As filed with the Securities and Exchange Commission on October 18, 2024.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
iSpecimen Inc.
(Exact name of registrant as specified in its charter)
| |
Delaware
|
|
|
8731
|
|
|
27-0480143
|
|
| |
(State or other jurisdiction of
incorporation or organization)
|
|
|
(Primary Standard Industrial
Classification Code Number)
|
|
|
(I.R.S. Employer
Identification Number)
|
|
8 Cabot Road, Suite 1800
Woburn, MA 01801
(781) 301-6700
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Tracy Curley
Chief Executive Officer
8 Cabot Road, Suite 1800
Woburn, MA 01801
(781) 301-6700
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| |
Ross D. Carmel, Esq.
Barry P. Biggar, Esq.
Sichenzia Ross Ference Carmel LLP
1185 Avenue of the Americas
New York, New York 10036
(212) 930-9700
|
|
|
Joseph M. Lucosky
Steven A. Lipstein
Lucosky Brookman LLP
101 Wood Ave South
Woodbridge, NJ 08830
(732) 395-4400
|
|
Approximate date of commencement of proposed sale to public:
As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| |
Large accelerated filer
☐
|
|
|
Accelerated filer
☐
|
|
| |
Non-accelerated filer
☒
|
|
|
Smaller reporting company
☒
|
|
| |
|
|
|
Emerging growth company
☒
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS, SUBJECT TO COMPLETION, DATED OCTOBER 18, 2024
UP TO 1,216,545 SHARES OF COMMON STOCK
PRE-FUNDED WARRANTS TO PURCHASE UP TO 1,216,545 SHARES OF COMMON STOCK
UP TO 1,216,545 SHARES OF COMMON STOCK ISSUABLE UPON THE EXERCISE OF THE PRE-FUNDED WARRANTS
iSpecimen Inc.
We are offering on a best-efforts basis up to 1,216,545 shares of our common stock, par value $0.0001 per share (the “Shares”) at an assumed public offering price of $4.11 per share, which was the reported closing price of our common stock on the Nasdaq Capital Market (“Nasdaq”), on October 15, 2024.
We are also offering to each purchaser of Shares that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% of our outstanding shares of common stock immediately following the consummation of this offering the opportunity to purchase one Pre-Funded Warrant (in lieu of one share of common stock) (each a “Pre-Funded Warrant”). A holder of Pre-Funded Warrants will not have the right to exercise any portion of its Pre-Funded Warrants if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, such limit may be increased to up to 9.99%) of the number of shares of common stock outstanding immediately after giving effect to such exercise. Each Pre-Funded Warrant will be exercisable for one share of common stock. The purchase price of each Pre-Funded Warrant will be equal to the price per Share, minus $0.0001, and the remaining exercise price of each Pre-Funded Warrant will equal $0.0001 per share. The Pre-Funded Warrants will be immediately exercisable (subject to the beneficial ownership cap) and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. For each Pre-Funded Warrant we sell (without regard to any limitation on exercise set forth therein), the number of Shares we are offering will be decreased on a one-for-one basis.
We are registering the Shares and the shares of common stock issuable from time to time upon the exercise of the Pre-Funded Warrants hereby.
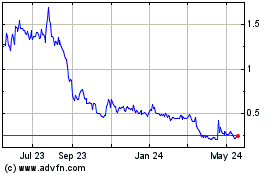
Our common stock is listed on Nasdaq under the symbol “ISPC”. On October 15, 2024, the reported closing price of our common stock was $4.11 per share. There is no established public trading market for the Pre-Funded Warrants. We do not intend to apply for listing of the Pre-Funded Warrants on any securities exchange or recognized trading system. Without an active trading market, the liquidity of the Pre-Funded Warrants will be limited.
The public offering price for the Shares in this offering will be determined at the time of pricing and may be at a discount to the then current market price. Therefore, the assumed public offering price used throughout this prospectus may not be indicative of the final offering price. The final public offering price will be determined through negotiation between us and the investors based upon a number of factors, including our history and our prospects, the industry in which we operate, our past and present operating results, the previous experience of our executive officers and the general condition of the securities markets at the time of this offering.
The Shares and Pre-Funded Warrants, if any, will be offered at a fixed price and are expected to be issued in a single closing. We expect this offering to be completed not later than two business days following the effective date of the registration statement of which this prospectus forms a part (the “Registration Statement”), and we will deliver all securities to be issued in connection with this offering delivery versus payment/receipt versus payment upon receipt of investor funds received by us. Accordingly, neither we nor WestPark Capital, Inc., whom we have engaged as the exclusive placement agent for this offering (“WestPark” or the “Placement
Agent”) have made any arrangements to place investor funds in an escrow account or trust account since the Placement Agent will not receive investor funds in connection with the sale of the securities offered hereunder.
We have engaged WestPark as our exclusive placement agent to use its reasonable best efforts to solicit offers to purchase our securities in this offering. The Placement Agent is not purchasing or selling any of the securities we are offering and is not required to arrange for the purchase or sale of any specific number or dollar amount of the securities. Because there is no minimum offering amount required as a condition to closing in this offering the actual public offering amount, Placement Agent’s fee, and proceeds to us, if any, are not presently determinable and may be substantially less than the total maximum offering amounts set forth above and throughout this prospectus. We have agreed to pay the Placement Agent the Placement Agent fees set forth in the table below. See “Plan of Distribution” in this prospectus for more information.
| |
|
|
Per Share
|
|
|
Total(2)
|
|
|
Public Offering Price
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
|
|
Placement Agent fees(1)
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
|
|
Proceeds, before expenses, to us
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
|
(1)
In connection with this offering, we have agreed to pay to WestPark as placement agent a cash fee equal to 4% of the gross proceeds received by us in the offering. We have also agreed to reimburse WestPark for all expenses related to the offering up to $[ ] for reimbursement of legal expenses and other out-of-pocket expenses in connection with its engagement as placement agent See “Plan of Distribution.”
(2)
Assumes investors purchase only Shares and no Pre-Funded Warrants in this offering.
Investing in our securities involves a high degree of risk. See the section entitled “Risk Factors” beginning on page 16 of the prospectus. You should carefully consider these risk factors, as well as the information contained in this prospectus before you invest.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
WestPark Capital, Inc.
The date of this prospectus is , 2024
TABLE OF CONTENTS
| |
|
|
Page
|
|
|
|
|
|
|
|
ii |
|
|
|
|
|
|
|
|
1 |
|
|
|
|
|
|
|
|
13 |
|
|
|
|
|
|
|
|
15 |
|
|
|
|
|
|
|
|
16 |
|
|
|
|
|
|
|
|
42 |
|
|
|
|
|
|
|
|
43 |
|
|
|
|
|
|
|
|
44 |
|
|
|
|
|
|
|
|
46 |
|
|
|
|
|
|
|
|
52 |
|
|
|
|
|
|
|
|
57 |
|
|
|
|
|
|
|
|
60 |
|
|
|
|
|
|
|
|
65 |
|
|
|
|
|
|
|
|
65 |
|
|
|
|
|
|
|
|
65
|
|
|
|
|
|
|
|
|
66
|
|
|
This prospectus contains forward-looking statements that are subject to a number of risks and uncertainties, many of which are beyond our control. See “Risk Factors” and “Special Note Regarding Forward-Looking Statements.”
ABOUT THIS PROSPECTUS
The information contained in this prospectus is not complete and may be changed. You should rely only on the information provided in or incorporated by reference in this prospectus, or in a related free writing prospectus, or documents to which we otherwise refer you. We have not authorized anyone else to provide you with different information.
We have not authorized any dealer, agent or other person to give any information or to make any representation other than those contained or incorporated by reference in this prospectus or any related free writing prospectus. You must not rely upon any information or representation not contained or incorporated by reference in this prospectus or any related free writing prospectus. This prospectus and any related free writing prospectus, if any, do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus and any related free writing prospectus, if any, constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction. You should not assume that the information contained in this prospectus and any related free writing prospectus, if any, is accurate on any date subsequent to the date set forth on the front of such prospectus, even though this prospectus and any related free writing prospectus is delivered or securities are sold on a later date.
We have not done anything that would permit this offering or possession or distribution of this prospectus or any free writing prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourself about and to observe any restrictions relating as to this offering and the distribution of this prospectus and any such free writing prospectus outside the United States.
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference in this prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
Except where the context otherwise requires or where otherwise indicated, the terms “iSpecimen,” “ISPC,” “we,” “us,” “our,” “our company,” “Company” and “our business” refer to iSpecimen.
PROSPECTUS SUMMARY
This summary highlights, and is qualified in its entirety by, the more detailed information and financial statements included elsewhere in this prospectus. This summary does not contain all of the information that may be important to you in making your investment decision. You should read this entire prospectus carefully, especially the “Risk Factors” section beginning on page 20 and our financial statements and the related notes included elsewhere in this prospectus, before making an investment decision.
Unless otherwise noted, the share and per share information in this prospectus reflects a reverse stock split of the outstanding common stock of the Company at a ratio of 1-for-20, which was effected on September 13, 2024.
Our Mission, Vision, and Core Values
iSpecimen’s mission is to accelerate life science research, discovery and development with a global marketplace platform that connects researchers to subjects, specimens, and associated data. Our vision is to create an “Amazon-like” global Marketplace of patients, biospecimens, and data for research to improve the quality of human life. We implement employee programs that foster a company culture predicated on the core values of corporate and individual growth, results and accountability, team before self; a can-do positive attitude, and the perseverance to succeed.
Overview
iSpecimen is a technology-driven company founded to address a critical challenge: how to connect life science researchers who need human biofluids, tissues, and living cells (“biospecimens”) for their research, with the billions of biospecimens available (but not easily accessible) in healthcare provider organizations worldwide. Our ground-breaking iSpecimen Marketplace platform was designed to solve this problem and transform the biospecimen procurement process to accelerate medical discovery.
The iSpecimen Marketplace brings new capabilities to a highly fragmented and inefficient biospecimen procurement market. Our technology consolidates the biospecimen buying experience in a single, online marketplace that brings together healthcare providers who have biospecimens and researchers across industry, academia, and government institutions who need them. We are seeking to transform the world of biospecimen procurement much like the way travel websites changed the consumer buying process for flights, hotels, and rental cars.
The iSpecimen Marketplace Solution
The iSpecimen Marketplace offers single-source access to millions of human biospecimens and patients across a diverse network of specimen providers quickly and compliantly, saving researchers time and money in their specimen procurement process while making it easier and more efficient for providers to get their specimens in the hands of researchers who need them. Our iSpecimen Marketplace technology makes it as easy to find specimens for research as it is to find flights on a travel website. We have adopted many of the same ease-of-use characteristics of these business-to-consumer, or B2C, marketplaces, from simple guided searches to the ability to refine search criteria with sliders and checkboxes, to the ability to add chosen items to a cart in order to purchase them, to online order management. Our two-sided marketplace platform makes it easy for researchers and healthcare providers to connect and transact, introducing efficiencies into what is otherwise a very time-consuming and manual process.
Our iSpecimen Marketplace technology is groundbreaking in the human biospecimen procurement space. In a world where there are thousands of biospecimen providers who typically rely upon e-mail and spreadsheets to communicate with customers to manage the bioprocurement process, our iSpecimen Marketplace offers a more efficient user experience to life science researchers who are looking for better ways to access research subjects, specimens, and data, and to healthcare provider organizations, who are looking to realize their missions of supporting research while augmenting their bottom line.
Planned Developments of our Marketplace
While the iSpecimen Marketplace currently supports our business model of providing access to search, find, and acquire human biospecimens and associated data from “inquiry to invoice” and positions us for future
expanded business model exploration, there are a number of areas in which the iSpecimen Marketplace functionality could be enhanced to better support our stakeholders, including our prospects and customers, iSpecimen sales and operations staff, and our supply partners. We believe with additional investment in technology development resources, we could make significant progress in scaling our iSpecimen Marketplace and, in addition to increased patient and specimen data integration, we expect to continue to improve the matchmaking across the platform and have capabilities such as more direct support for our prospective collections, deeper search and workflow capabilities, increased automation, and direct pricing availability in the platform.
As investment allows, we plan to continue to better connect healthcare researchers with our network of suppliers to enable the acquisition of human biospecimens and data to help accelerate research and expand the impact of our iSpecimen Marketplace platform from “inquiry to invoice” through the following key approaches:
•
Enhance the customer experience. By working with our prospects and customers to understand their needs, we strive to provide a platform that more easily enables them to specify and find human biospecimens and data that meet the requirements of their research.
•
Increase our supplier engagement. By continuing to engage with our supply partners to deliver solutions that make their interactions with us more fulfilling, we become more seamlessly integrated into their workflows and daily operations.
•
Improve operational efficiency. By measuring the results of our operational workflows, we endeavor to reduce the friction and manual efforts in our processes and systems.
We continue to prioritize and release updated versions of the iSpecimen Marketplace platform in alignment with these areas and believe that continuing to focus on these approaches will enable us to scale our business model more effectively. As part of this continued platform evolution, iSpecimen continues to explore adjacencies that leverage the platform including a data as a product model.
Our Technology
Technology Components
The iSpecimen Marketplace technology is comprised of four major functional areas: search; workflow; data; and administrative, compliance and reporting. We continue to invest in the evolution of these areas to improve customer and supplier engagement with the platform; provide operational efficiencies for our suppliers, our customers, and our internal operations; and increase the liquidity of products and services obtained through the platform. Our core business objective is to retain and grow both researcher and supplier usage of our platform to support biospecimen procurement, as well as to position our Company to explore other adjacent business opportunities that can benefit from the use of the iSpecimen Marketplace.
•
Search. The primary purpose of the iSpecimen Marketplace is to matchmake between those with access to subjects, specimens, and data, and those with a need for them to power their research. By entering subject and sample selection requests through the iSpecimen Marketplace, researchers can instantly search across the available medical records of large populations within iSpecimen’s healthcare provider network to create customized patient and specimen cohorts. Researchers can specify their criteria and either refine and review results to select specific specimens instantly, or they can request that iSpecimen find patients, specimens, and associated data to satisfy their needs when specimens do not currently exist in our network. Using our own proprietary algorithms, we enable researchers to explore both biospecimens that are currently available and view projections of those that are likely to become available in the future based on historic statistical analysis of data. This allows researchers to quickly and easily determine how we can fulfill their requirements, which is especially useful for project planning and budgeting.
•
Workflow. Our workflow engine supports the unique bioprocurement workflows of our suppliers, customers, and internal iSpecimen operations users. For our suppliers, our ability to easily integrate into their environments and automate key parts of their bioprocurement workflow enables us to maintain a level of engagement and responsiveness necessary to successfully deliver on specimen
requests from our research customers. We make it easy for suppliers to list their specimens in our iSpecimen Marketplace by receiving their data in the most commonly used data transmission formats for healthcare data, such as HL7 feeds (a healthcare data interchange standard), JSON files (a standard data interchange format), and CSV files (a comma separated values file used for tabular data), and then by harmonizing this data into standard terminology sets that allows their specimens to be searchable by our research customers. We provide these onboarding services at no charge to our supply partners. Additionally, our iSpecimen Marketplace technology enables suppliers to track and manage all of their specimen requests from feasibility assessment through the ordering and fulfillment process in a single web application, thereby streamlining their bioprocurement workflow. Because the work that we do with our suppliers is often a secondary concern to their primary mission of providing patient care, we believe that seamlessly integrating into their workflow is critical to its use and ongoing success.
•
Data. We power search and orchestrate the procurement workflow through our ability to acquire, ingest, generate, and use big data from our healthcare provider partners. Working with a global, centralized set of healthcare providers, we receive this data in a variety of different formats and quality levels. We de-identify, normalize, and harmonize our supplier network’s data for usage in our iSpecimen Marketplace, ensuring the highest level of patient privacy and compliance with HIPAA and other applicable regulations that govern the research use of patient specimens and data.
•
Administrative, Compliance, and Reporting. Administrative, compliance, and reporting functions are critical components to enable users to properly evaluate and manage the bioprocurement process. Our administrative capabilities include functions such as user management to assign users and roles and password management to ensure passwords are updated regularly, among other capabilities. Compliance management includes manual and technology-based processes that allow iSpecimen to track and manage unique regulatory and legal requirements across customers and suppliers (such as consent requirements versus consents granted, required specimen and data uses versus allowable specimen and data uses, resale or distribution requirements versus resale or distribution rights) to make sure that customer requirements and supplier requirements match before transferring specimens and data. Additionally, we conduct regular audits of supply sites capabilities and confirm that supply sites have Institutional Review Board (or equivalent) protocols in place where required by law. Our reporting tools turn operational data into useful information by enabling users to view operational data in tables and other visualizations. Together, they help manage and streamline administrative, compliance, and operational functions.
Technology Development
The iSpecimen Marketplace software was developed over ten years with more than 80 staff years invested in research, development, implementation, maintenance and support. It comprises an orchestration of software as a service, or SaaS, solutions, commercial and open-source components, and custom developed software deployed in the cloud on a third-party hosting platform built and maintained through a combination of full-time staff and outsourced partners. The team uses agile practices to develop and improve the platform. We continue to enhance and improve the performance, functionality, and reliability of the iSpecimen Marketplace platform based on a user-informed roadmap that is actively updated based on internal and external feedback aligned with our goals.
The iSpecimen Marketplace relies on third parties for certain technology to support development, delivery, and operations of the platform including product management, software development, cloud hosting, data processing, content mapping, and security services. iSpecimen uses software (including source code) and other materials that are distributed under a “free,” “open source,” or similar licensing model, including software distributed under the Apache License, Version 2.0, The MIT License, Mozilla Public License 2.0 (MPL-2.0), GNU General Public License version 2, GNU Lesser General Public License version 2.1, Eclipse Public License 1.0 (EPL-1.0), Common Development and Distribution License 1.0. In addition, iSpecimen uses software and services from commercial providers. We do not believe any of them are not generally commercially available to us from other parties. iSpecimen does not have any technology licensing contracts signed within the last two years upon which our business is substantially dependent. We continue to evaluate partners whose capabilities can help us deliver our iSpecimen Marketplace solution in areas such as
functionality, efficiency, and security and expect to continue to leverage and consider additional third-party capabilities in our ongoing Marketplace development.
Our Competitive Advantages
When successfully implemented, online marketplaces are a highly efficient supply chain that offer many advantages to both suppliers and customers, including lower costs, reduced procurement timeframes, increased revenue (for suppliers), increased access to a large and growing supply network (for customers), and reduced risks. While our iSpecimen Marketplace is providing these benefits now, we believe they will become even more apparent when the iSpecimen Marketplace achieves greater capabilities and scale as additional investment is made into the platform.
Our Products and Services
The iSpecimen Marketplace currently supports the supply chain management and bioprocurement process for specimens and associated data. We derive our revenue by procuring specimens from our healthcare provider network and then distributing these annotated biospecimens to our research client base. Revenue flows from the researchers who pay our Company to provide the specimens and we share that revenue back with the healthcare providers who supplied them. Revenue share back to the supplying organization is generally 20% to 50%, depending upon the sample type, collection requirements, and data provided. We are flexible and allow our suppliers to work with us using a number of revenue share constructs, including a fixed percent revenue share arrangement (whereby we share a fixed percentage of the revenue back with them), a fixed pricing schedule (whereby they set their pricing per specimen type), or on a project-based pricing (whereby the supply site sets fees on a per project basis). We have derived substantially all of our revenue from annotated biospecimen procurement and to date, have not charged our customers or suppliers fees for the use of the iSpecimen Marketplace platform, or for marketing, sales, contracting, or compliance functions that we provide as part of the specimen procurement process.
We generally operate in a “just in time” fashion, meaning we procure specimens from our suppliers and distribute specimens to our customers after we obtain an order for specimens from a research client. Generally, we do not speculatively purchase and bank samples in anticipation of future, unspecified needs. We believe our approach offers many advantages over a more traditional inventory-based supplier business model where biorepositories take inventory risks, and where turnover and cash conversion cycles can be lengthy, depending on market demand for certain specimen types.
Currently, we provide access to the following types of human biospecimens from healthy and diseased-state subjects:
•
Biofluids — such as whole blood, plasma, serum, urine, saliva, sputum, nasopharyngeal material, and cerebral spinal fluid;
•
Solid tissue — such as fresh, fixed, and cryopreserved tissue; and formalin-fixed paraffin embedded blocks, slides, and curls; and
•
Hematopoietic stem and immune cells — such as bone marrow, cord blood, whole blood, or sub-components of these tissues such as peripheral blood mononuclear cells (including normal or mobilized leukapheresis collections) and other isolated cell types (CD34+,T cells, NK cells, B cells, and monocytes).
For each of the biospecimen types, we offer:
•
Remnant specimens — specimens collected originally for clinical testing purposes but are no longer needed for clinical care of that patient. These samples typically are sourced from clinical laboratories and pathology laboratories prior to their disposal; and
•
Research use only specimens — specimens collected specifically for research via a direct intervention with a research subject, under a protocol that has been reviewed and approved by an ethics committee such as an Institutional Review Board (“IRB”) and with such research subject’s consent. These samples are typically sourced at healthcare providers or commercial partners that are a part of our supply network.
The cross product of all these categories (i.e., remnant or research use only and biofluids, tissues, or hematopoietic stem or immune cells) describes the product types we use to track and manage the business. These groupings include:
•
Remnant biofluids — These leftover clinical samples are procured from our clinical lab partners and are typically available days after specimen collection. They are generally priced to the researcher per specimen, depending upon specimen type, rarity, and requested data. These specimens contributed to approximately 13% of our revenue in 2023 and 12% of our revenue for the six months ended June 30, 2024, respectively.
•
Remnant tissue — These leftover anatomic pathology samples are procured from our pathology lab partners and typically are available years after they were first collected for clinical care. They are generally priced depending upon specimen type, rarity, and requested data.
•
Remnant hematopoietic stem and immune cells — Remnant hematopoietic stem and immune cells includes bone marrow, cord blood, whole blood, or their viable cellular components, that are left over from a clinical testing process. These samples may be obtained from clinical and anatomic pathology labs.
•
Next generation sequenced (“NGS”) tissues — NGS tissues include various cancer types that have been fully DNA/RNA sequenced to identify specific biomarkers of interest. The tissues screened are tumor only FFPE specimens. Results are analyzed and paired with clinical annotation to create a robust data package that has some utility even without the need for the specimen itself. Tissues used for the program are a combination of remnant waiver of consent tissue blocks along with RUO fully consented blocks.
•
Research use only biofluids — Research use only biofluids are collected directly from subjects, with their consent, and under an IRB (or equivalent) protocol. We obtain these samples via a variety of sources, including our biorepository and clinical research center partners. They are generally priced to the researcher per collection, depending upon specimen type, rarity, and requested data. These specimens contributed to approximately 39% of our revenue in 2023 and 52% of our revenue for the six months ended June 30, 2024, respectively.
•
Research use only tissue — Research use only tissues are collected directly from subjects, with their consent, and under an IRB (or equivalent) protocol. They are typically collected during a clinically required surgical procedure. We obtain these specimens from our biorepository partners, anatomic pathology laboratories, or clinical research centers that have relationships with surgical facilities. These samples are priced to the researcher per sample, depending upon specimen type, rarity, and requested data. These specimens contributed to approximately 47% of our revenue in 2023 and 34% of our revenue for the six months ended June 30, 2024, respectively.
•
Research use only hematopoietic stem and immune cells — Research use only hematopoietic stem and immune cells includes bone marrow, cord blood, whole blood, or their cellular components, which are collected from subjects with their consent and under an IRB (or equivalent) protocol. Some of the aforementioned products are collected from healthy subjects or diagnosed (diseased) subjects and may be offered to researchers in fresh or cryopreserved format. They are prospectively collected primarily from our blood donor center partners or picked from banked inventory maintained by our supply site partners. The collection of these samples may require subjects to undergo apheresis procedures, bone marrow extraction procedures, and/or hematopoietic stem cell (HSC) mobilization therapies. These products are generally priced to the researcher per collection depending upon collection type, specimen type, rarity (subject phenotype or attributes selected), required procedures, and requested data.
For each of these product types, biospecimens may already exist in laboratory archives or banked in our network of biorepositories (“banked”) or may be collected in the future from our network of healthcare providers and commercial specimen providers (“prospectively-collected” or “custom collections”).
Our Supply Partners
Critical to the success of the iSpecimen Marketplace is the network of healthcare providers who make their patients, samples, and data available to researchers. This supply network was built over a ten-year period and
as of June 30, 2024, our supply network consisted of approximately 105 unique healthcare organizations and biospecimen providers under agreement, including healthcare systems, community hospitals, clinics, private practice groups, commercial laboratories, blood centers, commercial biobanks, clinical research sites, and cadaveric donation centers.
Our suppliers are located in nine (9) countries across the Americas, Europe, and Asia and our cost of revenue for the six months ended June 30, 2024 and the year ended December 31, 2023, break out as follows geographically:
| |
|
|
June 30,
2024
|
|
|
December 31,
2023
|
|
|
Americas
|
|
|
|
|
70.01% |
|
|
|
|
|
64.87% |
|
|
|
Europe, Middle East and Africa
|
|
|
|
|
28.41% |
|
|
|
|
|
23.08% |
|
|
|
Asia Pacific
|
|
|
|
|
1.58% |
|
|
|
|
|
12.05% |
|
|
There was one supplier that accounted for 11.6% of our total cost of revenue during the period ended June 30, 2024. There was one supplier that accounted for 12.7% of our total cost of revenue during the year ended December 31, 2023.
Each supplier organization may give us access to one or more of the following environments within their organization where specimens may be obtained:
•
Clinical labs — This environment provides access to remnant biofluids and is typically found in hospitals, commercial laboratories, clinics, and private practice groups. As of June 30, 2024, approximately 40 of our healthcare supply sites provided us with access to remnant biofluids originating in clinical labs;
•
Pathology labs — This environment provides access to remnant tissue and remnant hematopoietic stem and immune cells and typically exists within hospitals or commercial laboratories. As of June 30, 2024, approximately three of our healthcare supply sites provided us with access to remnant tissue or cells originating in pathology labs;
•
Biorepositories — These organizations typically reside within larger healthcare systems or commercial organizations. Generally, they collect and store specimens for unspecified future research purposes. As of June 30, 2024, approximately 58 of our supply sites provided us with access to specimens stored in biorepositories;
•
Blood donor centers — These organizations typically collect large volumes of blood and derivatives for therapeutic or research purposes. They own and operate donor centers and may manufacture broad selection of isolated cell types (fresh or cryopreserved) from consented donors for research use. As of June 30, 2024, eight of our supply sites provided us with access to large volume blood products;
•
Cadaveric donation centers — These organizations receive whole cadavers and provide access to cadaveric tissues, biofluids, and stem cells, specifically for research purposes. As of June 30, 2024, two of our supply sites provided us with cadaveric tissues and biofluids; and
•
Clinical research centers — These organizations generally reside within healthcare facilities such as hospitals or clinics, or they operate as standalone entities providing access to subjects for research programs. Subjects may be approached and consented to provide specimens when they are in for healthcare appointments (i.e., patient encounters) or may be called in to specifically participate in research projects. As of June 30, 2024, approximately 58 of our healthcare supply sites provided us with access to patients directly from over 1,000 hospitals and thousands of clinics and practice groups.
Supply sites may provide specimens from one or all these environments, depending on their practices and capabilities. Each supply site can select how it will work with our Company.
In addition to obtaining specimens and data directly from healthcare organizations, we work with several commercial biobanks and biospecimen brokers who have their own network of healthcare provider supply partners and wish to make their samples available to our research clients as well. While these organizations are generally considered our competitors, they are willing to work with us because we provide value by acting as
both a distribution channel for them and a supply partner to them to increase their revenues. Moreover, the inclusion of competitors’ specimens in our iSpecimen Marketplace platform further strengthens our competitive position and value to our customers by further de-fragmenting our customers’ buying experience.
Our Customers
Our customer base is primarily comprised of three main segments: biopharmaceutical companies, in vitro diagnostic companies, and government/academic institutions. As of June 30, 2024, we had distributed our specimens to approximately 701 customers, such as the Centers for Disease Control and Prevention. Since entering the regenerative medicine market late 2019, we have acquired 33 customers representing 0.7% of our total revenue in 2023, and 1.2% of our total revenue in the six months ended June 30, 2024.
From our inception through June 30, 2024, we had distributed more than 220,000 specimens to 23 countries and our geographical revenues distribution for the six months ended June 30, 2024 and the year ended December 31, 2023 were as follows:
| |
|
|
June 30,
2024
|
|
|
December 31,
2023
|
|
|
Americas
|
|
|
|
|
85.79% |
|
|
|
|
|
89.93% |
|
|
|
Europe, Middle East and Africa
|
|
|
|
|
12.54% |
|
|
|
|
|
9.10% |
|
|
|
Asia Pacific
|
|
|
|
|
1.67% |
|
|
|
|
|
0.97% |
|
|
During the six months ended June 30, 2024, there was one customer that accounted for approximately 26% of our total revenue generated. During the year ended December 31, 2023, there was one customer that accounted for approximately 25% of our total revenue generated. We continuously engage with all customers when we receive inbound requests from them, whether they are within or outside of the Americas. Year-over-year, our top customers have been different because their specimen needs tend to be project-based and depending upon where they are in their research and development cycle, they may not need large numbers of specimens each year. Regardless, our customer retention rates are high, with 22 of our top 25 customers (88%) in the year ended December 31, 2022 also procuring specimens in the year ended December 31, 2023, and 16 of our top 25 customers (64%) in the year ended December 31, 2023 also procuring specimens in the six months ended June 30, 2024.
Biospecimens have broad utility within the healthcare and life science industries, as they are collected and used throughout nearly every stage of diagnostic and therapeutic product discovery and development. For diagnostic products, they are used consistently for preclinical discovery, clinical validation, and post-market validation, as well as surveillance. For therapeutic products, these samples are most often used during preclinical research involving drug target identification and validation, compound screening, lead optimization, predictive toxicology, and pharmacokinetic studies. They are also used for biomarker companion diagnostic discovery and development, which has been shown to reduce the costs of drug clinical trials by 30 to 60% according to Ark Research. In the case of regenerative medicine applications, hematologic samples are used for research and development of engineered cell therapies (e.g., CAR-T, CAR-NK), stem cell therapies (e.g., hematopoietic stem cells, mesenchymal stem cells), exosome therapies, identification of cell immunophenotypes for allogeneic therapies, and for developing and scaling-up cell therapy manufacturing processes.
Given recent advances in technology that now allow for the identification of molecular determinants of disease, the role of the patient’s biospecimen has become even more important in all these endeavors and is essential to the development of precision medicine. This pursuit of precision medicine by the healthcare and life science industries has further increased the already high demand for human biospecimens and the clinical data that describe them.
Recent Developments
September 2024 Debt Financing
On September 19, 2024, we entered into a Note Purchase Agreement (the “Purchase Agreement”) with a lender (the “Lender”). Pursuant to the provisions of the Purchase Agreement, the Lender agreed to provide a
loan to us in the amount of $1,000,000 (the “Loan”) and we agreed to issue to the Lender a promissory note in the principal amount of $1,000,000 payable within 12 months after the date of issuance, with interest accruing and payable at a rate of 18% per annum (the “Note”). The Purchase Agreement contains customary representations and warranties and obligates the Lender to provide an additional loan to us, in the form of a revolving line of credit of up to $1,000,000, upon our initial filing of a Registration Statement for an underwritten or best-efforts public offering for gross proceeds of at least $5,000,000. On September 25, 2024, we and the Lender closed the transactions (“Closing”) described in the Purchase Agreement, the Lender provided funds to the Company in the net amount of $960,000 and we issued the Note to the Lender in the principal amount of $1,000,000. WestPark served as the placement agent in connection with the Loan and was paid a placement agent fee in the amount of $40,000 for its services.
Reverse Stock Split
On July 19, 2024, our stockholders approved a proposal to amend our Fourth Amended and Restated Certificate of Incorporation (the “Certificate of Incorporation”) to effect a reverse stock split of our issued and outstanding shares of common stock, as well as any shares of common stock held by the Company in treasury, at a ratio in the range from 1-for-10 to 1-for-20.
On August 19, 2024, our board of directors approved a one-for-twenty (1:20) reverse stock split of our issued and outstanding shares of common stock (the “Reverse Stock Split”). On September 13, 2024, we filed with the Secretary of State of the State of Delaware a Certificate of Amendment to our Certificate of Incorporation to effect the Reverse Stock Split. The Reverse Stock Split became effective on September 13, 2024, and our common stock began trading on a split-adjusted basis on Nasdaq on September 16, 2024.
Except as otherwise indicated, all references to our common stock, share data, per share data and related information has been adjusted for the Reverse Stock Split ratio of 1-for-20 as if they had occurred at the beginning of the earliest period presented. The Reverse Stock Split combined each 20 shares of our outstanding common stock and treasury shares into one share of common stock without any change in the par value per share, and the Reverse Stock Split correspondingly adjusted, among other things, the exercise rate of our warrants and options into our common stock. No fractional shares were issued in connection with the Reverse Stock Split, and any fractional shares resulting from the Reverse Stock Split were rounded up to the nearest whole share.
New Office Lease
On July 2, 2024, we entered into a commercial lease (the “Woburn Lease”) with Cummings Properties, LLC (the “Landlord”) for an office space of approximately 2,273 square feet at 8 Cabot Road, Suite 1800, Woburn, MA 01801 (the “Woburn Premises”) for a term of five years and two months, commencing on September 1, 2024, and terminating on October 30, 2029. We have a one-time option to extend the term of the Woburn Lease for one additional term of five years, provided that we are not in arrears in any payment of rent, the payment of any outstanding invoice, or otherwise in default. We terminated the lease at the office space we previously occupied at 450 Bedford Street, Suite 1010, Lexington, MA 02420 effective August 31, 2024.
Changes to Board of Directors
On July 17, 2024, the Company received a written notice from Andrew L. Ross that he will be resigning as a director of the Company, effective July 25, 2024. Mr. Ross’s decision to resign as a director was not the result of any disagreements between Mr. Ross, on the one hand, and the Company’s management or Board of Directors, on the other hand, as to any matter relating to the Company’s operations, policies, or practices.
Pursuant to the Purchase Agreement, among other things, as a condition to Closing, three of the five directors serving on the Board of Director of the Company resigned from the Board and were replaced by three new directors designated by the Lender, which became effective immediately upon Closing. The Company received letters of resignation from each of Steven Gullans and Theresa Mock, each as a member of the Board, and from Elizabeth A. Graham, as a member and the chairperson of the Board, effective upon Closing. None of Mr. Gullans’, Ms. Mock’s or Ms. Graham’s decisions to resign as a director was the result of any disagreements between such director, on the one hand, and the Company’s management or Board, on the other hand, as to any matter relating to the Company’s operations, policies, or practices.
In addition, effective upon Closing on September 25, 2024, Richard Paolone, Avtar Dhaliwal and Katharyn (Katie) Field were each appointed to serve on the Board as a Class I director, a Class II director and a Class III director of the Company, respectively, with Ms. Field appointed as the chairperson of the Board. Mr. Paolone’s, Mr. Dhaliwal’s, and Ms. Field’s term of office will expire at the Company’s 2025 2026 and 2027 annual meetings of stockholders, respectively, or until the election and qualification of his or her successor, subject to his or her earlier death, resignation or removal. Also, effective upon Closing, each of Mr. Paolone and Ms. Field was appointed as a member of the Audit Committee of the Board each of Mr. Paolone and Mr. Dhaliwal was appointed as a member of the Compensation Committee of the Board (the “Compensation Committee”), with Mr. Paolone appointed as Chair of the Compensation Committee, and each of Mr. Dhaliwal and Ms. Field was appointed as a member of the Nominating and Corporate Governance Committee of the Board (the “Nominating Committee”), with Mr. Dhaliwal appointed as Chair of the Nominating Committee.
Resignation of Chief Information Officer and Filing of Demand for Arbitration
On July 25, 2024, Benjamin Bielak, the Company’s Chief Information Officer, until his resignation on July 14, 2024, initiated a Demand for Arbitration against the Company with the American Arbitration Association, pursuant to the dispute resolution provisions contained in Mr. Bielak’s employment agreement. The terms and conditions of Mr. Bielak’s employment with the Company were governed by his employment agreement.
In his Demand for Arbitration Mr. Bielak claims that the Company failed to provide him with certain bonus payments allegedly due to him for work performed in 2023 and 2024. Mr. Bielak also claims that the Company failed to provide him with severance payments allegedly due pursuant to the provisions of his employment agreement. The total amount of Mr. Bielak’s claim for alleged damages is $586,800 plus attorneys’ fees and interest.
The Company believes that Mr. Bielak’s claims are without legal or factual basis, and intends to vigorously defend these claims. An arbitrator has not yet been selected, and a schedule for the arbitration has not yet been set.
Summary of Risk Factors
Investing in our common stock involves risks. In addition, our business and operations are subject to a number of risks, which you should be aware of prior to making a decision to invest in our common stock. These risks are discussed more-fully in the “Risk Factors” section of this prospectus immediately following this prospectus summary. Below is a summary of these risks.
Risks Related to Our Business
•
We have incurred losses since inception and anticipate that we will continue to incur losses for the foreseeable future. We are not currently profitable, and we may never achieve or sustain profitability.
•
There is substantial doubt about our ability to continue as a going concern.
•
During the year ended December 31, 2023, we identified a material weakness in our internal control over financial reporting that may cause us to fail to meet our reporting obligations or result in material misstatements of our financial statements. If we fail to remediate this material weakness or if we otherwise fail to establish and maintain effective control over financial reporting, our ability to accurately and timely report our financial results could be adversely affected.
•
Our revenue trend is not predictive, which can lead to difficulty in accurately forecasting future results.
•
International operation expansion could expose us to additional risks which could harm our business, prospects, results of operation, and financial condition.
•
We rely upon our technology solution for the operation of our business and if our technology platform contains defects or fails to perform as expected, we may need to suspend its availability and divert development resources, and our business and reputation may be harmed.
•
If our security measures are breached, or if our services are subject to attacks that degrade or deny the ability of users to access our platforms, our platforms and applications may be perceived as not being
secure, customers and suppliers may curtail or stop using our services, and we may incur significant legal and financial exposure.
•
Challenges or unanticipated costs in establishing the sales, marketing, and distribution capabilities necessary to successfully commercialize our products globally could affect profitability.
•
We rely upon relatively few customers for a significant portion of revenue and do not have a recurring revenue business model. A loss of large customers could affect our ability to operate.
•
Customers and customer prospects may be averse to using a self-service marketplace to procure specimens and may continue to require iSpecimen personnel in the procurement process, impacting our scalability and profitability.
•
The adoption cycle of our supply network tends to be very lengthy, which may adversely affect our ability to scale rapidly and increase revenues.
•
Potential adverse effects from changes in the healthcare industry, including consolidations and regulatory changes, could affect access to subjects, samples, and data and affect our growth.
•
We do not control the end-to-end quality of specimens and data collected in our supply chain and quality issues can affect our reputation, revenue, and profitability.
•
Reliance on relatively few supply partners for significant supplies and services could affect our ability to operate and grow.
•
We may lose business to competitors which have or develop their own biorepositories and/or collection centers that can meet customers’ needs.
•
We have incurred losses from sales tax obligations owed to various jurisdictions by us because we did not collect taxes on taxable sales in prior years, and we may never be able to recover the prior sales taxes from the customers.
•
We may incur additional debt, and we are subject to the covenants and conditions contained in our Purchase Agreement and any other agreement governing our debt, which may restrict our operations and ability to make investments and distributions.
Risks Related to Intellectual Property
•
We do not have any patents protecting our intellectual property and if we are unable to protect the confidentiality of our trade secrets, know-how and other proprietary and internally developed technology, our business could be adversely affected.
Risks Related to Regulatory Environment
•
Failure to comply with federal and state data protection regulations could result in fines, penalties, and litigation, and have a material adverse effect upon our business.
•
Failure to comply with international laws related to data protection, such as the General Data Protection Regulation (“GDPR”) could result in fines, penalties, and litigation, and have a material adverse effect upon the Company’s business.
•
Failure to comply with federal and state laws around environmental, health and safety, biohazards and dangerous goods, and imports/exports could result in fines, penalties, and litigation, and have a material adverse effect upon our business.
•
Failure to comply with other international laws around environmental, health and safety, biohazards and dangerous goods, imports/exports, and other regulations could result in fines, penalties, and litigation, and have a material adverse effect upon our business.
•
Failure to comply with laws and regulations related to the protection of research subjects could result in fines, penalties, and litigation, and have a material adverse effect upon our business.
•
Failure to comply with governmental export and import regulations could result in fines, penalties, and litigation, and have a material adverse effect upon the Company’s business
•
Product safety and product liability, including bio-hazard risks, could provide exposure to claims and litigation.
Risks Related to This Offering and our Securities
•
This is a reasonable best efforts offering, with no minimum amount of securities required to be sold, and we may sell fewer than all of the securities offered hereby.
•
We have broad discretion in the use of the net proceeds we receive from this offering and may not use them effectively.
•
There can be no assurance that an active and liquid trading market for our common stock will continue or that we will be able to continue to comply with Nasdaq’s continued listing standards.
•
Our share price may be volatile, and purchasers of our common stock could incur substantial losses.
•
If we are not able to comply with the applicable continued listing requirements or standards of Nasdaq, our common stock could be delisted from Nasdaq.
•
We may need additional capital, and the sale of additional shares of common stock or other equity securities could result in additional dilution to our stockholders.
•
We do not expect to pay dividends in the foreseeable future. Any return on investment may be limited to the value of our common stock.
•
Our bylaws, as amended, designate certain courts as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or employees.
•
Our quarterly revenue tends to fluctuate, making it harder to forecast and meet investor expectations.
Implications of Being an Emerging Growth Company and a Smaller Reporting Company
We qualify as an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). As an “emerging growth company” we may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
•
The option to present only two years of audited financial statements and only two years of related “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this prospectus;
•
Not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended (the “Sarbanes-Oxley Act”);
•
Not being required to comply with any requirements that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis);
•
Reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and
•
Exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
We may take advantage of these provisions until December 31, 2026, which is the last day of our fiscal year following the fifth anniversary of the consummation of our initial public offering (“IPO”). However, if any of the following events occur prior to the end of such five-year period, (i) our annual gross revenue exceeds $1.235 billion, (ii) we issue more than $1.0 billion of non-convertible debt in any three-year period, or (iii) we become a “large accelerated filer,” (as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), we will cease to be an emerging growth company prior to the end of such five-year period. We will be deemed to be a “large accelerated filer” at such time that we (a) have an aggregate
worldwide market value of common equity securities held by non-affiliates of $700.0 million or more as of the last business day of our most recently completed second fiscal quarter, (b) have been required to file annual and quarterly reports under the Exchange Act for a period of at least 12 months and (c) have filed at least one annual report pursuant to the Exchange Act. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company,” which would allow us to take advantage of many of the same exemptions from disclosure requirements including reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements.
We have elected to take advantage of certain of the reduced disclosure obligations in the registration statement of which this prospectus is a part (the “Registration Statement”) and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
We are also a “smaller reporting company” as defined in the Exchange Act, and have elected to take advantage of certain of the scaled disclosures available to smaller reporting companies. To the extent that we continue to qualify as a “smaller reporting company” as such term is defined in Rule 12b-2 under the Exchange Act, after we cease to qualify as an emerging growth company, certain of the exemptions available to us as an “emerging growth company” may continue to be available to us as a “smaller reporting company,” including exemption from compliance with the auditor attestation requirements pursuant to SOX and reduced disclosure about our executive compensation arrangements. We will continue to be a “smaller reporting company” until we have $250 million or more in public float (based on our common stock) measured as of the last business day of our most recently completed second fiscal quarter or, in the event we have no public float (based on our common stock) or a public float (based on our common stock) that is less than $700 million, annual revenues of $100 million or more during the most recently completed fiscal year.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. We have elected to take advantage of this extended transition period.
Corporate Information
We were formed as a Delaware corporation in July 2009. Our headquarters are in Woburn, MA, and our principal executive offices are located at 8 Cabot Road, Suite 1800, Woburn, MA 01801, and our telephone number is (781)301-6700. Our website address is www.ispecimen.com. The information contained in, or accessible through, our website does not constitute a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
THE OFFERING
Up to 1,216,545 Shares on a best-efforts basis, at an assumed public offering price of $4.11 per share, which was the reported closing price of our common stock on Nasdaq on October 15, 2024.
We are also offering to each purchaser, with respect to the purchase of Shares that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% of our outstanding shares of common stock immediately following the consummation of this offering, the opportunity to purchase one Pre-Funded Warrant in lieu of one share of common stock. A holder of Pre-Funded Warrants will not have the right to exercise any portion of its Pre-Funded Warrant if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, such limit may be increased to up to 9.99%) of the number of shares of common stock outstanding immediately after giving effect to such exercise. Each Pre-Funded Warrant will be exercisable for one share of common stock. The purchase price per Pre-Funded Warrant will be equal to the price per Share, minus $0.0001, and the exercise price of each Pre-Funded Warrant will equal $0.0001 per share. The Pre-Funded Warrants will be immediately exercisable (subject to the beneficial ownership cap) and may be exercised at any time in perpetuity until all of the Pre-Funded Warrants are exercised in full. For more information regarding the Pre-Funded Warrants, you should carefully read the section titled “Description of Our Securities” in this prospectus.
Up to $5,000,000 of Shares and/or Pre-Funded Warrants.
Assumed Subscription Price
Per Share
$4.11 (or $4.1100 per Pre-Funded Warrant in lieu of one share of common stock), which was the reported closing price of our common stock on Nasdaq on October 15, 2024.
Common Stock Outstanding Prior to This Offering
829,812 shares (as of October 15, 2024)
Common Stock Outstanding after This Offering
Up to 2,046,072 shares (assuming no issuance or exercise of Pre-Funded Warrants) and an assumed public offering price of $4.11 per share, which was the reported closing price of our common stock on Nasdaq on October 15, 2024
Assuming that all 1,216,545 Shares are sold in this offering at an assumed public offering price of $4.11 per share, which was the reported closing price of our common stock on Nasdaq on October 15, 2024, and assuming no issuance of Pre-Funded Warrants, we estimate the net proceeds of the Offering, after the deduction of placement agent fees and expenses and other offering expenses will be approximately $4,485,000. However, this is a best efforts offering with no minimum number of securities or amount of proceeds as a condition to closing, and we may not sell all or any of these securities offered pursuant to this prospectus; as a result, we may receive significantly less in net proceeds. If we raise the full offering amount of $5,000,000, we intend to use the net proceeds from this offering for the (i) repayment of outstanding debt, (ii) potential acquisitions of assets or investments in businesses, products and technologies that complement our business, although we have no present commitments or agreements to make any such acquisitions or
investments as of the date of this prospectus, (iii) $2,000,000 to IR Agency LLC for marketing and advertising services to communicate information about the Company to the financial community including, but not limited to, creating company profiles, media distribution and building a digital community with respect to the Company, and (iv) the remaining amount for working capital and general corporate purposes. Our management will have broad discretion in the application of the net proceeds, and investors will be relying on our judgment regarding the application of the net proceeds from this offering. In the event that we raise less than the full offering amount of $5,000,000, we may be required to revise the allocation of the net proceeds received in this offering. See “Use of Proceeds” Also see “Risk Factors” for a discussion of certain risks that may affect our intended use of the net proceeds from this offering.
Our common stock is listed on Nasdaq under the symbol “ISPC”.
Market for Pre-Funded
Warrants
There is no established public trading market for the Pre-Funded Warrants, and we do not expect a market to develop. In addition, we do not intend to apply for listing of the Pre-Funded Warrants, if any, on any securities exchange or recognized trading system. Without an active trading market, the liquidity of the Pre-Funded Warrants, if any, will be limited.
An investment in our securities is highly speculative and involves a significant degree of risk. See “Risk Factors” and other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in our securities.
We have agreed to offer and sell the securities offered hereby to the purchasers through the Placement Agent. The Placement Agent is not required to buy or sell any specific number or dollar amount of the securities offered hereby, but it will use its reasonable best efforts to solicit offers to purchase the securities offered by this prospectus. See “Plan of Distribution” on page 57 of this prospectus.
Except as otherwise indicated, all references to our common stock, share data, per share data and related information has been adjusted for the Reverse Stock Split based on a ratio of 1-for-20 as if it had occurred at the beginning of the earliest period presented.
The number of shares of common stock outstanding prior to this offering and to be outstanding after this offering is based on $4.11 shares of common stock outstanding as of October 15, 2024, and excludes:
•
1,921 shares of our common stock issuable upon vesting of restricted stock units outstanding under our stock incentive plans;
•
17,206 shares of our common stock issuable upon exercise of stock options outstanding under our stock incentive plans, 10,591 of which are currently exercisable, which have a weighted average exercise price of $46.05 per share; and
•
5,125 shares of our common stock issuable upon exercise of our outstanding warrants which have a weighted average exercise price of $195.12.
SUMMARY FINANCIAL DATA
The following tables set forth a summary of our historical financial data as of, and for the periods ended on, the dates indicated. We have derived the statements of operations data for the six months ended June 30, 2024, and 2023 and our balance sheet data at June 30, 2024 from our unaudited interim financial statements included elsewhere in this prospectus. We have derived the statements of operations data for the years ended December 31, 2023, and 2022 and our balance sheet data at December 31, 2023 and 2022 from our audited financial statements included elsewhere in this prospectus. In the opinion of the management, the audited data reflects all adjustments, consisting of normal and recurring adjustments, necessary for a fair presentation of results as of and for these periods. You should read this data together with our consolidated financial statements and related notes included elsewhere in this prospectus and the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our condensed consolidated financial statements and related notes appearing in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2024, and our Annual Report on Form 10-K for the fiscal years ended December 31, 2023 and 2022, which are incorporated by reference in this prospectus. Our historical results for any prior period are not indicative of our future results.
| |
|
|
Years Ended December 31,
|
|
|
Six Months Ended June 30,
|
|
| |
|
|
2023
|
|
|
2022
|
|
|
2024
|
|
|
2023
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(unaudited)
|
|
|
(unaudited)
|
|
|
Revenue
|
|
|
|
$ |
9,928,184 |
|
|
|
|
$ |
10,402,303 |
|
|
|
|
$ |
5,153,672 |
|
|
|
|
$ |
4,575,339 |
|
|
|
Total operating expenses
|
|
|
|
|
21,097,533 |
|
|
|
|
|
20,588,385 |
|
|
|
|
|
10,335,857 |
|
|
|
|
|
10,679,584 |
|
|
|
Loss from operations
|
|
|
|
|
(11,169,349) |
|
|
|
|
|
(10,186,082) |
|
|
|
|
|
(5,182,185) |
|
|
|
|
|
(6,104,245) |
|
|
|
Other income (expense), net
|
|
|
|
|
69,861 |
|
|
|
|
|
(59,840) |
|
|
|
|
|
171,044 |
|
|
|
|
|
188,819 |
|
|
|
Net loss
|
|
|
|
$ |
(11,099,488) |
|
|
|
|
$ |
(10,245,922) |
|
|
|
|
$ |
(5,011,141) |
|
|
|
|
$ |
(5,915,426) |
|
|
|
Net loss per share – basic and diluted
|
|
|
|
$ |
(24.55) |
|
|
|
|
$ |
(23.17) |
|
|
|
|
$ |
(9.79) |
|
|
|
|
$ |
(13.13) |
|
|
|
Weighted average shares of common stock outstanding – basic and diluted
|
|
|
|
|
452,189 |
|
|
|
|
|
442,235 |
|
|
|
|
|
511,827 |
|
|
|
|
|
450,582 |
|
|
| |
|
|
June 30, 2024
|
|
| |
|
|
(unaudited)
|
|
|
Balance Sheet Data:
|
|
|
|
|
|
|
|
|
Cash
|
|
|
|
$ |
2,151,243 |
|
|
|
Working capital(1)
|
|
|
|
|
(577,321) |
|
|
|
Total assets
|
|
|
|
|
11,901,858 |
|
|
|
Total liabilities
|
|
|
|
|
5,812,574 |
|
|
|
Accumulated deficit
|
|
|
|
|
(64,375,953) |
|
|
|
Total stockholders’ equity
|
|
|
|
|
6,089,284 |
|
|
(1)
We define working capital as current assets less deferred offering costs and less current liabilities.
RISK FACTORS
You should carefully consider the risks and uncertainties described below and the other information in this prospectus, including our financial statements and related notes appearing elsewhere in this prospectus and in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” before deciding whether to invest in our common stock. Our business, financial condition, results of operations or prospects could be materially and adversely affected if any of these risks occurs, and as a result, the market price of our common stock could decline and you could lose all or part of your investment. This prospectus also contains forward-looking statements that involve risks and uncertainties. See “Special Note Regarding Forward-Looking Statements.” Our actual results could differ materially and adversely from those anticipated in these forward-looking statements as a result of certain factors, including those set forth below. For a summary of these risk factors, please see “Summary of Risk Factors” in the section titled “Prospectus Summary” beginning on page 1 of this prospectus.
Risks Related to Our Business
We have incurred losses since inception and anticipate that we will continue to incur losses for the foreseeable future. We are not currently profitable, and we may never achieve or sustain profitability.
We were founded in 2009 and completed our first commercial sale in 2012. We did not start generating revenues until 2016. We are not profitable and have incurred losses in each period since our inception in 2009. For the years ended December 31, 2023 and 2022, we reported net losses of $11,099,488 and $10,245,922, respectively. For the six months ended June 30, 2024 and 2023, we reported net losses of $5,011,141 and $5,915,426, respectively. We had an accumulated deficit of $59,364,812 as of December 31, 2023 and $64,375,953 as of June 30, 2024.
We expect to continue to incur losses for the foreseeable future, and we expect these losses to increase as we continue to invest in the growth of our business. We may encounter unforeseen expenses, difficulties, complications, delays, and other unknown factors that may adversely affect our business. The magnitude of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate and grow revenue. Even if we achieve profitability in a future period, we may not be able to sustain profitability in subsequent periods. Our prior losses and expected future losses have had and will continue to have adverse effects on our stockholders’ equity and working capital.
There is substantial doubt about our ability to continue as a going concern.
Our audited financial statements included in this prospectus include an explanatory paragraph that indicates that they were prepared assuming that we would continue as a going concern. We have suffered recurring net losses and accumulated deficits as of December 31, 2023 and June 30, 2024. These conditions raise substantial doubts about our ability to continue as a going concern. Our plan for continuing as a going concern includes improving our profitability and obtaining additional financing, including public and private placements of capital stock for additional funding to meet our operating needs. There can be no assurance that we will be successful in our plans described above or in attracting equity or alternative financing on acceptable terms, or if at all. These consolidated financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
During the year ended December 31, 2023, we identified a material weakness in our internal control over financial reporting that may cause us to fail to meet our reporting obligations or result in material misstatements of our financial statements. If we fail to remediate this material weakness or if we otherwise fail to establish and maintain effective control over financial reporting, our ability to accurately and timely report our financial results could be adversely affected.
We are required to comply with the SEC’s rules implementing Sections 302 and 404 of the Sarbanes-Oxley Act, which requires management to certify financial and other information in our quarterly and annual reports and provide an annual management report on the effectiveness of our controls over financial reporting. We are also required to make assessment of our internal controls over financial reporting pursuant to Section 404. We have included in this prospectus management’s assessment disclosure of any material weaknesses in our
internal control over financial reporting. Our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting until our first annual report required to be filed with the SEC, following the later of the date we are deemed to be an “accelerated filer” or a “large accelerated filer,” each as defined in the Exchange Act. We could be an emerging growth company for up to five years after the date of our IPO.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our financial statements will not be prevented or detected on a timely basis.
As described elsewhere in this prospectus, we identified a material weakness in our internal control over financial reporting related to a failure to design and maintain adequate controls to maintain appropriate documentation for the tax exempt status of its customers, calculate and collect sales tax at point of sale, and subsequently report and remit in a timely manner to the relevant tax jurisdictions sales tax obligations.
We initiated and implemented several remediation measures including, but not limited to, (i) engaging external tax advisors to complement internal resources and efforts and provide support in assessing the appropriate sales tax treatment associated with the Company’s products for all prior years in which the Company had generated revenue, (ii) obtaining sales tax exemption letters, representation letters or proof of payments of compensating use tax from our customers and we have started a collection effort of these sales taxes from certain customers who have notified the Company that they do not have a sales tax exemption letter, (iii) implementing a sales tax software platform solution for the calculation, communication, collection, and remittance of sales tax for all non-exempt future sales, and assisting with the collection and tracking of Voluntary Disclosure Agreements received from states where a potential sales tax liability may exist, (iv) designing and implementing enhanced policies, procedures and controls related to the calculation, communication, collection, and remittance of sales tax to relevant jurisdictions, and (v) training appropriate personnel in the effective design and execution of our enhanced policies, procedures, and controls, including the importance of the ongoing, consistent effective execution of such procedures and controls.
We believe the measures described above should address the material weakness identified and strengthen our internal control over financial reporting. These measures are expected to result in future costs for us. While we continue the process to implement our plan to remediate the material weakness, we cannot predict the success of such plan or the outcome of our assessment of this plan until the remediation initiatives have been completed and have been operating effectively for a sufficient period of time. We can give no assurance that these measures will remediate the deficiencies in internal control or that additional material weaknesses or significant deficiencies in our internal control over financial reporting will not be identified in the future. Our failure to implement and maintain effective internal control over financial reporting could result in errors in our financial statements that may lead to a restatement of our financial statements or cause us to fail to meet our reporting obligations, any of which could diminish investor confidence in us and cause a decline in our stock price.
We may identify future material weaknesses in our internal controls over financial reporting or fail to meet the demands that will be placed upon us as a public company, including the requirements of the Sarbanes-Oxley Act, and we may be unable to accurately report our financial results, or report them within the timeframes required by law or stock exchange regulations. We cannot assure that additional material weaknesses will not exist or otherwise be discovered, any of which could adversely affect our reputation, financial condition and results of operations.
We may likely require additional capital in the future and an inability to meet future capital needs could adversely impact our ability to operate.
We require substantial capital to fund our business growth and we will likely need additional capital in the future to fund our operations. In addition to investing in personnel growth commensurate with business growth, we believe we must continue to invest in the development of our iSpecimen Marketplace platform to enhance and improve its performance, functionality, ease of use, and reliability to carry out our business strategies. New industry standards, the availability of alternative products, and evolving life science research needs could render our products and services obsolete and/or new third-party marketplace technology may be introduced that makes it easier for our competitors to create their own marketplace platforms. Our success will
depend, in part, on our ability to develop new products and services and make corresponding technology enhancements that address the increasingly sophisticated and varied needs of our suppliers and customers and respond to technological advances and emerging industry standards and practices on a cost-effective and timely basis. We cannot be certain that additional financing will be available to us if required on favorable terms or at all. To the extent that we cannot raise capital if needed, we may not be able to continue operations.
Our revenue trend is not predictive, which can lead to difficulty in accurately forecasting future results.
Our revenue trend is not predictive and our ability to accurately forecast future results is limited and is impacted by a number of factors, including:
•
Our revenue is transactional and not recurring. Researchers pay us to provide specimens when they have a need for specimens. We do not currently charge our customer or supply chain for access to the iSpecimen Marketplace;
•
Our revenue is significantly concentrated and varies by customer year-over-year. There was one customer that accounted for approximately 25% of our revenue in 2023. In the six months ended June 30, 2024, there was one customer that represented approximately 26% of our revenue;
•
Researcher needs may change over the lifetime of a project, based on the stage of the project. A research customer in one time period may not have a need for specimens again in the next;
•
Research projects get terminated or suspended for a variety of reasons, including funding issues or unexpected results. Any termination or suspension of a project may cause a corresponding cancellation or delay in purchase orders we have received for specimens; and
•
Suppliers may not accurately estimate how long it will take them to fulfill specimen requests, making it more difficult to accurately forecast when we will recognize revenue on these specimen requests.
Many of these are outside of our control and all of which may change from time to time. Our historical revenue results should not be taken as predictive of future performance. There are many risks that could impact future performance resulting in variations in expected results which could lead to a negative business impact.
Our growth strategy may not prove viable and we may not realize expected results.
Our business strategy is to grow by improving and expanding iSpecimen’s Marketplace platform. This growth is expected to come through: (i) expansion of our platform capabilities to drive increased acquisition of annotated biospecimens through the platform, (ii) further expansion of our customer and supplier base in and outside the United States, and (iii) expansion into new lines of business such as patient recruitment and data licensing. Expansion of our existing business and entry into new lines of business will require a significant investment in technology development, supply development, operations, and marketing and sales. We may not achieve market expansion and acceptance and we may incur problems introducing new solutions and services. We may experience losses related to these investments, which could have a material adverse effect on our results of operations.
Our growth strategy involves a number of risks and uncertainties, including:
•
We may not successfully enter into contracts with healthcare providers to gain access to specimens, subjects, and data on terms favorable to us or at all. This can limit our ability to grow in existing lines of business and expand into new lines of business;
•
We may not obtain new customers or may lose existing customers if we cannot offer products and services that they need on a timely basis or at all;
•
We may fail in the development of our technology and it may not adequately keep pace to support an expansion of our existing line of business or our entry into new lines of businesses;
•
The market adoption rate of our marketplace technology may be too slow, and we may fail to get our customers and suppliers to transact for products and services using our technology;
•
We may fail to continue to expand outside of the United States, especially if we are required to comply with laws and regulations that differ from geographies in which we currently operate;
•
We may fail to gain market acceptance for new products or services; and/or
•
We may lose to competitors, some of whom may have greater resources than we do. This competition may intensify due to the ongoing consolidation in the biospecimen industry, which may increase our costs to pursue opportunities.
If we fail to properly evaluate and execute existing and new business opportunities properly, we may not achieve anticipated benefits and may incur increased costs. There can be no assurance that we will be able to successfully capitalize on growth opportunities, which may adversely impact our business model, revenues, results of operations, and financial condition.
International operation expansion could expose us to additional risks which could harm our business, prospects, results of operation, and financial condition.
We operate internationally and expect to expand internationally. For example, we procure specimens from sites outside of the United States and we also distribute samples to organizations located around the world. As of June 30, 2024, we had customers in 26 countries and supply sites in nine (9) countries. International expansion exposes us to additional risks, including:
•
changes in local political, economic, social, and labor conditions, which may adversely affect our business;
•
risks associated with trade restrictions and foreign import requirements, including the importation and exportation of our solutions, as well as changes in trade, tariffs, restrictions or requirements;
•
heightened risks of unethical, unfair or corrupt business practices, actual or claimed, in certain geographies;
•
fluctuations in currency exchange rates, which may make doing business with us less appealing as our contracts are generally denominated in U.S. dollars;
•
greater difficulty in enforcing contracts;
•
lack of brand awareness that can make commercializing our products more difficult and expensive;
•
management communication and integration problems resulting from cultural differences and geographic dispersion;
•
the uncertainty and limitation of protection for intellectual property rights in some countries;
•
increased financial accounting and reporting burdens and complexities as a result of being a public company;
•
lack of familiarity with local laws, customs and practices, and laws and business practices favoring local competitors or partners;
•
potentially different pricing environments, longer payment cycles in some countries, increased credit risk, and higher levels of payment fraud;
•
uncertainty regarding liability for products and services, including uncertainty as a result of local laws and lack of legal precedent;
•
different employee/employer relationships, existence of workers’ councils and labor unions, and other challenges caused by distance, language, and cultural differences, making it harder to do business in certain jurisdictions;
•
compliance with complex foreign and U.S. laws and regulations applicable to international operations may increase the cost of doing business in international jurisdictions. These numerous and sometimes conflicting laws and regulations include internal control and disclosure rules, data privacy requirements, research ethics and compliance laws, anti-corruption laws, and anti-competition regulations, among others. Violations of these laws and regulations could result in fines and penalties, criminal sanctions against us, our officers, or our employees, prohibitions on the conduct of our business and on our ability to offer our products and services in one or more countries, and could also materially affect our brand, our international expansion efforts, our ability to attract and retain employees, our business, and our operating results; and
•
instability, disruption or destruction in a significant geographic region, regardless of cause, including war, terrorism, riot, civil insurrection or social unrest; and natural or man-made disasters, including famine, flood, fire, earthquake, storm or disease, including without limitation, the war between Russia and Ukraine which started in February 2022, regions from which we obtain specimen supplies.
The occurrence of any one of these risks could harm our international business and, consequently, our results of operations. Additionally, operating in international markets requires significant management attention and financial resources. We cannot be certain that the investment and additional resources required to operate in other countries will produce desired levels of revenue or profitability.
We, or the third parties who provide services for us, may be adversely affected by external events for which our business continuity plans may not adequately prepare us.
The occurrence of severe weather, natural disasters, health epidemics, acts of war or terrorism, military conflicts such as the war between Russia and Ukraine, and other adverse external events or conditions that impact us or the operations of third parties who provide services for us have the potential to significantly impact our ability to conduct business. Although we have business continuity plans in place, including an emergency succession plan, there is no guarantee that our plans can be successfully implemented. Even if we were to successfully implement our continuity plans, we may incur substantial expenses and there is no guarantee that our business, financial condition, and results of operations will not be materially impacted.
We rely upon our technology solution for the operation of our business and if our technology platform contains defects or fails to perform as expected, we may need to suspend its availability and divert development resources, and our business and reputation may be harmed.
Technology as complex as ours may contain unknown and undetected errors or performance problems. There could be numerous reasons for performance and quality issues including new and updated features, defects in integrated commercial and open source technologies, outages and disruptions in the cloud infrastructure on which our platform relies, human error or malfeasance, scale constraints, design flaws, and bad actions by external factors including security and performance related incidents. Many serious defects are frequently found during the period immediately following introduction and initial release of new capabilities or enhancements to existing platforms. Although we attempt to resolve errors that we believe would be considered serious by our users before making our platforms available to them, our products are not error-free. If a significant failure occurs that prevents our customers, suppliers, or our Company from using the iSpecimen Marketplace, our operations may be disrupted, and it may be difficult or, in certain cases, impossible for us to continue our business for a period of time until the failure is corrected. Any performance or quality problem could result in lost revenues or delays in user acceptance that would be detrimental to our business and reputation. We may not be able to detect and correct errors before releasing our product commercially. Undetected errors or performance problems in our existing or future products may be discovered in the future and known errors, considered minor by us, may be considered serious by our customers, resulting in a loss of customers and a decrease in our revenues.
Sustainable future revenue growth is dependent upon the development of technology solutions that enable scale and address new markets.
Our iSpecimen Marketplace technology consists of four major functional areas: data ingestion and harmonization, search, workflow management, and administration, compliance and reporting. Each of these functional areas need continual development to both enable our current business to scale and to enable us to enter new markets. As financial resources become available, our intention is to focus most of our engineering resources on the development of the iSpecimen Marketplace platform for the foreseeable future. In fiscal year 2023, we incurred $5,386,165 in technology expenses, and capitalized $3,767,332 for internally developed software. For the six months ended June 30, 2024, we incurred $1,196,026 in technology expenses, and capitalized $447,687 for internally developed software. While we have spent a significant amount of time and resources on the development of this platform, we cannot provide any assurances of our iSpecimen Marketplace’s short or long-term success or growth and there is no assurance that the resources being allocated for the platform will be sufficient to complete planned additional capabilities, or that such completion will
result in significant revenues or profit for us. If our customers or suppliers do not perceive this platform to be of high value and quality, we may not be able to retain them or acquire new customers or suppliers.
Our platform may become technologically obsolete or commoditized.
We must continue to enhance and improve the performance, functionality, ease of use, and reliability of our iSpecimen Marketplace platform or it may become obsolete or commoditized. New industry standards, the availability of alternative products, and evolving life science research needs could render our products and services obsolete and/or new third-party marketplace technology may be introduced that makes it easier for our competitors to create their own marketplace platforms. Our success will depend, in part, on our ability to develop new products and services that address the increasingly sophisticated and varied needs of our suppliers and customers and respond to technological advances and emerging industry standards and practices on a cost-effective and timely basis. The development of our technology involves significant technical and business risks. We may fail to use new technologies effectively or to adapt our proprietary technology and systems to user requirements or emerging industry standards. If we are unable to adapt to changing market conditions, user requirements, or emerging industry standards, we may not be able to increase our revenue and expand our business. Additionally, if existing or future competitors develop or offer products or services that provide significant performance, price, creative or other advantages over this platform, demand for our services through the iSpecimen Marketplace may decrease and our business, prospects, results of operations and financial condition could be adversely affected.
If our security measures are breached, or if our services are subject to attacks that degrade or deny the ability of users to access our platforms, our platforms and applications may be perceived as not being secure, customers and suppliers may curtail or stop using our services, and we may incur significant legal and financial exposure.
Our platforms and the network infrastructure that are hosted by third-party providers involve the storage and transmission of healthcare data as well as proprietary information about organizations and programs, and security breaches could expose us to a risk of loss of this information, litigation, and potential liability. Our security measures may be breached due to the actions of outside parties, employee error, malfeasance, security flaws in the third party hosting service that we rely upon, or any number of other reasons and, as a result, an unauthorized party may obtain access to our suppliers’ or customers’ data. Although we have never had any breach of data in our third-party provider’s environment, any future breach or unauthorized access could result in significant legal and financial exposure, damage to our reputation, and a loss of confidence in the security of our platforms and applications that could potentially have an adverse effect on our business. Because the techniques used to obtain unauthorized access, disable or degrade service, or sabotage systems change frequently and often are not recognized until launched against a target, we may be unable to anticipate these techniques or to implement adequate preventative measures on a timely basis. If an actual or perceived breach of our security occurs, the market perception of the effectiveness of our security measures could be harmed and we could lose suppliers and customers and we may have difficulty obtaining merchant processors or insurance coverage essential for our operations.
We, and the third-party providers upon which we rely, have experienced, and may in the future experience, cybersecurity threats, including threats or attempts to disrupt our information technology infrastructure and unauthorized attempts to gain access to sensitive or confidential information. Our and our third-party vendors’ technology systems may be damaged or compromised by malicious events, such as cyberattacks (including computer viruses, malicious and destructive code, phishing attacks, and denial of service attacks), physical or electronic security breaches, natural disasters, fire, power loss, telecommunications failures, personnel misconduct, and human error. Such attacks or security breaches may be perpetrated by internal bad actors, such as employees or contractors, or by third parties (including traditional computer hackers, persons involved with organized crime, or foreign state or foreign state-supported actors). Cybersecurity threats can employ a wide variety of methods and techniques, which may include the use of social engineering techniques, are constantly evolving, and have become increasingly complex and sophisticated; all of which increase the difficulty of detecting and successfully defending against them. Furthermore, because the techniques used to obtain unauthorized access or sabotage systems change frequently and generally are not identified until after they are launched against a target, we and our third-party providers may be unable to anticipate these techniques or implement adequate preventative measures. Although prior cyberattacks directed at us have not had a material impact on our financial results, and we are continuing to bolster our threat detection and
mitigation processes and procedures, we cannot guarantee that future cyberattacks, if successful, will not have a material impact on our business or financial results. While we have security measures in place to protect our information and our customers’ and suppliers’ information and to prevent data loss and other security breaches, there can be no assurance that in the future we will be able to anticipate or prevent security breaches or unauthorized access of our information technology systems or the information technology systems of the third-party providers upon which we rely. Despite our implementation of network security measures and internal information security policies, data stored on personnel computer systems is also vulnerable to similar security breaches, unauthorized tampering or human error.
Many governments and other regulatory bodies including the SEC have enacted laws requiring companies to provide notice of data security incidents involving certain types of data, including personal data. If an actual or perceived breach of security measures, unauthorized access to our system or the systems of the third-party providers that we rely upon, or any other cybersecurity threat occurs, we may face direct or indirect liability, costs, or damages, contract termination, our reputation in the industry and with current and potential customers may be compromised, our ability to attract new customers could be negatively affected, and our business, financial condition, and results of operations could be materially and adversely affected.
We maintain cybersecurity insurance and other types of insurance, subject to applicable deductibles and policy limits, but our insurance may not be sufficient to cover all costs associated with a potential data security incident. We also cannot be sure that our existing general liability insurance coverage and coverage for cyber liability or errors or omissions will continue to be available on acceptable terms or will be available in sufficient amounts to cover one or more large claims or that the insurer will not deny coverage as to any future claim. The successful assertion of one or more large claims against us that exceed available insurance coverage, or the occurrence of changes in our insurance policies, including premium increases or the imposition of large deductible or co-insurance requirements, could harm our financial condition.
Changes in demand for our products and services could affect profitability.
We are fundamentally a matchmaking service provider between researchers who have needs for access to subjects, samples, and data, and healthcare providers and other organizations that have them. Any change that either reduces the demand for our services or changes the composition of the demand could adversely impact our financial results.
Overall customer demand could change for many reasons outside of our control, reducing demand or making it more difficult to match up to our supply chain’s capabilities. These reasons include:
•
general economic downturn that impacts the research and development budgets of biopharma;
•
changes in the disease landscape, like COVID-19, that affect the types of products and services needed;
•
changes in drugs and therapies and the desire to study subjects on these drugs and therapies;
•
changes in diagnostic tests performed (like genomic sequencing) that drive the need for subjects and samples with these new or novel test results;
•
changes in data requirements, such as the need to know specific outcomes data;
•
overall changes in biomarker research, such as emerging liquid biopsy or cell therapy research, that drives the need for different products and services;
•
leadership changes within our customers resulting in loss of sponsorship;
•
new (alternative) products introduced by competitors and/or developed by customers, which may have potential to reduce or replace the need for certain types of biospecimens that we provide;
•
competitive forces, which make it easier for customers to find products and services elsewhere; and/or
•
cancellation or delay of research programs, due to funding issues or preliminary research result issues.
If we fail to address these factors in a timely manner or at all, our financial results could be adversely affected.
Additionally, overall customer demand could decrease if we fail to:
•
provide high quality products and services;
•
provide products and services at a competitive price;
•
deliver products and services in a reasonable amount of time;
•
offer high levels of customer service;
•
offer adjacent services that researchers want to procure along with our existing products and services;
•
adequately invest in sales and marketing programs and teams to drive demand or operational support to fulfill requests;
•
develop a large and diverse supply network to satisfy demand; or
•
provide a technology solution that simplifies the biospecimen procurement process for researchers and specimen providers alike.
We incur credit risk with our customers, and we may provide them with products and services for which we do not get paid.
Our customers generally place orders for our products and services using a purchase order and we invoice our customers after they have received the products or services from us. During this procurement process, we become obligated to pay our suppliers for any products or services we procure from them on behalf of our customers regardless of whether our customers ultimately pay us for these products or services. Therefore, we bear the responsibility for the credit risk of our customers. We mitigate this credit risk through procedures that evaluate the creditworthiness of customers prior to accepting a purchase order from them. However, our procedures may not successfully identify all those who ultimately fail to pay us for our products and services and any non-payments may negatively impact our revenues, results of operations, and financial condition.
Our customer mix increases the risk of customers not paying our invoices.
We derive, and believe that we may continue to derive, a significant portion of our revenues from privately held, investor-backed biopharma companies that are not profitable and have little operating history. These organizations may be at a higher risk of not paying for provided products and services on a timely basis or at all. If these companies fail to pay our invoices, our profitability will be adversely impacted.
We rely upon relatively few customers for a significant portion of revenue and do not have a recurring revenue business model. A loss of large customers could affect our ability to operate.
We have derived, and believe that we may continue to derive, a significant portion of our revenue from a limited number of customers that vary each year. During the year ended December 31, 2023, one customer represented 25% of the Company’s revenues, and during the year ended December 31, 2022, two customers represented 14% and 12% of our revenue, respectively. During the six months ended June 30, 2024, one customer represented 26% of the Company’s revenues, and during the six months ended June 30, 2023, one customer represented 21% of our revenue, respectively. We do not have a recurring revenue model and our customers may buy less of our products or services depending on their research and development cycles, internal budget cycles, product and service requirements, and competitive offerings. A major customer in one year may not purchase any of our products or services in another year, which may adversely affect our financial performance.
Customers and customer prospects may be averse to using a self-service marketplace to procure specimens and may continue to require iSpecimen personnel in the procurement process, impacting our scalability and profitability.
The iSpecimen Marketplace functions as a lead generation system to capture customer requests for specimens and as a workflow engine to allow customers, suppliers, and our Company to track and manage specimen requests. Currently, it does not fully support self-service eCommerce because key capabilities required to satisfy these transactions across all of our product lines, such as a pricing engine and patient-level search, have yet to be incorporated. Therefore, currently all customer requests for specimens require assistance from iSpecimen sales personnel. At a minimum, our sales personnel are involved in the generation of customer quotes, but they often also act in a consulting role to help develop specimen request specifications on more complex projects or to perform searches on the customer or customer prospect’s behalf.
While we continue to invest in capabilities to support customer self-service in the iSpecimen Marketplace, we do not know when we will consider these capabilities to be fully developed. Additionally, we do not know if researchers will utilize the iSpecimen Marketplace to transact without the intervention of iSpecimen personnel which could limit our scalability. We may continue to invest in software which may never provide a return on its investment and diverts resources from the development of software that drives other parts of our procurement workflow.
Our business may be materially and adversely impacted by the reduction, delay or cancellation of orders from our customers.
Our contracts with our customers generally allow them to reduce, delay, or cancel the unfulfilled portion of their specimen order with a two-week notice. Customers may reduce, delay, or cancel their unfulfilled orders due to a variety of reasons including they make changes to project requirements and the open request no longer meets their needs; their budgets change or projects get cancelled; they place orders with multiple specimen providers and cancel open orders when they have procured sufficient quantity of samples across all their sources; or we are unable to fulfill the entire order before the project deadline. For orders received in 2023 and 2022, we fulfilled approximately 77% and 76%, respectively, of the total value of these orders. For orders received in the six months ended June 30, 2024, we fulfilled approximately 63% of the total value of these orders. Our orders are a mix of specimens for immediate fulfillment to long term studies, that can take up to a year or longer to fulfill. We expect this six-month value to continue increasing as long running projects continue. The six-month actual percentage has not been annualized to take into consideration long term or open-ended projects that are not intended to be completely fulfilled at year end. Our business, financial condition, results of operations and cash flows may be materially and adversely impacted by the reduction, delay or cancellation of orders.
We have entered into contracts with U.S. government agencies and contractors which subjects us to federal contract and audit risks.
We entered into contracts with U.S. government agencies and contractors, representing approximately 1.0% and 8.3% of our total revenue for 2023 and 2022, respectively, and approximately 0.1% and 0.6% of our total revenue for the six months ended June 30, 2024 and 2023, respectively, that may contain unfavorable termination provisions and are subject to audit and modification by the government at its sole discretion, which subjects us to additional risks. These risks include the ability of the U.S. government to unilaterally:
•
suspend or prevent us for a set period of time from receiving new contracts or extending existing contracts;
•
terminate our existing contracts;
•
reduce the scope and value of our existing contracts;
•
audit and object to our contract-related costs and fees, including allocated indirect costs; and
•
change certain terms and conditions in our contracts.
The U.S. government may terminate any of its contracts with us either for its convenience or if we default by failing to perform in accordance with the contract schedule and terms. Termination for convenience provisions may enable us to recover only our costs incurred or committed, and settlement expenses and profit on the work completed prior to termination. Termination for default provisions may not permit these recoveries and make us liable for excess costs incurred by the U.S. government in procuring undelivered items from another source.
As a U.S. government contractor and subcontractor, we may become subject to periodic audits and reviews. Based on the results of these audits, the U.S. government may adjust our contract-related costs and fees, including allocated indirect costs. As part of any such audit or review, the U.S. government may review the adequacy of, and our compliance with, our internal control systems and policies, including those relating to our purchasing, property, compensation, and/or management information systems. In addition, if an audit or review uncovers any improper or illegal activity, we may be subject to civil and criminal penalties and administrative sanctions, including termination of our contracts, forfeiture of profits, suspension of payments, fines and suspension or prohibition from doing business with the U.S. government.
We could also suffer serious harm to our reputation if allegations of impropriety were made against us. Although we have not had any government audits and reviews to date, future audits and reviews could cause adverse effects.
Sustainable future revenue growth is dependent on growth in the capabilities of our supply network which we may not be able to achieve.
Our business is fundamentally a match-making business between healthcare providers who have access to subjects, samples, and data and life science researchers who need them. Currently, we receive more requests for our products and services than we have access to in our supply network and we are therefore supply constrained. Although we continue to allocate resources to supply development and commensurately grow our supply network capabilities to keep pace with demand, this supply-demand imbalance could increase in the future if we do not continue or increase our investment in this area.
Additionally, demand for specimens we receive is becoming more specific, requiring access to a greater population of subjects, samples, and data to find those that meet a researcher’s inclusion and exclusion criteria. It takes a larger network of subjects, samples, and data to access a wide enough population of subjects to meet a growing number of requests with more stringent criteria. Delays, difficulties, or unanticipated costs in developing our supply network capabilities necessary to successfully procure products and services could adversely affect revenue and profitability.
Sustainable future revenue growth is dependent upon gaining access to more healthcare data from our supply network and a failure to obtain this data may adversely affect our growth.
Key to our growth strategy is the accessibility and availability of deep medical record data from our healthcare provider supply sites. This data is used to automate the process of matching researchers to subjects, samples, and data, and also used to automate the procurement workflow. Currently, we have gained access to laboratory data to support the distribution of clinical lab specimens as well as biorepository data to support the distribution of banked specimens. However, we have not gained access to deeper medical record data sets from a broad set of healthcare providers to support custom specimen collections, clinical trial recruitment, or data licensing. Should we fail in our ability to access deeper healthcare data, we may not be able to effectively compete in our served markets or grow as anticipated and our business may suffer.
The adoption cycle of our supply network tends to be very lengthy, which may adversely affect our ability to scale rapidly and increase revenues.
The business development cycle for the adoption of our technology solution at healthcare provider supply partners can take up to 18 months or more from initial contact with the prospect through execution of a contract. We may spend significant resources to attempt to secure a new supply partner without successfully engaging the supply partner. Even if we are successful in securing a new supply partner, once a contract is executed, implementation of our technology in the supply partner’s environment can take another several months to a year or more. Because of the lengthy adoption cycle, we may fail to expand our supply network quickly enough to reach our revenue growth targets.
Potential adverse effects from changes in the healthcare industry, including consolidations and regulatory changes, could affect access to subjects, samples, and data and affect our growth.
Changing healthcare-related legislation and regulation may impact the fiscal stability and sustainability of our supply partners. Additionally, many healthcare providers are consolidating to create larger healthcare systems and/or integrated healthcare delivery systems. These changes can divert resources at our healthcare provider supply sites away from the evaluation or implementation of the iSpecimen solution to the adoption of new infrastructure, policies, and procedures to support the changes, thereby extending their timeline to adopt the iSpecimen solution. We cannot predict whether or when future healthcare reform initiatives at the international, federal, or state level, consolidations, or other initiatives affecting healthcare providers’ businesses will be proposed, enacted, or implemented or what impact those initiatives may have on our business, results of operations, and financial condition.
Our supply chain may not provide adequate resources to quickly respond to requests for specimens and delays in the procurement process can affect our reputation, revenue, and profitability.
Many of the healthcare providers in our supply network are not-for-profit organizations whose primary business is to provide clinical care to patients. Supporting biospecimen research may be an adjunct activity for them. These organizations may lack adequate resources to quickly respond to our requests for specimens now and into the future. Should we and our customers experience slow turnaround times on specimen requests, our reputation may be damaged and there may be an adverse impact on our revenue and profitability.
We do not control the end-to-end quality of specimens and data collected in our supply chain and quality issues can affect our reputation, revenue, and profitability.
We rely upon our supply sites and their quality control processes to provide us with products and services that meet order specifications. In certain situations, products are shipped directly from the supply sites to our customers. When we receive products from our supply sites, we perform a visual inspection of the products, but we do not perform an in-depth quality control check to ensure that products meet all specifications.
Instead, we rely upon our customers to perform quality checks themselves and offer refunds or replacements for products that do not meet specification. We receive products from supply sites and ship them to our customers. In 2023, the percent of specimens that met specifications was 99% for clinical remnant specimens, 97% for banked research specimens and 99% for custom research collections. In 2022, the percent of specimens that met specifications was 99% for clinical remnant specimens, 99% for banked research specimens and 99% for custom research collections. For the six months ended June 30, 2024, the percent of specimens that met specifications was 99% for clinical remnant specimens, 87% for banked research specimens and 99% for custom research collections. Percentage of banked research specimens that met specifications decreased year over year from 2022 and then again during the six months ended June 30, 2024. Following feedback from our customers, we implemented a robust return and exchange program to better meet customer needs. iSpecimen also has terminating contracts with suppliers with lower quality specimens. Any issues with quality from our supply sites can adversely affect our reputation, revenue, and profitability.
Reliance on relatively few supply partners for significant supplies and services could affect our ability to operate and grow.
We have derived, and believe that we may continue to derive, a significant portion of our revenues from products we procure from a limited number of supply sites. For the year ended December 31, 2023, there was one supplier who accounted for 13% of our total cost of revenue and three other suppliers who, together, accounted for an additional 23% of our total cost of revenue. For the year ended December 31, 2022, there were two suppliers who each accounted for 12% of our total cost of revenue and two other suppliers who, together, accounted for an additional 16% of our total cost of revenue. For the six months ended June 30, 2024, there was one supplier who accounted for 12% of our total cost of revenue and three other suppliers who, together, accounted for an additional 20% of our total cost of revenue. Any change in the ability of a major supply site to provide us with products and services (such as financial health of the supply site, key leadership, research focus, information technology, competitive demand for specimens from third-parties, pricing structures, contract status and changes in the general economy) may adversely affect our financial performance.
Our supply partners’ inventories may become obsolete, which could have a material adverse effect upon our ability to generate revenue.
During the year ended December 31, 2023, approximately 52% of our revenue was derived from specimens that were procured from our supply partners’ existing sample inventories in their biobanks. For the six months ended June 30, 2024, approximately 42% of our revenue was derived from specimens that were procured from our supply partners’ existing sample inventories in their biobanks. These inventories may become obsolete due to changes in regulatory requirements such as a requirement for new consent form disclosures; changes in researcher requirements for the types of specimens, subjects, and data they need for their studies; and/or general degradation in the quality of stored specimens. Any change in regulations, researcher needs, or specimen quality could render our supply partners’ inventories obsolete and may adversely affect our financial performance.
Specimen collection from human subjects, including the possible occurrence of adverse events during or after tissue collection, could provide exposure to claims and litigation.
There are inherent risks associated with collecting specimens from human subjects. Although specimen collections are completed by certified staff according to established industry standards, specimen donors vary in their ability to tolerate specimen collection protocols and such donors may potentially have an adverse health reaction either during or following a specimen collection. Research subjects or their legally authorized representative may file claims related to a specimen collection and these claims could result in litigation that could be expensive, and time consuming to defend or result in judgements that exceed the resources of the Company and its insurance coverage.
We procure specimens and data from organizations outside of the U.S. and as such, we rely upon these organizations to collect and distribute specimens and data in accordance with their local regulations as well as our contractual requirements. A failure by our sites to comply with both applicable regulations and our contractual requirements could introduce us to compliance risk.
Some of the organizations from which we procure specimens and data reside outside of the U.S. in jurisdictions that may have data protection rules, human research protection rules, and other pertinent rules that relate to the collection and distribution of specimens and data that vary from U.S. regulations. We, as an organization are not knowledgeable about all the pertinent rules and regulations of all of the jurisdictions in which these sites operate, and therefore we rely upon our contractual relationships with supply sites to ensure that they have legal responsibility for compliance with their own jurisdiction-specific regulations.
Should any site fail to comply with the applicable regulations, we may suffer reputational risks if we have distributed specimens and data from that site. Additionally, any compliance failure on the part of our supply sites that impacts our research customers’ ability to utilize specimens and data they previously obtained from us, as well as utilize any research results, they derived from these specimens and data, may subject us to claims by these customers. These claims could result in litigation that could be expensive to defend or result in judgements that exceed our resources and our insurance coverage. Any such litigations and judgement could adversely affect our business, financial condition, and results of operations.
We may experience delays or interruption in the shipments of our specimens due to factors outside of our control, and such disruption could lead to lost revenue and customer satisfaction issues.
We distribute biological specimens to customers around the world. These specimens need to be delivered over a range of temperatures from ambient to cryogenic and delivery timeframes that can be as quick as hours. We rely on third-party shipping materials (such as thermal containers) as well as shipping services (such as FedEx) to transport specimens to our customers. Shipping materials may be defective and third-party shipping services, including international shipping services, could become disrupted by adverse weather conditions, natural disasters, military conflicts, flight cancellations, ground logistics issues, customs delays, and other service interruptions. Any defect in our shipping materials or delays in shipping service times could cause damage to these specimens and render them unusable by our customers. If we are unable to deliver our specimens in a timely matter and without damage, our revenue could be negatively impacted and our reputation with our customers could suffer, resulting in material harm to our business.
The Company’s business was negatively impacted during the first half of 2022 by the ongoing war between Russia and Ukraine. At the start of the war, the Company had approximately $1 million of purchase orders that were slated to be fulfilled by the Company’s supply network in Ukraine and Russia. This supply network shut down quickly at the start of the war. Ukrainian suppliers were disabled due to war conditions and evacuations and some of the Company’s Russian suppliers were disabled by sanctions. While the Company mobilized to shift these purchase orders to other suppliers in the network, the process of getting specimen collections from other supply sites took time, which caused a delay in the fulfillment of such purchase orders.
As of December 31, 2023 and June 30, 2024, the Company’s supply sites in Russia that had not been under sanctions were now accessible and the Company’s supply sites in Ukraine had mostly reopened. However, due to the uncertainty caused by the ongoing war, Ukraine suppliers may again become inaccessible to the Company. Therefore, as long as the uncertainty continues, the Company does not use them as sole specimen sources at a purchase order level. Alternate suppliers do not have the same favorable unit economics or
specimen collection rates. The short and long-term implications of the war are difficult to predict at this time. The imposition of more sanctions and counter sanctions may have an adverse effect on the economic markets generally and could impact the Company’s business and the businesses of the Company’s supply partners, especially those in Ukraine and Russia. Because of the highly uncertain and dynamic nature of these events, it is not currently possible to estimate the impact of the war on the Company’s business and the companies from which the Company obtains supplies and distributes specimens.
Our future success depends on our ability to retain our key personnel and to attract, retain and motivate qualified personnel.
Our future success will depend upon our ability to retain our key management and other personnel and will also depend in large part on our ability to attract and retain additional qualified software developers, bioinformaticists, operations personnel, sales and marketing personnel, and business development personnel. Competition for these types of employees is intense due to the limited number of qualified professionals and the high demand for them, particularly in the Boston, Massachusetts area where our headquarters are located. We have in the past experienced difficulty in recruiting qualified personnel, especially in the area of sales. Failure to attract, assimilate, and retain personnel would have a material adverse effect on our business and potential growth.
Our senior management team has limited experience managing a public company.
Our senior management team has limited experience managing a public company, and regulatory compliance may divert its attention from the day-to-day management of our business. Our management team may not successfully or efficiently manage our continued transition to a public company that will be subject to significant regulatory oversight and reporting obligations under the federal securities laws. In particular, these obligations will require substantial attention from our senior management and could divert their attention away from the day-to-day management of our business, which could materially and adversely impact our business operations.
Our competitors may have greater resources than us and may outspend us to grow more quickly.
Our competitors are highly fragmented and comprise of thousands of biobanks, healthcare providers, and commercial biospecimen organizations. We expect to continue to experience significant and increasing levels of competition in the future, especially from several larger biospecimen providers who have consolidated via mergers and acquisitions and who are well-capitalized by private equity. These organizations are currently acquiring smaller biospecimen businesses and have larger customer bases, their own collection centers, biospecimen inventories, larger marketing and sales budgets, and an international presence. They may also be developing their own technology solution that could be better or less costly to develop than our own iSpecimen Marketplace, thereby eliminating one of our key competitive advantages. They may continue to outspend us to grow more quickly and we may not be able to successfully compete with a competitor that has greater resources; hence such competition may adversely affect our business.
We may lose business to competitors which have or develop their own biorepositories and/or collection centers that can meet customers’ needs.
Many of our competitors have their own biorepository of specimens that they have collected or procured over time. These inventories, when they meet a customer’s needs for product, almost always provide our competitors with a time-to-delivery advantage because they can directly fulfill requests from their own inventories, whereas we must procure products through our supply network after an order has been received from our customers. Additionally, some competitors have their own collection facilities and direct access to eligible research subjects, which also provides a time-to-delivery advantage. We have lost and will continue to lose business to competitors when they can provide samples more quickly than we can from our supply network.
We may face pricing pressure from competitors who may lower prices to reduce biorepository inventories or because they have more favorable specimen acquisition costs.
Many competitors invest in biorepositories of specimens and data. These competitors may be incented to drop prices in order to more quickly recoup their inventory carrying costs, especially when they have held
inventory for longer periods of time. This may cause downward pricing pressure on us. Additionally, some competitors may have cost advantages on some types of collections either because of more favorable supply relationships or because they have their own collection centers, and they can likewise exert pricing pressure in the market. Lower prices will adversely impact our revenue and gross margins.
Our overall business results may suffer from an economic downturn.
We rely upon researchers from biopharma companies as the primary source of our revenue. During an economic downturn, the biopharma industry typically experiences a drop in the annual growth rate of research and development spending and allocates fewer resources towards it. An economic downturn could adversely affect the demand for our products and services and have a corresponding impact on our revenue and profitability. A prolonged economic downturn may cause us to reduce investment in the longer-term growth of our Company in order to reduce short term costs.
Our operations and performance depend on economic conditions in the United States and other countries where we do business. Deterioration in general economic conditions, whether due to COVID-19 or otherwise, could negatively affect our and our customers’ purchasing power.
Our results of operations and financial condition may be adversely impacted from high inflation rates.
We have experienced negative effects from inflation in certain areas of our business due to the recent high rates of inflation in the U.S. and around the world. Inflation is causing the cost of employee salaries to rise and our salaries account for a significant portion of our overall operating costs. Additionally, costs of supplies and other sales, marketing and general and administrative costs have increased due to inflation.
Inflation has not had a significant adverse impact on the cost of specimens due to our long-term contracts maintained with vendors, which include revenue sharing plans. However, if inflation continues, it may have an adverse impact on the costs of our samples in the future.
Our timely fulfillment of customer orders may be adversely impacted due to constraints in the supply chain.
Our operations are heavily reliant on specimen availability and delays or shortages in obtaining specimens caused by constraints in the supply chain, may adversely impact the timing and extent of our ability to fulfill our customer orders which could adversely impact our results of operations and financial condition.
We may have difficulty managing growth in our business, which could adversely affect our financial condition and results of operations.
Significant growth in the size and scope of our operations could place a strain on our financial, technical, operational, and management resources. The failure to continue to upgrade our technical, administrative, operating and financial control systems, or the occurrences of unexpected expansion difficulties, could have a material adverse effect on our financial condition and our ability to timely execute our business plans.
We have incurred losses from sales tax obligations owed to various jurisdictions by us because we did not collect taxes on taxable sales in prior years, and we may never be able to recover the prior sales taxes from the customers.
States and other jurisdictions have varying policies regarding when a company has a taxable presence in their locale. We are required to collect taxes on taxable sales in prior years but we failed to do so and thus have incurred losses from sales tax obligations owed to various jurisdictions. We are in discussions with those tax jurisdictions to rectify and have made tax payments to some of those jurisdictions. We have also reached out to our customers who owe sales taxes and recovered partial tax payments from certain customers. However, we may never be able to recover the prior sales taxes from all the customers, which could have a material adverse effect on our financial condition.
Our ability to utilize net operating loss carryforwards may be limited, resulting in income taxes sooner than currently anticipated.
As of June 30, 2024, we had federal net operating loss carryforwards (“NOLs”) of approximately $55.2 million for federal income tax purposes of which approximately $13 million expires at various periods through 2037
and approximately $42.2 million can be carried forward indefinitely. These NOLs may be used to offset future taxable income, to the extent we generate any taxable income, and thereby reduce or eliminate our future federal income taxes otherwise payable. Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, imposes limitations on a corporation’s ability to utilize NOLs if it experiences an ownership change as defined in Section 382. In general terms, an ownership change may result from transactions increasing the ownership of certain stockholders in the stock of a corporation by more than 50% over a three-year period. In the event that an ownership change has occurred, or were to occur, utilization of our NOLs would be subject to an annual limitation under Section 382 determined by multiplying the value of our stock at the time of the ownership change by the applicable long-term tax-exempt rate as defined in the Code. Any unused annual limitation may be carried over to later years. We may be found to have experienced an ownership change under Section 382 as a result of events in the past or the issuance of shares of common stock in the future. If so, the use of our NOLs, or a portion thereof, against our future taxable income may be subject to an annual limitation under Section 382, which may result in expiration of a portion of our NOLs before utilization.
We may acquire other businesses, products, or technologies that could disrupt our business, reduce our financial resources, or cause dilution to our stockholders.
As part of our business strategy, we may, in the future, pursue acquisitions of businesses and assets or pursue strategic alliances and joint ventures that leverage our core technology and industry experience to expand our offerings, increase our customer base, or increase our supply base. We have limited experience with acquiring other companies or assets, with forming strategic alliances and joint ventures. We may not be able to find suitable partners or acquisition candidates, and we may not be able to complete such transactions on favorable terms, if at all. If we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business, and we could assume unknown or contingent liabilities. Any future acquisitions also could result in significant write-offs or the incurrence of debt and contingent liabilities, any of which could have a material adverse effect on our financial condition, results of operations, and cash flows. Integration of an acquired company also may disrupt ongoing operations and require management resources that would otherwise focus on developing our existing business. We may experience losses related to acquisitions of other companies, which could have a material adverse effect on our results of operations. We may not identify or complete these transactions in a timely manner, on a cost-effective basis, or at all, and we may not realize the anticipated benefits of any acquisition, technology license, strategic alliance, or joint venture.
To finance any acquisitions or joint ventures, we may choose to issue shares of our common stock as consideration, which would dilute the ownership of our stockholders. If the price of our common stock is low or volatile, we may not be able to acquire other companies or fund a joint venture project using our stock as consideration. Alternatively, it may be necessary for us to raise additional funds for acquisitions through public or private financings. Additional funds may not be available on terms that are favorable to us, or at all.
We may incur significant debt, and our governing documents contain no limit on the amount of debt we may incur.
Subject to market conditions and availability, we may incur significant debt through our Purchase Agreement or other repurchase or credit facilities (including term loans and revolving facilities), public and private debt issuances or otherwise. The amount of leverage we use will vary depending on our available capital, our ability to obtain and access financing arrangements with lenders and the lenders’ and our estimate of the stability of our cash flow. Our governing documents contain no limit on the amount of debt we may incur, and we may significantly increase the amount of leverage we utilize at any time without approval of our shareholders. The amount of leverage on individual assets may vary, with leverage on some assets substantially higher than others. Leverage can enhance our potential returns but can also exacerbate our losses.
Incurring substantial debt could subject us to many risks that, if realized, would materially and adversely affect us, including the risk that:
•
our cash flow from operations may be insufficient to make required payments of principal of and interest on the debt or we may fail to comply with covenants contained in our debt instruments, including our Purchase Agreement, which would likely result in (1) acceleration of such debt (and any other debt arrangements containing a cross default or cross acceleration provision) that we may be unable to repay from internal funds or to refinance on favorable terms, or at all, and/or (2) our inability
to borrow under other financing arrangements, even if we are current in payments on borrowings under those arrangements;
•
our debt may increase our vulnerability to adverse economic, market and industry conditions with no assurance that our investment yields will increase to match our higher financing costs;
•
we may be required to dedicate a substantial portion of our cash flow from operations to payments on our debt, thereby reducing funds available for operations, future business opportunities, distributions to our shareholders or other purposes; and
•
we may not be able to refinance maturing debts.
We cannot be sure that our leverage strategies will be successful.
A failure to comply with restrictive covenants in our Purchase Agreement or our other financing arrangements would have a material adverse effect on us, and any future financings may require us to provide additional collateral or pay down debt.
We are subject to various restrictive covenants contained in our Purchase Agreement and we may be subject to additional covenants in connection with future financing arrangements. Financing arrangements that we may enter into in the future may contain similar or more restrictive covenants. These covenants may limit our flexibility to pursue certain investments or incur additional debt. If we fail to meet or satisfy any of these covenants, we may be in default under the agreements governing the applicable arrangements, and our lenders could elect to accelerate our obligation to repurchase certain assets, declare outstanding amounts due and payable, terminate their commitments, require the posting of additional collateral or enforce their rights against existing collateral. We may also be subject to cross default and acceleration rights and, with respect to collateralized debt, the posting of additional collateral or foreclosure upon default.
Risks Related to Intellectual Property
We use third-party technology licenses as part of our technology solution.
The iSpecimen Marketplace uses third parties for certain technology to support development, delivery, and operations of the platform including product management, software development, cloud hosting, data processing, content mapping, and security services and may need to license additional technology in the future for use in the ongoing operations as part of our technology solution. Most of the software (including source code) and other materials we use are distributed under a “free,” “open source,” or similar licensing model. We also use software and services from commercial providers. However, we believe all of them are generally commercially available to us from other parties. We continue to evaluate partners whose capabilities can help us deliver our iSpecimen Marketplace solution in areas such as functionality, efficiency, and security and expect to continue to leverage and consider additional third-party capabilities in our ongoing Marketplace development. However, there is no assurance that these third-party technology licenses will continue to be available to us on acceptable commercial terms or at all, which could significantly harm our business, financial condition, and operating results.
We use open source licenses as part of our technology solution, which may subject us to claims from third parties claiming ownership and unauthorized use.
We use open source software in our software solutions and technology-enabled services. We may encounter claims from third parties claiming ownership and unauthorized use of the software purported to be licensed under the open source terms, demanding release of derivative works of open source software that could include our proprietary source code, or otherwise seeking to enforce the terms of the applicable open source licenses. These claims could result in litigation that could be expensive to defend. If we become liable to third parties for such claims, we could be required to make our software source code available under the applicable open source license, utilize or develop alternative technology, or cease using, selling, offering for sale, licensing, implementing or supporting the applicable solutions or technology-enabled services. In addition, use of certain open source software may pose greater risks than use of third-party commercial software, as most open source licensors and distributors do not provide commercial warranties or indemnities or controls on the origin of the software.
We may become subject to third parties’ claims alleging infringement of their patents and proprietary rights, which could be costly, time consuming, and prevent the use of our technology solution.
We cannot assure you that third parties will not claim our current or future products or services infringe their intellectual property rights. Any such claims, with or without merit, could cause costly litigation that could consume significant management time. As the number of product and services offerings in our market increases and functionalities increasingly overlap, companies such as ours may become increasingly subject to infringement claims. These claims also might require us to enter into royalty or license agreements. If required, we may not be able to obtain such royalty or license agreements or obtain them on terms acceptable to us.
We do not have any patents protecting our intellectual property and if we are unable to protect the confidentiality of our trade secrets, know-how and other proprietary and internally developed technology, our business could be adversely affected.
Our success depends upon our proprietary technology. We do not have registered patents on any of our technology because we do not believe that we could obtain blocking patents and that the costs of patent monitoring and prosecution outweigh the benefits. Instead, we rely upon software copyright laws, service marks, trade secret laws, confidentiality procedures, and contractual provisions to establish and protect our proprietary rights as well as the skills, knowledge and experience of our technical and operational personnel, our consultants and advisors, and contractors. Because we operate in a highly competitive industry, we rely in part on trade secrets to protect our proprietary technology and processes. However, trade secrets are difficult to protect.
We enter into confidentiality or non-disclosure agreements with our corporate partners, employees, consultants, collaborators, and other advisors. These agreements generally require that the receiving party keep confidential and not disclose to third-parties confidential information developed by the receiving party or made known to the receiving party by us during the course of the receiving party’s relationship with us. These agreements also generally provide that inventions conceived by the receiving party in the course of rendering services to us will be our exclusive property, and we enter into assignment agreements to protect our rights. These confidentiality, inventions and assignment agreements may be breached and may not effectively assign intellectual property rights to us. Our trade secrets also could be independently discovered by competitors, in which case we may not be able to prevent the use of such trade secrets by our competitors. The enforcement of a claim alleging that a party illegally obtained and was using our trade secrets could be difficult, expensive and time consuming and the outcome would be unpredictable. In addition, effective protection of intellectual property rights is unavailable or limited in certain foreign countries. The failure to obtain or maintain meaningful trade secret protection could adversely affect our competitive position.
Risks Related to Regulatory Environment
Failure to comply with federal and state data protection regulations could result in fines, penalties, and litigation, and have a material adverse effect upon our business.
Because we may gain access to protected healthcare or personal data, we must comply with various data protection regulations worldwide, including the Health Insurance Portability and Accountability Act of 1996, as amended by HITECH, and their implementing regulations at 45 CFR Parts 160-164 (collectively, “HIPAA”). As part of the operation of our business, we act in the capacity of a HIPAA business associate with respect to protected health information (“PHI”), we receive from our healthcare provider partners. As a HIPAA business associate, we are required to protect the privacy and confidentiality of PHI, and we are required to comply with HIPAA security regulations requiring certain administrative, physical, and technical safeguards to ensure the confidentiality, integrity, and availability of electronic PHI (“ePHI”). To comply with our regulatory and contractual obligations, which may change over time, we may have to reorganize processes and invest in new technologies. We are also required to train personnel regarding data protection requirements. If we, or any of our employees or agents, are unable to maintain the privacy, confidentiality, and security of the PHI that is entrusted to us, we could be subject to civil and criminal fines and sanctions imposed by the HHS or state regulatory authorities, and we could be found to have breached our HIPAA business associate agreements with our healthcare provider suppliers. In addition to the HIPAA requirements that we are subject to, we may be subject to similar state laws and regulations, which regulate the collection, handling, processing,
and storage of sensitive personal information. While we have never had a data breach, we cannot guarantee that it will not happen in the future nor can we guarantee that we will always be in compliance with these regulations. Failure to comply with federal, state and local laws and regulations could subject the Company to denial of the right to conduct business, fines, criminal penalties, and/or other enforcement actions which would have a material adverse effect on its business. In addition, compliance with future legislation could impose additional requirements on the Company, which may be costly.
Failure to comply with international laws related to data protection, such as the General Data Protection Regulation (“GDPR”) could result in fines, penalties, and litigation, and have a material adverse effect upon the Company’s business.
We may be required to comply with international laws, such as the GDPR. The GDPR took effect in May 2018 and regulates the collection, storage, use, disclosure, transfer, and/or other processing of personal data of identified or identifiable individuals located in the European Economic Area (“EEA”), including the EU. This data specifically includes personal health data that generally is provided as part of biospecimen collection studies. The GDPR imposes numerous requirements on companies that process personal data, including requirements relating to processing health and other sensitive data, obtaining consent of the individuals to whom the personal data relates for processing (with some exceptions), allowing individuals to revoke consents granted, enabling individuals the right to have their data erased (with some exceptions), amended, or transferred to another data controller (known as “data portability”), providing information to individuals regarding data processing activities, implementing safeguards to protect the security and confidentiality of personal data, limiting the transfer of data to countries outside of the EU, providing notification of data breaches, and taking certain measures when engaging third-parties who may also use or process the data. In addition, EU member states may make their own further laws and regulations limiting the processing of personal data, including biometric, genetic or health data.
The GDPR covers areas where we may not have expertise and the GDPR and the regulatory guidance enforcing GDPR may be actively evolving. We, or our other third-party customers, suppliers and/or distribution partners, may not be able to maintain regulatory compliance with the GDPR or may incur significant costs in obtaining or maintaining regulatory compliance. Any action brought against us for violations of this law, even if successfully defended, could cause us to incur significant legal expenses, reputational risks, and divert our management’s attention from the operation of our business. In addition, compliance with future legislation could impose additional requirements on the Company, which may be costly.
Failure to comply with federal and state laws around environmental, health and safety, biohazards and dangerous goods, and imports/exports could result in fines, penalties, and litigation, and have a material adverse effect upon our business.
Because we receive, store, and ship specimens, we are subject to regulation under federal, state, and local laws and regulations relating to the protection of the environment and human health and safety, including laws and regulations relating to the handling, transportation, and disposal of specimens and infectious and hazardous waste materials, as well as regulations relating to the safety and health of laboratory employees. Our laboratory is subject to applicable federal and state laws and regulations relating to biohazard disposal of all laboratory specimens, and we utilize outside vendors for disposal of such specimens. In addition, the federal Occupational Safety and Health Administration has established extensive requirements relating to workplace safety for healthcare employers whose workers may be exposed to blood-borne pathogens such as HIV, COVID-19, and the hepatitis B virus. These requirements, among other things, require work practice controls, protective clothing and equipment, training, medical follow-up, vaccinations, and other measures designed to minimize exposure to, and transmission of, blood-borne pathogens. There are also federal laws related to import and export of biospecimens and related data.
Failure to comply with federal, state and local laws and regulations could subject us to denial of the right to conduct business, fines, criminal penalties, and/or other enforcement actions which would have a material adverse effect on our business. In addition, compliance with future legislation could impose additional requirements on us which may be costly.
Failure to comply with other international laws around environmental, health and safety, biohazards and dangerous goods, imports/exports, and other regulations could result in fines, penalties, and litigation, and have a material adverse effect upon our business.
Because we procure specimens from and distribute specimens to countries outside of the United States, we are subject to international and foreign rules similar to any of the aforementioned U.S. rules, including those related to environmental, health and safety, biohazards, and imports/exports. We may be unaware of those international and foreign rules.
These laws cover areas where we may not have expertise and, in many areas, these laws are actively evolving. We, or our other third-party customers, suppliers and/or distribution partners, may not be able to maintain regulatory compliance in such countries or may incur significant costs in obtaining or maintaining our foreign regulatory compliance. Any action brought against us for violations of these laws or regulations, even if successfully defended, could cause us to incur significant legal expenses, reputational risks, and divert our management’s attention from the operation of our business. In addition, compliance with future legislation could impose additional requirements on us which may be costly.
Failure to comply with laws and regulations related to the protection of research subjects could result in fines, penalties, and litigation, and have a material adverse effect upon our business.
We are subject to regulation under international, federal, state, and local laws and regulations relating to the protection of research subjects. Federally-funded human-subject research in the United States, including the collection of identifiable human biospecimens, is governed by 45 CFR Part 46, also known as the Health and Human Services Policy for Protection of Human Research Subjects or the “Common Rule.” Use of biospecimens in certain other research is subject to FDA regulations for the Protection of Human Subjects and Institutional Review Boards at 21 CFR Parts 50 and 56. Research funded by the National Institutes of Health (“NIH”) may be subject to grant or contract requirements, as well as NIH Certificates of Confidentiality. When collecting specimens for research in the United States, iSpecimen and its collection sites are responsible for ensuring that specimens are collected in accordance with these regulations. In addition, other countries have their own regulations around the ethical collection of human specimens for research. While we believe that we are in compliance with these laws, we may not be aware of all such laws or may fail to properly audit and identify gaps in compliance. Similarly, we may find errors in our technology and processes and may fail to properly match the compliance requirements of our researchers to the compliance requirements of our suppliers. Failure of our Company or our suppliers to comply with international, federal, state, and local laws and regulations could subject us to denial of the right to conduct business, fines, criminal penalties, and/or other enforcement actions which could have a material adverse effect on our business.
Our failure to comply with other laws and regulations related to our business operations also have a material adverse effect upon our business.
In addition to the above-described laws and regulations, there are many other federal, state and international laws and regulations applicable to iSpecimen. The following list contains some of the other laws and regulations that could directly or indirectly affect our ability to operate the business:
•
Occupational Safety and Health regulations and requirements;
•
Centers for Disease Control Import Permit Program rules related to biological agents;
•
Shipping rules such as IATA Dangerous Goods regulations;
•
State and local laws and regulations for the disposal and handling of medical waste and biohazardous material;
•
Export laws such as the U.S. Department of Commerce’s Bureau of Industry and Security Export Administration Regulations, U.S. State Department’s Directorate of Defense Trade Controls, and the U.S. Department of the Treasury’s Office of Foreign Assets Control in export licensing;
•
Import laws such as the Customs and Border Protection Trade Act of 2002 and the Customs Modernization Act;
•
The federal Anti-Kickback Statute, which prohibits, among other things, any person from knowingly and willfully offering, soliciting, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs;
•
Federal, state, and local tax and tariff rules;
•
Other laws and regulations administered by the FDA;
•
Other laws and regulations administered by HHS; and
•
State and local laws and regulations governing human subject research and clinical trials.
These laws cover several areas of our business and are actively evolving. We, or our other third-party customers, suppliers and/or distribution partners, may not be able to maintain regulatory compliance or may incur significant costs in obtaining or maintaining regulatory compliance. Any action brought against us for violations of these laws or regulations, even if successfully defended, could cause us to incur significant legal expenses, reputational risks, and divert our management’s attention from the operation of our business. In addition, compliance with future legislation could impose additional requirements on us which may be costly.
Failure to comply with governmental export and import regulations could result in fines, penalties, and litigation, and have a material adverse effect upon the Company’s business.
Our products and services are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, and various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls. Exports of our products and services must be made in compliance with these laws and regulations. If we fail to comply with these laws and regulations, we and certain of our employees could be subject to substantial civil or criminal penalties, including the possible loss of export or import privileges; fines, which may be imposed on us and responsible employees or managers; and, in extreme cases, the incarceration of responsible employees or managers.
In addition, changes in our products and services or changes in applicable export or import laws and regulations may create delays in the introduction and sale of our products and services to international markets, prevent our customers from procuring our products and services or, in some cases, prevent the export or import of our products and services to certain countries, governments or persons altogether. Any change in export or import laws and regulations, shift in the enforcement or scope of existing laws and regulations, or change in the countries, governments, persons or technologies targeted by such laws and regulations could also result in decreased use of our products and services, or in our decreased ability to export or sell our products and services to existing or potential customers. Any decreased use of our products and services or limitation on our ability to export or sell our products and services could adversely affect our business, financial condition and results of operations.
Product safety and product liability, including bio-hazard risks, could provide exposure to claims and litigation.
Specimens may have hazardous properties and may carry transmissible infectious agents. There are inherent risks in connection with the handling, storage, disposal, distribution, and/or use of the specimens.
Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by federal, state and local regulation and regulations of foreign jurisdictions, the risk of accidental contamination or injury from these materials cannot be completely eliminated. Individuals who use or come in contact with the specimens may file claims related to their use and these claims could result in litigation that could be expensive to defend or result in judgements that exceed our resources and our insurance coverage. Any such litigations and judgement could adversely affect our business, financial condition and results of operations.
Risks Related to This Offering and our Securities
This is a reasonable best efforts offering, with no minimum amount of securities required to be sold, and we may sell fewer than all of the securities offered hereby.
The placement agent has agreed to use its reasonable best efforts to solicit offers to purchase the Shares and Pre-Funded Warrants, if any, in this offering. The placement agent has no obligation to buy any of the Shares
from us or to arrange for the purchase or sale of any specific number or dollar amount of the Shares. There is no required minimum number of Shares that must be sold as a condition to completion of this offering. As there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, placement agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth above. We may sell fewer than all of the Shares offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive a refund in the event that we do not sell all of the Shares offered in this offering. The success of this offering will impact our ability to use the proceeds to execute our business plans. We may have insufficient capital to implement our business plans and satisfy current obligations, potentially resulting in greater operating losses or dilution unless we are able to raise the required capital from alternative sources. There is no assurance that alternative capital, if needed, would be available on terms acceptable to us, or at all.
We have broad discretion in the use of the net proceeds we receive from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds we receive in this offering, including for any of the purposes described in the section entitled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess whether our management is using the net proceeds appropriately. Because of the number and variability of factors that will determine our use of our net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business and cause the price of our common stock to decline. Pending their use, we may invest our net proceeds from this offering in short-term, investment-grade, interest-bearing securities. These investments may not yield a favorable return to our stockholders.
There can be no assurance that an active and liquid trading market for our common stock will continue or that we will be able to continue to comply with Nasdaq’s continued listing standards.
Our common stock began trading on Nasdaq in June 2021, as a result of our consummation of an initial public offering of our shares of common stock. Our common stock is currently listed on Nasdaq under the symbol “ISPC”. There can be no assurance an active and liquid trading market in our common stock will continue.
There is no guarantee that we will be able to maintain such listing for any period of time by perpetually satisfying the Nasdaq’s continued listing requirements. Our failure to continue to meet these requirements may result in our common stock being delisted from Nasdaq.
If we are not able to comply with the applicable continued listing requirements or standards of Nasdaq, our common stock could be delisted from Nasdaq.
Our common stock is currently listed on Nasdaq. In order to maintain that listing, we must satisfy minimum financial and other continued listing requirements and standards, including those regarding director independence and independent committee requirements, minimum stockholders’ equity, minimum share price, and certain corporate governance requirements. There can be no assurances that we will be able to comply with the applicable listing standards of Nasdaq.
In the event that our common stock is delisted from Nasdaq and is not eligible for quotation on another market or exchange, trading of our common stock could be conducted in the over-the-counter market established for unlisted securities, such as the OTC Markets. In such event, it could become more difficult to dispose of, or obtain accurate price quotations for, our common stock, and there would likely also be a reduction in our coverage by securities analysts and the news media, which could cause the price of our common stock to decline further. Also, it may be difficult for us to raise additional capital if we are not listed on a major exchange.
In the event that our common stock is delisted from Nasdaq, U.S. broker-dealers may be discouraged from effecting transactions in shares of our common stock because it may be considered a penny stock and thus be subject to the penny stock rules.
The SEC has adopted a number of rules to regulate a “penny stock” that restricts transactions involving stock which is deemed to be a penny stock. Such rules include Rules 3a51-1, 15g-1, 15g-2, 15g-3, 15g-4, 15g-5, 15g-6,
15g-7, and 15g-9 under the Exchange Act. These rules may have the effect of reducing the liquidity of penny stocks. “Penny stocks” generally are equity securities with a price of less than $5.00 per share (other than securities registered on certain national securities exchanges or traded on Nasdaq if current price and volume information with respect to transactions in such securities is provided by the exchange or system). Our shares of common stock may, in the future constitute, a “penny stock” within the meaning of the rules. The additional sales practice and disclosure requirements imposed upon U.S. broker-dealers may discourage such broker-dealers from effecting transactions in shares of our common stock, which could severely limit the market liquidity of such shares of common stock and impede their sale in the secondary market.
A U.S. broker-dealer selling a penny stock to anyone other than an established customer or “accredited investor” (generally, an individual with a net worth in excess of $1,000,000 or an annual income exceeding $200,000, or $300,000 together with his or her spouse) must make a special suitability determination for the purchaser and must receive the purchaser’s written consent to the transaction prior to sale, unless the broker-dealer or the transaction is otherwise exempt. In addition, the “penny stock” regulations require the U.S. broker-dealer to deliver, prior to any transaction involving a “penny stock”, a disclosure schedule prepared in accordance with SEC standards relating to the “penny stock” market, unless the broker-dealer or the transaction is otherwise exempt. A U.S. broker-dealer is also required to disclose commissions payable to the U.S. broker-dealer and the registered representative and current quotations for the securities. Finally, a U.S. broker-dealer is required to submit monthly statements disclosing recent price information with respect to any “penny stock” held in a customer’s account and information with respect to the limited market in “penny stocks”.
You should be aware that, according to the SEC, the market for “penny stocks” has suffered in recent years from patterns of fraud and abuse. Such patterns include (i) control of the market for the security by one or a few broker-dealers that are often related to the promoter or issuer; (ii) manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; (iii) “boiler room” practices involving high-pressure sales tactics and unrealistic price projections by inexperienced sales persons; (iv) excessive and undisclosed bid-ask differentials and markups by selling broker-dealers; and (v) the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, resulting in investor losses. Our management is aware of the abuses that have occurred historically in the penny stock market. Although we do not expect to be in a position to dictate the behavior of the market or of broker-dealers who participate in the market, management will strive within the confines of practical limitations to prevent the described patterns from being established with respect to our securities.
Our share price may be volatile, and purchasers of our common stock could incur substantial losses.
Our share price has been volatile in the past and may continue to be so in the future. Since our IPO, our common stock has traded at prices ranging from $3.41 to $579.60, adjusted for the Reverse Stock Split based on a ratio of 1-for-20 as if it had occurred at the beginning of the earliest period presented. The stock market in general has experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their common stock at or above the price paid for such shares. The market price for our common stock may be influenced by many factors, including, but not limited to:
•
changes in our industry;
•
ability to enhance our platform or to add new functionality;
•
regulatory changes;
•
competitive pricing or other pressures;
•
failures of our suppliers to deliver product on time;
•
loss of supply partners;
•
additions or departures of key personnel;
•
sales of our common stock;
•
our ability to execute our business plan;
•
operating results that fall below expectations;
•
loss of any strategic relationship including customers, suppliers and channel partners; and/or
•
economic and other external factors.
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our common stock.
Our directors, officers and principal stockholders have significant voting power and may take actions that may not be in the best interests of our other stockholders.
As of October 15, 2024, our officers, directors and principal stockholders each holding more than 5% of our common stock collectively controlled approximately 20.98% of our outstanding common stock. As a result, these stockholders, if they act together, will be able to control the management and affairs of our Company and most matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change of control, impeding a merger, consolidation or other business combination transaction involving us and discouraging a potential acquiror from making a tender offer or otherwise attempting to obtain control of the Company and might adversely affect the market price of our common stock. This concentration of ownership may not be in the best interests of our other stockholders.
Certain provisions of our certificate of incorporation, as amended, and our bylaws, as amended, may make it more difficult for a third party to affect a change-of-control.
Our certificate of incorporation, as amended, authorizes the board of directors (the “Board”) to issue up to 50,000,000 shares of preferred stock. The preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by the Board without further action by the stockholders.
These terms may include preferences as to dividends and liquidation, conversion rights, redemption rights and sinking fund provisions. The issuance of any preferred stock could diminish the rights of holders of our common stock, and therefore could reduce the value of such common stock. In addition, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell assets to, a third party. The ability of the Board to issue preferred stock could make it more difficult, delay, discourage, prevent or make it more costly to acquire or effect a change-in-control, which in turn could prevent our stockholders from recognizing a gain in the event that a favorable offer is extended and could materially and negatively affect the market price of our common stock. In addition, our certificate of incorporation, as amended, provides for a staggered Board. As a consequence, only a minority of the Board will be considered for election at every annual meeting of stockholders, which may make the removal of management more difficult and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our securities. Additional provisions that may discourage unsolicited takeover proposals include (i) board vacancies may be filled by a majority of the remaining board members, (ii) the board may adopt, repeal, rescind, alter or amend our bylaws without stockholder approval, (iii) stockholders holding more than 15% of the outstanding shares may call a special meeting, (iv) a director may be removed from office only by the affirmative vote of a majority of the issued and outstanding stock entitled to vote; and (v) no cumulative voting in the election of directors, which would allow holders of less than a majority of the stock to elect some directors.
Our bylaws, as amended, designate certain courts as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or employees.
Our bylaws, as amended, provide that, unless we consent in writing to an alternative forum, the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have jurisdiction, the federal district court for the District of Delaware) will be the exclusive forum for: (i) any derivative action or proceeding brought on behalf of the Company; (ii) any action asserting a claim for breach of a fiduciary duty owed by any director, officer, employee, or agent of ours to us or our stockholders; (iii) any action asserting a claim
arising pursuant to any provision of the Delaware General Corporation Law, the certificate of incorporation, or the bylaws; and (iv) any action asserting a claim governed by the internal affairs doctrine (the “Delaware Forum Provision”). Our bylaws further provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America shall be the sole and exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act (the “Federal Forum Provision”). In addition, our bylaws provide that any person or entity purchasing or otherwise acquiring any interest in shares of our common stock is deemed to have notice of and consented to the Delaware Forum Provision and the Federal Forum Provision.
Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. As a result, the Delaware Forum Provision will not apply to suits brought to enforce any duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. We note, however, that there is uncertainty as to whether a court would enforce this provision and that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder.
We recognize that the Delaware Forum Provision and the Federal Forum Provision in our bylaws may impose additional litigation costs on stockholders in pursuing any such claims, particularly if the stockholders do not reside in or near the State of Delaware. Additionally, the Delaware Forum Provision and the Federal Forum Provision may limit our stockholders’ ability to bring a claim in a forum that they find favorable for disputes with us or our directors, officers or employees, which may discourage such lawsuits against us and our directors, officers and employees even though an action, if successful, might benefit our stockholders. In addition, while the Delaware Supreme Court ruled in March 2020 that federal forum selection provisions purporting to require claims under the Securities Act be brought in federal court were “facially valid” under Delaware law, there is uncertainty as to whether other courts will enforce the Federal Forum Provision. If the Federal Forum Provision is found to be unenforceable, we may incur additional costs associated with resolving such matters. The Federal Forum Provision may also impose additional litigation costs on stockholders who assert that the provision is not enforceable or invalid. The Court of Chancery of the State of Delaware and the United States District Court may also reach different judgments or results than would other courts, including courts where a stockholder considering an action may be located or would otherwise choose to bring the action, and such judgments may be more or less favorable to us than our stockholders.
Limitations on director and officer liability and indemnification of our officers and directors by us may discourage stockholders from bringing suit against an officer or director.
Our certificate of incorporation, as amended, and bylaws, as amended, provide that, to the fullest extent permitted by Delaware law, as it presently exists or may be amended from time to time, a director shall not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duty as a director. Under Delaware law, this limitation of liability does not extend to, among other things, acts or omissions which involve intentional misconduct, fraud or knowing violation of law, or unlawful payments of dividends. These provisions may discourage stockholders from bringing suit against a director or officer for breach of fiduciary duty and may reduce the likelihood of derivative litigation brought by stockholders on our behalf against a director or officer.
We are responsible for the indemnification of our officers and directors.
Should our officers and/or directors require us to contribute to their defense, we may be required to spend significant amounts of our capital. Our certificate of incorporation, as amended, and bylaws, as amended, also provide for the indemnification of our directors, officers, employees, and agents, under certain circumstances, against attorney’s fees and other expenses incurred by them in any litigation to which they become a party arising from their association with or activities on behalf of our Company. This indemnification policy could result in substantial expenditures, which we may be unable to recoup. If these expenditures are significant or involve issues which result in significant liability for our key personnel, we may be unable to continue operating as a going concern.
We do not expect to pay dividends in the foreseeable future. Any return on investment may be limited to the value of our common stock.
We have never paid cash dividends on our common stock and do not anticipate doing so in the foreseeable future. The payment of dividends on our common stock will depend on earnings, financial condition and
other business and economic factors affecting us at such time as our Board may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on an investment will only occur if our stock price appreciates.
We may need additional capital, and the sale of additional shares of common stock or other equity securities could result in additional dilution to our stockholders.
We may need to raise additional funds sooner than expected, after this offering, to fund our current operating plans. We may finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances, licensing arrangements, or other sources. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest may be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect rights as a common stockholder. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances, or licensing arrangements with third parties, we may be required to relinquish valuable rights to our technologies or future revenue streams or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate technology development or future commercialization efforts.
Our quarterly revenue tends to fluctuate, making it harder to forecast and meet investor expectations.
Quarterly revenue has been difficult to predict, has historically fluctuated, and may vary from quarter to quarter due to a variety of factors, many of which are beyond our control. Accordingly, comparing our operating results on a period-to-period basis may not be meaningful. Factors that may affect our quarterly revenue and operating results may include: any material changes in demand for our products and services; changes in our supply sites’ ability to collect and ship specimens or our ability to retain them; changes in the number, availability, and quality of competing products; our ability to maintain a timely delivery of high quality products and services; the timing and amount of sales and marketing expenses incurred by us to attract new customers; changes in the economic or business prospects of our customers or the economy generally; changes in the pricing policies of our competitors; unforeseen defects in our technology; changes in the regulatory environment; and unforeseen costs necessary to improve and maintain our technology.
These factors affecting our future earnings are difficult to forecast and could harm our quarterly and/or annual operating results. The change in our earnings or general economic conditions may cause the market price of our common stock to fluctuate.
General Risk Factors
Our status as an “emerging growth company” under the JOBS Act may make it more difficult to raise capital when we need to do it or make our common stock less attractive to investors.
Because of the exemptions from various reporting requirements provided to us as an “emerging growth company,” and because we will have an extended transition period for complying with new or revised financial accounting standards, we may be less attractive to investors and it may be difficult for us to raise additional capital as and when we need it. Investors may be unable to compare our business with other companies in our industry if they believe that our financial accounting is not as transparent as other companies in our industry. If we are unable to raise additional capital as and when we need it, our financial condition and results of operations may be materially and adversely affected.
We have limited insurance which may not cover claims by third parties against us or our officers and directors.
We have limited directors’ and officers’ liability insurance and commercial liability insurance policies. Claims by third parties against us may exceed policy amounts and we may not have amounts to cover these claims.
Also, due to high self-insured retention costs and deductibles, we may incur significant costs from any claim made against us before insurance policies provide coverage. Any significant claims would have a material adverse effect on our business, financial condition, and results of operations. In addition, our limited directors’ and officers’ liability insurance may affect our ability to attract and retain directors and officers.
The requirements of being a U.S. public company may strain our resources and divert management’s attention.
As a public company, we are subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 (the “Dodd-Frank Act”) and Nasdaq rules. The requirements of these rules and regulations result in significant legal and financial compliance costs, including costs associated with the employment of personnel, making some activities more difficult, time-consuming or costly, and may also place undue strain on our personnel, systems and resources and divert management’s attention.
The Exchange Act requires, among other things, that we file annual, quarterly, and current reports with respect to our business and financial condition. The Sarbanes-Oxley Act requires, among other things, that we maintain disclosure controls and procedures and internal control over financial reporting. Ensuring that we have adequate internal financial and accounting controls and procedures in place, as well as maintaining these controls and procedures, is a costly and time-consuming effort that needs to be re-evaluated frequently.
Additionally, various rules and regulations applicable to public companies make it more difficult and more expensive for us to maintain directors’ and officers’ liability insurance, and we may be required to accept reduced coverage or higher deductibles or incur substantially higher costs to maintain coverage.
Evaluation of internal control and remediation of potential problems will be costly and time consuming and could expose weaknesses in financial reporting.
Section 404 of the Sarbanes-Oxley Act (“Section 404”) requires that we evaluate our internal control over financial reporting to enable management to report on the effectiveness of those controls annually. In connection with the Section 404 requirements, we could, as part of that documentation, identify material weaknesses, significant deficiencies, or other areas for further attention or improvement.
Implementing any appropriate changes to our internal controls may require specific compliance training for our directors, officers, and employees, require the hiring of additional finance, accounting and other personnel, entail substantial costs to modify our existing accounting systems, and take a significant period of time to complete. Such changes may not, however, be effective in maintaining the adequacy of our internal controls, and any failure to maintain that adequacy, or consequent inability to produce accurate financial statements on a timely basis, could increase our operating costs and could materially impair our ability to operate our business. Moreover, adequate internal controls are necessary for us to produce reliable financial reports and are important to help prevent fraud. As a result, our failure to satisfy the requirements of Section 404 on a timely basis could result in the loss of investor confidence in the reliability of our financial statements, which in turn could cause the market value of our common stock to decline.
Public company compliance may make it more difficult to attract and retain officers and directors.
The Sarbanes-Oxley Act and new rules subsequently implemented by the SEC have required changes in corporate governance practices of public companies. As a public company, we are expected to follow Sarbanes-Oxley Act regulations and other public company rules, and these rules and regulations will increase our compliance costs and make certain activities more time consuming and costly. As a result, these rules and regulations may make it more difficult and expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult and costly for us to attract and retain qualified persons to serve on our Board or as executive officers.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Exchange Act. These forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from the results projected in any forward-looking statement. In addition to the factors specifically noted in the forward-looking statements, other important factors, risks and uncertainties that could result in those differences include, but are not limited to, those discussed under “Risk Factors” in this prospectus. The forward-looking statements are made as of the date of this prospectus, and we assume no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements. You should consult all of the information set forth in this prospectus and the other information set forth from time to time in our reports filed with the Securities and Exchange Commission (the “SEC”) pursuant to the Securities Act and the Exchange Act, including our reports on Forms 10-K, 10-Q and 8-K.
You can identify some of these forward-looking statements by words or phrases such as “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “is/are likely to,” “potential,” “continue” or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements include statements relating to:
•
our ability to enter into contracts with healthcare providers to gain access to specimens, subjects, and data on favorable terms;
•
our ability to obtain new customers and keep existing customers;
•
development of our technology to adequately keep pace to support expansion of our existing line of business or our entry into new lines of businesses;
•
market adoption rate of our marketplace technology;
•
our ability to continue to expand outside of the United States in compliance with local laws and regulations;
•
acceptance of the products and services that we market;
•
the viability of our current intellectual property;
•
government regulations and our ability to comply with government regulations;
•
our ability to retain key employees;
•
adverse changes in general market conditions for biospecimens;
•
our ability to generate cash flow and profitability and continue as a going concern;
•
our future financing plans; and
•
our ability to adapt to changes in market conditions which could impair our operations and financial performance.
These forward-looking statements involve numerous risks and uncertainties. Although we believe that our expectations expressed in these forward-looking statements are reasonable, our expectations may later be found to be incorrect. Our actual results of operations or the results of other matters that we anticipate could be materially different from our expectations. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the section titled “Risk Factors” and elsewhere in this prospectus.
USE OF PROCEEDS
We estimate that the net proceeds from this offering will be approximately $4,485,000 (assuming the sale of all Shares offered hereby at the assumed public offering price of $4.11 per share, which was the reported closing sale price of our common stock on Nasdaq on October 15, 2024, and assuming no issuance or exercise of Pre-Funded Warrants), after deducting expenses relating to this offering payable by us estimated at approximately $515,000, including placement agent fees and expenses, for certain financial advisor services provided in connection with this offering.
| |
|
|
100% of
Shares Sold
|
|
|
% of
Total
|
|
|
50% of
Shares Sold
|
|
|
% of
Total
|
|
|
25% of
Shares Sold
|
|
|
% of
Total
|
|
|
Gross Proceeds from Offering
|
|
|
|
$ |
5,000,000 |
|
|
|
|
|
|
|
|
|
|
$ |
2,500,000 |
|
|
|
|
|
|
|
|
|
|
$ |
1,250,000 |
|
|
|
|
|
|
|
|
|
Use of Proceeds
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Placement Agent Fees and Expenses
|
|
|
|
$ |
315,000 |
|
|
|
|
|
6.3% |
|
|
|
|
$ |
215,000 |
|
|
|
|
|
8.6% |
|
|
|
|
$ |
165,000 |
|
|
|
|
|
13.2% |
|
|
|
Offering Expenses
|
|
|
|
|
200,000 |
|
|
|
|
|
4.0% |
|
|
|
|
|
200,000 |
|
|
|
|
|
8.0% |
|
|
|
|
|
200,000 |
|
|
|
|
|
16.0% |
|
|
|
Outstanding Debt
|
|
|
|
|
1,030,000(1) |
|
|
|
|
|
20.6% |
|
|
|
|
|
1,030,000(1) |
|
|
|
|
|
41.2% |
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
|
Potential Acquisitions of Assets or Investments in Businesses
|
|
|
|
|
550,000 |
|
|
|
|
|
11.0% |
|
|
|
|
|
550,000 |
|
|
|
|
|
22.0% |
|
|
|
|
|
550,000 |
|
|
|
|
|
44.0% |
|
|
|
Marketing and Advertising
|
|
|
|
|
2,000,000 |
|
|
|
|
|
40.0% |
|
|
|
|
|
350,000 |
|
|
|
|
|
14.0% |
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
|
General Corporate and Working Capital
|
|
|
|
|
905,000 |
|
|
|
|
|
18.1% |
|
|
|
|
|
155,000 |
|
|
|
|
|
6.2% |
|
|
|
|
|
335,000 |
|
|
|
|
|
26.8% |
|
|
|
Total Use of Proceeds
|
|
|
|
$ |
5,000,000 |
|
|
|
|
|
100% |
|
|
|
|
$ |
2,500,000 |
|
|
|
|
|
100% |
|
|
|
|
$ |
1,250,000 |
|
|
|
|
|
100% |
|
|
(1)
This includes the principal amount of $1,000,000 outstanding with respect to the Loan and accrued and unpaid interest of approximately $10,000 through October 15, 2024. We estimate that the unpaid interest will be $30,000 on the loan pay-off date.
We believe that the net proceeds of this offering, together with our existing cash, will enable us to fund our operations for at least six months following the completion of this offering. We have based this estimate on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we expect. If we raise $5,000,000 in this offering, it is our intent to use approximately $2,000,000 of the net proceeds for marketing and advertising services provided by IR Agency LLC, which will include creating company profiles, media distribution, and building a digital community to communicate information about the Company to the financial community. Additionally, approximately $1,030,000, or 22.9% of the $4,485,000 in net proceeds, will be used to repay outstanding indebtedness. If we raise $2,500,000 in this offering, it is our intent to use approximately $350,000 for marketing and advertising services provided by IR Agency LLC, and the remaining net proceeds to repay outstanding indebtedness.
Although we currently anticipate that we will use the net proceeds from this offering as described above, there may be circumstances where a reallocation of funds is necessary. The amounts and timing of our actual expenditures will depend upon numerous factors, including permits, and the accessibility of rigs and other equipment, our operating costs and the other factors described under “Risk Factors” in this prospectus. Accordingly, our management will have flexibility in applying the net proceeds from this offering. An investor will not have the opportunity to evaluate the economic, financial or other information on which we base our decisions on how to use the proceeds.
Each $1.00 increase (decrease) in the assumed public offering price of $4.11 per share, which was the reported closing price of our common stock on Nasdaq on October 15, 2024, would increase (decrease) the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $1,216,545, assuming that the number of Shares and/or Pre-Funded Warrants offered by us, as set forth on the cover page of this prospectus, remains the same. Similarly, each increase (decrease) of 100,000 Shares and/or Pre-Funded Warrants offered by us at the assumed public offering price of $4.11 per share, which was the reported closing price of our common stock on Nasdaq on October 15, 2024, would increase (decrease) the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $411,000, assuming that the assumed public offering price of $4.11 per share, which was the reported closing price of our common stock on Nasdaq on October 15, 2024, stays the same.
Pending these uses, we intend to invest the funds in short-term, investment grade, interest-bearing securities. It is possible that, pending their use, we may invest the net proceeds in a way that does not yield a favorable, or any, return for us.
CAPITALIZATION
The following table sets forth our cash and capitalization as of June 30, 2024, as follows:
•
on an actual basis;
•
on a pro forma basis to give effect to (i) the receipt, after June 30, 2024, of the Loan, resulting in net proceeds of $859,980 to the Company; and (ii) an adjustment to reduce existing sales tax liability by $343,000 upon determination that the Company’s products are not taxable in certain states; and
•
on a pro forma, as adjusted, basis to further reflect the issuance and sale of 1,216,545 Shares in this offering at an assumed public offering price of $4.11 per share, which was the reported closing price of our common stock on the Nasdaq on October 15, 2024, resulting in net proceeds to the Company of approximately $4,485,000 after deducting placement agent fees and estimated offering expenses payable by us.
You should read this information in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our condensed consolidated financial statements and related notes appearing in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2024, and our Annual Report on Form 10-K for the fiscal years ended December 31, 2023 and 2022, which are incorporated by reference in this prospectus. All share and per share information below reflects the Reverse Stock Split effected on September 13, 2024, in a ratio of 1-for-20.
| |
|
|
As of June 30, 2024
(Unaudited)
|
|
|
Pro Forma as
Adjusted(1)
|
|
| |
|
|
Actual
|
|
|
Pro Forma
|
|
|
Cash
|
|
|
|
$ |
2,151,243 |
|
|
|
|
$ |
3,011,223(2) |
|
|
|
|
$ |
7,496,223(3) |
|
|
|
Accounts receivable
|
|
|
|
$ |
892,814 |
|
|
|
|
$ |
993,510 |
|
|
|
|
$ |
993,510 |
|
|
|
Accrued expense
|
|
|
|
$ |
1,319,201 |
|
|
|
|
$ |
1,171,240 |
|
|
|
|
$ |
1,171,240 |
|
|
|
Debt
|
|
|
|
$ |
— |
|
|
|
|
$ |
859,980 |
|
|
|
|
$ |
859,980 |
|
|
|
Stockholders’ equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock, par value $0.0001, 200,000,000 shares authorized; 829,527, 829,527 and 2,046,072 shares outstanding on an actual basis, pro forma basis and on a pro forma, adjusted basis, respectively
|
|
|
|
$ |
83 |
|
|
|
|
$ |
83 |
|
|
|
|
$ |
205 |
|
|
|
Additional paid-in capital
|
|
|
|
$ |
70,465,326 |
|
|
|
|
$ |
70,465,326 |
|
|
|
|
$ |
74,950,204 |
|
|
|
Treasury stock, 1,550 shares, at cost
|
|
|
|
$ |
(172) |
|
|
|
|
$ |
(172) |
|
|
|
|
$ |
(172) |
|
|
|
Accumulated deficit
|
|
|
|
$ |
(64,375,953) |
|
|
|
|
$ |
(64,127,296) |
|
|
|
|
$ |
(64,127,296) |
|
|
|
Total stockholders’ equity
|
|
|
|
$ |
6,089,284 |
|
|
|
|
$ |
6,337,941 |
|
|
|
|
$ |
10,822,941 |
|
|
|
Total capitalization
|
|
|
|
$ |
11,901,858 |
|
|
|
|
$ |
12,862,534 |
|
|
|
|
$ |
17,347,534 |
|
|
(1)
Assumes no sale of Pre-Funded Warrants
(2)
Amount includes net cash proceeds from the Loan in the amount of $859,980.
(3)
Reflects net proceeds of $4,485,000 from the offering after payment of placement agency fees and estimated offering expenses of $515,000.
The number of shares of our common stock issued and outstanding on a pro forma basis and a pro forma, as adjusted, basis set forth in the table above is based on 829,527 shares of our common stock outstanding as of June 30, 2024, and excludes:
•
2,775 shares of our common stock issuable upon vesting of restricted stock units outstanding under our stock incentive plans;
•
17,606 shares of our common stock issuable upon exercise of stock options outstanding under our stock incentive plans, 9,858 of which are currently exercisable, which have a weighted average exercise price of $48.22 per share; and
•
5,125 shares of our common stock issuable upon exercise of our outstanding warrants which have a weighted average exercise price of $195.12.
Each $1.00 increase (decrease) in the assumed public offering price of $4.11 per share, which was the reported closing price of our common stock on the Nasdaq on October 15, 2024, would increase (decrease) the as adjusted amount of each of cash and cash equivalents, total equity and total capitalization by $1,216,545, assuming that the number of Shares and/or Pre-Funded Warrants offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of 100,000 shares in the number of Shares and/or Pre-Funded Warrants offered by us at the assumed public offering price of $4.11 per share, which was the reported closing price of our common stock on Nasdaq on October 15, 2024, would increase (decrease) the as adjusted amount of each of cash and cash equivalents, total equity and total capitalization by approximately $411,000 after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
MANAGEMENT
Executive Officers and Directors
The following table sets forth the name and age as of October 18, 2024, and position of the individuals who currently serve as directors and executive officers of the Company. The following also includes certain information regarding the individual experience, qualifications, attributes and skills of our directors and executive officers as well as brief statements of those aspects of our directors’ backgrounds that led us to conclude that they are qualified to serve as directors.
|
Name
|
|
|
Age
|
|
|
Position
|
|
|
Tracy Curley
|
|
|
62
|
|
|
Chief Executive Officer, Chief Financial Officer, Treasurer and Director
|
|
|
John L. Brooks III
|
|
|
73
|
|
|
Director
|
|
|
Katharyn (Katie) Field
|
|
|
41
|
|
|
Chairperson and Director
|
|
|
Avtar Dhaliwal
|
|
|
30
|
|
|
Director
|
|
|
Richard J. Paolone
|
|
|
34
|
|
|
Director
|
|
Tracy Curley has been serving as our Chief Executive Officer, since January 2023, our Chief Financial Officer since August 2020, as Treasurer since July 2021 and director since May 2023. Ms. Curley also served as our Interim Chief Executive Officer from September 2022 to January 2023. Ms. Curley is a Class II director and will serve for a three-year term that expires at our 2027 annual meeting of stockholders, or until the election and qualification of her successor in office, subject to an event or death, resignation, or removal. She was a partner at CohnReznick LLP, a national accounting firm, from September 2017 to June 2020. During her time at CohnReznick, LLP, Ms. Curley led the creation and development of an emerging markets commercial audit practice for the firm in their Boston, MA office. Her practice focused on recruiting and providing audit services to private and public emerging growth companies in the technology and life sciences industries. From November 2014 to August 2017, she also served as a partner at Marcum LLP, a national accounting firm. Ms. Curley led the northeast regional high-tech practice for the firm. She focused on expanding the client base to provide a full range of accounting, tax and advisory services for private and public emerging growth companies in high tech industries such as technology, life sciences and advanced manufacturing. From March 2010 to October 2014, Ms. Curley served as a partner at Moody, Famiglietti & Andronico, LLP (“MFA”), a proactive consulting firm in the greater Boston, MA area with national and global reach. During her time at MFA, Ms. Curley led the creation and development of a public company audit practice focused on recruiting and providing audit services to public emerging growth companies. Ms. Curley serves as the Co-Chair and a board member of Project Green Schools. Ms. Curley serves as Past President and a board member of the North Shore Technology Council. Ms. Curley serves on the audit committee of the Girl Scouts of Eastern Massachusetts. Ms. Curley received her Master of Accountancy and Bachelor of Science in Business Administration with a concentration in accounting from Kansas State University. She also attended the United States Military Academy. She is a certified public accountant licensed in the Commonwealth of Massachusetts. Ms. Curley is well-qualified to serve on the Board due to her extensive experience in operations and finance.
John L. Brooks III has been serving as our director since June 2021. Mr. Brooks serves as a Class II Director and his current term will expire at our 2026 annual meeting of stockholders. He currently serves as a director of Noxilizer since March 2009, Theromics since February 2021, AltrixBio since December 2021, Basys.ai since 2022, Alertgy since 2023, Sharp Tx since 2023, and Senscio since 2024. Mr. Brooks was the President of the NTT division of L-Nutra Inc., a company focused on nutrition and fasting mimicking technologies from March 2021 to May 2022. In January 2011, Mr. Brooks founded Ammonett Pharma and continues to serve on its board of directors since then. He is also a co-founder of Rocky Mountain Biphasic and serves as a director since April 2022. He has also served as the managing director of Healthcare Capital LLC since February 2007. Previously, Mr. Brooks served as the Chief Executive Officer, President and a director of NeuroBo Pharmaceuticals, Inc. from March 2018 to December 2019 and as the chairman of Cellnovo, Ltd. from 2012 to December 2019. Mr. Brooks is also involved with several non-profit organizations. He currently serves as the Chief Executive Officer and President of Worldwide Network for Innovation in Clinical Education and Research (WNICER) since January 2019 and serves as a director of T1D Exchange since March 2020, the ADA New England Chapter since January 2015, The Diabetes Link since January 2010, and the University of Massachusetts Amherst Foundation since January 2012. Mr. Brooks received his BBA and MSBA in
Accounting from the University of Massachusetts Amherst. Mr. Brooks is well-qualified to serve on the Board due to his expertise in healthcare and life sciences.
Katharyn (Katie) Field has been serving as our director since September 2024. Ms. Field has a background which includes positions spanning both the private and public sectors and brings a wealth of experience and expertise in strategy consulting and executive leadership. Ms. Field is currently the chief executive officer and Chairman of Halo Collective Inc., a cannabis company, where she has served since May 2019, an Executive Director at Akanda Corporation, a medical cannabis company, where she has served since June 2022, and a director and Vice President of Virpax Pharmaceuticals, Inc. (Nasdaq: VRPX), a preclinical-stage pharmaceutical company, where she has served as director since July 2024. Previously, she served as a director of Elegance Brands from March 2021 until March 2022. She has held prominent positions at renowned organizations such as The White House in the office of the public liaison, The Brookings Institution as a manager of operations, and Bain & Company as a consultant. In 2014, Ms. Field entered the cannabis industry and played a pivotal role in the procurement, build-out, and sale of one of the original vertically integrated licensed medical marijuana treatment centers in Florida. Subsequently, she operated a strategy consulting practice focused on cannabis and served as Executive Vice President of Corporate Development at MariMed from 2018 to 2019. Ms. Field holds an MBA in Economics from Columbia Business School and a BA in Public Policy with honors from Stanford University. Ms. Field is well-qualified to serve on the Board due to her experience and expertise in strategy consulting and executive leadership.
Avtar Dhaliwal has been serving as our director since September 2024. Mr. Dhaliwal has served as a director and a member of the compensation committee of the board of Halo Collective Inc. since March 2022, which is a cannabis extraction company that develops and manufactures quality cannabis oils and concentrates, and he has been a member of its audit committee of the board in August 2024. Since August 2024, Mr. Dhaliwal has been a director of Advent Technologies Holdings Inc., a US corporation that develops, manufactures, and assembles complete fuel cell systems as well as supplying customers with critical components for fuel cells in the renewable energy sector. Since December 2021, Mr. Dhaliwal has been the Chief Executive Officer of Modern Plant Based Foods, a Canadian food company that offers a portfolio of plant-based products. Previously, Mr. Dhaliwal worked in operations and logistics with Modern Plant Based Foods Inc. beginning in October 2019, and has also been the Chief Executive Officer of Pontus Protein Ltd., an agricultural food and technology company focused on creating and acquiring the best technology, since March 2022. From January 2024 until May 2024, Mr. Dhaliwal was the Chief Executive Officer and a director of Trilogy AI, a company committed to transforming the beauty industry through its artificial intelligence technology. Mr. Dhaliwal holds a Bachelor of Science in Biology from the University of British Columbia Okanagan. Mr. Dhaliwal is well-qualified to serve on the Board due to his management experience across multiple industries.
Richard J. Paolone has been serving as our director since September 2024. Mr. Paolone is a Toronto-based securities lawyer where his work focuses on securities, corporate finance, and mergers and acquisitions. He has a wide range of corporate experience from representing companies in private and public offerings of debt and equity securities. In June 2020, Mr. Paolone founded Paolone Law Professional Corporation, where he has been the principal since such date. From February 2019 to October 2019, and again from September 2020 to January 2021, Mr. Paolone was a director of Evolution Global Frontier Ventures Corp. (formerly Ascension Exploration Inc.), a company that is listed on the Canadian Securities Exchange. Mr. Paolone also serves as Director and CEO of several private and reporting companies. Since February 2019, Mr. Paolone has also been the CEO and director of Rotonda Ventures Corp., a public company in Canada. Since February 2021, Mr. Paolone has also been the CEO, CFO, and director of Republic Goldfields Inc., a public company in Canada. Also, since February 2021, Mr. Paolone has been the CEO, CFO, and director of Emerald Isle Resources Inc., a public company in Canada. Since April 2022, Mr. Paolone has also served as a director of Critical Infrastructure Technologies Ltd., a mining technology company listed on the Canadian Securities Exchange. Since December 2022, Mr. Paolone has also served as a director of SBD Capital Inc., a company listed on the Canadian Securities Exchange. Since June 2023, Mr. Paolone has also served as a director of Xander Resources Inc., a mining company listed on the Canadian Securities Exchange. Since November 2023, he has also served as a director of Ashington Innovations Plc., a special purpose acquisition company listed on London Stock Exchange. Since September 2024, Mr. Paolone has served on the board of Safe Supply Streaming Co Ltd., an investment issuer listed on the Canadian Securities Exchange. Since May 2019, he has served as a director of Red Pine Petroleum Ltd., a company listed on the Toronto Stock Exchange, and also
served as its CEO from October 2020 until September 2021. Mr. Paolone has been integral to multiple mergers and acquisitions and reverse takeover transactions in the industries of mining, cannabis, carbon credits, oil and gas, technology, and plant-based food. Mr. Paolone holds a B.A. in criminal justice from Mount Royal University and a J.D. from Bond University. He is a licensed barrister and solicitor lawyer in Ontario. Mr. Paolone is well-qualified to serve on the Board due to his corporate experiences in mergers and acquisition and private and public offerings of debt and equity securities.
Family Relationships
There are no family relationships among any of our executive officers or directors.
Composition of our Board of Directors
Our Board currently consists of five directors. Our certificate of incorporation, as amended, and bylaws, as amended, provide that our Board can consist of any number of directors as voted on and approved by the Board. Our Board is divided into three classes, designated as Class I, Class II and Class III directors, with only one class of directors being elected in each year and each class serving a three-year term. The term of office of the Class I director, consisting of Mr. Paolone, will expire at our 2025 annual meeting of stockholders. The term of office of the Class II directors, consisting of Mr. Brooks and Mr. Dhaliwal, will expire at our 2026 annual meeting of stockholders. The term of office of the Class III directors, consisting of Ms. Curley and Ms. Fields, will expire at our 2027 annual meeting of stockholders. When considering whether directors have the experience, qualifications, attributes or skills, taken as a whole, to enable our Board to satisfy its oversight responsibilities effectively in light of our business and structure, the Board focuses primarily on each person’s background and experience as reflected in the information discussed in each of the directors’ individual biographies set forth above. We believe that our directors provide an appropriate mix of experience and skills relevant to the size and nature of our business.
Director Independence
As our common stock is listed on the Nasdaq Capital Market, our determination of the independence of directors is made using the definition of “independent director” contained in Nasdaq Listing Rule 5605(a)(2). Our Board has affirmatively determined that each of Mr. Brooks, Mr. Dhaliwal and Ms. Field are “independent directors,” as that term is defined in the Nasdaq rules. Under the Nasdaq rules, our Board must be composed of a majority of “independent directors.” Additionally, subject to certain limited exceptions, our Board’s audit, compensation, and nominating and corporate governance committees also must be composed of all independent directors.
Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act. Under the rules of Nasdaq, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
To be considered to be independent for purposes of Rule 10A-3 of the Exchange Act, a member of an audit committee of a listed company may not, other than in his capacity as a member of our audit committee, our Board, or any other committee of our Board: (1) accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or (2) be an affiliated person of the listed company or any of its subsidiaries.
Role of the Board in Risk Oversight
One of the key functions of our board of directors is informed oversight of our risk management process. Our board of directors does not have a standing risk management committee, but rather administers this oversight function directly through our board of directors as a whole, as well as through various standing committees of our board of directors that address risks inherent in their respective areas of oversight. In particular, our board of directors is responsible for monitoring and assessing strategic risk exposure and our Audit Committee has the responsibility to consider and discuss our major financial risk exposures and the steps our management has taken to monitor and control these exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. Our Audit Committee also monitors compliance with
legal and regulatory requirements. Our Nominating and Corporate Governance Committee monitors the effectiveness of our corporate governance practices, including whether they are successful in preventing illegal or improper liability-creating conduct. Our Compensation Committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking. While each committee is responsible for evaluating certain risks and overseeing the management of such risks, our entire board of directors is regularly informed through committee reports about such risks.
Committees of Our Board of Directors
Our Board directs the management of our business and affairs, as provided by Delaware law, and conducts its business through meetings of the Board and standing committees. We have a standing audit committee, compensation committee, and nominating and corporate governance committee. In addition, from time to time, special committees may be established under the direction of the Board when necessary to address specific issues.
Audit Committee
We have established an audit committee of the Board. Mr. Brooks, Me. Paolone and Ms. Field serve as members of our audit committee, and Mr. Brooks chairs the audit committee. Each member of the audit committee is financially literate, and our Board has determined that Mr. Brooks qualifies as an “audit committee financial expert” as defined in applicable SEC rules and has accounting or related financial management expertise.
We have adopted an audit committee charter that is available to stockholders on the Company’s website at https://investors.ispecimen.com/governance-documents, which details the principal functions of the audit committee, including:
•
reviewing and discussing with management and the independent auditor the annual audited financial statements, and recommending to the Board whether the audited financial statements should be included in our Form 10-K;
•
discussing with management and the independent auditor significant financial reporting issues and judgments made in connection with the preparation of our financial statements;
•
discussing with management major risk assessment and risk management policies;
•
monitoring the independence of the independent auditor;
•
verifying the rotation of the lead (or coordinating) audit partner having primary responsibility for the audit and the audit partner responsible for reviewing the audit as required by law;
•
reviewing and approving all related-party transactions;
•
inquiring and discussing with management our compliance with applicable laws and regulations;
•
pre-approving all audit services and permitted non-audit services to be performed by our independent auditor, including the fees and terms of the services to be performed;
•
appointing or replacing the independent auditor;
•
determining the compensation and oversight of the work of the independent auditor (including resolution of disagreements between management and the independent auditor regarding financial reporting) for the purpose of preparing or issuing an audit report or related work;
•
establishing procedures for the receipt, retention and treatment of complaints received by us regarding accounting, internal accounting controls or reports which raise material issues regarding our financial statements or accounting policies; and
•
approving reimbursement of expenses incurred by our management team in identifying potential target businesses.
The Board reviews the Nasdaq listing standards definition of independence for audit committee members on an annual basis and has determined that all current members of our audit committee are independent (as independence is currently defined in Rule 5605(c)(2)(A)(i) and (ii) of the Nasdaq listing standards).
Compensation Committee
We have established a compensation committee of the Board. Mr. Paolone, Mr. Brooks and Mr. Dhaliwal serve as members of our compensation committee. Mr. Paolone chairs the compensation committee.
We have adopted a compensation committee charter that is available to stockholders on the Company’s website at https://investors.ispecimen.com/governance-documents, which details the principal functions of the compensation committee, including:
•
reviewing and approving on an annual basis the corporate goals and objectives relevant to our Chief Executive Officer’s compensation, evaluating our Chief Executive Officer’s performance in light of such goals and objectives and determining and approving the remuneration (if any) of our Chief Executive Officer based on such evaluation;
•
reviewing and approving the compensation of all our other executive officers;
•
reviewing our executive compensation policies and plans;
•
implementing and administering our incentive compensation equity-based remuneration plans;
•
assisting management in complying with our proxy statement and annual report disclosure requirements;
•
approving all special perquisites, special cash payments and other special compensation and benefit arrangements for our executive officers and employees;
•
if required, producing a report on executive compensation to be included in our annual proxy statement; and
•
reviewing, evaluating and recommending changes, if appropriate, to the remuneration for directors.
The charter also provides that the compensation committee may, in its sole discretion, retain or obtain the advice of a compensation consultant, independent legal counsel or other adviser and will be directly responsible for the appointment, compensation and oversight of the work of any such adviser. However, before engaging or receiving advice from a compensation consultant, external legal counsel or any other adviser, the compensation committee will consider the independence of each such adviser, including the factors required by Nasdaq and the SEC.
Nominating and Corporate Governance Committee
We have established a nominating and corporate governance committee of the Board. Mr. Dhaliwal and Ms. Field serve as members of our nominating and corporate governance committee. Mr. Dhaliwal chairs the nominating and corporate governance committee.
We have adopted a nominating and corporate governance committee charter that is available to stockholders on the Company’s website at https://investors.ispecimen.com/governance-documents, which details the principal functions of the nominating and corporate governance committee, and which provides that persons to be nominated to serve as directors:
•
should have demonstrated notable or significant achievements in business, education or public service;
•
should possess the requisite intelligence, education and experience to make a significant contribution to the Board and bring a range of skills, diverse perspectives and backgrounds to its deliberations; and
•
should have the highest ethical standards, a strong sense of professionalism and intense dedication to serving the interests of the stockholders.
The nominating and corporate governance committee considers several qualifications relating to management and leadership experience, background and integrity and professionalism in evaluating a person’s candidacy for membership on the Board. The nominating committee may require certain skills or attributes, such as financial or accounting experience, to meet specific Board needs that arise from time to time and will also consider the overall experience and makeup of its members to obtain a broad and diverse mix of Board members. The nominating committee does not distinguish among nominees recommended by stockholders and other persons.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee is or has been an officer or employee of our company. None of our executive officers currently serves, or in the past year has served, as a member of the Board’s compensation committee (or other board committee performing equivalent functions) of any entity that has one or more of its executive officers serving on our Board or compensation committee. See the section titled “Item 13. Certain Relationships and Related Transactions, and Director Independence” in our Annual Report on Form 10-K for the fiscal years ended December 31, 2023, which is incorporated by reference in this prospectus for information about related party transactions involving members of our compensation committee or their affiliates.
Code of Conduct and Ethics
We have adopted a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. A copy of the code of business conduct and ethics has been posted on our website, www.ispecimen.com. In addition, we post on our website all disclosures that are required by law or the Nasdaq listing standards concerning any amendments to, or waivers from, any provision of the code. The information on or accessed through our website is deemed not to be incorporated in this Annual Report or to be part of this Annual Report.
Director or Officer Involvement in Certain Prior Legal Proceedings
None of our directors and executive officers were involved in any legal proceedings as described in Item 401(f) of Regulation S-K in the past ten years.
DESCRIPTION OF OUR SECURITIES
General
The following description is not complete and may not contain all the information you should consider before investing in our common stock. For a more detailed description of these securities, you should read the applicable provisions of Delaware law and our Fourth Amended and Restated Certificate of Incorporation, as may be amended from time to time, referred to herein as our certificate of incorporation, and our Second Amended and Restated Bylaws, as may be amended from time to time.
Our authorized capital stock consists of 250,000,000 shares, par value $0.0001 per share, consisting of: 200,000,000 shares of common stock and 50,000,000 shares of blank check preferred stock. Our authorized but unissued shares of common stock and preferred stock are available for issuance without further action by our stockholders unless such action is required by applicable law or the rules of any stock exchange or automated quotation system on which our securities may be listed or traded in the future.
Common Stock
Authorization; Outstanding Shares. We are authorized to issue 200,000,000 shares of common stock, par value $0.0001 per share, of which 829,812 shares were outstanding as of October 15, 2024. In addition, there were 5,125 shares of common stock issuable upon exercise of outstanding warrants, 17,206 shares of common Stock issuable upon exercise of outstanding stock options and 1,921 shares of common stock issuable upon vesting of restricted stock units. We may amend from time to time our certificate to increase the number of authorized shares of common stock. Any such amendment would require the approval of the holders of a majority of the voting power of the shares present in person or represented by proxy at the meeting and entitled to vote.
Voting Rights. Holders of our common stock are entitled to one (1) vote for each share on all matters submitted to a stockholder vote. The common stock does not have cumulative voting rights. Therefore, holders of a majority of the shares of common stock voting for the election of directors can elect all of the directors. Holders of our common stock representing a majority of the voting power of our capital stock issued, outstanding and entitled to vote, represented in person or by proxy, are necessary to constitute a quorum at any meeting of stockholders.
Dividends. Subject to limitations under Delaware law and the rights of holders of any class of stock having preference over our common stock, holders of our common stock are entitled to share in all dividends that our board of directors, in its discretion, declares from legally available funds.
Liquidation; Dissolution. In the event of a liquidation, dissolution or winding up, each outstanding share entitles its holder to participate pro rata in all assets that remain after payment of liabilities and after providing for each class of stock, if any, having preference over the common stock.
Other Rights and Restrictions. Our common stock has no pre-emptive rights, no conversion rights and there are no redemption provisions or sinking fund provisions applicable to the common stock.
Listing. Our common stock is listed on the Nasdaq Capital Market under the symbol “ISPC”.
Transfer Agent and Registrar. The transfer agent and registrar for our common stock is Broadridge Corporate Issuer Solutions LLC. The transfer agent and registrar’s address is 1717 Arch Street, Suite 1300, Philadelphia, PA 19103 and its telephone number is 1-800 353-0103.
Description of Securities Included in this Offering
Pre-Funded Warrants
We are offering to each purchaser whose purchase of shares of common stock in this offering would otherwise result in the purchaser, together with its affiliates, beneficially owning more than 4.99% (or, at the election of the holder, 9.99%) of our outstanding shares of common stock immediately following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, Pre-Funded Warrants, in lieu of shares
of common stock that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the holder, 9.99%) of our outstanding shares of common stock. For each Pre-Funded Warrant we sell (without regard to any limitation on exercise set forth therein), the number of shares of common stock we are offering will be decreased on a one-for-one basis.
We are also registering the shares of common stock issuable from time to time upon exercise of the Pre-Funded Warrants offered hereby.
The following summary of certain terms and provisions of the Pre-Funded Warrants offered hereby is not complete and is subject to, and qualified in its entirety by the provisions of the form of Pre-Funded Warrant which is filed as an exhibit to the Registration Statement. Prospective investors should carefully review the terms and provisions set forth in the form of Pre-Funded Warrant.
Exercisability. The Pre-Funded Warrants are exercisable at any time after their original issuance until they are exercised in full. The Pre-Funded Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and, at any time a registration statement registering the issuance of the shares of common stock underlying the Pre-Funded Warrants, under the Securities Act is effective and available for the issuance of such shares, by payment in full in immediately available funds for the number of shares of common stock purchased upon such exercise. If a registration statement registering the issuance of the shares of common stock underlying the Pre-Funded Warrants under the Securities Act is not effective or available, the holder may, in its sole discretion, elect to exercise the Pre-Funded Warrant through a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of common stock determined according to the formula set forth in the Pre-Funded Warrant. We may be required to pay certain amounts as liquidated damages as specified in the Pre-Funded Warrants in the event we do not deliver shares of common stock upon exercise of any of the Pre-Funded Warrants within the time periods specified in the Pre-Funded Warrants. No fractional shares of common stock will be issued in connection with the exercise of a Pre-Funded Warrant.
Exercise Limitation. A holder will not have the right to exercise any portion of the Pre-Funded Warrants if the holder (together with its affiliates) would beneficially own in excess of 4.99% (or, upon election by a holder prior to the issuance of any warrants, 9.99%) of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-Funded Warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99%, upon at least 61 days’ prior notice from the holder to us with respect to any increase in such percentage.
Exercise Price. The exercise price for the Pre-Funded Warrants is $0.0001 per share. The exercise price and number of shares of common stock issuable on exercise are subject to appropriate adjustments in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock.
Transferability. Subject to applicable laws, the Pre-Funded Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. We do not intend to list the Pre-Funded Warrants on any securities exchange or other trading market. Without an active trading market, the liquidity of the Pre-Funded Warrants will be limited.
Fundamental Transactions. In the event of a fundamental transaction, as described in the Pre-Funded Warrants and generally including, with certain exceptions, any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding shares of common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding shares of common stock, the holders of the Pre-Funded Warrants will be entitled to receive upon exercise of the Pre-Funded Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Pre-Funded Warrants immediately prior to such fundamental transaction.
Rights as a Shareholder. Except as otherwise provided in the Pre-Funded Warrants or by virtue of such holder’s ownership of our shares of common stock, the holder of a Pre-Funded Warrant does not have the
rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the Pre-Funded Warrant. Holders of Pre-Funded Warrants have the right to participate in dividends and certain distributions as specified in the Pre-Funded Warrants.
Governing Law. The Pre-Funded Warrants and warrant agreement are governed by New York law.
Anti-Takeover Effects of Certain Provisions of Our Certificate of Incorporation and Bylaws
Provisions of our bylaws could make it more difficult to acquire us by means of a merger, tender offer, proxy contest, open market purchases, removal of incumbent directors and otherwise. These provisions, which are summarized below, are expected to discourage types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of us to first negotiate with us. We believe that the benefits of increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging takeover or acquisition proposals because negotiation of these proposals could result in an improvement of their terms.
Vacancies. Newly created directorships resulting from any increase in the number of directors and any vacancies on the Board resulting from death, resignation, disqualification, removal or other cause shall be filled by a majority of the remaining directors on the Board.
Bylaws. Our certificate of incorporation and bylaws authorizes the Board to adopt, repeal, rescind, alter or amend our bylaws without stockholder approval.
Removal. Except as otherwise provided, a director may be removed from office only by the affirmative vote of the holders of not less than a majority of the voting power of the issued and outstanding stock entitled to vote.
Calling of Special Meetings of Stockholders. Our bylaws provide that special meetings of stockholders for any purpose or purposes may be called at any time only by the Board or by our Secretary following receipt of one or more written demands from stockholders of record who own, in the aggregate, at least 15% the voting power of our outstanding stock then entitled to vote on the matter or matters to be brought before the proposed special meeting.
Cumulative Voting. Our certificate of incorporation does not provide for cumulative voting in the election of directors, which would allow holders of less than a majority of the stock to elect some directors.
Staggered Board. Our bylaws provided that our Board is divided into three classes with only one class of directors being elected in each year and each class (except for those directors appointed prior to the Annual Meeting) serving a three-year term. As a result, only a minority of the Board will be considered for election at every annual meeting of stockholders, which may make the removal of management more difficult and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our securities.
Choice of Forum
Our bylaws provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have jurisdiction, the federal district court for the District of Delaware) will be the exclusive forum for: (i) any derivative action or proceeding brought on behalf of the Company; (ii) any action asserting a claim for breach of a fiduciary duty owed by any director, officer, employee, or agent of ours to us or our stockholders; (iii) any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, the certificate of incorporation, or the bylaws; and (iv) any action asserting a claim governed by the internal affairs doctrine. The bylaws further provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America shall be the sole and exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. In addition, the bylaws provide that any person or entity purchasing or otherwise acquiring any interest in shares of our common stock is deemed to have notice of and consented to the Delaware Forum Provision and the Federal Forum Provision.
Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. As a result, the Delaware
Forum Provision will not apply to suits brought to enforce any duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. We note, however, that there is uncertainty as to whether a court would enforce this provision and that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder.
We recognize that the Delaware Forum Provision and the Federal Forum Provision in the bylaws may impose additional litigation costs on stockholders in pursuing any such claims, particularly if the stockholders do not reside in or near the State of Delaware. Additionally, the Delaware Forum Provision and the Federal Forum Provision may limit our stockholders’ ability to bring a claim in a forum that they find favorable for disputes with us or our directors, officers or employees, which may discourage such lawsuits against us and our directors, officers and employees even though an action, if successful, might benefit our stockholders. In addition, while the Delaware Supreme Court ruled in March 2020 that federal forum selection provisions purporting to require claims under the Securities Act be brought in federal court were “facially valid” under Delaware law, there is uncertainty as to whether other courts will enforce the Federal Forum Provision. If the Federal Forum Provision is found to be unenforceable, we may incur additional costs associated with resolving such matters. The Federal Forum Provision may also impose additional litigation costs on stockholders who assert that the provision is not enforceable or invalid. The Court of Chancery of the State of Delaware and the United States District Court may also reach different judgments or results than would other courts, including courts where a stockholder considering an action may be located or would otherwise choose to bring the action, and such judgments may be more or less favorable to us than our stockholders.
Indemnification of Directors and Officers
We are incorporated in the State of Delaware. The certificate of incorporation and bylaws provide that, to the fullest extent permitted by Delaware law, as it presently exists or may be amended from time to time, a director shall not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duty as a director. And under Delaware law, this limitation of liability does not extend to, among other things, acts or omissions which involve intentional misconduct, fraud or knowing violation of law, or unlawful payments of dividends. So these provisions may discourage stockholders from bringing suit against a director or officer for breach of fiduciary duty and may reduce the likelihood of derivative litigation brought by stockholders on our behalf against a director or officer.
The certificate of incorporation and bylaws also provide for the indemnification of our directors, officers, employees, and agents, under certain circumstances, against attorney’s fees and other expenses incurred by them in any litigation to which they become a party arising from their association with or activities on behalf of the Company. As such, should our officers and/or directors require us to contribute to their defense, we may be required to spend significant amounts of our capital. This indemnification policy could therefore result in substantial expenditures, which we may be unable to recoup. If these expenditures are significant or involve issues which result in significant liability for our key personnel, we may be unable to continue operating as a going concern.
Furthermore, we intend to enter into indemnification agreements with our directors and executive officers that require us to indemnify them against expenses, judgments, fines, settlements and other amounts that any such person becomes legally obligated to pay (including with respect to a derivative action) in connection with any proceeding, whether actual or threatened, to which such person may be made a party by reason of the fact that such person is or was a director or officer of us or any of our affiliates, provided such person acted in good faith and in a manner such person reasonably believed to be in, or not opposed to, our best interests. We maintain a directors’ and officers’ liability insurance policy. The policy insures directors and officers against unindemnified losses arising from certain wrongful acts in their capacities as directors and officers and reimburses us for those losses for which we have lawfully indemnified the directors and officers. The policy contains various exclusions.
Listing
Our common stock is listed on Nasdaq under the symbol “ISPC”.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Broadridge Corporate Issuer Solutions LLC. .
PLAN OF DISTRIBUTION
We are offering up to 1,216,545 shares of common stock, based on an assumed public offering price of $4.11 per share, which was the reported closing price of our common stock on Nasdaq on October 15, 2024, for gross proceeds of up to approximately $5,000,000 before deduction of placement agent commissions and offering expenses, in a best-efforts offering. There is no minimum amount of proceeds that is a condition to closing of this offering. The actual amount of gross proceeds, if any, in this offering could vary substantially from the gross proceeds from the sale of the maximum amount of securities being offered in this prospectus.
Pursuant to a placement agency agreement (the “Placement Agency Agreement”) to be signed by and between the Company and WestPark Capital Inc. to solicit offers to purchase the securities offered by this prospectus, WestPark will act as our exclusive placement agent. The Placement Agent is not purchasing or selling any securities, nor is it required to arrange for the purchase and sale of any specific number or dollar amount of securities, other than to use its “reasonable best efforts” to arrange for the sale of the securities by us. Therefore, we may not sell the entire amount of securities being offered. There is no minimum amount of proceeds that is a condition to closing of this offering. We will enter into a securities purchase agreement directly with the investors, at the investor’s option, who purchase our securities in this offering. Investors who do not enter into a securities purchase agreement shall provide verbal orders and rely solely on this prospectus in connection with the purchase of our securities in this offering. The Placement Agent may engage one or more subagents or selected dealers in connection with this offering.
The Placement Agency Agreement provides that the Placement Agent’s obligations are subject to conditions contained in the Placement Agency Agreement.
We will deliver the securities being issued to the investors upon receipt of investor funds for the purchase of the securities offered pursuant to this prospectus. We expect that investors in this offering may enter into an agreement, substantially in the form of the securities purchase agreement attached hereto as Exhibit 10.46 (the “Form of Securities Purchase Agreement”), with the Company to purchase shares of common stock or Pre-Funded Warrants, or a combination of both securities, to participate in the offering. We expect to deliver the securities being offered pursuant to this prospectus on or about , 2024. The Form of Securities Purchase Agreement is incorporated herein by reference.
Placement Agent Fees, Commissions and Expenses
Upon the closing of this offering, we will pay the Placement Agent a cash transaction fee equal to 4% of the aggregate gross cash proceeds to us from the sale of the securities in the offering. Pursuant to the Placement Agency Agreement, we will agree to pay WestPark a maximum of $15,000 for reasonable out-of-pocket accountable expenses, including “road show”, diligence, escrow agent or clearing agent fees and reasonable fees and expenses of the Placement Agent’s counsel, in an amount up to $100,000. The Placement Agency Agreement, however, will provide that in the event this offering is terminated, the Placement Agent will only be entitled to the reimbursement of out-of-pocket accountable expenses actually incurred in accordance with Financial Industry Regulatory Authority, Inc. (“FINRA”) Rule 5110(g).
The following table shows the public offering price, Placement Agent fees and proceeds, before expenses, to us.
| |
|
|
Per Share
|
|
|
Per
Pre-Funded
Warrant
|
|
|
Total
|
|
|
Public offering price
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
|
|
Placement agent fees (4%)
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
|
|
Proceeds, before expenses, to us
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
|
We estimate that the total expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the Placement Agent commissions, will be approximately $200,000, all of which are payable by us. This figure does not include, among other things, the Placement Agent’s fees and expenses (including the legal fees, costs and expenses for the Placement Agent’s legal counsel) up to $315,000.
Tail Financing
Subject to certain exceptions, the Placement Agent shall be entitled to a cash fee equal to four percent (4%) of the gross proceeds received by the Company with respect to any public or private offering or other financing or capital-raising transaction of any kind for a period of twelve months from the closing of this offering, to the extent that such financing or capital is provided to the Company by an investor whom the Placement Agent introduced to the Company and conducted discussions with respect to the Offering or with respect to the Loan that closed on September 25, 2024.
Lock-Up
The Company, on behalf of itself and any successor entity, will agree that, without the prior written consent of the Placement Agent, it will not, for a period of ninety (90) days after the date of the Placement Agency Agreement, other than certain exempt issuances, (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of capital stock of the Company or any securities convertible into or exercisable or exchangeable for shares of capital stock of the Company; (ii) file or caused to be filed any registration statement with the Commission relating to the offering of any shares of capital stock of the Company or any securities convertible into or exercisable or exchangeable for shares of capital stock of the Company; (iii) complete any offering of debt securities of the Company, other than entering into a line of credit with a traditional bank, or (iv) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of capital stock of the Company, whether any such transaction described in clause (i), (ii), (iii) or (iv) above is to be settled by delivery of shares of capital stock of the Company or such other securities, in cash or otherwise.
The directors and executive officers of the Company will not until the date that is ninety (90) days after the date of this prospectus, subject to certain customary exceptions, directly or indirectly, (a) offer, sell, agree to offer or sell, solicit offers to purchase, grant any call option or purchase any put option with respect to, pledge, encumber, assign, borrow or otherwise dispose of (each a “Transfer”) any shares of common stock, any unit, any warrant to purchase shares of common stock or any other security of the Company or any other entity that is convertible into, or exercisable or exchangeable for, common stock or any other equity security of the Company (each a “Relevant Security”), or (b) establish or increase any “put equivalent position” or liquidate or decrease any “call equivalent position” with respect to any Relevant Security (in each case within the meaning of Section 16 of the Exchange Act, and the rules and regulations thereunder) with respect to any Relevant Security or otherwise enter into any swap, derivative or other transaction or arrangement that Transfers to another, in whole or in part, any economic consequence of ownership of a Relevant Security, whether or not such transaction is to be settled by the delivery of Relevant Securities, other securities, cash or other consideration, with respect to the undersigned’s holdings, or otherwise publicly disclose the intention to do so.
The foregoing description of the compensation arrangements between the Company and the Placement Agent are set forth in the Placement Agency Agreement, and is qualified in its entirety by reference to the form of Placement Agency Agreement, a copy of which is attached hereto as Exhibit 1.1 and which is incorporated herein by reference.
Indemnification
We have agreed to indemnify the Placement Agent against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the Placement Agent may be required to make for these liabilities.
Regulation M
The Placement Agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit realized on the resale of the securities sold by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. As an underwriter, the Placement Agent would be required to comply with the requirements of the Securities Act and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of our securities by the Placement Agent acting as principal. Under these rules and regulations, the Placement Agent (i) may not engage in any stabilization activity in connection with our securities and (ii) may not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until it has completed its participation in the distribution.
Determination of Offering Price
The actual offering price of the Shares we are offering were negotiated between us, the Placement Agent and the investors in the offering based on the trading of our shares of common stock prior to the offering, among other things. Other factors considered in determining the public offering price of the Shares we are offering, include our history and prospects, the stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment of our management, the general conditions of the securities markets at the time of the offering and such other factors as were deemed relevant.
Electronic Distribution
A prospectus in electronic format may be made available on a website maintained by the Placement Agent. In connection with the offering, the Placement Agent or selected dealers may distribute prospectuses electronically. No forms of electronic prospectus other than prospectuses that are printable as Adobe® PDF will be used in connection with this offering.
Other than the prospectus in electronic format, the information on the Placement Agent’s website and any information contained in any other website maintained by the Placement Agent is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the Placement Agent in its capacity as Placement Agent and should not be relied upon by investors.
Other Activities and Certain Relationships
The Placement Agent and its affiliates have and may in the future provide, from time to time, investment banking and financial advisory services to us in the ordinary course of business, for which they may receive customary fees and commissions. The Placement Agent served as the placement agent in connection with a $1,000,000 loan transaction the Company entered into in September 2024. The Placement Agent was paid a placement agent fee in the amount of $40,000 for its services.
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES
The following is a summary of the material U.S. federal income tax considerations arising from and relating to the acquisition, ownership and disposition of the Shares, the ownership, exercise and disposition of Pre-Funded Warrants acquired pursuant to this prospectus and the shares of common stock received upon the exercise of the Pre-Funded Warrants. The Shares and Pre-Funded Warrants may be referred to in this summary as the “securities.”
This discussion is limited to certain U.S. federal income tax considerations to beneficial owners of our securities who are initial purchasers of our Shares and Pre-Funded Warrants pursuant to this offering and hold our securities as capital assets within the meaning of Section 1221(a) of the U.S. Internal Revenue Code of 1986, as amended (the “Code”) (generally, property held for investment). This discussion assumes that any distributions made (or deemed made) by us on the Shares and any consideration received (or deemed received) by a holder in consideration for the sale or other disposition of our securities will be in U.S. dollars. This discussion is a summary only and does not consider all aspects of U.S. federal income taxation that may be relevant to the acquisition, ownership and disposition of our securities by a prospective investor in light of its particular circumstances or that is subject to special rules under the U.S. federal income tax laws, including, but not limited to:
•
banks and other financial institutions or financial services entities;
•
broker-dealers;
•
mutual funds;
•
retirement plans, individual retirement accounts or other tax-deferred accounts;
•
taxpayers that are subject to the mark-to-market tax accounting rules;
•
tax-exempt entities;
•
S-corporations, partnerships or other flow-through entities and investors therein;
•
governments or agencies or instrumentalities thereof;
•
insurance companies;
•
regulated investment companies;
•
real estate investment trusts;
•
passive foreign investment companies;
•
controlled foreign corporations;
•
qualified foreign pension funds;
•
expatriates or former long-term residents of the United States;
•
persons that actually or constructively own five percent or more of our voting shares;
•
persons that acquired our securities pursuant to an exercise of employee share options, in connection with employee share incentive plans or otherwise as compensation or in connection with services;
•
persons required for U.S. federal income tax purposes to conform the timing of income accruals to their financial statements under Section 451 of the Code;
•
persons subject to the alternative minimum tax;
•
persons that hold our securities as part of a straddle, constructive sale, hedging, conversion or other integrated or similar transaction; or
•
U.S. Holders (as defined below) whose functional currency is not the U.S. dollar.
The discussion below is based upon current provisions of the Code, applicable U.S. Treasury regulations promulgated under the Code (“Treasury Regulations”), judicial decisions and administrative rulings of the Internal Revenue Service (“IRS”), all as in effect on the date hereof, and all of which are subject to differing
interpretations or change, possibly on a retroactive basis. Any such differing interpretations or change could alter the U.S. federal income tax consequences discussed below. Furthermore, this discussion does not address any aspect of U.S. federal non-income tax laws, such as gift, estate or Medicare contribution tax laws, or state, local or non-U.S. tax laws.
We have not sought, and will not seek, a ruling from the IRS as to any U.S. federal income tax consequence described herein. The IRS may disagree with the discussion herein, and its determination may be upheld by a court. Moreover, there can be no assurance that future legislation, regulations, administrative rulings or court decisions will not adversely affect the accuracy of the statements in this discussion.
As used herein, the term “U.S. Holder” means a beneficial owner of our securities that is for U.S. federal income tax purposes: (i) an individual who is a citizen or resident of the United States, (ii) a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) that is created or organized (or treated as created or organized) in or under the laws of the United States, any state thereof or the District of Columbia, (iii) an estate the income of which is subject to U.S. federal income taxation regardless of its source or (iv) a trust if (A) a court within the United States is able to exercise primary supervision over the administration of the trust and one or more United States persons have the authority to control all substantial decisions of the trust, or (B) it has in effect a valid election under Treasury Regulations to be treated as a United States person.
This discussion does not consider the tax treatment of partnerships or other pass-through entities (including branches) or persons who hold our securities through such entities. If a partnership (or other entity or arrangement classified as a partnership for U.S. federal income tax purposes) is the beneficial owner of our securities, the U.S. federal income tax treatment of a partner in the partnership generally will depend on the status of the partner and the activities of the partner and the partnership. If you are a partner or a partnership holding our securities, we urge you to consult your own tax advisor.
THIS DISCUSSION IS ONLY A SUMMARY OF CERTAIN U.S. FEDERAL INCOME TAX CONSIDERATIONS ASSOCIATED WITH THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR SECURITIES. EACH PROSPECTIVE INVESTOR IN OUR SECURITIES IS URGED TO CONSULT ITS OWN TAX ADVISOR WITH RESPECT TO THE PARTICULAR TAX CONSEQUENCES TO SUCH INVESTOR OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR SECURITIES, INCLUDING THE APPLICABILITY AND EFFECT OF ANY STATE, LOCAL, AND NON-UNITED STATES TAX LAWS.
Treatment of Pre-Funded Warrants
Although it is not entirely free from doubt, applicable authority indicates that, and we intend to take the position that, the Pre-Funded Warrants should be treated as a separate class of our shares of common stock for U.S. federal income tax purposes and a U.S. Holder of Pre-Funded Warrants should generally be taxed in the same manner as a holder of shares of common stock except as described below. Accordingly, no gain or loss should be recognized upon the exercise of a Pre-Funded Warrant and, upon exercise, the holding period of the shares of common stock received upon exercise of the Pre-Funded Warrant should include the holding period of the Pre-Funded Warrant. The tax basis of the Pre-Funded Warrant should carry over to the shares of common stock received upon exercise, increased by the exercise price of $0.0001 per share. However, such characterization is not binding on the IRS, and the IRS may treat the Pre-Funded Warrants as warrants to acquire shares of common stock. If so, the amount and character of a U.S. Holder’s gain with respect to an investment in Pre-Funded Warrants could change. Accordingly, each U.S. Holder should consult its own tax advisor regarding the risks associated with the acquisition of a Pre-Funded Warrant pursuant to this prospectus (including potential alternative characterizations). The balance of this discussion generally assumes that the characterization described above is respected for U.S. federal income tax purposes.
U.S. Holders
Taxation of Distributions
If we pay distributions in cash or other property (other than certain distributions of our stock or rights to acquire our stock) to U.S. Holders of our shares of common stock, such distributions will constitute dividends
for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Distributions in excess of current and accumulated earnings and profits will constitute a return of capital that will be applied against and reduce (but not below zero) the U.S. Holder’s adjusted tax basis in our shares of common stock. Any remaining excess will be treated as gain realized on the sale or other disposition of the shares of our common stock and will be treated as described under “U.S. Holders — Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Our Shares and Pre-Funded Warrants” below.
Dividends we pay to a corporate U.S. Holder generally will qualify for the dividends received deduction if certain holding period requirements are met. With certain exceptions (including, but not limited to, dividends treated as investment income for purposes of investment interest deduction limitations), and provided certain holding period requirements are met, dividends we pay to a non-corporate U.S. Holder will generally be taxed as qualified dividend income at the preferential tax rate for long-term capital gains.
Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Our Shares and Pre-Funded Warrants
A U.S. Holder generally will recognize capital gain or loss on a sale or other taxable disposition of the Shares or Pre-Funded Warrants. Any such capital gain or loss generally will be long-term capital gain or loss if the U.S. Holder’s holding period for such Shares or Pre-Funded Warrants exceeds one year. Long-term capital gains recognized by a non-corporate U.S. holder are currently eligible to be taxed preferential rates. The deductibility of capital losses is subject to limitations.
The amount of gain or loss recognized on a sale or other taxable disposition generally will be equal to the difference between (i) the sum of the amount of cash and the fair market value of any property received in such disposition and (ii) the U.S. Holder’s adjusted tax basis in our shares of common stock so disposed of. A U.S. Holder’s adjusted tax basis in the Shares generally will equal the U.S. Holder’s acquisition cost reduced by any prior distributions treated as a return of capital.
Non-U.S. Holders
This section applies to “Non-U.S. Holders.” As used herein, the term “Non-U.S. Holder” means a beneficial owner of our shares of common stock that is not a U.S. Holder and is not a partnership or other entity classified as a partnership for U.S. federal income tax purposes, but such term generally does not include an individual who is present in the United States for 183 days or more in the taxable year of disposition. If you are such an individual, you should consult your tax advisor regarding the U.S. federal income tax consequences of the acquisition, ownership or sale or other disposition of our securities.
Taxation of Distributions
In general, any distributions we make to a Non-U.S. Holder of shares of our common stock, to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles), will constitute dividends for U.S. federal income tax purposes. Provided such dividends are not effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (or, if required pursuant to an applicable income tax treaty, are not attributable to a permanent establishment of fixed base maintained by the Non-U.S. Holder in the United States), we will be required to withhold tax from the gross amount of the dividend at a rate of 30%, unless such Non-U.S. Holder is eligible for a reduced rate of withholding tax under an applicable income tax treaty and provides proper certification of its eligibility for such reduced rate (usually on an IRS Form W-8BEN or W-8BEN-E, as applicable). Any distribution not constituting a dividend will be treated first as reducing (but not below zero) the Non-U.S. Holder’s adjusted tax basis in our shares of common stock and, to the extent such distribution exceeds the Non-U.S. Holder’s adjusted tax basis, as gain realized from the sale or other disposition of our shares of common stock, which will be treated as described under “Non-U.S. Holders — Gain on Sale, Taxable Exchange or Other Taxable Disposition of Our Shares and Pre-Funded Warrants” below. In addition, if we determine that we are or are likely to be classified as a “United States real property holding corporation” (see “Non-U.S. Holders — Gain on Sale, Taxable Exchange or Other Taxable Disposition of Our Shares and Pre-Funded Warrants” below), we will withhold 15% of any distribution that exceeds our current and accumulated earnings and profits, including a distribution in redemption of our shares of common stock.
Dividends that we pay to a Non-U.S. Holder that are effectively connected with such Non-U.S. Holder’s conduct of a trade or business within the United States (and, if a tax treaty applies, are attributable to a permanent establishment or fixed base maintained by the Non-U.S. Holder in the United States) will not be subject to U.S. withholding tax, provided such Non-U.S. Holder complies with certain certification and disclosure requirements (usually by providing an IRS Form W-8ECI). Instead, the effectively connected dividends will be subject to regular U.S. federal income tax as if the Non-U.S. Holder were a U.S. resident, unless an applicable income tax treaty provides otherwise. A Non-U.S. Holder that is a foreign corporation receiving effectively connected dividends may also be subject to an additional “branch profits tax” imposed at a rate of 30% (or a lower treaty rate).
Gain on Sale, Taxable Exchange or Other Taxable Disposition of Our Shares and Pre-Funded Warrants
Subject to the discussion of FATCA and backup withholding below, a Non-U.S. Holder generally will not be subject to U.S. federal income or withholding tax in respect of gain recognized on a sale, taxable exchange or other taxable disposition of our Shares or Pre-Funded Warrants, unless:
•
the gain is effectively connected with the conduct of a trade or business by the Non-U.S. Holder within the United States (and, under certain income tax treaties, is attributable to a permanent establishment or fixed base maintained by the Non-U.S. Holder in the United States); or
•
we are or have been a “United States real property holding corporation” for U.S. federal income tax purposes at any time during the shorter of the five-year period ending on the date of disposition or the period that the Non-U.S. Holder held our shares of common stock, and, in the case where shares of our common stock are regularly traded on an established securities market, the Non-U.S. Holder has owned, directly or constructively, more than 5% of our shares of common stock at any time within the shorter of the five-year period preceding the disposition or such Non-U.S. Holder’s holding period for our shares of common stock. There can be no assurance that our shares of common stock will be treated as regularly traded on an established securities market for this purpose.
Unless an applicable treaty provides otherwise, gain described in the first bullet point above will generally be subject to tax at the applicable U.S. federal income tax rates as if the Non-U.S. Holder were a U.S. resident. Any gains described in the first bullet point above of a Non-U.S. Holder that is a foreign corporation may also be subject to an additional “branch profits tax” at a 30% rate (or lower treaty rate).
If the second bullet point above applies to a Non-U.S. Holder, gain recognized by such holder on the sale, exchange or other disposition of our Shares or Pre-Funded Warrants will generally be subject to tax at applicable U.S. federal income tax rates as if the Non-U.S. Holder were a U.S. resident. In addition, a buyer of our Shares or Pre-Funded Warrants from such holder may be required to withhold U.S. federal income tax at a rate of 15% of the amount realized upon such disposition. We cannot determine whether we will be a United States real property holding corporation in the future. In general, we would be classified as a United States real property holding corporation if the fair market value of our “United States real property interests” equals or exceeds 50% of the sum of the fair market value of our worldwide real property interests plus our other assets used or held for use in a trade or business, as determined for U.S. federal income tax purposes.
Information Reporting and Backup Withholding
Dividend payments with respect to our shares of common stock and proceeds from the sale, exchange or redemption of shares of our common stock or Pre-Funded Warrants may be subject to information reporting to the IRS and possible United States backup withholding. Backup withholding will not apply, however, to payments made to a U.S. Holder who furnishes a correct taxpayer identification number and makes other required certifications, or who is otherwise exempt from backup withholding and establishes such exempt status. Payments made to a Non-U.S. Holder generally will not be subject to backup withholding if the Non-U.S. Holder provides certification of its foreign status, under penalties of perjury, on a duly executed applicable IRS Form W-8 or by otherwise establishing an exemption.
Backup withholding is not an additional tax. Amounts withheld under the backup withholding rules may be credited against a holder’s U.S. federal income tax liability, and a holder generally may obtain a refund of any excess amounts withheld by timely filing the appropriate claim for refund with the IRS and furnishing any
required information. All holders should consult their tax advisors regarding the application of information reporting and backup withholding to them.
FATCA Withholding Taxes
Sections 1471 through 1474 of the Code and the Treasury Regulations and administrative guidance promulgated thereunder (commonly referred to as the “Foreign Account Tax Compliance Act” or “FATCA”) generally impose withholding of 30% in certain circumstances on payments of dividends and, subject to the proposed Treasury Regulations discussed below, on proceeds from sales or other disposition of our securities paid to “foreign financial institutions” (which is broadly defined for this purpose and includes investment vehicles) and certain other non-U.S. entities unless various U.S. information reporting and due diligence requirements (relating to ownership by U.S. persons of interests in or accounts with those entities) have been satisfied or an exemption applies (typically certified as to by the delivery of a properly completed IRS Form W-8BEN-E). If FATCA withholding is imposed, a beneficial owner that is not a foreign financial institution will be entitled to a refund of any amounts withheld by filing a U.S. federal income tax return (which may entail significant administrative burden). Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules. Similarly, dividends and, subject to the proposed Treasury Regulations discussed below, proceeds from sales or other disposition in respect of our securities held by an investor that is a non-financial non-U.S. entity that does not qualify under certain exceptions generally will be subject to withholding at a rate of 30%, unless such entity either (i) certifies to us or the applicable withholding agent that such entity does not have any “substantial United States owners” or (ii) provides certain information regarding the entity’s “substantial United States owners,” which will in turn be provided to the U.S. Department of the Treasury. The U.S. Department of the Treasury has proposed regulations which eliminate the federal withholding tax of 30% applicable to the gross proceeds of a sale or other disposition of our securities. Withholding agents may rely on the proposed Treasury Regulations until final regulations are issued. Prospective investors should consult their tax advisors regarding the possible effects of FATCA on their investment in our securities.
THE U.S. FEDERAL INCOME TAX DISCUSSION SET FORTH ABOVE IS INCLUDED FOR GENERAL INFORMATION ONLY AND MAY NOT BE APPLICABLE DEPENDING UPON A HOLDER’S PARTICULAR SITUATION. HOLDERS ARE URGED TO CONSULT THEIR OWN TAX ADVISORS WITH RESPECT TO THE TAX CONSEQUENCES TO THEM OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR SECURITIES, INCLUDING THE TAX CONSEQUENCES UNDER STATE, LOCAL, ESTATE, NON-U.S. AND OTHER TAX LAWS AND TAX TREATIES AND THE POSSIBLE EFFECTS OF CHANGES IN U.S. OR OTHER TAX LAWS.
LEGAL MATTERS
The validity of the securities offered hereby will be passed upon for us by Sichenzia Ross Ference Carmel LLP, New York, New York. The placement agent is being represented by Lucosky Brookman LLP, Woodbridge, New Jersey.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to incorporate by reference into this prospectus certain information we file with it, which means that we can disclose important information by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Because we are incorporating by reference future filings with the SEC, this prospectus is continually updated and those future filings may modify or supersede some of the information included or incorporated in this prospectus. We incorporate by reference the documents listed below and all documents subsequently filed with the SEC (excluding any portions of any Form 8-K that are not deemed “filed” pursuant to the General Instructions of Form 8-K) pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act, after the date of this prospectus and prior to the date this offering is terminated or we issue all of the securities under this prospectus:
•
•
our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2024 and June 30, 2024, filed with the SEC on May 7, 2024 and August 6, 2024, respectively;
•
our Current Reports on Form 8-K filed with the SEC on February 15, 2024, March 5, 2024, March 26, 2024, April 11, 2024, May 17, 2024, July 3, 2024, July 8, 2024, July 22, 2024, September 11, 2024 and September 25, 2024 and October 2, 2024;
•
•
the description of our common stock contained in the registration statement on Form 8-A, filed with the SEC on June 14, 2021, and any amendment or report filed for the purpose of updating such description (including Exhibit 4.3 to the our Current Report on Form 10-K filed with the SEC on March 22, 2022).
To obtain copies of these filings, see “Where You Can Find More Information” in this prospectus. Nothing in this prospectus shall be deemed to incorporate information furnished, but not filed, with the SEC, including pursuant to Item 2.02 or Item 7.01 of Form 8-K and any corresponding information or exhibit furnished under Item 9.01 of Form 8-K.
Information in this prospectus supersedes related information in the documents listed above and information in subsequently filed documents supersedes related information in both this prospectus and the incorporated documents.
EXPERTS
The financial statements of the Company, as of and for the years ended December 31, 2023 and 2022 incorporate by reference in this prospectus have been audited by Wolf & Company, P.C., independent registered public accounting firm, as set forth in their report thereon (which contains an explanatory paragraph relating to substantial doubt about the ability of the Company to continue as a going concern as described in Note 1 to the financial statements), appearing elsewhere in this prospectus, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and other reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at http://www.sec.gov. Our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K, including any amendments to those reports, and other information that we file with or furnish to the SEC pursuant to Section 13(a) or 15(d) of the Exchange Act can also be accessed free of charge through the Internet. These filings will be available as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. You may also access these filings through our website at www.sintx.com.
We have filed with the SEC a registration statement under the Securities Act relating to the offering of these securities. The registration statement, including the attached exhibits, contains additional relevant information about us and the securities. This prospectus does not contain all of the information set forth in the registration statement. You can obtain a copy of the registration statement, at prescribed rates, from the SEC at the address listed above. The registration statement, along with our most recent annual report on Form 10-K, subsequent reports on Form 10-Q and current reports on Form 8-K, as well as other filings that we make with the SEC, are also available on our Internet website, www.sintx.com. We have not incorporated by reference into this prospectus the information on our website, and you should not consider it to be a part of this prospectus.
UP TO [ ] SHARES OF COMMON STOCK
[ ] PRE-FUNDED WARRANTS TO PURCHASE UP TO [ ] SHARES OF COMMON STOCK
UP TO [ ] SHARES OF COMMON STOCK ISSUABLE UPON THE EXERCISE OF THE PRE-FUNDED WARRANTS
iSpecimen Inc.
PROSPECTUS
WestPark Capital, Inc.
, 2024
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following table sets forth the expenses in connection with this registration statement. All of such expenses are estimates, other than the filing fees payable to the Securities and Exchange Commission and to FINRA.
|
Description
|
|
|
Amount
to be Paid
|
|
|
Filing Fee – Securities and Exchange Commission
|
|
|
|
$ |
765.50 |
|
|
|
Legal fees and expenses
|
|
|
|
|
75,000.00 |
|
|
|
Filing Fee – FINRA
|
|
|
|
|
1,250.00 |
|
|
|
Accounting fees and expenses
|
|
|
|
|
75,000.00 |
|
|
|
Transfer agent’s and registrar fees and expenses
|
|
|
|
|
5,000.00 |
|
|
|
Printing fees and expenses
|
|
|
|
|
12,000.00 |
|
|
|
Miscellaneous fees and expenses
|
|
|
|
|
32,234.50 |
|
|
|
Total
|
|
|
|
$ |
201,250.00 |
|
|
Item 14. Indemnification of Directors and Officers.
Section 145 of the Delaware General Corporation Law provides that a corporation may indemnify directors and officers as well as other employees and individuals against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with any threatened, pending or completed actions, suits or proceedings in which such person is made a party by reason of such person being or having been a director, officer, employee or agent of the corporation. Section 145 of the Delaware General Corporation Law also provides that expenses (including attorneys’ fees) incurred by a director or officer in defending an action may be paid by a corporation in advance of the final disposition of an action if the director or officer undertakes to repay the advanced amounts if it is determined such person is not entitled to be indemnified by the corporation. The Delaware General Corporation Law provides that Section 145 is not exclusive of other rights to which those seeking indemnification may be entitled under any bylaw, agreement, vote of stockholders or disinterested directors or otherwise. Our second amended and restated bylaws provide that, to the fullest extent permitted by law, we shall indemnify and hold harmless any person who was or is made or is threatened to be made a party or is otherwise involved in any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative by reason of the fact that such person, or the person for whom he is the legally representative, is or was a director or officer of ours, against all liabilities, losses, expenses (including attorney’s fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such proceeding.
Section 102(b)(7) of the Delaware General Corporation Law permits a corporation to provide in its Certificate of Incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except for liability (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) for unlawful payments of dividends or unlawful stock repurchases, redemptions or other distributions, or (iv) for any transaction from which the director derived an improper personal benefit.
Our fourth amended and restated certificate of incorporation provides that we shall, to the maximum extent permitted from time to time under the law of the State of Delaware, indemnify and upon request shall advance expenses to any person who is or was a party or is threatened to be made a party to any threatened, pending or completed action, suit, proceeding or claim, whether civil, criminal, administrative or investigative, by reason of the fact that such person is or was or has agreed to be a director or officer of ours or while a director or officer is or was serving at our request as a director, officer, partner, trustee, employee or agent of any corporation, partnership, joint venture, trust or other enterprise, including service with respect to employee
benefit plans, against expenses (including attorneys’ fees and expenses), judgments, fines, penalties and amounts paid in settlement incurred in connection with the investigation, preparation to defend or defense of such action, suit, proceeding or claim; provided, however, that the foregoing shall not require us to indemnify or advance expenses to any person in connection with any action, suit, proceeding or claim initiated by or on behalf of such person or any counterclaim against us initiated by or on behalf of such person. Such indemnification shall not be exclusive of other indemnification rights arising under any by-law, agreement, vote of directors or stockholders or otherwise and shall inure to the benefit of the heirs and legal representatives of such person. Any person seeking indemnification shall be deemed to have met the standard of conduct required for such indemnification unless the contrary shall be established. Any repeal or modification of our fourth amended and restated certificate of incorporation shall not adversely affect any right or protection of a director or officer of ours with respect to any acts or omissions of such director or officer occurring prior to such repeal or modification.
Our fourth amended and restated certificate of incorporation and second amended and restated bylaws provide we shall, to the fullest extent permitted under the laws of the State of Delaware, as amended and supplemented from time to time, indemnify each person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that such party is or was, or has agreed to become, a director or officer of ours, or is or was serving, or has agreed to serve, at our request, as a director, officer or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, including any employee benefit plan, or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such party or on such party’s behalf in connection with such action, suit or proceeding and any appeal therefrom.
Expenses incurred by such a person in defending a civil or criminal action, suit or proceeding by reason of the fact that such person is or was, or has agreed to become, a director or officer of ours, or is or was serving, or has agreed to serve, at our request, as a director, officer or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, including any employee benefit plan, or by reason of any action alleged to have been taken or omitted in such capacity shall be paid by us in advance of the final disposition of such action, suit or proceeding upon receipt of an undertaking by or on behalf of such person to repay such amount if it shall ultimately be determined that he is not entitled to be indemnified by us as authorized by relevant sections of the Delaware General Corporation Law. Notwithstanding the foregoing, we shall not be required to advance such expenses to a person who is a party to an action, suit or proceeding brought by us and approved by a majority of our Board of Directors that alleges willful misappropriation of corporate assets by such person, disclosure of confidential information in violation of such person’s fiduciary or contractual obligations to us or any other willful and deliberate breach in bad faith of such person’s duty to us or our stockholders.
We shall not indemnify any such person seeking indemnification in connection with a proceeding (or part thereof) initiated by such person unless the initiation thereof was approved by our Board of Directors.
The indemnification rights provided in our fourth amended and restated certificate of incorporation and second amended and restated bylaws shall not be deemed exclusive of any other rights to which those indemnified may be entitled under any by-law, agreement or vote of stockholders or disinterested directors or otherwise, both as to action in their official capacities and as to action in another capacity while holding such office, continue as to such person who has ceased to be a director or officer, and inure to the benefit of the heirs, executors and administrators of such a person.
If the Delaware General Corporation Law is amended to expand further the indemnification permitted to indemnitees, then we shall indemnify such persons to the fullest extent permitted by the Delaware General Corporation Law, as so amended.
We may, to the extent authorized from time to time by our Board of Directors, grant indemnification rights to other employees or agents of ours or other persons serving us and such rights may be equivalent to, or greater or less than, those set forth in our second amended and restated bylaws.
Our obligation to provide indemnification under our second amended and restated bylaws shall be offset to the extent of any other source of indemnification or any otherwise applicable insurance coverage under a policy maintained by us or any other person.
To assure indemnification under our second amended and restated bylaws of all directors, officers, employees or agents who are determined by us or otherwise to be or to have been “fiduciaries” of any employee benefit plan of ours that may exist from time to time, Section 145 of the Delaware General Corporation Law shall, for the purposes of our second amended and restated bylaws, be interpreted as follows: an “other enterprise” shall be deemed to include such an employee benefit plan, including without limitation, any plan of ours that is governed by the Act of Congress entitled “Employee Retirement Income Security Act of 1974,” as amended from time to time; we shall be deemed to have requested a person to serve an employee benefit plan where the performance by such person of his duties to us also imposes duties on, or otherwise involves services by, such person to the plan or participants or beneficiaries of the plan; and excise taxes assessed on a person with respect to an employee benefit plan pursuant to such Act of Congress shall be deemed “fines.”
Our second amended and restated bylaws shall be deemed to be a contract between us and each person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that person is or was, or has agreed to become, a director or officer of ours, or is or was serving, or has agreed to serve, at our request, as a director, officer or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, including any employee benefit plan, or by reason of any action alleged to have been taken or omitted in such capacity, at any time while this bylaw is in effect, and any repeal or modification thereof shall not affect any rights or obligations then existing with respect to any state of facts then or theretofore existing or any action, suit or proceeding theretofore or thereafter brought based in whole or in part upon any such state of facts.
The indemnification provision of our second amended and restated bylaws does not affect directors’ responsibilities under any other laws, such as the federal securities laws or state or federal environmental laws.
We may purchase and maintain insurance on behalf of any person who is or was a director, officer or employee of ours, or is or was serving at our request as a director, officer, employee or agent of another company, partnership, joint venture, trust or other enterprise against liability asserted against him and incurred by him in any such capacity, or arising out of his status as such, whether or not we would have the power to indemnify him against liability under the provisions of this section. We currently maintain such insurance. The right of any person to be indemnified is subject to our right, in lieu of such indemnity, to settle any such claim, action, suit or proceeding at our expense of by the payment of the amount of such settlement and the costs and expenses incurred in connection therewith.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling our Company pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities (other than the payment of expenses incurred or paid by a director, officer or controlling person in a successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered herewith, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to the court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
Item 15. Recent Sales of Unregistered Securities.
All share and price per share information in Part II of this registration statement has been adjusted to reflect the Company’s 1-for-20 reverse stock split of its outstanding common stock, which became effective for the trading of its common stock on September 13, 2024.
On December 1, 2021, the Company completed a private placement (the “December 2021 Private Placement”) in which it sold to certain accredited investors (i) an aggregate of 87,500 shares of common stock and (ii) warrants to purchase up to an aggregate 65,625 shares of common stock (the “Warrants”), at a combined price per share of common stock and .75 of a warrant of $240.00. The shares of common stock and the Warrants were issued pursuant to a Securities Purchase Agreement, dated November 28, 2021, by and among
the Company and the each of the Selling Stockholders, as well as a placement agency agreement, dated November 28, 2021, between the Company and ThinkEquity LLC, the placement agent for such offering.
On February 13, 2024, the Company entered into certain warrant repurchase and termination agreements (the “Repurchase Agreement”) with certain holders (each, the “Warrant Holder”) of Warrants, and the resale of the shares of common stock issuable upon exercise of the Warrants were registered under an effective Registration Statement on Form S-1, as amended (File Number: 333-261640), filed by the Company.
Pursuant to the Repurchase Agreement, the Company agreed to repurchase from the Warrant Holders, who agreed to sell and transfer to the Company, the Warrants for an amount in cash equal to $0.04 multiplied by the total maximum number of shares of common stock issuable to the Warrant Holders upon the full exercise of the Warrants. In addition, upon full payment for the Warrants sold under the Repurchase Agreement, the Warrants and any warrants agreements or any other agreements, documents or instruments relating to the Warrants (including that certain Securities Purchase Agreements, dated November 28, 2021, by and between the Company and the Warrant Holders, that certain Registration Rights Agreements, dated November 28, 2021 by and between the Company and the Warrant Holders) (collectively, the “Warrant Documentation”) would terminate, and all past, current and future obligations of the Company relating to the Warrants and Warrant Documentation would be released, discharged and of no further force or effect.
The sale and the issuance of the foregoing securities were offered and sold in reliance upon exemptions from registration pursuant to Section 4(a)(2) of the Securities Act and Rule 506 of Regulation D promulgated under the Securities Act (“Regulation D”). We made this determination based on the representations of each investor which included, in pertinent part, that each such investor was either (a) an “accredited investor” within the meaning of Rule 501 of Regulation D or (b) a “qualified institutional buyer” within the meaning of Rule 144A under the Securities Act and upon such further representations from each investor that (i) such investor acquired the securities for his, her or its own account for investment and not for the account of any other person and not with a view to or for distribution, assignment or resale in connection with any distribution within the meaning of the Securities Act, (ii) such investor agreed not to sell or otherwise transfer the purchased securities unless they are registered under the Securities Act and any applicable state securities laws, or an exemption or exemptions from such registration are available, (iii) such investor had knowledge and experience in financial and business matters such that he, she or it was capable of evaluating the merits and risks of an investment in us, (iv) such investor had access to all of our documents, records, and books pertaining to the investment and was provided the opportunity to ask questions and receive answers regarding the terms and conditions of the offering and to obtain any additional information which we possessed or were able to acquire without unreasonable effort and expense, and (v) such investor had no need for the liquidity in its investment in us and could afford the complete loss of such investment. In addition, there was no general solicitation or advertising for securities issued in reliance upon these exemptions.
|
Exhibit No.
|
|
|
Description of Exhibit
|
|
|
10.14
|
|
|
|
|
|
10.15
|
|
|
|
|
|
10.16
|
|
|
|
|
|
10.17
|
|
|
|
|
|
10.18
|
|
|
|
|
|
10.19
|
|
|
|
|
|
10.20
|
|
|
|
|
|
10.21
|
|
|
|
|
|
10.22
|
|
|
|
|
|
10.23
|
|
|
|
|
|
10.24
|
|
|
|
|
|
10.25
|
|
|
|
|
|
10.26
|
|
|
|
|
|
10.27
|
|
|
|
|
|
Exhibit No.
|
|
|
Description of Exhibit
|
|
|
10.28
|
|
|
|
|
|
10.29
|
|
|
|
|
|
10.30#
|
|
|
|
|
|
10.31#
|
|
|
|
|
|
10.32#
|
|
|
|
|
|
10.33#
|
|
|
|
|
|
10.34
|
|
|
|
|
|
10.35
|
|
|
|
|
|
10.36#
|
|
|
|
|
|
10.37#
|
|
|
|
|
|
10.38#
|
|
|
|
|
|
10.39#
|
|
|
|
|
|
10.40+#
|
|
|
|
|
|
10.41
|
|
|
|
|
|
10.42+#
|
|
|
|
|
|
10.43
|
|
|
|
|
*
Filed herewith.
+
Schedules and exhibits have been omitted pursuant to Items 601(a)(5) and 601(b)(2) of Regulation S-K. The Company agrees to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon request.
#
Indicates management contract or compensatory plan or arrangement.
Item 17. Undertakings.
Insofar as indemnification for liabilities arising under the Securities Act “may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(a)
The undersigned registrant hereby undertakes:
(1)
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i)
To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii)
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii)
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2)
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3)
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(i)
The undersigned Registrant hereby undertakes that it will:
a.
for determining any liability under the Securities Act of 1933, treat the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant under Rule 424(b)(1), or (4) or 497(h) under the Securities Act of 1933 as part of this registration statement as of the time the SEC declared it effective.
b.
for determining any liability under the Securities Act of 1933, treat each post-effective amendment that contains a form of prospectus as a new registration statement for the securities offered in the registration statement, and that offering of the securities at that time as the initial bona fide offering of those securities.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Woburn, State of Massachusetts, on the 18th day of October 2024.
iSPECIMEN INC.
By:
/s/ Tracy Curley
Tracy Curley
Chief Executive Officer
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose individual signature appears below hereby authorizes and appoints Tracy Curley with full power of substitution and resubstitution and full power to act as his or her true and lawful attorney-in-fact and agent to act in his or her name, place and stead, and to execute in the name and on behalf of each person, individually and in each capacity stated below, and to file any and all amendments to this registration statement, any related registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and any or all pre- or post-effective amendments thereto, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the U.S. Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully for all intents and purposes as he or she might or could do in person, hereby ratifying and confirming that said attorney-in-fact and agents, and each of them, or any substitute or substitutes for each of them, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement on Form S-1 has been signed by the following persons in the capacities set forth opposite their names.
| |
Signature
|
|
|
Title
|
|
|
Date
|
|
| |
/s/ Tracy Curley
Tracy Curley
|
|
|
Chief Executive Officer, Chief Financial Officer, Treasurer and Director (Principal Executive Officer and Principal Financial and Accounting Officer)
|
|
|
October 18, 2024
|
|
| |
/s/ Katharyn Field
Katharyn Field
|
|
|
Chairperson and Director
|
|
|
October 18, 2024
|
|
| |
/s/ John L. Brooks III
John L. Brooks III
|
|
|
Director
|
|
|
October 18, 2024
|
|
| |
/s/ Richard J. Paolone
Richard J. Paolone
|
|
|
Director
|
|
|
October 18, 2024
|
|
| |
/s/ Avtar Dhaliwal
Avtar Dhaliwal
|
|
|
Director
|
|
|
October 18, 2024
|
|
Exhibit 1.1
WestPark Capital, Inc.
1800 Century Park East, Suite 220
Los Angeles, CA 90067
[ ] [ ], 2024
iSpecimen, Inc.
8 Cabot Road
Woburn, MA 01801
Attention: Tracy Curley
Dear Ms. Curley:
Subject to the terms and conditions
of this letter agreement (the “Agreement”), between WestPark Capital, Inc., as lead placement agent (“Placement
Agent”), and iSpecimen, Inc., a company organized under the laws of the state of Nevada (the “Company”),
the parties hereby agree the Placement Agent shall serve as the placement agent for the Company, on a “reasonable best efforts”
basis, in connection with the proposed placement (the “Placement”) of (i) shares (the “Shares”)
of common stock of the Company, par value, $0.0001 per share (the “Common Stock”) and (ii) pre-funded warrants
to purchase Common Stock (the “Pre-Funded Warrants”). The Shares and Pre-Funded Warrants actually sold by the Placement
Agent are referred to herein as the “Securities.” The Securities and shares of Common Stock issuable upon the exercise
of the Pre-Funded Warrants have been registered and shall be offered and sold pursuant to the Company’s registration statement on
Form S-1 (File No. 333-[ ]) (the “Registration Statement”), which was declared effective by the Securities
and Exchange Commission (the “Commission”) on [ ], 2024. The documents executed and delivered by the Company and the
Purchasers (as defined below), as applicable, in connection with the Placement, including, without limitation, a securities purchase agreement
(the “Purchase Agreement”) and Pre-Funded Warrant certificates, shall be collectively referred to herein as the “Transaction
Documents.”
The Placement Agent may retain
other brokers or dealers to act as sub-agents or selected-dealers on its behalf in connection with the Placement.
The terms of the Placement
shall be mutually agreed upon by the Company and the purchasers listed in the Purchase Agreement (each, a “Purchaser”
and collectively, the “Purchasers”), and nothing herein shall imply that the Placement Agent would have the power or
authority to bind the Company or any Purchaser, or shall imply that the Company has an obligation to issue any Securities or complete
the Placement. The Company expressly acknowledges and agrees that the Placement Agent’s obligations hereunder are on a reasonable
best efforts basis only and that the execution of this Agreement does not constitute a commitment by the Placement Agent to purchase the
Securities and does not ensure the successful placement of the Securities or any portion thereof or the success of the Placement Agent
with respect to securing any other financing on behalf of the Company. Certain affiliates of the Placement Agent may participate in the
Placement by purchasing some of the Securities. The sale of Securities to any Purchaser will be evidenced by the Purchase Agreement between
the Company and such Purchaser, in a form reasonably acceptable to the Company and the Purchaser, provided, however, certain Purchasers
may purchase Securities via verbal orders placed with the Placement Agent. Capitalized terms that are not otherwise defined herein have
the meanings given to such terms in the Purchase Agreement. Prior to the signing of any Purchase Agreement, officers of the Company will
be available to answer inquiries from prospective Purchasers.
SECTION 1.
REPRESENTATIONS AND WARRANTIES OF THE COMPANY; COVENANTS OF THE COMPANY.
A. Representations of the
Company. With respect to the Securities, each of the representations and warranties and covenants made by the Company to the Purchasers
in the Purchase Agreement in connection with the Placement, is hereby incorporated herein by reference into this Agreement (as though
fully restated herein) and is, as of the date of this Agreement and as of the date of the closing of the sale of the Securities hereunder
(the “Closing Date”), hereby made to, and in favor of, the Placement Agent. In addition to the foregoing, the Company
represents and warrants that, to its knowledge, there are no affiliations with any Financial Industry Regulatory Authority (“FINRA”)
member firm among the Company’s officers, directors or any holders of five percent (5.0%) or more of the outstanding shares of Common
Stock.
B. Covenants of the Company.
The Company covenants and agrees to continue to retain (i) a firm of Public Company Accounting Oversight Board independent registered
public accountants for a period of at least two (2) years after the Closing Date and (ii) a competent transfer agent with respect
to the Shares for a period of two (2) years after the Closing Date.
The Company agrees that the
Purchase Agreement as in effect on the date hereof may not be amended or waived without the prior written consent of the Placement Agent.
SECTION 2.
REPRESENTATIONS OF THE PLACEMENT AGENT. The Placement Agent represents and warrants that it (i) is a member in good standing
of FINRA, (ii) is registered with the Commission as a broker/dealer under the Securities Exchange Act of 1934, as amended (the “Exchange
Act”), (iii) is licensed as a broker/dealer under the laws of the United States of America, applicable to the offers and
sales of the Securities by the Placement Agent, (iv) is and will be a corporate body validly existing under the laws of its place
of incorporation, and (v) has full power and authority to enter into and perform its obligations under this Agreement. The Placement
Agent will immediately notify the Company in writing of any change in its status with respect to subsections (i) through (v) above.
The Placement Agent covenants that it will use its reasonable best efforts to conduct the Placement hereunder in compliance with the provisions
of this Agreement and the requirements of applicable law.
SECTION 3.
COMPENSATION. In consideration of the services to be provided for hereunder, the Company shall pay to the Placement Agent or its
respective designees on the Closing Date a total cash fee equal to four percent (4.0%) of gross proceeds from the Placement of the total
amount of Securities sold. The Placement Agent reserves the right to reduce any item of compensation or adjust the terms thereof as specified
herein in the event that a determination shall be made by FINRA that the Placement Agent’s aggregate compensation exceeds the amount
allowable under FINRA Rule 5110 or that the terms thereof require adjustment.
SECTION 4.
EXPENSES. The Company agrees to pay all costs, fees and expenses incurred by the Company in connection with the performance of
its obligations hereunder and in connection with the transactions contemplated hereby, including, without limitation: (i) all expenses
incident to the issuance, delivery and qualification of the Securities (including all printing and engraving costs); (ii) all filing
fees and communication expenses associated with the review of the Placement by FINRA; (iii) all fees and expenses of the registrar
and transfer agent of the Shares; (iv) all necessary issue, transfer and other stamp taxes in connection with the issuance and sale
of the Securities; (v) all fees and expenses of the Company’s counsel, independent public or certified public accountants and
other advisors; (vi) all costs and expenses incurred in connection with the preparation, printing, filing, shipping and distribution
of the Registration Statement (including financial statements, exhibits, schedules, consents and certificates of experts), the Prospectus
and all amendments and supplements thereto, and this Agreement; (vii) all filing fees, reasonable attorneys’ fees and expenses
incurred by the Company in connection with qualifying or registering (or obtaining exemptions from the qualification or registration of)
all or any part of the Securities for offer and sale under the state securities or blue sky laws or the securities laws of any other country;
(viii) the fees and expenses associated with including the Shares on the Trading Market; and (ix) all fees and expenses incurred
by the Placement Agent, in connection with the Placement, other than the reasonable fees and expenses of the Placement Agent’s counsel,
in an amount not to exceed $15,000, and with regard to the reasonable fees and expenses of the Placement Agent’s counsel, in an
amount that shall not exceed $100,000 without the Company’s prior written consent. Notwithstanding the foregoing, any advance received
by the Placement Agent will be reimbursed to the Company to the extent not actually incurred in compliance with FINRA Rule 5110(g)(4)(A).
In the event that this Agreement shall not be carried out for any reason whatsoever, within the time specified herein or any extensions
thereof pursuant to the terms herein, the Company shall be obligated to pay to the Placement Agent their actual and accountable out-of-pocket
expenses related to the transactions contemplated herein then due and payable (including the fees and disbursements of the Placement Agent’s
counsel) and upon demand the Company shall pay the full amount thereof to the Placement Agent; provided, however, that such
expense payment in no way limits or impairs the indemnification and contribution provisions of this Agreement.
SECTION 5.
INDEMNIFICATION.
A. To the extent permitted
by law, with respect to the Securities, the Company will indemnify the Placement Agent and its affiliates, stockholders, directors, officers,
employees, members and controlling persons (within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange
Act) (each such entity or person, an “Indemnified Person”) from and against all claims, actions, suits, proceedings
(including those of stockholders), damages, costs and liabilities (collectively, “Claims”), and shall reimburse each
Indemnified Person for all reasonable fees and expenses (including the reasonable fees and expenses of counsel) (collectively, the “Expenses”)
as they are incurred by an Indemnified Person in investigating, preparing, pursuing or defending any Claim that is caused by, arises out
of, or is based upon (i) any untrue statements made or any statements omitted to be made in the Registration Statement, the Preliminary
Prospectus or the Prospectus, or by any omission or alleged omission to state therein a material fact necessary to make the statements
therein, in light of the circumstances under which they were made, not misleading (other than untrue statements or alleged untrue statements
in, or omissions or alleged omissions from, information relating to an Indemnified Person furnished in writing by or on behalf of such
Indemnified Person for use in the Registration Statement, Preliminary Prospectus or any Prospectus) or (ii) any other actions taken
or omitted to be taken by the Company or any Indemnified Person in connection with this Agreement; provided, however, the Company will
not be responsible for any Claims or Expenses of any Indemnified Person that are judicially determined to have resulted primarily from
such Indemnified Person’s (x) willful misconduct, violation of law or gross negligence in connection with any of the action,
inaction or the services described herein, or (y) use of any offering materials or information concerning the Company in connection
with the offer or sale of the Securities in the Placement, which were not authorized for such use by the Company and which use constitutes
gross negligence, violation of law or willful misconduct..
B. Promptly after receipt
by the Placement Agent of notice of any claim or the commencement of any action or proceeding with respect to which the Placement Agent
is entitled to indemnity hereunder, the Placement Agent will promptly notify the Company in writing of such claim or of the commencement
of such action or proceeding, but failure to so notify the Company shall not relieve the Company from any obligation it may have hereunder,
except and only to the extent such failure results in the forfeiture by the Company of substantial rights and defenses. If the Company
so elects or is requested by the Placement Agent, the Company will assume the defense of such action or proceeding and will employ counsel
reasonably satisfactory to the Placement Agent and will pay the fees and expenses of such counsel. Notwithstanding the preceding sentence,
the Placement Agent will be entitled to employ its own counsel separate from counsel for the Company and from any other party in such
action if counsel for the Placement Agent reasonably determines that it would be inappropriate under the applicable rules of professional
responsibility for the same counsel to represent both the Company and the Placement Agent. In such event, the reasonable fees and disbursements
of no more than one such separate counsel will be paid by the Company, in addition to fees of local counsel. The Company will have the
right to settle the claim or proceeding, provided that the Company will not settle any such claim, action or proceeding without the prior
written consent of the Placement Agent.
C. The Company may not settle, compromise or consent
to the entry of any judgment in any pending or threatened Claim, in which indemnification may be sought hereunder (whether or not any
Indemnified Person is an actual or potential party thereto), without the prior written consent of the Placement Agents (which will not
be unreasonably delayed or withheld) unless such settlement, compromise or consent provides for an unconditional and irrevocable release
of each Indemnified Person from any and all liability arising out of such Claim.
D. The Company agrees to notify
the Placement Agent promptly of the assertion against it or any other person of any claim or the commencement of any action or proceeding
relating to a transaction contemplated by this Agreement.
E. If for any reason the foregoing
indemnity is unavailable to the Placement Agents or insufficient to hold the Placement Agents harmless, then the Company shall contribute
to the amount paid or payable by the Placement Agents as a result of such Claim or Expenses in such proportion as is appropriate to reflect
(a) the relative benefits to the Company on the one hand, and the Placement Agents on the other hand, in connection with the Placement,
(b) the relative fault of the parties, and (c) other equitable considerations; provided, however, that in no event shall the
amount to be contributed by the Placement Agents exceed the fees actually received by the Placement Agents under this Agreement. Notwithstanding
the immediately preceding sentence, to the extent the exception to indemnification contemplated by Paragraph A of this Section applies
with respect to the Placement Agent, the Company shall contribute to the amount paid or payable by the Placement Agents as a result of
such Claim or Expenses in such proportion as is appropriate to reflect the relative fault of the Company, on the one hand, and the Placement
Agent, on the other hand, in connection with the matters contemplated by the Agreement; provided, however, that in no event shall the
amount to be contributed by Placement Agents exceed the fees actually received by Placement Agents under the Agreement. The Company agrees
that for the purposes of this paragraph the relative benefits to the Company and the Placement Agents of the contemplated transaction
(whether or not such transaction is consummated) shall be deemed to be in the same proportion that the aggregate cash consideration payable
(or contemplated to be payable) in such transaction bears to the fees paid or payable to the Placement Agents under the Agreement.
F. These indemnification provisions
shall remain in full force and effect whether or not the transaction contemplated by this Agreement is completed and shall survive the
termination of this Agreement, and shall be in addition to any liability that the Company might otherwise have to any indemnified party
under this Agreement or otherwise.
SECTION 6.
LOCK-UP AGREEMENTS.
A. Officer, Director, and
5% Holder Lock-Up Agreements. On the Closing Date, Placement Agent shall have received signed Lock-Up Agreements, in the form of Exhibit A
annexed hereto, addressed to the Placement Agent by each of the Company’s directors, officers, and holders of five percent (5.0%)
or more of the outstanding shares of Common Stock, as of the date of this Agreement.
B. Restriction on Sales
of Capital Stock. The Company, on behalf of itself and any successor entity, agrees that, without the prior written consent of the
Placement Agent, which will not be unreasonably withheld, it will not, for a period beginning on the Closing Date and ending on the date
that is ninety (90) days after the Closing Date, (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase,
purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly
or indirectly, any shares of capital stock of the Company or any securities convertible into or exercisable or exchangeable for shares
of capital stock of the Company; (ii) file or cause to be filed any registration statement with the Commission relating to the offering
of any shares of capital stock of the Company or any securities convertible into or exercisable or exchangeable for shares of capital
stock of the Company; (iii) complete any offering of debt securities of the Company, (iv) enter into any swap or other arrangement
that transfers to another, in whole or in part, any of the economic consequences of ownership of capital stock of the Company, whether
any such transaction described in clause (i), (ii), (iii) or (iv) above is to be settled by delivery of shares of capital stock
of the Company or such other securities, in cash or otherwise, (v) effect or enter into an agreement to effect any issuance by the
Company or any of its subsidiaries of capital stock of the Company or its subsidiaries or any securities convertible into or exercisable
or exchangeable for shares of capital stock of the Company or its subsidiaries involving a Variable Rate Transaction (as defined herein)
or (vi) purchase any shares of its capital stock other than repurchases at cost or without cost pursuant to the terms of the Company’s
stock option and restricted stock purchase agreements. For the purposes of this Agreement, “Variable Rate Transaction”
means a transaction in which the Company or its subsidiaries (i) issues or sells any debt or equity securities that are convertible
into, exchangeable or exercisable for, or include the right to receive, additional shares of capital stock of the Company or its subsidiaries
either (A) at a conversion price, exercise price or exchange rate or other price that is based upon, and/or varies with, the trading
prices of or quotations for the shares of the Company’s capital stock at any time after the initial issuance of such debt or equity
securities or (B) with a conversion, exercise or exchange price that is subject to being reset at some future date after the initial
issuance of such debt or equity security or upon the occurrence of specified or contingent events directly or indirectly related to the
business of the Company or the market for the Company’s capital stock or (ii) enters into, or effects a transaction under,
any agreement, including, but not limited to, an equity line of credit, whereby the Company or its subsidiaries may issue securities at
a future determined price.
The restrictions contained
in this Section 6(B) shall not apply to (i) the Securities, (ii) the issuance of shares of capital stock of the Company
in connection with any warrant, option or convertible security of the Company that is outstanding prior to the date of this Agreement,
and (ii) the adoption of an equity incentive plan approved by the Company’s independent directors, and the grant of awards
or equity pursuant to any such equity incentive plan or existing plan disclosed in the Registration Statement to officers, directors,
employees or consultants of the Company or any of its subsidiaries, and the filing of a registration statement on Form S-8 relating
thereto.
SECTION 7.
ENGAGEMENT TERM. The Placement Agent’s engagement hereunder will be until the earlier of (i) the 90th
day after the date of this Agreement and (ii) the Closing Date (such date, the “Termination Date”). Either party
may terminate this Agreement at any time upon ten (10) days’ written notice to the other party, effective upon receipt of such
notice by the other party. Unless such termination by the Company is for cause (the Placement’s Agent material failure to provide
the services contemplated in this Agreement), the Company will remain responsible for fees pursuant to Section 3 hereof with respect
to the Securities if sold in the Placement. If the Company terminates the engagement hereunder, including for cause, the Company will
remain responsible to reimburse expenses actually incurred and reimbursable pursuant to Section 4 hereof. Notwithstanding anything
to the contrary contained herein, the provisions concerning the Company’s obligation to pay any fees actually earned pursuant to
Section 3 hereof and any reimbursable expenses actually incurred and reimbursable pursuant to Section 4 hereof and the provisions
concerning confidentiality, indemnification and contribution contained herein will survive any expiration or termination of this Agreement.
If this Agreement is terminated prior to the completion of the Placement (other than, with regard to fees set forth in Section 3,
termination by the Company for cause) all fees due to the Placement Agent as set forth in Section 3 and 4 shall be paid by the Company
to the Placement Agent on or before the Termination Date (in the event such fees are earned or owed as of the Termination Date). The Placement
Agent agrees not to use any confidential information concerning the Company provided to the Placement Agent by the Company for any purposes
other than those contemplated under this Agreement.
SECTION 8.
PLACEMENT AGENT INFORMATION. The Company agrees that any information or advice rendered by the Placement Agent in connection with
this engagement is for the confidential use of the Company only in their evaluation of the Placement and, except as otherwise required
by law, the Company will not disclose or otherwise refer to the advice or information in any manner without the Placement Agent’s
prior written consent.
SECTION 9.
NO FIDUCIARY RELATIONSHIP. This Agreement does not create, and shall not be construed as creating rights enforceable by any person
or entity not a party hereto, except those entitled hereto by virtue of the indemnification provisions hereof. The Company acknowledges
and agrees that the Placement Agent is not and shall not be construed as a fiduciary of the Company and shall have no duties or liabilities
to the equity holders or the creditors of the Company or any other person by virtue of this Agreement or the retention of the Placement
Agent hereunder, all of which are hereby expressly waived.
SECTION 10.
CLOSING. The obligations of the Placement Agent, and the closing of the sale of the Securities hereunder are subject to the accuracy,
when made and on the Closing Date, of the representations and warranties on the part of the Company contained herein and in the Purchase
Agreement, to the performance by the Company of its obligations hereunder and under the Purchase Agreement, and to each of the following
additional terms and conditions, except as otherwise disclosed to and acknowledged and waived by the Placement Agent:
A. All corporate proceedings
and other legal matters incident to the authorization, form, execution, delivery and validity of each of this Agreement, the Securities,
and all other legal matters relating to this Agreement and the transactions contemplated hereby with respect to the Securities shall be
reasonably satisfactory in all material respects to the Placement Agent.
B. The Placement Agent shall
not have discovered and disclosed to the Company on or prior to the Closing Date that the Registration Statement or any amendment or supplement
thereto contains an untrue statement of a fact which, in the reasonable opinion of counsel for the Placement Agent, is material or omits
to state any fact which, in the reasonable opinion of such counsel, is material and is required to be stated therein or is necessary to
make the statements therein not misleading and was not remedied prior to the Closing Date by the filing of an amendment to the Registration
Statement.
C. The Placement Agent shall
have received from outside counsel to the Company such counsel’s written opinion dated as of the Closing Date, including without
limitation, a negative assurance letter, in each case in form and substance reasonably satisfactory to the Placement Agent.
D. On the date of the signing
of the Purchase Agreement, the Placement Agent shall have received, and the Company shall have caused to be delivered to the Placement
Agent, a letter from Wolf & Company, P.C. (the independent registered public accounting firm of the Company), addressed to the
Placement Agent, dated as of the date thereof, in form and substance satisfactory to the Placement Agent. The letter shall not disclose
any change in the condition (financial or other), earnings, operations, business or prospects of the Company from that set forth in the
Incorporated Documents or the applicable Prospectus, which, in the Placement Agent's sole judgment, is material and adverse and that makes
it, in the Placement Agent's sole judgment, impracticable or inadvisable to proceed with the offering of the Securities as contemplated
by such Prospectus.
E. On the Closing Date, the
Placement Agent shall have received from Wolf & Company, P.C., a letter dated as of such Closing Date, in form and substance
satisfactory to the Placement Agent, to the effect that they reaffirm the statements made in the letter furnished pursuant to subsection
C of this Section except that the specified date referred to therein for the carrying out of procedures shall be no more than three
business days prior to such Closing Date.
F. The Securities sold in
the Placement and the shares of Common Stock issuable upon the exercise of the Pre-Funded Warrants, shall have been registered under the
Exchange Act. The Company shall have taken no action designed to, or likely to have the effect of, terminating the registration of the
Common Stock under the Exchange Act or delisting or suspending from trading the Common Stock from the Trading Market or other applicable
U.S. national exchange, nor has the Company received any information suggesting that the Commission or the Trading Market or other U.S.
applicable national exchange is contemplating terminating such registration or listing, except as disclosed in the Registration Statement,
the Preliminary Prospectus and the Prospectus.
G. No action shall have been
taken and no statute, rule, regulation or order shall have been enacted, adopted or issued by any governmental agency or body which would,
as of the Closing Date, prevent the issuance or sale of the Securities or materially and adversely affect or potentially and adversely
affect the business or operations of the Company; and no injunction, restraining order or order of any other nature by any federal or
state court of competent jurisdiction shall have been issued as of the Closing Date which would prevent the issuance or sale of the Securities
or materially and adversely affect or potentially and adversely affect the business or operations of the Company.
H. The Company shall have
entered into a Purchase Agreement with each of the Purchasers of the Securities and such agreements shall be in full force and effect
and shall contain representations, warranties and covenants of the Company as agreed upon between the Company and the Purchasers.
I. FINRA shall have raised
no objection to the fairness and reasonableness of the terms and arrangements of this Agreement.
J. The Prospectus (in accordance
with Rule 424(b)) and “free writing prospectus” (as defined in Rule 405 of the Securities Act), if any, shall
have been duly filed with the Commission, as appropriate; no stop order suspending the effectiveness of the Registration Statement or
any part thereof shall have been issued and no proceeding for that purpose shall have been initiated or threatened by the Commission;
no order preventing or suspending the use of the Prospectus shall have been issued and no proceeding for that purpose shall have been
initiated or threatened by the Commission; no order having the effect of ceasing or suspending the distribution of the Securities or any
other securities of the Company shall have been issued by any securities commission, securities regulatory authority or stock exchange
and no proceedings for that purpose shall have been instituted or shall be pending or, to the knowledge of the Company, contemplated by
any securities commission, securities regulatory authority or stock exchange; and all requests for additional information on the part
of the Commission shall have been complied with.
K. Subsequent to the execution
and delivery of this Agreement and prior to the Closing Date, in the Placement Agent’s sole judgment after consultation with the
Company, there shall not have occurred any material adverse effect or development involving a prospective material adverse change in the
condition or the business activities, financial or otherwise, of the Company from the latest dates as of which such condition is set forth
in the Registration Statement and Prospectus.
L. Officers’ Certificate.
The Placement Agent shall have received on the Closing Date a certificate of the Company, dated as of such Closing Date, signed by the
Chief Executive Officer and Chief Financial Officer of the Company, to the effect that, and the Placement Agent shall be satisfied that,
the signers of such certificate have reviewed the Registration Statement, the documents incorporated by reference therein (the “Incorporated
Documents”), any prospectus supplement, and this Agreement and to the further effect that:
(i) The representations
and warranties of the Company in this Agreement are true and correct, as if made on and as of such Closing Date, and the Company has
complied with all the agreements and satisfied all the conditions on its part to be performed or satisfied at or prior to such Closing
Date;
(ii) No stop order suspending
the effectiveness of the Registration Statement or the use of the Prospectus or any prospectus supplement has been issued and no proceedings
for that purpose have been instituted or are pending or, to the Company’s knowledge, threatened under the Securities Act; no order
having the effect of ceasing or suspending the distribution of the Securities or any other securities of the Company has been issued by
any securities commission, securities regulatory authority or stock exchange in the United States and no proceedings for that purpose
have been instituted or are pending or, to the knowledge of the Company, contemplated by any securities commission, securities regulatory
authority or stock exchange in the United States;
(iii) When the Registration
Statement became effective, at the time of sale, and at all times subsequent thereto up to the delivery of such certificate, the Registration
Statement and the Incorporated Documents, if any, when such documents became effective or were filed with the Commission, the Prospectus,
and any prospectus supplement, contained all material information required to be included therein by the Securities Act and the Exchange
Act and the applicable rules and regulations of the Commission thereunder, as the case may be, and in all material respects conformed
to the requirements of the Securities Act and the Exchange Act and the applicable rules and regulations of the Commission thereunder,
as the case may be, and the Registration Statement and the Incorporated Documents, if any, and the Prospectus, and any prospectus supplement,
did not and do not include any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary
to make the statements therein, in the light of the circumstances under which they were made, not misleading (provided, however, that
the preceding representations and warranties contained in this paragraph (iii) shall not apply to any statements or omissions made
in reliance upon and in conformity with information furnished in writing to the Company by the Placement Agent expressly for use therein)
and, since the effective date of the Registration Statement, there has occurred no event required by the Securities Act and the rules and
regulations of the Commission thereunder to be set forth in the Incorporated Documents which has not been so set forth; and
(iv) Subsequent to the
respective dates as of which information is given in the Registration Statement, the Incorporated Documents, the Prospectus, and any prospectus
supplement, there has not been: (a) any Material Adverse Effect; (b) any transaction that is material to the Company and the
subsidiaries taken as a whole, except transactions entered into in the ordinary course of business; (c) any obligation, direct or
contingent, that is material to the Company and the subsidiaries taken as a whole, incurred by the Company or any subsidiary, except obligations
incurred in the ordinary course of business; (d) any material change in the capital stock (except changes thereto resulting from
the exercise of outstanding stock options or warrants) or outstanding indebtedness of the Company or any subsidiary; (e) any dividend
or distribution of any kind declared, paid or made on the capital stock of the Company; or (f) any loss or damage (whether or not
insured) to the property of the Company or any subsidiary which has been sustained or will have been sustained which has a Material Adverse
Effect.
M. On or before the Closing
Date, the Placement Agent and counsel for the Placement Agent shall have received such information and documents as they may reasonably
require for the purposes of enabling them to pass upon the issuance and sale of the Securities as contemplated herein, or in order to
evidence the accuracy of any of the representations and warranties, or the satisfaction of any of the conditions or agreements, herein
contained.
If any of the conditions specified
in this Section 10 shall not have been fulfilled when and as required by this Agreement, all obligations of the Placement Agent hereunder
may be cancelled by the Placement Agent at, or at any time prior to, the Closing Date. Notice of such cancellation shall be given to the
Company in writing or orally. Any such oral notice shall be confirmed promptly thereafter in writing.
SECTION 11.
GOVERNING LAW. This Agreement will be governed by, and construed in accordance with, the laws of the State of New York applicable
to agreements made and to be performed entirely in such State. This Agreement may not be assigned by either party without the prior written
consent of the other party. This Agreement shall be binding upon and inure to the benefit of the parties hereto, and their respective
successors and permitted assigns. Any right to trial by jury with respect to any dispute arising under this Agreement or any transaction
or conduct in connection herewith is waived. Any dispute arising under this Agreement may be brought into the courts of the State of
New York or of the United States of America, in each case sitting in the County and State of New York and, by execution and delivery
of this Agreement, the Company hereby accepts for itself and in respect of its property, generally and unconditionally, the jurisdiction
of such courts. Each party hereto hereby irrevocably waives personal service of process and consents to process being served in any such
suit, action or proceeding by delivering a copy thereof via overnight delivery (with evidence of delivery) to such party at the address
in effect for notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process
and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by
law. If either party shall commence an action or proceeding to enforce any provisions of this Agreement, then the prevailing party in
such action or proceeding shall be reimbursed by the other party for its attorney’s fees and other costs and expenses incurred
with the investigation, preparation and prosecution of such action or proceeding.
SECTION 12.
ENTIRE AGREEMENT/MISCELLANEOUS. This Agreement embodies the entire agreement and understanding between the parties hereto, and
supersedes all prior agreements and understandings, relating to the subject matter hereof. If any provision of this Agreement is determined
to be invalid or unenforceable in any respect, such determination will not affect such provision in any other respect or any other provision
of this Agreement, which will remain in full force and effect. This Agreement may not be amended or otherwise modified or waived except
by an instrument in writing signed by the Placement Agent and the Company. The representations, warranties, agreements and covenants contained
herein shall survive the Closing Date of the Placement and delivery of the Securities. This Agreement may be executed in two or more counterparts,
all of which when taken together shall be considered one and the same agreement and shall become effective when counterparts have been
signed by each party and delivered to the other party, it being understood that both parties need not sign the same counterpart. In the
event that any signature is delivered by facsimile transmission or a .pdf format file, such signature shall create a valid and binding
obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such facsimile
or .pdf signature page were an original thereof.
SECTION 13.
NOTICES. Any and all notices or other communications or deliveries required or permitted to be provided hereunder shall be in writing
and shall be deemed given and effective on the earliest of (a) the date of transmission, if such notice or communication is sent
to the email address specified on the signature pages attached hereto prior to 5:30 p.m. (New York City time) on a business
day, (b) the next business day after the date of transmission, if such notice or communication is sent to the email address on the
signature pages attached hereto on a day that is not a business day or later than 5:30 p.m. (New York City time) on any business
day, (c) the third business day following the date of mailing, if sent by U.S. internationally recognized air courier service, or
(d) upon actual receipt by the party to whom such notice is required to be given. The address for such notices and communications
shall be as set forth on the signature pages hereto.
SECTION 14.
Press Announcements. The Company agrees that the Placement Agent shall, on and after
the Closing Date, have the right to reference the Placement and the Placement Agent’s role in connection therewith in the Placement
Agent’s marketing materials and on its website and to place advertisements in financial and other newspapers and journals, in each
case at its own expense.
SECTION 15.
TAIL FINANCING. The Placement Agent shall be entitled to compensation in the form
of the cash fees calculated in the manner described in Section 3 hereto with respect to any public or private offering or other financing
or capital-raising transaction of any kind (each, a "Tail Financing") for a period of twelve months from the Closing
Date, to the extent that such financing or capital is provided to the Company by an investor whom the Placement Agent introduced to the
Company and conducted discussions with respect to the Placement or with respect to that certain loan transaction that closed on September 25,
2024. Notwithstanding anything herein to the contrary, the right to receive a Tail Financing fee shall be subject to FINRA Rule 5110(g)(5)(B),
and the Company shall have a right of termination for cause in connection with this Agreement. The Company’s exercise of its right
to terminate for cause will eliminate any obligations with respect to the payment of any termination fee or provision of any tail financing
fee, including the Tail Financing.
[The remainder of this page has been intentionally
left blank.]
Please confirm that the foregoing
correctly sets forth our agreement by signing and returning to the Placement Agent the enclosed copy of this Agreement.
Very truly yours,
| WESTPARK
CAPITAL, INC. |
|
| |
|
| By: |
|
|
| |
Name: |
|
| |
Title: |
|
Address
for notice:
1800 Century Park East, Suite 220
Los Angeles, CA 90067
Attention:
Email:
with a copy (which shall not constitute notice)
to:
Lucosky Brookman LLP
101 Wood Avenue South, 5th Floor
Woodbridge, NJ 08830
Attention: Joseph M. Lucosky
E-mail: jlucosky@lucbro.com
[Signature Page to Placement
Agent Agreement]
Accepted and agreed to as of the date first written above:
| |
|
| ISPECIMEN, INC. |
|
| |
|
| By: |
|
|
| |
Name: |
|
| |
Title: |
|
Address for notice:
8 Cabot Road
Woburn, MA 01801
Attention:
E-mail:
with a copy (which shall not constitute notice) to:
Sichenzia Ross Ference Carmel LLP
1185 Avenue of the Americas, 31st Floor
New York, New York 10036
Attention: Ross D. Carmel, Esq.
Email: rcarmel@srfc.law
[Signature Page to Placement Agent Agreement]
EXHIBIT A
Form of Lock-Up Agreement
EXHIBIT A
FORM OF LOCK-UP AGREEMENT
_____, 2024
WestPark Capital, Inc.
1800 Century Park East, Suite 220
Los Angeles, CA 90067
Ladies and Gentlemen:
The undersigned understands
WestPark Capital, Inc. (the “Placement Agent”) proposes to enter into a placement agency agreement (the “Placement
Agency Agreement”) with iSpecimen Inc., a company incorporated under the law of the State of Delaware (the “Company”),
providing for the public offering (the “Public Offering”) of [●] shares of common stock (the “Common
Stock”), par value $0.0001 per share, of the Company (each, a “Closing Share”) and/or pre-funded warrants
to purchase shares of Common Stock (the “Pre-Funded Warrants,” together with the Common Stock, the “Securities”).
To induce the Placement Agent
to continue its efforts in connection with the Public Offering, the undersigned hereby agrees that, without the prior written consent
of the Placement Agent, the undersigned will not, during the period commencing on the date hereof and ending ninety (90) days after
the date of the closing of the sale of the Securities under the Placement Agency Agreement (the “Lock-Up Period”),
(1) offer, pledge, sell, contract to sell, grant, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of
Common Stock or any securities convertible into or exercisable or exchangeable for shares of Common Stock, whether now owned or hereafter
acquired by the undersigned or with respect to which the undersigned has or hereafter acquires the power of disposition (collectively,
the “Lock-Up Securities”); (2) enter into any swap or other arrangement that transfers to another, in whole or
in part, any of the economic consequences of ownership of the Lock-Up Securities, whether any such transaction described in clause (1) or
(2) above is to be settled by delivery of Lock-Up Securities, in cash or otherwise; (3) make any demand for or exercise any
right with respect to the registration of any Lock-Up Securities; or (4) publicly disclose the intention to make any offer, sale,
pledge or disposition, or to enter into any transaction, swap, hedge or other arrangement relating to any Lock-Up Securities.
Notwithstanding the foregoing,
and subject to the conditions below, the undersigned may transfer Lock-Up Securities without the prior written consent of the Placement
Agent in connection with
| |
(a) |
transactions relating to Lock-Up Securities acquired in open market transactions after the completion of the Public Offering; provided that no filing under Section 13 or Section 16(a) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or other public announcement shall be required or shall be voluntarily made in connection with subsequent sales of Lock-Up Securities acquired in such open market transactions; |
| |
|
|
| |
(b) |
transfers of Lock-Up Securities as a bona fide gift, by will or intestacy or to a family member or trust for the benefit of the undersigned (for purposes of this lock-up agreement, “family member” means any relationship by blood, marriage or adoption, not more remote than first cousin); |
| |
|
|
| |
(c) |
transfers of Lock-Up Securities to a charity or educational institution; |
| |
|
|
| |
(d) |
if the undersigned is a corporation, partnership, limited liability company or other business entity, (i) any transfers of Lock-Up Securities to another corporation, partnership or other business entity that controls, is controlled by or is under common control with the undersigned or (ii) distributions of Lock-Up Securities to members, partners, stockholders, subsidiaries or affiliates (as defined in Rule 405 promulgated under the Securities Act of 1933, as amended) of the undersigned; |
| |
(e) |
if the undersigned is a trust, to a trustee or beneficiary of the trust; provided that in the case of any transfer pursuant to the foregoing clauses (b), (c) (d) or (e), (i) any such transfer shall not involve a disposition for value, (ii) each transferee shall sign and deliver to the Placement Agent a lock-up agreement substantially in the form of this agreement and (iii) no filing under Section 13 or Section 16(a) of the Exchange Act or other public announcement shall be required or shall be voluntarily made during the Lock-Up Period; |
| |
(f) |
the receipt by the undersigned from the Company of shares of Common Stock upon the vesting of restricted stock awards or stock units or upon the exercise of options to purchase the Company’s shares of Common Stock issued under an equity incentive plan of the Company or an employment arrangement described in the Registration Statement (as defined in the Placement Agency Agreement) (the “Plan Shares”) or the transfer or withholding of shares of Common Stock or any securities convertible into shares of Common Stock to the Company upon a vesting event of the Company’s securities or upon the exercise of options to purchase the Company’s securities, in each case on a “cashless” or “net exercise” basis or to cover tax obligations of the undersigned in connection with such vesting or exercise, provided that if the undersigned is required to file a report under Section 13 or Section 16(a) of the Exchange Act reporting a reduction in beneficial ownership of shares of Common Stock during the Lock-Up Period, the undersigned shall include a statement in such schedule or report to the effect that the purpose of such transfer was to cover tax withholding obligations of the undersigned in connection with such vesting or exercise and, provided further, that the Plan Shares shall be subject to the terms of this agreement; |
| |
|
|
| |
(g) |
the transfer of Lock-Up Securities pursuant to agreements described in the Registration Statement under which the Company has the option to repurchase such securities or a right of first refusal with respect to the transfer of such securities, provided that if the undersigned is required to file a report under Section 13 or Section 16(a) of the Exchange Act reporting a reduction in beneficial ownership of shares of Common Stock during the Lock-Up Period, the undersigned shall include a statement in such schedule or report describing the purpose of the transaction; |
| |
|
|
| |
(h) |
the establishment of a trading plan pursuant to Rule 10b5-1 under the Exchange Act for the transfer of Lock-Up Securities, provided that (i) such plan does not provide for the transfer of Lock-Up Securities during the Lock-Up Period and (ii) to the extent a public announcement or filing under the Exchange Act, if any, is required of or voluntarily made by or on behalf of the undersigned or the Company regarding the establishment of such plan, such public announcement or filing shall include a statement to the effect that no transfer of Lock-Up Securities may be made under such plan during the Lock-Up Period; |
| |
|
|
| |
(i) |
the transfer of Lock-Up Securities that occurs by operation of law, such as pursuant to a qualified domestic order or in connection with a divorce settlement, provided that the transferee agrees to sign and deliver an agreement substantially in the form of this agreement for the balance of the Lock-Up Period, and provided further, that any filing under Section 13 or Section 16(a) of the Exchange Act that is required to be made during the Lock-Up Period as a result of such transfer shall include a statement that such transfer has occurred by operation of law; and |
| |
|
|
| |
(j) |
the transfer of Lock-Up Securities pursuant to a bona fide third party tender offer, merger, consolidation or other similar transaction made to all holders of shares of Common Stock involving a change of control (as defined below) of the Company after the closing of the Public Offering and approved by the Company’s board of directors; provided that in the event that the tender offer, merger, consolidation or other such transaction is not completed, the Lock-Up Securities owned by the undersigned shall remain subject to the restrictions contained in this agreement. “Change of control” means the consummation of any bona fide third party tender offer, merger, amalgamation, consolidation or other similar transaction the result of which is that any “person” or “group” of persons (as defined in Section 13(d)(3) of the Exchange Act) becomes the beneficial owner (as defined in Rules 13d-3 and 13d-5 of the Exchange Act) of a majority of total voting power of the voting stock of the Company. |
The undersigned also agrees
and consents to the entry of stop transfer instructions with the Company’s transfer agent and registrar against the transfer of
the undersigned’s Lock-Up Securities except in compliance with this lock-up agreement.
If the undersigned is an officer
or director of the Company, (i) the undersigned agrees that the foregoing restrictions shall be equally applicable to any issuer-directed
or “friends and family” securities that the undersigned may purchase in the Public Offering; (ii) the Placement Agent
in the Placement Agency Agreement has agreed that, at least three (3) business days before the effective date of any release or waiver
of the foregoing restrictions in connection with a transfer of Lock-Up Securities, the Placement Agent will notify the Company of the
impending release or waiver; and (iii) the Company has agreed in the Placement Agency Agreement to announce the impending release
or waiver by press release through a major news service at least two (2) business days before the effective date of the release or
waiver. Any release or waiver granted by the Placement Agent hereunder to any such officer or director shall only be effective two (2) business
days after the publication date of such press release. The provisions of this paragraph will not apply if (a) the release or waiver
is effected solely to permit a transfer of Lock-Up Securities not for consideration and (b) the transferee has agreed in writing
to be bound by the same terms described in this lock-up agreement to the extent and for the duration that such terms remain in effect
at the time of such transfer.
The undersigned understands
that the Company and the Placement Agent are relying upon this lock-up agreement in proceeding toward consummation of the Public Offering.
The undersigned further understands that this agreement is irrevocable and shall be binding upon the undersigned’s heirs, legal
representatives, successors and assigns.
The undersigned understands
that, if the Placement Agency Agreement is not executed by [●], 2024 or if the Placement Agency Agreement (other than the provisions
thereof which survive termination) shall terminate or be terminated prior to payment for and delivery of the shares of Common Stock to
be sold thereunder, then this lock-up agreement shall be void and of no further force or effect.
| |
Very truly yours, |
| |
|
| |
(Name - Please Print) |
| |
|
| |
|
| |
|
| |
(Signature) |
| |
|
| |
|
| |
|
| |
|
| |
|
| |
(Name of Signatory, in the case of entities - Please
Print) |
| |
|
| |
|
| |
|
| |
(Title of Signatory, in the case of entities - Please
Print) |
Exhibit 4.4
PRE-FUNDED COMMON STOCK PURCHASE WARRANT
ISPECIMEN INC.
| Warrant Shares:_______________ |
|
Initial Exercise Date: _____________, 2024 |
THIS PRE-FUNDED COMMON STOCK
PURCHASE WARRANT (“Warrant”) certifies that, for value received, _____________ or its assigns (the “Holder”)
is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after
the date hereof (the “Initial Exercise Date”) and until this Warrant is exercised in full (the “Termination
Date”) but not thereafter, to subscribe for and purchase from iSpecimen Inc., a Delaware corporation (the “Company”),
up to ______ shares (as subject to adjustment hereunder, the “Warrant Shares”) of Common Stock. The purchase price
of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(b).
Section 1.
Definitions. In addition to the terms defined elsewhere in this Warrant, the following terms have the meanings indicated in this
Section 1:
“Affiliate”
means any Person that, directly or indirectly through one or more intermediaries, controls or is controlled by or is under common control
with a Person, as such terms are used in and construed under Rule 405 under the Securities Act.
“Alternate
Consideration” shall have the meaning ascribed to such term in Section 3(d).
“Attribution
Parties” shall have the meaning ascribed to such term in Section 2(e).
“Beneficial
Ownership Limitation” shall have the meaning ascribed to such term in Section 2(e).
“Bid Price”
means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed
or quoted on a Trading Market, the bid price of the Common Stock for the time in question (or the nearest preceding date) on the Trading
Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (“Bloomberg”) (based on a Trading
Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not a Trading Market,
the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c) if
the Common Stock is not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Stock are then reported on the
Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per
share of the Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined
by an independent appraiser selected in good faith by the Holders of a majority in interest of the Warrants then outstanding and reasonably
acceptable to the Company, the fees and expenses of which shall be paid by the Company.
“Bloomberg”
shall have the meaning ascribed to such term in definition of “Bid Price.”
“Business
Day” means any day other than Saturday, Sunday or other day on which commercial banks in The City of New York are authorized
or required by law to remain closed; provided, however, for clarification, commercial banks shall not be deemed to be authorized
or required by law to remain closed due to “stay at home,” “shelter-in-place,” “non-essential employee”
or any other similar orders or restrictions or the closure of any physical branch locations at the direction of any governmental authority
so long as the electronic funds transfer systems (including for wire transfers) of commercial banks in The City of New York generally
are open for use by customers on such day.
“Buy-In”
shall have the meaning ascribed to such term in Section 2(d)(iv).
“Commission”
means the United States Securities and Exchange Commission.
“Common
Stock” means the common stock of the Company, par value $0.0001 per share, and any other class of securities into which such
securities may hereafter be reclassified or changed.
“Common
Stock Equivalents” means any securities of the Company or the Subsidiaries which would entitle the holder thereof to acquire
at any time Common Stock, including, without limitation, any debt, preferred stock, right, option, warrant or other instrument that is
at any time convertible into or exercisable or exchangeable for, or otherwise entitles the holder thereof to receive, Common Stock.
“Company”
shall have the meaning ascribed to such term in the Preamble.
“Distribution”
shall have the meaning ascribed to such term in Section 3(c).
“DWAC”
shall have the meaning ascribed to such term in Section 2(d)(i).
“Exercise
Price” shall have the meaning ascribed to such term in Section 2(b).
“Exchange
Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“Fundamental
Transaction” shall have the meaning ascribed to such term in Section 3(d).
“Holder”
shall have the meaning ascribed to such term in the Preamble.
“Initial
Exercise Date” shall have the meaning ascribed to such term in the Preamble.
“Notice
of Exercise” shall have the meaning ascribed to such term in Section 2(a).
“Person”
means an individual or corporation, partnership, trust, incorporated or unincorporated association, joint venture, limited liability company,
joint stock company, government (or an agency or subdivision thereof) or other entity of any kind.
“Purchase
Rights” shall have the meaning ascribed to such term in Section 3(b).
“Registration
Statement” means the Company’s registration statement on Form S-1 (File No. 333-[●]).
“Securities
Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Standard
Settlement Period” shall have the meaning ascribed to such term in Section 2(d)(i).
“Subsidiary”
means any subsidiary of the Company and shall, where applicable, also include any direct or indirect subsidiary of the Company formed
or acquired after the date hereof.
“Successor
Entity” shall have the meaning ascribed to such term in Section 3(d).
“Termination
Date” shall have the meaning ascribed to such term in the Preamble.
“Trading
Day” means a day on which the Common Stock is traded on a Trading Market.
“Trading
Market” means any of the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date
in question: the NYSE American, The Nasdaq Capital Market, The Nasdaq Global Market, The Nasdaq Global Select Market or the New York Stock
Exchange (or any successors to any of the foregoing).
“Transfer
Agent” means Broadridge Corporate Issuer Solutions, LLC, the current transfer agent of the Company, with a mailing address
of 51 Mercedes Way, Edgewood, NY 11717, and a telephone number of 1-631-254-7400, and any successor transfer agent of the Company.
“VWAP”
means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed
or quoted on a Trading Market, the daily volume weighted average price of the Common Stock for such date (or the nearest preceding date)
on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg (based on a Trading Day from 9:30 a.m. (New
York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not a Trading Market, the volume weighted average
price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c) if the Common Stock
is not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Stock are then reported on the Pink Open Market
(or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common
Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined by an independent appraiser
selected in good faith by the holders of a majority in interest of the Warrants then outstanding and reasonably acceptable to the Company,
the fees and expenses of which shall be paid by the Company.
“Warrant
Register” shall have the meaning ascribed to such term in Section 4(c).
“Warrant
Share Delivery Date” shall have the meaning ascribed to such term in Section 2(d)(i).
“Warrant
Shares” shall have the meaning ascribed to such term in the Preamble.
“Warrants”
means this Warrant and other Common Stock purchase warrants issued by the Company pursuant to the Registration Statement.
Section 2.
Exercise.
a) Exercise of
Warrant. Exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or
after the Initial Exercise Date and on or before the Termination Date by delivery to the Company of a duly executed PDF copy submitted
by e-mail (or e-mail attachment) of the Notice of Exercise in the form annexed hereto as Exhibit A (the “Notice of
Exercise”). Within the earlier of (i) two (2) Trading Days and (ii) the number of Trading Days comprising the
Standard Settlement Period (as defined in Section 2(d)(i) herein) following the date of exercise as aforesaid, the Holder
shall deliver the aggregate Exercise Price for the Warrant Shares specified in the applicable Notice of Exercise by wire transfer or cashier’s
check drawn on a United States bank unless the cashless exercise procedure specified in Section 2(c) below is specified
in the applicable Notice of Exercise. No ink-original Notice of Exercise shall be required, nor shall any medallion guarantee (or other
type of guarantee or notarization) of any Notice of Exercise be required. Notwithstanding anything herein to the contrary, the Holder
shall not be required to physically surrender this Warrant to the Company until the Holder has purchased all of the Warrant Shares available
hereunder and the Warrant has been exercised in full, in which case, the Holder shall surrender this Warrant to the Company for cancellation
within three (3) Trading Days of the date on which the final Notice of Exercise is delivered to the Company. Partial exercises of
this Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering
the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased.
The Holder and the Company shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. The Company
shall deliver any objection to any Notice of Exercise within one (1) Business Day of receipt of such notice. The Holder and any
assignee, by acceptance of this Warrant, acknowledge and agree that, by reason of the provisions of this paragraph, following the purchase
of a portion of the Warrant Shares hereunder, the number of Warrant Shares available for purchase hereunder at any given time may be less
than the amount stated on the face hereof.
b) Exercise Price.
The aggregate exercise price of this Warrant, except for a nominal exercise price of $0.0001 per Warrant Share, was pre-funded to the
Company on or prior to the Initial Exercise Date and, consequently, no additional consideration (other than the nominal exercise price
of $0.0001 per Warrant Share) shall be required to be paid by the Holder to any Person to effect any exercise of this Warrant. The Holder
shall not be entitled to the return or refund of all, or any portion, of such pre-paid aggregate exercise price under any circumstance
or for any reason whatsoever. The remaining unpaid exercise price per share of Common Stock under this Warrant shall be $0.0001, subject
to adjustment hereunder (the “Exercise Price”).
c) Cashless Exercise.
This Warrant may also be exercised, in whole or in part, at such time by means of a “cashless exercise” in which the Holder
shall be entitled to receive a number of Warrant Shares equal to the quotient obtained by dividing [(A-B) (X)] by (A), where:
| (A) = |
as applicable: (i) the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise if such Notice of Exercise is (1) both executed and delivered pursuant to Section 2(a) hereof on a day that is not a Trading Day or (2) both executed and delivered pursuant to Section 2(a) hereof on a Trading Day prior to the opening of “regular trading hours” (as defined in Rule 600(b) of Regulation NMS promulgated under the federal securities laws) on such Trading Day, (ii) at the option of the Holder, either (y) the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise or (z) the Bid Price of the Common Stock on the principal Trading Market as reported by Bloomberg as of the time of the Holder’s execution of the applicable Notice of Exercise if such Notice of Exercise is executed during “regular trading hours” on a Trading Day and is delivered within two (2) hours thereafter (including until two (2) hours after the close of “regular trading hours” on a Trading Day) pursuant to Section 2(a) hereof or (iii) the VWAP on the date of the applicable Notice of Exercise if the date of such Notice of Exercise is a Trading Day and such Notice of Exercise is both executed and delivered pursuant to Section 2(a) hereof after the close of “regular trading hours” on such Trading Day; |
| (B) = |
the Exercise Price of this Warrant, as adjusted hereunder; and |
| (X) = |
the number of Warrant Shares that would be issuable upon exercise of this Warrant in accordance with the terms of this Warrant if such exercise were by means of a cash exercise rather than a cashless exercise. |
If Warrant Shares
are issued in such a cashless exercise, the parties acknowledge and agree that in accordance with Section 3(a)(9) of the Securities
Act, the Warrant Shares shall take on the registered characteristics of the Warrants being exercised. The Company agrees not to take any
position contrary to this Section 2(c).
d) Mechanics
of Exercise.
i. Delivery of
Warrant Shares Upon Exercise. The Company shall cause the Warrant Shares purchased hereunder to be transmitted by the Transfer Agent
to the Holder by crediting the account of the Holder’s or its designee’s balance account with The Depository Trust Company
through its Deposit or Withdrawal at Custodian system (“DWAC”) if the Company is then a participant in such system
and either (A) there is an effective registration statement permitting the issuance of the Warrant Shares to or resale of the Warrant
Shares by Holder or (B) this Warrant is being exercised via cashless exercise, and otherwise by physical delivery of a certificate,
registered in the Company’s share register in the name of the Holder or its designee, for the number of Warrant Shares to which
the Holder is entitled pursuant to such exercise to the address specified by the Holder in the Notice of Exercise by the date that is
the earliest of (i) two (2) Trading Days after the delivery to the Company of the Notice of Exercise, (ii) one (1) Trading
Day after delivery of the aggregate Exercise Price to the Company and (iii) the number of Trading Days comprising the Standard Settlement
Period after the delivery to the Company of the Notice of Exercise (such date, the “Warrant Share Delivery Date”).
Upon delivery of the Notice of Exercise, the Holder shall be deemed for all corporate purposes to have become the holder of record of
the Warrant Shares with respect to which this Warrant has been exercised, irrespective of the date of delivery of the Warrant Shares,
provided that payment of the aggregate Exercise Price (other than in the case of a cashless exercise) is received within the earlier of
(i) two (2) Trading Days and (ii) the number of Trading Days comprising the Standard Settlement Period following delivery
of the Notice of Exercise. If the Company fails for any reason to deliver to the Holder the Warrant Shares subject to a Notice of Exercise
by the Warrant Share Delivery Date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each
$1,000 of Warrant Shares subject to such exercise (based on the VWAP of the Common Stock on the date of the applicable Notice of Exercise),
$10 per Trading Day (increasing to $20 per Trading Day on the fifth Trading Day after the Warrant Share Delivery Date) for each Trading
Day after such Warrant Share Delivery Date until such Warrant Shares are delivered or Holder rescinds such exercise. The Company agrees
to maintain a transfer agent that is a participant in the Fast Automated Securities Transfer Program so long as this Warrant remains outstanding
and exercisable. As used herein, “Standard Settlement Period” means the standard settlement period, expressed in a
number of Trading Days, on the Company’s primary Trading Market with respect to the Common Stock as in effect on the date of delivery
of the Notice of Exercise.
ii. Delivery of
New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the request of a Holder and upon
surrender of this Warrant certificate, at the time of delivery of the Warrant Shares, deliver to the Holder a new Warrant evidencing the
rights of the Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects
be identical with this Warrant.
iii. Rescission
Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares pursuant to Section 2(d)(i) by
the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
iv. Compensation
for Buy-In on Failure to Timely Deliver Warrant Shares Upon Exercise. In addition to any other rights available to the Holder, if
the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares in accordance with the provisions of Section 2(d)(i) above
pursuant to an exercise on or before the Warrant Share Delivery Date, and if after such date the Holder is required by its broker to purchase
(in an open market transaction or otherwise) or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver
in satisfaction of a sale by the Holder of the Warrant Shares which the Holder anticipated receiving upon such exercise (a “Buy-In”),
then the Company shall (A) pay in cash to the Holder the amount, if any, by which (x) the Holder’s total purchase price
(including brokerage commissions, if any) for the shares of Common Stock so purchased exceeds (y) the amount obtained by multiplying
(1) the number of Warrant Shares that the Company was required to deliver to the Holder in connection with the exercise at issue
times (2) the price at which the sell order giving rise to such purchase obligation was executed, and (B) at the option of the
Holder, either reinstate the portion of the Warrant and equivalent number of Warrant Shares for which such exercise was not honored (in
which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock that would have been
issued had the Company timely complied with its exercise and delivery obligations hereunder. For example, if the Holder purchases Common
Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise of shares of Common Stock with
an aggregate sale price giving rise to such purchase obligation of $10,000, under clause (A) of the immediately preceding sentence
the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating the amounts payable
to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing herein shall limit
a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree
of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver shares of Common Stock
upon exercise of the Warrant as required pursuant to the terms hereof.
v. No Fractional
Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As
to any fraction of a share which the Holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election,
either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or
round up to the next whole share.
vi. Charges, Taxes
and Expenses. Issuance of Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental
expense in respect of the issuance of such Warrant Shares, all of which taxes and expenses shall be paid by the Company, and such Warrant
Shares shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however,
that, in the event that Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for
exercise shall be accompanied by the Assignment Form attached hereto as Exhibit B duly executed by the Holder and the
Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto.
The Company shall pay all Transfer Agent fees required for same-day processing of any Notice of Exercise and all fees to The Depository
Trust Company (or another established clearing corporation performing similar functions) required for same-day electronic delivery of
the Warrant Shares.
vii. Closing of
Books. The Company will not close its stockholder books or records in any manner which prevents the timely exercise of this Warrant,
pursuant to the terms hereof.
e)
Holder’s Exercise Limitations. The Company shall not effect any exercise of this Warrant, and a Holder shall not have the
right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, to the extent that after giving effect
to such issuance after exercise as set forth on the applicable Notice of Exercise, the Holder (together with the Holder’s Affiliates,
and any other Persons acting as a group together with the Holder or any of the Holder’s Affiliates (such Persons, “Attribution
Parties”)), would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of the
foregoing sentence, the number of shares of Common Stock beneficially owned by the Holder and its Affiliates and Attribution Parties shall
include the number of shares of Common Stock issuable upon exercise of this Warrant with respect to which such determination is being
made, but shall exclude the number of shares of Common Stock which would be issuable upon (i) exercise of the remaining, nonexercised
portion of this Warrant beneficially owned by the Holder or any of its Affiliates or Attribution Parties and (ii) exercise or conversion
of the unexercised or nonconverted portion of any other securities of the Company (including, without limitation, any other Common Stock
Equivalents) subject to a limitation on conversion or exercise analogous to the limitation contained herein beneficially owned by the
Holder or any of its Affiliates or Attribution Parties. Except as set forth in the preceding sentence, for purposes of this Section 2(e),
beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations
promulgated thereunder, it being acknowledged by the Holder that the Company is not representing to the Holder that such calculation is
in compliance with Section 13(d) of the Exchange Act and the Holder is solely responsible for any schedules required to be filed
in accordance therewith. To the extent that the limitation contained in this Section 2(e) applies, the determination
of whether this Warrant is exercisable (in relation to other securities owned by the Holder together with any Affiliates and Attribution
Parties) and of which portion of this Warrant is exercisable shall be in the sole discretion of the Holder, and the submission of a Notice
of Exercise shall be deemed to be the Holder’s determination of whether this Warrant is exercisable (in relation to other securities
owned by the Holder together with any Affiliates and Attribution Parties) and of which portion of this Warrant is exercisable, in each
case subject to the Beneficial Ownership Limitation, and the Company shall have no obligation to verify or confirm the accuracy of such
determination. In addition, a determination as to any group status as contemplated above shall be determined in accordance with Section 13(d) of
the Exchange Act and the rules and regulations promulgated thereunder. For purposes of this Section 2(e), in determining
the number of outstanding shares of Common Stock, a Holder may rely on the number of outstanding shares of Common Stock as reflected in
(A) the Company’s most recent periodic or annual report filed with the Commission, as the case may be, (B) a more recent
public announcement by the Company or (C) a more recent written notice by the Company or the Transfer Agent setting forth the number
of shares of Common Stock outstanding. Upon the written or oral request of a Holder, the Company shall within one (1) Trading Day
confirm orally and in writing to the Holder the number of shares of Common Stock then outstanding. In any case, the number of outstanding
shares of Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including this
Warrant, by the Holder or its Affiliates or Attribution Parties since the date as of which such number of outstanding shares of Common
Stock was reported. The “Beneficial Ownership Limitation” shall be 4.99% (or, upon election by a Holder prior to the
issuance of any Warrants, 9.99%) of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance
of shares of Common Stock issuable upon exercise of this Warrant. The Holder, upon notice to the Company, may increase or decrease the
Beneficial Ownership Limitation provisions of this Section 2(e), provided that the Beneficial Ownership Limitation
in no event exceeds 9.99% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of shares
of Common Stock upon exercise of this Warrant held by the Holder and the provisions of this Section 2(e) shall continue
to apply. Any increase in the Beneficial Ownership Limitation will not be effective until the sixty-first (61st)
day after such notice is delivered to the Company. The provisions of this paragraph shall be construed and implemented in a manner otherwise
than in strict conformity with the terms of this Section 2(e) to correct this paragraph (or any portion hereof) which
may be defective or inconsistent with the intended Beneficial Ownership Limitation herein contained or to make changes or supplements
necessary or desirable to properly give effect to such limitation. The limitations contained in this paragraph shall apply to a successor
holder of this Warrant.
Section 3.
Certain Adjustments.
a) Stock Dividends
and Splits. If the Company, at any time while this Warrant is outstanding: (i) pays a stock dividend or otherwise makes a distribution
or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock (which,
for avoidance of doubt, shall not include any shares of Common Stock issued by the Company upon exercise of this Warrant), (ii) subdivides
outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way of reverse stock split) outstanding
shares of Common Stock into a smaller number of shares, or (iv) issues by reclassification of shares of the Common Stock any shares
of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be
the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such event and of which the denominator
shall be the number of shares of Common Stock outstanding immediately after such event, and the number of shares issuable upon exercise
of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant shall remain unchanged. Any adjustment
made pursuant to this Section 3(a) shall become effective immediately after the record date for the determination of
stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the
case of a subdivision, combination or re-classification.
b) Subsequent
Rights Offerings. In addition to any adjustments pursuant to Section 3(a) above, if at any time the Company grants,
issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata to the record
holders of any class of shares of Common Stock (the “Purchase Rights”), then the Holder will be entitled to acquire,
upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder could have acquired if the Holder had
held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise
hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date on which a record is taken for
the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of shares
of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, that, to the
extent that the Holder’s right to participate in any such Purchase Right would result in the Holder exceeding the Beneficial Ownership
Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such
shares of Common Stock as a result of such Purchase Right to such extent) and such Purchase Right to such extent shall be held in abeyance
for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation).
c) Pro Rata Distributions.
During such time as this Warrant is outstanding, if the Company shall declare or make any dividend or other distribution of its assets
(or rights to acquire its assets) to holders of shares of Common Stock, by way of return of capital or otherwise (including, without limitation,
any distribution of cash, stock or other securities, property or options by way of a dividend, spin off, reclassification, corporate rearrangement,
scheme of arrangement or other similar transaction) (a “Distribution”), at any time after the issuance of this Warrant,
then, in each such case, the Holder shall be entitled to participate in such Distribution to the same extent that the Holder would have
participated therein if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without
regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the
date of which a record is taken for such Distribution, or, if no such record is taken, the date as of which the record holders of shares
of Common Stock are to be determined for the participation in such Distribution (provided, however, that, to the extent
that the Holder’s right to participate in any such Distribution would result in the Holder exceeding the Beneficial Ownership Limitation,
then the Holder shall not be entitled to participate in such Distribution to such extent (or in the beneficial ownership of any shares
of Common Stock as a result of such Distribution to such extent) and the portion of such Distribution shall be held in abeyance for the
benefit of the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership
Limitation).
d) Fundamental
Transaction. If, at any time while this Warrant is outstanding, (i) the Company, directly or indirectly, in one or more related
transactions effects any merger or consolidation of the Company with or into another Person, (ii) the Company or any Subsidiary,
directly or indirectly, effects any sale, lease, license, assignment, transfer, conveyance or other disposition of all or substantially
all of its assets in one or a series of related transactions, (iii) any, direct or indirect, purchase offer, tender offer or exchange
offer (whether by the Company or another Person) is completed pursuant to which holders of Common Stock are permitted to sell, tender
or exchange their shares for other securities, cash or property and has been accepted by the holders of 50% or more of the outstanding
Common Stock or 50% or more of the voting power of the common equity of the Company, (iv) the Company, directly or indirectly, in
one or more related transactions effects any reclassification, reorganization or recapitalization of the Common Stock or any compulsory
share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property, or
(v) the Company, directly or indirectly, in one or more related transactions consummates a stock or share purchase agreement or other
business combination (including, without limitation, a reorganization, recapitalization, spin-off, merger or scheme of arrangement) with
another Person or group of Persons whereby such other Person or group acquires 50% or more of the outstanding shares of Common Stock or
50% or more of the voting power of the common equity of the Company (each a “Fundamental Transaction”), then, upon
any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable
upon such exercise immediately prior to the occurrence of such Fundamental Transaction, at the option of the Holder (without regard to
any limitation in Section 2(e) on the exercise of this Warrant), the number of shares of Common Stock of the successor
or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate
Consideration”) receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for
which this Warrant is exercisable immediately prior to such Fundamental Transaction (without regard to any limitation in Section 2(e) on
the exercise of this Warrant). For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted
to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one (1) share of Common
Stock in such Fundamental Transaction, and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable
manner reflecting the relative value of any different components of the Alternate Consideration. If holders of Common Stock are given
any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holder shall be given the same
choice as to the Alternate Consideration it receives upon any exercise of this Warrant following such Fundamental Transaction.
The Company shall
cause any successor entity in a Fundamental Transaction in which the Company is not the survivor (the “Successor Entity”)
to assume in writing all of the obligations of the Company under this Warrant in accordance with the provisions of this Section 3(d) pursuant
to written agreements in form and substance reasonably satisfactory to the Holder and approved by the Holder (without unreasonable delay)
prior to such Fundamental Transaction and shall, at the option of the Holder, deliver to the Holder in exchange for this Warrant a security
of the Successor Entity evidenced by a written instrument substantially similar in form and substance to this Warrant which is exercisable
for a corresponding number of shares of capital stock of such Successor Entity (or its parent entity) equivalent to the shares of Common
Stock acquirable and receivable upon exercise of this Warrant (without regard to any limitations on the exercise of this Warrant) prior
to such Fundamental Transaction, and with an exercise price which applies the exercise price hereunder to such shares of capital stock
(but taking into account the relative value of the shares of Common Stock pursuant to such Fundamental Transaction and the value of such
shares of capital stock, such number of shares of capital stock and such exercise price being for the purpose of protecting the economic
value of this Warrant immediately prior to the consummation of such Fundamental Transaction), and which is reasonably satisfactory in
form and substance to the Holder. Upon the occurrence of any such Fundamental Transaction, the Successor Entity shall be added to the
term “Company” under this Warrant (so that from and after the occurrence or consummation of such Fundamental Transaction,
each and every provision of this Warrant and the other Transaction Documents referring to the “Company” shall refer instead
to each of the Company and the Successor Entity or Successor Entities, jointly and severally), and the Successor Entity or Successor Entities,
jointly and severally with the Company, may exercise every right and power of the Company prior thereto and the Successor Entity or Successor
Entities shall assume all of the obligations of the Company prior thereto under this Warrant and the other Transaction Documents with
the same effect as if the Company and such Successor Entity or Successor Entities, jointly and severally, had been named as the Company
herein. For the avoidance of doubt, the Holder shall be entitled to the benefits of the provisions of this Section 3(d) regardless
of (i) whether the Company has sufficient authorized shares of Common Stock for the issuance of Warrant Shares and/or (ii) whether
a Fundamental Transaction occurs prior to the Initial Exercise Date.
e) Calculations.
All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may
be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as of a given
date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
f) Notice to
Holder.
i. Adjustment
to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3, the Company shall
promptly deliver to the Holder by email a notice setting forth the Exercise Price after such adjustment and any resulting adjustment to
the number of Warrant Shares and setting forth a brief statement of the facts requiring such adjustment.
ii. Notice to
Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever form) on the Common
Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock, (C) the Company
shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital
stock of any class or of any rights, (D) the approval of any stockholders of the Company shall be required in connection with any
reclassification of the Common Stock, any consolidation or merger to which the Company (or any of its Subsidiaries) is a party, any sale
or transfer of all or substantially all of its assets, or any compulsory share exchange whereby the Common Stock is converted into other
securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding
up of the affairs of the Company, then, in each case, the Company shall cause to be delivered by email to the Holder at its last email
address as it shall appear upon the Warrant Register of the Company, at least twenty (20) calendar days prior to the applicable record
or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend,
distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock
of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on
which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the
date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock
for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange;
provided that the failure to deliver such notice or any defect therein or in the delivery thereof shall not affect the validity of the
corporate action required to be specified in such notice. To the extent that any notice provided in this Warrant constitutes, or contains,
material, non-public information regarding the Company or any of the Subsidiaries, the Company shall simultaneously file such notice with
the Commission pursuant to a Current Report on Form 8-K. The Holder shall remain entitled to exercise this Warrant during the period
commencing on the date of such notice to the effective date of the event triggering such notice except as may otherwise be expressly set
forth herein.
Section 4.
Transfer of Warrant.
a) Transferability. This Warrant and all
rights hereunder (including, without limitation, any registration rights) are transferable, in whole or in part, upon surrender of this
Warrant at the principal office of the Company or its designated agent, together with a written assignment of this Warrant substantially
in the form attached hereto duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable
upon the making of such transfer. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant
or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified in such instrument
of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant
shall promptly be cancelled. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender
this Warrant to the Company unless the Holder has assigned this Warrant in full, in which case, the Holder shall surrender this Warrant
to the Company within three (3) Trading Days of the date on which the Holder delivers an assignment form to the Company assigning
this Warrant in full. The Warrant, if properly assigned in accordance herewith, may be exercised by a new holder for the purchase of Warrant
Shares without having a new Warrant issued.
b) New Warrants.
This Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together
with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent
or attorney. Subject to compliance with Section 4(a), as to any transfer which may be involved in such division or combination,
the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance
with such notice. All Warrants issued on transfers or exchanges shall be dated the initial issuance date of this Warrant and shall be
identical with this Warrant except as to the number of Warrant Shares issuable pursuant thereto.
c) Warrant Register.
The Company shall register this Warrant, upon records to be maintained by the Company for that purpose (the “Warrant Register”),
in the name of the record Holder hereof from time to time. The Company may deem and treat the registered Holder of this Warrant as the
absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual
notice to the contrary.
Section 5.
Miscellaneous.
a) No Rights
as Stockholder Until Exercise; No Settlement in Cash. This Warrant does not entitle the Holder to any voting rights, dividends or
other rights as a stockholder of the Company prior to the exercise hereof as set forth in Section 2(d)(i), except as expressly
set forth in Section 3. Without limiting any rights of a Holder to receive Warrant Shares on a “cashless exercise”
pursuant to Section 2(c) or to receive cash payments pursuant to Section 2(d)(i) and Section 2(d)(iv) herein,
in no event shall the Company be required to net cash settle an exercise of this Warrant.
b) Loss, Theft,
Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence reasonably satisfactory to
it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case
of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant, shall not include
the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make
and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
c) Saturdays,
Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or
granted herein shall not be a Business Day, then such action may be taken or such right may be exercised on the next succeeding Business
Day.
d) Authorized
Shares.
The Company covenants
that, during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock a sufficient number
of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. The Company further
covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of issuing the
necessary Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action
as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation,
or of any requirements of the Trading Market upon which the Common Stock may be listed. The Company covenants that all Warrant Shares
which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented
by this Warrant and payment for such Warrant Shares in accordance herewith, be duly authorized, validly issued, fully paid and nonassessable
and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than taxes in respect of any
transfer occurring contemporaneously with such issue).
Except and to the
extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its certificate
of incorporation or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or
any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all
times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate
to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the
Company will (i) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately
prior to such increase in par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly
and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable
efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may
be, necessary to enable the Company to perform its obligations under this Warrant.
Before taking any
action which would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price,
the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory
body or bodies having jurisdiction thereof.
e) Governing
Law. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be governed by and
construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles of conflicts of
law thereof. Each party agrees that all legal proceedings concerning the interpretations, enforcement and defense of the transactions
contemplated by this Warrant (whether brought against a party hereto or their respective affiliates, directors, officers, shareholders,
partners, members, employees or agents) shall be commenced exclusively in the state and federal courts sitting in the City of New York.
Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the City of New York, Borough
of Manhattan for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed
herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally
subject to the jurisdiction of any such court, that such suit, action or proceeding is improper or is an inconvenient venue for such proceeding.
Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding
by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address
in effect for notices to it under this Warrant and agrees that such service shall constitute good and sufficient service of process and
notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any other manner permitted
by law. If either party shall commence an action, suit or proceeding to enforce any provisions of this Warrant, the prevailing party in
such action, suit or proceeding shall be reimbursed by the other party for their reasonable attorneys’ fees and other costs and
expenses incurred with the investigation, preparation and prosecution of such action or proceeding.
f) Restrictions.
The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered, and the Holder does not
utilize cashless exercise, will have restrictions upon resale imposed by state and federal securities laws.
g) Nonwaiver
and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as
a waiver of such right or otherwise prejudice the Holder’s rights, powers or remedies. Without limiting any other provision of this
Warrant, if the Company willfully and knowingly fails to comply with any provision of this Warrant, which results in any material damages
to the Holder, the Company shall pay to the Holder such amounts as shall be sufficient to cover any costs and expenses including, but
not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by the Holder in collecting any amounts
due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder.
h)
Notices. Any and all notices or other communications or deliveries to be provided by the Holders hereunder including, without limitation,
any Notice of Exercise, shall be in writing and delivered personally, by e-mail, or sent by a nationally recognized overnight courier
service, addressed to the Company, at 8 Cabot Road, Woburn, MA 01801, Attention: [●], Chief Executive Officer, email address: [●],
or such other email address or address as the Company may specify for such purposes by notice to the Holders. Any and all notices or other
communications or deliveries to be provided by the Company hereunder shall be in writing and delivered personally, by e-mail, or sent
by a nationally recognized overnight courier service addressed to each Holder at the e-mail address or address of such Holder appearing
on the books of the Company. Any notice or other communication or deliveries hereunder shall be deemed given and effective on the earliest
of (i) the time of transmission, if such notice or communication is delivered via e-mail at the e-mail address set forth in this
Section prior to 5:30 p.m. (New York City time) on any date, (ii) the next Trading Day after the time of transmission,
if such notice or communication is delivered via e-mail at the e-mail address set forth in this Section on a day that is not a Trading
Day or later than 5:30 p.m. (New York City time) on any Trading Day, (iii) the second (2nd)
Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier service, or (iv) upon actual receipt
by the party to whom such notice is required to be given. To the extent that any notice provided hereunder constitutes, or contains, material,
non-public information regarding the Company or any Subsidiaries, the Company shall simultaneously file such notice with the Commission
pursuant to a Current Report on Form 8-K.
i) Limitation
of Liability. No provision hereof, in the absence of any affirmative action by the Holder to exercise this Warrant to purchase Warrant
Shares, and no enumeration herein of the rights or privileges of the Holder, shall give rise to any liability of the Holder for the purchase
price of any Common Stock or as a stockholder of the Company, whether such liability is asserted by the Company or by creditors of the
Company.
j) Remedies.
The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific
performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss
incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive and not to assert the defense in any
action for specific performance that a remedy at law would be adequate.
k) Successors
and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the
benefit of and be binding upon the successors and permitted assigns of the Company and the successors and permitted assigns of Holder.
The provisions of this Warrant are intended to be for the benefit of any Holder from time to time of this Warrant and shall be enforceable
by the Holder or holder of Warrant Shares.
l) Amendment.
This Warrant may be modified or amended or the provisions hereof waived with the written consent of the Company, on the one hand, and
the Holder, on the other hand.
m) Severability.
Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law,
but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the
extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
n) Headings.
The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
********************
(Signature Page Follows)
IN WITNESS WHEREOF, the Company
has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
| ISPECIMEN INC. |
|
| |
|
|
|
| By: |
|
|
| |
Name: |
Tracy Curley |
|
| |
Title: |
Chief Executive Officer |
|
EXHIBIT A
NOTICE OF EXERCISE
To: ISPECIMEN INC.
(1) The undersigned hereby
elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant (only if exercised in full), and
tenders herewith payment of the exercise price in full, together with all applicable transfer taxes, if any.
(2) Payment shall take
the form of (check applicable box):
[ ] in lawful money of the United States;
or
[ ] if permitted the cancellation of such
number of Warrant Shares as is necessary, in accordance with the formula set forth in subsection 2(c), to exercise this Warrant with respect
to the maximum number of Warrant Shares purchasable pursuant to the cashless exercise procedure set forth in subsection 2(c).
(3) Please issue said
Warrant Shares in the name of the undersigned or in such other name as is specified below:
_______________________________
The Warrant Shares shall be delivered to the following
DWAC Account Number:
_______________________________
_______________________________
_______________________________
[SIGNATURE OF HOLDER]
Name of Investing Entity: ____________________________________________________________________________________________________
Signature of Authorized Signatory of Investing
Entity: ______________________________________________________________________________
Name of Authorized Signatory: ________________________________________________________________________________________________
Title of Authorized Signatory: _________________________________________________________________________________________________
Date: ____________________________________________________________________________________________________________________
EXHIBIT B
ASSIGNMENT FORM
(To assign the foregoing Warrant, execute this
form and supply required information. Do not use this form to purchase shares.)
FOR VALUE RECEIVED, the foregoing Warrant and
all rights evidenced thereby are hereby assigned to
| Name: |
|
|
| |
|
(Please Print) |
| Address: |
|
|
| |
|
(Please Print) |
| Phone Number: |
|
|
| Email Address: |
|
|
| |
|
|
| Dated: _______________ __, ______ |
|
|
| Holder’s Signature:__________________________ |
|
|
| Holder’s Address:__________________________ |
|
|
EXHIBIT 5.1

October 18, 2024
iSpecimen Inc.
8 Cabot Road, Suite 1800
Woburn, MA, 08101
| Re: |
iSpecimen Inc. |
| |
Registration Statement on Form S-1, Registration
No. 333-__
|
Ladies and Gentlemen:
We have acted as counsel to iSpecimen Inc., a
Delaware corporation (the “Company”), in connection with the preparation of the Company’s registration
statement on Form S-1 (Registration No. 333-__ ) and the preliminary prospectus forming a part of the registration statement (the “Prospectus”),
under the Securities Act of 1933, as amended (the “Securities Act”), initially filed by the Company with the
Securities and Exchange Commission (the “Commission”) on October 10, 2024, as thereafter amended or supplemented
(the “Registration Statement”). The Prospectus relates to the registration of the proposed offering of (i)
up to 1,030,928 shares (the “Shares”) of common stock of the Company, par value $0.0001 per share (the “Common
Stock”) and (ii) up to an aggregate of 1,030,928 pre-funded warrants (the “Pre-Funded Warrants”)
to purchase one share of Common Stock (the “Pre-Funded Warrant Shares”). For each Pre-Funded Warrant the Company
sells, the number of Shares offered will be decreased on a one-for-one basis. The Shares, the Pre-Funded Warrants, and the Pre-Funded
Warrant Shares are collectively referred to as the “Securities.”
In rendering the opinion set forth herein, we
have examined the originals, or photostatic or certified copies, of (i) the Amended and Restated Articles of Incorporation and Amended
and Restated Bylaws of the Company, each as amended and/or restated as of the date hereof (together, the “Company Charter
Documents”); (ii) certain resolutions of the Board of Directors of the Company (the “Board”) related
to the filing of the Registration Statement and related matters; (iii) the Registration Statement and all exhibits thereto; (iv) the form
of securities purchase agreement to be entered into by and among the Company and the purchasers named therein (the “Securities
Purchase Agreement”); (v) the form of Pre-Funded Warrant; and (vi) such other records, documents and instruments as we deemed
relevant and necessary for purposes of the opinion stated herein.
We have examined such documents and have reviewed
such questions of law as we have considered necessary or appropriate for the purposes of our opinions set forth below. In rendering our
opinions set forth below, we have assumed the authenticity of all documents submitted to us as originals, the genuineness of all signatures
and the conformity to authentic originals of all documents submitted to us as copies. We have also assumed the legal capacity for all
purposes relevant hereto of all natural persons. As to questions of fact material to our opinions, we have relied upon certificates or
comparable documents of officers and other representatives of the Company and of public officials.
We have also assumed that, at the time of the
issuance of the Securities: (i) the Company will continue to be incorporated and in existence and good standing in its jurisdiction of
organization; (ii) the resolutions of the Board referred to above will not have been modified or rescinded; (iii) all Securities will
be offered, issued and sold in compliance with applicable federal and state securities laws and in the manner stated in the Prospectus;
and (iv) the Securities Purchase Agreement will have been duly authorized and validly executed and delivered by the parties thereto and
will be enforceable against the parties thereto in accordance with its terms.
The opinion expressed herein is limited to the
Delaware General Corporation Law. We have not considered, and express no opinion, as to the laws of any other state or jurisdiction.

Based on the foregoing, and subject to the assumptions,
qualifications, limitations and exceptions set forth herein, we are of the opinion that:
| |
1. |
When the Securities Purchase Agreement has
been duly executed and delivered by the respective parties thereto and the Shares have been issued and delivered in accordance with the
Securities Purchase Agreement against payment in full of the consideration payable therefor as determined by the Board or a duly authorized
committee thereof and as contemplated by the Securities Purchase Agreement, the Shares will be validly issued, fully paid and non-assessable. |
| |
2. |
When the Securities Purchase Agreement has been duly executed and delivered by the respective parties thereto and the Pre-Funded Warrants have been issued and delivered in accordance with the Securities Purchase Agreement against payment in full of the consideration payable therefor as determined by the Board or a duly authorized committee thereof and as contemplated by the Securities Purchase Agreement, the Pre-Funded Warrants will constitute valid and legally binding obligations of the Company. |
| |
3. |
When the Securities Purchase Agreement has
been duly executed and delivered by the respective parties thereto, the Pre-Funded Warrants have been duly executed by the Company and
delivered to and paid for by the investors pursuant to the terms of the Securities Purchase Agreement against payment in full of the
consideration payable therefor as determined by the Board or a duly authorized committee thereof and as contemplated by the Securities
Purchase Agreement (a) the Pre-Funded Warrant Shares will have been duly authorized for issuance, and (b) if, as and when issued against
payment in full of the consideration payable therefor in accordance with the terms of the Pre-Funded Warrants, the Pre-Funded Warrant
Shares will be validly issued, fully paid and non-assessable. |
The opinions expressed herein as to the validity
and legally binding obligation of the Pre-Funded Warrants are subject to and qualified and limited (i) by applicable bankruptcy, insolvency,
fraudulent transfer and conveyance, reorganization, moratorium and similar laws affecting creditors’ rights and remedies generally;
(ii) as enforceability of any indemnification or contribution provision may be limited under the federal and state securities laws; and
(iii) by general principles of equity, including without limitation, concepts of materiality, reasonableness, good faith and fair dealing
and the possible unavailability of specific performance or injunctive relief (regardless of whether considered in a proceeding in equity
or at law).
We hereby consent to the filing of this opinion
with the Commission as an exhibit to the Registration Statement. We further consent to the reference to our firm under the caption “Legal
Matters” in the prospectus constituting a part of the Registration Statement. In giving this consent, we are not admitting that
we are within the category of persons whose consent is required under Section 7 of the Securities Act or the rules and regulations of
the Commission. This opinion is given as of the date hereof and we assume no obligation to update or supplement such opinion after the
date hereof to reflect any facts or circumstances that may thereafter come to our attention or any changes that may thereafter occur.
| |
Very truly yours, |
| |
|
| |
/s/ Sichenzia Ross Ference Carmel LLP |
| |
Sichenzia Ross Ference Carmel LLP |
Exhibit 10.46
SECURITIES
PURCHASE AGREEMENT
This Securities Purchase Agreement
(this “Agreement”) is dated as of _, 2024, between iSpecimen Inc., a Delaware corporation (the “Company”),
and each purchaser identified on the signature pages hereto (each, including its successors and assigns, a “Purchaser”
and collectively the “Purchasers”).
WHEREAS, subject to the terms
and conditions set forth in this Agreement and pursuant to an effective registration statement under the Securities Act of 1933, as amended
(the “Securities Act”), the Company desires to issue and sell to each Purchaser, and each Purchaser, severally and
not jointly, desires to purchase from the Company, securities of the Company as more fully described in this Agreement.
NOW, THEREFORE, IN CONSIDERATION
of the mutual covenants contained in this Agreement, and for other good and valuable consideration the receipt and adequacy of which are
hereby acknowledged, the Company and each Purchaser agree as follows:
ARTICLE I.
DEFINITIONS
1.1 Definitions. In
addition to the terms defined elsewhere in this Agreement, for all purposes of this Agreement, the following terms have the meanings set
forth in this Section 1.1:
“Acquiring Person”
shall have the meaning ascribed to such term in Section 4.5.
“Action”
shall have the meaning ascribed to such term in Section 3.1(j).
“Affiliate”
means any Person that, directly or indirectly through one or more intermediaries, controls or is controlled by or is under common control
with a Person as such terms are used in and construed under Rule 405 under the Securities Act.
“Board
of Directors” means the board of directors of the Company.
“Business
Day” means any day except any Saturday, any Sunday, any day which is a federal legal holiday in the United States or any day
on which banking institutions in the State of New York are authorized or required by law or other governmental action to close.
“Closing”
means the closing of the purchase and sale of the Securities pursuant to Section 2.1.
“Closing
Date” means the Trading Day on which all of the Transaction Documents have been executed and delivered by the applicable parties
thereto, and all conditions precedent to (i) the Purchasers’ obligations to pay the Subscription Amount and (ii) the Company’s
obligations to deliver the Securities, in each case, have been satisfied or waived, but in no event later than the second (2nd)
Trading Day following the date hereof.
“Commission”
means the United States Securities and Exchange Commission.
“Common
Stock” means the common stock of the Company, par value $0.0001 per share, and any other class of securities into which such
securities may hereafter be reclassified or changed.
“Common
Stock Equivalents” means any securities of the Company or the Subsidiaries which would entitle the holder thereof to acquire
at any time Common Stock, including, without limitation, any debt, preferred stock, right, option, warrant or other instrument that is
at any time convertible into or exercisable or exchangeable for, or otherwise entitles the holder thereof to receive, Common Stock.
“Company
Counsel” means Sichenzia Ross Ference Carmel LLP, with offices located at 1185 Avenue of the Americas, 31st Floor,
New York, NY 10036.
“Disclosure Schedules” means the Disclosure Schedules
of the Company delivered concurrently herewith.
“Disclosure
Time” means, (i) if this Agreement is signed on a day that is not a Trading Day or after 9:00 a.m. (New York City
time) and before midnight (New York City time) on any Trading Day, 9:01 a.m. (New York City time) on the Trading Day immediately
following the date hereof, unless otherwise instructed as to an earlier time by the Placement Agent, and (ii) if this Agreement is
signed between midnight (New York City time) and 9:00 a.m. (New York City time) on any Trading Day, no later than 9:01 a.m. (New
York City time) on the date hereof, unless otherwise instructed as to an earlier time by the Placement Agent.
“Disqualification
Event” shall have the meaning ascribed to such term in Section 3.1(oo).
“Evaluation
Date” shall have the meaning ascribed to such term in Section 3.1(s).
“Exchange
Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“Exempt
Issuance” means (i) the adoption of an equity incentive plan approved by the Company’s independent directors, and
the grant of awards or equity pursuant to any such equity incentive plan or existing plan disclosed in the Registration Statement to officers,
directors, employees or consultants of the Company or any of its subsidiaries, and the filing of a registration statement on Form S-8
relating thereto; (ii) the issuance of securities pursuant to the conversion or exercise of Common Stock Equivalents outstanding
prior to the date of this Agreement; and (iii) the issuance of equity securities in connection with an acquisition or a strategic
relationship, which may include the sale of securities, and the filing of a registration statement on Form S-4 relating thereto,
provided that any such issuance shall only be to an entity (or to the equity holders of an entity) which is, itself or through its subsidiaries,
an operating company or an owner of an asset in a business synergistic with the business of the Company and shall provide to the Company
additional benefits in addition to the investment of funds, but shall not include a transaction in which the Company is issuing securities
primarily for the purpose of raising capital or to an entity whose primary business is investing in securities.
“FCPA”
means the Foreign Corrupt Practices Act of 1977, as amended.
“GAAP”
shall have the meaning ascribed to such term in Section 3.1(h).
“Indebtedness”
shall have the meaning ascribed to such term in Section 3.1(aa).
“Intellectual
Property Rights” shall have the meaning ascribed to such term in Section 3.1(p).
“Issuer
Covered Person” shall have the meaning ascribed to such term in Section 3.1(oo).
“LB”
means Lucosky Brookman LLP, counsel to the Underwriters, with offices located at 101 Wood Ave South, 5th Floor, Woodbridge, NJ 08830.
“Liens”
means a lien, charge, pledge, security interest, encumbrance, right of first refusal, preemptive right or other restriction, other than
Permitted Liens.
“Lock-Up
Agreements” means the Lock-Up Agreements, dated as of the date hereof, by and between the Company and each of the officers,
and directors of the Company and holders of five percent (5.0%) or more of the outstanding shares of Common Stock, as of the date of this
Agreement, in the form of Exhibit B attached hereto.
“Material
Adverse Effect” shall have the meaning assigned to such term in Section 3.1(b).
“Material
Permits” shall have the meaning ascribed to such term in Section 3.1(n).
“Permitted Liens” means (i) any Lien for taxes
not yet due or delinquent or being contested in good faith by appropriate proceedings for which adequate reserves have been established
in accordance with GAAP, (ii) any statutory Lien arising in the ordinary course of business by operation of law with respect to
a liability that is not yet due or delinquent, (iii) any Lien created by operation of law, such as materialmen’s liens, mechanics’
liens and other similar liens, arising in the ordinary course of business with respect to a liability that is not yet due or delinquent
or that are being contested in good faith by appropriate proceedings, (iv) Liens (A) upon or in any equipment acquired or held
by the Company or any of its Subsidiaries to secure the purchase price of such equipment or Indebtedness incurred solely for the purpose
of financing the acquisition or lease of such equipment, or (B) existing on such equipment at the time of its acquisition, provided
that the Lien is confined solely to the property so acquired and improvements thereon, and the proceeds of such equipment, in either
case, with respect to Indebtedness in an aggregate amount not to exceed $100,000, (v) Liens incurred in connection with the extension,
renewal or refinancing of the Indebtedness secured by Liens of the type described in clause (iv) above, provided that any extension,
renewal or replacement Lien shall be limited to the property encumbered by the existing Lien and the principal amount of the Indebtedness
being extended, renewed or refinanced does not increase, (vi) Liens in favor of customs and revenue authorities arising as a matter
of law to secure payments of custom duties in connection with the importation of goods, and (vii) Liens arising from judgments,
decrees or attachments in circumstances not constituting a default under this Agreement.
“Per Share
Purchase Price” equals $[●], subject to adjustment for reverse and forward stock splits, stock dividends, stock combinations
and other similar transactions of the Common Stock that occur after the date of this Agreement.
“Person”
means an individual or corporation, partnership, trust, incorporated or unincorporated association, joint venture, limited liability company,
joint stock company, government (or an agency or subdivision thereof) or other entity of any kind.
“Placement
Agent” means WestPark Capital, Inc.
“Placement
Agency Agreement” means the Placement Agency Agreement by and between the Company and the Placement Agent dated the date hereof.
“Pre-Funded
Warrant” means, collectively, the Pre-Funded Common Stock purchase warrants delivered to the Purchasers at the Closing in accordance
with Section 2.2(a), which Pre-Funded Warrants shall be exercisable immediately and shall expire when exercised in full, in the form
of Exhibit A attached hereto.
“Pre-Funded
Warrant Shares” means Common Stock issuable upon exercise of the Pre-Funded Warrants.
“Pre-Funded
Warrant Purchase Price” equals $0.0001 per each Pre-Funded Warrant, subject to adjustment for reverse and forward stock splits,
stock dividends, stock combinations and other similar transactions of the Common Stock that occur after the date of this Agreement.
“Pre-Funded
Warrant Subscription Amount” means, as to each Purchaser, the aggregate amount to be paid for the Pre-Funded Warrants purchased
hereunder as specified below such Purchaser’s name on the signature page of this Agreement and next to the heading “Pre-Funded
Warrant Subscription Amount,” in immediately available funds.
“Proceeding”
means an action, claim, suit, investigation or proceeding (including, without limitation, an informal investigation or partial proceeding,
such as a deposition), whether commenced or, to the Company’s knowledge, threatened.
“Prospectus”
means the final prospectus filed for the Registration Statement.
“Purchaser
Party” shall have the meaning ascribed to such term in Section 4.8.
“Registration
Statement” means the effective registration statement with Commission File No. 333-[●], which registers the sale
of the Shares, the Pre-Funded Warrants and the Pre-Funded Warrant Shares to the Purchasers.
“Required
Approvals” shall have the meaning ascribed to such term in Section 3.1(e).
“Rule 144”
means Rule 144 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from
time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect
as such Rule.
“Rule 424”
means Rule 424 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from
time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect
as such Rule.
“SEC Reports”
shall have the meaning ascribed to such term in Section 3.1(h).
“Securities”
means the Shares, the Pre-Funded Warrants and the Pre-Funded Warrant Shares.
“Securities
Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Shares”
means the shares of Common Stock issued or issuable to each Purchaser pursuant to this Agreement.
“Short
Sales” means all “short sales” as defined in Rule 200 of Regulation SHO under the Exchange Act (but shall not
be deemed to include locating and/or borrowing shares of Common Stock).
“Subscription
Amount” means, as to each Purchaser, the Common Stock Subscription Amount and/or Pre-Funded Warrant Subscription Amount as applicable,
specified below such Purchaser’s name on the signature page of this Agreement and next to the heading “Subscription Amount,”
in United States dollars and in immediately available funds.in accordance with Section 2.1.
“Subsidiary”
means any subsidiary of the Company as set forth on Section 3.1(a), and shall, where applicable, also include any direct or indirect
subsidiary of the Company formed or acquired after the date hereof.
“Trading
Day” means a day on which the principal Trading Market is open for trading.
“Trading
Market” means any of the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date
in question: the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market or the New York Stock
Exchange (or any successors to any of the foregoing).
“Transaction
Documents” means this Agreement, the Pre-Funded Warrants, the Placement Agency Agreement, the Lock-Up Agreement and all exhibits
and schedules thereto and hereto.
“Transfer
Agent” means Broadridge Corporate Issuer Solutions, LLC, the current transfer agent of the Company, with a mailing address of
51 Mercedes Way, Edgewood, NY 11717, and a telephone number of 1-631-254-7400, and any successor transfer agent of the Company.
“VWAP”
means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed
or quoted on a Trading Market, the daily volume weighted average price of the Common Stock for such date (or the nearest preceding date)
on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30
a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not a Trading Market, the volume
weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c) if
the Common Stock is not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Stock are then reported in The
Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per
share of the Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined
by an independent appraiser selected in good faith by the Purchasers of a majority in interest of the Securities then outstanding and
reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
ARTICLE II.
PURCHASE AND SALE
2.1 Closing. On the
Closing Date, upon the terms and subject to the conditions set forth herein, substantially concurrent with the execution and delivery
of this Agreement by the parties hereto, the Company agrees to sell, and the Purchasers, severally and not jointly, agree to purchase,
up to an aggregate of $5,000.000 Shares determined pursuant to Section 2.2(a); provided, however, that, to the extent that a Purchaser
determines, in its sole discretion, that such Purchaser (together with such Purchaser’s Affiliates, and any Person acting as a group
together with such purchaser or any of such Purchaser’s Affiliates) would beneficially own in excess of the Beneficial Ownership
Limitation, or as such Purchaser may otherwise choose, in lieu of purchasing the Shares such Purchaser may elect to purchase Pre-Funded
Warrants at the Pre-Funded Warrant Purchase Price in lieu of the Shares. The “Beneficial Ownership Limitation” shall be 4.99%
(or, at the election of the Purchaser at Closing, 9.99%) of the number of shares of the Common Stock outstanding immediately after giving
effect to the issuance of the Securities on the Closing Date. Unless otherwise directed by the Placement Agent, each Purchaser’s
Subscription Amount as set forth on the signature page hereto executed by such Purchaser shall be made available for “Delivery
Versus Payment” settlement with the Company or its designee. The Company shall deliver to each Purchaser its respective Shares and/or
Pre-Funded Warrants (as applicable to such purchaser), as determined pursuant to Section 2.2(a), and the Company and each Purchaser
shall deliver the other items set forth in Section 2.2 deliverable at the Closing. Upon satisfaction of the covenants and conditions
set forth in Sections 2.2 and 2.3, the Closing shall occur at the offices of LB, such other location as the parties shall mutually agree
or may be closed remotely by electronic delivery of documents. Unless otherwise directed by the Placement Agent, settlement of the Shares
shall occur via “Delivery Versus Payment” (“DVP”) (i.e., on the Closing Date, the Company shall issue the
Shares registered in the Purchasers’ names and addresses and released by the Transfer Agent directly to the account(s) at the
Placement Agent identified by each Purchaser; upon receipt of such Shares, the Placement Agent shall promptly electronically deliver such
Shares to the applicable Purchaser, and payment therefor shall be made by the Placement Agent (or its clearing firm) by wire transfer
to the Company). Notwithstanding anything to the contrary herein and a Purchaser’s Subscription Amount set forth on the signature
pages attached hereto, the number of Shares purchased by a Purchaser (and its Affiliates) hereunder shall not, when aggregated with
all other Common Stock owned by such Purchaser (and its Affiliates) at such time, result in such Purchaser beneficially owning (as determined
in accordance with Section 13(d) of the Exchange Act) in excess of 9.9% of the then issued and outstanding Common Stock outstanding
at the Closing (the “Beneficial Ownership Maximum”), and such Purchaser’s Subscription Amount, to the extent it would
otherwise exceed the Beneficial Ownership Maximum immediately prior to the Closing, shall be conditioned upon the issuance of Shares at
the Closing to the other Purchasers signatory hereto. To the extent that a Purchaser’s beneficial ownership of the Shares would
otherwise be deemed to exceed the Beneficial Ownership Maximum, such Purchaser’s Subscription Amount shall automatically be reduced
as necessary in order to comply with this paragraph. Notwithstanding the foregoing, with respect to any Notice(s) of Exercise (as
defined in the Pre-Funded Warrants) delivered on or prior to 12:00 p.m. (New York City time) on the Closing Date, which may be delivered
at any time after the time of execution of the this Agreement, the Company agrees to deliver the Pre-Funded Warrant Shares subject to
such notice(s) by 4:00 p.m. (New York City time) on the Closing Date and the Closing Date shall be the Warrant Share Delivery
Date (as defined in the Pre-Funded Warrants) for purposes hereunder.
2.2 Deliveries.
(a) On or prior
to the Closing Date, the Company shall deliver or cause to be delivered to each Purchaser the following:
(i) on the date
hereof, this Agreement duly executed by the Company;
(ii) a legal
opinion of Company Counsel, including without limitation, a negative assurance letter, in each case in the form and substance reasonably
satisfactory to the Placement Agent and each of the Purchasers;
(iii) on the
date hereof, a comfort letter, addressed to the Placement Agent in form and substance reasonably satisfactory in all material respects
from Wolf & Company, P.C. (as applicable);
(iv) on the Closing
Date, a comfort letter from Wolf & Company, P.C., addressed to the Placement Agent dated as of such Closing Date, in form and
substance satisfactory to the Placement Agent, to the effect that they reaffirm the statements made in the letter furnished pursuant to
Section 2.2(a)(iii) except that the specified date referred to therein for the carrying out of procedures shall be no more than
three business days prior to such Closing Date;
(v) subject to
the last sentence of Section 2.1, the Company shall have provided each Purchaser with the Company’s wire instructions, on Company
letterhead and executed by the Chief Executive Officer or Chief Financial Officer;
(vi) subject
to the last sentence of Section 2.1, a copy of the irrevocable instructions to the Transfer Agent instructing the Transfer Agent
to deliver, on an expedited basis via The Depository Trust Company Deposit or Withdrawal at Custodian system (“DWAC”),
Shares equal to such Purchaser’s Subscription Amount divided by the Per Share Purchase Price, registered in the name of such Purchaser;
(vii) for each
Purchaser of Pre-Funded Warrants pursuant to Section 2.1, a Pre-Funded Warrant registered in the name of such Purchaser to purchase
up to a number of shares of Common Stock equal to the portion of such Purchaser’s Subscription Amount applicable to Pre-Funded Warrant
divided by the Pre-Funded Warrant Purchase Price, with an exercise price equal to $0.0001 subject to adjustment therein;
(viii) on the
date hereof, the duly executed Lock-Up Agreements; and
(ix) the Prospectus
(which may be delivered in accordance with Rule 172 under the Securities Act).
(b) On or prior
to the Closing Date, each Purchaser shall deliver or cause to be delivered to the Company, the following:
(i) on the date
hereof, this Agreement duly executed by such Purchaser; and
(ii) such Purchaser’s
Subscription Amount, which shall be made available for “Delivery Versus Payment” settlement with the Company or its designee.
2.3 Closing Conditions.
(a) The obligations
of the Company hereunder in connection with the Closing are subject to the following conditions being met:
(i) the accuracy
in all material respects (or, to the extent representations or warranties are qualified by materiality or Material Adverse Effect, in
all respects) on the Closing Date of the representations and warranties of the Purchasers contained herein (unless as of a specific date
therein in which case they shall be accurate as of such date);
(ii) all obligations,
covenants and agreements of each Purchaser required to be performed at or prior to the Closing Date shall have been performed; and
(iii) the delivery
by each Purchaser of the items set forth in Section 2.2(b) of this Agreement.
(b) The respective
obligations of the Purchasers hereunder in connection with the Closing are subject to the following conditions being met:
(i) the accuracy
in all material respects (or, to the extent representations or warranties are qualified by materiality or Material Adverse Effect, in
all respects) when made and on the Closing Date of the representations and warranties of the Company contained herein (unless as of a
specific date therein in which case they shall be accurate as of such date);
(ii) all obligations,
covenants and agreements of the Company required to be performed at or prior to the Closing Date shall have been performed;
(iii) the delivery
by the Company of the items set forth in Section 2.2(a) of this Agreement;
(iv) there shall
have been no Material Adverse Effect with respect to the Company since the date hereof; and
(v) from the
date hereof to the Closing Date, trading in the Common Stock shall not have been suspended by the Commission or the Company’s principal
Trading Market, and, at any time prior to the Closing Date, trading in securities generally as reported by Bloomberg L.P. shall not have
been suspended or limited, or minimum prices shall not have been established on securities whose trades are reported by such service,
or on any Trading Market, nor shall a banking moratorium have been declared either by the United States or New York State authorities
nor shall there have occurred any material outbreak or escalation of hostilities or other national or international calamity of such magnitude
in its effect on, or any material adverse change in, any financial market which, in each case, in the reasonable judgment of such Purchaser,
makes it impracticable or inadvisable to purchase the Securities at the Closing.
ARTICLE III.
REPRESENTATIONS AND WARRANTIES
3.1 Representations and
Warranties of the Company. Except as set forth in the Disclosure Schedules, which Disclosure Schedules shall be deemed a part hereof
and shall qualify any representation or otherwise made herein to the extent of the disclosure contained in the corresponding section of
the Disclosure Schedules, the Company hereby makes the following representations and warranties to each Purchaser:
(a) Subsidiaries.
The Company has no subsidiaries, except as set forth in the SEC Reports.
(b) Organization
and Qualification. The Company and each of the Subsidiaries is an entity duly incorporated or otherwise organized, validly existing
and in good standing (if a good standing concept exists in such jurisdiction) under the laws of the jurisdiction of its incorporation
or organization, with the requisite power and authority to own and use its properties and assets and to carry on its business as currently
conducted. Neither the Company nor any Subsidiary is in violation or default of any of the provisions of its respective certificate or
articles of incorporation, bylaws or other organizational or charter documents. Each of the Company and the Subsidiaries is duly qualified
to conduct business and is in good standing (if a good standing concept exists in such jurisdiction) as a foreign corporation or other
entity in each jurisdiction in which the nature of the business conducted or property owned by it makes such qualification necessary,
except where the failure to be so qualified or in good standing, as the case may be, could not have or reasonably be expected to result
in: (i) a material adverse effect on the legality, validity or enforceability of any Transaction Document, (ii) a material adverse
effect on the results of operations, assets, business, prospects or condition (financial or otherwise) of the Company and the Subsidiaries,
taken as a whole, or (iii) a material adverse effect on the Company’s ability to perform in any material respect on a timely
basis its obligations under any Transaction Document (any of (i), (ii) or (iii), a “Material Adverse Effect”)
and no Proceeding has been instituted in any such jurisdiction revoking, limiting or curtailing or seeking to revoke, limit or curtail
such power and authority or qualification.
(c) Authorization;
Enforcement. The Company has the requisite corporate power and authority to enter into and to consummate the transactions contemplated
by this Agreement and each of the other Transaction Documents and otherwise to carry out its obligations hereunder and thereunder. The
execution and delivery of this Agreement and each of the other Transaction Documents by the Company and the consummation by it of the
transactions contemplated hereby and thereby have been duly authorized by all necessary action on the part of the Company and no further
action is required by the Company, the Board of Directors or the Company’s stockholders in connection herewith or therewith other
than in connection with the Required Approvals as set forth in Section 3.1(e) of this Agreement. This Agreement and each other
Transaction Document to which it is a party has been (or upon delivery will have been) duly executed by the Company and, when delivered
in accordance with the terms hereof and thereof, will constitute the valid and binding obligation of the Company enforceable against the
Company in accordance with its terms, except (i) as limited by general equitable principles and applicable bankruptcy, insolvency,
reorganization, moratorium and other laws of general application affecting enforcement of creditors’ rights generally, (ii) as
limited by laws relating to the availability of specific performance, injunctive relief or other equitable remedies and (iii) insofar
as indemnification and contribution provisions may be limited by applicable law.
(d) No Conflicts.
Except as set forth on Schedule 3.1(d), the execution, delivery and performance by the Company of this Agreement and the other
Transaction Documents to which it is a party, the issuance and sale of the Securities and the consummation by it of the transactions contemplated
hereby and thereby do not and will not (i) conflict with or violate any provision of the Company’s or any Subsidiary’s
certificate or articles of incorporation, bylaws or other organizational or charter documents, or (ii) conflict with, or constitute
a default (or an event that with notice or lapse of time or both would become a default) under, result in the creation of any Lien upon
any of the properties or assets of the Company or any Subsidiary, or give to others any rights of termination, amendment, acceleration
or cancellation (with or without notice, lapse of time or both) of, any agreement, credit facility, debt or other instrument (evidencing
a Company or Subsidiary debt or otherwise) or other understanding to which the Company or any Subsidiary is a party or by which any property
or asset of the Company or any Subsidiary is bound or affected, or (iii) subject to the Required Approvals, conflict with or result
in a violation of any law, rule, regulation, order, judgment, injunction, decree or other restriction of any court or governmental authority
to which the Company or a Subsidiary is subject (including federal and state securities laws and regulations), or by which any property
or asset of the Company or a Subsidiary is bound or affected; except in the case of each of clauses (ii) and (iii), such as could
not have or reasonably be expected to result in a Material Adverse Effect.
(e) Filings,
Consents and Approvals. The Company is not required to obtain any consent, waiver, authorization or order of, give any notice to,
or make any filing or registration with, any court or other federal, state, local or other governmental authority or other Person in connection
with the execution, delivery and performance by the Company of the Transaction Documents, other than: (i) the filings required pursuant
to Section 4.4 of this Agreement, (ii) the filing with the Commission of the Prospectus, (iii) application(s) to each
applicable Trading Market for the listing of the Shares and Pre-Funded Warrant Shares for trading thereon in the time and manner required
thereby (iv) such filings as are required to be made under applicable state securities laws and (v) the filing of Form D
with the Commission, if applicable, and such filings as are required to be made under applicable state securities laws (collectively,
the “Required Approvals”).
(f) Issuance
of the Securities; Registration. The Securities are duly authorized and, when issued and paid for in accordance with the applicable
Transaction Documents, will be duly and validly issued, fully paid and nonassessable, free and clear of all Liens imposed by the Company.
The Pre-Funded Warrant Shares, when issued in accordance with the terms of the Pre-Funded Warrants, will be validly issued, fully paid
and nonassessable, free and clear of all Liens imposed by the Company. The Company has reserved from its duly authorized capital stock
the maximum number of shares of Common Stock issuable pursuant to this Agreement and the Pre-Funded Warrants. The Company has prepared
and filed the Registration Statement in conformity with the requirements of the Securities Act, which initially became effective on [●],
2024, including the Prospectus, and such amendments and supplements thereto as may have been required to the date of this Agreement. The
Registration Statement is effective under the Securities Act and no stop order preventing or suspending the effectiveness of the Registration
Statement or suspending or preventing the use of the Prospectus has been issued by the Commission and no proceedings for that purpose
have been instituted or, to the knowledge of the Company, are threatened by the Commission. The Company, if required by the rules and
regulations of the Commission, shall file the Prospectus with the Commission pursuant to Rule 424(b). At the time the Registration
Statement and any amendments thereto became effective, at the date of this Agreement and at the Closing Date, the Registration Statement
and any amendments thereto conformed and will conform in all material respects to the requirements of the Securities Act and did not and
will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary
to make the statements therein not misleading; and the Prospectus and any amendments or supplements thereto, at the time the Prospectus
or any amendment or supplement thereto was issued and at the Closing Date, conformed and will conform in all material respects to the
requirements of the Securities Act and did not and will not contain an untrue statement of a material fact or omit to state a material
fact necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading.
The Company was at the time of the filing of the Registration Statement eligible to use Form S-3. The Company is eligible to use
Form S-3 under the Securities Act and it meets the transaction requirements with respect to the aggregate market value of securities
being sold pursuant to this offering and during the twelve (12) months prior to this offering, as set forth in General Instruction I.B.6
of Form S-3.
(g) Capitalization.
The capitalization of the Company as of the date hereof is as set forth on Schedule 3.1(g), which Schedule 3.1(g) shall
also include the number of shares of Common Stock owned beneficially, and of record, by Affiliates of the Company as of the date hereof.
Other than as set forth on Schedule 3.1(g), the Company has not issued any capital stock since its most recently filed periodic
report under the Exchange Act, other than pursuant to the exercise of employee stock options under the Company’s stock option plans,
the issuance of shares of Common Stock to employees pursuant to the Company’s employee stock purchase plans and pursuant to the
conversion and/or exercise of Common Stock Equivalents outstanding as of the date of the most recently filed periodic report under the
Exchange Act. Except as set forth on Schedule 3.1(g), no Person has any right of first refusal, preemptive right, right of participation,
or any similar right to participate in the transactions contemplated by the Transaction Documents. Except as set forth on Schedule
3.1(g) and as a result of the purchase and sale of the Securities, there are no outstanding options, warrants, scrip rights to
subscribe to, calls or commitments of any character whatsoever relating to, or securities, rights or obligations convertible into or exercisable
or exchangeable for, or giving any Person any right to subscribe for or acquire, any shares of Common Stock or the capital stock of any
Subsidiary, or contracts, commitments, understandings or arrangements by which the Company or any Subsidiary is or may become bound to
issue additional shares of Common Stock or Common Stock Equivalents or capital stock of any Subsidiary. The issuance and sale of the Securities
will not obligate the Company or any Subsidiary to issue shares of Common Stock or other securities to any Person (other than the Purchasers).
Except as set forth on Schedule 3.1(g), there are no outstanding securities or instruments of the Company or any Subsidiary with
any provision that adjusts the exercise, conversion, exchange or reset price of such security or instrument upon an issuance of securities
by the Company or any Subsidiary. There are no outstanding securities or instruments of the Company or any Subsidiary that contain any
redemption or similar provisions, and there are no contracts, commitments, understandings or arrangements by which the Company or any
Subsidiary is or may become bound to redeem a security of the Company or such Subsidiary. The Company does not have any stock appreciation
rights or “phantom stock” plans or agreements or any similar plan or agreement. All of the outstanding shares of capital stock
of the Company are duly authorized, validly issued, fully paid and nonassessable, have been issued in material compliance with all applicable
federal and state securities laws, and none of such outstanding shares was issued in violation of any preemptive rights or similar rights
to subscribe for or purchase securities. No further approval or authorization of any stockholder, the Board of Directors or others is
required for the issuance and sale of the Securities. There are no stockholders agreements, voting agreements or other similar agreements
with respect to the Company’s capital stock to which the Company is a party or, to the knowledge of the Company, between or among
any of the Company’s stockholders.
(h) SEC
Reports; Financial Statements. The Company has filed all reports, schedules, forms, statements and other documents required to be
filed by the Company under the Securities Act and the Exchange Act, including pursuant to Section 13(a) or 15(d) thereof,
for the two years preceding the date hereof (or such shorter period as the Company was required by law or regulation to file such material)
(the foregoing materials, including the exhibits thereto and documents incorporated by reference therein, together with the Prospectus,
being collectively referred to herein as the “SEC Reports”) on a timely basis or has received a valid extension of
such time of filing and has filed any such SEC Reports prior to the expiration of any such extension. As of their respective dates, the
SEC Reports complied in all material respects with the requirements of the Securities Act and the Exchange Act, as applicable, and none
of the SEC Reports, when filed, contained any untrue statement of a material fact or omitted to state a material fact required to be stated
therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading.
The Company has never been an issuer subject to Rule 144(i) under the Securities Act. The financial statements of the Company
included in the SEC Reports comply in all material respects with applicable accounting requirements and the rules and regulations
of the Commission with respect thereto as in effect at the time of filing. Such financial statements have been prepared in accordance
with United States generally accepted accounting principles (“GAAP”) applied on a consistent basis during the periods
involved, except as may be otherwise specified in such financial statements or the notes thereto and except that unaudited financial statements
may not contain all footnotes required by GAAP, and fairly present in all material respects the financial position of the Company and
its consolidated Subsidiaries as of and for the dates thereof and the results of operations and cash flows for the periods then ended,
subject, in the case of unaudited statements, to normal, immaterial, year-end audit adjustments.
(i) Material
Changes; Undisclosed Events, Liabilities or Developments. Since the date of the latest audited financial statements included within
the SEC Reports, except as set forth on Schedule 3.1(i), (i) there has been no event, occurrence or development that has had
or that could reasonably be expected to result in a Material Adverse Effect, (ii) the Company has not incurred any material liabilities
(contingent or otherwise) other than (A) trade payables and accrued expenses incurred in the ordinary course of business consistent
with past practice and (B) liabilities not required to be reflected in the Company’s financial statements pursuant to GAAP
or disclosed in filings made with the Commission, (iii) the Company has not altered its method of accounting, (iv) the Company
has not declared or made any dividend or distribution of cash or other property to its stockholders or purchased, redeemed or made any
agreements to purchase or redeem any shares of its capital stock and (v) the Company has not issued any equity securities to any
officer, director or Affiliate, except pursuant to existing Company stock option plans. The Company does not have pending before the Commission
any request for confidential treatment of information. Except for the issuance of the Securities contemplated by this Agreement or as
set forth on Schedule 3.1(i), no event, liability, fact, circumstance, occurrence or development has occurred or exists or is reasonably
expected to occur or exist with respect to the Company or its Subsidiaries or their respective businesses, prospects, properties, operations,
assets or financial condition that would be required to be disclosed by the Company under applicable securities laws at the time this
representation is made or deemed made that has not been publicly disclosed at least one (1) Trading Day prior to the date that this
representation is made.
(j) Litigation.
Except as set forth in the SEC Reports, there is no action, suit, inquiry, notice of violation, proceeding or investigation pending or,
to the knowledge of the Company, threatened against or affecting the Company, any Subsidiary or any of their respective properties before
or by any court, arbitrator, governmental or administrative agency or regulatory authority (federal, state, county, local or foreign)
(collectively, an “Action”) which such Action, (i) adversely affects or challenges the legality, validity or enforceability
of any of the Transaction Documents or the Securities or (ii) could, if there were an unfavorable decision, have or reasonably be
expected to result in a Material Adverse Effect. Neither the Company nor any Subsidiary, nor any current director or current officer thereof,
is or has been the subject of any Action involving a claim of violation of or liability under federal or state securities laws or a claim
of breach of fiduciary duty. There has not been, and to the knowledge of the Company, there is not pending or contemplated, any investigation
by the Commission involving the Company or, to the Company’s knowledge, any current or former director or officer of the Company.
The Commission has not issued any stop order or other order suspending the effectiveness of any registration statement filed by the Company
or any Subsidiary under the Exchange Act or the Securities Act.
(k) Labor
Relations. No material labor dispute exists or, to the knowledge of the Company, is imminent with respect to any of the employees
of the Company, which could reasonably be expected to result in a Material Adverse Effect. None of the Company’s or its Subsidiaries’
employees is a member of a union that relates to such employee’s relationship with the Company or such Subsidiary, and neither the
Company nor any of its Subsidiaries is a party to a collective bargaining agreement, and the Company and its Subsidiaries believe that
their relationships with their employees are good. To the knowledge of the Company, no executive officer of the Company or any Subsidiary,
is, or is now expected to be, in violation of any material term of any employment contract, confidentiality, disclosure or proprietary
information agreement or non-competition agreement, or any other contract or agreement or any restrictive covenant in favor of any third
party, and the continued employment of each such executive officer does not subject the Company or any of its Subsidiaries to any liability
with respect to any of the foregoing matters. The Company and its Subsidiaries are in compliance with all applicable U.S. federal, state,
local and foreign laws and regulations relating to employment and employment practices, terms and conditions of employment and wages and
hours, except where the failure to be in compliance could not, individually or in the aggregate, reasonably be expected to have a Material
Adverse Effect.
(l) Compliance.
Neither the Company nor any Subsidiary: (i) is in default under or in violation of (and no event has occurred that has not been waived
that, with notice or lapse of time or both, would result in a default by the Company or any Subsidiary under), nor has the Company or
any Subsidiary received notice of a claim that it is in default under or that it is in violation of, any indenture, loan or credit agreement
or any other agreement or instrument to which it is a party or by which it or any of its properties is bound (whether or not such default
or violation has been waived), (ii) is in violation of any judgment, decree or order of any court, arbitrator or other governmental
authority or (iii) is or has been in violation of any statute, rule, ordinance or regulation of any governmental authority, including,
without limitation, all foreign, federal, state and local laws relating to taxes, environmental protection, occupational health and safety,
product quality and safety and employment and labor matters, except in each case as could not have or reasonably be expected to result
in a Material Adverse Effect.
(m) Environmental
Laws. The Company and its Subsidiaries (i) are in compliance with all federal, state, local and foreign laws relating to pollution
or protection of human health or the environment (including ambient air, surface water, groundwater, land surface or subsurface strata),
including laws relating to emissions, discharges, releases or threatened releases of chemicals, pollutants, contaminants, or toxic or
hazardous substances or wastes (collectively, “Hazardous Materials”) into the environment, or otherwise relating to
the manufacture, processing, distribution, use, treatment, storage, disposal, transport or handling of Hazardous Materials, as well as
all authorizations, codes, decrees, demands, or demand letters, injunctions, judgments, licenses, notices or notice letters, orders, permits,
plans or regulations, issued, entered, promulgated or approved thereunder (“Environmental Laws”); (ii) have received
all permits licenses or other approvals required of them under applicable Environmental Laws to conduct their respective businesses; and
(iii) are in compliance with all terms and conditions of any such permit, license or approval where in each clause (i), (ii) and
(iii), the failure to so comply could be reasonably expected to have, individually or in the aggregate, a Material Adverse Effect. To
the Company’s knowledge, the costs and liabilities (including, without limitation, any capital or operating expenditures required
for clean-up, closure of properties or compliance with Environmental Laws, or any permit, license or approval, any related constraints
on operating activities and any potential liabilities to third parties) would not, singly or in the aggregate, have a Material Adverse
Effect on the Company.
(n) Regulatory
Permits. The Company and the Subsidiaries possess all certificates, authorizations and permits issued by the appropriate federal,
state, local or foreign regulatory authorities necessary to conduct their respective businesses as described in the SEC Reports, except
where the failure to possess such permits could not reasonably be expected to result in a Material Adverse Effect (“Material
Permits”), and neither the Company nor any Subsidiary has received any notice of proceedings relating to the revocation or modification
of any Material Permit.
(o) Title
to Assets. The Company and the Subsidiaries have good and marketable title in fee simple to all real property owned by them and good
and marketable title in all personal property owned by them that is material to the business of the Company and the Subsidiaries, in each
case free and clear of all Liens, except for Permitted Liens. Any real property and facilities held under lease by the Company and the
Subsidiaries are held by them under valid, subsisting and enforceable leases with which the Company and the Subsidiaries are in compliance,
except where the failure to be in compliance could not reasonably be expected to have a Material Adverse Effect.
(p) Intellectual
Property. To the knowledge of the Company, the Company and the Subsidiaries have, or have rights to use, all patents, patent applications,
trademarks, trademark applications, service marks, trade names, trade secrets, inventions, copyrights, licenses and other intellectual
property rights and similar rights necessary or required for use in connection with their respective businesses as described in the SEC
Reports and which the failure to so have could have a Material Adverse Effect (collectively, the “Intellectual Property Rights”).
None of, and neither the Company nor any Subsidiary has received a notice (written or otherwise) that any of, the Intellectual Property
Rights has expired, terminated or been abandoned, or is expected to expire or terminate or be abandoned, within two (2) years from
the date of this Agreement. Neither the Company nor any Subsidiary has received, since the date of the latest audited financial statements
included within the SEC Reports, a written notice of a claim or otherwise has any knowledge that the Intellectual Property Rights violate
or infringe upon the rights of any Person, except as could not have or reasonably be expected to not have a Material Adverse Effect. To
the knowledge of the Company, all such Intellectual Property Rights are enforceable and there is no existing infringement by another Person
of any of the Intellectual Property Rights. The Company and its Subsidiaries have taken reasonable security measures to protect the secrecy,
confidentiality and value of all of their intellectual properties, except where failure to do so could not, individually or in the aggregate,
reasonably be expected to have a Material Adverse Effect.
(q) Insurance.
The Company and the Subsidiaries are insured by insurers of recognized financial responsibility against such losses and risks and in such
amounts as are prudent and customary in the businesses in which the Company and the Subsidiaries are engaged, including, but not limited
to, directors and officers insurance coverage. Neither the Company nor any Subsidiary has any reason to believe that it will not be able
to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as may
be necessary to continue its business without a significant increase in cost.
(r) Transactions
With Affiliates and Employees. Except as set forth in any of the Company’s SEC Reports, none of the officers or directors of
the Company or any Subsidiary and, to the knowledge of the Company, none of the employees of the Company or any Subsidiary is presently
a party to any transaction with the Company or any Subsidiary (other than for services as employees, officers and directors), including
any contract, agreement or other arrangement providing for the furnishing of services to or by, providing for rental of real or personal
property to or from, providing for the borrowing of money from or lending of money to or otherwise requiring payments to or from any officer,
director or such employee or, to the knowledge of the Company, any entity in which any officer, director, or any such employee has a substantial
interest or is an officer, director, trustee, stockholder, member or partner, in each case in excess of $120,000 other than for (i) payment
of salary or consulting fees for services rendered, (ii) reimbursement for expenses incurred on behalf of the Company and (iii) other
employee benefits, including stock option agreements under any stock option plan of the Company.
(s) Sarbanes-Oxley;
Internal Accounting Controls. The Company and the Subsidiaries are in material compliance with any and all applicable requirements
of the Sarbanes-Oxley Act of 2002 that are effective as of the date hereof, and any and all applicable rules and regulations promulgated
by the Commission thereunder that are effective as of the date hereof and as of the Closing Date. Except as disclosed in the Company’s
SEC Reports, the Company and the Subsidiaries maintain a system of internal accounting controls sufficient to provide reasonable assurance
that: (i) transactions are executed in accordance with management’s general or specific authorizations, (ii) transactions
are recorded as necessary to permit preparation of financial statements in conformity with GAAP and to maintain asset accountability,
(iii) access to assets is permitted only in accordance with management’s general or specific authorization, and (iv) the
recorded accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect
to any differences. The Company and the Subsidiaries have established disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and
15d-15(e)) for the Company and the Subsidiaries and designed such disclosure controls and procedures to ensure that information required
to be disclosed by the Company in the reports it files or submits under the Exchange Act is recorded, processed, summarized and reported,
within the time periods specified in the Commission’s rules and forms. The Company’s certifying officers have evaluated
the effectiveness of the disclosure controls and procedures of the Company and the Subsidiaries as of the end of the period covered by
the most recently filed periodic report under the Exchange Act (such date, the “Evaluation Date”). The Company presented
in its most recently filed periodic report under the Exchange Act the conclusions of the certifying officers about the effectiveness of
the disclosure controls and procedures based on their evaluations as of the Evaluation Date. Since the Evaluation Date, there have been
no changes in the internal control over financial reporting (as such term is defined in the Exchange Act) of the Company and its Subsidiaries
that have materially affected, or is reasonably likely to materially affect, the internal control over financial reporting of the Company
and its Subsidiaries.
(t) Certain
Fees. Except as set forth in the Prospectus , no brokerage or finder’s fees or commissions are or will be payable by the Company
or any Subsidiary to any broker, financial advisor or consultant, finder, placement agent, investment banker, bank or other Person with
respect to the transactions contemplated by the Transaction Documents. The Purchasers shall have no obligation with respect to any fees
or with respect to any claims made by or on behalf of other Persons for fees of a type contemplated in this Section that may be due
in connection with the transactions contemplated by the Transaction Documents.
(u) Investment
Company. The Company is not, and is not an Affiliate of, and immediately after receipt of payment for the Securities, will not be
or be an Affiliate of, an “investment company” within the meaning of the Investment Company Act of 1940, as amended. The Company
shall conduct its business in a manner so that it will not become an “investment company” subject to registration under the
Investment Company Act of 1940, as amended.
(v) Registration
Rights. Except as set forth in any of the Company’s SEC Reports, no Person has any right to cause the Company or any Subsidiary
to effect the registration under the Securities Act of any securities of the Company or any Subsidiary, which rights have not been satisfied
prior to the date hereof.
(w) Listing
and Maintenance Requirements. The Common Stock is registered pursuant to Section 12(b) or 12(g) of the Exchange Act,
and the Company has taken no action designed to, or which to its knowledge is likely to have the effect of, terminating the registration
of the Common Stock under the Exchange Act nor has the Company received any notification that the Commission is contemplating terminating
such registration. Except as set forth in any of the SEC Reports, the Company has not, in the twelve (12) months preceding the date hereof,
received notice from any Trading Market on which the Common Stock is or has been listed or quoted to the effect that the Company is not
in compliance with the listing or maintenance requirements of such Trading Market. Except as set forth in any of the SEC Reports, the
Company is in compliance with all such listing and maintenance requirements. The Common Stock is currently eligible for electronic transfer
through the Depository Trust Company or another established clearing corporation and the Company is current in payment of the fees to
the Depository Trust Company (or such other established clearing corporation) in connection with such electronic transfer, except where
such nonpayment would not reasonably be expected to have a Material Adverse Effect.
(x) [RESERVED].
(y) Disclosure.
Except with respect to the material terms and conditions of the transactions contemplated by the Transaction Documents, the Company confirms
that neither it nor any other Person acting on its behalf has provided any of the Purchasers or their agents or counsel with any information
that it believes constitutes material, non-public information which is not otherwise disclosed in the Prospectus. The Company understands
and confirms that the Purchasers will rely on the foregoing representation in effecting transactions in securities of the Company. All
of the disclosure furnished by or on behalf of the Company to the Purchasers regarding the Company and its Subsidiaries, their respective
businesses and the transactions contemplated hereby, including the Disclosure Schedules to this Agreement, is true and correct in all
material respects and does not contain any untrue statement of a material fact or omit to state any material fact necessary in order to
make the statements made therein, in the light of the circumstances under which they were made, not misleading. The press releases disseminated
by the Company during the twelve (12) months preceding the date of this Agreement taken as a whole do not contain any untrue statement
of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statement therein,
in the light of the circumstances under which they were made and when made, not misleading. The Company acknowledges and agrees that no
Purchaser makes or has made any representations or warranties with respect to the transactions contemplated hereby other than those specifically
set forth in Section 3.2 hereof.
(z) No Integrated
Offering. Assuming the accuracy of the Purchasers’ representations and warranties set forth in Section 3.2, neither the
Company, nor any of its Affiliates, nor any Person acting on its or their behalf has, directly or indirectly, made any offers or sales
of any security or solicited any offers to buy any security, under circumstances that would cause this offering of the Securities to be
integrated with prior offerings by the Company for purposes of (i) the Securities Act which would require the registration of the
Securities under the Securities Act or (ii) any applicable shareholder approval provisions of any Trading Market on which any of
the securities of the Company are listed or designated.
(aa) Solvency.
Based on the consolidated financial condition of the Company as of the Closing Date, after giving effect to the receipt by the Company
of the proceeds from the sale of the Securities hereunder, (i) the fair saleable value of the Company’s assets exceeds the
amount that will be required to be paid on or in respect of the Company’s existing debts and other liabilities (including known
contingent liabilities) as they mature, (ii) the Company’s assets do not constitute unreasonably small capital to carry on
its business as now conducted and as proposed to be conducted including its capital needs taking into account the particular capital requirements
of the business conducted by the Company, consolidated and projected capital requirements and capital availability thereof, and (iii) the
current cash flow of the Company, together with the proceeds the Company would receive, were it to liquidate all of its assets, after
taking into account all anticipated uses of the cash, would be sufficient to pay all amounts on or in respect of its liabilities when
such amounts are required to be paid. The Company does not intend to incur debts beyond its ability to pay such debts as they mature (taking
into account the timing and amounts of cash to be payable on or in respect of its debt). The Company has no knowledge of any facts or
circumstances which lead it to believe that it will file for reorganization or liquidation under the bankruptcy or reorganization laws
of any jurisdiction within one year from the Closing Date. Schedule 3.1(aa) sets forth as of the date hereof all outstanding secured
and unsecured Indebtedness of the Company or any Subsidiary, or for which the Company or any Subsidiary has commitments. For the purposes
of this Agreement, “Indebtedness” means (x) any liabilities for borrowed money or amounts owed in excess of $100,000
(other than trade accounts payable incurred in the ordinary course of business), (y) all guaranties, endorsements and other contingent
obligations in respect of indebtedness of others, whether or not the same are or should be reflected in the Company’s consolidated
balance sheet (or the notes thereto), except guaranties by endorsement of negotiable instruments for deposit or collection or similar
transactions in the ordinary course of business; and (z) the present value of any lease payments in excess of $100,000 due under
leases required to be capitalized in accordance with GAAP. Neither the Company nor any Subsidiary is in default with respect to any Indebtedness.
(bb) Tax Status.
Except as set forth in any of the Company’s SEC Reports, for matters that would not, individually or in the aggregate, have or reasonably
be expected to result in a Material Adverse Effect, the Company and its Subsidiaries each (i) has made or filed all United States
federal, state and local income and all foreign income and franchise tax returns, reports and declarations required by any jurisdiction
to which it is subject, subject to permitted extensions, (ii) has paid all taxes and other governmental assessments and charges that
are material in amount, shown or determined to be due on such returns, reports and declarations and (iii) has set aside on its books
provision reasonably adequate for the payment of all material taxes for periods subsequent to the periods to which such returns, reports
or declarations apply. There are no unpaid taxes in any material amount claimed to be due by the taxing authority of any jurisdiction,
and the officers of the Company or of any Subsidiary know of no basis for any such claim.
(cc) Foreign
Corrupt Practices. Neither the Company nor any Subsidiary, nor to the knowledge of the Company or any Subsidiary, any agent or other
person acting on behalf of the Company or any Subsidiary, has (i) directly or indirectly, used any funds for unlawful contributions,
gifts, entertainment or other unlawful expenses related to foreign or domestic political activity, (ii) made any unlawful payment
to foreign or domestic government officials or employees or to any foreign or domestic political parties or campaigns from corporate funds,
(iii) failed to disclose fully any contribution made by the Company or any Subsidiary (or made by any person acting on its behalf
of which the Company is aware) which is in violation of law, or (iv) violated in any material respect any provision of FCPA.
(dd) Accountants.
The Company’s independent registered accounting firm is set forth on Schedule 3.1(dd) of the Disclosure Schedules. To the
knowledge and belief of the Company, such accounting firm (i) is a registered public accounting firm as required by the Exchange
Act and (ii) shall express its opinion with respect to the financial statements included in the Company’s Annual Report for
the fiscal year ending December 31, 2024.
(ee) Acknowledgment
Regarding Purchasers’ Purchase of Securities. The Company acknowledges and agrees that each of the Purchasers is acting solely
in the capacity of an arm’s length purchaser with respect to the Transaction Documents and the transactions contemplated thereby.
The Company further acknowledges that no Purchaser is acting as a financial advisor or fiduciary of the Company (or in any similar capacity)
with respect to the Transaction Documents and the transactions contemplated thereby and any advice given by any Purchaser or any of their
respective representatives or agents in connection with the Transaction Documents and the transactions contemplated thereby is merely
incidental to the Purchasers’ purchase of the Securities. The Company further represents to each Purchaser that the Company’s
decision to enter into this Agreement and the other Transaction Documents has been based solely on the independent evaluation of the transactions
contemplated hereby by the Company and its representatives.
(ff) Acknowledgment
Regarding Purchaser’s Trading Activity. Anything in this Agreement or elsewhere herein to the contrary notwithstanding (except
for Sections 3.2(f) and 4.13 hereof), it is understood and acknowledged by the Company that: (i) none of the Purchasers has
been asked by the Company to agree, nor has any Purchaser agreed, to desist from purchasing or selling, long and/or short, securities
of the Company, or “derivative” securities based on securities issued by the Company or to hold the Securities for any specified
term; (ii) past or future open market or other transactions by any Purchaser, specifically including, without limitation, Short Sales
or “derivative” transactions, before or after the closing of this or future private placement transactions, may negatively
impact the market price of the Company’s publicly-traded securities; (iii) any Purchaser, and counter-parties in “derivative”
transactions to which any such Purchaser is a party, directly or indirectly, presently may have a “short” position in the
Common Stock, and (iv) each Purchaser shall not be deemed to have any affiliation with or control over any arm’s length counter-party
in any “derivative” transaction. The Company further understands and acknowledges that (y) one or more Purchasers may
engage in hedging activities at various times during the period that the Securities are outstanding, including, without limitation, during
the periods that the value of the Pre-Funded Warrant Shares deliverable with respect to Securities are being determined, and (z) such
hedging activities (if any) could reduce the value of the existing stockholders' equity interests in the Company at and after the time
that the hedging activities are being conducted. The Company acknowledges that such aforementioned hedging activities do not constitute
a breach of any of the Transaction Documents.
(gg) Regulation
M Compliance. The Company has not, and to its knowledge no one acting on its behalf has, (i) taken, directly or indirectly, any
action designed to cause or to result in the stabilization or manipulation of the price of any security of the Company to facilitate the
sale or resale of any of the Securities, (ii) sold, bid for, purchased, or, paid any compensation for soliciting purchases of, any
of the Securities, or (iii) paid or agreed to pay to any Person any compensation for soliciting another to purchase any other securities
of the Company, other than, in the case of clauses (ii) and (iii), compensation paid to the Company’s Placement Agent in connection
with the placement of the Securities.
(hh) [RESERVED].
(ii) Stock
Option Plans. Each stock option granted by the Company under the Company’s stock option plan was granted (i) in accordance
with the terms of the Company’s stock option plan and (ii) with an exercise price at least equal to the fair market value of
the Common Stock on the date such stock option would be considered granted under GAAP and applicable law. No stock option granted under
the Company’s stock option plan has been backdated. The Company has not knowingly granted, and there is no and has been no Company
policy or practice to knowingly grant, stock options prior to, or otherwise knowingly coordinate the grant of stock options with, the
release or other public announcement of material information regarding the Company or its Subsidiaries or their financial results or prospects.
(jj) Office of
Foreign Assets Control. Neither the Company nor any Subsidiary nor, to the Company's knowledge, any director, officer, agent, employee
or affiliate of the Company or any Subsidiary is currently subject to any U.S. sanctions administered by the Office of Foreign Assets
Control of the U.S. Treasury Department (“OFAC”).
(kk) U.S. Real
Property Holding Corporation. The Company is not and has never been a U.S. real property holding corporation within the meaning of
Section 897 of the Internal Revenue Code of 1986, as amended, and the Company shall so certify upon Purchaser’s request.
(ll) Bank Holding
Company Act. Neither the Company nor any of its Subsidiaries or Affiliates is subject to the Bank Holding Company Act of 1956, as
amended (the “BHCA”) and to regulation by the Board of Governors of the Federal Reserve System (the “Federal
Reserve”). Neither the Company nor any of its Subsidiaries or Affiliates owns or controls, directly or indirectly, five percent
(5%) or more of the outstanding shares of any class of voting securities or twenty-five percent or more of the total equity of a bank
or any entity that is subject to the BHCA and to regulation by the Federal Reserve. Neither the Company nor any of its Subsidiaries or
Affiliates exercises a controlling influence over the management or policies of a bank or any entity that is subject to the BHCA and to
regulation by the Federal Reserve.
(mm) Money Laundering.
The operations of the Company and to the extent applicable to non US entities, its Subsidiaries, are and have been conducted at all times
in material compliance with applicable financial record-keeping and reporting requirements of the Currency and Foreign Transactions Reporting
Act of 1970, as amended, applicable money laundering statutes and applicable rules and regulations thereunder (collectively, the
“Money Laundering Laws”), and no Action or Proceeding by or before any court or governmental agency, authority or body
or any arbitrator involving the Company or any Subsidiary with respect to the Money Laundering Laws is pending or, to the knowledge of
the Company or any Subsidiary, threatened.
(nn) Reserved.
(oo) Reserved
(pp) Brokers.
Other than the Placement Agent, the Company is not aware of any person that has been or will be paid (directly or indirectly) remuneration
for solicitation of purchasers in connection with the sale of any Securities.
(qq) Reserved
(rr) Forward
Looking Statements. Each financial or operational projection or other “forward-looking statement” (as defined by Section 27A
of the Securities Act or Section 21E of the Exchange Act) contained in the Registration Statement or the Prospectus (i) was
so included by the Company in good faith and with reasonable basis after due consideration by the Company of the underlying assumptions,
estimates and other applicable facts and circumstances and (ii) is accompanied by meaningful cautionary statements identifying those
factors that would reasonably be expected to cause actual results to differ materially from those in such forward-looking statement. No
such statement was made with the knowledge of an executive officer or director of the Company that was false or misleading.
(ss) Sources
of Data. Nothing has come to the attention of the Company that has caused the Company to believe that the statistical or market-related
data included or incorporated by reference in the Registration Statement or the Prospectus, or included in the marketing materials, are
not based on or derived from sources that the Company reasonably believes to be reliable and accurate, and the Company has obtained the
written consent to the use of such data from such sources, to the extent required.
Each purchaser acknowledges and agrees that the
representations contained in this Section 3.1 shall not modify, amend or affect the Company’s right to rely on each Purchaser’s
representations and warranties contained in this Agreement or any representations and warranties contained in any other Transaction Document
or any other document or instrument executed and/or delivered in connection with this Agreement or the consummation of the transactions
contemplated hereby.
3.2 Representations and
Warranties of the Purchasers. Each Purchaser, for itself and for no other Purchaser, hereby represents and warrants as of the date
hereof and as of the Closing Date to the Company as follows (unless as of a specific date therein, in which case they shall be accurate
as of such date):
(a) Organization;
Authority. Such Purchaser is either an individual or an entity duly incorporated or formed, validly existing and in good standing
under the laws of the jurisdiction of its incorporation or formation with full right, corporate, partnership, limited liability company
or similar power and authority to enter into and to consummate the transactions contemplated by the Transaction Documents and otherwise
to carry out its obligations hereunder and thereunder. The execution and delivery of the Transaction Documents and performance by such
Purchaser of the transactions contemplated by the Transaction Documents have been duly authorized by all necessary corporate, partnership,
limited liability company or similar action, as applicable, on the part of such Purchaser. Each Transaction Document to which it is a
party has been duly executed by such Purchaser, and when delivered by such Purchaser in accordance with the terms hereof, will constitute
the valid and legally binding obligation of such Purchaser, enforceable against it in accordance with its terms, except: (i) as limited
by general equitable principles and applicable bankruptcy, insolvency, reorganization, moratorium and other laws of general application
affecting enforcement of creditors’ rights generally, (ii) as limited by laws relating to the availability of specific performance,
injunctive relief or other equitable remedies and (iii) insofar as indemnification and contribution provisions may be limited by
applicable law.
(b) Understandings
or Arrangements. Such Purchaser is acquiring the Securities as principal for its own account and has no direct or indirect arrangement
or understandings with any other persons to distribute or regarding the distribution of such Securities (this representation and warranty
not limiting such Purchaser’s right to sell the Securities pursuant to the Registration Statement or otherwise in compliance with
applicable federal and state securities laws). Such Purchaser is acquiring the Securities hereunder in the ordinary course of its business.
(c) Purchaser
Status. At the time such Purchaser was offered the Securities, it was, and as of the date hereof it is, and on each date on which
it exercises its Pre-Funded Warrants for cash, it will be an “accredited investor” as defined in Rule 501(a)(1), (a)(2),
(a)(3), (a)(7) or (a)(8) under the Securities Act.
(d) Experience
of Such Purchaser. Such Purchaser, either alone or together with its representatives, has such knowledge, sophistication and experience
in business and financial matters so as to be capable of evaluating the merits and risks of the prospective investment in the Securities,
and has so evaluated the merits and risks of such investment. Such Purchaser is able to bear the economic risk of an investment in the
Securities and, at the present time, is able to afford a complete loss of such investment.
(e) Access
to Information. Such Purchaser acknowledges that it has had the opportunity to review the Transaction Documents (including all exhibits
and schedules thereto) and the SEC Reports and has been afforded, (i) the opportunity to ask such questions as it has deemed necessary
of, and to receive answers from, representatives of the Company concerning the terms and conditions of the offering of the Securities
and the merits and risks of investing in the Securities; (ii) access to information about the Company and its financial condition,
results of operations, business, properties, management and prospects sufficient to enable it to evaluate its investment; and (iii) the
opportunity to obtain such additional information that the Company possesses or can acquire without unreasonable effort or expense that
is necessary to make an informed investment decision with respect to the investment. Such Purchaser acknowledges and agrees that neither
the Placement Agent nor any Affiliate of the Placement Agent has provided such Purchaser with any information or advice with respect to
the Securities nor is such information or advice necessary or desired. Neither the Placement Agent nor any Affiliate has made or makes
any representation as to the Company or the quality of the Securities and the Placement Agent and any Affiliate may have acquired non-public
information with respect to the Company which such Purchaser agrees need not be provided to it. In connection with the issuance of the
Securities to such Purchaser, neither the Placement Agent nor any of its Affiliates has acted as a financial advisor or fiduciary to such
Purchaser.
(f) Certain
Transactions and Confidentiality. Other than consummating the transactions contemplated hereunder, such Purchaser has not, nor has
any Person acting on behalf of or pursuant to any understanding with such Purchaser, directly or indirectly executed any purchases or
sales, including Short Sales, of the securities of the Company during the period commencing as of the time that such Purchaser first received
a term sheet (written or oral) from the Company or any other Person representing the Company setting forth the material terms of the transactions
contemplated hereunder and ending immediately prior to the execution hereof. Notwithstanding the foregoing, in the case of a Purchaser
that is a multi-managed investment vehicle whereby separate portfolio managers manage separate portions of such Purchaser’s assets
and the portfolio managers have no direct knowledge of the investment decisions made by the portfolio managers managing other portions
of such Purchaser’s assets, the representation set forth above shall only apply with respect to the portion of assets managed by
the portfolio manager that made the investment decision to purchase the Securities covered by this Agreement. Other than to other Persons
party to this Agreement or to such Purchaser’s representatives, including, without limitation, its officers, directors, partners,
legal and other advisors, employees, agents and Affiliates, such Purchaser has maintained the confidentiality of all disclosures made
to it in connection with this transaction (including the existence and terms of this transaction). Notwithstanding the foregoing, for
the avoidance of doubt, nothing contained herein shall constitute a representation or warranty, or preclude any actions, with respect
to locating or borrowing shares in order to effect Short Sales or similar transactions in the future.
(g) General
Solicitation. Such Purchaser is not purchasing the Securities as a result of any advertisement, article, notice or other communication
regarding the Securities published in any newspaper, magazine or similar media or broadcast over television or radio or presented at any
seminar or, to the knowledge of such Purchaser, any other general solicitation or general advertisement.
The Company acknowledges and agrees that the representations
contained in this Section 3.2 shall not modify, amend or affect such Purchaser’s right to rely on the Company’s representations
and warranties contained in this Agreement or any representations and warranties contained in any other Transaction Document or any other
document or instrument executed and/or delivered in connection with this Agreement or the consummation of the transactions contemplated
hereby. Notwithstanding the foregoing, for the avoidance of doubt, nothing contained herein shall constitute a representation or warranty,
or preclude any actions, with respect to locating or borrowing shares in order to effect Short Sales or similar transactions in the future.
ARTICLE IV.
OTHER AGREEMENTS OF THE PARTIES
4.1 Pre-Funded Warrant
Shares. If all or any portion of a Pre-Funded Warrant is exercised at a time when there is an effective registration statement to
cover the issuance or resale of the Pre-Funded Warrant Shares or if the Pre-Funded Warrant is exercised via cashless exercise, the Pre-Funded
Warrant Shares issued pursuant to any such exercise shall be issued free of all legends. If at any time following the date hereof the
Registration Statement (or any subsequent registration statement registering the sale or resale of the Pre-Funded Warrant Shares) is not
effective or is not otherwise available for the sale or resale of the Pre-Funded Warrant Shares, the Company shall promptly notify the
holders of the Pre-Funded Warrants in writing that such registration statement is not then effective and thereafter shall promptly notify
such holders when the registration statement is effective again and available for the sale or resale of the Pre-Funded Warrant Shares
(it being understood and agreed that the foregoing shall not limit the ability of the Company to issue, or any Purchaser to sell, any
of the Pre-Funded Warrant Shares in compliance with applicable federal and state securities laws). The Company shall use best efforts
to keep a registration statement (including the Registration Statement) registering the issuance or resale of the Pre-Funded Warrant Shares
effective during the term of the Pre-Funded Warrants.
4.2 Furnishing of Information.
Until the earliest of the time that (i) no Purchaser owns Securities or (ii) the Securities can be sold by the Purchaser pursuant
to Rule 144, the Company covenants to timely file (or obtain extensions in respect thereof and file within the applicable grace period)
all reports required to be filed by the Company after the date hereof pursuant to the Exchange Act even if the Company is not then subject
to the reporting requirements of the Exchange Act, except in connection with a merger or consolidation of the Company where the Company
is not the surviving entity, or the acquisition of, or any other going private transaction involving, the Company.
4.3 Integration. The
Company shall not sell, offer for sale or solicit offers to buy or otherwise negotiate in respect of any security (as defined in Section 2
of the Securities Act) that would be integrated with the offer or sale of the Securities for purposes of the rules and regulations
of any Trading Market such that it would require shareholder approval prior to the closing of such other transaction unless shareholder
approval is obtained before the closing of such subsequent transaction.
4.4 Securities Laws Disclosure;
Publicity. The Company shall (a) by the Disclosure Time, issue a press release disclosing the material terms of the transactions
contemplated hereby, and (b) file a Current Report on Form 8-K, including the Transaction Documents as exhibits thereto, with
the Commission within the time required by the Exchange Act. From and after the issuance of such press release, the Company represents
to the Purchasers that it shall have publicly disclosed all material, non-public information delivered to any of the Purchasers by the
Company or any of its Subsidiaries, or any of their respective officers, directors, employees or agents in connection with the transactions
contemplated by the Transaction Documents. In addition, effective upon the issuance of such press release, the Company acknowledges and
agrees that any and all confidentiality or similar obligations under any agreement, whether written or oral, between the Company, any
of its Subsidiaries or any of their respective officers, directors, agents, employees or Affiliates on the one hand, and any of the Purchasers
or any of their Affiliates on the other hand, shall terminate. The Company and each Purchaser shall consult with each other in issuing
any other press releases with respect to the transactions contemplated hereby, and neither the Company nor any Purchaser shall issue any
such press release nor otherwise make any such public statement without the prior consent of the Company, with respect to any press release
of any Purchaser, or without the prior consent of each Purchaser, with respect to any press release of the Company, which consent shall
not unreasonably be withheld or delayed, except if such disclosure is required by law, in which case the disclosing party shall promptly
provide the other party with prior notice of such public statement or communication. Notwithstanding the foregoing, the Company shall
not publicly disclose the name of any Purchaser, or include the name of any Purchaser in any filing with the Commission or any regulatory
agency or Trading Market, without the prior written consent of such Purchaser, except (a) as required by federal securities law in
connection with the filing of final Transaction Documents with the Commission and (b) to the extent such disclosure is required by
law or Trading Market regulations, in which case the Company shall provide the Purchasers with prior notice of such disclosure permitted
under this clause (b).
4.5 Shareholder Rights
Plan. No claim will be made or enforced by the Company or, with the consent of the Company, any other Person, that any Purchaser is
an “Acquiring Person” under any control share acquisition, business combination, poison pill (including any distribution
under a rights agreement) or similar anti-takeover plan or arrangement in effect or hereafter adopted by the Company, or that any Purchaser
could be deemed to trigger the provisions of any such plan or arrangement, by virtue of receiving Securities under the Transaction Documents
or under any other agreement between the Company and the Purchasers.
4.6 Non-Public Information.
Except with respect to the material terms and conditions of the transactions contemplated by the Transaction Documents, which shall be
disclosed pursuant to Section 4.4, the Company covenants and agrees that neither it, nor any other Person acting on its behalf will
provide any Purchaser or its agents or counsel with any information that constitutes, or the Company reasonably believes constitutes,
material non-public information, unless prior thereto such Purchaser shall have consented to the receipt of such information and agreed
with the Company to keep such information confidential. The Company understands and confirms that each Purchaser shall be relying on the
foregoing covenant in effecting transactions in securities of the Company. To the extent that the Company delivers any material, non-public
information to a Purchaser without such Purchaser’s consent, the Company hereby covenants and agrees that such Purchaser shall not
have any duty of confidentiality to the Company, any of its Subsidiaries, or any of their respective officers, directors, agents, employees
or Affiliates, or a duty to the Company, any of its Subsidiaries or any of their respective officers, directors, agents, employees or
Affiliates not to trade on the basis of, such material, non-public information, provided that the Purchaser shall remain subject to applicable
law. To the extent that any notice provided pursuant to any Transaction Document constitutes, or contains, material, non-public information
regarding the Company or any Subsidiaries, the Company shall simultaneously file such notice with the Commission pursuant to a Current
Report on Form 8-K. The Company understands and confirms that each Purchaser shall be relying on the foregoing covenant in effecting
transactions in securities of the Company.
4.7 Use of Proceeds.
The Company shall use the net proceeds from the sale of the Securities hereunder for working capital purposes and shall not use such proceeds:
(a) except as set forth in the Prospectus, for the satisfaction of any portion of the Company’s debt (other than payment of
trade payables), (b) for the redemption of any Common Stock or Common Stock Equivalents, (c) for the settlement of any outstanding
litigation or (d) in violation of FCPA or OFAC regulations.
4.8 Indemnification of
Purchasers. Subject to the provisions of this Section 4.8, the Company will indemnify and hold each Purchaser and its directors,
officers, shareholders, members, partners, employees and agents (and any other Persons with a functionally equivalent role of a Person
holding such titles notwithstanding a lack of such title or any other title), each Person who controls such Purchaser (within the meaning
of Section 15 of the Securities Act and Section 20 of the Exchange Act), and the directors, officers, shareholders, agents,
members, partners or employees (and any other Persons with a functionally equivalent role of a Person holding such titles notwithstanding
a lack of such title or any other title) of such controlling persons (each, a “Purchaser Party”) harmless from any
and all losses, liabilities, obligations, claims, contingencies, damages, costs and expenses, including all judgments, amounts paid in
settlements, court costs and reasonable attorneys’ fees and costs of investigation that any such Purchaser Party may suffer or incur
as a result of or relating to (a) any breach of any of the representations, warranties, covenants or agreements made by the Company
in this Agreement or in the other Transaction Documents or (b) any action instituted against the Purchaser Parties in any capacity,
or any of them or their respective Affiliates, by any stockholder of the Company who is not an Affiliate of such Purchaser Party, with
respect to any of the transactions contemplated by the Transaction Documents (unless such action is solely based upon a material breach
of such Purchaser Party’s representations, warranties or covenants under the Transaction Documents or any agreements or understandings
such Purchaser Party may have with any such stockholder or any violations by such Purchaser Party of state or federal securities laws
or any conduct by such Purchaser Party which is finally judicially determined to constitute fraud, gross negligence or willful misconduct).
If any action shall be brought against any Purchaser Party in respect of which indemnity may be sought pursuant to this Agreement, such
Purchaser Party shall promptly notify the Company in writing, and the Company shall have the right to assume the defense thereof with
counsel of its own choosing reasonably acceptable to the Purchaser Party. Any Purchaser Party shall have the right to employ separate
counsel in any such action and participate in the defense thereof, but the fees and expenses of such counsel shall be at the expense of
such Purchaser Party except to the extent that (x) the employment thereof has been specifically authorized by the Company in writing,
(y) the Company has failed after a reasonable period of time to assume such defense and to employ counsel or (z) in such action
there is, in the reasonable opinion of counsel, a material conflict on any material issue between the position of the Company and the
position of such Purchaser Party, in which case the Company shall be responsible for the reasonable fees and expenses of no more than
one such separate counsel. The Company will not be liable to any Purchaser Party under this Agreement (1) for any settlement by a
Purchaser Party effected without the Company’s prior written consent, which shall not be unreasonably withheld or delayed; or (2) to
the extent, but only to the extent that a loss, claim, damage or liability is attributable to any Purchaser Party’s breach of any
of the representations, warranties, covenants or agreements made by such Purchaser Party in this Agreement or in the other Transaction
Documents. The indemnification required by this Section 4.8 shall be made by periodic payments of the amount thereof during the course
of the investigation or defense, as and when bills are received or are incurred. The indemnity agreements contained herein shall be in
addition to any cause of action or similar right of any Purchaser Party against the Company or others and any liabilities the Company
may be subject to pursuant to law.
4.9 Reservation of Common
Stock. As of the date hereof, the Company has reserved and the Company shall continue to reserve and keep available at all times,
free of preemptive rights, a sufficient number of shares of Common Stock for the purpose of enabling the Company to issue Shares pursuant
to this Agreement and Pre-Funded Warrant Shares pursuant to any exercise of the Pre-Funded Warrants.
4.10 Listing of Common
Stock. The Company hereby agrees to use best efforts to maintain the listing or quotation of the Common Stock on the Trading Market
on which it is currently listed, and concurrently with the Closing, the Company shall apply to list or quote all of the Shares and Pre-Funded
Warrant Shares on such Trading Market and promptly secure the listing of all of the Shares and Pre-Funded Warrant Shares on such Trading
Market. The Company further agrees, if the Company applies to have the Common Stock traded on any other Trading Market, it will then include
in such application all of the Shares and Pre-Funded Warrant Shares, and will take such other action as is necessary to cause all of the
Shares and Pre-Funded Warrant Shares to be listed or quoted on such other Trading Market as promptly as possible. The Company will then
take all action reasonably necessary to continue the listing and trading of its Common Stock on a Trading Market and will comply in all
material respects with the Company’s reporting, filing and other obligations under the bylaws or rules of the Trading Market.
The Company agrees to use best efforts to maintain the eligibility of the Common Stock for electronic transfer through the Depository
Trust Company or another established clearing corporation, including, without limitation, by timely payment of fees to the Depository
Trust Company or such other established clearing corporation in connection with such electronic transfer.
4.11 Subsequent Equity
Sales.
(a) The Company,
on behalf of itself and any successor entity, agrees that, without the prior written consent of the Placement Agent, neither it nor its
Subsidiaries will, for a period beginning on the date of this Agreement and ending on the date that is the 90th day after the date of
this Agreement (the “Lock-Up Period”), (i) offer, pledge, sell, contract to sell, sell any option or contract
to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose
of, directly or indirectly, any shares of capital stock or any Common Stock Equivalents; (ii) other than the Prospectus or filing
a registration statement on Form S-8 in connection with any employee benefit plan and the filing of a registration statement for
a public offering that names the Placement Agent as underwriter or placement agent for the offering covered thereby, file or cause to
be filed any registration statement with the Commission or any amendments or supplements thereto (other than the Prospectus), (iii) complete
any offering of debt securities, other than entering into a line of credit with a traditional bank or (iv) enter into any swap or
other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of capital stock of the
Company or any of its Subsidiaries, whether any such transaction described in clause (i), (ii), (iii) or (iv) above is to be
settled by delivery of shares of capital stock or such other securities, in cash or otherwise.
(b) Notwithstanding
the foregoing, this Section 4.11 shall not apply in respect of an Exempt Issuance.
4.12 Equal Treatment of
Purchasers. No consideration (including any modification of any Transaction Document) shall be offered or paid to any Person to amend
or consent to a waiver or modification of any provision of the Transaction Documents unless the same consideration is also offered to
all of the parties to the Transaction Documents. For clarification purposes, this provision constitutes a separate right granted to each
Purchaser by the Company and negotiated separately by each Purchaser, and is intended for the Company to treat the Purchasers as a class
and shall not in any way be construed as the Purchasers acting in concert or as a group with respect to the purchase, disposition or voting
of Securities or otherwise.
4.13 Certain Transactions
and Confidentiality. Each Purchaser, severally and not jointly with the other Purchasers, covenants that neither it nor any Affiliate
acting on its behalf or pursuant to any understanding with it will execute any purchases or sales, including Short Sales of any of the
Company’s securities during the period commencing with the execution of this Agreement and ending at such time that the transactions
contemplated by this Agreement are first publicly announced pursuant to the initial press release as described in Section 4.4. Each
Purchaser, severally and not jointly with the other Purchasers, covenants that until such time as the transactions contemplated by this
Agreement are publicly disclosed by the Company pursuant to the initial press release as described in Section 4.4, such Purchaser
will maintain the confidentiality of the existence and terms of this transaction and the information included in the Transaction Documents
and the Disclosure Schedules. Notwithstanding the foregoing and notwithstanding anything contained in this Agreement to the contrary,
the Company expressly acknowledges and agrees that (i) no Purchaser makes any representation, warranty or covenant hereby that it
will not engage in effecting transactions in any securities of the Company after the time that the transactions contemplated by this Agreement
are first publicly announced pursuant to the initial press release as described in Section 4.4, (ii) no Purchaser shall be restricted
or prohibited from effecting any transactions in any securities of the Company in accordance with applicable securities laws from and
after the time that the transactions contemplated by this Agreement are first publicly announced pursuant to the initial press release
as described in Section 4.4 and (iii) no Purchaser shall have any duty of confidentiality or duty not to trade in the securities
of the Company to the Company or its Subsidiaries after the issuance of the initial press release as described in Section 4.4. Notwithstanding
the foregoing, in the case of a Purchaser that is a multi-managed investment vehicle whereby separate portfolio managers manage separate
portions of such Purchaser’s assets and the portfolio managers have no direct knowledge of the investment decisions made by the
portfolio managers managing other portions of such Purchaser’s assets, the covenant set forth above shall only apply with respect
to the portion of assets managed by the portfolio manager that made the investment decision to purchase the Securities covered by this
Agreement.
4.14 Lock-Up Agreements.
The Company will enforce the terms of the Lock-Up Agreements and not agree to any amendment to, or modification of, the Lock-Up Agreements
absent the prior written consent of the Placement Agent.
4.15 [RESERVED].
4.16 Exercise Procedures.
The form of Notice of Exercise included in the Pre-Funded Warrants set forth the totality of the procedures required of the Purchasers
in order to exercise the Pre-Funded Warrants. No additional legal opinion, other information or instructions shall be required of the
Purchasers to exercise their Pre-Funded Warrants. Without limiting the preceding sentences, no ink-original Notice of Exercise shall be
required, nor shall any medallion guarantee (or other type of guarantee or notarization) of any Notice of Exercise form be required in
order to exercise the Pre-Funded Warrants. The Company shall honor exercises of the Pre-Funded Warrants and shall deliver Pre-Funded Warrant
Shares in accordance with the terms, conditions and time periods set forth in the Transaction Documents.
4.17 Sales During Pre-Settlement
Period. Notwithstanding anything herein to the contrary, if at any time on or after the time of execution of this Agreement by the
Company and an applicable Purchaser, through, and including the time immediately prior to the Closing (the “Pre-Settlement Period”),
such Purchaser sells to any Person all, or any portion, of any Shares to be issued hereunder to such Purchaser at the Closing (collectively,
the “Pre-Settlement Shares”), such Purchaser shall, automatically hereunder (without any additional required actions
by such Purchaser or the Company), be deemed to be unconditionally bound to purchase, and the Company shall be deemed unconditionally
bound to sell, such Pre-Settlement Shares to such Purchaser at the Closing; provided, that the Company shall not be required to deliver
any Pre-Settlement Shares to such Purchaser prior to the Company’s receipt of the purchase price of such Pre-Settlement Shares hereunder;
and provided further that the Company hereby acknowledges and agrees that the forgoing shall not constitute a representation or covenant
by such Purchaser as to whether or not during the Pre-Settlement Period such Purchaser shall sell any Shares to any Person and that any
such decision to sell any Shares by such Purchaser shall solely be made at the time such Purchaser elects to effect any such sale, if
any.
ARTICLE V.
MISCELLANEOUS
5.1 Termination. This
Agreement may be terminated by any Purchaser, as to such Purchaser’s obligations hereunder only and without any effect whatsoever
on the obligations between the Company and the other Purchasers, by written notice to the other parties, if the Closing has not been consummated
on or before the fifth (5th) Trading Day following the date hereof; provided, however,
that no such termination will affect the right of any party to sue for any breach by any other party (or parties).
5.2 Fees and Expenses.
Except as expressly set forth in the Transaction Documents to the contrary, each party shall pay the fees and expenses of its advisers,
counsel, accountants and other experts, if any, and all other expenses incurred by such party incident to the negotiation, preparation,
execution, delivery and performance of this Agreement. The Company shall pay all Transfer Agent fees (including, without limitation, any
fees required for same-day processing of any instruction letter delivered by the Company and any exercise notice delivered by a Purchaser),
stamp taxes and other taxes and duties levied in connection with the delivery of any Securities to the Purchasers.
5.3 Entire Agreement.
The Transaction Documents, together with the exhibits and schedules thereto, the Prospectus, contain the entire understanding of the parties
with respect to the subject matter hereof and thereof and supersede all prior agreements and understandings, oral or written, with respect
to such matters, which the parties acknowledge have been merged into such documents, exhibits and schedules.
5.4 Notices. Any and
all notices or other communications or deliveries required or permitted to be provided hereunder shall be in writing and shall be deemed
given and effective on the earliest of: (a) the time of transmission, if such notice or communication is delivered via facsimile
at the facsimile number or email attachment at the email address as set forth on the signature pages attached hereto at or prior
to 5:30 p.m. (New York City time) on a Trading Day, (b) the next Trading Day after the time of transmission, if such notice
or communication is delivered via facsimile at the facsimile number or email attachment at the email address as set forth on the signature
pages attached hereto on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading Day, (c) the
second (2nd) Trading Day following the date of mailing, if sent by U.S. nationally recognized
overnight courier service or (d) upon actual receipt by the party to whom such notice is required to be given. The address for such
notices and communications shall be as set forth on the signature pages attached hereto. To the extent that any notice provided
pursuant to any Transaction Document constitutes, or contains, material, non-public information regarding the Company or any Subsidiaries,
the Company shall simultaneously file such notice with the Commission pursuant to a Current Report on Form 8-K.
5.5 Amendments; Waivers.
Prior to Closing, no provision of this Agreement may be waived, modified, supplemented or amended except in a written instrument signed,
in the case of an amendment, by the Company and all of the Purchasers. Thereafter, no provision of this Agreement may be waived, modified,
supplemented or amended except in a written instrument signed, in the case of an amendment, by the Company and Purchasers which purchased
at least 51.0% in interest of the Shares and Pre-Funded Warrants based on the initial Subscription Amounts hereunder (or, prior to the
Closing, by the Company and each Purchaser) or, in the case of a waiver, by the party against whom enforcement of any such waived provision
is sought, provided that if any amendment, modification or waiver disproportionately and adversely impacts a Purchaser (or group of Purchasers),
the consent of such disproportionately impacted Purchaser (or group of Purchasers) shall also be required. No waiver of any default with
respect to any provision, condition or requirement of this Agreement shall be deemed to be a continuing waiver in the future or a waiver
of any subsequent default or a waiver of any other provision, condition or requirement hereof, nor shall any delay or omission of any
party to exercise any right hereunder in any manner impair the exercise of any such right. Any proposed amendment or waiver that disproportionately,
materially and adversely affects the rights and obligations of any Purchaser relative to the comparable rights and obligations of the
other Purchasers shall require the prior written consent of such adversely affected Purchaser. Any amendment effected in accordance with
this Section 5.5 shall be binding upon each Purchaser and holder of Securities and the Company.
5.6 Headings. The headings
herein are for convenience only, do not constitute a part of this Agreement and shall not be deemed to limit or affect any of the provisions
hereof.
5.7 Successors and Assigns.
This Agreement shall be binding upon and inure to the benefit of the parties and their successors and permitted assigns. The Company may
not assign this Agreement or any rights or obligations hereunder without the prior written consent of each Purchaser (other than by merger).
Any Purchaser may assign any or all of its rights under this Agreement to any Person to whom such Purchaser assigns or transfers any Securities,
provided that such transferee agrees in writing to be bound, with respect to the transferred Securities, by the provisions of the Transaction
Documents that apply to the “Purchasers.”
5.8 No Third-Party Beneficiaries.
The Placement Agent shall be the third-party beneficiary of the representations and warranties of the Company in Section 3.1 and
the representations and warranties of the Purchasers in Section 3.2. This Agreement is intended for the benefit of the parties hereto
and their respective successors and permitted assigns and is not for the benefit of, nor may any provision hereof be enforced by, any
other Person, except as otherwise set forth in Section 4.8 and this Section 5.8.
5.9 Governing Law.
All questions concerning the construction, validity, enforcement and interpretation of the Transaction Documents shall be governed by
and construed and enforced in accordance with the internal laws of the State of New York. Each party agrees that all legal Proceedings
concerning the interpretations, enforcement and defense of the transactions contemplated by this Agreement and any other Transaction Documents
(whether brought against a party hereto or its respective affiliates, directors, officers, shareholders, partners, members, employees
or agents) shall be commenced exclusively in the state and federal courts sitting in the City of New York. Each party hereby irrevocably
submits to the exclusive jurisdiction of the state and federal courts sitting in the City of New York, Borough of Manhattan for the adjudication
of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein (including with respect
to the enforcement of any of the Transaction Documents), and hereby irrevocably waives, and agrees not to assert in any Action or Proceeding,
any claim that it is not personally subject to the jurisdiction of any such court, that such Action or Proceeding is improper or is an
inconvenient venue for such Proceeding. Each party hereby irrevocably waives personal service of process and consents to process being
served in any such Action or Proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence
of delivery) to such party at the address in effect for notices to it under this Agreement and agrees that such service shall constitute
good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve
process in any other manner permitted by law. If any party shall commence an Action or Proceeding to enforce any provisions of the Transaction
Documents, then, in addition to the obligations of the Company under Section 4.8, the prevailing party in such Action or Proceeding
shall be reimbursed by the non-prevailing party for its reasonable attorneys’ fees and other costs and expenses incurred with the
investigation, preparation and prosecution of such Action or Proceeding.
5.10 Survival. The
representations and warranties contained herein shall survive the Closing and the delivery of the Securities.
5.11 Execution. This
Agreement may be executed in two or more counterparts, all of which when taken together shall be considered one and the same agreement
and shall become effective when counterparts have been signed by each party and delivered to each other party, it being understood that
the parties need not sign the same counterpart. In the event that any signature is delivered by facsimile transmission or by e-mail delivery
of a “.pdf” format data file, such signature shall create a valid and binding obligation of the party executing (or on whose
behalf such signature is executed) with the same force and effect as if such facsimile or “.pdf” signature page were
an original thereof.
5.12 Severability.
If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction to be invalid, illegal,
void or unenforceable, the remainder of the terms, provisions, covenants and restrictions set forth herein shall remain in full force
and effect and shall in no way be affected, impaired or invalidated, and the parties hereto shall use their commercially reasonable efforts
to find and employ an alternative means to achieve the same or substantially the same result as that contemplated by such term, provision,
covenant or restriction. It is hereby stipulated and declared to be the intention of the parties that they would have executed the remaining
terms, provisions, covenants and restrictions without including any of such that may be hereafter declared invalid, illegal, void or unenforceable.
5.13 Rescission and Withdrawal
Right. Notwithstanding anything to the contrary contained in (and without limiting any similar provisions of) any of the other Transaction
Documents, whenever any Purchaser exercises a right, election, demand or option under a Transaction Document and the Company does not
timely perform its related obligations within the periods therein provided, then such Purchaser may rescind or withdraw, in its sole discretion
from time to time upon written notice to the Company, any relevant notice, demand or election in whole or in part without prejudice to
its future actions and rights; provided, however, that in the case of a rescission of an exercise of a Pre-Funded Warrant,
the applicable Purchaser shall be required to return any shares of Common Stock subject to any such rescinded exercise notice concurrently
with the return to such Purchaser of the aggregate exercise price paid to the Company for such shares and the restoration of such Purchaser’s
right to acquire such shares pursuant to such Purchaser’s Pre-Funded Warrant (including, issuance of a replacement warrant certificate
evidencing such restored right).
5.14 Replacement of Securities.
If any certificate or instrument evidencing any Securities is mutilated, lost, stolen or destroyed, the Company shall issue or cause to
be issued in exchange and substitution for and upon cancellation thereof (in the case of mutilation), or in lieu of and substitution therefor,
a new certificate or instrument, but only upon receipt of evidence reasonably satisfactory to the Company of such loss, theft or destruction.
The applicant for a new certificate or instrument under such circumstances shall also pay any reasonable third-party costs (including
customary indemnity) associated with the issuance of such replacement Securities.
5.15 Remedies. In addition
to being entitled to exercise all rights provided herein or granted by law, including recovery of damages, each of the Purchasers and
the Company will be entitled to specific performance under the Transaction Documents. The parties agree that monetary damages may not
be adequate compensation for any loss incurred by reason of any breach of obligations contained in the Transaction Documents and hereby
agree to waive and not to assert in any Action for specific performance of any such obligation the defense that a remedy at law would
be adequate.
5.16 Payment Set Aside.
To the extent that the Company makes a payment or payments to any Purchaser pursuant to any Transaction Document or a Purchaser enforces
or exercises its rights thereunder, and such payment or payments or the proceeds of such enforcement or exercise or any part thereof are
subsequently invalidated, declared to be fraudulent or preferential, set aside, recovered from, disgorged by or are required to be refunded,
repaid or otherwise restored to the Company, a trustee, receiver or any other Person under any law (including, without limitation, any
bankruptcy law, state or federal law, common law or equitable cause of action), then to the extent of any such restoration the obligation
or part thereof originally intended to be satisfied shall be revived and continued in full force and effect as if such payment had not
been made or such enforcement or setoff had not occurred.
5.17 Independent Nature
of Purchasers’ Obligations and Rights. The obligations of each Purchaser under any Transaction Document are several and not
joint with the obligations of any other Purchaser, and no Purchaser shall be responsible in any way for the performance or non-performance
of the obligations of any other Purchaser under any Transaction Document. Nothing contained herein or in any other Transaction Document,
and no action taken by any Purchaser pursuant hereto or thereto, shall be deemed to constitute the Purchasers as a partnership, an association,
a joint venture or any other kind of entity, or create a presumption that the Purchasers are in any way acting in concert or as a group
with respect to such obligations or the transactions contemplated by the Transaction Documents. Each Purchaser shall be entitled to independently
protect and enforce its rights including, without limitation, the rights arising out of this Agreement or out of the other Transaction
Documents, and it shall not be necessary for any other Purchaser to be joined as an additional party in any Proceeding for such purpose.
Each Purchaser has been represented by its own separate legal counsel in its review and negotiation of the Transaction Documents. For
reasons of administrative convenience only, each Purchaser and its respective counsel have chosen to communicate with the Company through
LB. LB does not represent any of the Purchasers and only represents the Placement Agent. The Company has elected to provide all Purchasers
with the same terms and Transaction Documents for the convenience of the Company and not because it was required or requested to do so
by any of the Purchasers. It is expressly understood and agreed that each provision contained in this Agreement and in each other Transaction
Document is between the Company and a Purchaser, solely, and not between the Company and the Purchasers collectively and not between and
among the Purchasers.
5.18 Liquidated Damages.
The Company’s obligations to pay any partial liquidated damages or other amounts owing under the Transaction Documents is a continuing
obligation of the Company and shall not terminate until all unpaid partial liquidated damages and other amounts have been paid notwithstanding
the fact that the instrument or security pursuant to which such partial liquidated damages or other amounts are due and payable shall
have been canceled.
5.19 Saturdays, Sundays,
Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein
shall not be a Trading Day, then such action may be taken or such right may be exercised on the next succeeding Trading Day.
5.20 Construction.
The parties agree that each of them and/or their respective counsel have reviewed and had an opportunity to revise the Transaction Documents
and, therefore, the normal rule of construction to the effect that any ambiguities are to be resolved against the drafting party
shall not be employed in the interpretation of the Transaction Documents or any amendments thereto. In addition, each and every reference
to share prices and shares of Common Stock in any Transaction Document shall be subject to adjustment for reverse and forward stock splits,
stock dividends, stock combinations and other similar transactions of the Common Stock that occur after the date of this Agreement.
5.21 WAIVER OF JURY
TRIAL. IN ANY ACTION, SUIT, OR PROCEEDING IN ANY JURISDICTION BROUGHT BY ANY PARTY AGAINST ANY OTHER PARTY, THE PARTIES EACH KNOWINGLY
AND INTENTIONALLY, TO THE GREATEST EXTENT PERMITTED BY APPLICABLE LAW, HEREBY ABSOLUTELY, UNCONDITIONALLY, IRREVOCABLY AND EXPRESSLY
WAIVES FOREVER TRIAL BY JURY.
(Signature Pages Follow)
IN WITNESS WHEREOF, the parties
hereto have caused this Securities Purchase Agreement to be duly executed by their respective authorized signatories as of the date first
indicated above.
| ISPECIMEN, INC. |
|
Address for Notice: |
| By: |
|
|
8 Cabot Road
Woburn, MA 01801
E-Mail: tcurley@ispecimen.com |
| |
Name: Tracy Curley |
|
| |
Title: Chief Executive Officer |
|
With a copy to (which shall not constitute notice):
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK.
SIGNATURE PAGE FOR PURCHASER FOLLOWS.]
[PURCHASER SIGNATURE PAGES TO ISPECIMEN SECURITIES
PURCHASE AGREEMENT]
IN WITNESS WHEREOF, the undersigned
have caused this Securities Purchase Agreement to be duly executed by their respective authorized signatories as of the date first indicated
above.
Name of Purchaser: ________________________________________________________
Signature of Authorized Signatory of Purchaser:
_________________________________
Name of Authorized Signatory: _______________________________________________
Title of Authorized Signatory: ________________________________________________
Email Address of Authorized Signatory:_________________________________________
Facsimile Number of Authorized Signatory: __________________________________________
Address for Notice to Purchaser:
Address for Delivery of Warrants to Purchaser (if not same as address
for notice):
DWAC for Shares: _________________
Subscription Amount: $_________________
Shares: _________________
Pre-Funded Warrants: __________________ Beneficial Ownership Blocker ¨ 4.99% or ¨ 9.99%
EIN Number: _______________________
¨
Notwithstanding anything contained in this Agreement to the contrary, by checking this box (i) the obligations of the above-signed
to purchase the securities set forth in this Agreement to be purchased from the Company by the above-signed, and the obligations of the
Company to sell such securities to the above-signed, shall be unconditional and all conditions to Closing shall be disregarded, (ii) the
Closing shall occur on the second (2nd) Trading Day following the date of this Agreement
and (iii) any condition to Closing contemplated by this Agreement (but prior to being disregarded by clause (i) above) that
required delivery by the Company or the above-signed of any agreement, instrument, certificate or the like or purchase price (as applicable)
shall no longer be a condition and shall instead be an unconditional obligation of the Company or the above-signed (as applicable) to
deliver such agreement, instrument, certificate or the like or purchase price (as applicable) to such other party on the Closing Date.
Exhibit A
Pre-Funded Warrant
Exhibit B
Form of Lock-Up Agreement
Exhibit 10.47

CONSULTING AGREEMENT
IR Agency LLC (the “Consultant” or
“IR Agency”) is pleased to provide certain consulting services to Ispecimen inc (“you,” the “Client”
or the “Company”) as more fully described in this agreement (the “Agreement”). This Agreement sets forth the terms
and conditions pursuant to which the Company engages the Consultant to provide such services.
| (a) | Commencing on ________, 2024 (the “Start Date”), Consultant will provide marketing and advertising
services (“Advertising” or “Services”) to communicate information about the Company (trading symbol: “ISPC”)
to the financial community. |
| (b) | Consultant does not make any representation about the response, if any, to the public release of Advertising
for the Company. |
| (c) | Client acknowledges that the Consultant carries no professional licenses. Consultant will not participate
in discussions or negotiations with potential investors. Consultant will not solicit orders, make recommendations or give investment advice.
Consultant will not affect transactions of Company securities for potential investors or anyone else. Consultant and Client agree that
Consultant is not being engaged for, and is not permitted to engage in, activities that would give rise to Consultant being required to
register federally or in any state or other jurisdiction as a broker or an investment advisor. If a financial intermediary expresses interest
in the Company to Consultant, Consultant will refer the intermediary to the Company. In providing the Services under the Agreement, Consultant
agrees to comply in all materials respects with all applicable U.S. securities laws. The Client acknowledges and agrees that (a) it
and its affiliates each have relied and will continue to rely on the advice of its own legal, regulatory, and securities law advisors
for all matters and (b) neither the Client nor any of its affiliates has received, or has relied upon, the advice of Consultant or
any of its affiliates regarding legal, regulatory, or securities law matters. |
| (d) | The Services of the Consultant shall not be exclusive to the Client, and the Client acknowledges that
Consultant will be performing similar Services for other clients and Consultant shall be free to perform Services for such other persons. |
| 2. | Independent Contractor. Client and Consultant agree that Consultant shall perform its duties under
this Agreement as an independent contractor. Nothing contained herein shall be considered as creating a relationship of agent-principal,
employer-employee or joint venturers between the Consultant and the Client. |
| (a) | As consideration for the performance of the Services hereunder during the Term (defined below), the
Client shall pay to the Consultant the sum of Two Million US Dollars $2,000,000 (the “Fee”) by________2024 in cash via Bank
Wire Transfer. This fee and the performance of the services is contingent upon the successful completion of a $5 million at the market,
best efforts capital raise. Such consideration shall be deemed earned in full upon receipt. |
| (b) | Unless otherwise provides in this Agreement, all other services, including out-of-scope assignments, rendered
by Consultant shall be subject to additional compensation under a separate agreement between Consultant and Company. Consultant shall
be responsible for all out-of-pocket expenses incurred or paid in connection with its performance of the Services hereunder. |
| (a) | The term of this Agreement shall commence on the Start Date and continue for a period of 1 month(s) (the
“Term”) unless otherwise extended by mutual agreement of the parties (the “Extended Term”). This Agreement may
be terminated, with or without cause, by either Client or Consultant at any time by written notice to the other Party. If the Agreement
is terminated by Client during the Term for any reason, Client will not be entitled to return of any of the Fee. If the Client files for
bankruptcy, becomes insolvent or is in material breach of this Agreement (“Cause”), Consultant may terminate the Agreement
and Client will not be entitled to the return of any of the Fee. If the Consultant terminates the Agreement without Cause, then Consultant
must return the unused portion (if any) of the Fee. Within ten days after the termination or expiration of this Agreement, each party
shall return to the other all Proprietary or Confidential Information (defined below) of the other party (and any copies thereof) in the
party’s possession or, with the approval of the party, destroy all such Proprietary or Confidential Information. |
| (b) | In the event the Client elects to purchase and the Consultant agrees to supply additional Services during
the Term or the Extended Term of this Agreement, the terms and condition of this Agreement will apply to such additional Services. |
| (a) | In connection with Consultant’s performance of its Services, Consultant will rely on the Company’s
press releases and the Company’s most recent reports, if any, filed with the Securities and Exchange Commission (collectively, the
“Company Information”). In this regard, Company agrees to use its best efforts to make all filings required by the exchange
act and all other applicable laws, in each case on a timely basis in accordance with such laws. Client hereby grants to Consultant the
right to use the name and service marks of Company in its Services. Company will be entitled to require that certain or all materials
created by Consultant in performing its Services be submitted to Company for its review and approval, such approval not to be unreasonably
withheld, conditioned or delayed. |
| (b) | The Client hereby acknowledges and agrees that, in performing its Services hereunder, Consultant will
be using and relying on the Company Information without independent verification thereof. Consultant will also be under no obligation
to determine whether there have been, or to investigate any changes in, such information. Consultant will be entitled to submit any materials
created by Consultant to Company for its review and approval. Client represents and warrants that that the Company Information and all
information provided by Company or its affiliate or representatives to Consultant shall, at the time provided, not contain any untrue
statement or material fact or omit to state a material fact necessary in order to make the statement made, in light of the circumstances
under which they were made, not misleading. |
| (c) | The Client, by its authorization or approval of the Advertising, represents and warrants to Consultant
that, to its knowledge, the Advertising is complete and correct in all material respects and does not contain any untrue statement of
a material fact or omit to state a material fact necessary to make the statements therein not misleading. The Client agrees to promptly
notify Consultant upon the occurrence of any material adverse change in the business or affairs of the Company or upon the occurrence
of any event which causes Client to believe that the Advertising contains any untrue statement of a material fact or omits to state a
material fact necessary to make the statements therein not misleading. |
| 6. | Securities Laws. The Client represents and warrants that the Company Information and all information
provided by the Company or its affiliate or representatives complies in all material respects with the U.S. federal and applicable state
securities laws, and are not and will not be or constitute a part of any activity that is or may be deemed to be illegal under the U.S.
federal or applicable state securities laws, including, without limitation, being a part of any illegal offering, illegal pump-and-dump,
illegal scalping, illegal touting schemes, or an effort to assist with a violation of any court order including, but not limited to, any
order banning or limiting a person’s involvement in the securities markets. |
| 7. | Work Product. All information and materials produced for the Client shall be the property of the
Consultant, free and clear of all claims thereto by the Client, and the Client shall have no claim of authorship therein. Consultant shall
retain all right, title, and interest in and to, including any intellectual property rights with respect to, any data, designs, processes,
specifications, software, applications, course, code, object code, utilities, methodologies, know-how, materials, information and skills
(and any derivative works, modifications and enhancements thereto) owned, acquired or developed by or for Consultant’s databases. |
| 8. | Confidentiality. The parties agree to hold each other's Proprietary or Confidential Information
in strict confidence. “Proprietary or Confidential Information” shall include, but is not limited to, written or oral contracts,
trade secrets, know-how, business methods, business policies, memoranda, reports, records, computer retained information, notes, or financial
information. Proprietary or Confidential Information shall not include any information which: (i) is or becomes generally known to
the public by any means other than a breach of the obligations of the receiving party; (ii) was previously known to the receiving
party or rightly received by the receiving party from a third party not under a duty of confidentiality to the disclosing party; (iii) is
independently developed by the receiving party without use of, or reference to, any Proprietary or Confidential Information; or (iv) is
subject to disclosure under applicable law, regulation, stock exchange rule, court order or other lawful process. The parties agree not
to make each other's Proprietary or Confidential Information available in any form to any third party or to use each other's Proprietary
or Confidential Information for any purpose other than as specified in this Agreement. Each party's Proprietary or Confidential Information
shall remain the sole and exclusive property of that party. The parties agree that in the event of use or disclosure by the other party
other than as specifically provided for in this Agreement, the non-disclosing party may be entitled to equitable relief. Notwithstanding
termination or expiration of this Agreement, the parties acknowledge and agree that their obligations of confidentiality with respect
to Proprietary or Confidential Information shall continue in effect for a total period of three (3) years from the termination date. |
| 9. | Non-Public Material Information. Consultant acknowledges that in order to prepare appropriate
Advertising in a timely manner it may be made aware of price sensitive or confidential information that has not been publicly disclosed
yet. Consultant confirms that it is fully aware of its obligations in relation to such information and will ensure that the confidentiality
of such information is maintained at all times and that it, and its employees and contractors, are all fully aware of and comply with,
all appropriate securities laws and regulations in relation to insider trading and related matters. |
| 10. | Limitation of Liability. Consultant shall not be liable to Client or any other person for any damages
in connection with the provision of Services under the Agreement, whether because of Consultant’s negligence or otherwise, and regardless
of the form of action, except in the event of Consultant’s deliberate fault, will full misconduct or gross negligence. Nevertheless,
regardless of the form of action, whether in contract, tort or otherwise, Consultant shall not be liable to Client for any lost profits,
business interruption, or for any indirect, incidental, special, consequential, exemplary or punitive damages arising out of or relating
to this Agreement, nor shall Consultant’s aggregate liability for any other damages arising out of this Agreement exceed the Fee
paid by Company to Consultant. |
| 11. | Indemnification. Client shall indemnify and hold Consultant harmless from and against any and all
actions, claims, investigations (including but not limited to any formal or informal investigations brought by any state or federal regulator
and any subpoenas or requests for documents issued in connection therewith), liabilities, losses, or damages arising from the preparation,
presentation or dissemination of any Advertising covered by this Agreement including, but limited to, the costs of defense and attorneys’
fees except for any claims as a result of Consultant’s deliberate fault, willful misconduct or gross negligence. |
| 12. | Notices. Any notice or other communication required or permitted to be given to either party hereunder
shall be in writing and shall be given to such party at such party’s address set forth below or such other address as such party
may hereafter specify by notice in writing to the other party. Any such notice or other communication shall be addressed as aforesaid
and given by (a) certified mail, return receipt requested, with first class postage prepaid, (b) hand delivery, or (c) via
electronic communication (i.e., e-mail) or reputable overnight courier. Any notice or other communication will be deemed to have been
duly given (i) on the fifth (5) day after mailing, provided receipt of delivery is confirmed, if mailed by certified mail, return
receipt requested, with first class postage prepaid, (ii) on the date of Service if served personally or (iii) on the business
day after delivery to an overnight courier service or by sending of an electronic communication, provided the notifying party specifies
next day delivery and receipt of delivery has been confirmed: |
If to the Client:
Email:
If
to Consultant:
IR Agency LLC
23 Downing Street, Newark NJ 07105 E-mail: [Raf@ir.agency]
_____________________________________
| 13. | Waiver of Breach. Any waiver by either party of a breach of any provision of this Agreement by
the other party shall not operate or be construed as a waiver of any subsequent breach by any party. |
| 14. | Assignment. Neither this Agreement nor any of the rights, interests or obligations hereunder may
be assigned by either party hereto without the prior written consent of the other party, which will not be delayed or withheld unreasonably;
provided that the Client shall not be required to consent to any assignment by Consultant of its cash and compensation payable pursuant
to this Agreement. Any assignment without such consent, when required, shall have no legal validity; subject to the foregoing, this Agreement
and all of the provisions hereof will be binding upon and inure to the benefit of the parties to this Agreement and their respective successors
and permitted assigns. |
| 15. | Governing Law and Jurisdiction. This Agreement shall be governed and construed under State of New
Jersey. The parties consent to the exclusive jurisdiction of the federal and state courts located in New Jersey, to hear and determine
any dispute that may arise under this Agreement. |
| 16. | Entire Agreement. This Agreement contains the complete agreement between the parties with respect
to the subject matter hereof and supersedes any prior proposals, understandings, agreements or representations by or between the parties,
written or oral. |
| 17. | Severability. Whenever possible, each provision of this Agreement will be interpreted in such manner
as to be effective and valid under applicable law, but if any provision of this Agreement is held by any court of competent jurisdiction
to be prohibited by or invalid under applicable law, such provision will be ineffective only to the extent of such prohibition or invalidity,
without invalidating the remainder of such provision or the remaining provisions of this Agreement. |
| 18. | Waiver and Modification. Any waiver, alteration, or modification of any of the provisions of this
Agreement shall be valid only if made in writing through an amendment of this Agreement and signed by the parties hereto. |
| 19. | Acceptance.
Please confirm that the foregoing is in accordance with the Company’s understanding
by signing and returning this Agreement, which will thereupon constitute a binding Agreement
between the Company and IR Agency, LLC as of _______. The undersigned officers of IR Agency,
LLC and the Company represent that they have the authority to bind IR Agency and the Company,
respectively. This Agreement may be executed in counterparts and with electronic or facsimile
signatures. |
| IR Agency LLC |
|
| By: |
|
|
| Print Name: Rafael Pereira |
|
Ispecimen Inc
By:
Print Name:
Position:
IR Agency wire Instructions
Capital One Bank
Account Beneficiary- IR Agency LLC
Address: 23 Downing Street, Newark NJ 07105
Wire Acct #: 7057541044
Wire Routing #- 021407912
SWIFT# HIBKUS44
Exhibit 23.1
Consent of Independent Registered
Public Accounting Firm
We consent to the incorporation by reference in this Registration Statement
on Form S-1 and related Prospectus of iSpecimen Inc. of our report dated March 13, 2024, relating to the financial statements of iSpecimen
Inc., appearing in the Annual Report on Form 10-K for the years ended December 31, 2023, and 2022.
We also consent to the reference to our firm under the heading "Experts"
in such Prospectus.
/s/ Wolf & Company, P.C.
Boston, Massachusetts
October 18, 2024
Exhibit 107
Calculation of Filing Fee Tables
S-1
(Form Type)
iSpecimen Inc.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered Securities
| |
|
Security
Type |
|
Security
Class
Title |
|
Fee
Calculation
or Carry
Forward
Rule |
|
Amount
Registered |
|
|
Proposed
Maximum
Offering
Price Per
Unit |
|
|
Maximum
Aggregate
Offering
Price(1)(2) |
|
|
Fee
Rate |
|
|
Amount
of
Registration
Fee |
| Fees
to Be Paid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Equity |
|
Common Stock, $0.0001 par
value per share (2) |
|
Rule 457(0) |
|
|
— |
|
|
|
— |
|
|
$ |
5,000,000 |
|
|
|
$153.10 per $1,000,000 |
|
|
$ |
765.5 |
| |
|
Equity |
|
Pre-Funded
Warrants(4) |
|
Rule 457(g) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
| |
|
Equity |
|
Common Stock underlying Pre-Funded
Warrants(3)(5) |
|
Rule 457(o) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
— |
| Fees Previously
Paid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Carry Forward
Securities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Total Offering
Amounts |
|
|
|
|
|
|
|
|
|
$ |
5,000,000 |
|
|
|
|
|
|
$ |
765.5 |
| |
|
Total Fees
Previously Paid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Total Fee
Offsets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Net Fee Due |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
765.5 |
| (1) |
Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(a) under the Securities Act of 1933, as amended (the “Securities Act”). |
| (2) |
Pursuant to Rule 416 of the Securities Act, there are also being registered an indeterminable number of additional securities as may be issued to prevent dilution resulting from stock splits, stock dividends or similar transactions. |
| (3) |
The proposed maximum aggregate offering price of the common stock will be reduced on a dollar-for-dollar basis based on the offering price of any Pre-Funded Warrants issued in the offering, and the proposed maximum aggregate offering price of the Pre-Funded Warrants to be issued in the offering will be reduced on a dollar-for-dollar basis based on the offering price of any common stock issued in the offering. Accordingly, the proposed maximum aggregate offering price of the common stock and Pre-Funded Warrants (including the common stock issuable upon exercise of the Pre-Funded Warrants), if any, is $5,000,000. |
| (4) |
No fee pursuant to Rule 457(g) of the Securities Act. |
| (5) |
The Registrant may issue pre-funded warrants to purchase shares of common stock in the offering. The purchase price of each Pre-Funded Warrant will equal the price per share at which shares of common stock are being sold to the public in this offering, minus $0.0001, which constitutes the pre-funded portion of the exercise price, and the remaining unpaid exercise price of the Pre-Funded Warrant will equal $0.0001 per share (subject to adjustment as provided for therein). |
iSpecimen (NASDAQ:ISPC)
Historical Stock Chart
From Nov 2024 to Dec 2024
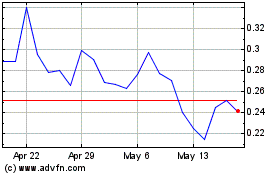
iSpecimen (NASDAQ:ISPC)
Historical Stock Chart
From Dec 2023 to Dec 2024