As filed with the Securities and Exchange Commission on February 28, 2024
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-3
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
AVIDITY BIOSCIENCES, INC.
(Exact Name of Registrant as Specified in Its Charter)
| | | | | | | | |
| Delaware | 10578 Science Center Drive, Suite 125 San Diego, CA 92121 (858) 401-7900 | 46- 1336960 |
| (State or other jurisdiction of incorporation or organization) | (Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices) | (I.R.S. Employer Identification No.) |
Sarah Boyce
President and Chief Executive Officer
Avidity Biosciences, Inc.
10578 Science Center Drive, Suite 125
San Diego, CA 92121
(858) 401-7900
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| | | | | |
| Cheston J. Larson | John W. Wallen III, Ph.D., J.D. |
| Matthew T. Bush | General Counsel |
| Latham & Watkins LLP | Avidity Biosciences, Inc. |
| 12670 High Bluff Drive | 10578 Science Center Drive, Suite 125 |
| San Diego, CA 92130 | San Diego, CA 92121 |
| (858) 523-5400 | (858) 401-7900 |
APPROXIMATE DATE OF COMMENCEMENT OF PROPOSED SALE TO THE PUBLIC: From time to time after the effective date of this registration statement.
If the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check the following box. ☐
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ☒
If this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | |
Large accelerated filer ☒ | Accelerated filer ☐ |
Non-accelerated filer ☐ | Smaller reporting company ☐ |
| Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of Securities Act. ☐
PROSPECTUS
5,075,304 Shares of Common Stock
This prospectus relates to the proposed resale or other disposition by the selling stockholder identified in this prospectus of up to 5,075,304 shares of our common stock, par value $0.0001 per share, including by the selling stockholder’s transferees, pledgees, donees or successors. The shares being offered were issued and sold to Bristol-Myers Squibb Company, or BMS, in a private placement pursuant to a purchase agreement between BMS and us, or the Purchase Agreement, which closed on November 27, 2023. We are not selling any shares of common stock under this prospectus and will not receive any of the proceeds from the sale or other disposition of common stock by the selling stockholder. All expenses of registration incurred in connection with this offering are being borne by us. All selling and other expenses incurred by the selling stockholder will be borne by the selling stockholder.
The selling stockholder may, from time to time, sell, transfer, or otherwise dispose of any or all of the shares of common stock on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale, in the over-the-counter market, in one or more transactions otherwise than on these exchanges or systems, such as privately negotiated transactions, or using a combination of these methods, and at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. See the disclosure under the heading “Plan of Distribution” elsewhere in this prospectus for more information about how the selling stockholder may sell or otherwise dispose of its shares of common stock hereunder. The selling stockholder may sell any, all or none of the securities offered by this prospectus and we do not know when or in what amount the selling stockholder may sell its shares of common stock hereunder following the effective date of the registration statement of which this prospectus forms a part.
INVESTING IN OUR COMMON STOCK INVOLVES RISKS. SEE THE “RISK FACTORS” ON PAGE 5 OF THIS PROSPECTUS AND ANY SIMILAR SECTION CONTAINED IN OTHER DOCUMENTS THAT ARE INCORPORATED BY REFERENCE INTO THIS PROSPECTUS CONCERNING FACTORS YOU SHOULD CONSIDER BEFORE INVESTING IN OUR COMMON STOCK. Our common stock is listed on the Nasdaq Global Market under the symbol “RNA.” On February 23, 2024, the last reported sale price of our common stock on the Nasdaq Global Market was $14.04 per share.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2024.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the U.S. Securities and Exchange Commission, or the SEC, as a “well-known seasoned issuer” as defined in Rule 405 under the Securities Act of 1933, as amended, or the Securities Act, using a “shelf” registration process. By using a shelf registration statement, the selling stockholder may sell the shares of common stock from time to time and in one or more offerings as described in this prospectus. Before purchasing any securities, you should carefully read both this prospectus and any applicable free writing prospectuses, together with the additional information described under the heading “Where You Can Find More Information; Incorporation by Reference.”
Neither we nor the selling stockholder have authorized anyone to provide you with any information or to make any representations other than those contained or incorporated by reference in this prospectus. We and the selling stockholder take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. The selling stockholder will not make an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus is accurate only as of the date on the cover and that any information incorporated by reference is accurate only as of the date of the document incorporated by reference, unless we indicate otherwise. Our business, financial condition, results of operations and prospects may have changed since those dates. This prospectus incorporates by reference market data and industry statistics and forecasts that are based on independent industry publications and other publicly available information. Although we believe these sources are reliable, we do not guarantee the accuracy or completeness of this information and we have not independently verified this information. In addition, the market and industry data and forecasts that may be included or incorporated by reference in this prospectus may involve estimates, assumptions and other risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” contained in this prospectus, and under similar headings in other documents that are incorporated by reference into this prospectus. Accordingly, investors should not place undue reliance on this information.
A prospectus supplement may add to, update or change the information contained in this prospectus. Before purchasing any securities, you should carefully read both this prospectus and any applicable prospectus supplement, together with additional information described below under the heading “Where You Can Find More Information; Incorporation by Reference.”
When we refer to “Avidity,” “we,” “our,” “us” and the “Company” in this prospectus, we mean Avidity Biosciences, Inc., unless otherwise specified. When we refer to “you,” we mean the potential holders of the shares of common stock.
This prospectus also includes trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience, trademarks and tradenames referred to in this prospectus appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or that the applicable owner will not assert its rights, to these trademarks and tradenames.
WHERE YOU CAN FIND MORE INFORMATION; INCORPORATION BY REFERENCE
Available Information
We file reports, proxy statements and other information with the SEC. The SEC maintains a website that contains reports, proxy and information statements and other information about issuers, such as us, who file electronically with the SEC. The address of that website is http://www.sec.gov.
Our website address is www.aviditybiosciences.com. The information on, or accessible through, our website, however, is not, and should not be deemed to be, a part of this prospectus.
This prospectus is part of a registration statement that we filed with the SEC and does not contain all of the information in the registration statement. The full registration statement may be obtained from the SEC or us, as provided below. Other documents establishing the terms of the offered securities are or may be filed as exhibits to the registration statement or documents incorporated by reference in the registration statement. Statements in this prospectus about these documents are summaries and each statement is qualified in all respects by reference to the document to which it refers. You should refer to the actual documents for a more complete description of the relevant matters. You may inspect a copy of the registration statement through the SEC’s website, as provided above.
Incorporation by Reference
The SEC’s rules allow us to “incorporate by reference” information into this prospectus, which means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is deemed to be part of this prospectus, and subsequent information that we file with the SEC will automatically update and supersede that information. Any statement contained in this prospectus or a previously filed document incorporated by reference will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus or a subsequently filed document incorporated by reference modifies or replaces that statement.
This prospectus incorporates by reference the documents set forth below that have previously been filed with the SEC:
•our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on February 28, 2024; •our Definitive Proxy Statement on Schedule 14A filed with the SEC on April 28, 2023 (solely to the extent specifically incorporated by reference into our Annual Report on Form 10-K for the fiscal year ended December 31, 2022); •the description of our common stock contained in our registration statement on Form 8-A, filed with the SEC on June 9, 2020, as updated by Exhibit 4.3 to our Annual Report on Form 10-K for the year ended December 31, 2020, and any amendment or report filed with the SEC for the purpose of updating the description. All reports and other documents we subsequently file pursuant to Section 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, prior to the termination of this offering, but excluding any information furnished to, rather than filed with, the SEC, will also be incorporated by reference into this prospectus and deemed to be part of this prospectus from the date of the filing of such reports and documents.
You may request a free copy of any of the documents incorporated by reference in this prospectus (other than exhibits, unless they are specifically incorporated by reference in the documents) by writing or telephoning us at the following address:
Avidity Biosciences, Inc.
Attention: Corporate Secretary
10578 Science Center Drive, Suite 125
San Diego, California 92121
(858) 401-7900
Exhibits to the filings will not be sent, however, unless those exhibits have specifically been incorporated by reference in this prospectus or any accompanying prospectus supplement.
THE COMPANY
We are a biopharmaceutical company committed to delivering a new class of RNA therapeutics called Antibody Oligonucleotide Conjugates, or AOCs. Our proprietary AOC platform is designed to combine the specificity of monoclonal antibodies, or mAbs, with the precision of RNA therapeutics to target the root cause of diseases previously untreatable with RNA therapeutics. Our advancing and expanding pipeline currently has three programs in clinical development. AOC 1001 is designed to treat people with myotonic dystrophy type 1, or DM1, and is currently in Phase 1/2 development with the ongoing MARINA open label extension study, or MARINA-OLE™. In mid-2024, we plan to initiate the global Phase 3 HARBORTM trial of AOC 1001 for adults living with DM1. AOC 1020 is designed to treat people living with facioscapulohumeral muscular dystrophy, or FSHD, and is currently in Phase 1/2 development with the FORTITUDE™ trial. AOC 1044 is designed for people with Duchenne muscular dystrophy and is currently in Phase 1/2 development with the EXPLORE44™ trial. AOC 1044 is specifically designed for people with mutations amenable to exon 44 skipping, or DMD44, and is the first of multiple AOCs we are developing for DMD. AOC 1001, AOC 1020 and AOC 1044 have all been granted Orphan Designation by the FDA and the European Medicines Agency, or EMA, and Fast Track Designation by the FDA. The FDA has also granted AOC 1044 Rare Pediatric Disease Designation. We continue to advance and expand our internal discovery pipeline with the addition of new research and development candidates to treat conditions in skeletal muscle and cardiology as we continue to deliver on the RNA revolution. In addition to our own internal research programs, we continue to explore the full potential of our AOC platform through collaborations and partnerships that we have initiated, including programs in cardiology, immunology, and other select indications outside of muscle.
We were originally founded as a Delaware limited liability company on November 13, 2012, under the name Avidity NanoMedicines LLC. On June 4, 2016, we changed our name to Avidity Biosciences LLC, and on April 1, 2019, we converted into a Delaware corporation under the name Avidity Biosciences, Inc. Our principal executive offices are located at 10578 Science Center Drive, Suite 125, San Diego, California 92121, and our telephone number is (858) 401-7900.
RISK FACTORS
Investment in any securities offered pursuant to this prospectus involves risks. You should carefully consider the risk factors incorporated by reference to our most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q or Current Reports on Form 8-K we file after the date of this prospectus, and all other information contained or incorporated by reference into this prospectus, as updated by our subsequent filings under the Exchange Act, and the risk factors and other information contained in any applicable free writing prospectus before acquiring any of such securities. The occurrence of any of these risks might cause you to lose all or part of your investment in the offered securities.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference herein contain forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act. All statements other than statements of historical facts contained in this prospectus and the documents incorporated by reference herein, including statements regarding our future results of operations and financial position, business strategies and plans, research and development plans, the timing and likelihood of resolution of the partial clinical hold related to AOC 1001, the anticipated timing, costs, design and conduct of our ongoing and planned preclinical studies and clinical trials for our product candidates, the anticipated timing of release of data from our ongoing clinical trials, the timing and likelihood of regulatory filings and approvals for our product candidates, the timing and likelihood of success, plans and objectives of management for future operations and future results of anticipated product development efforts, and the anticipated impacts of any pandemics or epidemics, inflationary pressures, and the ongoing hostilities in Ukraine and the Middle East on our business, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. This prospectus and the documents incorporated by reference herein also contain estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this prospectus and the documents incorporated by reference herein are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements speak only as of the date of this prospectus and are subject to a number of risks, uncertainties and assumptions, which we discuss in greater detail in the documents incorporated by reference herein, including under the heading “Risk Factors” and elsewhere in this prospectus. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. Given these risks and uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained in this prospectus or the documents incorporated by reference herein, whether as a result of any new information, future events, changed circumstances or otherwise. For all forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
USE OF PROCEEDS
We are registering the shares of our common stock covered by this prospectus pursuant to registration rights granted to the selling stockholder in the Purchase Agreement. We are not selling any securities under this prospectus and will not receive any proceeds from the sale or other disposition of the shares covered hereby.
We have agreed to pay all costs relating to the registration of the shares of our common stock covered by this prospectus.
DESCRIPTION OF SECURITIES
General
The following description summarizes some of the terms of our common stock. Because it is only a summary, it does not contain all the information that may be important to you and is subject to and qualified in its entirety by reference to our amended and restated certificate of incorporation, and amended and restated bylaws, which are filed as exhibits to our most recent Annual Report on Form 10-K and are incorporated by reference herein. We encourage you to read our certificate of incorporation and our bylaws for additional information.
Our authorized capital stock consists of 400,000,000 shares of common stock, $0.0001 par value per share, and 40,000,000 shares of preferred stock, $0.0001 par value per share.
Common Stock
Under the terms of our amended and restated certificate of incorporation, holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders, including the election of directors, and do not have cumulative voting rights. Accordingly, the holders of a majority of the outstanding shares of common stock entitled to vote in any election of directors can elect all of the directors standing for election, if they so choose, other than any directors that holders of any preferred stock we may issue may be entitled to elect. Subject to the supermajority votes for some matters, other matters shall be decided by the affirmative vote of our stockholders having a majority in voting power of the votes cast by the stockholders present or represented and voting on such matter. Our amended and restated certificate of incorporation and amended and restated bylaws also provide that our directors may be removed only for cause and only by the affirmative vote of the holders of at least two-thirds in voting power of the outstanding shares of capital stock entitled to vote thereon. In addition, the affirmative vote of the holders of at least two-thirds in voting power of the outstanding shares of capital stock entitled to vote thereon is required to amend or repeal, or to adopt any provision inconsistent with, several of the provisions of our amended and restated certificate of incorporation.
Subject to preferences that may be applicable to any then outstanding preferred stock, holders of common stock are entitled to receive ratably those dividends, if any, as may be declared by the board of directors out of legally available funds. In the event of our liquidation, dissolution or winding up, the holders of common stock will be entitled to share ratably in the assets legally available for distribution to stockholders after the payment of or provision for all of our debts and other liabilities, subject to the prior rights of any preferred stock then outstanding. Holders of common stock have no preemptive or conversion rights or other subscription rights and there are no redemption or sinking funds provisions applicable to the common stock. All outstanding shares of common stock are, and the common stock to be outstanding upon the closing of this offering will be, duly authorized, validly issued, fully paid and nonassessable. The rights, preferences and privileges of holders of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A. The transfer agent and registrar’s address is 150 Royall Street, Canton, Massachusetts 02021.
Preferred Stock
As of December 31, 2023, there were no shares of our preferred stock outstanding. Under the terms of our amended and restated certificate of incorporation, our board of directors has the authority, without further action by our stockholders, to issue up to 40,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the dividend, voting and other rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions thereon, and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding. Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the common stock.
The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in our control and may adversely affect the market price of the common stock and the voting and other rights of the holders of common stock. We have no current plans to issue any shares of preferred stock.
Prior to the issuance of shares of each series, the board of directors is required by the General Corporation Law of the State of Delaware, or the DGCL, and our amended and restated certificate of incorporation to adopt resolutions and file a certificate of designation with the Secretary of State of the State of Delaware. The certificate of designation fixes for each class or series the designations, powers, preferences, rights, qualifications, limitations and restrictions, including dividend rights, conversion rights, redemption privileges and liquidation preferences.
Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in our control and may adversely affect the market price of the common stock and the voting and other rights of the holders of common stock.
Registration Rights
We agreed to file a registration statement with the SEC by no later than April 25, 2024 to register for resale under the Securities Act all of the shares of our common stock issued pursuant to the Purchase Agreement, and we have further agreed to use commercially reasonable efforts to cause such registration statement to be declared effective as soon as practicable but no later than the tenth business day after we are notified by the SEC that the registration statement will not be reviewed or will not be subject to further comments from the SEC. This registration statement on Form S-3 is being filed with the SEC in accordance with the aforementioned obligations.
Anti-Takeover Effects of Delaware Law and Our Certificate of Incorporation and Bylaws
Some provisions of Delaware law, our amended and restated certificate of incorporation and our amended and restated bylaws contain provisions that could make the following transactions more difficult: an acquisition of us by means of a tender offer; an acquisition of us by means of a proxy contest or otherwise; or the removal of our incumbent officers and directors. It is possible that these provisions could make it more difficult to accomplish or could deter transactions that stockholders may otherwise consider to be in their best interest or in our best interests, including transactions which provide for payment of a premium over the market price for our shares.
These provisions, summarized below, are intended to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We believe that the benefits of the increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging these proposals because negotiation of these proposals could result in an improvement of their terms.
Undesignated Preferred Stock
The ability of our board of directors, without action by the stockholders, to issue up to 40,000,000 shares of undesignated preferred stock with voting or other rights or preferences as designated by our board of directors could impede the success of any attempt to change control of us. These and other provisions may have the effect of deferring hostile takeovers or delaying changes in control or management of our company.
Stockholder Meetings
Our amended and restated bylaws provide that a special meeting of stockholders may be called only by our board of directors, chairperson of the board of directors, or chief executive officer or president (in the absence of a chief executive officer).
Requirements for Advance Notification of Stockholder Nominations and Proposals
Our amended and restated bylaws establish advance notice procedures with respect to stockholder proposals to be brought before a stockholder meeting and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors or a committee of the board of directors.
No Stockholder Action by Written Consent
Our amended and restated certificate of incorporation and amended and restated bylaws do not allow for the right of stockholders to act by written consent without a meeting.
Staggered Board
Our board of directors is divided into three classes. The directors in each class will serve for a three-year term, with one class being elected each year by our stockholders. This system of electing directors may tend to discourage a third party from attempting to obtain control of us, because it generally makes it more difficult for stockholders to replace a majority of the directors.
Removal of Directors
Our amended and restated certificate of incorporation provides that no member of our board of directors may be removed except for cause and, in addition to any other vote required by law, upon the approval of not less than two thirds of the total voting power of all of our outstanding voting stock then entitled to vote in the election of directors.
Stockholders Not Entitled to Cumulative Voting
Our amended and restated certificate of incorporation does not permit stockholders to cumulate their votes in the election of directors. Accordingly, the holders of a majority of the outstanding shares of our common stock entitled to vote in any election of directors can elect all of the directors standing for election, if they choose, other than any directors that holders of our preferred stock may be entitled to elect.
Delaware Anti-Takeover Statute
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits persons deemed to be “interested stockholders” from engaging in a “business combination” with a publicly held Delaware corporation for three years following the date these persons become interested stockholders unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another prescribed exception applies. Generally, an “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporation’s voting stock. Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. The existence of this provision may have an anti-takeover effect with respect to transactions not approved in advance by the board of directors.
Choice of Forum
Our amended and restated certificate of incorporation provides that, unless we consent in writing to the selection of an alternative form, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for the following types of actions or proceedings under Delaware statutory or common law: (i) any derivative action or proceeding brought on our behalf; (ii) any action asserting a claim of breach of a fiduciary duty or other wrongdoing by any of our directors, officers, employees or agents to us or our stockholders, creditors or other constituents; (iii) any action asserting a claim against us arising pursuant to any provision of the General Corporation Law of the State of Delaware or our amended and restated certificate of incorporation or amended and restated bylaws; (iv) any action to interpret, apply, enforce or determine the validity of our certificate of incorporation or bylaws; or (v) any action asserting a claim governed by the internal affairs doctrine. The provision would not apply to suits brought to enforce a duty or liability created by the Exchange Act. Furthermore, our amended and restated certificate of incorporation will also provide that unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States shall be the exclusive forum for the resolution
of any complaint asserting a cause of action arising under the Securities Act. In any case, stockholders will not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder. The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that a court could find these types of provisions to be inapplicable or unenforceable. Our amended and restated certificate of incorporation also provides that any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock will be deemed to have notice of and to have consented to this choice of forum provision.
Amendment of Charter Provisions
The amendment of any of the above provisions, except for the provision making it possible for our board of directors to issue preferred stock, would require approval by holders of at least two thirds of the total voting power of all of our outstanding voting stock.
The provisions of Delaware law, our amended and restated certificate of incorporation and our amended and restated bylaws could have the effect of discouraging others from attempting hostile takeovers and, as a consequence, they may also inhibit temporary fluctuations in the market price of our common stock that often result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing changes in the composition of our board of directors and management. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
SELLING STOCKHOLDER
This prospectus covers the resale or other disposition from time to time by the selling stockholder identified in the table below of up to an aggregate of 5,075,304 shares of our common stock sold to BMS on November 27, 2023.
On November 27, 2023, or the Effective Date, we entered into the Purchase Agreement with BMS. Pursuant to, and subject to the terms and conditions of, the Purchase Agreement, BMS purchased 5,075,304 unregistered shares of our common stock for approximately $40.0 million, representing a purchase price per share equal to $7.8813. This prospectus covers the resale or other disposition by the selling stockholder or its transferees of up to the total number of shares of common stock issued to the selling stockholder pursuant to the Purchase Agreement.
We are registering the above-referenced shares to permit the selling stockholder and their pledgees, donees, transferees or other successors-in-interest that receive their shares after the date of this prospectus to resell or otherwise dispose of the shares in the manner contemplated under “Plan of Distribution” herein.
Except as otherwise disclosed in this prospectus, the selling stockholder does not have, and within the past three years has not had, any position, office or other material relationship with us.
The following table sets forth the name of the selling stockholder, the number of shares owned by the selling stockholder, the number of shares that may be offered under this prospectus and the number of shares of our common stock owned by the selling stockholder assuming all of the shares registered for resale hereby are sold. The number of shares in the column “Maximum Number of Shares to be Sold Pursuant to this Prospectus” represents all of the shares that the selling stockholder may offer under this prospectus. The selling stockholder may sell some, all or none of its shares. We do not know how long the selling stockholder will hold the shares before selling them, and we currently have no agreements, arrangements or understandings with the selling stockholder regarding the sale or other disposition of any of the shares. The shares covered hereby may be offered from time to time by the selling stockholder.
The information set forth below is based upon information obtained from the selling stockholder and upon information in our possession regarding the issuance of shares of common stock to the selling stockholder in connection with the Purchase Agreement. The percentages of shares owned after the offering are based on 79,839,993 shares of our common stock outstanding as of February 27, 2024, including the shares of common stock registered for resale hereby. Beneficial ownership is determined in accordance with Section 13(d) of the Exchange Act and generally includes voting or investment power with respect to securities and including any securities that grant the selling stockholder the right to acquire shares of common stock within 60 days of February 28, 2024.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Shares Beneficially Owned before this Offering | | Maximum Number of Shares to be Sold Pursuant to this Prospectus | | Shares Beneficially Owned after this Offering(1) |
| Name of Selling Securityholder | | Shares | | Percentage | | | Shares | | Percentage |
Bristol-Myers Squibb Company(2) | | 5075304 | | 6.4 | % | | 5,075,304 | | | — | | | — | |
__________________
(1)Assumes the selling stockholder sells all of its shares of our common stock offered pursuant to this prospectus.
(2)The address for BMS is Route 206 & Province Line Road, Princeton, NJ 08543-400.
Relationship with the Selling Stockholder
Research Collaboration and License Agreement
In connection with the foregoing private placement, on the Effective Date we entered into a Research Collaboration and License Agreement, or the Collaboration Agreement, with BMS for the development of certain cardiovascular treatments. The Collaboration Agreement replaced the previous research agreement between us and MyoKardia, Inc., a wholly-owned subsidiary of BMS.
Research; Development
Under the terms of the Collaboration Agreement, BMS will have the right to select up to five cardiovascular targets, or each a Target, for collaborative research programs under which we will utilize our proprietary AOC platform to conduct research and development activities in order to identify, generate and optimize AOC compounds directed to such Targets with the goal of generating an applicable development candidate. On a Target-by-Target basis, after we complete specified research activities in accordance with a research plan, BMS will have the right to develop, manufacture and commercialize such compounds generated during the research term, and products containing such compounds, worldwide.
Licenses; Exclusivity
We will grant BMS exclusive licenses under certain of our intellectual property rights (including our AOC platform) for the purpose of developing, manufacturing and commercializing the compounds developed under the Collaboration Agreement directed to the Targets. We will not pursue or engage in the discovery of any product competitive to the Targets, and BMS will not enter into a collaboration with any third party for the purpose of researching or developing certain AOC products competitive to the Targets.
Governance
The research and activities conducted under the Collaboration Agreement will be governed by a joint steering committee comprised of representatives from us and BMS.
Financial Terms
BMS paid us an upfront payment of $60.0 million under the terms of the Collaboration Agreement. We are also eligible to receive up to approximately $1.35 billion in research and development milestone payments, up to approximately $825.0 million in commercial milestone payments and tiered royalties up to low double-digits on net sales.
Termination
Either party may impose termination on a Target-by-Target or research program-by-research program basis for the other party’s uncured, material breach of such Target or research program. Subject to certain limitations, (1) BMS may terminate, on an at will basis, the Collaboration Agreement in its entirety, (in which case it would not be entitled to recoup any of the amounts paid under the “Financial Terms” subheading above) or on a Target-by-Target or research program-by-research program basis, and (2) we may terminate the Collaboration Agreement in its entirety if BMS challenges a patent that is licensed to BMS under the Collaboration Agreement.
Lock-up Restrictions
The shares of common stock purchased by BMS pursuant to the Purchase Agreement are subject to lock-up restrictions, which, without our prior approval, prohibit BMS from transferring such shares prior to May 25, 2024. These lockup restrictions are subject to certain exceptions and shall terminate upon the occurrence of certain trigger events, including a change of control of us.
Right of First Negotiation
We also granted BMS a 90-calendar day right of first negotiation, or ROFN, with respect to any potential grant by us of rights to acquire, develop, commercialize or promote any of our current or future cardiovascular programs, products or product candidates, and excluding any change of control transaction. The ROFN remains in effect only during the term of the Collaboration Agreement, and provided that BMS continues to beneficially own at least 50% of the shares of common stock purchased pursuant to the Purchase Agreement.
PLAN OF DISTRIBUTION
The selling stockholder and any of its pledgees, donees, transferees, assignees or other successors-in-interest may, from time to time, sell, transfer or otherwise dispose of any or all of its shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale, or at negotiated prices. The selling stockholder may use one or more of the following methods when disposing of the shares or interests therein:
•ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
•block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
•through brokers, dealers or underwriters that may act solely as agents;
•purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
•an exchange distribution in accordance with the rules of the applicable exchange;
•privately negotiated transactions;
•settlement of short sales entered into after the effective date of the registration statement of which this prospectus is a part;
•distributions to the selling stockholder’s employees, partners, members or stockholders;
•through the writing or settlement of options or other hedging transactions entered into after the effective date of the registration statement of which this prospectus is a part, whether through an options exchange or otherwise;
•broker-dealers may agree with the selling stockholder to sell a specified number of such shares at a stipulated price per share;
•a combination of any such methods of disposition; and
•any other method permitted pursuant to applicable law.
The selling stockholder may also sell shares under Rule 144 or Rule 904 under the Securities Act, if available, or Section 4(a)(1) under the Securities Act, rather than under this prospectus.
Broker-dealers engaged by the selling stockholder may arrange for other broker-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling stockholder (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. The selling stockholder does not expect these commissions and discounts to exceed what is customary in the types of transactions involved.
The selling stockholder may, from time to time, pledge or grant a security interest in some or all of the shares of common stock owned by it and, if it defaults in the performance of its secured obligations, the pledgees or secured parties may offer and sell shares of common stock from time to time under this prospectus, or under a supplement or amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus.
Upon being notified in writing by the selling stockholder that any material arrangement has been entered into with a broker-dealer for the sale of common stock through a block trade, special offering, exchange distribution or secondary distribution or a purchase by a broker or dealer, we will file a supplement to this prospectus, if required, pursuant to Rule 424(b) under the Securities Act, disclosing (i) the name of each such selling stockholder and of the participating broker-dealer(s), (ii) the number of shares involved, (iii) the price at which such shares of common
stock were sold, (iv) the commissions paid or discounts or concessions allowed to such broker-dealer(s), where applicable, (v) that such broker-dealer(s) did not conduct any investigation to verify the information set out or incorporated by reference in this prospectus, and (vi) other facts material to the transaction. In addition, upon being notified in writing by a selling stockholder that a donee or pledgee intends to sell more than 500 shares of common stock, we will file a supplement to this prospectus if then required in accordance with applicable securities law.
The selling stockholder also may transfer the shares of common stock in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
In connection with the sale of the shares of common stock, the selling stockholder may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the common stock in the course of hedging the positions they assume. The selling stockholder may also sell shares of common stock short and deliver these securities to close out its short positions, or loan or pledge the common stock to broker-dealers that in turn may sell these securities. The selling stockholder may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The selling stockholder and any broker-dealers or agents that are involved in selling the shares may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales (it being understood that the selling stockholder shall not be deemed to be an underwriter solely as a result of their participation in this offering). In such event, any profits realized by such selling stockholder or compensation received by such broker-dealers or agents may be deemed to be underwriting commissions or discounts under the Securities Act. The maximum commission or discount to be received by any member of the Financial Industry Regulatory Authority, or FINRA, or independent broker-dealer will not be greater than 8% of the initial gross proceeds from the sale of any security being sold.
We have advised the selling stockholder that it is required to comply with Regulation M promulgated under the Exchange Act during such time as it may be engaged in a distribution of the shares. The foregoing may affect the marketability of the common stock.
The aggregate proceeds to the selling stockholder from the sale of the common stock offered by it will be the purchase price of the common stock less discounts or commissions, if any. The selling stockholder reserves the right to accept and, together with its agents from time to time, to reject, in whole or in part, any proposed purchase of common stock to be made directly or through agents. We will not receive any of the proceeds from this offering.
We are required to pay all fees and expenses incident to the registration of the shares. We have agreed to indemnify the selling stockholder against certain losses, claims, damages and liabilities, including liabilities under the Securities Act or otherwise.
We have agreed with the selling stockholder to keep the registration statement of which this prospectus constitutes a part effective until the earliest of (a) such time as all of the shares covered by this prospectus have been resold and (b) such time as all of the shares covered by this prospectus no longer remain “Registrable Securities” pursuant to the terms of the Purchase Agreement.
LEGAL MATTERS
The validity of the shares of common stock offered hereby will be passed upon for us by Latham & Watkins LLP, San Diego, California.
EXPERTS
The financial statements of Avidity Biosciences, Inc. (the Company) as of December 31, 2023 and 2022 and for each of the three years in the period ended December 31, 2023 and management's assessment of the effectiveness of internal control over financial reporting as of December 31, 2023 incorporated by reference in this Prospectus and in the Registration Statement have been so incorporated in reliance on the reports of BDO USA, P.C., an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting. The report on the effectiveness of internal control over financial reporting expresses an adverse opinion on the effectiveness of the Company's internal control over financial reporting as of December 31, 2023.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 14. Other Expenses of Issuance and Distribution
The following is an estimate of the expenses (all of which are to be paid by the registrant) that we may incur in connection with the securities being registered hereby.
| | | | | |
| SEC registration fee | $ | 10,600 | |
| Legal fees and expenses | $ | 40,000 | |
| Accounting fees and expenses | $ | 10,000 | |
| Miscellaneous | $ | — | |
| Total | $ | 60,600 | |
Item 15. Indemnification of Directors and Officers
Section 102 of the General Corporation Law of the State of Delaware permits a corporation to eliminate the personal liability of directors of a corporation to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our amended and restated certificate of incorporation provides that no director of the Registrant shall be personally liable to it or its stockholders for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except to the extent that the General Corporation Law of the State of Delaware prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
Section 145 of the General Corporation Law of the State of Delaware provides that a corporation has the power to indemnify a director, officer, employee or agent of the corporation, or a person serving at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise in related capacities against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with an action, suit or proceeding to which he was or is a party or is threatened to be made a party to any threatened, ending or completed action, suit or proceeding by reason of such position, if such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
Our amended and restated bylaws provide that we will indemnify each person who was or is a party or threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than an action by or in the right of us) by reason of the fact that he or she is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (all such persons being referred to as an “Indemnitee”), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding and any appeal therefrom, if such Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her conduct was unlawful. Our amended and restated bylaws provide that we will indemnify any Indemnitee who was or is a party to an action or suit by or in the right of us to procure a judgment in our favor by reason of the
fact that the Indemnitee is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees) and, to the extent permitted by law, amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, and any appeal therefrom, if the Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to us, unless a court determines that, despite such adjudication but in view of all of the circumstances, he or she is entitled to indemnification of such expenses. Notwithstanding the foregoing, to the extent that any Indemnitee has been successful, on the merits or otherwise, he or she will be indemnified by us against all expenses (including attorneys’ fees) actually and reasonably incurred in connection therewith. Expenses must be advanced to an Indemnitee under certain circumstances.
We have entered into indemnification agreements with each of our directors and officers. These indemnification agreements may require us, among other things, to indemnify our directors and officers for some expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or officer in any action or proceeding arising out of his or her service as one of our directors or officers, or any of our subsidiaries or any other company or enterprise to which the person provides services at our request.
We maintain a general liability insurance policy that covers certain liabilities of directors and officers of our corporation arising out of claims based on acts or omissions in their capacities as directors or officers.
Item 16. Exhibits
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit Number | | Exhibit Description | | Incorporated by Reference | | Field Herewith |
| Form | | Date | | Number |
| 4.1 | | | | S-1 | | 5/22/2020 | | 4.1 | | |
| 5.1 | | | | | | | | | | X |
| 10.1 | | | | 10-K | | 2/28/2024 | | 10.14 | | |
| 23.1 | | | | | | | | | | X |
| 23.2 | | | | | | | | | | X |
| 24.1 | | | | | | | | | | X |
| 107 | | | | | | | | | | X |
Item 17. Undertakings
(a)The undersigned registrant hereby undertakes:
(1)To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i)To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii)To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement; and
(iii)To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; provided, however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) above
do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Securities and Exchange Commission by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is a part of the registration statement.
(2)That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3)To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4)That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(A)Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(B)Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5) or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by Section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date.
(6)That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities:
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i)Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii)Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii)The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv)Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b)The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(h)Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(j)The undersigned registrant hereby undertakes to file an application for the purpose of determining the eligibility of the trustee to act under subsection (a) of Section 310 of the Trust Indenture Act, or the Act, in accordance with the rules and regulations prescribed by the SEC under Section 305(b)(2) of the Act.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Diego, State of California, on February 28, 2024.
| | | | | |
| AVIDITY BIOSCIENCES, INC. |
| By: | /s/ Sarah Boyce |
| Sarah Boyce |
| President and Chief Executive Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints, jointly and severally, Sarah Boyce and Michael F. MacLean, and each one of them, his or her true and lawful attorneys-in-fact and agents, each with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to file and sign any and all amendments (including post-effective amendments) to this registration statement, and to sign any registration statement for the same offering covered by this registration statement that is to be effective upon filing pursuant to Rule 462(b) promulgated under the Securities Act of 1933, as amended, and all post-effective amendments thereto, and to file the same, with all exhibits thereto and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming that each of said attorneys-in-fact and agents or any of them, or his or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof. This power of attorney shall be governed by and construed with the laws of the State of Delaware and applicable federal securities laws.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
| | | | | | | | | | | | | | |
| Signature | | Title | | Date |
| | | | |
| /s/ Sarah Boyce | | President, Chief Executive Officer and Director
(Principal Executive Officer) | | February 28, 2024 |
| Sarah Boyce | | |
| | | | |
| /s/ Michael F. MacLean | | Chief Financial and Chief Business Officer
(Principal Financial and Accounting Officer) | | February 28, 2024 |
| Michael F. MacLean | | |
| | | | |
| /s/ Troy Wilson | | Chair of the Board of Directors | | February 28, 2024 |
| Troy Wilson, Ph.D., J.D. | | |
| | | | |
| /s/ Arthur A. Levin | | Director | | February 28, 2024 |
| Arthur A. Levin, Ph.D. | | |
| | | | |
| /s/ Carsten Boess | | Director | | February 28, 2024 |
| Carsten Boess | | |
| | | | |
| /s/ Noreen Henig | | Director | | February 28, 2024 |
| Noreen Henig, M.D. | | |
| | | | |
| /s/ Edward Kaye | | Director | | February 28, 2024 |
| Edward Kaye, M.D. | | |
| | | | |
| /s/ Jean Kim | | Director | | February 28, 2024 |
| Jean Kim | | |
| | | | |
| /s/ Tamar Thompson | | Director | | February 28, 2024 |
| Tamar Thompson | | |
Calculation of Filing Fee Table
Form S-3
(Form Type)
Avidity Biosciences, Inc.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered and Carry Forward Securities
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Security
Type | | Security
Class Title | | Fee
Calculation
or Carry
Forward
Rule | | Amount Registered | | Proposed
Maximum
Offering
Price Per
Unit | | Maximum
Aggregate
Offering Price | | Fee Rate | | Amount of Registration Fee |
| Fees to Be Paid | | Equity | | Common Stock, par value $0.0001 per share | | 457(c) | | 5,075,304(1) | | $14.15(2) | | $71,815,551.60(2) | | 0.0001476 | | $10,600 |
| | Total Offering Amounts | | | | 71,815,551.60 | | | | 10,600 |
| | Total Fees Previously Paid | | | | | | | | - |
| | Total Fee Offsets | | | | | | | | |
| | Net Fee Due | | | | | | | | 10,600 |
(1)Consists of 5,075,304 shares of the registrant’s common stock issued to the selling stockholder. Pursuant to Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”), the registrant is also registering an indeterminate number of additional shares of common stock issuable by reason of any stock dividend, stock split, recapitalization or other similar transaction.
(2)Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) of the Securities Act, and based upon the average of the high and low prices for a share of the registrant’s common stock as reported on the Nasdaq Global Market on February 23, 2024 (such date being within five business days of the date that this registration statement was filed with the U.S. Securities and Exchange Commission).
Exhibit 5.1
| | | | | | | | |
| San Diego, California 92130 Tel: +1.858.523.5400 Fax: +1.858.523.5450 www.lw.com FIRM / AFFILIATE OFFICES |
| Austin
Beijing
Boston
Brussels
Century City
Chicago
Dubai
Düsseldorf
Frankfurt
Hamburg
Hong Kong
Houston
London
Los Angeles
Madrid | Milan Munich New York Orange County Paris Riyadh San Diego San Francisco Seoul Shanghai Silicon Valley Singapore Tel Aviv Tokyo Washington, D.C. |
February 28, 2024
Avidity Biosciences, Inc.
10578 Science Center Drive, Suite 125
San Diego, CA 92121
Re: Registration Statement on Form S-3; 5,075,304 Shares of Common Stock, Par Value $0.0001 Per Share
To the addressee set forth above:
We have acted as special counsel to Avidity Biosciences, Inc., a Delaware corporation (the “Company”), in connection with the resale from time to time by the selling securityholders named in the Registration Statement (as defined below) of up to 5,075,304 shares (the “Shares”) of the Company’s common stock, par value $0.0001 per share (the “Common Stock”). The Shares are included in a registration statement on Form S-3 under the Securities Act of 1933, as amended (the “Act”), filed with the U.S. Securities and Exchange Commission (the “Commission”) on February 28, 2024 (the “Registration Statement”).
This opinion is being furnished in connection with the requirements of Item 601(b)(5) of Regulation S-K under the Act, and no opinion is expressed herein as to any matter pertaining to the contents of the Registration Statement or the prospectus contained therein, other than as expressly stated herein with respect to the issuance of the Shares.
As such counsel, we have examined such matters of fact and questions of law as we have considered appropriate for purposes of this letter. With your consent, we have relied upon certificates and other assurances of officers of the Company and others as to factual matters without having independently verified such factual matters. We are opining herein as to the General Corporation Law of the State of Delaware (the “DGCL”), and we express no opinion with respect to any other laws.
Subject to the foregoing and the other matters set forth herein, it is our opinion that, as of the date hereof, the issuance of the Shares has been duly authorized by all necessary corporate action of the Company, and the Shares are validly issued, fully paid and non-assessable.
This opinion is for your benefit in connection with the Registration Statement and may be relied upon by you and by persons entitled to rely upon it pursuant to the applicable provisions of the Act. We consent to your filing this opinion as an exhibit to the Registration Statement and to the reference to our firm in the prospectus contained therein under the heading “Legal Matters.” In giving such consent, we do not thereby admit that we are in the category of persons whose
consent is required under Section 7 of the Act or the rules and regulations of the Commission thereunder.
| | |
| Sincerely, |
|
| /s/ Latham & Watkins LLP |
Consent of Independent Registered Public Accounting Firm
We hereby consent to the incorporation by reference in the Prospectus constituting a part of this Registration Statement of our reports dated February 28, 2024, relating to the financial statements and the effectiveness of internal control over financial reporting of Avidity Biosciences, Inc. (the Company) appearing in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023. Our report on the effectiveness of internal control over financial reporting expresses an adverse opinion on the effectiveness of the Company’s internal control over financial reporting as of December 31, 2023.
We also consent to the reference to us under the caption “Experts” in the Prospectus.
| | |
/s/ BDO USA, P.C. |
|
| San Diego, California |
| February 28, 2024 |
Avidity Biosciences (NASDAQ:RNA)
Historical Stock Chart
From Mar 2024 to May 2024
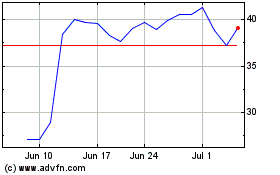
Avidity Biosciences (NASDAQ:RNA)
Historical Stock Chart
From May 2023 to May 2024