Anavex Life Sciences to Announce Fiscal 2024 Fourth Quarter Financial Results on Monday, December 23, 2024
16 December 2024 - 11:30PM

Anavex Life Sciences Corp. (“Anavex” or the “Company”) (Nasdaq:
AVXL), a clinical-stage biopharmaceutical company focused on
developing innovative treatments for Alzheimer's disease,
Parkinson's disease, schizophrenia, neurodevelopmental,
neurodegenerative, and rare diseases, including Rett syndrome, and
other central nervous system (CNS) disorders, today announced that
it will issue financial results for its fiscal year ended September
30, 2024, on Monday, December 23, 2024.
Management will host a conference call on
Monday, December 23, at 8:30 am ET to review financial results and
provide an update on the execution of the Company’s growth
strategy. Following management’s remarks, there will be a
question-and-answer session.
Webcast / Conference Call Information:
The live webcast of the conference call will be available on
Anavex’s website at www.anavex.com.
The conference call can be also accessed by
dialing 1 929 205 6099 for participants in the U.S. using the
Meeting ID# 814 6482 4038 and reference passcode 336064. A replay
of the conference call will also be available on Anavex’s website
for up to 30 days.
About Anavex Life Sciences
Corp.Anavex Life Sciences Corp. (Nasdaq: AVXL) is a
publicly traded biopharmaceutical company dedicated to the
development of novel therapeutics for the treatment of
neurodegenerative, neurodevelopmental, and neuropsychiatric
disorders, including Alzheimer's disease, Parkinson's disease,
schizophrenia, Rett syndrome, and other central nervous system
(CNS) diseases, pain, and various types of cancer. Anavex's lead
drug candidate, ANAVEX®2-73 (blarcamesine), has successfully
completed a Phase 2a and a Phase 2b/3 clinical trial for
Alzheimer's disease, a Phase 2 proof-of-concept study in
Parkinson's disease dementia, and both a Phase 2 and a Phase 3
study in adult patients and one Phase 2/3 study in pediatric
patients with Rett syndrome. ANAVEX®2-73 is an orally available
drug candidate designed to restore cellular homeostasis by
targeting SIGMAR1 and muscarinic receptors. Preclinical studies
demonstrated its potential to halt and/or reverse the course of
Alzheimer's disease. ANAVEX®2-73 also exhibited anticonvulsant,
anti-amnesic, neuroprotective, and anti-depressant properties in
animal models, indicating its potential to treat additional CNS
disorders, including epilepsy. The Michael J. Fox Foundation for
Parkinson's Research previously awarded Anavex a research grant,
which fully funded a preclinical study to develop ANAVEX®2-73 for
the treatment of Parkinson's disease. We believe that ANAVEX®3-71,
which targets SIGMAR1 and M1 muscarinic receptors, is a promising
clinical stage drug candidate demonstrating disease-modifying
activity against the major hallmarks of Alzheimer's disease in
transgenic (3xTg-AD) mice, including cognitive deficits, amyloid,
and tau pathologies. In preclinical trials, ANAVEX®3-71 has shown
beneficial effects on mitochondrial dysfunction and
neuroinflammation. Further information is available at
www.anavex.com. You can also connect with the Company on Twitter,
Facebook, Instagram, and LinkedIn.
Forward-Looking Statements
Statements in this press release that are not
strictly historical in nature are forward-looking statements. These
statements are only predictions based on current information and
expectations and involve a number of risks and uncertainties.
Actual events or results may differ materially from those projected
in any of such statements due to various factors, including the
risks set forth in the Company’s most recent Annual Report on Form
10-K filed with the SEC. Readers are cautioned not to place undue
reliance on these forward-looking statements, which speak only as
of the date hereof. All forward-looking statements are qualified in
their entirety by this cautionary statement and Anavex Life
Sciences Corp. undertakes no obligation to revise or update this
press release to reflect events or circumstances after the date
hereof.
For Further Information:Anavex
Life Sciences Corp.Research & Business DevelopmentToll-free:
1-844-689-3939Email: info@anavex.com
Investors:Andrew J.
BarwickiInvestor RelationsTel: 516-662-9461Email:
andrew@barwicki.com
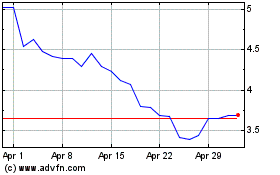
Anavex Life Sciences (NASDAQ:AVXL)
Historical Stock Chart
From Nov 2024 to Dec 2024
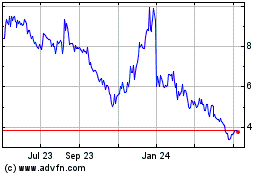
Anavex Life Sciences (NASDAQ:AVXL)
Historical Stock Chart
From Dec 2023 to Dec 2024