Filed Pursuant to Rule 424(b)(5)
Registration No. 333-274816
PROSPECTUS SUPPLEMENT
(To Prospectus dated October 25, 2023)
| |
ALTERITY THERAPEUTICS LIMITED
US$6,000,000
Ordinary Shares represented by American Depositary
Shares |
|
This prospectus supplement relates to the offer
and sale of ordinary shares from time to time, represented by American Depositary Shares, or ADSs, having an aggregate offering price
of up to US$6,000,000. The ADSs are evidenced by American Depositary Receipts, or ADRs. We have entered into an Sales Agreement, dated
February 15, 2024, which we refer to as the Sales Agreement, with JonesTrading Institutional Services LLC, or the “Agent”.
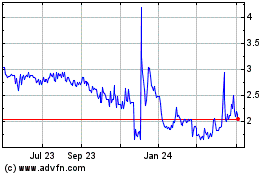
Our ADSs are listed on The NASDAQ Capital Market
under the symbol “ATHE.” On February 14, 2024, the closing price of an ADS of Alterity Therapeutics Limited on The NASDAQ
Capital Market was US$1.98.
Sales of our ADSs under this prospectus supplement
and the accompanying prospectus, if any, may be made in sales deemed to be “at the market offerings” as defined in Rule 415
promulgated under the Securities Act of 1933, as amended, or the Securities Act. The Agent will act as sales agent on a best efforts basis
using commercially reasonable efforts consistent with its normal trading and sales practices. There is no arrangement for funds to be
received in any escrow, trust or similar arrangement.
The Agent will be entitled to compensation at a
fixed commission rate of equal to 3.0% of the gross sales price per share sold. In connection with the sale of ADSs on our behalf, the
Agent will be deemed to be an “underwriter” within the meaning of the Securities Act, and the compensation of the Agent will
be deemed to be underwriting commissions or discounts. We have agreed to provide indemnification and contribution to the Agent against
certain liabilities, including liabilities under the Securities Act.
As of the date of this prospectus supplement, the aggregate market
value of our outstanding ordinary shares held by non-affiliates is US$20,989,187, based on 4,382,754,741 ordinary shares outstanding,
represented by 7,304,592 ADSs, of which 4,342,590,393 ordinary shares, represented by 7,237,651 ADSs, are held by non-affiliates, and
a per share price of US$2.90, which was the closing sale price of our common stock as quoted on Nasdaq on December 18, 2023. As of the
date of this prospectus supplement, we have offered and sold US$118,061 of our securities pursuant to General Instruction I.B.5. of Form
F-3 during the prior 12 calendar month period that ends on, and includes, the date of this prospectus supplement. Pursuant to General
Instruction I.B.5 of Form F-3, in no event will we sell our ordinary shares in a public primary offering with a value exceeding more than
one-third of our public float in any 12-month period so long as our public float remains below US$75,000,000.
Investing in the ADSs involves a high degree of
risk. Before buying any securities, you should carefully consider the risk factors described in “Risk Factors” beginning on
page S-3 of this prospectus supplement and on page 2 of the accompanying prospectus.
Neither the Securities and Exchange Commission nor
any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement and the accompanying
prospectus are truthful or complete. Any representation to the contrary is a criminal offense.

The date of this prospectus supplement is February 15, 2024.
TABLE OF CONTENTS
PROSPECTUS SUPPLEMENT
BASE PROSPECTUS
Unless expressly stated otherwise, all references in
this prospectus supplement and the accompanying prospectus to “Alterity” “we,” “us,” “our,”
or similar references mean Alterity Therapeutics Limited and its subsidiaries, unless otherwise indicated.
All references to “U.S. dollars” or “US$”
in this supplement and the accompanying prospectus are to U.S. dollars, and all references to “Australian dollars” or “A$”
are to the currency of Australia.
This document is in two parts. The first part is this
prospectus supplement, which describes the terms of this offering of our ADSs and supplements information contained in the accompanying
prospectus and the documents incorporated by reference into the accompanying prospectus. The second part is the accompanying prospectus,
which gives more general information about us and the ADSs that we may offer from time to time under our shelf registration statement.
To the extent there is a conflict between the information contained in this prospectus supplement, on the one hand, and the information
contained in the accompanying prospectus or any document incorporated by reference therein, on the other hand, the information in this
prospectus supplement shall control.
You should read this document together with the
additional information described under the headings “Where You Can Find More Information” and “Incorporation of Certain
Information by Reference” in this prospectus supplement. We have not authorized any dealer, salesperson or other person to give
any information or to make any representation other than those contained or incorporated by reference in this prospectus supplement, the
accompanying prospectus and any related free writing prospectus. You should not rely upon any information or representation not contained
or incorporated by reference in this prospectus supplement, the accompanying prospectus or any free writing prospectus that we may authorize
to be provided to you. This prospectus supplement, the accompanying prospectus and any related free writing prospectus do not constitute
an offer to sell or the solicitation of an offer to buy ADSs, nor does this prospectus supplement, the accompanying prospectus and any
related free writing prospectus constitute an offer to sell or the solicitation of an offer to buy ADSs in any jurisdiction to any person
to whom it is unlawful to make such offer or solicitation in such jurisdiction. You should not assume that the information contained in
this prospectus supplement, the accompanying prospectus and any related free writing prospectus is accurate on any date subsequent to
the date set forth on the front of the document or that any information we have incorporated by reference is correct on any date subsequent
to the date of the document incorporated by reference, even though this prospectus supplement, the accompanying prospectus and any related
free writing prospectus is delivered or ADS is sold on a later date.
We further note that the representations, warranties
and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference into the accompanying
prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating
risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such
representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and
covenants should not be relied on as accurately representing the current state of our affairs.
FORWARD-LOOKING STATEMENTS
This prospectus supplement, the accompanying prospectus
and the documents incorporated in it by reference contain forward-looking statements which involve known and unknown risks and uncertainties.
We include this notice for the express purpose of permitting us to obtain the protections of the safe harbor provided by the Private Securities
Litigation Reform Act of 1995 with respect to all such forward-looking statements. Examples of forward-looking statements include: projections
of capital expenditures, competitive pressures, revenues, growth prospects, product development, financial resources and other financial
matters. You can identify these and other forward-looking statements by the use of words such as “may,” “plans,”
“anticipates,” “believes,” “estimates,” “predicts,” “intends,” “potential”
or the negative of such terms, or other comparable terminology.
Our ability to predict the results of our operations
or the effects of various events on our operating results is inherently uncertain. Therefore, we caution you to consider carefully the
matters described under the caption “Risk Factors” and certain other matters discussed in this prospectus supplement, the
accompanying prospectus, the documents incorporated by reference in the accompanying prospectus, and other publicly available sources.
Such factors and many other factors beyond the control of our management could cause our actual results, performance or achievements to
be materially different from any future results, performance or achievements that may be expressed or implied by the forward-looking statements.
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights selected information contained
elsewhere or incorporated by reference in this prospectus supplement and the accompanying prospectus. The summary may not contain all
the information that you should consider before investing in our ADSs. You should read the entire prospectus supplement and the accompanying
prospectus carefully, including “Risk Factors” contained in this prospectus supplement and the accompanying prospectus and
the documents incorporated by reference in the accompanying prospectus, before making an investment decision. This prospectus supplement
may add to, update or change information in the accompanying prospectus.
Alterity Therapeutics Limited
We were incorporated under the laws of the Commonwealth of Australia on
November 11, 1997 and began limited operations shortly thereafter. Our mission is to develop therapeutic drugs designed to treat neurodegenerative
diseases, currently focusing on Parkinsonian and other movement disorders.
Corporate Information
Our registered office is located at Level 3, 62 Lygon Street, Carlton,
Victoria 3053 Australia and our telephone number is 011-61-3-9824-5254. Our principal executive office is located at Level 14, 350 Collins
Street, Melbourne, Victoria 3052, Australia and our telephone number is 011-61-3-9349-4906. Our address on the internet is www.alteritytherapeutics.com. The
information in our website is not incorporated by reference into this prospectus supplement and should not be considered as part of this
prospectus supplement.
The Offering
|
Securities offered
|
Ordinary shares represented by ADSs having an aggregate offering price of up to US$6,000,000. |
| |
|
| The ADSs |
Each ADS represents six hundred ordinary shares, no par value. The offered ADSs are evidenced by ADRs. |
| |
|
| Depositary |
The Bank of New York Mellon |
| |
|
| Manner of Offering |
“At the market offering” that may be made from time to time through the Agent. See “Plan of Distribution” on page S-6. |
| |
|
| Ordinary Shares outstanding as of February 14, 2024 |
4,382,754,741 ordinary shares (which excludes 2,768,018,796 ordinary
shares issuable upon the exercise of options having exercise prices ranging from A$0.032 to A$0.007 per share). |
| |
|
| Use of proceeds |
We intend to use the net proceeds from this offering for ongoing and future clinical trials, and future research programs into the development of our proprietary compounds, including our lead compound, ATH434, and for working capital purposes. See “Use of Proceeds” on page S-4. |
| |
|
| NASDAQ Capital Market symbol |
“ATHE” |
| |
|
| Risk Factors |
This investment involves a high degree of risk. See “Risk Factors” beginning on page S-3 of this prospectus supplement as well as the other information included in or incorporated by reference in this prospectus supplement and the accompanying prospectus for a discussion of risks you should consider carefully before making an investment decision. |
Unless otherwise stated, all information contained
in this prospectus supplement reflects the assumed public offering price of US$1.98 per ADS, which was the last reported sale price of
an ADS representing our ordinary shares on The NASDAQ Capital Market on February 14, 2024.
RISK FACTORS
Investing in our securities involves significant risks.
Before making an investment decision, you should carefully consider the risks described under “Risk Factors” in the applicable
prospectus supplement and under Item 3.D. – “Risk Factors” in our most recent Annual Report on Form 20-F, or any updates
in our Reports on Form 6-K, together with all of the other information appearing in this prospectus or incorporated by reference into
this prospectus and any applicable prospectus supplement, in light of your particular investment objectives and financial circumstances.
The risks so described are not the only risks facing us. Additional risks not presently known to us or that we currently deem immaterial
may also impair our business operations. Our business, financial condition and results of operations could be materially adversely affected
by any of these risks. The trading price of our securities could decline due to any of these risks, and you may lose all or part of your
investment. The discussion of risks includes or refers to forward-looking statements; you should read the explanation of the qualifications
and limitations on such forward-looking statements discussed elsewhere in this prospectus.
Risks Relating to the Offering
We have broad discretion in the use of the net proceeds from this
offering.
Our management will have broad discretion in the application of the net
proceeds from this offering and could spend the proceeds in ways with which you may not agree. Accordingly, you will be relying on the
judgment of our management with regard to the use of the net proceeds, and you will not have the opportunity, as part of your investment
decision, to assess whether the proceeds are being used appropriately. It is possible that the net proceeds will be invested or otherwise
used in a way that does not yield a favorable, or any, return for the Company.
Investors in this offering will experience immediate and substantial
dilution in the net tangible book value per ADS they purchase.
Since the price per share of our ordinary shares represented by ADSs being
offered is higher than the net tangible book value per share of our ordinary shares represented by ADSs, you will suffer substantial dilution
in the net tangible book value of the common stock you purchase in this offering. See the section entitled “Dilution” in this
prospectus for a more detailed discussion of the dilution you will incur if you purchase ADSs in this offering. In addition, we have a
significant number of options outstanding. If the holders of these options exercise such options, you may incur further dilution.
Our stockholders may experience significant dilution as a result
of future equity offerings and exercise of outstanding options.
In order to raise additional capital, we may in the future offer additional
ordinary shares represented by ADSs or other securities convertible into or exchangeable for our ordinary shares. We cannot assure you
that we will be able to sell shares or other securities in any other offering at a price per share that is equal to or greater than the
price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights
superior to existing stockholders. The price per share at which we sell additional ordinary shares represented by ADSs or other securities
convertible into or exchangeable for our ordinary shares in future transactions may be higher or lower than the price per share in this
offering.
USE OF PROCEEDS
Except as otherwise provided in the applicable prospectus
supplement, we intend to use the net proceeds from the sale of the securities covered by this prospectus for ongoing and future clinical
trials and research programs into the development of our proprietary compounds, including our compound ATH434 for Parkinsonian disorders,
and for working capital purposes. Additional information on the use of net proceeds from the sale of securities covered by this
prospectus may be set forth in the prospectus supplement relating to the specific offering.
DILUTION
If you invest in our securities, your ownership interest
will be immediately diluted to the extent of the difference between the offering price per ordinary share paid by purchasers in this offering
and the pro forma as adjusted net tangible book value per ordinary share after completion of the offering. Dilution results from the fact
that the per ordinary share offering price is substantially in excess of the book value per ordinary share attributable to the existing
holders of our presently outstanding ordinary shares.
Our net tangible book value as of June 30, 2023 was approximately US$15.1
million, or US$0.0062 per ordinary share (US$3.72 per ADS). Net tangible book value per share or per ADS represents the amount of our
total tangible assets less total liabilities divided by the total number of ordinary shares or ADSs outstanding. Each ADS represents 600
ordinary shares.
After giving effect to the placement of 1,942,857,123 shares in placements
on November 29, 2023 (362,462,763 shares), January 8, 2024 (1,008,965,809 shares) and February 2, 2024 (571,428,556 shares), our adjusted
proforma net tangible book value as of June 30, 2023, would have been US$21.92 million or US$0.005 per ordinary share (US$3.00 per ADS).
After giving further effect to the sale of 1,764,705,882 ordinary shares (2,941,176 ADSs) in this offering of US$6,000,000 at an assumed
offering price of US$0.0034 per ordinary share, or US$2.04 per ADS, which was our closing price of our ADSs on the NASDAQ Capital Market
on February 13, 2024 and after deducting estimated offering commissions and other estimated offering expenses, our as-adjusted proforma
net tangible book value as of June 30, 2023 would have been US$27.62 million or US$0.0045 per ordinary share (US$2.70 per ADS). This represents
an immediate dilution in the proforma net tangible book value of US$0.0005 per ordinary share (US$0.31 per ADS) to our existing stockholders
and an increase in proforma net tangible book value of US$0.0011 per ordinary share (US$0.66 per ADS) to new investors.
The following table sets forth the estimated net tangible book value
per ordinary share in US$ after the offering and the dilution to persons purchasing ordinary shares based on the foregoing offering assumptions.
Assumed offering price per ordinary share | |
| | | |
$ | 0.0034 | |
| Adjusted net tangible book value per ordinary share as of June 30,
2023 | |
$ | 0.0050 | | |
| | |
| Dilution per ordinary share attributable to new investors | |
$ | 0.0005 | | |
| | |
| Pro forma as-adjusted net tangible book value per ordinary share after
this offering | |
| | | |
$ | 0.0045 | |
| Increase per share to new investors purchasing shares in this offering | |
| | | |
$ | 0.0011 | |
This discussion of dilution, and the table quantifying it, assumes
no exercise of any outstanding options or warrants over our ordinary shares. The table above contains a translation of net tangible book
value at June 30, 2023 from Australian dollar amounts into U.S. dollar amounts at specified rates solely for the convenience of the reader,
which was A$1 to US$0.663. Thereafter the proforma has been made at the exchange rate of A$1 to US$0.6516, which was the exchange rate
at February 13, 2024.
PLAN OF DISTRIBUTION
We have entered into an Sales Agreement,
dated February 15, 2024, or the Sales Agreement, with JonesTrading Institutional Services LLC, or the Agent, under which we may issue
and sell ADSs for up to $6,000,000 of our ordinary shares from time to time pursuant to this Prospectus Supplement through the Agent.
The Agent may sell the ADSs
by any method permitted by law deemed to be an “at the market offering” as defined in Rule 415 under the Securities Act. The
ADSs are evidenced by ADRs.
Each time that we wish to issue and sell ADSs under
the Sales Agreement, we will provide the Agent with a placement notice describing the number of ADSs to be issued, the time period during
which sales are requested to be made, any limitation on the number of ADSs that may be sold in any one day and any minimum price below
which sales may not be made.
Upon receipt of a placement notice from us, and subject
to the terms and conditions of the Sales Agreement, the Agent has agreed to use its commercially reasonable efforts consistent with its
normal trading and sales practices to sell such ADSs up to the amount specified on such terms. Unless otherwise specified, the settlement
between us and the Agent of our ADSs will occur on the second trading day following the date on which the sale was made. The obligation
of the Agent under the Sales Agreement to sell our ADSs pursuant to a placement notice is subject to a number of conditions. There is
no arrangement for funds to be received in an escrow, trust or similar arrangement.
We will pay the Agent a commission of equal to
3.0% of the gross proceeds of the sales price of all ADSs sold through it as sales agent under the Sales Agreement. Because there is no
minimum offering amount required as a condition to closing this offering, the actual total public offering amount, commissions and proceeds
to us, if any, are not determinable at this time. We estimate that the total expenses for the offering, excluding compensation payable
to the Agent under the terms of the Sales Agreement, will be approximately US$100,000.
In connection with the sale of our ADSs contemplated
in this prospectus supplement, the Agent is an “underwriter” within the meaning of the Securities Act, and the compensation
paid to it will be deemed to be underwriting commissions or discounts. We have agreed to indemnify the Agent against certain civil liabilities,
including liabilities under the Securities Act.
Sales of our ADSs as contemplated in this prospectus
supplement will be settled through the facilities of The Depository Trust Company or by such other means as we and the Agent may agree
upon.
The offering of our ADSs pursuant to this prospectus
supplement will terminate on the earlier of (1) the sale of all of the ordinary shares subject to this prospectus supplement, or (2) termination
of the Sales Agreement by us or the Agent.
This summary
of the material provisions of the Sales Agreement does not purport to be a complete statement of its terms and conditions. A copy of the
Sales Agreement, as amended, was filed with the SEC as an exhibit to a Report on Form 6-K filed under the Securities Exchange Act of 1934,
or the Exchange Act, and is incorporated by reference in this prospectus supplement and the accompanying prospectus.
The Agent and its affiliates have in the past and may
in the future provide various investment banking and other financial services for us, for which services they may in the future receive
customary fees.
LEGAL MATTERS
The validity of the securities offered hereunder will be passed upon
for us by QR Lawyers Pty Ltd., Melbourne, Australia, our Australian counsel. Carter Ledyard & Milburn LLP, New York, New York, will
be passing upon matters of United States law for us with respect to securities offered by this prospectus and any accompanying prospectus
supplement. The Agent is being represented in connection with this offering by Duane Morris LLP, New York, New York.
EXPERTS
The financial statements incorporated in this Prospectus
by reference to the Annual Report on Form 20-F for the year ended June 30, 2023 have been so incorporated in reliance on the report (which
contains an explanatory paragraph relating to the Company's ability to continue as a going concern as described in Note 1 to the financial
statements) of PricewaterhouseCoopers, an independent registered public accounting firm, given on the authority of said firm as experts
in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We filed a registration statement on Form F-3 to register
with the SEC the securities described in this prospectus. This prospectus is part of that registration statement. This prospectus, which
constitutes a part of the registration statement, summarizes material provisions of contracts and other documents that we refer to in
the prospectus. Since this prospectus does not contain all of the information contained in the registration statement, you should read
the registration statement and its exhibits and schedules for further information with respect to us and our ordinary shares and the ADSs.
Our SEC filings, including the registration statement, are also available to you on the SEC’s Web site at http://www.sec.gov.
We are subject to the information reporting requirements
of the Exchange Act that are applicable to foreign private issuers, and under those requirements we file reports with the SEC. As a foreign
private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our
officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16
of the Exchange Act. In addition, we are not required under the Exchange Act to file annual, quarterly and current reports and financial
statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act.
However, we file with the SEC, within four months after the end of each fiscal year, or such applicable time as required by the SEC, an
annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm, and submit
to the SEC, on Form 6-K, unaudited quarterly financial information for the first three quarters of each fiscal year within 60 days
after the end of each such quarter, or such applicable time as required by the SEC.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference”
information into this prospectus, which means that we can disclose important information to you by referring you to other documents which
we have filed or will file with the SEC. We are incorporating by reference in this prospectus the documents listed below and all amendments
or supplements we may file to such documents, as well as any future filings we may make with the SEC on Form 20-F under the Exchange Act
before the time that all of the securities offered by this prospectus have been sold or de-registered.
| |
● |
Our Annual Report on Form 20-F for the fiscal year ended June 30, 2023, as filed with the Commission on August 31, 2023; |
| |
● |
Our Reports on Form 6-K filed with or furnished to the SEC on August
31, 2023, August 31,
2023, August
31, 2023, September 13,
2023, October 30,
2023, October 31,
2023, November 8,
2023, November 16,
2023, November 20,
2023, November 22,
2023, November 22,
2023, November 22,
2023, November 22,
2023, November 22,
2023, November 27,
2023, November 29,
2023, November 29,
2023, November
29, 2023, November 29,
2023, November 29,
2023, November 29,
2023, November 30,
2023, November 30,
2023, December 4,
2023, December 8, 2023, December
11, December 18, December
21, 2023, December 21,
2023, December 29,
2023, December 29,
2023, January 2, 2024, January
2, 2024, January 2,
2024, January 5, 2024, January
5, 2024, January 8,
2024, January 8, 2024, January
8, 2024, January 8,
2024, January 10, 2024, January
11, 2024, January 11,
2024, January 18, 2024, January
22, 2024, January 31, 2024, February 2, 2024, February 2, 2024, February 2, 2024, and February 6, 2024; |
| |
● |
The description of our ADRs contained in our Form 20-F for the fiscal year ended June 30, 2023. |
In addition, we may incorporate by reference
into this prospectus our reports on Form 6-K filed after the date of this prospectus (and before the time that all of the securities offered
by this prospectus have been sold or de-registered) if we identify in the report that it is being incorporated by reference in this prospectus.
Certain statements in and portions of this prospectus
update and replace information in the above listed documents incorporated by reference. Likewise, statements in or portions of a future
document incorporated by reference in this prospectus may update and replace statements in and portions of this prospectus or the above
listed documents.
We will provide you without charge, upon your written
or oral request, a copy of any of the documents incorporated by reference in this prospectus, other than exhibits to such documents which
are not specifically incorporated by reference into such documents. Please direct your written or telephone requests to:
Alterity Therapeutics Limited
Level 14, 350 Collins Street
Melbourne, Victoria 3000 Australia
Attn.: Phillip Hains, Chief Financial Officer
Telephone number +61-3-9349-4906.
You may also obtain information about us by visiting
our website at http://www.alteritytherapeutics.com. Information contained in our website is not part of this prospectus.
We are an Australian company and are a “foreign
private issuer” as defined in Rule 3b-4 under the Exchange Act. As a result, (1) our proxy solicitations are not subject to the
disclosure and procedural requirements of Regulation 14A under the Exchange Act, (2) transactions in our equity securities by our officers
and directors are exempt from Section 16 of the Exchange Act, and (3) we are not required under the Exchange Act to file periodic reports
and financial statements as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. We make
all required filings with the SEC electronically, and these filings are available over the Internet at the SEC’s website at http://www.sec.gov.
PROSPECTUS
ALTERITY
THERAPEUTICS LIMITED
$50,000,000
Ordinary Shares represented by American Depositary Shares
Warrants
Units
We
may offer to the public from time to time in one or more series or issuances:
| ● | American
depositary shares, or ADSs, with each ADS representing six hundred ordinary shares; or |
| |
● |
warrants
to purchase ADSs; or |
| |
● |
a
combination of the above as units. |
We
refer to the ordinary shares, ADSs, warrants and units collectively as “securities” in this prospectus.
The
ADSs, are listed on the NASDAQ Capital Market under the symbol “ATHE.” On September 27, 2023, the closing price of an ADS
representing ordinary shares of Alterity Therapeutics Limited on the NASDAQ Capital Market was US$2.51.
The
securities will have a total public offering price not to exceed $50,000,000. This prospectus provides a general description of the securities
we may offer. Each time we sell securities, we will provide specific terms of the securities offered in a supplement to this prospectus.
The prospectus supplement may also add, update, or change information contained in this prospectus. This prospectus may not be used to
consummate a sale of securities unless accompanied by the applicable prospectus supplement. You should read both this prospectus and
any prospectus supplement together with additional information described under the heading “Where You Can Find More Information”
and the documents incorporated or deemed to be incorporated by reference carefully before you make your investment decision.
We
will sell these securities directly to our shareholders or to purchasers or through agents on our behalf or through underwriters or dealers
as designated from time to time. If any agents or underwriters are involved in the sale of any of these securities, the applicable prospectus
supplement will provide the names of the agents or underwriters and any applicable fees, commissions, or discounts. The prospectus supplement
for each offering of securities will describe in detail the plan of distribution for that offering. For general information about the
distribution of securities offered, please see “Plan of Distribution” in this prospectus on page 7.
The
aggregate market value of our outstanding Ordinary Shares held by non-affiliates on 21 September 2023, as calculated in accordance with
General Instruction I.B.5. of Form F-3, was approximately $3.96 million. During the prior 12 calendar month period that ends on, and
includes, the date of this prospectus, we have offered securities with an aggregate market value of approximately $0.12 million pursuant
to General Instruction I.B.5 of Form F-3. Pursuant to General Instruction I.B.5, in no event will we sell securities pursuant to this
prospectus with a value of more than one-third of the aggregate market value of our Ordinary Shares held by non-affiliates in any 12-month
period, so long as the aggregate market value of our Ordinary Shares held by non-affiliates is less than $75,000,000.
INVESTING
IN OUR SECURITIES INVOLVES A HIGH DEGREE OF RISK. SEE “RISK FACTORS” BEGINNING ON PAGE 2 AND UNDER SIMILAR HEADINGS IN THE
OTHER DOCUMENTS THAT ARE INCORPORATED BY REFERENCE INTO THIS PROSPECTUS FOR A DISCUSSION OF CERTAIN FACTORS THAT SHOULD BE CONSIDERED
BY PROSPECTIVE PURCHASERS OF THE SECURITIES OFFERED HEREBY.
NEITHER
THE U.S. SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED
UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The
date of this prospectus is October 25, 2023
TABLE
OF CONTENTS
You
should rely only on the information contained or incorporated by reference in this prospectus. We have not authorized any other person
to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on
it. We are not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume
that the information appearing in this prospectus is accurate only as of the date on the front cover of this prospectus. Our business,
financial condition, results of operation and prospects may have changed since that date.
In
this prospectus, the terms “we,” “us,” “Alterity and “our” mean Alterity Therapeutics Limited
and its subsidiaries, unless otherwise indicated
All
references to “U.S. dollars” or “US$” in this prospectus are to U.S. dollars, and all references to “Australian
dollars” or “A$” are to the currency of Australia.
SUMMARY
This
prospectus is part of a registration statement on Form F-3 that we filed with the Securities and Exchange Commission, or SEC, using a
“shelf” registration process. Under this process, we may sell from time to time any combination of the securities described
in this prospectus in one or more offerings up to a total U.S. dollar amount of $50,000,000 or the equivalent denominated in foreign
currencies or foreign currency units. This prospectus does not contain all of the information included in the registration statement.
For a more complete understanding of the offering of the securities, you should refer to the registration statement, including its exhibits.
This
prospectus provides you with a general description of the securities we may offer. Each time we sell securities, we will provide a prospectus
supplement that will contain specific information about the terms of that offering. The prospectus supplement may also add, update or
change information contained in this prospectus, and may also contain information about any material federal income tax considerations
relating to the securities covered by the prospectus supplement. You should read both this prospectus and any prospectus supplement together
with additional information under the headings “Where You Can Find More Information” and “Incorporation of
Certain Information by Reference.”
This
summary may not contain all of the information that may be important to you. You should read this entire prospectus, including the financial
data and related notes incorporated by reference in this prospectus, before making an investment decision. This summary contains forward-looking
statements that involve risks and uncertainties. Our actual results may differ significantly from the results discussed in the forward-looking
statements. Factors that might cause or contribute to such differences include those discussed in “Risk Factors” and “Forward-Looking
Statements.”
Alterity
Therapeutics Limited
We
were incorporated under the laws of the Commonwealth of Australia on November 11, 1997 and began limited operations shortly thereafter.
Our mission is to develop therapeutic drugs designed to treat neurodegenerative diseases, currently focusing on Parkinsonian and other
movement disorders.
Corporate
Information
Our
registered office is located at Level 3, 62 Lygon Street, Carlton, Victoria 3053 Australia and our telephone number is 011-61-3-9824-5254.
Our principal executive office is located at Level 14, 350 Collins Street, Melbourne, Victoria 3000, Australia and our telephone number
is 011-61-3-9349-4906. Our address on the internet is www.alteritytherapeutics.com. The information in our website is not incorporated
by reference into this prospectus and should not be considered as part of this prospectus.
RISK
FACTORS
Investing
in our securities involves significant risks. Before making an investment decision, you should carefully consider the risks described
under “Risk Factors” in the applicable prospectus supplement and under Item 3.D. – “Risk Factors” in our
most recent Annual Report on Form 20-F, or any updates in our Reports on Form 6-K, together with all of the other information appearing
in this prospectus or incorporated by reference into this prospectus and any applicable prospectus supplement, in light of your particular
investment objectives and financial circumstances. The risks so described are not the only risks facing us. Additional risks not presently
known to us or that we currently deem immaterial may also impair our business operations. Our business, financial condition and results
of operations could be materially adversely affected by any of these risks. The trading price of our securities could decline due to
any of these risks, and you may lose all or part of your investment. The discussion of risks includes or refers to forward-looking statements;
you should read the explanation of the qualifications and limitations on such forward-looking statements discussed elsewhere in this
prospectus.
FORWARD-LOOKING
STATEMENTS
Some
of the statements contained in this prospectus and the documents incorporated by reference are forward-looking statements. Forward-looking
statements involve risks and uncertainties, such as statements about our plans, objectives, expectations, assumptions or future events.
In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “estimate,” “plan,”
“project,” “continuing,” “ongoing,” “expect,” “we believe,” “we intend,”
“may,” “should,” “will,” “could” and similar expressions denoting uncertainty or an action
that may, will or is expected to occur in the future. These statements involve estimates, assumptions, known and unknown risks, uncertainties
and other factors that could cause actual results to differ materially from any future results, performances or achievements expressed
or implied by the forward-looking statements. Examples of forward-looking statements include, but are not limited to, statements about:
| ● | statements
of expected future economic performance; |
| ● | the
future impact of pandemics on the economy and our operations; |
| ● | product
and technology development and rapid technological change; |
| ● | the
potential attributes and benefit of our products and their competitive position; |
| ● | our
estimates regarding expenses, future revenues, capital requirements and our need for additional financing; |
| ● | statements
of our plans and objectives; |
| ● | statements
regarding the capabilities of our business operations; |
| ● | statements
regarding competition in our market; and |
| ● | assumptions
underlying statements regarding us or our business. |
Forward-looking
statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations
and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy
and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks
and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial
condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these
forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those
indicated in the forward-looking statements include, among others, the following:
Risks
Related to Our Financial Condition
| ● | We
have a history of operating losses and our management has concluded that factors raise substantial
doubt about our ability to continue as a going concern and our auditor has included an explanatory
paragraph relating to our ability to continue as a going concern in its audit report for
the fiscal year ended June 30, 2023. |
| ● | We
will need additional funding to complete our clinical trials and to operate our business;
such funding may not be available or, if it is available, such financing is likely to substantially
dilute our existing shareholders. |
Risks
Related to Our Business
| ● | We
are a development stage company engaged in the development of pharmaceutical products and
our success is uncertain. |
| ● | We
rely on research institutions to conduct our clinical trials and we may not be able to secure
and maintain research institutions to conduct our future trials. The institutions that we
work with have their own limits and procedures that will influence or limit our ability to
conduct research and development and the conduct of clinical trials. |
| ● | We
are faced with uncertainties related to our research. |
| ● | Clinical
trials as they relate to our business are expensive and time consuming and their outcome
is uncertain. |
| ● | We
may experience delays in our clinical trials that could adversely affect our business and
operations. |
| ● | We
may not be able to complete the development of our products candidates or develop other pharmaceutical
products. |
| ● | We
may need to prioritise the development of our most promising candidates at the expense of
the development of other products. |
| ● | Our
research and development efforts will be seriously jeopardised if we are unable to retain
key personnel and cultivate key academic and scientific collaborations. |
| ● | If
we are unable to successfully keep pace with technological change or with the advances of
our competitors, our technology and products may become obsolete or non-competitive. |
| ● | Acceptance
of our products in the marketplace is uncertain and failure to achieve market acceptance
will negatively impact our business and operations. |
| ● | We
have limited large scale manufacturing experience with our product candidates. Delays in
manufacturing sufficient quantities of such materials to the required standards for pre-clinical
and clinical trials may negatively impact our business and operations. |
| ● | The
failure to establish sales, marketing and distribution capability would materially impair
our ability to successfully market and sell our pharmaceutical products. |
| ● | If
healthcare insurers and other organisations do not pay for our products, or impose limits
on reimbursement, our future business may suffer. |
| ● | We
may be exposed to product liability claims, which could harm our business. |
| ● | Breaches
of network or information technology security, natural disasters or terrorist attacks could
have an adverse effect on our business. |
Risks
Related to Government Regulation
| ● | If
we do not obtain the necessary governmental approvals, we will be unable to develop or commercialise
our pharmaceutical products. |
| ● | We
will not be able to commercialise any current or future product candidates if we fail to
adequately demonstrate their safety and efficacy. |
| ● | Positive
results in previous clinical trials of product candidates may not be replicated in future
clinical trials, which could result in development delays or a failure to obtain marketing
approval. |
| ● | Even
if approved, any product candidates that we or our subsidiaries may develop and market may
be later withdrawn from the market or subject to promotional limitations. |
| ● | Healthcare
reform measures and other statutory or regulatory changes could adversely affect our business |
| ● | We
could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act. |
Risks
Related to Intellectual Property
| ● | Our
success depends upon our ability to protect our intellectual property and our proprietary
technology, to operate without infringing the proprietary rights of third parties and to
obtain marketing exclusivity for our products and technologies. |
| ● | We
may face difficulties in certain jurisdictions in protecting our intellectual property rights,
which may diminish the value of our intellectual property rights in those jurisdictions. |
| ● | Intellectual
property rights do not address all potential threats to our competitive advantage. |
| ● | Changes
in patent laws or patent jurisprudence could diminish the value of our patents, thereby impairing
our ability to protect our products or product candidates. |
| ● | Confidentiality
agreements with employees and others may not adequately prevent disclosure of our trade secrets
and protect our other proprietary information. |
Risks
Related to Our Compliance with the Sarbanes-Oxley Act of 2002
| ● | We
may fail to maintain effective internal control over financial reporting in accordance with
Section 404 of the Sarbanes-Oxley Act of 2002, which could adversely affect our operating
results, investor confidence in our reported financial information, and the market price
of our ordinary shares and ADSs. |
| ● | Material
weaknesses in our disclosure controls and procedures could negatively affect shareholder
and customer confidence. |
Risks
Related to Ownership of Our Securities
| ● | Our
stock price may be volatile and the trading markets for our securities is limited. |
| ● | Ownership
interest in our company may be further diluted as a result of additional financings. |
| ● | There
is a substantial risk that we are a passive foreign investment company, or PFIC, to some
U.S. investors which will subject those investors to adverse tax rules |
| ● | We
do not anticipate paying dividends on our ordinary shares. |
| ● | Currency
fluctuations may adversely affect the price of our securities. |
| ● | If
we fail to maintain compliance with NASDAQ’s continued listing requirements, our shares
may be delisted from the NASDAQ Capital Market. |
Risks
Related to Our Location in Australia
| ● | It
may be difficult to enforce a judgment in the United States against us and our officers and
directors or to assert U.S. securities laws claims in Australia or serve process on our officers
and directors. |
| ● | As
a foreign private issuer whose shares are listed on The NASDAQ Capital Market, we may follow
certain home country corporate governance practices instead of certain NASDAQ requirements. |
| ● | We
currently do not have a majority of independent directors serving on our Board of Directors,
which may afford less protection to our shareholders than if our Board of Directors had a
majority of independent directors. |
| ● | Australian
takeovers laws may discourage takeover offers being made for us or may discourage the acquisition
of large numbers of our ordinary shares. |
| ● | Our
Constitution and other Australian laws and regulations applicable to us may adversely affect
our ability to take actions that could be beneficial to our shareholders. |
OFFER
STATISTICS AND EXPECTED TIMETABLE
We
may sell from time to time pursuant to this prospectus (as may be detailed in prospectus supplements) an indeterminate number of securities
as shall have a maximum aggregate offering price of $50,000,000. The actual per share price of the securities that we will
offer pursuant hereto will depend on a number of factors that may be relevant as of the time of offer (see “Plan of Distribution”
below).
CAPITALIZATION
The
table below sets forth our capitalization as of June 30, 2023.
| | |
As of
June 30,
2023 | |
| | |
(In A$, except number of shares) | |
| Ordinary Shares, no par value, 2,439,897,618 shares issued and outstanding (1) | |
| |
| Issued capital | |
| 213,971,323 | |
| Reserves | |
| 3,972,475 | |
| Accumulated deficit | |
| (195,130,889 | ) |
| Total shareholders’ equity | |
| 22,812,909 | |
| (1) | The
number of shares issued and outstanding excludes 844,737,659 ordinary shares issuable upon the exercise of 844,737,659 options, having
exercise prices ranging from $A0.02 to $A0.09 per ordinary share having a weighted average exercise price of $A0.[ ] per ordinary share. |
MARKET
FOR OUR ORDINARY SHARES
Our
ordinary shares have traded on the ASX since our initial public offering on March 29, 2000. Since September 5, 2002 our ADRs have traded
on the NASDAQ Capital Market and since April 8, 2019, when we changed our name to Alterity Therapeutics Limited, under the symbol “ATHE.”
USE
OF PROCEEDS
Except
as otherwise provided in the applicable prospectus supplement, we intend to use the net proceeds from the sale of the securities covered
by this prospectus for ongoing and future clinical trials and research programs into the development of our proprietary compounds, including
our compound ATH434 for Parkinsonian disorders, and for working capital purposes. Additional information on the use of net proceeds
from the sale of securities covered by this prospectus may be set forth in the prospectus supplement relating to the specific offering.
PLAN
OF DISTRIBUTION
We
may sell securities in any of the ways described below, including any combination thereof:
| ● | to
or through underwriters or dealers; |
| ● | through
one or more agents; or |
| ● | directly
to one or more purchasers. |
The
distribution of the securities may be effected from time to time in one or more transactions:
| ● | at
a fixed price, or prices, which may be changed from time to time; |
| ● | at
market prices prevailing at the time of sale; |
| ● | at
prices related to such prevailing market prices; or |
Each
prospectus supplement will describe the method of distribution of the securities and any applicable restrictions. The prospectus supplement
with respect to the securities of a particular series will describe the terms of the offering of the securities, including the following:
| ● | the
name or names of any underwriters, dealers or agents, and the amounts of securities underwritten or purchased by each of them; |
| ● | the
initial public offering price of the securities and the proceeds to us and any discounts, commissions, or concessions allowed or reallowed
or paid to dealers; and |
| ● | any
securities exchanges on which the securities may be listed. |
Any
public offering price and any discounts or concessions allowed or reallowed or paid to dealers may be changed from time to time. In no
event will any underwriter or dealer receive fees, commissions, and markups which, in the aggregate, would exceed 8% of the price of
the shares being registered.
Only
the agents or underwriters named in the prospectus supplement are agents or underwriters in connection with the securities being offered.
We
may authorize underwriters, dealers, or other persons acting as our agents to solicit offers by certain institutions to purchase securities
from us pursuant to delayed delivery contracts providing for payment and delivery on the date stated in the prospectus supplement. Each
contract will be for an amount not less than, and the aggregate amount of securities sold pursuant to such contracts shall not be less
nor more than, the respective amounts stated in the prospectus supplement. Institutions with whom the contracts, when authorized, may
be made include commercial and savings banks, insurance companies, pension funds, investment companies, educational and charitable institutions,
and other institutions, but shall in all cases be subject to our approval. Delayed delivery contracts will be subject only to those conditions
set forth in the prospectus supplement, and the prospectus supplement will set forth any commissions we pay for solicitation of these
contracts.
Agents,
underwriters and other third parties described above may be entitled to indemnification by us against certain civil liabilities, including
liabilities under the Securities Act of 1933, or to contribution with respect to payments which the agents or underwriters may be required
to make in respect thereof. Agents, underwriters and such other third parties may be customers of, engage in transactions with, or perform
services for us in the ordinary course of business.
One
or more firms, referred to as “remarketing firms,” may also offer or sell the securities, if the prospectus supplement so
indicates, in connection with a remarketing arrangement upon their purchase. Remarketing firms will act as principals for their own accounts
or as our agents. These remarketing firms will offer or sell the securities in accordance with the terms of the securities. The prospectus
supplement will identify any remarketing firm and the terms of its agreement, if any, with us and will describe the remarketing firm’s
compensation. Remarketing firms may be deemed to be underwriters in connection with the securities they remarket. Remarketing firms may
be entitled under agreements that may be entered into with us to indemnification by us against certain civil liabilities, including liabilities
under the Securities Act of 1933, and may be customers of, engage in transactions with, or perform services for us in the ordinary course
of business.
Certain
of the underwriters may use this prospectus and the accompanying prospectus supplement for offers and sales related to market making
transactions in the securities. These underwriters may act as principal or agent in these transactions, and the sales will be made at
prices related to prevailing market prices at the time of sale.
The
securities may be new issues of securities and may have no established trading market. The securities may or may not be listed on a national
securities exchange. Underwriters may make a market in these securities, but will not be obligated to do so and may discontinue any market
making at any time without notice. We can make no assurance as to the liquidity of or the existence of trading markets for any of the
securities.
Certain
persons participating in this offering may engage in overallotment, stabilizing transactions, short covering transactions, and penalty
bids in accordance with rules and regulations under the Securities Exchange Act of 1934, or the Exchange Act. Overallotment involves
sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying
security so long as the stabilizing bids do not exceed a specified maximum. Short covering transactions involve purchase of the securities
in the open market after the distribution is completed to cover short positions. Penalty bids permit the underwriters to reclaim a selling
concession from a dealer when the securities originally sold by the dealer are purchased in a covering transaction to cover short positions.
Those activities may cause the price of the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue
any of the activities at any time.
DESCRIPTION
OF OUR SHARE CAPITAL
The
concept of authorized share capital no longer exists in Australia and as a result, our authorized share capital is unlimited. All our
outstanding ordinary shares are validly issued, fully paid and non-assessable. The rights attached to our ordinary shares are as follows:
Dividend
rights. If our board of directors recommends a dividend, registered holders of our ordinary shares may declare a dividend by ordinary
resolution in a general meeting. The dividend, however, cannot exceed the amount recommended by our board of directors. Our board of
directors may declare an interim dividend. No dividend may be paid except out of our profits.
Voting
rights. Holders of ordinary shares have one vote for each ordinary share held on all matters submitted to a vote of shareholders.
Such voting rights may be affected by the grant of any special voting rights to the holders of a class of shares with preferential rights
that may be authorized in the future.
The
quorum required for an ordinary meeting of shareholders consists of at least two shareholders represented in person or by proxy who hold
or represent, in the aggregate, at least one third of the voting rights of the issued share capital. A meeting adjourned for lack of
a quorum generally is adjourned to the same day in the following week at the same time and place or any time and place as the directors
designate in a notice to the shareholders. At the reconvened meeting, the required quorum consists of any two members present in person
or by proxy.
An
ordinary resolution, such as a resolution for the declaration of dividends, requires approval by the holders of a majority of the voting
rights represented at the meeting, in person, by proxy or by written ballot and voting thereon. Under our Constitution, a special resolution,
such as amending our Constitution, approving any change in capitalization, winding-up, authorization of a class of shares with special
rights, or other changes as specified in our Constitution, requires approval of a special majority, representing the holders of no less
than 75% of the voting rights represented at the meeting in person, by proxy or by written ballot, and voting thereon.
Pursuant
to our Constitution, our directors are elected at our annual general meeting of shareholders by a vote of the holders of a majority of
the voting power represented and voting at such meeting.
Rights
in our profits. Our shareholders have the right to share in our profits distributed as a dividend and any other permitted distribution.
Rights
in the event of liquidation. In the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be
distributed to the holders of ordinary shares in proportion to the nominal value of their holdings. This right may be affected by the
grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized
in the future.
Changing
Rights Attached to Shares
According
to our Constitution, in order to change the rights attached to any class of shares, unless otherwise provided by the terms of the class,
such change must be adopted by a general meeting of the shareholders and by a separate general meeting of the holders of the affected
class with a majority of 75% of the voting power participating in such meeting.
Annual
and Extraordinary Meetings
Our
Board of Directors must convene an annual meeting of shareholders at least once every calendar year, within five months of our last fiscal
year-end balance sheet data. Notice of at least twenty-eight (28) days prior to the date of the meeting is required. An extraordinary
meeting may be convened by the board of directors, if it decides or upon a demand of any directors, or of one or more shareholders holding
in the aggregate at least five percent (5%) of our issued capital. An extraordinary meeting must be called not more than twenty-one (21)
days after the request is made. The meeting must be held not later than two months after requested.
Limitations
on the Rights to Own Securities in Our Company
Neither
our Constitution nor the laws of the Commonwealth of Australia restrict in any way the ownership or voting of our shares.
Changes
in Our Capital
Pursuant
to the Listing Rules of the Australian Securities Exchange, our directors may in their discretion issue securities equal to not more
than 25% of our issued capital within a 12-month period. Issuances of securities in excess of such amount require the approval of our
shareholders by an ordinary resolution, unless made under an exception contained in the Listing Rules of the Australian Securities Exchange
which includes, among other things, a pro rata offer to shareholders, offers or issues made under previously approved employee incentive
schemes and share purchase plans under Australian law involving an offer of up to A$30,000 of shares at the applicable price.
DESCRIPTION
OF OUR AMERICAN DEPOSITARY SHARES
American
Depositary Shares
The
Bank of New York Mellon, as depositary, will register and deliver ADSs. Each ADS represents six hundred ordinary shares (or a right to
receive six hundred ordinary shares) deposited with HSBC Custody Nominees (Australia) Limited, as custodian for the depositary. Each
ADS also represents any other securities, cash or other property which may be held by the depositary. The depositary’s corporate
trust office at which the ADSs are administered is located at 101 Barclay Street, New York, New York 10286. The Bank of New York
Mellon’s principal executive office is located at 240 Greenwich Street, New York, New York 10286.
You
may hold ADSs either (A) directly (i) by having an American depositary receipt, which is a certificate evidencing a specific
number of ADSs, registered in your name, or (ii) by holding ADSs in the Direct Registration System, or (B) indirectly through
your broker or other financial institution. If you hold ADSs directly, you are an ADS holder. This description assumes you hold your
ADSs directly. If you hold the ADSs indirectly, you must rely on the procedures of your broker or other financial institution to assert
the rights of ADR holders described in this section. You should consult with your broker or financial institution to find out what those
procedures are.
The
Direct Registration System is a system administered by DTC pursuant to which the depositary may register the ownership of uncertificated
ADSs, which ownership shall be confirmed by periodic statements issued by the depositary to the ADS holders entitled thereto.
As
an ADS holder, we will not treat you as one of our shareholders and you will not have shareholder rights. Australian law governs shareholder
rights. The depositary will be the holder of the shares underlying your ADSs. As a holder of ADSs, you will have ADS holder rights. A
deposit agreement among us, the depositary and you, as an ADS holder, and the beneficial owners of ADSs set out ADS holder rights as
well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs.
The
following is a summary of the material provisions of the deposit agreement. For more complete information, you should read the entire
deposit agreement and the form of American depositary receipt. Directions on how to obtain copies of those documents are provided under
“Where You Can Find Additional Information.”
Dividends
and Other Distributions
If
We Pay a Dividend or Other Distribution, How Will You Receive Dividends and Other Distributions on the Shares?
In
the event that we pay a cash dividend or make another distribution, the depositary has agreed to pay to you the cash dividends or other
distributions it or the custodian receives on shares or other deposited securities, after deducting its fees and expenses. You will receive
these distributions in proportion to the number of shares your ADSs represent.
| ● | Cash. The
depositary will convert any cash dividend or other cash distribution we pay on the shares into U.S. dollars, if it can do so on
a reasonable basis and can transfer the U.S. dollars to the United States. If that is not possible or if any government approval
is needed and cannot be obtained, the deposit agreement allows the depositary to distribute the foreign currency only to those ADR holders
to whom it is possible to do so. It will hold the foreign currency it cannot convert for the account of the ADS holders who have not
been paid. It will not invest the foreign currency and it will not be liable for any interest. |
Before
making a distribution, any withholding taxes, or other governmental charges that must be paid will be deducted. The depositary will distribute
only whole U.S. dollars and cents and will round fractional cents to the nearest whole cent. If exchange rates fluctuate during
a time when the depositary cannot convert the foreign currency, you may lose some or all of the value of the distribution.
| |
● |
Shares. The
depositary may distribute additional ADSs representing any shares we distribute as a dividend or free distribution. The depositary
will only distribute whole ADSs. It will sell shares which would require it to deliver a fractional ADS and distribute the net proceeds
in the same way as it does with cash. If the depositary does not distribute additional ADSs, the outstanding ADSs will also represent
the new shares. |
| |
● |
Rights
to Purchase Additional Shares. If we offer holders of our securities any rights to subscribe for additional shares or any
other rights, the depositary may make these rights available to you. If the depositary decides it is not legal and practical to make
the rights available but that it is practical to sell the rights, the depositary will use reasonable efforts to sell the rights and
distribute the proceeds in the same way as it does with cash. The depositary will allow rights that are not distributed or sold to
lapse. In that case, you will receive no value for them. |
If
the depositary makes rights available to you, it will exercise the rights and purchase the shares on your behalf. The depositary will
then deposit the shares and deliver ADSs to you. It will only exercise rights if you pay it the exercise price and any other charges
the rights require you to pay.
U.S. securities
laws may restrict transfers and cancellation of the ADSs represented by shares purchased upon exercise of rights. For example, you may
not be able to trade these ADSs freely in the United States. In this case, the depositary may deliver restricted depositary shares that
have the same terms as the ADSs described in this section except for changes needed to put the necessary restrictions in place.
| |
● |
Other
Distributions. The depositary will send to you anything else we distribute on deposited securities by any means
it thinks is legal, fair and practical. If it cannot make the distribution in that way, the depositary has a choice. It may decide
to sell what we distributed and distribute the net proceeds, in the same way as it does with cash. Or, it may decide to hold what
we distributed, in which case ADSs will also represent the newly distributed property. However, the depositary is not required to
distribute any securities (other than ADSs) to you unless it receives satisfactory evidence from us that it is legal to make that
distribution. |
The
depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any ADS holders. We
have no obligation to register ADSs, shares, rights or other securities under the Securities Act. We also have no obligation to take
any other action to permit the distribution of ADSs, shares, rights or anything else to ADS holders. This means that you may
not receive the distributions we make on our shares or any value for them if it is illegal or impractical for us to make them available
to you.
Deposit,
Withdrawal and Cancellation
How
Are ADSs Issued?
The
depositary will deliver ADSs if you or your broker deposits shares or evidence of rights to receive shares with the custodian. Upon payment
of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will register
the appropriate number of ADSs in the names you request and will deliver the ADSs to or upon the order of the person or persons entitled
thereto.
How
Do ADS Holders Cancel an ADS?
You
may turn in your ADSs at the depositary’s corporate trust office. Upon payment of its fees and expenses and of any taxes or charges,
such as stamp taxes or stock transfer taxes or fees, the depositary will deliver the shares and any other deposited securities underlying
the ADSs to you or a person you designate at the office of the custodian. Or, at your request, risk and expense, the depositary will
deliver the deposited securities at its corporate trust office, if feasible.
How
Do ADS Holders Interchange Between Certificated ADSs and Uncertificated ADSs?
You
may surrender your ADR to the depositary for the purpose of exchanging your ADR for uncertificated ADSs. The depositary will cancel that
ADR and will send you a statement confirming that you are the owner of uncertificated ADSs. Alternatively, upon receipt by the depositary
of a proper instruction from a holder of uncertificated ADSs requesting the exchange of uncertificated ADSs for certificated ADSs, the
depositary will execute and deliver to you an ADR evidencing those ADSs.
Voting
Rights
How
Do You Vote?
You
may instruct the depositary to vote the deposited securities, but only if we ask the depositary to ask for your instructions. Otherwise,
you won’t be able to exercise your right to vote unless you withdraw the shares. However, you may not know about the meeting enough
in advance to withdraw the shares.
If
we ask for your instructions, the depositary will notify you of the upcoming vote and arrange to deliver our voting materials to you.
The materials will (1) describe the matters to be voted on and (2) explain how you may instruct the depositary to vote the
shares or other deposited securities underlying your ADSs as you direct. For instructions to be valid, the depositary must receive them
on or before the date specified. The depositary will try, as far as practical, subject to the laws of Australia and our Constitution,
to vote or to have its agents vote the shares or other deposited securities as you instruct. The depositary will only vote or attempt
to vote as you instruct.
We
cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your shares.
In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying
out voting instructions. This means that you may not be able to exercise your right to vote and there may be nothing you can do if
your shares are not voted as you requested.
In
order to give you a reasonable opportunity to instruct the depositary as to the exercise of voting rights relating to deposited securities,
if we request the depositary to act, we will try to give the depositary notice of any such meeting and details concerning the matters
to be voted upon sufficiently in advance of the meeting date.
Fees
and Expenses
| Persons
Depositing or Withdrawing Shares Must Pay: |
|
For: |
| ● US$3.00
(or less) per 100 ADSs (or portion of 100 ADSs) |
|
● Issuance
of ADSs, including issuances resulting from a distribution of shares or rights or other property
● Cancellation
of ADSs for the purpose of withdrawal, including if the deposit agreement terminates |
| |
|
|
| ● US$0.03
(or less) per ADS |
|
● Any
cash distribution to you |
| |
|
|
| ● A
fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited
for issuance of ADSs |
|
● Distribution
of securities distributed to holders of deposited securities which are distributed by the depositary to ADS holders |
| |
|
|
| ● US$1.50
(or less) per ADR |
|
● Transfers,
combination and split-up of ADRs |
| |
|
|
| ● Expenses
of the depositary |
|
● Cable,
telex and facsimile transmissions (when expressly provided in the deposit agreement)
● Converting
foreign currency to U.S. dollars |
| |
|
|
| ● Taxes
and other governmental charges the depositary or the custodian have to pay on any ADS or share underlying an ADS, for example,
stock transfer taxes, stamp duty or withholding taxes |
|
● As
necessary |
| |
|
|
| ● Any
charges incurred by the depositary or its agents for servicing the deposited securities |
|
● As
necessary |
The
Bank of New York Mellon, as depositary, has agreed to reimburse us for expenses we incur that are related to establishment and maintenance
of the ADR program, including investor relations expenses and Nasdaq application and listing fees. There are limits on the amount of
expenses for which the depositary will reimburse us, but the amount of reimbursement available to us is not related to the amount of
fees the depositary collects from investors.
The
depositary collects its fees for issuance and cancellation of ADSs directly from investors depositing shares or surrendering ADSs for
the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors
by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary
may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging
the book-entry system accounts of participants acting for them. The depositary may generally refuse to provide fee-attracting services
until its fees for those services are paid.
Payment
of Taxes
You
will be responsible for any taxes or other governmental charges payable on your ADSs or on the deposited securities represented by any
of your ADSs. The depositary may refuse to register any transfer of your ADSs or allow you to withdraw the deposited securities represented
by your ADSs until such taxes or other charges are paid. It may apply payments owed to you or sell deposited securities represented by
your ADSs to pay any taxes owed and you will remain liable for any deficiency. If the depositary sells deposited securities, it will,
if appropriate, reduce the number of ADSs to reflect the sale and pay to you any proceeds, or send to you any property, remaining after
it has paid the taxes.
Reclassifications,
Recapitalizations and Mergers
| If
we: |
|
Then: |
● Change
the nominal or par value of our shares
● Reclassify,
split up or consolidate any of the deposited securities
● Recapitalize,
reorganize, merge, liquidate, sell all or substantially all of our assets, or take any similar action |
|
● The
securities received by the depositary will become deposited securities. Each ADS will automatically
represent its equal share of the new deposited securities
● The
depositary may, and will if we ask it to, deliver new ADRs or ask you to surrender your outstanding ADRs in exchange for new ADRs
identifying the new deposited securities. |
Amendment
and Termination
How
May the Deposit Agreement Be Amended?
We
may agree with the depositary to amend the deposit agreement and the ADSs without your consent for any reason. If an amendment adds or
increases fees or charges, except for taxes and other governmental charges or expenses of the depositary for registration fees, facsimile
costs, delivery charges or similar items, or prejudices a substantial right of ADS holders, it will not become effective for outstanding
ADSs until 30 days after the depositary notifies ADS holders of the amendment. At the time an amendment becomes effective, you
are considered, by continuing to hold your ADS, to agree to the amendment and to be bound by the ADRs and the deposit agreement as amended.
How
May the Deposit Agreement Be Terminated?
The
depositary will terminate the deposit agreement at our direction by mailing a notice of termination to the ADS holders then outstanding
at least 90 days prior to the date fixed in such notice for such termination. The depositary may also terminate the deposit agreement
by mailing a notice of termination to us and the ADS holders then outstanding if at any time 90 days shall have expired after the
depositary shall have delivered to our company a written notice of its election to resign and a successor depositary shall not have been
appointed and accepted its appointment.
After
termination, the depositary and its agents will do the following under the deposit agreement but nothing else: collect dividends and
other distributions on the deposited securities, sell rights and other property, and deliver shares and other deposited securities upon
cancellation of ADSs. One year after termination, the depositary may sell any remaining deposited securities by public or private sale.
After that, the depositary will hold the money it received on the sale, as well as any other cash it is holding under the deposit agreement
for the pro rata benefit of the ADS holders that have not surrendered their ADSs. It will not invest the money and has no liability
for interest. The depositary’s only obligations will be to account for the money and other cash. After termination our only obligations
will be to indemnify the depositary and to pay fees and expenses of the depositary that we agreed to pay.
Limitations
on Obligations and Liability
Limits
on Our Obligations and the Obligations of the Depositary; Limits on Liability to Holders of ADSs
The
deposit agreement expressly limits our obligations and the obligations of the depositary. It also limits our liability and the liability
of the depositary. We and the depositary:
| ● | are
only obligated to take the actions specifically set forth in the deposit agreement without negligence or bad faith; |
| ● | are
not liable if either of us is prevented or delayed by law or circumstances beyond our control from performing our obligations under the
deposit agreement; |
| ● | are
not liable if either of us exercises discretion permitted under the deposit agreement; |
| ● | have
no obligation to become involved in a lawsuit or other proceeding related to the ADSs or the deposit agreement on your behalf or on behalf
of any other party if it involves expenses or liability unless you furnish satisfactory indemnity; |
| ● | may
rely upon the advice of or information from legal counsel, accountants, any person presenting shares for deposit and any other holder
of ADSs or any other person if we believe in good faith such person is competent to give such advice or information. |
In
the deposit agreement, we and the depositary agree to indemnify each other under certain circumstances.
Requirements
for Depositary Actions
Before
the depositary will deliver or register a transfer of an ADS, make a distribution on an ADS, or permit withdrawal of shares, the depositary
may require:
| ● | payment
of stock transfer or other taxes or other governmental charges and transfer or registration fees charged by third parties for the transfer
of any shares or other deposited securities; |
| ● | satisfactory
proof of the identity and genuineness of any signature or other information it deems necessary; and |
| ● | compliance
with regulations it may establish, from time to time, consistent with the deposit agreement, including presentation of transfer documents. |
The
depositary may refuse to deliver ADSs or register transfers of ADSs generally when the transfer books of the depositary or our transfer
books are closed or at any time if the depositary or we think it advisable to do so.
Your
Right to Receive the Shares Underlying Your ADRs
You
have the right to cancel your ADSs and withdraw the underlying shares at any time except:
| ● | When
temporary delays arise because: (i) the depositary has closed its transfer books or we have closed our transfer books; (ii) the
transfer of shares is blocked to permit voting at a shareholders’ meeting; or (iii) we are paying a dividend on our shares. |
| ● | When
you or other ADS holders seeking to withdraw shares owe money to pay fees, taxes and similar charges. |
| ● | When
it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal
of shares or other deposited securities. |
This
right of withdrawal may not be limited by any other provision of the deposit agreement.
Pre-Release
of ADSs
The
deposit agreement permits the depositary to deliver ADSs before deposit of the underlying shares. This is called a pre-release of the
ADSs. The depositary may also deliver shares upon cancellation of pre-released ADSs (even if the ADSs are cancelled before the pre-release
transaction has been closed out). A pre-release is closed out as soon as the underlying shares are delivered to the depositary. The depositary
may receive ADSs instead of shares to close out a pre-release. The depositary may pre-release ADSs only under the following conditions:
(1) before or at the time of the pre-release, the person to whom the pre-release is being made represents to the depositary in writing
that it or its customer owns the shares or ADSs to be deposited and assigns all beneficial rights, title and interest in such shares
or ADSs to the depositary; (2) the pre-release is fully collateralized with cash or other collateral that the depositary considers
appropriate; and (3) the depositary must be able to close out the pre-release on not more than five business days’ notice.
In addition, the depositary will limit the number of ADSs that may be outstanding at any time as a result of pre-release to 30% of the
deposited shares, although the depositary may disregard the limit from time to time, if it thinks it is appropriate to do so.
DESCRIPTION
OF WARRANTS
We
may issue warrants to purchase ordinary shares represented by ADSs in one or more series together with other securities or separately,
as described in the applicable prospectus supplement. Below is a description of certain general terms and provisions of the warrants
that we may offer. Particular terms of the warrants will be described in the warrant agreements and the prospectus supplement for the
warrants.
The
applicable prospectus supplement will contain, where applicable, the following terms of and other information relating to the warrants:
| ● | the
specific designation and aggregate number of, and the price at which we will issue, the warrants; |
| ● | the
currency or currency units in which the offering price, if any, and the exercise price are payable; |
| ● | the
designation, amount, and terms of the securities purchasable upon exercise of the warrants; |
| ● | if
applicable, the exercise price for ordinary shares and the number of ordinary shares to be received upon exercise of the warrants; |
| ● | the
date on which the right to exercise the warrants will begin and the date on which that right will expire or, if you may not continuously
exercise the warrants throughout that period, the specific date or dates on which you may exercise the warrants; |
| ● | whether
the warrants will be issued in fully registered form or bearer form, in definitive or global form, or in any combination of these forms,
although, in any case, the form of a warrant included in a unit will correspond to the form of the unit and of any security included
in that unit; |
| ● | any
applicable material U.S. federal or Australian income tax consequences; |
| ● | the
identity of the warrant agent for the warrants and of any other depositaries, execution or paying agents, transfer agents, registrars,
or other agents; |
| ● | the
proposed listing, if any, of the warrants or any securities purchasable upon exercise of the warrants on any securities exchange; |
| ● | if
applicable, the date from and after which the warrants and the ordinary shares will be separately transferable; |
| ● | if
applicable, the minimum or maximum amount of the warrants that may be exercised at any other time; |
| ● | information
with respect to book-entry procedures, if any; |
| ● | the
anti-dilution provisions of the warrants, if any; |
| ● | any
redemption or call provisions; |
| |
● |
whether
the warrants are to be sold separately or with other securities as parts of units; and |
| ● | any
additional terms of the warrants, including terms, procedures, and limitations relating to the exchange and exercise of the warrants. |
DESCRIPTION
OF UNITS
We
may, from time to time, issue units comprised of one or more of the other securities that may be offered under this prospectus, in any
combination.
Each
unit will be issued so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit
will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide
that the securities included in the unit may not be held or transferred separately at any time, or at any time before a specified date.
Any
applicable prospectus supplement will describe:
| |
● |
the
material terms of the units and of the securities comprising the units, including whether and under what circumstances those securities
may be held or transferred separately; |
| |
● |
any
material provisions relating to the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising
the units; and |
| |
● |
any
material provisions of the governing unit agreement that differ from those described above. |
The
description in the applicable prospectus supplement of any units we offer will not necessarily be complete and will be qualified in its
entirety by reference to the applicable unit agreement, which will be filed with the SEC if we offer units. For more information on how
you can obtain copies of the applicable unit agreement if we offer units, see the sections entitled “Where You Can Find More Information”
and “Incorporation of Certain Information by Reference.” We urge you to read the applicable unit agreement and any applicable
prospectus supplement in their entirety.
FOREIGN
EXCHANGE CONTROLS AND OTHER LIMITATIONS
Australia
has largely abolished exchange controls on investment transactions. The Australian dollar is freely convertible into U.S. dollars. In
addition, there are currently no specific rules or limitations regarding the export from Australia of profits, dividends, capital, or
similar funds belonging to foreign investors, except that certain payments to non-residents must be reported to the Australian Cash Transaction
Reports Agency, which monitors such transactions, and amounts on account of potential Australian tax liabilities may be required to be
withheld unless a relevant taxation treaty can be shown to apply.
The
Foreign Acquisitions and Takeovers Act 1975
Under
Australian law, in certain circumstances foreign persons are prohibited from acquiring more than a limited percentage of the shares in
an Australian company without approval from the Australian Treasurer. These limitations are set forth in the Australian Foreign Acquisitions
and Takeovers Act, or the Takeovers Act.
An
acquisition of an interest in 10% or more of our issued voting shares by a foreign government investor (unless a country specific exception
applies) requires notification to and approval by the Australian Treasurer (usually through the Foreign Investments Review Board (“FIRB”)).
Under
the Takeovers Actany private foreign person (that is, a person who is not a foreign government investor), together with associates, is
prohibited from acquiring 20% or more of the shares in any company having total assets of A$310 million or more without notification
to and approval by the Australian Treasurer. However for investors from the U.S. and certain other countries, a threshold of A$1,339
million applies (except in certain circumstances).
If
the necessary approval is not obtained, the Treasurer may make an order requiring the acquirer to dispose of the shares it has acquired
within a specified period of time. At present, we do not have total assets of A$310 million.
The
notification and approval requirements may also apply to increases in interests above these levels (including passive increases without
an acquisition).
If
the necessary approvals are not obtained, the Treasurer may make an order requiring the acquirer to dispose of the shares it has acquired
within a specified period of time.
If
the level of foreign ownership exceeds 40% at any time, we would be considered a foreign person under the Takeovers Act. In such event,
we would be required to obtain the approval of the Treasurer for our company, together with our associates, to acquire (i) more than
20% of an Australian company or business of an Australian company or business with assets totaling over A$310 million; or (ii) any direct
or indirect ownership interest in Australian land or an agribusiness.
The
percentage of foreign ownership in our company would also be included in determining the foreign ownership of any Australian company
or business in which it may choose to invest. Since we have no current plans for any such acquisitions and do not own any property, any
such approvals required to be obtained by us as a foreign person under the Takeovers Act will not affect our current or future ownership
or lease of property in Australia.
Our
Constitution does not contain any additional limitations on a non-resident’s right to hold or vote our securities.
Australian
law requires the transfer of shares in our company to be made in writing. No stamp duty will be payable in Australia on the transfer
of ADSs.
TAXATION
If
required, the material Australian and U.S. federal income tax consequences relating to the purchase, ownership and disposition of any
of the securities offered by this prospectus will be set forth in the prospectus supplement offering those securities.
AUTHORIZED
REPRESENTATIVE
Our
authorized representative in the United States for this offering as required pursuant to Section 6(a) of the Securities Act of 1933,
is Puglisi & Associates; 850 Library Avenue, Suite 204; P.O. Box 885; Newark, Delaware 19715. We have agreed to indemnify the authorized
representative against liabilities under the Securities Act of 1933.
OFFERING
EXPENSES
The
following is a statement of expenses in connection with the distribution of the securities registered. All amounts shown are estimates
except the SEC registration fee. The estimates do not include expenses related to offerings of particular securities. Each prospectus
supplement describing an offering of securities will reflect the estimated expenses related to the offering of securities under that
prospectus supplement.
| SEC registration fee | |
| TBA | |
| FINRA fee | |
| TBA | |
| EDGAR and printing fees | |
| 1,000 | |
| Legal fees and expenses | |
| 10,000 | |
| Accounting fees and expenses | |
| 20,000 | |
| Depositary fees and
expenses | |
| 4,000 | |
| Miscellaneous | |
| 2,000 | |
| Total | |
US$ | | |
LEGAL
MATTERS
The
validity of the securities offered hereunder will be passed upon for us by QR Lawyers Pty Ltd., Melbourne, Australia, our Australian
counsel. Carter Ledyard & Milburn LLP, New York, New York, will be passing upon matters of United States law for us with respect
to securities offered by this prospectus and any accompanying prospectus supplement.
EXPERTS
The
financial statements incorporated in this pospectus by reference to the Annual Report on Form 20-F for the year ended June 30, 2023 have
been so incorporated in reliance on the report (which contains an explanatory paragraph relating to the Company's ability to continue
as a going concern as described in Note 1 to the financial statements) of PricewaterhouseCoopers, an independent registered public accounting
firm, given on the authority of said firm as experts in auditing and accounting.
WHERE
YOU CAN FIND MORE INFORMATION
We
filed a registration statement on Form F-3 to register with the SEC the securities described in this prospectus. This prospectus is part
of that registration statement. This prospectus, which constitutes a part of the registration statement, summarizes material provisions
of contracts and other documents that we refer to in the prospectus. Since this prospectus does not contain all of the information contained
in the registration statement, you should read the registration statement and its exhibits and schedules for further information with
respect to us and our ordinary shares and the ADSs. Our SEC filings, including the registration statement, are also available to you
on the SEC’s Web site at http://www.sec.gov.
We
are subject to the information reporting requirements of the Exchange Act that are applicable to foreign private issuers, and under those
requirements we file reports with the SEC. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to
the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting
and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the
Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as United
States companies whose securities are registered under the Exchange Act. However, we file with the SEC, within four months after the
end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements
audited by an independent registered public accounting firm, and submit to the SEC, on Form 6-K, unaudited quarterly financial information
for the first three quarters of each fiscal year within 60 days after the end of each such quarter, or such applicable time as required
by the SEC.
INCORPORATION
OF CERTAIN INFORMATION BY REFERENCE
The
SEC allows us to “incorporate by reference” information into this prospectus, which means that we can disclose important
information to you by referring you to other documents which we have filed or will file with the SEC. We are incorporating by reference
in this prospectus the documents listed below and all amendments or supplements we may file to such documents, as well as any future
filings we may make with the SEC on Form 20-F under the Exchange Act before the time that all of the securities offered by this prospectus
have been sold or de-registered.
| |
● |
Our
Annual Report on Form 20-F for the fiscal year ended June 30, 2023, as filed with the Commission on August 31, 2023; |
| |
● |
The
description of our ADRs contained in our Form
20-F for the fiscal year ended June 30, 2023. |
In
addition, we may incorporate by reference into this prospectus our reports on Form 6-K filed after the date of this prospectus (and before
the time that all of the securities offered by this prospectus have been sold or de-registered) if we identify in the report that it
is being incorporated by reference in this prospectus.
Certain
statements in and portions of this prospectus update and replace information in the above listed documents incorporated by reference.
Likewise, statements in or portions of a future document incorporated by reference in this prospectus may update and replace statements
in and portions of this prospectus or the above listed documents.
We
will provide you without charge, upon your written or oral request, a copy of any of the documents incorporated by reference in this
prospectus, other than exhibits to such documents which are not specifically incorporated by reference into such documents. Please direct
your written or telephone requests to:
Alterity
Therapeutics Limited
Level
14, 350 Collins Street
Melbourne,
Victoria 3000 Australia
Attn.:
Kathryn Andrews, Chief Financial Officer
Telephone
number +61-3-9349-4906.
You
may also obtain information about us by visiting our website at http://www.alteritytherapeutics.com. Information contained in our website
is not part of this prospectus.
We
are an Australian company and are a “foreign private issuer” as defined in Rule 3b-4 under the Exchange Act. As a result,
(1) our proxy solicitations are not subject to the disclosure and procedural requirements of Regulation 14A under the Exchange Act, (2)
transactions in our equity securities by our officers and directors are exempt from Section 16 of the Exchange Act, and (3) we are not
required under the Exchange Act to file periodic reports and financial statements as frequently or as promptly as U.S. companies whose
securities are registered under the Exchange Act. We make all required filings with the SEC electronically, and these filings are available
over the Internet at the SEC’s website at http://www.sec.gov.
ENFORCEABILITY
OF CIVIL LIABILITIES
Service
of process upon us and upon our directors and officers and the Australian experts named in this prospectus, most of whom reside outside
the United States, may be difficult to obtain within the United States. Furthermore, because substantially all of our assets and substantially
all of our directors and officers are located outside the United States, any judgment obtained in the United States against us or any
of such directors and officers may not be collectible within the United States.
We
have irrevocably appointed Puglisi & Associates as our agent to receive service of process in any action against us in the state
and federal courts sitting in the City of New York, Borough of Manhattan arising out of this offering or any purchase or sale of securities
in connection therewith. We have not given consent for this agent to accept service of process in connection with any other claim.
US$6,000,000
Alterity Therapeutics Limited
Ordinary Shares represented by American Depositary
Shares
PROSPECTUS SUPPLEMENT

February 15, 2024
Alterity Therapeutics (NASDAQ:ATHE)
Historical Stock Chart
From Mar 2024 to Apr 2024
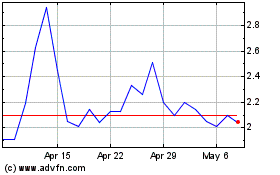
Alterity Therapeutics (NASDAQ:ATHE)
Historical Stock Chart
From Apr 2023 to Apr 2024