SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
6-K
REPORT
OF FOREIGN PRIVATE ISSUER
PURSUANT
TO RULE 13a-16 OR 15d-163
UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For
the month of January 2024
Alterity
Therapeutics Limited
(Name
of Registrant)
Level 14, 350 Collins Street,
Melbourne, Victoria 3000 Australia
(Address
of Principal Executive Office)
Indicate
by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form
20-F ☒ Form 40-F ☐
This
Form 6-K is being incorporated by reference into our Registration Statement on Form S-8 (Files No. 333-251073, 333-248980
and 333-228671) and our
Registration Statements on Form F-3 (Files No. 333-274816, 333-251647, 333-231417
and 333-250076)
ALTERITY
THERAPEUTICS LIMITED
(a
development stage enterprise)
The
following exhibits are submitted:
SIGNATURE
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned, thereunto duly authorized.
| |
Alterity Therapeutics Limited |
| |
|
| |
By: |
/s/ Geoffrey P. Kempler |
| |
|
Geoffrey P. Kempler |
| |
|
Chairman |
Date:
January 8, 2024
2
Exhibit 99.1

Not for release to US wire services
or distribution in the United States
Alterity Therapeutics Completes
Tranche Two of Placement Raising A$3.5M
MELBOURNE, AUSTRALIA AND SAN FRANCISCO,
USA – 8 January 2024: Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) (“Alterity” or “the Company”),
a biotechnology company dedicated to developing disease modifying treatments for neurodegenerative diseases, today announced that the
Company has completed Tranche Two of a $4.8M (before costs) Placement to Australian and international institutions and other unrelated
sophisticated, professional or other exempt investors first announced on 22 November 2023. This second tranche raised approximately A$3.5M.
As announced on 29 November 2023,
Tranche One of the Placement raised approximately A$1.3M in accordance with the Company’s available placement capacity pursuant
to ASX Listing Rules 7.1 (362,462,762 shares). Tranche Two was approved by shareholders at the Extraordinary General Meeting (EGM) on
29 December 2023 and raised approximately A$3.5M (1,008,965,805 shares and all free attaching options). The new shares rank equally with
ATH fully paid ordinary shares.
As approved by shareholders at the
EGM on 29 December 2023, eligible shareholders with a registered address in Australia and New Zealand at the record date (7.00 pm on 21
November 2023) will be invited to participate in a Securities Purchase Plan (SPP) under which shares and free-attaching options will be
offered on the same terms as the Placement up to a maximum of A$30,000 per eligible shareholder. The maximum total subscription under
the SPP is A$2 million. The SPP offer is only being made to shareholders with a registered address in Australia or New Zealand in the
register of members of the Company, having regard to the compliance costs of making the SPP offer in other jurisdictions.
Key Dates for SPP
| Event |
Time and Date (AEST) |
| 2023 |
| Record Date for SPP |
7:00 pm Tue 21 November |
| SPP Prospectus lodged with ASIC and ASX |
Fri 29 December |
| 2024 |
| Dispatch of the SPP offer booklet |
Wed 10 January |
| SPP offer opens |
Wed 10 January |
| SPP offer closes |
5.00 pm, Thu 25 January |
| Announcement of results of SPP (including scale back, if any) |
Fri 2 February |
| Allotment of Shares under the SPP |
Fri 2 February |
| Normal trading of SPP shares and dispatch of holding statements |
Mon 5 February |
Note: The timetable above is indicative
only and subject to variation. The Company reserves the right to alter the timetable as its absolute discretion and without notice, subject
to the ASX Listing Rules and Corporations Act 2001 (Cth).
The proceeds from this financing
will provide ongoing funding of Alterity’s Phase 2 clinical trials in Multiple System Atrophy (MSA), planning for a potential Phase
3 clinical trial in MSA, continuing discovery and research efforts in neurodegenerative diseases, including Parkinson’s Disease,
and general working capital. The placement was managed by MST Financial Pty Ltd.
About Alterity Therapeutics Limited
Alterity Therapeutics is a clinical
stage biotechnology company dedicated to creating an alternate future for people living with neurodegenerative diseases. The Company’s
lead asset, ATH434, has the potential to treat various Parkinsonian disorders and is currently being evaluated in two Phase 2 clinical
trials in Multiple System Atrophy. Alterity also has a broad drug discovery platform generating patentable chemical compounds to treat
the underlying pathology of neurological diseases. The Company is based in Melbourne, Australia, and San Francisco, California, USA. For
further information please visit the Company’s web site at www.alteritytherapeutics.com.
Authorisation & Additional information
This announcement was authorized by David Stamler, CEO of Alterity
Therapeutics Limited.
Investor and Media Contacts:
Australia
Hannah Howlett
we-aualteritytherapeutics@we-worldwide.com
+61 450 648 064
U.S.
Remy Bernarda
remy.bernarda@iradvisory.com
+1 (415) 203-6386
Forward Looking Statements
This press release contains “forward-looking
statements” within the meaning of section 27A of the Securities Act of 1933 and section 21E of the Securities Exchange Act of 1934.
The Company has tried to identify such forward-looking statements by use of such words as “expects,” “intends,” “hopes,”
“anticipates,” “believes,” “could,” “may,” “evidences” and “estimates,” and
other similar expressions, but these words are not the exclusive means of identifying such statements.
Important factors that could
cause actual results to differ materially from those indicated by such forward-looking statements are described in the sections titled
“Risk Factors” in the Company’s filings with the SEC, including its most recent Annual Report on Form 20-F as well as
reports on Form 6-K, including, but not limited to the following: statements relating to the Company’s drug development program, including,
but not limited to the initiation, progress and outcomes of clinical trials of the Company’s drug development program, including, but
not limited to, ATH434, and any other statements that are not historical facts. Such statements involve risks and uncertainties, including,
but not limited to, those risks and uncertainties relating to the difficulties or delays in financing, development, testing, regulatory
approval, production and marketing of the Company’s drug components, including, but not limited to, ATH434, the ability of the Company
to procure additional future sources of financing, unexpected adverse side effects or inadequate therapeutic efficacy of the Company’s
drug compounds, including, but not limited to, ATH434, that could slow or prevent products coming to market, the uncertainty of obtaining
patent protection for the Company’s intellectual property or trade secrets, the uncertainty of successfully enforcing the Company’s
patent rights and the uncertainty of the Company freedom to operate.
Any forward-looking statement
made by us in this press release is based only on information currently available to us and speaks only as of the date on which it is
made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time
to time, whether as a result of new information, future developments or otherwise.
Alterity Therapeutics (NASDAQ:ATHE)
Historical Stock Chart
From Mar 2024 to May 2024
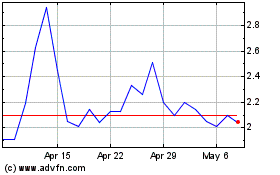
Alterity Therapeutics (NASDAQ:ATHE)
Historical Stock Chart
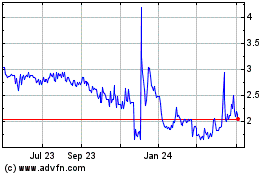
From May 2023 to May 2024