false000153496900015349692025-01-132025-01-13
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): January 13, 2025 |
Sera Prognostics, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-40606 |
26-1911522 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
2749 East Parleys Way Suite 200 |
|
Salt Lake City, Utah |
|
84109 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (801) 990-0520 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Class A Common Stock, $0.0001 par value per share |
|
SERA |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On January 13, 2025, Sera Prognostics, Inc. (the “Company”) will post a new investor presentation to its corporate website. Slides from this presentation are also scheduled to be shared at the 43rd Annual J.P. Morgan Healthcare Conference on January 16, 2025 at 9:45 a.m. PT. A copy of the investor presentation is filed as Exhibit 99.1 hereto and incorporated by reference herein.
The information contained in Item 7.01, including Exhibit 99.1 furnished herewith, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities under that section, nor shall it be deemed incorporated by reference into any registration statement or other document pursuant to the Securities Act of 1933, as amended, or into any filing or other document pursuant to the Exchange Act, except to the extent required by applicable law or regulation.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
SERA PROGNOSTICS, INC. |
|
|
|
|
Date: |
January 13, 2025 |
By: |
/s/ Austin Aerts |
|
|
|
Austin Aerts
Chief Financial Officer |

©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. In order to transform the experience into one with fewer uncertainties { WE AT SERA AIM TO CHANGE PREGNANCY CARE }

This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The company has no obligation to provide any updates to these forward-looking statements, even if its expectations change, whether as a result of new information, future events or otherwise, except as required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. Further information on potential factors, risks and uncertainties that could affect operating and financial results is included in the company’s Registration Statement on Form S-1, most recent Annual Report on Form 10-K, and/or subsequent Forms 10-Q, including in each case under the heading RISK FACTORS, and in the company’s other filings with the SEC. The information in this presentation should be considered in conjunction with a review of the company’s filings with the SEC including the information in the company’s Registration Statement on Form S-1, most recent Annual Report on Form 10-K, and/or subsequent Forms 10-Q, under the heading MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIALCONDITION AND RESULTS OF OPERATIONS. Safe Harbor Statement

Preterm Birth is a Persistent Clinical & Economic Challenge Preterm birth contributes to of newborn deaths2 Preterm birth causes numerous medical issues requiring more hospital time and pediatric visits4,5 References: 1. Osterman MJK, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. Births: Final Data for 2022. Natl Vital Stat Rep. 2024 Apr;73(2):1-56. PMID: 38625869. 2. Callaghan WM, et al. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006 Oct;118(4):1566-73. 3. March of Dimes 2024 report,. 4. Howson CP, et al. Born Too Soon: Preterm birth matters. Reprod Health 10, S1 (2013). 5. Crump C, et al. Prevalence of Survival Without Major Comorbidities Among Adults Born Prematurely. JAMA. 2019 Oct 22;322(16):1580-1588. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. The US rate rose from �9.4% to 10.4% �2013-20233 34.2% 1 in 10 BABIES DID YOU KNOW? ARE BORN �TOO SOON1

Preterm Birth References: 1. Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2022. NCHS Data Brief 2023; (477): 1-8.. 2. Callaghan WM, et al. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006 Oct;118(4):1566-73. 3. Manuck TA, et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol. 2016;215(1):103. 4. Beam et al, Estimates of healthcare spending for preterm and low-birthweight infants in a commercially insured population: 2008-2016, Journal of Perinatology (2020) 40:1091–1099. Median hospital length of stay by gestational week (Aetna)4 Neonatal outcomes are dependent on gestational age at birth3 Major Morbidities: Persistent pulmonary hypertension; Intraventricular hemorrhage grade II/IV; Seizures; Hypoxic-ischemic encephalopathy; Necrotizing enterocolitis stage II/III; Bronchopulmonary dysplasia Minor Morbidities: Intraventricular hemorrhage grade I/II; Necrotizing enterocolitis stage I; Respiratory distress syndrome; Hyperbilirubinemia requiring treatment; Hypotension requiring treatment Prevalence Mortality 34.2% of newborn deaths are attributed to preterm birth2 Any birth before 37 weeks of gestation 10.4% in 20221 Preterm birth is a prevalent complication and a leading cause of neonatal morbidity and mortality ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

References: �1. Waitzman NJ, et al. Updating National Preterm Birth Costs to 2016 with Separate Estimates for Individual States: Final Report to the March of Dimes. Available from: marchofdimes.org/peristats/documents/Cost_of_Prematurity_2019.pdf �2. Phibbs CS, et al. Birth Hospitalization Costs and Days of Care for Mothers and Neonates in California, 2009-2011. J Pediatr. 2019 Jan; 204:118-125.e14. �3. Beam et al, Estimates of healthcare spending for preterm and low-birthweight infants in a commercially insured population: 2008-2016, Journal of Perinatology (2020) 40:1091–1099. In the U.S., the cost to manage the complications of prematurity in 2016 was on average, �$64,815 �per preterm birth1 The lifetime costs associated with preterm birth are estimated to be �10 times higher �than those associated with a full-term birth in the U.S.2 Preterm births account for �61% of neonatal costs for in-hospital deliveries2 The health impacts of preterm birth drive increased expenditures, with significant cost of care reductions as gestational age at birth increases ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Expenditure distributions stratified by gestational age3 $500 1K 5K 10K 25K 50K 100K 250K Gestational age at birth (weeks) Full Term 35-36 33-34 31-32 29-30 27-28 25-26 24 1M 2.5M 5M 500K TOTAL 6-MONTH EXPENDITURE

OF PREGNANT �WOMEN who deliver prematurely have no known risk factors1 References: 1. Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press; 2007. 2. Iams JD, et al. Prevention of Preterm Parturition N Engl J Med 2014;370:254-61. DOI: 10.1056/NEJMcp1103640 3. American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Obstetrics. Prediction and Prevention of Spontaneous Preterm Birth: ACOG Practice Bulletin, Number 234. Obstetrics Gynecol. 2021 Aug 1;138(2):e65-e90. doi: 10.1097/AOG.0000000000004479. PMID: 34293771. 50% “ ACOG defined characteristics of an effective screening program IDENTIFYING PATIENTS AT HIGHER RISK OF PRETERM BIRTH IS A CLINICAL CHALLENGE ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Importantly, an effective treatment to reduce PTB should be available, and the screening program should be feasible, cost-effective, and accessible to all patients.3 ”

Given the limitations of current screening practices for pregnancy complications, scientists at Sera discovered a biomarker risk predictor that fills a critical diagnostic gap Proteomics provide insights into the biology of each pregnancy and go beyond genetic inheritance Proteins reflect the functions of specific cells allowing for early detection and intervention before symptoms arise The answers are �provided by PROTEOMICS ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Biologically important proteins are powerful predictors of spontaneous preterm birth, PreTRM® looks at each and their interaction 1. Sitar T, et al. Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc Natl Acad Sci USA 2006 103: 13028-13033. 2. Grishkovskaya I, et al. Resolution of a disordered region at the entrance of the human sex hormone-binding globulin steroid-binding site. J Mol Biol. 2002 318: 621-626. 3. Internal data on file.

SERA’S PRETRM® TEST IS VALIDATED FOR PREDICTION DURING WEEKS 18-20 OF PREGNANCY PAPR �Validation Study1 (n=5501, 11 centers) The PreTRM® Test is highly predictive of spontaneous preterm birth with a single blood draw between 18 and 20 6/7 weeks of gestation2 Three Independent Studies (discovery, verification, validation) reported in a large, multi-center trial Published as Editor’s Choice article in American Journal of Obstetrics & Gynecology (May 2016) References �1. Saade GR, et al. development and validation of spontaneous preterm delivery predictor in asymptomatic women. Am J Obstet Gynecol. 2016;214:633e1-24. �2. Burchard, J., et al. Better Estimation of Spontaneous Preterm Birth Prediction Performance through Gestational Age Dating. J. Clin. Med. 2022, 11, 2885. doi.org/10.3390/jcm11102885 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

Results delivered in an average of five days from a CLIA-certified, CAP-accredited lab Blood sample kits are provided for testing during 180/7-206/7 weeks with prepaid shipment to Sera’s lab Sample collection can be performed by staff and aligns with preexisting appointments ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. { OUR TESTING PROCESS SEAMLESSLY INTEGRATES INTO WOMEN’S HEALTH CLINICS } PreTRM® was designed to meet ACOG’s criteria to screen women without obvious risk factors for preterm birth

KIT CONTENTS THE PRETRM® TEST The first of its kind to detect premature birth The PreTRM® Test predicts a patient’s individual risk of spontaneous preterm delivery in asymptomatic singleton pregnancies through a single blood draw performed during weeks 18 through 20 of gestational age. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

Patients identified as higher risk by the PreTRM Test are at increased risk for*: Final Communicates a patient’s risk of spontaneous preterm birth & helps physicians engage with patients TEST REPORT Spontaneous preterm birth Severe adverse neonatal outcomes Longer neonatal hospital length of stay Reference: Burchard J, et al. Clinical Validation of a Proteomic Biomarker Threshold for Increased Risk of Spontaneous Preterm Birth and Associated Clinical Outcomes: A Replication Study. J. Clin. Med. 2021, 10, 5088. doi: 10.3390/jcm10215088. Available at https://www.mdpi.com/2077-0383/10/21/5088 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

Follow Patient with Regularly �Scheduled Office Visits NO Is Patient <21 Weeks Gestation? Does Patient Meet PreTRM® Testing Criteria*? Provider Orders PreTRM® Blood Test Drawn �at 18 Weeks to 20 Weeks-6 Days Results Sent to Provider Office Within 5 Days Do Test Results Show Higher Risk �for Spontaneous Preterm Birth? NO NO Patient Presents to Prenatal Care Provider This is an example clinical protocol from a large integrated system. Sera does not give medical advice or endorse any specific protocol for any specific practice or patient. You should customize this document to fit your practice and its treatment protocols. CARE MANAGEMENT Refer patient to care management �services for weekly phone �outreach & assessment PROGESTERONE SUPPLEMENTATION Start daily vaginal progesterone 200mg TARGETED �INTERVENTION �BUNDLE LOW-DOSE �ASPIRIN Start LDA 81mg/day �until 36 Weeks YES PreTRM® identifies those who may benefit most from targeted preterm birth interventions AS A RISK STRATIFICATION BIOMARKER TEST PreTRM TESTING CRITERIA Single Fetus No History of Preterm Birth Unknown or Normal �Cervical Length Intact Membranes No Signs of Preterm Labor

Clinical Utility Validation PreTRM value has been demonstrated in multiple studies Biomarker Validation �Studies PAPR Study (2016) 5,501 patients 11 sites Historical Controlled �Trial AVERT PRETERM TRIAL (2024) 1,460 patients screened compared to 10,000 historical controls single site ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Randomized �Controlled Trial PRIME TRIAL (2025) 5,018 patients 19 sites

Neonatal outcomes after proteomic biomarker �guided intervention: The AVERT PRETERM TRIAL STUDY DESIGN Care management Daily open label vaginal progesterone 200mg Daily aspirin 81mg Screened with the �PreTRM® Test Historical �Controls High Risk Treated with �Interventions N=279 (mITT and ITT) Normal Care N=939 �(mITT and ITT) Historical �Controls N=10,000 Declined Treatment N=214 (ITT) The AVERT PRETERM Trial studied the health impacts of the PreTRM® test-and-treat strategy June 2018 — September 2020�ChristianaCare Hospital (Newark, DE) Black women comprised 26.5% of study participants, reflecting racial diversity as compared to the U.S. population The AVERT study characterized the benefits of the PreTRM test-and-treat strategy. Reference: Hoffman MK, Kitto C, Zhang Z, Shi J, Walker MG, Shahbaba B, Ruhstaller K. Neonatal Outcomes after Maternal Biomarker-Guided Preterm Birth Intervention: The AVERT PRETERM Trial. Diagnostics. 2024; 14(14):1462. https://doi.org/10.3390/diagnostics14141462 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Low Risk

AVERT STUDY RESULTS PreTRM helped reduce the length of time a baby spent in the hospital on average by one week Reference: Hoffman MK, Kitto C, Zhang Z, Shi J, Walker MG, Shahbaba B, Ruhstaller K. Neonatal Outcomes after Maternal Biomarker-Guided Preterm Birth Intervention: The AVERT PRETERM Trial. Diagnostics. 2024; 14(14):1462. https://doi.org/10.3390/diagnostics14141462 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Reduction in neonatal �hospital length of stay 7 Days Length of stay (days) Neonatal length of hospital stay (days)�for babies with the longest hospital stays

0-4 score given (4 = infant mortality) The score increases by 1 point �for each additional diagnosis of: Respiratory distress syndrome Bronchopulmonary dysplasia Intraventricular hemorrhage grade III or IV All stages of necrotizing enterocolitis Periventricular leukomalacia Proven severe sepsis *Severe neonatal morbidity and mortality are defined as Neonatal Composite Morbidity & Mortality Index (NMI) ≥3 NMI scores were significantly reduced in the prospective arm vs the historical arm (OR 0.81; 95% CI 0.67-0.98; P=0.03) 18% reduction in severe neonatal morbidity �and mortality* (probability of NMI ≥ 3) AVERT STUDY RESULTS Severe morbidity and mortality rates were significantly reduced 18% Reduction NMI Scores Reference: Hoffman MK, Kitto C, Zhang Z, Shi J, Walker MG, Shahbaba B, Ruhstaller K. Neonatal Outcomes after Maternal Biomarker-Guided Preterm Birth Intervention: The AVERT PRETERM Trial. Diagnostics. 2024; 14(14):1462. https://doi.org/10.3390/diagnostics14141462 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. The scale uses NICU stays to determine index scores if the length of stay gives a higher score than concomitant diagnoses: 1-4 days give a score of �1, 5-20 days a score of 2 and >20 days a score of 3.1

Mother A �without PreTRM Mother B �with PreTRM The PreTRM® Test is the first step to reduce the harms of preterm birth Medical Management Gestational Age at Birth Outcomes Time Spent in Hospital Standard care 30 0/7 weeks Necrotizing enterocolitis stage II/III Respiratory distress syndrome1 40 days1 Weekly nurse calls Daily open label vaginal progesterone 200mg Daily aspirin 81mg 32 0/7 weeks Necrotizing enterocolitis stage I 12 days References: 1. Manuck TA, et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol. 2016;215(1):103. 2. Hoffman MK, Kitto C, Zhang Z, Shi J, Walker MG, Shahbaba B, Ruhstaller K. Neonatal Outcomes after Maternal Biomarker-Guided Preterm Birth Intervention: The AVERT PRETERM Trial. Diagnostics. 2024; 14(14):1462. https://doi.org/10.3390/diagnostics14141462 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Risk of Singleton Spontaneous Preterm Birth Perceived as low-risk Normal cervical length No history of preterm birth Higher-risk result from the PreTRM Test AVERT OUTCOMES: Increased gestational age at birth on average by 2.48 weeks for babies born before 32 weeks gestation)2 18% reduction in severe neonatal morbidity and mortality)2 An average of 28-day reduction in neonatal hospital length of stay for babies born before �32 weeks gestation)2

In the full mITT and ITT population neonates were discharged earlier from the hospital. NMI, significant in the full mITT primary analysis, remained significant in the full ITT population. NICU LOS was observed to be significantly reduced in the mITT and ITT populations. The number of patients needed to screen to reduce one NICU day was calculated to be between 3 and 4** The odds of PTB and sPTB at several gestational age cut-offs was either significantly reduced or showed a trend in the direction of benefit in both the mITT and ITT populations. AVERT Study Results The AVERT study achieved statistically significant reductions in neonatal length of stay (NNLOS)* and neonatal morbidity/mortality (NMI), its two co-primary outcomes, in the mITT population *Among those babies with the longest stays **Internal data on file NNLOS: Neonatal hospital length of stay NICU LOS: NICU length of stay GAB: Gestational age at birth NMI: Neonatal morbidity and mortality, evaluated using a composite index PTB: Preterm birth sPTB: Spontaneous preterm birth mITT: Modified intent-to-treat ITT: Intent-to-treat Reference: Hoffman MK, Kitto C, Zhang Z, Shi J, Walker MG, Shahbaba B, Ruhstaller K. Neonatal Outcomes after Maternal Biomarker-Guided Preterm Birth Intervention: The AVERT PRETERM Trial. Diagnostics. 2024; 14(14):1462. https://doi.org/10.3390/diagnostics14141462 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

A pioneering clinical trial assessing the efficacy of the PreTRM Test and preventive interventions in lowering the occurrence of adverse pregnancy outcomes ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. PRIME STUDY In 2024, Sera completed a pivotal multicenter national trial: PRIME Prematurity Risk Assessment Combined With Clinical Interventions for Improving Neonatal outcoMEs (PRIME) Achievement of a primary endpoint resulted in the early termination of the study

Care management Daily open label vaginal progesterone 200mg Daily aspirin 81mg Control Arm Full Analysis Set Not-Higher Risk Higher-Risk mITT Analysis Treated with Interventions Screened Arm The Randomized Controlled Trial Involved: 19 U.S. sites, including community practices and university-based or -associated medical centers From November 2020 – December 2023 Full Analysis mITT Analysis The mITT population includes all intention-to-treat participants: (1) for whom both co-primary outcomes are known; and (2) who have been randomized to the control arm or received a not-higher-risk test result in the prevention arm, or consented to and initiated treatment, before 24 weeks and 0 days of gestation, after receiving a higher-risk test result in the prevention arm. Enrolled individuals who were subsequently diagnosed with COVID-19 and evaluated in a hospital setting during pregnancy were also excluded from mITT analyses. Enrolled and Randomized �1:1 N=5018 Full Analysis Full Analysis: �All participants and their neonates with complete data through follow-up mITT= modified intention-to-treat Reference: Internal data on file. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. PRIME TRIAL Study design of the Prematurity Risk Assessment Combined with Clinical Interventions for Improving Neonatal Outcomes

TABLE 1 Modified intent-to-treat analysis of co-primary endpoints TABLE 2 Neonatal outcomes in the intent-to-treat population Variable Measure N Adjusted Rate (95% CI)* P NMI Odds Ratio 4468 0.75 (0.62-0.91) 0.003 NNLOS Hazard Ratio 437 0.82 (0.68-0.99) 0.044 NMI, neonatal morbidity index; NNLOS, neonatal hospital length of stay. *Controlled for parity, pre-enrollment aspirin use, and COVID-19 positivity. 'Prespecified alpha reserved for final analysis was 0.0482 post-interim analysis. 'Ordinal logistic regression analysis in entire mITT population; prevention versus control arm. An odds ratio <1 reflects lower NMI scores in the prevention arm. *Cox regression analysis in a prespecified quantile (~10%) of longest stays; control versus prevention arm. A hazard ratio <1 reflects shorter length of hospital stay in the prevention arm. Full Analysis Set of the Intent-to-Treat-Population* Variable Measure Adjusted Rate (95% CI)* P NMI Odds ratio of increased NMI 0.80 (0.66-0.96) 0.015 NNLOS Ratio of average days in hospital 0.91 (0.88-0.94) <0.001 NICULOS Ratio of average days in NICU 0.92 (0.88-0.97) 0.003 NICU Admission Odds ratio of NICU admission 0.78 (0.65-0.93) 0.006 Intent-to-Treat Population with Multiple Imputation (N=5018) NMI Odds ratio of increased NMI 0.80 (0.67-0.96) 0.019 NNLOS Ratio of average days in hospital 0.92 (0.98-0.95) <0.001 NNLOS Ratio of average days in NICU 0.94 (0.89-0.99) 0.017 NICU Admission Odds ratio of NICU admission 0.80 (0.67-0.96) 0.018 Full analysis set is the intent-to-treat population minus 4.9% with missing data. Prevention versus control; adjusted for parity, pre-enrollment aspirin use, and COVID-19 positivity. 'Odds ratio; ordinal logistic regression (NMI), logistic regression (NICU admission). 'incidence rate ratio; Poisson regression. Incidence rate ratio; zero-inflated Poisson regression. *Reductions in NICU admissions and NICULOS (time spent in the NICU) contribute independently to overall NICU day reductions across Reference: Internal data on file. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. PRIME TRIAL Excerpt from Abstract, The Pregnancy Journal: The Prematurity Risk Assessment Combined with Clinical Interventions for Improving Neonatal Outcomes

Number Needed to Screen to prevent NICU admission is a critical metric of test performance for commercialization: PreTRM® Test shows powerful results The number of patients needed to screen (NNS) to improve a clinical outcome is a convenient and widely used metric that informs both the economic and clinical impact of a screening test. The lower the NNS the better. The NNS established evidence-based tests recommended by societies can be compared to PreTRM's screen-and-treat approach References: 1. Am J Obstet Gynecol. 2016 February ; 214(2): 235–242. doi:10.1016/j.ajog.2015.09.102. 2. JAMA. 2021;326(6):531-538. doi:10.1001/jama.2021.11922. 3. Estimate based on 20% admission reduction reported in abstract of PRIME study, The Pregnancy Journal, and public information on national prevalence of NICU admission. 4. Data on File. Number Needed to Screen to Prevent NICU Admissions: TVCL + Progesterone1 Glucose Testing2 Number Needed to Screen for PreTRM to prevent 1 NICU Day4 150 50 ~4 ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. PreTRM Test3 40

Number Needed to Screen �to prevent NICU admission is a critical metric of test performance for Commercialization: PreTRM® Test shows powerful results The number of patients needed to screen (NNS) to improve a clinical outcome is a convenient and widely used metric that informs both the economic and clinical impact of a screening test Number Needed to Screen to prevent �NICU admission: widely adopted tests v PreTRM Transvaginal Ultrasound Cervical Length + Progesterone to prevent NICU Admission1 150 Demonstrated in AVERT study3 ~4 References: 1. Am J Obstet Gynecol. 2016 February ; 214(2): 235–242. doi:10.1016/j.ajog.2015.09.102. 2.JAMA. 2021;326(6):531-538. doi:10.1001/jama.2021.11922. 3. Data on file ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. The lower the NNS the better The NNS for established evidence-based tests recommended by the professional societies can be compared to PreTRM's screen-and-treat approach Glucose screening test to prevent NICU Admission2 50 Number Needed to Screen for PreTRM to prevent 1 NICU day Sera PreTRM risk screening test to prevent NICU Admission 40

Economic value of PreTRM® from a Typical Fee-for-Service Payer’s Perspective Test costs�$29,790 5 MILLION COVERED LIVES Source: Sera Economic Model leveraging inputs of costs of care from Elevance model: Burchard J, et al. Clinical and economic utility of a preterm birth predictor derived from an analysis of a large and diverse pregnancy cohort. medRxiv. 2021.09.08.21262940. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. Total cost saving ~$15M 39,733 pregnancies eligible for PreTRM® and screened 13,920 higher-risk 7,656 accept interventions Intervention costs�$9,187 Cost offsets�$53,750 10,750 NICU days reduced (NNS=3.7)

Elevance Claims Data Results Analysis of >40,000 mothers and babies within commercially insured Elevance membership Evaluated screening with PreTRM along with proactive interventions given to PreTRM-higher risk patients vs. standard care Demonstrated robust clinical and economic impacts of the PreTRM test-and-treat strategy 20% reduction in preterm birth rates $1,608 gross savings, excluding a $745 PreTRM list price per pregnant woman (amortized over all pregnancies including non-tested) 10% reduction in neonatal intensive care admissions 7% reduction in overall hospital length-of-stay 33% reduction in births <32 weeks The test-and-treat strategy is dominant with respect to cost savings across all conservative probabilistic sensitivity analyses and scenarios Economic Utility Study Published Elevance Cost-Effectiveness Model ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. References: 1. Burchard J, et al. Clinical and economic utility of a preterm birth predictor derived from an analysis of a large and diverse pregnancy cohort. medRxiv. 2021.09.08.21262940.

©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. At a 6-week post-partum appointment, I walked into the exam room and my patient greeted me with a big smile and said… “I’m so grateful for the PreTRM test!” Because after she took it, the results showed an extremely high risk for preterm labor. We worked together during the next 4 months and the outcome was a vaginal delivery, at term, with a healthy baby. My patient then told me… “I came home from the hospital with a healthy baby I can hold. My friend can only touch her baby in an incubator.” My patient then explained she’d just returned from a visit with a friend who was also pregnant—with a due date close to her own. The friend had a preterm delivery. She did not have the PreTRM test available to her and now both mom and baby were in the NICU. “I’m so glad we did the PreTRM test.” Dr. Barbi Phelps-Sandall shares a patient pregnancy story “ ”

Next Milestones Publications Commercialization: Driving Next Inflection Point Reimbursement Guidelines Regulatory Additional Evidence ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

PRIME and AVERT Publications Awareness campaigns Early adopter cadre Institutional focus Awareness Building & Evidence Dissemination OUR FOCUS Pathway to Reaching Commercial Inflection Physician �Demand & Early Payer Coverage Guidelines Inclusion Broad Payer Coverage & Reimbursement PRIME and AVERT Publications Awareness campaigns Early adopter cadre Institutional focus ACOG Bulletin 234 Engagement with SMFM leadership Development of PreTRM champions Review when evidence is sufficient for recommendation PRIME and AVERT publications Health Economics publication RWE study publication GRADE assessment of evidence ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

Source: ACOG website, expert interviews. 1Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311-1320. doi:10.1056/NEJMoa1516783 Chronic Hypertension �and Pregnancy (CHAP) Study led to an update �in ACOG guidance Study published in April 2022 in NEJM: demonstrated that utilizing a treatment threshold of 140/90 (vs. 160/105) for pregnant people with chronic hypertension provides improved outcomes ACOG recommended NIPT as an option to be “discussed and offered to all patients early in pregnancy, regardless of maternal age or baseline risk” in Oct ‘20 via practice bulletin with 87 references: 1 Level I (Harmony/Roche RCT) 23 Level II-2 27 Level II-3 27 Level III & 9 systemic revie/meta-analysis ACOG is conservative with their approach to recommending novel diagnostics ACOG recommended antenatal steroids in October 2017 with one pivotal study: SMFM presentation January 2016 by Cynthia Gyamfi-Bannerman, published in NEJM later that year1 There are examples of a pivotal RCT leading to a speedy ACOG practice advisory announcement...and then others that required more time & evidence to change guidelines. ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States. HERE ARE (3) CASE STUDIES Time to commercial inflection point influenced by guideline inclusion, can be supported by continued physician awareness building & community 1 2 3

Q3 2024 earnings release highlights balance of ~$75M cash on Sera’s balance sheet Source: Sera Q3 2024 10Q. SERA’S STRONG BALANCE SHEET Sera cash position extends well into 2027 to support reaching seminal revenue inflection point ~$75M With <$30M annual operating spend, strong balance sheet allows Sera to push full steam ahead on Commercialization to reach seminal inflection point

Sera Leadership Team Zhenya Lindgardt�President & CEO • Former VP of Platform and Customer Engagement, Uber Technologies • Former Senior Partner and Managing Director, The Boston Consulting Group • Former CEO, The Commons Project Foundation • MBA, Harvard University Austin Aerts�Chief Financial Officer • Former VP, Finance and Corporate Controller, Sera • Former finance team member, Myriad • Former auditor, Ernst & Young LLP • Master of Accounting University of Utah; CPA Jay Boniface, Ph.D. Chief Scientific Officer Robert G. Harrison Chief Information Officer Benjamin Jackson General Counsel Paul Kearney, Ph.D, Chief Data Officer Michael Foley, M.D. Medical Advisor Doug Roach VP of Commercial ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

As a result of the weekly check-ins & overall care management, I was �well-informed and empowered to advocate for myself and my baby. “ ” BONNIE �Actual PreTRM® patient ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.

Educate Providers. Empower MOMS. �HELP Babies. Advance Science. �Elevate Care. Reach Maternity Deserts. Inform Payers. Evoke Change. TOGETHER, WE CAN DO BETTER ©2025 Sera Prognostics, Inc. All rights reserved. PreTRM, Sera Prognostics and their logos are trademarks or registered trademarks of Sera Prognostics, Inc. in the United States.
v3.24.4
Document And Entity Information
|
Jan. 13, 2025 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 13, 2025
|
| Entity Registrant Name |
Sera Prognostics, Inc.
|
| Entity Central Index Key |
0001534969
|
| Entity Emerging Growth Company |
true
|
| Entity File Number |
001-40606
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
26-1911522
|
| Entity Address, Address Line One |
2749 East Parleys Way
|
| Entity Address, Address Line Two |
Suite 200
|
| Entity Address, City or Town |
Salt Lake City
|
| Entity Address, State or Province |
UT
|
| Entity Address, Postal Zip Code |
84109
|
| City Area Code |
(801)
|
| Local Phone Number |
990-0520
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Ex Transition Period |
false
|
| Title of 12(b) Security |
Class A Common Stock, $0.0001 par value per share
|
| Trading Symbol |
SERA
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
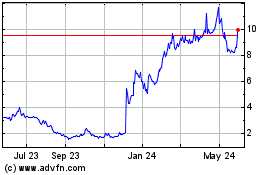
Sera Prognostics (NASDAQ:SERA)
Historical Stock Chart
From Dec 2024 to Jan 2025
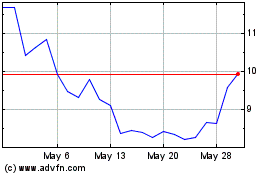
Sera Prognostics (NASDAQ:SERA)
Historical Stock Chart
From Jan 2024 to Jan 2025