TIDMAREC
RNS Number : 3775M
Arecor Therapeutics PLC
14 September 2023
Arecor Therapeutics plc
("Arecor", the "Company" or the "Group")
INTERIM RESULTS FOR THE SIX MONTHSED 30 JUNE 2023
Strong progress across proprietary portfolio and partnered
revenue-generating collaborations
Cambridge, UK, 14 September 2023: Arecor Therapeutics plc (AIM:
AREC), a globally focused biopharmaceutical company advancing
today's therapies to enable healthier lives, today announces its
interim results for the six months ended 30 June 2023.
Sarah Howell, Chief Executive Officer of Arecor, said : "We have
made further, strong progress across the business towards our
ambition to transform patient care by enhancing existing
therapeutic medicines and, in doing so, building a significant
self-sustaining biopharmaceutical company.
"Our revenues increased by 141%, compared to 1H 22, and our
belief in the growth potential of the business is reinforced by
significant progress by our partners under license, as well as
development progress across our in-house proprietary product
portfolio. We have seen significant partnering traction with the
first product incorporating the Arestat(TM) technology, AT220,
progressing towards commercialisation, positive clinical and
development advances from Inhibrx and Hikma, as well as the signing
of new revenue-generating collaborations.
"Through the remainder of 2023 and into 2024, we expect key data
from AT278, achievement of anticipated further partnering
milestones and continued commercial traction for Ogluo(R). We look
forward, with confidence, to further material progress in the near-
and medium-term towards our long-term ambitions for the Group."
Operational highlights (including post period events)
-- AT278 - Second Phase I clinical trial of ultra-rapid acting,
ultra-concentrated insulin in people with Type 2 diabetes ongoing,
with good progress in recruitment following first patient dosing in
March
-- AT247 - Phase I clinical data for ultra-rapid acting insulin
delivered by continuous subcutaneous infusion over three days via
an insulin pump, reinforcing potential to enable a fully closed
loop artificial pancreas system, presented at American Diabetes
Association (ADA) 83rd Scientific Sessions meeting in June
-- AT307 - the specialty hospital, ready-to-use injectable
medicine, transferred to Hikma in January, and which triggered a
milestone payment to the Group in the period; achieved a recent
positive meeting with Food and Drug Administration confirming
abbreviated 505(b)(2) regulatory pathway
-- Initiation of Inhibrx' registration-enabling clinical trial of AT292 (INBRX-101)
-- Regulatory approval by partner expected in coming months for
first product incorporating Arestat(TM) technology, AT220
-- Three additional revenue generating technology partnerships
entered into with new and existing pharmaceutical and biotech
partners, bringing the total number of new partnerships signed
since IPO to 11
-- Continued sales growth of Ogluo(R) with roll-out across
additional key European territories, adding Denmark, Norway and
Austria to existing markets of Germany and the UK; exclusive
commercialisation agreement signed with Goodlife in the BeNeLux
region
-- Strengthening of robust IP portfolio with key patents granted
in US, Europe, China and India protecting proprietary diabetes
portfolio, enhanced monoclonal antibody platform and high value
biologics formulations
-- Appointment of Dr. Manjit Rahelu as Chief Business Officer
Financial highlights
-- Revenue of GBP1.7 million increased by 141% (H1 2022: GBP0.7 million)
-- Total income of GBP2.3 million, increased by 103% (H1 2022: GBP1.1 million)
-- Investment in R&D of GBP2.9 million (H1 2022: GBP4.8 million)
-- Loss after tax for the period of GBP4.5 million (H1 2022: GBP4.4 million)
-- Cash, cash equivalents and short-term investments of GBP8.2
million at 30 June 2023 (30 June 2022: GBP13.7 million)
-- Post period: R&D tax credit of GBP1.3 million received on 3 August 2023
Analyst conference call today
Dr Sarah Howell, Chief Executive Officer, and Susan Lowther,
Chief Financial Officer, will host a meeting and webcast for
analysts and investors at 11.00 am UK time today. Join the webcast
here . A copy of the interim results presentation will be released
later this morning on the Company website at www.arecor.com. Please
contact Consilium Strategic Communications for details on
arecor@consilium-comms.com / +44 203709 5700.
For more information, please contact:
Arecor Therapeutics plc www.arecor.com
Dr Sarah Howell, Chief Executive Tel: +44 (0) 1223 426060
Officer Email: info@arecor.com
Susan Lowther, Chief Financial Officer Tel: +44 (0) 1223 426060
Email: info@arecor.com
Mo Noonan, Communications Tel: +44 (0) 7876 444977
Email: mo.noonan@arecor.com
Panmure Gordon (UK) Limited (NOMAD
and Broker)
Freddy Crossley, Emma Earl (Corporate Tel: +44 (0) 20 7886 2500
Finance)
Rupert Dearden (Corporate Broking)
Consilium Strategic Communications
Chris Gardner, David Daley, Lindsey Tel: +44 (0) 20 3709 5700
Neville Email: arecor@consilium-comms.com
Notes to Editors
About Arecor
Arecor Therapeutics plc is a globally focused biopharmaceutical
group transforming patient care by bringing innovative medicines to
market through the enhancement of existing therapeutic products. By
applying our innovative proprietary formulation technology
platform, Arestat(TM) , we are developing an internal portfolio of
proprietary products in diabetes and other indications, as well as
working with leading pharmaceutical and biotechnology companies to
deliver enhanced formulations of their therapeutic products. The
Arestat (TM) platform is supported by an extensive patent portfolio
. For further details please see our website, www.arecor.com
This announcement contains inside information for the purposes
of the retained UK version of the EU Market Abuse Regulation (EU)
596/2014 ("UK MAR").
Corporate overview
Arecor's ambition is to transform patient care by enhancing
existing therapeutic medicines and, in doing so, build a
significant self-sustaining biopharmaceutical company. We have
continued to make significant progress towards this goal across all
areas of the business during the first half of 2023. We have
further built the foundations for future growth through our
portfolio of proprietary and partnered programmes which is
progressing strongly, and our commercial subsidiary, Tetris Pharma,
with its scalable sales, marketing and distribution platform.
Revenues have increased by 141% versus H1 2022. Trading for the
full year remains in line with the Board's expectations subject to
continued sales momentum and partnership milestones anticipated in
the remainder of 2023.
We continue to deliver on our strategy for our lead proprietary
diabetes product candidates AT278 and AT247, generating additional
clinical data to further demonstrate their superior profiles
compared with gold standard insulins available to patients today.
There remains a very significant patient need for even faster
acting and ultra-concentrated insulins which are key unmet needs in
the pursuit of the development of a fully closed loop artificial
pancreas system, as well as enabling longer wear miniaturised
insulin delivery devices, and their use in high insulin users. In
this field, Arecor's insulins have the potential to significantly
improve healthcare outcomes for people living with diabetes. We
continue to build the value of our insulin programmes through the
development of clinical strategies and data packages which would
best realise their future potential and maximise partnering
opportunities and value in the growing diabetes market.
We have also further progressed our in-house proprietary
pipeline of speciality hospital products underpinned by our
extensive know-how and expertise in the delivery of novel
ready-to-use ("RTU") and ready-to-administer ("RTA") formulations
for highly complex point-of-care medicines. There is a growing
demand for these superior RTA and RTU products to enable fast, safe
and effective treatment options for patients and caregivers in the
hospital setting. Our proven expertise and track record, utilising
our proprietary technology platform, Arestat(TM), in developing
these difficult to achieve stable and efficacious liquid
formulation product formats allows us to bring superior products to
patients and caregivers, and presents a clear opportunity for
Arecor to negotiate high-value co-development and commercialisation
license collaborations with pharmaceutical partners.
Our three programmes which have progressed through to license -
one from our internal Specialty Hospital Products franchise and two
from our technology partnership model, where we apply the platform
to develop novel formulations of our partners proprietary products
- are advancing well under our partners' control. These
partnerships with leading biotech and biopharma companies validate
the need, and demonstrate the opportunity, for the Arestat(TM)
platform and are testament to our world-leading expertise and
innovation in formulation science.
The expansion of our pipeline of revenue-generating technology
partnership deals with leading healthcare companies further
validates the strength of our technology and its value to our
partners in the development of enhanced formulations of their
proprietary products which would otherwise be unachievable. These
partnerships, which have continued to build steadily since Arecor's
IPO, provide both near-term revenue at the pre-license stage with
significant future license upside potential.
In Tetris Pharma, the speciality pharmaceutical company we
acquired in August 2022, we have an opportunity to accelerate our
commercially driven strategy. Its lead commercial stage diabetes
product, Ogluo(R), is a ready-to-use glucagon auto-injector pen to
treat severe hypoglycaemia and meets a key patient need. The sales
momentum seen in the UK market, and progress with its pan-European
commercial roll-out, continues to support our belief in the
complementary nature of the Tetris Pharma acquisition and the
growth potential of this important product for people with
diabetes.
Operational Review (including post period events)
Internal proprietary pipeline
The Arestat(TM) enabled novel formulations of insulin within our
diabetes franchise are designed to accelerate insulin absorption
post injection, enabling more precise and effective management of
blood glucose levels for people living with diabetes, particularly
around difficult to manage mealtimes.
Recruitment of Type 2 subjects for the second Phase I clinical
study of AT278, our ultra-rapid, ultra-concentrated insulin
candidate, is progressing well. We are currently considering
increasing the number of subjects within the study from 32 to 42 to
increase the power of the study, and in turn, to increase the value
of the results for patients with high insulin needs. If
implemented, this will result in a short delay in the reporting of
results into early 2024 and we will provide more details in the
coming weeks. The randomised, double-blind Phase I cross-over study
in people with Type 2 diabetes receiving one subcutaneous dose (0.5
U/kg) of AT278, in a euglycemic clamp setting, compares the
pharmacokinetic (PK) and pharmacodynamic (PD) profile with
NovoRapid(R) and Humulin(R) R U-500. AT278 has the potential to
disrupt the market for insulin treatment as the first concentrated,
yet very rapid acting insulin, and thereby become the gold standard
insulin for the growing population of people with diabetes with
high daily insulin needs. It has the potential to be a critical
enabler in the development of next generation miniaturised insulin
delivery systems that are beginning to dominate segments of the
market.
In June, the positive results from the second Phase I clinical
trial investigating Arecor's ultra-rapid acting insulin product
candidate, AT247, when delivered by continuous subcutaneous
infusion, were shared in a poster presentation at the American
Diabetes Association (ADA) 83rd Scientific Sessions meeting. The
data clearly demonstrates faster insulin absorption than the
currently available, gold standard, rapid acting insulins,
NovoRapid(R) and Fiasp(R), reinforcing AT247's potential to enable
even more effective disease management for people with Type I
diabetes. The availability of a truly ultra-rapid acting insulin is
a critical step towards a fully closed loop artificial pancreas
system, a potentially life changing treatment option for people
living with diabetes that has the potential to improve health
outcomes and reduce the significant burden of managing this chronic
disease.
Our Specialty Hospital Products franchise is developing
medicines that are administered within the hospital setting by
health care professionals, particularly during the treatment of
serious infections, cancer and emergency care. Leveraging our
Arestat(TM) technology, we are developing RTU and RTA medicines
within this franchise, which provide significant benefits at the
point of care by avoiding complex reconstitutions procedures and in
doing so enabling fast, safe and effective treatment options for
patients and caregivers. These products provide future high-value
licensing opportunities to Arecor.
Partnership agreements
The Group's three licensed programmes, under milestone and
royalty-based agreements or equivalent, have also advanced. Arecor
continues to expect the first product incorporating its Arestat(TM)
technology, AT220, to be commercialised by its partner under a
royalty-generating license agreement in a multi-billion dollar
market. Our partner has taken key regulatory steps towards
commercialisation and, while timing of launch is in our partner's
control, we anticipate first sales could come in late 2023 or the
early months of 2024.
Hikma continues to progress AT307, a RTU injectable medicine
after its milestone-triggering transfer from Arecor in January. A
positive pre-investigational new drug application (pre-IND) meeting
between Hikma and the FDA confirms development of AT307 in the US
under the FDA's abbreviated 505(b)(2) regulatory pathway. This
pathway provides companies with an abbreviated regulatory review
process when evidence of safety and clinical efficacy generated for
an originator product is deemed suitable to be relied upon in new
marketing applications. This also further validates a fundamental
assumption within our business that the abbreviated 505(b)(2)
pathway can be utilised across our specialty hospital portfolio,
where we are developing enhanced, RTU and RTA formulations of
existing therapeutic products.
Inhibrx is advancing towards dosing of the first patient in a
registration-enabling trial of AT292 (INBRX-101), an Arestat(TM)
formulated optimized recombinant human AAT-Fc fusion protein, for
treatment of patients with emphysema due to alpha-1 antitrypsin
deficiency, which would trigger a milestone to Arecor under the
terms of the license agreement. The initial read-out from the
Inhibrx ElevAATe trial is expected to occur in late 2024, and if
successful, will potentially provide the final data required for a
regulatory submission for this product.
We continue to build a strong portfolio of pre-license
technology partnerships in which our partners fully fund the
development work with the option for each partner to acquire rights
to the new proprietary formulation and associated intellectual
property under a technology licensing model. This offers the
potential for Arecor to generate significant future revenue through
associated milestone and royalty payments, or equivalent. We have
established three new revenue generating portfolio collaborations
so far in 2023 bringing the total technology partnerships with the
Group to 11 since IPO, including:
1. In February, we entered into an additional formulation study
agreement with an existing top five global pharmaceutical partner,
building on a collaboration formed in 2022, to develop improved,
stable, high concentration, liquid formulations of its proprietary
product.
2. In July, we entered into a collaboration to support the
ongoing development of a biosimilar product with a leading
biopharmaceutical company. This follows an earlier technology
partnership with the same company.
3. In August, we signed an agreement with a top 10
pharmaceutical company to develop an enhanced antibody formulation
for one of its investigational drugs.
Tetris Pharma
The European commercial roll out of key diabetes product,
Ogluo(R), continues to gain traction with growing sales in the UK,
and additional launches in Austria, Denmark and Norway during the
first half of 2023 complementing existing markets in Germany and
the UK. As planned, Ogluo(R) sales now represent a significant
proportion of total Tetris Pharma sales and, as a result of our
commercial strategies and focus, we have seen continuing growth in
the UK as our primary market. We anticipate this momentum in
Ogluo(R) sales to continue through the remainder of 2023 and
beyond.
The team at Tetris Pharma is focused on accelerating market
adoption of Ogluo(R) to maximise its value in launched countries.
Earlier this month, Tetris Pharma established an exclusive
commercialisation agreement with Goodlife, who will act as sole
partner for the import, marketing and distribution of Ogluo(R) in
the BeNeLux region. Goodlife is expected to launch the product in
The Netherlands during H1 2024.
With Ogluo(R), Tetris Pharma is targeting market share within an
existing c.GBP100 million market across the licensed territory. The
momentum we are seeing, and the increasingly pan-European focus of
our commercial efforts, provide the Group with continued confidence
in Ogluo(R). Sales of Gvoke(R) in the US ( Ogluo(R) is sold by
Xeris Pharmaceuticals, Inc. in the US under the registered name
Gvoke(R)) also remain strong with latest quarter on quarter growth
of 36% and, while the market dynamics clearly differ, the US
experience provides further support for the Group's belief in the
growth potential of the product in the UK and Europe.
Intellectual Property portfolio
Arecor's broad and robust global patent portfolio has >75
granted patents across key territories protecting both the
Arestat(TM) technology platform as well as the enhanced versions of
therapeutic medicines that we develop leveraging Arestat(TM). To
date, during 2023, the portfolio was bolstered with the addition of
five key patents:
-- In February, the Indian Patent Office granted a patent
(IN412485) protecting novel formulations of the Group's proprietary
insulin products, AT247 and AT278, until 2038.
-- In February, the United States Patent and Trademark Office
granted two patents (US11534402 and US11534403) protecting the
novel formulations of high-concentration adalimumab until 2038.
-- In June, the European Patent Office granted a key patent
(EP3518892), protecting novel formulations of AT278 and AT247.
-- In June, the China National Intellectual Property
Administration granted a further patent (CN110582285) protecting
AT278 and AT247.
Finance
The consolidated financial results for the period ended 30 June
2023 reflect the performance of Arecor Therapeutics plc and its
trading subsidiaries; Arecor Limited and Tetris Pharma Ltd.
The acquisition of Tetris Pharma Ltd occurred on 4 August 2022
and therefore its results are not included in the comparatives for
the six-month period to 30 June 2022. The full year comparatives to
31 December 2022 include five months of trading by Tetris Pharma
Ltd for the post-acquisition period.
Total income for the six months to 30 June 2023 of GBP2.3
million (H1 2022: GBP1.1 million) reflected increased revenue and
grant income. Revenue recognised in the period of GBP1.7 million
(H1 2022: GBP0.7 million), included sales of pharmaceutical
products of GBP1.2 million (H1 2022: Nil).
Other operating income of GBP0.6 million (H1 2022: GBP0.4
million) was grant income received from a GBP2.8 million grant
awarded by Innovate UK in March 2021.
Investment in R&D of GBP2.9 million (H1 2022: GBP4.8
million) was lower than the prior period and reflected the costs of
the current clinical trial for AT278. R&D expenditure in the
period ended 30(th) June 2022 included the US Phase I clinical
trial for AT247 and the second clinical study for AT278 which was
initiated in the first quarter of 2023.
Sales, General and Administrative costs of GBP4.4 million (H1
2022: GBP1.6 million) increased over the prior period and included
expenditure on product sales and distribution by Tetris Pharma Ltd
which was nil in the six months to 30 June 2022. On a like for like
basis the SG&A costs for the period excluding Tetris Pharma Ltd
were GBP1.9 million (H1 2022: GBP1.6 million)
The total loss after tax for the six-month period was GBP4.5
million (H1 2022: GBP4.4 million).
The Group ended the first half of the year with cash, cash
equivalents and short-term investments of GBP8.2 million (30 June
2022: GBP13.7 million).
We continue to manage cash carefully as part of our working
capital. There were two notable post period end receipts; GBP0.4
million grant receivable in early July (2022: GBP0.1 million) for
costs incurred in H1 and GBP1.3 million R&D tax credit for the
year ended 31 December 2022 (2022: GBP0.8 million) which was
received in early August. The increased R&D tax credit was due
to the level of R&D expenditure in the year ended 31 December
2022 which included costs of the US AT247 clinical study and AT278
preparatory work for the study initiated in 2023.
Contingent consideration
The acquisition of Tetris Pharma Ltd included up to a further
GBP4.0 million contingent consideration which may become payable,
consisting of three earn out payments, subject to Tetris Pharma Ltd
achieving sales and EBITDA targets in each of the three years
following completion.
The additional consideration is considered to be contingent on
future performance which is uncertain and therefore was not
included in the assessment of goodwill in the audited financial
statements for the year ended 31 December 2022.
The first earn out payment was subject to Tetris Pharma Ltd
achieving mid-single-digit million-pound net sales and a low
single-digit million-pound EBITDA loss in the 12-month period
following completion.
Earn out accounts, prepared by an independent accountant, have
determined that the first earn out target was not achieved and
therefore a payment of GBP1.0 million contingent consideration for
the first earn out period was not triggered.
Summary and outlook
We have seen further substantial progress across our in-house
proprietary products pipeline, partnered portfolio and commercial
operations during the period as we build a broad, robust platform
from which to become a significant self-sustaining
biopharmaceutical company.
We are pleased to report that revenue recognised in H1 has
materially increased compared to the comparative period to 30 June
2022. Trading for the full year remains in line with the Board's
expectations subject to continued sales momentum and partnership
milestones anticipated in the remainder of 2023.
Revenue in the period included milestone and sales of
pharmaceutical products in addition to formulation development
revenue reported in the comparative period to 30 June 2022. This
gives a broad revenue mix without a dependency on a single product
or revenue stream.
As our partnered programmes progress we will recognise further
milestone revenue and with the expected commercial launch of AT220,
annually recurring royalties which would bring a solid revenue
foundation and more clarity to forward looking forecasts. Our
partnered programmes are under the direction of our partners and
the timing of revenue recognition is outside of our direct control
which can result in recognition occurring in different financial
periods compared to our expectations.
We anticipate a number of further milestones from these existing
partnerships through the remainder of the year and beyond. Whilst
we continue to actively engage with our partners to understand
their plans, our focus is on delivering consistent year on year
revenue growth.
With the expected commercial launch of the first product
incorporating the Arestat(TM) technology, AT220, additional
milestones from existing partnerships, and the signing of new
revenue-generating agreements anticipated through the remainder of
2023 and into 2024, as well as key data from AT278 and continued
commercial traction for Ogluo(R) , we look forward with confidence
to further material progress in the near- and medium-term towards
our long-term ambitions for the Group.
Arecor Therapeutics plc
INTERIM RESULTS FOR THE SIX MONTHSED 30 JUNE 2023
Consolidated Statement of Comprehensive Income
Notes Period ended Period Year ended
30 June 2023 ended 30 31 December
June 2022 2022
Unaudited Unaudited Audited
GBP000 GBP000 GBP000
Revenue 3 1,669 693 2,403
Other operating income 609 429 1,132
--------------- ------------ --------------
Total Income 2,278 1,122 3,535
--------------- ------------ --------------
Research and Development (2,858) (4,763) (8,613)
Sales, General and Administrative 4 (4,375) (1,587) (5,552)
Operating loss (4,955) (5,228) (10,630)
--------------- ------------ --------------
Finance income 164 3 109
Finance expense 6 (10) (9) (21)
Loss before tax (4,801) (5,234) (10,542)
--------------- ------------ --------------
Taxation 7 273 867 1,282
Loss for the period (4,528) (4,367) (9,260)
=============== ============ ==============
Basic and diluted loss per
share (GBP) 8 (0.15) (0.16) (0.32)
There were no other items of comprehensive income during the
periods under review.
Arecor Therapeutics plc
INTERIM RESULTS FOR THE SIX MONTHSED 30 JUNE 2023
Consolidated Statement of Financial Position
Notes 30 June 30 June 2022 31 December
2023 2022
Unaudited Unaudited Audited
GBP000 GBP000 GBP000
Assets
Non-current assets
Intangible Assets 1,815 26 1,918
Goodwill 1,484 - 1,484
Property, Plant and Equipment 720 346 838
Other receivables 48 48 48
4,067 420 4,288
Current assets
Trade and other receivables 9 4,671 1,466 2,215
Inventory 1,564 68 1,131
Current tax receivable 1,598 1,642 1,325
Cash and cash equivalents 10 6,610 13,717 4,765
Short term investments 10 1,619 - 8,041
--------- ------------ -----------
16,062 16,893 17,477
Current liabilities
Trade and other payables 11 (6,254) (2,568) (3,526)
Lease liabilities (116) (127) (202)
(6,370) (2,695) (3,728)
Non-current liabilities
Lease liabilities (51) (42) (86)
Deferred tax (496) - (496)
(547) (42) (582)
Net Assets 13,212 14,576 17,455
========= ============ ===========
Equity
Share capital 12 306 278 306
Share premium account 28,976 23,348 28,976
Share-based payment reserve 1,143 912 893
Other reserves 11,455 11,455 11,455
Merger relief reserve 2,014 - 2,014
Foreign exchange reserve 14 - (8)
Retained earnings (30,696) (21,417) (26,181)
--------- ------------ -----------
Shareholder's funds 13,212 14,576 17,455
Arecor Therapeutics plc
INTERIM RESULTS FOR THE SIX MONTHSED 30 JUNE 2023
Consolidated Statement of Changes in Equity
Share-based Merger Foreign
Share Share payment relief Other exchange Retained Total
capital premium reserve reserve reserves reserve losses equity
GBP000 GBP000 GBP000 GBP000 GBP000 GBP000 GBP000 GBP000
For the period ended
30
June 2022
Balance at 1 January
2022 278 23,348 519 - 11,455 - (17,051) 18,549
Loss for the period - - - - - - (4,367) (4,367)
Total comprehensive
loss
for the period - - - - - - (4,367) (4,367)
Transactions with
owners:
Share-based
compensation - - 393 - - - - 393
----------------------- ---------- ---------- ------------ --------- ---------- ---------- --------- ---------
Total transactions
with
owners - - 393 - - - (4,367) (3,974)
Balance at 30 June
2022
(Unaudited) 278 23,348 912 - 11,455 - (21,417) 14,576
For the period ended
31
December 2022
Balance at 1 July 2022 278 23,348 912 - 11,455 - (21,417) 14,576
Loss for the period (4,894) (4,894)
Total comprehensive
loss
for the period (4,894) (4,894)
Transactions with
owners
Share-based
compensation 111 111
Issue of shares on
acquisition
of Tetris Pharma Ltd 7 2,014 2,021
Issue of shares for
working
capital purposes 20 5,980 6,000
Share Issue expense (352) (352)
Issue of shares on
exercise
of share options 1 1
Reserve Transfer (130) 130 -
Foreign Exchange
movements (8) (8)
----------------------- ---------- ---------- ------------ --------- ---------- ---------- --------- ---------
Total transactions
with
owners 28 5,628 (19) 2,014 - (8) 130 7,773
Balance at 31 December
2022 (audited) 306 28,976 893 2,014 11,455 (8) (26,181) 17,455
Arecor Therapeutics plc
INTERIM RESULTS FOR THE SIX MONTHSED 30 JUNE 2023
Consolidated Statement of Changes in Equity (continued)
Share-based Merger Foreign
Share Share payment relief Other exchange Retained Total
capital premium reserve reserve reserves reserve losses equity
GBP000 GBP000 GBP000 GBP000 GBP000 GBP000 GBP000 GBP000
For the period ended
30
June 2023
Balance at 1 January
2023 306 28,976 893 2,014 11,455 (8) (26,181) 17,455
Loss for the period - - - - - - (4,528) (4,528)
Total comprehensive
loss
for the period - - - - - - (4,528) (4,528)
Transactions with
owners:
Share-based
compensation - - 263 - - - - 263
Reserve Transfer - - (13) - - - 13 -
Foreign Exchange
movements - - - - - 22 - 22
----------------------- ---------- ---------- ------------ --------- ---------- ---------- --------- ---------
Total transactions
with
owners - - 250 - - 22 (4,515) 7,773
Balance at 30 June
2023
(unaudited) 306 28,976 1,143 2,014 11,455 14 (30,696) 13,212
Arecor Therapeutics plc
INTERIM RESULTS FOR THE SIX MONTHSED 30 JUNE 2023
Consolidated Statement of Cash Flows
Period ended 30 June 2023 Period ended 30 June 2022 Year ended 31 December 2022
Unaudited Unaudited Audited
GBP000 GBP000 GBP000
Cash flow from operating
activities
Loss before tax (4,801) (5,234) (10,542)
Finance income (164) (3) (109)
Finance costs 10 9 21
Share-based compensation 263 393 503
Depreciation 198 85 248
Amortisation 103 4 93
Foreign exchange movements 132 76 (69)
-------------------------- -------------------------- ----------------------------
(4,259) (4,670) (9,855)
Changes in working capital
(Increase)/ decrease in
inventory (433) (68) 587
(Increase)/ decrease in trade
and other receivables (2,456) (43) (48)
Increase/(decrease) in trade and
other payables 2,728 427 (2,198)
Tax received - - 734
-------------------------- -------------------------- ----------------------------
(161) 316 (925)
Net cash used in operating
activities (4,420) (4,354) (10,780)
Cash flow from investing
activities
Acquisition of subsidiary net of
cash acquired - - 284
Purchase of property, plant &
equipment (73) (100) (299)
Purchase of intangible assets - - (46)
Short term investments 6,422 - (8,041)
Interest received 164 3 109
Net cash used in investing
activities 6,513 (97) (7,993)
Cash flow from financing
activities
Issue of ordinary shares - - 6,000
Share issue costs - - (352)
Capital payments on lease
liabilities (114) (63) (165)
Interest paid on lease
liabilities (10) (9) (21)
Repayment of working capital
facility - - (295)
Other interest paid - - (7)
Net cash (used in) / generated
by financing activities (124) (72) 5,160
Net (decrease) / increase in
cash and cash equivalents 1,969 (4,523) (13,613)
Exchange (losses) / gains on
cash and cash equivalents (124) (76) 62
Cash and cash equivalents at
beginning of period or
financial year 4,765 18,316 18,316
Cash and cash equivalents at end
of period or financial year 6,610 13,717 4,765
========================== ========================== ============================
Arecor Therapeutics plc
INTERIM RESULTS FOR THE SIX MONTHSED 30 JUNE 2023
Notes to the financial information
COMPANY INFORMATION
Arecor Therapeutics plc ("Arecor" or the "Company") is a public
limited company registered in England and Wales at Chesterford
Research Park, Little Chesterford, Saffron Walden, CB10 1XL with
registered number 13331147.
The principal activity of the Company is to act as a holding
company. The Group has two wholly owned trading subsidiaries;
Arecor Limited and Tetris Pharma Ltd.
Tetris Pharma Ltd and its wholly owned subsidiary Tetris Pharma
B.V were acquired on 4th August 2022.
1. BASIS OF PREPARATION
The financial statements for the period ended 30 June 2023
incorporate the results of Arecor Therapeutics plc and its trading
subsidiaries. The consolidated interim financial statements for the
period to 30 June 2023 are unaudited and were approved by the board
of directors on 13 September 2023.
The consolidated interim financial statements have been prepared
in accordance with UK-adopted International Accounting Standards
("IFRS") in conformity with the requirements of the Companies Act
2006. The financial information has been prepared on the basis of
IFRS that the Directors expect to be applicable at 31 December
2023.
The financial information contained in these interim financial
statements does not constitute statutory accounts as defined in
section 434 of the Companies Act 2006. These interim financial
statements do not include all of the information and disclosures
required in the annual financial statements. The financial
information for the six months ended 30 June 2023 and 30 June 2022
is unaudited.
Financial statements for year ended 31 December 2022 have been
filed with the Registrar of Companies for Arecor Therapeutics plc
(Company registration number 13331147). The audit report for this
period, previously filed, was unmodified.
All intra-Group transactions, balances, income and expenses have
been eliminated in full on consolidation.
Tetris Pharma Ltd was acquired by Arecor Therapeutics plc on
4(th) August 2022 and is therefore not included in the comparatives
for the six-month period to 30 June 2022. The full year
comparatives to 31 December 2022 contain five months of trading by
Tetris Pharma Ltd for the post-acquisition period.
The financial information is presented in Sterling, which is the
functional currency of the Group and has been rounded to the
nearest GBP000.
2. PRINCIPAL ACCOUNTING POLICIES
The interim financial statements have been prepared in
accordance with the accounting policies set out in the audited
financial statements for the period ended 31 December 2022 and
IFRS. There have been no changes to the accounting policies or the
application of the accounting standards during the period of
review.
a) Going Concern
The Directors have reviewed current cash and short- term
investments together with forecast receivables to support forecast
operating expenditure and planned investment in R&D.
Sensitivities included the impact of reduced receivables and
mitigating actions. The review indicated that in potential downside
scenarios, cash flow forecasts extended to a period beyond 12
months from the date of approval of the consolidated interim
results.
In reaching their decision to prepare these unaudited interim
financial statements on a going concern basis, the Directors have a
reasonable expectation that the Group has adequate resources to
continue in operational existence for the foreseeable future.
Accordingly, they continue to adopt the going concern basis in
preparing these unaudited interim financial statements.
3. REVENUE AND OPERATING SEGMENTS
Year ended
Period ended Period ended 31 December
30 June 2023 30 June 2022 2022
UK 1,190 101 1,136
Switzerland 77 33 240
Rest of Europe 124 22 108
USA 248 492 784
India 30 135
Rest of World - 45 -
Total revenue 1,669 693 2,403
Operating segments are reported in a manner consistent with the
internal reporting provided to the chief operating decision makers.
Information reported includes revenue by project, expenditure by
type and department, cashflows and EBITDA for the Group.
The Board of Directors has been identified as the chief
operating decision makers, who are responsible for allocating
resources, assessing the performance of the operating segment and
making strategic decisions. Accordingly, the Directors consider
there to be a single operating segment.
Year ended
Period ended Period ended 31 December
30 June 2023 30 June 2022 2022
Formulation development projects 342 693 1,352
Milestones from licence agreements 108 - -
Sale of pharmaceuticals 1,219 - 1,051
Total revenue 1,669 693 2,403
Revenue from formulation development projects has been
recognised as the performance obligations set out in agreements are
satisfied over time.
Revenue from Milestones defined in license agreements has been
recognised when a milestone is achieved.
Sales of pharmaceuticals are product sales which have been
recognised as the rights and obligations pertaining to those items
are transferred to the buyer.
4. SALES, GENERAL AND ADMINISTRATIVE COSTS
Operating expenditure which is not considered as Research and
Development is treated as Sales, General and Administrative costs.
This includes Finance, HR, Administrative and sales and marketing
and Business Development teams, building facilities, sale of
pharmaceutical products and costs relating to the Board of
Directors.
5. SHARE BASED COMPENSATION
The Company operates an All-Employee Share Option Plan (AESOP)
and grants share options to eligible employees. The options vest
over time.
The Company's Long Term Incentive Plan (LTIP) is principally
used to grant options to Executive directors and senior management.
The LTIP options vest after three years subject to meeting
performance criteria as defined in the option agreement. These can
be a combination of both operational objectives and share price
performance compared to a benchmark. These performance conditions
are approved by the Board on each occasion prior to the grant of
the options. Ordinary shares acquired on exercise of the LTIP
options are subject to a holding period of a minimum of one year
from the date of vesting.
The movement in share options in the period was as follows:
Number of Options
Balance at 31 December 2021 1,414,944
Options lapsed (13,497)
Balance at 30 June 2022 1,401,447
AESOP options granted 312,750
LTIP options granted 270,000
AESOP options exercised (131,433)
Options lapsed (224,961)
Balance at 31 December 2022 1,627,803
AESOP options granted 86,250
LTIP options granted 190,000
AESOP options exercised (7,471)
Options lapsed (235,167)
Balance at 30 June 2023 1,661,415
Shared Based Payment charges to the Statement GBP000
of Comprehensive Income
Period to June 2023 263
Period to June 2022 393
Year to December 2022 504
6. FINANCE EXPENSES
In the period ended 30 June 2023, the finance expenses of
GBP10,000 were interest costs on finance leases (period ended 30
June 2022: GBP9,000).
7. TAXATION
Year ended
Period ended Period ended 31 December
30 June 2023 30 June 2022 2022
R&D Tax credit receivable 273 867 1,282
Total taxation 273 867 1,282
On 1 April 2023 the UK Governments rates of tax relief for loss
making SME R&D tax credits decreased from 14.5% to 10%. On the
same date, the tax relief for the RDEC scheme increased from 13% to
20%. The Group utilises both schemes and has calculated the balance
receivable based on the applicable rates for expenditure incurred
before and after the date of transition.
8. EARNINGS PER SHARE
Basic earnings per share is calculated by dividing the loss
attributable to ordinary shareholders by the
weighted average number of ordinary shares outstanding during
the period.
Given the Company's reported loss for the periods and financial
year, share options were not taken into account when determining
the weighted average number of ordinary shares in issue during the
year as they would be anti-dilutive, and therefore the basic and
diluted loss per share are the same.
Basic and diluted loss per share
Period ended Period ended Year ended
30 June 2023 30 June 31 December 2023
2022
Loss for the period (GBP000) (4,528) (4,367) (9,260)
Weighted average number of ordinary shares (number) 30,619,091 27,835,024 28,936,088
Loss per share from continuing operations (GBP per share) (0.15) (0.16) (0.32)
============== ============= ==================
9. TRADE AND OTHER RECEIVABLES
Year ended
Period ended Period ended 31 December
30 June 2023 30 June 2022 2022
Trade receivables 3,688 157 664
Other receivables 175 273 273
Grant receivables 423 83 562
Prepayments 385 953 716
Total Trade and other receivables 4,671 1,466 2,215
The growth in Trade receivables of GBP3.7 million reflects the
gross sales of pharmaceutical products by Tetris Pharma Ltd, which
were nil in the period ended 30 June 2022.
Trade receivables for pharmaceutical products are gross of
rebates payable to wholesalers. Rebates are reported in Trade
payables and accruals.
Grant receivables of GBP0.4 million reflect the timing of
reimbursement of expenditure incurred in the first half of the year
and an increase over the prior period grant receivable of GBP0.1
million.
10. CASH AND CASH EQUIVALENTS AND SHORT TERM INVESTMENTS
Year ended
Period ended Period ended 31 December
30 June 2023 30 June 2022 2022
Cash and cash equivalents 6,610 13,717 4,765
Short term investments 1,619 - 8,041
Total cash, cash equivalents
and short term investments 8,229 13,717 12,806
Short term investments relate to balances held in either fixed
term accounts with a six-month maturity or notice accounts with a
95 day notice period.
All significant cash, cash equivalents and short-term
investments are deposited in the UK with large international
banks.
11. TRADE AND OTHER PAYABLES
Year ended
Period ended Period ended 31 December
30 June 2023 30 June 2022 2022
Trade payables 2,779 1,508 1,709
Other tax and social security 123 97 120
Other creditors 1,172 - 217
Contract liabilities 682 188 206
Accruals 1,498 775 1,274
Total Trade and other payables 6,254 2,568 3,526
The growth in Trade payables and Accruals include rebate amounts
due to wholesalers on the sales of pharmaceutical products by
Tetris Pharma Ltd, which were nil in the prior period ended 30 June
2022.
Other creditors of GBP1.2 million includes VAT payable and stock
provisions which were nil in the prior period.
12. EQUITY
Share Capital
At 30 June At 30 June At 31 December
2023 2022 2022
Number Number Number
Allotted, called up and fully
paid
Ordinary shares of GBP0.01 30,625,654 27,835,024 30,618,183
Total share capital 30,625,654 27,835,024 30,618,183
=========== =========== ===============
At 30 June At 30 June At 31 December
2023 2022 2022
GBP'000 GBP'000 GBP'000
Allotted, called up and fully
paid
Ordinary shares of GBP0.01 306 278 306
Total share capital 306 278 306
=========== =========== ===============
13. EVENTS AFTER THE BALANCE SHEET DATE
In accordance with a Sale and Purchase Agreement dated 1(st)
August 2022, the acquisition of Tetris Pharma Ltd included
contingent consideration of three earn out payments, which may
become payable on the first, second and third anniversary following
completion.
The first earn out payment was subject to Tetris Pharma Ltd
achieving mid-single-digit million-pound net sales and a low
single-digit million-pound EBITDA loss in the 12-month period
following completion.
Earn out accounts were prepared by an independent accountant and
have been provided to the previous shareholders of Tetris Pharma
Ltd. The earn out accounts determined that the first earn out
target was not achieved and therefore contingent consideration of
GBP1,000,000 for the first earn out period was not triggered.
14. COPIES OF THE INTERIM REPORT
Copies of the consolidated interim financial statements are
available to the public free of charge from the Company at
Chesterford Research Park, Little Chesterford, Saffron Walden, CB10
1 XL during normal business hours for 14 days from today.
Copies are also available on the Company's website at
www.arecor.com.
This information is provided by RNS, the news service of the
London Stock Exchange. RNS is approved by the Financial Conduct
Authority to act as a Primary Information Provider in the United
Kingdom. Terms and conditions relating to the use and distribution
of this information may apply. For further information, please
contact rns@lseg.com or visit www.rns.com.
RNS may use your IP address to confirm compliance with the terms
and conditions, to analyse how you engage with the information
contained in this communication, and to share such analysis on an
anonymised basis with others as part of our commercial services.
For further information about how RNS and the London Stock Exchange
use the personal data you provide us, please see our Privacy
Policy.
END
IR EANNDFEPDEFA
(END) Dow Jones Newswires
September 14, 2023 02:00 ET (06:00 GMT)
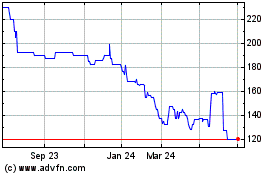
Arecor Therapeutics (LSE:AREC)
Historical Stock Chart
From Apr 2024 to May 2024
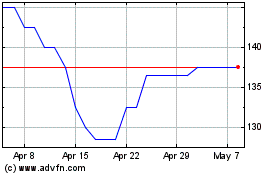
Arecor Therapeutics (LSE:AREC)
Historical Stock Chart
From May 2023 to May 2024