UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
6-K
REPORT
OF FOREIGN PRIVATE ISSUER
PURSUANT
TO RULE 13a-16 OR 15d-16
UNDER
THE SECURITIES EXCHANGE ACT OF 1934
February
2025
Commission
File Number: 001-41386
OKYO
Pharma LTD
(Exact
Name of Registrant as Specified in Its Charter)
9th
Floor
107
Cheapside
London
EC2V
6DN
(Address
of registrant’s principal executive office)
Indicate
by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form
20-F ☒ Form 40-F ☐
Indicate
by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate
by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
INFORMATION
CONTAINED IN THIS REPORT ON FORM 6-K
On
February 12, 2025, OKYO Pharma LTD (the “Company”) issued this 6K announcing today, that its lead asset, OK-101, has
been officially assigned the United States Adopted Name (USAN) “urcosimod”.
The
Announcement is furnished herewith as Exhibit 99.1 to this Report on Form 6-K. The information in the attached Exhibits 99.1 is being
furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, or otherwise
subject to the liabilities of that Section, nor shall it be deemed incorporated by reference in any filing made by the Company under
the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, except as otherwise set forth herein or as shall be expressly
set forth by specific reference in such a filing.
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned, thereunto duly authorized.
| |
OKYO
Pharma LTD |
| |
|
|
| Date:
February 12, 2025 |
By: |
/s/
Keeren Shah |
| |
Name: |
Keeren
Shah |
| |
Title: |
Chief
Financial Officer |
EXHIBIT
INDEX
Exhibit
99.1

OKYO
Pharma Announces OK-101 Officially Assigned USAN : Urcosimod
London
and New York, NY, February 12, 2025. OKYO Pharma Limited (NASDAQ: OKYO), a clinical-stage biopharmaceutical company developing innovative
therapies for the treatment of neuropathic corneal pain (NCP), a severe ocular condition without an FDA approved therapy, and for inflammatory
dry eye disease (DED), a multi-billion-dollar market, is pleased to announce that its lead asset, OK-101, has been officially assigned
the United States Adopted Name (USAN) “urcosimod”.
The
USAN designation reflects Okyo Pharma’s commitment to developing new therapies for unmet medical needs in ophthalmology. The suffix
“-mod” in urcosimod denotes its classification as a modulator of key inflammatory and neuropathic pathways, critical to addressing
ocular conditions such as neuropathic corneal pain (NCP) and dry eye disease (DED).
The
USAN program, jointly managed by the American Medical Association (AMA), the United States Pharmacopeial Convention (USP), and the American
Pharmacists Association (APhA), assigns unique nonproprietary names to pharmaceutical substances to ensure clarity in medical communication.
This naming milestone underscores the progress of urcosimod (OK-101) in its clinical development program.
Gary
S. Jacob, Ph.D., Chief Executive Officer of Okyo Pharma, commented:
“We
are thrilled to announce that OK-101 has been granted the name urcosimod, marking an important step in its development as a therapeutic
option for patients suffering from serious ocular conditions. Urcosimod is currently in a Phase 2 clinical trial for neuropathic corneal
pain, an area of significant unmet medical need. This follows the encouraging results observed in our prior Phase 2 trial of urcosimod
for dry eye disease, where the drug demonstrated strong pain reducing effects and a favorable safety profile.”
Dr.
Jacob continued: “Neuropathic corneal pain is a debilitating condition for which there are currently no FDA-approved treatments.
We believe urcosimod’s dual anti-inflammatory and analgesic properties uniquely position it to address both the symptoms and the
underlying causes of this condition. With the USAN name granted, we are further cementing the path forward for this promising therapeutic
candidate.”
Okyo
Pharma’s innovative lipid-conjugated small molecule platform enables urcosimod to target and modulate ocular G-protein coupled
receptors (GPCRs), reducing inflammation and pain at the source. The drug’s design, combining anti-inflammatory and pain-modulating
properties, sets it apart from traditional approaches in ocular disease treatment.
The
ongoing Phase 2 trial for neuropathic corneal pain was initiated in October 2024. The company is planning to release top-line results
in Q4 2025.
Okyo
Pharma remains committed to addressing the unmet needs of patients with sight-threatening and quality-of-life-impacting conditions.
About
NCP
Neuropathic
corneal pain (NCP) is a condition that causes pain and sensitivity of the eyes, face, or head. The exact cause of NCP is unknown but
thought to result from nerve damage to the cornea combined with inflammation. NCP, which can exhibit as a severe, chronic, or debilitating
condition in patients suffering from a host of ophthalmic conditions, is presently treated by various topical and systemic treatments
in an off-label fashion. Notably, there is no FDA approved drug to treat this debilitating condition.
About
Urcosimod (Formerly called OK-101)
Urcosimod
is a lipid conjugated chemerin peptide agonist of the ChemR23 G-protein coupled receptor which is typically found on immune cells of
the eye responsible for the inflammatory response, as well as on neurons and glial cells in the dorsal root ganglion. Urcosimod was developed
using a membrane-anchored-peptide technology to produce a novel long-acting drug candidate for treating dry eye disease. Urcosimod has
been shown to produce anti-inflammatory and pain-reducing activities in mouse models of dry eye disease and corneal neuropathic pain
(NCP), respectively, and is designed to combat washout through the inclusion of the lipid anchor built into the drug molecule to potentially
enhance the residence time of urcosimod within the ocular environment. Urcosimod showed clear statistical significance in multiple endpoints
in a recently completed Phase 2, multi-center, double-masked, placebo-controlled trial to treat DED, and is presently being evaluated
in a randomized, placebo-controlled, double-masked Phase 2 trial to treat 48 NCP patients.
About
OKYO
OKYO
Pharma Limited (NASDAQ: OKYO) is a clinical stage biopharmaceutical company developing innovative therapies for the treatment of NCP
and DED, with ordinary shares listed for trading on the NASDAQ Capital Market. OKYO is focused on the discovery and development of novel
molecules to treat NCP and inflammatory DED. In addition to the completed Phase 2 trial of urcosimod to treat DED patients, OKYO is also
currently evaluating urcosimod to treat NCP patients in a Phase 2 trial.
For
further information, please visit www.okyopharma.com.
Enquiries:
| OKYO
Pharma Limited |
|
Gary
S. Jacob, Chief Executive Officer |
|
917-497-7560 |
| Business
Development & Investor Relations |
|
Paul
Spencer |
|
+44
(0)20 7495 2379
|
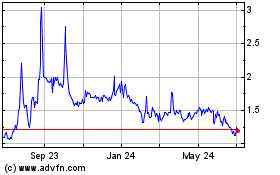
OKYO Pharma (NASDAQ:OKYO)
Historical Stock Chart
From Jan 2025 to Feb 2025
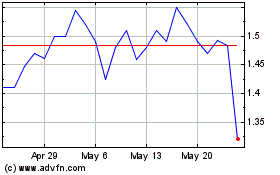
OKYO Pharma (NASDAQ:OKYO)
Historical Stock Chart
From Feb 2024 to Feb 2025