Form 6-K - Report of foreign issuer [Rules 13a-16 and 15d-16]
18 October 2024 - 7:45AM
Edgar (US Regulatory)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of October 2024
Commission File Number: 001-39032
PROFOUND MEDICAL CORP.
(Translation of registrant's name into English)
2400 Skymark Avenue, Unit 6, Mississauga, Ontario L4W 5K5
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F [ ] Form 40-F [ X ]
EXHIBIT INDEX
The following document is attached as an exhibit hereto and is incorporated by reference herein:
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | PROFOUND MEDICAL CORP. |
| | | (Registrant) |
| | | |
| | | |
| Date: October 17, 2024 | | /s/ Rashed Dewan |
| | | Rashed Dewan |
| | | Chief Financial Officer |
| | | |
EXHIBIT 99.1
Profound Medical to Release Third Quarter 2024 Financial Results on November 7 – Conference Call to Follow
TORONTO, Oct. 17, 2024 (GLOBE NEWSWIRE) -- Profound Medical Corp. (NASDAQ:PROF; TSX:PRN) (“Profound” or the “Company”), a commercial-stage medical device company that develops and markets customizable, incision-free therapies for the ablation of diseased tissue, will announce its third quarter 2024 financial results after market close on Thursday, November 7, 2024.
Profound management will host a conference call at 4:30 p.m. ET to review the financial results and discuss business developments in the period.
Third Quarter 2024 Results Conference Call Details:
| Date: | | Thursday, November 7, 2024 |
| | | |
| Time: | | 4:30 p.m. ET |
| | | |
| Live Call Registration: | | https://register.vevent.com/register/BI0b43a811a8b64fc1a7f58af602ccc933 |
The call will also be broadcast live and archived on the Company's website at www.profoundmedical.com under "Webcasts" in the Investors section.
About Profound Medical Corp.
Profound is a commercial-stage medical device company that develops and markets customizable, incision-free therapies for the ablation of diseased tissue.
Profound is commercializing TULSA-PRO®, a technology that combines real-time MRI, robotically-driven transurethral ultrasound and closed-loop temperature feedback control. The TULSA procedure, performed using the TULSA-PRO® system, has the potential of becoming a mainstream treatment modality across the entire prostate disease spectrum; ranging from low-, intermediate-, or high-risk prostate cancer; to hybrid patients suffering from both prostate cancer and benign prostatic hyperplasia (“BPH”); to men with BPH only; and also, to patients requiring salvage therapy for radio-recurrent localized prostate cancer. TULSA employs real-time MR guidance for pixel-by-pixel precision to preserve prostate disease patients’ urinary continence and sexual function, while killing the targeted prostate tissue via a precise sound absorption technology that gently heats it to kill temperature (55-57°C). TULSA is an incision- and radiation-free “one-and-done” procedure performed in a single session that takes a few hours. Virtually all prostate shapes and sizes can be safely, effectively, and efficiently treated with TULSA. There is no bleeding associated with the procedure; no hospital stay is required; and most TULSA patients report quick recovery to their normal routine. TULSA-PRO® is CE marked, Health Canada approved, and 510(k) cleared by the U.S. Food and Drug Administration (“FDA”).
Profound is also commercializing Sonalleve®, an innovative therapeutic platform that is CE marked for the treatment of uterine fibroids and palliative pain treatment of bone metastases. Sonalleve® has also been approved by the China National Medical Products Administration for the non-invasive treatment of uterine fibroids and has FDA approval under a Humanitarian Device Exemption for the treatment of osteoid osteoma. The Company is in the early stages of exploring additional potential treatment markets for Sonalleve® where the technology has been shown to have clinical application, such as non-invasive ablation of abdominal cancers and hyperthermia for cancer therapy.
For further information, please contact:
Stephen Kilmer
Investor Relations
skilmer@profoundmedical.com
T: 647.872.4849
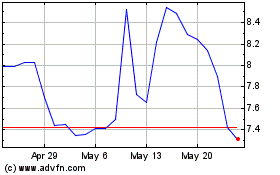
Profound Medical (NASDAQ:PROF)
Historical Stock Chart
From Oct 2024 to Nov 2024
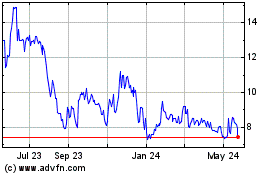
Profound Medical (NASDAQ:PROF)
Historical Stock Chart
From Nov 2023 to Nov 2024