Anesthesiologists recommend Covalon’s gentle
and effective silicone solution over acrylic dressings for securing
vascular insertion sites in Epidermolysis Bullosa patients prone to
skin injury
Covalon Technologies Ltd. (the "Company" or "Covalon") (TSXV:
COV; OTCQX: CVALF), an advanced medical technologies company, today
announced that its IV Clear® line of dual antimicrobial silicone
dressings has been recommended by physicians at a Top 10 U.S.
Children’s Hospital in Colorado, which is also an EB
(“Epidermolysis Bullosa”) Center of Excellence, as a novel solution
to effectively secure and protect vascular access insertion sites
on patients with fragile skin, including those with EB.
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20230613612362/en/
Covalon's IV Clear® is the world's only
dual antimicrobial silicone adhesive vascular access dressing.
(Photo: Business Wire)
EB, sometimes known as “Butterfly Skin”, is a little-known skin
disorder with a prevalence of 1 in 20,000 births. There is no cure
to the debilitating connective tissue disorder that causes
extremely fragile skin prone to blisters and tears from minor
friction. EB patients often require surgical procedures, and
securement of intravenous (IV) catheters and peripheral nerve
catheters on EB patients present major challenges to
anesthesiologists.
In a statement, Kim Strupp MD, FAAP and Norah Janosy, MD, said,
“As pediatric anesthesiology physicians who care for patients with
Epidermolysis Bullosa (EB) routinely when they need surgical
interventions or procedural care, IV Clear® has been transformative
in our practice. We are now able to place this transparent,
occlusive dressing over intravenous, central and nerve block
catheters without damaging our EB patients’ fragile skin. Having a
clear, transparent, occlusive dressing decreases the risk of
infection, increases the visibility of the catheter, and has
improved our care and patient and family satisfaction.”
The recommendation follows research completed by physicians at
Children’s Hospital of Colorado (“CHCO”), where they observed how
the use of IV Clear® in EB patients improved the safety of
indwelling catheter placement and securement without causing
injury. The findings were presented by Drs. Mancone, Strupp,
Janosy, and Brooks Peterson at the International Anesthesia
Research Society meeting in Denver, Colorado on April 14, 2023.1 To
date, IV Clear® dressings have been used on approximately 20 EB
patients at CHCO without incidence of skin damage or other adverse
events. One patient who spent several months in the hospital
underwent several consecutive dressing applications without
incident.
“At Covalon, our mission has been driven by compassion and a
commitment to improving patient care,” said Ron Hebert, SVP
Marketing of Covalon. “Healing should never be hindered by
setbacks, trauma, or tears caused by dressings. IV Clear® is
designed to ensure that every step of a patient’s journey is one of
comfort and compassion.”
Traditional securement dressings made with acrylic cause
substantial injury upon removal. Covalon’s IV Clear® contains
atraumatic silicone adhesive and its gentle, dual antimicrobial
properties make it ideal for use on patients at risk of skin
injury.
Covalon’s vascular access infection prevention solutions also
help to prevent central line-associated bloodstream infections
(CLABSIs) as part of an effective CLABSI prevention bundle, while
ensuring patient comfort and include:
- CovaClear® IV – utilizes soft silicone adhesive technology to
help protect patients from skin injuries, but does not incorporate
antimicrobials, for use with patients who either don't require, or
cannot tolerate, antimicrobials.
- IV Clear® - the world’s only dual-antimicrobial vascular access
dressing that offers complete transparency at and around the
insertion site for easy daily assessment. It also utilizes soft
silicone adhesive technology to minimize skin injuries and preserve
skin barrier functions, and incorporates safe amounts of
antimicrobials, without sacrificing efficacy, to protect against
chemical irritation.
- VALGuard® - an FDA listed, transparent, environmental barrier
designed to protect catheter hubs and line connections from
external contaminants and gross contamination, including body
fluids and other secretions. It incorporates a quick-release pull
strip for fast access to infusion hubs and for easy removal.
Those interested in learning more about Covalon’s solutions may
visit www.covalon.com or follow Covalon on LinkedIn, Facebook,
Instagram or Twitter.
Reference
- Mancone, A. et al. 2023. Novel Approach to Catheter Securement
for a patient with Epidermolysis Bullosa. [Poster]. IARS Annual
Meeting 2023, 14 April, Denver.
About Covalon
Covalon Technologies Ltd. is a patient-driven medical device
company, built on the relentless pursuit to help the most
vulnerable patients have a better chance at healing. Through a
strong portfolio of patented technologies and solutions for
advanced wound care, infection prevention, and medical device
coatings, we offer innovative, gentler, and more compassionate
options for patients to heal with less infections, less pain, and
better outcomes. Our solutions are designed for patients and made
for care providers. Covalon leverages its patented medical
technology platforms and expertise in two ways: (i) by developing
products that are sold under Covalon’s name; and (ii) by developing
and commercializing medical products for other medical companies
under development and license contracts. The Company is listed on
the TSX Venture Exchange, having the symbol COV and trades on the
OTCQX Market under the symbol CVALF. To learn more about Covalon,
visit our website at www.covalon.com.
Anesthesiologists at a Top 10 U.S. Children’s Hospital in
Colorado, which is also an Epidermolysis Bullosa Center of
Excellence, recommend Covalon’s gentle and effective silicone
solution over acrylic dressings for securing vascular insertion
sites in EB patients prone to skin injury.
Neither the TSX Venture Exchange nor its Regulation Services
Provider (as that term is defined in the policies of the TSX
Venture Exchange) accepts responsibility for the adequacy or
accuracy of this release.
This news release may contain forward-looking statements which
reflect the Company's current expectations regarding future events.
The forward-looking statements are often, but not always,
identified by the use of words such as "seek", "anticipate", "plan,
"estimate", "expect", "intend", or variations of such words and
phrases or state that certain actions, events, or results “may”,
“could”, “would”, “might”, “will” or “will be taken”, “occur”, or
“be achieved”. In addition, any statements that refer to
expectations, projections or other characterizations of future
events or circumstances contain forward-looking information.
Statements containing forward-looking information are not
historical facts, but instead represent management’s expectations,
estimates, and projections regarding future events. Forward-looking
statements involve risks and uncertainties, including, but not
limited to, the factors described in greater detail in the “Risks
and Uncertainties” section of our management’s discussion and
analysis of financial condition and results of operations for the
year ended September 30, 2022, which is available on the Company’s
profile at www.sedar.com, any of which could cause results,
performance, or achievements to differ materially from the results
discussed or implied in the forward-looking statements. Investors
should not place undue reliance on any forward-looking statements.
The forward-looking statements contained in this news release are
made as of the date of this news release, and the Company assumes
no obligation to update or alter any forward-looking statements,
whether as a result of new information, further events, or
otherwise, except as required by law.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20230613612362/en/
To learn more about Covalon: Brian Pedlar, CEO, Covalon
Technologies Ltd. Email: bpedlar@covalon.com Phone: 905.568.8400 x
233 Toll-Free: 1.877.711.6055 Website: https://covalon.com/
Twitter: @covalon
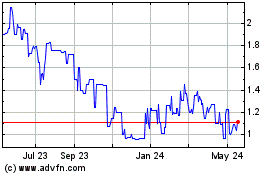
Covalon Technologies (TSXV:COV)
Historical Stock Chart
From Mar 2025 to Apr 2025
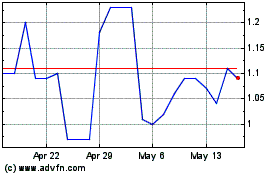
Covalon Technologies (TSXV:COV)
Historical Stock Chart
From Apr 2024 to Apr 2025