UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
Form
6-K
REPORT
OF FOREIGN PRIVATE ISSUER
PURSUANT
TO RULE 13a-16 OR 15d-16 UNDER
THE
SECURITIES EXCHANGE ACT OF 1934
For
the Month of March 2025
Commission
File Number: 001-38104
IMMURON
LIMITED
(Name
of Registrant)
Level
3, 62 Lygon Street, Carlton South, Victoria, 3053, Australia
(Address
of principal executive office)
Indicate
by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form
20-F ☒ Form 40-F ☐
Indicate
by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information
to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes
☐ No ☒
If
“Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b): 82-
IMMURON
LIMITED
EXPLANATORY
NOTE
Immuron
Limited (the “Company”) published one announcement (the “Public Notices”) to the Australian Securities Exchange
on March 7, 2025 titled:
| - | “Immuron
CEO presentation to Coffee Microcaps Conference” |
A
copy of the Public Notice is attached as an exhibit to this report on Form 6-K.
This
report on Form 6-K (including the exhibit hereto) shall not be deemed to be “filed” for purposes of the Securities Exchange
Act of 1934, as amended (the “Exchange Act”) and shall not be incorporated by reference into any filing under the Securities
Act of 1933, as amended, except as shall be expressly set forth by specific reference in such filing.
EXHIBITS
SIGNATURE
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned, thereunto duly authorized.
| |
IMMURON LIMITED |
| |
|
|
| Date: March 7, 2025 |
By: |
/s/ Phillip
Hains |
| |
|
Phillip Hains |
| |
|
Company Secretary |
Exhibit
99.1

Immuron
CEO, Steven Lydeamore presentation to Coffee Microcaps Conference
Melbourne,
Australia, March 7, 2025: Immuron Limited (ASX: IMC; NASDAQ: IMRN) is pleased to advise our Chief Executive Officer, Steven Lydeamore
will be presenting virtually at the Coffee Microcaps Conference on Friday 7th March 2025 (11am Australian Eastern time).
A
copy of the presentation being made is included below.
This
release has been authorised by the directors of Immuron Limited.
-
- - END - - -
COMPANY
CONTACT:
Steven
Lydeamore
Chief Executive Officer
steve@immuron.com
About
Immuron
Immuron
Limited (ASX: IMC, NASDAQ: IMRN), is an Australian biopharmaceutical company focused on developing and commercializing orally delivered
targeted polyclonal antibodies for the treatment of infectious diseases.
About
Travelan®
Travelan®
is an orally administered passive immunotherapy that prophylactically reduces the likelihood of contracting travelers’ diarrhea,
a digestive tract disorder that is commonly caused by pathogenic bacteria and the toxins they produce. Travelan® is a
highly purified tabletized preparation of hyper immune bovine antibodies and other factors, which when taken with meals bind to diarrhea-causing
bacteria and prevent colonization and the pathology associated with travelers’ diarrhea. In Australia, Travelan®
is a listed medicine on the Australian Register for Therapeutic Goods (AUST L 106709) and is indicated to reduce the risk of Travelers’
Diarrhea, reduce the risk of minor gastro-intestinal disorders and is antimicrobial. In Canada, Travelan® is a licensed
natural health product (NPN 80046016) and is indicated to reduce the risk of Travelers’ Diarrhea. In the U.S., Travelan®
is sold as a dietary supplement for digestive tract protection.
Travelers’
diarrhea (TD)
TD
is generally defined as the passage of ≥ 3 unformed stools per 24 hours plus at least one additional symptom (such as nausea, vomiting,
abdominal cramps, fever, blood/mucus in the stools, or fecal urgency) that develop while abroad or within 10 days of returning from any
resource-limited destinations (Leung et al., 2006). Diarrhea continues to be the most frequent health problem among travelers to destinations
in lower- and middle-income regions (Steffen, 2017). Deployed US military personnel, essentially representing a long-term traveller population,
are particularly affected given their population dynamics and the context in which they seek care and treatment (Connor et al., 2012).
Diarrhea is the leading infectious disease threat to the overall health and preparedness of deployed US armed forces, with diarrheagenic
E. coli, Campylobacter spp., and Shigella spp. among the most commonly reported etiologies (Riddle et al., 2006).


Immuron
Platform Technology
Immuron’s
proprietary technology is based on polyclonal immunoglobulins (IgG) derived from engineered hyper-immune bovine colostrum. Immuron
has the capability of producing highly specific immunoglobulins to any enteric pathogen and our products are orally active. Bovine
IgG can withstand the acidic environment of the stomach and is resistant to proteolysis by the digestive enzymes found in the
Gastrointestinal (GI) tract. Bovine IgG also possesses this unique ability to remain active in the human GI tract delivering its
full benefits directly to the bacteria found there. The underlying nature of Immuron’s platform technology enables the
development of medicines across a large range of infectious diseases. The platform can be used to block viruses or bacteria at
mucosal surfaces such as the Gastrointestinal tract and neutralize the toxins they produce.
IMM-124E
(Travelan®)
IMM-124E
was developed using Immuron’s platform technology. IMM-124E is produced from the colostrum of birthing cattle that have been immunised
during pregnancy with a vaccine containing the outer antigens of multiple human derived ETEC. A total of 13 ETEC strains are used in
the vaccine to produce high levels of antibodies against selected surface antigens from the most common strains of ETEC. (Otto et al.,
2011)
The
resultant hyperimmune colostrum IMM-124E from ETEC vaccinated cows contains significant levels of polyclonal antibodies specific for
ETEC antigens LPS, CFA-I and Flagellin (Sears et al., 2017).
The
antibodies produced in IMM-124E have been found to have a stronger binding and neutralizing activity (than the antibodies of unvaccinated
cattle) against a wide range of LPS antigens including both the variable O-polysaccharide region and the preserved oligosaccharide core
‘R’ region of LPS from the 13 serotypes used in the ETEC vaccine.
IMM-124E
is manufactured into a tablet form referred to as Travelan®.
IMM-529
Immuron
is developing IMM-529 as an adjunctive therapy in combination with standard of care antibiotics for the prevention and/or treatment of
recurrent Clostridioides difficile infection (CDI). IMM-529 antibodies targeting Clostridioides difficile (C. diff) may help to clear
CDI infection and promote a quicker re-establishment of normal gut flora, providing an attractive oral preventative for recurrent CDI.
Immuron
is collaborating with Dr. Dena Lyras and her team at Monash University, Australia to develop vaccines to produce bovine colostrum-derived
antibodies. Dairy cows were immunised to generate hyperimmune bovine colostrum (HBC) that contains antibodies targeting three essential
C. diff virulence components. IMM-529 targets Toxin B (TcB), the spores and the surface layer proteins of the vegetative cells.
This
unique 3-target approach has yielded promising results in pre-clinical infection and relapse models, including (1) Prevention of primary
disease (80% P =0.0052); (2) Protection of disease recurrence (67%, P <0.01) and (3) Treatment of primary disease (78.6%, P<0.0001;
TcB HBC). Importantly IMM-529 antibodies cross-react with whole cell lysates of many different human strains of C. diff including hypervirulent
strains.
To
our knowledge, IMM-529 is, to date, the only investigational drug that has shown therapeutic potential in all three phases of the disease
(Hutton et al., 2017).
ProIBS®
Immuron
has an exclusive distribution agreement with Calmino goup AB for the territories of Australia and New Zealand for ProIBS®.
ProIBS® - to help patients treat IBS symptoms ProIBS® is a certified medical device for the treatment of
IBS symptoms such as abdominal pain, bloating and unsettled bowel movements (diarrhoea and/or constipation). ProIBS® contains
AVH200®, derived from the plant Aloe barbadensis. Mill. AVH200® has gel forming components which support
the intestinal mucosal barrier. As IBS is known to affect individuals for a long period of time, it is essential to have a treatment
appropriate for long-term use –as ProIBS® is. The product is safe, and no interactions with other medications are
known. Science-driven innovative Calmino group AB, the developer of ProIBS®, conducted a usability study among 1,003 users.
PROIBS® was helpful for 94% of them. 91% of the users experienced an improvement in daily life and 98% would recommend
PROIBS® to someone else. To learn more please check: www.proibs.eu.


Irritable
bowel syndrome (IBS) is a common condition where you experience symptoms related to your digestive system. This is sometimes linked to
certain foods, lifestyle habits and stress levels or mood. IBS affects around 3 out of every 10 people.
Females
are more likely than males to be affected. Some key symptoms of IBS include: abdominal pain or discomfort; stomach bloating and wind;
chronic diarrhoea or constipation, or alternating between the two.(healthdirect.gov.au) According to available data, the IBS treatment
market in Australia is estimated to be a part of the broader “Digestives & Intestinal Remedies” market, generating a revenue
of around AU$221.14 million in 2025, with a projected annual growth rate of 3.28%.(Statista)
References
Connor
P, Porter CK, Swierczewski B and Riddle MS. Diarrhea during military deployment: current concepts and future directions. Curr Opin Infect
Dis. 25(5): 546-54; 2012.
Hutton,
M.L., Cunningham, B.A., Mackin, K.E. et al. Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent
antibiotic alternative. Sci Rep 7, 3665 (2017). https://doi.org/10.1038/s41598-017-03982-5
Leung
AK, Robson WL, Davies HD. Travelers’ diarrhea. Adv Ther. Jul-Aug; 23(4): 519-27; 2006
Otto
W, Najnigier B, Stelmasiak T and Robins-Browne RM. Randomized control trials using a tablet formulation of hyperimmune bovine colostrum
to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers Scandinavian Journal of Gastroenterology 46: 862–
868; 2011.
Riddle
MS, Sanders JW, Putnam SD, and Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers’ (US military
and similar populations): A systematic review. American Journal of Tropical Medicine and Hygiene. 74(5): 891-900; 2006.
Sears
KT, Tennant SM, Reymann MK, Simon R, Konstantopolos N, Blackwelder WC, Barry EM and Pasetti MF. Bioactive Immune Components of Anti-Diarrheagenic
Enterotoxigenic Escherichia coli Hyperimmune Bovine Colostrum products. Clinical and Vaccine Immunology. 24 (8) 1-14; 2017.
Steffen
R. Epidemiology of travelers’ diarrhea. J Travel Med. 24(suppl_1): S2-S5; 2017.
For
more information visit: https://www.immuron.com.au/ and https://www.travelan.com
Subscribe
for Immuron News: Here
FORWARD-LOOKING
STATEMENTS:
This
press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and
Section 21E of the Securities Exchange Act of 1934, each as amended. Such statements include, but are not limited to, any statements
relating to our growth strategy and product development programs and any other statements that are not historical facts. Forward-looking
statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect
our business, operating results, financial condition, and stock value. Factors that could cause actual results to differ materially from
those currently anticipated include: risks relating to our growth strategy; our ability to obtain, perform under and maintain financing
and strategic agreements and relationships; risks relating to the results of research and development activities; risks relating to the
timing of starting and completing clinical trials; uncertainties relating to preclinical and clinical testing; our dependence on third-party
suppliers; our ability to attract, integrate and retain key personnel; the early stage of products under development; our need for substantial
additional funds; government regulation; patent and intellectual property matters; competition; as well as other risks described in our
SEC filings. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking
statements contained herein to reflect any change in our expectations or any changes in events, conditions, or circumstances on which
any such statement is based, except as required by law.


7 March 2025 Steven Lydeamore Chief Executive Officer Coffee Microcaps NASDAQ: IMRN ASX: IMC

Certain statements made in this presentation are forward - looking statements and are based on Immuron’s current expectations, estimates and projections. Words such as “ anticipates,” “expects,” “intends,” “plans,” “believes,” “ seeks,” “estimates,” “guidance” and s imilar expressions are intended to identify forward - looking statements. Although Immuron believes the forward - looking statements are based on reasonable assumptions, they are subject to certain r i sks and uncertainties, some of which are beyond Immuron’s control, including those r i sks or uncertainties inherent in the process of both developing and commercializing technology. As a result, actual results could materially differ f rom those expressed or forecasted in the forward - looking statements. The forward - looking statements made in this presentation relate only to events as of the date on which the statements are made. Immuron will not undertake any obligation to release publicly any revisions or updates to these forward - looking statements to reflect events, circumstances or unanticipated events occurring after the date of this presentation except as required by l aw or by any appropriate regulatory authority. YTD FY 2025 results in this presentation are subject to audit review. SAFE HARBOR STATEMENT

3 Executive summary Immuron Ltd (NASDAQ:IMRN) (ASX:IMC) is a globally integrated biopharmaceutical company focused on developing, and commercialising, oral immunotherapeutics for the treatment of gut mediated diseases 3 as of 5 March 2025 IMM - 529 Immuron completes pre - IND meeting with FDA on the development of IMM - 529 New Antimicrobial resistance (AMR) collaboration with Monash University to develop new therapeutic drug candidates against Vancomycin - resistant enterococci (VRE) New distribution agreement ; IMC to launch ProIBS in Australia Major Shareholders Financial Snapshot 233,526,869 Shares on Issue 14,418,566 Total Options IMC: A$0.076 Last Traded Price IMC: A$0.17/0.066 IMRN: $5.96/1.65 52 week High/Low IMC: A$17.7m Market Cap A$7.7m Cash & Cash Equivalents (31 December 2024) % of CSO Units Holder 34.0 % 79,299,224 HSBC Custody Nominees (Australia) 2.4 % 5,500,000 Authentics Australia Pty. Ltd. 1.7 % 3,846,712 Grandlodge 1.4 % 3,234,153 Management & Board Company Overview Two commercially available oral immunotherapeutic products – Travelan® and Protectyn® 3 clinical programs: Travelan®(IMC: Phase 2 CHIM trial), Travelan®(USU: Phase 4 field study), IMM - 529 (IMC: preparing IND, Phase 2 trial) Business Update Travelan® (IMM - 124E) Phase 2 Clinical Study Report submitted to the FDA Travelan® (IMM - 124E) Phase 2 Clinical Study statistically significant immunology and microbiome responses Travelan® (IMM - 124E) Travelan® Uniformed Services University IMM - 124E trial recruited 100% of 866 participants Results & Outlook December 2024 Half Yearly revenue of A$4.0 million, up 70% on pcp December 2024 Half Yearly North American revenue of A$1.1 million, up 130% on pcp Evaluating options to enter international markets Evaluating additional options to add to marketed products portfolio

Immuron Ltd is an Australian integrated biopharmaceutical company with global scale, focused on developing, and commercialising, oral immunotherapeutic for the treatment of gut mediated diseases Immuron Limited Vancouver, BC Canada Immuron Inc New York USA Immuron HQ Melbourne Australia Research & Development Ongoing clinical trials 3 pipeline assets in 3 clinical programmes Global footprint Australia, US, Canada and expanding Market Penetration New markets & expanding distribution channels Technology Platform Developing oral immunoglobulin technology Our Products Coming Soon • E.U. certified medical device • Reduce occurrence of symptoms of medically diagnosed Irritable Bowel Syndrome • Relief of symptoms of medically diagnosed Irritable Bowel Syndrome • Reduce the risk of Traveller’s Diarrhoea • Sold in pharmacies Australia - wide • Available in Australia, Canada and U.S.A. • Clinically proven to support Liver Health and promote healthy Digestive Function • A dietary supplement scientifically formulated to contain high levels of active antibodies • Established as a Practioner Only brand since 2014

TRAVELLERS DIARRHOEA: FACTS Travellers’ Diarrhoea – What is it? • Travellers’ Diarrhoea is more commonly referred to as Bali Belly, Delhi Belly, Montezuma’s Revenge, Tourist Trot, Rangoon Runs or just TD. • Characterised by fever, belly cramps and profuse watery diarrhoea that can last for several days and can lead to severe dehydration. Travellers’ Diarrhoea – How common is it? • The most predictable travel - related illness with attack rates ranging from 30% to 70% of travellers, depending on the destination and season of travel. 2 Travellers’ Diarrhoea – Causes? • Poor standards of hygiene, sanitation and food handling practices are likely the largest contributor to the risk for Travellers’ Diarrhoea. • ~ 80% - 90% of Travellers’ Diarrhoea is caused by bacteria pathogens, the most common being Enterotoxigenic Escherichia coli (ETEC). 1

Travel statistics: • Statistics show that diarrhea and symptoms associated with gastrointestinal disorders is one of the leading travel health problems, affecting up to 70% of travelers (in medium to high risk area’s) • Travellers Diarrhoea frequently occurs in environments where there is poor or no access to plumbing or latrines, poorly functioning refrigeration, resulting in unsafe food storage and an increased risk for disease. Lack of safe water may lead to contaminated foods and drinks prepared with such water; inadequate water supply may lead to shortcuts in cleaning hands, surfaces, utensils, and foods such as fruits and vegetables. • High - risk travel areas: South and Southeast Asia, Central America, West and North Africa, South America, East Africa. High risk areas for traveler’s diarrhoea – CDC

7 Opportunity to Convert Billion Dollar Traveller’s Diarrhoea Market from Relief to Prevention by Travelan® Billion Dollar Market Traveller’s diarrhoea treatment market is large and growing at a CAGR of ~7% 1 Industry tailwinds International travel continues to grow Travel to high - risk destinations from Australia exceeds pre - pandemic levels and still growing Frequent Symptom 30% - 70% of travelers experience traveller’s diarrhoea 2 1 https://www.transparencymarketresearch.com/travelers - diarrhea - market.html ; Centers for Disease Control and Prevention CDC.gov 2024 Proprietary Vaccine Dairy cows inoculated with proprietary vaccines covering 13 strains of enterotoxigenic E.coli (ETEC) Product Differentiation • Colostrum has some antibacterial and immune modulatory properties. • However, Travelan® has in addition to the colostrum - derived compounds very high concentration of anti - E.coli antibodies. • Travelan® utilises specific antibodies to bind the bacteria and the toxins they produce effectively neutralising them and inhibiting their attachment to the gastrointestinal tract reducing LPS - related inflammation and bacterial. • These antibodies target the major bacteria which cause Traveller’s Diarrhoea. • Travelan® has a unique synergistic effect between the colostrum - derived products and the high concentration antibodies for suppressing the inflammation and targeting the bacteria which cause Traveller’s Diarrhoea in the gastrointestinal system. Bind and Neutralise to Prevent According to the Centers for Disease Control and Prevention Traveller’s Diarrhoea is a clinical syndrome resulting from microbial contamination of ingested food and water. Travelan® utilises specific antibodies to bind the bacteria and the toxins they produce effectively neutralising them and inhibiting their attachment to the gastrointestinal tract reducing LPS - related inflammation and bacterial colonisation.

CONTINUED STRONG SALES GROWTH o Global sales increased by 172% in the 2024 fiscal year to a record A$4.9 million compared to A$1.8 million in FY23 o Half Yearly sales record in Dec 24 A$4.0 million, up 70% pcp o Australia o FY24: a record A$3.75 million; up 223% o Half Yearly sales in December 2024 of A$2.9 million; up 54% o USA o FY24: a record A$1.08 million; up 67% o Half Yearly sales in December of A$0.73 million o Canada o FY24: A$0.08 million o Half Yearly sales in December of A$0.38 million

EXPANSION OF TRAVELAN® DISTRIBUTION

STRONG PIPELINE WITH NEAR TERM MILESTONES 10 2H 2025 1H 2025 Current Status Collaborator Registration Phase 3 Phase 2 Phase 1 Preclinical Peak U.S. sales Compound Indication Topline Data Recruitment Completed 100% of 866 participants recruited Uniformed Services University US$ 102 m Travelan® Traveller's Diarrhoea Initiate Phase 3 Clinical Study End of Phase 2 FDA meeting Completed Naval Medical Research Command IMM - 124E Initiate Phase 2 Clinical Study IND submission to FDA Pre - IND submission to FDA US$ 400 m IMM - 529 Clostridioides Difficile Complete Preclinical Studies Initiated Preclinical activities IMM - 926 Vancomycin Resistant Enterococci IMM - 529 Clostridioides Difficile Infection Uniformed Services University Traveller’s Diarrhoea Trial Travelan® (IMM - 124E) Preclinical studies: 1. Prevention of primary disease (80% P =0.0052) 2. Protection of disease recurrence (67%, P <0.01) and 3. Treatment of primary disease (78.6%, P<0.0001; TcB HBC) Phase 4 (n=866, placebo - controlled trial) Participants U.S. and U.K. military personnel Phase 2 (n=2; placebo - controlled challenge study) Phase 2 (n=60; planned placebo - controlled trial) 100% recruitment – January 2025 Statistically significant endpoints (immunology and microbiome) – January 2025

11 Scientific references Travelan® (IMM - 124E) Scandinavian Journal of Gastroenterology, 46:7 - 8, 862 - 868, DOI: 10.3109/00365521.2011.574726 Travelan® has been shown to reduce both the incidence and severity of ETEC - induced diarrhea in up to 90% of volunteers Military Health System Research Symposium 14 - 17 Aug 2023_Abstract 1 Clinical Evaluation of Travelan® an Oral Prophylactic for Prevention of Travelers’ Diarrhea in Active Duty Military Service Assigned Abroad. Immuron Limited, 29 April, 2011 Travelan as a broad Spectrum anti - bacterial US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 4 September, 2019 Travelan® demonstrates broad reactivity to Vibrio cholera strains from Southeast Asia indicating broad potential for prevention of traveler’s diarrhea US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 5 September, 2018 Travelan® prevented clinical shigellosis (bacillary dysentery) in 75% of Travelan® treated animals compared to placebo and demonstrated a significant clinical benefit US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 30 January, 2017 Travelan® able to bind and was reactive to 60 clinical isolates of each bacteria, Campylobacter, ETEC, and Shigella Islam D, Ruamsap N, Imerbsin R, Khanijou P, Gonwong S, Wegner MD, et al. (2023) Bioactivity and efficacy of a hyperimmune bovine colostrum product - Travelan, against shigellosis in a non - Human primate model (Macaca mulatta). PLoS ONE 18(12): e0294021. Bioactivity and efficacy of a hyperimmune bovine colostrum product - Travelan, against shigellosis in a non - Human primate model (Macaca mulatta) Clin Vaccine Immunol 24:e00186 - 16. https://doi.org/10.1128/CVI.00186 - 16 Bioactive Immune Components of Travelan® Infect Immun. 2023 Nov; 91(11): e00097 - 23. Hyperimmune bovine colostrum containing lipopolysaccharide antibodies (IMM - 124E) has a non - detrimental effect on gut microbial communities in unchallenged mice Journal of Crohn's and Colitis, Volume 13, Issue 6, June 2019, Pages 785 – 797, https://doi.org/10.1093/ecco - jcc/jjy213 Administration of the Hyper - immune Bovine Colostrum Extract IMM - 124E Ameliorates Experimental Murine Colitis IMM - 529 Sci Rep 7, 3665 (2017). https://doi.org/10.1038/s41598 - 017 - 03982 - 5 Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative

EMAIL: STEVE@IMMURON.COM PHONE: AUSTRALIA: +61 438 027 172 STEVEN LYDEAMORE CHIEF EXECUTIVE OFFICER IMMURON LIMITED CONTACT INFORMATION:
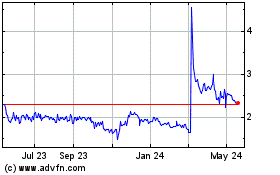
Immuron (NASDAQ:IMRN)
Historical Stock Chart
From Feb 2025 to Mar 2025
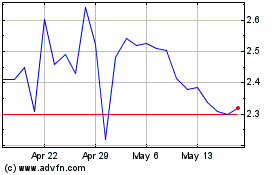
Immuron (NASDAQ:IMRN)
Historical Stock Chart
From Mar 2024 to Mar 2025