Merck to Help Johnson & Johnson Make Its Covid-19 Vaccine--3rd Update
03 March 2021 - 9:55AM
Dow Jones News
By Sabrina Siddiqui and Tarini Parti
President Biden said the U.S. would have enough Covid-19
vaccines for all American adults by the end of May, two months
earlier than he had previously said, after regulators authorized
the one-shot Johnson & Johnson vaccine and Merck & Co.
agreed to help produce it.
Mr. Biden also called on states to give priority to teachers,
school staff and child-care workers for vaccinations, as virtual
learning continues for many students across the country. Several
teachers unions have made vaccinations part of their negotiations
for returning to in-person teaching. Mr. Biden said 30 states are
giving priority to such workers for the shot.
Mr. Biden said the federal pharmacy program would give priority
to teachers, and he set a goal for those workers to get at least
one dose of the vaccine by the end of March.
"We're moving in the right direction," he said Tuesday. "And
today's announcements are a huge step in our effort to beat this
pandemic."
Mr. Biden said the partnership to make the new J&J vaccine,
which was cleared by regulators on Saturday, is "the type of
collaboration between companies we saw in World War II." He said
the U.S. will have enough supply for all adults by the end of May,
but it wasn't immediately clear when everyone will be able to get
the shot.
J&J said it expected the collaboration with Merck to
"enhance our production capacity so that we can supply beyond our
current commitments." In a statement Tuesday, a Merck spokesman
said the company "remains steadfast in our commitment to contribute
to the global response to the pandemic and to preparing to address
future pandemics."
White House press secretary Jen Psaki said one of the Merck
facilities will help "fill-finish" the vaccine -- a step in which
vials are filled with vaccines, capped and readied for shipment --
and the other will help produce the vaccine.
She said conversations between J&J and Merck were under way
and the administration helped in completing the deal, in part by
providing Merck with a commitment to help with upgrading its
facilities for vaccine production. An administration official said
Mr. Biden was invoking the Defense Production Act to give Merck
priority access to supplies, including purchase of machinery, tubes
and filtration systems.
Ms. Psaki declined to detail the role administration officials
played, but she said they took steps to help expedite manufacturing
after learning J&J was behind on its production. J&J
executives have said they ran into challenges in scaling
manufacturing output but that they would deliver 100 million
vaccines to the U.S. in the first half of 2021.
White House Covid-19 coordinator Jeff Zients worked on the deal
with the two companies' chief executives and officials at the
Department of Health and Human Services, a person familiar with the
situation said.
J&J, based in New Brunswick, N.J., had made nearly four
million doses for shipments that began going out this week. The
Biden administration said it expected about 20 million doses to be
delivered by the end of March.
Moderna, Pfizer and J&J, without the supplement from Merck,
are scheduled to supply enough doses in the U.S. in March to
vaccinate about 80 million people, according to analysts from
Evercore ISI. In April, enough doses will be supplied for 125
million people, assuming shots from AstraZeneca PLC and Novavax
Inc. are cleared for use, according to Evercore. By the end of May,
the analysts projected the U.S. will have received enough Covid-19
vaccine doses since December to fully vaccinate 345 million
people.
Merck, a Kenilworth, N.J., firm, is a pioneer in vaccines, such
as those to prevent mumps and shingles, but it scrapped two
programs to develop a Covid-19 vaccine in January after
disappointing clinical studies. The company was also slower to
pursue Covid-19 vaccines, The Wall Street Journal reported last
year.
The partnership between J&J and Merck would mark the latest
example of pharmaceutical rivals working together to make Covid-19
vaccines. Sanofi SA and Novartis AG are helping to make the shot
from Pfizer and its partner BioNTech SE.
The partnership comes as Mr. Biden's administration has
emphasized the urgency of vaccinating the public against Covid-19
and warned of a new and more transmissible variant that is rapidly
spreading across the country.
Mr. Zients said Monday that the administration had begun
distributing 3.9 million doses of the J&J vaccine to states,
tribes and territories, as well as to pharmacies and community
health centers. Mr. Zients said the company had communicated to the
administration that supply "will be limited for the next couple of
weeks" after the initial distribution but expected to deliver
additional doses by the end of March.
The $1 billion contract J&J signed with the U.S. government
called for it to have 12 million doses ready by the end of
February. The contract allowed the company to make the deliveries
up to 30 days late if it ran into delays.
--Jared Hopkins and Stephanie Armour contributed to this
article.
Write to Sabrina Siddiqui at Sabrina.Siddiqui@wsj.com and Tarini
Parti at Tarini.Parti@wsj.com
(END) Dow Jones Newswires
March 02, 2021 17:40 ET (22:40 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
Johnson and Johnson (NYSE:JNJ)
Historical Stock Chart
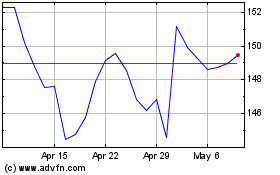
From Mar 2024 to May 2024
Johnson and Johnson (NYSE:JNJ)
Historical Stock Chart
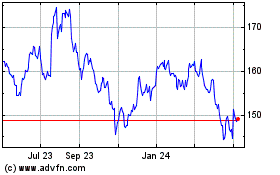
From May 2023 to May 2024