Profound Medical to Release Third Quarter 2024 Financial Results on November 7 – Conference Call to Follow
18 October 2024 - 7:30AM

Profound Medical Corp. (NASDAQ:PROF; TSX:PRN) (“Profound” or the
“Company”), a commercial-stage medical device company that develops
and markets customizable, incision-free therapies for the ablation
of diseased tissue, will announce its third quarter 2024 financial
results after market close on Thursday, November 7, 2024.
Profound management will host a conference call
at 4:30 p.m. ET to review the financial results and discuss
business developments in the period.
Third Quarter 2024 Results Conference
Call Details:
| Date: |
|
Thursday, November 7, 2024 |
| |
|
|
| Time: |
|
4:30 p.m. ET |
| |
|
|
| Live Call Registration: |
|
https://register.vevent.com/register/BI0b43a811a8b64fc1a7f58af602ccc933 |
The call will also be broadcast live and
archived on the Company's website at www.profoundmedical.com under
"Webcasts" in the Investors section.
About Profound Medical
Corp.
Profound is a commercial-stage medical device
company that develops and markets customizable, incision-free
therapies for the ablation of diseased tissue.
Profound is commercializing TULSA-PRO®, a
technology that combines real-time MRI, robotically-driven
transurethral ultrasound and closed-loop temperature feedback
control. The TULSA procedure, performed using the TULSA-PRO®
system, has the potential of becoming a mainstream treatment
modality across the entire prostate disease spectrum; ranging from
low-, intermediate-, or high-risk prostate cancer; to hybrid
patients suffering from both prostate cancer and benign prostatic
hyperplasia (“BPH”); to men with BPH only; and also, to patients
requiring salvage therapy for radio-recurrent localized prostate
cancer. TULSA employs real-time MR guidance for pixel-by-pixel
precision to preserve prostate disease patients’ urinary continence
and sexual function, while killing the targeted prostate tissue via
a precise sound absorption technology that gently heats it to kill
temperature (55-57°C). TULSA is an incision- and radiation-free
“one-and-done” procedure performed in a single session that takes a
few hours. Virtually all prostate shapes and sizes can be safely,
effectively, and efficiently treated with TULSA. There is no
bleeding associated with the procedure; no hospital stay is
required; and most TULSA patients report quick recovery to their
normal routine. TULSA-PRO® is CE marked, Health Canada approved,
and 510(k) cleared by the U.S. Food and Drug Administration
(“FDA”).
Profound is also commercializing Sonalleve®, an
innovative therapeutic platform that is CE marked for the treatment
of uterine fibroids and palliative pain treatment of bone
metastases. Sonalleve® has also been approved by the China National
Medical Products Administration for the non-invasive treatment of
uterine fibroids and has FDA approval under a Humanitarian Device
Exemption for the treatment of osteoid osteoma. The Company is in
the early stages of exploring additional potential treatment
markets for Sonalleve® where the technology has been shown to have
clinical application, such as non-invasive ablation of abdominal
cancers and hyperthermia for cancer therapy.
For further information, please
contact:
Stephen KilmerInvestor
Relationsskilmer@profoundmedical.com T: 647.872.4849
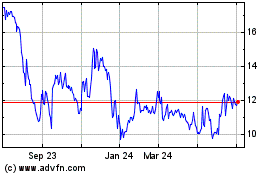
Profound Medical (TSX:PRN)
Historical Stock Chart
From Nov 2024 to Dec 2024
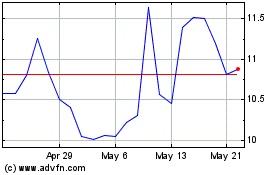
Profound Medical (TSX:PRN)
Historical Stock Chart
From Dec 2023 to Dec 2024