Roche Says Data From Lung Cancer Trial Was Inadvertently Disclosed
23 August 2023 - 4:56PM
Dow Jones News
By Adria Calatayud
Roche Holding said data from a late-stage clinical trial
evaluating a lung-cancer treatment has been inadvertently
disclosed, and that the study will continue until a final analysis
for overall survival has been completed.
The Swiss pharmaceutical giant said Wednesday that there was an
inadvertent disclosure of the second interim analysis of the phase
3 trial when interim results for the overall survival rate--a
primary goal of the trial--weren't mature.
The study is continuing and remains blinded to patients and
investigators, the company said.
The trial evaluates immunotherapy tiragolumab in combination
with antibody Tecentriq against Tecentriq alone as an initial
treatment for people with a particular case of advanced or
metastatic non-small cell lung cancer, Roche said.
The interim results showed an estimated median overall survival
of 22.9 months for patients who received the combination, against
16.7 months for those who were in the monotherapy arm, the company
said. The combination of tiragolumab plus Tecentriq was well
tolerated, it said.
Write to Adria Calatayud at adria.calatayud@dowjones.com
(END) Dow Jones Newswires
August 23, 2023 02:41 ET (06:41 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
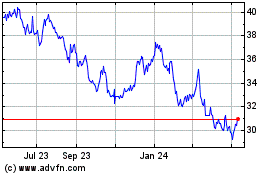
Roche (QX) (USOTC:RHHBY)
Historical Stock Chart
From Apr 2024 to May 2024
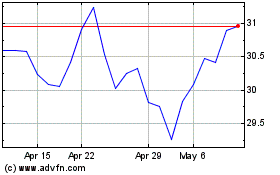
Roche (QX) (USOTC:RHHBY)
Historical Stock Chart
From May 2023 to May 2024