Ardelyx Appeal of Xphozah Denial Granted by FDA
30 December 2022 - 12:25AM
Dow Jones News
By Dean Seal
Ardelyx Inc. said Thursday that the U.S. Food and Drug
Administration has granted an appeal of its earlier rejection of
the company's Xphozah drug candidate.
The Waltham, Mass.-based biopharmaceutical company said the FDA
has directed its Division of Cardiology and Nephrology to work with
Ardelyx on the development of an appropriate label for Xphozah, a
drug used to control of serum phosphorus in adults with chronic
kidney disease who are on dialysis.
The FDA has also advised Ardelyx to request a meeting with the
Division of Cardiology and Nephrology to determine what specific
information the company will need to furnish in its resubmission of
a New Drug Application for Xphozah.
The agency rejected Ardelyx's previous application last year,
but an advisory committee for the FDA voted in mid-November that
the benefits of Xphozah outweigh its risks, paving the way for an
appeal.
Ardelyx said it plans to resubmit the application in the first
half of next year.
The company's shares rose 9.1%, to $2.74, in premarket
trading.
Write to Dean Seal at dean.seal@wsj.com
(END) Dow Jones Newswires
December 29, 2022 08:10 ET (13:10 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
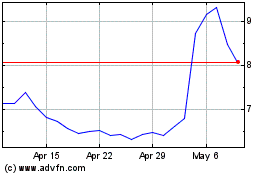
Ardelyx (NASDAQ:ARDX)
Historical Stock Chart
From Mar 2024 to Apr 2024
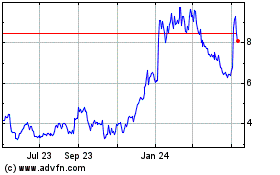
Ardelyx (NASDAQ:ARDX)
Historical Stock Chart
From Apr 2023 to Apr 2024