Roche Gets FDA Approval for Columvi Lymphoma Treatment
16 June 2023 - 3:44PM
Dow Jones News
By Adria Calatayud
Roche Holding said Friday that it has received approval from the
U.S. Food and Drug Administration for Columvi, a treatment for
adult patients with relapsed or refractory diffuse large B-cell
lymphoma.
The Swiss pharmaceutical giant said it obtained an accelerated
approval based on data from an early-stage Phase 1 and 2 study, and
that continued approval for this indication may be contingent upon
verification of clinical benefit in a confirmatory trial.
In the Phase 1 and 2 study, the drug showed a 56% overall
response rate and a median duration of response of more than 18
months, the company said.
Roche said Columvi will be available in the U.S. in the coming
weeks.
Write to Adria Calatayud at adria.calatayud@dowjones.com
(END) Dow Jones Newswires
June 16, 2023 01:29 ET (05:29 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
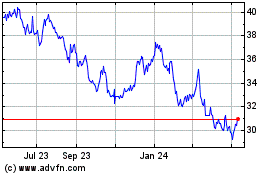
Roche (QX) (USOTC:RHHBY)
Historical Stock Chart
From Apr 2024 to May 2024
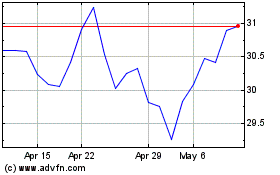
Roche (QX) (USOTC:RHHBY)
Historical Stock Chart
From May 2023 to May 2024