Assumed Options”) and (ii) all ImmunogenX warrants immediately outstanding prior to the First Merger, each
becoming a warrant to purchase Common Stock subject to adjustment pursuant to the terms of the Merger Agreement (the “Assumed Warrants”).
The Assumed Options are exercisable for an aggregate of 200,652 shares of Common Stock, have an exercise price of $0.81 and expire between
February 1, 2031 and June 6, 2033. The Assumed Warrants are exercisable for and aggregate of 127,680 shares of Common Stock, have exercise
prices ranging from $3.02 to $3.92 and expire between September 30, 2032 and September 6, 2033.
Pursuant to the Merger Agreement, the Company
has agreed to hold a stockholders’ meeting to submit the following matters to its stockholders for their consideration: (i) the
approval of the conversion of shares of Series G Preferred Stock into shares of Common Stock in accordance with the rules of the Nasdaq
Stock Market LLC (the “Conversion Proposal”) and (ii) if deemed necessary or appropriate by the Company or as otherwise required
by applicable law or contract, the approval of an amendment to the Company’s certificate of incorporation, as amended (the “Charter”),
to authorize sufficient shares of Common Stock for the conversion of Series G Preferred Stock issued pursuant to the Merger Agreement
(the “Share Increase Proposal” and together with the Conversion Proposal, the “Meeting Proposals”).
UNAUDITED PRO FORMA COMBINED
STATEMENT OF OPERATIONS
FOR THE THREE-MONTHS
ENDED MARCH 31, 2024
| | |
Entero
formerly
known as First
Wave BioPharma,
Inc. | | |
ImmunogenX
1/1/24 -
3/13/24 | | |
Transaction
Accounting
Adjustments | | |
Notes | | |
Pro Forma
Combined | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | | |
| | |
| General and administrative | |
$ | 8,659,692 | | |
$ | 943,828 | | |
$ | — | | |
| | | |
$ | 9,603,520 | |
| Research and development | |
| 565,962 | | |
| 669,624 | | |
| — | | |
| | | |
| 1,235,586 | |
| Total operating expenses | |
| 9,225,654 | | |
| 1,613,452 | | |
| — | | |
| | | |
| 10,839,106 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | | |
| | | |
| | | |
| | |
| Interest expense | |
| (66,202 | ) | |
| (690,634 | ) | |
| — | | |
| | | |
| (756,836 | ) |
| Interest income | |
| 228 | | |
| — | | |
| — | | |
| | | |
| 228 | |
| Other income, net | |
| — | | |
| 617,397 | | |
| — | | |
| | | |
| 617,397 | |
| Other expense | |
| (653 | ) | |
| — | | |
| (21,667 | ) | |
| A | | |
| (22,320 | ) |
| Change in fair value of convertible notes | |
| — | | |
| (5,687,225 | ) | |
| — | | |
| | | |
| (5,687,225 | ) |
| Total other expense, net | |
| (66,627 | ) | |
| (5,760,462 | ) | |
| (21,667 | ) | |
| | | |
| (5,848,756 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss before benefit for income taxes | |
| (9,292,281 | ) | |
| (7,373,914 | ) | |
| (21,667 | ) | |
| | | |
| (16,687,862 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Benefit for state income taxes | |
| 14,859,887 | | |
| — | | |
| — | | |
| | | |
| 14,859,887 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net income (loss) | |
| 5,567,606 | | |
| (7,373,914 | ) | |
| (21,667 | ) | |
| | | |
| (1,827,975 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Preferred stock dividends | |
| (66,144 | ) | |
| — | | |
| — | | |
| | | |
| (66,144 | ) |
| Net income (loss) applicable to common shareholders | |
$ | 5,501,462 | | |
$ | (7,373,914 | ) | |
$ | (21,667 | ) | |
| | | |
$ | (1,894,119 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Basic weighted average shares outstanding | |
| 1,761,953 | | |
| 1,921,897 | | |
| | | |
| | | |
| 1,761,953 | |
| Diluted weighted average shares outstanding | |
| 14,511,461 | | |
| 1,921,897 | | |
| | | |
| B | | |
| 1,761,953 | |
| Income (loss) per share - basic | |
$ | 3.12 | | |
$ | (3.84 | ) | |
| | | |
| | | |
$ | (1.08 | ) |
| Income (loss) per share - diluted | |
$ | 0.38 | | |
$ | (3.84 | ) | |
| | | |
| | | |
$ | (1.08 | ) |
NOTES TO UNAUDITED PRO FORMA COMBINED FINANCIAL INFORMATION
Note 1. Basis of Presentation
Entero’s unaudited historical financial
information has been derived from its Quarterly Report on Form 10-Q for the three-months ended March 31, 2024. Such unaudited historical
financial information includes the results of ImmunogenX operations from the acquisition date of March 13, 2024 through March 31, 2024.
ImmunogenX results for the period from January 1, 2024 through March 13, 2024 were based on the unaudited historical statement of operations
for that period. Pro forma adjustments have been made to reflect the Transaction and certain transaction accounting adjustments, as discussed
further in Note 2—Pro Forma Adjustments and Assumptions. The pro forma statement for the three-months ended March 31, 2024 gives
pro forma effect to the Transaction as if it had occurred on January 1, 2024, the beginning of the earliest period presented.
The unaudited pro forma combined financial information
was prepared using the acquisition method of accounting and is based on the historical financial statements of Entero and ImmunogenX.
The acquisition method of accounting is based on Accounting Standards Codification (“ASC”) 805, Business Combinations, with
the Company as the accounting acquirer, and uses the fair value concepts defined in ASC 820, Fair Value Measurement. For additional information
about the Transaction and the accounting methodology used, refer to the Current Report on Form 8-K/A issued on May 8, 2024.
In the opinion of management, all material adjustments have been made
that are necessary to present fairly, in accordance with Article 11 of Regulation S-X, the pro forma financial statement. The pro forma
financial statement is provided for illustrative purposes only and does not purport to be indicative of what Entero’s actual results
of operations would have been on a consolidated basis if the Transaction had occurred on the dates indicated, nor is it indicative of
the future results of operations or financial position.
The pro forma basic and diluted earnings per share amounts presented
in the unaudited pro forma statement are based on the weighted average number of Entero’s common shares outstanding, assuming the
Transaction occurred on January 1, 2024.
Note 2. Pro Forma Adjustments
The unaudited pro forma combined financial information
includes pro forma adjustments that are (1) directly attributable to the Transaction (2) factually supportable, and (3) with respect to
the unaudited pro forma combined statements of operations, expected to have a continuing impact on the results of operations of the combined
company.
The pro forma adjustments reflecting the completion
of the transaction are based upon the accounting analysis conclusion that the Transaction should be accounted for under the acquisition
method of accounting and upon the assumptions set forth below.
The pro forma adjustments, based on preliminary
estimates that may change significantly as additional information is obtained, are as follows:
| A. | Reflects amortization expense for patents and trade names/trademarks
with useful lives of two and six years, respectively. |
| B. | Anti-dilutive common share equivalents excluded from the computation
of diluted net loss per share at March 31, 2024 consisted of 118,084 warrants, 169,536 stock options, 88,523 restricted stock units,
12,373 Series G Convertible Preferred shares convertible into 12,373,226 common shares, and 139 Series B Convertible Preferred shares. |
UNAUDITED PRO FORMA COMBINED
BALANCE SHEET
AS OF DECEMBER 31, 2023
| |
|
Entero
(Historical) |
|
|
ImmunogenX
(Historical) |
|
|
Transaction
Accounting
Adjustments |
|
|
Notes |
|
Pro
Forma
Combined |
|
| ASSETS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Current
assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash
and cash equivalents |
|
$ |
3,711,770 |
|
|
$ |
251,266 |
|
|
$ |
— |
|
|
|
|
$ |
3,963,036 |
|
| Accounts
receivable |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
| Prepaid
expenses and other current assets |
|
|
1,244,466 |
|
|
|
3,068,344 |
|
|
|
— |
|
|
|
|
|
4,312,810 |
|
| Total
current assets: |
|
|
4,956,236 |
|
|
|
3,319,610 |
|
|
|
— |
|
|
|
|
|
8,275,846 |
|
| Property,
equipment, and leasehold improvements, net |
|
|
14,565 |
|
|
|
20,853 |
|
|
|
— |
|
|
|
|
|
35,418 |
|
| Restricted
cash |
|
|
21,522 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
21,522 |
|
| Operating
lease right-of-use asset, net |
|
|
195,440 |
|
|
|
10,704 |
|
|
|
— |
|
|
|
|
|
206,144 |
|
| Patents,
net |
|
|
— |
|
|
|
668,451 |
|
|
|
(528,451 |
) |
|
I |
|
|
140,000 |
|
| In
process R&D assets |
|
|
— |
|
|
|
— |
|
|
|
63,000,000 |
|
|
I |
|
|
63,000,000 |
|
| Other
intangible assets |
|
|
— |
|
|
|
19,200 |
|
|
|
210,800 |
|
|
I |
|
|
230,000 |
|
| Goodwill |
|
|
1,684,182 |
|
|
|
— |
|
|
|
17,962,939 |
|
|
C |
|
|
19,647,121 |
|
| Deposits |
|
|
11,250 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
11,250 |
|
| Other
assets |
|
|
— |
|
|
|
63,558 |
|
|
|
(17,000 |
) |
|
I |
|
|
46,558 |
|
| Total
assets |
|
$ |
6,883,195 |
|
|
$ |
4,102,376 |
|
|
$ |
80,628,288 |
|
|
|
|
$ |
91,613,859 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| LIABILITIES
AND STOCKHOLDERS' DEFICIT |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Current
liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accounts
payable |
|
$ |
554,277 |
|
|
$ |
399,283 |
|
|
$ |
— |
|
|
|
|
$ |
953,560 |
|
| Accrued
expenses and other current liabilities |
|
|
825,290 |
|
|
|
2,008,888 |
|
|
|
1,444,763 |
|
|
A |
|
|
4,278,941 |
|
| Accrued
dividend payable |
|
|
1,069,616 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
1,069,616 |
|
| Current
portion of operating lease liability |
|
|
67,111 |
|
|
|
9,129 |
|
|
|
— |
|
|
|
|
|
76,240 |
|
| Short-term
note payable |
|
|
612,784 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
612,784 |
|
| Related
party promissory note |
|
|
— |
|
|
|
393,282 |
|
|
|
— |
|
|
|
|
|
393,282 |
|
| Revolving
line of credit |
|
|
— |
|
|
|
6,360,000 |
|
|
|
— |
|
|
|
|
|
6,360,000 |
|
| Other
current liabilities |
|
|
4,239 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
4,239 |
|
| Total
current liabilities |
|
|
3,133,317 |
|
|
|
9,170,582 |
|
|
|
1,444,763 |
|
|
|
|
|
13,748,662 |
|
| Non-current
operating lease liabilities |
|
|
146,949 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
146,949 |
|
| Deferred
tax liability |
|
|
— |
|
|
|
— |
|
|
|
15,431,108 |
|
|
I |
|
|
571,221 |
|
| |
|
|
|
|
|
|
|
|
|
|
(14,859,887 |
) |
|
L |
|
|
|
|
| Convertible
notes |
|
|
— |
|
|
|
8,663,804 |
|
|
|
(8,663,804 |
) |
|
D |
|
|
— |
|
| Note
payable |
|
|
— |
|
|
|
500,000 |
|
|
|
(462,000 |
) |
|
I |
|
|
38,000 |
|
| Total
liabilities |
|
|
3,280,266 |
|
|
|
18,334,386 |
|
|
|
(7,109,820 |
) |
|
|
|
|
14,504,832 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Temporary
equity: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Series
G convertible preferred stock |
|
|
— |
|
|
|
— |
|
|
|
57,790,474 |
|
|
E |
|
|
57,790,474 |
|
| |
|
|
— |
|
|
|
— |
|
|
|
3,890,626 |
|
|
B |
|
|
3,890,626 |
|
| Total
temporary equity |
|
|
— |
|
|
|
— |
|
|
|
61,681,100 |
|
|
|
|
|
61,681,100 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stockholders'
deficit: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Preferred
stock |
|
|
— |
|
|
|
226 |
|
|
|
(226 |
) |
|
F |
|
|
— |
|
| Common
stock |
|
|
156 |
|
|
|
101 |
|
|
|
(101 |
) |
|
F |
|
|
162 |
|
| |
|
|
|
|
|
|
|
|
|
|
4 |
|
|
G |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
2 |
|
|
B |
|
|
|
|
| Additional
paid-in capital |
|
|
187,931,445 |
|
|
|
4,608,364 |
|
|
|
(4,608,364 |
) |
|
F |
|
|
190,352,587 |
|
| |
|
|
|
|
|
|
|
|
|
|
240,496 |
|
|
G |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
2,060,000 |
|
|
H |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
120,646 |
|
|
B |
|
|
|
|
| Accumulated
deficit |
|
|
(184,328,672 |
) |
|
|
(18,840,701 |
) |
|
|
18,840,701 |
|
|
F |
|
|
(174,924,823 |
) |
| |
|
|
|
|
|
|
|
|
|
|
(1,444,763 |
) |
|
A |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
(4,011,275 |
) |
|
B |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
14,859,887 |
|
|
L |
|
|
|
|
| Total
stockholders' deficit |
|
|
3,602,929 |
|
|
|
(14,232,010 |
) |
|
|
26,057,008 |
|
|
|
|
|
15,427,927 |
|
| Total
liabilities, temporary equity and stockholders' deficit |
|
$ |
6,883,195 |
|
|
$ |
4,102,376 |
|
|
$ |
80,628,288 |
|
|
|
|
$ |
91,613,859 |
|
See accompanying notes to the unaudited pro forma
combined financial statements.
UNAUDITED PRO FORMA COMBINED
STATEMENTS OF OPERATIONS
FOR THE YEAR ENDED DECEMBER
31, 2023
| |
|
Entero
(Historical) |
|
|
ImmunogenX
(Historical) |
|
|
Transaction
Accounting
Adjustments |
|
|
Notes |
|
Pro
Forma
Combined |
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| General
and administrative |
|
$ |
10,737,609 |
|
|
$ |
2,308,851 |
|
|
$ |
1,444,763 |
|
|
A |
|
$ |
14,491,223 |
|
| |
|
|
|
|
|
|
|
|
|
|
4,011,275 |
|
|
B |
|
|
4,011,275 |
|
| Research
and development |
|
|
5,033,218 |
|
|
|
2,828,531 |
|
|
|
— |
|
|
|
|
|
7,861,749 |
|
| Total operating expenses
(recovery) |
|
|
15,770,827 |
|
|
|
5,137,382 |
|
|
|
5,456,038 |
|
|
|
|
|
26,364,247 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Grant
income |
|
|
— |
|
|
|
910,577 |
|
|
|
— |
|
|
|
|
|
910,577 |
|
| Change
in fair value of convertible notes |
|
|
— |
|
|
|
(3,615,230 |
) |
|
|
— |
|
|
|
|
|
(3,615,230 |
) |
| Interest
expense |
|
|
(22,463 |
) |
|
|
(1,083,056 |
) |
|
|
— |
|
|
|
|
|
(1,105,519 |
) |
| Interest
income |
|
|
2,531 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
2,531 |
|
| Other
income, net |
|
|
— |
|
|
|
67,886 |
|
|
|
— |
|
|
|
|
|
67,886 |
|
| Other
expense |
|
|
(4,224 |
) |
|
|
— |
|
|
|
(108,333 |
) |
|
J |
|
|
(112,557 |
) |
| Total other income
(expense), net |
|
|
(24,156 |
) |
|
|
(3,719,823 |
) |
|
|
(108,333 |
) |
|
|
|
|
(3,852,312 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Loss before provision (benefit) for income taxes |
|
|
(15,794,983 |
) |
|
|
(8,857,205 |
) |
|
|
(5,564,371 |
) |
|
|
|
|
(30,216,559 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Provision (benefit)
for state income taxes |
|
|
— |
|
|
|
791 |
|
|
|
(14,859,887 |
) |
|
L |
|
|
(14,859,096 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
|
(15,794,983 |
) |
|
|
(8,857,996 |
) |
|
|
9,295,516 |
|
|
|
|
|
(15,357,463 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Preferred stock dividends |
|
|
(308,128 |
) |
|
|
— |
|
|
|
— |
|
|
|
|
|
(308,128 |
) |
| Net loss applicable
to common shareholders |
|
$ |
(16,103,111 |
) |
|
$ |
(8,857,996 |
) |
|
$ |
9,295,516 |
|
|
|
|
$ |
(15,665,591 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Basic weighted average shares outstanding |
|
|
336,342 |
|
|
|
1,007,696 |
|
|
|
|
|
|
|
|
|
391,648 |
|
| Diluted weighted average shares outstanding |
|
|
336,342 |
|
|
|
1,007,696 |
|
|
|
|
|
|
K |
|
|
391,648 |
|
| Net (loss) income per share - basic |
|
$ |
(47.88 |
) |
|
$ |
(8.79 |
) |
|
|
|
|
|
|
|
$ |
(40.00 |
) |
| Net (loss) income per share - diluted |
|
$ |
(47.88 |
) |
|
$ |
(8.79 |
) |
|
|
|
|
|
|
|
$ |
(40.00 |
) |
See accompanying notes to the unaudited pro forma
combined financial statements.
NOTES TO THE UNAUDITED
PRO FORMA CONSOLIDATED
FINANCIAL INFORMATION
Note 1. Basis of Presentation
The unaudited pro forma
combined financial information was prepared using the acquisition method of accounting and is based on the historical financial statements
of Entero and ImmunogenX. The acquisition method of accounting is based on Accounting Standards Codification (“ASC”) 805,
Business Combinations, with the Company as the accounting acquirer, and uses the fair value concepts defined in ASC 820, Fair Value Measurement.
ASC 805 requires, among other
things, that most assets acquired and liabilities assumed be recognized at their fair values as of the acquisition date. In addition,
ASC 805 requires that the consideration transferred be measured at the date the acquisition is completed at the then-current market price.
ASC 820 defines the term
“fair value,” sets forth the valuation requirements for any asset or liability measured at fair value, expands related disclosure
requirements and specifies a hierarchy of valuation techniques based on the nature of the inputs used to develop the fair value measures.
Fair value is defined in ASC 820 as “the price that would be received to sell an asset or paid to transfer a liability in an orderly
transaction between market participants at the measurement date.” This is an exit price concept for the valuation of the asset
or liability. In addition, market participants are assumed to be buyers and sellers in the principal (or the most advantageous) market
for the asset or liability. Fair value measurements for an asset assume the highest and best use by these market participants. As a result
of these standards, Entero may be required to record the fair value of assets which are not intended to be used or sold and/or to value
assets at fair value measures that do not reflect Entero’s intended use of those assets. Many of these fair value measurements can be highly
subjective, and it is possible that other professionals, applying reasonable judgment to the same facts and circumstances, could develop
and support a range of alternative estimated amounts.
Under the acquisition
method of accounting, the assets acquired and liabilities assumed are recorded, as of the completion of the acquisition, primarily at
their respective fair values, with the excess of the purchase consideration over the fair value of ImmunogenX’s net assets, allocated
to goodwill, if any, and added to those of Entero. Financial statements and reported results of operations of Entero issued after completion
of the acquisition will reflect these values and will not be retroactively restated to reflect the historical financial position or results
of operations of ImmunogenX. The pro forma allocation of the purchase price reflected in the unaudited pro forma combined financial information
is preliminary and thus subject to adjustment and may vary materially from the final purchase price allocation that will be completed
within the measurement period, but in no event later than one year following the Closing Date.
Under ASC 805, acquisition-related
transaction costs (e.g., advisory, legal and other professional fees) are not included as a component of consideration transferred but
are accounted for as expenses in the periods in which such costs are incurred. Total acquisition-related transaction costs expected to
be incurred by Entero are estimated to be $5,456,038 (including amounts paid in cash and amounts paid through the issuance of Series
G preferred stock and common stock) and incurred after December 31, 2023. These acquisition related transaction costs are reflected as
a pro forma adjustment to the unaudited pro forma combined statements of operations.
The unaudited pro forma combined
financial statements do not include any adjustments to the realization of any costs (or cost savings) from operating efficiencies, synergies,
or other restructuring activities that might result from the Transaction. The pro forma adjustments represent management’s best
estimates and are based upon currently available information and certain assumptions that the Company believes are reasonable under the
circumstances.
The unaudited pro forma combined
financial information is presented for informational purposes only and does not necessarily indicate the financial results of the combined
company had the companies been combined at the beginning of the period presented, nor does it necessarily indicate the results of operations
in future periods or the future financial position of the combined company.
Note 2. Accounting Policies and Reclassifications
Upon consummation of the Transaction,
the Company performed a comprehensive review of the two entities’ accounting policies. Based on its analysis, the Company did not
identify any differences that would have a material impact on the unaudited pro forma combined financial information. As a result, the
unaudited pro forma combined financial information does not assume any differences in accounting policies.
As part of the preparation
of these unaudited pro forma combined financial statements, certain reclassifications were made to align ImmunogenX’s financial
statement presentation with that of Entero.
Accounting for Stock Option and Warrants
Conversion
The Company accounts for
stock-based compensation arrangements with employees and non-employee consultants using a fair value method which requires the recognition
of compensation expense for costs related to all stock-based payments, including stock options. As of the Closing Date, each ImmunogenX
option prior to the business combination that is then outstanding will be converted into an option to purchase shares of Entero common
stock upon substantially the same terms and conditions as are in effect with respect to such option immediately prior to the Closing
Date, subject to specific terms and conditions. Depending on the fair value measurement of the replacement awards and vesting conditions,
either all or a portion of the fair value-based measure of the replacement awards will be included in measuring the consideration transferred
in the asset acquisition. As the fair value measurement of the replacement awards is the same as compared to the historical awards, the
fair value of the awards will be included in the consideration.
Note 3. Consideration Transferred
The preliminary fair value of the consideration
totaled $60,090,974, summarized as follows:
| | |
Shares | | |
Amount | |
| Common Stock of Entero issued to ImmunogenX
shareholders | |
| 36,830 | | |
$ | 240,500 | |
| Series G Preferred Stock of Entero issued to ImmunogenX
shareholders | |
| 11,777 | | |
| 57,790,474 | |
| ImmunogenX stock options and warrants
allocated to total consideration paid | |
| | | |
| 2,060,000 | |
| Total consideration | |
| | | |
$ | 60,090,974 | |
Note 4. Preliminary Estimates of Assets Acquired and Liabilities
Assumed
The Company recorded the assets
acquired and liabilities assumed as of the date of the acquisition based on the information available at that date. The following table
presents the preliminary allocation of the total consideration paid to the estimated fair values of the assets acquired and liabilities
assumed as of the acquisition date, and includes a reconciliation to the total consideration transferred:
| | |
Amount | |
| Assets acquired | |
| | |
| Cash and cash equivalents | |
| 88,000 | |
| Prepaid expenses and other current assets | |
| 3,132,000 | |
| Property, plant and equipment, net | |
| 19,000 | |
| Other long term assets | |
| 4,000 | |
| Patents | |
| 140,000 | |
| Trade names and trademarks | |
| 230,000 | |
| In process research and development assets | |
| 63,000,000 | |
| Goodwill | |
| 18,351,108 | |
| Total assets acquired | |
| 84,964,108 | |
| | |
| | |
| Liabilities assumed | |
| | |
| Accounts payable | |
| 916,000 | |
| Accrued expenses and other current liabilities | |
| 855,000 | |
| Deferred tax liability | |
| 15,431,108 | |
| Short term debt | |
| 7,633,000 | |
| Long term debt | |
| 38,000 | |
| Total liabilities assumed | |
| 24,873,108 | |
| | |
| | |
| Net assets acquired | |
$ | 60,091,000 | |
The above allocation of the
purchase price is based upon certain valuations and other analyses that have not been completed as of the date of this filing. Any changes
in the estimated fair values of the net assets recorded for this business combination upon the finalization of more detailed analyses
of the facts and circumstances that existed at the date of the Transaction will change the allocation of the purchase price. As such,
the purchase price allocations for the acquisition are preliminary estimates, which are subject to change within the measurement period.
As of the completion of the
acquisition, identifiable intangible assets are required to be measured at fair value, and these acquired assets could include assets
that are not intended to be used or sold or that are intended to be used in a manner other than their highest and best use. For purposes
of these unaudited pro forma combined financial statements and consistent with the ASC 820 requirements for fair value measurements, it
is assumed that all acquired assets will be used, and that all acquired assets will be used in a manner that represents the highest and
best use of those acquired assets.
The goodwill recorded related
to the acquisition is the excess of the fair value of the consideration transferred by the acquirer over the fair value of the net identifiable
assets acquired and liabilities assumed at the date of acquisition. The goodwill recorded is not deductible for tax purposes.
Note 5. Pro Forma Adjustments
The unaudited pro forma combined
financial information includes pro forma adjustments that are (1) directly attributable to the Transaction (2) factually supportable,
and (3) with respect to the unaudited pro forma combined statements of operations, expected to have a continuing impact on the results
of operations of the combined company.
The pro forma adjustments
reflecting the completion of the transaction are based upon the accounting analysis conclusion that the Transaction should be accounted
for under the acquisition method of accounting and upon the assumptions set forth below.
The pro forma adjustments,
based on preliminary estimates that may change significantly as additional information is obtained, are as follows:
| |
A. |
Reflects the accrual
of Entero transaction costs of $1,444,763 to be paid in cash upon consummation of the Transaction. |
| |
B. |
Reflects Entero Acquisition
Transaction advisory fee of $4,011,275 that was paid in the form of common and preferred stock. |
| |
C. |
Reflects the excess consideration paid to acquire ImmunogenX over the net assets acquired. |
| |
D. |
Reflects the conversion of the ImmunogenX convertible note into common shares of ImmunogenX immediately prior to the Transaction. |
| |
E. |
Issuance of 11,777 shares of Series G convertible preferred stock upon consummation of the Transaction. |
| |
F. |
Elimination of ImmunogenX historical equity carrying values. |
| |
G. |
Issuance of 36,830 shares of common stock upon consummation of the Transaction. |
| |
H. |
Issuance of 127,680 replacement warrants with a fair value of $789,000 and 200,652 options with a fair value of $1,271,000 included in the consideration transferred. |
| |
I. |
Reflects application of purchase accounting under the acquisition method. |
| |
J. |
Reflects amortization expense for patents and trade names/trademarks with useful lives of two and fifteen years, respectively. |
| |
K. |
Anti-dilutive common share equivalents excluded from the computation of diluted net loss per share at December 31, 2023 consisted of 127,680 warrants, 200,652 stock options, and 11,777 Series G Convertible Preferred shares convertible into 11,777,418 common shares. |
| |
L. |
Reflects reversal of the valuation allowance and recognition of an income tax benefit as a result of the transaction. |
THE ANNUAL MEETING
This proxy statement
is being provided to Entero stockholders in connection with the solicitation of proxies by the Entero Board for use at the Annual
Meeting and at any adjournments or postponements thereof. Entero stockholders are encouraged to read this entire document carefully,
including its annexes and the documents incorporated by reference herein, for more detailed information regarding the share exchange
agreement and the transactions contemplated thereby.
Date, Time and Place of the Annual Meeting
The
Annual Meeting is scheduled to be held on [●], 2024, beginning at [●] a.m., Eastern Time.
The
Annual Meeting will be held at our corporate headquarters, located at 777 Yamato Rd., Suite 502, Boca Raton, FL 33431.
Voting
The specific proposals to
be considered and acted upon at our Annual Meeting are each described in this proxy statement. Only stockholders holding shares of Common
Stock as of the close of business on the Record Date are entitled to notice of and to vote at the Annual Meeting. As of the Record Date,
there were [●] shares of Common Stock issued and outstanding. Holders of record of shares of Common Stock have the right to vote
on all matters brought before the Annual Meeting. Each holder of record of our Common Stock is entitled to one vote per share of Common
Stock on each matter to be acted upon at the Annual Meeting.
Matters
to be Considered at the Annual Meeting
|
Required
Vote for
Approval
No. |
|
Proposal |
| 1. |
|
Election of Directors (the “Director Election Proposal”). The seven director nominees who receive the highest number of affirmative votes cast by shares of our Common Stock present or represented by proxy and entitled to vote at the Annual Meeting will be elected. |
| |
|
|
| 2. |
|
Approval of an amendment to our 2020 Omnibus Equity Incentive Plan to: (1) increase the number of shares of Common Stock authorized for issuance thereunder from 58,374 shares to 1,500,000 shares and to increase the number of shares that otherwise become available under the plan for grants as incentive stock options (“ISOs”) from 250,000 to 750,000, and (2) to increase the number of shares that may be granted to any one non-employee director of the Board from 5 to 250,000 (the “Equity Plan Amendment Proposal”). This proposal requires the affirmative (“FOR”) vote of a majority of votes cast by shares of our Common Stock present or represented by proxy and entitled to vote at the Annual Meeting. Shares that are not represented at the Annual Meeting, abstentions and broker non-votes, if any, with respect to this proposal are not counted as votes cast and will not affect the outcome of the voting on this proposal. |
| |
|
|
| 3. |
|
To
approve, in accordance with Nasdaq Listing Rule 5635, the issuance of: (i) up to 12,373,226 shares of Common Stock, subject to adjustment,
upon conversion of the Company’s Series G Non-Voting Convertible Preferred Stock, par value $0.0001 per share, (ii) the issuance
of shares upon exercise of certain assumed ImmunogenX, Inc. (“ImmunogenX” or “IMGX”) stock options exercisable
for an aggregate of 200,652 shares of Common Stock (the “Assumed Options”), (iii) the issuance of shares upon exercise
of certain assumed ImmunogenX warrants exercisable for an aggregate of 127,680 shares of Common Stock (the “Assumed Warrants”),
and (iv) the prior issuance of 36,830 shares of Common Stock to the stockholders of ImmunogenX (the “Nasdaq Proposal”).
Entero agreed to all of such issuances in connection with its acquisition of ImmunogenX (the “IMGX Transaction”) pursuant
to that certain Agreement and Plan of Merger, dated March 13, 2024 (the “Merger Agreement”), by and among Entero, IMMUNO
Merger Sub I, Inc., IMMUNO Merger Sub II, LLC, and ImmunogenX, all as described in further detail herein. This proposal
requires the affirmative (“FOR”) vote of a majority of votes cast by shares of our Common Stock present or represented
by proxy and entitled to vote at the Annual Meeting. Shares that are not represented at the Annual Meeting, abstentions and broker
non-votes, if any, with respect to this proposal are not counted as votes cast and will not affect the outcome of the voting on this
proposal. |
| |
|
|
| 4. |
|
Adoption and approval of an amendment to our Amended and Restated Certificate of Incorporation, as amended (the “Charter”), to effect a reverse stock split of our issued and outstanding shares of Common Stock, as a specific ratio, ranging from [●] to [●], at any time prior to the one-year anniversary date of the Annual Meeting, with the exact ratio to be determined by the Board without further approval or authorization of our stockholders (the “Reverse Split Proposal”). This proposal requires the affirmative (“FOR”) vote of a majority of votes cast by shares of our Common Stock present or represented by proxy and entitled to vote at the Annual Meeting. Shares that are not represented at the Annual Meeting, abstentions and broker non-votes, if any, with respect to this proposal are not counted as votes cast and will not affect the outcome of the voting on this proposal. |
| 5. |
|
Approval, on an advisory basis, of the executive compensation of the Company’s named executive officers as described in this proxy statement (the “Say on Pay Proposal”). This proposal requires the affirmative (“FOR”) vote of a majority of votes cast by shares of our Common Stock present or represented by proxy and entitled to vote at the Annual Meeting. Shares that are not represented at the Annual Meeting, abstentions and broker non-votes, if any, with respect to this proposal are not counted as votes cast and will not affect the outcome of the voting on this proposal. |
| |
|
|
| 6. |
|
Ratification
of Forvis Mazars, LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2024 (the “Auditor
Ratification Proposal”). To approve the ratification of Forvis Mazars, LLP as our independent registered public
accounting firm for the current fiscal year. This proposal requires the affirmative (“FOR”) vote of a majority of votes
cast by shares of our Common Stock present or represented by proxy and entitled to vote at the Annual Meeting. Shares that are not
represented at the Annual Meeting and abstentions, if any, with respect to this proposal are not counted as votes cast and will not
affect the outcome of the voting on this proposal. |
| |
|
|
| 7. |
|
Approval of the Adjournment of the Annual Meeting to the Extent There Are Insufficient Proxies at the Annual Meeting to Approve Any One or More of the Foregoing Proposals. To approve the adjournment of the Annual Meeting in the event that the number of shares of Common Stock present or represented by proxy at the Annual Meeting and voting “FOR” the adoption of any one or more of the foregoing proposals are insufficient to approve any proposal. This proposal requires the affirmative (“FOR”) vote of a majority of votes cast by shares of our Common Stock present or represented by proxy and entitled to vote at the Annual Meeting. Shares that are not represented at the Annual Meeting, abstentions and broker non-votes, if any, with respect to this proposal are not counted as votes cast and will not affect the outcome of the voting on this proposal. |
Recommendation of the Entero Board
After careful consideration,
the Entero Board unanimously recommends that Entero’s stockholders vote “FOR” each of the proposals.
Required Votes
Assuming a quorum is present
at the Annual Meeting, the Equity Plan Amendment Proposal, the Nasdaq Proposal, the Reverse Split Proposal, the Say on Pay Proposal,
the Auditor Ratification Proposal and the Adjournment Proposal require the affirmative vote of the majority of the votes cast by stockholders
present or represented by proxy and entitled to vote on the matter at the Annual Meeting. The Director Election Proposal requires the
affirmative vote of the plurality of the votes cast by stockholders present or represented by proxy and entitled to vote on the matter
at the Annual Meeting. An Entero stockholder’s failure to vote by proxy or to vote in person at the Annual Meeting will have no
effect on such proposals, provided that a quorum is otherwise present. An abstention or other failure of any shares present or represented
by proxy to vote on such proposals will have no effect on such proposals.
Quorum; Abstentions and Broker Non-Votes
A
quorum of Entero stockholders is necessary to conduct business at the Annual Meeting. In order for any business to be conducted at the
Annual Meeting, both (i) the holders of one-third of the voting power of the shares of the capital stock of the Company issued and outstanding
and entitled to vote at the Annual Meeting, and (ii) the holders of at least one-third of the shares of Common Stock issued and outstanding
and entitled to vote at the Annual Meeting must be represented at the Annual Meeting, either in person, by means of remote communication
in a manner, if any, authorized by the Board in its sole discretion, or represented by proxy. Shares of Common Stock present at
the Annual Meeting or represented by proxy and entitled to vote, including shares for which an Entero stockholder directs an “abstention”
from voting, will be counted for purposes of determining a quorum. Since the Auditor Ratification Proposal is considered a routine matter,
shares held in “street name” through a broker, bank or other nominee will be counted as present for the purpose of determining
the existence of a quorum if such broker, bank or other nominee does not have instructions to vote on such proposal.
If a quorum is not present,
the Annual Meeting will be adjourned or postponed until the holders of the number of shares of Common Stock required to constitute a quorum
attend.
Under Nasdaq rules, banks,
brokers or other nominees who hold shares in “street name” on behalf of the beneficial owner of such shares have the authority
to vote such shares in their discretion on certain “routine” proposals when they have not received voting instructions from
the beneficial owners. However, banks, brokers or other nominees are not allowed under Nasdaq rules to exercise their voting discretion
with respect to matters that are “non-routine.” This can result in a “broker non-vote,” which occurs on a proposal
when (i) a bank, broker or other nominee has discretionary authority to vote on one or more “routine” proposals to be
voted on at a meeting of stockholders, but is not permitted to vote on other “non-routine” proposals without instructions
from the beneficial owner of the shares, and (ii) the beneficial owner fails to provide the bank, broker or other nominee with voting
instructions on a “non-routine” matter. All proposals other than the Auditor Ratification Proposal are considered “non-routine”
matters, and banks, brokers or other nominees will not have discretionary authority to vote on such matters before the Annual Meeting.
As a result, Entero only expects broker non-votes with respect to the Auditor Ratification Proposal. If you hold your shares of Common
Stock in “street name,” your shares will not be voted on any matter other than the Auditor Ratification Proposal unless you
affirmatively instruct your bank, broker or other nominee how to vote your shares in accordance with the voting instructions provided
by your bank, broker or other nominee. It is therefore critical that you cast your vote by instructing your bank, broker or other nominee
on how to vote. Brokers will not be able to vote on any of the non-routine proposals before the Annual Meeting unless they have received
voting instructions from the beneficial owners.
Vote of Entero Directors and Executive Officers
As of the Record Date,
Entero directors and executive officers and their affiliates beneficially owned and were entitled to vote in the aggregate [●] shares
of Common Stock, which represented [●]% of the Common Stock issued and outstanding on the record date. Entero currently expects
that all Entero directors and executive officers will vote their shares “FOR” each of the proposals. See the section
titled “Interests of Entero Directors and Executive Officers” in this proxy statement.
Methods of Voting
Stockholders of Record
If you are an Entero stockholder
of record, you may vote at the Annual Meeting by proxy over the internet or telephone or by mail, or by attending and voting at the Annual
Meeting, as described below.
| |
● |
By Internet: To
vote via the Internet, go to www.aalvote.com/ENTO to complete an electronic proxy card. You will be asked to provide
the 11-digit control number from the proxy card you receive. Your vote must be received by 11:59 p.m. Eastern Time on the day
before the Annual Meeting to be counted. If you vote via the Internet, you do not need to return a proxy card by mail. |
| |
● |
By Telephone: To
vote by telephone, dial 1-(866)-804-9616 (the call is toll-free in the United States and Canada; toll charges apply to
calls from other countries) and follow the recorded instructions. You will be asked to provide the 11-digit control number from the
proxy card. Your vote must be received by 11:59 p.m., Eastern Time, on the day before the Annual Meeting to be counted. If you
vote by telephone, you do not need to return a proxy card by mail. |
| |
● |
By Mail: To vote by mail using the proxy card (if you requested paper copies of the proxy materials to be mailed to you), you need to complete, date and sign the proxy card and return it promptly by mail in the envelope provided so that it is received no later than the day before the annual meeting. The persons named in the proxy card will vote the shares you own in accordance with your instructions on the proxy card you mail. |
| |
● |
In Person at the Annual Meeting: To vote in person at the Annual Meeting, please attend the Annual Meeting at [●] a.m. on [●] at our corporate headquarters, located at 777 Yamato Road, Suite 502
Boca Raton, FL 33431. |
Unless revoked, all duly executed
proxies representing shares of Common Stock entitled to vote at the Annual Meeting will be voted at the Annual Meeting and, where a choice
has been specified on the proxy card, will be voted in accordance with such specification. If you submit an executed proxy without providing
instructions for any proposal, your shares will be voted “FOR” each of the proposals.
Beneficial (Street Name) Stockholders
If you hold your shares of
Common Stock through a bank, broker or other nominee in “street name” instead of as a registered holder, you must follow the
voting instructions provided by your bank, broker or other nominee in order to vote your shares. Your voting instructions must be received
by your bank, broker or other nominee prior to the deadline set forth in the information from your bank, broker or other nominee on how
to submit voting instructions. If you do not provide voting instructions to your bank, broker or other nominee for a proposal, your shares
of Common Stock will not be voted on any proposals other than the Auditor Ratification Proposal because your bank, broker or other nominee
does not have discretionary authority to vote on such proposals. See the section titled “The Annual Meeting — Quorum;
Abstentions and Broker Non-Votes.”
Attending the Annual Meeting
If you wish to attend
the Annual Meeting, you must (i) be an Entero stockholder of record at the close of business on [●], 2024, the record date,
(ii) hold your shares of Common Stock beneficially in the name of a broker, bank or other nominee as of the record date or (iii) hold
a valid proxy for the Annual Meeting.
If you plan to attend
and vote at the Annual Meeting, Entero still encourages you to vote in advance by the internet, telephone or (if you received a paper
copy of the proxy materials) by mail so that your vote will be counted even if you later decide not to attend the Annual Meeting. Voting
your proxy by the internet, telephone or mail will not limit your right to attend and vote at the Annual Meeting if you later decide
to do so.
Revocability of Proxies
Any Entero stockholder
giving a proxy has the right to revoke it at any time before the proxy is voted at the Annual Meeting. If you are an Entero stockholder
of record, you may revoke your proxy by any one of the following actions:
| |
● |
by sending a signed
written notice of revocation to Entero’s Chief Financial Officer , provided
such notice is received no later than the close of business on the day before the Annual Meeting; |
| |
● |
by voting again over the internet or telephone as instructed on your proxy card before the closing of the voting facilities at 11:59 p.m., Eastern Time, on the day before the Annual Meeting; |
| |
● |
by submitting a properly
signed and dated proxy card with a later date that is received by Entero’s Chief Financial Officer no
later than the close of business the day before the Annual Meeting; or |
| |
● |
by attending the Annual Meeting and requesting that your proxy be revoked, as described above. |
Only your last submitted proxy
will be considered.
Execution or revocation
of a proxy will not in any way affect an Entero stockholder’s right to attend and vote at the Annual Meeting.
Written notices of revocation
and other communications relating to the revocation of proxies should be addressed to:
Entero Therapeutics, Inc.
Attention: Chief Financial Officer 777 Yamato Road, Suite 502
Boca Raton, FL 33431
If your shares of Common Stock
are held in “street name” and you previously provided voting instructions to your broker, bank or other nominee, you should
follow the instructions provided by your broker, bank or other nominee to revoke or change your voting instructions. You may also change
your vote by obtaining your specific control number and instructions from your bank, broker or other nominee and voting your shares at
the Annual Meeting.
Proxy Solicitation Costs
Entero is soliciting proxies
on behalf of the Entero Board. Entero will bear the entire cost of soliciting proxies from Entero stockholders. Proxies may be solicited
on behalf of Entero or by Entero directors, officers and other employees in person or by mail, telephone, facsimile, messenger, the internet
or other means of communication, including electronic communication. Entero directors, officers and employees will not be paid any additional
amounts for their services or solicitation in this regard.
Entero will request that
banks, brokers and other nominee record holders send proxies and proxy material to the beneficial owners of Entero common stock and secure
their voting instructions, if necessary. Entero may be required to reimburse those banks, brokers and other nominees on request for their
reasonable expenses in taking those actions.
Entero has also retained
Alliance to assist in soliciting proxies and in communicating with Entero stockholders and estimates that it will pay Alliance a fee
of approximately $34,200, plus reimbursement for certain out-of-pocket fees and expenses. Entero also has agreed to indemnify Alliance
against various liabilities and expenses that relate to or arise out of its solicitation of proxies (subject to certain exceptions).
Householding
SEC rules permit companies
and intermediaries such as brokers to satisfy delivery requirements for proxy statements and notices with respect to two or more stockholders
sharing the same address by delivering a single proxy statement or a single notice addressed to those stockholders. This process, which
is commonly referred to as “householding,” provides cost savings for companies. Entero has previously adopted householding
for Entero stockholders of record. As a result, Entero stockholders with the same address and last name may receive only one copy of
this proxy statement. Registered Entero stockholders (those who hold shares of Common Stock directly in their name with Entero’s
transfer agent) may opt out of householding and receive a separate proxy statement or other proxy materials by sending a written request
to Entero at the address below.
Some brokers household
proxy materials, delivering a single proxy statement or notice to multiple Entero stockholders sharing an address unless contrary instructions
have been received from the affected stockholders. Once you have received notice from your broker that they will be householding materials
to your address, householding will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no
longer wish to participate in householding and would prefer to receive a separate proxy statement or notice, or if your receiving multiple
copies of these documents and you wish to request that future deliveries be limited to a single copy, please notify your broker.
Entero
will promptly deliver a copy of this proxy statement to any Entero stockholder who only received one copy of these materials due to householding
upon request in writing to: Entero Therapeutics, Inc., Attn: Chief Financial Officer at 777 Yamato Road, Suite 502,
Boca Raton, FL 33431 or by calling (561) 589-7020.
Adjournments
If a quorum is present
at the Annual Meeting but there are insufficient votes at the time of the Annual Meeting to approve the Director Election Proposal, the
Equity Plan Amendment Proposal, the Nasdaq Proposal, the Reverse Split Proposal, the Say on Pay Proposal or the Auditor Ratification
Proposal, then Entero stockholders may be asked to vote on the Adjournment Proposal. If a quorum is not present, the presiding officer
may adjourn the Annual Meeting, from time to time, without notice other than announcement at the meeting of the hour, date and place,
if any, to which the meeting is adjourned, and the means of remote communications, if any, by which Entero stockholders and proxyholders
may be deemed to be present in person and vote at such adjourned meeting. The presiding officer may also adjourn the meeting to another
hour, date or place, even if a quorum is present.
At any subsequent reconvening
of the Annual Meeting at which a quorum is present, any business may be transacted that might have been transacted at the original meeting,
and all proxies will be voted in the same manner as they would have been voted at the original convening of the Annual Meeting, except
for any proxies that have been effectively revoked or withdrawn prior to the time the proxy is voted at the reconvened meeting.
No Appraisal Rights
Our stockholders have no dissenter’s or appraisal
rights in connection with any of the proposals described herein.
Assistance
If you need assistance
voting or completing your proxy card, or if you have questions regarding the Annual Meeting, please contact Alliance, Entero’s
proxy solicitor for the Annual Meeting, at:
Alliance Advisors LLC
200 Broadacres Drive, 3rd Floor, Bloomfield,
NJ 07003
866-407-1875
ENTERO STOCKHOLDERS SHOULD CAREFULLY READ
THIS PROXY STATEMENT IN ITS ENTIRETY FOR MORE DETAILED INFORMATION CONCERNING THE NASDAQ PROPOSAL AND THE IMGX TRANSACTION. IN PARTICULAR,
ENTERO STOCKHOLDERS ARE DIRECTED TO THE MERGER AGREEMENT, WHICH IS ATTACHED AS ANNEX B HERETO.
PROPOSAL NO. 1: ELECTION OF DIRECTORS
General
Our Bylaws provide that the
Board shall consist of one or more members, and that any newly created directorship that results from an increase in the number of directors
or any vacancy on the Board will be filled solely by the affirmative vote of a majority of the directors then in office; provided that
a vacancy created by the removal of a director by the stockholders may be filled by the stockholders. A director elected by the Board
in the case of a newly created directorship will hold office for his or her full term until his or her successor is elected and qualified.
A director elected by the Board in the case of a vacancy will hold office for the remaining term of his or her predecessor until his or
her successor is elected and qualified.
Our Board currently consists
of seven directors. Each of the director nominees identified below has confirmed that he or she is able and willing to serve as a director
if elected. If any of the director nominees becomes unable or unwilling to serve, your proxy will be voted for the election of a substitute
director nominee recommended by the current Board.
Upon recommendation of the
Corporate Governance and Nominating Committee, the Board has nominated James Sapirstein, Edward J. Borkowski, Charles J. Casamento, Terry
Coelho, Alastair Riddell, Jack Syage, and Chaitan Khosla for election at the Annual Meeting, each to serve for a one-year term until the
conclusion of the 2025 annual meeting of stockholders or until their successor is duly elected and qualified.
Please see “Director
Compensation” below for more information, including the background and business experience of each director nominee taken into
consideration by the Corporate Governance and Nominating Committee.
Required Vote and Recommendation
The election of directors
requires the affirmative (“FOR”) vote of a plurality of votes cast by shares of our Common Stock present or represented by
proxy and entitled to vote at the Annual Meeting. The seven director nominees receiving the highest number of affirmative votes cast will
be elected. Unless otherwise instructed on the proxy or unless authority to vote is withheld, shares represented by executed proxies will
be voted “FOR” the election of each of the below director nominees. Any abstentions or broker non-votes are not counted as
votes cast and will not affect the outcome of this proposal, although they will be counted for purposes of determining whether there is
a quorum present.
OUR BOARD RECOMMENDS A VOTE “FOR”
THE ELECTION OF
MESSRS. SAPIRSTEIN, BORKOWSKI, CASAMENTO AND
CHAITAN, MS. COELHO AND
DRS. RIDDELL AND SYAGE UNDER PROPOSAL ONE.
DIRECTOR COMPENSATION
The following section sets
forth certain information regarding the nominees for election as directors. There are no family relationships between any of the directors
and our Named Executive Officers.
| Director,
Title |
|
Age |
|
James Sapirstein
– Chief Executive Officer, Chairman and Non-Independent Director |
|
62 |
|
Edward J.
Borkowski – Lead Independent Director |
|
64 |
|
Charles J.
Casamento – Independent Director |
|
78 |
|
Alastair
Riddell, MSc., MBChB., DSc. – Independent Director |
|
74 |
|
Terry Coelho
– Independent Director |
|
62 |
|
Jack Syage,
Ph.D. – President, Chief Scientific Officer and Director |
|
69 |
|
Chaitan
Khosla, Ph.D. – Independent Director |
|
59 |
|
James
Sapirstein was appointed to the Board on October 8, 2019 and as our President and Chief Executive Officer effective that
same day. Mr. Sapirstein was appointed Chair of the Board effective February 19, 2021. Mr. Sapirstein served as the Company’s President
until the appointment of Dr. Syage in March 2024. Prior to joining us, Mr. Sapirstein served as Chief Executive Officer and as a director
of ContraVir Pharmaceuticals, Inc. (now known as Hepion Pharmaceuticals, Inc.) from March 2014 to October 2018. Previously, Mr. Sapirstein
was the Chief Executive Officer of Alliqua Therapeutics from October 2012 to February 2014. He founded and served as Chief Executive Officer
of Tobira Therapeutics from October 2006 to April 2011 and served as Executive Vice President, Metabolic and Endocrinology for Serono
Laboratories from June 2002 to May 2005. Mr. Sapirstein’s earlier career included a number of senior level positions in the area
of marketing and commercialization, including as Global Marketing Lead for Viread (tenofovir) while at Gilead Sciences and as Director
of International Marketing of the Infectious Disease Division at Bristol Myers Squibb. Mr. Sapirstein is currently the Chair Emeritus
of BioNJ, the New Jersey affiliate of the Biotechnology Innovation Organization, and also serves on the Emerging Companies and Health
Section Boards of the Biotechnology Innovation Organization. Mr. Sapirstein received his bachelor’s degree in pharmacy from Rutgers
University and holds an MBA degree in management from Fairleigh Dickinson University.
Mr. Sapirstein’s nearly
36 years of pharmaceutical industry experience which spans areas such as drug development and commercialization, including participation
in 23 product launches, six of which were global launches led by him makes him a valuable asset to the Board and in his oversight and
execution of our business plan.
Edward
J. Borkowski was appointed to the Board in May 2015, and currently serves as our Lead Independent Director. Mr. Borkowski
served as Chair of the Board from 2015 through his resignation effective as of February 19, 2021. Mr. Borkowski is a healthcare executive
who currently serves as Executive Vice President for Therapeutics MD. He served as Executive Vice President of MiMedx Group, Inc. (Nasdaq:
MDGX) from April 2018 until December 2019. Mr. Borkowski also served as a director for Co-Diagnostics, Inc. (Nasdaq: CODX), from May 2017
until June 2019. Previously, he served as the Chief Financial Officer of Aceto Corporation (Nasdaq: ACET) from February 2018 to April
2018, and has held several executive positions with Concordia International, an international specialty pharmaceutical company, between
May 2015 to February 2018. Mr. Borkowski has also served as Chief Financial Officer of Amerigen Pharmaceuticals, a generic pharmaceutical
company with a focus on oral, controlled release products and as the Chief Financial Officer and Executive Vice President of Mylan N.V.
In addition, Mr. Borkowski previously held the position of Chief Financial Officer with Convatec, a global medical device company focused
on wound care and ostomy, and Carefusion, a global medical device company for which he helped lead its spin-out from Cardinal Health into
an independent public company. Mr. Borkowski has also served in senior financial positions at Pharmacia and American Home Products (Wyeth).
He started his career with Arthur Andersen & Co. after receiving his MBA in accounting from Rutgers University subsequent to having
earned his degree in Economics and Political Science from Allegheny College. Mr. Borkowski is currently a Trustee and a member of the
Executive Committee of Allegheny College.
Mr. Borkowski’s extensive
healthcare and financial expertise, together with his public company experience provides the Board and management with valuable insight
in the growth of our business plan.
Charles
J. Casamento was appointed to the Board in March 2017. Since 2007, Mr. Casamento has been executive director and principal
of The Sage Group, a health care advisory group. Prior to that, Mr. Casamento was president and Chief Executive Officer of Osteologix,
a startup company which he oversaw going public, from October 2004 until April 2007. Mr. Casamento was the founder of Questcor Pharmaceuticals
where he was President, Chief Executive Officer and Chair from 1999 through 2004. During his time at Questcor, the company acquired Acthar,
a product with sales that would eventually exceed $1.0 billion. Mr. Casamento also served as President, Chief Executive Officer and Chair
of RiboGene Inc. until 1999 when RiboGene was merged another company to form Questcor. He was also the Co-Founder, President and Chief
Executive Officer of Indevus (formerly Interneuron Pharmaceuticals) and has held senior management positions at Genzyme Corporation, where
he was Senior Vice President, American Hospital Supply, where he was Vice President of Business Development for the Critical Care division,
Johnson & Johnson, Hoffmann-LaRoche and Sandoz. He currently serves as Chairman of the Board of Directors of Relmada Therapeutics
(OTCQB: RLMD), serves on the Board of Directors of Eton Pharmaceuticals (Nasdaq: ETON), and serves on the Board of Directors of PaxMedica,
Inc. (Nasdaq: PXMD), and was previously a Director and Vice Chair of the Catholic Medical Missions Board, a large not for profit international
organization. Mr. Casamento holds a bachelor’s degree in Pharmacy from Fordham University and an MBA from Iona College.
Mr. Casamento’s expertise
and knowledge of the financial community combined with his experience in the healthcare sector makes him a valued member of the Board.
Terry
Coelho was appointed to the Board on August 11, 2021. Ms. Coelho is currently serving as the CFO of Gamida Cell, a public,
commercial – stage biotechnology company focused on cell therapy. She serves as a member of the Audit Committee and the Compensation
Committee, and as a member and the Chair of the Nominating and Corporate Governance Committee for INOTIV (NASDAQ: NOTV). Additionally,
Ms. Coelho serves as the Chair of the Audit Committee and member of the Compensation Committee for the Board of HOOKIPA (NASDAQ: HOOK).
Previously, Ms. Coelho served as the Executive Vice President, Chief Financial Officer & Chief Business Development Officer of CinCor
Pharma, Inc. (NASDAQ: CINC), a clinical stage cardiorenal therapeutics company from November 2021 to November 2022. Prior to this, Ms.
Coelho served as Executive Vice President and Chief Financial Officer at BioDelivery Sciences International, Inc. (NASDAQ: BDSI), a commercial-stage
specialty pharmaceutical company, from January 2019 to November 2021. Prior to her tenure at BDSI, Ms. Coelho served as Chief Financial
Officer and Treasurer at Balchem Corporation (NASDAQ: BCPC) from October 2017 to October 2018. Previous to her role at Balchem she served
as Chief Operating Officer for Diversey, Inc., a multi-billion-dollar global private equity carve-out from Sealed Air Corporation. She
additionally held senior finance positions at Diversey Care, including that of Division Chief Financial Officer and VP of Global Commercial
Excellence, from October 2014 through August 2017. Ms. Coelho has also served in senior finance and operational leadership roles of increasing
responsibility with leading global organizations, including Mars, Incorporated, and Novartis Pharmaceuticals from 2007 to 2014, including
serving as Global Head of Oncology Development Finance. Ms. Coelho earned an MBA in Finance from IBMEC in Brazil and a Bachelor of Arts
degree in both Economics and International Relations, summa cum laude, from The American University School of International Service in
Washington, DC. She has led Women’s Networking ERGs and is a founding Steering Committee Member of the CFO Leadership Council –
Charlotte, North Carolina, chapter.
Ms. Coelho was selected as
a director due to her financial background and experience as a senior financial officer of public companies.
Dr.
Alastair Riddell was appointed to the Board in September 2015. From June 2016 to February 2023, Dr. Riddell served as Chair
and Director of Nemesis Biosciences Ltd and previously Chair of Feedback plc (LON: FDBK). He has also served as Chair of the South West
Academic Health Science network in the UK since January 2016. Since his appointment in December 2015, Dr. Riddell has served as Non-Executive
Director of Cristal Therapeutics in The Netherlands. From September 2012 to February 2016, he served as Chair of Definigen Ltd., and from
November 2013 to September 2015 as Chair of Silence Therapeutics Ltd., and from October 2009 to November 2012 as Chair of Procure Therapeutics.
Between 2007 to 2009, Dr. Riddell served as the Chief Executive Officer of Stem Cell Sciences plc. And between 2005 to 2007, served at
Paradigm Therapeutics Ltd. as the Chief Executive Officer. Between 1998 to 2005, Dr. Riddell also served as the Chief Executive Officer
of Pharmagene plc. Dr. Riddell began his career as a doctor in general practice in a variety of hospital specialties and holds a Master
of Science and a Bachelor of Medicine and Surgery degrees. He was awarded a Doctorate of Science, Honoris Causa by Aston University in
2016.
Dr. Riddell’s medical
background coupled with his expertise in the life sciences industry, directing all phases of clinical trials, before moving to sales,
marketing and general management, makes him a well-qualified member of the Board.
Jack
Syage was appointed to the Board on March 13, 2024, and as our Chief Operating Officer effective the same
day. On June 17, 2024, Dr. Syage transitioned from the office of Chief Operating Officer to be appointed to the office of Chief Scientific
Officer. Dr. Syage served as the Chief Executive Officer of ImmunogenX from July 2013 until the Closing of the Merger and as a member
of the board of directors of ImmunogenX from January 2021 until the Closing of the Merger. Dr. Syage has over 30 years of experience in
creating and leading the development of innovative technologies in the analytical instrumentation field. He is regarded as a leading expert
in mass spectrometry and trace chemical detection. Dr. Syage served as CEO and Co – Founder of ImmunogenX, a global leader in the
development of treatments for celiac disease, which developed Latiglutenase before its combination with Entero Therapeutics. Previously
he founded Syagen Technology, Inc. and led its growth culminating in its successful acquisition by French multinational Safran S.A. in
2011. Dr. Syage sits on the Board of Directors of Advanced Telesensors, PhageTech, Analytical Detection, and Appellation Ventures. He
has published over 130 papers, delivered about 100 invited talks, has over 30 U.S. patents issued or pending, and appears on the list
of the Most Highly Cited Chemists. His honorary positions include Fellow of the American Physical Society, Visiting Professor at UC Irvine,
Visiting Professor at the Université de Paris – Sud, Editorial Board member of the Journal of Physical Chemistry, and invitee
to the Nobel Symposium in Chemistry. He has received a Tibbets Award (SBIR) and was recently inducted into the Orange County OC 500 Directory
of Influence. He received BA and PhD degrees from Hamilton College and Brown University, respectively, where he won several academic awards
including Best Thesis. He was a postdoctoral fellow at Caltech under Nobel Laureate Ahmed Zewail.
Chaitan Khosla
was appointed to the Board on March 13, 2024. Dr. Khosla has served as a Professor in the Departments of Chemistry and Chemical Engineering
at Stanford University since 1992 and is founding director of the university’s Innovative Medicines Accelerator. He served as a
member of the board of directors of ImmunogenX from 2021 until the Closing of the Merger. He received his Ph.D. in 1990 at Caltech. After
completing postdoctoral studies in 1992, he joined the faculty of Stanford University. In 2002 he and his collaborators discovered that
the immunotoxicity of dietary gluten in celiac disease is causatively correlated to its intestinal proteolytic resistance, a finding
that led to the invention of Latiglutenase by his laboratory. He has co – authored over 400 peer – reviewed publications
and is an inventor on more than 80 issued U.S. patents. Dr. Khosla is an elected member of the American Academy for Arts and Science,
the National Academy of Engineering, and the National Academy of Sciences. Over the past three decades, he has helped launch and build
four biotechnology companies including Kosan Biosciences (NASDAQ: KOSN; acquired by Bristol Myers Squibb in 2008) and Sitari Pharmaceuticals
(acquired by GlaxoSmithKline in 2019).
Involvement in Certain Legal Proceedings
On March 27, 2024, Gamida Cell Ltd., of which our
director, Terry Coelho, serves as the chief financial officer, filed a voluntary petition for restructuring in Israeli court.
Board Diversity Matrix
In accordance with Nasdaq’s
board diversity listing standards, we are also disclosing aggregated statistical information about our Board’s self-identified gender
and racial characteristics and LGBTQ+ status as voluntarily confirmed to us by each of our directors.
Board
Diversity Matrix (As of March 29, 2024)
Total Number of Directors – 7
| |
|
Female |
|
Male |
|
Non-Binary |
|
Did Not
Disclose
Gender |
| Directors |
|
1 |
|
6 |
|
- |
|
- |
| Demographic Information: |
|
|
|
|
|
|
|
|
| African American or Black |
|
- |
|
- |
|
- |
|
- |
| Alaskan Native or Native American |
|
- |
|
- |
|
- |
|
- |
| Asian |
|
- |
|
1 |
|
- |
|
- |
| Hispanic or Latinx |
|
1 |
|
1 |
|
- |
|
- |
| Native Hawaiian or Pacific Islander |
|
- |
|
- |
|
- |
|
- |
| White |
|
1 |
|
3 |
|
- |
|
- |
| Two or More Races or Ethnicities |
|
1 |
|
- |
|
- |
|
- |
| LGBTQ+ |
|
- |
| Did Not Disclose Demographic Background |
|
1 |
Non-Executive Director Compensation
Effective October 1, 2022,
following an independent review of external benchmarks, our Board adopted an updated Non-Executive Director Compensation Policy under
which each of our non-executive directors is entitled to receive the following cash compensation for their service on the Board (paid
quarterly): (i) an annual retainer of $60,000, (ii) the lead independent director an annual retainer of $20,000, (iii) the chair of the
Audit Committee is entitled to receive an additional annual retainer in the amount of $15,000, (iv) each non-chairperson member of the
Audit Committee is entitled to receive an additional annual retainer in the amount of $7,500, (v) the chair of the Compensation Committee
is entitled to receive an additional annual retainer in the amount of $12,500, (vi) each non-chairperson member of the Compensation Committee
is entitled to receive an additional annual retainer in the amount of $6,000, (vii) the chair of the Corporate Governance and Nominating
Committee is entitled to receive an additional annual retainer in the amount of $10,000, and (viii) each non-chairperson member of the
Corporate Governance and Nominating Committee is entitled to receive an additional annual retainer in the amount of $5,000. Additionally,
under this policy, each of our non-executive directors is entitled to receive an annual grant, effective on the date of the Company’s
annual meeting of stockholders, of restricted stock unit awards for their service on the Board equivalent to $75,000, which vests in equal
quarterly installments.
Our Board will review the
Non-Executive Director Compensation Policy on an annual basis prior to September 1 of each year.
The following table provides
information regarding compensation paid to non-employee directors for the year ended December 31, 2023. Mr. Sapirstein did not receive
compensation for his service on the Board as employee director for the year ended December 31, 2023. Information regarding executive compensation
paid to Mr. Sapirstein during 2023 is reflected in the Summary Compensation table under “Executive Compensation.”
| | |
| | |
| | |
| | |
| | |
| |
| | |
Fees Earned or | | |
Stock | | |
Option | | |
All Other | | |
| |
| Non-Executive Directors | |
Paid in Cash(2) | | |
Award (3) | | |
Award | | |
Compensation | | |
Total | |
| Edward J. Borkowski | |
$ | 102,500 | | |
$ | 75,020 | | |
$ | - | | |
$ | - | | |
$ | 177,520 | |
| Charles J. Casamento | |
$ | 75,833 | | |
$ | 75,020 | | |
$ | - | | |
$ | - | | |
$ | 150,853 | |
| Alastair Riddell | |
$ | 71,000 | | |
$ | 75,020 | | |
$ | - | | |
$ | - | | |
$ | 146,020 | |
| David Hoffman (1) | |
$ | 30,000 | | |
$ | 75,020 | | |
$ | - | | |
$ | - | | |
$ | 105,020 | |
| Terry Coelho | |
$ | 83,167 | | |
$ | 75,020 | | |
$ | - | | |
$ | - | | |
$ | 158,187 | |
| (1) |
Mr. Hoffman resigned from the board effective June 22, 2023. |
| (2) |
Represents amounts of accrued and unpaid cash compensation for board services through December 31, 2023. |
| (3) |
Represents the aggregate grant date fair value of 605 restricted stock units issued to each of Messrs. Borkowski, Casamento, Riddell, Hoffman, and Ms. Coelho on January 3, 2023, our non-employee directors, calculated in accordance with ASC Topic 718. |
CORPORATE GOVERNANCE AND BOARD MATTERS
Board Leadership Structure
Effective as of February 19,
2021, our President and Chief Executive Officer, Mr. James Sapirstein, was appointed to serve as Chairman of our Board. The Board has
chosen this structure because it believes Mr. Sapirstein serves as a bridge between management and the Board, ensuring that both groups
act with a common purpose. Mr. Sapirstein has provided strong leadership to the Board and management, instilling a clear focus on our
strategy and business plans. The Board believes that the combined role of Chairman and Chief Executive Officer better assists management
in developing strategic direction and then more effectively holds management accountable for the execution of strategy once it is developed.
Accordingly, the Board believes, at this time, that the combined role of Chairman and Chief Executive Officer, together with independent
directors, is in the best interest of stockholders, because it provides the appropriate balance between strategy development and independent
oversight of management. All directors, other than Mr. Sapirstein, are independent as defined under Nasdaq and SEC rules, and all committees
of the Board are comprised entirely of independent directors.
Mr. Edward J. Borkowski currently serves as lead
independent director and has the following responsibilities:
| |
· |
providing leadership to the Board in any situation where the Chairman’s role may be, or may be perceived to be, in conflict, and also chairing meetings when the Chairman is absent; |
| |
· |
serving as liaison between the Chairman and the independent directors; |
| |
· |
approving information sent to the Board; and |
| |
· |
approving meeting agendas for the Board. |
The Board believes that the
lead independent director further strengthens the Board’s independence and autonomous oversight of our business and also enhances
Board communication and effectiveness. The role of lead independent director serves as a bridge between the independent directors and
management.
Director Independence
The Board has
determined that all of its members, other than Mr. Sapirstein, our Chief Executive Officer and Chair of our Board, and Dr. Syage,
our President and Chief Scientific Officer, are “independent” within the meaning of Nasdaq Listing Rule 5605(a)(2) under
the rules of Nasdaq, and the Securities and Exchange Commission rules regarding independence.
Director Nomination Process
The Corporate Governance and
Nominating Committee identifies director nominees by first considering those current members of the Board who are willing to continue
service. Current members of the Board with skills and experience that are relevant to our business and are willing to continue their service
as a director are considered for re-election, balancing the value of continuity of service by existing members of the Board with that
of obtaining a new perspective. Nominees for director are selected by a majority of the members of the Board. Although we do not have
a formal diversity policy, in considering the suitability of director nominees, the Corporate Governance and Nominating Committee considers
such factors as it deems appropriate to develop a Board and its committees that are diverse in nature and comprised of experienced and
seasoned advisors. Factors considered by the Corporate Governance and Nominating Committee include sound judgment, knowledge, skill, diversity,
integrity, experience with businesses and other organizations of comparable size, including experience in the biopharma industry, clinical
studies, FDA compliance, intellectual property, business, finance, administration or public service, the relevance of a candidate’s
experience to our needs and experience of other Board members, experience with accounting rules and practices, the desire to balance the
considerable benefit of continuity with the periodic injection of the fresh perspective provided by new members, and the extent to which
a director candidate would be a desirable addition to the Board and its committees.
Nominations of persons for
election to the Board may be made at an annual meeting of stockholders only (a) pursuant to our notice of meeting, (b) by or at the direction
of the Board or any committee thereof or (c) by any stockholder of the Company who was a stockholder of record of the Company at the time
the notice is delivered by such stockholder to the secretary of the Company, who is entitled to vote at the meeting upon such election
of directors or upon such other business, as the case may be, and who complies with the notice procedures set forth in our bylaws. For
any nominations to be properly brought before an annual meeting by a stockholder, the stockholder must have given timely notice, which
must be delivered to the secretary of the Company at our principal executive offices not later than the close of business on the 90th
day, nor earlier than the close of business on the 120th day, prior to the first anniversary of the preceding year’s
annual meeting; provided however, that in the event that the date of the annual meeting is more than 30 days before or more than 70 days
after such anniversary date, notice by the stockholder must be so delivered not earlier than the close of business on the 120th
day prior to such annual meeting and not later than the close of business on the later of the 90th day prior to such annual meeting or
the 10th day following the day on which public announcement of the date of such meeting is first made by the Company. In no event shall
the public announcement of an adjournment, postponement or recess of an annual meeting commence a new time period (or extend any time
period) for the giving of a stockholder’s notice as described above. The number of nominees a stockholder may nominate for election
at the annual meeting (or in the case of a stockholder giving the notice on behalf of a beneficial owner, the number of nominees a stockholder
may nominate for election at the annual meeting on behalf of such beneficial owner) shall not exceed the number of directors to be elected
at such annual meeting.
To be in proper form, such
stockholder’s notice shall set forth: (a) as to each person whom the stockholder proposes to nominate for election as a director
(i) the name, age, business and residence address, and principal occupation or employment of the nominee, (ii) and all other information
relating to such nominee that is required to be disclosed in solicitations of proxies for election of directors in an election contest,
or is otherwise required, in each case pursuant to and in accordance with Section 14(a) of the Exchange Act, and the rules and regulations
promulgated thereunder, (iii) a reasonably detailed description of any compensatory, payment or other financial agreement, arrangement
or understanding that such nominee has with any other person or entity other than the Company including the amount of any payment or payments
received or receivable thereunder, In each case in connection with candidacy or service as a director of the Company, (iv) such person’s
written consent to being named in the Company’s proxy statement and associated proxy card as a nominee of the stockholder and to
serving as a director if elected and (v) all information with respect to such nominee that would be required to be set forth in a stockholder’s
notice if such nominee were the stockholder giving notice hereunder and (b) as to the stockholder giving the notice and the beneficial
owner, if any, on whose behalf the nomination or proposal is made (i) the name and address of such stockholder, as they appear on the
Company’s books, and of such beneficial owner, (ii) the class or series and number of shares of capital stock of the Company which
are, directly or indirectly, owned beneficially (within the meaning of Rule 13d-3 under the Exchange Act) or of record by such stockholder
and such beneficial owner (provided, that such stockholder and the beneficial owner, if any, on whose behalf the nomination or proposal
is made shall in all events be deemed to beneficially own any shares of any class or series and number of shares of capital stock of the
Company as to which such stockholder or beneficial owner, if any, has a right to acquire beneficial ownership at any time in the future),
(iii) a description of any agreement, arrangement or understanding with respect to the nomination between or among such stockholder and/or
such beneficial owner, any of their respective affiliates or associates, and any others acting in concert with any of the foregoing (including
their names), including the nominee, (iv) a description of any agreement, arrangement or understanding (including any derivative or short
positions, profit interests, options, warrants, convertible securities, stock appreciation or similar rights, hedging transactions, and
borrowed or loaned shares) that has been entered into as of the date of the stockholder’s notice by, or on behalf of, such stockholder
and such beneficial owners, whether or not such instrument or right shall be subject to settlement in underlying shares of capital stock
of the Company, the effect or intent of which is to mitigate loss to, manage risk or benefit of share price changes for, or increase or
decrease the voting power of, such stockholder or such beneficial owner, with respect to securities of the Company, (v) a representation
that the stockholder is a holder of record of stock of the Company entitled to vote at such meeting upon such business or nomination,
as the case may be, and intends to appear in person or by proxy at the meeting to propose such business or nomination, (vi) a representation
as to whether the stockholder or the beneficial owner, if any, intends or is part of a group which intends (a) to deliver a proxy statement
and/or form of proxy to holders of at least the percentage of the Company’s outstanding capital stock required to elect the nominee
and/or (b) otherwise to solicit proxies or votes from stockholders in support of such nomination, and (vii) any other information relating
to such stockholder and beneficial owner, if any, required to be disclosed in a proxy statement or other filings required to be made in
connection with solicitations of proxies for the election of directors in an election contest pursuant to and in accordance with Section
14(a) of the Exchange Act and the rules and regulations promulgated thereunder. The Company may require any proposed nominee to furnish
such other information as the Company may reasonably require to determine the eligibility of such proposed nominee to serve as a director
of the Company. If requested by the Company, the information required on such nominee shall be supplemented by such stockholder and any
such beneficial owner not later than 10 days after the record date for the meeting to disclose such information as of the record date.
In addition, a stockholder seeking to nominate a director candidate shall promptly provide any other information reasonably requested
by the Company.
Provided that stockholders
provide the information above required for candidates recommended by stockholders, the Corporate Governance and Nominating Committee will
evaluate those candidates by following substantially the same process, and applying substantially the same criteria, as for candidates
submitted by members of the Board or other persons, as described above and as set forth in its charter.
The Role of the Board in Risk Oversight
Our Board oversees a company-wide
approach to risk management, determines our appropriate risk level in general, assesses the specific risks faced by us and reviews steps
taken by management to manage those risks. Although our Board has ultimate oversight responsibility for the risk management process, specific
areas of risk are overseen by designation of such duties and responsibilities to certain committees of the Board.
Specifically, the Board has
designated certain fiduciary duties to its Compensation Committee, which is responsible for overseeing the management of risks relating
to our executive compensation plans and arrangements, and the incentives created by the compensation awards it administers. The Board
has also designated specific fiduciary duties to its Audit Committee, which is responsible for overseeing the management of enterprise
risks and financial risks, as well as potential conflicts of interests. The Board is responsible for overseeing the management of risks
associated with the independence of the Board.
Code of Business Conduct and Ethics
The Board adopted a code
of ethics (the “Code”) that applies to our directors, officers and employees. A copy of this Code is available on our website
at www.enterothera.com/investors. We intend to disclose on our website any amendments to and waivers of the Code that apply to
our principal executive officer, principal financial officer, principal accounting officer, controller, or persons performing similar
functions.
Insider Trading Policy
Our board of directors has
adopted an insider trading policy, the full text of which is incorporated herein by reference to Exhibit 19.1 of the Company’s Annual
Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March 29, 2024.
Employee, Officer and Director Hedging
Our Insider Trading Policy
expressly prohibits direct and indirect short selling of our securities by our employees, officers, directors, consultants, contractors
and members of their immediate families and households (collectively, “Insiders”). Insiders are prohibited from hedging transactions
if they have a trading plan in place under Rule 10b5-1. All transactions in our securities by Insiders must be pre-cleared by the Chief
Financial Officer.
Stockholder Communications
If you wish to communicate
with the Board, you may send your communication in writing to Entero Therapeutics, Inc., Attention: Chief Financial Officer–- 777
Yamato Road, Suite 502, Boca Raton, Florida 33431.
You must include your name
and address in the written communication and indicate whether you are a stockholder of the Company. The Chief Financial Officer will review
any communication received from a stockholder, and all material and appropriate communications from stockholders will be forwarded to
the appropriate director or directors or committee of the Board based on the subject matter.
Meetings of the Board
Each of our directors who
served during the year ended December 31, 2023 attended or participated in no less than 75% or more of the aggregate of (i) the total
number of meetings of the Board; and (ii) the total number of meetings held by all committees of the Board on which such director served
as a member during such year. Although directors are not required to attend our annual meeting of stockholders, they are encouraged to
attend. At the 2023 Annual Meeting, all of our then-serving directors were in attendance.
The following table represents
the composition of each committee of the Board and meetings held as well as actions taken by unanimous written consent (“UWC”)
in lieu of holding a meeting, during the fiscal year ended December 31, 2023:
| | |
| |
Committees |
| Director | |
Board | |
Audit | |
Compensation(1) | |
Corporate
Governance and
Nominating |
| Edward J. Borkowski | |
X | |
X | |
| |
CC |
| Charles J. Casamento | |
X | |
X | |
CC | |
|
| David Hoffman | |
X | |
| |
| |
|
| Alastair Riddell | |
X | |
| |
X | |
X |
| James Sapirstein | |
C | |
| |
| |
|
| Terry Coelho | |
X | |
CC | |
X | |
|
| Meetings Held During 2023 | |
9 | |
5 | |
3 | |
1 |
| Actions Taken by UWC During 2023 | |
18 | |
0 | |
0 | |
0 |
C–- Chairman of the Board
CC–- Committee Chairman
X – Member
| (1) |
Effective April 20, 2023, Mr. Casamento was appointed to the Compensation Committee as Chairman. |
Board Committees
The standing committees
of the Board consist of the Audit Committee, Compensation Committee, and Corporate Governance and Nominating Committee. Our Board has
adopted written charters for each of these committees, copies of which are available on our website at www.enterothera.com/investors.
Our Board may establish other committees as it deems necessary or appropriate from time to time.
Audit Committee
The duties and responsibilities
of the Audit Committee include but are not limited to:
| |
· |
appointing, compensating, retaining, evaluating, terminating, and overseeing our independent registered public accounting firm; |
| |
· |
discussing with our independent registered public accounting firm the independence of its members from its management; |
| |
· |
reviewing with our independent registered public accounting firm the scope and results of their audit; |
| |
· |
approving all audit and permissible non-audit services to be performed by our independent registered public accounting firm; |
| |
· |
overseeing the financial reporting process and discussing with management and our independent registered public accounting firm the interim and annual financial statements that are filed with the SEC; |
| |
· |
reviewing and monitoring our accounting principles, accounting policies, financial and accounting controls, and compliance with legal and regulatory requirements; |
| |
· |
coordinating oversight of the Code and our disclosure controls and procedures on behalf of the Board; |
| |
· |
establishing procedures for the confidential and/or anonymous submission of concerns regarding accounting, internal controls or auditing matters; and |
| |
· |
reviewing and approving related-person transactions. |
The rules of Nasdaq require
our Audit Committee to consist of at least three directors, all of whom must be deemed to be independent directors under Nasdaq rules.
The Board has affirmatively determined that Ms. Coelho and Messrs. Borkowski and Casamento, each meet the definition of “independent
director” for purposes of serving on an Audit Committee under Nasdaq rules. Additionally, the Board has determined that Ms. Coelho
and Messrs. Borkowski and Casamento each qualify as an “audit committee financial expert,” as such term is defined in Item
407(d)(5) of Regulation S-K.
Compensation Committee
The duties and responsibilities
of the Compensation Committee include but are not limited to:
| |
· |
reviewing key employee compensation goals, policies, plans and programs; |
| |
· |
reviewing and approving the compensation of our directors and executive officers; |
| |
· |
reviewing and approving employment agreements and other similar arrangements between us and our executive officers; and |
| |
· |
appointing and overseeing any compensation consultants or advisors to the Company. |
The Compensation Committee
may form subcommittees for any purpose that the Committee deems appropriate and may delegate to such subcommittees such power and authority
as the Compensation Committee deems appropriate; provided, however, that the Compensation Committee shall not delegate to a subcommittee
any power or authority required by any law, regulation or listing standard to be exercised by the Compensation Committee as a whole. The
Compensation Committee may also delegate to one or more executive officers of the Company the authority to make grants of equity-based
compensation to eligible individuals who are not executive officers. Any executive officer to whom the Compensation Committee grants such
authority shall regularly report to the Compensation Committee grants so made and the Compensation Committee may revoke any delegation
of authority at any time.
The rules of Nasdaq require
our Compensation Committee to consist entirely of independent directors. The Board has affirmatively determined that Mr. Casamento, Ms.
Coelho and Dr. Riddell meet the definition of “independent director” for purposes of serving on the Compensation Committee
under Nasdaq rules. The executive officers of the Company have no determinative authority over the amount or form of executive and director
compensation, though they may make recommendations to the Compensation Committee for consideration.
Corporate Governance and Nominating Committee
The duties and responsibilities
of the Corporate Governance and Nominating Committee include but are not limited to:
| |
· |
assisting the Board in identifying qualified individuals to become members of the Board; |
| |
· |
determining the composition of the Board and monitoring the activities of the Board to assess overall effectiveness; and |
| |
· |
developing and recommending to our Board corporate governance guidelines applicable to the Company and advising our Board on corporate governance matters. |
The rules of Nasdaq require
our Corporate Governance and Nominating Committee to consist entirely of independent directors. The Board has affirmatively determined
that Messrs. Borkowski and Dr. Riddell meet the definition of “independent director” for purposes of serving on the Corporate
Governance and Nominating Committee under Nasdaq rules.
EXECUTIVE COMPENSATION
The following table sets forth
information regarding our current executive officers as appointed by the Board, each to serve in such position until their respective
successors have been duly appointed and qualified or until their earlier death, resignation or removal from office.
| Executive Officer | |
Age | |
Title |
| James Sapirstein | |
62 | |
Chief Executive Officer and Non-Independent Director |
| Sarah Romano | |
44 | |
Chief Financial Officer |
| Jack Syage, Ph.D. | |
69 | |
President and Chief Scientific Officer |
Our executive officers are
appointed by and serve at the discretion of the Board, subject to the terms of any employment agreements they may have with us. The following
is a brief description of the qualifications and business experience of each of our current executive officers.
James
Sapirstein. Please see Mr. Sapirstein’s biography under the “Director Compensation” section of this proxy
statement.
Sarah
Romano was appointed to serve as our Chief Financial Officer on March 1, 2022. Ms. Romano previously served as Chief Financial
Officer of Kiora Pharmaceuticals, Inc. (NASDAQ: KPRX) (formerly EyeGate Pharmaceuticals, Inc.), a clinical-stage specialty pharmaceutical
company developing products for treating ophthalmic diseases, from February 2017 through February 2022 and as its Corporate Controller
from August 2016 to January 2017. Prior to joining Kiora, Ms. Romano served as Assistant Controller at TechTarget from June 2015 through
August 2016 and Corporate Controller at Bowdoin Group, a healthcare-focused executive recruiting firm, from September 2013 through May
2015. Previously, she held financial reporting positions of increasing responsibility at SoundBite Communications from 2008 until its
acquisition by Genesys in 2013, and at Cognex Corporation from 2004 through 2008. Ms. Romano began her career as an auditor in the Boston
office of PricewaterhouseCoopers. A licensed CPA in Massachusetts, she holds a Bachelor of Arts in Accounting from College of the Holy
Cross and a Master of Accounting from Boston College.
Jack
Syage. Please see Dr. Sayge’s biography under the “Director Compensation” section of this proxy statement.
Summary Compensation
The table set forth below
reflects certain information regarding the compensation paid or accrued during the years ended December 31, 2023 and 2022 to our Chief
Executive Officer and our executive officers, other than our Chief Executive Officer, who were serving as an executive officer as of December
31, 2022, and whose annual compensation exceeded $100,000 during such year (collectively the “Named Executive Officers”).
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Stock |
|
Option |
|
All Other |
|
|
|
| Named Executive Officers |
|
Year |
|
Salary |
|
Bonus |
|
Award |
|
Award |
|
Compensation |
|
Total |
| James Sapirstein |
|
2023 |
|
$ |
513,600 |
|
$ |
192,600 |
(1) |
$ |
339,566 |
(3) |
$ |
- |
|
$ |
200,000 |
(5) |
$ |
1,245,766 |
| Chief Executive Officer |
|
2022 |
|
$ |
480,000 |
|
$ |
96,000 |
(2) |
$ |
- |
|
$ |
165,720 |
(4) |
$ |
- |
|
$ |
741,720 |
| Sarah Romano |
|
2023 |
|
$ |
390,550 |
|
$ |
117,165 |
(1) |
$ |
185,676 |
(3) |
$ |
- |
|
$ |
117,165 |
(5) |
$ |
810,556 |
| Chief Financial Officer |
|
2022 |
|
$ |
304,166 |
|
$ |
54,000 |
(2) |
$ |
- |
|
$ |
135,503 |
(4) |
$ |
- |
|
$ |
493,669 |
| (1) |
Represents accrued and unpaid bonuses during 2023, as of December 31, 2023. |
| (2) |
Represents accrued and unpaid bonuses during 2022, as of December 31, 2022. |
| (3) |
Represents the grant date fair value of restricted stock units issued during the year ended December 31, 2023.The assumptions used in the calculation of these amounts are included in Note 11 of the notes to the consolidated financial statements contained in this Annual Report. |
| (4) |
Represents the grant date fair value of stock options issued during the year ended December 31, 2022, calculated in accordance with ASC Topic 718. The assumptions used in the calculation of these amounts are included in Note 11 of the notes to the consolidated financial statements contained in the Company’s Annual Report, filed with the SEC on March 20, 2023. |
| (5) |
Represents retention bonus paid during the year ended December 31, 2023. |
Employment Arrangements and Potential Payments upon Termination
or Change of Control
Current Named Executive Officers
Sapirstein Employment
Agreement. Effective October 8, 2019, we entered into an employment agreement with Mr. Sapirstein to serve
as our President and Chief Executive Officer for a term of three years, subject to further renewal upon agreement of the parties. The
employment agreement with Mr. Sapirstein originally provided for a base salary of $450,000 per year, which was subsequently increased
to $480,000 per year during the year ended December 31, 2020, to $513,600 during the year ended December 31, 2023, to $539,280 in January,
2024, and to $600,000 in June, 2024. In addition to the base salary, Mr. Sapirstein is eligible to receive (i) an initial bonus of up
to 40% of his base salary on an annual basis, based on certain milestones that are yet to be determined, that was increased to a bonus
of up to 50% of his base salary effective January 1, 2023, and increased to a bonus of up to 55% of his base salary, effective June 1,
2024; (ii) 1% of net fees received by us upon entering into license agreements with any third-party with respect to any product currently
in development or upon the sale of all or substantially all of our assets; (iii) a grant of 33 restricted shares of our Common Stock
which are subject to vesting as follows (a) 16 upon the first commercial sale of Adrulipase in the U.S., and (b) 17 upon our total market
capitalization exceeding $1.0 billion for 20 consecutive trading days; (iv) a grant of 50 10-year stock options to purchase shares of
our Common Stock which are subject to vesting as follows (a) 8 upon us initiating our next Phase 2 clinical trial in the U.S. for Adrulipase,
(b) 8 upon us completing our next or subsequent Phase 2 clinical trial in the U.S. for Adrulipase, (c) 17 upon us initiating a Phase
2 clinical trial in the U.S. for Adrulipase, and (d) 17 upon us initiating a Phase 1 clinical trial in the U.S. for any product other
than Adrulipase. Mr. Sapirstein is entitled to receive 20 days of paid vacation, participate in full employee health benefits and receive
reimbursement for all reasonable expenses incurred in connection with his services to us.
In the event that Mr. Sapirstein’s
employment is terminated by us for Cause, as defined in his employment agreement, or by Mr. Sapirstein voluntarily, then will not be entitled
to receive any payments beyond amounts already earned, and any unvested equity awards will terminate. In the event that Mr. Sapirstein’s
employment is terminated as a result of an Involuntary Termination Other than for Cause, as defined in the Agreement, Mr. Sapirstein will
be entitled to receive the following compensation: (i) severance in the form of continuation of his salary (at the Base Salary rate in
effect at the time of termination, but prior to any reduction triggering Good Reason) for a period of 12 months following the termination
date; (ii) payment of Executive’s premiums to cover COBRA for a period of 12 months following the termination date; and (iii) a
prorated annual bonus.
Romano Employment
Agreement. Effective March 1, 2022, we entered into an employment agreement with Ms. Romano to serve as our Chief Financial
Officer for a term of three years, subject to further renewal upon agreement of the parties. The employment agreement with Ms. Romano
provides for a base salary of $365,000 per year, which was subsequently increased to $390,550 during the year ended December 31, 2023,
increased to $410,078 in January, 2024, and increased to $435,000 in June, 2024. In addition to the base salary, Ms. Romano is eligible
to receive an annual milestone cash bonus of 35% of her annual salary based on certain milestones that will be established by our Board
or the Compensation Committee, which was subsequently increased to 40% on January 1, 2023. On March 1, 2022, Ms. Romano was granted stock
options to purchase 250 shares of Common Stock on March 1, 2022, with an exercise price of $708.00 per share, which shall vest in over
a term of three years pursuant to her employment agreement. Ms. Romano is entitled to receive 20 days of paid vacation, participate in
full employee health benefits and receive reimbursement for all reasonable expenses incurred in connection with her service to us. We
may terminate Ms. Romano’s employment agreement at any time, with or without Cause, as such term is defined in her employment agreement.
In the event that Ms. Romano’s
employment is terminated by us for Cause, as defined in Ms. Romano’s employment agreement, or by Ms. Romano voluntarily, she will
not be entitled to receive any payments beyond amounts already earned, and any unvested equity awards will terminate. If we terminate
her employment agreement without Cause, not in connection with a Change of Control, as such term is defined in Ms. Romano’s employment
agreement, she will be entitled to (i) all salary owed through the date of termination; (ii) any unpaid annual milestone bonus; (iii)
severance in the form of continuation of her salary for the greater of a period of six months following the termination date or the remaining
term of the employment agreement; (iv) payment of premiums to cover COBRA for a period of six months following the termination date; (v)
a prorated annual bonus equal to the target annual milestone bonus, if any, for the year of termination multiplied by the formula set
forth in the agreement. If we terminate Ms. Romano’s employment agreement without Cause, in connection with a Change of Control,
she will be entitled to the above and immediate accelerated vesting of any unvested options or other unvested awards.
New Executive Officers since December 31, 2023
Syage
Offer Letter. In connection with Dr. Syage’s appointment as an officer, on March 13, 2024, we entered into an offer
letter with Dr. Syage, pursuant to which he will receive a base annual salary of $350,000 and will be entitled to an incentive bonus of
up to 40% of his annual salary, dependent on meeting pre-determined objectives. Dr. Syage is entitled to receive 15 days of paid vacation,
participate in full employee health benefits, and is eligible for other employee benefits we offer.
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth
information regarding unexercised options, stock that has not vested and equity incentive awards held by each of the Named Executive Officers
outstanding as of December 31, 2023:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Market |
|
|
|
Market |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
value |
|
Number |
|
or payout |
| |
|
|
|
|
|
|
|
|
|
|
|
|
Number |
|
of |
|
of |
|
value of |
| |
|
|
|
|
|
|
|
|
|
|
|
|
of |
|
shares |
|
unearned |
|
unearned |
| |
|
|
|
|
|
|
|
|
|
|
|
|
Shares |
|
or units |
|
shares, |
|
shares, |
| |
|
|
|
Number of |
|
|
|
|
|
|
|
|
or units |
|
of stock |
|
units or |
|
units or |
| |
|
|
|
Securities |
|
Number of |
|
|
|
|
|
|
of stock |
|
that |
|
other |
|
other |
| |
|
|
|
underlying |
|
underlying |
|
|
|
|
|
|
that |
|
have |
|
rights |
|
rights |
| |
|
|
|
unexercised |
|
unexercised |
|
Option |
|
Option |
|
have not |
|
not |
|
that have |
|
that have |
| |
|
|
|
options (#) |
|
unearned |
|
exercise |
|
expiration |
|
vested |
|
vested |
|
not vested |
|
not |
|
Name |
|
Grant Date |
|
exercisable |
|
options (#) |
|
price ($) |
|
date |
|
(#) |
|
($) |
|
(#) |
|
vested ($) |
|
Named Executive
Officers |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
James Sapirstein |
|
10/08/2019 |
|
4 |
|
3 |
(1) |
$ |
23,520.00 |
|
10/07/2029 |
|
- |
|
$ |
- |
|
- |
|
$ |
- |
| |
|
10/08/2019 |
|
- |
|
- |
|
|
- |
|
10/07/2029 |
|
- |
|
$ |
- |
|
4 |
(2) |
$ |
112,000 |
| |
|
07/16/2020 |
|
7 |
|
- |
|
$ |
35,700.00 |
|
07/15/2030 |
|
- |
|
$ |
- |
|
- |
|
$ |
- |
| |
|
06/30/2021 |
|
14 |
|
5 |
(3) |
$ |
35,700.00 |
|
07/15/2030 |
|
- |
|
$ |
- |
|
- |
|
$ |
- |
| |
|
01/03/2022 |
|
23 |
|
11 |
(4) |
$ |
6,090.00 |
|
01/02/2032 |
|
- |
|
$ |
- |
|
- |
|
$ |
- |
| |
|
01/03/2023 |
|
- |
|
- |
|
|
- |
|
01/02/2033 |
|
- |
|
$ |
- |
|
535 |
(5) |
$ |
66,286 |
| |
|
12/29/2023 |
|
- |
|
- |
|
|
- |
|
12/28/2033 |
|
- |
|
$ |
- |
|
17,660 |
(6) |
$ |
74,172 |
|
Sarah Romano |
|
03/01/2022 |
|
12 |
|
23 |
(7) |
$ |
4,956.00 |
|
02/28/2032 |
|
- |
|
$ |
- |
|
- |
|
|
- |
| |
|
01/03/2023 |
|
- |
|
- |
|
|
- |
|
01/02/2033 |
|
- |
|
$ |
- |
|
267 |
(8) |
$ |
33,081 |
| |
|
12/29/2023 |
|
- |
|
- |
|
|
- |
|
12/28/2033 |
|
- |
|
$ |
- |
|
12,614 |
(9) |
$ |
52,978 |
| (1) |
Represents stock options issued to Mr. Sapirstein on October 8, 2019 under the terms of his employment agreement, which options will vest as to 3 shares upon our initiating a Phase 3 clinical trial in the U.S. for Adrulipase. |
| (2) |
Represents the restricted stock unit (“RSU”) award issued to Mr. Sapirstein on October 8, 2019 under the terms of his employment agreement, which RSU will vest as follows: (i) as to 2 shares upon the first commercial sale in the U.S. of Adrulipase, and (ii) as to 2 shares upon our total market capitalization exceeding $1.0 billion for 20 consecutive trading days. |
| (3) |
On June 30, 2021, the Board rescinded and cancelled certain stock option awards previously made under the 2014 Omnibus Equity Incentive Plan (the “2014 Plan”) (the “Prior Sapirstein Awards”) to Mr. Sapirstein and issued new stock options awards (the “New Sapirstein Awards”) under the 2020 Omnibus Equity Incentive Plan (the “2020 Plan”) in an equivalent amount and with equivalent exercise price, vesting and expiration terms to the Prior Sapirstein Awards. The terms of the New Sapirstein Awards covering 19 shares of the Common Stock at an exercise price of $35,700.00 per share comprised of (i) stock options to purchase 6 shares of Common Stock that vest over a term of 18 months in 18 equal monthly installments starting on February 16, 2022, (ii) stock options to purchase 4 shares of Common Stock that vested immediately upon the grant of such stock options, and (iii) stock options to purchase 9 shares of Common Stock subject to milestone-based vesting based upon the achievement of certain strategic milestones specified by the Board. |
| (4) |
Represents stock options issued to Mr. Sapirstein on January 3, 2022, which options will vest over a term of three years, in 36 equal monthly installments on each monthly anniversary of January 3, 2022. |
| (5) |
Represents the RSU award issued to Mr. Sapirstein on January 3, 2023, which RSU will vest over a term of one year on each three-month anniversary. |
| (6) |
Represents the RSU award issued to Mr. Sapirstein on December 29, 2023, which RSU vested upon the closing of the Merger. |
| |
|
| (7) |
Represents stock options issued to Ms. Romano on March 1, 2022, which options will vest over a term of three years, in three equal annual installments on each yearly anniversary of March 1, 2022. |
| |
|
| (8) |
Represents the RSU award issued to Ms. Romano on January 3, 2023, which RSU will vest over a term of one year on each three-month anniversary. |
| |
|
| (9) |
Represents the RSU award issued to Ms. Romano on December 29, 2023, which RSU vested upon the closing of the Merger. |
PAY VERSUS PERFORMANCE
The following tables and related
disclosures provide information about (i) the “total compensation” of our principal executive officer (“PEO”),
and our other named executive officers (the “Other NEOs” or the “Non-PEO NEOs”) as presented in
the table under “Executive Compensation – Summary Compensation”, (ii) the “compensation actually paid” to
our PEO and our Other NEOs, as calculated pursuant to the SEC’s pay-versus-performance rules, (iii) certain financial performance
measures, and (iv) the relationship of the “compensation actually paid” to those financial performance measures.
This disclosure has been prepared
in accordance with Item 402(v) of Regulation S-K under the Securities Exchange Act of 1934, as amended, and does not necessarily reflect
value actually realized by the executives or how our compensation committee evaluates compensation decisions in light of company or individual
performance.
| Year | | |
Summary
Compensation
Table Total for
PEO (1) | | |
Compensation
Actually Paid
to PEO (3) | | |
Average Summary
Compensation
Table Total for
Non-PEO NEOs(2) | | |
Average
Compensation
Actually Paid to Non-
PEO NEOs(3) | | |
Value of Initial Fixed
$100 Investment
Based On Total
Shareholder Return(4) | | |
Net Loss(5) | |
| | 2023 | | |
$ | 1,245,766 | | |
$ | 671,314 | | |
$ | 810,556 | | |
$ | 549,587 | | |
$ | (28.20 | ) | |
$ | (15,794,983 | ) |
| | 2022 | | |
$ | 741,720 | | |
$ | 184,270 | | |
$ | 389,870 | | |
$ | 171,330 | | |
$ | (48.49 | ) | |
$ | (14,629,642 | ) |
| | 2021 | | |
$ | 1,294,380 | | |
$ | 423,078 | | |
$ | 507,764 | | |
$ | 147,668 | | |
$ | (5.71 | ) | |
$ | (58,537,849 | ) |
| (1) |
The dollar amounts reported are the amounts of total compensation reported for James Sapirstein, our Chief Executive Officer / PEO, for each corresponding year in the “Total” column of the Summary Compensation Table. Refer to “Executive Compensation—Summary Compensation Table.” |
| |
|
| (2) |
The dollar amounts reported represent the average of the amounts reported for our company’s Non-PEO NEOs as a group (excluding Mr. Sapirstein) in the “Total” column of the Summary Compensation Table in each applicable year. The names of each of the named executive officers (excluding Mr. Sapirstein) included for purposes of calculating the average amounts in each applicable year are as follows: for 2023, Ms. Romano, for 2022, Ms. Romano, Mr. Schneiderman and Mr. Pennington, and for 2021, Mr. Schneiderman and Mr. Pennington. For 2022, the information reported under this heading combines compensation earned by Ms. Romano and Mr. Schneiderman as one NEO in 2022. Mr. Schneiderman resigned as Chief Financial Officer on February 28, 2022. Ms. Romano was appointed Chief Financial Officer on March 1, 2022. |
| |
|
| (3) |
The dollar amounts reported represent the amount of “compensation actually paid” to our PEO and Non-PEO NEOs as computed in accordance with Item 402(v) of Regulation S-K. The dollar amounts do not reflect the actual amount of compensation earned by or paid during the applicable year. In accordance with the requirements of Item 402(v) of Regulation S-K, the following adjustments were made to total compensation for each year to determine the compensation actually paid: |
| | |
2023 | | |
2022 | | |
2021 | |
| | |
PEO | | |
Average
Non-PEO
NEOs | | |
PEO | | |
Average
Non-PEO
NEOs | | |
PEO | | |
Average
Non-PEO
NEOs | |
| Summary Compensation Table Totals for Non-PEO NEOs | |
$ | 1,245,766 | | |
$ | 810,556 | | |
$ | 741,720 | | |
$ | 389,870 | | |
$ | 1,294,380 | | |
$ | 507,764 | |
| Add (Subtract): | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Fair value of equity awards granted during the year from the Summary Compensation Table | |
| (339,566 | ) | |
| (185,676 | ) | |
| (165,720 | ) | |
| (77,237 | ) | |
| (628,380 | ) | |
| (131,265 | ) |
| Fair value at year end of equity awards granted during the year | |
| 83,168 | | |
| 57,477 | | |
| 2,138 | | |
| 1,192 | | |
| 86,517 | | |
| 18,529 | |
| Change in fair value of equity awards granted in prior years that were unvested as of the end of the year | |
| (208,370 | ) | |
| (87,249 | ) | |
| (282,671 | ) | |
| - | | |
| (124,650 | ) | |
| (125,382 | ) |
| Change in fair value of equity awards granted in prior years that vested during the year | |
| (109,684 | ) | |
| (45,521 | ) | |
| (111,197 | ) | |
| (142,495 | ) | |
| (204,789 | ) | |
| (121,979 | ) |
| Compensation Actually Paid Totals | |
$ | 671,314 | | |
$ | 549,587 | | |
$ | 184,270 | | |
$ | 171,330 | | |
$ | 423,078 | | |
$ | 147,667 | |
| (4) |
Cumulative total shareholder return (“TSR”) is calculated by dividing the sum of the cumulative amount of dividends for the measurement period, assuming dividend reinvestment, and the difference between our company’s share price at the end and the beginning of the measurement period by our company’s share price at the beginning of the measurement period. No dividends were paid on stock or option awards in 2021, 2022 or 2023. |
| |
|
| (5) |
The dollar amounts reported represent the amount of net loss reflected in our consolidated audited financial statements for the applicable year. |
Analysis of the
Information Presented in the Pay Versus Performance Table
In
accordance with Item 402(v) of Regulation S-K, the graphs below compare the compensation actually paid to our PEO and the average of the
compensation actually paid to our remaining NEOs, with (i) our TSR, and (ii) our net income, in each case, for the fiscal years ended
December 31, 2021, 2022 and 2023. TSR amounts reported in the graph assume an initial fixed investment of $100.

A portion of our NEOs’
compensation consists of equity awards. As a result, the change between the values disclosed in our Summary Compensation Table and Compensation
Actually Paid tends to be directionally aligned with changes in our TSR.

While we are required
by SEC rules to disclose the relationship between our net income and Compensation Actually Paid to our NEOs, this is not a metric our
compensation committee currently uses in evaluating our NEOs’ compensation as we are a GI disease company that has not generated
any revenues from the sale of products.
All information provided
above under the “Pay Versus Performance” heading will not be deemed to be incorporated by reference in any filing of our company
under the Securities Act of 1933, as amended, whether made before or after the date hereof and irrespective of any general incorporation
language in any such filing.
Related Party Transactions
As of May 12, 2022, Messrs. Sapirstein
and Borkowski have entered into waiver agreements with the Company pursuant to which they have agreed to permanently waive the exchange
right related to their Series B Preferred Stock for any offering by the Company of its securities for cash consideration occurring
on or after January 1, 2022.
ImmunogenX Merger
In consideration for the
Merger, our company issued approximately $240,500 in Common Stock and $76.9 million in Series G Preferred Stock to the shareholders of
IMGX. As a result of this payment, Dr. Syage received 15,400 shares of our Common Stock and 4,920.037 shares of Series G Preferred Stock,
in exchange for shares of IMGX common stock that he held immediately prior to the Merger. The shares issued to Dr. Syage were valued
at approximately $32.2 million. Dr. Syage is a related party as a director. Dr. Khosla received 440 shares of our Common Stock and 140.70
shares of Series G Preferred Stock, in exchange for shares of IMGX common stock that he held immediately prior to the Merger. The shares
issued to Dr. Khosla were valued at approximately $0.9 million. Dr. Khosla is a related party as a director.
Stockholder Notes
On March 13, 2024, in
connection with the Merger and in order to fund the payment of $1 million to Lender, IMGX entered into the Syage Note. The Syage Note
bears interest at a per annum floating rate equal to the prime rate plus 4.5% and matures on October 13, 2025. Dr. Syage entered into
a patent and trademark security agreement dated March 13, 2024 (the “Shareholder Security Agreement”), pursuant to
which the obligations under the Syage Note are secured by, among other things, the patents and trademarks owned by IMGX.
The foregoing descriptions
of the Syage Note and the Shareholder Security Agreement do not purport to be complete and are qualified in their entirety by reference
to the Form of Shareholder Note and Form of Shareholder Security Agreement, which are incorporated by reference to Exhibits 10.58 and
10.59, respectively, of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC
on March 29, 2024.
Policy and Procedures Governing Related Party Transactions
The Board is committed to
upholding the highest legal and ethical conduct in fulfilling its responsibilities and recognizes that related party transactions can
present a heightened risk of potential or actual conflicts of interest.
The SEC rules define a related
party transaction to include any transaction, arrangement or relationship which: (i) we are a participant; (ii) the amount involved exceeds
$120,000; and (iii) executive officer, director or director nominee, or any person who is known to be the beneficial owner of more than
5% of our Common Stock, or any person who is an immediate family member of an executive officer, director or director nominee or beneficial
owner of more than 5% of our Common Stock had or will have a direct or indirect material interest.
Although we do not maintain
a formal written procedure for the review and approval of transactions with such related persons, it is our policy for the disinterested
members of our Board to review all related party transactions on a case-by-case basis. To receive approval, a related-party transaction
must have a legitimate business purpose for us and be on terms that are fair and reasonable to us and our stockholders and as favorable
to us and our stockholders as would be available from non-related entities in comparable transactions.
All related party transactions must be disclosed
in our applicable filings with the SEC as required under SEC rules.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange
Act, requires our officers, directors, and persons who beneficially own more than 10% of our Common Stock to file reports of ownership
and changes in ownership with the SEC. Officers, directors, and greater-than-ten-percent stockholders are also required by the SEC to
furnish us with copies of all Section 16(a) forms that they file.
Based solely upon a review
of these forms that were furnished to us, we believe that all reports required to be filed by these individuals and persons under Section
16(a) were filed during the year ended December 31, 2023 and that such filings were timely.
Report of the Audit Committee
The following Report of the Audit Committee
of the Board of Directors shall not be deemed to be soliciting material or to be incorporated by reference by any general statement incorporating
by reference this proxy statement into any filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934,
as amended, except to the extent the Company specifically incorporates this Report therein, and shall not otherwise be deemed filed under
such Acts.
The Audit Committee has
reviewed and discussed with management and Mazars USA LLP, our independent registered public accounting firm, the audited consolidated
financial statements in the Entero Therapeutics, Inc. (formerly AzurRx BioPharma, Inc.) Annual Report on Form 10-K for the year ended
December 31, 2023 (filed with the SEC on March 29, 2024). The Audit Committee has also discussed with Mazars USA LLP those matters required
to be discussed by Public Company Accounting Oversight Board (“PCAOB”) Auditing Standard 1301.
Mazars USA LLP also provided the Audit Committee
with the written disclosures and the letter required by the applicable requirements of the PCAOB regarding the independent auditor’s
communication with the Audit Committee concerning independence. The Audit Committee has discussed with the registered public accounting
firm their independence from our Company.
Based on its discussions with
management and the independent registered public accounting firm, and its review of the representations and information provided by management
and the independent registered public accounting firm, including as set forth above, the Audit Committee recommended to our Board of Directors
that the audited consolidated financial statements be included in our Annual Report on Form 10-K for the year ended December 31, 2023
(filed with the SEC on March 29, 2024).
| RESPECTFULLY SUBMITTED, |
| |
| Terry Coelho, Chair |
| |
| Edward J. Borkowski |
| |
| Charles J. Casamento |
BENEFICIAL OWNERSHIP OF PRINCIPAL STOCKHOLDERS
AND
MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Beneficial Ownership Prior to Reverse Split and Series G Conversion
The following table sets forth information
regarding shares of our capital stock beneficially owned as of June 12, 2024, prior to giving effect to the proposed Reverse Split and the conversion of any Series G Preferred Stock by:
| |
· |
each of our officers and directors; |
| |
· |
all officers and directors as a group; and |
| |
· |
each person known by
us to beneficially own five percent or more of the outstanding shares of our capital stock. Percentage of ownership is calculated
based on 2,750,108 shares of Common Stock outstanding as of June 12, 2024. |
| | |
| | |
Percent | |
| | |
Number | | |
Ownership | |
| | |
of Shares | | |
of Class | |
| Name and Address
of Beneficial Owner (1) | |
(2) | | |
(3) | |
| Current Named Executive Officers and Directors | |
| | | |
| | |
| James Sapirstein, Chief Executive Officer and
Chairman (4) | |
| 27,162 | | |
| * | % |
| Sarah Romano, Chief Financial Officer (5) | |
| 15,635 | | |
| * | |
| Edward J. Borkowski, Director (6) | |
| 5,552 | | |
| * | |
| Charles J. Casamento, Director (7) | |
| 5,222 | | |
| * | |
| Alastair Riddell, Director (8) | |
| 5,525 | | |
| * | |
| Terry Coelho, Director (9) | |
| 5,521 | | |
| * | |
| Jack Syage, President, Chief Scientific Officer, President
and Director (10) | |
| 15,400 | | |
| * | |
| Chaitan Khosla, Director (11) | |
| 61,716 | | |
| 2.20 | |
| All directors and executive officer as a group (8 persons) | |
| 141,733 | | |
| 5.06 | % |
All Directors and Executive Officers as a group (8 persons)
| (1) |
Unless
otherwise indicated, the address of such individual is c/o Entero Therapeutics, Inc., 777 Yamato Rd., Suite 502, Boca Raton, FL 33431. |
| (2) |
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. All entries exclude beneficial ownership of shares issuable pursuant to warrants, options or other derivative securities that have not vested or that are not otherwise exercisable as of the date hereof or which will not become vested or exercisable within 60 days. |
| (3) |
Percentages are rounded to nearest tenth of a percent. Percentages are based on 2,475,090 shares of Common Stock outstanding. Warrants, options or other derivative securities that are presently exercisable or exercisable within 60 days are deemed to be beneficially owned by the person holding such securities for the purpose of computing the percentage ownership of that person but are not treated as outstanding for the purpose of computing the percentage of any other person. |
| (4) |
Includes
(i) 56 shares of Common Stock issuable upon exercise of vested options; (ii) 27,101 shares of Common Stock issuable upon vested Restricted
Stock Units (“RSUs”); (iii) 4 shares of Common Stock issuable upon conversion of approximately 13.53 shares of
Series B Preferred Stock, which includes accrued and unpaid dividends through June 12, 2024; and (iv) 1 share of Common Stock issuable
upon exercise of warrants. Excludes (i) 11 shares of Common Stock issuable upon exercise of unvested options and (ii) 10,004 shares
of Common Stock issuable upon unvested RSUs. Pursuant to the Series B Exchange Right, Mr. Sapirstein has the right to exchange the
stated value, plus accrued and unpaid dividends, of the shares of Series B Preferred Stock beneficially owned by him for shares of
Common Stock on a dollar-for-dollar basis. |
| (5) |
Includes
(i) 24 shares of Common Stock issuable upon exercise of vested options and (ii) 15,611 shares of Common Stock issuable upon vested
RSUs. Excludes (i) 11 shares of Common Stock issuable upon exercise of unvested options and (ii) 3,500 shares of Common Stock issuable
upon unvested RSUs. |
| (6) |
Includes
(i) 10 shares of Common Stock; (ii) 6 shares of Common Stock issuable upon the exercise of warrants; (iii) 9 shares of Common Stock
issuable upon exercise of vested options; (iv) 5,513 shares of Common Stock issuable upon vested RSUs; and (v) 14 shares of Common
Stock issuable upon conversion of approximately 48.043 shares of Series B Preferred Stock, which includes accrued and unpaid dividends
through June 12, 2024. Excludes 9,818 unvested and unissued restricted shares of Common Stock and RSUs. Pursuant to the Series B
Exchange Right, Mr. Borkowski has the right to exchange the stated value, plus accrued and unpaid dividends, of the shares of Series
B Preferred Stock beneficially owned by him for shares of Common Stock on a dollar-for-dollar basis. |
| (7) |
Includes
(i) 2 shares of Common Stock; (ii) 9 shares of Common Stock issuable upon exercise of vested options; and (iii) 5,211 shares of Common
Stock issuable upon vested RSUs. Excludes 9,817 shares of Common Stock issuable upon unvested RSUs. |
| (8) |
Includes
(i) 3 shares of Common Stock; (ii) 9 shares of Common Stock issuable upon exercise of vested options; and (iii) 5,513 shares of Common
Stock issuable upon vested RSUs. Excludes (i) 9,817 unvested restricted shares of Common Stock and RSUs. |
| (9) |
Includes
(i) 8 shares of Common Stock issuable upon exercise of vested options and (ii) 5,513 shares of Common Stock issuable upon vested
RSUs. Excludes 9,817 shares of Common Stock issuable upon unvested RSUs. |
| (10) |
Includes
15,400 shares of Common Stock. Excludes 4,920,037 shares of Common Stock issuable upon conversion of 4,920.037 shares of Series G
Preferred Stock. |
| (11) |
Includes
(i) 440 shares of Common Stock; (ii) 8,296 shares of Common Stock issuable upon vested RSUs; and (iii) 52,980 shares of Common Stock
issuable upon exercise of vested options. Excludes 140,700 shares of Common Stock issuable upon conversion of 140.70 shares of Series
G Preferred Stock. |
Beneficial Ownership After Series G Conversion
The following table sets forth information
regarding shares of our capital stock beneficially owned as of June 12, 2024, assuming conversion of 12,373.226 shares of our Series
G Preferred Stock into 12,373,226 shares of our Common Stock, by:
| |
· |
each of our current officers
and directors; |
| |
· |
all current officers and directors as a group; and |
| |
· |
each person currently
known by us to beneficially own five percent or more of the outstanding shares of our capital stock. Percentage of ownership is
calculated based on 15,123,334 shares, which includes 2,750,108 shares of Common Stock outstanding as of June 12, 2024 plus the
assumed conversion of 12,373.226 shares of Series G Preferred Stock into 12,373,226
shares of Common Stock. |
| | |
| | |
Percent | |
| | |
Number | | |
Ownership | |
| | |
of Shares | | |
of Class | |
| Name and Address
of Beneficial Owner (1) | |
(2) | | |
(3) | |
| Current Named Executive Officers and Directors | |
| | | |
| | |
| James Sapirstein, Chief Executive Officer and
Chairman (4) | |
| 27,162 | | |
| * | % |
| Sarah Romano, Chief Financial Officer (5) | |
| 15,635 | | |
| * | |
| Edward J. Borkowski, Director (6) | |
| 5,552 | | |
| * | |
| Charles J. Casamento, Director (7) | |
| 5,222 | | |
| * | |
| Alastair Riddell, Director (8) | |
| 5,525 | | |
| * | |
| Terry Coelho, Director (9) | |
| 5,521 | | |
| * | |
| Jack Syage, President, Chief Scientific Officer, President
and Director (10) | |
| 4,935,437 | | |
| 32.63 | |
| Chaitan Khosla, Director (11) | |
| 219,009 | | |
| 1.33 | |
| All directors and executive officer as a group (8 persons) | |
| 5,271,858 | | |
| 34.28 | % |
All Directors and Executive Officers as a group (8 persons)
| (1) |
Unless otherwise
indicated, the address of such individual is c/o Entero Therapeutics, Inc., 777 Yamato Rd., Suite 502, Boca Raton, FL 33431. |
| (2) |
Beneficial
ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to
securities. All entries exclude beneficial ownership of shares issuable pursuant to warrants, options or other derivative securities
that have not vested or that are not otherwise exercisable as of the date hereof or which will not become vested or exercisable within
60 days. |
| (3) |
Percentages
are rounded to nearest tenth of a percent. Percentages are based on 2,750,108 shares of Common Stock outstanding on June 12, 2024,
plues the assumed conversion of 12,373.226 shares of Series G Preferred Stock into 12,373,226 shares of common stock, for an assumed
aggregate of 15,123,334 shares of Common Stock outstanding. Warrants, options or other derivative securities that are presently exercisable
or exercisable within 60 days are deemed to be beneficially owned by the person holding such securities for the purpose of computing
the percentage ownership of that person but are not treated as outstanding for the purpose of computing the percentage of any other
person. |
| (4) |
Includes
(i) 56 shares of Common Stock issuable upon exercise of vested options; (ii) 27,101 shares of Common Stock issuable upon vested Restricted
Stock Units (“RSUs”); (iii) 4 shares of Common Stock issuable upon conversion of approximately 13.53 shares of
Series B Preferred Stock, which includes accrued and unpaid dividends through June 12, 2024; and (iv) 1 share of Common Stock issuable
upon exercise of warrants. Excludes (i) 11 shares of Common Stock issuable upon exercise of unvested options and (ii) 10,004 shares
of Common Stock issuable upon unvested RSUs. Pursuant to the Series B Exchange Right, Mr. Sapirstein has the right to exchange the
stated value, plus accrued and unpaid dividends, of the shares of Series B Preferred Stock beneficially owned by him for shares of
Common Stock on a dollar-for-dollar basis. |
| (5) |
Includes
(i) 24 shares of Common Stock issuable upon exercise of vested options and (ii) 15,611 shares of Common Stock issuable upon vested
RSUs. Excludes (i) 11 shares of Common Stock issuable upon exercise of unvested options and (ii) 3,500 shares of Common Stock issuable
upon unvested RSUs. |
| (6) |
Includes
(i) 10 shares of Common Stock; (ii) 6 shares of Common Stock issuable upon the exercise of warrants; (iii) 9 shares of Common Stock
issuable upon exercise of vested options; (iv) 5,513 shares of Common Stock issuable upon vested RSUs; and (v) 14 shares of Common
Stock issuable upon conversion of approximately 48.043 shares of Series B Preferred Stock, which includes accrued and unpaid dividends
through June 12, 2024. Excludes 9,818 unvested and unissued restricted shares of Common Stock and RSUs. Pursuant to the Series B
Exchange Right, Mr. Borkowski has the right to exchange the stated value, plus accrued and unpaid dividends, of the shares of Series
B Preferred Stock beneficially owned by him for shares of Common Stock on a dollar-for-dollar basis. |
| (7) |
Includes
(i) 2 shares of Common Stock; (ii) 9 shares of Common Stock issuable upon exercise of vested options; and (iii) 5,211 shares of Common
Stock issuable upon vested RSUs. Excludes 9,817 shares of Common Stock issuable upon unvested RSUs. |
| (8) |
Includes
(i) 3 shares of Common Stock; (ii) 9 shares of Common Stock issuable upon exercise of vested options; and (iii) 5,513 shares of Common
Stock issuable upon vested RSUs. Excludes (i) 9,817 unvested restricted shares of Common Stock and RSUs. |
| (9) |
Includes
(i) 8 shares of Common Stock issuable upon exercise of vested options and (ii) 5,513 shares of Common Stock issuable upon vested
RSUs. Excludes 9,817 shares of Common Stock issuable upon unvested RSUs. |
| (10) |
Includes
(i) 15,400 shares of Common Stock and (ii) 4,920,037 shares of Common Stock upon conversion of 4,920.037 shares of Series G Preferred
Stock. |
| (11) |
Includes
(i) 440 shares of Common Stock; (ii) 8,296 shares of Common Stock issuable upon vested RSUs; (iii) 52,980 shares of Common
Stock issuable upon exercise of vested options; and (iv) 140,700 shares of Common Stock upon conversion of 140.70 shares of Series
G Preferred Stock. |
PROPOSAL NO. 2: APPROVAL OF AN AMENDMENT TO
OUR 2020 OMNIBUS EQUITY INCENTIVE PLAN (i) TO INCREASE THE NUMBER OF SHARES OF COMMON STOCK AUTHORIZED FOR ISSUANCE THEREUNDER FROM 58,374
TO 1,500,000, (ii) TO INCREASE THE NUMBER OF SHARES THAT OTHERWISE BECOME AVAILABLE UNDER THE PLAN FOR GRANTS AS INCENTIVE STOCK OPTIONS
(ISOs) FROM 250,000 TO 750,000 MILLION, AND (iii) TO INCREASE THE NUMBER OF SHARES THAT MAY BE GRANTED TO ANY ONE NON-EMPLOYEE DIRECTOR
OF THE BOARD FROM 5 TO 250,000.
General
The general purpose of our
2020 Omnibus Equity Incentive Plan (the “Plan”), is to benefit our Company and its shareholders by assisting the Company
and its subsidiaries to attract, retain and provide incentives to key management employees, officers, directors, and consultants of the
Company and its affiliates, and to align the interests of such service providers with those of the Company’s shareholders.
Our Board believes that the
granting of stock options, restricted stock awards, restricted stock units, stock appreciation rights and similar kinds of cash-based
and equity-based compensation promotes continuity of management and provides a critical incentive to align the interests of those who
are primarily responsible for shaping and carrying out our long range plans and securing our growth and financial success with the interests
of our shareholders.
On
May 7, 2024 our Board approved an amendment (i) increasing the number of
shares of our common stock available for issuance under the Plan from 58,374 to 1,500,000, (ii) increasing the number of shares of our
common stock otherwise available under the Plan that may be granted as “incentive stock options” (“ISOs”)
under Section 422 of the Internal Revenue Code of 1986, as amended (the “Code”) from 250,000 to 750,000 shares, and
(iii) increasing the number of shares that may be granted to any one non-employee director of the Board from 5 to 250,000. The Board directed
that the amendment be submitted to the stockholders for approval at the Annual Meeting. A copy of the amendment is attached as Annex
A.
If the Company’s stockholders
do not approve the increase in the number of shares available for issuance under the Plan, the Company will continue to operate the Plan
under its current provisions, but will be limited in its ability to make future grants and incentives under the Plan to individuals we
believe are and in the future will be critical to the Company’s success.
Description of the Existing Plan
The following description
of the material terms of the Plan is intended to be a summary only. This summary is qualified in its entirety by the full text of the
Plan, as amended, which is incorporated herein by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed
with the SEC on November 16, 2020 and by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC
on June 23, 2023.
Administration. The
Plan is administered by a committee of our Board (the “Committee”). The Committee has authority to determine the persons
to whom options to purchase shares of our common stock, stock appreciation rights (“SARs”), restricted stock units,
restricted or unrestricted shares of our common stock, performance stock awards, performance units, other cash-based awards and other
stock-based awards (collectively, “Awards”) may be granted. The Committee may also establish rules and regulations
for the administration of the Plan and amendments or modifications of outstanding Awards. The Committee may delegate authority to one
or more “reporting persons” (as defined in the Plan), including officers, to grant options and other Awards to employees (other
than themselves), subject to applicable law and the Plan. No options, stock purchase rights or other Awards may be made under the Plan
on or after August 10, 2030, but the Plan will continue thereafter while previously granted options, SARs or other Awards remain outstanding.
Eligibility. Persons
eligible to receive options, SARs or other Awards under the Plan are all employees, officers, directors, consultants, advisors or other
individual service providers of our Company and our affiliates, or any person who is determined by the Committee to be a prospective employee,
officer, director, consultant, advisor or other individual service provider of the Company or any affiliate (including prospective employees,
officers, directors, consultants, advisors or other individual services providers as long as they commence employment or engagement with
the Company or any affiliate).
As of June 12, 2024, the
Company and its affiliates had a total of fifteen employees, zero officers, three executive officers (who are not included in the number
of officers), five non-employee directors, and approximately six consultants, other advisors, and individual service providers.
Shares Subject to
the Plan. As of June 12, 2024, after giving effect to four reverse stock splits, previous amendments
to the Plan, and increases in the shares available under the Plan in accordance with the “evergreen provision” discussed
below, an aggregate of 334,078 shares of our common stock were reserved for issuance under the Plan, of which approximately 125,315 shares
remain available for issuance as of the date of this proxy statement. If this Proposal No. 2 is approved by our stockholders, the amendment
adopted by the Board will increase the number of shares of our common stock available under the Plan to 1,500,000 (subject to future
increase in accordance with the Plan’s evergreen provision).
The Plan contains an “evergreen
provision” providing for an annual increase in the number of shares of our common stock available for issuance under the Plan on
each January 1st in an amount equal to ten percent (10%) of the total number of shares of common stock outstanding on each December 31st
of the immediately preceding year on an “as converted basis”. As converted shares include all shares of our outstanding common
stock and all shares of our common stock issuable upon the conversion of outstanding preferred stock, warrants and other convertible securities,
but do not include any shares of common stock issuable upon the exercise of options and other Awards pursuant to the Plan or the Company’s
Amended and Restated 2014 Omnibus Equity Incentive Plan. The Board or Committee may determine that no increase in the share reserve will
be made for any year or determine that a lesser number of shares to be added.
If any option or SAR granted
under the Plan terminates without having been exercised in full or if any other Award is forfeited, or if shares of common stock are withheld
to cover withholding taxes on options or other Awards or applied to the payment of the exercise price of an option or purchase price of
an Award, the number of shares of common stock as to which such option or Award was forfeited, withheld or paid, will be available for
future grants under the Plan.
All 334,078 shares of our
common stock currently reserved for issuance under the Plan may be granted as ISO. If this Proposal No.2 is approved by stockholders,
up to 750,000 shares of common stock that may otherwise become available for Awards under the Plan may be granted as ISOs.
The number of shares authorized
for issuance under the Plan and the foregoing share limitations are subject to customary adjustments for stock splits, stock dividends
or similar transactions.
Terms
and Conditions of Options. Options granted under the Plan may be either ISOs or “nonstatutory
stock options” that do not meet the requirements of Section 422 of the Code. The Committee will determine the exercise price of
options granted under the Plan. The exercise price of stock options may not be less than the fair market value per share of our common
stock on the date of grant (or 110% of fair market value in the case of ISOs granted to a ten-percent stockholder).
If on the date of grant
our common stock is listed on a stock exchange or is quoted on the automated quotation system of Nasdaq, the fair market value will generally
be the closing sale price on the date of grant (or the last trading day before the date of grant if no trades occurred on the date of
grant). If no such prices are available, the fair market value will be determined in good faith by the Committee based on the reasonable
application of a reasonable valuation method. On June 13, 2024, the closing sale price of a share of our common stock on Nasdaq was $2.25.
No option may be exercisable
for more than ten years (five years in the case of an ISO granted to a ten-percent stockholder) from the date of grant. Options granted
under the Plan will be exercisable at such time or times as the Committee prescribes at the time of grant. No employee may receive ISOs
that first become exercisable in any calendar year in an amount exceeding $100,000. The Committee may, in its discretion, permit a holder
of an option to exercise the option before it has otherwise become exercisable, in which case the shares of our common stock issued to
the recipient will continue to be subject to the vesting requirements that applied to the option before exercise.
Generally, the option price
may be paid (a) in cash or by certified check, bank draft or money order, (b) through delivery of shares of our common stock having a
fair market value equal to the purchase price, or (c) a combination of these methods. The Committee is also authorized to establish a
cashless exercise program and to permit the exercise price (or tax withholding obligations) to be satisfied by reducing from the shares
otherwise issuable upon exercise a number of shares having a fair market value equal to the exercise price.
No option may be transferred
other than by will or by the laws of descent and distribution, and during a recipient’s lifetime an option may be exercised only
by the recipient or the recipient’s guardian or legal representative. However, the Committee may permit the holder of an option,
SAR or other Award to transfer the option, right or other award to family members or a family trust for estate planning purposes. The
Committee will determine the extent to which a holder of a stock option may exercise the option following termination of service with
us.
Stock Appreciation
Rights. The Committee may grant SARs, independent of or in connection with an option. The Committee
will determine the other terms applicable to SARs. The exercise price per share of a SAR will not be less than 100% of the fair market
value of a share of our common stock on the date of grant, as determined by the Committee. The maximum term of any SAR granted under
the Plan is ten years from the date of grant. Generally, each SAR will entitle a participant upon exercise to an amount equal to:
| · | the
excess of the fair market value on the exercise date of one share of our common stock over the exercise price, multiplied by |
| · | the
number of shares of common stock covered by the SAR. |
Payment may be made in shares
of our common stock, in cash, or partly in common stock and partly in cash, all as determined by the Committee.
Restricted
Stock, Unrestricted Stock and Restricted Stock Units. The Committee may award restricted
common stock, unrestricted shares of common stock and/or restricted stock units under the Plan. Restricted stock awards consist of shares
of common stock that are transferred to a participant subject to restrictions that may result in forfeiture if specified conditions are
not satisfied. Unrestricted stock awards consist of shares of common stock that are not subject to any restrictions. Restricted stock
units confer the right to receive shares of our common stock, cash, or a combination of shares and cash, at a future date upon or following
the attainment of certain conditions specified by the Committee. The restrictions and conditions applicable to each award of restricted
stock or restricted stock units may include performance-based conditions. Dividends with respect to restricted stock or unrestricted stock
may be paid to the holder of the shares as and when dividends are paid to stockholders or at the time that the restricted stock vests,
if applicable, as determined by the Committee. Dividend equivalent amounts may be paid with respect to restricted stock units either when
cash dividends are paid to stockholders or when the units vest. Unless the Committee determines otherwise, holders of restricted stock
and unrestricted stock will have the right to vote the shares.
Performance
Stock Awards and Performance Units. The Committee may award performance stock awards
and/or performance units under the Plan. Performance stock awards and performance units are awards, denominated in either shares of common
stock or U.S. dollars, which are earned during a specified performance period subject to the attainment of performance criteria, as established
by the Committee. The Committee will determine the restrictions and conditions applicable to each award of performance stock awards and
performance units.
Incentive
Bonus Awards, Other Stock-Based and Cash-Based Awards. The Committee may award other
types of equity-based or cash-based awards under the Plan, including the grant or offer for sale of shares of our common stock that do
not have vesting requirements and the right to receive one or more cash payments subject to satisfaction of such conditions as the Committee
may impose.
Effect
of Certain Corporate Transactions. The Committee may, at the time of the grant of an
Award provide for the effect of a change of control (as defined in the Plan) on any Award, including (i) accelerating or extending the
time periods for exercising, vesting in, or realizing gain from any award, (ii) eliminating or modifying the performance or other conditions
of an award, or (iii) providing for the cash settlement of an award for an equivalent cash value, as determined by the committee. The
Committee may, in its discretion and without the need for the consent of any recipient of an award, also take one or more of the following
actions contingent upon the occurrence of a change of control: (a) cause any or all outstanding options and SARs to become immediately
exercisable, in whole or in part; (b) cause any other Awards to become non-forfeitable, in whole or in part; (c) cancel any
option or SAR in exchange for a substitute option; (d) cancel any Award of restricted stock, restricted stock units, performance stock
awards or performance units in exchange for a similar Award of the capital stock of any successor corporation; (e) redeem any restricted
stock, restricted stock unit, performance share or performance unit for cash and/or other substitute consideration with a value equal
to the fair market value of an unrestricted share of our common stock on the date of the change of control; or (f) cancel any option or
SAR in exchange for cash and/or other substitute consideration based on the value of our common stock on the date of the change of control,
and cancel any option or SAR without any payment if its exercise price exceeds the value of our common stock on the date of the change
of control.
Amendment,
Termination. Our Board may at any time amend the Plan for the purpose of satisfying the
requirements of the Code, or other applicable law or regulation or for any other legal purpose, provided that, without the consent of
our stockholders, the Board may not (a) increase the number of shares of common stock available under the Plan, or (b) change the group
of individuals eligible to receive options, SARs and/or other awards.
New Plan Benefits
Grants of Awards under the Plan are discretionary
and we cannot determine now the number of type of options or other awards to be granted in the future to any particular person or group.
Material Federal Income Tax Consequences
The following is a summary
of the principal federal income tax consequences of option and other Awards under the Plan. Optionees and recipients of other rights and
Awards granted under the Plan are advised to consult their personal tax advisors before exercising an option or SAR or disposing of any
stock received pursuant to the exercise of an option or SAR or following vesting of a restricted stock award or restricted stock unit
or upon grant of an unrestricted stock award. In addition, the following summary is based upon an analysis of the Code as currently in
effect, existing laws, judicial decisions, administrative rulings, regulations and proposed regulations, all of which are subject to change
and does not address state, local or other tax laws.
Treatment of Options
The Code treats incentive
stock options and nonstatutory stock options differently. However, as to both types of options, no income will be recognized to the optionee
at the time of the grant of the options under the Plan, nor will our Company be entitled to a tax deduction at that time.
Generally, upon exercise of
a nonstatutory stock option (including an option intended to be an incentive stock option but which has not continued to so qualify at
the time of exercise), an optionee will recognize ordinary income tax on the excess of the fair market value of the stock on the exercise
date over the option price. Our Company will be entitled to a tax deduction in an amount equal to the ordinary income recognized by the
optionee in the fiscal year which includes the end of the optionee’s taxable year. We will be required to satisfy applicable withholding
requirements in order to be entitled to a tax deduction. In general, if an optionee, in exercising a nonstatutory stock option, tenders
shares of our common stock in partial or full payment of the option price, no gain or loss will be recognized on the tender. However,
if the tendered shares were previously acquired upon the exercise of an incentive stock option and the tender is within two years from
the date of grant or one year after the date of exercise of the incentive stock option, the tender will be a disqualifying disposition
of the shares acquired upon exercise of the incentive stock option.
For incentive stock options,
there is no taxable income to an optionee at the time of exercise. However, the excess of the fair market value of the stock on the date
of exercise over the exercise price will be taken into account in determining whether the “alternative minimum tax” will apply
for the year of exercise. If the shares acquired upon exercise are held until at least two years from the date of grant and more than
one year from the date of exercise, any gain or loss upon the sale of such shares, if held as capital assets, will be long-term capital
gain or loss (measured by the difference between the sales price of the stock and the exercise price). Under current federal income tax
law, a long-term capital gain will be taxed at a rate which is less than the maximum rate of tax on ordinary income. If the two-year and
one year holding period requirements are not met (a “disqualifying disposition”), an optionee will recognize ordinary income
in the year of disposition in an amount equal to the lesser of (i) the fair market value of the stock on the date of exercise minus the
exercise price or (ii) the amount realized on disposition minus the exercise price. The remainder of the gain will be treated as long-term
capital gain, depending upon whether the stock has been held for more than a year. If an optionee makes a disqualifying disposition, our
Company will be entitled to a tax deduction equal to the amount of ordinary income recognized by the optionee.
In general, if an optionee,
in exercising an incentive stock option, tenders shares of common stock in partial or full payment of the option price, no gain or loss
will be recognized on the tender. However, if the tendered shares were previously acquired upon the exercise of another incentive stock
option and the tender is within two years from the date of grant or one year after the date of exercise of the other option, the tender
will be a disqualifying disposition of the shares acquired upon exercise of the other option.
As noted above, the exercise
of an incentive stock option could subject an optionee to the alternative minimum tax. The application of the alternative minimum tax
to any particular optionee depends upon the particular facts and circumstances which exist with respect to the optionee in the year of
exercise. However, as a general rule, the amount by which the fair market value of the common stock on the date of exercise of an option
exceeds the exercise price of the option will constitute an item of “adjustment” for purposes of determining the alternative
minimum taxable income on which the alternative tax may be imposed. As such, this item will enter into the tax base on which the alternative
minimum tax is computed and may therefore cause the alternative minimum tax to become applicable in any given year.
Treatment of Stock Appreciation Rights
Generally, the recipient of
a SAR will not recognize any income upon grant of the SAR, nor will our Company be entitled to a deduction at that time. Upon exercise
of a SAR, the holder will recognize ordinary income, and our Company generally will be entitled to a corresponding deduction equal to
the fair market value of our common stock at that time.
Treatment of Stock Awards
Generally, absent an election
to be taxed currently under Section 83(b) of the Code (a “Section 83(b) Election”), there will be no federal income tax consequences
to either the recipient or our Company upon the grant of a restricted stock award. At the expiration of the restriction period and the
satisfaction of any other restrictions applicable to the restricted shares, the recipient will recognize ordinary income and our Company
generally will be entitled to a corresponding deduction equal to the fair market value of the common stock at that time. If a Section
83(b) Election is made within 30 days after the date the restricted stock award is granted, the recipient will recognize an amount of
ordinary income at the time of the receipt of the restricted shares, and our Company generally will be entitled to a corresponding deduction,
equal to the fair market value (determined without regard to applicable restrictions) of the shares at such time, less any amount paid
by the recipient for the shares. If a Section 83(b) Election is made, no additional income will be recognized by the recipient upon the
lapse of restrictions on the shares (and prior to the sale of such shares), but, if the shares are subsequently forfeited, the recipient
may not deduct the income that was recognized pursuant to the Section 83(b) Election at the time of the receipt of the shares.
The recipient of an unrestricted
stock award will recognize ordinary income, and our Company generally will be entitled to a corresponding deduction, equal to the fair
market value of our common stock that is the subject of the award when the award is made.
The recipient of a restricted
stock unit will recognize ordinary income as and when the units vest and shares of our common stock are issued. The amount of the income
will be equal to the fair market value of the shares of our common stock issued at that time, and our Company will be entitled to a corresponding
deduction. The recipient of a restricted stock unit will not be permitted to make a Section 83(b) Election with respect to such award.
The federal income tax consequences
of performance share awards, performance unit awards, other cash-based awards and other stock-based awards will depend on the terms and
conditions of those awards but, in general, participants will be required to recognize ordinary income in an amount equal to the cash
and the fair market value of any fully vested shares of our common stock paid, determined at the time of such payment, in connection with
such awards.
Section
409A. If an award is subject to Section 409A of the Code but does not comply with the requirements
of Section 409A of the Code, the taxable events as described above could apply earlier than described and could result in the imposition
of additional taxes and penalties. Participants are urged to consult with their tax advisors regarding the applicability of Section 409A
of the Code to their awards.
Potential Limitation on Company Deductions
Section 162(m) of the Code
generally disallows a tax deduction for compensation in excess of $1 million paid in a taxable year by a publicly held corporation to
its chief executive officer and certain other “covered employees”. The Committee intends to consider the potential impact
of Section 162(m) on grants made under the Plan but reserves the right to approve grants of options and other awards for an executive
officer that exceeds the deduction limit of Section 162(m).
Tax Withholding
As and when appropriate, we
shall have the right to require each optionee purchasing shares of common stock and each grantee receiving an award of shares of common
stock under the Plan to pay any federal, state or local taxes required by law to be withheld.
Equity Compensation Plan Information
Securities Authorized for Issuance Under Equity Compensation Plans
The following table sets forth the aggregate information
of our equity compensation plans in effect as of March 31, 2024:
| | |
| | |
| | |
Number of | |
| | |
| | |
| | |
securities | |
| | |
Number of | | |
| | |
remaining | |
| | |
securities to be | | |
| | |
available for | |
| | |
issued upon | | |
Weighted-average | | |
future issuance | |
| | |
exercise of | | |
exercise price of | | |
under equity | |
| | |
outstanding | | |
outstanding | | |
compensation | |
| | |
options, warrants | | |
option, warrants | | |
plans reflected | |
| Plan category | |
and rights | | |
and rights | | |
in column (a) | |
| | |
(a) | | |
(b) | | |
(c) | |
| Equity compensation plans approved by security holders (1) (2) | |
| 201,078 | | |
$ | 13.74 | | |
| - | |
| Equity compensation plans not approved by security holders | |
| - | | |
| - | | |
| - | |
| Total | |
| 201,078 | | |
$ | 13.74 | | |
| - | |
| (1) |
Excludes 120,541 shares of Common Stock reserved under the 2014 Plan and 2020 Plan as of March 31, 2024, subject to the issuance of restricted stock and RSUs. |
| (2) |
Represents
outstanding stock options granted to our current or former employees, directors and consultants pursuant to the 2014 Plan, 2020 Plan,
and 2021 Plan. |
2021 IMGX Stock Option Plan
In connection with the IMGX Transaction, we
assumed the ImmunogenX, Inc. 2021 Stock Option Plan, which was initially adopted by ImmunogenX stockholders (the “2021 Plan”).
The material terms of the 2021 Plan are summarized below.
Purpose
The purpose of the 2021 Plan is to attract
and retain the best available personnel for positions of substantial responsibility, to provide additional incentive to our employees
and consultants, and to promote the success of our business.
Eligibility
The Administrator may grant awards to any
employee or consultant of the Company, its parents, affiliates or its subsidiaries. Only employees are eligible to receive incentive
stock options.
Administration
The 2021 Plan will be administered by our
Board of Directors or one more committees or subcommittees of the Board consisting of two or more directors appointed by the Board to
administer the 2021 Plan (a “Committee” and together with the Board, the “Administrator”). The
Administrator, has the full power to (i) determine fair market value; (ii) select the employees and consultants to whom awards may from
time to time be granted; (iii) determine the number of shares to be covered by each award; (iv) approve the form(s) of agreement(s) and
other related documents used under the 2021 Plan; (v) determine the terms and conditions of any award granted under the 2021 Plan, including,
the exercise or purchase price, the time or times when awards may vest and/or be exercised (which may be based on performance criteria),
the circumstances (if any) when vesting will be accelerated or forfeiture restrictions will be waived, and any restriction or limitation
regarding any award; (vi) amend any outstanding award or agreement related to any award, including any amendment adjusting vesting (e.g.,
in connection with a change in the terms or conditions under which such person is providing services to the Company), provided that no
amendment may be made that would materially and adversely affect the rights of any participant without his or her consent; (vii) determine
whether and under what circumstances an option may be settled in cash; (viii) subject to applicable laws, to implement an option exchange
program and establish the terms and conditions of such option exchange program without consent of the holders of capital stock of the
Company, provided that no amendment or adjustment to an option that would materially and adversely affect the rights of any participant
may be made without his or her consent; (ix) approve addenda or grant awards to, or to modify the terms of, any outstanding option agreement
or restricted stock purchase agreement or any agreement related to any optioned stock or restricted stock held by participants who are
foreign nationals or employed outside of the United States with such terms and conditions as the Administrator deems necessary or appropriate
to accommodate differences in local law, tax policy or custom which deviate from the terms and conditions set forth in the 2021 Plan
to the extent necessary or appropriate to accommodate such differences; and (x) construe and interpret the terms of the 2021 Plan, any
option agreement or restricted stock purchase agreement, and any agreement related to any optioned stock or restricted stock.
Share Reserve
The maximum aggregate number of shares that
may be issued under the 2021 Plan is 350,000 shares. All 350,000 shares available under the 2021 Plan may be issued upon the exercise
of incentive stock options.
The shares issued under the 2021 Plan may
be authorized, but unissued, or reacquired shares. If an award expires or becomes unexercisable for any reason without having been exercised
in full, or is surrendered pursuant to an option exchange program, the unissued shares that were subject to such award will, unless the
2021 Plan is terminated, continue to be available under the 2021 Plan for issuance pursuant to future awards. In addition, any shares
which are retained by the Company upon exercise of an award in order to satisfy the exercise or purchase price for such award or any
withholding taxes due with respect to such award shall be treated as not issued and shall continue to be available under the 2021 Plan
for issuance pursuant to future awards. Shares issued under the 2021 Plan and later forfeited to the Company due to the failure to vest
or which are repurchased by the Company at the original purchase price paid to the Company for the shares (including, without limitation,
upon forfeiture to or repurchase by the Company in connection with the termination of a participant’s service) shall again be available
for future grant under the 2021 Plan. Notwithstanding the foregoing, in no event shall the maximum aggregate number of shares that may
be issued under the 2021 Plan pursuant to incentive stock options exceed 350,000 plus, to the extent allowable under Section 422 of the
Internal Revenue Code and the Treasury Regulations promulgated there under, any shares that again become available for issuance.
The share reserve described herein may be
subject to certain adjustments in the event of certain changes in the capitalization of the Company (see Equitable Adjustments below).
Types of Awards
The 2021 Plan provides for the grant of stock
options and restricted stock (collectively, “awards”).
Stock
Options. The 2021 Plan permits the granting of both options intended to qualify as incentive stock options under Section 422
of the Internal Revenue Code of 1986, as amended (the “Code”) and options that do not so qualify. Options granted under the
2021 Plan will be nonqualified options if they fail to qualify as incentive stock options or exceed the annual limit on incentive stock
options. Incentive stock options may only be granted to employees of the Company and its subsidiaries. Nonqualified options may be granted
to any persons eligible to receive awards under the 2021 Plan.
The exercise price of each option will be
determined by the Administrator, but such exercise price generally may not be less than 100% of the fair market value of one share of
Company common stock on the date of grant (except if less than fair market value, then the option must comply with Code Section 409A)
or, in the case of an incentive stock option granted to a 10% or greater stockholder, 110% of such share’s fair market value. The
term of each option will be set by the Administrator and may not exceed ten (10) years from the date of grant (or five (5) years for
an incentive stock option granted to a 10% or greater stockholder). The Administrator will determine at what time or times each option
may be exercised, including the ability to accelerate the vesting of such options.
The consideration to be paid for the shares
to be issued upon exercise of an option, including the method of payment, shall be determined by the Administrator (and, in the case
of an incentive stock option and to the extent required by applicable laws, shall be determined at the time of grant) and may consist
entirely of (1) cash; (2) check; (3) to the extent permitted under, and in accordance with, applicable laws, delivery
of a promissory note with such recourse, interest, security and redemption provisions as the Administrator determines to be appropriate
(subject to the provisions of Section 152 of the Delaware General Corporation Law); (4) cancellation of indebtedness; (5) other
previously owned shares of Company stock that have a fair market value on the date of surrender equal to the aggregate exercise price
of the shares as to which the option is exercised; (6) a cashless exercise; (7) such other consideration and method of payment
permitted under applicable laws; or (8) any combination of the foregoing methods of payment. In making its determination as to the type
of consideration to accept, the Administrator may consider if acceptance of such consideration may be reasonably expected to benefit
the Company and the Administrator may, in its sole discretion, refuse to accept a particular form of consideration at the time of any
option exercise.
The Administrator may at
any time offer to buy out for a payment in cash or shares of the Company an option previously granted under the 2021 Plan based on such
terms and conditions as the Administrator shall establish and communicate to the optionee at the time that such offer is made.
Restricted
Stock. A restricted stock award is an award of shares of Company stock that vests in accordance with the terms and conditions
established by the Administrator. The Administrator will determine the persons to whom grants of restricted stock awards are made, the
number of restricted shares to be awarded, the price (if any) to be paid for the restricted shares, the time or times within which awards
of restricted stock may be subject to forfeiture, the vesting schedule and rights to acceleration thereof, and all other terms and conditions
of restricted stock awards. Unless otherwise provided in the applicable award agreement, a participant generally will have the rights
and privileges of a stockholder as to such restricted shares, including without limitation the right to vote such restricted shares and
the right to receive cash dividends, if applicable.
When a right to purchase or receive restricted
stock is granted under the 2021 Plan, the Company will advise the recipient in writing of the terms, conditions and restrictions related
to the offer, including the number of shares that such person shall be entitled to purchase, the price to be paid, if any (which shall
be as determined by the Administrator, subject to applicable laws, including any applicable securities laws), and the time within which
such person must accept such offer. The permissible consideration for restricted stock shall be determined by the Administrator and shall
be the same as with respect to the exercise of options. The offer to purchase shares shall be accepted by execution of a restricted stock
purchase agreement in the form determined by the Administrator.
Repricing
The 2021 Plan authorizes the Administrator
to take the following repricing actions without stockholder approval: (i) modify the purchase price or the exercise price of any outstanding
option or (ii) cancel any option in exchange for cash or another award or property.
Tax Withholding
As
a condition of the grant, vesting and exercise of an award under the 2021 Plan, a participant (or in the case of the participant’s
death or a permitted transferee, the person holding or exercising the award) must make such arrangements as the Administrator may require
for the satisfaction of any applicable U.S. federal, state, local or foreign tax, withholding, and any other required deductions or payments
that may arise in connection with such award. The Company is not required to issue any shares under the 2021 Plan until such obligations
are satisfied. The Administrator may, to the extent permitted under applicable laws, permit a participant (or in the case of the
participant’s death or a permitted transferee, the person holding or exercising the award) to satisfy all or part of his or her
tax, withholding, or any other required deductions or payments by cashless exercise or by surrendering shares (either directly or by
stock attestation) that he or she previously acquired; provided that, unless specifically permitted by the Company, any such cashless
exercise must be an approved broker-assisted cashless exercise or the shares withheld in the cashless exercise must be limited to avoid
financial accounting charges under applicable accounting guidance and any such surrendered shares must have been previously held for
any minimum duration required to avoid financial accounting charges under applicable accounting guidance. Any payment of taxes by surrendering
shares to the Company may be subject to restrictions, including, but not limited to, any restrictions required by rules of the Securities
and Exchange Commission.
Equitable Adjustments
In the event of a stock split, reverse stock
split, stock dividend, combination, consolidation, reclassification of the shares or subdivision of the shares, the Company will automatically
proportionately adjust (i) the numbers and class of shares or other stock or securities, including securities : (x) available for future
awards under the 2021 Plan and (y) covered by each outstanding award, (ii) the exercise price per share of each such outstanding option,
and (iii) any repurchase price per share applicable to shares issued pursuant to any award. In the event of any increase or decrease
in the number of issued shares effected without receipt of consideration by the Company, a declaration of an extraordinary dividend with
respect to the shares payable in a form other than shares in an amount that has a material effect on the fair market value, a recapitalization
(including a recapitalization through a large nonrecurring cash dividend), a rights offering, a reorganization, merger, a spin-off, split-up,
change in corporate structure or a similar occurrence, the Administrator shall make appropriate adjustments, in its discretion, in one
or more of (i) the numbers and class of shares or other stock or securities: (x) available for future awards under the 2021 Plan and
(y) covered by each outstanding award, (ii) the exercise price per share of each outstanding option and (iii) any repurchase price per
share applicable to shares issued pursuant to any award.
Change in Control
In the event of a “Corporate Transaction”
(as defined in the 2021 Plan), each outstanding award (vested or unvested) will be treated as the Administrator determines, which determination
may be made without the consent of any participant and need not treat all outstanding awards (or portion thereof) in an identical manner.
Such determination, without the consent of any participant, may provide (without limitation) for one or more of the following in the
event of a Corporate Transaction: (A) the continuation of such outstanding awards by the Company (if the Company is the surviving corporation);
(B) the assumption of such outstanding awards by the surviving corporation or its parent; (C) the substitution by the surviving corporation
or its parent of new options or equity awards for such awards; (D) the cancellation of such awards in exchange for a payment to the participants
equal to the excess of (1) the fair market value of the shares subject to such awards as of the closing date of such Corporate Transaction
over (2) the exercise price or purchase price paid or to be paid for the shares subject to the awards; or (E) the cancellation of any
outstanding options or an outstanding right to purchase restricted stock, in either case, for no consideration.
Transferability of Awards
Unless determined otherwise by the Administrator,
an award may not be sold, pledged, assigned, hypothecated, transferred, or disposed of in any manner, except to a participant’s
estate or legal representative, and may be exercised, during the lifetime of the participant, only by the participant or certain permitted
transferees.
Term
The 2021 Plan became effective upon the Board’s
adoption of the 2021 Plan, and the 2021 Plan will continue in effect for a term of ten (10) years.
Amendment and Termination
Our Board may amend, alter, suspend or terminate
the 2021 Plan at any time. No amendment or termination of the 2021 Plan will materially impair the rights of any participant, unless
mutually agreed otherwise between the participant and the Company. Approval of the stockholders shall be required for any amendment,
where required by applicable law.
Required Vote and Recommendation
In accordance with our Charter,
Bylaws and Delaware law, and as further discussed above under “The Annual Meeting — Quorum; Abstentions and Broker
Non-Votes,” approval and adoption of this Equity Plan Amendment Proposal requires the affirmative (“FOR”) vote of
a majority of votes cast by shares of our Common Stock present or represented by proxy and entitled to vote at the Annual Meeting and
voting affirmatively or negatively on such matter. Unless otherwise instructed on the proxy or unless authority to vote is withheld, shares
represented by executed proxies will be voted “FOR” this proposal. Abstentions and broker non-votes, if any, with respect
to this proposal are not counted as votes cast and will not affect the outcome of such proposal.
OUR BOARD RECOMMENDS A VOTE “FOR”
PROPOSAL 2.
PROPOSAL NO.3: NASDAQ PROPOSAL
Overview
On March 13, 2024, the
Company acquired ImmunogenX, Inc., a Delaware corporation (“ImmunogenX”), in accordance with the terms of an Agreement
and Plan of Merger, dated March 13, 2024 (the “Merger Agreement”), by and among the Company, IMMUNO Merger Sub I,
Inc., a Delaware corporation (“First Merger Sub”), IMMUNO Merger Sub II, LLC, a Delaware limited liability company
(“Second Merger Sub”), and ImmunogenX. Pursuant to the Merger Agreement, First Merger Sub merged with and into ImmunogenX,
pursuant to which ImmunogenX was the surviving corporation (the “First Merger”). Immediately following the First Merger,
ImmunogenX merged with and into Second Merger Sub, pursuant to which Second Merger Sub was the surviving entity and a wholly owned subsidiary
of the Company (the “Second Merger” and together with the First Merger, the “Merger”). The Second
Merger Sub amended its certificate of formation concurrently with the Second Merger to change its company name to ImmunogenX, LLC. The
Merger is intended to qualify as a tax-free reorganization for U.S. federal income tax purposes.
Under the terms of the
Merger Agreement, upon the consummation of the Merger on March 13, 2024, in exchange for the outstanding shares of capital stock of ImmunogenX
immediately prior to the effective time of the First Merger, the Company issued to the stockholders of ImmunogenX an aggregate of (A)
36,830 shares of common stock of the Company, par value $0.0001 per share (the “Common Stock”), and (B) 11,777.418
shares of Series G Preferred Stock (as defined and described below), each share of which is convertible into 1,000 shares of Common Stock,
subject to certain conditions described below. In addition, the Company assumed (i) all ImmunogenX stock options immediately outstanding
prior to the First Merger, each becoming an option to purchase Common Stock subject to adjustment pursuant to the terms of the Merger
Agreement (the “Assumed Options”) and (ii) all ImmunogenX warrants immediately outstanding prior to the First Merger,
each becoming a warrant to purchase Common Stock subject to adjustment pursuant to the terms of the Merger Agreement (the “Assumed
Warrants”). The Assumed Options are exercisable for an aggregate of 200,652 shares of Common Stock, have an exercise price
of $0.81 and expire between February 1, 2031 and June 6, 2033. The Assumed Warrants are exercisable for and aggregate of 127,682 shares
of Common Stock, have exercise prices ranging from $3.02 to $3.92 and expire between September 30, 2032 and September 6, 2033
Tungsten acted as financial
advisor to the Company in connection with the Merger. As partial compensation for services rendered by Tungsten, the Company issued to
Tungsten or its affiliates or designees an aggregate of 18,475 shares of Common Stock and 595.808 shares of Series G Preferred Stock.
The Series G Preferred Stock
issued to stockholders of ImmunogenX is initially convertible, in the aggregate, into approximately 12,373,226 shares of the Company’s
common stock, subject to adjustment and assuming approval of this Proposal No. 3. If the Nasdaq Proposal is approved, it will result
in the automatic conversion of the 12,373.226 shares of Series G Preferred Stock into 12,373,226 shares of the Company’s Common
Stock.
Purpose of the Nasdaq Proposal
We are subject to the Nasdaq
Rules because our Common Stock is currently listed on the Nasdaq Capital Market.
Pursuant to Nasdaq Rule 5635(a),
stockholder approval is required prior to the issuance by the Company of Common Stock (or securities convertible into or exercisable for
Common Stock) in connection with the acquisition of the stock or assets of another company if, due to the present or potential issuance
of common stock, including shares issued pursuant to an earn-out provision or similar type of provision, or securities convertible into
or exercisable for common stock, other than a public offering for cash: (A) the common stock has or will have upon issuance voting power
equal to or in excess of 20% of the voting power outstanding before the issuance of stock or securities convertible into or exercisable
for common stock; or (B) the number of shares of common stock to be issued is or will be equal to or in excess of 20% of the number of
shares of common stock outstanding before the issuance of the stock or securities. Additionally, pursuant to Nasdaq Rule 5635(b), stockholder
approval is required prior to certain issuances with respect to Common Stock or securities convertible into Common Stock which could result
in a change of control of the issuer. Although Nasdaq has not adopted any rule on what constitutes a “change of control” for
purposes of Rule 5635(b), Nasdaq has previously indicated that the acquisition of, or right to acquire, by a single investor or affiliated
investor group, as little as 20% of the common stock (or securities convertible into or exercisable for common stock) or voting power
of an issuer could constitute a change of control.
Combined with shares of Common
Stock issued in the IMGX Transaction as well as the shares of Common Stock underlying the Assumed Options and Assumed Warrants, the shares
issuable upon conversion of the Series G Preferred Stock would result in the issuance of more than 20% of the voting power and the number
of shares of Common Stock outstanding as of the issuance of the Series G Preferred Stock. As a result of the foregoing, in accordance
with Nasdaq Rule 5635(a) and (b), the Series G Certificate of Designation provides that the Series G Preferred Stock will not be convertible
into Common Stock until such time as we obtain stockholder approval for their removal, as discussed in “Proposal 3: Nasdaq Proposal.”
If stockholders do not approve
the Nasdaq Proposal, the Company will not be able to honor any conversions of Series G Preferred Stock held by ImmunogenX.
Description of Series G Preferred Stock
The
terms of the Series G Non-Voting Convertible Preferred Stock are set forth in a Certificate of Designations of Preferences, Rights and
Limitations of Series G Non-voting Convertible Preferred Stock of the Company (the “Certificate of Designations”), which was
filed with the State of Delaware on March 13, 2024. The Certificate of Designations provides for the designation of shares of the Company’s
Series G Non-Voting Convertible Preferred Stock, par value $0.0001 per share (the “Series G Preferred Stock”). Pursuant to
the Certificate of Designations, holders of Series G Preferred Stock are entitled to receive dividends on shares of Series G Preferred
Stock equal to, on an as-if-converted-to-Common-Stock basis, and in the same form as dividends actually paid on shares of the Common Stock.
Except as otherwise required by law, the Series G Preferred Stock does not have voting rights. However, as long as any shares of Series
G Preferred Stock are outstanding, the Company will not, without the affirmative vote of the holders of a majority of the then-outstanding
shares of the Series G Preferred Stock, (i) alter or change adversely the powers, preferences or rights given to the Series G Preferred
Stock or alter or amend the Certificate of Designation, amend or repeal any provision of, or add any provision to, the Charter or bylaws
of the Company, or file any articles of amendment, certificate of designations, preferences, limitations and relative rights of any series
of preferred stock, in each case if any such action would adversely alter or change the preferences, rights, privileges or powers of,
or restrictions provided for the benefit of the Series G Preferred Stock, regardless of whether any of the foregoing actions shall be
by means of amendment to the Charter or by merger, consolidation, recapitalization, reclassification, conversion or otherwise, (ii) issue
further shares of Series G Preferred Stock, (iii) prior to the earlier of stockholder approval of the Conversion or the six-month anniversary
of the Closing, consummate either: (A) any Fundamental Transaction (as in the Certificate of Designation) or (B) any stock sale to, or
any merger, consolidation or other business combination of the Company with or into, another entity in which the stockholders of the Company
immediately before such transaction do not hold at least a majority of the capital stock of the Company immediately after such transaction,
or (iv) enter into any agreement with respect to any of the foregoing.
Background of the IMGX Transaction
The following chronology
summarizes certain meetings and other events between representatives of Entero and representatives of ImmunogenX during the period preceding
the closing of the Merger. The following chronology does not purport to catalogue every conversation among Entero, ImmunogenX, and their
respective representatives.
In Q1 2023, the Board,
as part of its effort to continually evaluate how to best maximize stockholder value, believed it in the best interest of Entero and
its stockholders to conduct a preliminary assessment to identify and evaluate potential strategic transactions that would allow Entero
to further develop its pipeline assets, add new pipeline assets or otherwise increase value for stockholders.
In April 2023, James Sapirstein,
Sarah Romano, Dr. Jim Pennington, CMO, Ted Stover, VP of Product Development, Amy Stover, VP and Martin Krusin, SVP of Corporate Development,
constituting Entero’s senior management team (“Entero Senior Management”) initiated discussions with a Canadian
biopharmaceutical third party (“Party A”) to potentially in-license and co-develop Party A’s drug candidates.
On May 1, 2023, Entero
formally engaged Tungsten to assist and advise with respect to its strategic process. Tungsten began its outreach to potential target
companies and other sources. Approximately 10 companies were contacted as part of this initial outreach process, including a number of
private companies in the life sciences industry seeking a Nasdaq listing, public companies in the life sciences industry that were believed
to have a strategic fit with Entero, and a broad set of investors and service providers which included venture capital firms, institutional
healthcare funds, and debt funds to garner additional interest in a reverse merger transaction with Entero.
In total, Tungsten presented
nine potential strategic partners to Entero. The candidate companies were presented to Entero’s Board on June 22, 2023; September
20, 2023; November 16, 2023; December 12, 2023; and during routine calls with Board members. During each meeting, the Board and management
team reviewed the candidates and discussed the status of term sheet negotiations and potential back-ups.
Entero was introduced
to ImmunogenX by Tungsten in February 2023 and both parties provided access to their respective virtual data rooms. Subsequently, Entero
conducted due diligence on ImmunogenX, held several diligence calls with senior management at ImmunogenX between February 2023 and June
2023, and had an in-person meeting with the Chief Executive Officer and co-founders of ImmunogenX, Jack Syage and Jennifer Sealey-Voyksner,
at the May 2023 Digestive Disease Week conference.
Between March and June
2023, several draft term sheets were circulated between Entero and ImmunogenX. The initial draft term sheet was prepared by Tungsten
and Entero and sent to ImmunogenX and contemplated an equity purchase, proposed transaction structure, minimum investment, board composition,
continuation of management, full and satisfactory business and financial due diligence, exclusivity, and other customary terms. On June
21, 2023, the Board approved, and Entero entered into, a non-binding business combination term sheet (the “June Term Sheet”)
with ImmunogenX, which provided for a $110 million valuation of ImmunogenX, with no working capital adjustment contemplated and an approximate
$3 million valuation for Entero based upon Entero’s share price as of that date. Pursuant to the June Term Sheet, Entero was to
issue the ImmunogenX securityholders an aggregate of $110 million in Entero common stock and for Entero to assume no debt.
On June 28, 2023, Entero
announced that the final patient dosed in its Phase 2 Span Clinical Trial of Enhanced Adrulipase Formulation.
On June 29-30, 2023, the
Entero Senior Management and Jack Syage, Gail Stover, David Crean, Jennifer Sealey-Voyksner, and Matt Dickason (“ImmunogenX
Senior Management”) held a hybrid meeting (both in person at ImmunogenX’s offices and virtually) and discussed diligence
and potential merger related issues.
On July 18, 2023, Entero
consummated a public offering of common stock and warrants in which it entered into a placement agency agreement with Roth Capital Partners,
LLC (“Roth”) and a securities purchase agreement with certain purchasers, with net proceeds of approximately $1.8
million.
On September 13, 2023,
Entero entered into a license agreement with Sanofi, pursuant to which Entero received a license to obtain certain exclusive worldwide
rights to develop and commercialize Capeserod, a selective 5-HT4 receptor partial agonist which Entero intends to repurpose and develop
for gastrointestinal indications.
On September 14, 2023,
Entero announced the exercise of certain warrants and the issuances of new warrants in a private placement with gross proceeds of approximately
$4 million.
On November 16, 2023 and
December 12, 2023, with consideration given to Entero’s then-current financial and operational considerations, the Board approved
drafts of an updated non-binding business combination term sheet with ImmunogenX. On December 18, 2023, Entero entered into the term
sheet (the “December Term Sheet”), which contained a binding exclusivity agreement until June 2, 2024, for a payment
of $500,000, and issued a press release announcing the same. The December Term Sheet again provided for a $110 million valuation of ImmunogenX,
subject to a to a dollar-for-dollar adjustment for net indebtedness and working capital of ImmunogenX. Pursuant to the December Term
Sheet, Entero was to issue the ImmunogenX securityholders an aggregate of $110 million of secured convertible notes convertible into
shares of Entero common stock, the conversion of which would be subject to the approval of Entero’s stockholders. Additionally,
the December Term Sheet provided for the Entero board of directors and management maintaining control of the combined company until stockholder
approval is received.
On December 12, 2023,
the stockholders of Entero approved an increase in the total authorized shares of Entero from 50,000,000 shares to 100,000,000 shares,
granted the Board discretion to affect a reverse stock split at a ratio of not less than 1:10 and no more than 1:20 and ratified Entero’s
entry into the July 18, 2023 securities purchase agreements.
On December 23, 2023,
Entero entered into a non-binding letter of interest for a licensing agreement with a strategic global pharmaceutical company that contemplates
a cash payment to Entero upon the closing of the licensing and certain milestone and royalty payments as consideration for the exclusive
license of latiglutenase within the United States and Canada.
In parallel with seeking
a merger partner, Entero continued outreach to parties potentially interested in acquiring or forming a new private company to invest
in Entero’s programs. These activities are ongoing. In 2023, Entero held discussions with more than 30 companies to out-license
or sell its Phase 2 adrulipase program and held discussions with approximately 10 companies to out-license or sell Phase 2 niclosamide
IBD program. On December 27, 2023, the Company announced that it had signed a non-binding term sheet to sell its Phase 2 niclosamide
IBD program.
On December 27, 2023,
Entero announced the exercise of certain warrants and the issuances of new warrants in a private placement with gross proceeds of approximately
$4.8 million.
On January 3, 2024, Lowenstein
Sandler LLP (“Lowenstein”), counsel to Entero, circulated a due diligence request list and tentative transaction timeline
to Fortis LLP (“Fortis”), counsel to ImmunogenX.
On January 9, 2024, Lowenstein
and Fortis participated in a teleconference to discuss the structure of the transaction in light of tax considerations and Nasdaq rules.
On January 11, 2024, Lowenstein
coordinated the scheduling of a diligence call between Entero Senior Management, ImmunogenX Senior Management, Fortis and Lowenstein.
Lowenstein circulated a diligence call agenda.
On January 15, 2024, Lowenstein,
Entero Senior Management, ImmunogenX Senior Management, Fortis and Orrick, Herrington & Sutcliffe, LLP (“Orrick”),
counsel to ImmunogenX, participated in a teleconference to discuss open diligence questions and requests that Lowenstein had for ImmunogenX,
Fortis and Orrick.
On January 18, 2024, Lowenstein
circulated a first draft of the Merger Agreement to Fortis and Orrick.
On January 19, 2024, Lowenstein,
Fortis and Orrick participated in a teleconference to discuss treatment of ImmunogenX’s outstanding debt and outstanding options
to purchase common stock, the structure of Entero’s board after closing and the Merger Agreement generally.
On January 24, 2024, Lowenstein
circulated a supplemental due diligence request list to ImmunogenX, Fortis and Orrick.
On January 29, 2024, Lowenstein,
Entero senior management and Ladenburg Thalmann & Co. Inc. (“Ladenburg”) participated in a teleconference to discuss
the retention of Ladenburg to evaluate the transaction and draft a fairness opinion for this transaction.
On January 30, 2024, Entero
entered into a Confidentiality and Non-Disclosure Agreement with Ladenburg.
On January 31, 2024, Lowenstein,
Fortis and Orrick participated in a teleconference to discuss certain material issues in the Merger Agreement, the primary topic of this
teleconference being the treatment of ImmunogenX’s outstanding debt.
On February 2, 2024, Orrick
circulated a revised draft of the merger agreement. On that same day, Lowenstein circulated drafts of the lock-up agreement and certificate
of designations.
On February 5, 2024, Lowenstein,
Fortis, Orrick and Ladenburg met via teleconference to discuss certain other outstanding issues related to the Merger Agreement, the
primary topic of this teleconference being to ensure the structure of the transaction was compliant with Nasdaq requirements.
On February 8, 2024, Lowenstein
circulated a revised draft of the Merger Agreement to Fortis and Orrick addressing issues discussed during the February 5, 2024 meeting.
On February 8, 2024, Lowenstein
and Orrick participated in a teleconference to discuss the tax implications of this transaction and ensure that the transaction qualifies
as a tax-free reorganization.
On February 12, 2024,
Entero Senior Management, Lowenstein, Fortis, Orrick, ImmunogenX Senior Management and Tungsten participated in a teleconference to again
discuss treatment of ImmunogenX’s outstanding debt, and a potential timeline to consummate a private investment in public equity
(“PIPE”) for the combined company.
On February 14, 2024,
Lowenstein circulated a proposal on behalf of Entero on how ImmunogenX’s outstanding debt be treated in connection with this transaction,
pursuant to which certain indebtedness would remain outstanding at the ImmunogenX level upon closing of the transaction. Pursuant to
such proposal, $20 million of Entero common stock would be placed into an escrow account for the benefit of satisfaction of such indebtedness.
Additionally, as a result of the assumption of the indebtedness, the valuation of the combined company would be $110 million, of which
$86 million would be ascribed to ImmunogenX and $24 million would be ascribed to Entero. On February 16, 2024, Lowenstein and Orrick
discussed potential revisions to Lowenstein’s proposal, and shortly after, Lowenstein provided an updated proposal which provided
for the release of the lender’s liens on the ImmunogenX collateral upon certain conditions, including the closing of a $25 million
PIPE. On February 17, 2024, Orrick circulated a revised draft of the proposal, which included comments from ImmunogenX’s lender.
That day, Lowenstein, Fortis and Orrick participated in a teleconference to the lender’s comments to the proposal.
On February 19, 2024,
Entero Senior Management, Lowenstein, Fortis, Orrick and ImmunogenX Senior Management participated in a teleconference to discuss treatment
of ImmunogenX’s outstanding debt, the valuation of each of Entero and ImmunogenX, the draft Certificate of Designation and the
treatment of the outstanding options of ImmunogenX. Lowenstein and Orrick more thoroughly discussed the issuance of replacement options
on a teleconference call on February 20, 2024.
On February 22, 2024, Lowenstein
circulated a list of outstanding diligence questions for ImmunogenX to Fortis and Orrick.
On February 22, 2024, Lowenstein circulated an updated proposal
on the treatment of ImmunogenX’s outstanding debt, and the valuation of each of ImmunogenX and Entero. Under such proposal, the
valuation of the combined company would be $110 million, of which $90 million would be ascribed to ImmunogenX and $21 million would be
ascribed to Entero.
On February 23, 2024,
at a meeting duly called and held, and attended by representatives of Entero Senior Management and Lowenstein, the Board of Directors
was provided an update on the negotiations of the transaction. At the meeting, the Board discussed the transaction, including the proposed
valuation of each of ImmunogenX and Entero. Shortly thereafter, Lowenstein participated in a teleconference with Fortis, Orrick and Armstrong
Teasdale LLP, counsel to ImmunogenX’s lender (“Armstrong”).
On March 1, 2024, Orrick circulated
revised drafts of the Merger Agreement and related documents.
On March 2, 2024, Lowenstein
circulated a revised draft of the Merger Agreement to Fortis and Orrick, which provided for the assumption of the 2021 Plan by Entero
as well as the outstanding options thereunder.
On March 4, 2024, Lowenstein,
Fortis, Orrick and Tungsten participated in a teleconference to discuss certain open items related to the transactions, such as a target
closing date and the allocation of the merger consideration.
On March 6, 2024, Orrick circulated
a first draft of the Second Modification of Loan Documents and a revised draft of the Voting Agreement to Lowenstein, and Lowenstein circulated
a first draft of the Letter of Transmittal to Fortis and Orrick.
On March 7, 2024, Orrick circulated a first draft of the Syage Note and Shareholder Security
Agreement. On March 8, 2024, Lowenstein circulated a revised draft of the Syage Note and Shareholder Security Agreement . The primary
revisions in Lowenstein’s draft of the Syage Note included adding a five (5) business day cure period upon the occurrence of an
Event of Default before the amount owed under the Syage Note became due and payable, and revised the definition of “Event of Default”
such that if ImmunogenX defaulted under the terms of any other debt obligation, it would not automatically provide for a default under
the Syage Note. Lowenstein’s revised draft of the Shareholder Security Agreement provided for a thirty (30) day cure period upon
an Event of Default under the Shareholder Security Agreement that results from Entero not observing a convenant or agreement binding on
it as well as updates to treflect that Entero’s obligations under the Shareholder Security Agreement are subject to its obligatins
under the Amended Credit Agreement. In an email exchange on March 9, 2024, Lowenstein, Fortis and Orrick agreed to add a default under
the Note as an Event of Default under the Syage Note and on march 10, 2024, the parties agreed to Lowenstein’s updates to the Shaerholder
Security Agreement.
From February 22, 2024
to March 8, 2024, the parties exchanged several valuation proposals which varied as to the per share price and the
valuation of ImmunogenX. On March 8, 2024, the parties agreed to an $85 million valuation of ImmunogenX pursuant to which the number of
securities of Entero to be issued to the ImmunogenX shareholders would be based on a $7.00 share price, a slight discount to the $7.16
closing price of Entero Common Stock on Nasdaq on March 7, 2024. The closing price of Entero Common Stock on Nasdaq on the day of Closing,
March 13, 2024, was $6.53,
On March 9, 2024, Entero
Senior Management, Lowenstein and Tungsten participated in a teleconference to discuss the allocation of the merger consideration. Later
that day, Lowenstein circulated a revised draft of the Lock-Up Agreement and a notice to ImmunogenX warrant holders and option holders
regarding the treatment of outstanding warrants and options in connection with this transaction. Orrick approved the drafts shortly thereafter.
On March 11, 2024, at a meeting duly called and held, and attended
by representatives of Entero Senior Management, the Board of Directors was provided an update on the near-final terms of the transaction.
On March 13, 2024, Entero
Senior Management shared a draft offer letter with Dr. Syage pursuant to which he (i) would receive a base annual salary of $350,000,
(ii) would be entitled to an incentive bonus of up to 40% of his annual salary, dependent on meeting pre-determined objectives and (iii)
would be eligible to participate in Entero benefits in connection with his appointment as the Entero’s President and Chief Operating
Officer. Upon discussion, the terms were accepted.
On
March 13, 2024, at a meeting duly called and held, and attended by representatives of Entero Senior Management, Ladenberg and Lowenstein,
the Board of Directors received an update on the transaction. Representatives of Lowenstein made a presentation regarding the director’s
fiduciary duties in connection with the transaction. Lowenstein then provided an overview of the negotiations that had transpired related
to the Merger Agreement and the outstanding indebtedness of ImmunogenX. Lowenstein then reviewed the final key terms of the Merger Agreement
and related transactions, but not limited to (i) the assumption of the indebtedness of ImmunogenX, (ii)
the treatment of the ImmunogenX options and warrants and (iii) the requirement to obtain stockholder approval to above the issuance of
the shares of common stock upon conversion of the Series G Preferred Stock. Thereafter, at the request of the Board, Ladenburg rendered
its oral opinion to the Board (which was subsequently confirmed in writing by delivery of Ladenburg’s written opinion addressed
to the Board dated of the same date) as to the fairness, and subject to the factors, limitations, qualifications and other matters set
forth in its written opinion, from a financial point of view, to the stockholders of the Company of the merger consideration to be paid
by the Company pursuant to the Merger Agreement. After considering the foregoing, and taking into consideration the factors described
under “Nasdaq Proposal – Entero Board’s Reasons for the Approval of the IMGX Transaction” beginning on
page 157 of this proxy statement, the Board unanimously (i) determined that the Merger Agreement and the transactions contemplated
by the Merger Agreement, including the Merger (collectively, the “Transactions”), are fair to and in the best interests
of Entero and its stockholders and (ii) adopted, approved and declared the Merger Agreement other transactions contemplated thereby advisable.
Accordingly, the Board unanimously authorized and approved the Merger Agreement and related transactions.
On March 13, 2024, following
the meeting of the Board, Entero, First Merger Sub and Second Merger Sub executed the Merger Agreement. On the morning of March 14, 2024,
prior to market open on Nasdaq, Entero issued a press release announcing entry into the Merger Agreement and the Closing of the Merger.
Entero Board’s Reasons for the Approval
of the IMGX Transaction
In approving the Transactions, the Board of Directors considered a
wide range of factors, including those discussed below, and the Board of Directors compared the Transactions to other alternatives, including
continuing to focus our resources on the Company’s legacy research and development pipeline and other potential business development
opportunities reviewed by the Board of Directors and the opportunities and risks presented with the Transactions. Based on these considerations
and analyses, including the reasons and factors discussed below, the Board of Directors concluded that entering into the Merger Agreement
and consummating the Transactions would yield the highest value reasonably available for our stockholders and would be fair to, and in
the best interests of, the Company and our stockholders.
In
particular, the Board of Directors took into account the following reasons, facts and circumstances in approving the Transactions:
| · | Available Alternatives. The process to
seek other potential transactions (including potential financings, license or collaborations, and alternative acquisitions by another
buyer) and the Company’s consideration of such alternative transactions. |
| · | Value to Company Equityholders. The valuation
of the Company implied by the Merger, the ownership of the legacy Company stockholders of the pro forma company, and the liquidity retained
by Company stockholders through continuing holding shares of Common Stock, which will remain listed and publicly traded on Nasdaq. |
| · | Certainty of Closing. The fact that pursuant
to the terms of the Merger Agreement, the Merger was to be consummated immediately following the execution of the Merger Agreement. The
structure of the Transactions whereby the Closing and the effectiveness of the Merger were to occur immediately following the execution
of the Merger Agreement, as opposed to a structure in which the satisfaction of certain closing conditions could result in a delayed closing. |
| · | ImmunogenX’s Business and Prospects.
The business, operations, assets, business strategy, and prospects of ImmunogenX, taking into account the discussions with the Company’s
management. |
| |
· |
Phase 3 Ready Asset. The Merger would allow Entero to acquire Latiglutenase, a Phase 3 ready, first in class, asset for the treatment of CeD as well as a strategic partner to support development and commercialization of the asset. |
| · | Current and Future Financing Needs of the
Company. The Company’s substantial financing needs to further the development and commercialization of the Company’s legacy
product candidates, for which the Company would have been reliant upon raising additional capital, which was expected to have come with
substantial uncertainty of success in addition to the likelihood that such financing may have had a substantial dilutive effect to the
existing stockholders of the Company, may only have been available on unfavorable terms, or may not have been available at all. |
| · | The Company’s Historical Difficulties
in Raising Funds. The Company’s difficulty in raising funds prior to the Merger, which cast doubt on its ability to raise adequate
funds in the future. |
| · | Continuity of Company Senior Management.
The confidence the Board had in the operations of the combined company due to the fact that the Company’s seasoned, public company
chief executive officer and chief financial officer would both remain in these roles for the combined public company going forward. |
| · | Arms’-length Negotiations with ImmunogenX.
The Merger Agreement was the product of extensive arms’-length negotiations with the assistance of experienced legal and financial
advisors and reflected the belief of the Board of Directors that the valuation ascribed to the Company following the Merger and the resulting
ownership of the legacy Company stockholders of the pro forma company, is fair to and in the best interests of the Company and its legacy
stockholders. |
| · | Opinion of Ladenburg Thalman. The opinion
of Ladenburg Thalman, rendered orally to the Board of Directors on March 13, 2024 (and subsequently confirmed in writing by delivery of
Ladenburg Thalman’s written opinion, dated March 13, 2024) that, as of such date and based upon and subject to the various assumptions
made, and the qualifications and limitations upon the review undertaken by Ladenburg Thalman in preparing its opinion, the consideration
proposed to be paid by the Company pursuant to the terms of the Merger Agreement was fair, from a financial point of view, to the Company,
as more fully described below in the section titled “The Merger—Opinion of Ladenburg Thalman.” |
| · | Fully Informed Board of Directors. The
involvement of the Board of Directors during the negotiation of the Merger Agreement and their belief that they were fully informed about
the key terms and conditions thereof and all factors thereunder that may impact the financial terms in the Transactions. |
| · | Assumption of Indebtedness of ImmunogenX.
Upon the consummation of the Merger, the Company assumed approximately $9.2 million of indebtedness of ImmunogenX, which is secured
by substantially all of the assets of ImmunogenX, including the patents and trademarks owned by ImmunogenX. If the Company is not able
to repay or refinance such indebtedness as it becomes due, it may be required to adopt one or more alternatives, such as selling assets,
restructuring debt or obtaining additional debt or equity on terms that may be onerous or highly dilutive. Additionally, in an event of
default under any of such indebtedness, the lenders could elect to foreclose on the Company’s assets securing such debt. In such
an event, the Company may not hold the necessary intellectual property rights to conduct its business. |
| · | Uncertainties Regarding Stand-alone Plan.
The risk that prolonging the transaction search process further could result in the loss of the opportunity to consummate the Transactions
with ImmunogenX. Given the lack of other desirable alternative transactions, the Company may not have otherwise had a viable path forward
for developing its legacy pipeline assets or operating on a standalone basis in light of its insufficient cash on hand and its low market
capitalization. |
| · | Effect of Potential Redemption of the Series
G Preferred Stock Due to Failure to Consummate the Conversion. The risk that a holder of Series A Preferred Stock issued in connection
with the Merger may elect to require the Company to redeem such holder’s shares of Series G Preferred Stock for cash if the Company’s
stockholders fail to vote to approve the conversion of the Series g Preferred Stock into Common Stock within 6 months following the consummation
of the Merger. If such failure occurs and all or a significant portion of holders of Series G Preferred Stock exercise such optional redemption
right, the Company could be required to use a significant amount of its cash resources on hand to satisfy such redemption obligation,
which could materially limit the amount of cash that the Company would have available to fund its operations and result in a need to raise
additional capital to satisfy the redemption obligation. |
| · | Litigation. The risk of stockholder lawsuits
against the Company or the Board of Directors in connection with the Transactions. |
Opinion of Entero’s Financial Advisor
As
stated above, pursuant to an engagement letter dated January 29, 2024 (referred to as the “Engagement Letter”), Entero
retained Ladenburg to render the Opinion to the Entero Board as to the fairness of the Merger Consideration to be paid by Entero, from
a financial point of view, to the stockholders of Entero. On March 13, 2024, at the request of the Entero Board, Ladenburg rendered the
oral opinion, subsequently confirmed by delivery of the written opinion dated March 13, 2024, to the Entero Board, that the Merger Consideration
to be paid by Entero pursuant to the Merger Agreement was fair, from a financial point of view, to the stockholders of Entero as of the
date of such Opinion and based upon the various assumptions, qualifications and limitations set forth therein.
The
full text of the Opinion is attached as Annex C to this proxy statement and is incorporated herein by reference. Entero encourages
its stockholders to read the Opinion in its entirety for the assumptions made, procedures followed, other matters considered and limits
of the review by Ladenburg. The summary of the Opinion set forth herein is qualified by reference to the full text of the Opinion. Ladenburg
provided its Opinion for the sole benefit and use by the Entero Board in its consideration of the merger. The Opinion is not a recommendation
to the Entero Board or to any stockholder as to how to vote with respect to the proposed merger or to take any other action in connection
with the merger or otherwise.
In
connection with the Opinion, Ladenburg took into account an assessment of general economic, market and financial conditions as well as
its experience in connection with similar transactions and securities valuations generally and, among other things:
| |
· |
Reviewed the Merger Agreement; |
| |
· |
Reviewed and analyzed certain publicly available
financial and other information for each of Entero and ImmunogenX, respectively, including equity research on comparable companies,
and certain other relevant financial and operating data furnished to us by the management of Entero; |
| |
· |
Reviewed and analyzed certain relevant historical
financial and operating data concerning ImmunogenX furnished to us by the management of Entero; |
| |
· |
Discussed with certain members of the management
of Entero and ImmunogenX the historical and current business operations, financial condition and prospects of Entero and ImmunogenX,
respectively; |
| |
· |
Reviewed and analyzed certain operating results of ImmunogenX as compared to operating results and the reported price and trading histories of certain publicly traded companies that we deemed relevant; |
| |
· |
Reviewed and analyzed certain financial terms of the Merger Agreement as compared to the publicly available financial terms of certain selected business combinations that we deemed relevant; |
| |
· |
Reviewed and analyzed certain financial terms of completed initial public offerings for certain companies that we deemed relevant; |
| |
· |
Reviewed certain pro forma financial effects of the Mergers; and |
| |
· |
Reviewed and analyzed such other information and such other factors, and conducted such other financial studies, analyses and investigations, as we deemed relevant for the purposes of rendering our Opinion. |
In
conducting Ladenburg’s review and arriving at Ladenburg’s Opinion, Ladenburg has, with Entero’s consent, assumed and
relied, without independent verification or investigation, upon the accuracy and completeness of all financial and other information
provided to or discussed with Ladenburg by Entero and ImmunogenX, respectively (for their respective employees, representatives or affiliates),
or which is publicly available or was otherwise reviewed by Ladenburg. Ladenburg has not undertaken any responsibility for the accuracy,
completeness or reasonableness of, or independent verification of, such information. Ladenburg has relied upon, without independent verifications,
the assessment of Entero management and ImmunogenX management as to the viability of, and risks associated with, the current and future
products and services of ImmunogenX (including without limitation, the development, testing and marketing of such products and services,
the receipt of all necessary governmental and other regulatory approvals for the development, testing and marketing thereof, and the
life and enforceability of all relevant patents and other intellectual and other property rights associated with such products and services).
In addition, Ladenburg has not conducted, nor has it assumed any obligation to conduct any physical inspection of the properties or facilities
of Entero or ImmunogenX. Furthermore, Ladenburg has assumed, with Entero’s consent, that there will be no further adjustments to
the Merger Consideration between the date hereof and the date the final Merger Consideration is determined. Ladenburg has, with Entero’s
consent, relied upon the assumption that all information provided to Ladenburg by Entero and ImmunogenX is accurate and complete in all
material respects.
Ladenburg
expressly disclaimed any undertaking or obligation to advise any person of any change in any fact or matter affecting Ladenburg’s
Opinion of which Ladenburg has become aware after the date of its Opinion. Ladenburg assumed there were no material changes in the assets,
liabilities, financial condition, results of operations, business or prospects of Entero or ImmunogenX since the date of the last financial
statements made available to Ladenburg. Ladenburg has not obtained any independent evaluations, valuations or appraisals of the assets
or liabilities of Entero or ImmunogenX, nor has Ladenburg been furnished with such materials. In addition, Ladenburg has not evaluated
the solvency or fair value of Entero or ImmunogenX under any state or federal laws relating to bankruptcy, insolvency or similar matters.
Ladenburg’s Opinion does not address any legal, tax or accounting matters related to the merger, as to which Ladenburg has assumed
that Entero and the Entero Board have received such advice from legal, regulatory, tax and accounting advisors as each has determined
appropriate. Ladenburg’s Opinion addresses only the fairness of the Merger Consideration, from a financial point of view, to the
Entero Stockholders. Ladenburg expresses no view as to any other aspect or implication of the merger or any other agreement or arrangement
entered into in connection with the merger. Ladenburg’s Opinion is necessarily based upon economic and market conditions and other
circumstances as they existed and could be evaluated by Ladenburg on the date of its Opinion. It should be understood that although subsequent
developments may affect Ladenburg’s Opinion, Ladenburg does not have any obligation to update, revise or reaffirm its Opinion and
Ladenburg expressly disclaims any responsibility to do so.
Ladenburg
did not consider any potential legislative or regulatory changes currently being considered or recently enacted by the United States or
any foreign government, or any domestic or foreign regulatory body, or any changes in accounting methods or generally accepted accounting
principles that may be adopted by the Securities and Exchange Commission, the Financial Accounting Standards Board, or any similar foreign
regulatory body or board.
For
purposes of rendering the Opinion, Ladenburg assumed in all respects material to Ladenburg’s analysis, that the representations
and warranties of each party contained in the merger agreement were true and correct, that each party will perform all of the standards
of the covenants and agreements required to be performed by it under the merger agreement and that all conditions to the consummation
of the merger will be satisfied without waiver or amendment of any term or condition thereof. Ladenburg has assumed that the final form
of the merger agreement will be substantially similar to the last draft reviewed by Ladenburg. Ladenburg has also assumed that all governmental,
regulatory and other consents and approvals contemplated by the merger agreement or otherwise required for the transactions contemplated
thereby will be obtained and that in the course of obtaining any of those consents no restrictions will be imposed or waivers made that
would have an adverse effect on Entero, the Company or the contemplated benefits of the merger. Ladenburg has assumed that the merger
will be consummated in a manner that complies with the applicable provisions of the Securities Act of 1933, as amended, the Securities
Exchange Act of 1934, as amended, and all other applicable federal and state statutes, rules and regulations. Entero has informed Ladenburg,
and Ladenburg has assumed, that the merger is intended to constitute a reorganization within the meaning of Section 368(a) of the
Code and the Treasury Regulations promulgated thereunder.
It
is understood that the Ladenburg Opinion is intended for the benefit and use of the Entero Board in its consideration of the financial
terms of the merger and, except as set forth in Ladenburg’s engagement letter with Entero, dated January 29, 2024, may not be used
for any other purpose or reproduced, disseminated, quoted or referred to at any time, in any manner or for any purpose without Ladenburg’s
prior written consent, unless pursuant to applicable law or regulations or required by other regulatory authority by the order or ruling
of a court or administrative body, except that this Opinion may be included in its entirety in any filing related to the merger to be
filed with the Securities and Exchange Commission and the proxy statement to be mailed to the Entero stockholders. The Opinion does not
constitute a recommendation to the Entero Board of whether or not to approve the merger or to any Entero stockholder or any other person
as to how to vote with respect to the merger or to take any other action in connection with the merger or otherwise. Ladenburg’s
Opinion does not address Entero’s underlying business decision to proceed with the merger or the relative merits of the merger
compared to other alternatives available to Entero. Ladenburg expressed no opinion as to the prices or ranges of prices at which shares
or the securities of any person, including Entero, will trade at any time, including following the announcement or consummation of the
merger. Ladenburg has not been requested to opine as to, and its Opinion does not in any manner address, the amount or nature of compensation
to any of the officers, directors or employees of any party to the merger, or any class of such persons, relative to the compensation
to be paid to the Entero stockholders in connection with the merger or with respect to the fairness of any such compensation.
The
Opinion may not be published or otherwise used or referred to, nor shall any public reference to Ladenburg be made, without Ladenburg’s
prior written consent.
Principal Financial
Analyses
The
following is a summary of the principal financial analyses performed by Ladenburg to arrive at its Opinion. Some of the summaries of
financial analyses include information presented in tabular format. In order to fully understand the financial analyses, the tables must
be read together with the text of each summary. The tables alone do not constitute a complete description of the financial analyses.
Considering the data set forth in the tables without considering the full narrative description of the financial analyses, including
the methodologies and assumptions underlying the analyses, could create a misleading or incomplete view of the financial analyses. Ladenburg
performed certain procedures, including each of the financial analyses described below and reviewed with the Entero Board the assumptions
on which such analyses were based and other factors, including the historical and projected financial results of Entero and ImmunogenX.
Transaction Overview as of the Date
of the Opinion
Based
upon the Exchange Ratio of 2.649 at the time of the signing of the merger agreement, it was estimated that at the closing: (a) ImmunogenX
equity holders as of immediately prior to the merger will own approximately 81.9% of the fully-diluted shares of Entero common stock
at the closing of the merger, and (b) the Entero equity holders as of immediately prior to the merger (excluding for this purpose
certain out-of-the-money Entero options) will own approximately 18.1% of the fully-diluted shares of Entero common stock at
the closing of the merger, in each case, subject to adjustment of the Exchange Ratio as set forth in the merger agreement and described
herein.
ImmunogenX Valuation
Ladenburg utilized an
implied equity value for ImmunogenX of $85.0 million, which was calculated by adding the negotiated pre-money Enterprise Value of ImmunogenX
of $95.0 million minus $10.0 million of ImmunogenX’s debt on hand.
Analysis of Selected Initial Public Offering
Transactions
Ladenburg
reviewed certain publicly available information for the IPOs of 13 gastrointestinal / inflammation / autoimmune-focused biopharmaceutical
companies that have completed an IPO since January 2018 and whose lead product at the time of IPO was in a Phase 2 or Phase 3 stage of
clinical development. Although the companies referred to below were used for comparison purposes, none of these companies are directly
comparable to ImmunogenX. Accordingly, an analysis of the results of such a comparison is not purely mathematical but instead involves
complex considerations and judgments concerning differences in historical and projected financial and operating characteristics of the
selected companies below. These companies, which are referred to as the “Selected Precedent IPO Companies,” were:
| |
· |
Abivax |
| |
· |
Acelyrin |
| |
· |
Annexon |
| |
· |
Connect Biopharma Holdings Limited |
| |
· |
Finch Therapeutics |
| |
· |
Gossamer Bio |
| |
· |
Kiniksa Pharmaceuticals, Ltd. |
| |
· |
Landos BioPharma, Inc. |
| |
· |
Menlo Therapeutics Inc. |
| |
· |
Pliant Therapeutics, Inc. |
| |
· |
Sol-Gel Technologies Ltd |
| |
· |
Ventyx Biosciences |
| |
· |
Virios Therapeutics |
The
total enterprise value at IPO is defined as the pre-money equity value plus indebtedness, liquidation value of preferred stock
and non-controlling interest, minus cash and cash equivalents at the time of its IPO. The Selected Precedent IPO Companies
had total enterprise values between $180.2 million and $889.1 million. Ladenburg derived a median total enterprise value of $493.8 million
for the Selected Precedent IPO Companies. Using the 25th percentile and the 75th percentile of the implied total enterprise values, Ladenburg
then calculated a range of implied total equity values for ImmunogenX (by subtracting an estimated $10.0 million in debt at closing),
which was $249.3 million to $534.9 million. This compares to ImmunogenX’s implied equity value as per the merger agreement
of approximately $85.0 million.
Selected Precedent
IPO Companies
| Filing Date | |
Issuer | |
Enterprise Value ($M) | |
| 10/19/2023 | |
Abivax | |
$ | 414.0 | |
| 5/4/2023 | |
Acelyrin | |
| 840.7 | |
| 10/20/2021 | |
Ventyx Biosciences | |
| 489.4 | |
| 3/19/2021 | |
Connect Biopharma Holdings Limited | |
| 889.1 | |
| 3/18/2021 | |
Finch Therapeutics | |
| 576.1 | |
| 2/3/2021 | |
Landos BioPharma, Inc. | |
| 498.5 | |
| 12/16/2020 | |
Virios Therapeutics | |
| 52.0 | |
| 7/23/2020 | |
Annexon | |
| 236.6 | |
| 6/2/2020 | |
Pliant Therapeutics, Inc. | |
| 240.2 | |
| 2/12/2019 | |
Gossamer Bio | |
| 507.8 | |
| 5/23/2018 | |
Kiniksa Pharmaceuticals, Ltd. | |
| 498.2 | |
| 2/1/2018 | |
Sol-Gel Technologies Ltd | |
| 185.9 | |
| 1/24/2018 | |
Menlo Therapeutics Inc. | |
| 180.2 | |
Analysis of Selected Publicly Traded Companies
Based
on its experience and professional judgment and using financial screening sources and databases to find companies that share similar business
characteristics to ImmunogenX within the biopharmaceutical industry, Ladenburg selected financial data of 27 publicly traded companies
(referred to as the “Selected Publicly Traded Companies”). Each of the Selected Publicly Traded
Companies had a lead candidate in clinical development and focused on the gastrointestinal / inflammation / autoimmune space. Although
the companies referred to below were used for comparison purposes, none of those companies is directly comparable to ImmunogenX. Accordingly,
an analysis of the results of such a comparison is not purely mathematical but instead involves complex considerations and judgments concerning
differences in historical and projected financial and operating characteristics of the selected companies below. The total enterprise
values are based on closing stock prices on March 12, 2024. The Selected Publicly Traded Companies were:
| |
· |
89bio, Inc. |
| |
· |
Abivax |
| |
· |
Acelyrin |
| |
· |
Annexon Biosciences |
| |
· |
ASLAN Pharmaceuticals Limited |
| |
· |
aTyr Pharma |
| |
· |
CalciMedica |
| |
· |
Conduit Pharmaceuticals |
| |
· |
Edesa Biotech |
| |
· |
Evelo Biosciences, Inc. |
| |
· |
Galmed Pharmaceuticals |
| |
· |
Gossamer bio |
| |
· |
GRI Bio |
| |
· |
Immunic Therapeutics |
| |
· |
Immunovant |
| |
· |
Incannex Healthcare |
| |
· |
Kymera Therapeutics |
| |
· |
Landos Biopharma, Inc. |
| |
· |
Morphic Holding, Inc. |
| |
· |
Pliant Therapeutics, Inc. |
| |
· |
RAPT Therapeutics, Inc. |
| |
· |
Selecta Biosciences |
| |
· |
Synact Pharma |
| |
· |
Third Harmonic Bio |
| |
· |
Ventyx Biosciences |
| |
· |
vTv Therapeutics Inc. |
| |
· |
Zura Bio Limited |
The
Selected Publicly Traded Companies had implied total enterprise values between negative $22.1 million and $3.9 billion. Ladenburg
derived a median implied total enterprise value of $51.8 million for the Selected Publicly Traded Companies. Using the 25th percentile
and the 75th percentile of the implied total enterprise values, Ladenburg then calculated a range of implied total equity values for
ImmunogenX (by subtracting an estimated $10.0 million in debt at closing), which was $13.9 million to $250.2 million.
This compares to ImmunogenX’s implied equity value as per the merger agreement of approximately $85.0 million.
| Selected Publicly Traded Companies |
| |
| Company Name | |
Enterprise Value ($M) | |
| Immunovant | |
$ | 3,886.1 | |
| Kymera Therapeutics | |
| 2,267.3 | |
| Morphic Holding, Inc. | |
| 1,073.8 | |
| Abivax | |
| 773.3 | |
| 89bio, Inc. | |
| 651.6 | |
| Pliant Therapeutics, Inc. | |
| 429.1 | |
| Ventyx Biosciences | |
| 259.1 | |
| Conduit Pharmaceuticals | |
| 241.4 | |
| Gossamer bio | |
| 231.9 | |
| Annexon Biosciences | |
| 225.8 | |
| RAPT Therapeutics, Inc. | |
| 125.4 | |
| Third Harmonic Bio | |
| 119.3 | |
| Immunic Therapeutics | |
| 77.0 | |
| Incannex Healthcare | |
| 51.8 | |
| Zura Bio Limited | |
| 38.3 | |
| CalciMedica | |
| 33.7 | |
| Selecta Biosciences (Cartesian) | |
| 29.3 | |
| Acelyrin | |
| 19.9 | |
| Evelo Biosciences, Inc. | |
| 17.8 | |
| Synact Pharma | |
| 17.5 | |
| Edesa Biotech | |
| 10.2 | |
| ASLAN Pharmaceuticals Limited | |
| 8.9 | |
| aTyr Pharma | |
| 8.0 | |
| GRI Bio | |
| (2.9 | ) |
| Galmed Pharmaceuticals | |
| (11.9 | ) |
| vTv Therapeutics Inc. | |
| (18.5 | ) |
| Landos Biopharma, Inc. | |
| (22.1 | ) |
Analysis of Selected Precedent M&A
Transactions
Ladenburg
reviewed the financial terms, to the extent the information was publicly available, of the 13 most recent qualifying merger transactions
of companies in the biopharmaceutical industry, which had a lead candidate in clinical development and focused on the gastrointestinal
/ inflammation / autoimmune space (referred to as the “Selected Precedent M&A Transactions”). Although
the precedent transactions referred to below were used for comparison purposes, none of the target companies is directly comparable to
ImmunogenX. Accordingly, an analysis of the results of such a comparison is not purely mathematical, but instead involves complex considerations
and judgments concerning differences in historical and projected financial and operating characteristics of the companies involved and
other factors that could affect the merger value of such companies and ImmunogenX to which they are being compared. Ladenburg reviewed
the total enterprise values of the target companies (not including downstream milestone payments). These transactions, including the date
each was closed, were as follows below.
The
Selected Precedent M&A Transactions had total implied enterprise values between $4.4 million and $976.0 million. Ladenburg derived
a median total enterprise value of $356.0 million for the Selected Precedent M&A Transactions. Using the 25th percentile and
the 75th percentile of the implied total enterprise values, Ladenburg then calculated a range of implied total enterprise values for ImmunogenX
(by subtracting an estimated $10.0 million in debt at closing), which was $277.5 million to $432.1 million. This compares to
ImmunogenX’s implied equity value as per the merger agreement of approximately $85.0 million.
| Selected Precedent M&A Transactions |
| |
| Announced Date | |
Target | |
Acquirer | |
Implied Enterprise
Value ($M) | |
| 5/22/2023 | |
VectivBio Holding | |
Ironwood Pharmaceuticals | |
$ | 976.0 | |
| 4/16/2023 | |
Prometheus Biosciences | |
Merck & Co | |
| 382.0 | |
| 9/27/2022 | |
ViaCyte, Inc. | |
Vertex Pharmaceuticals, Inc. | |
| 320.0 | |
| 5/7/2020 | |
Naia Rare Diseases | |
9 Meters Biopharma | |
| 4.4 | |
| 2/26/2020 | |
PVP Biologics | |
Takeda Pharmaceutical | |
| 330.0 | |
| 2/13/2020 | |
Promedior, Inc. | |
Roche Holding AG | |
| 390.0 | |
| 12/31/2019 | |
PharmAkea, Inc. | |
Galecto, Inc. | |
| 17.4 | |
| 12/19/2017 | |
Eloxx Pharmaceuticals Ltd. | |
Sevion Therapeutics, Inc. | |
| 22.6 | |
| 1/20/2017 | |
Ziarco Group Limited | |
Novartis AG | |
| 325.0 | |
| 12/6/2016 | |
Creabilis SA | |
Sienna Biopharmaceuticals, Inc. | |
| 150.0 | |
| 11/1/2016 | |
Tobira Therapeutics, Inc. | |
Allergan plc | |
| 723.6 | |
| 10/25/2016 | |
Vitae Pharmaceuticals, Inc. | |
Allergan plc | |
| 528.2 | |
| 5/17/2016 | |
Nimbus Apollo, Inc. | |
Gilead Sciences, Inc. | |
| 400.0 | |
Discounted Cash Flow Analysis
Ladenburg
estimated a range of total enterprise values for ImmunogenX based upon the present value of ImmunogenX’s estimated after-tax unlevered
free cash flows. In conducting its diligence, Entero utilized the projections and estimates provided by ImmunogenX and then added certain
adjustments to derive the financial projections for ImmunogenX. Ladenburg received the projections on February 2, 2024, and reviewed
and analyzed the revenue and expense projections for ImmunogenX as prepared by the management of Entero.
Management Forecasts
for Latiglutenase
Entero
estimated a market opportunity for the Latiglutenase program, based on the following data in the table below concerning celiac disease
prevalence, diagnosis rate, and seroactivity, which is based on the clinical literature:
Population Group
(2022 numbers****) | |
US | | |
Europe | | |
ROTW | |
| Total population | |
| 331,000,000 | | |
| 749,000,000 | | |
| 6,920,000,000 | |
| CD
Patients as % of Population | |
| 1.00 | % | |
| 1.00 | % | |
| 1.00 | % |
| Celiac disease | |
| 3,310,000 | | |
| 7,490,000 | | |
| 69,200,000 | |
| CD
Diagnosis Rate % | |
| 30.0 | %* | |
| 30.0 | % | |
| 2.5 | % |
| Diagnosed CD | |
| 993,000 | | |
| 2,247,000 | | |
| 1,730,000 | |
| Seroactive
% of Diagnosed CD | |
| 25.0 | %** | |
| 25.0 | % | |
| 25.0 | % |
| Symptomatic
% of Seroactives | |
| 75.0 | %*** | |
| 75.0 | % | |
| 75.0 | % |
| Seroactive and Symptomatic | |
| 18.75 | % | |
| 18.75 | % | |
| 18.75 | % |
| Current Seropositive and Symptomatic | |
| 186,188 | | |
| 421,313 | | |
| 324,375 | |
* Celiac Disease Foundation
2024
**Silvester JA, Kurada
S, Szwajcer A, Kelly CP, Leffler DA, Duerksen DR. Tests for Serum Transglutaminase and Endomysial Antibodies Do Not Detect Most Patients
With Celiac Disease and Persistent Villous Atrophy on Gluten-free Diets: A Meta-analysis. Gastroenterology 2017;153:689-701.
Nachman F, Sugai E, Vazques
H, et al. Serological tests for celiac disease as indicators of long-term compliance with the gluten-free diet. Eur J Gastroenterol Hepatol
2011;23:473-480.
***from Latiglutenase historical
studies (ALV003-1221 trial)
****High single digit growth
rate of Diagnosed CD patients - https://www.beyondceliac.org/celiac-disease/facts-and-figures/
Entero
is targeting celiac disease patients who are both symptomatic and seroactive, and projects 30% market penetration of the diagnosed seroactive
population in the United States by 2036.
Estimated Growth Rate and Market Capture of Diagnosed CD Patients
| | |
2023 | | |
2024 | | |
2025 | | |
2026 | | |
2027 | | |
2028 | | |
2029 | | |
2030 | | |
2031 | | |
2032 | | |
2033 | | |
2034 | | |
2035 | | |
2036 | | |
2037 | | |
2038 | | |
2039 | | |
2040 | | |
2041 | |
| Diagnosed CD
growth rate - US | |
10.0 | % | |
9.5 | % | |
9.0 | % | |
8.5 | % | |
8.0 | % | |
7.5 | % | |
7.0 | % | |
6.8 | % | |
6.5 | % | |
6.3 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % |
| Diagnosed CD growth rate
- Europe | |
8.0 | % | |
7.6 | % | |
7.2 | % | |
6.8 | % | |
6.4 | % | |
6.0 | % | |
5.6 | % | |
5.4 | % | |
5.2 | % | |
5.0 | % | |
4.8 | % | |
4.8 | % | |
4.8 | % | |
4.8 | % | |
4.8 | % | |
4.8 | % | |
4.8 | % | |
4.8 | % | |
4.8 | % |
| Diagnosed CD growth rate
- ROTW | |
10.0 | % | |
9.5 | % | |
9.0 | % | |
8.5 | % | |
8.0 | % | |
7.5 | % | |
7.0 | % | |
6.8 | % | |
6.5 | % | |
6.3 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % | |
6.0 | % |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Diagnosed CD Patients | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| US | |
1,092,300 | | |
1,196,069 | | |
1,303,715 | | |
1,414,530 | | |
1,527,693 | | |
1,642,270 | | |
1,757,229 | | |
1,875,842 | | |
1,997,771 | | |
2,122,632 | | |
2,249,990 | | |
2,384,989 | | |
2,528,089 | | |
2,679,774 | | |
2,840,560 | | |
3,010,994 | | |
3,191,654 | | |
3,383,153 | | |
3,586,142 | |
| Europe | |
2,426,760 | | |
2,611,194 | | |
2,799,200 | | |
2,989,545 | | |
3,180,876 | | |
3,371,729 | | |
3,560,546 | | |
3,752,815 | | |
3,947,961 | | |
4,145,359 | | |
4,344,337 | | |
4,552,865 | | |
4,771,402 | | |
5,000,430 | | |
5,240,450 | | |
5,491,992 | | |
5,755,608 | | |
6,031,877 | | |
6,321,407 | |
| ROTW | |
1,903,000 | | |
2,083,785 | | |
2,271,326 | | |
2,464,388 | | |
2,661,539 | | |
2,861,155 | | |
3,061,436 | | |
3,268,083 | | |
3,480,508 | | |
3,698,040 | | |
3,919,922 | | |
4,155,117 | | |
4,404,424 | | |
4,668,690 | | |
4,948,811 | | |
5,245,740 | | |
5,560,484 | | |
5,894,113 | | |
6,247,760 | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Diagnosed Seroactive +
Symptomatic CD Patients | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| US | |
204,806 | | |
224,263 | | |
244,446 | | |
265,224 | | |
286,442 | | |
307,926 | | |
329,480 | | |
351,720 | | |
374,582 | | |
397,994 | | |
421,873 | | |
447,186 | | |
474,017 | | |
502,458 | | |
532,605 | | |
564,561 | | |
598,435 | | |
634,341 | | |
672,402 | |
| Europe | |
455,018 | | |
489,599 | | |
524,850 | | |
560,540 | | |
596,414 | | |
632,199 | | |
667,602 | | |
703,653 | | |
740,243 | | |
777,255 | | |
814,563 | | |
853,662 | | |
894,638 | | |
937,581 | | |
982,584 | | |
1,029,748 | | |
1,079,176 | | |
1,130,977 | | |
1,185,264 | |
| ROTW | |
356,813 | | |
390,710 | | |
425,874 | | |
462,073 | | |
499,039 | | |
536,467 | | |
574,019 | | |
612,765 | | |
652,595 | | |
693,382 | | |
734,985 | | |
779,085 | | |
825,830 | | |
875,379 | | |
927,902 | | |
983,576 | | |
1,042,591 | | |
1,105,146 | | |
1,171,455 | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Latiglutenase Market Capture | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| US | |
- | | |
- | | |
- | | |
| | |
| | |
2.0 | % | |
5.0 | % | |
10.0 | % | |
12.0 | % | |
15.0 | % | |
18.0 | % | |
25.0 | % | |
25.0 | % | |
30.0 | % | |
30.0 | % | |
30.0 | % | |
30.0 | % | |
30.0 | % | |
30.0 | % |
| Europe | |
- | | |
- | | |
- | | |
- | | |
- | | |
- | | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| ROTW | |
- | | |
- | | |
- | | |
- | | |
- | | |
- | | |
- | | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
Market
growth is driven by the increase in natural population and assumes a 6.8% average annual increase in diagnosed celiac disease patients
between 2024-2041 (Source: https://www.beyondceliac.org/celiac-disease/facts-and-figures/).
Entero
provided certain assumptions that supported the market opportunity for the Latiglutenase program that assumes a commercial launch in
2027 following a successful post-Phase 3 BLA, and only included U.S. sales from the current Latiglutenase license. Revenue projections
assume patients receiving the treatment receive 50% compliance with three doses per day at $15.17 unit price per dose. Unit pricing was
based on a current pricing quote and did not assume an annual price increase. The projections also assume that Entero would enter into
a licensing agreement for Latiglutenase with a strategic partner, based on terms agreed to in a non-binding letter of intent from a strategic
pharmaceutical company in December 2023. Such terms include an 8-figure non-dilutive payment to be paid in installments, at closing and
upon satisfaction of certain regulatory and commercial milestones, and high single-digit to double-digit royalties. The major out-licensed
geographies contemplated by the term sheet include only exclusive rights to the United States and Canada, along with a right of first
refusal to commercial rights in the following countries: Poland, Czech republic, Slovakia, Slovenia, Hungary, Croatia, Bulgaria, Serbia,
Montenegro, Russia, Kazakhstan, Ukraine, Romania, Greece, Cyprus, Malta, Mexico, Colombia, Guatemala, El Salvador, Panama, Honduras,
Costa Rica, Nicaragua, and the Dominican Republic. The Company would retain full commercial rights for the rest of the world. Since December
2023, we have been in discussions with the strategic licensee to advance towards a definitive agreement. ImmunogenX provided costs related
to commercialization expenditures for Latiglutenase, which were further adjusted by the Entero management team based on extensive knowledge
in the industry. If any of these assumptions, or the projections as a whole, prove to be inaccurate, the net present value of Latiglutenase
could be lower than presently forecasted, which could result in a lower amount of future royalties being paid to Entero over the forecasted
time period.
After
arriving at a set of projections, Entero further adjusted the revenue assumptions downward in the years 2028 to 2040 to reflect a 51.9%
probability of success (calculated by determining the likelihood of approval of each program) given the clinical stage of development
of ImmunogenX’s products. Specifically, such projections were based solely on Latiglutenase therapeutic sales following a successful
post-Phase 3 BLA. Future life cycle Latiglutenase products, such as potential new formulations and therapeutic indications, were excluded
from these revenue and cost projections. Potential revenue from the drug candidates in our portfolio that are in development, capeserod
and adrulipase, were also excluded. Entero also applied this probability adjustment to expenses, which consisted of cost of goods sold,
research and development costs, general and administrative and commercialization expenses and then subtracted all the risk-adjusted expenses
in the projection period from risk-adjusted revenue. Entero then assumed a 21.0% corporate tax rate when calculating unlevered free cash
flow.
In
performing this discounted cash flow analysis, Ladenburg utilized discount rates ranging from 10.4% to 12.4%, which were selected based
on the capital asset pricing model and the estimated weighted average cost of capital of the Selected Publicly Traded Companies. This
discounted cash flow analysis assumed that ImmunogenX will have no terminal value after 2040, does not take into account ImmunogenX’s
available net operating losses, if any, does not take into account stock-based compensation costs, if any, and assigns no value to revenues
beyond 2040.
Using
the range of discount rates of 10.4% to 12.4%, Ladenburg then calculated a range of implied total equity values for ImmunogenX (by subtracting
an estimated $10.0 million in debt at closing), which was $383.3 million to $506.0 million. This compares to ImmunogenX’s
implied equity value of approximately $85.0 million.
The
following table presents a summary of the Entero-prepared ImmunogenX financial projections that were made available to Ladenburg and
the Entero Board in February 2024. Entero management chose to provide projections from 2024 through 2041 to provide a full picture of
the Latiglutenase program during the period of biologic exclusivity, beginning 12 years from receipt of a BLA in 2028.
Neither
Entero nor ImmunogenX, as a matter of course, publicly discloses forecasts, internal projections as to future performance, revenues,
earnings or other results of operations due to the inherent unpredictability and subjectivity of underlying assumptions and projections.
ImmunogenX’s
future financial results may materially differ from those expressed in the projections due to factors that are beyond ImmunogenX’s
ability to control or predict. ImmunogenX cannot make any assurances that the projections will be realized or that ImmunogenX’s
future financial results will not materially vary from the projections. These forecasts are dependent entirely on assumptions about a
variety of events and circumstances that may or may not occur and are subject, in all upon a number of assumptions respects, to actual
results and to risks and contingencies, known and unknown, many of which are outside of Entero’s and ImmunogenX’s control,
in many cases, and which cannot be predicted in advance. Forward-looking information, including information about future business plans,
are inherently subject to significant uncertainties and contingencies. Forward-looking statements are also susceptible to multiple interpretations
and inherently reflect assumptions with respect to general business, economic, regulatory, market and financial conditions and other
future events, all of which are difficult to predict and many of which are beyond the control of Entero and ImmunogenX. Additionally,
the longer the time frame for the projections, the more such estimates, particularly those estimates farthest in the future, are subject
to variation. Such estimates include, but are not limited to, the costs associated with CMC scale up. Investors are encouraged to read
carefully the information contained in this proxy statement, including the financial information and the descriptions about various risks
and uncertainties concerning Entero’s and ImmunogenX’s business described herein, including risks relating to our product
candidates and our ability to successfully commercialize our assets, please see the section entitled “Risks – Risks Related
to Clinical Development, Regulatory Approval and Commercialization”, “Entero’s Management’s Discussion and
Analysis of Financial Condition and Results of Operations,” and “ImmunogenX’s Management’s Discussion and Analysis
of Financial Condition and Results of Operations.
The
projections were prepared for internal use, and were not prepared with a view toward public disclosure, or with a view toward compliance
with published guidelines of the SEC regarding projections, the guidelines established by the American Institute of Certified Public
Accountants for preparation and presentation of prospective financial information, or GAAP. At the time of preparation, Entero and ImmunogenX’s
management considered these projections to be reasonable, based on management’s observations, expertise, and industry knowledge,
taking into account that all of ImmunogenX’s planned business activities for Latiglutenase, as reflected in the forecasts, are
speculative, and the costs and expenses ImmunogenX incurs may be different, potentially substantially, from the estimates thereof incorporated
in the assumptions. Moreover, Entero and ImmunogenX’s plans may, and can be expected, to change over the timeline of the forecast
periods, potentially materially, as underlying facts and circumstances specific to Latiglutenase, and more generally to Entero and ImmunogenX’s
operations, change. Neither ImmunogenX’s nor Entero’s independent registered public accounting firm, nor any other independent
accountants, have compiled, examined or performed any procedures with respect to the prospective financial information included below,
or expressed any opinion or any other form of assurance with respect thereto or the achievability of the results reflected in such projections,
and none of the foregoing assumes any responsibility for such information.
The
projections included below are not being included herein to influence Entero’s stockholders' decision whether to vote in favor
of any proposal contained in this proxy statement/prospectus/information statement. In light of the foregoing factors and the uncertainties
inherent in the projections, stockholders are cautioned not to place undue reliance on the projections included in this proxy statement/prospectus/information
statement.
| |
2024 | |
2025 | |
2026 | |
2027 | |
2028 | |
2029 | |
2030 | |
2031 | |
2032 | |
2033 | |
2034 | |
2035 | |
2036 | |
2037 | |
2038 | |
2039 | |
2040 | |
| Earnings Before
Interest & Taxes |
$ | (46.6 | ) |
$ | (45.8 | ) |
$ | (45.5 | ) |
$ | (13.7 | ) |
$ | 8.5 | |
$ | 45.1 | |
$ | 126.2 | |
$ | 165.0 | |
$ | 223.5 | |
$ | 319.5 | |
$ | 477.1 | |
$ | 506.3 | |
$ | 647.8 | |
$ | 687.3 | |
$ | 729.1 | |
$ | 773.5 | |
$ | 820.5 | |
| Probability of Success |
| - | |
| - | |
| - | |
| - | |
| 51.9 | % |
| 51.9 | % |
| 51.9 | % |
| 51.9 | % |
| 51.9 | % |
| 51.9 | % |
| 51.9 | % |
| 51.9 | % |
| 51.9 | % |
| 51.9 | % |
| 51.9 | % |
| 51.9 | % |
| 51.9 | % |
| PoS Adjusted
EBIT |
| - | |
| - | |
| - | |
| - | |
$ | 4.4 | |
$ | 23.4 | |
$ | 65.5 | |
$ | 85.6 | |
$ | 116.0 | |
$ | 165.8 | |
$ | 247.6 | |
$ | 262.8 | |
$ | 336.2 | |
$ | 356.7 | |
$ | 378.4 | |
$ | 401.4 | |
$ | 425.8 | |
| Less: Income Tax (21%) |
| - | |
| - | |
| - | |
| - | |
| 0.9 | |
| 4.9 | |
| 13.8 | |
| 18.0 | |
| 24.4 | |
| 34.8 | |
| 52.0 | |
| 55.2 | |
| 70.6 | |
| 74.9 | |
| 79.5 | |
| 84.3 | |
| 89.4 | |
| Cash Flow
From Operations |
$ | (46.6 | ) |
$ | (45.8 | ) |
$ | (45.5 | ) |
$ | (13.7 | ) |
$ | 7.6 | |
$ | 18.5 | |
$ | 51.8 | |
$ | 67.6 | |
$ | 91.6 | |
$ | 131.0 | |
$ | 195.6 | |
$ | 207.6 | |
$ | 265.6 | |
$ | 281.8 | |
$ | 299.0 | |
$ | 317.1 | |
$ | 336.4 | |
| Risk Adjusted
Free Cash Flow |
$ | (46.6 | ) |
$ | (45.8 | ) |
$ | (45.5 | ) |
$ | (13.7 | ) |
$ | 7.6 | |
$ | 18.5 | |
$ | 51.8 | |
$ | 67.6 | |
$ | 91.6 | |
$ | 131.0 | |
$ | 195.6 | |
$ | 207.6 | |
$ | 265.6 | |
$ | 281.8 | |
$ | 299.0 | |
$ | 317.1 | |
$ | 336.4 | |
The
summary set forth above does not purport to be a complete description of all the analyses performed by Ladenburg. The preparation of
a fairness opinion involves various determinations as to the most appropriate and relevant methods of financial analysis and the application
of these methods to the particular circumstances. Therefore, such an opinion is not readily susceptible to partial analysis or summary
description. Ladenburg did not attribute any particular weight to any analysis or factor considered by it, but rather made qualitative
judgments as to the significance and relevance of each analysis and factor. Accordingly, notwithstanding the separate factors summarized
above, Ladenburg believes, and advised the Entero Board, that its analyses must be considered as a whole. Selecting portions of its analyses
and the factors considered by it without considering all analyses and factors could create an incomplete view of the process underlying
its Opinion. In performing its analyses, Ladenburg made numerous assumptions with respect to industry performance, business and economic
conditions and other matters, many of which are beyond the control of Entero and ImmunogenX. These analyses performed by Ladenburg are
not necessarily indicative of actual values or future results, which may be significantly more or less favorable than suggested by such
analyses. In addition, analyses relating to the value of businesses do not purport to be appraisals or to reflect the prices at which
businesses or securities may actually be sold. Accordingly, such analyses and estimates are inherently subject to uncertainty, being
based upon numerous factors or events beyond the control of the parties or their respective advisors. None of Entero, ImmunogenX, Ladenburg
or any other person assumes responsibility if future results are materially different from those projected. The analyses supplied by
Ladenburg and its Opinion were among several factors taken into consideration by the Entero Board in making its decision to enter into
the merger agreement and should not be considered as determinative of such a decision.
Other Items Relating
to Fairness Opinion
Ladenburg
was selected by the Entero Board to render an opinion to the Entero Board because Ladenburg is a nationally recognized investment banking
firm and because, as part of its investment banking business, Ladenburg is continually engaged in the valuation of businesses and their
securities in connection with mergers, negotiated underwritings, secondary distributions of listed and unlisted securities, private placements
and valuations for corporate and other purposes. In addition, in the ordinary course of its business, Ladenburg and its affiliates may
trade the equity securities of Entero for its own account and for the accounts of their customers, and, accordingly, may at any time
hold a long or short position in such securities. In the two years preceding the date hereof, Ladenburg has not received any fees from
Entero. In the two years preceding the date hereof, Ladenburg has not had a relationship with ImmunogenX and has not received any fees
from ImmunogenX. Ladenburg and its affiliates may in the future seek to provide investment banking or financial advisory services to
Entero and ImmunogenX and/or certain of their respective affiliates and expect to receive fees for the rendering of these services.
Pursuant
to the engagement letter between Ladenburg and Entero, Entero paid Ladenburg an Opinion fee of $1,750,000 upon delivery of its Opinion.
The terms of the fee arrangement with Ladenburg, which are customary in transactions of this nature, were negotiated at arm’s length
between Entero and Ladenburg, and the Entero Board was aware of the arrangement.
Required Vote and Recommendation
In accordance with our Charter,
Bylaws and Delaware law, and as further discussed above under “The Annual Meeting — Quorum; Abstentions and Broker
Non-Votes,” approval and adoption of this Nasdaq Proposal requires the affirmative (“FOR”) vote of a majority of
votes cast by shares of our Common Stock present or represented by proxy and entitled to vote at the Annual Meeting and voting affirmatively
or negatively on such matter. Unless otherwise instructed on the proxy or unless authority to vote is withheld, shares represented by
executed proxies will be voted “FOR” this proposal. Abstentions and broker non-votes, if any, with respect to this proposal
are not counted as votes cast and will not affect the outcome of such proposal.
THE ENTERO BOARD OF DIRECTORS UNANIMOUSLY
RECOMMENDS THAT ENTERO STOCKHOLDERS VOTE “FOR” PROPOSAL 3.
PROPOSAL NO. 4:
ADOPTION AND APPROVAL OF AN AMENDMENT TO OUR CHARTER TO EFFECT A REVERSE STOCK SPLIT OF OUR ISSUED AND OUTSTANDING SHARES OF COMMON
STOCK, AT A SPECIFIC RATIO, RANGING FROM [●] TO [●], AT ANY TIME PRIOR TO THE ONE-YEAR ANNIVERSARY DATE OF THE ANNUAL
MEETING, WITH THE EXACT RATIO TO BE DETERMINED BY THE BOARD
Overview
Our Board has determined that
it is advisable and in the best interests of the Company and its stockholders, for us to amend our Charter to authorize our Board to effect
a reverse stock split (the “Charter Amendment”) of our issued and outstanding shares of Common Stock at a specific ratio,
ranging from [●] to [●] (the “Approved Split Ratios”), to be determined by the Board (the “Reverse Split”).
A vote for this Reverse Split Proposal will constitute approval of the Reverse Split that, once authorized by the Board and effected by
filing the Charter Amendment with the Secretary of State of the State of Delaware, will combine between [●] and [●] shares
of our Common Stock into one share of our Common Stock. If implemented, the Reverse Split will have the effect of decreasing the number
of shares of our Common Stock issued and outstanding.
Accordingly, stockholders
are asked to adopt and approve the Charter Amendment set forth in Annex D to effect the Reverse Split as set forth in the Charter Amendment,
subject to the Board’s determination, in its sole discretion, whether or not to implement the Reverse Split, as well as the specific
ratio within the range of the Approved Split Ratios, and provided that the Reverse Split must be effected on or prior to the one-year
anniversary date of the Annual Meeting. The text of Annex D remains subject to modification to include such changes as may be required
by the Secretary of State of the State of Delaware and as our Board deems necessary or advisable to implement the Reverse Split.
If adopted and approved by
the holders of our outstanding voting securities, the Reverse Split would be applied at an Approved Split Ratio approved by the Board
prior to the one-year anniversary date of the Annual Meeting. The Board reserves the right to elect to abandon the Reverse Split if it
determines, in its sole discretion, that the Reverse Split is no longer in the best interests of the Company and its stockholders.
Purpose and Rationale for the Reverse Split
On March 28, 2024, Entero
received a letter from the Staff stating that the Staff had determined Entero’s acquisition of ImmunogenX constitutes a business
combination that results in a “Change of Control” pursuant to Nasdaq Listing Rule 5110(a), and that, as a result, Entero
will be required to satisfy all of Nasdaq’s initial listing criteria and to complete Nasdaq’s initial listing process prior
to shareholder approval of the conversion of the Series G Preferred Stock, or other material changes triggering a change of control.
Failure to approve the
Reverse Split Proposal may have serious, adverse effects on Entero and its stockholders. Our Common Stock could be delisted from
Nasdaq because shares of our Common Stock may trade below the requisite per share price needed to maintain our listing. Our shares may
then be quoted on the OTC Bulletin Board or other small trading markets, which are generally considered to have less volume and be less
efficient markets. We believe an investor likely would find it less convenient to sell, or to obtain accurate quotations in seeking to
buy, our Common Stock on an over-the-counter market. Many investors likely would not buy or sell our Common Stock due to difficulty in
accessing over-the-counter markets, policies preventing them from trading in securities not listed on a national exchange, or other reasons.
In that event, the Common Stock could trade thinly as a microcap or penny stock, adversely decrease to nominal levels of trading and
may be avoided by retail and institutional investors, resulting in the impaired liquidity of our Common Stock.
In addition, an investment
in our Common Stock may not appeal to brokerage firms that are reluctant to recommend lower-priced securities to their clients. Investors
may also be dissuaded from purchasing lower-priced stocks because the brokerage commissions, as a percentage of the total transaction,
tend to be higher for such stocks. Moreover, the analysts at many brokerage firms do not monitor the trading activity or otherwise provide
research coverage of lower-priced stocks. Also, the Company’s Board of Directors believes that most investment funds are reluctant
to invest in lower-priced stocks.
As of June 13, 2024, our
Common Stock closed at $2.25 per share on Nasdaq. The Reverse Split, if effected, should have the immediate effect of increasing the
price of our Common Stock as reported on Nasdaq, therefore reducing the risk that our Common Stock could be delisted from Nasdaq.
Our Board strongly believes
that the Reverse Split may be necessary to maintain our listing on Nasdaq. Accordingly, the Board has recommended that our stockholders
approve the Reverse Split Proposal to effect the Reverse Split, if needed, at an Approved Split Ratio to be determined by the
Board, and directed that this proposal be submitted to our stockholders for approval at the Annual Meeting.
Risks of the Proposed Reverse Split
A
decline in the market price of our Common Stock after the Reverse Split is implemented may result in a greater percentage decline than
would occur in the absence of a reverse stock split.
If the Reverse Split is implemented
and the market price of our Common Stock declines, the percentage decline may be greater than would occur in the absence of a reverse
stock split. The market price of our Common Stock will, however, also be based upon our performance and other factors, which are unrelated
to the number of shares of Common Stock outstanding.
The
proposed Reverse Split may decrease the liquidity of our Common Stock.
The liquidity of our Common
Stock may be harmed by the proposed Reverse Split given the reduced number of shares of Common Stock that would be outstanding after the
Reverse Split, particularly if the stock price does not increase as a result of the Reverse Split.
Determination of the Ratio for the Reverse
Split
If the Reverse Split Proposal
is approved by stockholders and the Board determines that it is in the best interests of the Company and its stockholders to move forward
with the Reverse Split, the Approved Split Ratio will be selected by the Board, in its sole discretion. However, the Approved Split Ratio
will not be less than a ratio of [●] or exceed a ratio of [●]. In determining which Approved Split Ratio to use, the Board
will consider numerous factors, including the historical and projected performance of our Common Stock, the effect of the Approved Split
Ratio on our compliance with other Nasdaq listing requirements, prevailing market conditions and general economic trends, and will place
emphasis on the expected closing price of our Common Stock in the period following the effectiveness of the Reverse Split. The Board will
also consider the impact of the Approved Split Ratios on investor interest. The purpose of selecting a range is to give the Board the
flexibility to meet business needs as they arise, to take advantage of favorable opportunities and to respond to a changing corporate
environment. Based on the number of shares of Common Stock issued and outstanding as of [●], 2024, after completion of the Reverse
Split, we will have between [●] and [●] shares of Common Stock issued and outstanding, depending on the Approved Split Ratio
selected by the Board.
Principal Effects of the Reverse Split
After the effective date of
the proposed Reverse Split, each stockholder will own a reduced number of shares of Common Stock. Except for adjustments that may result
from the treatment of fractional shares as described below, the proposed Reverse Split will affect all stockholders uniformly. The proportionate
voting rights and other rights and preferences of the holders of our Common Stock will not be affected by the proposed Reverse Split (other
than as a result of the payment of cash in lieu of fractional shares). For example, a holder of 2% of the voting power of the outstanding
shares of our Common Stock immediately prior to a Reverse Split would continue to hold 2% of the voting power of the outstanding shares
of our Common Stock immediately after such Reverse Split. The number of stockholders of record also will not be affected by the proposed
Reverse Split, except to the extent that any stockholder holds only a fractional share interest and receives cash for such interest after
the Reverse Split.
The following table contains
approximate number of issued and outstanding shares of Common Stock, and the estimated per share trading price following a [●]
to [●] Reverse Split, without giving effect to any adjustments for fractional shares of Common Stock or the issuance of any derivative
securities, as of June 12, 2024.
After Each Reverse Split Ratio
| |
|
Current |
|
|
[Ratio 1] |
|
|
[Ratio 2] |
|
| Common Stock Authorized |
|
|
100,000,000 |
|
|
|
[ ] |
|
|
|
[ ] |
|
| Common Stock Issued and Outstanding |
|
|
2,750,108 |
|
|
|
[ ] |
|
|
|
[ ] |
|
| Number of Shares of Common Stock Reserved
for Issuance |
|
|
18,825,787 |
|
|
|
[ ] |
|
|
|
[ ] |
|
| Number of Shares of Common Stock Authorized
but Unissued and Unreserved |
|
|
81,174,213 |
|
|
|
[ ] |
|
|
|
[ ] |
|
| Price per share, based
on the closing price of our Common Stock on the Record Date |
|
|
$ [ ] |
|
|
|
$ [ ] |
|
|
|
$ [ ] |
|
After the effective date of
the Reverse Split, our Common Stock would have a new committee on uniform securities identification procedures (CUSIP) number, a number
used to identify our Common Stock.
Our Common Stock is currently
registered under Section 12(b) of the Exchange Act, and we are subject to the periodic reporting and other requirements of the Exchange
Act. The proposed Reverse Split will not affect the registration of our Common Stock under the Exchange Act. Our Common Stock would continue
to be reported on Nasdaq under the symbol “ENTO”, assuming that we are able to regain compliance with the minimum bid price
requirement, although it is likely that Nasdaq would add the letter “D” to the end of the trading symbol for a period of
twenty trading days after the effective date of the Reverse Split to indicate that the Reverse Split had occurred.
Effect on Outstanding Derivative Securities
The Reverse Split will
require that proportionate adjustments be made to the conversion rate, the per share exercise price and the number of shares issuable
upon the exercise or conversion of the following outstanding derivative securities issued by us, in accordance with the Approved Split
Ratio (all figures are as of June 12, 2024, and are on a pre-Reverse Split basis), including:
| |
● |
27 shares of Common Stock issuable upon exercise of stock options, with a weighted average exercise price of $35,062 per share, under our Amended and Restated 2014 Omnibus Equity Incentive Plan (the “2014 Plan”); |
| |
|
|
| |
● |
9 shares of awarded but unissued restricted stock under our 2014 Plan; |
| |
|
|
| |
● |
119,539 shares of awarded but unissued restricted stock units under
our 2020 Plan; |
| |
|
|
| |
● |
398 shares of Common Stock issuable upon exercise of stock options, with a weighted average exercise price of $3,970 per share, under our Amended and Restated 2020 Omnibus Equity Incentive Plan (the “2020 Plan”); |
| |
|
|
| |
● |
125,315 shares of Common Stock available for future issuance under
our 2020 Plan; |
| |
|
|
| |
● |
200,652 shares of Common Stock issuable upon exercise of stock options,
with a weighted average exercise price of $0.81 per share, under our IMGX 2021 Equity Incentive Plan (the “2021 Plan”); |
| |
|
|
| |
● |
3,256,085 shares of Common Stock issuable upon exercise of outstanding
warrants, with a weighted average exercise price of $12.50 per share; |
| |
|
|
| |
● |
134 shares of Common Stock issuable upon conversion of 484.52 shares
of Series B Convertible Preferred Stock (the “Series B Preferred Stock”), including in respect of accrued and
unpaid dividends of approximately $1,157,260 through June 12, 2024 at a conversion price of $32,340 per share; and |
| |
|
|
| |
● |
up to 294 additional shares of Common Stock issued pursuant to an
exchange right in excess of amounts currently underlying Series B Preferred Stock if the holders of Series B Preferred Stock elect
to exchange into our sale of shares of Common Stock at $10,986 per share under our At The Market Offering Agreement, dated November
30, 2021 (the “ATM Agreement”). |
| |
|
|
| |
● |
12,373,226 shares of Common Stock issuable upon conversion of 12.373 shares of Series G Convertible Preferred Stock. |
The adjustments to the above
securities, as required by the Reverse Split and in accordance with the Approved Split Ratio, would result in approximately the same aggregate
price being required to be paid under such securities upon exercise, and approximately the same value of shares of Common Stock being
delivered upon such exercise or conversion, immediately following the Reverse Split as was the case immediately preceding the Reverse
Split.
Effect
on Equity Incentive Plans
As of June 12, 2024, we
had 27 shares of Common Stock reserved for issuance pursuant to the exercise of outstanding options issued under our 2014 Plan. Further,
as of June 12, 2024, we had 398 shares of Common Stock reserved for issuance pursuant to the exercise of outstanding options issued under
our 2020 Plan, as well as 125,315 shares of Common Stock available for issuance under the 2020 Plan. Additionally, as of June 12, 2024,
we had 200,652 shares of Common Stock reserved for issuance pursuant to the exercise of outstanding options issued under the 2021 Plan.
Pursuant to the terms of the 2014 Plan and the 2020 Plan, the Board, or a designated committee thereof, as applicable, will adjust the
number of shares of Common Stock underlying outstanding awards, the exercise price per share of outstanding stock options and other terms
of outstanding awards issued pursuant to the 2014 Plan and the 2020 Plan to equitably reflect the effects of the Reverse Split. The number
of shares subject to vesting under restricted stock awards and the number of shares issuable as contingent consideration as part of an
acquisition by the Company will be similarly adjusted, subject to our treatment of fractional shares. The number of shares available
for future grant under the 2014 Plan and the 2020 Plan will be similarly adjusted.
Pursuant to the terms
of the 2021 Plan, the number of shares of Common Stock underlying outstanding awards and the exercise price per share of each outstanding
option will be automatically proportionately adjusted.
Effective
Date
The proposed Reverse Split
would become effective on the date of filing of the Charter Amendment with the office of the Secretary of State of the State of Delaware
unless another effective date is set forth in the Charter Amendment. On the effective date, shares of Common Stock issued and outstanding
shares of Common Stock held in treasury, in each case, immediately prior thereto will be combined and reclassified, automatically and
without any action on the part of our stockholders, into new shares of Common Stock in accordance with the Approved Split Ratio set forth
in this Reverse Split Proposal. If the proposed Charter Amendment is not adopted and approved by our stockholders, the Reverse Split will
not occur.
Treatment
of Fractional Shares
No fractional shares of Common
Stock will be issued as a result of the Reverse Split. Instead, in lieu of any fractional shares to which a stockholder of record would
otherwise be entitled as a result of the Reverse Split, we will pay cash (without interest) equal to such fraction multiplied by the average
of the closing sales prices of our Common Stock on The Nasdaq Capital Market during regular trading hours for the five consecutive trading
days immediately preceding the effective date of the Reverse Split (with such average closing sales prices being adjusted to give effect
to the Reverse Split). After the Reverse Split, a stockholder otherwise entitled to a fractional interest will not have any voting, dividend
or other rights with respect to such fractional interest except to receive payment as described above.
Upon stockholder adoption
and approval of this Reverse Split Proposal, if the Board elects to implement the proposed Reverse Split, stockholders owning fractional
shares will be paid out in cash for such fractional shares. For example, assuming the Board elected to consummate an Approved Split Ratio
of 1:10, if a stockholder held eleven shares of Common Stock immediately prior to the Reverse Split, then such stockholder would be paid
in cash for the one share of Common Stock but will maintain ownership of the remaining shares of Common Stock.
Record
and Beneficial Stockholders
If the Reverse Split is authorized
by our stockholders and our Board elects to implement the Reverse Split, stockholders of record holding some or all of their shares of
Common Stock electronically in book entry form under the direct registration system for securities will receive a transaction statement
at their address of record indicating the number of shares of Common Stock they hold after the Reverse Split along with payment in lieu
of any fractional shares. Non-registered stockholders holding Common Stock through a bank, broker or other nominee should note that such
banks, brokers or other nominees may have different procedures for processing the consolidation and making payment for fractional shares
than those that would be put in place by us for registered stockholders. If you hold your shares with such a bank, broker or other nominee
and if you have questions in this regard, you are encouraged to contact your nominee.
If the Reverse Split is authorized
by the stockholders and our Board elects to implement the Reverse Split, stockholders of record holding some or all of their shares in
certificate form will receive a letter of transmittal, as soon as practicable after the effective date of the Reverse Split. Our transfer
agent will act as “exchange agent” for the purpose of implementing the exchange of stock certificates. Holders of pre-Reverse
Split shares will be asked to surrender to the exchange agent certificates representing pre-Reverse Split shares in exchange for post-Reverse
Split shares and payment in lieu of fractional shares (if any) in accordance with the procedures to be set forth in the letter of transmittal.
Until surrender, each certificate representing shares before the Reverse Split would continue to be valid and would represent the adjusted
number of whole shares based on the approved exchange ratio of the Reverse Split selected by the Board. No new post-Reverse Split share
certificates will be issued to a stockholder until such stockholder has surrendered such stockholder’s outstanding certificate(s)
together with the properly completed and executed letter of transmittal to the exchange agent.
STOCKHOLDERS
SHOULD NOT DESTROY ANY PRE-REVERSE SPLIT STOCK CERTIFICATE AND SHOULD NOT SUBMIT ANY CERTIFICATES UNTIL THEY ARE REQUESTED TO DO SO.
Accounting
Consequences
The par value per share of
Common Stock would remain unchanged at $0.0001 per share after the Reverse Split. As a result, on the effective date of the Reverse Split,
the stated capital on our balance sheet attributable to the Common Stock will be reduced proportionally, based on the Approved Split Ratio
selected by the Board, from its present amount, and the additional paid-in capital account shall be credited with the amount by which
the stated capital is reduced. The per share Common Stock net income or loss and net book value will be increased because there will be
fewer shares of Common Stock outstanding. The shares of Common Stock held in treasury, if any, will also be reduced proportionately based
on the Approved Split Ratio selected by the Board. Retroactive restatement will be given to all share numbers in the financial statements,
and accordingly all amounts including per share amounts will be shown on a post-split basis. We do not anticipate that any other accounting
consequences would arise as a result of the Reverse Split.
No
Appraisal Rights
Our stockholders are not entitled
to dissenters’ or appraisal rights under the Delaware General Corporation Law with respect to this Reverse Split Proposal and we
will not independently provide our stockholders with any such right if the Reverse Split is implemented.
Material
Federal U.S. Income Tax Consequences of the Reverse Split
The following is a summary
of certain material U.S. federal income tax consequences of a Reverse Split to our stockholders. The summary is based on the Internal
Revenue Code of 1986, as amended (the “Code”), applicable Treasury Regulations promulgated thereunder, judicial authority
and current administrative rulings and practices as in effect on the date of this Proxy Statement. Changes to the laws could alter the
tax consequences described below, possibly with retroactive effect. We have not sought and will not seek an opinion of counsel or a ruling
from the Internal Revenue Service regarding the federal income tax consequences of a Reverse Split. This discussion only addresses stockholders
who hold Common Stock as capital assets. It does not purport to be complete and does not address stockholders subject to special tax treatment
under the Code, including, without limitation, financial institutions, tax-exempt organizations, insurance companies, dealers in securities,
foreign stockholders, stockholders who hold their pre-reverse stock split shares as part of a straddle, hedge or conversion transaction,
and stockholders who acquired their pre-reverse stock split shares pursuant to the exercise of employee stock options or otherwise as
compensation. If a partnership (or other entity treated as a partnership for U.S. federal income tax purposes) is the beneficial owner
of our Common Stock, the U.S. federal income tax treatment of a partner in the partnership will generally depend on the status of the
partner and the activities of the partnership. Accordingly, partnerships (and other entities treated as partnerships for U.S. federal
income tax purpose) holding our Common Stock and the partners in such entities should consult their own tax advisors regarding the U.S.
federal income tax consequences of the proposed Reverse Split to them. In addition, the following discussion does not address the tax
consequences of the Reverse Split under state, local and foreign tax laws. Furthermore, the following discussion does not address any
tax consequences of transactions effectuated before, after or at the same time as the Reverse Split, whether or not they are in connection
with the Reverse Split.
In general, the federal income
tax consequences of a Reverse Split will vary among stockholders depending upon whether they receive cash for fractional shares or solely
a reduced number of shares of Common Stock in exchange for their old shares of Common Stock. We believe that because the Reverse Split
is not part of a plan to increase periodically a stockholder’s proportionate interest in our assets or earnings and profits, the
Reverse Split should have the following federal income tax effects. The Reverse Split is expected to constitute a “recapitalization”
for U.S. federal income tax purposes pursuant to Section 368(a)(1) (E) of the Code. A stockholder who receives solely a reduced number
of shares of Common Stock will not recognize gain or loss. In the aggregate, such a stockholder’s basis in the reduced number of
shares of Common Stock will equal the stockholder’s basis in its old shares of Common Stock and such stockholder’s holding
period in the reduced number of shares will include the holding period in its old shares exchanged. The Treasury Regulations provide detailed
rules for allocating the tax basis and holding period of shares of common stock surrendered in a recapitalization to shares received in
the recapitalization. Stockholders of our Common Stock acquired on different dates and at different prices should consult their tax advisors
regarding the allocation of the tax basis and holding period of such shares.
A stockholder that, pursuant
to the proposed Reverse Split, receives cash in lieu of a fractional share of our Common Stock should recognize capital gain or loss in
an amount equal to the difference, if any, between the amount of cash received and the portion of the stockholder’s aggregate adjusted
tax basis in the shares of our Common Stock surrendered that is allocated to such fractional share. Such capital gain or loss will be
short term if the pre-Reverse Split shares were held for one year or less at the effective time of the Reverse Split and long term if
held for more than one year. Stockholders should consult their own tax advisors regarding the tax consequences to them of a payment for
fractional shares.
We will not recognize any
gain or loss as a result of the proposed Reverse Split.
A stockholder of our Common
Stock may be subject to information reporting and backup withholding on cash paid in lieu of a fractional share in connection with the
proposed Reverse Split. A stockholder of our Common Stock will be subject to backup withholding if such stockholder is not otherwise exempt
and such stockholder does not provide its taxpayer identification number in the manner required or otherwise fails to comply with backup
withholding tax rules. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be refunded
or allowed as a credit against a stockholder’s U.S. federal income tax liability, if any, provided the required information is timely
furnished to the Internal Revenue Service. Stockholders of our Common Stock should consult their own tax advisors regarding their qualification
for an exemption from backup withholding and the procedures for obtaining such an exemption.
THE PRECEDING DISCUSSION IS
INTENDED ONLY AS A SUMMARY OF CERTAIN FEDERAL U.S. INCOME TAX CONSEQUENCES OF THE REVERSE SPLIT AND DOES NOT PURPORT TO BE A COMPLETE
ANALYSIS OR DISCUSSION OF ALL POTENTIAL TAX EFFECTS RELEVANT THERETO. YOU SHOULD CONSULT YOUR OWN TAX ADVISORS AS TO THE PARTICULAR FEDERAL,
STATE, LOCAL, FOREIGN AND OTHER TAX CONSEQUENCES OF THE REVERSE SPLIT IN LIGHT OF YOUR SPECIFIC CIRCUMSTANCES.
Required
Vote and Recommendation
Pursuant to changes to Section
242 of the Delaware General Corporation Law which became effective on August 1, 2023 (the “DGCL Change”), the necessary stockholder
vote to approve reverse stock splits and an increase in authorized share capital was reduced from a majority of outstanding shares entitled
to vote, to a majority of votes actually cast at a meeting. In addition to reducing the required shareholder vote for approval of these
actions, the DGCL Change has the effect of causing abstentions to have no effect on a stockholder vote. This reduced vote requirement
only applies to companies (like ours) whose stock is listed on a national securities exchange and who would continue to meet the listing
requirements of the exchange immediately after giving effect to such actions.
Pursuant to the DCGL Change,
approval and adoption of this Reverse Split Proposal requires the affirmative vote of at least a majority of votes actually cast at the
meeting. Abstentions and broker non-votes, if any, with respect to this proposal are not counted as votes cast and will not affect the
outcome of such proposal.
OUR BOARD RECOMMENDS A VOTE “FOR”
THE REVERSE SPLIT PROPOSAL.
PROPOSAL NO. 5: APPROVAL, ON AN ADVISORY BASIS,
OF THE EXECUTIVE COMPENSATION
OF THE COMPANY’S NAMED EXECUTIVE OFFICERS
AS DESCRIBED IN THIS PROXY STATEMENT
At our 2022 annual meeting
of stockholders, we conducted a non-binding stockholder vote on the frequency of future Say-on-Pay votes (commonly known as a “Say-When-on-Pay”
vote). We recommended that such votes be conducted annually and our stockholders approved that recommendation. We will hold a Say-on-Pay
vote at each annual meeting until the time our stockholders vote to hold a Say-on-Pay vote at a different frequency.
Q: What are you voting on?
A:
In accordance with Section 14A of the Securities Exchange Act of 1934, we are asking stockholders to vote, on an advisory basis, on:
Say-on-pay.
Approval of the compensation of our named executive officers as disclosed in this proxy statement, including the various compensation
tables and the related narrative disclosures.
Q: Why does your Board recommend a vote “FOR” the
say-on-pay proposal (Proposal 5)?
A.
The Board believes that the Company’s compensation policies and practices are effective in achieving our goals of motivating
and retaining our executives by:
| |
· |
rewarding excellence in leadership and sustained financial performance; and |
| |
|
|
| |
· |
aligning our executives’ interests with those of our stockholders to create long-term value. |
Q: What are the effects of these votes?
A:
The Say on Pay Proposal is advisory and non-binding on our Board. However, the Board and the Compensation Committee will review
and consider the results of this vote when evaluating our executive compensation program.
The Say on Pay Proposal is as follows:
“Resolved, that the
compensation of the Company’s named executive officers, as described in the Company’s proxy statement for the 2024 Annual
Meeting of Stockholders, including the various compensation tables and the related narrative disclosures, is hereby APPROVED.”
Required Vote and Recommendation
In accordance with our Charter,
Bylaws and Delaware law, and as further discussed above under “The Annual Meeting — Quorum; Abstentions and Broker
Non-Votes,” approval and adoption of this Say on Pay Proposal requires the affirmative (“FOR”) vote of a majority
of votes cast by shares of our Common Stock present or represented by proxy and entitled to vote at the Annual Meeting and voting affirmatively
or negatively on such matter. Unless otherwise instructed on the proxy or unless authority to vote is withheld, shares represented by
executed proxies will be voted “FOR” this proposal. Abstentions and broker non-votes, if any, with respect to this proposal
are not counted as votes cast and will not affect the outcome of such proposal.
OUR BOARD RECOMMENDS A VOTE “FOR”
PROPOSAL FIVE.
PROPOSAL NO. 6: RATIFICATION
OF THE APPOINTMENT OF FORVIS MAZARS, LLP
TO SERVE TO SERVE AS OUR REGISTERED PUBLIC
ACCOUNTING FIRM
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2024
General
The
Company was notified that Mazars USA LLP (“Mazars”), the Company’s independent registered public accounting
firm, entered into a transaction with FORVIS, LLP (“FORVIS”) whereby substantially all of the partners and employees
of Mazars joined FORVIS. As a result, on the effective date of June 1, 2024, FORVIS, LLP changed its name to Forvis Mazars, LLP (“Forvis
Mazars”) and Mazars resigned as the Company’s independent public accounting firm.
The Audit Committee of
the Board has appointed Forvis Mazars as our independent registered public accounting firm for the year ending December 31, 2024, effective
June 1, 2024, subject to ratification by the Company’s stockholders, and the Board hereby recommends that the stockholders ratify
such appointment. The Board may terminate the appointment of Forvis Mazars as our independent registered public accounting firm without
the approval of our stockholders whenever the Board deems such termination necessary or appropriate.
Representatives of Forvis
Mazars will be present at the Annual Meeting or available by telephone and will have an opportunity to make a statement if they so desire
and to respond to any appropriate questions from stockholders.
Audit Fees
The following table represents
fees for professional services billed by Mazars for the fiscal years ended December 31, 2023 and 2022 in relation to services rendered
in connection with the audit of our consolidated financial statements and for tax services rendered with respect to tax-related compliance,
advice and planning.
| |
|
For the years ended
December 31, |
|
| |
|
2023 |
|
|
2022 |
|
| Audit fees(1) |
|
$ |
185,000 |
|
|
$ |
152,700 |
|
| Audit-related fees(2) |
|
|
204,600 |
|
|
|
154,236 |
|
| Tax fees(3) |
|
|
— |
|
|
|
— |
|
| All other fees(4) |
|
|
11,451 |
|
|
|
16,104 |
|
| Total |
|
$ |
401,051 |
|
|
$ |
323,040 |
|
| (1) |
Professional services rendered by Mazars USA LLP for the audit of our annual financial statements and review of financial statements included in our Form 10-Q’s. |
| (2) |
The aggregate fees billed for assurance and related services by Mazars USA LLP that are reasonably related to the performance of the audit or review of our financial statements and are not reported under Note 1 above, principally related to registration statement filings. |
| (3) |
The aggregate fees billed for professional services rendered by Mazars USA LLP (when also acting as auditor) for tax compliance, tax advice, and tax planning. |
| (4) |
The aggregate fees billed for products and services provided by Mazars USA LLP other than the services reported in Notes 1 through 3 above. |
Audit Committee Pre-Approval Policies and Procedures
The Audit Committee has the
sole authority for the appointment, compensation and oversight of the work of our independent auditors. The Audit Committee has established
a policy regarding pre-approval of all auditing services and the terms thereof and non-audit services (other than non-audit services prohibited
under Section 10A(g) of the Exchange Act or the applicable rules of the SEC or the Public Company Accounting Oversight Board) to be provided
to us by the independent auditor. However, the pre-approval requirement may be waived with respect to the provision of non-audit services
for us if the “de minimis” provisions of Section 10A(i)(1)(B) of the Exchange Act are satisfied.
The Audit Committee has considered
whether the provision of audit-related fees, tax fees, and all other fees as described above is compatible with maintaining Mazars USA
LLP’s independence and has determined that such services for fiscal year 2023 were compatible. All such services were approved by
the Audit Committee pursuant to Rule 2-01 of Regulation S-X under the Exchange Act to the extent that rule was applicable.
The Audit Committee is responsible
for reviewing and discussing the audited financial statements with management, discussing with the independent registered public accountants
the matters required in Auditing Standards No. 16, receiving written disclosures from the independent registered public accountants required
by the applicable requirements of the Public Company Accounting Oversight Board regarding the independent registered public accountants’
communications with the Audit Committee concerning independence and discussing with the independent registered public accountants their
independence, and recommending to our board of directors that the audited financial statements be included in our annual report on Form
10-K.
Changes in and Disagreements with Accountants on Accounting and
Financial Disclosure
Dismissal of Mazars
On
April 27, 2022, the Audit Committee of the Board approved the dismissal of Mazars, as the Company’s independent registered public
accounting firm, effective immediately, and the engagement of Marcum LLP (“Marcum”) as the Company’s new independent
registered public accounting firm as of and for the year ending December 31, 2022. As described below, the change in independent registered
public accounting firm is not the result of any disagreement with Mazars.
Mazars’
audit report on the financial statements for the years ended December 31, 2020 and 2021 did not provide an adverse opinion or disclaimer
of opinion to the Company’s financial statements, nor modify or qualify its opinion as to uncertainty, audit scope or accounting
principles, except that Mazars’ reports dated March 31, 2021 and March 31, 2022 contained an explanatory paragraph stating there
was substantial doubt about the Company’s ability to continue as a going concern.
During
the fiscal years ended December 31, 2020 and 2021 and through April 27, 2022, the date of dismissal, there were: (i) no disagreements
within the meaning of Item 304(a)(1)(iv) of Regulation S-K and the related instructions between the Company and Mazars
on any matters of accounting principles or practices, financial statement disclosure, or auditing scope or procedure which, if not resolved
to Mazars’s satisfaction, would have caused Mazars to make reference thereto in its report; and (ii) no “reportable events”
within the meaning of Item 304(a)(1)(v) of Regulation S-K.
Appointment of Marcum
On
April 27, 2022, the Audit Committee of the Board approved the engagement of Marcum as the Company’s new independent registered public
accounting firm as of and for the year ending December 31, 2022.
During
the fiscal years ended December 31, 2020 and 2021 and through April 27, 2022, neither the Company nor anyone on its behalf has consulted
with Marcum regarding: (i) the application of accounting principles to a specific transaction, either completed or proposed, or the type
of audit opinion that might be rendered on the Company’s financial statements, and neither a written report nor oral advice was
provided to the Company that Marcum concluded was an important factor considered by the Company in reaching a decision as to any accounting,
auditing, or financial reporting issue; (ii) any matter that was the subject of a disagreement within the meaning of Item 304(a)(1)(iv) of Regulation
S-K and the related instructions; or (iii) any reportable event within the meaning of Item 304(a)(1)(v) of Regulation
S-K.
Dismissal of Marcum
On July 5, 2022, the Audit
Committee of the Board approved the dismissal of Marcum as the Company’s independent registered public accounting firm, effective
immediately, and the re-engagement of Mazars as the Company’s independent registered public accounting firm as of and for the year
ending December 31, 2022. As described below, the change in independent registered public accounting firm is not the result of any disagreement
with Marcum.
Marcum was engaged as the
Company’s independent registered public accounting firm from April 27, 2022 to July 5, 2022 (the “Marcum Engagement Period”).
During the Marcum Engagement Period, Marcum had not audited the Company’s financial statements or issued any reports on the Company’s
financial statements.
During the Marcum Engagement
Period, there were: (i) no disagreements within the meaning of Item 304(a)(1)(iv) of Regulation S-K and the related
instructions between the Company and Marcum on any matters of accounting principles or practices, financial statement disclosure, or auditing
scope or procedure which, if not resolved to Marcum’s satisfaction, would have caused Marcum to make reference thereto in its report;
and (ii) no “reportable events” within the meaning of Item 304(a)(1)(v) of Regulation S-K.
Re-Appointment of Mazars
On July 5, 2022, the Audit
Committee of the Board approved the re-engagement of Mazars as the Company’s independent registered public accounting firm as of
and for the year ending December 31, 2022.
Mazars previously served as
the Company’s independent registered public accounting firm from 2014 to April 27, 2022 (the “Prior Mazars Engagement Period”).
During the Prior Mazars Engagement Period, Mazars audited the Company’s financial statements as of and for the years ended December
31, 2020 and December 31, 2021.
Mazars’ audit report
on the financial statements for the years ended December 31, 2020 and 2021 did not provide an adverse opinion or disclaimer of opinion
to the Company’s financial statements, nor modify or qualify its opinion as to uncertainty, audit scope or accounting principles,
except that Mazars’ reports dated March 31, 2021 and March 31, 2022 contained an explanatory paragraph stating there was substantial
doubt about the Company’s ability to continue as a going concern.
During the fiscal years ended
December 31, 2020 and 2021 and through April 27, 2022, the date of Mazars’ prior dismissal, there were: (i) no disagreements within
the meaning of Item 304(a)(1)(iv) of Regulation S-K and the related instructions between the Company and Mazars on
any matters of accounting principles or practices, financial statement disclosure, or auditing scope or procedure which, if not resolved
to Mazars’s satisfaction, would have caused Mazars to make reference thereto in its report; and (ii) no “reportable events”
within the meaning of Item 304(a)(1)(v) of Regulation S-K.
During the fiscal years ended
December 31, 2020 and 2021 and through July 5, 2022, neither the Company nor anyone on its behalf has consulted with Mazars regarding:
(i) the application of accounting principles to a specific transaction, either completed or proposed, or the type of audit opinion that
might be rendered on the Company’s financial statements, and neither a written report nor oral advice was provided to the Company
that Mazars concluded was an important factor considered by the Company in reaching a decision as to any accounting, auditing, or financial
reporting issue; (ii) any matter that was the subject of a disagreement within the meaning of Item 304(a)(1)(iv) of Regulation
S-K and the related instructions; or (iii) any reportable event within the meaning of Item 304(a)(1)(v) of Regulation
S-K.
Appointment of Forvis Mazars, LLP
The
Company was notified that Mazars, the Company’s independent registered public accounting firm, entered into a transaction with
FORVIS whereby substantially all of the partners and employees of Mazars joined FORVIS. As a result, on the effective date of June 1,
2024, FORVIS changed its name to Forvis Mazars, LLP and Mazars resigned as the Company’s independent public accounting firm. The
Audit Committee of the Company’s Board of Directors appointed Forvis Mazars to serve as the Company’s independent registered
public accounting firm effective June 1, 2024, subject to ratification by the Company’s stockholders at the Company’s annual
meeting of stockholders.
The
audit reports of Mazars on the financial statements of the Company for the fiscal years ended December 31, 2023 and 2022 did not contain
an adverse opinion or a disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope, or accounting principles.
During
the Company’s fiscal years ended December 31, 2023 and 2022, and the subsequent interim period preceding the engagement of Forvis
Mazars, there were no disagreements between the Company and Mazars on any matter of accounting principles or practices, financial statement
disclosure, or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of Mazars, would have cause Mazars
to make reference to any subject matter of the disagreements in connection with its audit reports on the Company’s financial statements.
During the Company’s previous fiscal years ended December 31, 2023 and 2022, and the interim period through the engagement of Forvis
Mazars, Mazars did not advise the Company of any matters specified in Item 304(a)(1)(v) of Regulation S-K.
During the Company’s
two most recently completed fiscal years and through the date of engagement of Forvis Mazars, neither the Company nor anyone on behalf
of the Company consulted with Mazars regarding (a) the application of accounting principles to a specific transaction, either completed
or proposed, or the type of audit opinion that might be rendered on the Company’s financial statements as to which the Company
received a written report or oral advice that was an important factor in reaching a decision on any accounting, auditing or financial
reporting issue; or (b) any matter that was the subject of a disagreement or a reportable event as defined in Items 304(a)(1)(iv) and
(v), respectively, of Regulation S-K. During the Company’s fiscal years ended December 31, 2023 and 2022, and the subsequent period
through the date of this Current Report on Form 8-K, the Company did not consult with Forvis Mazars regarding any of the matters set
forth in Items 304(a)(2)(i) and (ii) of Regulation S-K.
Required Vote and Recommendation
This proposal requires the
affirmative (“FOR”) vote of a majority of votes cast by shares present or represented by proxy and entitled to vote at the
Annual Meeting and voting affirmatively or negatively on such matter. Unless otherwise instructed on the proxy or unless authority to
vote is withheld, shares represented by executed proxies will be voted “FOR” this proposal. Any abstentions are not counted
as votes cast and will not affect the outcome of this proposal, although they will be counted for purposes of determining whether there
is a quorum present. There are no broker non-votes for this Proposal.
OUR BOARD RECOMMENDS A VOTE “FOR”
PROPOSAL SIX.
PROPOSAL NO. 7: APPROVAL OF THE ADJOURNMENT
OF THE ANNUAL MEETING
TO THE EXTENT THERE ARE INSUFFICIENT PROXIES
AT THE MEETING
TO APPROVE ANY ONE OR MORE OF THE FOREGOING
PROPOSALS.
Adjournment of the Annual Meeting
In the event that the number
of shares of Common Stock and Preferred Stock present or represented by proxy at the Annual Meeting and voting “FOR” the adoption
of any one or more of the foregoing proposals are insufficient to approve any such proposal, we may move to adjourn the Annual Meeting
in order to enable us to solicit additional proxies in favor of the adoption of any such proposal. In that event, we will ask stockholders
to vote only upon the adjournment proposal and not on any other proposal discussed in this proxy statement. If the adjournment is for
more than thirty (30) days, a notice of the adjourned meeting shall be given to each stockholder of record entitled to vote at the meeting.
For the avoidance of doubt,
any proxy authorizing the adjournment of the Annual Meeting shall also authorize successive adjournments thereof, at any meeting so adjourned,
to the extent necessary for us to solicit additional proxies in favor of the adoption of any such proposal.
Required Vote and Recommendation
In accordance with our Charter,
Bylaws and Delaware law, and as further discussed above under “The Annual Meeting — Quorum; Abstentions and Broker
Non-Votes,” approval and adoption of this Adjournment Proposal requires the affirmative (“FOR”) vote of a majority
of votes cast by shares of our Common Stock present or represented by proxy and entitled to vote at the Annual Meeting and voting affirmatively
or negatively on such matter. Unless otherwise instructed on the proxy or unless authority to vote is withheld, shares represented by
executed proxies will be voted “FOR” this proposal. Abstentions and broker non-votes, if any, with respect to this proposal
are not counted as votes cast and will not affect the outcome of this proposal.
OUR BOARD RECOMMENDS A VOTE “FOR”
PROPOSAL SEVEN.
INTERESTS OF DIRECTORS AND EXECUTIVE OFFICERS
IN THE PROPOSALS
As of the date of this
proxy statement, Entero directors and executive officers do not have interests in the proposals that are different from, or in addition
to, the interests of other Entero stockholders generally, except that:
| |
● |
Dr. Jack Syage, our President, Chief Scientific Officer, and Director, is a holder of 15,400 shares of Common Stock and 4,920.037 shares of Series G Preferred Stock. |
| |
● |
Dr. Chaitan Khosla,
a Director, is a holder of 440 shares of Common Stock and 140.70 shares of Series G Preferred Stock. |
ADDITIONAL INFORMATION
Deadline for Receipt of Stockholder Proposals for the 2024 Annual
Meeting
Pursuant to Rule 14a-8
under the Exchange Act, stockholder proposals to be included in our next proxy statement must be received by our Chief Financial Officer
by writing to Entero Therapeutics, Inc., Attention: Chief Financial Officer - 777 Yamato Road, Suite 502, Boca Raton, Florida 33431,
no later than 90 days, or [●], 2025, nor more than 120 days, or v, 2025, prior to the first anniversary of the preceding year’s
annual meeting. Submitted proposals must comply with applicable Delaware law, the rules and regulations promulgated by the SEC and the
procedures set forth in our Bylaws.
Our Bylaws state that
a stockholder must provide timely written notice of any nominations of persons for election to our Board or any other proposal to be
brought before the meeting together with supporting documentation as well as be present at such meeting, either in person or by a representative.
For our 2025 Annual Meeting of Stockholders, a stockholder’s notice shall be timely received by us at our principal executive office
no later than [●], 2024 and no earlier than [●], 2024; provided, however, that in the event the Annual Meeting
is scheduled to be held on a date more than thirty (30) days before the anniversary date of the immediately preceding Annual Meeting
of Stockholders (the “Anniversary Date”) or more than seventy (70) days after the Anniversary Date, a stockholder’s
notice shall be timely if received by the Company at our principal executive office not earlier than the close of business on the one
hundred twentieth (120th) day prior to such annual meeting and not later than the close of business on the later of the ninetieth
(90th) day prior to the scheduled date of such annual meeting; and (ii) the tenth (10th) day following the day on which such public announcement
of the date of such Annual Meeting is first made by the Company. Proxies solicited by our Board will confer discretionary voting authority
with respect to these nominations or proposals, subject to the SEC’s rules and regulations governing the exercise of this authority.
Any such nomination or proposal shall be mailed to: Entero Therapeutics, Inc., Attention: Chief Financial Officer - 777 Yamato Road,
Suite 502, Boca Raton, Florida 33431.
In addition, to comply with
the universal proxy rules, stockholders who intend to solicit proxies in support of director nominees other than our nominees must provide
notice that sets forth the information required by Rule 14a-19 under the Exchange Act.
We reserve the right to reject,
rule out of order, or take other appropriate action with respect to any proposal that does not comply with these and all other applicable
requirements.
Householding of Proxy Materials
The SEC has adopted rules
that permit companies and intermediaries (e.g., brokers) to satisfy the delivery requirements for proxy statements and annual reports
with respect to two or more stockholders sharing the same address by delivering a single proxy statement and annual report addressed to
those stockholders. This process, which is commonly referred to as “householding,” potentially means extra convenience for
stockholders and cost savings for companies.
A number of brokers with
account holders who are stockholders of the Company will be “householding” our proxy materials. A single set of our proxy
materials will be delivered to multiple stockholders sharing an address unless contrary instructions have been received from the affected
stockholders. Once you have received notice from your broker that they will be “householding” communications to your address,
“householding” will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer
wish to participate in “householding” and would prefer to receive a separate set of our proxy materials at no charge, please
notify your broker or direct a written request to Entero Therapeutics, Inc., Attention: Chief Financial Officer - 777 Yamato Road, Suite
502, Boca Raton, Florida 33431, or contact us at (561) 589-7020. We undertake to deliver promptly, upon any such verbal or written request,
a separate copy of our proxy materials to a stockholder at a shared address to which a single copy of these documents was delivered.
Stockholders who currently receive multiple copies of our proxy materials at their address and would like to request “householding”
of their communications should contact their broker, bank or other nominee, or contact us at the above address or phone number.
Other Matters
At the date of this proxy
statement, we know of no other matters, other than those described above, that will be presented for consideration at the Annual Meeting.
If any other business should come before the Annual Meeting, it is intended that the proxy holders will vote all proxies using their best
judgment in the interest of the Company and the stockholders.
Solicitation of Proxies
The solicitation of proxies
pursuant to this proxy statement is being made by us. Proxies may be solicited, among other methods, by mail, facsimile, telephone, telegraph,
Internet and in person.
The expenses of preparing,
printing and distributing this proxy statement and the accompanying form of proxy and the cost of soliciting proxies will be borne by
us.
Copies of soliciting materials
will be furnished to banks, brokerage houses and other custodians, nominees and fiduciaries for forwarding to the beneficial owners of
shares of Common Stock and Preferred Stock for whom they hold shares, and we will reimburse them for their reasonable out-of-pocket expenses
in connection therewith.
We have engaged Alliance Advisors
LLC to assist in the solicitation of proxies and provide related advice and informational support, for a services fee, plus customary
disbursements, which are not expected to exceed $50,000 in total. Alliance Advisors LLC will solicit proxies on our behalf from individuals,
brokers, bank nominees and other institutional holders in the same manner described above. We have also agreed to indemnify Alliance Advisors
LLC against certain claims.
WHERE YOU CAN FIND MORE INFORMATION
We are “incorporating
by reference” in this proxy statement certain documents we file with the SEC, which means that we can disclose important information
to you by referring you to those documents. The information in the documents incorporated by reference is considered to be part of this
proxy statement. We have filed the following documents with the SEC and they are incorporated herein by reference as of their respective
dates of filing.
| |
(i) |
our Annual Report on Form 10-K for the fiscal year ended December 31, 2023 as filed with the SEC on March 29, 2024; |
| |
|
|
| |
(ii) |
our Current Reports
on Form 8-K, filed on March
5, 2024, March
14, 2024 (as amended on May 8, 2024, and May 30, 2024), March
18, 2024, March
22, 2024, May 13, 2024, May 16, 2024, and June 5, 2024 (other than any portions thereof deemed furnished and not filed); |
| |
|
|
| |
(iii) |
our Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2024 as filed with the SEC on May 14, 2024. |
| |
|
|
| |
(iv) |
the description of our
securities registered under Section 12 of the Exchange Act as filed as Exhibit
4.31 on Annual Report on Form 10-K for the year ended December 31, 2023 as filed with the SEC on March 29, 2024. |
Any statement contained in
a document incorporated or deemed to be incorporated by reference in this proxy statement shall be deemed modified, superseded or replaced
for purposes of this proxy statement to the extent that a statement contained in this proxy statement, or in any subsequently filed document
that also is deemed to be incorporated by reference in this proxy statement, modifies, supersedes or replaces such statement. Any statement
so modified, superseded or replaced shall not be deemed, except as so modified, superseded or replaced, to constitute a part of this proxy
statement. None of the information that we disclose under Items 2.02 or 7.01 of any Current Report on Form 8-K or any corresponding information,
either furnished under Item 9.01 or included as an exhibit therein, that we may from time to time furnish to the SEC will be incorporated
by reference into, or otherwise included in, this proxy statement, except as otherwise expressly set forth in the relevant document. Subject
to the foregoing, all information appearing in this proxy statement is qualified in its entirety by the information appearing in the documents
incorporated by reference.
You may request, orally
or in writing, a copy of these documents, which will be provided to you at no cost (other than exhibits, unless such exhibits are specifically
incorporated by reference), by contacting Entero Therapeutics, Inc., Attn: Chief Financial Officer at 777 Yamato Road, Suite 502, Boca
Raton, FL 33431. Our telephone number is (561) 589-7020. Information about us is also available at our website at http://www.enterothera.com.
However, the information on our website is not a part of this proxy statement and is not incorporated by reference.
REGARDLESS OF WHETHER YOU PLAN TO ATTEND THE
ANNUAL MEETING, PLEASE READ THE PROXY STATEMENT AND THEN SUBMIT A PROXY TO VOTE BY INTERNET, TELEPHONE OR MAIL AS PROMPTLY AS POSSIBLE
TO ENSURE THAT YOUR SHARES ARE REPRESENTED AT THE ANNUAL MEETING.
| |
BY ORDER OF THE BOARD OF DIRECTORS, |
| |
|
| |
|
| Boca Raton, Florida |
JAMES SAPIRSTEIN |
| [●], 2024 |
Chief Executive Officer and
Chairman of the Board of Directors |
If you have any questions or require any assistance
in voting your shares, please call:
Alliance Advisors LLC
200 Broadacres Drive, 3rd Floor, Bloomfield, NJ
07003
866-407-1875
ANNEX A
AMENDMENT TO THE
azurrx
biopharma, inc.
2020 OMNIBUS EQUITY INCENTIVE PLAN
azurrx
biopharma, inc.
2020 OMNIBUS EQUITY INCENTIVE PLAN
Dated: [ ], 2024
WHEREAS, the Board
of Directors (the “Board”) of Entero Therapeutics, Inc. (formerly, AzurRx BioPharma, Inc.), a Delaware corporation
(the “Company”) heretofore established the AzurRx BioPharma, Inc. 2020 Omnibus Equity Incentive Plan, as amended and
restated as of August 11, 2020 (the “Plan”); and
WHEREAS,
after giving effect to four reverse stock splits and increases in accordance with the “evergreen” provisions of the Plan,
the maximum number of shares of common stock of the Company (“Common Stock”) currently available for grants of “Awards”
(as defined under the Plan) is 58,374 (not counting shares of Common Stock that have previously been issued pursuant to the Plan or that
are the subject of outstanding Awards under the Plan), all of which are available as grants as Incentive Stock Options, and the number
of shares that may be granted to any one non-employee director of the Board in a calendar year is 5 (not counting shares of Common Stock
that have previously been issued pursuant to the Plan or that are the subject of outstanding Awards under the Plan); and
WHEREAS,
in order to ensure that a sufficient number of shares of Common Stock are available under the Plan in order to properly incentivize those
eligible to participate in the Plan, including future eligible participants, the Board believes it to be in the best interests of the
Company and its shareholders to increase the maximum number of shares of Common Stock available for grants of Awards thereunder by 1,441,626
additional shares of Common Stock (the “Additional Reserved Shares”) (from 58,374 to 1,500,000 shares), not counting
shares of Common Stock that have previously been issued pursuant to the Plan or that are the subject of outstanding Awards under the Plan;
and
WHEREAS,
the Board further believes it to be in the best interests of the Company and its shareholders to increase the number of shares of Common
Stock that may be granted under the Plan as “incentive stock options” (“ISOs”), within the meaning of Section
422 of the Internal Revenue Code of 1986, as amended, to 750,000 shares in order to allow the Additional Reserved Shares and such other
additional shares of Common Stock that may become available under the Plan in accordance with the Plan’s evergreen provisions to
be granted as ISOs; and
WHEREAS,
the Board further believes it to be in the best interests of the Company and its shareholders to increase the number of shares of Common
Stock that may be granted to any one non-employee director of the Board to 250,000 shares; and
WHEREAS,
Article XIX of the Plan authorizes the Board to amend the Plan, subject to stockholder approval to the extent that such approval is required
by applicable law;
NOW,
THEREFORE, subject to approval of the Company’s stockholders, effective the date hereof, the Plan is hereby amended as
follows:
Section 5.1 of the Plan is
hereby amended in its entirety, to read as follows:
“Authorized
Shares and Award Limits. The Committee may from time to time grant Awards to one or more Employees, Directors and/or Consultants determined
by it to be eligible for participation in the Plan in accordance with the provisions of Article VI. Subject to Article XVIII, the number
of Shares that may be issued under all Awards granted under the Plan shall be 1,500,000 (the “Share Reserve”); provided
that, subject to Article XVIII, the Share Reserve (as adjusted as set forth herein) shall automatically be increased, but not decreased,
on January 1 of each calendar year (beginning January 1, 2021) so as to be equal to ten percent (10%) of the issued and outstanding shares
of the Company’s common stock on December 31 of the preceding calendar year on an as converted basis (the “As Converted
Shares”). Notwithstanding the foregoing, the Board may in its discretion, determine that no increase in the Share Reserve shall
be made for any year or determine that a lesser number of Shares shall be added to the Share Reserve than would otherwise have been added
pursuant to the preceding sentence. For calculation purposes, the As Converted Shares shall include all shares of the Company’s
outstanding common stock and all shares of the Company’s common stock issuable upon the conversion of outstanding preferred stock,
warrants and other convertible securities, but shall not include any shares of common stock issuable upon the exercise of options and
other convertible securities issued pursuant to the Plan or the Company’s Amended and Restated 2014 Omnibus Equity Incentive Plan.
Subject to Article XVIII, the maximum number of Shares available for issuance in respect of Incentive Stock Options is 750,000, but in
no event may Incentive Stock Options be granted in excess of the Share Reserve as the same may be increased in accordance with the foregoing
provisions of this Section. To the extent that an Award lapses, expires, is canceled, is terminated unexercised or ceases to be exercisable
for any reason, or the rights of its Holder terminate, any Shares subject to such Award shall again be available for the grant of a new
Award. Shares that otherwise would have been issued upon the exercise of an Option or Stock Appreciation Right or in payment with respect
to any other form of Award, that are surrendered in payment or partial payment of the exercise price thereof and/or taxes withheld with
respect to the exercise thereof or the making of such payment, will no longer be counted against the foregoing maximum share limitations
and may again be made subject to Awards under the Plan pursuant to such limitations.”
Section 5.2 of the Plan is
hereby amended in its entirety, to read as follows:
“Individual
Holder Limitations. Subject to adjustment as provided in Article XVIII, the number of Shares with respect to which Awards may be granted
during any calendar year to any one Director who is a non-employee director of the Board shall not exceed 250,000.”
[Signature Page Follows]
IN WITNESS WHEREOF, the undersigned
has executed this Amendment as evidence of its adoption by the Board of Directors of the Company on the date set forth above.
Entero
Therapeutics, Inc.
| |
|
| By: |
|
|
| |
|
|
| Name: |
James Sapirstein |
|
| |
|
|
| Title: |
Chief Executive Officer and Chairman of the Board |
|
| |
|
|
| Date: |
[ ], 2024 |
|
ANNEX B
AGREEMENT AND PLAN OF MERGER, BY AND AMONG FIRST
WAVE BIOPHARMA, INC., IMMUNO MERGER SUB I, INC., IMMUNO MERGER SUB II, LLC, AND IMMUNOGENX, INC.
Execution Version
AGREEMENT AND PLAN OF MERGER
among:
FIRST WAVE BIOPHARMA, INC.;
IMMUNO MERGER SUB I, INC.;
IMMUNO MERGER SUB II, LLC;
and
IMMUNOGENX, INC.
Dated
as of March 13, 2024
TABLE OF CONTENTS
Page
| Section 1. |
Definitions and Interpretative Provisions |
3 |
| |
|
|
| 1.1 |
Definitions |
3 |
| 1.2 |
Other Definitional and Interpretative Provisions |
14 |
| |
|
|
| Section 2. |
Description of Transaction |
15 |
| |
|
|
| 2.1 |
The Merger |
15 |
| 2.2 |
Effects of the Merger |
15 |
| 2.3 |
Closing; First Effective Time; Second Effective Time |
15 |
| 2.4 |
Certificate of Designation; Organizational Documents; Directors and Officers |
16 |
| 2.5 |
Merger Consideration; Effect of Merger on Company Capital Stock |
17 |
| 2.6 |
Conversion of Stock |
17 |
| 2.7 |
Closing of the Company’s Transfer Books |
18 |
| 2.8 |
Exchange of Shares |
18 |
| 2.9 |
Stock Options |
19 |
| 2.10 |
Company Warrants |
20 |
| 2.11 |
Other Company Convertible Securities |
20 |
| 2.12 |
Appraisal Rights |
20 |
| 2.13 |
Further Action |
21 |
| 2.14 |
Intended Tax Treatment |
21 |
| 2.15 |
Withholding |
21 |
| |
|
|
| Section 3. |
Representations and Warranties of the Company |
22 |
| |
|
|
| 3.1 |
Due Organization; Subsidiaries |
22 |
| 3.2 |
Organizational Documents |
22 |
| 3.3 |
Authority; Binding Nature of Agreement |
22 |
| 3.4 |
Vote Required |
23 |
| 3.5 |
Non-Contravention; Consents |
23 |
| 3.6 |
Capitalization |
24 |
| 3.7 |
Financial Statements |
25 |
| 3.8 |
Absence of Changes |
26 |
| 3.9 |
Absence of Undisclosed Liabilities |
28 |
| 3.10 |
Title to Assets |
28 |
| 3.11 |
Real Property; Leasehold |
28 |
| 3.12 |
Intellectual Property |
29 |
| 3.13 |
Agreements, Contracts and Commitments |
32 |
| 3.14 |
Compliance; Permits; Restrictions |
34 |
| 3.15 |
Legal Proceedings; Orders |
37 |
| 3.16 |
Tax Matters |
37 |
| 3.17 |
Employee and Labor Matters; Benefit Plans |
39 |
| 3.18 |
Environmental Matters |
42 |
| 3.19 |
Insurance |
42 |
| 3.20 |
No Financial Advisors |
42 |
| 3.21 |
Transactions with Affiliates |
43 |
| 3.22 |
Privacy and Data Security |
43 |
| 3.23 |
Accredited Investors |
43 |
| 3.24 |
No Other Representations or Warranties |
43 |
| |
|
|
| Section 4. |
Representations and Warranties of Parent and Merger Subs |
44 |
| |
|
|
| 4.1 |
Due Organization; Subsidiaries |
44 |
| 4.2 |
Organizational Documents |
45 |
| 4.3 |
Authority; Binding Nature of Agreement |
45 |
| 4.4 |
Vote Required |
45 |
| 4.5 |
Non-Contravention; Consents |
46 |
| 4.6 |
Capitalization |
47 |
| 4.7 |
SEC Filings; Financial Statements |
48 |
| 4.8 |
Absence of Changes |
50 |
| 4.9 |
Absence of Undisclosed Liabilities |
52 |
| 4.10 |
Title to Assets |
52 |
| 4.11 |
Real Property; Leasehold |
52 |
| 4.12 |
Intellectual Property |
52 |
| 4.13 |
Agreements, Contracts and Commitments |
56 |
| 4.14 |
Compliance; Permits; Restrictions |
56 |
| 4.15 |
Legal Proceedings; Orders |
59 |
| 4.16 |
Tax Matters |
60 |
| 4.17 |
Employee and Labor Matters; Benefit Plans |
62 |
| 4.18 |
Environmental Matters |
64 |
| 4.19 |
Insurance |
65 |
| 4.20 |
Transactions with Affiliates |
65 |
| 4.21 |
No Financial Advisors |
65 |
| 4.22 |
Valid Issuance; No Bad Actor |
65 |
| 4.23 |
Privacy and Data Security |
66 |
| 4.24 |
No Other Representations or Warranties |
66 |
| |
|
|
| Section 5. |
Agreements of the Parties |
66 |
| |
|
|
| 5.1 |
Stockholder Notice |
66 |
| 5.2 |
Proxy Statement |
67 |
| 5.3 |
Employee Benefits; Employment Agreements |
68 |
| 5.4 |
Parent Stockholder Meeting |
68 |
| 5.5 |
Indemnification of Officers and Directors |
69 |
| 5.6 |
Tax Matters |
70 |
| 5.7 |
Legends |
71 |
| 5.8 |
Officers and Directors |
71 |
| 5.9 |
Termination of Certain Agreements and Rights |
71 |
| 5.10 |
Section 16 Matters |
72 |
| 5.11 |
Listing |
72 |
| 5.12 |
Allocation Certificate |
72 |
| 5.13 |
Obligations of Merger Subs |
72 |
| 5.14 |
Reservation of Parent Common Stock; Issuance of Shares of Parent Common Stock |
72 |
| 5.15 |
Takeover Statutes |
72 |
| 5.16 |
Private Placement |
73 |
| |
|
|
| Section 6. |
Conditions Precedent to Obligations of Each Party |
73 |
| |
|
|
| 6.1 |
No Restraints |
73 |
| 6.2 |
Company Stockholder Approval |
73 |
| |
|
|
| Section 7. |
Closing Deliveries |
73 |
| |
|
|
| 7.1 |
Closing Deliveries of the Company |
73 |
| 7.2 |
Closing Deliveries of Parent |
74 |
| |
|
|
| Section 8. |
Miscellaneous Provisions |
74 |
| |
|
|
| 8.1 |
Non-Survival of Representations and Warranties |
74 |
| 8.2 |
Amendment |
75 |
| 8.3 |
Waiver |
75 |
| 8.4 |
Entire Agreement; Counterparts; Exchanges by Electronic Transmission or Facsimile |
75 |
| 8.5 |
Applicable Law; Jurisdiction |
75 |
| 8.6 |
Assignability |
76 |
| 8.7 |
Notices |
76 |
| 8.8 |
Cooperation |
77 |
| 8.9 |
Severability |
77 |
| 8.10 |
Other Remedies; Specific Performance |
77 |
| 8.11 |
No Third-Party Beneficiaries |
78 |
| 8.12 |
Expenses |
78 |
| Exhibits: |
|
| |
|
| Exhibit A |
Form of Voting Agreement |
| |
|
| Exhibit B |
Form of Parent Stockholder Support Agreement |
| |
|
| Exhibit C |
Form of Lock-Up Agreement |
| |
|
| Exhibit D |
Form of Restrictive Covenants Agreement |
| |
|
| Exhibit E |
Form of Certificate of Designation |
| |
|
| Exhibit F |
Form of Letter of Transmittal |
| |
|
| Exhibit G |
Form of Accredited Investor Questionnaire |
AGREEMENT AND PLAN OF MERGER
THIS AGREEMENT AND PLAN OF
MERGER (this “Agreement”) is made and entered into as of March 13, 2024, by and among First Wave BioPharma, Inc.,
a Delaware corporation (“Parent”), IMMUNO Merger Sub I, Inc., a Delaware corporation and wholly owned subsidiary
of Parent (“First Merger Sub”), IMMUNO Merger Sub II, LLC, a Delaware limited liability company and wholly owned
subsidiary of Parent (“Second Merger Sub” and together with First Merger Sub, “Merger Subs”), and
ImmunogenX, Inc., a Delaware corporation (the “Company”). Certain capitalized terms used in this Agreement are
defined in Section 1.
RECITALS
A. Parent
and the Company intend to effect a merger of First Merger Sub with and into the Company (the “First Merger”) in accordance
with this Agreement and the DGCL. Upon consummation of the First Merger, First Merger Sub will cease to exist and the Company will become
a wholly owned subsidiary of Parent.
B. Immediately
following the First Merger and as part of the same overall transaction as the First Merger, the Company will merge with and into Second
Merger Sub (the “Second Merger” and, together with the First Merger, the “Merger”), with Second
Merger Sub being the surviving entity of the Second Merger.
C. The
Parties intend, for U.S. federal (and applicable state and local) income Tax purposes, that (a) this Agreement constitutes, and is
hereby adopted by the parties hereto as a, “plan of reorganization” for purposes of Section 368 of the Code and within
the meaning of Treasury Regulations Sections 1.368-2(g) and 1.368-3(a), and (b) the First Merger and Second Merger, taken together,
will constitute a single integrated transaction that qualifies as a “reorganization” within the meaning of Section 368(a)(1)(A) of
the Code and the Treasury Regulations as described in Rev. Rul. 2001-46, 2001-2 C.B. 321, to which Parent and the Company are parties
under Section 368(b) of the Code.
D. The
Parent Board has (i) determined that the Contemplated Transactions are fair to, advisable, and in the best interests of Parent and
its stockholders, (ii) approved and declared advisable this Agreement and the Contemplated Transactions, including the issuance of
shares of Parent Capital Stock to the stockholders of the Company pursuant to the terms of this Agreement and (iii) determined to
recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of Parent vote to approve (a) the
conversion of the Parent Convertible Preferred Stock issued pursuant to this Agreement into shares of Parent Common Stock in accordance
with Nasdaq Listing Rule 5635(a) (the “Conversion Proposal”), (b) if deemed necessary or appropriate
by Parent or as otherwise required by applicable Law or Contract, to authorize the amendment of Parent’s certificate of incorporation
to authorize sufficient Parent Common Stock in Parent’s certificate of incorporation for the conversion of Parent Convertible Preferred
Stock issued pursuant to this Agreement (the “Charter Amendment Proposal” and together with the Conversion Proposal,
the “Parent Stockholder Matters”) and (c) the Certificate of Designation.
E. A
majority of the holders of Parent Series B Preferred Stock outstanding as of the date of this Agreement have authorized the execution,
delivery and performance of this Agreement and the consummation of the Contemplated Transactions (including the Conversion Proposal).
F. The
First Merger Sub Board has (i) determined that the Contemplated Transactions are fair to, advisable, and in the best interests of
First Merger Sub and its sole stockholder, (ii) approved and declared advisable this Agreement and the Contemplated Transactions
and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the sole stockholder
of First Merger Sub votes to adopt this Agreement and thereby approve the Contemplated Transactions.
G. The
Second Merger Sub Board has (i) determined that the Contemplated Transactions are fair to, advisable, and in the best interests of
Second Merger Sub and its sole member, (ii) approved and declared advisable this Agreement and the Contemplated Transactions and
(iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the member of Second
Merger Sub votes to adopt this Agreement and thereby approve the Contemplated Transactions.
H. In
connection with the transactions contemplated hereby, each Convertible Note of the Company shall convert in accordance with its terms,
or be deemed to convert in accordance with an applicable agreement, into shares of Company Common Stock, which shares shall be entitled
to the right to receive the consideration set forth herein.
I. The
Company Board has (i) determined that the Contemplated Transactions are fair to, advisable, and in the best interests of the Company
and its stockholders, (ii) approved and declared advisable this Agreement and the Contemplated Transactions and (iii) determined
to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of the Company vote to adopt
this Agreement and thereby approve the Contemplated Transactions (“Company Board Approval”).
J. Subsequent
to Company Board Approval, but prior to the execution and delivery of this Agreement, the requisite Company stockholders by written consent
and in accordance with the Company’s certificate of incorporation, the Company’s bylaws and the DGCL (i) approved and
adopted this Agreement and the Contemplated Transactions, (ii) acknowledged that the approval given thereby is irrevocable and that
such stockholder is aware of its rights to demand appraisal for its shares pursuant to Section 262 of the DGCL, a true and correct
copy of which was attached thereto, and that such stockholder has received and read a copy of Section 262 of the DGCL, and (iii) acknowledged
that by its approval of the Merger it is not entitled to appraisal rights with respect to its shares in connection with the Merger and
thereby waives any rights to receive payment of the fair value of its capital stock under the DGCL (such consent, the “Company
Stockholder Written Consent”), and the Company Stockholder Written Consent is to become effective by its terms immediately following
the execution of this Agreement by the parties hereto.
K. Concurrently
with the execution and delivery of this Agreement and as a condition and inducement to Parent’s willingness to enter into this Agreement,
the persons set forth on Section A of the Company Disclosure Schedule are executing voting agreements in substantially the
form attached hereto as Exhibit A (the “Voting Agreement” and collectively, the “Voting Agreements”).
L. Concurrently
with the execution and delivery of this Agreement and as a condition and inducement to the Company’s willingness to enter into this
Agreement, certain stockholders of Parent set forth on Section A of the Parent Disclosure Schedule (solely in their capacity
as stockholders) are executing support agreements in favor of the Company in substantially the form attached hereto as Exhibit B
(the “Parent Stockholder Support Agreement”), pursuant to which such Persons have, subject to the terms and conditions
set forth herein, agreed to vote all of their shares of Parent Capital Stock in favor of the Parent Stockholder Matters.
M. Concurrently
with the execution and delivery of this Agreement and as a condition and inducement to Parent’s and the Company’s willingness
to enter into this Agreement, all of the stockholders of the Company set forth on Section B of the Company Disclosure Schedule
(the “Company Signatories”) and all of the stockholders of the Parent set forth on Section B of the Parent
Disclosure Schedule (the “Parent Signatories”) are executing lock-up agreements in substantially the form attached
hereto as Exhibit C (the “Lock-Up Agreements”).
N. Concurrently
with the execution and delivery of this Agreement and as a condition and inducement to Parent’s willingness to enter into this Agreement,
the Persons set forth on Section C of the Company Disclosure Schedule are executing restrictive covenants agreements in substantially
the form attached hereto as Exhibit D (the “Restrictive Covenants Agreement” and collectively, the “Restrictive
Covenants Agreements”).
O. Immediately
following the execution and delivery of this Agreement, but prior to the filing of the Certificate of Merger, Parent will file the Certificate
of Designation with the office of the Secretary of State of the State of Delaware.
AGREEMENT
The Parties, intending to
be legally bound, agree as follows:
Section 1. Definitions
and Interpretative Provisions.
1.1 Definitions.
(a) For
purposes of the Agreement (including this Section 1):
“Affiliate”
shall have the meaning given to such term in Rule 145 under the Securities Act.
“Affordable Care
Act” means the Patient Protection and Affordable Care Act.
“Allocation Certificate”
shall have the meaning set forth in Section 5.11.
“Business Day”
means any day other than a day on which banks in the State of New York are authorized or obligated to be closed.
“Certificate of Designation”
means a certificate of designation for Parent Preferred Stock Payment Shares in the form attached hereto as Exhibit E.
“COBRA”
means the Consolidated Omnibus Budget Reconciliation Act of 1985, as set forth in Section 4980B of the Code and Part 6 of Title
I of ERISA.
“Code”
means the Internal Revenue Code of 1986, as amended.
“Company Audited
Financial Statements” means the audited balance sheet of the Company, and the related audited statement of income and comprehensive
income, consolidated statement of changes in stockholders’ equity and statement of cash flows of the Company for the years ended
December 31, 2021, December 31, 2022 and December 31, 2023, together with the notes thereto.
“Company Board”
means the board of directors of the Company.
“Company Capital
Stock” means the Company Common Stock and Company Preferred Stock.
“Company Capitalization
Representations” means the representations and warranties of the Company set forth in Sections 3.6(a) and 3.6(d).
“Company Common Stock”
means the common stock, $0.0001 par value per share, of the Company.
“Company Contract”
means any Contract: (a) to which the Company is a Party, (b) by which the Company is or may become bound or under which the
Company or any of its Subsidiaries has, or may become subject to, any obligation or (c) under which the Company has or may acquire
any right or interest.
“Company Employee
Plan” means any Employee Plan that the Company (i) sponsors, maintains, administers, or contributes to, or has any obligation
to contribute to or provide benefits under or through, or may reasonably be expected to have any Liability, and (ii) which provides
benefits to or otherwise covers any current or former employee, officer, director or other service provider of the Company or any of its
Subsidiaries (or their spouses, dependents, or beneficiaries).
“Company IP Rights”
means all Intellectual Property owned, licensed, or controlled by the Company that is necessary for, or used or held for use in, the operation
of the business of the Company as presently conducted.
“Company IP Rights
Agreement” means any Contract governing, related to or pertaining to any Company IP Rights other than any confidential information
provided under confidentiality agreements.
“Company Material
Adverse Effect” means any Effect that, considered together with all other Effects that have occurred prior to the date of determination
of the occurrence of a Company Material Adverse Effect, has or would reasonably be expected to have a material adverse effect on (a) the
business, condition (financial or otherwise), assets, liabilities or results of operations of the Company, or (b) the Company’s
ability to consummate the Contemplated Transactions; provided, however, that Effects arising or resulting from the following
shall not be taken into account in determining whether there has been a Company Material Adverse Effect: (i) the announcement of
this Agreement or the pendency of the Contemplated Transactions, (ii) the taking of any action, or the failure to take any action,
by the Company that is required to comply with the terms of the Agreement, (iii) any natural disaster or epidemics, pandemics or
other force majeure events, or any act or threat of terrorism or war, any armed hostilities or terrorist activities (including any escalation
or general worsening of any of the foregoing) anywhere in the world or any governmental or other response or reaction to any of the foregoing,
or (iv) any change in GAAP or applicable Law or the interpretation thereof, (v) general economic or political conditions or
conditions generally affecting the industries in which the Company operates; except in each case with respect to clauses (iii), (iv) and
(v), to the extent disproportionately affecting the Company, relative to other similarly situated companies in the industries in which
the Company operates.
“Company Merger Shares”
means the total number of shares of Parent Common Stock issuable to the Company shareholders determined by (i) dividing the Company
Valuation by (y) the Parent Share Price.
“Company Options”
means options or other rights to purchase shares of Company Common Stock granted by the Company, including pursuant to any Company Option
Plan, as an “inducement” award.
“Company Outstanding
Shares” means the total number of shares of Company Common Stock outstanding immediately prior to the First Effective Time expressed
on a fully diluted basis, and assuming, without limitation or duplication, the issuance of shares of Company Common Stock in respect of
all Company Options, Company Warrants and the conversion of each share of Company Preferred Stock into shares of Company Common Stock
pursuant to the terms of the Company’s certificate of incorporation, whether conditional or unconditional, that will be outstanding
as of immediately prior to the First Effective Time.
“Company Preferred
Stock” means the preferred stock, par value $0.0001 per share, of the Company.
“Company Registered
IP” means all Company IP Rights that are owned or exclusively licensed by the Company that are registered, filed or issued under
the authority of, with or by any Governmental Authority, including all patents, registered copyrights and registered trademarks and all
applications and registrations for any of the foregoing.
“Company Stockholder
Written Consent” shall have the meaning set forth in the recitals.
“Company Unaudited
Balance Sheet” means the estimated unaudited statement of assets, liabilities and equity of the Company for December 31,
2023.
“Company Valuation”
means eighty-five million dollars ($85,000,000).
“Company Warrants”
means that certain (1) Warrant to Purchase Common Stock of ImmunogenX, Inc. dated as of September 6, 2023, by and between
Mattress Liquidators, Inc. and the Company, and (2) Warrant to Purchase Common Stock of ImmunogenX, Inc. dated as of October 1,
2022, by and between Mattress Liquidators, Inc. and the Company.
“Confidentiality
Agreement” means that certain Mutual Non-Disclosure Agreement, dated as of April 19, 2023, by and between Parent and the
Company.
“Consent”
means any approval, consent, ratification, permission, waiver or authorization (including any Governmental Authorization).
“Contemplated Transactions”
means the Merger and the other transactions contemplated by the Agreement, including the Conversion Proposal and the Charter Amendment
Proposal.
“Convertible Note”
means each note set forth on Schedule 1.1(a)(ii), which collectively constitute all of the outstanding convertible notes entered
into by the Company, as debtor, the principal and accrued interest of which shall convert, or be deemed to convert in accordance with
an applicable agreement, into shares of Company Common Stock immediately prior to the Closing.
“Contract”
means, with respect to any Person, any written agreement, contract, subcontract, lease (whether for real or personal property), mortgage,
license, or other legally binding commitment or undertaking of any nature to which such Person is a party or by which such Person or any
of its assets are bound or affected under applicable Law.
“DGCL”
means the General Corporation Law of the State of Delaware.
“DLLCA”
means the Delaware Limited Liability Company Act.
“Effect”
means any effect, change, event, circumstance, or development.
“Employee Plan”
means (A) an “employee benefit plan” within the meaning of Section 3(3) of ERISA whether or not subject to
ERISA; (B) any individual employment, consulting, change in control, severance pr other agreement or arrangements; or (C) any
plan, program, policy or arrangement providing for stock options, stock purchases, equity-based compensation, bonuses (including any annual
bonuses and retention bonuses) or other incentives, severance pay, deferred compensation, employment, compensation, change in control
or transaction bonuses, supplemental, vacation, retirement benefits (including post-retirement health and welfare benefits), pension benefits,
profit-sharing benefits, fringe benefits, life insurance benefits, perquisites, health benefits, medical benefits, dental benefits, vision
benefits, and all other employee benefit plans, agreements, and arrangements, not described in (A) or (B) above; and (D) all
other plans, programs, policies or arrangements providing compensation to employees, consultants and non-employee directors.
“Encumbrance”
means any lien, pledge, hypothecation, charge, mortgage, security interest, lease, exclusive license, option, easement, reservation, servitude,
adverse title, claim, infringement, interference, option, right of first refusal, preemptive right, community property interest or restriction
or encumbrance of any nature (including any restriction on the voting of any security, any restriction on the transfer of any security
or other asset, any restriction on the receipt of any income derived from any asset, any restriction on the use of any asset and any restriction
on the possession, exercise or transfer of any other attribute of ownership of any asset).
“Enforceability Exceptions”
means the (a) Laws of general application relating to bankruptcy, insolvency and the relief of debtors and (b) rules of
law governing specific performance, injunctive relief and other equitable remedies.
“Entity”
means any corporation (including any nonprofit corporation), partnership (including any general partnership, limited partnership or limited
liability partnership), joint venture, estate, trust, company (including any company limited by shares, limited liability company or joint
stock company), firm, society or other enterprise, association, organization or entity, and each of its successors.
“Environmental Law”
means any federal, state, local or foreign Law relating to pollution or protection of human health or the environment (including ambient
air, surface water, ground water, land surface or subsurface strata), including any law or regulation relating to emissions, discharges,
releases or threatened releases of Hazardous Materials, or otherwise relating to the manufacture, processing, distribution, use, treatment,
storage, disposal, transport or handling of Hazardous Materials.
“ERISA”
means the Employee Retirement Income Security Act of 1974, as amended.
“ERISA Affiliate”
means, with respect to any Entity, any other Person that would be treated as a single employer with such Entity or part of the same “controlled
group” as such Entity under Sections 414(b),(c),(m) or (o) of the Code.
“Exchange Act”
means the Securities Exchange Act of 1934, as amended.
“Exchange Ratio”
means the quotient (rounded to three decimal places) obtained by dividing (x) the Company Merger Shares by (b) the Company Outstanding
Shares. The Parties agree that the Exchange Ratio is 2.649.
“First Merger Sub
Board” means the board of directors of First Merger Sub.
“Governmental Authority”
means any: (a) nation, state, commonwealth, province, territory, county, municipality, district or other jurisdiction of any nature,
(b) federal, state, local, municipal, foreign, supra-national or other government, (c) governmental or quasi-governmental authority
of any nature (including any governmental division, department, agency, commission, bureau, instrumentality, official, ministry, fund,
foundation, center, organization, unit, body or Entity and any court or other tribunal, and for the avoidance of doubt, any taxing authority)
or (d) self-regulatory organization (including Nasdaq).
“Governmental Authorization”
means any: (a) permit, license, certificate, franchise, permission, variance, exception, order, approval, clearance, registration,
qualification or authorization issued, granted, given or otherwise made available by or under the authority of any Governmental Authority
or pursuant to any Law or (b) right under any Contract with any Governmental Authority.
“Hazardous Materials”
means any pollutant, chemical, substance and any toxic, infectious, carcinogenic, reactive, corrosive, ignitable or flammable chemical,
or chemical compound, or hazardous substance, material or waste, whether solid, liquid or gas, that is subject to regulation, control
or remediation under any Environmental Law, including without limitation, crude oil or any fraction thereof, and petroleum products or
by-products.
“Intellectual Property”
means: (a) United States, foreign and international patents, patent applications, including all provisionals, nonprovisionals, substitutions,
divisionals, continuations, continuations-in-part, reissues, extensions, supplementary protection certificates, reexaminations, term extensions,
certificates of invention and the equivalents of any of the foregoing, statutory invention registrations, invention disclosures and inventions
(collectively, “Patents”), (b) trademarks, service marks, trade names, domain names, corporate names, brand names,
URLs, trade dress, logos and other source identifiers, including registrations and applications for registration thereof and goodwill
associated therewith, (c) copyrights, including registrations and applications for registration thereof, (d) software, including
all source code, object code and related documentation, (e) trade secrets, know how, inventions (including conceptions and/or reductions
to practice), invention disclosures, methods, processes, protocols, specifications, techniques, discoveries and improvements, formulae,
confidential and proprietary information, technical information, designs, drawings, procedures, models, formulations, manuals and systems,
whether or not patentable or copyrightable, including all biological, chemical, biochemical, toxicological, pharmacological and metabolic
material and information and data relating thereto and formulation, clinical, analytical and stability information and data, in each case
which are not available in the public domain and have actual or potential commercial value that is derived, in whole or in part, from
such secrecy, and (f) all United States and foreign rights arising under or associated with any of the foregoing.
“IRS” means
the United States Internal Revenue Service.
“Key Employee”
means (i) an executive officer of Parent; and (ii) any employee of Parent that reports directly to the board of directors of
Parent or to an executive officer of Parent.
“Knowledge”
means, with respect to an individual, that such individual is actually aware of the relevant fact or such individual would reasonably
be expected to know such fact in the ordinary course of the performance of such individual’s employment responsibilities. Any Person
that is an Entity shall have Knowledge if any executive officer or director of such Person as of the date such knowledge is imputed has
or should reasonably be expected to have Knowledge of such fact or other matter. With respect to any matters relating to Intellectual
Property, such awareness or reasonable expectation to have knowledge does not require any such individual to conduct or have conducted
or obtain or have obtained any freedom to operate opinions of counsel or any Intellectual Property rights clearance searches.
“Law” means
any federal, state, national, supra-national, foreign, local or municipal or other law, statute, constitution, principle of common law,
resolution, ordinance, code, edict, decree, rule, regulation, ruling or requirement issued, enacted, adopted, promulgated, implemented
or otherwise put into effect by or under the authority of any Governmental Authority (including under the authority of Nasdaq or the Financial
Industry Regulatory Authority).
“Legal Proceeding”
means any action, suit, litigation, arbitration, proceeding (including any civil, criminal, administrative, investigative or appellate
proceeding), hearing, inquiry, audit, examination or investigation commenced, brought, conducted or heard by or before, or otherwise involving,
any court or other Governmental Authority or any arbitrator or arbitration panel.
“Multiemployer Plan”
means a “multiemployer plan,” as defined in Section 3(37) or 4001(a)(3) of ERISA.
“Multiple Employer
Plan” means a “multiple employer plan” within the meaning of Section 413(c) of the Code or Section 3(40)
of ERISA.
“Multiple Employer
Welfare Arrangement” means a “multiple employer welfare arrangement” within the meaning of Section 3(40) of
ERISA.
“Nasdaq”
means The Nasdaq Stock Market.
“Order”
means any judgment, order, writ, injunction, ruling, decision or decree of (that is binding on a Party), or any plea agreement, corporate
integrity agreement, resolution agreement, or deferred prosecution agreement with, or any settlement under the jurisdiction of, any court
or Governmental Authority.
“Ordinary Course
of Business” means, in the case of each of the Company and Parent, such actions taken in the ordinary course of its normal operations
and consistent with its past practices.
“Organizational Documents”
means, with respect to any Person (other than an individual), (a) the certificate or articles of association or incorporation or
organization or limited partnership or limited liability company, and any joint venture, limited liability company, operating or partnership
agreement and other similar documents adopted or filed in connection with the creation, formation or organization of such Person and (b) all
bylaws, regulations and similar documents or agreements relating to the organization or governance of such Person, in each case, as amended
or supplemented.
“Parent Associate”
means any current or former employee, independent contractor, officer or director of Parent or any of its Subsidiaries.
“Parent Unaudited
Interim Balance Sheet” means the unaudited balance sheet of Parent as of September 30, 2023, included in Parent’s
Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, as filed with the SEC.
“Parent Board”
means the board of directors of Parent.
“Parent Capital Stock”
means the Parent Common Stock and Parent Preferred Stock.
“Parent Common Stock”
means the common stock, $0.0001 par value per share, of Parent.
“Parent Contract”
means any Contract: (a) to which Parent is a party, (b) by which Parent or any Parent IP Rights or any other asset of Parent
is or may become bound or under which Parent has, or may become subject to, any obligation or (c) under which Parent has or may acquire
any right or interest.
“Parent Convertible
Preferred Stock” means Parent’s non-voting convertible preferred stock, par value $0.0001 per share, with the
rights, preferences, powers and privileges specified in the Certificate of Designation.
“Parent Covered Person”
means, with respect to Parent as an “issuer” for purposes of Rule 506 promulgated under the Securities Act, any Person
listed in the first paragraph of Rule 506(d)(1).
“Parent Employee
Plan” means any Employee Plan that Parent (i) sponsors, maintains, administers, or contributes to, or has any obligation
to contribute to or provide benefits under or through, or may reasonably be expected to have any Liability, and (ii) which provides
benefits to or otherwise covers any current or former employee, officer, director or other service provider of Parent or any of its Subsidiaries
(or their spouses, dependents, or beneficiaries).
“Parent Warrants”
means the warrants to purchase shares of Parent Common Stock issued by Parent.
“Parent IP Rights”
means all Intellectual Property owned, licensed or controlled by Parent or any of its Subsidiaries that is necessary for, or used or held
for use in, the operation of the business of Parent or any of its Subsidiaries as presently conducted.
“Parent IP Rights
Agreement” means any Contract governing, related or pertaining to any Parent IP Rights.
“Parent Material
Adverse Effect” means any Effect that, considered together with all other Effects that have occurred prior to the date of determination
of the occurrence of the Parent Material Adverse Effect, has or would reasonably be expected to have a material adverse effect on (a) the
business, condition (financial or otherwise), assets, liabilities or results of operations of Parent, taken as a whole, or (b) Parent’s
ability to consummate the Contemplated Transactions; provided, however, that Effects arising or resulting from the following
shall not be taken into account in determining whether there has been a Parent Material Adverse Effect: (i) the announcement of the
Agreement or the pendency of the Contemplated Transactions, (ii) any change in the stock price or trading volume of Parent Common
Stock (it being understood, however, that any Effect causing or contributing to any change in stock price or trading volume of Parent
Common Stock may be taken into account in determining whether a Parent Material Adverse Effect has occurred, unless such Effects are otherwise
excepted from this definition), (iii) the taking of any action, or the failure to take any action, by Parent that is required to
comply with the terms of the Agreement, (iv) any natural disaster or epidemics, pandemics or other force majeure events, or any act
or threat of terrorism or war, any armed hostilities or terrorist activities (including any escalation or general worsening of any of
the foregoing) anywhere in the world, or any governmental or other response or reaction to any of the foregoing, (v) any change in
GAAP or applicable Law or the interpretation thereof or (vi) general economic or political conditions or conditions generally affecting
the industries in which Parent or any of its Subsidiaries operates; except, in each case with respect to clauses (iv), (v) and (vi),
to the extent materially and disproportionately affecting Parent and any its Subsidiaries, taken as a whole, relative to other similarly
situated companies in the industries in which Parent or any of its Subsidiaries operates.
“Parent Options”
means options or other rights to purchase shares of Parent Common Stock granted by Parent, including pursuant to any Parent Stock Plan,
as an “inducement” award.
“Parent Preferred
Stock” means Parent Series B Preferred Stock, Parent Series C Preferred Stock, Parent Series D Preferred Stock,
Parent Series E Preferred Stock and Parent Series F Preferred Stock, together with the Parent Convertible Preferred Stock.
“Parent Registered
IP” means all Parent IP Rights that are owned or exclusively licensed by Parent or any of its Subsidiaries that are registered,
filed or issued under the authority of, with or by any Governmental Authority, including all patents, registered copyrights and registered
trademarks and all applications for any of the foregoing.
“Parent Series B
Preferred Stock” means Series B Convertible Preferred Stock of Parent, $0.0001 par value per share.
“Parent Series C
Preferred Stock” means Series C Convertible Preferred Stock of Parent, $0.0001 par value per share.
“Parent Series D
Preferred Stock” means Series D Convertible Preferred Stock of Parent, $0.0001 par value per share.
“Parent Series E
Preferred Stock” means Series E Convertible Preferred Stock of Parent, $0.0001 par value per share.
“Parent Series F
Preferred Stock” means Series F Convertible Preferred Stock of Parent, $0.0001 par value per share.
“Parent Share Price”
means seven dollars ($7.00).
“Parent Restricted
Stock Units” means any equity award with respect to Parent Common Stock that represents the right to receive in the future shares
of Parent Common Stock pursuant to any Parent Stock Plan.
“Party”
or “Parties” means the Company, Merger Subs and Parent.
“PEO Plan”
means a plan, program, policy or arrangement sponsored or maintained by a third party “professional employer organization.”
“Permitted Encumbrance”
means (a) any statutory liens for current Taxes not yet due and payable or for Taxes that are being contested in good faith and for
which adequate reserves have been made on the Company Unaudited Balance Sheet or the Parent Unaudited Interim Balance Sheet, as applicable,
in accordance with GAAP, (b) minor liens that have arisen in the Ordinary Course of Business and that do not (in any case or in the
aggregate) materially detract from the value of the assets subject thereto or materially impair the operations of the Company or Parent,
as applicable, (c) statutory liens to secure obligations to landlords, lessors or renters under leases or rental agreements, (d) deposits
or pledges made in connection with, or to secure payment of, workers’ compensation, unemployment insurance or similar programs mandated
by Law, (e) statutory liens in favor of carriers, warehousemen, mechanics and materialmen, to secure claims for labor, materials
or supplies and (f) liens arising under applicable securities Law.
“Person”
means any individual, Entity or Governmental Authority.
“Personal Information”
means data and information concerning an identifiable natural person.
“Privacy Laws”
mean Laws relating to privacy, security and/or collection, use or other processing of Personal Information.
“Representatives”
means directors, officers, employees, agents, attorneys, accountants, investment bankers, advisors and representatives.
“Sarbanes-Oxley Act”
means the Sarbanes-Oxley Act of 2002.
“SEC” means
the United States Securities and Exchange Commission.
“Second Merger Sub
Board” means the board of managers of Second Merger Sub.
“Securities Act”
means the Securities Act of 1933, as amended.
An Entity shall be deemed
to be a “Subsidiary” of a Person if such Person directly or indirectly owns or purports to own, beneficially or of
record, (a) an amount of voting securities or other interests in such Entity that is sufficient to enable such Person to elect at
least a majority of the members of such entity’s board of directors or other governing body or (b) at least 50% of the outstanding
equity, voting, beneficial or financial interests in such Entity.
“Tax” means
(i) any federal, state, local, foreign or other tax, including any income tax, franchise tax, capital gains tax, gross receipts tax,
value-added tax, surtax, estimated tax, unemployment tax, national health insurance tax, excise tax, ad valorem tax, transfer tax, stamp
tax, sales tax, use tax, property tax, business tax, withholding tax, payroll tax, customs duty, alternative or add-on minimum or other
tax or similar charge (whether imposed directly or through withholding and whether or not disputed), and including any fine, penalty,
addition to tax, interest or additional amount imposed by a Governmental Authority with respect thereto (or attributable to the nonpayment
thereof) and (ii) any liability for payment of amounts described in clause (i) whether as a result of transferee or successor
liability, of being a member of an affiliated, consolidated, combined or unitary group for any period, pursuant to a Contract, through
operation of Law or otherwise.
“Tax Return”
means any return (including any information return), report, statement, declaration, claim or refund, estimate, schedule, notice, notification,
form, election, certificate or other document or information, and any amendment or supplement to any of the foregoing, filed or required
to be filed with any Governmental Authority (or provided to a payee) in connection with the determination, assessment, collection or payment
of any Tax or in connection with the administration, implementation or enforcement of or compliance with any Law relating to any Tax.
“Treasury Regulations”
means the United States Treasury regulations promulgated under the Code.
“WARN Act”
means the Worker Adjustment and Retraining Notification Act of 1988, as amended, or any similar Laws.
(b) Each
of the following terms is defined in the Section set forth opposite such term:
| Agreement |
Preamble |
|
First Effective Time |
2.3 |
| Allocation Certificate |
5.10 |
|
First Merger |
Recitals |
| Assumed Options |
2.9 |
|
First Merger Sub |
Preamble |
| Business Associate Agreement |
3.14(i) |
|
GAAP |
3.7(a) |
| Capitalization Date |
4.6(a) |
|
HIPAA |
3.14(i) |
| Certifications |
4.7(a) |
|
Intended Tax Treatment |
2.13 |
| Closing |
2.3 |
|
Investor Agreements |
5.8 |
| Closing Date |
2.3 |
|
Lock-Up Agreement |
Recitals |
| Closing Distribution |
2.6(e) |
|
Merger |
Recitals |
| Company |
Preamble |
|
Merger Consideration |
2.5 |
| Company Board Approval |
Recitals |
|
Nasdaq Listing Application |
5.11 |
| Company Consultant |
3.17(a) |
|
Non-Scheduled Company IP Contracts |
3.12(b) |
| Company Disclosure Schedule |
Section 3 |
|
Old Stock Options |
2.9 |
| Company Employee |
3.17(a) |
|
Parent |
Preamble |
| Company Financials |
3.7(a) |
|
Parent 2014 Plan |
4.6(c) |
| Company Material Contract |
3.13(a) |
|
Parent 2020 Plan |
4.6(c) |
| Company Material Contracts |
3.13(a) |
|
Parent Board Recommendation |
5.4(b) |
| Company Option Plan |
3.6(c) |
|
Parent Common Stock Consideration Cap |
2.5 |
| Company Permits |
3.14(b) |
|
Parent Common Stock Payment Shares |
2.5 |
| Company Product Candidates |
3.14(b) |
|
Parent Consultant |
4.17(a) |
| Company Real Estate Leases |
3.11 |
|
Parent Disclosure Schedule |
Section 4 |
| Company Stockholder Written Consent |
Recitals |
|
Parent Employee |
4.17(a) |
| Contingent Workers |
3.17(a) |
|
Parent Grant Date |
4.6(f) |
| Conversion Proposal |
Recitals |
|
Parent Material Contracts |
4.13(a) |
| Costs |
5.5(a) |
|
Parent Permits |
4.14(b) |
| D&O Indemnified Parties |
5.5(a) |
|
Parent Preferred Stock Payment Shares |
2.5 |
| Disqualifying Event |
4.22 |
|
Parent Product Candidates |
4.14(b) |
| Dissenting Shares |
2.12 |
|
Parent Real Estate Leases |
4.11 |
| Drug Regulatory Agency |
3.14(c) |
|
Parent Regulatory Permits |
4.14(d) |
| EDGAR |
Section 4 |
|
Parent SEC Documents |
4.7(a) |
| Exchange Agent |
2.8(a) |
|
Parent Stock Plans |
4.6(c) |
| FDA |
3.14(c) |
|
Parent Stockholder Matters |
5.4(a) |
| FDCA |
3.14(c) |
|
Parent Stockholder Meeting |
5.4(a) |
| First Certificate of Merger |
2.3 |
|
PHSA |
3.14(c) |
| Privacy Policies |
3.22 |
|
Second Effective Time |
2.3 |
| Proxy Statement |
5.2(a) |
|
Second Merger |
Recitals |
| Required Company Stockholder Vote |
3.4 |
|
Second Merger Sub |
Preamble |
| Required Parent Stockholder Vote |
4.4 |
|
Stockholder Notice |
5.1 |
| Restricted Covenants Agreement |
Recitals |
|
Transfer Taxes |
5.6(b) |
| Second Certificate of Merger |
2.3 |
|
Voting Agreement |
Recitals |
| |
|
|
Withholding Agent |
2.14 |
1.2 Other
Definitional and Interpretative Provisions. The words “hereof,” “herein” and “hereunder” and words
of like import used in this Agreement shall refer to this Agreement as a whole and not to any particular provision of this Agreement.
The captions herein are included for convenience of reference only and shall be ignored in the construction or interpretation hereof.
References to Sections, Exhibits and Schedules are to Sections, Exhibits and Schedules of this Agreement unless otherwise specified. Any
capitalized terms used in any Exhibit or Schedule but not otherwise defined therein shall have the meaning as defined in this Agreement.
Any singular term in this Agreement shall be deemed to include the plural, and any plural term the singular, the masculine gender shall
include the feminine and neuter genders; the feminine gender shall include the masculine and neuter genders; and the neuter gender shall
include masculine and feminine gender. Whenever the words “include,” “includes” or “including” are
used in this Agreement, they shall be deemed to be followed by the words “without limitation,” whether or not they are in
fact followed by those words or words of like import. “Writing,” “written” and comparable terms refer to printing,
typing and other means of reproducing words (including electronic media) in a visible form. References to any agreement or Contract are
to that agreement or Contract as amended, modified or supplemented from time to time in accordance with the terms hereof and thereof.
References to any Person include the successors and permitted assigns of that Person. References to any statute are to that statute and
to the rules and regulations promulgated thereunder, in each case as amended, modified, re-enacted thereof, substituted, from time
to time. References to “$” and “dollars” are to the currency of the United States. All accounting terms used herein
will be interpreted, and all accounting determinations hereunder will be made, in accordance with GAAP unless otherwise expressly specified.
References from or through any date shall mean, unless otherwise specified, from and including or through and including, respectively.
All references to “days” shall be to calendar days unless otherwise indicated as a “Business Day.” Except as otherwise
specifically indicated, for purposes of measuring the beginning and ending of time periods in this Agreement (including for purposes of
“Business Day” and for hours in a day or Business Day), the time at which a thing, occurrence or event shall begin or end
shall be deemed to occur in the Eastern time zone of the United States. The Parties agree that any rule of construction to the effect
that ambiguities are to be resolved against the drafting Party shall not be applied in the construction or interpretation of this Agreement.
The Parties agree that the Company Disclosure Schedule or Parent Disclosure Schedule shall be arranged in sections and subsections corresponding
to the numbered and lettered sections and subsections contained in Section 3 or Section 4, respectively. The disclosures
in any section or subsection of the Company Disclosure Schedule or the Parent Disclosure Schedule shall qualify other sections and subsections
in Section 3 or Section 4, respectively, to the extent it is readily apparent from a reading of the disclosure
that such disclosure is applicable to such other sections and subsections. The words “delivered” or “made available”
mean, with respect to any documentation, (a) that prior to 5:00 p.m. (New York City time) on the date that is the day prior
to the date of this Agreement, a copy of such material has been posted to and made available by a Party to the other Party and its Representatives
in the electronic data room maintained by such disclosing Party for the purposes of the Contemplated Transactions or (b) delivered
by or on behalf of a Party or its Representatives to the other Party or its Representatives via electronic mail or in hard copy form prior
to the execution of this Agreement.
Section 2. Description
of Transaction.
2.1 The
Merger. Upon the terms and subject to the conditions set forth in this Agreement, at the First Effective Time, First Merger Sub shall
be merged with and into the Company, and the separate existence of First Merger Sub shall cease. As a result of the First Merger, the
Company will continue as the surviving company of the First Merger (the “First Step Surviving Company”). Upon the terms
and subject to the conditions set forth in this Agreement, at the Second Effective Time, the First Step Surviving Company will merge with
and into Second Merger Sub, and the separate existence of the First Step Surviving Company shall cease. As a result of the Second Merger,
Second Merger Sub will continue as the surviving company in the Second Merger (the “Surviving Company”).
2.2 Effects
of the Merger. At and after the First Effective Time, the First Merger shall have the effects set forth in this Agreement, the First
Certificate of Merger, and in the applicable provisions of the DGCL. As a result of the First Merger, the First Step Surviving Company
will become a wholly owned subsidiary of Parent. At and after the Second Effective Time, the Second Merger shall have the effects set
forth in this Agreement, the Second Certificate of Merger, and in the applicable provisions of the DGCL and DLLCA.
2.3 Closing;
First Effective Time; Second Effective Time. Subject to the satisfaction or waiver of the conditions set forth in Section 7,
Section 8 and Section 9 the consummation of the Merger (the “Closing”) shall take place remotely,
on the date of this Agreement, or at such other time, date and place as Parent and the Company may mutually agree in writing. The date
on which the Closing actually takes place is referred to as the “Closing Date.” At the Closing, (i) the Parties
shall cause the First Merger to be consummated by executing and filing with the Secretary of State of the State of Delaware a certificate
of merger with respect to the First Merger, satisfying the applicable requirements of the DGCL and in form and substance to be agreed
upon by the Parties (the “First Certificate of Merger”) and (ii) the Parties shall cause the Second Merger to
be consummated by executing and filing with the Secretary of State of the State of Delaware a certificate of merger with respect to the
Second Merger, satisfying the applicable requirements of the DLLCA and in form and substance to be agreed upon by the Parties (the “Second
Certificate of Merger”). The First Merger shall become effective at the time of the filing of such First Certificate of Merger
with the Secretary of State of the State of Delaware or at such later time as may be specified in such First Certificate of Merger with
the consent of Parent and the Company (the time as of which the First Merger becomes effective being referred to as the “First
Effective Time”). The Second Merger shall become effective at the time of the filing of such Second Certificate of Merger with
the Secretary of State of the State of Delaware or at such later time as may be specified in such Second Certificate of Merger with the
consent of Parent and the Company (the time as of which the Second Merger becomes effective being referred to as the “Second
Effective Time”).
2.4 Certificate
of Designation; Organizational Documents; Directors and Officers.
(a) Prior
to the First Effective Time, Parent will file the Certificate of Designation with the office of the Secretary of State of the State of
Delaware.
(b) As
of the First Effective Time:
(i) the
certificate of incorporation of the First Step Surviving Company shall be amended and restated as set forth in an exhibit to the First
Certificate of Merger, until thereafter amended as provided by the DGCL and such certificate of incorporation;
(ii) the
bylaws of the First Step Surviving Company shall be identical to the bylaws of the Company as in effect immediately prior to the First
Effective Time, until thereafter amended as provided by the DGCL and such bylaws; and
(iii) the
directors and officers of the First Step Surviving Company, each to hold office in accordance with the certificate of incorporation and
bylaws of the First Step Surviving Company, shall be such persons as mutually agreed to by Parent and the Company.
(c) As
of the Second Effective Time:
(i) the
manager of the Surviving Company in accordance with the certificate of formation and limited liability company agreement of the Surviving
Company, shall be Parent;
(ii) the
limited liability company agreement of the Surviving Company shall be identical to the limited liability company agreement of Second Merger
Sub as in effect immediately prior to the Second Effective Time, until thereafter amended as provided by the DLLCA and such limited liability
company agreement; provided that (I) the limited liability company agreement of the Surviving Company shall comply with Section 5.5
and (II) all references to Second Merger Sub in the limited liability company agreement of the Surviving Company shall be changed
to reference to ImmunogenX, LLC; and
(iii) the
certificate of formation of the Second Merger Sub as in effect immediately prior to the Second Effective Time shall be the certificate
of formation of the Surviving Company, until thereafter further amended in accordance with DLLCA and as provided in such certificate of
formation, except that Article I of the certificate of formation of the Surviving Company shall be amended and restated in its entirety
to read as follows: “The name of this limited liability company is ImmunogenX, LLC”.
(iv) the
officers of the Surviving Company, each to hold office in accordance with certificate of formation and limited liability company agreement
of the Surviving Company, shall be such persons as mutually agreed to by Parent and the Company.
2.5 Merger
Consideration; Effect of Merger on Company Capital Stock. The aggregate merger consideration (the “Merger Consideration”)
to be paid by Parent for all of the outstanding shares of Company Capital Stock at the Closing and amounts reserved for Company Options
shall be (a) 36,830 shares of Parent Common Stock (“Parent Common Stock Payment Shares”), which shares shall represent
a number of shares equal to no more than 19.9% of the outstanding shares of Parent Common Stock as of immediately before the First Effective
Time (the “Parent Common Stock Consideration Cap”), and (b) in the event the aggregate number of shares of Parent
Common Stock Payment Shares issued to any Company stockholder at Closing would result in the issuance of shares of Parent Common Stock
in an amount in excess of the Parent Common Stock Consideration Cap, Parent shall issue to such Company stockholders shares of Parent
Common Stock up to the Parent Common Stock Consideration Cap and shall issue the remaining balance to such stockholders a total of 11,777.418
shares of Parent Convertible Preferred Stock (“Parent Preferred Stock Payment Shares”); provided, that, as required
under certain Nasdaq Stock Market Rules that are applicable to Parent, none of the shares of Parent Preferred Stock Payment Shares
may be converted into shares of Parent Common Stock unless and until the Conversion Proposal is approved by the approval of the Parent
Stockholder Matters at the Parent Stockholders’ Meeting pursuant to Section 5.3. Each Parent Preferred Stock Payment
Share shall be convertible into one thousand (1,000) shares of Parent Common Stock, subject to and contingent upon the approval of the
Conversion Proposal and the terms of the Certificate of Designation.
2.6 Conversion
of Stock.
(a) At
the First Effective Time, by virtue of the First Merger and without any further action on the part of Parent, Merger Subs, the Company
or any stockholder of the Company or Parent:
(i) any
shares of Company Common Stock held as treasury stock or held or owned by the Company immediately prior to the First Effective Time shall
be canceled and retired and shall cease to exist, and no consideration shall be delivered in exchange therefor; and
(ii) subject
to Section 2.5 and Section 2.6(c), each share of Company Capital Stock outstanding immediately prior to the First
Effective Time (excluding shares to be canceled pursuant to Section 2.6(a)(i) and excluding Dissenting Shares) shall
be automatically converted solely into the right to receive the number of Parent Common Stock Payment Shares and Parent Preferred Stock
Payment Shares as set forth on the Allocation Certificate.
(b) If
any shares of Company Common Stock outstanding immediately prior to the First Effective Time are subject to a repurchase option or a risk
of forfeiture under any applicable restricted stock purchase agreement or other similar agreement with the Company, such shares of Company
Common Stock shall no longer be subject to any right of repurchase, risk of forfeiture or other such conditions.
(c) No
fractional shares of Parent Common Stock shall be issued in connection with the First Merger, and no certificates or scrip for any such
fractional shares shall be issued, with no cash being paid for any fractional share eliminated by such rounding. Any fractional shares
of Parent Common Stock a holder of Company Capital Stock would otherwise be entitled to receive shall be aggregated together first prior
to eliminating any remaining fractional share.
(d) At
the First Effective Time, by virtue of the First Merger and without any further action on the part of Parent, Merger Subs, the Company
or any stockholder of the Company or stockholder of Parent, each share of First Merger Sub issued and outstanding immediately prior to
the First Effective Time shall be converted into and exchanged for one share of common stock of the First Step Surviving Company. If applicable,
each stock certificate of First Merger Sub evidencing ownership of any such share shall, as of the First Effective Time, evidence ownership
of such shares of common stock of the First Step Surviving Company.
(e) At
the Second Effective Time, by virtue of the Second Merger and without any action on the part of Parent, the First Step Surviving Company,
Second Merger Sub or their respective members, each share of common stock of the First Step Surviving Company issued and outstanding immediately
prior to the Second Effective Time shall be canceled and extinguished without any conversion thereof and no payment or distribution shall
be made with respect thereto.
2.7 Closing
of the Company’s Transfer Books. At the First Effective Time: (a) all holders of (i) certificates representing shares
of Company Capital Stock and (ii) book-entry shares representing shares of Company Capital Stock, in each case, that were outstanding
immediately prior to the First Effective Time shall cease to have any rights as stockholders of the Company; and (b) the stock transfer
books of the Company shall be closed with respect to all shares of Company Capital Stock outstanding immediately prior to the First Effective
Time. No further transfer of any such shares of Company Capital Stock shall be made on such stock transfer books after the First Effective
Time. If, after the first Effective Time, a valid certificate previously representing any shares of Company Capital Stock outstanding
immediately prior to the First Effective Time is presented to the Exchange Agent or to the Surviving Entity, such Company Stock Certificate
shall be canceled and shall be exchanged as provided in Sections 2.5 and 2.8.
2.8 Exchange
of Shares.
(a) On
or prior to the Closing Date, Parent and the Company shall jointly select a reputable bank, transfer agent or trust company to act as
exchange agent in the Merger (the “Exchange Agent”). At the First Effective Time, Parent shall deposit with the Exchange
Agent evidence of book-entry shares representing the shares of Parent Capital Stock issuable pursuant to Section 2.6(a) in
exchange for Company Capital Stock.
(b) Promptly
after the First Effective Time, the Parties shall cause the Exchange Agent to mail to the Persons who were record holders of Company Capital
Stock that were converted into the right to receive Merger Consideration: (i) a letter of transmittal in substantially the form attached
hereto as Exhibit F (the “Letter of Transmittal”) and an Accredited Investor Questionnaire
in customary form and containing such provisions as Parent may reasonably specify and (ii) instructions for effecting the surrender
of Company Capital Stock in exchange for book-entry shares of Parent Capital Stock representing Merger Consideration. Upon surrender of
a duly executed Letter of Transmittal, Accredited Investor Questionnaire and such other documents as may be reasonably required by the
Exchange Agent or Parent, the Exchange Agent shall issue and the holder of such Company Capital Stock shall be entitled to receive in
exchange therefor book-entry shares representing Parent Capital Stock representing Merger Consideration that such holder has the right
to receive pursuant to the provisions of Section 2.6(a).
(c) No
dividends or other distributions declared or made with respect to Parent Capital Stock with a record date after the First Effective Time
shall be paid to the holder of any Company Capital Stock with respect to the shares of Parent Capital Stock that such holder has the right
to receive in the Merger until such holder delivers a duly executed Letter of Transmittal (at which time (or, if later, on the applicable
payment date) such holder shall be entitled, subject to the effect of applicable abandoned property, escheat or similar Laws, to receive
all such dividends and distributions, without interest).
(d) Any
shares of Parent Capital Stock deposited with the Exchange Agent that remain undistributed to holders of Company Capital Stock as of the
date that is 180 days after the Closing Date shall be delivered to Parent upon demand, and any holders of Company Capital Stock who have
not theretofore delivered a duly executed Letter of Transmittal in accordance with this Section 2.8 shall thereafter look
only to Parent for satisfaction of their claims for Parent Capital Stock and any dividends or distributions with respect to shares of
Parent Capital Stock.
(e) No
Party shall be liable to any holder of any Company Capital Stock or to any other Person with respect to any shares of Parent Capital Stock
(or dividends or distributions with respect thereto) or for any cash amounts delivered to any public official pursuant to any applicable
abandoned property Law, escheat Law or similar Law.
2.9 Stock
Options. At the First Effective Time, each Company Option which is outstanding immediately prior to the First Effective Time, whether
vested or unvested (“Old Stock Options”), shall be accelerated and deemed vested and shall automatically be converted
as of the First Effective Time into options to purchase Parent Common Stock (such options as so converted, “Assumed Options”),
which Assumed Options shall be identical to the Old Stock Options in all material respects, except that (i) upon exercise of the
Assumed Option, the optionholder will receive Parent Common Stock rather than Company Common Stock, (ii) the number of shares of
Parent Common Stock covered by each Assumed Option shall equal the number of shares of Company Common Stock covered by the corresponding
Old Stock Option multiplied by the Exchange Ratio (rounded down to the nearest whole share), (iii) the exercise price of each Assumed
Option shall equal the exercise price applicable to the corresponding Old Stock Option divided by the Exchange Ratio (rounded down to
the nearest whole penny) and (iv) the committee that administers the plan by which such Assumed Options are governed shall be a committee
established by the Parent Board. In all other material respects, the Assumed Options shall be governed by the terms of the Company Option
Plan at and after the First Effective Time, subject to such additional modifications as the Parent Board or such committee deems appropriate
to reflect the Merger, to the extent permissible under the terms of the Company Option Plan without the consent of the holder of the Assumed
Options. Promptly after the First Effective Time, Parent shall use its reasonable best efforts to register the shares issuable upon exercise
of the New Stock Options under the Securities Act of 1933, and to keep such registration in effect until such time as all New Stock Options
have been exercised, expire or otherwise are no longer outstanding. As soon as practicable after the First Effective Time, Parent shall
deliver a notice to holders of Assumed Options describing the adjustments set forth in this Section 2.9. To the extent necessary
to effect Parent’s obligations under this Section 2.9, Parent shall assume sponsorship of the Company Option Plan, effective
as of the First Effective Time.
2.10 Company
Warrants. Each outstanding Company Warrant shall be assumed by Parent and automatically converted into a warrant for shares of Parent
Common Stock (each, an “Assumed Warrant”). Each Assumed Warrant shall: (i) have the right to acquire a number
of shares of Parent Common Stock equal to (as rounded down to the nearest whole number) the product of (A) the number of shares of
Company Common Stock which the Company Warrant had the right to acquire immediately prior to the First Effective Time, multiplied by (B) the
Exchange Ratio; and (ii) have an exercise price equal to (as rounded up to the nearest whole cent) the quotient of (A) the exercise
price of the Company Warrant (in U.S. Dollars), divided by (B) the Exchange Ratio. Parent shall take all corporate action necessary
to reserve for future issuance, and shall maintain such reservation for so long as any of the Assumed Warrants remain outstanding, a sufficient
number of shares of Parent Common Stock for delivery upon the exercise of such Assumed Warrant.
2.11 Other
Company Convertible Securities. Any other securities of the Company other than the Company Options, if not exercised or converted
at or prior to the First Effective Time, shall be cancelled, retired and terminated and cease to represent a right to acquire, be exchanged
for or convert into shares of Company Capital Stock.
2.12 Appraisal
Rights.
(a) Notwithstanding
any provision of this Agreement to the contrary, shares of Company Capital Stock that are outstanding immediately prior to the First Effective
Time and which are held by stockholders who have exercised and perfected appraisal rights for such shares of Company Capital Stock in
accordance with the DGCL (collectively, the “Dissenting Shares”) shall not be converted into or represent the right
to receive the Parent Capital Stock representing Merger Consideration described in Section 2.5 attributable to such Dissenting
Shares. Such stockholders shall be entitled to receive payment of the appraised value of such shares of Company Capital Stock held by
them in accordance with the DGCL, unless and until such stockholders fail to perfect or effectively withdraw or otherwise lose their appraisal
rights under the DGCL. All Dissenting Shares held by stockholders who shall have failed to perfect or shall have effectively withdrawn
or lost their right to appraisal of such shares of Company Capital Stock under the DGCL (whether occurring before, at or after the First
Effective Time) shall thereupon be deemed to be converted into and to have become exchangeable for, as of the First Effective Time, the
right to receive the Parent Capital Stock representing Merger Consideration, without interest, attributable to such Dissenting Shares
upon their surrender in the manner provided in Sections 2.6 and 2.8.
(b) The
Company shall give Parent prompt written notice of any demands by dissenting stockholders received by the Company, withdrawals of such
demands and any other instruments served on the Company and any material correspondence received by the Company in connection with such
demands, and Parent shall have the right to direct all negotiations and proceedings with respect to such demands; provided that the Company
shall have the right to participate in such negotiations and proceedings. Neither the Parent nor the Company shall, except with the other
party’s prior written consent, voluntarily make any payment with respect to, or settle or offer to settle, any such demands, or
approve any withdrawal of any such demands or agree to do any of the foregoing.
2.13 Further
Action. If, at any time after the First Effective Time, any further action is determined by the Surviving Company to be necessary
or desirable to carry out the purposes of this Agreement or to vest the Surviving Company with full right, title and possession of and
to all rights and property of the Company, then the officers and manager of the Surviving Company shall be fully authorized, and shall
use their and its commercially reasonable efforts (in the name of the Company, in the name of Merger Subs, in the name of the Surviving
Company and otherwise) to take such action.
2.14 Intended
Tax Treatment. The Parties acknowledge and agree that, for U.S. federal (and applicable state and local), income tax purposes (a) this
Agreement constitutes, and is hereby adopted by the parties hereto as a, “plan or reorganization” for purposes of Section 368
of the Code and within the meaning of Treasury Regulations Sections 1.368-2(g) and 1.368-3(a), and (b) the First Merger and
the Second Merger, taken together, are intended to constitute a single integrated transaction that qualifies as a reorganization within
the meaning of Section 368(a)(1)(A) of the Code and Treasury Regulations as described in Rev. Rul. 2001-46, 2001-2 C.B. 321,
to which the Parent and Company are parties under Section 368(b) of the Code (the “Intended Tax Treatment").
2.15 Withholding.
Each of the Exchange Agent, Parent, First Step Surviving Company and the Surviving Company (each, a “Withholding Agent”)
shall be entitled to deduct and withhold from any consideration deliverable pursuant to this Agreement (including the Closing Distribution)
such amounts as are required to be deducted or withheld from such consideration under the Code or under any other applicable Law; provided
that if a Withholding Agent determines that any payment to any stockholder of the Company hereunder is subject to deduction and/or withholding,
then, except with respect to compensatory payments or as a result of a failure to deliver the certificate described in Section 5.6(c),
such Withholding Agent shall (i) provide notice to such stockholder as soon as reasonably practicable after such determination (no
later than three (3) Business Days prior to undertaking such deduction and/or withholding) and (ii) use commercially reasonable
efforts to cooperate with such stockholder prior to Closing to reduce or eliminate any such deduction and/or withholding. To the extent
such amounts are so deducted or withheld, and remitted to the appropriate taxing authority, such amounts shall be treated for all purposes
under this Agreement as having been paid to the Person to whom such amounts would otherwise have been paid.
Section 3. Representations
and Warranties of the Company. Subject to Section 3, except as set forth in the written disclosure schedule delivered
by the Company to Parent (the “Company Disclosure Schedule”), the Company represents and warrants to Parent and Merger
Subs as follows:
3.1 Due
Organization; Subsidiaries.
(a) The
Company is a corporation duly incorporated, validly existing and in good standing under the Laws of the jurisdiction of its incorporation
and has all necessary power and authority: (i) to conduct its business in the manner in which its business is currently being conducted,
(ii) to own or lease and use its property and assets in the manner in which its property and assets are currently owned or leased
and used and (iii) to perform its obligations under all Contracts by which it is bound.
(b) The
Company is duly licensed and qualified to do business, and is in good standing (to the extent applicable in such jurisdiction), under
the Laws of all jurisdictions where the nature of its business in the manner in which its business is currently being conducted requires
such licensing or qualification other than in jurisdictions where the failure to be so qualified individually or in the aggregate would
not be reasonably expected to have a Company Material Adverse Effect.
(c) The
Company has no Subsidiaries and the Company does not own any capital stock or membership interests of, or any equity, ownership or profit
sharing interest of any nature in, or controls directly or indirectly, any other Entity. The Company is not and has never otherwise been,
directly or indirectly, a party to, member of or participant in any partnership, joint venture or similar business entity. The Company
has not agreed to, and is not obligated to make, nor is bound by any Contract under which it may become obligated to make, any future
investment in or capital contribution to any other Entity. The Company has not, at any time, been a general partner of, or has otherwise
been liable for any of the debts or other obligations of, any general partnership, limited partnership or other Entity.
3.2 Organizational
Documents. The Company has made available to Parent accurate and complete copies of the Organizational Documents of the Company in
effect as of the date of this Agreement. The Company is not in breach or violation of its Organizational Documents in any material respect.
3.3 Authority;
Binding Nature of Agreement. The Company has all necessary corporate power and authority to enter into and to perform its obligations
under this Agreement and to consummate the Contemplated Transactions. The Company Board has (i) determined that the Contemplated
Transactions are fair to, advisable and in the best interests of the Company and its stockholders, (ii) approved and declared advisable
this Agreement and the Contemplated Transactions and (iii) determined to recommend, upon the terms and subject to the conditions
set forth in this Agreement, that the stockholders of the Company vote to adopt this Agreement and thereby approve the Contemplated Transactions.
This Agreement has been duly executed and delivered by the Company and assuming the due authorization, execution and delivery by Parent
and Merger Subs, constitutes the legal, valid and binding obligation of the Company, enforceable against the Company in accordance with
its terms, subject to the Enforceability Exceptions.
3.4 Vote
Required. The affirmative vote of a majority of the outstanding shares of Company Common Stock and Company Preferred Stock, voting
on an as-converted basis, voting together as a single class (the “Required Company Stockholder Vote”) is the only vote
of the holders of any class or series of Company Capital Stock necessary to adopt and approve this Agreement and approve the Contemplated
Transactions. The Company has obtained approval by written consent from the stockholders of the Company sufficient for the Required Company
Stockholder Vote in lieu of a meeting pursuant to Section 228 of the DGCL, for purposes of adopting and approving this Agreement
and the Contemplated Transactions.
3.5 Non-Contravention;
Consents.
(a) Subject
to obtaining the Required Company Stockholder Vote and the filing of the First Certificate of Merger and Second Certificate of Merger
required by the DGCL and DLLCA, neither (x) the execution, delivery or performance of this Agreement by the Company, nor (y) the
consummation of the Contemplated Transactions, will directly or indirectly (with or without notice or lapse of time):
(i) contravene,
conflict with or result in a violation of any of the provisions of the Company’s Organizational Documents;
(ii) contravene,
conflict with or result in a material violation of, or give any Governmental Authority or other Person the right to challenge the Contemplated
Transactions or to exercise any remedy or obtain any relief under, any Law or any Order by which the Company, or any of the assets owned
or used by the Company, is subject;
(iii) contravene,
conflict with or result in a material violation of any of the terms or requirements of, or give any Governmental Authority the right to
revoke, withdraw, suspend, cancel, terminate or modify, any Governmental Authorization that is held by the Company or otherwise relates
to the business of the Company, or any assets owned, leased or used by the Company, except as would not reasonably be expected to be material
to the Company or its business, except as would not reasonably be expected to be material to the Company or its business;
(iv) contravene,
conflict with or result in a violation or breach of, or result in a default under, any provision of any Company Material Contract, or
give any Person the right to: (A) declare a default or exercise any remedy under any Company Material Contract, (B) any material
payment, rebate, chargeback, penalty or change in delivery schedule under any Company Material Contract, (C) accelerate the maturity
or performance of any Company Material Contract or (D) cancel, terminate or modify any term of any Company Material Contract, except
in the case of any nonmaterial breach, default, penalty or modification; or
(v) result
in the imposition or creation of any Encumbrance upon or with respect to any asset owned or used by the Company (except for Permitted
Encumbrances).
(b) Except
for (i) the Required Company Stockholder Vote, (ii) the filing of the First Certificate of Merger and Second Certificate of
Merger with the Secretary of State of the State of Delaware pursuant to the DGCL and DLLCA, (iii) the filing of the Certificate of
Designation with the Secretary of State of the State of Delaware pursuant to the DGCL and DLLCA, and (iv) such consents, waivers,
approvals, orders, authorizations, registrations, declarations and filings as may be required under applicable federal and state securities
Laws, the Company is not required to make any filing with or give any notice to, or to obtain any Consent from, any Person in connection
with (x) the execution, delivery or performance of this Agreement or (y) the consummation of the Contemplated Transactions.
(c) The
Company Board has taken and will take all actions necessary to ensure that the restrictions applicable to business combinations contained
in Section 203 of the DGCL, to the extent applicable to the Company, are, and will be, inapplicable to the execution, delivery and
performance of this Agreement and to the consummation of the Contemplated Transactions. No other state takeover statute or similar Law
applies or purports to apply to the Merger, this Agreement or any of the Contemplated Transactions.
3.6 Capitalization.
(a) Section 3.6(a) of
the Company Disclosure Schedule sets forth an accurate and complete capitalization table of the Company as of the date of this Agreement.
The Company does not hold any shares of Company Capital Stock in its treasury.
(b) All
of the outstanding Company Capital Stock as set out in Section 3.6(a) of the Company Disclosure Schedule have been duly
authorized and validly issued, and are fully paid and nonassessable and are free of any Encumbrances other than Encumbrances set forth
in the Organizations Documents or under applicable securities Laws. None of the outstanding Company Capital Stock is entitled or subject
to any preemptive right, right of participation, right of maintenance or any similar right and none of the outstanding Company Capital
Stock is subject to any right of first refusal in favor of the Company. Except as contemplated herein, there is no Company Contract relating
to the voting or registration of, or restricting any Person from purchasing, selling, pledging or otherwise disposing of (or granting
any option or similar right with respect to), any Company Capital Stock. The Company is not under any obligation, nor is it bound by any
Contract pursuant to which it may become obligated, to repurchase, redeem or otherwise acquire any outstanding Company Capital Stock or
other securities. Section 3.6(b) of the Company Disclosure Schedule accurately and completely lists all repurchase rights
held by the Company with respect to Company Capital Stock (including shares issued pursuant to the exercise of options) and specifies
which of those repurchase rights are currently exercisable and whether the holder of such shares of Company Capital Stock timely filed
an election with the relevant Governmental Authority under Section 83(b) of the Code with respect to such shares.
(c) Except
for the Company’s 2021 Stock Option Plan (the “Company Option Plan”), the Company does not have any stock option
plan or any other plan, program, agreement or arrangement providing for any equity-based compensation for any Person. As of the date of
this Agreement, the Company has reserved 200,000 shares of Company Common Stock for issuance under the Company Option Plan, of which 118,050
shares have been issued and are currently outstanding, 75,750 shares have been reserved for issuance upon exercise of Company Options
previously granted and currently outstanding under the Company Option Plan, and 6,200 shares of Company Common Stock remain available
for future issuance of awards pursuant to the Company Option Plan. Section 3.6(c) of the Company Disclosure Schedule
sets forth the following information with respect to each Company Option outstanding as of the date of this Agreement: (i) the name
of the optionee; (ii) the number of shares of Company Common Stock subject to such Company Option at the time of grant; (iii) the
number of shares of Company Common Stock subject to such Company Option as of the date of this Agreement; (iv) the exercise price
of such Company Option; (v) the date on which such Company Option was granted; (vi) the applicable vesting schedule, including
the number of vested and unvested shares as of the date of this Agreement and any acceleration provisions; (vii) the date on which
such Company Option expires; and (viii) whether such Company Option is intended to constitute an “incentive stock option”
(as defined in the Code) or a non-qualified stock option. The Company has made available to Parent an accurate and complete copy of the
Company Option Plan and a form of stock option agreement that is consistent in all material respects with the stock option agreements
evidencing outstanding options granted thereunder.
(d) Except
for the Company Options set forth on Section 3.6(c) of the Company Disclosure Schedule, there is no: (i) outstanding
subscription, option, call, warrant or right (whether or not currently exercisable) to acquire any shares of common stock or other securities
of the Company, (ii) outstanding security, instrument or obligation that is or may become convertible into or exchangeable for any
shares of the capital stock or other securities of the Company, or (iii) condition or circumstance that could be reasonably likely
to give rise to or provide a basis for the assertion of a claim by any Person to the effect that such Person is entitled to acquire or
receive any shares of capital stock or other securities of the Company. There are no outstanding or authorized stock appreciation, phantom
stock, profit participation or other similar rights with respect to the Company.
(e) All
outstanding Company Common Stock, Company Preferred Stock, Company Options and other securities of the Company have been issued and granted
in material compliance with (i) all applicable securities Laws and other applicable Law and (ii) all requirements set forth
in applicable Contracts.
3.7 Financial
Statements.
(a) Section 3.7(a) of
the Company Disclosure Schedule includes true and complete copies of the Company Unaudited Balance Sheet, and the Company’s Audited
Financial Statements (collectively, the “Company Financials”). The Company Financials (A) were prepared in accordance
with United States generally accepted accounting principles (“GAAP”) (except that the Company Financials may not have
notes thereto and other presentation items that may be required by GAAP and are subject to normal and recurring year-end adjustments,
none of which is material) applied on a consistent basis unless otherwise noted therein throughout the periods indicated and (B) fairly
present, in all material respects, the financial position and operating results of the Company as of the dates and for the periods indicated
therein.
(b) The
Company maintains a system of internal accounting controls designed to provide reasonable assurance that: (i) transactions are executed
in accordance with management’s general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation
of the financial statements of the Company in conformity with GAAP and to maintain accountability of the Company’s assets, (iii) access
to the Company’s assets is permitted only in accordance with management’s general or specific authorization, (iv) the
recorded accountability for the Company’s assets is compared with the existing assets at regular intervals and appropriate action
is taken with respect to any differences, and (v) accounts, notes and other receivables and inventory are recorded accurately, and
proper and adequate procedures are implemented which are designed to effect the collection thereof on a current and timely basis. The
Company maintains internal controls consistent with the practices of similarly situated private companies over financial reporting that
provides reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external
purposes in accordance with GAAP.
(c) Section 3.7(c) of
the Company Disclosure Schedule lists, and the Company has delivered to Parent accurate and complete copies of the documentation creating
or governing, all securitization transactions and “off-balance sheet arrangements” (as defined in Item 303(c) of Regulation
S-K under the Exchange Act) effected by the Company.
(d) There
have been no formal internal investigations regarding financial reporting or accounting policies and practices discussed with, reviewed
by or initiated at the direction of the chief executive officer or chief financial officer of the Company, the Company Board or any committee
thereof. Neither the Company nor its independent auditors have identified (i) any significant deficiency or material weakness in
the design or operation of the system of internal accounting controls utilized by the Company, (ii) any fraud, whether or not material,
that involves the Company, the Company’s management or other employees who have a role in the preparation of financial statements
or the internal accounting controls utilized by the Company or (iii) any claim or allegation regarding any of the foregoing.
3.8 Absence
of Changes. Except as set forth on Section 3.8 of the Company Disclosure Schedule, after the date of the Company Unaudited
Balance Sheet, the Company has conducted its business only in the Ordinary Course of Business (except for the execution and performance
of this Agreement and the discussions, negotiations and transactions related thereto) and there has not been any (a) Company Material
Adverse Effect or (b) actions to do any of the following:
(a) declare,
accrue, set aside or pay any dividend or make any other distribution in respect of any shares of capital stock; or repurchase, redeem
or otherwise reacquire any shares of its capital stock or other securities (except for shares of Company Common Stock from terminated
employees, directors or consultants of the Company or in connection with the payment of the exercise price and/or withholding Taxes incurred
upon the exercise, settlement or vesting of any award granted under the Company Option Plan);
(b) except
as required to give effect to anything in contemplation of the Closing, amend any of its Organizational Documents, or effect or be a party
to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse
stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions;
(c) sell,
issue, grant, pledge or otherwise dispose of or encumber or authorize any of the foregoing actions with respect to: (A) any capital
stock or other security of the Company (except for Company Common Stock issued upon the valid exercise or settlement of outstanding Company
Options or Company Restricted Stock Units, as applicable), (B) any option, warrant or right to acquire any capital stock or any other
security, or (C) any instrument convertible into or exchangeable for any capital stock or other security of the Company;
(d) form
any Subsidiary or acquire any equity interest or other interest in any other Entity or enter into a joint venture with any other Entity;
(e) (A) lend
money to any Person, (B) incur or guarantee any indebtedness for borrowed money, or (C) guarantee any debt securities of others;
(f) other
than as required by applicable Law: (A) adopt, establish or enter into any new Employee Plan, including, for the avoidance of doubt,
any equity awards plans, (B) cause or permit any Company Employee Plan to be, (C) pay any bonus or make any profit-sharing or
similar payment to (except with respect to obligations in place on the date of this Agreement pursuant to any Company Employee Plan),
or increase the amount of the wages, salary, commissions, fringe benefits or other compensation or remuneration payable to, any of its
directors, officers, employees or consultants (D) grant any new equity awards or increase the coverage or benefits available under
any Company Employee Plan, (E) increase the severance or change of control benefits offered to any current or new employees, directors
or consultants, or (F) hire, terminate or give notice of termination (other than for cause) to any officer, employee or consultant;
(g) enter
into any material transaction outside the Ordinary Course of Business, other than in connection with the Contemplated Transactions;
(h) acquire
any material asset or sell, lease or otherwise irrevocably dispose of any of its assets or properties, or grant any Encumbrance (other
than Permitted Encumbrances) with respect to such assets or properties;
(i) sell,
assign, transfer, license, sublicense or otherwise dispose of any material Company IP Rights (other than pursuant to nonexclusive licenses
in the Ordinary Course of Business);
(j) make,
change or revoke any material Tax election; file any material amendment to any Tax Return; settle or compromise any material Tax claim;
waive or extend any statute of limitations in respect of a period within which an assessment or reassessment of material Taxes may be
issued (other than any extension pursuant to an extension to file any Tax Return); enter into any “closing agreement” as described
in Section 7121 of the Code (or any similar Law) with any Governmental Authority; or adopt or change any material accounting method
in respect of Taxes;
(k) initiate
or settle any Legal Proceeding;
(l) delay
or fail to repay when due any material obligation, including accounts payable and accrued expenses, other than in the Ordinary Course
of Business;
(m) forgive
any loans to any Person, including its employees, officers, directors or Affiliates;
(n) make
any expenditures, incur any Liabilities or discharge or satisfy any Liabilities, in each case, in amounts that exceed $100,000;
(o) terminate
or modify in any material respect, or fail to exercise renewal rights with respect to, any material insurance policy;
(p) enter
into, amend, terminate, or waive any material option or right under, any Company Material Contract;
(q) (A) materially
change pricing or royalties or other payments set or charged by the Company to its customers or licensees or (B) agree to materially
change pricing or royalties or other payments set or charged by Persons who have licensed Intellectual Property to the Company; or
(r) agree,
resolve or commit to do any of the foregoing.
3.9 Absence
of Undisclosed Liabilities. The Company does not have any liability, indebtedness, obligation, expense, claim, deficiency, guaranty
or endorsement of any kind, whether accrued, absolute, contingent, matured, unmatured or otherwise required to be reflected in the financial
statements in accordance with GAAP (each a “Liability”), except for: (a) Liabilities disclosed, reflected or reserved
against in the Company Financials or in the notes thereto, (b) Liabilities that have been incurred by the Company since the date
of the Company Unaudited Balance Sheet in the Ordinary Course of Business (none of which relates to any breach of contract, breach of
warranty, tort, infringement, or violation of Law), (c) Liabilities for performance of obligations of the Company under Company Contracts
in the Ordinary Course of Business, (d) Liabilities incurred in connection with the Contemplated Transactions and (f) Liabilities
listed in Section 3.9 of the Company Disclosure Schedule.
3.10 Title
to Assets. The Company owns, and has good and valid title to, or, in the case of leased properties and assets, valid leasehold interests
in, all tangible properties or tangible assets and equipment used or held for use in its business or operations or purported to be owned
by it, including: (a) all tangible assets reflected on the Company Unaudited Balance Sheet and (b) all other tangible assets
reflected in the books and records of the Company as being owned by the Company. All of such assets are owned or, in the case of leased
assets, leased by the Company free and clear of any Encumbrances, other than Permitted Encumbrances.
3.11 Real
Property; Leasehold. The Company does not own and has never owned any real property. The Company has made available to Parent (a) an
accurate and complete list of all real properties with respect to which the Company directly or indirectly holds a valid leasehold interest
as well as any other real estate that is in the possession of, or occupied or leased by, the Company and (b) copies of all leases
under which any such real property is possessed, occupied or leased (the “Company Real Estate Leases”), each of which
is in full force and effect, with no existing material default thereunder. Except as set forth on Section 3.11 of the Company
Disclosure Schedule, the Company’s possession, occupancy, lease, use and/or operation of each such leased property conforms to all
applicable Laws in all material respects, and the Company has exclusive possession of each such leased property and leasehold interest
and has not granted any occupancy rights to tenants or licensees with respect to such leased property or leasehold interest. In addition,
each such leased property and leasehold interest is free and clear of all Encumbrances other than Permitted Encumbrances. The Company
has not received any written notice from its landlords or any Governmental Body that: (i) relates to violations of building, zoning,
safety or fire ordinances or regulations; (ii) claims any defect or deficiency with respect to any of such properties; or (iii) requests
the performance of any repairs, alterations or other work to such properties.
3.12 Intellectual
Property.
(a) Section 3.12(a) of
the Company Disclosure Schedule is an accurate, true and complete listing of all Company Registered IP.
(b) Section 3.12(b) of
the Company Disclosure Schedule accurately identifies (i) all Company Contracts pursuant to which any material Company IP Rights
are licensed to the Company (other than (A) any non-customized software that (1) is so licensed solely in executable or object
code form pursuant to a nonexclusive, internal use software license and other Intellectual Property associated with such software and
(2) is not incorporated into, or material to the development, manufacturing, or distribution of, any of the Company’s products
or services, (B) any Intellectual Property licensed on a nonexclusive basis in the Ordinary Course of Business ancillary to the purchase
or use of equipment, reagents or other materials or that is otherwise not material to the Company’s business, (C) any confidential
information provided under confidentiality agreements and (D) agreements between Company and its employees in Company’s standard
form thereof ((A) through (D), “Non-Scheduled Company IP Contracts”) and (ii) whether the license or licenses
granted to the Company are exclusive or nonexclusive.
(c) Section 3.12(c) of
the Company Disclosure Schedule accurately identifies each Company Contract pursuant to which any Person has been granted by the Company
any license, sublicense or covenant not to sue under, or otherwise has received or acquired any right (whether or not currently exercisable)
or interest in, any material Company IP Rights (other than (i) any confidential information provided under confidentiality agreements
and (ii) any Company IP Rights nonexclusively licensed to academic collaborators, suppliers or service providers for the sole purpose
of enabling such academic collaborator, supplier or service providers to provide services for the Company’s benefit).
(d) The
Company is not bound by, and no Company IP Rights are subject to, any Contract containing any covenant or other provision that in any
way limits or restricts the ability of the Company to use, exploit, assert, or enforce any Company IP Rights anywhere in the world.
(e) The
Company solely and exclusively owns all right, title, and interest to and in Company IP Rights (other than Company IP Rights licensed
to the Company pursuant to a Company Contract set forth on Section 3.12(b) of the Company Disclosure Schedule or pursuant
to a Non-Scheduled Company IP Contract), in each case, free and clear of any Encumbrances (other than Permitted Encumbrances). Without
limiting the generality of the foregoing:
(i) All
documents and instruments necessary to register or apply for or renew registration of material Company Registered IP have been validly
executed, delivered, and filed in a timely manner with the appropriate Governmental Authority.
(ii) Each
Person who is or was an employee or contractor of the Company and who is or was involved in the creation or development of any material
Intellectual Property for the Company has signed a valid, enforceable agreement containing a present assignment of such Intellectual Property
to the Company and confidentiality provisions protecting trade secrets and confidential information of the Company.
(iii) To
the Knowledge of the Company, no current or former member, officer, director, or employee of the Company has any claim, right (whether
or not currently exercisable), or interest to or in any Company IP Rights purported to be owned by the Company. To the Knowledge of the
Company, no employee of the Company is (a) bound by or otherwise subject to any Contract restricting him or her from performing his
or her duties for the Company or (b) in breach of any Contract with any former employer or other Person concerning Company IP Rights
purported to be owned by the Company or confidentiality provisions protecting trade secrets and confidential information comprising such
Company IP Rights purported to be owned by the Company.
(iv) No
funding, facilities, or personnel of any Governmental Authority were used, directly or indirectly, to develop or create, in whole or in
part, any Company IP Rights in which the Company has an ownership interest, except for any such funding or use of facilities or personnel
that does not result in such Governmental Authority obtaining ownership rights to such Company IP Rights and does not require or otherwise
obligate the Company or its Subsidiaries to grant or offer to any such Governmental Authority or institution any material license or other
material right to such Company IP Right (except for non-commercial use rights during the term of the applicable agreement between the
Company or one of its Subsidiaries and such Governmental Authority) or the right to receive royalties for the practice of such Company
IP Right.
(v) The
Company has taken reasonable steps to maintain the confidentiality of and otherwise protect and enforce its rights in all proprietary
information that the Company holds, or purports to hold, as confidential or a trade secret.
(vi) The
Company has not assigned or otherwise transferred ownership of, or agreed to assign or otherwise transfer ownership of, any Company IP
Rights to any other Person.
(vii) (vii) To
the Knowledge of the Company, the Company IP Rights constitute all Intellectual Property necessary for the Company to conduct its business
as currently conducted; provided, however, that the foregoing representation is not a representation with respect to noninfringement
of Intellectual Property.
(f) The
Company has delivered or made available to Parent, a complete and accurate copy of all Company IP Rights Agreements that are required
to be listed on the Company Disclosure Schedule. With respect to each of the material Company IP Rights Agreements: (i) each such
agreement is valid and binding on the Company and in full force and effect, (ii) the Company has not received any written notice
of termination or cancellation under such agreement, or received any written notice of breach or default under such agreement, which breach
has not been cured or waived and (iii) the Company, and to the Knowledge of the Company, no other party to any such agreement, is
not in breach or default thereof in any material respect.
(g) The
manufacture, marketing, sale, offering for sale, importation, use or intended use or other disposal of any product or proposed product
as currently sold or under development by the Company does not violate any license or agreement between the Company and any other third
party in any material respect, and, to the Knowledge of the Company, does not infringe or misappropriate (and, when made commercially
available, will not infringe or misappropriate) any valid and issued Patent right or other Intellectual Property of any other Person,
which infringement or misappropriation would reasonably be expected to have a Company Material Adverse Effect. To the Knowledge of the
Company, no third party is infringing upon any Patents within the Company IP Rights or is violating any Company IP Rights Agreement.
(h) As
of the date of this Agreement, (i) the Company is not a party to any Legal Proceeding (including, but not limited to, opposition,
interference or other proceeding in any patent or other government office) contesting the validity, enforceability, ownership or right
to use, sell, offer for sale, license or dispose of any Company IP Rights, (ii) the Company has not received any written notice asserting
that any Company IP Rights or the proposed use, sale, offer for sale, license or disposition of any products, methods, or processes claimed
or covered thereunder infringes or misappropriates or violates the rights of any other Person or that the Company has otherwise infringed,
misappropriated or otherwise violated any Intellectual Property of any Person, and (iii) none of the Company IP Rights is subject
to any outstanding order of, judgment of, decree of or agreement with any Governmental Authority that limits the ability of the Company
to exploit any Company IP Rights.
(i) Each
item of material Company Registered IP (other than any Company Registered IP that the Company in its business judgment has elected to
abandon) is and at all times has been filed and maintained in compliance in all material respects with all applicable Law and all filings,
payments, and other actions required to be made or taken to maintain such item of Company Registered IP in full force and effect have
been made by the applicable deadline. To the Knowledge of the Company, all Company Registered IP that is issued or granted is valid and
enforceable.
(j) To
the Knowledge of the Company, no trademark (whether registered or unregistered) or trade name owned, used, or applied for by the Company
conflicts or interferes with any trademark (whether registered or unregistered) or trade name owned, used, or applied for by any other
Person, except as would not reasonably be expected to have a Company Material Adverse Effect. None of the goodwill associated with or
inherent in any trademark (whether registered or unregistered) in which the Company has or purports to have an ownership interest has
been impaired as determined by the Company in accordance with GAAP.
(k) Except
as set forth in the Company Contracts on Sections 3.12(b) or 3.12(c) of the Company Disclosure Schedule or as
contained in license, distribution or service agreements entered into in the Ordinary Course of Business by the Company, (i) the
Company is not bound by any Contract to indemnify, defend, hold harmless, or reimburse any other Person with respect to any Intellectual
Property infringement, misappropriation, or similar claim which is material to the Company, taken as a whole and (ii) the Company
has never assumed, or agreed to discharge or otherwise take responsibility for, any existing or potential liability of another Person
for infringement, misappropriation, or violation of any Intellectual Property right, which assumption, agreement or responsibility remains
in force as of the date of this Agreement.
(l) The
Company is not party to any Contract that, as a result of such execution, delivery and performance of this Agreement, will cause the grant
of any license or other right to any Company IP Rights, result in breach of, default under or termination of such Contract with respect
to any Company IP Rights, or impair the right of the Company or the Surviving Company and its Subsidiaries to use, sell or license or
enforce any Company IP Rights or portion thereof.
3.13 Agreements,
Contracts and Commitments.
(a) Section 3.13(a) of
the Company Disclosure Schedule lists the following Company Contracts in effect as of the date of this Agreement (each, a “Company
Material Contract” and collectively, the “Company Material Contracts”):
(i) each
Company Contract relating to any material bonus, deferred compensation, severance, incentive compensation, pension, profit-sharing or
retirement plans, or any other employee benefit plans or arrangements;
(ii) each
Company Contract requiring payments by the Company after the date of this Agreement in excess of $75,000 relating to the employment of,
or the performance of employment-related services by, any Person, including any employee, consultant or independent contractor, or Entity
providing employment related, consulting or independent contractor services, not terminable by the Company on ninety (90) calendar days’
or less notice without liability, except to the extent general principles of wrongful termination Law may limit the Company’s, or
such successor’s ability to terminate employees at will;
(iii) each
Company Contract relating to any agreement of indemnification or guaranty not entered into in the Ordinary Course of Business;
(iv) each
Company Contract containing (A) any covenant limiting the freedom of the Company or the Surviving Company to engage in any line of
business or compete with any Person, or limiting the development, manufacture, or distribution of the Company’s products or services
(B) any most-favored pricing arrangement, (C) any exclusivity provision or (D) any non-solicitation provision except for
Company Contracts with Persons that are employees or independent contractors;
(v) each
Company Contract (A) pursuant to which any Person granted the Company an exclusive license under any Intellectual Property, or (B) pursuant
to which the Company granted any Person an exclusive license under any Company IP Rights;
(vi) each
Company Contract relating to capital expenditures and requiring payments after the date of this Agreement in excess of $100,000 pursuant
to its express terms and not cancelable without penalty;
(vii) each
Company Contract relating to the disposition or acquisition of material assets or any ownership interest in any Entity;
(viii) each
Company Contract relating to any mortgages, indentures, loans, notes or credit agreements, security agreements or other agreements or
instruments relating to the borrowing of money or extension of credit in excess of $50,000 or creating any material Encumbrances with
respect to any assets of the Company or any loans or debt obligations with officers or directors of the Company;
(ix) each
Company Contract requiring payment by or to the Company after the date of this Agreement in excess of $50,000 pursuant to its express
terms relating to: (A) any distribution agreement (identifying any that contain exclusivity provisions), (B) any agreement involving
provision of services or products with respect to any pre-clinical or clinical development activities of the Company, (C) any dealer,
distributor, joint marketing, alliance, joint venture, cooperation, development or other agreement currently in force under which the
Company has continuing obligations to develop or market any product, technology or service, or any agreement pursuant to which the Company
has continuing obligations to develop any Intellectual Property that will not be owned, in whole or in part, by the Company or (D) any
Contract to license any patent, trademark registration, service mark registration, trade name or copyright registration to or from any
third party to manufacture or produce any product, service or technology of the Company or any Contract to sell, distribute or commercialize
any products or service of the Company, in each case, except for Company Contracts entered into in the Ordinary Course of Business;
(x) each
Company Contract with any Person, including any financial advisor, broker, finder, investment banker or other Person, providing advisory
services to the Company in connection with the Contemplated Transactions;
(xi) each
Company Contract with a Governmental Authority;
(xii) each
Company Contract to which the Company is a party or by which any of its assets and properties is currently bound, which involves annual
obligations of payment by, or annual payments to, the Company in excess of $50,000;
(xiii) a
Company Real Estate Lease; or
(xiv) any
other Company Contract that is not terminable at will (with no penalty or payment) by the Company, and (A) which involves payment
or receipt by the Company after the date of this Agreement under any such agreement, contract or commitment of more than $25,000 in the
aggregate, or obligations after the date of this Agreement in excess of $25,000 in the aggregate or (B) that is material to the business
or operations of the Company taken as a whole.
(b) The
Company has delivered or made available to Parent accurate and complete copies of all Company Material Contracts, including all amendments
thereto. There are no Company Material Contracts that are not in written form. The Company has not, nor to the Company’s Knowledge,
as of the date of this Agreement has any other party to a Company Material Contract, breached, violated or defaulted under, or received
notice that it breached, violated or defaulted under, any of the terms or conditions of any Company Material Contract in such manner as
would permit any other party to cancel or terminate any such Company Material Contract, or would permit any other party to seek damages
which would reasonably be expected to have a Company Material Adverse Effect. As to the Company, as of the date of this Agreement, each
Company Material Contract is valid, binding, enforceable and in full force and effect, subject to the Enforceability Exceptions. No Person
is renegotiating, or has a right pursuant to the terms of any Company Material Contract to change, any material amount paid or payable
to the Company under any Company Material Contract or any other material term or provision of any Company Material Contract, and no Person
has indicated in writing to the Company that it desires to renegotiate, modify, not renew or cancel any Company Material Contract.
3.14 Compliance;
Permits; Restrictions.
(a) The
Company is in material compliance with all applicable Laws. No investigation, claim, suit, proceeding, audit, Order, or other action by
any Governmental Authority is pending or, to the Knowledge of the Company, threatened against the Company. There is no agreement or Order
binding upon the Company which (i) has or would reasonably be expected to have the effect of prohibiting or materially impairing
any business practice of the Company, any acquisition of material property by the Company or the conduct of business by the Company as
currently conducted, (ii) is reasonably likely to have an adverse effect on the Company’s ability to comply with or perform
any covenant or obligation under this Agreement or (iii) is reasonably likely to have the effect of preventing, delaying, making
illegal or otherwise interfering with the Contemplated Transactions.
(b) The
Company holds all required Governmental Authorizations which are material to the operation of the business of the Company as currently
conducted (the “Company Permits”), including Governmental Authorizations related to the development, testing, manufacturing,
processing, storage, labeling, sale, marketing, advertising, distribution and importation or exportation, as currently conducted, of any
of its products or product candidates (the “Company Product Candidates”). Section 3.14(b) of the Company
Disclosure Schedule identifies each Company Permit. The Company has timely maintained and is in material compliance with the terms of
the Company Permits and no Company Permit has been (i) revoked, withdrawn, suspended, cancelled or terminated or (ii) modified
in any adverse manner, other than immaterial adverse modifications. No Legal Proceeding is pending or, to the Knowledge of the Company,
threatened, which seeks to revoke, substantially limit, suspend, or materially modify any Company Permit. The rights and benefits of each
Company Permit (other than INDs with the Company) will be available to the Surviving Company or its Subsidiaries, as applicable, immediately
after the Second Effective Time on terms substantially identical to those enjoyed by the Company as of the date of this Agreement and
immediately prior to the First Effective Time.
(c) There
are no Legal Proceedings pending or, to the Knowledge of the Company, threatened with respect to an alleged material violation by the
Company of the Federal Food, Drug, and Cosmetic Act (“FDCA”), the Public Health Service Act (“PHSA”),
Food and Drug Administration (“FDA”) regulations adopted thereunder, the Controlled Substances Act or any other similar
Law promulgated by the FDA or other comparable Governmental Authority responsible for regulation of the development, testing, manufacturing,
processing, storage, labeling, sale, marketing, advertising, distribution and importation or exportation of drug products (“Drug
Regulatory Agency”).
(d) The
Company has made available to Parent all information requested by Parent in the Company’s possession or control relating to the
Company Product Candidates and the development, testing, manufacturing, processing, storage, labeling, sale, marketing, advertising, distribution
and importation or exportation of the Company Product Candidates, including but not limited to complete copies of the following (to the
extent there are any): (x) adverse event reports; preclinical, clinical and other study reports and material study data; inspection
reports, notices of adverse findings, untitled letters, warning letters, filings and letters and other written correspondence to and from
any Drug Regulatory Agency; and meeting minutes with any Drug Regulatory Agency and (y) similar reports, material study data, notices,
letters, filings, correspondence and meeting minutes with any other Governmental Authority. All such information is accurate and complete
in all material respects.
(e) All
clinical, preclinical and other studies and tests conducted by or on behalf of, or sponsored by, the Company, or in which the Company
or its current products or product candidates, including the Company Product Candidates, have participated, were and, if still pending,
are being conducted in all material respects in accordance with standard medical and scientific research procedures and in compliance
in all material respects with the applicable regulations of the Drug Regulatory Agencies and other applicable Law, including 21 C.F.R.
Parts 50, 54, 56, 58 and 312. The Company has not received any written notices, correspondence, or other communications from any Drug
Regulatory Agency requiring, or to the Knowledge of the Company threatening to initiate, any action to place a clinical hold order on,
or otherwise terminate, delay, or suspend any clinical studies conducted by or on behalf of, or sponsored by, the Company or in which
the Company or its current products or product candidates, including the Company Product Candidates, have participated. Further, no clinical
investigator, researcher, or clinical staff participating in any clinical study conducted by or, to the Knowledge of the Company, on behalf
of the Company has been disqualified from participating in studies involving the Company Product Candidates, and to the Knowledge of the
Company, no such administrative action to disqualify such clinical investigators, researchers or clinical staff has been threatened or
is pending.
(f) The
Company is not, and to the Knowledge of the Company, no contract manufacturer with respect to any Company Product Candidate, is the subject
of any pending or, to the Knowledge of the Company, threatened investigation in respect of its business or products by the FDA pursuant
to its “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg.
46191 (September 10, 1991) and any amendments thereto. To the Knowledge of the Company, the Company has not, and no contract manufacturer
with respect to any Company Product Candidate has committed any acts, made any statement, or failed to make any statement, in each case
in respect of its business or products that would violate the FDA’s “Fraud, Untrue Statements of Material Facts, Bribery,
and Illegal Gratuities” Final Policy, and any amendments thereto. None of the Company, and to the Knowledge of the Company, any
contract manufacturer with respect to any Company Product Candidate, or any of their respective officers, employees or agents has been
convicted of any crime or engaged in any conduct that could result in a debarment or exclusion under (i) 21 U.S.C. Section 335a
or (ii) any similar applicable Law. To the Knowledge of the Company, no debarment or exclusionary claims, actions, proceedings or
investigations in respect of their business or products are pending or threatened against the Company, and to the Knowledge of the Company,
any contract manufacturer with respect to any Company Product Candidate, or any of their respective officers, employees or agents.
(g) All
manufacturing operations conducted by, or to the Knowledge of the Company, for the benefit of, the Company in connection with any Company
Product Candidate, since April 28, 2020, have been and are being conducted in compliance in all material respects with applicable
Laws, including the FDA’s standards for current good manufacturing practices, including applicable requirements contained in 21
C.F.R. Parts 210, 211, 600-680, and 1271, and the respective counterparts thereof promulgated by Governmental Authorities in countries
outside the United States.
(h) No
laboratory or manufacturing site owned by the Company, and to the Knowledge of the Company, no manufacturing site of a contract manufacturer
or laboratory, with respect to any Company Product Candidate, (i) is subject to a Drug Regulatory Agency shutdown or import or export
prohibition or (ii) has received any Form FDA 483, notice of violation, warning letter, untitled letter, or similar correspondence
or notice from the FDA or other Governmental Authority alleging or asserting noncompliance with any applicable Law, in each case, that
have not been complied with or closed to the satisfaction of the relevant Governmental Authority, and, to the Knowledge of the Company,
neither the FDA nor any other Governmental Authority is considering such action.
(i) The
Company has complied with all Laws relating to patient, medical or individual health information, including the Health Insurance Portability
and Accountability Act of 1996 and its implementing regulations promulgated thereunder, all as amended from time to time (collectively
“HIPAA”), including the standards for the privacy of Individually Identifiable Health Information at 45 C.F.R. Parts
160 and 164, Subparts A and E, the standards for the protection of Electronic Protected Health Information set forth at 45 C.F.R. Part 160
and 45 C.F.R. Part 164, Subpart A and Subpart C, the standards for transactions and code sets used in electronic transactions at
45 C.F.R. Part 160, Subpart A and Part 162, and the standards for Breach Notification for Unsecured Protected Health Information
at 45 C.F.R. Part 164, Subpart D, all as amended from time to time. The Company has entered into, where required, and is in compliance
in all material respects with the terms of all Business Associate (as defined in HIPAA) agreements (“Business Associate Agreements”)
to which the Company is a party or otherwise bound. The Company has created and maintained written policies and procedures to protect
the privacy of all Protected Health Information, has provided training to all employees and agents as required under HIPAA, and has implemented
security procedures, including physical, technical and administrative safeguards, to protect all personal information and Protected Health
Information stored or transmitted in electronic form. The Company has not received written notice from the Office for Civil Rights for
the U.S. Department of Health and Human Services or any other Governmental Authority of any allegation regarding its failure to comply
with HIPAA or any other federal or state law or regulation applicable to the protection of individually identifiable health information
or personally identifiable information. No successful Security Incident, Breach of Unsecured Protected Health Information, unpermitted
disclosure of Personal Health Information or breach of personally identifiable information under applicable Laws has occurred with respect
to information maintained or transmitted to the Company or an agent or third party subject to a Business Associate Agreement with the
Company. The Company is currently submitting, receiving and handling or is capable of submitting, receiving and handling transactions
in accordance with the Transactions and Code Sets Rule. All capitalized terms in this Section 3.14(i) not otherwise defined
in this Agreement shall have the meanings set forth under HIPAA.
3.15 Legal
Proceedings; Orders.
(a) There
is no pending Legal Proceeding and, to the Knowledge of the Company, no Person has threatened in writing to commence any Legal Proceeding:
(i) that involves the Company or any of the material assets owned or used by Company or (ii) that challenges, or that may have
the effect of preventing, delaying, making illegal or otherwise interfering with, the Contemplated Transactions.
(b) There
is no Order to which the Company, or any of the material assets owned or used by the Company, is subject. To the Knowledge of the Company,
no officer or employees of the Company is subject to any Order that prohibits such officer or employee from engaging in or continuing
any conduct, activity or practice relating to the business of the Company or to any material assets owned or used by the Company.
3.16 Tax
Matters.
(a) The
Company has timely filed all income Tax Returns and all other material Tax Returns that it was required to file under applicable Law.
All such Tax Returns were correct and complete in all material respects and have been prepared in material compliance with all applicable
Law. No claim has ever been made by a Governmental Authority in a jurisdiction where the Company does not file Tax Returns that the Company
is subject to taxation by that jurisdiction.
(b) All
material amounts of Taxes due and owing by (or on behalf of) the Company (whether or not shown on any Tax Return) have been timely paid.
The unpaid Taxes of the Company for periods (or portions thereof) ending on or prior to the date of the Company Unaudited Balance Sheet
do not materially exceed the accruals for current Taxes set forth on the Company Unaudited Balance Sheet. Since the date of the Company
Unaudited Balance Sheet, the Company has not incurred any material Liability for Taxes outside the Ordinary Course of Business or otherwise
inconsistent with past custom and practice.
(c) The
Company has withheld and paid to the appropriate Governmental Authority all material Taxes required to have been withheld and paid in
connection with any amounts paid or owing to any employee, independent contractor, creditor, stockholder or other third party.
(d) There
are no Encumbrances for material Taxes (other than Encumbrances described in clause (a) of the definition of “Permitted Encumbrances”)
upon any of the assets of the Company.
(e) No
deficiencies for a material amount of Taxes with respect to the Company have been claimed, proposed or assessed by any Governmental Authority
in writing that have not been timely paid in full. There are no pending (or, based on written notice, threatened) material audits, assessments,
examinations or other actions for or relating to any liability in respect of Taxes of the Company. The Company (or any of its predecessors)
has not waived any statute of limitations in respect of material Taxes or agreed to any extension of time with respect to a material Tax
assessment or deficiency.
(f) The
Company has not been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code in
the last five years.
(g) The
Company is not a party to any Tax allocation, Tax sharing or similar agreement (including indemnity arrangements), other than customary
indemnification provisions in commercial Contracts entered into in the Ordinary Course of Business with vendors, customers, lenders, or
landlords (an “Ordinary Course Agreement”).
(h) The
Company has never been a member of an affiliated group filing a consolidated U.S. federal income Tax Return (other than a group the common
parent of which is the Company). The Company has no material Liability for the Taxes of any Person (other than the Company) under Treasury
Regulations Section 1.1502-6 (or any similar provision of state, local, or foreign law), as a transferee or successor, by Contract
(other than an Ordinary Course Agreement) or otherwise.
(i) The
Company has not distributed stock of another Person, or has had its stock distributed by another Person, in a transaction that was purported
or intended to be governed in whole or in part by Section 355 of the Code or Section 361 of the Code.
(j) The
Company has not entered into any transaction identified as a “reportable transaction” for purposes of Treasury Regulations
Sections 1.6011-4(b)(2) or 301.6111-2(b)(2).
(k) The
Company will not be required to include any item of income in, or exclude any item of deduction from, taxable income for any taxable period
(or portion thereof) ending after the Closing Date as a result of any: (i) change in, or use of improper, method of accounting for
a taxable period ending on or prior to the Closing Date; (ii) “closing agreement” as described in Section 7121 of
the Code (or any corresponding or similar provision of state, local or foreign income Tax law) executed on or prior to the Closing Date;
(iii) installment sale or open transaction disposition made on or prior to the Closing Date; (iv) prepaid amount, advance payments
or deferred revenue received or accrued on or prior to the Closing Date other than in respect of such amounts reflected in the Company
Unaudited Balance Sheet or received in the Ordinary Course of Business since the date of the Company Unaudited Balance Sheet; or (v) intercompany
transaction or excess loss amount described in Treasury Regulations under Section 1502 of the Code (or any corresponding or similar
provision of state, local or foreign income Tax Law).
(l) The
Company has never had a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise had an office or fixed place
of business in a country other than the country in which it is organized.
(m) The
Company is, and has been since its date of incorporation, a C corporation for U.S. federal income tax purposes.
(n) The
Company is not aware of any facts and has not knowingly taken or agreed to take any action, in each case, that would reasonably be expected
to prevent or impede the Merger from qualifying as a “reorganization” within the meaning of Section 368(a)(1)(A) of
the Code.
3.17 Employee
and Labor Matters; Benefit Plans.
(a) Section 3.17(a) of
the Company Disclosure Schedule sets forth a true and complete listing of the employees and consultants of the Company as of the Closing
Date (each, respectively, a “Company Employee” or a “Company Consultant”), including, as applicable,
each such person’s name, job title(s) or function(s) (including board positions) and job location, current salary or wage,
and current status (as to full time or part time, exempt or nonexempt and temporary or leave status and as to classification as an employee,
consultant or independent contractor). The Company has delivered or made available to Parent a true and complete copy of each Company
Employee Plan and the employee handbook for the Company, if any, and all other employment policies, if any, currently applicable to any
Company Employee or Company Consultant.
(b) To
the Company’s Knowledge, no officer or executive of the Company has disclosed any plans to terminate his or her employment with
the Company or, following the Merger, with Parent.
(c) Except
as set forth in Section 3.17(c) of the Company Disclosure Schedule:
(i) The
Company has paid or made provision for payment of all salaries and wages, which became payable to any Company Employee prior to the Closing
Date and is in compliance in all material respects with all applicable Laws respecting employment and employment practices, terms and
conditions of employment, collective bargaining, immigration, wages, hours and benefits, non-discrimination in employment, workers’
compensation, including Title VII of the Civil Rights Act of 1964, the Age Discrimination in Employment Act of 1967, the Equal Employment
Opportunity Act of 1972, ERISA, the Equal Pay Act of 1963, the National Labor Relations Act of 1935, the Fair Labor Standards Act of 1938,
the Americans with Disabilities Act of 1990, the Vietnam Era Veterans’ Reemployment Act, the Family and Medical Leave Act of 1993,
the Occupational Safety and Health Act and any and all similar applicable state and local Laws;
(ii) the
Company has not received a notice, citation, complaint or charge asserting any violation or liability under the Occupational Safety and
Health Act or any similar applicable Law regulating employee health and safety;
(iii) (a) no
Company Employee is represented by any labor union or other labor representative with respect to his or her employment with the Company;
(b) the Company is not bound by any labor, collective bargaining agreement or similar arrangement; (c) to the Company’s
Knowledge, no petition has been filed nor has any proceeding been instituted by any employee or group of employees with the National Labor
Relations Board or similar Governmental Authority seeking recognition of a collective bargaining agreement; (d) to the Company’s
Knowledge, there are no Persons attempting to represent or organize or purporting to represent for bargaining purposes any employees of
the Company; and (e) since January 1, 2021, there has not occurred and, to the Company’s Knowledge, there has not been
threatened any strikes, slowdowns, picketing, work stoppages or concerted refusals to work or other similar labor activities with
respect to employees of the Company;
(iv) the
Company has not received notice of any charge or complaint pending before the Equal Employment Opportunity Commission or similar Governmental
Authority alleging unlawful discrimination in employment practices, or before the National Labor Relations Board or similar Governmental
Authority alleging any unfair labor practice, by the Company, nor, to the Knowledge of the Company, has any such charge been threatened;
(v) all
Company Employees are employed on an at-will basis and their employment can be terminated at any time for any reason without any amounts
being owed to such individual other than with respect to wages, compensation and benefits accrued before such termination; and all Company
Consultants can be terminated at any time for any reason without notice or any amounts being owed to such individual other than with respect
to compensation or payments accrued before such termination;
(vi) since
January 1, 2023, the Company has not effectuated a “plant closing” or a “mass layoff” (as such terms are
defined in the WARN Act); and
(vii) to
the Company’s Knowledge, any individual performing services for the Company who has been classified as an independent contractor
has been correctly classified as such;
(viii) the
Company (a) has withheld and reported all material amounts required by Law or by agreement to be withheld and reported with respect
to wages, salaries and other payments to employees, and (b) is not liable for any material payment to any trust or other fund governed
by or maintained by or on behalf of any Governmental Authority, with respect to unemployment compensation benefits, social security or
other benefits or obligations for employees (other than routine payments to be made in the Ordinary Course of Business); and
(ix) no
current or former employee of the Company has, since January 1, 2021, asserted any legal claims either orally or in writing to the
Company concerning violations of any of the following Laws or regulations: labor relations, equal employment opportunities, fair employment
practices, employment discrimination, harassment, retaliation, reasonable accommodation, disability rights or benefits, immigration, wages,
hours, overtime compensation, child labor, hiring, promotion and termination of employees, working conditions, meal and break periods,
privacy, health and safety, workers’ compensation, leaves of absence and unemployment insurance.
(d) Each
Company Employee Plan that is intended to be qualified under Section 401(a) of the Code has received a favorable determination
or opinion letter with respect to such qualified status from the IRS. To the Knowledge of the Company, nothing has occurred that would
reasonably be expected to adversely affect the qualified status of any such Company Employee Plan or the exempt status of any related
trust.
(e) Each
Company Employee Plan has been established, maintained and operated in compliance, in all material respects, with its terms all applicable
Law, including, without limitation, the Code, ERISA and the Affordable Care Act. No Legal Proceeding (other than those relating to routine
claims for benefits) is pending or, to the Knowledge of the Company, threatened with respect to any Company Employee Plan. All payments
and/or contributions required to have been made with respect to all Company Employee Plans either have been made or have been accrued
in accordance with the terms of the applicable Company Employee Plan and applicable Law.
(f) Neither
the Company nor any of its ERISA Affiliates maintains, contributes to or is required to contribute to, or has, in the past six (6) years,
maintained, contributed to, or been required to contribute to (i) any “employee benefit plan” that is or was subject
to Title IV or Section 302 of ERISA or Section 412 of the Code, (ii) a Multiemployer Plan, (iii) any funded welfare
benefit plan within the meaning of Section 419 of the Code, (iv) any Multiple Employer Plan, or (v) any Multiple Employer
Welfare Arrangement. Neither the Company nor any of its ERISA Affiliates has ever incurred any liability under Title IV of ERISA.
(g) No
Company Employee Plan provides for medical or other welfare benefits to any service provider beyond termination of service or retirement,
other than (1) pursuant to COBRA or an analogous state law requirement or (2) continuation coverage through the end of the month
in which such termination or retirement occurs. The Company does not sponsor or maintain any self-funded medical or long-term disability
benefit plan.
(h) Except
as otherwise provided in this Agreement or under applicable Law, neither the execution and delivery of this Agreement nor the consummation
of the Merger shall result in (i) any payment becoming due to any Company Employee or Company Consultant, (ii) the provision
of any benefits or other rights to any Company Employee or Company Consultant, (iii) the increase, acceleration or provision of any
payments, benefits or other rights to any Company Employee or Company Consultant, whether or not any such payment, right or benefit would
constitute a “parachute payment” within the meaning of Section 280G of the Code, (iv) require any contributions
or payments to fund any obligations under any Company Employee Plan, or (v) the forgiveness in whole or in part of any outstanding
loans made by the Company to any Company Employee or Company Consultant. No payment, right or benefit that becomes due or accelerated
as a result of the execution and delivery of this Agreement or the consummation of the Merger is an “excess parachute payment”
within the meaning of Section 280G of the Code with respect to any current or former employee of the Company.
(i) Each
Company Employee Plan that constitutes in any part a nonqualified deferred compensation plan within the meaning of Section 409A of
the Code has been operated and maintained in all material respects in operational and documentary compliance with Section 409A of
the Code and applicable guidance thereunder.
(j) No
Company Employee Plan is subject to the laws of any jurisdiction other than the United States of America.
3.18 Environmental
Matters. Since December 31, 2020, the Company has complied with all applicable Environmental Laws, which compliance includes
the possession by the Company of all permits and other Governmental Authorizations required under applicable Environmental Laws and compliance
with the terms and conditions thereof, except for any failure to be in compliance that, individually or in the aggregate, would not result
in a Company Material Adverse Effect. The Company has not received since December 31, 2020, any written notice or other communication
(in writing or otherwise), whether from a Governmental Authority, citizens group, employee or otherwise, that alleges that the Company
is not in compliance with any Environmental Law and, to the Knowledge of the Company, there are no circumstances that may prevent or interfere
with the Company’s compliance with any Environmental Law in the future, except where such failure to comply would not reasonably
be expected to have a Company Material Adverse Effect. To the Knowledge of the Company: (i) no current or prior owner of any property
leased or controlled by the Company has received since December 31, 2020, any written notice or other communication relating to property
owned or leased at any time by the Company, whether from a Governmental Authority, citizens group, employee or otherwise, that alleges
that such current or prior owner or the Company is not in compliance with or violated any Environmental Law relating to such property
and (ii) the Company has no material liability under any Environmental Law.
3.19 Insurance.
The Company has delivered to or made available to Parent copies of all material insurance policies and all material self-insurance programs
and arrangements relating to the business, assets, liabilities and operations of the Company. Each of such insurance policies is in full
force and effect and the Company is in compliance in all material respects with the terms thereof. Other than customary end of policy
notifications from insurance carriers, since December 31, 2020, the Company has not received any notice or other communication regarding
any actual or possible: (i) cancellation or invalidation of any insurance policy or (ii) refusal or denial of any coverage,
reservation of rights or rejection of any material claim under any insurance policy. The Company has provided timely written notice to
the appropriate insurance carrier(s) of each Legal Proceeding pending against the Company, and no such carrier has issued a denial
of coverage or a reservation of rights with respect to any such Legal Proceeding, or informed the Company of its intent to do so.
3.20 No
Financial Advisors. No broker, finder or investment banker is entitled to any brokerage fee, finder’s fee, opinion fee, success
fee, transaction fee or other fee or commission in connection with the Contemplated Transactions based upon arrangements made by or on
behalf of the Company.
3.21 Transactions
with Affiliates. Section 3.21 of the Company Disclosure Schedule describes any material agreements between, on one hand,
the Company and, on the other hand, any (a) executive officer or director of the Company or any of such executive officer’s
or director’s immediate family members (other than with respect to wages or benefits payable in the Ordinary Course of Business),
(b) owner of more than five percent (5%) of the voting power of the outstanding Company Capital Stock or (c) to the Knowledge
of the Company, any “related person” (within the meaning of Item 404 of Regulation S-K under the Securities Act) of any such
officer, director or owner (other than the Company) in the case of each of (a), (b) or (c) that is of the type that would be
required to be disclosed under Item 404 of Regulation S-K under the Securities Act.
3.22 Privacy
and Data Security. The Company has complied in all material respects with all applicable Privacy Laws and the applicable terms of
any Company Contracts relating to privacy, security, collection or use of Personal Information of any individuals (including clinical
trial participants, patients, patient family members, caregivers or advocates, physicians and other health care professionals, clinical
trial investigators, researchers, pharmacists) that interact with the Company in connection with the operation of the Company’s
business, except for such noncompliance as has not had, and would not reasonably be expected to have, individually or in the aggregate,
a Company Material Adverse Effect. To the Knowledge of the Company, the Company has implemented and maintains reasonable written policies
and procedures, satisfying the requirements of applicable Privacy Laws, concerning the privacy, security, collection and use of Personal
Information (the “Privacy Policies”) and has complied with the same, except for such noncompliance as has not to the
Knowledge of the Company had, and would not reasonably be expected to have, individually or in the aggregate, a Company Material Adverse
Effect. To the Knowledge of the Company, as of the date hereof, no claims have been asserted or threatened against the Company by any
Person alleging a violation of Privacy Laws, Privacy Policies and/or the applicable terms of any Company Contracts relating to privacy,
security, collection or use of Personal Information of any individuals. To the Knowledge of the Company, there have been no data security
incidents, personal data breaches or other adverse events or incidents related to Personal Information or Company data in the custody
or control of the Company or any service provider acting on behalf of the Company, in each case where such incident, breach or event would
result in a notification obligation to any Person under applicable law or pursuant to the terms of any Company Contract.
3.23 Accredited
Investors. Except as provided on Section 3.23 of the Company Disclosure Schedule, each holder of any Company Capital Stock as
of immediately prior to the First Effective time is an accredited investor, as that term is defined in Regulation D promulgated by the
SEC.
3.24 No
Other Representations or Warranties.
(a) Except
for the representations and warranties expressly set forth in this Section 3 or in any certificate delivered by the Company
to Parent or Merger Subs pursuant to this Agreement, neither the Company, nor any of its Affiliates or Representatives, nor any other
Person makes any representation or warranty, express or implied, at Law or in equity, with respect to the Company or any of the Company’s
assets, liabilities or operations, and any such other representations or warranties are hereby expressly disclaimed.
(b) The
Company acknowledges and agrees that, except for the representations and warranties of Parent and Merger Subs set forth in Section 4
or in any certificate delivered by Parent or Merger Subs to the Company pursuant to this Agreement, neither the Company nor any of its
Affiliates or Representatives is relying on any other representation or warranty of Parent, Merger Subs, any of their Affiliates or Representatives
or any other Person made outside of Section 4 or such certificate, including regarding the accuracy or completeness of any
such other representations or warranties or the omission of any material information, whether express or implied.
Section 4. Representations
and Warranties of Parent and Merger Subs.
Except (i) as set forth
in the written disclosure schedule delivered by Parent to the Company (the “Parent Disclosure Schedule”) or (ii) as
disclosed in the Parent SEC Documents filed with the SEC prior to the date hereof and publicly available on the SEC’s Electronic
Data Gathering Analysis and Retrieval system (“EDGAR”) (but (A) without giving effect to any amendment thereof
filed with, or furnished to the SEC on or after the date hereof and (B) excluding any disclosures contained under the heading “Risk
Factors” and any disclosure of risks included in any “forward-looking statements” disclaimer or in any other section
to the extent they are forward-looking statements or cautionary, predictive or forward-looking in nature), Parent and Merger Subs represent
and warrant to the Company as follows:
4.1 Due
Organization; Subsidiaries.
(a) Each
of Parent and its Subsidiaries (including Merger Subs) is a corporation or limited liability company duly incorporated or formed, validly
existing and in good standing under the Laws of the jurisdiction of its incorporation or organization and has all necessary corporate
power and authority: (i) to conduct its business in the manner in which its business is currently being conducted, (ii) to own
or lease and use its property and assets in the manner in which its property and assets are currently owned or leased and used and (iii) to
perform its obligations under all Contracts by which it is bound. Since the date of their formation, Merger Subs have not engaged in any
activities other than in connection with or as contemplated by this Agreement. All of Parent’s Subsidiaries are wholly owned by
Parent.
(b) Each
of Parent and its Subsidiaries is licensed and qualified to do business, and is in good standing (to the extent applicable in such jurisdiction),
under the Laws of all jurisdictions where the nature of its business in the manner in which its business is currently being conducted
requires such licensing or qualification other than in jurisdictions where the failure to be so qualified individually or in the aggregate
would not be reasonably expected to have a Parent Material Adverse Effect.
(c) Except
as set forth on Section 4.1(c) of the Parent Disclosure Schedule, Parent has no Subsidiaries other than Merger Subs and
Parent does not own any capital stock of, or any equity ownership or profit sharing interest of any nature in, or control directly or
indirectly, any other Entity other than Merger Subs. Parent is not and has not otherwise been, directly or indirectly, a party to, member
of or participant in any partnership, joint venture or similar business entity. Parent has not agreed and is not obligated to make, nor
is Parent bound by any Contract under which it may become obligated to make, any future investment in or capital contribution to any other
Entity. Parent has not, at any time, been a general partner of, and has not otherwise been liable for any of the debts or other obligations
of, any general partnership, limited partnership or other Entity.
4.2 Organizational
Documents. Accurate and complete copies of Parent’s Organizational Documents in effect as of the date of this Agreement are
disclosed in the Parent SEC Documents filed with the SEC prior to the date of this Agreement and publicly available on EDGAR. Parent is
not in breach or violation of its Organizational Documents in any material respect.
4.3 Authority;
Binding Nature of Agreement. Each of Parent and Merger Subs has all necessary corporate power and authority to enter into and to perform
its obligations under this Agreement and subject, with respect to Parent, to receipt of the approval of the Parent Stockholder Matters
and, with respect to Merger Subs, to the adoption of this Agreement by Parent in its capacity as sole stockholder or sole member of Merger
Subs, to consummate the Contemplated Transactions. The Parent Board (at meetings duly called and held) has: (a) determined that the
Contemplated Transactions are fair to, advisable and in the best interests of Parent and its stockholders, (b) approved and declared
advisable this Agreement and the Contemplated Transactions, including the issuance of shares of Parent Capital Stock to the stockholders
of the Company pursuant to the terms of this Agreement and (c) determined to recommend, upon the terms and subject to the conditions
set forth in this Agreement, that the stockholders of Parent vote to approve the Parent Stockholder Matters pursuant to the terms of this
Agreement. The First Merger Sub Board (by unanimous written consent) has: (a) determined that the Contemplated Transactions are fair
to, advisable, and in the best interests of First Merger Sub and its sole stockholder, (b) deemed advisable and approved this Agreement
and the Contemplated Transactions and (c) determined to recommend, upon the terms and subject to the conditions set forth in this
Agreement, that the sole stockholder of First Merger Sub vote to adopt this Agreement and thereby approve the Contemplated Transactions.
The Second Merger Sub Board (by unanimous written consent) has: (a) determined that the Contemplated Transactions are fair to, advisable,
and in the best interests of Second Merger Sub and its sole member, (b) deemed advisable and approved this Agreement and the Contemplated
Transactions and (c) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the
sole member of Second Merger Sub vote to adopt this Agreement and thereby approve the Contemplated Transactions. This Agreement has been
duly executed and delivered by Parent and Merger Subs and, assuming the due authorization, execution and delivery by the Company, constitutes
the legal, valid and binding obligation of Parent and Merger Subs, enforceable against each of Parent and Merger Subs in accordance with
its terms, subject to the Enforceability Exceptions.
4.4 Vote
Required. The affirmative vote of a majority of the votes cast at the Parent Stockholder Meeting by the holders of Parent Common Stock
is the only vote of the holders of any class or series of Parent Capital Stock necessary to approve the Conversion Proposal. The affirmative
vote of a majority of shares of Parent Common Stock entitled to vote thereon is the only vote of the holders of any class or series of
Parent Capital Stock necessary to approve the Charter Amendment Proposal.
4.5 Non-Contravention;
Consents.
(a) Subject
to obtaining approval of the Parent Stockholder Matters, the filing of the First Certificate of Merger and Second Certificate of Merger
required by the DGCL and DLLCA and the filing of the Certificate of Designation, neither (x) the execution, delivery or performance
of this Agreement by Parent or Merger Subs, nor (y) the consummation of the Contemplated Transactions, will directly or indirectly
(with or without notice or lapse of time):
(i) contravene,
conflict with or result in a violation of any of the provisions of the Organizational Documents of Parent or Merger Subs;
(ii) contravene,
conflict with or result in a material violation of, or give any Governmental Authority or other Person the right to challenge the Contemplated
Transactions or to exercise any remedy or obtain any relief under, any Law or any Order to which Parent or Merger Subs or any of the assets
owned or used by Parent or Merger Subs, is subject;
(iii) contravene,
conflict with or result in a material violation of any of the terms or requirements of, or give any Governmental Authority the right to
revoke, withdraw, suspend, cancel, terminate or modify, any Governmental Authorization that is held by Parent or Merger Subs or that otherwise
relates to the business of Parent, or any of the assets owned, leased or used by Parent, except as would not reasonably be expected to
be material to Parent or it business;
(iv) contravene,
conflict with or result in a violation or breach of, or result in a default under, any provision of any Parent Material Contract, or give
any Person the right to: (A) declare a default or exercise any remedy under any Parent Material Contract, (B) any material payment,
rebate, chargeback, penalty or change in delivery schedule under any such Parent Material Contract, (C) accelerate the maturity or
performance of any Parent Material Contract or (D) cancel, terminate or modify any term of any Parent Material Contract, except in
the case of any nonmaterial breach, default, penalty or modification; or
(v) result
in the imposition or creation of any Encumbrance upon or with respect to any asset owned or used by Parent or its Subsidiaries (except
for Permitted Encumbrances).
(b) Except
for (i) any Consent set forth on Section 4.5 of the Parent Disclosure Schedule under any Parent Contract, (ii) the
approval of the Parent Stockholder Matters, (iii) the filing of the First Certificate of Merger and Second Certificate of Merger
with the Secretary of State of the State of Delaware pursuant to the DGCL and DLLCA, (iv) the filing of the Certificate of Designation
with the Secretary of State of the State of Delaware pursuant to the DGCL and (v) such consents, waivers, approvals, orders, authorizations,
registrations, declarations and filings as may be required under applicable federal and state securities Laws, neither Parent nor any
of its Subsidiaries was, is or will be required to make any filing with or give any notice to, or to obtain any Consent from, any Person
in connection with (x) the execution, delivery or performance of this Agreement or (y) the consummation of the Contemplated
Transactions.
(c) The
Parent Board, the First Merger Sub Board and the Second Merger Sub Board have taken and will take all actions necessary to ensure that
the restrictions applicable to business combinations contained in Section 203 of the DGCL are, and will be, inapplicable to the execution,
delivery and performance of this Agreement and to the consummation of the Contemplated Transactions. No other state takeover statute or
similar Law applies or purports to apply to the Merger, this Agreement or any of the other Contemplated Transactions.
4.6 Capitalization.
(a) The
authorized capital stock of Parent consists of (i) 100,000,000 shares of Parent Common Stock, par value $0.0001 of which 1,835,914
shares have been issued and are outstanding as of March 9, 2024 (the “Capitalization Date”) and (ii) 10,000,000
shares of Parent Preferred Stock, consisting of, as of the Capitalization Date, (A) 5,194.81 shares of Parent Series B Preferred
Stock, 521.72 of which are issued and outstanding, (B) 75,000 shares of Parent Series C Preferred Stock, none of which are issued
and outstanding, (C) 150 shares of Parent Series D Preferred Stock, none of which are issued and outstanding, (D) 150 shares
of Parent Series E Preferred Stock, none of which are issued and outstanding, and (E) 7,000 shares of Parent Series F Preferred
Stock, none of which are issued and outstanding.
(b) All
of the outstanding shares of Parent Capital Stock have been duly authorized and validly issued, and are fully paid and nonassessable.
None of the outstanding shares of Parent Capital Stock is entitled or subject to any preemptive right, right of participation, right of
maintenance or any similar right. None of the outstanding shares of Parent Capital Stock is subject to any right of first refusal in favor
of Parent. Except as contemplated herein, there is no Parent Contract relating to the voting or registration of, or restricting any Person
from purchasing, selling, pledging or otherwise disposing of (or granting any option or similar right with respect to), any shares of
Parent Capital Stock. Parent is not under any obligation, nor is Parent bound by any Contract pursuant to which it may become obligated,
to repurchase, redeem or otherwise acquire any outstanding shares of Parent Capital Stock or other securities. Section 4.6(b) of
the Parent Disclosure Schedule accurately and completely lists all repurchase rights held by Parent with respect to shares of Parent Capital
Stock (including shares issued pursuant to the exercise of stock options) and specifies which of those repurchase rights are currently
exercisable.
(c) Except
for the Amended and Restated 2014 Omnibus Equity Incentive Plan (the “Parent 2014 Plan”) and the 2020 Omnibus Equity
Incentive Plan, as amended (the “Parent 2020 Plan” and, together with the Parent 2014 Plan, the “Parent Stock
Plans”), and except as set forth on Section 4.6(c) of the Parent Disclosure Schedule, Parent does not have
any stock option plan or any other plan, program, agreement or arrangement providing for any equity-based compensation for any Person.
As of the date of this Agreement, Parent has reserved 334,078 shares of Parent Common Stock for issuance under the Parent Stock Plans,
of which 1,562,814 shares have been issued and are currently outstanding, 178,874 shares have been reserved for issuance upon exercise
or settlement of Parent Options and Parent Restricted Stock Units, as applicable, granted under the Parent Stock Plans, and 155,204 shares
remain available for future issuance pursuant to the Parent Stock Plans. Section 4.6(c) of the Parent Disclosure Schedule
sets forth the following information with respect to each Parent Option and Parent Restricted Stock Unit outstanding as of the date of
this Agreement, as applicable: (i) the name of the holder, (ii) the number of shares of Parent Common Stock subject to such
Parent Option and Parent Restricted Stock Units at the time of grant, (iii) the number of shares of Parent Common Stock subject to
such Parent Option and Parent Restricted Stock Units as of the date of this Agreement, (iv) the exercise price of such Parent Option,
(v) the date on which such Parent Option and Parent Restricted Stock Units was granted, (vi) the applicable vesting schedule,
including any acceleration provisions and the number of vested and unvested shares as of the date of this Agreement, and (vii) whether
such Parent Option is intended to be an “incentive stock option” (as defined in the Code) or a nonqualified stock option.
Accurate and complete copies of the Parent Stock Plans are disclosed in the Parent SEC Documents filed with the SEC prior to the date
of this Agreement and publicly available on EDGAR.
(d) Except
for the outstanding Parent Options and Parent Restricted Stock Units or as set forth on Section 4.6(d) of the Parent
Disclosure Schedule, there is no: (i) outstanding subscription, option, call, warrant or right (whether or not currently exercisable)
to acquire any shares of the capital stock or other securities of Parent, (ii) outstanding security, instrument or obligation that
is or may become convertible into or exchangeable for any shares of the capital stock or other securities of Parent, (iii) stockholder
rights plan (or similar plan commonly referred to as a “poison pill”) or Contract under which Parent is or may become obligated
to sell or otherwise issue any shares of its capital stock or any other securities or (iv) condition or circumstance that may give
rise to or provide a basis for the assertion of a claim by any Person to the effect that such Person is entitled to acquire or receive
any shares of capital stock or other securities of Parent. There are no outstanding or authorized stock appreciation, phantom stock, profit
participation or other similar rights with respect to Parent.
(e) All
outstanding shares of Parent Common Stock, Parent Options, Parent Restricted Stock Units and other securities of Parent have been issued
and granted in material compliance with (i) all applicable securities laws and other applicable Law and (ii) all requirements
set forth in applicable Contracts.
(f) All
distributions, dividends, repurchases and redemptions of Parent Common Stock or other equity interests of Parent were undertaken in material
compliance with (i) the Organizational Documents of Parent in effect as of the relevant time and all applicable securities Laws and
other applicable Laws, and (ii) all requirements set forth in applicable Contracts.
4.7 SEC
Filings; Financial Statements.
(a) Parent
has filed or furnished, as applicable, on a timely basis all forms, statements, Certifications (as defined below), reports and documents
required to be filed or furnished by it with the SEC under the Exchange Act or the Securities Act since January 1, 2022 (the “Parent
SEC Documents”). As of the time it was filed with the SEC, or, if amended or superseded by a filing prior to the date of this
Agreement, then on the date of such filing (and, in the case of registration statements, on the dates of effectiveness), each of the Parent
SEC Documents complied in all material respects with the applicable requirements of the Securities Act or the Exchange Act (as the case
may be) and as of the time they were filed, none of the Parent SEC Documents contained any untrue statement of a material fact or omitted
to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances
under which they were made, not misleading. The certifications and statements required by (i) Rule 13a-14 under the Exchange
Act and (ii) 18 U.S.C. §1350 (Section 906 of the Sarbanes-Oxley Act) relating to the Parent SEC Documents (collectively,
the “Certifications”) are accurate and complete and comply as to form and content with all applicable Laws. As used
in this Section 4.7, the term “file” and variations thereof shall be broadly construed to include any manner in
which a document or information is furnished, supplied or otherwise made available to the SEC.
(b) The
financial statements (including any related notes) contained or incorporated by reference in the Parent SEC Documents: (i) complied
as to form in all material respects with the Securities Act and the Exchange Act, as applicable, and the published rules and regulations
of the SEC applicable thereto, (ii) were prepared in accordance with GAAP (except as may be indicated in the notes to such financial
statements or, in the case of unaudited financial statements, as permitted by Form 10-Q of the SEC, and except that the unaudited
financial statements may not contain footnotes and are subject to normal and recurring year-end adjustments) applied on a consistent basis
unless otherwise noted therein throughout the periods indicated and (iii) fairly present, in all material respects, the financial
position of Parent as of the respective dates thereof and the results of operations and cash flows of Parent for the periods covered thereby.
Other than as expressly disclosed in the Parent SEC Documents filed prior to the date hereof, there has been no material change in Parent’s
accounting methods or principles that would be required to be disclosed in Parent’s financial statements in accordance with GAAP.
(c) Parent’s
independent registered public accounting firm has at all times since the date of enactment of the Sarbanes-Oxley Act been: (i) a
registered public accounting firm (as defined in Section 2(a)(12) of the Sarbanes-Oxley Act), (ii) to the Knowledge of Parent,
“independent” with respect to Parent within the meaning of Regulation S-X under the Exchange Act and (iii) to the Knowledge
of Parent, in compliance with subsections (g) through (l) of Section 10A of the Exchange Act and the rules and regulations
promulgated by the SEC and the Public Company Accounting Oversight Board thereunder.
(d) Since
January 1, 2022, Parent has not received any comment letter from the SEC or the staff thereof or any correspondence from Nasdaq or
the staff thereof relating to the delisting or maintenance of listing of the Parent Common Stock on Nasdaq. Parent has not disclosed any
unresolved comments in the Parent SEC Documents.
(e) Since
January 1, 2022, there have been no formal internal investigations regarding financial reporting or accounting policies and practices
discussed with, reviewed by or initiated at the direction of the chief executive officer or chief financial officer of Parent, the Parent
Board or any committee thereof, other than ordinary course audits or reviews of accounting policies and practices or internal controls
required by the Sarbanes-Oxley Act.
(f) Parent
is in compliance in all material respects with the applicable provisions of the Sarbanes-Oxley Act, the Exchange Act and the applicable
listing and governance rules and regulations of Nasdaq.
(g) Parent
maintains a system of internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange
Act) that is sufficient to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial
statements for external purposes in accordance with GAAP. Parent has disclosed to Parent’s auditors and the Audit Committee of the
Parent Board (and made available to the Company a summary of the significant aspects of such disclosure) (A) all significant deficiencies
and material weaknesses in the design or operation of internal control over financial reporting that are reasonably likely to adversely
affect Parent’s ability to record, process, summarize and report financial information and (B) any fraud, whether or not material,
that involves management or other employees who have a significant role in Parent’s or its Subsidiaries’ internal control
over financial reporting. Except as disclosed in the Parent SEC Documents filed prior to the date hereof, Parent’s internal control
over financial reporting is effective and Parent has not identified any material weaknesses in the design or operation of Parent’s
internal control over financial reporting.
(h) Parent
maintains “disclosure controls and procedures” (as defined in Rules 13-15(e) and 15d-15(e) of the Exchange
Act) that are reasonably designed to ensure that all information required to be disclosed by Parent in the reports that it files or submits
under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of
the SEC, and that all such information is accumulated and communicated to Parent’s principal executive officer and principal financial
officer as appropriate to allow timely decisions regarding required disclosure.
(i) Parent
has not been and is not currently a “shell company” as defined under Section 12b-2 of the Exchange Act.
4.8 Absence
of Changes. Except as set forth on Section 4.8 of the Parent Disclosure Schedule, after the date of the Parent Unaudited
Balance Sheet, Parent has conducted its business only in the Ordinary Course of Business (except for the execution and performance of
this Agreement and the discussions, negotiations and transactions related thereto) and there has not been any (a) Parent Material
Adverse Effect or (b) actions to do any of the following:
(a) declare,
accrue, set aside or pay any dividend or make any other distribution in respect of any shares of its capital stock or repurchase, redeem
or otherwise reacquire any shares of its capital stock or other securities (except in connection with the payment of the exercise price
and/or withholding Taxes incurred upon the exercise, settlement or vesting of any award granted under the Parent Stock Plans);
(b) sell,
issue, grant, pledge or otherwise dispose of or encumber or authorize the issuance of: (A) any capital stock or other security (except
for Parent Common Stock issued upon the valid exercise or settlement of outstanding Parent Options or Parent Restricted Stock Units as
applicable), (B) any option, warrant or right to acquire any capital stock or any other security, other than options grants to employees
in the Ordinary Course of Business or (C) any instrument convertible into or exchangeable for any capital stock or other security;
(c) except
as required to give effect to anything in contemplation of the Closing, amend any of its Organizational Documents, or effect or be a party
to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse
stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions;
(d) form
any Subsidiary or acquire any equity interest or other interest in any other Entity or enter into a joint venture with any other Entity;
(e) (A) lend
money to any Person (except for the advance of reasonable business expenses to employees, directors and consultants in the Ordinary Course
of Business), (B) incur or guarantee any indebtedness for borrowed money, or (C) guarantee any debt securities of others;
(f) other
than as required by applicable Law: (A) adopt, establish or enter into any new Employee Plan, including, for the avoidance of doubt,
any equity awards plans, (B) cause or permit any Parent Employee Plan to be amended, (C) pay any bonus or make any profit-sharing
or similar payment to (except with respect to obligations in place on the date of this Agreement pursuant to any Parent Employee Plan),
or increase the amount of the wages, salary, commissions, fringe benefits or other compensation or remuneration payable to, any of its
directors, officers, employees or consultants, (D) grant any new equity awards or increase the coverage or benefits available under
any Parent Employee Plan, (E) increase the severance or change of control benefits offered to any current or new employees, directors
or consultants, or (F) hire, terminate or give notice of termination (other than for cause) to any officer, employee or consultant;
(g) enter
into any material transaction other than (A) in the Ordinary Course of Business or (B) in connection with the Contemplated Transactions;
(h) acquire
any material asset or sell, lease or otherwise irrevocably dispose of any of its assets or properties, or grant any Encumbrance (other
than Permitted Encumbrances) with respect to such assets or properties, except in the Ordinary Course of Business;
(i) sell,
assign, transfer, license, sublicense or otherwise dispose of any material Parent IP Rights (other than in the Ordinary Course of Business);
(j) make
(other than consistent with past practice), change or revoke any material Tax election; file any material amendment to any Tax Return;
settle or compromise any material Tax claim; waive or extend any statute of limitations in respect of a period within which an assessment
or reassessment of material Taxes may be issued (other than any extension pursuant to an extension to file any Tax Return); enter into
any “closing agreement” as described in Section 7121 of the Code (or any similar Law) with any Governmental Authority;
or adopt or change any material accounting method in respect of Taxes;
(k) initiate,
waive, settle or compromise any pending or threatened Legal Proceeding;
(l) delay
or fail to repay when due any material obligation, including accounts payable and accrued expenses, other than in the Ordinary Course
of Business;
(m) terminate
or modify in any material respect, or fail to exercise renewal rights with respect to, any material insurance policy;
(n) enter
into, amend, terminate, or waive any material option or right under, any Parent Material Contract; or
(o) agree,
resolve or commit to do any of the foregoing.
4.9 Absence
of Undisclosed Liabilities. As of the date hereof, neither Parent nor any of its Subsidiaries has any Liability of a type required
to be reflected or reserved for on a balance sheet prepared in accordance with GAAP, except for: (a) Liabilities disclosed, reflected
or reserved against in the Parent Unaudited Interim Balance Sheet, (b) Liabilities that have been incurred by Parent or its Subsidiaries
since the date of the Parent Unaudited Interim Balance Sheet in the Ordinary Course of Business (none of which relates to any breach of
contract, breach of warranty, tort, infringement, or violation of Law), (c) Liabilities for performance of obligations of Parent
or any of its Subsidiaries under Parent Contracts, (d) Liabilities incurred in connection with the Contemplated Transactions; (e) Liabilities
which would not, individually or in the aggregate, reasonably be expected to be material to Parent and (f) Liabilities described
in Section 4.9 of the Parent Disclosure Schedule.
4.10 Title
to Assets. Each of Parent and its Subsidiaries owns, and has good and valid title to, or, in the case of leased properties and assets,
valid leasehold interests in, all tangible properties or tangible assets and equipment used or held for use in its business or operations
or purported to be owned by it, including: (a) all tangible assets reflected on the Parent Unaudited Interim Balance Sheet and (b) all
other tangible assets reflected in the books and records of Parent as being owned by Parent. All of such assets are owned or, in the case
of leased assets, leased by Parent or any of its Subsidiaries free and clear of any Encumbrances, other than Permitted Encumbrances.
4.11 Real
Property; Leasehold. Neither Parent nor any of its Subsidiaries owns or has ever owned any real property. Parent has made available
to the Company (a) an accurate and complete list of all real properties with respect to which Parent directly or indirectly holds
a valid leasehold interest as well as any other real estate that is in the possession of or leased by Parent or any of its Subsidiaries
and (b) copies of all leases under which any such real property is possessed (the “Parent Real Estate Leases”),
each of which is in full force and effect, with no existing material default thereunder. Parent’s possession, occupancy, lease,
use and/or operation of each such leased property conforms to all applicable Laws in all material respects, and Parent has exclusive possession
of each such leased property and leasehold interest and has not granted any occupancy rights to tenants or licensees with respect to such
leased property or leasehold interest. In addition, each such leased property and leasehold interest is free and clear of all Encumbrances
other than Permitted Encumbrances. Parent has not received any written notice from its landlords or any Governmental Body that: (i) relates
to violations of building, zoning, safety or fire ordinances or regulations; (ii) claims any defect or deficiency with respect to
any of such properties; or (iii) requests the performance of any repairs, alterations or other work to such properties.
4.12 Intellectual
Property.
(a) Section 4.12(a) of
the Parent Disclosure Schedule is an accurate, true and complete listing of all Parent Registered IP.
(b) Section 4.12(b) of
the Parent Disclosure Schedule accurately identifies (i) all Parent Contracts pursuant to which any material Parent IP Rights are
licensed to Parent or any of its Subsidiaries (other than (A) any non-customized software that (1) is so licensed solely in
executable or object code form pursuant to a nonexclusive, internal use software license and other Intellectual Property associated with
such software and (2) is not incorporated into, or material to the development, manufacturing, or distribution of, any of Parent
products or services, (B) any Intellectual Property licensed on a nonexclusive basis in the Ordinary Course of Business ancillary
to the purchase or use of equipment, reagents or other materials or that is otherwise not material to the business of Parent or any of
its Subsidiaries, (C) any confidential information provided under confidentiality agreements and (D) agreements between Parent
or any of its Subsidiaries and its or their respective employees in Parent’s standard form thereof ((A) through (D), “Non-Scheduled
Parent IP Contracts”)) and (ii) whether the license or licenses granted to Parent or its Subsidiary are exclusive or nonexclusive.
(c) Section 4.12(c) of
the Parent Disclosure Schedule accurately identifies each Parent Contract pursuant to which any Person has been granted by Parent or any
of its Subsidiaries any license, sublicense or covenant not to sue under, or otherwise has received or acquired any right (whether or
not currently exercisable) or interest in, any material Parent IP Rights (other than (i) any confidential information provided under
confidentiality agreements and (ii) any Parent IP Rights nonexclusively licensed to academic collaborators, suppliers or service
providers for the sole purpose of enabling such academic collaborator, supplier or service providers to provide services for Parent’s
or any of its Subsidiaries’ benefit).
(d) Neither
Parent nor any of its Subsidiaries is bound by, and no Parent IP Rights are subject to, any Contract containing any covenant or other
provision that in any way limits or restricts the ability of Parent or any of its Subsidiaries to use, exploit, assert, or enforce any
Parent IP Rights anywhere in the world.
(e) Parent
or one of its Subsidiaries solely and exclusively owns all right, title, and interest to and in all Parent IP Rights (other than Parent
IP Rights licensed to the Parent or its Subsidiary pursuant to a Parent Contract set forth on Section 4.12(b) of the
Parent Disclosure Schedule or pursuant to a Non-Scheduled Parent IP Contract), in each case, free and clear of any Encumbrances (other
than Permitted Encumbrances). Without limiting the generality of the foregoing:
(i) All
documents and instruments necessary to register or apply for or renew registration of material Parent Registered IP have been validly
executed, delivered, and filed in a timely manner with the appropriate Governmental Authority.
(ii) Each
Person who is or was an employee or contractor of Parent or any of its Subsidiaries and who is or was involved in the creation or development
of any material Intellectual Property for Parent or any of its Subsidiaries has signed a valid, enforceable agreement containing a present
assignment of such Intellectual Property to Parent or such Subsidiary and confidentiality provisions protecting trade secrets and confidential
information of Parent and its Subsidiaries.
(iii) To
the Knowledge of Parent, no current or former officer, director, or employee of Parent or any of its Subsidiaries has any claim, right
(whether or not currently exercisable), or interest to or in any Parent IP Rights purported to be owned by Parent or any of its Subsidiaries.
To the Knowledge of Parent, no employee of Parent or any of its Subsidiaries is (a) bound by or otherwise subject to any Contract
restricting him or her from performing his or her duties for Parent or such Subsidiary or (b) in breach of any Contract with any
former employer or other Person concerning Parent IP Rights purported to be owned by Parent or such Subsidiary or confidentiality provisions
protecting trade secrets and confidential information comprising such Parent IP Rights purported to be owned by Parent or such Subsidiary.
(iv) No
funding, facilities, or personnel of any Governmental Authority were used, directly or indirectly, to develop or create, in whole or in
part, any Parent IP Rights in which Parent or any of its Subsidiaries has an ownership interest, except for any such funding or use of
facilities or personnel that does not result in such Governmental Authority obtaining ownership rights to such Parent IP Rights and does
not require or otherwise obligate the Parent or its Subsidiaries to grant or offer to any such Governmental Authority or institution any
material license or other material right to such Parent IP Right (except for non-commercial use rights during the term of the applicable
agreement between the Parent or one of its Subsidiaries and such Governmental Authority), including the right to receive royalties for
the practice of such Parent IP Right.
(v) Parent
and each of its Subsidiaries has taken reasonable steps to maintain the confidentiality of and otherwise protect and enforce its rights
in all proprietary information that Parent or such Subsidiary holds, or purports to hold, as confidential or a trade secret.
(vi) Neither
Parent nor any of its Subsidiaries has assigned or otherwise transferred ownership of, or agreed to assign or otherwise transfer ownership
of, any material Parent IP Rights to any other Person.
(vii) To
the Knowledge of Parent, the Parent IP Rights constitute all Intellectual Property necessary for Parent to conduct its business as currently
conducted; provided, however, that the foregoing representation is not a representation with respect to non-infringement
of Intellectual Property.
(f) Parent
has delivered, or made available to the Company, a complete and accurate copy of all Parent IP Rights Agreements that are required to
be listed on the Parent Disclosure Schedule. With respect to each of the material Parent IP Rights Agreements: (i) each such agreement
is valid and binding on the Parent or its Subsidiary (as applicable) and in full force and effect, (ii) neither the Parent nor any
of its Subsidiaries has received any written notice of termination or cancellation under such agreement, or received any written notice
of breach or default under such agreement, which breach has not been cured or waived and (iii) neither the Parent nor any of its
Subsidiaries, nor to the Knowledge of the Parent, any other party to any such agreement, is not in breach or default thereof in any material
respect.
(g) The
manufacture, marketing, sale, offering for sale, importation, use or intended use or other disposal of any product or proposed product
as currently sold or under development by Parent does not violate any license or agreement between Parent or any of its Subsidiaries and
any third party in any material respect, and, to the Knowledge of Parent, does not infringe or misappropriate (and, when made commercially
available, will not infringe or misappropriate) any valid and issued Patent right or other Intellectual Property of any other Person,
which infringement or misappropriation would reasonably be expected to have a Parent Material Adverse Effect. To the Knowledge of Parent,
no third party is infringing upon any Patents within the Parent IP Rights, or violating any Parent IP Rights Agreement.
(h) As
of the date of this Agreement, (i) neither Parent nor any of its Subsidiaries is a party to any Legal Proceeding (including, but
not limited to, opposition, interference or other proceeding in any patent or other government office) contesting the validity, enforceability,
ownership or right to use, sell, offer for sale, license or dispose of any Parent IP Rights, (ii) neither Parent nor any of its Subsidiaries
has received any written notice asserting that any Parent IP Rights or the proposed use, sale, offer for sale, license or disposition
of any products, methods, or processes claimed or covered thereunder infringes or misappropriates or violates the rights of any other
Person or that Parent or any of its Subsidiaries has otherwise infringed, misappropriated or otherwise violated any Intellectual Property
of any Person, and (iii) none of the Parent IP Rights is subject to any outstanding order of, judgment of, decree of or agreement
with any Governmental Authority that limits the ability of the Parent or any of its Subsidiaries to exploit any Parent IP Rights.
(i) Each
item of material Parent Registered IP (other than any Parent Registered IP that the Parent in its business judgment has elected to abandon)
is and at all times has been filed and maintained in compliance in all material respects with all applicable Law and all filings, payments,
and other actions required to be made or taken to maintain such item of Parent Registered IP in full force and effect have been made by
the applicable deadline. To the Knowledge of Parent, all Parent Registered IP that is issued or granted is valid and enforceable.
(j) To
the Knowledge of Parent, no trademark (whether registered or unregistered) or trade name owned, used, or applied for by Parent or any
of its Subsidiaries conflicts or interferes with any trademark (whether registered or unregistered) or trade name owned, used, or applied
for by any other Person, except as would not reasonably be expected to have a Parent Material Adverse Effect. None of the goodwill associated
with or inherent in any trademark (whether registered or unregistered) in which Parent or any of its Subsidiaries has or purports to have
an ownership interest has been impaired as determined by Parent in accordance with GAAP.
(k) Except
as set forth in the Contracts listed on Section 4.12(b) or 4.12(c) of the Parent Disclosure Schedule or as
contained in license, distribution or service agreements entered into in the Ordinary Course of Business by Parent or any of its Subsidiaries,
(i) neither Parent nor any of its Subsidiaries is bound by any Contract to indemnify, defend, hold harmless, or reimburse any other
Person with respect to any Intellectual Property infringement, misappropriation, or similar claim which is material to Parent and its
Subsidiaries, taken as a whole and (ii) neither Parent nor any of its Subsidiaries has ever assumed, or agreed to discharge or otherwise
take responsibility for, any existing or potential liability of another Person for infringement, misappropriation, or violation of any
Intellectual Property right, which assumption, agreement or responsibility remains in force as of the date of this Agreement.
(l) Neither
Parent nor any of its Subsidiaries is party to any Contract that, as a result of such execution, delivery and performance of this Agreement,
will cause the grant of any license or other right to any Parent IP Rights, result in breach of, default under or termination of such
Contract with respect to any Parent IP Rights, or impair the right of Parent or any of its Subsidiaries, or the Surviving Company and
its Subsidiaries to use, sell or license or enforce any Parent IP Rights or portion thereof.
4.13 Agreements,
Contracts and Commitments.
(a) Neither
Parent nor any of its Subsidiaries is a party to or is bound by any “material contract” (as such term is defined
in Item 601(b)(10) of Regulation S-K under the Securities Act, excluding, however, any Parent Stock Plan) (all such Contracts “Parent
Material Contracts”).
(b) (i) Each
Parent Material Contract is valid and binding on Parent and any of its Subsidiaries to the extent such Subsidiary is a party thereto,
as applicable, and to the knowledge of Parent, each other party thereto, and is in full force and effect and enforceable in accordance
with its terms; (ii) Parent and each of its Subsidiaries, and, to the knowledge of Parent, each other party thereto, has performed
all material obligations required to be performed by it under each Parent Material Contract; and (iii) there is no material breach,
violation, or default under any Parent Material Contract by Parent or any of its Subsidiaries or, to the knowledge of Parent, any other
party thereto, and no event or condition has occurred that constitutes, or, after notice or lapse of time or both, would constitute, a
material breach, violation, or default on the part of Parent or any of its Subsidiaries or, to the knowledge of Parent, any other party
thereto under any such Parent Material Contract, nor has Parent or any of its Subsidiaries received any notice of any such material breach,
violation, default, event or condition. Parent has made available to the Company true and complete copies of all Parent Material Contracts,
including all amendments thereto.
4.14 Compliance;
Permits; Restrictions.
(a) Parent
and each of its Subsidiaries is, and since January 1, 2022, has been in material compliance with all applicable Laws. No investigation,
claim, suit, proceeding, audit, Order, or other action by any Governmental Authority is pending or, to the Knowledge of Parent, threatened
against Parent or any of its Subsidiaries. There is no agreement or Order binding upon Parent or any of its Subsidiaries which (i) has
or could reasonably be expected to have the effect of prohibiting or materially impairing any business practice of Parent or any of its
Subsidiaries, any acquisition of material property by Parent or any of its Subsidiaries or the conduct of business by Parent or any of
its Subsidiaries as currently conducted, (ii) is reasonably likely to have an adverse effect on Parent’s ability to comply
with or perform any covenant or obligation under this Agreement or (iii) is reasonably likely to have the effect of preventing, delaying,
making illegal or otherwise interfering with the Contemplated Transactions.
(b) Each
of Parent and its Subsidiaries holds all required Governmental Authorizations that are material to the operation of the business of Parent
and Merger Subs as currently conducted (collectively, the “Parent Permits”), including Governmental Authorizations
related to the development, testing, manufacturing, processing, storage, labeling, sale, marketing, advertising, distribution and importation
or exportation, as currently conducted, of any of its products or product candidates (the “Parent Product Candidates”).
Section 4.14(b) of the Parent Disclosure Schedule identifies each Parent Permit. Each of Parent and its Subsidiaries
have timely maintained and is in material compliance with the terms of the Parent Permits and no Parent Permit has been (i) revoked,
withdrawn, suspended, cancelled or terminated or (ii) modified in any adverse manner, other than immaterial adverse modifications.
No Legal Proceeding is pending or, to the Knowledge of Parent, threatened, which seeks to revoke, substantially limit, suspend, or materially
modify any Parent Permit.
(c) There
are no Legal Proceedings pending or, to the Knowledge of Parent, threatened with respect to an alleged material violation by Parent or
any of its Subsidiaries of the FDCA, PHSA, FDA regulations adopted thereunder, the Controlled Substances Act or any other similar Law
promulgated by a Drug Regulatory Agency.
(d) Except
for the information and files identified in Section 4.14(d) of the Parent Disclosure Schedule, Parent has made available
to the Company all information requested by the Company in Parent’s or its Subsidiaries’ possession or control relating to
the Parent Product Candidates and the development, testing, manufacturing, processing, storage, labeling, sale, marketing, advertising,
distribution and importation or exportation of the Parent Product Candidates, including, but not limited to, complete copies of the following
(to the extent there are any): (x) adverse event reports; pre-clinical, clinical and other study reports and material study data;
inspection reports, notices of adverse findings, untitled letters, warning letters, filings and letters and other written correspondence
to and from any Drug Regulatory Agency; and meeting minutes with any Drug Regulatory Agency and (y) similar reports, material study
data, notices, letters, filings, correspondence and meeting minutes with any other Governmental Authority. All such information are accurate
and complete in all material respects.
(e) All
clinical, pre-clinical and other studies and tests conducted by or on behalf of, or sponsored by, Parent or its Subsidiaries, in which
Parent or its Subsidiaries or their respective product candidates, including the Parent Product Candidates, have participated were and,
if still pending, are being conducted in all material respects in accordance with standard medical and scientific research procedures
and in compliance in all material respects with the applicable regulations of the Drug Regulatory Agencies and other applicable Law, including,
without limitation, 21 C.F.R. Parts 50, 54, 56, 58 and 312. Other than as set forth on Section 4.14(e) of the Parent
Disclosure Schedule, neither Parent nor any of its Subsidiaries has received any written notices, correspondence, or other communications
from any Drug Regulatory Agency requiring or, to the Knowledge of Parent, any action to place a clinical hold order on, or otherwise terminate,
delay, or suspend any clinical studies conducted by or on behalf of, or sponsored by, Parent or any of its Subsidiaries or in which Parent
or any of its Subsidiaries or its current product candidates, including the Parent Product Candidates, have participated. Further, no
clinical investigator, researcher, or clinical staff participating in any clinical study conducted by or, to the Knowledge of Parent,
on behalf of Parent or any of its Subsidiaries has been disqualified from participating in studies involving the Parent Product Candidates,
and to the Knowledge of Parent, no such administrative action to disqualify such clinical investigators, researchers or clinical staff
has been threatened or is pending.
(f) Neither
Parent nor any of its Subsidiaries and, to the Knowledge of Parent, any contract manufacturer with respect to any Parent Product Candidate
is the subject of any pending or, to the Knowledge of Parent, threatened investigation in respect of its business or products by the FDA
pursuant to its “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56
Fed. Reg. 46191 (September 10, 1991) and any amendments thereto. To the Knowledge of Parent, neither Parent nor any of its Subsidiaries
and no contract manufacturer with respect to any Parent Product Candidate has committed any acts, made any statement, or failed to make
any statement, in each case in respect of its business or products that would violate FDA’s “Fraud, Untrue Statements of Material
Facts, Bribery, and Illegal Gratuities” Final Policy, and any amendments thereto. None of Parent, any of its Subsidiaries, and to
the Knowledge of Parent, any contract manufacturer with respect to any Parent Product Candidate, or any of their respective officers,
employees or agents has been convicted of any crime or engaged in any conduct that could result in a material debarment or exclusion under
(i) 21 U.S.C. Section 335a or (ii) any similar applicable Law. To the Knowledge of Parent, no material debarment or exclusionary
claims, actions, proceedings or investigations in respect of their business or products are pending or threatened against Parent, any
of its Subsidiaries, and to the Knowledge of the Parent, any contract manufacturer with respect to any Parent Product Candidate, or any
of its officers, employees or agents.
(g) All
manufacturing operations conducted by, or to the Knowledge of Parent, for the benefit of, Parent or its Subsidiaries in connection with
any Parent Product Candidate, since January 1, 2018, have been and are being conducted in compliance in all material respects with
applicable Laws, including the FDA’s standards for current good manufacturing practices, including applicable requirements contained
in 21 C.F.R. Parts 210 and 211, and the respective counterparts thereof promulgated by Governmental Authorities in countries outside the
United States.
(h) No
laboratory or manufacturing site owned by Parent or its Subsidiaries, and to the Knowledge of Parent, no manufacturing site of a contract
manufacturer or laboratory, with respect to any Parent Product Candidate, (i) is subject to a Drug Regulatory Agency shutdown or
import or export prohibition or (ii) has received any Form FDA 483, notice of violation, warning letter, untitled letter, or
similar correspondence or notice from the FDA or other Governmental Authority alleging or asserting noncompliance with any applicable
Law, in each case, that have not been complied with or closed to the satisfaction of the relevant Governmental Authority, and, to the
Knowledge of Parent, neither the FDA nor any other Governmental Authority is considering such action.
(i) Parent
and its Subsidiaries have complied with all Laws relating to patient, medical or individual health information, including HIPAA, including
the standards for the privacy of Individually Identifiable Health Information at 45 C.F.R. Parts 160 and 164, Subparts A and E, the standards
for the protection of Electronic Protected Health Information set forth at 45 C.F.R. Part 160 and 45 C.F.R. Part 164, Subpart
A and Subpart C, the standards for transactions and code sets used in electronic transactions at 45 C.F.R. Part 160, Subpart A and
Part 162, and the standards for Breach Notification for Unsecured Protected Health Information at 45 C.F.R. Part 164, Subpart
D, all as amended from time to time. Parent and its Subsidiaries have entered into, where required, and is in compliance in all material
respects with the terms of all Business Associate Agreements to which Parent or its Subsidiaries are a party or otherwise bound. Parent
and its Subsidiaries have, if required, created and maintained written policies and procedures to protect the privacy of all Protected
Health Information, have provided training to all employees and agents as required under HIPAA, and have, if required, implemented security
procedures, including physical, technical and administrative safeguards, to protect all personal information and Protected Health Information
stored or transmitted in electronic form. Parent and its Subsidiaries have not received written notice from the Office for Civil Rights
for the U.S. Department of Health and Human Services or any other Governmental Authority of any allegation regarding its failure to comply
with HIPAA or any other federal or state law or regulation applicable to the protection of individually identifiable health information
or personally identifiable information. No successful Security Incident, Breach of Unsecured Protected Health Information, unpermitted
disclosure of Personal Health Information or breach of personally identifiable information under applicable Laws has occurred with respect
to information maintained or transmitted to Parent and its Subsidiaries or an agent or third party subject to a Business Associate Agreement
with Parent or its Subsidiaries. Parent and its Subsidiaries are, if required, currently submitting, receiving and handling or is capable
of submitting, receiving and handling transactions in accordance with the Transactions and Code Sets Rule. All capitalized terms in this
Section 4.14 not otherwise defined in this Agreement shall have the meanings set forth under HIPAA.
4.15 Legal
Proceedings; Orders.
(a) Except
as set forth in Section 4.15 of the Parent Disclosure Schedule, there is no pending Legal Proceeding and, to the Knowledge
of Parent, no Person has threatened in writing to commence any Legal Proceeding: (i) that involves Parent or any of its Subsidiaries
or any Parent Associate (in his or her capacity as such) or any of the material assets owned by or used by Parent or (ii) that challenges,
or that may have the effect of preventing, delaying, making illegal or otherwise interfering with, the Contemplated Transactions.
(b) There
is no Order to which Parent or any of its Subsidiaries, or any of the material assets owned or used by Parent or any of its Subsidiaries
is subject. To the Knowledge of Parent, no officer or other Key Employee of Parent or any of its Subsidiaries is subject to any Order
that prohibits such officer or employee from engaging in or continuing any conduct, activity or practice relating to the business of Parent
or any of its Subsidiaries or to any material assets owned or used by Parent or any of its Subsidiaries.
4.16 Tax
Matters.
(a) Each
of Parent and each of its Subsidiaries has timely filed all income Tax Returns and all other material Tax Returns that were required to
be filed by or with respect to it under applicable Law. All such Tax Returns were correct and complete in all material respects and have
been prepared in material compliance with all applicable Law. No claim has ever been made by a Governmental Authority in a jurisdiction
where Parent or any of its Subsidiaries does not file Tax Returns that Parent or any of its Subsidiaries is subject to taxation by that
jurisdiction.
(b) All
material amounts of Taxes due and owing by Parent and each of its Subsidiaries (whether or not shown on any Tax Return) have been timely
paid. The unpaid Taxes of Parent and each of its Subsidiaries for periods (or portions thereof) ending on or prior to the date of the
Parent Unaudited Interim Balance Sheet do not materially exceed the accruals for current Taxes set forth on the Parent Unaudited Interim
Balance Sheet. Since the date of the Parent Unaudited Interim Balance Sheet, neither Parent nor any of its Subsidiaries has incurred any
material Liability for Taxes outside the Ordinary Course of Business or otherwise inconsistent with past custom and practice.
(c) Each
of Parent and each of its Subsidiaries has withheld and paid to the appropriate Governmental Authority all material Taxes required to
have been withheld and paid in connection with any amounts paid or owing to any employee, independent contractor, creditor, stockholder,
or other third party.
(d) There
are no Encumbrances for material Taxes (other than Encumbrances described in clause (a) of the definition of “Permitted Encumbrances”)
upon any of the assets of Parent or any of its Subsidiaries.
(e) No
deficiencies for a material amount of Taxes with respect to Parent or any of its Subsidiaries have been claimed, proposed or assessed
by any Governmental Authority in writing that have not been timely paid in full. There are no pending (or, based on written notice, threatened)
material audits, examinations assessments or other actions for or relating to any liability in respect of Taxes of Parent or any of its
Subsidiaries. Neither Parent nor any of its Subsidiaries has waived any statute of limitations in respect of material Taxes or agreed
to any extension of time with respect to a material Tax assessment or deficiency.
(f) Neither
Parent nor any of its Subsidiaries has been a United States real property holding corporation within the meaning of Section 897(c)(2) of
the Code in the last five years.
(g) Neither
Parent nor any of its Subsidiaries is a party to any Tax allocation, Tax sharing or similar agreement (including indemnity arrangements),
other than Ordinary Course Agreements.
(h) Neither
Parent nor any of its Subsidiaries has been a member of an affiliated group filing a consolidated U.S. federal income Tax Return (other
than a group the common parent of which is Parent). Neither Parent nor any of its Subsidiaries has any material Liability for the Taxes
of any Person (other than Parent and Merger Subs) under Treasury Regulations Section 1.1502-6 (or any similar provision of state,
local, or foreign law), as a transferee or successor, by Contract (other than an Ordinary Course Agreement) or otherwise.
(i) Neither
Parent nor any of its Subsidiaries has distributed stock of another Person, or had its stock distributed by another Person, in a transaction
that was purported or intended to be governed in whole or in part by Section 355 of the Code or Section 361 of the Code.
(j) Neither
Parent nor any of its Subsidiaries has entered into any transaction identified as a “reportable transaction” for purposes
of Treasury Regulations Sections 1.6011-4(b)(2) or 301.6111-2(b)(2).
(k) Neither
Parent nor any of its Subsidiaries will be required to include any item of income in, or exclude any item of deduction from, taxable income
for any taxable period (or portion thereof) ending after the Closing Date as a result of any: (i) change in, or use of improper,
method of accounting for a taxable period ending on or prior to the Closing Date; (ii) “closing agreement” as described
in Section 7121 of the Code (or any corresponding or similar provision of state, local or foreign income Tax law) executed on or
prior to the Closing Date; (iii) installment sale or open transaction disposition made on or prior to the Closing Date; (iv) prepaid
amount, advance payments or deferred revenue received or accrued on or prior to the Closing Date other than in respect of such amounts
reflected in the Parent Unaudited Interim Balance Sheet or received in the Ordinary Course of Business since the date of the Parent Unaudited
Interim Balance Sheet; or (v) intercompany transaction or excess loss amount described in Treasury Regulations under Section 1502
of the Code (or any corresponding or similar provision of state, local or foreign income Tax Law).
(l) Neither
Parent nor any of its Subsidiaries has ever had a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise
had an office or fixed place of business in a country other than the country in which it is organized.
(m) Parent
is, and has been since its date of formation, a C corporation for U.S. federal income tax purposes.
(n) Neither
Parent nor any of its Subsidiaries is aware of any facts or has knowingly taken or agreed to take any action, in each case, that would
reasonably be expected to prevent or impede the Merger from qualifying as a “reorganization” within the meaning of Section 368(a)(1)(A) of
the Code.
(o) The
stock in First Merger Sub and the membership interests in Second Merger Sub are each directly and wholly owned by Parent, and (i) First
Merger Sub is, and has been since its date of formation, a C corporation for U.S. federal income tax purposes, and (ii) Second Merger
Sub is, and has been since its date of formation, disregarded as an entity (within the meaning of Section 301.7701-3 of the Treasury
Regulations) separate from Parent for U.S. federal income tax purposes. Each of First Merger Sub and Second Merger Sub was formed solely
for the purpose of engaging in the Merger and has not engaged in any business activity, incurred any Liabilities or entered into any agreements
or arrangements, in each case other than as contemplated by this Agreement.
4.17 Employee
and Labor Matters; Benefit Plans.
(a) Section 4.17(a) of
the Parent Disclosure Schedule sets forth a true and complete listing of the employees and consultants of Parent as of the Closing Date
(each, respectively, a “Parent Employee” or a “Parent Consultant”), including, as applicable, each
such person’s name, job title(s) or function(s) (including board positions) and job location, current salary or wage,
and current status (as to full time or part time, exempt or nonexempt and temporary or leave status and as to classification as an employee,
consultant or independent contractor). Parent has delivered or made available to the Company a true and complete copy of each Parent Employee
Plan that is not a PEO Plan and each Parent Employee Plan that is a PEO Plan that has been provided to the Company and the employee handbook
for Parent, if any, and all other employment policies, if any, currently applicable to any Parent Employee or Parent Consultant.
(b) To
Parent’s Knowledge, no officer or executive of Parent has disclosed any plans to terminate his or her employment with Parent.
(c) Except
as set forth in Section 4.17(c) of the Parent Disclosure Schedule:
(i) Parent
has paid or made provision for payment of all salaries and wages, which became payable to any Parent Employee prior to the Closing Date
and is in compliance in all material respects with all applicable Laws respecting employment and employment practices, terms and conditions
of employment, collective bargaining, immigration, wages, hours and benefits, non-discrimination in employment, workers’ compensation,
including Title VII of the Civil Rights Act of 1964, the Age Discrimination in Employment Act of 1967, the Equal Employment Opportunity
Act of 1972, ERISA, the Equal Pay Act of 1963, the National Labor Relations Act of 1935, the Fair Labor Standards Act of 1938, the Americans
with Disabilities Act of 1990, the Vietnam Era Veterans’ Reemployment Act, the Family and Medical Leave Act of 1993, the Occupational
Safety and Health Act and any and all similar applicable state and local Laws;
(ii) Parent
has not received a notice, citation, complaint or charge asserting any violation or liability under the Occupational Safety and Health
Act or any similar applicable Law regulating employee health and safety;
(iii) (a) no
Parent Employee is represented by any labor union or other labor representative with respect to his or her employment with Parent; (b) Parent
is not bound by any labor, collective bargaining agreement or similar arrangement; (c) to Parent’s Knowledge, no petition has
been filed nor has any proceeding been instituted by any employee or group of employees with the National Labor Relations Board or similar
Governmental Authority seeking recognition of a collective bargaining agreement; (d) to Parent’s Knowledge, there are no Persons
attempting to represent or organize or purporting to represent for bargaining purposes any employees of Parent; and (e) since January 1,
2021, there has not occurred and, to Parent’s Knowledge, there has not been threatened any strikes, slowdowns, picketing, work stoppages or
concerted refusals to work or other similar labor activities with respect to employees of Parent;
(iv) Parent
has not received notice of any charge or complaint pending before the Equal Employment Opportunity Commission or similar Governmental
Authority alleging unlawful discrimination in employment practices, or before the National Labor Relations Board or similar Governmental
Authority alleging any unfair labor practice by Parent, nor, to Parent’s Knowledge, has any such charge been threatened;
(v) all
Parent Employees are employed on an at-will basis and their employment can be terminated at any time for any reason without any amounts
being owed to such individual other than with respect to wages, compensation and benefits accrued before such termination; and all Parent
Consultants can be terminated at any time for any reason without notice or any amounts being owed to such individual other than with respect
to compensation or payments accrued before such termination;
(vi) since
January 1, 2023, Parent has not effectuated a “plant closing” or a “mass layoff” (as such terms are defined
in the WARN Act); and
(vii) to
Parent’s Knowledge, any individual performing services for Parent who has been classified as an independent contractor has been
correctly classified as such;
(viii) Parent
(a) has withheld and reported all material amounts required by Law or by agreement to be withheld and reported with respect to wages,
salaries and other payments to employees, and (b) is not liable for any material payment to any trust or other fund governed by or
maintained by or on behalf of any Governmental Authority, with respect to unemployment compensation benefits, social security or other
benefits or obligations for employees (other than routine payments to be made in the Ordinary Course of Business); and
(ix) no
current or former employee of Parent has, since January 1, 2021, asserted any legal claims either orally or in writing to Parent
concerning violations of any of the following Laws or regulations: labor relations, equal employment opportunities, fair employment practices,
employment discrimination, harassment, retaliation, reasonable accommodation, disability rights or benefits, immigration, wages, hours,
overtime compensation, child labor, hiring, promotion and termination of employees, working conditions, meal and break periods, privacy,
health and safety, workers’ compensation, leaves of absence and unemployment insurance.
(d) Each
Parent Employee Plan other than a PEO Plan, and to Parent’s Knowledge, each Parent Employee Plan that is a PEO Plan, that is intended
to be qualified under Section 401(a) of the Code has received a favorable determination or opinion letter with respect to such
qualified status from the IRS. To Parent’s Knowledge, nothing has occurred that would reasonably be expected to adversely affect
the qualified status of any such Parent Employee Plan or the exempt status of any related trust.
(e) Each
Parent Employee Plan other than a PEO Plan, and to Parent’s Knowledge, each Parent Employee Plan that is a PEO Plan, has been established,
maintained and operated in compliance, in all material respects, with its terms all applicable Law, including, without limitation, the
Code, ERISA and the Affordable Care Act. No Legal Proceeding (other than those relating to routine claims for benefits) is pending or,
to Parent’s Knowledge, threatened with respect to any Parent Employee Plan (other than any PEO Plan). All payments and/or contributions
required to have been made with respect to all Parent Employee Plans other than PEO Plans, and to the Parent’s Knowledge, PEO Plans,
either have been made or have been accrued in accordance with the terms of the applicable Parent Employee Plan and applicable Law.
(f) Neither
Parent nor any of its ERISA Affiliates maintains, contributes to or is required to contribute to, or has, in the past six (6) years,
maintained, contributed to, or been required to contribute to (i) any “employee benefit plan” that is or was subject
to Title IV or Section 302 of ERISA or Section 412 of the Code, (ii) a Multiemployer Plan, (iii) any funded welfare
benefit plan within the meaning of Section 419 of the Code, (iv) any Multiple Employer Plan, or (v) any Multiple Employer
Welfare Arrangement. Neither Parent nor any of its ERISA Affiliates has ever incurred any liability under Title IV of ERISA.
(g) No
Parent Employee Plan provides for medical or other welfare benefits to any service provider beyond termination of service or retirement,
other than (i) pursuant to COBRA or an analogous state law requirement or (ii) continuation coverage through the end of the
month in which such termination or retirement occurs. Parent does not sponsor or maintain any self-funded medical or long-term disability
benefit plan that is not a PEO Plan.
(h) Except
as otherwise provided in this Agreement or under applicable Law, neither the execution and delivery of this Agreement nor the consummation
of the Merger shall result in (i) any payment becoming due to any Parent Employee or Parent Consultant, (ii) the provision of
any benefits or other rights to any Parent Employee or Parent Consultant, (iii) the increase, acceleration or provision of any payments,
benefits or other rights to any Parent Employee or Parent Consultant, whether or not any such payment, right or benefit would constitute
a “parachute payment” within the meaning of Section 280G of the Code, (iv) require any contributions or payments
to fund any obligations under any Parent Employee Plan, or (v) the forgiveness in whole or in part of any outstanding loans made
by Parent to any Parent Employee or Parent Consultant. No payment, right or benefit that becomes due or accelerated as a result of the
execution and delivery of this Agreement or the consummation of the Merger is an “excess parachute payment” within the meaning
of Section 280G of the Code with respect to any current or former employee of Parent.
(i) Each
Parent Employee Plan that constitutes in any part a nonqualified deferred compensation plan within the meaning of Section 409A of
the Code has been operated and maintained in all material respects in operational and documentary compliance with Section 409A of
the Code and applicable guidance thereunder.
(j) No
Parent Employee Plan is subject to the laws of any jurisdiction other than the United States of America.
4.18 Environmental
Matters. Since December 31, 2020, Parent and each of its Subsidiaries has complied with all applicable Environmental Laws, which
compliance includes the possession by Parent of all permits and other Governmental Authorizations required under applicable Environmental
Laws and compliance with the terms and conditions thereof, except for any failure to be in compliance that, individually or in the aggregate,
would not result in a Parent Material Adverse Effect. Neither Parent nor any of its Subsidiaries has received since December 31,
2020, any written notice or other communication (in writing or otherwise), whether from a Governmental Authority, citizens group, employee
or otherwise, that alleges that Parent or any of its Subsidiaries is not in compliance with any Environmental Law, and, to the Knowledge
of Parent, there are no circumstances that may prevent or interfere with Parent’s or any of its Subsidiaries’ compliance with
any Environmental Law in the future, except where such failure to comply would not reasonably be expected to have a Parent Material Adverse
Effect. To the Knowledge of Parent: (i) no current or prior owner of any property leased or controlled by Parent or any of its Subsidiaries
has received since December 31, 2020, any written notice or other communication relating to property owned or leased at any time
by Parent or any of its Subsidiaries, whether from a Governmental Authority, citizens group, employee or otherwise, that alleges that
such current or prior owner or Parent or any of its Subsidiaries is not in compliance with or violated any Environmental Law relating
to such property and (ii) neither Parent nor any of its Subsidiaries has any material liability under any Environmental Law.
4.19 Insurance.
Parent has made available to the Company accurate and complete copies of all material insurance policies and all material self-insurance
programs and arrangements relating to the business, assets, liabilities and operations of Parent and its Subsidiaries (including Merger
Subs). Each of such insurance policies is in full force and effect and Parent and its Subsidiaries (including Merger Subs) are in compliance
in all material respects with the terms thereof. Other than customary end of policy notifications from insurance carriers, since December 31,
2020, neither Parent nor any of its Subsidiaries has received any notice or other communication regarding any actual or possible: (i) cancellation
or invalidation of any insurance policy or (ii) refusal or denial of any coverage, reservation of rights or rejection of any material
claim under any insurance policy. Each of Parent and its Subsidiaries (including Merger Subs) has provided timely written notice to the
appropriate insurance carrier(s) of each Legal Proceeding pending against Parent or such Subsidiary for which Parent or such Subsidiary
has insurance coverage, and no such carrier has issued a denial of coverage or a reservation of rights with respect to any such Legal
Proceeding, or informed Parent or any of its Subsidiaries of its intent to do so.
4.20 Transactions
with Affiliates. Except as set forth in the Parent SEC Documents filed prior to the date of this Agreement, since the date of Parent’s
last proxy statement filed in June 2023 with the SEC, no event has occurred that would be required to be reported by Parent pursuant
to Item 404 of Regulation S-K promulgated by the SEC. Section 4.20 of the Parent Disclosure Schedule identifies each Person
who is (or who may be deemed to be) an Affiliate of Parent as of the date of this Agreement.
4.21 No
Financial Advisors. Except as set forth on Section 4.21 of the Parent Disclosure Schedule, no broker, finder or investment
banker is entitled to any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee or other fee or commission in connection
with the Contemplated Transactions based upon arrangements made by or on behalf of Parent.
4.22 Valid
Issuance; No Bad Actor. The Parent Capital Stock to be issued in the First Merger will, when issued in accordance with the provisions
of this Agreement, be validly issued, fully paid and nonassessable. To the Knowledge of Parent as of the date of this Agreement, no “bad
actor” disqualifying event described in Rule 506(d)(1)(i)-(viii) of the Securities Act (a “Disqualifying Event”)
is applicable to Parent or, to Parent’s Knowledge, any Parent Covered Person, except for a Disqualifying Event as to which Rule 506(d)(2)(ii-iv)
or (d)(3) of the Securities Act is applicable.
4.23 Privacy
and Data Security. Parent and its Subsidiaries have complied with all applicable Privacy Laws and the applicable terms of any Parent
Contracts relating to privacy, security, collection or use of Personal Information of any individuals (including clinical trial participants,
patients, patient family members, caregivers or advocates, physicians and other health care professionals, clinical trial investigators,
researchers, pharmacists) that interact with Parent or any of its Subsidiaries in connection with the operation of Parent’s and
its Subsidiaries’ business, except for such noncompliance as has not had, and would not reasonably be expected to have, individually
or in the aggregate, a Parent Material Adverse Effect. To the Knowledge of Parent, Parent has implemented and maintains reasonable Privacy
Policies and has complied with its Privacy Policies, except for such noncompliance as has not to the Knowledge of the Parent had, and
would not reasonably be expected to have, individually or in the aggregate, a Parent Material Adverse Effect. To the Knowledge of Parent,
as of the date hereof, no claims have been asserted or threatened against Parent by any Person alleging a violation of Privacy Laws, Privacy
Policies and/or the applicable terms of any Parent Contracts relating to privacy, security, collection or use of Personal Information
of any individuals. To the Knowledge of Parent, there have been no data security incidents, personal data breaches or other adverse events
or incidents related to Personal Information or Parent data in the custody or control of Parent or any service provider acting on behalf
of Parent, in each case where such incident, breach or event would result in a notification obligation to any Person under applicable
law or pursuant to the terms of any Parent Contract.
4.24 No
Other Representations or Warranties.
(a) Except
as previously set forth in this Section 4 or in any certificate delivered by Parent or Merger Subs to the Company pursuant
to this Agreement, neither Parent nor any Merger Sub makes any representation or warranty, express or implied, at law or in equity, with
respect to it or any of its assets, liabilities or operations, and any such other representations or warranties are hereby expressly disclaimed.
(b) Each
of Parent, First Merger Sub and Second Merger Sub acknowledges and agrees that, except for the representations and warranties of the Company
set forth in Section 3 or in any certificate delivered by the Company to Parent or the Merger Subs pursuant to this Agreement,
none of the Company or any of their respective Representatives is relying on any other representation or warranty of the Company or any
other Person made outside of Section 3 or such certificates, including regarding the accuracy or completeness of any such
other representations or warranties or the omission of any material information, whether express or implied, in each case, with respect
to the Contemplated Transactions.
Section 5. Agreements
of the Parties.
5.1 Stockholder
Notice. Promptly following receipt of the Required Company Stockholder Vote, the Company shall prepare and mail a notice (the “Stockholder
Notice”) to every stockholder of the Company that did not execute the Company Stockholder Written Consent. The Stockholder Notice
shall (i) be a statement to the effect that the Company Board determined that the Merger is advisable in accordance with Section 251(b) of
the DGCL and in the best interests of the stockholders of the Company and approved and adopted this Agreement, the Merger and the other
Contemplated Transactions, (ii) provide the stockholders of the Company to whom it is sent with notice of the actions taken in the
Company Stockholder Written Consent, including the adoption and approval of this Agreement, the Merger and the other Contemplated Transactions
in accordance with Section 228(e) of the DGCL and the certificate of incorporation and bylaws of the Company and (iii) include
a description of the appraisal rights of the Company’s stockholders available under the DGCL, along with such other information
as is required thereunder and pursuant to applicable Law.
5.2 Proxy
Statement.
(a) As
promptly as practicable after the Closing Date, but in any case, no later than ten (10) Business Days following the filing of Parent’s
annual report on Form 10-K for the year ended December 31, 2023, Parent shall prepare and file with the SEC a proxy statement
relating to the Parent Stockholder Meeting to be held in connection with the Parent Stockholder Matters (together with any amendments
thereof or supplements thereto, the “Proxy Statement”). Parent shall use its reasonable best efforts to (i) cause
the Proxy Statement to comply with applicable rules and regulations promulgated by the SEC and (ii) respond promptly to any
comments or requests of the SEC or its staff related to the Proxy Statement.
(b) Parent
covenants and agrees that the Proxy Statement (and the letter to stockholders, notice of meeting and form of proxy included therewith)
will (i) comply as to form in all material respects with the requirements of applicable U.S. federal securities Laws and the DGCL,
and (ii) will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein
or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading.
(c) Parent
shall use reasonable best efforts to cause the Proxy Statement to be mailed to Parent’s stockholders as promptly as practicable
after the Proxy Statement has been filed with the SEC and either (i) the SEC has indicated that it does not intend to review the
Proxy Statement or that its review of the Proxy Statement has been completed or (ii) at least ten (10) days shall have passed
since the Proxy Statement was filed with the SEC without receiving any correspondence from the SEC commenting upon, or indicating that
it intends to review, the Proxy Statement, all in compliance with applicable U.S. federal securities laws and the DGCL. If Parent, First
Merger Sub, Second Merger Sub or the Company become aware of any event or information that, pursuant to the Securities Act or the Exchange
Act, should be disclosed in an amendment or supplement to the Proxy Statement, as the case may be, then such Party, as the case may be,
shall promptly inform the other Parties thereof and shall cooperate with such other Parties in Parent filing such amendment or supplement
with the SEC and, if appropriate, in mailing such amendment or supplement to the Parent stockholders.
(d) The
Parties shall reasonably cooperate with each other and provide, and shall use reasonable best efforts to cause their respective Representatives
to provide, the other Party and its Representatives, with all true, correct and complete information regarding such Party that is required
by Law to be included in the Proxy Statement or reasonably requested by the Other Party to be included in the Proxy Statement. If at any
time the information provided in the Proxy Statement has or will become “stale” and new information should, as determined
by Parent acting reasonably, be disclosed in an amendment or supplement to the Proxy Statement, the Parent shall promptly inform the Company
thereof and each such Party shall cooperate with one another, and shall use reasonable best efforts to cause their accounting and other
outside professionals to so cooperate, (i) in providing the financial reporting necessary for such filing and (ii) in filing
such amendment or supplement with the SEC (and, if related to the Proxy Statement, mailing such amendment or supplement to the Parent
stockholders).
5.3 Employee
Benefits; Employment Agreements. Parent shall comply with the terms of any employment, severance, retention, change of control, or
similar agreement specified on Section 4.17(c) of the Parent Disclosure Schedule, subject to the provisions of such agreements.
5.4 Parent
Stockholder Meeting.
(a) Parent
shall take all action necessary under applicable Law to call, give notice of and hold a meeting of the holders of Parent Common Stock
to consider and vote to approve the Parent Stockholder Matters pursuant to the terms of this Agreement (such meeting, the “Parent
Stockholder Meeting”). The Parent Stockholder Meeting shall be held as promptly as practicable after the date that the definitive
Proxy Statement is filed with the SEC, and in any event no later than sixty (60) days after such date. Parent shall take reasonable measures
to ensure that all proxies solicited in connection with the Parent Stockholder Meeting are solicited in compliance with all applicable
Law. Notwithstanding anything to the contrary contained herein, if on the date of the Parent Stockholder Meeting, or a date preceding
the date on which the Parent Stockholder Meeting is scheduled, Parent reasonably believes that (i) it will not receive proxies sufficient
to obtain the approval of the Parent Stockholder Matters, whether or not a quorum would be present or (ii) it will not have sufficient
shares of Parent Common Stock represented (whether in person or by proxy) to constitute a quorum necessary to conduct the business of
the Parent Stockholder Meeting, Parent may postpone or adjourn, or make one or more successive postponements or adjournments of, the Parent
Stockholder Meeting as long as the date of the Parent Stockholder Meeting is not postponed or adjourned more than an aggregate of 30 days
in connection with any postponements or adjournments.
(b) Parent
agrees that (i) the Parent Board shall recommend that the holders of Parent Common Stock vote to approve the Parent Stockholder Matters
and shall use reasonable best efforts to solicit such approval within the timeframe set forth in Section 5.4(a) above,
and in any event to obtain such approvals at the next occurring annual meeting of the stockholders of Parent or, if such meeting is not
scheduled to be held within six (6) months after the Parent Stockholder Meeting, a special meeting of the stockholders of Parent
to be held within six (6) months after the Parent Stockholder Meeting, and (ii) the Proxy Statement shall include a statement
to the effect that the Parent Board recommends that Parent’s stockholders vote to approve the Parent Stockholder Matters (the recommendation
of the Parent Board being referred to as the “Parent Board Recommendation”).
(c) The
Company and Parent acknowledge that, under the Nasdaq Stock Market Rules, the Parent Common Stock Payment Shares and the Parent Preferred
Stock Payment Shares will not be entitled to vote on the Conversion Proposal.
5.5 Indemnification
of Officers and Directors.
(a) From
the First Effective Time through the sixth anniversary of the date on which the First Effective Time occurs, each of Parent and the Surviving
Company shall indemnify and hold harmless each person who is now, or has been at any time prior to the date hereof, or who becomes prior
to the First Effective Time, a director or officer of Parent or the Company, respectively (the “D&O Indemnified Parties”),
against all claims, losses, liabilities, damages, judgments, fines and reasonable fees, costs and expenses, including attorneys’
fees and disbursements (collectively, “Costs”), incurred in connection with any claim, action, suit, proceeding or
investigation, whether civil, criminal, administrative or investigative, arising out of or pertaining to the fact that the D&O Indemnified
Party is or was a director or officer of Parent or of the Company, whether asserted or claimed prior to, at or after the First Effective
Time, in each case, to the fullest extent permitted under the DGCL and the DLLCA. Each D&O Indemnified Party will be entitled to advancement
of expenses incurred in the defense of any such claim, action, suit, proceeding or investigation from each of Parent and the Surviving
Company, jointly and severally, upon receipt by Parent or the Surviving Company from the D&O Indemnified Party of a request therefor;
provided that any such person to whom expenses are advanced provides an undertaking to Parent, to the extent then required by the DGCL
and the DLLCA, to repay such advances if it is ultimately determined that such person is not entitled to indemnification.
(b) The
provisions of the certificate of incorporation and bylaws of Parent with respect to indemnification, advancement of expenses and exculpation
of present and former directors and officers of Parent that are presently set forth in the certificate of incorporation and bylaws of
Parent shall not be amended, modified or repealed for a period of six years from the First Effective Time in a manner that would adversely
affect the rights thereunder of individuals who, at or prior to the First Effective Time, were officers or directors of Parent, unless
such modification is required by applicable Law. The certificate of formation and limited liability company agreement of the Surviving
Company shall contain, and Parent shall cause the limited liability company agreement of the Surviving Company to so contain, provisions
no less favorable with respect to indemnification, advancement of expenses and exculpation of present and former directors and officers
as those presently set forth in the certificate of incorporation and bylaws of Parent.
(c) From
and after the First Effective Time, (i) the Surviving Company shall fulfill and honor in all respects the obligations of the Company
to its D&O Indemnified Parties as of immediately prior to the Closing pursuant to any indemnification provisions under the Company’s
Organizational Documents and pursuant to any indemnification agreements between the Company and such D&O Indemnified Parties, with
respect to claims arising out of matters occurring at or prior to the First Effective Time and (ii) Parent shall fulfill and honor
in all respects the obligations of Parent to its D&O Indemnified Parties as of immediately prior to the Closing pursuant to any indemnification
provisions under Parent’s Organizational Documents and pursuant to any indemnification agreements between Parent and such D&O
Indemnified Parties, with respect to claims arising out of matters occurring at or prior to the First Effective Time.
(d) From
and after the First Effective Time, Parent shall maintain directors’ and officers’ liability insurance policies, with an effective
date as of the Closing Date, on commercially available terms and conditions and with coverage limits customary for U.S. public companies
similarly situated to Parent.
(e) From
and after the First Effective Time, Parent shall pay all expenses, including reasonable attorneys’ fees, that are incurred by the
persons referred to in this Section 5.5 in connection with their successful enforcement of the rights provided to such persons
in this Section 5.5.
(f) The
provisions of this Section 5.5 are intended to be in addition to the rights otherwise available to the current and former
officers and directors of Parent and the Company by Law, charter, statute, bylaw or agreement, and shall operate for the benefit of, and
shall be enforceable by, each of the D&O Indemnified Parties, their heirs and their Representatives.
(g) In
the event Parent or the Surviving Company or any of their respective successors or assigns (i) consolidates with or merges into any
other Person and shall not be the continuing or surviving corporation or entity of such consolidation or merger or (ii) transfers
all or substantially all of its properties and assets to any Person, then, and in each such case, proper provision shall be made so that
the successors and assigns of Parent or the Surviving Company, as the case may be, shall succeed to the obligations set forth in this
Section 5.5. Parent shall cause the Surviving Company to perform all of the obligations of the Surviving Company under this
Section 5.5.
5.6 Tax
Matters.
(a) The
parties intend that, for U.S. federal (and applicable state and local) income Tax purposes, the First Merger and Second Merger, taken
together, shall constitute a single integrated transaction that qualifies as a “reorganization” within the meaning of Section 368(a)(1)(A) of
the Code as described in Rev. Rul. 2001-46, 2001-2 C.B. 321to which each of the Parent and Company are parties under Section 368(b) of
the Code. The Parties shall not file any U.S. federal, state or local Tax Return in a manner that is inconsistent with the treatment described
in this Section 5.7(a), and shall not take any inconsistent position during the course of any audit, litigation or other proceeding
with respect to Taxes, in each case, unless otherwise required by a determination within the meaning of Section 1313(a) of the
Code (or corresponding provision of state or local Law). The parties hereto shall, and shall cause their Affiliates to, cooperate with
each other and their respective counsel to document and support the Intended Tax Treatment. Notwithstanding the foregoing, (i) Parent
makes no representations or warranties to any other Person or to any Stockholder regarding the Intended Tax Treatment of the Merger or
any of the transactions or agreements contemplated hereby (except for the specific representations and warranties set forth in Section 4.16(n) and
Section 4.16(o) of this Agreement) and (ii) the Company and each Stockholder will rely solely on their own Tax advisors
for Tax advice in connection with this Agreement and any of the transactions or agreements contemplated hereby.
(b) The
parties hereby adopt this Agreement, as a “plan of reorganization” within the meaning of Treasury Regulations Sections 1.368-2(g) and
1.368-3(a). From the date hereof through the Closing, and following the Closing, the parties shall not, and shall not permit or cause
their respective Affiliates to, take any action, or knowingly fail to take any action, which action or failure to act prevents or impedes,
or would reasonably be expected to prevent or impede the Merger from qualifying for the Intended Tax Treatment.
(c) All
transfer, documentary, sales, use, stamp, registration, excise, recording, registration value added and other such similar Taxes and fees
(including any penalties and interest) that become payable in connection with or by reason of the execution of this Agreement and the
transactions contemplated hereby (collectively, “Transfer Taxes”) shall be borne and paid by Parent. The Company, First
Merger Sub, Second Merger Sub and Parent further agree to reasonably cooperate, and join in the execution of any Tax Returns, in each
case with respect to Transfer Taxes, and to further reasonably cooperate to reduce or eliminate the amount of any such Transfer Taxes
in accordance with applicable Law.
(d) Following
the Closing, each of the parties shall use commercially reasonable efforts to (and shall cause their respective Affiliates to) cooperate
fully, as and to the extent reasonably requested by another party, in connection with the filing of relevant Tax Returns, and any audit
or Tax proceeding. Such cooperation shall include the retention and (upon the other party’s request) the provision (with the right
to make copies) of records and information reasonably relevant to any Tax proceeding or audit, and making employees available on a mutually
convenient basis to provide additional information and explanation of any material provided hereunder.
5.7 Legends.
Parent shall be entitled to place appropriate legends on the book entries and/or certificates evidencing any shares of Parent Capital
Stock to be received in the First Merger by equityholders of the Company and to issue appropriate stop transfer instructions to the transfer
agent for Parent Capital Stock.
5.8 Officers
and Directors.
(a) The
Parties shall use reasonable best efforts and take all necessary action (including, to the extent necessary, procuring the resignation
or removal of any directors on the Parent Board immediately prior to such time) so that immediately after the Closing Date, two (2) individuals
nominated by unanimous agreement of the individuals set forth on Section 5.8(a) of the Company Disclosure Schedule and
approved by the Nominating and Corporate Governance Committee of the Parent Board (the “Company Directors”) shall be
appointed to the Parent Board; provided, that a sufficient number of directors shall qualify as “independent directors”
to the extent necessary to ensure that the composition of the Parent Board complies with applicable SEC and Nasdaq rules.
(b) The
Parties shall use reasonable best efforts and take all necessary action so that immediately after the Closing Date, the Persons set forth
on Schedule 5.8(b) of the Company Disclosure Schedule are elected or appointed, as applicable, to the positions of officers
of Parent and the Surviving Entity, as set forth therein, to serve in such positions until successors are duly appointed and qualified
in accordance with applicable Law.
5.9 Termination
of Certain Agreements and Rights. The Company shall cause any stockholder agreements, voting agreements, registration rights agreements,
co-sale agreements and any other similar Contracts between the Company and any holders of Company Capital Stock, including any such Contract
granting any Person investor rights, rights of first refusal, registration rights or director registration rights (collectively, the “Investor
Agreements”), to be terminated immediately prior to the First Effective Time, without any liability being imposed on the part
of Parent or the Surviving Company.
5.10 Section 16
Matters. Prior to the First Effective Time, Parent shall take all such steps as may be required (to the extent permitted under applicable
Laws) to cause any acquisitions of Parent Capital Stock and any options to purchase Parent Common Stock in connection with the Contemplated
Transactions, by each individual who is reasonably expected to become subject to the reporting requirements of Section 16(a) of
the Exchange Act with respect to Parent, to be exempt under Rule 16b-3 promulgated under the Exchange Act.
5.11 Listing.
Parent shall use its reasonable best efforts to prepare and submit to Nasdaq a notification form for the listing of the shares of Parent
Common Stock Payment Shares and the Parent Common Stock to be issued upon conversion of the Parent Preferred Stock Payment Shares to be
issued in connection with the Contemplated Transactions. The Parties will use reasonable best efforts to coordinate with respect to compliance
with Nasdaq rules and regulations. Each Party will promptly inform the other Party of all verbal or written communications between
Nasdaq and such Party or its representatives.
5.12 Allocation
Certificate. The Company will prepare and deliver to Parent prior to the Closing a certificate signed by an executive officer of the
Company in a form reasonably acceptable to Parent setting forth (as of immediately prior to the First Effective Time) (a) each holder
of Company Capital Stock, (b) such holder’s name, address, phone number and e-mail (c) the number or percentage and type
of Company Capital Stock held as of the Closing Date for each such holder and (d) the number of shares of Parent Capital Stock to
be issued to such holder pursuant to this Agreement in respect of the Company Capital Stock held by such holder as of immediately prior
to the First Effective Time, which shall be calculated using the Exchange Ratio (the “Allocation Certificate”).
5.13 Obligations
of Merger Subs. Parent will take all action necessary to cause Merger Subs to perform their obligations under this Agreement and to
consummate the Merger on the terms and conditions set forth in this Agreement.
5.14 Reservation
of Parent Common Stock; Issuance of Shares of Parent Common Stock. For so long as any Parent Preferred Stock Payment Shares remain
outstanding, Parent shall at all times reserve and keep available, free from preemptive rights, out of its authorized but unissued Parent
Common Stock or shares of Parent Common Stock held in treasury by Parent, for the purpose of effecting the conversion of the Parent Preferred
Stock Payment Shares, the full number of shares of Parent Common Stock then issuable upon the conversion of all Parent Preferred Stock
Payment Shares then outstanding. All shares of Parent Common Stock delivered upon conversion of the Parent Preferred Stock Payment Shares
shall be newly issued shares or shares held in treasury by Parent, shall have been duly authorized and validly issued and shall be fully
paid and nonassessable, and shall be free from preemptive rights and free of any Encumbrance.
5.15 Takeover
Statutes. If any Takeover Statute is or may become applicable to the Contemplated Transaction, each of the Company, the Company Board,
the Parent and the Parent Board, as applicable, shall grant such approvals and take such actions as are necessary so that the Contemplated
Transactions may be consummated as promptly as practicable on the terms contemplated by this Agreement and otherwise to eliminate or minimize
the effects of such statute or regulation on the Contemplated Transactions.
5.16 Private
Placement. Each of the Company and Parent shall take all reasonably necessary action on its part such that the issuance of Parent
Common Stock Payment Shares and Parent Preferred Stock Payment Shares pursuant to this Agreement constitutes a transaction exempt from
registration under the Securities Act in compliance with Rule 506 of Regulation D promulgated thereunder.
Section 6. Conditions
Precedent to Obligations of Each Party.
The obligations of each Party
to effect the Merger and otherwise consummate the Contemplated Transactions to be consummated at the Closing are subject to the satisfaction
or, to the extent permitted by applicable law, the written waiver by each of the Parties, at or prior to the Closing, of each of the following
conditions:
6.1 No
Restraints. No temporary restraining order, preliminary or permanent injunction or other Order preventing the consummation of the
Contemplated Transactions shall have been issued by any court of competent jurisdiction or other Governmental Authority of competent jurisdiction
and remain in effect and there shall not be any Law which has the effect of making the consummation of the Contemplated Transactions illegal.
6.2 Company
Stockholder Approval. The Company shall have obtained the Required Company Stockholder Vote.
Section 7. Closing
Deliveries.
7.1 Closing
Deliveries of the Company.
The obligations of Parent
and Merger Subs to effect the Merger and otherwise consummate the transactions to be consummated at the Closing are subject to Parent
receiving the following documents, each of which shall be in full force and effect, or the written waiver by Parent of delivery:
| (A) | The Company Stockholder Written Consent executed by the stockholders of the Company shall be in full force
and effect; |
| (B) | The Lock-Up Agreements duly executed by each of the Company Signatories, each of which shall be in full
force and effect; |
| (D) | The Voting Support Agreements; |
| (E) | The Restrictive Covenants Agreements; |
| (F) | (i) An original signed statement from the Company that the Company is not, and has not been at any
time during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code, a “United States real property holding
corporation,” as defined in Section 897(c)(2) of the Code, conforming to the requirements of Treasury Regulations Section 1.1445-2(c)(3) and
1.897-2(h), and (ii) an original signed notice to be delivered to the IRS in accordance with the provisions of Treasury Regulations
Section 1.897-2(h)(2), together with written authorization for Parent to deliver such notice to the IRS on behalf of the Company
following the Closing, each dated as of the Closing Date, duly executed by an authorized officer of the Company, and in form and substance
reasonably acceptable to Parent; provided, that, the Parent’s sole remedy for company’s failure to deliver such
documentation shall be to withhold pursuant to Section 2.14; |
| (G) | The Allocation Certificate; |
| (H) | A duly completed and executed accredited investor questionnaires in substantially the form attached hereto
as Exhibit G (the “Accredited Investor Questionnaire”) from each holder of Company Capital
Stock; |
| (I) | agreements to convert into equity all indebtedness of the Company set forth on Schedule 7.1(I), in form
and substance reasonably acceptable to Parent; and |
| (J) | agreements to convert each Convertible Note into Company Common Stock, in form and substance reasonably
acceptable to Parent. |
7.2 Closing
Deliveries of Parent.
The obligations of the Company
to effect the Merger and otherwise consummate the transactions to be consummated at the Closing are subject to the Company receiving the
following documents, each of which shall be in full force and effect, or the written waiver by the Company of delivery:
| (A) | a copy of the Certificate of Designation, certified by the Secretary of State of the State of Delaware; |
| (B) | a written consent of the holders of a majority of the outstanding Parent Series B Preferred Stock
authorizing the execution, delivery and performance of this Agreement and the consummation of the Contemplated Transactions (including
the Parent Stockholder Matters); |
| (C) | the Parent Stockholder Support Agreement duly executed by each required stockholder of Parent; |
| (D) | certified copies of the resolutions duly adopted by the Parent Board and in full force and effect as of
the Closing authorizing the appointment of the Directors and officers set forth in Section 5.8(a); and |
| (E) | the Lock-Up Agreements duly executed by each of the Parent Signatories, each of which shall be in full
force and effect. |
Section 8. Miscellaneous
Provisions.
8.1 Non-Survival
of Representations and Warranties. The representations and warranties of the Company, Parent and Merger Subs contained in this Agreement
or any certificate or instrument delivered pursuant to this Agreement shall terminate at the First Effective Time, and only the covenants
that by their terms survive the First Effective Time and this Section 8 shall survive the First Effective Time.
8.2 Amendment.
This Agreement may be amended with the approval of the respective boards of directors of the Company and Parent at any time (whether before
or after the adoption and approval of this Agreement by the Company’s stockholders or before or after obtaining the approval of
the Parent Stockholder Matters); provided, however, that after any such approval of this Agreement by a Party’s stockholders,
no amendment shall be made which by Law requires further approval of such stockholders without the further approval of such stockholders.
This Agreement may not be amended except by an instrument in writing signed on behalf of each of the Company and Parent.
8.3 Waiver.
(a) Any
provision hereof may be waived by the waiving Party solely on such Party’s own behalf, without the consent of any other Party. No
failure on the part of any Party to exercise any power, right, privilege or remedy under this Agreement, and no delay on the part of any
Party in exercising any power, right, privilege or remedy under this Agreement, shall operate as a waiver of such power, right, privilege
or remedy; and no single or partial exercise of any such power, right, privilege or remedy shall preclude any other or further exercise
thereof or of any other power, right, privilege or remedy.
(b) No
Party shall be deemed to have waived any claim arising out of this Agreement, or any power, right, privilege or remedy under this Agreement,
unless the waiver of such claim, power, right, privilege or remedy is expressly set forth in a written instrument duly executed and delivered
on behalf of such Party and any such waiver shall not be applicable or have any effect except in the specific instance in which it is
given.
8.4 Entire
Agreement; Counterparts; Exchanges by Electronic Transmission or Facsimile. This Agreement and the other schedules, exhibits, certificates,
instruments and agreements referred to in this Agreement constitute the entire agreement and supersede all prior agreements and understandings,
both written and oral, among or between any of the Parties with respect to the subject matter hereof and thereof; provided, however,
that the Confidentiality Agreement shall not be superseded and shall remain in full force and effect in accordance with its terms. This
Agreement may be executed in several counterparts, each of which shall be deemed an original and all of which shall constitute one and
the same instrument. The exchange of a fully executed Agreement (in counterparts or otherwise) by all Parties by facsimile or electronic
transmission in .PDF format shall be sufficient to bind the Parties to the terms and conditions of this Agreement.
8.5 Applicable
Law; Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the Laws of the State of Delaware, regardless
of the Laws that might otherwise govern under applicable principles of conflicts of laws. In any action or proceeding between any of the
Parties arising out of or relating to this Agreement or any of the Contemplated Transactions, each of the Parties: (a) irrevocably
and unconditionally consents and submits to the exclusive jurisdiction and venue of the Court of Chancery of the State of Delaware or,
to the extent such court does not have subject matter jurisdiction, the Superior Court of the State of Delaware or the United States District
Court for the District of Delaware, (b) agrees that all claims in respect of such action or proceeding shall be heard and determined
exclusively in accordance with clause (a) of this Section 8.5, (c) waives any objection to laying venue in any such
action or proceeding in such courts, (d) waives any objection that such courts are an inconvenient forum or do not have jurisdiction
over any Party, (e) agrees that service of process upon such Party in any such action or proceeding shall be effective if notice
is given in accordance with Section 8.7 of this Agreement and (f) irrevocably and unconditionally waives the right to
trial by jury.
8.6 Assignability.
This Agreement shall be binding upon, and shall be enforceable by and inure solely to the benefit of, the Parties and their respective
successors and permitted assigns; provided, however, that neither this Agreement nor any of a Party’s rights or obligations
hereunder may be assigned or delegated by such Party without the prior written consent of the other Party, and any attempted assignment
or delegation of this Agreement or any of such rights or obligations by such Party without the other Party’s prior written consent
shall be void and of no effect.
8.7 Notices.
All notices and other communications hereunder shall be in writing and shall be deemed to have been duly delivered and received hereunder
(a) one Business Day after being sent for next Business Day delivery, fees prepaid, via a reputable international overnight courier
service, (b) upon delivery in the case of delivery by hand or (c) on the date delivered in the place of delivery if sent by
email or facsimile (with a written or electronic confirmation of delivery) prior to 6:00 p.m. New York City time, otherwise on the
next succeeding Business Day, in each case to the intended recipient as set forth below:
if to Parent or Merger Subs:
First Wave BioPharma, Inc.
777 Yamato Road, Suite 502
Boca Raton, FL 33431
Attention: James Sapirstein
Email: jsapirstein@firstwavebio.com
with a copy to (which shall
not constitute notice):
Lowenstein Sandler LLP
One Lowenstein Drive
Roseland, New Jersey 07068
Attention: Michael Lerner
Email:
mlerner@lowenstein.com
if to the Company:
ImmunogenX, Inc.
1600 Dove Street, Suite 330
Newport Beach, CA 92660
Attention: Jack Syage
Email: jsyage@immunogenx.com
with a copy to (which shall
not constitute notice):
Orrick, Herrington & Sutcliffe LLP
Columbia Center
1152 15th Street, N.W.
Washington, D.C. 20005-1706
Attention: David Schulman
Email: dschulman@orrick.com
8.8 Cooperation.
Each Party agrees to cooperate fully with the other Party and to execute and deliver such further documents, certificates, agreements
and instruments and to take such other actions as may be reasonably requested by the other Party to evidence or reflect the Contemplated
Transactions and to carry out the intent and purposes of this Agreement.
8.9 Severability.
Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity
or enforceability of the remaining terms and provisions of this Agreement or the validity or enforceability of the offending term or provision
in any other situation or in any other jurisdiction. If a final judgment of a court of competent jurisdiction declares that any term or
provision of this Agreement is invalid or unenforceable, the Parties agree that the court making such determination shall have the power
to limit such term or provision, to delete specific words or phrases or to replace such term or provision with a term or provision that
is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term or provision, and this
Agreement shall be valid and enforceable as so modified. In the event such court does not exercise the power granted to it in the prior
sentence, the Parties agree to replace such invalid or unenforceable term or provision with a valid and enforceable term or provision
that will achieve, to the extent possible, the economic, business and other purposes of such invalid or unenforceable term or provision.
8.10 Other
Remedies; Specific Performance. Except as otherwise provided herein, any and all remedies herein expressly conferred upon a Party
will be deemed cumulative with and not exclusive of any other remedy conferred hereby, or by law or equity upon such Party, and the exercise
by a Party of any one remedy will not preclude the exercise of any other remedy. The Parties agree that irreparable damage for which monetary
damages, even if available, would not be an adequate remedy, would occur in the event that any of the provisions of this Agreement were
not performed in accordance with their specific terms (including failing to take such actions as are required of it hereunder to consummate
this Agreement) or were otherwise breached. It is accordingly agreed that the Parties shall be entitled to an injunction or injunctions
to prevent breaches of this Agreement and to enforce specifically the terms and provisions hereof in any court of the United States or
any state having jurisdiction, this being in addition to any other remedy to which they are entitled at law or in equity, and each of
the Parties waives any bond, surety or other security that might be required of any other Party with respect thereto. Each of the Parties
further agrees that it will not oppose the granting of an injunction, specific performance or other equitable relief on the basis that
any other Party has an adequate remedy at law or that any award of specific performance is not an appropriate remedy for any reason at
law or in equity.
8.11 No
Third-Party Beneficiaries. Nothing in this Agreement, express or implied, is intended to or shall confer upon any Person (other than
the Parties and the D&O Indemnified Parties to the extent of their respective rights pursuant to Section 5.5 any right,
benefit or remedy of any nature whatsoever under or by reason of this Agreement.
8.12 Expenses.
Except as otherwise expressly provided in this Agreement, all expenses incurred in connection with this Agreement and the Contemplated
Transactions will be paid by the Party incurring such expenses.
[Remainder of page intentionally left blank]
IN
WITNESS WHEREOF, the Parties have caused this Agreement to be executed as of the date first above written.
FIRST WAVE BIOPHARMA, INC.
| By: |
/s/ James Sapirstein |
|
| Name: |
James Sapirstein |
|
| Title: |
President and Chief Executive Officer |
|
IMMUNO MERGER SUB I, INC.
| By: |
/s/ James Sapirstein |
|
| Name: |
James Sapirstein |
|
| Title: |
President and Secretary |
|
IMMUNO MERGER SUB II, LLC
| By: |
/s/ James Sapirstein |
|
| Name: |
James Sapirstein |
|
| Title: |
President and Secretary |
|
IMMUNOGENX, INC.
| By: |
/s/ Jack Syage |
|
| Name: |
Jack Syage |
|
| Title: |
Chief Executive Officer |
|
EXHIBIT A
FORM OF VOTING AGREEMENT
Execution Version
VOTING AGREEMENT
THIS
VOTING AGREEMENT (this “Agreement”), dated as of March 13, 2024, is made by and among First
Wave BioPharma, Inc., a Delaware corporation (“Parent”), ImmunogenX, Inc., a Delaware corporation
(the “Company”), and the undersigned holder (“Stockholder”) of shares of voting capital
stock (the “Shares”) of Parent.
WHEREAS,
concurrently with the entry into of this Agreement, Parent, IMMUNO Merger Sub I, Inc., a Delaware corporation and a wholly owned
subsidiary of Parent (“First Merger Sub”), IMMUNO Merger Sub II, LLC, a Delaware limited liability company
and a wholly owned subsidiary of Parent (“Second Merger Sub”), and the Company, are entering into an Agreement
and Plan of Merger, dated of even date herewith (the “Merger Agreement”), providing for the merger of First
Merger Sub with and into the Company (the “First Merger”) and the merger of the Company with and into Second
Merger Sub (the “Second Merger” and together with the First Merger, the “Merger”);
WHEREAS,
in connection with the Merger, Stockholder received and now beneficially owns and has sole or shared voting power with respect to the
number of Shares indicated opposite Stockholder’s name on Schedule 1 attached hereto;
WHEREAS,
as an inducement and a condition to the willingness of Parent, First Merger Sub, Second Merger Sub and the Company to enter into the Merger
Agreement, and in consideration of the substantial expenses incurred and to be incurred by them in connection therewith, Stockholder has
agreed to enter into and perform this Agreement; and
WHEREAS,
all capitalized terms used in this Agreement without definition herein shall have the meanings ascribed to them in the Merger Agreement.
NOW,
THEREFORE, in consideration of, and as a condition to, Parent, First Merger Sub, Second Merger Sub and the Company’s
entering into the Merger Agreement and proceeding with the transactions contemplated thereby, and in consideration of the substantial
expenses incurred and to be incurred by them in connection therewith, Stockholder, Parent and the Company agree as follows:
1) Agreement
to Vote Shares. Unless otherwise prohibited by the Organizational Documents of Parent, the Merger Agreement or the agreements entered
into pursuant thereto, or any Nasdaq Stock Market Rules applicable to Parent, Stockholder agrees that, prior to the Expiration Date
(as defined in Section 2), at any meeting of the stockholders of Parent or any adjournment or postponement thereof, or in
connection with any written consent of the stockholders of Parent, with respect to the Charter Amendment Proposal, if the Charter Amendment
Proposal is put forth to a vote of the stockholders of Parent, Stockholder shall, or shall cause the holder of record on any applicable
record date to:
a) appear
at such meeting or otherwise cause the Shares and any New Shares (as defined in Section 3) to be counted as present thereat
(in person or by proxy) for purposes of calculating a quorum; and
b) from
and after the date hereof until the Expiration Date, vote (or cause to be voted), or deliver a written consent (or cause a written consent
to be delivered) covering all of the Shares and any New Shares that Stockholder shall be entitled to so vote: (i) in favor of the
Charter Amendment Proposal and any matter that would reasonably be expected to facilitate the Charter Amendment Proposal; (ii) against
any agreement, transaction or other matter that is intended to, or would reasonably be expected to, impede, interfere with, delay, postpone,
discourage or materially and adversely affect the consummation of the Charter Amendment Proposal; and (iii) to approve any proposal
to adjourn or postpone the applicable meeting to a later date, if there are not sufficient votes for the approval of the Charter Amendment
Proposal on the date on which such meeting is held. Stockholder shall not take or commit or agree to take any action inconsistent with
the foregoing.
2) Expiration
Date. As used in this Agreement, the term “Expiration Date” shall mean the earlier to occur of (a) the
effective time of the approval of the Charter Amendment Proposal or (b) the date as of which, by mutual written agreement of Parent
and Stockholder, this agreement is terminated, or (c) twelve (12) months following the date of this Agreement.
3) Additional
Purchases. Stockholder agrees that any shares of capital stock or other equity securities of Parent that Stockholder purchases or
with respect to which Stockholder otherwise acquires sole or shared voting power (including any proxy) after the execution of this Agreement
and prior to the Expiration Date, whether by the exercise of any Parent Stock Options, settlement of Parent Restricted Stock Units or
otherwise, including by gift, succession, in the event of a stock split or as a dividend or distribution of any Shares (“New
Shares”), shall be subject to the terms and conditions of this Agreement to the same extent as if they constituted the Shares.
4) Reserved.
5) Representations
and Warranties of Stockholder. Stockholder hereby represents and warrants to Parent and the Company as follows:
a) If
Stockholder is an Entity: (i) Stockholder is duly organized, validly existing and in good standing under the laws of the jurisdiction
in which it is incorporated, organized or constituted, (ii) Stockholder has all necessary power and authority to execute and deliver
this Agreement, to perform Stockholder’s obligations hereunder and to consummate the transactions contemplated hereby, and (iii) the
execution and delivery of this Agreement, performance of Stockholder’s obligations hereunder and the consummation of the transactions
contemplated hereby by Stockholder have been duly authorized by all necessary action on the part of Stockholder and no other proceedings
on the part of Stockholder are necessary to authorize this Agreement, or to consummate the transactions contemplated hereby. If Stockholder
is an individual, Stockholder has the legal capacity to execute and deliver this Agreement, to perform Stockholder’s obligations
hereunder and to consummate the transactions contemplated hereby;
b) this
Agreement has been duly executed and delivered by or on behalf of Stockholder and, and assuming this Agreement constitutes a valid and
binding agreement of the Company and Parent, constitutes a valid and binding agreement with respect to Stockholder, enforceable against
Stockholder in accordance with its terms, except as enforcement may be limited by general principles of equity whether applied in a court
of Law or a court of equity and by bankruptcy, insolvency and similar Laws affecting creditors’ rights and remedies generally;
c) Stockholder
beneficially owns the number of Shares and other rights with respect to Shares indicated opposite Stockholder’s name on Schedule
1, and will own any New Shares, free and clear of any liens, claims, charges or other encumbrances or restrictions of any kind whatsoever
(“Liens”), and has sole or shared, and otherwise unrestricted, voting power with respect to such Shares or New
Shares and none of the Shares or New Shares is subject to any voting trust or other agreement, arrangement or restriction with respect
to the voting of the Shares or the New Shares, except as contemplated by this Agreement;
d) the
execution and delivery of this Agreement by Stockholder does not, and the performance by Stockholder of his, her or its obligations hereunder
and the compliance by Stockholder with any provisions hereof will not, violate or conflict with, result in a material breach of or constitute
a default (or an event that with notice or lapse of time or both would become a material default) under, or give to others any rights
of termination, amendment, acceleration or cancellation of, or result in the creation of any Liens on any Shares or New Shares pursuant
to, any agreement, instrument, note, bond, mortgage, Contract, lease, license, permit or other obligation or any order, arbitration award,
judgment or decree to which Stockholder is a party or by which Stockholder is bound, or any Law, statute, rule or regulation to which
Stockholder is subject or, in the event that Stockholder is a corporation, partnership, trust or other Entity, any bylaw or other Organizational
Document of Stockholder; except for any of the foregoing as would not reasonably be expected to prevent or delay the performance by Stockholder
of his, her or its obligations under this Agreement in any material respect;
e) the
execution and delivery of this Agreement by Stockholder does not, and the performance of this Agreement by Stockholder does not and will
not, require any consent, approval, authorization or permit of, or filing with or notification to, any Governmental Body or regulatory
authority by Stockholder except for applicable requirements, if any, of the Exchange Act, and except where the failure to obtain such
consents, approvals, authorizations or permits, or to make such filings or notifications, would not prevent or delay the performance by
Stockholder of his, her or its obligations under this Agreement in any material respect;
f) no
investment banker, broker, finder or other intermediary is entitled to a fee or commission from Parent or the Company in respect of this
Agreement based upon any Contract made by or on behalf of Stockholder; and
g) as
of the date of this Agreement, there is no Legal Proceeding pending or, to the knowledge of Stockholder, threatened against Stockholder
that would reasonably be expected to prevent or delay the performance by Stockholder of his, her or its obligations under this Agreement
in any material respect.
6) Irrevocable
Proxy. Subject to the final sentence of this Section 6, by execution of this Agreement, Stockholder does hereby appoint
Parent and any of its designees with full power of substitution and resubstitution, as Stockholder’s true and lawful attorney and
irrevocable proxy, to the fullest extent of Stockholder’s rights with respect to the Shares or New Shares, to vote and exercise
all voting and related rights, including the right to sign Stockholder’s name (solely in its capacity as a stockholder) to any stockholder
consent, if Stockholder is unable to perform or otherwise does not perform his, her or its obligations under this Agreement, with respect
to such Shares and New Shares solely with respect to the matters set forth in Section 1. Stockholder intends this proxy to
be irrevocable and coupled with an interest hereunder until the Expiration Date, hereby revokes any proxy previously granted by Stockholder
with respect to the Shares or New Shares and represents that none of such previously-granted proxies are irrevocable. The irrevocably
proxy and power of attorney granted herein shall survive the death or incapacity of Stockholder and the obligations of Stockholder shall
be binding on Stockholder’s heirs, personal representatives, successors, transferees and assigns. Stockholder hereby agrees not
to grant any subsequent powers of attorney or proxies with respect to any Shares or New Shares with respect to the matters set forth in
Section 1 until after the Expiration Date. The Stockholder hereby affirms that the proxy set forth in this Section 6
is given in connection with and granted in consideration of and as an inducement to the Company, Parent, First Merger Sub and Second Merger
Sub to enter into the Merger Agreement and that such proxy is given to secure the obligations of the Stockholder under Section 1.
Notwithstanding anything contained herein to the contrary, this irrevocable proxy shall automatically terminate upon the Expiration Date.
7) Other
Remedies; Specific Performance. Except as otherwise provided herein, any and all remedies herein expressly conferred upon a party
will be deemed cumulative with, and not exclusive of, any other remedy conferred hereby, or by Law or equity upon such party, and the
exercise by a party of any one remedy will not preclude the exercise of any other remedy. The parties hereto agree that irreparable damage
would occur in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were
otherwise breached. It is accordingly agreed that the parties shall be entitled to an injunction or injunctions to prevent breaches of
this Agreement and to enforce specifically the terms and provisions hereof without the need of posting bond in any court of the United
States or any state having jurisdiction, this being in addition to any other remedy to which they are entitled at Law or in equity.
8) Directors
and Officers. This Agreement shall apply to Stockholder solely in Stockholder’s capacity as a stockholder of Parent, and not
in Stockholder’s capacity as a director, officer or employee of Parent or any of its Subsidiaries or in Stockholder’s capacity
as a trustee or fiduciary of any employee benefit plan or trust, as applicable. Notwithstanding any provision of this Agreement to the
contrary, nothing in this Agreement shall (or require Stockholder to attempt to) limit or restrict a director or officer of Parent in
the exercise of his or her fiduciary duties as a director or officer of Parent or in his or her capacity as a trustee or fiduciary of
any employee benefit plan or trust or prevent or be construed to create any obligation on the part of any director or officer of Parent
or any trustee or fiduciary of any employee benefit plan or trust from taking any action in his or her capacity as such director, officer,
trustee or fiduciary.
9) No
Ownership Interest. Nothing contained in this Agreement shall be deemed to vest in the Company or Parent any direct or indirect ownership
or incidence of ownership of or with respect to any Shares or New Shares. All rights, ownership and economic benefits of and relating
to the Shares or New Shares shall remain vested in and belong to Stockholder.
10) Termination.
This Agreement shall terminate and shall have no further force or effect as of the Expiration Date. Notwithstanding the foregoing, upon
termination or expiration of this Agreement, no party shall have any further obligations or liabilities under this Agreement; provided,
however, that nothing set forth in this Section 10 or elsewhere in this Agreement shall relieve any party from liability
for any fraud or for any willful and material breach of this Agreement prior to termination hereof.
11) Further
Assurances. Stockholder shall, from time to time, execute and deliver, or cause to be executed and delivered, such additional or further
consents, documents and other instruments as the Company or Parent may reasonably request for the purpose of effectively carrying out
the transactions contemplated by this Agreement, the Charter Amendment Proposal, and the transactions contemplated thereby.
12) Disclosure.
Stockholder hereby agrees that Parent and the Company may publish and disclose in any registration statement, any prospectus filed with
any regulatory authority in connection with the Contemplated Transactions and any related documents filed with such regulatory authority
and as otherwise required by Law, Stockholder’s identity and ownership of Shares and the nature of Stockholder’s commitments,
arrangements and understandings under this Agreement and may further file this Agreement as an exhibit to any registration statement or
prospectus or in any other filing made by Parent or the Company as required by Law or the terms of the Merger Agreement, including with
the SEC or other regulatory authority, relating to the Contemplated Transactions, all subject to prior review and an opportunity to comment
by Stockholder’s counsel. Prior to the Closing, Stockholder shall not, and shall use its reasonable best efforts to cause its representatives
not to, directly or indirectly, make any press release, public announcement or other public communication that criticizes or disparages
this Agreement, the Charter Amendment Proposal, or the transactions contemplated thereby, without the prior written consent of Parent
and the Company; provided that the foregoing shall not limit or affect any actions taken by Stockholder (or any affiliated officer
or director of Stockholder) that would be permitted to be taken by Stockholder, Parent or the Company pursuant to the Merger Agreement;
provided, further, that the foregoing shall not affect any actions of Stockholder the prohibition of which would be prohibited
under applicable Law.
13) Notice.
All notices and other communications hereunder shall be in writing and shall be deemed given if delivered personally or sent by overnight
courier (providing proof of delivery), by electronic transmission (providing confirmation of transmission) to the Company or Parent, as
the case may be, in accordance with Section 8.7 of the Merger Agreement and to Stockholder at his, her or its address or email
address (providing confirmation of transmission) set forth on Schedule 1 attached hereto (or at such other address for a party
as shall be specified by like notice).
14) Severability.
Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity
or enforceability of the remaining terms and provisions of this Agreement or the validity or enforceability of the offending term or provision
in any other situation or in any other jurisdiction. If a final judgment of a court of competent jurisdiction declares that any term or
provision of this Agreement is invalid or unenforceable, the parties hereto agree that the court making such determination shall have
the power to limit such term or provision, to delete specific words or phrases or to replace such term or provision with a term or provision
that is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term or provision, and
this Agreement shall be valid and enforceable as so modified. In the event such court does not exercise the power granted to it in the
prior sentence, the parties hereto agree to replace such invalid or unenforceable term or provision with a valid and enforceable term
or provision that will achieve, to the extent possible, the economic, business and other purposes of such invalid or unenforceable term
or provision.
15) Assignability.
This Agreement shall be binding upon, and shall be enforceable by and inure solely to the benefit of, the parties hereto and their respective
successors and assigns; provided, however, that neither this Agreement nor any of a party’s rights or obligations
hereunder may be assigned or delegated by such party without the prior written consent of the other parties hereto, and any attempted
assignment or delegation of this Agreement or any of such rights or obligations by such party without the other party’s prior written
consent shall be void and of no effect. Nothing in this Agreement, express or implied, is intended to or shall confer upon any Person
(other than the parties hereto) any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement.
16) No
Waivers. No waivers of any breach of this Agreement extended by the Company or Parent to Stockholder shall be construed as a waiver
of any rights or remedies of the Company or Parent, as applicable, with respect to any other stockholder of Parent who has executed an
agreement substantially in the form of this Agreement with respect to Shares or New Shares held or subsequently held by such stockholder
or with respect to any subsequent breach of Stockholder or any other stockholder of Parent. No waiver of any provisions hereof by any
party shall be deemed a waiver of any other provisions hereof by any such party, nor shall any such waiver be deemed a continuing waiver
of any provision hereof by such party.
17) Applicable
Law; Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the Laws of the state of Delaware, regardless
of the Laws that might otherwise govern under applicable principles of conflicts of Laws. In any action or Legal Proceeding between any
of the parties arising out of or relating to this Agreement, each of the parties: (a) irrevocably and unconditionally consents and
submits to the exclusive jurisdiction and venue of the Court of Chancery of the state of Delaware or to the extent such court does not
have subject matter jurisdiction, the Superior Court of the State of Delaware or the United States District Court for the District of
Delaware, (b) agrees that all claims in respect of such action or Legal Proceeding shall be heard and determined exclusively in accordance
with clause (a) of this Section 17, (c) waives any objection to laying venue in any such action or Legal Proceeding
in such courts, (d) waives any objection that such courts are an inconvenient forum or do not have jurisdiction over any party, and
(e) agrees that service of process upon such party in any such action or Legal Proceeding shall be effective if notice is given in
accordance with Section 13.
18) Waiver
of Jury Trial. THE PARTIES HERETO HEREBY WAIVE ANY RIGHT TO TRIAL BY JURY WITH RESPECT TO ANY ACTION OR LEGAL PROCEEDING RELATED TO
OR ARISING OUT OF THIS AGREEMENT, ANY DOCUMENT EXECUTED IN CONNECTION HEREWITH AND THE MATTERS CONTEMPLATED HEREBY AND THEREBY.
19) No
Agreement Until Executed. Irrespective of negotiations among the parties or the exchanging of drafts of this Agreement, this Agreement
shall not constitute or be deemed to evidence a Contract, agreement, arrangement or understanding between the parties hereto unless and
until (a) the Parent Board has approved, for purposes of any applicable anti-takeover Laws and regulations and any applicable provision
of the certificate of incorporation of Parent, the Merger Agreement and the Contemplated Transactions, (b) the Merger Agreement is
executed by all parties thereto, and (c) this Agreement is executed by all parties hereto.
20) Entire
Agreement; Counterparts; Exchanges by Electronic Transmission. This Agreement and the other agreements referred to in this Agreement
constitute the entire agreement and supersede all prior agreements and understandings, both written and oral, among or between any of
the parties with respect to the subject matter hereof and thereof. This Agreement may be executed in several counterparts, each of which
shall be deemed an original and all of which shall constitute one and the same instrument. The exchange of a fully executed Agreement
(in counterparts or otherwise) by all parties by electronic transmission via “.pdf” shall be sufficient to bind the parties
to the terms and conditions of this Agreement.
21) Amendment.
This Agreement may not be amended, supplemented or modified, and no provisions hereof may be modified or waived, except by an instrument
in writing signed on behalf of each party hereto; provided, however, that the rights or obligations of any Stockholder may
be waived, amended or otherwise modified in a writing signed by Parent, the Company and Stockholder.
22) Fees
and Expenses. Except as otherwise specifically provided herein, the Merger Agreement or any other agreement contemplated by the Merger
Agreement to which a party hereto is a party, each party hereto shall bear its own expenses in connection with this Agreement and the
transactions contemplated hereby.
23) Voluntary
Execution of Agreement. This Agreement is executed voluntarily and without any duress or undue influence on the part or behalf of
the parties. Each of the parties hereby acknowledges, represents and warrants that (a) it has read and fully understood this Agreement
and the implications and consequences thereof; (b) it has been represented in the preparation, negotiation, and execution of this
Agreement by legal counsel of its own choice, or it has made a voluntary and informed decision to decline to seek such counsel; and (c) it
is fully aware of the legal and binding effect of this Agreement.
24) Construction.
a) For
purposes of this Agreement, whenever the context requires: the singular number shall include the plural, and vice versa; the masculine
gender shall include the feminine and neuter genders; the feminine gender shall include the masculine and neuter genders; and the neuter
gender shall include masculine and feminine genders.
b) The
parties hereto agree that any rule of construction to the effect that ambiguities are to be resolved against the drafting party shall
not be applied in the construction or interpretation of this Agreement.
c) As
used in this Agreement, the words “include” and “including,” and variations thereof, shall not be deemed to be
terms of limitation, but rather shall be deemed to be followed by the words “without limitation.”
d) Except
as otherwise indicated, all references in this Agreement to “Sections,” and “Schedules” are intended to refer
to Sections of this Agreement and Schedules to this Agreement, respectively.
e) The
underlined headings contained in this Agreement are for convenience of reference only, shall not be deemed to be a part of this Agreement
and shall not be referred to in connection with the construction or interpretation of this Agreement.
[Remainder of Page has Intentionally Been
Left Blank]
EXECUTED as of the date first
above written.
| |
STOCKHOLDER |
| |
|
| |
STOCKHOLDER NAME: |
| |
|
| |
Name of signatory (if an Entity): |
|
[Signature Page to Voting
Agreement]
EXECUTED as of the date first
above written.
| |
PARENT: |
| |
|
| |
FIRST WAVE BIOPHARMA, INC. |
[Signature Page to Voting
Agreement]
EXECUTED as of the date first
above written.
| |
COMPANY: |
| |
|
| |
IMMUNOGENX, INC. |
[Signature Page to Voting Agreement]
SCHEDULE 1
| Name of Stockholder |
|
|
| Address: |
|
|
| |
|
|
| Attention: |
|
|
| Email: |
|
|
| |
|
|
| PARENT SECURITIES HELD |
| Shares: |
|
|
EXHIBIT B
FORM OF PARENT STOCKHOLDER SUPPORT AGREEMENT
Execution Version
STOCKHOLDER SUPPORT AGREEMENT
THIS
SUPPORT AGREEMENT (this “Agreement”), dated as of March 13, 2024, is made by and among
First Wave BioPharma, Inc., a Delaware corporation (“Parent”), ImmunogenX, Inc., a Delaware corporation
(the “Company”), and the undersigned holder (“Stockholder”) of shares of capital stock
(the “Shares”) of Parent.
WHEREAS,
concurrently with the entry into of this Agreement, Parent, IMMUNO Merger Sub I, Inc., a Delaware corporation and a wholly owned
subsidiary of Parent (“First Merger Sub”), IMMUNO Merger Sub II, LLC, a Delaware limited liability company
and a wholly owned subsidiary of Parent (“Second Merger Sub”), and the Company, are entering into an Agreement
and Plan of Merger, dated of even date herewith (the “Merger Agreement”), providing for the merger of First
Merger Sub with and into the Company (the “First Merger”) and the merger of the Company with and into Second
Merger Sub (the “Second Merger” and together with the First Merger, the “Merger”);
WHEREAS,
Stockholder beneficially owns and has sole or shared voting power with respect to the number of Shares, and holds options to purchase
shares of Parent Common Stock (“Parent Stock Options”), restricted stock units to acquire shares of Parent Common
Stock (“Parent Restricted Stock Units”), and such other rights to acquire shares of Parent Common Stock, as
the case may be, in each case in the number of Shares indicated opposite Stockholder’s name on Schedule 1 attached
hereto;
WHEREAS,
as an inducement and a condition to the willingness of Parent, First Merger Sub, Second Merger Sub and the Company to enter into the Merger
Agreement, and in consideration of the substantial expenses incurred and to be incurred by them in connection therewith, Stockholder has
agreed to enter into and perform this Agreement; and
WHEREAS,
all capitalized terms used in this Agreement without definition herein shall have the meanings ascribed to them in the Merger Agreement.
NOW,
THEREFORE, in consideration of, and as a condition to, Parent, First Merger Sub, Second Merger Sub and the Company’s
entering into the Merger Agreement and proceeding with the transactions contemplated thereby, and in consideration of the substantial
expenses incurred and to be incurred by them in connection therewith, Stockholder, Parent and the Company agree as follows:
1) Agreement
to Vote Shares. Stockholder agrees that, prior to the Expiration Date (as defined in Section 2), at any meeting of the
stockholders of Parent or any adjournment or postponement thereof, or in connection with any written consent of the stockholders of Parent,
with respect to the Parent Stockholder Matters, Stockholder shall, or shall cause the holder of record on any applicable record date to:
a) appear
at such meeting or otherwise cause the Shares and any New Shares (as defined in Section 3) to be counted as present thereat
(in person or by proxy) for purposes of calculating a quorum; and
b) from
and after the date hereof until the Expiration Date, vote (or cause to be voted), or deliver a written consent (or cause a written consent
to be delivered) covering all of the Shares and any New Shares that Stockholder shall be entitled to so vote: (i) in favor of the
Parent Stockholder Matters and any matter that would reasonably be expected to facilitate the Parent Stockholder Matters; (ii) against
any proposal to remove the limitation initially set at the discretion of holders of Parent Convertible Preferred Stock between 4.9% and
19.9% of the number of shares of Parent Common Stock outstanding immediately after giving effect to the issuance of shares of Parent Common
Stock upon conversion (the “Beneficial Ownership Limitation”) restricting such holders from beneficially
owning a number of shares of Parent Common Stock in excess of the Beneficial Ownership Limitation; (iii) against any agreement, transaction
or other matter that is intended to, or would reasonably be expected to, impede, interfere with, delay, postpone, discourage or materially
and adversely affect the consummation of the Parent Stockholder Matters; and (iv) to approve any proposal to adjourn or postpone
the applicable meeting to a later date, if there are not sufficient votes for the approval of the Parent Stockholder Matters on the date
on which such meeting is held. Stockholder shall not take or commit or agree to take any action inconsistent with the foregoing.
2) Expiration
Date. As used in this Agreement, the term “Expiration Date” shall mean the earlier to occur of (a) the
effective time of the approval of the Parent Stockholder Matters or (b) the date as of which, by mutual written agreement of Parent
and Stockholder, this agreement is terminated, or (c) twelve (12) months following the date of this Agreement.
3) Additional
Purchases. Stockholder agrees that any shares of capital stock or other equity securities of Parent that Stockholder purchases or
with respect to which Stockholder otherwise acquires sole or shared voting power (including any proxy) after the execution of this Agreement
and prior to the Expiration Date, whether by the exercise of any Parent Stock Options, settlement of Parent Restricted Stock Units or
otherwise, including by gift, succession, in the event of a stock split or as a dividend or distribution of any Shares (“New
Shares”), shall be subject to the terms and conditions of this Agreement to the same extent as if they constituted the Shares.
4) Share
Transfers. From and after the date hereof until the Expiration Date, Stockholder shall not, directly or indirectly, (a) sell,
assign, transfer, tender, or otherwise dispose of (including by the creation of any Liens (as defined in Section 5(c))) any
Shares or any New Shares acquired, (b) deposit any Shares or New Shares into a voting trust or enter into a voting agreement or similar
arrangement with respect to such Shares or New Shares or grant any proxy or power of attorney with respect thereto (other than this Agreement),
(c) enter into any Contract, option, commitment or other arrangement or understanding with respect to the direct or indirect sale,
transfer, assignment or other disposition of (including by the creation of any Liens) any Shares or New Shares, or (d) take any action
that would make any representation or warranty of Stockholder contained herein untrue or incorrect or have the effect of preventing or
disabling Stockholder from performing Stockholder’s obligations under this Agreement. Notwithstanding the foregoing, Stockholder
may make: (i) transfers by will or by operation of Law or other transfers for estate planning purposes, in which case this Agreement
shall bind the transferee; (ii) with respect to Stockholder’s Parent Stock Options which expire on or prior to the Expiration
Date, transfers, sale, or other disposition of Shares or New Shares to Parent as payment for the (A) exercise price of Stockholder’s
Parent Stock Options and (B) taxes applicable to the exercise of Stockholder’s Parent Stock Options; (iii) with respect
to Stockholder’s Parent Restricted Stock Units, (A) transfers for the net settlement of Stockholder’s Parent Restricted
Stock Units settled in Shares or New Shares (to pay any tax withholding obligations) or (B) transfers for receipt upon settlement
of Stockholder’s Parent Restricted Stock Units, and the sale of a sufficient number of such Shares acquired upon settlement of such
securities as would generate sales proceeds sufficient to pay the aggregate taxes payable by Stockholder as a result of such settlement;
(iv) if Stockholder is a partnership or limited liability company, a transfer to one or more partners or members of Stockholder or
to an Affiliated corporation, trust or other Entity under common control with Stockholder, or if Stockholder is a trust, a transfer to
a beneficiary, provided that in each such case the applicable transferee has signed a voting agreement in substantially the form hereof;
(v) transfers to another holder of the capital stock of Parent that has signed a support agreement in substantially the form hereof;
and (vi) transfers, sales or other dispositions as Parent may otherwise agree in writing as its sole discretion. If any voluntary
or involuntary transfer of any Shares or New Shares covered hereby shall occur (including a transfer or disposition permitted by clauses
(i) through (vi), sale by a Stockholder’s trustee in bankruptcy, or a sale to a purchaser at any creditor’s or court
sale), the transferee (which term, as used herein, shall include any and all transferees and subsequent transferees of the initial transferee)
shall take and hold such Shares or New Shares subject to all of the restrictions, liabilities and rights under this Agreement, which shall
continue in full force and effect, and, as condition for such transfer, the transferee shall agree in writing to be bound by the terms
and conditions of this Agreement and either the Stockholder or the transferee provides Parent with a copy of such agreement promptly upon
consummation of any such transfer.
5) Representations
and Warranties of Stockholder. Stockholder hereby represents and warrants to Parent and the Company as follows:
a) If
Stockholder is an Entity: (i) Stockholder is duly organized, validly existing and in good standing under the laws of the jurisdiction
in which it is incorporated, organized or constituted, (ii) Stockholder has all necessary power and authority to execute and deliver
this Agreement, to perform Stockholder’s obligations hereunder and to consummate the transactions contemplated hereby, and (iii) the
execution and delivery of this Agreement, performance of Stockholder’s obligations hereunder and the consummation of the transactions
contemplated hereby by Stockholder have been duly authorized by all necessary action on the part of Stockholder and no other proceedings
on the part of Stockholder are necessary to authorize this Agreement, or to consummate the transactions contemplated hereby. If Stockholder
is an individual, Stockholder has the legal capacity to execute and deliver this Agreement, to perform Stockholder’s obligations
hereunder and to consummate the transactions contemplated hereby;
b) this
Agreement has been duly executed and delivered by or on behalf of Stockholder and, and assuming this Agreement constitutes a valid and
binding agreement of the Company and Parent, constitutes a valid and binding agreement with respect to Stockholder, enforceable against
Stockholder in accordance with its terms, except as enforcement may be limited by general principles of equity whether applied in a court
of Law or a court of equity and by bankruptcy, insolvency and similar Laws affecting creditors’ rights and remedies generally;
c) Stockholder
beneficially owns the number of Shares and other rights with respect to Shares indicated opposite Stockholder’s name on Schedule
1, and will own any New Shares, free and clear of any liens, claims, charges or other encumbrances or restrictions of any kind whatsoever
(“Liens”), and has sole or shared, and otherwise unrestricted, voting power with respect to such Shares or New
Shares and none of the Shares or New Shares is subject to any voting trust or other agreement, arrangement or restriction with respect
to the voting of the Shares or the New Shares, except as contemplated by this Agreement;
d) the
execution and delivery of this Agreement by Stockholder does not, and the performance by Stockholder of his, her or its obligations hereunder
and the compliance by Stockholder with any provisions hereof will not, violate or conflict with, result in a material breach of or constitute
a default (or an event that with notice or lapse of time or both would become a material default) under, or give to others any rights
of termination, amendment, acceleration or cancellation of, or result in the creation of any Liens on any Shares or New Shares pursuant
to, any agreement, instrument, note, bond, mortgage, Contract, lease, license, permit or other obligation or any order, arbitration award,
judgment or decree to which Stockholder is a party or by which Stockholder is bound, or any Law, statute, rule or regulation to which
Stockholder is subject or, in the event that Stockholder is a corporation, partnership, trust or other Entity, any bylaw or other Organizational
Document of Stockholder; except for any of the foregoing as would not reasonably be expected to prevent or delay the performance by Stockholder
of his, her or its obligations under this Agreement in any material respect;
e) the
execution and delivery of this Agreement by Stockholder does not, and the performance of this Agreement by Stockholder does not and will
not, require any consent, approval, authorization or permit of, or filing with or notification to, any Governmental Body or regulatory
authority by Stockholder except for applicable requirements, if any, of the Exchange Act, and except where the failure to obtain such
consents, approvals, authorizations or permits, or to make such filings or notifications, would not prevent or delay the performance by
Stockholder of his, her or its obligations under this Agreement in any material respect;
f) no
investment banker, broker, finder or other intermediary is entitled to a fee or commission from Parent or the Company in respect of this
Agreement based upon any Contract made by or on behalf of Stockholder; and
g) as
of the date of this Agreement, there is no Legal Proceeding pending or, to the knowledge of Stockholder, threatened against Stockholder
that would reasonably be expected to prevent or delay the performance by Stockholder of his, her or its obligations under this Agreement
in any material respect.
6) Irrevocable
Proxy. Subject to the final sentence of this Section 6, by execution of this Agreement, Stockholder does hereby appoint
Parent and any of its designees with full power of substitution and resubstitution, as Stockholder’s true and lawful attorney and
irrevocable proxy, to the fullest extent of Stockholder’s rights with respect to the Shares or New Shares, to vote and exercise
all voting and related rights, including the right to sign Stockholder’s name (solely in its capacity as a stockholder) to any stockholder
consent, if Stockholder is unable to perform or otherwise does not perform his, her or its obligations under this Agreement, with respect
to such Shares and New Shares solely with respect to the matters set forth in Section 1. Stockholder intends this proxy to
be irrevocable and coupled with an interest hereunder until the Expiration Date, hereby revokes any proxy previously granted by Stockholder
with respect to the Shares or New Shares and represents that none of such previously-granted proxies are irrevocable. The irrevocably
proxy and power of attorney granted herein shall survive the death or incapacity of Stockholder and the obligations of Stockholder shall
be binding on Stockholder’s heirs, personal representatives, successors, transferees and assigns. Stockholder hereby agrees not
to grant any subsequent powers of attorney or proxies with respect to any Shares or New Shares with respect to the matters set forth in
Section 1 until after the Expiration Date. The Stockholder hereby affirms that the proxy set forth in this Section 6
is given in connection with and granted in consideration of and as an inducement to the Company, Parent, First Merger Sub and Second Merger
Sub to enter into the Merger Agreement and that such proxy is given to secure the obligations of the Stockholder under Section 1.
Notwithstanding anything contained herein to the contrary, this irrevocable proxy shall automatically terminate upon the Expiration Date.
7) Other
Remedies; Specific Performance. Except as otherwise provided herein, any and all remedies herein expressly conferred upon a party
will be deemed cumulative with, and not exclusive of, any other remedy conferred hereby, or by Law or equity upon such party, and the
exercise by a party of any one remedy will not preclude the exercise of any other remedy. The parties hereto agree that irreparable damage
would occur in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were
otherwise breached. It is accordingly agreed that the parties shall be entitled to an injunction or injunctions to prevent breaches of
this Agreement and to enforce specifically the terms and provisions hereof without the need of posting bond in any court of the United
States or any state having jurisdiction, this being in addition to any other remedy to which they are entitled at Law or in equity.
8) Directors
and Officers. This Agreement shall apply to Stockholder solely in Stockholder’s capacity as a stockholder of Parent and holder
of Parent Stock Options, Parent Restricted Stock Units and other rights to acquire shares of Parent Common Stock, as the case may be,
and not in Stockholder’s capacity as a director, officer or employee of Parent or any of its Subsidiaries or in Stockholder’s
capacity as a trustee or fiduciary of any employee benefit plan or trust. Notwithstanding any provision of this Agreement to the contrary,
nothing in this Agreement shall (or require Stockholder to attempt to) limit or restrict a director or officer of Parent in the exercise
of his or her fiduciary duties as a director or officer of Parent or in his or her capacity as a trustee or fiduciary of any employee
benefit plan or trust or prevent or be construed to create any obligation on the part of any director or officer of Parent or any trustee
or fiduciary of any employee benefit plan or trust from taking any action in his or her capacity as such director, officer, trustee or
fiduciary.
9) No
Ownership Interest. Nothing contained in this Agreement shall be deemed to vest in the Company or Parent any direct or indirect ownership
or incidence of ownership of or with respect to any Shares or New Shares. All rights, ownership and economic benefits of and relating
to the Shares or New Shares shall remain vested in and belong to Stockholder.
10) Termination.
This Agreement shall terminate and shall have no further force or effect as of the Expiration Date. Notwithstanding the foregoing, upon
termination or expiration of this Agreement, no party shall have any further obligations or liabilities under this Agreement; provided,
however, that nothing set forth in this Section 10 or elsewhere in this Agreement shall relieve any party from liability
for any fraud or for any willful and material breach of this Agreement prior to termination hereof.
11) Further
Assurances. Stockholder shall, from time to time, execute and deliver, or cause to be executed and delivered, such additional or further
consents, documents and other instruments as the Company or Parent may reasonably request for the purpose of effectively carrying out
the transactions contemplated by this Agreement, the Parent Stockholder Matters, and the Contemplated Transactions.
12) Disclosure.
Stockholder hereby agrees that Parent and the Company may publish and disclose in any registration statement, any prospectus filed with
any regulatory authority in connection with the Contemplated Transactions and any related documents filed with such regulatory authority
and as otherwise required by Law, Stockholder’s identity and ownership of Shares and the nature of Stockholder’s commitments,
arrangements and understandings under this Agreement and may further file this Agreement as an exhibit to any registration statement or
prospectus or in any other filing made by Parent or the Company as required by Law or the terms of the Merger Agreement, including with
the SEC or other regulatory authority, relating to the Contemplated Transactions, all subject to prior review and an opportunity to comment
by Stockholder’s counsel. Prior to the Closing, Stockholder shall not, and shall use its reasonable best efforts to cause its representatives
not to, directly or indirectly, make any press release, public announcement or other public communication that criticizes or disparages
this Agreement or the Merger Agreement or any of the Contemplated Transactions or Parent Stockholder Matters, without the prior written
consent of Parent and the Company; provided that the foregoing shall not limit or affect any actions taken by Stockholder (or any
affiliated officer or director of Stockholder) that would be permitted to be taken by Stockholder, Parent or the Company pursuant to the
Merger Agreement; provided, further, that the foregoing shall not affect any actions of Stockholder the prohibition of which
would be prohibited under applicable Law.
13) Notice.
All notices and other communications hereunder shall be in writing and shall be deemed given if delivered personally or sent by overnight
courier (providing proof of delivery), by electronic transmission (providing confirmation of transmission) to the Company or Parent, as
the case may be, in accordance with Section 8.7 of the Merger Agreement and to Stockholder at his, her or its address or email address
(providing confirmation of transmission) set forth on Schedule 1 attached hereto (or at such other address for a party as shall
be specified by like notice).
14) Severability.
Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity
or enforceability of the remaining terms and provisions of this Agreement or the validity or enforceability of the offending term or provision
in any other situation or in any other jurisdiction. If a final judgment of a court of competent jurisdiction declares that any term or
provision of this Agreement is invalid or unenforceable, the parties hereto agree that the court making such determination shall have
the power to limit such term or provision, to delete specific words or phrases or to replace such term or provision with a term or provision
that is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term or provision, and
this Agreement shall be valid and enforceable as so modified. In the event such court does not exercise the power granted to it in the
prior sentence, the parties hereto agree to replace such invalid or unenforceable term or provision with a valid and enforceable term
or provision that will achieve, to the extent possible, the economic, business and other purposes of such invalid or unenforceable term
or provision.
15) Assignability.
This Agreement shall be binding upon, and shall be enforceable by and inure solely to the benefit of, the parties hereto and their respective
successors and assigns; provided, however, that neither this Agreement nor any of a party’s rights or obligations
hereunder may be assigned or delegated by such party without the prior written consent of the other parties hereto, and any attempted
assignment or delegation of this Agreement or any of such rights or obligations by such party without the other party’s prior written
consent shall be void and of no effect. Nothing in this Agreement, express or implied, is intended to or shall confer upon any Person
(other than the parties hereto) any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement.
16) No
Waivers. No waivers of any breach of this Agreement extended by the Company or Parent to Stockholder shall be construed as a waiver
of any rights or remedies of the Company or Parent, as applicable, with respect to any other stockholder of Parent who has executed an
agreement substantially in the form of this Agreement with respect to Shares or New Shares held or subsequently held by such stockholder
or with respect to any subsequent breach of Stockholder or any other stockholder of Parent. No waiver of any provisions hereof by any
party shall be deemed a waiver of any other provisions hereof by any such party, nor shall any such waiver be deemed a continuing waiver
of any provision hereof by such party.
17) Applicable
Law; Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the Laws of the state of Delaware, regardless
of the Laws that might otherwise govern under applicable principles of conflicts of Laws. In any action or Legal Proceeding between any
of the parties arising out of or relating to this Agreement, each of the parties: (a) irrevocably and unconditionally consents and
submits to the exclusive jurisdiction and venue of the Court of Chancery of the state of Delaware or to the extent such court does not
have subject matter jurisdiction, the Superior Court of the State of Delaware or the United States District Court for the District of
Delaware, (b) agrees that all claims in respect of such action or Legal Proceeding shall be heard and determined exclusively in accordance
with clause (a) of this Section 17, (c) waives any objection to laying venue in any such action or Legal Proceeding
in such courts, (d) waives any objection that such courts are an inconvenient forum or do not have jurisdiction over any party, and
(e) agrees that service of process upon such party in any such action or Legal Proceeding shall be effective if notice is given in
accordance with Section 13.
18) Waiver
of Jury Trial. THE PARTIES HERETO HEREBY WAIVE ANY RIGHT TO TRIAL BY JURY WITH RESPECT TO ANY ACTION OR LEGAL PROCEEDING RELATED TO
OR ARISING OUT OF THIS AGREEMENT, ANY DOCUMENT EXECUTED IN CONNECTION HEREWITH AND THE MATTERS CONTEMPLATED HEREBY AND THEREBY.
19) No
Agreement Until Executed. Irrespective of negotiations among the parties or the exchanging of drafts of this Agreement, this Agreement
shall not constitute or be deemed to evidence a Contract, agreement, arrangement or understanding between the parties hereto unless and
until (a) the Parent Board has approved, for purposes of any applicable anti-takeover Laws and regulations and any applicable provision
of the certificate of incorporation of Parent, the Merger Agreement and the Contemplated Transactions, (b) the Merger Agreement is
executed by all parties thereto, and (c) this Agreement is executed by all parties hereto.
20) Entire
Agreement; Counterparts; Exchanges by Electronic Transmission. This Agreement and the other agreements referred to in this Agreement
constitute the entire agreement and supersede all prior agreements and understandings, both written and oral, among or between any of
the parties with respect to the subject matter hereof and thereof. This Agreement may be executed in several counterparts, each of which
shall be deemed an original and all of which shall constitute one and the same instrument. The exchange of a fully executed Agreement
(in counterparts or otherwise) by all parties by electronic transmission via “.pdf” shall be sufficient to bind the parties
to the terms and conditions of this Agreement.
21) Amendment.
This Agreement may not be amended, supplemented or modified, and no provisions hereof may be modified or waived, except by an instrument
in writing signed on behalf of each party hereto; provided, however, that the rights or obligations of any Stockholder may
be waived, amended or otherwise modified in a writing signed by Parent, the Company and Stockholder.
22) Fees
and Expenses. Except as otherwise specifically provided herein, the Merger Agreement or any other agreement contemplated by the Merger
Agreement to which a party hereto is a party, each party hereto shall bear its own expenses in connection with this Agreement and the
transactions contemplated hereby.
23) Voluntary
Execution of Agreement. This Agreement is executed voluntarily and without any duress or undue influence on the part or behalf of
the parties. Each of the parties hereby acknowledges, represents and warrants that (a) it has read and fully understood this Agreement
and the implications and consequences thereof; (b) it has been represented in the preparation, negotiation, and execution of this
Agreement by legal counsel of its own choice, or it has made a voluntary and informed decision to decline to seek such counsel; and (c) it
is fully aware of the legal and binding effect of this Agreement.
24) Construction.
a) For
purposes of this Agreement, whenever the context requires: the singular number shall include the plural, and vice versa; the masculine
gender shall include the feminine and neuter genders; the feminine gender shall include the masculine and neuter genders; and the neuter
gender shall include masculine and feminine genders.
b) The
parties hereto agree that any rule of construction to the effect that ambiguities are to be resolved against the drafting party shall
not be applied in the construction or interpretation of this Agreement.
c) As
used in this Agreement, the words “include” and “including,” and variations thereof, shall not be deemed to be
terms of limitation, but rather shall be deemed to be followed by the words “without limitation.”
d) Except
as otherwise indicated, all references in this Agreement to “Sections,” and “Schedules” are intended to refer
to Sections of this Agreement and Schedules to this Agreement, respectively.
e) The
underlined headings contained in this Agreement are for convenience of reference only, shall not be deemed to be a part of this Agreement
and shall not be referred to in connection with the construction or interpretation of this Agreement.
[Remainder of Page has Intentionally Been
Left Blank]
EXECUTED as of the date first
above written.
| |
STOCKHOLDER |
| |
|
| |
STOCKHOLDER NAME: |
| |
|
| |
Name of signatory (if an Entity): |
|
[Signature Page to Parent
Support Agreement]
EXECUTED as of the date first
above written.
| |
PARENT: |
| |
|
| |
FIRST WAVE BIOPHARMA, INC. |
| |
By: |
|
| |
Name: |
James Sapirstein |
| |
Title: |
President and Chief Executive Officer |
[Signature Page to Parent
Support Agreement]
EXECUTED as of the date first
above written.
| |
COMPANY: |
| |
|
| |
IMMUNOGENX, INC. |
| |
By: |
|
| |
Name: |
Jack Syage |
| |
Title: |
Chief Executive Officer |
[Signature Page to Parent
Support Agreement]
SCHEDULE 1
| Name of Stockholder |
|
|
| Address: |
|
|
| |
|
|
| Attention: |
|
|
| Email: |
|
|
| |
|
|
| PARENT SECURITIES HELD |
| Shares: |
|
|
| Parent Stock Options: |
|
|
| Parent Restricted Stock Units: |
|
|
| Shares underlying other rights (e.g., Parent Warrants): |
|
|
[Signature Page to Parent
Support Agreement]
EXHIBIT C
FORM OF LOCK-UP AGREEMENT
FORM OF LOCK-UP AGREEMENT
LOCK-UP AGREEMENT
March 13, 2024
First Wave BioPharma, Inc.
777 Yamato Road, Suite 502
Boca Raton, Florida 33431
Ladies and Gentlemen:
The
undersigned signatory of this lock-up agreement (this “Lock-Up Agreement”) understands that,
concurrently with the execution and delivery of this Lock-Up Agreement, First Wave BioPharma, Inc., a Delaware corporation (“Parent”),
has entered into an Agreement and Plan of Merger, dated as of March 13, 2024 (as the same may be amended from time to time, the “Merger
Agreement”) with IMMUNO Merger Sub I, Inc., a Delaware corporation and a wholly owned subsidiary of Parent, IMMUNO
Merger Sub II, LLC, a Delaware limited liability company and a wholly owned subsidiary of Parent, and ImmunogenX, Inc., a Delaware
corporation (the “Company”). Capitalized terms used but not otherwise defined herein shall have the respective
meanings ascribed to such terms in the Merger Agreement.
As
a condition and material inducement to each of the parties to enter into the Merger Agreement and to consummate the Contemplated Transactions,
and for other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the undersigned hereby irrevocably
agrees that, subject to the exceptions set forth herein, without the prior written consent of Parent, by a majority vote of the Parent
Board (including, with respect to periods after the Closing, at least two (2) Company Directors), and, solely prior to the Closing,
the Company (the “Requisite Vote”), the undersigned will not, during the period commencing upon the Closing
and ending on the date that is 180 days after the Closing Date (the “Restricted Period”):
| (i) | offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase, lend any option
or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any
shares of Parent Common Stock, Parent Preferred Stock or any securities convertible into or exercisable or exchangeable for Parent Common
Stock (including without limitation, Parent Common Stock or such other securities which may be deemed to be beneficially owned by the
undersigned in accordance with the rules and regulations of the SEC and securities of Parent which may be issued upon exercise of
an option to purchase Parent Common Stock or warrant or settlement of a Parent Restricted Stock Unit) that are currently or hereafter
owned by the undersigned (collectively, the “Undersigned’s Shares”); |
| (ii) | enter into any swap, short sale, hedge or other agreement that transfers, in whole or in part, any of
the economic consequences of ownership of the Undersigned’s Shares regardless of whether any such transaction described in clause
(i) above or this clause (ii) is to be settled by delivery of Parent Common Stock, Parent Preferred Stock or other securities,
in cash or otherwise; |
| (iii) | make any demand for, or exercise any right with respect to, the registration of any shares of Parent Common
Stock, Parent Preferred Stock or any security convertible into or exercisable or exchangeable for Parent Common Stock (other than such
rights set forth in the Merger Agreement); or |
| (iv) | publicly disclose the intention to do any of the foregoing. |
The restrictions and
obligations contemplated by this Lock-Up Agreement shall not apply to:
| (a) | transfers of the Undersigned’s Shares: |
| (i) | if the undersigned is a natural person, (A) to any person related to the undersigned (or to an ultimate
beneficial owner of the undersigned) by blood or adoption who is an immediate family member of the undersigned, or by marriage or domestic
partnership (a “Family Member”), or to a trust formed for the benefit of the undersigned or any of the undersigned’s
Family Members, (B) to the undersigned’s estate, following the death of the undersigned, by will, intestacy or other operation
of Law, (C) as a bona fide gift or a charitable contribution, (D) by operation of Law pursuant to a qualified domestic order
or in connection with a divorce settlement or (E) to any partnership, corporation or limited liability company which is controlled
by or under common control with the undersigned and/or by any such Family Member(s); |
| (ii) | if the undersigned is a corporation, partnership or other Entity, (A) to another corporation, partnership,
or other Entity that is an affiliate (as defined under Rule 12b-2 of the Exchange Act) of the undersigned, including investment funds
or other entities under common control or management with the undersigned, (B) as a distribution or dividend to equity holders, current
or former general or limited partners, members or managers (or to the estates of any of the foregoing), as applicable, of the undersigned
(including upon the liquidation and dissolution of the undersigned pursuant to a plan of liquidation approved by the undersigned’s
equity holders), (C) as a bona fide gift or a charitable contribution or otherwise to a trust or other entity for the direct or indirect
benefit of an immediate family member of a beneficial owner (as defined in Rule 13d-3 of the Exchange Act) of the Undersigned’s
Shares or (D) transfers or dispositions not involving a change in beneficial ownership; or |
| (iii) | if the undersigned is a trust, to any grantors or beneficiaries of the trust; |
provided that, in the
case of any transfer or distribution pursuant to this clause (a), such transfer is not for value and each donee, heir, beneficiary or
other transferee or distributee shall sign and deliver to Parent a lock-up agreement in the form of this Lock-Up Agreement
with respect to the shares of Parent Common Stock, Parent Preferred Stock or such other securities that have been so transferred or distributed;
| (b) | the exercise of an option to purchase Parent Common Stock (including a net or cashless exercise of an
option to purchase Parent Common Stock), and any related transfer of shares of Parent Common Stock to Parent for the purpose of paying
the exercise price of such options or for paying taxes (including estimated taxes) due as a result of the exercise of such options; provided that,
for the avoidance of doubt, the underlying shares of Parent Common Stock shall continue to be subject to the restrictions on transfer
set forth in this Lock-Up Agreement; |
| (c) | the disposition (including a forfeiture or repurchase) to Parent of any shares of restricted stock granted
pursuant to the terms of any employee benefit plan or restricted stock purchase agreement; |
| (d) | transfers to Parent in connection with the net settlement of any restricted stock unit or other equity
award that represents the right to receive in the future shares of Parent Common Stock settled in Parent Common Stock to pay any tax withholding
obligations; provided that, for the avoidance of doubt, the underlying shares of Parent Common Stock shall continue
to be subject to the restrictions on transfer set forth in this Lock-Up Agreement; |
| (e) | the establishment of a trading plan pursuant to Rule 10b5-1 under the Exchange Act for the transfer
of Parent Common Stock; provided that such plan does not provide for any transfers of Parent Common Stock during
the Restricted Period; |
| (f) | transfers by the undersigned of shares of Parent Common Stock purchased by the undersigned on the open
market or in a public offering by Parent, in each case following the Closing Date; |
| (g) | pursuant to a bona-fide third party tender offer, merger, consolidation or other similar transaction made
to all holders of Parent’ capital stock involving a change of control of Parent, provided that in the event that such tender offer,
merger, consolidation or other such transaction is not completed, the Undersigned’s Shares shall remain subject to the restrictions
contained in this Lock-Up Agreement; |
| (h) | pursuant to an order of a court or regulatory agency; |
| (i) | following the approval of the Conversion Proposal, the conversion of Parent Preferred Stock into shares
of Parent Common Stock in accordance with the Certificate of Designation; |
| (j) | [the pledge of the Undersigned’s Shares to Mattress Liquidators, Inc. (“ML”)
pursuant to that certain Limited Recourse Pledge Agreement dated [•], 2024 by and among the undersigned, as a grantor, ML, as lender,
and the other grantors party thereto (the “Pledge Agreement”);]1 or |
| (k) | [the sale of all or any portion of the Undersigned’s Shares to ML in connection with a Debt Purchase
Transaction (as defined in the Pledge Agreement).]2 |
and provided,
further, that, with respect to each of (a), (b), (c), (d) and (e) above, no filing by any party (including any donor,
donee, transferor, transferee, distributor or distributee) under Section 16 of the Exchange Act or other public announcement shall
be required or shall be made voluntarily in connection with such transfer or disposition during the Restricted Period (other than (i) any
exit filings or public announcements that may be required under applicable federal and state securities Laws or (ii) in respect of
a required filing under the Exchange Act in connection with the exercise of an option to purchase Parent Common Stock or in connection
with the net settlement of any restricted stock unit or other equity award that represents the right to receive in the future shares of
Parent Common Stock settled in Parent Common Stock that would otherwise expire during the Restricted Period, provided that (A) reasonable
notice shall be provided to Parent prior to any such filing and (B) such filing, report or announcement shall clearly indicate in
the footnotes therein, in reasonable detail, a description of the circumstances of the transfer and that the shares remain subject to
the lock-up agreement.
1 NTD: Include this in the lock-up agreement for any party
that is pledging shares pursuant to the Pledge Agreement.
2 NTD: Include this in the lock-up agreement for any party
that is pledging shares pursuant to the Pledge Agreement.
For
purposes of this Lock-Up Agreement, “change of control” shall mean the transfer (whether by tender offer, merger, consolidation
or other similar transaction), in one transaction or a series of related transactions, to a person or group of affiliated persons, of
Parent's voting securities if, after such transfer, Parent's stockholders as of immediately prior to such transfer do not hold a majority
of the outstanding voting securities of Parent (or the surviving entity) other than the Contemplated Transactions.
Any
attempted transfer in violation of this Lock-Up Agreement will be of no effect and null and void, regardless of whether the
purported transferee has any actual or constructive knowledge of the transfer restrictions set forth in this Lock-Up Agreement,
and will not be recorded on the share register of Parent. In furtherance of the foregoing, the undersigned agrees that Parent and
any duly appointed transfer agent for the registration or transfer of the securities described herein are hereby authorized to decline
to make any transfer of securities if such transfer would constitute a violation or breach of this Lock-Up Agreement. Parent
may cause the legend set forth below, or a legend substantially equivalent thereto, to be placed upon any certificate(s) or other
documents, ledgers or instruments evidencing the undersigned’s ownership of Parent Common Stock, Parent Preferred Stock or any securities
convertible into or exercisable or exchangeable for Parent Common Stock:
THE SHARES
REPRESENTED BY THIS CERTIFICATE ARE SUBJECT TO AND MAY ONLY BE TRANSFERRED IN COMPLIANCE WITH A LOCK-UP AGREEMENT, A COPY
OF WHICH IS ON FILE AT THE PRINCIPAL OFFICE OF THE COMPANY.
The
undersigned hereby represents and warrants that the undersigned has full power and authority to enter into this Lock-Up Agreement. All
authority herein conferred or agreed to be conferred and any obligations of the undersigned shall be binding upon the successors, assigns,
heirs or personal representatives of the undersigned.
The
undersigned understands that if the Merger Agreement is terminated for any reason, the undersigned shall be released from all obligations
under this Lock-Up Agreement. The undersigned understands that Parent and the Company are proceeding with the Contemplated
Transactions in reliance upon this Lock-Up Agreement.
Any
and all remedies herein expressly conferred upon Parent or the Company will be deemed cumulative with and not exclusive of any other remedy
conferred hereby, or by Law or equity, and the exercise by Parent or the Company of any one remedy will not preclude the exercise of any
other remedy. The undersigned agrees that irreparable damage would occur to Parent and/or the Company in the event that any provision
of this Lock-Up Agreement were not performed in accordance with its specific terms or were otherwise breached. It is accordingly
agreed that Parent and the Company shall be entitled to an injunction or injunctions to prevent breaches of this Lock-Up Agreement
and to enforce specifically the terms and provisions hereof in any court of the United States or any state having jurisdiction, this being
in addition to any other remedy to which Parent or the Company is entitled at Law or in equity, and the undersigned waives any bond, surety
or other security that might be required of Parent or the Company with respect thereto. Each of the parties further agrees that it will
not oppose the granting of an injunction, specific performance or other equitable relief on the basis that any other party has an adequate
remedy at law or that any award of specific performance is not an appropriate remedy for any reason at law or in equity.
Upon
the release of any of the Undersigned’s Shares from this Lock-Up Agreement, Parent will cooperate with the undersigned
to facilitate the timely preparation and delivery of certificates or the establishment of book-entry positions at Parent's transfer agent
representing the Undersigned’s Shares without the restrictive legend above or the withdrawal of any stop transfer instructions.
This Lock-Up Agreement
and any claim, controversy or dispute arising under or related to this Lock-Up Agreement shall be governed by and construed
in accordance with the Laws of the state of Delaware, without regard to the conflict of Laws principles thereof.
This
Lock-Up Agreement may not be amended except by an instrument in writing signed on behalf of each of the Parent and the Company (as applicable),
pursuant to Requisite Vote and the undersigned.
This Lock-Up Agreement
may be executed in several counterparts, each of which shall be deemed an original and all of which shall constitute one and the same
instrument. The exchange of a fully executed Lock-Up Agreement (in counterparts or otherwise) by Parent, the Company and
the undersigned by facsimile or electronic transmission in .pdf format shall be sufficient to bind such parties to the terms and conditions
of this Lock-Up Agreement.
(Signature Page Follows)
| |
Very truly yours, |
| |
|
| Print Name of Stockholder: |
[ ] |
| |
|
| |
Signature (for individuals): |
| |
|
| |
|
| |
|
| |
Signature (for entities): |
Accepted and Agreed
by FIRST WAVE BIOPHARMA, INC.:
| By: |
|
|
| |
Name: |
|
|
| |
Title: |
|
|
| |
|
| Accepted and Agreed by IMMUNOGENX, INC.: |
|
| |
|
|
| By: |
|
|
| |
Name: |
Jack Syage |
|
| |
Title: |
CEO |
|
[Signature Page to
Lock-up Agreement]
EXHIBIT D
FORM OF RESTRICTIVE COVENANTS AGREEMENT
RESTRICTIVE COVENANT AGREEMENT
This Restrictive Covenant
Agreement (this “Agreement”) is made as of March 13, 2024, by and among First Wave BioPharma, Inc.,
a Delaware corporation, (“Parent”), ImmunogenX, Inc., a Delaware corporation (the “Company”),
and [●], an individual (“Restricted Party” and together with Parent and the Company, the “Parties”).
Capitalized terms used but not otherwise defined herein shall have the meanings ascribed to them in the Merger Agreement (as defined below).
RECITALS
Pursuant
to that certain Agreement and Plan of Merger, dated as of the date hereof (as amended, restated, supplemented or otherwise modified, the
“Merger Agreement”), by and among Parent, the Company, IMMUNO Merger Sub I, Inc., a Delaware corporation
and wholly-owned subsidiary of Parent (“First Merger Sub”), and IMMUNO Merger Sub II, LLC, a Delaware limited
liability company and wholly-owned subsidiary of Parent (“Second Merger Sub”), among other things, (1) at
the First Effective Time, First Merger Sub will merge with and into the Company, with the Company surviving such merger as a wholly-owned
subsidiary of Parent (the “First Merger”) and (2) immediately following the First Merger, the Company will
merge with and into Second Merger Sub with Second Merger Sub surviving such merger (the “Surviving Company”).
Restricted Party is a direct
owner of the Company and, as a consequence of the transactions contemplated by the Merger Agreement, will receive, directly or indirectly
through the Company, substantial consideration (the “Consideration”). As a specific inducement for Parent, First
Merger Sub and Second Merger Sub to enter into and consummate the transactions contemplated by the Merger Agreement, Restricted Party
is entering into this Agreement intending to be legally bound hereby.
AGREEMENT
Now, therefore, in consideration of the mutual
covenants contained herein and other good and valuable consideration, including the Consideration to be paid to and received by Restricted
Party, the receipt and sufficiency of which are hereby acknowledged, the Parties hereby agree as follows:
1. Restrictive
Covenants.
(a) Confidentiality.
(i) Restricted
Party acknowledges that the Confidential Information to which they or their Affiliates had, has or will have access is confidential and
proprietary to the Company, that the unauthorized disclosure of any of the Confidential Information to any Person will result in immediate
and irreparable competitive injury to Parent, Surviving Company and their Affiliates, and that such injury cannot adequately be remedied
by an award of monetary relief. Restricted Party agrees that during the Restricted Period, they shall not, and shall cause their Affiliates
not to, disclose any Confidential Information or the terms of this Agreement to any Person, or use any Confidential Information, without
the prior written permission of Parent or as may be required by a court order in which case, the Restricted Party shall give Parent prompt
advanced notice.
(ii) Immediately
upon the request of Parent, Restricted Party agrees that they shall return to Parent all documents and other materials in any form, and
any copies thereof, that constitute, contain, or refer or relate to any Confidential Information.
(iii) Notwithstanding
the foregoing, the Restricted Party may disclose Confidential Information or the terms of this Agreement:
(A) In
furtherance of or pursuant to Restricted Party’s role or duties as an officer or director of Parent or the Surviving Company;
(B) To
the Restricted Party’s accountants, attorneys or other agents who are advising the Restricted Party with respect to the Restricted
Party’s interest in the Parent, the Company, or the Surviving Company or the Restricted Party’s rights and obligations under
this Agreement, the Merger Agreement, and the agreements expressly contemplated thereby, but only (i) for legitimate business purposes
related to the management of the Restricted Party’s interest in the Parent, the Company, or the Surviving Company, the Restricted
Party’s rights and obligations under this Agreement, the Merger Agreement, and the agreements expressly contemplated thereby or
the Restricted Party’s personal business, financial, accounting, tax or legal affairs and (ii) with such Person having been
instructed to maintain the confidentiality of such information;
(C) as
to the Restricted Party that is subject to mandatory reporting obligations under the Securities and Exchange Act of 1934, as amended,
and the rules and regulations promulgated thereunder (the “Exchange Act”), only to the extent disclosure
is required by the Restricted Party’s mandatory reporting obligations (including public disclosures made in accordance with the
Exchange Act);
(D) to
tax authorities to the extent required to be disclosed in conjunction with the filing of tax returns and other reports under applicable
tax laws; or
(E) if
such Confidential Information is required to be disclosed by applicable law, in which case, to the extent permitted by applicable law,
the disclosing Party shall give the other Party prompt advanced notice, the Parties agree not to disclose the terms of this Agreement
to any Person; provided a Party may disclose this Agreement and/or any of its terms to such Party’s attorneys, so long as such Party
instructs every such Person to whom the Party makes such disclosure (and receives such Person’s written agreement) not to disclose
the terms of this Agreement further.
(iv) “Affiliate”
means with respect to any Person, any other Person who directly or indirectly, through one or more intermediaries, controls, is controlled
by, or is under common control with, such Person, and, if such Person is an individual, “Affiliate” shall also include any
member of the immediate family (including parents, spouse and children) of such individual and any trust whose principal beneficiary is
such individual or one or more members of such immediate family and any Person who is controlled by any such member or trust. As used
in this definition, “control” (including with correlative meanings, “controlled by” and “under
common control with”) means possession, directly or indirectly, of power to direct or cause the direction of management or policies
(whether through ownership of securities or partnership or other ownership interests, by contract, trust or otherwise).
(v) “Business”
shall mean the business of the development of, obtaining regulatory approval for, and commercialization of, celiac disease therapeutics
and celiac disease diagnostics (including companion diagnostics and diagnostic assessment tools) for the treatment of patients with celiac
disease, based on the research, development and manufacture of the Company Product Candidates or alternative formulations which are currently
being developed by the Company.
(vi) “Company
Product Candidates” shall mean all products or product candidates developed by the Company or any its Affiliates (including
all predecessors).
(vii) “Confidential
Information” shall mean all confidential, proprietary and trade secret information (whether merely remembered or embodied
in a tangible or intangible form) furnished before, on or after the date hereof that is related to the Business, the Company or any of
its Affiliates (including their respective predecessors), including, but not limited to: (a) any data, agreements, documents, memoranda,
reports, “know-how”, interpretations, plans, studies, forecasts, projections, business methods, concepts, models, lists, procedures,
formulations, trade secrets, and records; (b) all, notes, analyses, compilations, studies, or other documents which were developed
based upon or which include any such Confidential Information, whether prepared by the Company, the Restricted Party or others; (c) the
Company’s or any of its Affiliates’ software, technology, developments, improvements and methods of operation; (d) the
descriptions, specifications, numbers, names, vendors, suppliers, characteristics, costs, anticipated pricing, performance, market potential,
suitability, and other information relating to the Company Product Candidates; and (e) the Company’s or any of its Affiliates’
results of operations, financial condition, projected financial performance, sales and profit performance, financial requirements or the
names and addresses of, or amounts of compensation paid to, the Company’s or its Affiliates’ employees, agents consultants
and business relationships; provided, however, it is understood that Confidential Information shall not include any information which
(i) is in the public domain at the time of disclosure to the Restricted Party, (ii) currently is or becomes known in the public
domain generally, in each case through no wrongful act of the part of Restricted Party, (iii) at the time of receipt is or was already
in the possession of the Restricted Party, or becomes known to the Restricted Party or any of its Affiliates or representatives on or
after the date hereof from a third party on a non-confidential basis who is not known by such recipient to be bound by any confidentiality
obligation, (iv) is specifically released in writing by Parent from confidential status or (v) is independently developed
by the Restricted Party without aid, application or use of any Confidential Information.
(viii) “Person”
means an individual, partnership, corporation, limited liability company, joint stock company, unincorporated organization or association,
trust, joint venture, association or other similar entity.
(ix) “Restricted
Period” shall mean a period of three (3) years after the date hereof.
(x) “Subsidiary”
means, with respect to any Person, any corporation of which a majority of the total voting power of shares of stock entitled (without
regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time owned or
controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of such Person or a combination thereof, or
any partnership, limited liability company, association or other business entity of which a majority of the partnership, limited liability
company or other similar ownership interest is at the time owned or controlled, directly or indirectly, by such Person or one or more
Subsidiaries of such Person or a combination thereof. For purposes of this definition, a Person is deemed to have a majority ownership
interest in a partnership, limited liability company, association or other business entity if such Person is allocated a majority of the
gains or losses of such partnership, limited liability company, association or other business entity or is or controls the managing member
or general partner or similar position of such partnership, limited liability company, association or other business entity.
(b) Non-Competition.
Except with respect to Restricted Party’s employment or service (including pursuant to board, committee or any advisory role) with
Parent and/or the Surviving Company, Restricted Party acknowledges and agrees that during the Restricted Period, Restricted Party will
not, and shall cause Restricted Party’s Affiliates not to, directly or indirectly, individually or with others, own, manage, operate
or control, or become engaged or serve as a shareholder, officer, director, partner, member, employee, agent, consultant, advisor, or
representative of, or give financial, technical or other assistance to, otherwise invest in, lend to, or have a financial interest in,
or in exchange for compensation otherwise associate with any Person that performs all or any part of the Business anywhere in the world
(the “Territory”). Nothing herein shall prohibit Restricted Party or Restricted Party’s Affiliates from
being a passive owner of not more than 5% of the outstanding stock of any class of a corporation that is publicly traded, so long as Restricted
Party or Restricted Party’s Affiliates has no active participation in the business of such corporation.
(c) Non-Solicitation.
Except with respect to Restricted Party’s employment or service (including pursuant to board, committee or any advisory role) with
Parent and/or the Surviving Company, Restricted Party agrees that during the Restricted Period, they shall not, directly or indirectly,
through an Affiliate or otherwise in any manner (whether on the Restricted Party’s own account, or as an employee, director consultant,
agent, partner, manager, joint venture, owner operator or officer of any other Person or in any other capacity, but in all such cases,
excluding any actions or omissions taken pursuant to such Restricted Party’s role or duties as an officer or director of Parent
or the Surviving Company): (i)(X) recruit, solicit, employ, retain or induce, or attempt to recruit, solicit, employ, retain or induce,
any current employee, consultant, or freelancer of the Company (each a “Restricted Employee”) to leave his or
her employment, consultancy or representative relationship with Parent or Surviving Company, (Y) in any way interfere with the relationship
between Parent or Surviving Company on the one hand and Restricted Employee on the other hand or (Z) hire, employ, engage or otherwise
retain or enter into any business relationship with any Restricted Employee; provided, however, that general solicitations not specifically
directed toward Restricted Employees will not constitute a breach of covenants in this Section; or (ii)(W) solicit or induce, or
attempt to solicit or induce, any Person that is a supplier, vendor, referral source, or other business relation of the Company thereof
to cease doing business with Parent or its Affiliates, or in any way materially adversely interfere with the relationship between any
such supplier, vendor, referral source or other business relation, on the one hand, and Parent or any of its Affiliates on the other hand,
(X) solicit any business related to all or any portion of the Business from any current or former supplier, vendor or other business
relation of the Company, Parent or any of its Affiliates; (Y) solicit, divert or take away, or attempt to solicit, divert or take
away, any trade, any business or good will related to all or any portion of Parent or its Affiliates, or otherwise compete in any manner
for any trade, business or good will which became known to Restricted Party through his/her employment with the Company related to the
Business; or (Z) request or influence, or attempt to influence, any supplier, vendor, referral source, lessor or other business relation
of the Company not to do business with Parent or any of its Affiliates.
(d) Non-Hire.
Restricted Party agrees that during the Restricted Period, they shall not, directly or indirectly, through an Affiliate or otherwise in
any manner (whether on the Restricted Party’s own account, or as an employee, director consultant, agent, partner, manager, joint
venture, owner operator or officer of any other Person or in any other capacity, but in all such cases, excluding any actions or omissions
taken pursuant to such Restricted Party’s role or duties as an officer or director of Parent or the Surviving Company) hire, engage
or otherwise retain the services of any Restricted Employee during the time such Restricted Employee is an employee or consultant of either
Parent or Surviving Company.
(e) Non-Disparagement.
(i) During
the Restricted Period, the Restricted Party agrees that Restricted Party shall not, and shall cause Restricted Party’s Affiliates
not to, directly or indirectly, make any statement that is intended to become public, or that should reasonably be expected to become
public, and that ridicules, disparages or is otherwise derogatory of the Company, Parent or any of their respective subsidiaries, Affiliates,
employees, officers, directors, members or stockholders. Notwithstanding the covenants contained in this Section 1(e)(i),
neither the Restricted Party nor its Affiliates shall be prohibited or restricted from (X) making any such statements that the Restricted
Party or any of its Affiliates, as applicable, reasonably believes in good faith to be necessary in responding to or initiating a bona
fide claim involving such Person (including in connection with asserting or responding to any claim related to the Merger Agreement, any
other agreement entered into in connection therewith or this Agreement) or (Y) answering truthfully if compelled to do so in a deposition,
lawsuit or similar dispute resolution process or as required by any Law, order, writ, or injunction, or as required under the Exchange
Act or similar reporting obligations.
(f) Remedies.
Restricted Party acknowledges the breach or threatened breach of any of the covenants undertaken herein would cause irreparable injury
to the Business, Parent and/or any of its Affiliates, for which no adequate remedy at law exists. Therefore, Restricted Party agrees that
in the event of such breach or threatened breach by Restricted Party of any of the terms and conditions of this Agreement, Parent and
each of its Affiliates or any of their successors or assigns shall be entitled, if it so elects and in addition to any other rights or
remedies available to it, to institute and prosecute proceedings in any court of competent jurisdiction to specifically enforce this Agreement
or enjoin Restricted Party from breaching or attempting to breach this Agreement. This remedy is in addition to and not in lieu of the
remedies otherwise available to Parent and its Affiliates, and Parent and its Affiliates may institute an action against Restricted Party
to obtain injunctive and/or declaratory relief while pursuing claims for damages based on the same set of facts in the jurisdiction specified
in Section 4(d). Restricted Party agrees that Parent and its Affiliates shall be entitled to injunctive or declaratory relief
without the necessity of posting any bond or Parent and its Affiliates having to prove irreparable harm or actual damages and that Restricted
Party waives any claim or right to posting of any such bond or proving irreparable harm or actual damages. The protections contained within
this Section 1 are in addition to, and not in lieu of, all protections afforded by applicable state and federal law, including
those relating to protection of trade secrets and protection of computer systems and electronic information. Parent’s or its Affiliates’
election to institute an action against Restricted Party in a court of its choosing under this Section 1 shall not constitute
a waiver of Parent’s or its Affiliates rights under Section 4(d) of this Agreement. In addition, in the event of
an alleged breach or violation by Restricted Party of this Section 1, the Restricted Period shall be tolled until such breach
or violation has been cured.
(g) Acknowledgment.
Restricted Party acknowledges that they have carefully read this Agreement and have given careful consideration to the restraints imposed
upon them and is in full accord as to their necessity for the reasonable and proper protection of Parent’s and its respective Affiliates’
interests. Restricted Party recognizes the importance of the covenants contained in this Section 1 and acknowledges that,
based on their past experience, the Business and the currently anticipated plans to expand the Business, the restrictions imposed herein
are (i) reasonable in scope, time and area; (ii) necessary for the protection of Parent’s and its Affiliates’ legitimate
business interests, including trade secrets, goodwill and relationships with Customers, Prospective Customers and suppliers and (iii) not
unduly restrictive of any rights of Restricted Party or unreasonable impositions of Restricted Party’s ability to work and earn
a living. In addition, Restricted Party agrees and acknowledges that the potential harm to the Business and Parent of the non-enforcement
of this Section 1 outweighs any harm to Restricted Party. Restricted Party acknowledges and agrees that the covenants contained
in this Section 1 are essential elements of this Agreement and that but for these covenants Parent, First Merger Sub and Second
Merger Sub would not have entered into the Merger Agreement. Restricted Party acknowledges that the Business has been conducted throughout
the Territory, that the restrictions contained within this Section 1 are reasonable and necessary to protect the goodwill
of the Business, and that Restricted Party shall not challenge the enforceability or reasonableness of these restrictive covenants. Restricted
Party understands and agrees that the restrictions and covenants contained in this Section 1 are in addition to, and not in
lieu of, any non-competition, non-solicitation or other similar obligations contained in any other agreements between Restricted Party
and Parent or any of its Affiliates.
(h) Enforcement.
It is the understanding and desire of the Parties that the covenants contained in this Section 1 shall be enforced to the
fullest extent possible under the laws and public policies applied in each jurisdiction in which enforcement may be sought, and that should
any particular provision(s) of such covenants be deemed invalid or unenforceable, such provision(s) shall be deemed amended
to delete therefrom the invalid portion(s) and in its reduced form, such covenant shall be enforceable. To the extent that any provision(s) in
Section 1 is (are) deemed unenforceable by virtue of its (their) scope, but shall be enforceable by limitation thereof, Parent
and its Affiliates will be entitled to enforce such provision(s) to the extent permissible under the laws and public policies applied
in the jurisdiction in which enforcement is sought, and such provision(s) may be so modified and enforced by a court of competent
jurisdiction.
2. Representations
and Warranties. Restricted Party has received and carefully reviewed the Merger Agreement, the schedules, exhibits and annexes thereto
and the documents contemplated thereby, is familiar with the transactions contemplated hereby and thereby and fully understands the terms
and conditions set forth herein and in the Merger Agreement. Restricted Party has consulted with independent advisors and counsel regarding
Restricted Party’s rights and obligations under this Agreement and intends for such terms to be binding upon and enforceable against
Restricted Party. Restricted Party hereby warrants and covenants that (i) Restricted Party’s execution, delivery and performance
of this Agreement does not and shall not result in a breach of the terms, conditions or provisions of any agreement, instrument, order,
judgment or decree to which Restricted Party is subject and (ii) upon the execution and delivery of this Agreement by Parent and
the Company, this Agreement shall be the valid and binding obligation of Restricted Party, enforceable in accordance with its terms (except
as such enforceability may be limited by bankruptcy, insolvency, fraudulent conveyance, reorganization, moratorium or other similar laws
now or hereafter in effect relating to creditors’ rights and by general equitable principles).
3. Notices.
All notices, requests, claims, demands and other communications hereunder shall be given and shall be deemed to have been duly received
(a) if delivered personally, when received, (b) on the next Business Day if transmitted by national overnight courier with confirmation
of delivery or (c) if sent by email, when dispatched provided that receipt is confirmed electronically, as follows:
|
Notices
to Restricted Party:
To the address set forth on the signature page hereto.
|
|
|
Notices
to Parent:
First Wave BioPharma, Inc.
777 Yamato Road, Suite 502
Boca Raton, FL 33431
Attention: James Sapirstein
E-mail: jsapirstein@firstwavebio.com |
with
a copy to (which shall not constitute notice):
Lowenstein Sandler LLP
1251 Avenue of the Americas
New York, NY 10020
Attention: Michael Lerner
Email: mlerner@lowenstein.com |
4. General
Provisions.
(a) Amendment
and Waiver. The provisions of this Agreement may be amended and waived only in writing by Parent, the Company and Restricted Party.
(b) Severability.
Whenever possible, each provision of this Agreement shall be interpreted in such manner as to be effective and valid under applicable
law, but if any provision of this Agreement is held to be invalid, illegal or unenforceable in any respect under any applicable law or
rule in any jurisdiction, such invalidity, illegality or unenforceability shall not affect any other provision or any other jurisdiction,
and this Agreement shall be reformed, construed and enforced in such jurisdiction as if such invalid, illegal or unenforceable provision
had never been contained herein.
(c) Complete
Agreement. This Agreement embodies the complete agreement and understanding among the Parties relating to the subject matter hereof
and supersedes and preempts any prior understandings, agreements or representations by or among the Parties, written or oral, which may
have related to the subject matter hereof in any way.
(d) Choice
of Law; Waiver; Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of this Agreement
shall be governed by, and construed in accordance with, the internal law, and not the law of conflicts, of the State of Delaware, without
giving effect to any choice of law or conflict of law rules or provisions (whether of the State of Delaware or any other jurisdiction)
that would cause the application of the laws of any jurisdiction other than the State of Delaware. The Parties hereby irrevocably waive
any and all right to trial by jury in any action or proceeding arising out of or related to this Agreement or the transactions contemplated
hereby. Each Party hereby submits to the jurisdiction of any state or federal court sitting in Delaware in any action or proceeding arising
out of or relating to this Agreement and agrees that all claims in respect of the action or proceeding may be heard and determined in
any such court and hereby expressly submits to the personal jurisdiction and venue of such court for the purposes hereof and expressly
waives any claim of improper venue and any claim that such courts are an inconvenient forum. Each Party hereby irrevocably consents to
the service of process of any of the aforementioned courts in any such action or proceeding by the mailing of copies thereof by registered
or certified mail, postage prepaid and return receipt requested, to its address set forth in Section 3, such service to become
effective ten days after such mailing.
(e) Successors
and Assigns; Assignability. This Agreement shall be binding upon, inure to the benefit of, and be enforceable by the respective
successors and permitted assigns of the Parties hereto. Parent and Surviving Company shall have the right to sell, assign or otherwise
transfer this Agreement, in whole or in part, to any Person that purchases all or substantially all of Parent, Surviving Company or all
or substantially all of their assets or the Business. Restricted Party may not assign this Agreement without the written consent of Parent
and any assignment by Restricted Party without such consent shall be void ab initio.
(f) Attorneys’
Fees and Costs. In the event of any action or proceeding regarding or arising from this Agreement, the losing Party shall bear its
own attorneys’ fees and costs as well as pay the attorney’s fees and costs of the prevailing Party.
(g) Headings;
Construction; Definitions. The descriptive headings of this Agreement are inserted for convenience only and do not constitute a part
of this Agreement. The Parties have participated jointly in the negotiation and drafting of this Agreement. In the event an ambiguity
or question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the Parties and no presumption
or burden of proof shall arise favoring or disfavoring any Party by virtue of the authorship of any of the provisions of this Agreement.
The recitals are hereby incorporated into this Agreement in their entirety. The word “including” shall mean “including
without limitation.”
(h) Counterparts;
Delivery. This Agreement may be executed and delivered in any number of counterparts (including by means of facsimile or electronically
transmitted signature pages), each of which will be deemed an original, but all of which together will constitute but one and the same
instrument. This Agreement will become effective when duly executed by each Party.
* * * * *
The undersigned have executed
this Restrictive Covenant Agreement on and as of the date first above written.
| |
PARENT: |
| |
|
| |
FIRST WAVE BIOPHARMA, INC. |
| |
By: |
|
| |
Name: |
James Sapirstein |
| |
Title: |
President and Chief Executive Officer |
Signature Page to Restrictive
Covenant Agreement
| |
COMPANY: |
| |
|
| |
IMMUNOGENX, INC. |
| |
By: |
|
| |
Name: |
Jack Syage |
| |
Title: |
Chief Executive Officer |
Signature Page to Restrictive Covenant Agreement
| |
RESTRICTED
PARTY: |
| |
|
| |
|
| |
[●] |
| |
|
| |
Address: |
Signature Page to Restrictive Covenant
Agreement
EXHIBIT E
FORM OF CERTIFICATE OF DESIGNATION
EXHIBIT F
FORM OF LETTER OF TRANSMITTAL
EXHIBIT G
FORM OF ACCREDITED INVESTOR QUESTIONNAIRE
ANNEX C
FAIRNESS OPINION OF LADENBURG THALMANN &
CO. INC.
March 13, 2024
First Wave BioPharma, Inc.
777 Yamato Road, Suite 502
Boca Raton, FL 33431
Attention: Board of Directors
Members of the Board of Directors of First Wave
BioPharma, Inc.:
We
have been advised that First Wave BioPharma, Inc., a Delaware corporation (“First Wave” or “Parent”), proposes
to enter into an Agreement and Plan of Merger (the “Agreement”), by and among First Wave, IMMUNO Merger Sub I, Inc., a Delaware
corporation and wholly owned subsidiary of First Wave (“First Merger Sub”), IMMUNO Merger Sub II, LLC, a Delaware limited
liability company and wholly owned subsidiary of First Wave (“Second Merger Sub” and together with First Merger Sub, the “Merger
Subs”), and ImmunogenX, Inc., a Delaware corporation (the “Company” or “ImmunogenX”). We understand
that pursuant to, upon the terms, and subject to the conditions set forth in the Agreement and in accordance with the Delaware General
Corporation Law and the Delaware Limited Liability Company Act, First Wave and the Company intend to effect a merger of First Merger Sub
with and into the Company (the “First Merger”). Upon consummation of the First Merger, the separate corporate existence of
First Merger Sub will cease, and the Company will continue as the surviving company and a wholly-owned subsidiary of First Wave. Immediately
following the First Merger and as part of the same overall transaction as the First Merger, the Company will merge with and into Second
Merger Sub (the “Second Merger” and, together with the First Merger, the “Mergers”). Upon consummation of the
Second Merger, the separate corporate existence of the Company will cease, and Second Merger Sub will continue as the surviving corporation
(the “Surviving Company”). Pursuant to the Agreement, at the "First Effective Time", each outstanding share of the
Company’s common stock, par value $0.0001 per share, and Company Preferred Stock, par value $0.0001 per share, will be converted,
in accordance with the negotiated exchange ratios, into shares of the Parent’s common stock, par value $0.0001 per share, and a
new series of Parent Convertible Preferred Stock, par value $0.0001 per share (“Parent Convertible Preferred Stock”). Capitalized
terms used herein without definition herein shall have the meanings ascribed to such terms in the Agreement, dated March 13, 2024 previously
provided to us (the “Merger Agreement”).
Immediately
following the consummation of the Mergers and without taking into effect the issuance of shares to First Wave’s financial advisor,
(i) the holders of Company Common Stock, Company Preferred Stock, Company Warrants and Company Options shall hold no more than 19.99%
of the fully diluted shares of Parent Common Stock outstanding (excluding certain Parent options and warrants issuable in exchange for
such Company Options and Company Warrants and without giving effect to the conversion of Parent Convertible Preferred Stock) immediately
following the Mergers and (ii) the holders of Parent Common Stock prior to the Mergers (the “Parent Stockholders”) shall hold
approximately 80.01% of the fully-diluted shares of Parent Common Stock outstanding (excluding certain Parent options and warrants issuable
in exchange for Company Options and Company Warrants and without giving effect to the conversion of Parent Convertible Preferred Stock).
Assuming the conversion of all Parent Convertible Preferred Stock issued pursuant to the Merger Agreement into Parent Common Stock, which
is subject to approval by the Parent Stockholders, and without taking into effect the issuance of shares to First Wave’s financial
advisor, (i) the holders of Company Common Stock, Company Preferred Stock and Company Options shall hold approximately 81.85% of the fully-diluted
shares of Parent Common Stock outstanding immediately following the Mergers and (ii) the Parent Stockholders shall hold approximately
18.15% of the fully-diluted shares of Parent Common Stock outstanding immediately following the Mergers.
With your consent, we
have relied upon the assumption that all information provided to us by First Wave and ImmunogenX is accurate and complete in all material
respects. We expressly disclaim any undertaking or obligation to advise any person of any change in any fact or matter affecting our Opinion
of which we become aware after the date hereof. We have assumed there were no material changes in the assets, liabilities, financial condition,
results of operations, business or prospects of First Wave or ImmunogenX since the date of the last financial statements made available
to us. We have not obtained any independent evaluations, valuations or appraisals of the assets or liabilities of First Wave or ImmunogenX,
nor have we been furnished with such materials. In addition, we have not evaluated the solvency or fair value of First Wave or ImmunogenX
under any state or federal laws relating to bankruptcy, insolvency or similar matters.
Our Opinion does not
address any legal, regulatory, tax or accounting matters related to the Mergers, as to which we have assumed that First Wave and the Board
of Directors have received such advice from legal, tax and accounting advisors as each has determined appropriate. Our Opinion addresses
only the fairness, from a financial point of view, to the stockholders of First Wave of the Merger Consideration to be paid by First Wave
pursuant to the Merger Agreement. We express no view as to any other aspect or implication of the Mergers or any other agreement or arrangement
entered into in connection with the Mergers. Our Opinion is necessarily based upon economic and market conditions and other circumstances
as they exist and can be evaluated by us on the date hereof. It should be understood that although subsequent developments may affect
our Opinion, we do not have any obligation to update, revise or reaffirm our Opinion and we expressly disclaim any responsibility to do
so.
We have not considered
any potential legislative or regulatory changes currently being considered or recently enacted by the United States or any foreign government,
or any domestic or foreign regulatory body, including the rules and regulations of the Nasdaq Stock Market, or any changes in accounting
methods or generally accepted accounting principles that may be adopted by the Securities and Exchange Commission (the “SEC”),
the Financial Accounting Standards Board, or any similar foreign regulatory body or board.
In your capacity as members
of the Board of Directors of First Wave (the “Board of Directors”), you have requested our opinion (our “Opinion”)
as to the fairness, from a financial point of view and as of the date hereof, to First Wave of the Merger Consideration to be paid by
First Wave pursuant to the Merger Agreement.
In connection with our
Opinion, we took into account an assessment of general economic, market and financial conditions as well as our experience in connection
with similar transactions and securities valuations generally and, among other things:
| · |
Reviewed the Merger Agreement; |
| · |
Reviewed and analyzed certain publicly available financial and other information for each of First Wave and ImmunogenX, respectively, including equity research on comparable companies, and certain other relevant financial and operating data furnished to us by the management of First Wave; |
| · |
Reviewed and analyzed certain relevant historical financial and operating data concerning ImmunogenX furnished to us by the management of First Wave; |
| · |
Discussed with certain members of the management of First Wave and ImmunogenX the historical and current business operations, financial condition and prospects of First Wave and ImmunogenX, respectively; |
| · |
Reviewed and analyzed certain operating results of ImmunogenX as compared to operating results and the reported price and trading histories of certain publicly traded companies that we deemed relevant; |
| · |
Reviewed and analyzed certain financial terms of the Merger Agreement as compared to the publicly available financial terms of certain selected business combinations that we deemed relevant; |
| · |
Reviewed and analyzed certain financial terms of completed initial public offerings for certain companies that we deemed relevant; |
| · |
Reviewed certain pro forma financial effects of the Mergers; and |
| · |
Reviewed and analyzed such other information and such other factors, and conducted such other financial studies, analyses and investigations, as we deemed relevant for the purposes of rendering our Opinion. |
For
purposes of rendering our Opinion we have assumed, with your consent, that except as would not be in any way meaningful to our analysis:
(i) the final form of the Merger Agreement will not differ from the draft that we have reviewed; (ii) the representations and warranties
of each party contained in the Merger Agreement are true and correct in all material respects; (iii) each party will perform all of the
covenants and agreements required to be performed by such party under the Merger Agreement; and (iv) the transactions contemplated by
the Merger Agreement will be consummated in accordance with the terms of the Merger Agreement, without any waiver or amendment of any
term or condition thereof. We have also assumed that all governmental, regulatory and other consents and approvals contemplated by the
Agreement or otherwise required for the transactions contemplated by the Agreement will be obtained and that in the course of obtaining
any of those consents no restrictions will be imposed, or waivers made that would have an adverse effect on First Wave, ImmunogenX, or
the contemplated benefits of the Mergers. We have assumed that the Mergers will be consummated in a manner that complies with the applicable
provisions of the Securities Act of 1933, as amended, the Securities Exchange Act of 1934, as amended, and all other applicable federal
and state statutes and the rules and regulations promulgated thereunder.
It is understood that
this letter is intended for the benefit and use of the First Wave Board of Directors (in its capacity as such) in its consideration of
the financial terms of the Merger and, except as set forth in our engagement letter with First Wave, dated as of January 29, 2024 (the
“Fairness Opinion Agreement”), may not be used for any other purpose or reproduced, disseminated, quoted or referred to at
any time, in any manner or for any purpose without our prior written consent, except that this Opinion may be included in its entirety
in any filing related to the Mergers or the First Wave stockholder approval required to be filed with the SEC and any proxy statement
to be mailed to holders of Parent Common Stock. This letter does not constitute a recommendation to the Board of Directors of whether
to approve the Mergers or to any stockholder of First Wave or any other person as to how to vote or act with respect to the transactions
contemplated by the Agreement (including the Mergers) or any other matter. Our Opinion does not address First Wave’s underlying
business decision to proceed with the Mergers or the relative merits of the Mergers compared to other alternatives available to First
Wave. We express no opinion as to the prices or ranges of prices at which shares or the securities of any person, including First Wave,
will trade at any time, including following the announcement or consummation of the Mergers, or as to the potential effects of volatility
in the credit, financial, and stock markets on First Wave, ImmunogenX or the transactions contemplated by the Agreement. We have not been
requested to opine as to, and our Opinion does not in any manner address, the amount or nature of compensation to any of the officers,
directors or employees of any party to the Mergers, or any class of such persons, relative to the compensation to be paid to the holders
of Parent Common Stock in connection with the Merger or with respect to the fairness of any such compensation.
Ladenburg is a full-service
investment bank providing investment banking, brokerage, equity research, institutional sales and trading, and asset management services.
As part of our investment banking services, we are regularly engaged in the valuation of businesses and their securities in connection
with mergers, negotiated underwritings, secondary distributions of listed and unlisted securities, private placements and valuations for
corporate and other purposes. In the ordinary course of business, Ladenburg or certain of our affiliates, as well as investment funds
in which we or our affiliates may have financial interests, may acquire, hold or sell long or short positions, or trade or otherwise effect
transactions in debt, equity, and other securities and financial instruments (including bank loans and other obligations) of, or investments
in, First Wave, ImmunogenX or any other party that may be involved in the Mergers and/or their respective affiliates.
In connection with our
services, and pursuant to the Fairness Opinion Agreement between us and the First Wave, we are entitled to receive a $175,000 fee in connection
with delivering our opinion, of which Ladenburg has received a $50,000 upfront retainer with the remaining $125,000 to be paid when we
issue our opinion to the Company. In the two years preceding the date hereof, Ladenburg has not had a relationship with First Wave and
has not received any fees from First Wave, other than the $50,000 up-front retainer which was paid to Ladenburg pursuant to the Fairness
Opinion Agreement in connection with its engagement by First Wave thereunder. In the two years preceding the date hereof, Ladenburg has
not had a relationship with ImmunogenX or any of its affiliates and has not received any fees from ImmunogenX or any of its affiliates.
Ladenburg and its affiliates may in the future seek to provide investment banking or financial advisory services to First Wave and ImmunogenX
and/or their respective affiliates and expect to receive fees for the rendering of these services.
Consistent with applicable
legal and regulatory requirements, Ladenburg has adopted policies and procedures to establish and maintain the independence of our research
department and personnel. As a result, our research analysts may hold views, make statements or investment recommendations and/or publish
research reports with respect to First Wave and the proposed Mergers that may differ from the views of Ladenburg’s investment banking
personnel.
The Opinion set forth
below was reviewed and approved by a fairness opinion committee of Ladenburg.
Based upon and subject
to the foregoing, including the various assumptions and limitations set forth herein and such other factors that we deem relevant, it
is our opinion that, as of the date hereof, the Merger Consideration to be paid by First Wave pursuant to the Agreement is fair, from
a financial point of view, to the holders of Parent Common Stock.
Very truly yours,
/s/ Ladenburg Thalmann & Co. Inc.
Ladenburg Thalmann & Co. Inc.
ANNEX D
CERTIFICATE OF AMENDMENT TO THE AMENDED
AND RESTATED CERTIFICATE OF INCORPORATION OF ENTERO THERAPEUTICS, INC.
[To be provided]
ANNEX E
AUDITED FINANCIAL STATEMENTS OF IMMUNOGENX,
INC. FOR THE FISCAL YEARS ENDED DECEMBER 31, 2023, 2022 AND 2021
INDEPENDENT AUDITOR’S REPORT
To the Board of Directors of
ImmunogenX, Inc.:
Opinion
We have audited the accompanying financial statements
of ImmunogenX, Inc. (the “Company”), which comprise the balance sheets as of December 31, 2023, 2022 and 2021, and
the related statements of operations, changes in stockholders’ deficit, and cash flows for the years then ended, and the related
notes to the financial statements.
In our opinion, the financial statements referred
to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023, 2022 and 2021,
and the results of its operations and cash flows for the years then ended in conformity with accounting principles generally accepted
in the United States of America.
Basis for Opinion
We conducted our audits in accordance with auditing
standards generally accepted in the United States of America. Our responsibilities under those standards are further described in the
Auditor’s Responsibilities for the Audit of the Financial Statements section of our report. We are required to be independent of
the Company and to meet our other ethical responsibilities in accordance with the relevant ethical requirements relating to our audits.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Emphasis of Matter Regarding Going Concern
The accompanying financial statements have been
prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company has
incurred recurring net losses and negative cash flows from operations because of the Company being in the pre-revenue stage. The Company’s
current obligations and net losses combined with the uncertainty regarding future financing raise substantial doubt about the Company’s
ability to continue as a going concern. The Company’s financial statements do not include any adjustments that might result from
the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
Change in Accounting Principle
As discussed in Note 2 to the financial statements,
the Company changed its method of accounting for leases effective January 1, 2021, due to the early adoption of Financial Accounting
Standards Board Accounting Standards Codification 842, Leases. Our opinion is not modified with respect to this matter.
Responsibilities of Management for the Financial
Statements
Management is responsible for the preparation
and fair presentation of the financial statements in accordance with accounting principles generally accepted in the United States of
America, and for the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of
financial statements that are free from material misstatement, whether due to fraud or error.
In preparing the financial statements, management
is required to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s
ability to continue as a going concern within one year after the date that the financial statements are available to be issued.
Auditor’s Responsibilities for the
Audit of the Financial Statements
Our objectives are to obtain reasonable assurance
about whether the financial statements as a whole are free from material misstatement, whether due to fraud or error, and to issue an
auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance but is not absolute assurance and
therefore is not a guarantee that an audit conducted in accordance with generally accepted auditing standards will always detect a material
misstatement when it exists. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from
error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control. Misstatements
are considered material if there is a substantial likelihood that, individually or in the aggregate, they would influence the judgment
made by a reasonable user based on the financial statements.
In performing an audit in accordance with generally accepted auditing
standards, we:
| |
· |
Exercise professional judgment and maintain professional skepticism throughout the audit. |
| |
|
|
| |
· |
Identify and assess the risks of material misstatement of the financial statements, whether due to fraud or error, and design and perform audit procedures responsive to those risks. Such procedures include examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. |
| |
|
|
| |
· |
Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control. Accordingly, no such opinion is expressed. |
| |
|
|
| |
· |
Evaluate the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluate the overall presentation of the financial statements. |
| |
|
|
| |
· |
Conclude whether, in our judgment, there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern for a reasonable period of time. |
We are required to communicate with those charged
with governance regarding, among other matters, the planned scope and timing of the audit, significant audit findings, and certain internal
control related matters that we identified during our audits.
/s/ Holthouse Carlin & Van Trigt LLP
Irvine, California
March 13, 2024
IMMUNOGENX INC.
BALANCE SHEETS
| AS OF DECEMBER 31, |
|
2023 |
|
|
2022 |
|
|
2021 |
|
| ASSETS |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
| Current assets: |
|
|
|
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
251,266 |
|
|
$ |
95,342 |
|
|
$ |
1,486,844 |
|
| Prepaid expenses and other current assets |
|
|
3,068,344 |
|
|
|
1,869,860 |
|
|
|
6,466 |
|
| Total current assets |
|
|
3,319,610 |
|
|
|
1,965,202 |
|
|
|
1,493,310 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Property and equipment, net |
|
|
20,853 |
|
|
|
26,454 |
|
|
|
39,691 |
|
| Operating lease right-of-use asset, net |
|
|
10,704 |
|
|
|
42,680 |
|
|
|
74,454 |
|
| Patents, net |
|
|
668,451 |
|
|
|
782,246 |
|
|
|
896,042 |
|
| Other intangible assets |
|
|
19,200 |
|
|
|
19,200 |
|
|
|
19,200 |
|
| Other assets |
|
|
63,558 |
|
|
|
96,137 |
|
|
|
- |
|
| Total assets |
|
$ |
4,102,376 |
|
|
$ |
2,931,919 |
|
|
$ |
2,522,697 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| LIABILITIES AND STOCKHOLDERS' DEFICIT |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Current liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
| Accounts payable |
|
$ |
399,283 |
|
|
$ |
626,127 |
|
|
$ |
207,926 |
|
| Accrued expenses and other current liabilities |
|
|
2,008,888 |
|
|
|
509,384 |
|
|
|
187,963 |
|
| Current portion of operating lease liability |
|
|
9,129 |
|
|
|
24,675 |
|
|
|
30,668 |
|
| Related party promissory note |
|
|
393,282 |
|
|
|
269,481 |
|
|
|
592,222 |
|
| Revolving line of credit |
|
|
6,360,000 |
|
|
|
2,810,000 |
|
|
|
- |
|
| Total current liabilities |
|
|
9,170,582 |
|
|
|
4,239,667 |
|
|
|
1,018,779 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Operating lease liability, net of current portion |
|
|
- |
|
|
|
9,129 |
|
|
|
33,805 |
|
| Convertible notes (at fair value) |
|
|
8,663,804 |
|
|
|
3,668,574 |
|
|
|
2,948,457 |
|
| Note payable |
|
|
500,000 |
|
|
|
500,000 |
|
|
|
500,000 |
|
| Total liabilities |
|
|
18,334,386 |
|
|
|
8,417,370 |
|
|
|
4,501,041 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Stockholders' deficit: |
|
|
|
|
|
|
|
|
|
|
|
|
| Preferred stock, $0.0001 par value; 2,260,054 shares authorized; 2,260,054 shares issued and outstanding |
|
|
226 |
|
|
|
226 |
|
|
|
226 |
|
| Common stock, $0.0001 par value; 2,000,000 shares authorized; 1,009,925 shares issued and outstanding |
|
|
101 |
|
|
|
100 |
|
|
|
99 |
|
| Additional paid-in capital |
|
|
4,608,364 |
|
|
|
4,496,928 |
|
|
|
4,380,877 |
|
| Accumulated deficit |
|
|
(18,840,701 |
) |
|
|
(9,982,705 |
) |
|
|
(6,359,546 |
) |
| Total stockholders' deficit |
|
|
(14,232,010 |
) |
|
|
(5,485,451 |
) |
|
|
(1,978,344 |
) |
| Total liabilities and stockholders' deficit |
|
$ |
4,102,376 |
|
|
$ |
2,931,919 |
|
|
$ |
2,522,697 |
|
See accompanying notes to the financial statements.
IMMUNOGENX INC.
STATEMENTS OF OPERATIONS
| FOR THE YEARS ENDED DECEMBER 31, |
|
2023 |
|
|
2022 |
|
|
2021 |
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
| General and administrative |
|
$ |
2,308,851 |
|
|
$ |
1,932,116 |
|
|
$ |
1,474,494 |
|
| Research and development |
|
|
2,828,531 |
|
|
|
2,268,205 |
|
|
|
1,892,303 |
|
| Total operating expenses |
|
|
5,137,382 |
|
|
|
4,200,321 |
|
|
|
3,366,797 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
| Grant income |
|
|
910,577 |
|
|
|
1,090,595 |
|
|
|
1,630,858 |
|
| Paycheck Protection Program loan forgiveness |
|
|
- |
|
|
|
- |
|
|
|
120,023 |
|
| Change in fair value of convertible notes |
|
|
(3,615,230 |
) |
|
|
(190,117 |
) |
|
|
(48,017 |
) |
| Interest expense |
|
|
(1,083,056 |
) |
|
|
(374,588 |
) |
|
|
(126,159 |
) |
| Other income, net |
|
|
67,886 |
|
|
|
52,072 |
|
|
|
131,627 |
|
| Total other income (expense), net |
|
|
(3,719,823 |
) |
|
|
577,962 |
|
|
|
1,708,332 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Income before provision for income taxes |
|
|
(8,857,205 |
) |
|
|
(3,622,359 |
) |
|
|
(1,658,465 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Provision for state income taxes |
|
|
791 |
|
|
|
800 |
|
|
|
1,722 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(8,857,996 |
) |
|
$ |
(3,623,159 |
) |
|
$ |
(1,660,187 |
) |
See accompanying notes to the financial statements.
IMMUNOGENX INC.
STATEMENTS OF CHANGES IN STOCKHOLDERS' DEFICIT
| FOR THE YEARS ENDED |
|
Preferred Stock |
|
|
Common Stock |
|
|
Paid-In |
|
|
Accumulated |
|
|
|
|
| DECEMBER 31, 2023, 2022, AND 2021 |
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Total |
|
| Balance as of December 31, 2020 |
|
|
2,260,054 |
|
|
$ |
226 |
|
|
|
980,450 |
|
|
$ |
98 |
|
|
$ |
4,322,473 |
|
|
$ |
(4,699,359 |
) |
|
$ |
(376,562 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock granted |
|
|
- |
|
|
|
- |
|
|
|
14,670 |
|
|
|
1 |
|
|
|
22,929 |
|
|
|
- |
|
|
|
22,930 |
|
| Stock-based compensation - stock options |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
35,475 |
|
|
|
- |
|
|
|
35,475 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(1,660,187 |
) |
|
|
(1,660,187 |
) |
| Balance as of December 31, 2021 |
|
|
2,260,054 |
|
|
$ |
226 |
|
|
|
995,120 |
|
|
$ |
99 |
|
|
$ |
4,380,877 |
|
|
$ |
(6,359,546 |
) |
|
$ |
(1,978,344 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock granted |
|
|
- |
|
|
|
- |
|
|
|
8,590 |
|
|
|
1 |
|
|
|
17,597 |
|
|
|
- |
|
|
|
17,598 |
|
| Stock-based compensation - stock options |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
48,583 |
|
|
|
- |
|
|
|
48,583 |
|
| Equity-classified stock warrant |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
49,871 |
|
|
|
- |
|
|
|
49,871 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(3,623,159 |
) |
|
|
(3,623,159 |
) |
| Balance as of December 31, 2022 |
|
|
2,260,054 |
|
|
$ |
226 |
|
|
|
1,003,710 |
|
|
$ |
100 |
|
|
$ |
4,496,928 |
|
|
$ |
(9,982,705 |
) |
|
$ |
(5,485,451 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock granted |
|
|
- |
|
|
|
- |
|
|
|
6,215 |
|
|
|
1 |
|
|
|
12,907 |
|
|
|
- |
|
|
|
12,908 |
|
| Stock-based compensation - stock options |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
66,235 |
|
|
|
- |
|
|
|
66,235 |
|
| Equity-classified stock warrant |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
32,294 |
|
|
|
- |
|
|
|
32,294 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(8,857,996 |
) |
|
|
(8,857,996 |
) |
| Balance as of December 31, 2023 |
|
|
2,260,054 |
|
|
$ |
226 |
|
|
|
1,009,925 |
|
|
$ |
101 |
|
|
$ |
4,608,364 |
|
|
$ |
(18,840,701 |
) |
|
$ |
(14,232,010 |
) |
See accompanying notes to the financial statements.
IMMUNOGENX INC.
STATEMENTS OF CASH FLOWS
| FOR THE YEARS ENDED DECEMBER 31, |
|
2023 |
|
|
2022 |
|
|
2021 |
|
| Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
|
$ |
(8,857,996 |
) |
|
$ |
(3,623,159 |
) |
|
$ |
(1,660,187 |
) |
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
| Reduction of operating right-of-use asset |
|
|
31,977 |
|
|
|
31,774 |
|
|
|
31,492 |
|
| Payment-in-kind interest |
|
|
23,801 |
|
|
|
27,259 |
|
|
|
51,325 |
|
| Depreciation and amortization of property and equipment |
|
|
9,510 |
|
|
|
13,237 |
|
|
|
13,282 |
|
| Amortization of patents |
|
|
113,796 |
|
|
|
113,796 |
|
|
|
113,796 |
|
| Amortization of deferred debt issuance costs |
|
|
30,000 |
|
|
|
7,500 |
|
|
|
- |
|
| Amortization of loan commitment fee |
|
|
34,873 |
|
|
|
6,234 |
|
|
|
- |
|
| Change in fair value of convertible notes |
|
|
3,615,230 |
|
|
|
190,117 |
|
|
|
48,017 |
|
| Common stock granted |
|
|
12,907 |
|
|
|
17,597 |
|
|
|
22,929 |
|
| Stock-based compensation - stock options |
|
|
66,235 |
|
|
|
48,583 |
|
|
|
35,475 |
|
| Paycheck Protection Program loan forgiveness |
|
|
- |
|
|
|
- |
|
|
|
(120,023 |
) |
| Change in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
| Prepaid expenses and other current assets |
|
|
(1,198,484 |
) |
|
|
(1,863,394 |
) |
|
|
(8,381 |
) |
| Accounts payable |
|
|
(226,844 |
) |
|
|
418,201 |
|
|
|
106,525 |
|
| Accrued expenses and other current liabilities |
|
|
1,499,503 |
|
|
|
321,422 |
|
|
|
93,157 |
|
| Operating lease liability |
|
|
(24,675 |
) |
|
|
(30,669 |
) |
|
|
(36,597 |
) |
| Net cash used in operating activities |
|
|
(4,870,167 |
) |
|
|
(4,321,502 |
) |
|
|
(1,309,190 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
| Purchases of property and equipment |
|
|
(3,909 |
) |
|
|
- |
|
|
|
(18,441 |
) |
| Cash used in investing activities |
|
|
(3,909 |
) |
|
|
- |
|
|
|
(18,441 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
| Proceeds from note payable |
|
|
- |
|
|
|
- |
|
|
|
350,000 |
|
| Proceeds from line of credit |
|
|
3,550,000 |
|
|
|
2,750,000 |
|
|
|
- |
|
| Proceeds from related party promissory notes |
|
|
200,000 |
|
|
|
- |
|
|
|
- |
|
| Repayments on promissory notes |
|
|
- |
|
|
|
(250,000 |
) |
|
|
(100,000 |
) |
| Proceeds from convertible notes |
|
|
1,280,000 |
|
|
|
430,000 |
|
|
|
2,375,000 |
|
| Proceeds from Paycheck Protection Program loan |
|
|
- |
|
|
|
- |
|
|
|
66,607 |
|
| Repayments on Paycheck Protection Program loan |
|
|
- |
|
|
|
- |
|
|
|
(28,569 |
) |
| Net cash provided by financing activities |
|
|
5,030,000 |
|
|
|
2,930,000 |
|
|
|
2,663,038 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Net change in cash and cash equivalents |
|
|
155,924 |
|
|
|
(1,391,502 |
) |
|
|
1,335,407 |
|
| Cash and cash equivalents, beginning of year |
|
|
95,342 |
|
|
|
1,486,844 |
|
|
|
151,437 |
|
| Cash and cash equivalents, end of year |
|
$ |
251,266 |
|
|
$ |
95,342 |
|
|
$ |
1,486,844 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Supplemental disclosures of cash flow information: |
|
|
|
|
|
|
|
|
|
|
|
|
| Cash paid during the year for: |
|
|
|
|
|
|
|
|
|
|
|
|
| Interest |
|
$ |
30,168 |
|
|
$ |
5,028 |
|
|
$ |
- |
|
| State taxes |
|
$ |
791 |
|
|
$ |
800 |
|
|
$ |
1,722 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Non-cash operating and financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
| Operating right-of-use asset obtained in exchange for lease liability |
|
$ |
- |
|
|
$ |
- |
|
|
$ |
105,946 |
|
| Issuance of equity-classified stock warrants |
|
$ |
32,294 |
|
|
$ |
49,871 |
|
|
$ |
- |
|
| Reclass of related party promissory notes to convertible notes |
|
$ |
100,000 |
|
|
$ |
100,000 |
|
|
$ |
400,000 |
|
| Borrowings on line of credit for deferred debt issuance costs |
|
$ |
- |
|
|
$ |
60,000 |
|
|
$ |
- |
|
See accompanying notes to the financial statements.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
| 1. |
ORGANIZATION AND NATURE OF BUSINESS |
Founded in 2013 as a California LLC, ImmunogenX, Inc.
(the “Company”) is a private, clinical stage, biotherapeutics company, headquartered in Newport Beach, California, with a
research facility in North Carolina, formed to address critical needs for individuals with suspected or diagnosed celiac disease. On January 1,
2021, the Company converted to a corporation and incorporated in the state of Delaware. Research efforts are focused on therapy, disease
management, and food safety. The Company has patented formulations related to the use of latiglutenase, a digestive enzyme that degrades
unintended ingestion of gluten in the body. Another key area of research involves a method of monitoring intestinal villi atrophy by measuring
the level of a drug metabolite that is strongly dependent on the villi heath.
Through December 31, 2023, the
Company has been primarily engaged in developing its biopharmaceutical products, research and development, and raising capital through
equity and debt offerings. The Company has not commenced principal revenue producing operations and has incurred significant expenditures
for the research and development of the Company’s biopharmaceutical products. Once the Company’s planned principal operations
commence, its focus will be on the manufacturing, refining, licensing, and marketing of its biopharmaceuticals and the continued research
and development of new associated biopharmaceutical products.
The Company's activities are subject
to significant risks and uncertainties, including being unable to secure additional funding to make the Company's current biopharmaceuticals
operational before another company develops similar biopharmaceutical products. In addition, FDA approval is required for the Company’s
products at the completion of successful human clinical trials.
| 2. |
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis
of Presentation The accompanying financial statements have been prepared on an accrual basis of accounting in accordance
with accounting principles generally accepted in the United States of America (“US GAAP”), where revenues and expenses are
recorded as earned and incurred, respectively.
Use
of Estimates The preparation of financial statements in conformity with US GAAP requires management to make estimates and
assumptions that affect the reported amounts of certain assets and liabilities, certain disclosures at the date of the financial statements,
as well as the reported amounts of revenues and expenses during the reporting period. Significant items subject to such estimates and
assumptions include certain prepaid expenses, certain accrued expenses, and estimates regarding the fair value of debt and equity instruments.
Actual results may differ from these estimates under different assumptions or conditions.
Fair
Value Measurements The Company accounts for the fair value of its financial instruments in accordance with Financial Accounting
Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 820, Fair Value Measurement (“ASC
820”). Non-recurring, nonfinancial assets and liabilities are also accounted for under the provisions of ASC 820.
ASC 820 defines fair value, establishes
a framework for measuring fair value under US GAAP and enhances disclosures about fair value measurements. Fair value is defined under
ASC 820 as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market
participants on the measurement date.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
Valuation techniques used to measure
fair value under ASC 820 must maximize the use of observable inputs and minimize the use of unobservable inputs. The standard describes
a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that
may be used to measure fair value.
The Company’s management used
the following methods and assumptions to estimate the fair value of its financial instruments:
| |
Levels |
|
Hierarchy |
| |
|
|
|
| |
1 |
|
Pricing inputs are quoted prices available in active markets for identical assets or liabilities as of the reporting date. |
| |
|
|
|
| |
2 |
|
Pricing inputs are quoted prices for similar investments, or inputs that are observable, either directly or indirectly, for substantially the full term through corroboration with observable market data. Level 2 includes assets and liabilities valued at quoted prices adjusted for legal or contractual restrictions specific to these assets and liabilities. |
| |
|
|
|
| |
3 |
|
Pricing inputs are unobservable, supported by little or no market activity, and reflect the reporting entity’s own assumptions about the assumptions market participants would use in pricing the asset or liability. |
The Company’s management incorporated
Level 3 inputs, including assumptions to estimate the fair value of its stock warrants (see Note 7), convertible notes (see Note 9), and
stock options (see Note 12).
Cash
and Cash Equivalents Cash and cash equivalents is comprised of cash on hand, deposits in banks, and highly liquid investments
with an original maturity of three months or less.
Financial
Instruments and Concentrations of Business and Credit Risk Financial instruments that potentially subject the Company to
concentrations of business and credit risks consist of cash and cash equivalents and accounts payable.
The Company maintains cash balances
that can, at times, exceed amounts insured by the Federal Deposit Insurance Corporation. The Company has not experienced any losses in
these accounts and believes it is not exposed to any significant credit risk in this area.
The Company’s accounts payable
can expose the Company to business risks such as supplier concentrations, which the Company mitigates by attempting to diversify its supply
chain. For the year ended December 31, 2023, one supplier accounted for 68% of purchases and 15% of accounts payable as of December 31,
2023. For the year ended December 31, 2022, one supplier accounted for 74% of total purchases and 54% of accounts payable as of December 31,
2022. For the year ended December 31, 2021, two suppliers accounted for 36% of total purchases and 1% of accounts payable as of December 31,
2021.
Pre-Launch
Inventories The Company does not capitalize pre-launch inventory costs, including raw materials and inventory production,
until future commercialization is considered probable and the future economic benefit is expected to be realized or if there is an alternative
future use. Capitalizing pre-launch inventory costs will not occur prior to obtaining regulatory approval, commercialization is considered
probable, and future economic benefit can be asserted. The Company records such costs as research and development expenses in the statement
of operations in the period incurred.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
Research
and Development Costs Research and development costs are charged to expense as costs are incurred in performing research
and development activities in accordance with FASB ASC Topic 730, Research and Development (“ASC 730”). Such costs
include overhead costs, clinical study and related clinical manufacturing costs, license and milestone fees, contract services, manufacturing
costs for pre-launch inventories, and other related costs. Up-front fees and milestones paid to third parties in connection with
research and development activities are expensed as incurred.
Property
and Equipment Property and equipment are stated at cost, net of accumulated depreciation and amortization. Depreciation
and amortization expense are calculated using the straight-line method over the estimated useful lives of the related assets, which range
from 3 to 7 years. Leasehold improvements are amortized over the shorter of their estimated useful lives or the term of the related lease.
Betterments, renewals, and extraordinary
repairs that materially extend the useful life of the asset are capitalized; other repairs and maintenance charges are expensed as incurred.
The cost and related accumulated depreciation and amortization applicable to assets retired are removed from the accounts, and the gain
or loss on disposition, if any, is recognized in the statements of income and operations.
Intangible
Assets Intangible assets are stated at acquisition cost less accumulated amortization. Intangible assets recorded in asset
acquisitions are measured based on their cost, which is generally allocated to the assets on a relative fair value basis on the date of
acquisition.
In accordance with FASB ASC Topic 350, Intangibles,
Goodwill and Other (“ASC 350”), identifiable intangible assets acquired in a business combination or asset acquisition
and determined to have an indefinite useful life are not amortized, but instead tested for impairment at least annually and more frequently
if events and circumstances indicate that the asset might be impaired. ASC 350 also requires that intangible assets with estimable useful
lives be amortized over their respective estimated useful lives to their estimated residual values and reviewed for impairment in accordance
with FASB ASC Topic 360, Property, Plant and Equipment (“ASC 360”).
ASC 350 requires that intangible assets
with estimable useful lives be amortized over their respective estimated useful lives to their estimated residual values and reviewed
for impairment in accordance with ASC 360. Based on the application of impairment testing as discussed above, management deemed that the
fair value of intangible assets with estimable useful lives exceeds their carrying values; accordingly, no impairment of these intangible
assets has been recorded for the years ended December 31, 2023, 2022, and 2021.
Patents
Patent costs related to filing and pursuing patent applications, including direct application fees and legal and consulting expenses,
are expensed as incurred, as the recoverability of such expenses is uncertain. Additionally, costs to maintain existing patents are also
expensed as incurred. These costs are included in general and administrative expenses on the accompanying statement of operations.
In accordance with FASB ASC Topic 805,
Business Combinations (“ASC 805”), costs to acquire filed patents as a part of an asset purchase or business combination
are capitalized and, subsequently, amortized over their estimated useful lives and reviewed for impairment in accordance with ASC 360.
For the years ended December 31, 2023, 2022, and 2021, no impairment of these patents has been recorded. Amortization is calculated
using the straight-line method over the estimated useful lives of the patents, which range from 8.5 to 18 years.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
Impairment
of Long-Lived Assets In accordance with FASB ASC Topic 360, Property, Plant and Equipment (“ASC 360”),
long-lived assets such as property and equipment are reviewed for impairment whenever events or changes in circumstances indicate that
the carrying amount of an asset may not be recoverable. An impairment loss is recognized on long-lived assets when indicators of impairment
are present and the undiscounted cash flows estimated to be generated by those assets are less than the carrying amount of the assets.
In such cases, the carrying value of these assets is adjusted to their estimated fair value and assets held for sale are adjusted to their
estimated fair value less estimated selling expenses. No impairment losses of long-lived assets or intangible assets with estimated useful
lives were recognized for the years ended December 31, 2023, 2022, and 2021.
Deferred
Debt Issuance Costs Deferred debt issuance costs represent costs paid in connection with obtaining long-term financing.
Fees associated with long-term debt and revolving lines of credit are capitalized and subsequently amortized using the straight-line method
which approximates the effective-interest method, over the term of the related debt agreement. In accordance with FASB Account Standard
Update (“ASU”) 2015-03, Interest-Imputation of Interest (Subtopic 835-30), deferred debt issuance costs are presented,
net of accumulated amortization, as an asset for amounts relating to revolving lines of credit and as direct deductions from the face
amounts of all other related long-term debt (see Note 7).
Convertible
Notes In accordance with FASB ASC Topic 815, Derivatives and Hedging (“ASC 815”), certain financial
instruments with characteristics of liabilities and equity may have embedded derivatives that are required to be bifurcated and accounted
for separately from the host contract, unless the fair value option is elected. With the election of the fair value option, an irrevocable
election is made to initially and subsequently measure the hybrid financial instrument in its entirety at fair value, with changes in
fair value reported in earnings as they occur, except for changes in value related to the Company’s own creditworthiness, which
would be recognized in other comprehensive income. The Company elected the fair value option, and accordingly, recognized losses related
to the change in fair value for the years ended December 31, 2023, 2022, and 2021 in the amounts of $3,615,230, $190,117, and $48,017,
respectively, which is included in other income or expenses on the accompanying statements of operations.
In August 2020, the FASB issued
ASU 2020-06, Debt–Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging–Contracts in Entity’s
Own Equity (Subtopic 815-40), which simplifies the accounting for convertible instruments by eliminating the cash conversion and the
beneficial conversion feature accounting models for convertible debt and convertible preferred stock. For privately held companies, ASU
2020-06 is effective for annual reporting periods beginning after December 15, 2023, with early adoption permitted. The Company elected
to early adopt ASU 2020-06 as of January 1, 2021 by applying the modified retrospective transition method. As of January 1,
2021, the Company did not have any financial instruments outstanding that falls within the scope of ASU 2020-06; accordingly, there was
no impact to opening equity.
Related
Party Promissory Note Promissory notes payable to a related party are stated at unpaid principal and interest balances.
Interest on the notes is payment-in-kind (PIK), accrued, compounded annually, and added to the principal balance of the underlying notes.
Paycheck
Protection Program Loan In May 2020 and March 2021, the Company received loans in the amounts of $81,985 (the
“1st Draw”) and $66,607 (the “2nd Draw”), respectively, pursuant to the Paycheck Protection
Program (“PPP”) under the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”), which was enacted
on March 27, 2020. The PPP is administered by the U.S. Small Business Administration (the “SBA”). The Company accounts
for the loans under the PPP (the “PPP Loan”) as a financial liability in accordance with FASB ASC Topic 470, Debt (“ASC
470”). The Company does not impute additional interest at a market rate even though the stated interest rate under the PPP may be
below market. Transactions, where interest rates are prescribed by governmental agencies, are excluded from the scope of FASB ASC Subtopic
835-30, Interest – Imputation of Interest (“ASC 835-30”). In accordance with ASC 470, the proceeds from
the PPP Loan remained recorded as a liability until the loan was wholly forgiven.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
On August 18, 2021, $53,416 along
with interest of $689 related to the 1st Draw was forgiven by the SBA, and a repayment was made for the remaining principal
balance of $28,569. On October 19, 2021, the entire principal balance of the 2nd Draw along with interest of $385 was
forgiven by the SBA. The forgiven loan balances and interest are included in other income and expenses on the accompanying statements
of operations for the year ended December 31, 2021.
Economic
Injury Disaster Loan On June 30, 2020, the Company received a loan from the SBA under its Economic Injury Disaster
Loan (“EIDL”) assistance program in light of the impact of the COVID-19 pandemic on the Company’s business. Pursuant
to the Loan Authorization and Agreement (the “SBA Loan Agreement”), the Company received a principal amount of $150,000. On
August 12, 2021, the Company received approval from the SBA to increase the principal loan amount to $500,000. In accordance with
ASC 470, the Company accounts for the loan as a financial liability. The Company does not input additional interest at a market rate even
though the stated interest rate under the EIDL may be below market. Transactions, where interest rates are prescribed by governmental
agencies, are excluded from the scope of ASC 835-30. In accordance with ASC 470, the proceeds from the EIDL will remain recorded as a
liability until either (1) the loan is, in part or wholly, forgiven and the Company has been legally released; or (2) the loan
has been repaid. As of December 31, 2023, 2022, and 2021, the Company had an outstanding balance of $500,000 at the end of each year,
which is included in note payable on the accompanying balance sheets.
Stock
Warrants Warrants that were issued to obtained a line of credit (the “LOC”) are measured at fair value as of
the grant date, classified as equity, and included in additional paid-in-capital on the accompanying balance sheets as of December 31,
2023 and 2022. The warrants are equivalent to a loan commitment fee, therefore; these costs are capitalized on the balance sheet and amortized
on a straight-line basis over the stated term of the LOC (see Note 7).
Stock-Based
Compensation The Company accounts for its stock-based compensation in accordance with FASB ASC Topic 718, Compensation
– Stock Compensation (“ASC 718”), which requires all stock-based compensation to employees and non-employees, including
grants of non-employee stock awards, to be recognized in the statement of operations based on the fair value of those awards calculated
using a valuation model on the grant date. In accordance with ASC 718, compensation expense related to stock-based payments are recorded
on a straight-line basis over the requisite service period based on the grant date fair value of the awards. In addition, the Company
has elected to account for forfeitures when they occur in accordance with ASC 718.
Leases
The Company incurs leasing costs in connection to various non-cancelable leases. Effective January 1, 2021, the Company elected to
early adopt FASB ASC Topic 842, Leases, (“ASC 842”).
In accordance with ASC 842, at lease
inception, the Company determines whether an arrangement is or contains a lease, and whether they will be classified as either finance
or operating, with classification affecting the pattern of expense recognition in the statement of operations. For operating and finance
leases: right-of-use (“ROU”) assets are recognized at the commencement date based on the present value of the future minimum
lease payments over the lease term using the rate implicit in the lease, plus unamortized initial direct costs, plus any prepayments,
less any unamortized lease incentives received; lease liabilities are recognized at the commencement date based on the present value of
the future minimum lease payments over the lease term using the rate implicit in the lease. ROU assets represent the Company’s right
to use leased assets over the term of the lease. Lease liabilities represent the Company’s contractual obligations to make lease
payments over the lease terms.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
Lease terms may include renewal or extension
options to the extent they are reasonably certain to be exercised at lease commencement. When the rate implicit in the lease is not determinable,
ASC 842 prescribes the use of the Company’s incremental borrowing rate at the commencement date, or a risk-free rate for privately
held companies.
Operating leases and short-term leases
recognize rent expense on a straight-line basis over the lease term. Finance leases recognize amortization and interest expense over the
lease term. ROU assets are measured at cost, less any accumulated amortization and/or impairment losses over the period from the commencement
date to the earlier of the end of the useful life of the ROU asset or the end of the lease term. Lease liabilities are measured on an
amortized cost basis, which are increased to reflect interest on the liability and decreased to reflect the lease payments made during
the period.
The Company made a policy election to
use the risk-free discount rate to determine the ROU assets and lease liabilities related to its operating leases; finance leases use
the rates implicit in the leases. In addition, the Company elected the short-term lease exception policy, permitting the Company to exclude
the recognition requirements for leases with terms of 12 months or less from lease inception.
Grant
Assistance Grant assistance from the government is recognized as income when grant is awarded or, if conditional, immediately
once the condition is substantially met. Since US GAAP does not contain specific authoritative accounting guidance that addresses the
recognition and measurement of government assistance received by a business entity, the Company accounted for the government grants by
analogy to FASB ASC Topic 958-605, Not-for-Profit Entities – Revenue Recognition (“ASC 958-605”). Additionally,
ASC 958-605 permits the recognition in earnings on a gross basis as grant revenue or other income. Accordingly, the Company elected to
recognize the grant proceeds in other income and expenses on the accompanying statements of operations. For the years ended December 31,
2023, 2022, and 2021, the Company recognized grant income in the amounts of $910,577, $1,090,595, and $1,630,858, respectively.
Advertising
and Marketing Advertising and marketing costs are expensed as incurred. The Company incurred advertising and marketing
costs of $50,042, $87,081, and $88,388, which are included in general and administrative expenses on the accompanying statements of operations
for the years ended December 31, 2023, 2022 and 2021, respectively.
Income
Taxes The Company is a registered C-corporation in the state of Delaware. Under federal and state laws, C-corporations
are subject to federal and state income taxes. The Company files income tax returns in the U.S. federal jurisdiction and California. The
Company is subject to U.S. federal and state and local income tax examinations by tax authorities for the prior three years for federal
returns and for the prior four years for state returns. No examinations are currently pending.
The Company uses the asset and liability
method of accounting for income taxes in accordance with FASB ASC Topic 740, Income Taxes, where deferred tax assets and liabilities
are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing
assets and liabilities and their respective tax bases and tax credit carryforwards. Deferred tax assets and liabilities are measured using
enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or
settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized as income or expense in the period that
includes the enactment date. The Company provides a valuation allowance against its deferred tax assets when circumstances indicate that
it will, more likely than not, no longer be realized.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
The Company follows the provisions of
uncertain tax positions as addressed in FASB ASC Subtopic 740-10, Income Taxes - Overall. The Company did not recognize any
liabilities for unrecognized tax benefits as of December 31, 2023, 2022, and 2021, and has taken no tax positions as of December 31,
2023, 2022, and 2021 for which the ultimate deductibility is highly certain but for which there is uncertainty about the timing of such
deductibility. The Company recognizes interest accrued related to unrecognized tax benefits in operating expenses. As of December 31,
2023, 2022, and 2021, the Company had no accruals for interest and penalties and no such interest or penalties were recognized during
the years ended December 31, 2023, 2022, and 2021.
Measurement
of Credit Losses on Financial Instruments In June 2016, the Financial Accounting Standards Board (“FASB”)
issued amended guidance which replaced the incurred loss impairment methodology for measurement of credit losses on financial instruments
with a methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information
to inform credit loss estimates (the “current expected credit losses model” or “CECL”). Under the CECL, the allowance
for losses on financial assets, measured at amortized cost, reflects management’s estimate of credit losses over the remaining expected
life of such assets.
The Company adopted CECL as of January 1,
2023, using the modified retrospective method, with the cumulative-effect adjustment to the opening balance of stockholders’ equity
as of the adoption date. As the Company is a clinical-stage company with minimal financial instruments that can be subject to credit losses,
the cumulative effect of adopting CECL is immaterial.
| 3. |
LIQUIDITY, RISKS, AND UNCERTAINTIES |
The Company is subject to the risks
common to start-up, pre-revenue companies, including, among other factors, undercapitalization, cash shortages, limitations with respect
to personnel, financial and other resources and lack of revenues. Drug development companies typically incur substantial losses during
the product development and FDA testing phase of operations, and do not generate revenues until after the drug has received FDA approval,
which cannot be assured, and the Company has started to sell the product.
For the years ended December 31,
2023, 2022, and 2021, the Company recorded net losses totaling $8,857,996, $3,623,159, $1,660,187, respectively, and net cash used in
operating activities totaled $4,870,167, $4,321,502, $1,309,190, respectively. Cash flows are primarily dependent on debt financing and
government grants.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
| 4. |
PROPERTY AND EQUIPMENT |
Property and equipment
consist of the following:
| As of December 31, |
|
2023 |
|
|
2022 |
|
|
2021 |
|
| Computer equipment |
|
$ |
50,587 |
|
|
$ |
46,678 |
|
|
$ |
46,678 |
|
| Furniture and equipment |
|
|
28,874 |
|
|
|
28,874 |
|
|
|
28,874 |
|
| Equipment |
|
|
23,881 |
|
|
|
23,881 |
|
|
|
23,881 |
|
| |
|
|
103,342 |
|
|
|
99,433 |
|
|
|
99,433 |
|
| Less: accumulated depreciation and amortization |
|
|
(82,489 |
) |
|
|
(72,979 |
) |
|
|
(59,742 |
) |
| Property and equipment, net |
|
$ |
20,853 |
|
|
$ |
26,454 |
|
|
$ |
39,691 |
|
Depreciation and amortization expense
related to property and equipment for the years ended December 31, 2023, 2022, and 2021, amounted to $9,510, $13,237, and $13,282,
respectively and are reported in general and administrative expenses on the accompanying statements of operations.
| 5. |
PATENTS AND OTHER INTANGIBLE ASSETS |
Patents and other intangible assets
were recorded in connection with an asset acquisition that occurred in 2016, The fair values of the patents and identifiable intangible
assets were determined on a non-recurring basis by a third-party valuation specialist, in accordance with ASC 805.
The fair values of the patents and identifiable
intangible assets acquired were determined using the income approach, specifically the risk-adjusted discounted cash flow method, and
the cost approach, specifically the replacement value method, respectively. Significant assumptions used in estimating the fair value
of the acquired intangible assets fall within Level 3 of the fair value hierarchy described by ASC 820, which includes forecasted revenue
and expenses, probability of technical success, and replacement costs.
Patents and other intangible assets
consisted of the following:
| As of December 31, 2023 |
|
Gross
Carrying
Amount |
|
|
Accumulated
Amortization |
|
|
Net Carrying
Amount |
|
| Patents |
|
$ |
1,569,333 |
|
|
$ |
(900,882 |
) |
|
$ |
668,451 |
|
| Intellectual property |
|
|
19,200 |
|
|
|
- |
|
|
|
19,200 |
|
| Total |
|
$ |
1,588,533 |
|
|
$ |
(900,882 |
) |
|
$ |
687,651 |
|
| As of December 31, 2022 |
|
Gross
Carrying
Amount |
|
|
Accumulated
Amortization |
|
|
Net Carrying
Amount |
|
| Patents |
|
$ |
1,569,333 |
|
|
$ |
(787,087 |
) |
|
$ |
782,246 |
|
| Intellectual property |
|
|
19,200 |
|
|
|
- |
|
|
|
19,200 |
|
| Total |
|
$ |
1,588,533 |
|
|
$ |
(787,087 |
) |
|
$ |
801,446 |
|
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
| As of December 31, 2021 |
|
Gross
Carrying
Amount |
|
|
Accumulated
Amortization |
|
|
Net Carrying
Amount |
|
| Patents |
|
$ |
1,569,333 |
|
|
$ |
(673,291 |
) |
|
$ |
896,042 |
|
| Intellectual property |
|
|
19,200 |
|
|
|
- |
|
|
|
19,200 |
|
| Total |
|
$ |
1,588,533 |
|
|
$ |
(673,291 |
) |
|
$ |
915,242 |
|
Patents are amortized over useful lives
ranging from 8.5 to 18 years. The intellectual property consists of acquired in-process research and development with an alternative future
use, which have an indefinite useful life until the in-development product is completed.
Amortization expense related to the
patents for the years ended December 31, 2023, 2022, and 2021 amounted to $113,796 for each year, and is included in research and
development expenses on the accompanying statements of operations.
Future amortization expense on the patents
as of December 31, is estimated to be as follows:
| For the Years Ending December 31, |
|
Amount |
|
| 2024 |
|
$ |
111,531 |
|
| 2025 |
|
|
90,344 |
|
| 2026 |
|
|
87,464 |
|
| 2027 |
|
|
87,464 |
|
| 2028 |
|
|
72,953 |
|
| Thereafter |
|
|
218,695 |
|
| Total |
|
$ |
668,451 |
|
| 6. |
LEASE AND RELATED PARTY LEASE |
Adoption
of ASC 842 The Company elected to apply the modified retrospective transition method, including the package of practical
expedients and transition provisions available for expired or existing contracts, which allowed the Company to not reassess existing leases
to determine 1) whether contracts are or contain leases, 2) lease classification and 3) if initial direct costs already capitalized meet
the new definition of initial direct costs under ASC 842, to adopt ASC 842 as of January 1, 2021. As a result, the Company recognized
an operating ROU lease asset in the amount of $105,946 and operating lease liability in the amount of $101,070, net of previously recognized
prepaid rent of $4,877. There was no impact to opening equity.
ASC
842 Operating Lease The Company leases an office building under a non-cancelable operating lease, which expires in April 2024.
A renewal option was not included in the lease; therefore, potential extensions were excluded from the Company’s ROU asset and lease
liability as any extensions would be at the discretion of the lessor. The remaining lease term for the Company’s operating lease
is 4 months. The discount rate is 0.78%.
For the years ended December 31,
2023, 2022, and 2021, rent expense related to the operating lease amounted to $32,155 each year. Variable lease payments related to the
operating lease amounted to $10,810 for each year for the years ended December 31, 2023 and 2022, and there were no variable lease
payments for the year ended December 31, 2021. Rent expense and variable lease payments are included in general and administrative
expenses on the accompanying statements of operations.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
Future minimum lease payments under
the non-cancellable operating lease are as follows:
| Years Ending December 31, |
|
Amount |
|
| 2024 |
|
$ |
9,144 |
|
| 2025 |
|
|
- |
|
| 2026 |
|
|
- |
|
| 2027 |
|
|
- |
|
| 2028 |
|
|
- |
|
| Thereafter |
|
|
- |
|
| Total |
|
|
9,144 |
|
| Less: amount representing interest |
|
|
(15 |
) |
| Present value of minimum lease payments |
|
|
9,129 |
|
| Less: current portion of operating lease liability |
|
|
(9,129 |
) |
| Operating lease liability, net of current portion |
|
$ |
- |
|
ASC
842 Short-Term Lease The Company leases a facility from a related-party under a short-term evergreen lease, in which the
fixed monthly payments are expensed as incurred. For the years ended December 31, 2023, 2022, and 2021, short-term rent expense amounted
to $12,000 each year, and is included in general and administrative expenses on the accompanying statements of operations.
| 7. |
REVOLVING LINE OF CREDIT |
Revolver
In October 2022, the Company entered into a credit agreement, which allows for a revolving line of credit (the “Revolver”),
with a related party that allows for maximum borrowings up to $6,000,000, matures on October 1, 2024, and is collateralized by substantially
all of the Company’s assets. The credit agreement was amended (the “Revolver Amendment”) in September 2023 to increase
the maximum borrowings of the Revolver to $7,500,000. As of December 31, 2023 and 2022, the Revolver had outstanding borrowings in
the amounts of $6,360,000 and $2,810,000, respectively, and available borrowings of $1,140,000 and $3,190,000, respectively. The Revolver
bears interest per annum at Prime Rate plus 4.5%.
Revolver
Deferred Debt Issuance Costs Upon execution of the Revolver, the Company incurred $60,000 of deferred debt issuance costs.
As of December 31, 2023 and 2022, these deferred debt issuance costs, net of accumulated amortization, amounted to $22,500 and $52,500,
respectively, and are included in other assets on the accompanying balance sheets. Amortization of deferred debt issuance costs for the
years ended December 31, 2023 and 2022 amounted to $30,000 and $7,500, respectively, and are included in interest expense on the
accompanying statements of operations.
Stock
Warrants On October 1, 2022 and September 6, 2023, the Company issued Warrants to Purchase Common Stock (the
“Warrants”) in conjunction with and pursuant to the terms of the Revolver to the lender. The Warrants issued on October 1,
2022 certifies that the holder is entitled to purchase from the Company 24,100 shares of common stock, and the Warrants issued on September 6,
2023 entitled the holder to purchase an additional 24,100 shares. In accordance with ASC 815, as the Warrants are not considered to be
derivatives and are considered to be indexed to the Company’s own stock, the Warrants are accordingly classified as equity. As the
Warrants were issued in connection with obtaining a line of credit, the Warrants were recorded at fair value as of the grant date. Issuing
warrants to obtain a line of credit is equivalent to paying loan commitment fees; therefore, an asset is recognized in the amount of the
fair value of the Warrants and amortized on a straight-line basis over the stated term of the Revolver.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
The Warrants were recognized at a fair
value of $32,294 and $49,871 on September 6, 2023 and October 1, 2022, respectively. The corresponding loan commitment fees,
net of accumulated amortization, amounted to $41,058 and $43,637 as of December 31, 2023 and 2022, respectively, and is included
in other assets on the accompanying balance sheets. Amortization expense related to the loan commitment fees amounted to $34,873 and $6,234
for the years ended December 31, 2023 and 2022, and is included in interest expense on the accompanying statements of operations.
The Company utilized the Black-Scholes
option pricing model to value the Warrants. Significant assumptions used in estimating the fair value of the Warrants fall within Level
3 of the fair value hierarchy described by ASC 820 and are summarized below:
| Stock Warrants Assumptions |
|
2023 |
|
|
2022 |
|
| Price of stock at grant date |
|
$ |
3.79 |
|
|
$ |
2.42 |
|
| Dividend yield |
|
|
0.00 |
% |
|
|
0.00 |
% |
| Contractual term of warrants |
|
|
10 |
|
|
|
10 |
|
| Exercise price of option |
|
$ |
8.00 |
|
|
$ |
10.38 |
|
| Risk-free interest rate |
|
|
4.62 |
% |
|
|
3.83 |
% |
| Volatility |
|
|
36.3 |
% |
|
|
108.00 |
% |
| 8. |
NOTE PAYABLE AND RELATED PARTY PROMISSORY NOTES |
Note payable and related party promissory
notes consists of the following:
| As of December 31, |
|
2023 |
|
|
2022 |
|
|
2021 |
|
| EIDL loan bearing interest at 3.75% per annum, with interest payable monthly in arrears. All unpaid principal and interest are due at maturity on June 30, 2050. The loan is collateralized by substantially all of the Company’s assets. |
|
$ |
500,000 |
|
|
$ |
500,000 |
|
|
$ |
500,000 |
|
| Total |
|
|
500,000 |
|
|
|
500,000 |
|
|
|
500,000 |
|
| Less: current portion |
|
|
- |
|
|
|
- |
|
|
|
- |
|
| Note payable |
|
$ |
500,000 |
|
|
$ |
500,000 |
|
|
$ |
500,000 |
|
| As of December 31, |
|
2023 |
|
|
2022 |
|
|
2021 |
|
| Promissory notes due to related party bearing PIK interest at rates ranging from 5% to 8% per annum with principal and interest payable at maturity. The notes mature on various dates through July 2024. The promissory notes are collateralized by substantially all of the Company’s assets. |
|
$ |
393,282 |
|
|
$ |
269,481 |
|
|
$ |
592,222 |
|
| Total |
|
|
393,282 |
|
|
|
269,481 |
|
|
|
592,222 |
|
| Less: current portion |
|
|
(393,282 |
) |
|
|
(269,481 |
) |
|
|
(592,222 |
) |
| Related party promissory note, net of current portion |
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
Aggregate future minimum principal maturities
of note payable and related party promissory notes are as follows:
| Years Ending December 31, |
|
Amount |
|
| 2024 |
|
$ |
393,282 |
|
| 2025 |
|
|
- |
|
| 2026 |
|
|
- |
|
| 2027 |
|
|
- |
|
| 2028 |
|
|
- |
|
| Thereafter |
|
|
500,000 |
|
| Total |
|
$ |
893,282 |
|
On various dates throughout the years
ended December 31, 2023, 2022, and 2021, the Company issued secured, convertible promissory notes (the “Convertible Notes”)
with principal amounts totaling $1,380,000, $530,000, and $2,900,440, respectively, pursuant to the Convertible Promissory Note Purchase
Agreements. The Convertible Notes provide for interest at a rate of 8% or 10% per annum, payable upon maturity. All principal and accrued
interest are due 30 months from the date of issuance of the Convertible Notes unless the Convertible Notes are converted or repaid prior
to the maturity date. The Convertible Notes mature on various dates through May 16, 2026, and upon maturity, the Convertible Notes
are intended by the Company to be extended or converted to equity; accordingly, all Convertible Notes are classified as long-term. The
Convertible Notes are collateralized by substantially all the assets of the Company.
At any time prior to the maturity date,
the holders can elect to convert the Convertible Notes into the Company’s preferred stock at a conversion price equal to $10.38
or $20.00. Additionally, in the event that the Company issues and sells shares of its equity securities to investors (the “Investors”)
on or before the date of the repayment in full of the Convertible Notes in an equity financing (a “Qualified Financing”) resulting
in gross proceeds to the Company of at least $3,000,000, excluding the conversion of the Convertible Notes and other debt, then 100% of
the outstanding principal balances, and any accrued but unpaid interest, shall automatically convert in whole into such equity securities
at a per share conversion price equal to the lesser of (i) 80% of the per share price paid by the Investors, and otherwise on the
same terms and conditions as given to the Investors or (ii) $10.38 or $20.00 per share. Furthermore, if a Qualified Financing has
not occurred and the Company elects to consummate a sale of the Company prior to the maturity date of the Convertible Notes, at the option
of the holder, the Company will (i) pay the holder of the Convertible Notes at closing an aggregate amount equal to 1.5 times principal
and accrued interest, or (ii) allow conversion of the principal amount and accrued interest into shares of the Company’s preferred
stock at the conversion price of $10.38 or $20.00 per share.
The conversion features in the event
of a Qualified Financing or sale of the Company are embedded derivatives that would require separation from the host contract. However,
the Company has elected the fair value option, and accordingly, the Convertible Notes were measured at fair value at issuance and subsequently
remeasured on December 31, 2023, 2022, and 2021.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
The fair values of the Convertible Notes
were determined by a third-party valuation specialist using the Monte Carlo simulation method. Significant assumptions used in estimating
the fair value of the Convertible Notes fall within Level 3 of the fair value hierarchy described by ASC 820, which includes the probability
of scenarios occurring during the term of the note that would impact conversion of the Convertible Notes. Other key assumptions used to
determine the fair value of the Convertible Notes are summarized below:
| Convertible Notes Assumptions |
|
2023 |
|
|
2022 |
|
|
2021 |
|
| Risk-free interest rate |
|
|
3.7% - 5.2% |
|
|
|
4.3% - 4.6% |
|
|
|
0.3% - 0.8% |
|
| Equity volatility |
|
|
68.0% - 89.0% |
|
|
|
85.0% - 117.0% |
|
|
|
97.0% - 113.0% |
|
| Discount rate/IRR |
|
|
16.5% - 17.0% |
|
|
|
13.8% |
|
|
|
11.3% - 15.7% |
|
| Return on invested capital multiple in dissolution |
|
|
0.35x |
|
|
|
0.35x |
|
|
|
0.11x - 0.25x |
|
The fair value of the Convertible Notes
are as follows:
| As of December 31, |
|
2023 |
|
|
2022 |
|
|
2021 |
|
| Convertible notes principal balance |
|
$ |
4,810,440 |
|
|
$ |
3,430,440 |
|
|
$ |
2,900,440 |
|
| Fair value adjustment |
|
|
3,853,364 |
|
|
|
238,134 |
|
|
|
48,017 |
|
| Convertible notes |
|
$ |
8,663,804 |
|
|
$ |
3,668,574 |
|
|
$ |
2,948,457 |
|
Loss related to the change in fair value
of the Convertible Notes for the years ended December 31, 2023, 2022, and 2021 amounted to $3,615,230, $190,117, and $48,017, respectively,
and is included in change in fair value of convertible notes on the accompanying statements of operations.
Interest expense related to the Revolver
(see Note 7), note payable (see Note 8), related party promissory notes (see Note 8), and the Convertible Notes amounted to $1,048,183,
$368,354, and $126,159 for the years ended December 31, 2023, 2022 and 2021, respectively, and is included in interest expense on
the accompanying statements of operations.
| 10. |
COMMITMENTS AND CONTINGENCIES |
Litigation
The Company operates in a highly regulated industry and is subject to various regulatory and governmental laws and regulations.
As a result, legal claims and regulatory proceedings may be instituted or asserted against the Company. The Company records accruals for
contingencies to the extent that it concludes that it is probable that a liability has been incurred and the amount of the loss can be
reasonably estimated. Additionally, the Company is subject to certain claims and legal matters that arise in the normal course of business.
Management does not expect any such claims and legal actions to have a material adverse on the Company’s financial position, results
of operations, or liquidity.
| 11. |
STOCKHOLDERS’ DEFICIT |
The Company is authorized to issue common
stock and preferred stock. Pursuant to the Company’s Certificate of Amendment of Certificate of Incorporation dated March 4,
2022, the Company is authorized to issue 2,000,000 shares of common stock (the “Common”), of which 1,009,925, 1,003,710, and
995,120 shares are issued and outstanding as of December 31, 2023, 2022, and 2021, respectively. In addition, the Company is authorized
to issue 2,260,054 shares of preferred stock (the “Preferred”), in which all 2,260,054 shares are issued and outstanding as
of December 31, 2023, 2022, and 2021. Both classes of shares have a par value of $0.0001 per share.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
The following is a summary of the preferences,
limitations, and rights of the holders of the Common and Preferred:
Preferred
Dividends Dividends are noncumulative and payable to preferred shareholders only when and if declared by the Company’s
Board of Directors in its sole discretion. To date, no dividends have been declared.
Liquidation
In the event of any liquidation, dissolution or winding up of the Company, the holders of the Preferred shall be entitled
to receive, prior and in preference to any distribution of the assets or surplus funds of the Company to the holders of the Common, an
amount equal to the Original Issue Price per share plus any declared but unpaid dividends (the “Liquidation Preference”).
After the payment of the Liquidation Preference to the holders of the Preferred, the holders of the Preferred and Common shall share pro
rata in all remaining assets and funds on an as-converted basis.
A sale of substantially all of the assets
of the Company, or a merger, consolidation, or reorganization or other transaction, in which holders of the Company’s voting power
prior to such transaction will hold, post-transaction, less than 50% of the surviving entity’s voting power, shall be deemed to
be a liquidation.
Optional
Conversion Each share of Preferred, at the option of its holder, at any time after the date of issuance of such share,
is convertible into such number of fully paid and nonassessable shares of common stock as is determined by dividing the Original Issue
Price for each share of Preferred by the conversion price.
Automatic
Conversion Each share of Preferred will automatically convert into common stock, at the then applicable conversion price
upon the effective date of an underwritten public offering, pursuant to an effective registration statement under the Securities Act of
1933, of shares of the Common, provided that the aggregate gross proceeds of such offering are at least $20,000,000.
Voting
Rights Each holder of shares of Common is entitled to one vote. Each holder of shares of Preferred is entitled to cast
the number of votes equal to the number of whole shares of common stock into which such share of Preferred is convertible. The Preferred
will vote together with the Common as a single class.
| 12. |
COMMON STOCK AWARD ACTIVITY |
On August 1, 2013, the Company
adopted the 2013 Profits Interest Award Plan (the “2013 Plan”), which provided for the grant of equity awards to employees
and consultants of the Company. Subsequent to the Company’s conversion to a C-corporation in 2021, the Company adopted a stock option
plan (the “2021 Plan”) pursuant to which the Company’s board of directors may grant stock options to executives, members
of the board, consultants, and key employees.
Restricted
Units Pursuant to the 2013 Plan, the Company granted restricted profit interest units (the “Profit Interest Units”)
which vest over a range of less than a year to 5 years. In addition, upon consummation of the conversion of the Company from an LLC to
a C-corporation, all awards granted under the 2013 Plan shall be converted into shares of the resulting corporation’s common stock
or other awards or securities on terms and conditions which are substantially equivalent to the terms and conditions of the Profit Interest
Units. In accordance with the 2013 Plan, upon conversion to a C-corporation, the Company issued one unit of common stock per unit of Profit
Interest Units vested.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
Under the 2013 Plan, the Company granted
a total of 118,050 Profit Interest Units. As of December 31, 2023, 2022, and 2021, the number of vested units amounted to 109,925,
103,710, and 95,120, respectively, and were issued common stocks. For the years ended December 31, 2023, 2022, and 2021, the Company
recognized $12,907, $17,597, and $22,929 of expense, respectively, based on the fair value of the common stock on the conversion date
January 1, 2021, which is included in general and administrative expenses on the accompanying statements of operations. As of December 31,
2023, 2022, and 2021, there remains $13,025, $25,950, and $43,574, respectively, of unrecognized expenses.
The fair value of the Company’s
common stock at the date of conversion was determined by a third-party valuation specialist using the Black-Scholes option pricing model.
Significant assumptions used in estimating the fair value of the common stock fall within Level 3 of the fair value hierarchy described
by ASC 820. The assumptions used and the conversion date fair value of the common stock are as follows:
| Common Stock Assumptions |
|
2021 |
|
| Dividend yield |
|
|
0.00 |
% |
| Time to liquidity (years) |
|
|
3 |
|
| Risk-free interest rate |
|
|
0.2 |
% |
| Volatility |
|
|
95.0 |
% |
| Fair value at January 1, 2021 |
|
$ |
2.14 |
|
Stock
Options The 2021 Plan authorizes grants consisting of the Company’s authorized but unissued common stock. The 2021
Plan allows for the issuance of a maximum of 350,000 non-transferrable options, which are subject to time-based vesting. Granted stock
options vest over a range of less than a year to 5 years. As of December 31, 2023, 2022, and 2021, there were 274,250, 291,050, and
299,800 shares, respectively, available for grant under the 2021 Plan. The 2021 Plan provide for incentive stock options for the issuance
of options for common stock to employees of the Company and non-statutory stock options for the issuance of options for common stock to
executives, members of the board, consultants, and employes. The options expire no more than 10 years after the date of grant or earlier
if employment or relationship with the Company is terminated. The Company accounts for any forfeitures of options when they occur. In
addition, previously recognized stock-based compensation expense for a non-vested award is reversed in the period that the award is forfeited.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
A summary of stock option activity for
the years ended December 31, 2023, 2022, and 2021 is presented below:
| |
|
Number of
Options |
|
|
Exercise
Price |
|
|
Weighted
Average
Exercise
Price |
|
|
Weighted
Average
Remaining
Contractual
Life (Years) |
|
| Balance, December 31, 2020 |
|
|
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
|
|
|
| Granted |
|
|
50,200 |
|
|
|
2.14 |
|
|
|
2.14 |
|
|
|
|
|
| Exercised |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
| Forfeited |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
| Balance, December 31, 2021 |
|
|
50,200 |
|
|
|
2.14 |
|
|
|
2.14 |
|
|
|
9.2 |
|
| Granted |
|
|
8,750 |
|
|
|
2.14 |
|
|
|
2.14 |
|
|
|
|
|
| Exercised |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
| Forfeited |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
| Balance, December 31, 2022 |
|
|
58,950 |
|
|
|
2.14 |
|
|
|
2.14 |
|
|
|
8.4 |
|
| Granted |
|
|
16,800 |
|
|
|
2.14 |
|
|
|
2.14 |
|
|
|
|
|
| Exercised |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
| Forfeited |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
| Balance, December 31, 2023 |
|
|
75,750 |
|
|
$ |
2.14 |
|
|
$ |
2.14 |
|
|
|
7.8 |
|
Options exercisable for the years ended
December 31, 2023, 2022, and 2021 are as follows:
| For the years ended December 31, |
|
Number of
Options |
|
|
Exercise
Price |
|
|
Weighted
Average
Exercise
Price |
|
|
Weighted
Average
Remaining
Contractual
Life (Years) |
|
| 2023 |
|
|
47,805 |
|
|
$ |
2.14 |
|
|
$ |
2.14 |
|
|
|
7.3 |
|
| 2022 |
|
|
28,690 |
|
|
$ |
2.14 |
|
|
$ |
2.14 |
|
|
|
8.2 |
|
| 2021 |
|
|
600 |
|
|
$ |
2.14 |
|
|
$ |
2.14 |
|
|
|
9.6 |
|
The Company utilized the Black-Scholes
option pricing model to value the stock options. Significant assumptions used in estimating the fair value of the stock options fall within
Level 3 of the fair value hierarchy described by ASC 820. The assumptions used and the weighted average grant date fair value of the stock
options are as follows:
| Stock Options Assumptions |
|
2023 |
|
|
2022 |
|
|
2021 |
|
| Price of stock at grant date |
|
|
$3.53 - $3.79 |
|
|
|
$3.34 - $3.90 |
|
|
$ |
3.34 |
|
| Dividend yield |
|
|
0.00 |
% |
|
|
0.00 |
% |
|
|
0.00 |
% |
| Years to maturity/liquidity event |
|
|
3 |
|
|
|
3 |
|
|
|
3 |
|
| Risk-free interest rate |
|
|
4.10% - 4.60% |
|
|
|
1.00% - 4.20% |
|
|
|
1.0 |
% |
| Volatility |
|
|
79.0% - 98.0% |
|
|
|
105.0% - 108.0% |
|
|
|
105.0 |
% |
| Weighted average fair value |
|
$ |
2.95 |
|
|
$ |
2.80 |
|
|
$ |
2.77 |
|
The Company recognized stock-based compensation
expense under the 2021 Plan totaling $66,235, $48,583, and $35,475 for the years ended December 31, 2023, 2022, and 2021, which is
included in general and administrative expenses on the accompanying statements of operations. As of December 31, 2023, 2022, and
2021, there remains $63,304, $79,512, and $103,579, respectively, of unrecognized stock-based compensation expense, and the weighted-average
period over which the stock-based compensation is expected to be recognized is over 1.2 years, 1.8 year, and 2.4 years, respectively.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
| 13. |
FEDERAL GRANTS AWARDED |
The Company was awarded a 5-year federal
grant (the “5-year Grant”) from the National Institute of Allergy and Infectious Diseases for Phase 1 and Phase 2 testing.
The 5-year Grant was intended for the project period from 2019 – 2023, and in total, the Company was awarded approximately $3,200,000
to cover salaries and wages, materials and supplies, and other indirect costs, such as employee benefits, consultant services, and travel,
for clinical testing trials. Additionally, the Company was awarded a 3-year federal grant (the “3-year Grant”) from the National
Institute of Diabetes and Digestive and Kidney Diseases for Phase 1 and Phase 2 testing. The 3-year Grant was intended for the project
period from 2019 – 2021, and in total, the Company was awarded $2,200,000 to cover salaries and wages, and other indirect costs,
such as employee benefits, consultant services, and travel, for clinical testing trials. The Company receives payouts from the grants
as reimbursements, which are recognized as other income when received, when previously agreed upon milestones and conditions of the grants
are substantially met. For the years ended December 31, 2023, 2022, and 2021, the Company recognized grant income in the amounts
of $910,577, $1,090,595, and $1,630,858, respectively, which are included in grant income on the accompanying statements of operations.
The income tax provision consists of
the following:
| For the Year Ended December 31, |
|
2023 |
|
|
2022 |
|
|
2021 |
|
| Current: |
|
|
|
|
|
|
|
|
|
|
|
|
| Federal |
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
| State |
|
|
791 |
|
|
|
800 |
|
|
|
1,722 |
|
| Total current |
|
|
791 |
|
|
|
800 |
|
|
|
1,722 |
|
| Deferred: |
|
|
|
|
|
|
|
|
|
|
|
|
| Federal |
|
|
- |
|
|
|
- |
|
|
|
- |
|
| State |
|
|
- |
|
|
|
- |
|
|
|
- |
|
| Total deferred |
|
|
- |
|
|
|
- |
|
|
|
- |
|
| Income tax expense |
|
$ |
791 |
|
|
$ |
800 |
|
|
$ |
1,722 |
|
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
The cumulative temporary differences
comprising the deferred tax assets (liabilities) are as follows:
| As of December 31, |
|
2023 |
|
|
2022 |
|
|
2021 |
|
| Deferred tax assets: |
|
|
|
|
|
|
|
|
|
|
|
|
| Accrued vacation |
|
$ |
20,986 |
|
|
$ |
19,964 |
|
|
$ |
15,570 |
|
| Intangible assets |
|
|
40,247 |
|
|
|
28,218 |
|
|
|
16,188 |
|
| Convertible note fair value adjustment |
|
|
1,149,844 |
|
|
|
71,059 |
|
|
|
14,328 |
|
| Stock-based compensation |
|
|
48,568 |
|
|
|
30,334 |
|
|
|
17,428 |
|
| Net operating loss |
|
|
2,917,127 |
|
|
|
835,577 |
|
|
|
440,007 |
|
| Total deferred tax assets |
|
|
4,176,772 |
|
|
|
985,131 |
|
|
|
503,521 |
|
| Deferred tax liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
| Operating lease ROU asset |
|
|
(470 |
) |
|
|
(2,649 |
) |
|
|
(2,978 |
) |
| Fixed assets |
|
|
(1,177 |
) |
|
|
(1,753 |
) |
|
|
(3,440 |
) |
| Federal effect of state deferred tax asset |
|
|
(251,993 |
) |
|
|
(60,820 |
) |
|
|
(31,222 |
) |
| Prepaid expenses |
|
|
- |
|
|
|
(2,053 |
) |
|
|
- |
|
| Total deferred tax liabilities |
|
|
(253,640 |
) |
|
|
(67,275 |
) |
|
|
(37,640 |
) |
| Net deferred tax asset |
|
|
3,923,132 |
|
|
|
917,877 |
|
|
|
465,881 |
|
| Less: valuation allowance |
|
|
(3,923,132 |
) |
|
|
(917,877 |
) |
|
|
(465,881 |
) |
| Total |
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
As of December 31, 2023, 2022,
and 2021, based on the projections for future reversal of existing taxable temporary differences and future taxable income over the periods
in which the deferred tax assets are deductible, management believes there is not sufficient evidence to conclude that it is more likely
than not that the results of future income will generate sufficient taxable income to realize all of the deferred tax assets. Therefore,
a full valuation allowance has been applied to the net deferred tax assets as of December 31, 2023, 2022, and 2021.
As of December 31, 2023, 2022,
and 2021, $9,945,405, $2,799,337, and $1,469,316, respectively, of federal net operating losses and $9,373,211, $2,802,220, and $1,486,993,
respectively, of state net operating losses were available to the Company to offset future taxable income, which will expire in various
years through 2044 for state tax purposes. Federal net operating losses originating after 2017 will carryforward indefinitely subject
to 80% of taxable income for taxable years beginning on or after January 1, 2021.
The Company has evaluated subsequent
events that have occurred from January 1, 2024 through the independent auditor’s report date, which is the date that the financial
statements were available to be issued, and determined that there were no subsequent events or transactions that required recognition
or disclosure in the financial statements, except as disclosed below:
Sale
of the Company In December 2023, the Company signed a non-binding term sheet for a business combination with First
Wave BioPharma, Inc. (“FWBI”), a publicly traded company. Pursuant to the term sheet, FWBI will acquire the Company in
an all-stock transaction. As of the date of the independent auditor’s report, the transaction had not yet closed.
ImmunogenX, Inc.
NOTES TO FINANCIAL STATEMENTS
December
31, 2023, 2022 AND 2021
Convertible
Notes Amendment In February 2024, the Company amended the terms of two of the Convertible Notes to align with the
terms of all other Convertible Notes. Terms amended includes the conversion price, which was changed from $20.00 per share to $10.38 per
share, and the interest rate, which was changed from 10% to 8%. All other terms remained the same.
Additionally, Convertible Notes with
a maturity date of December 31, 2023 and January 1, 2024 have been extended due to the pending sale of the Company mentioned
above, with the exception of one note with a principal balance of $300,000. Repayment of principal and accrued interest in the aggregate
amount of $362,000 were made on January 29, 2024, which was funded by the issuance of additional convertible notes (the “2024
Convertible Notes”) totaling $360,000 to existing investors on January 24, 2024. The 2024 Convertible Notes bears interest
at a rate of 8%, has a conversion price of $10.38, and matures on July 24, 2026.
First Wave BioPharma (NASDAQ:FWBI)
Historical Stock Chart
From Dec 2024 to Jan 2025
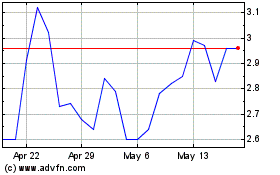
First Wave BioPharma (NASDAQ:FWBI)
Historical Stock Chart
From Jan 2024 to Jan 2025